Abstract
Lymph node metastasis (LNM) and perineural invasion (PNI) are regarded as two important prognostic factors in pancreatic cancer. The aim of this study was to identify the LNM-associated and PNI-associated markers in pancreatic cancer. We have constructed a formalin-fixed paraffin embedded pancreatic tissue microarrays containing 324 cylindrical tissue cores of human pancreatic cancer and its paracancerous nonmalignant pancreatic specimens (NMPs) from 162 patients. Among those patients, there were 83 of 162 with PNI, and 65 of 162 with LNM. The protein products of 2 genes encompassing a variety of functional classes, such as oncogenes (c-Myc), apoptosis (Fas), were analyzed by immunohistochemistry on the tissue microarray. There was marked increase in cancer tissues cytoplasmic c-Myc expression level in pancreatic cancer compared with the NMPs (P < 0.0001). Additionally, the expression level of c-Myc was also significant increase in pancreatic cancer with PNI compared with cancer without PNI (P < 0.01). In contrast, cytoplasmic Fas, low expressed in pancreatic cancer (P < 0.0001) was negatively correlated with PNI (P < 0.05). Logistic regression analysis showed that the c-Myc expression in the cancer tissues cytoplasm acted as a potential and independently predictive factor for PNI. However, there were no significant association between the expression of these two genes and LNM (P > 0.05). This study for the first time described expression levels of c-Myc and Fas played an important role in PNI of pancreatic cancer. Combined detection can be used as predictive factors, especially c-Myc.
Keywords: Pancreatic cancer, perineural invasion, c-Myc, Fas, tissue microarrays
Introduction
Pancreatic cancer is an aggressive and devastating disease, which is characterized by invasiveness, rapid progression and profound resistance to treatment. It ranks fourth on the list of cancer-related causes of death with an average 5-year survival rate of < 5% [1,2]. Surgical resection is currently the only chance for cure. Unfortunately, most patients first diagnosed with pancreatic cancer have already metastasized. Therefore, the results of surgical resection still remain poor due to the high incidence of local recurrence and distant metastasis. Despite cancer genetics have improved our understanding of this disease, and significant progress has been made in the management, the prognosis of this disease has not improved significantly over the past decades.
Generally, there are three primary ways tumors can spread - by local extension from the tumor to the adjacent tissues, through the circulatory system to distant sites or through the lymphatic vessels to local or distant lymph nodes. Lymph node metastasis (LNM) is one of the most important prognostic factors for various cancers. However, for some cancers, PNI may be the major route of metastatic spread [3]. Pancreatic cancer is one of the very few cancers that spread along nerves (PNI). PNI is considered as an important factor of aggressive tumor behavior and it is associated with local recurrence and poor outcome of pancreatic cancer [4,5]. Currently, previous researches have been carried out to assess the potential prognostic value of molecular markers in predicting whether the presence of LNM and PNI. However, to date, it appears little progress has been made in the understanding of molecular mechanisms involved in pathogenesis of metastasis and invasion.
Previous study has found that expression of the c-Myc oncogene or its protein product, c-Myc, was elevated in pancreatic cancer. It was reported, for instance, high frequencies of c-Myc over-expression ranging from 43.5% to 70.2% of primary pancreatic cancer [6], indicating that its activation may be essential during carcinogenesis. In addition, the correlation between the expression of c-Myc and either metastasis or a poor prognosis has been reported for various cancers [7,8]. However, a recent study showed that there was no correlation between c-Myc protein over-expression and tumor stage or lymph node status in pancreatic cancer [9]. In addition, to date, few studies reported on the research of the correlation between expression of c-Myc and PNI.
Defective apoptosis mechanism played an important role in carcinogenesis and progression in many human cancers, such as lung cancer [10], breast cancer [11] , colorectal carcinoma [12] and pancreatic cancer [13]. There were increasing experimental evidence showing that decreased expression of Fas favored malignant progression by reducing the cancer cell apoptosis [14]. Currently, Fas and Fas ligand were recognized as a major pathway for the induction of apoptosis. However, it has not yet been fully elucidated regarding whether Fas can be used as a significant prognostic biomarker for patients with pancreatic cancer. Furthermore, it was unclear whether there was a correlation between expression of Fas and PNI or not.
In the present study, we detected the expression of c-Myc, Fas in pancreatic cancer tissues and paracancerous tissues by immunohistochemistry, and investigated the role of the c-Myc and Fas in the carcinogenesis, LNM and PNI of pancreatic cancer.
Materials and methods
Patients and tissue microarray construction
Pancreatic cancer tissue samples, mainly adenocarcinoma, were collected from 162 patients who underwent pancreatic surgical resection between 1995 and 2009, and the samples were stored at the biobank center in National Engineering Center for Biochip at Shanghai. 162 patients with both complete clinical data and adequate tissue for inclusion in this study were identified. All of the tissue specimens were obtained for the present study with patient informed consent, and Ethical approval for the study was obtained from the ethical committee of biobank center related hospitals.
Pancreatic cancer tissue microarrays were constructed using tissue cores from formalin-fixed, paraffin-embedded specimens as previously described [15], Representative tumor regions and its paracancerous nonmalignant pancreatic specimens (NMPs) were selected by pathologists from each tissue block, and a single 0.6mm core was taken from every donor block. Microarray blocks were constructed using an automated tissue arrayer (Beecher Instruments, Sun Prarie, WI). Five-micron sections were cut from the array blocks. In all cases, cores were also from taken normal adjacent pancreas for use as internal controls. Sections were then stained with H&E to confirm the presence of tumor within each core, and immunohistochemical analysis of expression of c-Myc and Fas were performed as described below.
Immunohistochemistry and scoring
The TMA slides were heated at 55°for 15 min, deparaffinized and rehydrated in an ethanol gradient, washed with Tris-buffered saline, and processed using a streptavidin– biotin– peroxidase complex method. Antigen retrieval was performed 10 minutes at high temperature under high pressure in sodium citrate buffer (pH 6), followed by 3% hydrogen peroxide in PBS, followed by 3 x 5 min PBS. After quenching of endogenous peroxidase activity and blocking of nonspecific binding, 2 antibodies (c-Myc, Santa company, polyclone antibody, expression in cytoplasm and cytomembrane; Fas, Abcam company, monoclonal antibody, expression in cytoplasm) were added at a special dilution, 1:8000, 1:200 respectively, after which slides were incubated with primary antibody overnight in a humid chamber at 4°C. The corresponding secondary biotinylated rabbit antibody was used at a special dilution for 30 minutes at room temperature. After further washing with Tris-buffered saline, sections were incubated with StrepABComplex/horseradish peroxidase (1:100, DAKO) for 30 minutes at room temperature. Chromogenic immunolocalization was performed using 0.05% 3,3-diaminobenzidine tetrahydrochloride. Slides were counterstained with diluted hematoxylin before dehydration and mounting. Other cores containing pancreatic cancer served as positive controls for those genes expression. Normal serum was used in the place of primary antibody as a negative control.
The immunostained tissue microarray sections were analyzed by a pathologist. The expression of c-Myc and Fas was assessed according to the percentage of staining as follows: 1, 0 points for no staining; 2, 1 point for < 20%; 3, 2 points for 20-75%; 4, 3 points for > 75% of cancer tissue stained, as described previously [16]. The intensity of staining was graded on a scale of 0 to 3 (0, none; 1, weak, 2, intermediate; and 3 strong). The total score was the product of the score for the intensity and positive rate of staining (Staining index = intensity x positive rate). All slides were analyzed by two independent observers who were blinded to each other’s findings. In cases of disagreement, a consensus was reached by joint review.
Statistical analysis
The relationships between clinicopathological parameters and PNI and LNM, between expression levels of these proteins and PNI and LNM were studied using the χ2-test. Logistic regression analysis was used to determine the independent predictors of PNI. Statistical significance was determined by a two-tailed with P value < 0.05. Statistical analysis was carried out using the SPSS software package (version 17.0).
Results
Patient characteristics
As shown in Table 1, the sample consisted of 162 patients with a diagnosis of pancreatic cancer. These include 102 male and 60 female patients with a mean age of 59 year-old (range 34 to 85 year-old). The median tumor size was 4 cm (range 0.5 to 14 cm). According to the 6th edition of American Joint Committee on Cancer staging system (AJCC staging system), 80 cases were in stage I, 80 in stage II, none in stage III, and 2 in stage IV. 65 of 162 cases were with local and distant lymph node metastatic, 83 of 162 cases with PNI. Most tumors (117/162, 72%) were well differentiated, 8 (8%) were moderately differentiated, and 31 (19%) were poorly differentiated.
Table 1.
Characteristics of patients and tumors
| Median (range) | n(%) | |
|---|---|---|
| Age (years) | 59(34-85) | |
| <60 | 89 (54.9%) | |
| ≥60 | 73 (45.1%) | |
| Gender | ||
| female | 60 (37%) | |
| male | 102 (63%) | |
| Long diameter of tumor (cm) | 4(0.5-14) | |
| <4 | 74 (45.7%) | |
| ≥4 | 88 (54.3) | |
| Tumor location | ||
| head | 11 (72.2%) | |
| body and rear | 45 (27.8%) | |
| Nodal status | ||
| Negative | 97 (59.9%) | |
| Positive | 65 (40.1%) | |
| Perineural Invasion status | ||
| Negative | 79 (48.8%) | |
| Positive | 83 51.2%) |
A clinicopathologic analysis of these 162 patients was carried out. We evaluated the relationships of the clinicopathological factors with the presence of LNM and PNI. The following data were collected for each patient: gender, age (< 60 years or ≥ 60 years), tumor location (head or body and rear), long diameter of tumor (< 4.0 cm or ≥ 4.0 cm), and American Joint Committee on Cancer stage. Our results showed that there was a significant association between LNM and tumor location (P = 0.03). However, no significant association was observed between LNM and age, gender and long diameter of tumor (P > 0.05). In addition, there was no significant association between PNI and age, gender, tumor location and long diameter of tumor (P > 0.05) (Table 2).
Table 2.
Associations between the various clinicopathological factors and the presence of LNM and PNI
| LNM | PNI | |||||||
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | |||||
| Features | NO. of patients | n = 65 | n = 97 | P-value | n = 83 | n = 79 | P-value | |
| Age(years) | <60 | 89 | 39 | 50 | NS | 41 | 48 | NS |
| ≥60 | 73 | 26 | 47 | 42 | 31 | |||
| Gender | female | 60 | 22 | 38 | NS | 28 | 32 | NS |
| male | 102 | 43 | 59 | 55 | 47 | |||
| Localtion | head | 117 | 53 | 64 | P = 0.03 | 59 | 58 | NS |
| body/rear | 45 | 12 | 33 | 24 | 21 | |||
| Size | <4 | 74 | 33 | 41 | NS | 38 | 36 | NS |
| ≥4 | 88 | 32 | 56 | 45 | 43 | |||
| Differentiation | Poor | 31 | 11 | 20 | NS | 17 | 14 | NS |
| Moderate/Well | 131 | 54 | 77 | 66 | 65 | |||
LNM: Lymph node metastasis; PNI: perineural invasion; NS: not significant
Expression of c-Myc and Fas in pancreatic cancer tissues and paracancerous tissues
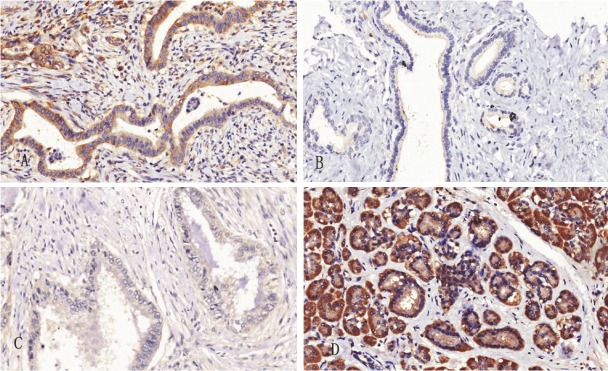
The c-Myc was mainly localized to the cytoplasm and minority of c-Myc staining was localized to the cytomembrane, and Fas staining was localized to the cytoplasm. There were significant differences in the expression levels of c-myc and Fas between cancer tissues and paracancerous tissues (P < 0.0001). The result showed that expression level of c-Myc was higher in cancer tissues than those in paracancerous tissues. In contrast, the expression level of Fas was lower in cancer tissues than that in paracancerous tissues (Figure 1).
Figure 1.
Immunohistochemical expression of c-Myc and Fas in pancreatic cancer tissue and paracancerous tissue. A shows positive expression of c-Myc in the cancer tissue (Original magnification x 200). B shows the negative expression of c-Myc in the paracancerous tissue (Original magnification x 200). C shows negative expression of Fas in the cancer tissue (Original magnification x 200). D shows positive expression of Fas in the paracancerous tissue (Original magnification x 200).
Correlation between perineural invasion and c-Myc and Fas staining in pancreatic cancer tissue.
The analysis showed that the cytoplasm expression of c-Myc was significantly positive correlation with PNI (P = 0.002). The difference remained significant even after adjusted for age, gender, cancer location and long diameter of tumor (P = 0.029). In contrast, cytoplasm expression level of Fas in cancer tissues was negatively correlated with PNI (P = 0.033). However, there was no significant difference between expression of Fas and PNI after adjusted for age, gender, cancer location and long diameter of tumor (P = 0.081). In addition, no correlation was observed between cytomembrane expression of c-Myc and PNI (P> 0.05) (Table 3).
Table 3.
Associations between the expression of genes and the presence of LNM and PNI
| PNI | LNM | ||||||
|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | ||||
| Variables | NO.of patients | n = 83 | n = 79 | P-value | n = 65 | n = 97 | P-value |
| c-Myc | |||||||
| high-expression | 127 | 73 | 54 | P = .002 | 46 | 81 | NS |
| low-expression | 35 | 10 | 25 | 19 | 16 | ||
| Fas | |||||||
| high-expression | 14 | 3 | 11 | P = .033 | 3 | 11 | NS |
| low-expression | 148 | 80 | 68 | 62 | 86 | ||
LNM: Lymph node metastasis; PNI: perineural invasion; NS: not significant
Correlation between lymph node metastasis and c-Myc and Fas staining in cancer tissue.
Between the two groups with or without LNM, we examined a possible association between LNM and expression of c-Myc and Fas. The result demonstrated that there was no significant association between LNM and expression levels of above two variables (P> 0.05) (Table 3).
Independent factors for perineural invasion
The statistical analysis showed that cytoplasm expression levels of c-Myc and Fas were all significantly related with PNI. In an attempt to identify which variable was the independent risk factor of PNI, we performed logistic regression analysis. The results showed that cytoplasm expression of c-Myc in cancer tissues acted as an independent risk factor for PNI (P< 0.05).
Discussion
In recent years, molecular biomarkers for metastasis have been indentified in various cancers. Because biomarkers can be analyzed in a noninvasive and economic way, it is worth to find out more potential biomarkers for early diagnosis and prognosis of pancreatic cancer. Recent studies have shown that deregulation of oncogenes, tumor suppressor and genome maintenance genes, up-regulation of growth factors/growth factor receptor signaling cascade systems, and aberrant expression of apoptosis-regulating genes play crucial roles in the process of metastasis.
We performed this study to verify whether the high-expression of c-Myc in pancreatic tumor tissue may give rise to a high rate of LNM and PNI or not. Our study showed that the expression level of c-Myc was higher in cancer tissues than paracancerous tissues, and cytoplasm expression of c-Myc was significantly positive correlation with PNI (P = 0.002). The result suggested that the over-expression of c-Myc played an important role in the process of pancreatic tumorigenesis and invasion. Furthermore, cytoplasm expression of c-Myc in cancer tissue acted as an independent risk factor for PNI (P = 0.022). Therefore, c-Myc may be considered as a useful marker for metastasis and prognosis of pancreatic cancer. The precise mechanism of the c-Myc in tumorigenesis and invasion has remained elusive. The proto-oncogene c-Myc belongs to the family of myc genes and encodes a transcription factor that regulates cell proliferation, growth and apoptosis. Dysregulated expression of c-Myc found in various human tumors including lung cancer [17,18], breast cancer [19], colon cancer [17], prostate cancer [20], liver cancer [21] and pancreatic cancer [22], and is often associated with poor prognosis [23]. The basic result showed that the effects of c-Myc on tumorigenesis have been mainly attributed to its ability to coordinate gene transcription [24].
In addition, c-Myc acted as a downstream transcriptional effector of many signaling pathways involved in pancreatic carcinogenesis. It was regulated at multiple levels and its overexpression contributed to the genesis of cancer [25]. For example Malte et al [26] demonstrated that ectopic activation of NFATc1 and the Ca2+/calcineurin signaling pathway was an important mechanism of oncogenic c-Myc activation in pancreatic cancer. However, so far only few research on the PNI, LNM and poor prognosis value of c-Myc over-expression have reported in pancreatic cancer. To the best knowledge, this was the first study to demonstrate overexpression of c-Myc was significantly positive correlation with PNI in pancreatic cancer. However, our study demonstrated that no significant association was observed between LNM and the expression level of c-Myc (P> 0.05), which was consistent with some previous studies [9]. These results indicated that different mechanisms may be involved in the pathogenesis of LNM and PIN.
Furthermore, our results showed that there was significant difference in the expression of Fas between cancer tissues and paracancerous tissues (P< 0.0001). Analysis showed that Fas was lower in cancer tissues than paracancerous tissues. The results in current study suggested that Fas played an important role in the development of pancreatic cancer. According to the biology behavior and function of Fas, The pathogenesis of cancer might be associated with the abnormal regulation of apoptosis of Fas. We all known that Fas was best known for its ability to induce apoptosis but can also promote tumorigenesis in apoptosis-resistant tumor cells [27]. Down-regulation of Fas expression in pancreatic cancer cells may be sufficient to confer resistance to Fas mediated apoptosis. Tang et al [28] reported that Fas up-regulated mediator of apoptosis promote tumorigenesis. However, in one study, the results suggested that pancreatic cancer cells were resistant to Fas-mediated apoptosis by mechanisms excluding receptor down-regulation or Fas-associated phosphatase up-regulation and raise the possibility that Fasmediated apoptosis may be dependent on the activation of the JNK/p38 MAPK pathway in these cells. In addition, Bernstorff et al [13] reported that loss of Fas expression in pancreatic adenocarcinoma significantly correlated with extrapancreatic spread of the tumors and was associated with a shorter overall survival.
Recently, a transcriptional analysis of perineural invasive PDAC cell lines has resulted in an extensive list of putative genes involved in perineural infiltration [29]. A series of studies found that Fas can activate a number of non-apoptotic pathways that stimulate invasion [30-32]. The present study showed that cytoplasm expression level of Fas in cancer tissues was negatively correlated with PNI (P = 0.033). Taken together, Fas may be also considered as a potential marker for PNI and prognosis of cancer. Currently, there were few studies reported on the association between the expression of Fas and the PNI. Therefore, our result in this study could promote to understand the molecular mechanism of PNI. However, in this study, the statistically analysis showed that there was no significant association between LNM and expression level of Fas (P> 0.05). The result was not consistent with a previous finding that significant association was observed between expression level of Fas and LNM (P< 0.0001 ) [33]. It may be partly due to small sample size involved in this study. Therefore, further studies will be necessary to determine whether Fas is a useful predictive factor for metastasis of pancreatic cancer.
Acknowledgement
HJ is supported by Key Discipline Construction Project of Pudong Health Bureau of Shanghai, China (Grant No: PWZxkq2010-05). HG is supported by China National 863 Project Foundation for Cancer Genomics (Pancreas Genomics) (Grant No: 1006AA02A302), respectively. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gong Z, Holly EA, Bracci PM. Survival in Population-based Pancreatic Cancer Patients: San Francisco Bay Area, 1995-1999. American Journal of Epidemiology. 2011;174:1373–1381. doi: 10.1093/aje/kwr267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu JQ, Ward E. Cancer Statistics, 2010. Ca-a Cancer Journal for Clinicians. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer: a review of the literature. Cancer. 2009;115:3379–3391. doi: 10.1002/cncr.24396. [DOI] [PubMed] [Google Scholar]
- 4.Koide N, Yamada T, Shibata R, Mori T, Fukuma M, Yamazaki K, Aiura K, Shimazu M, Hirohashi S, Nimura Y, Sakamoto M. Establishment of perineural invasion models and analysis of gene expression revealed an invariant chain (CD74) as a possible molecule involved in perineural invasion in pancreatic cancer. Clinical Cancer Research. 2006;12:2419–2426. doi: 10.1158/1078-0432.CCR-05-1852. [DOI] [PubMed] [Google Scholar]
- 5.Bapat AA, Hostetter G, Von Hoff DD, Han H. Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer. 2011;11:695–707. doi: 10.1038/nrc3131. [DOI] [PubMed] [Google Scholar]
- 6.Li YJ, Wei ZM, Meng YX, Ji XR. Beta-catenin up-regulates the expression of cyclinD1, c-myc and MMP-7 in human pancreatic cancer: relationships with carcinogenesis and metastasis. World J Gastroenterol. 2005;11:2117–2123. doi: 10.3748/wjg.v11.i14.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rapp UR, Korn C, Ceteci F, Karreman C, Luetkenhaus K, Serafin V, Zanucco E, Castro I, Potapenko T. Myc Is a Metastasis Gene for Non-Small-Cell Lung Cancer. Plos One. 2009;4:e6029. doi: 10.1371/journal.pone.0006029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsiatis AC, Herceg ME, Keedy VL, Halpern JL, Holt GE, Schwartz HS, Cates JM. Prognostic significance of c-Myc expression in soft tissue leiomyosarcoma. Mod Pathol. 2009;22:1432–1438. doi: 10.1038/modpathol.2009.113. [DOI] [PubMed] [Google Scholar]
- 9.Schleger C, Verbeke C, Hildenbrand R, Zentgraf H, Bleyl U. c-MYC activation in primary and metastatic ductal adenocarcinoma of the pancreas: incidence, mechanisms, and clinical significance. Mod Pathol. 2002;15:462–469. doi: 10.1038/modpathol.3880547. [DOI] [PubMed] [Google Scholar]
- 10.Myong NH. Tissue microarray analysis of Fas and FasL expressions in human non-small cell lung carcinomas; with reference to the p53 and bcl-2 overexpressions. Journal of Korean Medical Science. 2005;20:770–776. doi: 10.3346/jkms.2005.20.5.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mottolese M, Buglioni S, Bracalenti C, Cardarelli MA, Ciabocco L, Giannarelli D, Botti C, Natali PG, Concetti A, Venanzi FM. Prognostic relevance of altered Fas (CD95)-system in human breast cancer. International Journal of Cancer. 2000;89:127–132. doi: 10.1002/(sici)1097-0215(20000320)89:2<127::aid-ijc5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 12.Wang W-S, Chen P-M, Wang H-S, Liang W-Y, Su Y. Matrix metalloproteinase-7 increases resistance to Fas-mediated apoptosis and is a poor prognostic factor of patients with colorectal carcinoma. Carcinogenesis. 2006;27:1113–1120. doi: 10.1093/carcin/bgi351. [DOI] [PubMed] [Google Scholar]
- 13.Bernstorff WV, Glickman JN, Odze RD, Farraye FA, Joo HG, Goedegebuure PS, Eberlein TJ. Fas (CD95/APO-1) and Fas ligand expression in normal pancreas and pancreatic tumors. Implications for immune privilege and immune escape. Cancer. 2002;94:2552–2560. doi: 10.1002/cncr.10549. [DOI] [PubMed] [Google Scholar]
- 14.Houston A, O'Connell J. The Fas signalling pathway and its role in the pathogenesis of cancer. Curr Opin Pharmacol. 2004;4:321–326. doi: 10.1016/j.coph.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Lin MS, Chen WC, Huang JX, Gao HJ, Zhang BF, Fang J, Zhou Q, Hu Y. Tissue Microarrays in Chinese Human Rectal Cancer: Study of Expressions of the Tumor-Associated Genes. Hepato-gastroenterology. 2011;58:1937–42. doi: 10.5754/hge11262. [DOI] [PubMed] [Google Scholar]
- 16.Ohuchida K, Mizumoto K, Ishikawa N, Fujii K, Konomi H, Nagai E, Yamaguchi K, Tsuneyoshi M, Tanaka M. The role of S100A6 in pancreatic cancer development and its clinical implication as a diagnostic marker and therapeutic target. Clinical Cancer Research. 2005;11:7785–7793. doi: 10.1158/1078-0432.CCR-05-0714. [DOI] [PubMed] [Google Scholar]
- 17.Sertel S, Eichhorn T, Simon CH, Plinkert PK, Johnson SW, Efferth T. Pharmacogenomic identification of c-Myc/Max-regulated genes associated with cytotoxicity of artesunate towards human colon, ovarian and lung cancer cell lines. Molecules. 2010;15:2886–2910. doi: 10.3390/molecules15042886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu FY, Wang LP, Hou LL, Yin G, Wang HC. [The expressions of STAT3, WWOX and c-myc in human non small cell lung cancer tissue and correlativity analysis] . Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2010;26:1203–1205, 1209. [PubMed] [Google Scholar]
- 19.Musgrove EA, Sergio CM, Anderson LR, Inman CK, McNeil CM, Alles MC, Gardiner-Garden M, Ormandy CJ, Butt AJ, Sutherland RL. Identification of downstream targets of estrogen and c-myc in breast cancer cells. Adv Exp Med Biol. 2008;617:445–451. doi: 10.1007/978-0-387-69080-3_43. [DOI] [PubMed] [Google Scholar]
- 20.Sun C, Dobi A, Mohamed A, Li H, Thangapazham RL, Furusato B, Shaheduzzaman S, Tan SH, Vaidyanathan G, Whitman E, Hawksworth DJ, Chen Y, Nau M, Patel V, Vahey M, Gutkind JS, Sreenath T, Petrovics G, Sesterhenn IA, McLeod DG, Srivastava S. TMPRSS2-ERG fusion, a common genomic alteration in prostate cancer activates C-MYC and abrogates prostate epithelial differentiation. Oncogene. 2008;27:5348–5353. doi: 10.1038/onc.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaposi-Novak P, Libbrecht L, Woo HG, Lee YH, Sears NC, Coulouarn C, Conner EA, Factor VM, Roskams T, Thorgeirsson SS. Central role of c-Myc during malignant conversion in human hepatocarcinogenesis. Cancer Research. 2009;69:2775–2782. doi: 10.1158/0008-5472.CAN-08-3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazur PK, Einwachter H, Lee M, Sipos B, Nakhai H, Rad R, Zimber-Strobl U, Strobl LJ, Radtke F, Kloppel G, Schmid RM, Siveke JT. Notch2 is required for progression of pancreatic intraepithelial neoplasia and development of pancreatic ductal adenocarcinoma. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:13438–13443. doi: 10.1073/pnas.1002423107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vita M, Henriksson M. The Myc oncoprotein as a therapeutic target for human cancer. Seminars in Cancer Biology. 2006;16:318–330. doi: 10.1016/j.semcancer.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 24.Robinson K, Asawachaicharn N, Galloway DA, Grandori C. c-Myc accelerates S-phase and requires WRN to avoid replication stress. Plos One. 2009;4:e5951. doi: 10.1371/journal.pone.0005951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skoudy A, Hernandez-Munoz I, Navarro P. Pancreatic ductal adenocarcinoma and transcription factors: role of c-Myc. J Gastrointest Cancer. 2011;42:76–84. doi: 10.1007/s12029-011-9258-0. [DOI] [PubMed] [Google Scholar]
- 26.Buchholz M, Schatz A, Wagner M, Michl P, Linhart T, Adler G, Gress TM, Ellenrieder V. Overexpression of c-myc in pancreatic cancer caused by ectopic activation of NFATc1 and the Ca2+/calcineurin signaling pathway. EMBO J. 2006;25:3714–3724. doi: 10.1038/sj.emboj.7601246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nijkamp MW, Hoogwater FJH, Steller EJA, Westendorp BF, van der Meulen TA, Leenders MWH, Borel Rinkes IHM, Kranenburg O. CD95 is a key mediator of invasion and accelerated outgrowth of mouse colorectal liver metastases following radiofrequency ablation. Journal of Hepatology. 2010;53:1069–1077. doi: 10.1016/j.jhep.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 28.Tang D, Lotze MT, Kang R, Zeh HJ. Apoptosis promotes early tumorigenesis. Oncogene. 2011;30:1851–1854. doi: 10.1038/onc.2010.573. [DOI] [PubMed] [Google Scholar]
- 29.Abiatari I, DeOliveira T, Kerkadze V, Schwager C, Esposito I, Giese NA, Huber P, Bergman F, Abdollahi A, Friess H, Kleeff J. Consensus transcriptome signature of perineural invasion in pancreatic carcinoma. Molecular Cancer Therapeutics. 2009;8:1494–1504. doi: 10.1158/1535-7163.MCT-08-0755. [DOI] [PubMed] [Google Scholar]
- 30.Hoogwater FJH, Nijkamp MW, Smakman N, Steller EJA, Emmink BL, Westendorp BF, Raats DAE, Sprick MR, Schaefer U, Van Houdt WJ, De Bruijn MT, Schackmann RCJ, Derksen PWB, Medema JP, Walczak H, Rinkes IHMB, Kranenburg O. Oncogenic K-Ras Turns Death Receptors Into Metastasis-Promoting Receptors in Human and Mouse Colorectal Cancer Cells. Gastroenterology. 2010;138:2357–2367. doi: 10.1053/j.gastro.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 31.Kleber S, Sancho-Martinez I, Wiestler B, Beisel A, Gieffers C, Hill O, Thiemann M, Mueller W, Sykora J, Kuhn A, Schreglmann N, Letellier E, Zuliani C, Klussmann S, Teodorczyk M, Grone HJ, Ganten TM, Sultmann H, Tuttenberg J, von Deimling A, Regnier-Vigouroux A, Herold-Mende C, Martin-Villalba A. Yes and PI3K bind CD95 to signal invasion of glioblastoma. Cancer Cell. 2008;13:235–248. doi: 10.1016/j.ccr.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Wisniewski P, Ellert-Miklaszewska A, Kwiatkowska A, Kaminska B. Non-apoptotic Fas signaling regulates invasiveness of glioma cells and modulates MMP-2 activity via NFkappaB-TIMP-2 pathway. Cell Signal. 2010;22:212–220. doi: 10.1016/j.cellsig.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 33.Bebenek M, Dus D, Kozlak J. Fas and Fas ligand as prognostic factors in human breast carcinoma. Medical Science Monitor. 2006;12:Cr457–Cr461. [PubMed] [Google Scholar]