Abstract
KIT and PDGFRA in small cell lung carcinoma (SCLC) have been rarely examined in Japanese. The author investigated protein expression of KIT and PDGFRA in 54 Japanese cases of small cell lung carcinoma by immunohistochemistry, and gene mutations of KIT and PDGFRA in 20 Japanese cases of small cell lung carcinoma by the PCR-direct sequencing method. The molecular genetic analysis showed no mutations of KIT (exons 9, 11, 13, and 17) and PDGFRA (exons 12 and 18) genes in all 20 cases. KIT protein expression was recognized in all cases (100%). Membranous KIT expression was strong in 35 cases, moderate in 7 cases and weak in 12 cases. PDGFRA protein expression was noted in 35 cases (65%); the membranous expression was strong in 2 cases, moderate in 16 cases, and weak in 17 cases. The overall median survival was 13 months. There was no significant difference in the survival between KIT strongly positive cases (median, 12 months) and KIT moderately or weakly positive cases (median, 11 months). Likewise, there was no significant difference in the survival between PDGFRA-positive cases (median, 11 months) and PDGFRA-negative cases (median, 12 months). The protein expressions of KIT and PDGFRA did not correlate with gender, smoking, and disease stage. These findings suggest, in Japanese population, that mutations of KIT and PDGFRA were absent in small cell lung carcinoma of Japan, that KIT protein expression is present in 100%, that PDGFRA expression is present in 65%, and that KIT and PDGFRA protein expressions do not correlate with survival, gender, smoking, and disease stage.
Keywords: Small cell lung carcinoma, KIT, PDGFRA
Introduction
Small cell carcinoma can occur in any organ, but the great majority develops in the lung. Small cell lung carcinoma (SCLC) shows aggressive behavior and poor prognosis. Recent studies of SCLC have shown that KIT protein is expressed in 30-100% of SCLC [1-10]. The difference of the percentage may be due to different primary antibodies used, staining methods, incubation periods, and interpretation of the immunostaining. In addition, one report showed that a few KIT gene mutations were present in SCLC [8], but others did not [7,9,10]. Platelet-derived growth factor receptor-α (PDGFRA) protein in SCLC has not been reported. PDFFRA gene in SCLC has been investigated in only one study [10], which showed no mutations in 31 cases of SCLC. However, such studies have not been performed in the yellow race including Japanese.
KIT and PDGFRA genes, both mapped to 4q12, encode receptor tyrosine kinase oncoprotein called KIT (CD117) and PDGFRA, respectively [11-16]. Both molecules are transmembranous oncoprotein involved in tumorigenesis of some neoplasms including gastrointestinal stromal tumor (GIST), acute myeloid leukemia, mast cell neoplasms, germ cell tumors, melanoma, neuroendocrine carcinomas, large cell neuroendocrine carcinoma and SCLC [11-16]. The hot spots of gene mutations are exons 9, 11, 13, and 17 of KIT gene and exons 12 and 18 of PDGFRA gene [11-16].
The author retrospectively investigated the protein expression of KIT and PDGFRA, gene mutations of KIT and PDGFRA, and implications of clinical survival in 54 Japanese cases with SCLC.
Materials and methods
The author retrieved 54 consecutive lung biopsy samples (recent 20 years) of SCLC of 54 Japanese patients in our hospital. The mean age of the patients was 63.4±11.4 years (Table 1). Of the 54 cases, 52 were dead and 2 are alive (Table 1). Male to female ratio was 52:2. Smokers were 46 cases (85%). The 54 patients with SCLC accounted for almost 100% of patients with SCLC who underwent lung biopsy. All the 54 patients were treated in our hospital with cisplatin-based chemotherapy and radiation.
Table 1.
Results of immunohistochemistry and patients’ survival, smoking, and stage
| Case | Age | Gender | KIT | PDGFRA | Survival | Smoking | TNM | Stage |
|---|---|---|---|---|---|---|---|---|
| No. | (yrs) | (months) | ||||||
| 1 | 49 | male | +3 | +2 | 14 mo | + | T2N1M0 | II |
| 2 | 52 | male | +3 | +2 | 11 mo | + | T3N1Mx | III |
| 3 | 75 | male | +3 | +2 | 7 mo | + | T2M0N0 | I |
| 4 | 64 | male | +3 | +2 | 11mo | + | T2N2M1 | IV |
| 5 | 68 | male | +3 | +2 | 14mo | + | T1NxM1 | IV |
| 6 | 62 | male | +3 | +2 | 7 mo | + | T3N2M1 | IV |
| 7 | 46 | male | +3 | +2 | 9 mo | + | T2NxM1 | IV |
| 8 | 79 | male | +3 | +2 | 8 mo | + | T2N1M1 | IV |
| 9 | 63 | male | +3 | +1 | 14 mo | + | T2N0M0 | I |
| 10 | 71 | male | +3 | +1 | 12 mo | + | T2N0M0 | I |
| 11 | 68 | male | +3 | +1 | 9 mo | - | T3N2M0 | III |
| 12 | 51 | male | +3 | +1 | 21 mo | + | T1N0M0 | I |
| 13 | 67 | male | +3 | +1 | 9 mo | + | T2N0M0 | I |
| 14 | 82 | male | +3 | +1 | alive (5mo) | + | T2N1Mx | II |
| 15 | 53 | male | +3 | +1 | 21 mo | + | T1N0M0 | I |
| 16 | 49 | male | +3 | +1 | 12 mo | + | T2NxM1 | IV |
| 17 | 65 | male | +3 | +1 | 15 mo | + | T2N2M0 | III |
| 18 | 71 | male | +3 | +1 | 4 mo | - | T4N3M1 | IV |
| 19 | 60 | male | +3 | +1 | 15 mo | + | T2N0M0 | I |
| 20 | 77 | male | +3 | +1 | 19 mo | + | T1N0M0 | I |
| 21 | 82 | male | +3 | +1 | 6 mo | + | T3N2Mx | III |
| 22 | 81 | female | +3 | +1 | 8 mo | - | T2N1M0 | II |
| 23 | 57 | male | +3 | +1 | 14 mo | + | T1N1M0 | II |
| 24 | 67 | male | +3 | +1 | 17 mo | + | T1N0M0 | I |
| 25 | 73 | male | +3 | +1 | 8 mo | + | T2N0M0 | I |
| 26 | 59 | male | +3 | - | 11 mo | + | T2N0M0 | I |
| 27 | 78 | female | +3 | - | 12 mo | - | T2N0M0 | I |
| 28 | 56 | male | +3 | - | 12 mo | + | T2N1Mx | II |
| 29 | 68 | male | +3 | - | 17 mo | + | T1N0M0 | I |
| 30 | 55 | male | +3 | - | 26 mo | - | T1N0M0 | I |
| 31 | 62 | male | +3 | - | 10 mo | + | T2N1M1 | IV |
| 32 | 63 | male | +3 | - | alive (7mo) | + | T2N1M0 | II |
| 33 | 66 | male | +3 | - | 3 mo | + | T4N3M1 | IV |
| 34 | 65 | male | +3 | - | 12 mo | + | T2N0Mx | I |
| 35 | 71 | male | +3 | - | 16 mo | + | T2N0M0 | I |
| 36 | 64 | male | +2 | +3 | 21 mo | + | T1N0M0 | I |
| 37 | 52 | male | +2 | +3 | 11 mo | + | T2NxMx | I |
| 38 | 75 | male | +2 | +2 | 13 mo | + | T2N0M0 | I |
| 39 | 81 | male | +2 | +2 | 8 mo | + | T4NxM1 | IV |
| 40 | 59 | male | +2 | +2 | 17 mo | - | T2N0M0 | I |
| 41 | 35 | male | +2 | +2 | 9 mo | + | T2NoM0 | I |
| 42 | 43 | male | +2 | +2 | 8 mo | + | T3N2M1 | IV |
| 43 | 73 | male | +1 | +2 | 17 mo | - | T2N0M0 | I |
| 44 | 49 | male | +1 | +2 | 16 mo | + | T2N1M0 | II |
| 45 | 39 | male | +1 | +2 | 6 mo | + | T3N2M1 | IV |
| 46 | 59 | male | +1 | - | 15 mo | + | T1N0M0 | I |
| 47 | 75 | male | +1 | - | 7 mo | + | T3N2M1 | IV |
| 48 | 48 | male | +1 | - | 8 mo | + | T2N1M0 | II |
| 49 | 65 | male | +1 | - | 13 mo | + | T1N0M0 | I |
| 50 | 79 | male | +1 | - | 3 mo | + | T4N3M1 | IV |
| 51 | 64 | male | +1 | - | 26 mo | - | T1N0M0 | I |
| 52 | 73 | male | +1 | - | 15 mo | + | T2N0M0 | I |
| 53 | 62 | male | +1 | - | 6 mo | + | T3N1M1 | IV |
| 54 | 55 | male | +1 | - | 12 mo | + | T2N0M0 | I |
The samples had been embedded in paraffin blocks. Several 3-μm sections were cut, and one of them was stained with hematoxylin and eosin. The remaining sections were immunohistochemically examined, using the Dako’s Envision method as previously reported [17-20], for KIT (polyclonal, dilution=1:100, Dako, Glostrup, Denmark) and PDGFRA (polyclonal, 1:100, Santa Cruz, CA, USA). The incubation period of each primary antibody was overnight. The diagnosis of SCLC was also confirmed by immunohistochemical staining for neuron-specific enolase (clone BBS/NC/Vl-H14, 1:200, Dako), chromogranin (clone DAK-A3, 1:200, Dako), synaptophysin (polyclonal, 1:200, Dako), and CD56 (cone UJ13A, 1:150, Dako). The immunohistochemical scoring was performed according to the current scoring system of HER2/neu of breast cancer [21]. That is, in cases of positive cells < 10% was negative, and those > 10% was positive. In the positive cases, the scoring was performed according the strength of the immunostaining. Therefore, the immunohistochemical staining patterns of KIT and PDGFRA were categorized as follows: strong membranous expression 3+ (Figure 1A for KIT, and Figure 2A for PDGFRA), moderate membranous expression 2+ (Figure 1B for KIT, and Figure 2B for PDGFRA), weak membranous expression 1+ (Figure 1C for KIT, and Figure 2C for PDGFRA). Cases with positive cells < 10% was labeled as - (negative). Five cases of gastric GIST and five cases of uterine leiomyoma were employed as positive and negative controls, respectively.
Figure 1.
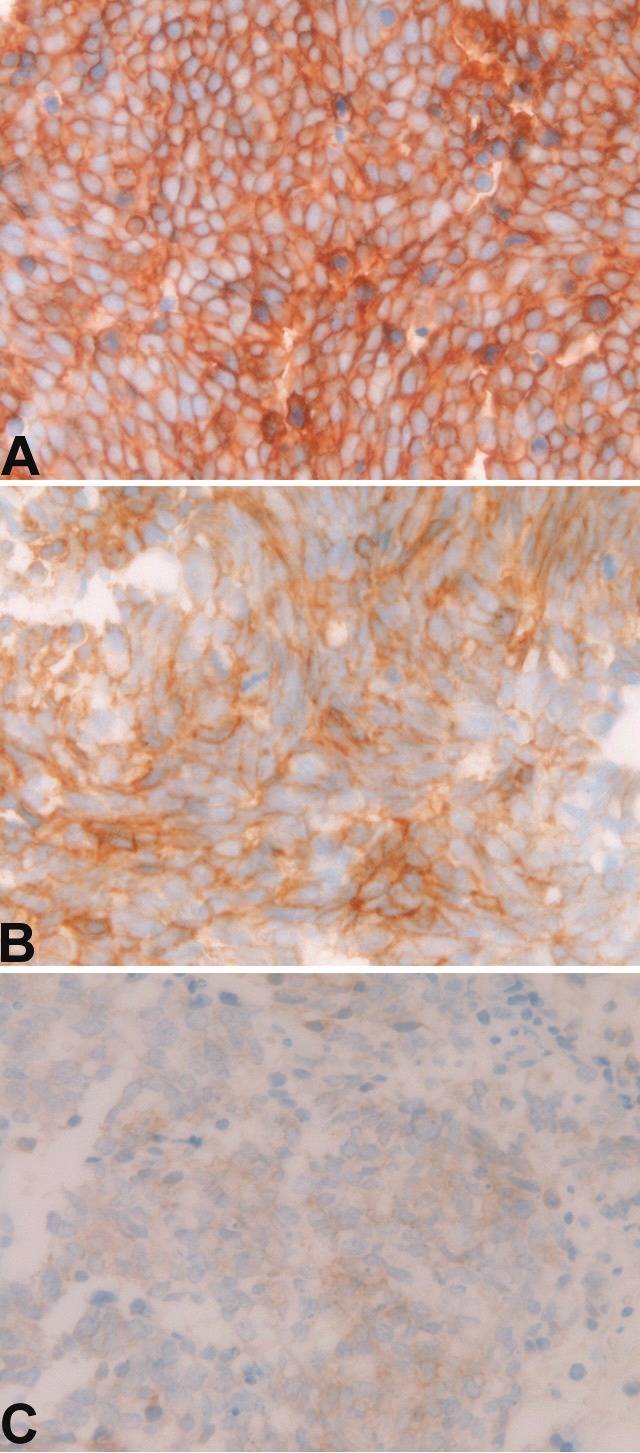
Expression of KIT. A: Strong membranous expression of KIT in small cell lung carcinoma. Immunostaining, X400. B: Moderate membranous expression of KIT in small cell lung carcinoma. Immunostaining, X400. C: Weak membranous expression of KIT in small cell lung carcinoma. Immunostaining, X400.
Figure 2.
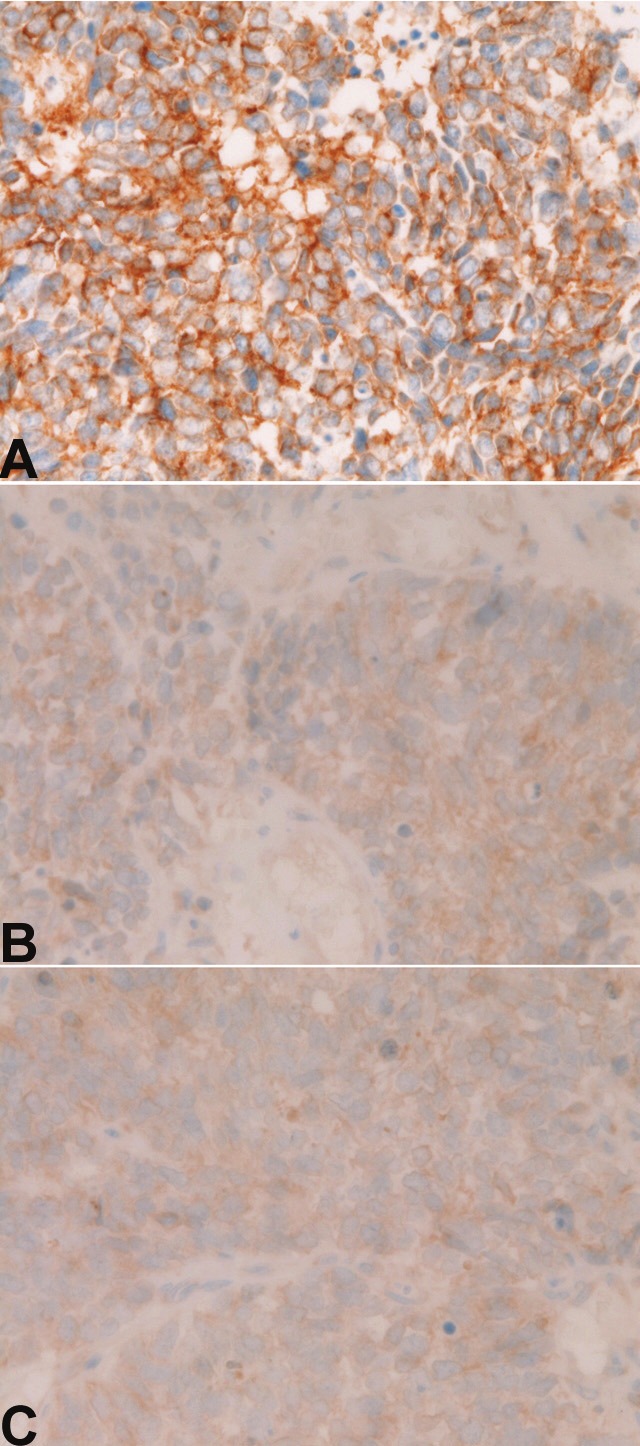
Expression of platelet-derived growth factor receptor-α (PDGFRA). A: Strong membranous expression of PDGFRA in small cell lung carcinoma. Immunostaining, X400. B: Moderate membranous expression of PDGFRA in small cell lung carcinoma. Immunostaining, X400. C: Weak membranous expression of PDGFRA in small cell lung carcinoma. Immunostaining, X400
A molecular genetic analysis of KIT gene (exons 9, 11, 13, and 17) and PDGFRA gene (exons 12 and 18) was performed in 20 cases by the PCR direct sequencing method, as previously reported [22-35]. The exons of both genes were selected because they are frequent mutation sites [11-16]. The primers are shown in Table 2. In brief, genomic DNA was extracted from paraffin sections with proteinase K digestion and phenol/chloroform extraction, and subjected to PCR for 40 cycles (94ºC for one minute, 52ºC for one minute, 72ºC for one minute), using a thermal cycler (GeneAmp PCR system 9700, Applied Biosystems, ABI, CA). The annealing temperature was 53ºC. PCR products were extracted, and subjected to a computed automatic DNA sequencer (ABI PRISM 3100 Genetic Analyzer, Applied Biosystems, ABI, CA). Two cases of gastric GIST and two cases of uterine leiomyoma were used as positive controls and negative controls, respectively.
Table 2.
Primer sequence
| Forward | Reverse |
|---|---|
| KIT exon 9 | |
| 5’-TCC TAG AGT AAG CCA GGG CTT-3’ | 5’-TGG TAG ACA GAG CCT AAA CAT CC-3’ |
| KIT exon 11 | |
| 5’-GAT CTA TTT TTC CCT TTC TC-3’ | 5’AGC CCC TGT TTC ATA CTG AC-3’ |
| KIT exon 13 | |
| 5’-GCT TGA CAT CAG TTT GCC AG -3’ | 5’-AAA GGC AGC TTG GAC ACG GCT TTA-3’ |
| KIT exon 17 | |
| 5’-CTC CTC CAA CCT AAT AGT GT-3’ | 5’-GTC AAG CAG AGA ATG GGT AC-3’ |
| PDGFRA exon 12 | |
| 5’-TTG GAT ATT CAC CAG TTA CCT GTC-3’ | 5’-CAA GGG AAA AGC TCT TGG-3’ |
| PDGFRA exon 18 | |
| 5’-ACC ATG GAT CAG CCA GTC TT-3’ | 5’-TGA AGG AGG ATG AGC CTG ACC-3’ |
Results
No mutations of KIT and PDGFRA genes were recognized in all the 20 cases of SCLC. The two GISTs used as positive controls showed two point mutations of KIT gene. The two uterine leiomyomas used as negative controls showed no mutations of KIT and PDGFRA genes.
Immunohistochemically, KIT protein expression (Figure 1A, 1B and 1C) was recognized in all the 54 cases (100%) (Table 1). Strong KIT expression (+3) (Figure 1A) was present in 35 cases (65%), moderate expression (2+) (Figure 1B) in 7 cases (13%), and weak expression (+1) (Figure 1C) in 12 cases (22%) (Table 1). PDGFRA expression (Figure 2A, 2B and 2C) was recognized in 35 cases (65%); strong expression (+3) (Figure 2A) in 2 cases (4%), moderate expression (+2) (Figure 2B) in 16 cases (30%), weak expression (+1) (Figure 2C) in 17 cases (31%), and negative expression (-) in 19 cases (35%) (Table 1). There was no significant difference between KIT scores and PDGFRA scores. The 5 gastric GISTs used as positive control showed strong KIT and moderate to weak PDGFRA expressions. The five uterine leiomyomas used as negative controls were negative for KIT and PDGFRA.
The survival is shown in Table 1. The overall median survival after initial diagnosis was 13 months (n=52). There was no significant difference in the survival between strong KIT (+3) expression cases (median survival=12 months, n=34) and cases of KIT expression less than +3 (median survival=11 months, n=18) (Figure 3). Likewise, there was no significant difference in the survival between PDGFRA-positive cases (median survival=11 months, n=34) and PDGFRA-negative cases (median survival=12 months, n=18) (Figure 4). There was a correlation between short survival and advanced stage. There were no correlations between KIT and PDGFRA expressions and smoking, gender, and disease stage (Table 3).
Figure 3.
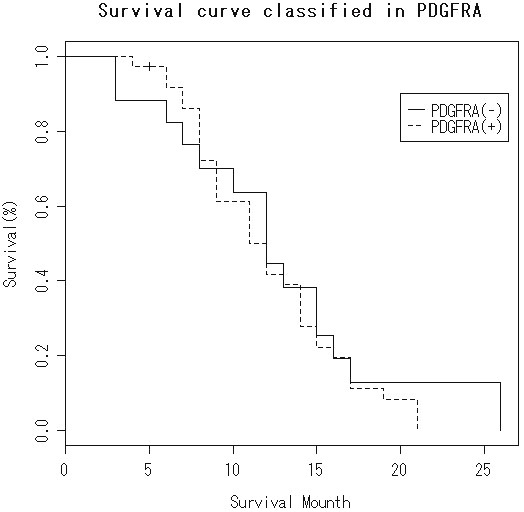
Kaplan-Meier survival in KIT 3+ group and KIT <3+ group. No significant difference is recognized (log-rank test).
Figure 4.
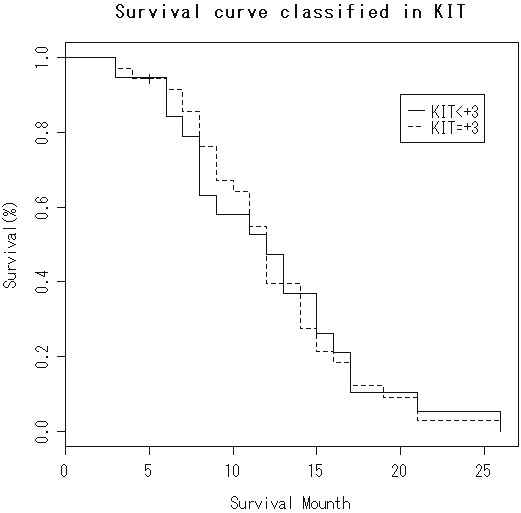
Kaplan-Meier survival in PDGFRA-positive group and PDGFRA-negative group. No significant difference is recognized (log-rank test).
Table 3.
Means and standard deviations of KIT and PDGFRA scores between smokers and non-smokers, between female and male, and between stages I+II and stages III+IV
| KIT score | PDGFRA score | |
|---|---|---|
| Non-smoker (n=8) | 2.375±0.875 | 1.000±0.707 |
| Smoker (n=46) | 2.434±0.825 | 1.066±0.904 |
| KIT score | PDGFRA score | |
| Female (n=2) | 3±0 | 0.5±0.5 |
| Male (n=52) | 2.432±0.822 | 1.039±0.906 |
| KIT score | PDGFRA score | |
| Stages I+II (n=34) | 2.382±0,840 | 0.911±0.9193 |
| Stages III+IV (n=20) | 2.500±0.806 | 1.200±0.8124 |
There are significant differences in the both scores between smokers and non-smokers, between female and male, and between stages I+II and stages III+IV. (p>0.05) (Student’s t test)
Discussion
The sensitivity and specificity of immunohistochemistry and gene mutational status in the present study appears confirmative. The author investigated the gene status in 20 cases. Further, the results of positive and negative controls for immunohistochemistry and gene analysis confirm the immunostaining and gene analysis of the SCLC in the present study. Furthermore, the author identified many gene mutations of KIT and PDGFRA in GIST, Extra-GISTs, and germ cell tumors [20-26] which were performed in the similar or same periods. In addition, the author could not detect mutations of KIT and PDGFRA genes in extra-pulmonary small cell carcinomas [25-30].
KIT is expressed in various tumors including GIST, mast cell neoplasm, melanoma, germ cell tumor, hematopoietic malignancies, and SCLC [13,16]. However, these reports are mainly from developed country of the white race, and such reports of the yellow race including Japan are very rare. Therefore, the author investigated KIT and PDGFRA in Japanese patients.
The KIT protein expression in SCLC varies among researchers [1-10]; it is reported to be 100% [1], 73% [2], 37% [3], 60% [4], 78% [5], 53% [6], 40% [8], 64% [9], and 30% [10]. The present case was 100%, similar to RaPoint et al. [1]. The various percentages may be due to antibody used, immunohistochemical procedures, or interpretation of the immunohistochemical stains. In the present study, the author employed the sensitive Dako’s Envision methods, the period of primary antibody was overnight, suggesting that the data of the present study is more accurate. In any way, the present study suggests that SCLC in Japanese patients shows high KIT protein expression. KIT expression without KIT gene mutations is thought to be due to KIT gene amplification [10].
The prognostic implications of positive KIT protein in SCLC have been controversial, and no definite conclusions have been obtained [2-6,8]. Some authors claimed that patients with KIT-positive SCLC showed good prognosis [36], while others, in contrast, demonstrated that patients with KIT-positive SCLC showed poor prognosis [3,6] and still others identified that there was no correlation between KIT positivity and prognosis [2]. The present study showed that there was no significant correlation in the survival between strong KIT-positive cases and weak KIT-positive cases in Japanese. More studies are required because if activating KIT mutations are present, treatment of imatinib mesylate may be effective [10,16].
KIT mutations are frequent in GIST, acute myeloid leukemia and mast cell neoplasms [16]. With regard to SCLC, one report showed a few KIT mutations [8], while others indicated no KIT mutations in SCLC [7,9,10]. Boldrini et al. [8] examined exons 9 and 11 of KIT gene, and reported that two mutations in exon 9 and three mutations in exon 11 were found in 60 SCLC. In contrast, Sihto et al. [10] showed no mutations (exons 9, 11, 13, and 17) in 31 SCLC. Mojika et al. [7] found no KIT mutations (exon 17) in 23 cases of KIT-positive SCLC. Burger et al. [9] identified no KIT gene mutations (exon 11) in 26 SCLC. The present Japanese study showed no KIT mutations (exons 9, 11, 13, and 17) in 20 SCLC. Taken together, it can be concluded that KIT gene mutations are none or very few in SCLC.
To the best of the author’s knowledge, there is only one study of PDGFRA mutations in SCLC [10]. Sihto et al. [10] found no PDGFRA mutations (exons 11 and 17) in 31 SCLC. The present case also identified no PDGFRA mutations (exons 12 and 18) in the 20 Japanese cases. Therefore, it can be concluded that PDGFRA mutations are absent in SCLC.
PDGFRA protein expression has not been reported in SCLC, to the best of the author’s knowledge. The present study, for the first time, demonstrated that 35 cases of the 54 cases (65%) were positive for PDGFRA protein. This suggests that a small amount of PDGFRA protein is present in 65 % of SCLC of Japanese patients. Clinical implications of PDGFRA have been unknown. The present study, for the first time, demonstrated that there was no correlation between PDGFRA expression and survival. Much more studies are needed in PDGFRA expression and PDFGRA gene mutations in SCLC.
Finally, the present study showed that there were no correlations between expressions of KIT and PDGFRA and gender, smoking, and disease stage.
In summary, the present study suggests, in Japanese population, that mutations of KIT and PDGFRA genes were absent in SCLC of Japan, that KIT protein expression is present in 100%, that PDGFRA protein expression is present in 65%, and that KIT and PDGFRA protein expressions do not correlate with survival, gender, smoking, and disease stage.
References
- 1.LaPoint RJ, Bourne PA, Wang HL, Xu H. Coexpression of c-kit and bcl-2 in small cell carcinoma and large cell neuroendocrine carcinoma of the lung. Appl Immunohistochem Mol Morphol. 2007;15:401–406. doi: 10.1097/01.pai.0000213153.41440.7d. [DOI] [PubMed] [Google Scholar]
- 2.Lopes-Martin A, Ballenstin C, Garcia-Carbonero R, Castano A, Lopez-Rios F, Lopes-Encuentra A, Sanchez-Cespedes M, Castellano D, Bartolomes A, Cortes Funes H, Paz-Ares L. Prognostic value of KIT expression in small cell lung carcinoma. Lung Cancer. 2007;56:405–413. doi: 10.1016/j.lungcan.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 3.Micke P, Basral M, Faldum A, Bittinger F, Ronnstrand L, Blaukat A, Beeh KM, Oesch F, Fischer B, Buhl R, Hengstler JG. Characterization of c-kit expression in small cell lung carcinoma: prognostic and therapeutic implications. Clin Cancer Res. 2003;9:188–194. [PubMed] [Google Scholar]
- 4.Camps C, Sirera R, Bremnes RM, Garde J, Safont MJ, Blasco A, Berrocal A, Sanchez JJ, Calabuig C, Marrorell M. Analysis of c-kit expression in small cell lung carcinoma: prevalence and prognostic implications. Lung Cancer. 2006;52:343–347. doi: 10.1016/j.lungcan.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Rossi G, Cavazza A, Marchioni A, Miggaldi M, Bavierli M, Fracciolongo N, Petruzzelli S, Longo L, Tamberi S, Crino L. KIT expression in small cell carcinoma of the lung: effects of chemotherapy. Mod Pathol. 2003;16:1041–1047. doi: 10.1097/01.MP.0000089780.30006.DE. [DOI] [PubMed] [Google Scholar]
- 6.Naeem M, Dahiya M, Clark JI, Creech SD, Alkin S. Analysis of c-kit protein expression in small-cell lung carcinoma and its implications for prognosis. Hum Pathol. 2002;33:1182–1187. doi: 10.1053/hupa.2002.129199. [DOI] [PubMed] [Google Scholar]
- 7.Mojica WD, Saxena R, Starostik P, Cheney RT. CD117 + small cell lung cancer lacks the asp 816 val point mutation in exon 17. Histopathology. 2005;47:517–522. doi: 10.1111/j.1365-2559.2005.02259.x. [DOI] [PubMed] [Google Scholar]
- 8.Boldrini L, Ursino S, Gisfredi S, Faviana P, Donati V, Camcci T, Lucchi M, Mussi A, Basolo F, Pingitore R, Fontanini G. Expression and mutational status of c-kit in small-cell lung cancer: prognostic relevance. Clin Cancer Res. 2004;15:4101–4108. doi: 10.1158/1078-0432.CCR-03-0664. [DOI] [PubMed] [Google Scholar]
- 9.Burger H, Den Bakker MA, Stoter G, Verweij J, Nooter K. Lack of c-kit exon 11 activating mutations in c-kit/CD117-positive SCLC tumor specimens. Eur J Cancer. 2003;39:793–799. doi: 10.1016/s0959-8049(03)00026-1. [DOI] [PubMed] [Google Scholar]
- 10.Sihto H, Sarlomo-Rikara M, Tynnienen O, Tanner M, Andersson LC, Franssila K, Nupponen NN, Joensuu H. KIT and platelet-derived growth factor receptor alpha tyrosine kinase gene mutations and KIT amplifications in human solid tumors. J Clin Oncol. 2005;23:49–57. doi: 10.1200/JCO.2005.02.093. [DOI] [PubMed] [Google Scholar]
- 11.Hirota S, Isozaki K. Pathology of gastrointestinal stromal tumor. Pathol Int. 2006;56:1–9. doi: 10.1111/j.1440-1827.2006.01924.x. [DOI] [PubMed] [Google Scholar]
- 12.Losota J, Miettinen M. KIT and PDGFRA mutations in gastrointestinal stromal tumors (GISTs) Semin Diang Pathol. 2006;23:91–101. doi: 10.1053/j.semdp.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Miettinen M, Lasota J. Gastrointestinal stromal tumor: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466–1478. doi: 10.5858/2006-130-1466-GSTROM. [DOI] [PubMed] [Google Scholar]
- 14.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shimomura Y, Kitamura Y. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumor. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 15.Hirota S, Ohashi A, Nishida T, Isozaki K, Kinoshita K, Shinomura Y, Kitamura Y. Gain-of-function mutations of platelet-derived growth factor receptor alpha gene in gastrointestinal stromal tumor. Gastroenterology. 2003;125:660–667. doi: 10.1016/s0016-5085(03)01046-1. [DOI] [PubMed] [Google Scholar]
- 16.Miettinen M, Lasota J. KIT (CD117): a review on expression in normal and neoplastic tissue, and mutations and their clinicopathologic correlation. Appl Immunohistochem Mol Morphol. 2005;13:205–220. doi: 10.1097/01.pai.0000173054.83414.22. [DOI] [PubMed] [Google Scholar]
- 17.Terada T, Kawaguchi M, Furukawa K, Sekido Y, Osamura Y. Minute mixed ductal-endocrine carcinoma of the pancreas with predominant intraductal growth. Pathol Int. 2002;52:740–746. doi: 10.1046/j.1440-1827.2002.01416.x. [DOI] [PubMed] [Google Scholar]
- 18.Terada T, Kawaguchi M. Primary clear cell adenocarcinoma of the peritoneum. Tohoku J Exp Med. 2005;271:271–275. doi: 10.1620/tjem.206.271. [DOI] [PubMed] [Google Scholar]
- 19.Terada T, Tanigichi M. Intraductal oncocytic papillary neoplasm of the liver. Pathol Int. 2004;54:116–123. doi: 10.1111/j.1440-1827.2004.01594.x. [DOI] [PubMed] [Google Scholar]
- 20.Terada T. Ductal adenoma of the breast: Immunohistochemistry of two cases. Pathol Int. 2008;58:801–805. doi: 10.1111/j.1440-1827.2008.02315.x. [DOI] [PubMed] [Google Scholar]
- 21.Gown AM, Goldstein LC, Barry TS, Kussick SJ, Kandalaft PL, Kim PM, Tse CC. High concordance between immunohistochemistry and fluolescent in situ hybridization testing for HER2 status in breast cancer requires a normalized IHC scoring system. Mod Pathol. 2008;21:1272–1277. doi: 10.1038/modpathol.2008.83. [DOI] [PubMed] [Google Scholar]
- 22.Terada T. Gastrointestinal stromal tumor of the uterus: a case report with genetic analyses of c-kit and PDGFRA genes. Int J Gynecol Pathol. 2009;28:29–34. doi: 10.1097/PGP.0b013e3181808000. [DOI] [PubMed] [Google Scholar]
- 23.Terada T. Primary extragastrointestinal stromal tumors of the transverse mesocolon without c-kit mutations but with PDGFRA mutations. Med Oncol. 2009;26:233–237. doi: 10.1007/s12032-008-9092-9. [DOI] [PubMed] [Google Scholar]
- 24.Terada T. Primary multiple extragastrointestinal stromal tumors of the omentum with different mutations of c-kit gene. Would J Gastroenterol. 2008;14:7256–7259. doi: 10.3748/wjg.14.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terada T. Mediastinal seminoma with multiple KIT gene mutations. Pathology. 2009;41:695–697. doi: 10.3109/00313020903305852. [DOI] [PubMed] [Google Scholar]
- 26.Terada T. Mutations and protein expression of KIT and PDGFRA genes in ipsilateral testicular seminomas: an immunohistochemical and molecular genetic study. Appl Immunohisto-Chem Mol Morphol. 2011;19:450–453. doi: 10.1097/PAI.0b013e31820d2872. [DOI] [PubMed] [Google Scholar]
- 27.Terada T. Primary small cell carcinoma of the mediastinum: A case report with immunohistochemical and molecular genetic analyses of KIT and PDGFRA genes. Med Oncol. 2009;26:247–250. doi: 10.1007/s12032-008-9116-5. [DOI] [PubMed] [Google Scholar]
- 28.Terada T. Primary small cell carcinoma of the ureter: A case report involving immunohistochemical and molecular genetic analyses of KIT and PDGFRA genes. Pathology. 2010;42:101–102. doi: 10.3109/00313020903443018. [DOI] [PubMed] [Google Scholar]
- 29.Terada T. Autopsy case of primary small cell carcinoma of the urinary bladder: KIT and PDGFRA expression and mutations. Pathol Int. 2009;59:247–250. doi: 10.1111/j.1440-1827.2009.02358.x. [DOI] [PubMed] [Google Scholar]
- 30.Terada T. KIT and PDGFRA in esophageal small cell carcinoma. Int J Clin Exp Pathol. 2011;4:718–721. [PMC free article] [PubMed] [Google Scholar]
- 31.Terada T. Low incidence of KIT gene mutations and no PDGFRA gene mutations in primary cutaneous melanoma: an immunohistochemical and molecular genetic study of Japanese cases. Int J Clin Oncol. 2010;15:453–456. doi: 10.1007/s10147-010-0087-0. [DOI] [PubMed] [Google Scholar]
- 32.Terada T. Amelanotic malignant melanoma of the esophagus: report of two cases with immunohistocheimcal and molecular genetic study of KIT and PDGFRA . World J Gastroenterol. 2009;15:2679–2683. doi: 10.3748/wjg.15.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terada T. Gastrointestinal stromal tumor of the digestive organs: a histopathologic study of 31 cases in a single Japanese institute. Int J Clin Exp Pathol. 2010;3:162–168. [PMC free article] [PubMed] [Google Scholar]
- 34.Terada T. Primary small cell carcinoma of the pleura: A case report with immunohistochemical and molecular genetic analyses of KIT and PDGFRA genes. Med Oncol. 2010;27:1119–1122. doi: 10.1007/s12032-009-9345-2. [DOI] [PubMed] [Google Scholar]
- 35.Terada T. Large cell neuroendocrine carcinoma with sarcomatous changes of the endometrium: a case report with immunohistochemical studies and molecular genetic study of KIT and PDGFRA. Pathol Res Pract. 2010;206:420–425. doi: 10.1016/j.prp.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 36.Rohr UP, Rehfeld N, Pflugfelder L, Geddert H, Muller W, Steidl U, Fenk R, Graf T, Schott M, Thiele KP, Gabbert HE, Germing U, Kronenwett R, Haas R. Expression of the tyrosine kinase c-kit is an independent prognostic factor in patients with small cell lung cancer. Int J Cancer. 2004;20:259–263. doi: 10.1002/ijc.20252. [DOI] [PubMed] [Google Scholar]


