Abstract
Human aldo-keto reductase family 1 member C3 (AKR1C3) was initially identified as a critical enzyme in reducing 5α-dihydrotestosterone (5α-DHT) to 5α-androstane-3α,17β-diol (3α-diol) and oxidizing 3α-diol to androsterone. Based on these enzymatic activities, AKR1C3 was originally named type 2 3α-hydroxysteroid dehydrogenase (HSD)/type 5 17β-HSD. Additionally, AKR1C3 was demonstrated to be capable of metabolizing other steroids including estrogen and progesterone. Subsequently, AKR1C3 was shown to possess 11-ketoprostaglandin reductase activity in metabolizing prostaglandins and dihydrodiol dehydrogenase x (DDx) activity in metabolizing xenobiotics. Tissue distribution of AKR1C3 has been detected in both sex hormone-dependent organs such as the testis, breast, endometrium, and prostate as well as sex hormone-independent organs including the kidney and urothelium. Although prominent expression of AKR1C isozymes has been reported in human non-small cell lung carcinoma (NSCLC), the expression of AKR1C3 in small cell carcinoma of the lung has not been described. Also, the expression of AKR1C3 in normal lung has not been described. In this study, we demonstrated strong AKR1C3 immunoreactivity in bronchial epithelium but not in bronchial glands or alveolar pneumocytes. Strong AKR1C3 immunoreactivity was also demonstrated in columnar epithelium but only weak immunoreactivity in squamous epithelium of the gastrointestinal junction. Although AKR1C3 immunoreactivity was absent in small cell carcinoma of the lung, positive AKR1C3 immunoreactivity was extensively present in both adenocarcinoma and squamous cell carcinoma arising from the lung and the gastroesophageal junction. AKR1C3 may serve as an adjunct marker for differentiating small cell carcinoma from NSCLC. However, roles of AKR1C3 in adenocarcinoma, squamous cell carcinoma, and small cell carcinoma pathogenesis require further studies.
Keywords: AKR1C3, small cell carcinoma, non-small cell carcinoma, adenocarcinoma, squamous cell carcinoma, lung, esophagus, stomach, gastroesophageal junction
Introduction
As a member of the aldo-keto reductase (AKR) superfamily (www.med.upenn.edu/akr) [1], AKR family 1 member C3 (AKR1C3) was originally cloned from human liver and prostate cDNA libraries [2,3] and named type 2 3α-hydroxysteroid dehydrogenase (3α-HSD)/type 5 17β-HSD. AKR1C3 is a monomeric cytoplasmic protein with 323 amino acids, a molecular weight of 37 kDa [4,5], and shares high sequence homology with related human AKR1C1 (20α-HSDs), AKR1C2 (type 3 3α-HSD) and AKR1C4 (type 1 3α-HSD) [6]. Recombinant AKR1C3 reduces aldehydes and/or ketones to their corresponding alcohols through oxidation of NADH or NADPH to NAD+ and NADP+ [3]. AKR1C3 was originally shown to have the capability to inactivate the most potent androgen, 5α-dihydrotestosterone (5α-DHT), to form weak androgens, 5α-androstane-3α,17β-diol (3α-diol) , 5α-androstan-3α-ol-17-one (androsterone), and 5α-androstan-3,17-dione (androstanedione) and may regulate ligand availability for the androgen receptor (AR).
In addition to androgen metabolism, AKR1C3 was shown to be an enzyme possessing 3α-HSD, 3β-HSD, 17β -HSD, and 11-ketoprostaglandin reductase activities [3,4,6,7]. These enzymatic properties allow AKR1C3 to metabolize estrogen, progesterone, and prostaglandins (PGs), and may indirectly govern the amount of ligands available to various nuclear receptors, including estrogen receptor (ER), progesterone receptor (PR), and nuclear orphan receptors such as the peroxisome proliferator-activated receptor (PPAR) [8,9]. As a result, AKR1C3 can be an intracellular modulator in regulating trans-activation activities of these nuclear receptors in target organs [10].
Tissue distribution of AKR1C3 has been demonstrated in sex hormone-dependent organs including breast [4], endometrium [11,12], prostate [4,5], and testis [13]. De-regulated expression of AKR1C3 has been established in multiple types of hormone-related cancers, including breast cancer [14], endometrial cancer [11,12,15], and prostate cancer [5,16-18]. In addition, AKR1C3 expression has been studied in sex hormone-independent tissues including urothelial epithelium [5] and epithelium of renal tubules [19]. Abnormal AKR1C3 expression has been observed in hormone-independent cancers, including urothelial carcinoma, renal cell carcinoma [5], brain tumor [20], and Wilms’ tumor [21]. Roles of AKR1C3 in the development and progression of these hormone-dependent and independent cancers remain unclear.
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous environmental pollutants including tobacco carcinogens implicated in the causation of human lung cancer. PAHs need to be metabolically activated to exert their carcinogenic effects. Dihydrodiol dehydrogenases (DDs), members of the AKR superfamily, represents a major pathway responsible for PAH activation [22], and divert PAH trans-dihydrodiols to corresponding o-quinones [23]. Homogenous recombinant AKR1C3 can oxidize a wide variety of PAH trans-dihydrodiols and yield of o-quinones [22]. While expression of AKR1C isozymes (DDs) in non-small cell lung carcinoma and cell lines have been described [24,25], AKR1C3-specific expression in lung cancer has not been reported.
In this investigation, we studied spatial distributions of AKR1C3 in normal lung and gastroesophageal junction as well as in small cell and non-small cell carcinoma residing in these locations. A total of 15 cases of small cell carcinoma, 14 cases of adenocarcinoma, and 18 cases of squamous cell carcinoma of the lung were studied. The expression of AKR1C3 in 15 cases of adenocarcinoma and 15 cases of squamous cell carcinoma of the gastroesophageal junction were also studied for comparison.
Materials and methods
Human normal and neoplastic tissue
A total of 77 separate cases of archival, formalin fixed, paraffin embedded biopsy or resection specimens were retrieved. All specimens were obtained through the Department of Pathology at the University of Oklahoma Health Sciences Center with Institutional Review Board (IRB) approval. Cases were limited to those with indisputable diagnostic features, containing sufficient amount of tissues, and free of excessive artifacts. All tumors were primary tumors and all except one were untreated tumors. Normal tissue sections were obtained from histological normal areas within these cases. This consortium included 15 small cell carcinomas, 14 adenocarcinomas, and 18 squamous cell carcinomas of the lung, as well as 15 of adenocarcinomas and squamous cell carcinomas respectively arising from the gastroesophageal junction (Table 1). The diagnosis was reconfirmed by a pathologist in this study (KMF).
Table 1.
Expression of AKR1C3 in carcinomas
| Case | Age | Sex | Procedure | Differentiation | Score |
|---|---|---|---|---|---|
| Lung, Squamous Cell Carcinoma | |||||
| 1 | 82 | M | Rs | MD | ●●●●● |
| 2 | 87 | F | Rs | MD | ●●●●● |
| 3 | 88 | M | Rs | MD | ●●●●● |
| 4 | 92 | M | Rs | MD | ●●●●● |
| 5 | 70 | M | Rs | MD | ●●●●● |
| 6 | 77 | F | Rs | MD | ●●●●● |
| 7 | 66 | M | Rs | MD | ●●●●● |
| 8 | 67 | M | Rs | PD | ●●●●● |
| 9 | 59 | F | Rs | MD | ●●●●● |
| 10 | 56 | F | Rs | PD | ●●●●● |
| 11 | 47 | M | Rs | MD | ●●●●● |
| 12 | 81 | F | Rs | PD | ●●●●● |
| 13 | 74 | F | Rs | PD | ●●●● |
| 14 | 73 | M | Rs | MD | ●●●● |
| 15 | 74 | F | Rs | PD | ●●● |
| 16 | 71 | F | Rs | PD | ● |
| 17 | 56 | M | Rs | MD | ● |
| 18 | 69 | F | Rs | PD | - |
| Lung, Adenocarcinoma | |||||
| 1 | 80 | F | Rs | PD | ●●●●● |
| 2 | 63 | M | Rs | MD | ●●●●● |
| 3 | 53 | F | Rs | MD | ●●●●● |
| 4 | 54 | M | Rs | PD | ●●●●● |
| 5 | 71 | M | Rs | MD | ●●●● |
| 6 | 59 | F | Rs | MD | ●●● |
| 7 | 83 | F | Rs | PD | ●●● |
| 8 | 76 | M | Rs | PD | ●●● |
| 9 | 81 | F | Rs | PD | ●● |
| 10 | 85 | F | Rs | PD | ●● |
| 11 | 62 | F | Rs | PD | ●● |
| 12 | 64 | F | Rs | WD | ●● |
| 13 | 70 | M | Rs | PD | ● |
| 14 | 86 | M | Rs | WD | - |
| Lung, Small Cell Carcinoma | |||||
| 1 | 73 | M | Bx | NA | - |
| 2 | 77 | M | Bx | NA | - |
| 3 | 50 | F | Bx | NA | - |
| 4 | 77 | F | Bx | NA | - |
| 5 | 86 | M | Bx | NA | - |
| 6 | 55 | M | Bx | NA | - |
| 7 | 63 | F | Bx | NA | - |
| 8 | 47 | M | Bx | NA | - |
| 9 | 71 | F | Bx | NA | - |
| 10 | 47 | M | Bx | NA | - |
| 11 | 58 | F | Bx | NA | - |
| 12 | 68 | M | Bx | NA | - |
| 13 | 57 | F | Bx | NA | - |
| 14 | 63 | M | Rs | NA | - |
| 15 | 56 | F | Bx | NA | - |
| Gastroesophageal Junction, Squamous cell Carcinoma | |||||
| 1 | 71 | M | Rs | PD | ●●●●● |
| 2 | 64 | F | Bx | MD | ●●●●● |
| 3 | 69 | F | Bx | MD | ●●●●● |
| 4 | 64 | F | Rs | MD | ●●●●● |
| 5 | 75 | F | Rs | MD | ●●●● |
| 6 | 71 | M | Rs | PD | ●●●● |
| 7 | 65 | M | Bx | MD | ●●●● |
| 8 | 59 | M | Bx | MD | ●●●● |
| 9 | 58 | M | Bx | MD | ●●● |
| 10 | 59 | F | Bx | MD | ●●● |
| 11 | 91 | M | Bx | PD | ● |
| 12 | 78 | F | Rs | PD | ● |
| 13 | 60 | F | Bx | MD | - |
| 14 | 81 | F | Bx | MD | - |
| 15 | 79 | M | Bx | MD | - |
| Gastroesophageal Junction, Adenocarcinoma | |||||
| 1 | 61 | M | Rs | MD | ●●●●● |
| 2 | 53 | M | Rs | MD | ●●●●● |
| 3 | 79 | M | Mr | MD | ●●●●● |
| 4 | 47 | M | Rs | MD | ●●●●● |
| 5 | 47 | M | Rs | PD | ●●●●● |
| 6 | 77 | F | Rs | PD | ●●●●● |
| 7 | 53 | M | Rs | MD | ●●●●● |
| 8 | 64 | M | Rs | PD | ●●●●● |
| 9 | 60 | M | Rs | PD | ●●●●● |
| 10 | 79 | M | Rs | PD | ●●●● |
| 11 | 84 | M | Rs | MD | ●●●● |
| 12 | 66 | M | Rs | PD | ●●●● |
| 13 | 83 | M | Rs | PD | ●●● |
| 14 | 37 | M | Rs | MD | ●●● |
| 15 | 55 | M | Rs | PD | ●●● |
Intensity of Immunoreactivity: 0: - (negative); 1: ● (weak); 2: ● (moderate); 3: ● (strong). Percentage of Immunoreactivity: - Totally negative; ●Positivity ≤5%; ●●Positivity >5% to ≤25%; ●●●Positivity >25% to ≤75%; ●●●●Positivity >75% to ≤100%; ●●●●●Positivity is 100%. Procedure: Bx: Biopsy; Rs: Resection (esophagogastrectomy or lobectomy); Mr: Mucosal resection. Differentiation: WD: Well-differentiated; MD: Moderately differentiated; PD: Poorly differentiated
Antibody and immunohistochemistry
Monospecific mouse anti-AKR1C3 monoclonal antibody was produced and characterized in our laboratory as described [4]. Paraffin sections were cut at 5 μm thick, deparaffinized and rehydrated. Immunohistochemistry was performed by an automated staining machine (Benchmark, Ventana, Tucson, AZ) with a CC1-medium antigen retrieval protocol described by the manufacture. Primary antibody was applied at 1:200 dilution and incubated for 90 min. Diaminobenzidine and hematoxylin were used as chromogen and counter stain, respectively.
Immunohistochemical evaluation and scoring
The stained tissue sections were evaluated with a conventional light microscope independently by two of the investigators (VLM, KMF). The percentage of positive cells within the entire tumors were evaluated and allocated to one of the following categories: completely negative, ≤ 5% positive immunoreactivity, > 5% but ≤ 25% positive immunoreactivity, > 25% but ≤ 75% positive immunoreactivity, > 75% but ≤ 100% positive immunoreactivity, and 100% positive immunoreactivity. The intensity of staining was also scored as weak, moderate, or strong for every case. In cases with more than one level of intensity, the staining intensity with the largest area was considered the final score.
Results
Normal lung
Bronchial epithelium (Figure 1A and 1B) including the cilia were immunoreactive for AKR1C3. Endothelial cells were immunoreactive but the supporting stroma was largely negative. Most of the seromucinous glands within the supporting mucosa were negative. Weak positive immunoreactivity was demonstrated in the cytoplasm and nuclei of scant glandular epithelial cells (Figure 1A and 1C). In the alveoli, type II pneumocytes were non-reactive but cytoplasmic immunoreactivity was noted in the alveolar macrophages (Figure 1D and 1E). Similar to our prior observations, the immunoreactivity was mainly cytoplasmic but some nuclear immunoreactivities were also noted.
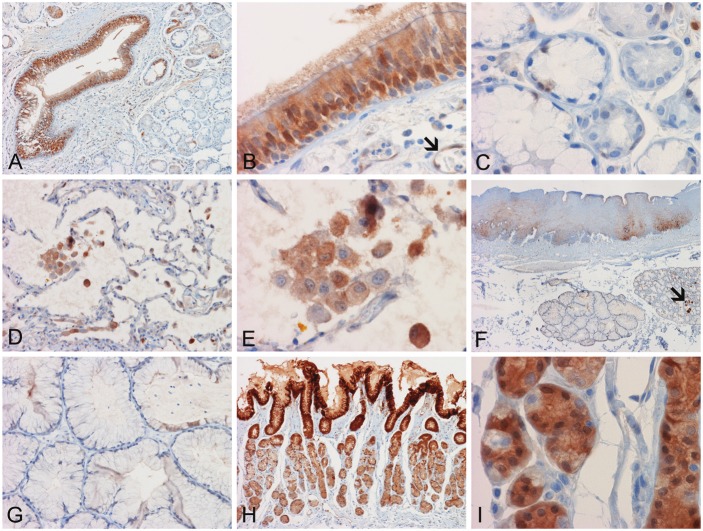
Figure 1.
Expression of AKR1C3 in normal tissue. (A) The bronchial epithelium is strongly positive but normal bronchial glands are negative. Occasional bronchial glands with atrophic changes are positive. (B) Positive cytoplasmic and nuclear immunoreactivity are noted in the bronchial epithelial cells. Endothelial cells are also positive (arrow). (C) Both serous and mucinous cells of the bronchial glands are negative in this field. (D) The type II pneumocytes lining the alveoli are negative while alveolar macrophages are positive. (E) Note the predominantly cytoplasmic and occasional nuclear positive immunoreactivity present in the macrophages. (F) Squamous epithelium of esophagus is negative in the more superficial layers but some weak and patchy positive immunoreactivity is noted in the more basally located layers. While the esophageal glands are negative, some of the secretions are positive (arrow). (G) The esophageal glands are negative. (H) The gastric type epithelium at the gastroesophageal junction is strongly positive. The more superficially located epithelial cells are more strongly positive than those in the deeper locations. (I) Gastric glandular epithelial cells in the deeper locations are positive but occasional cells are negative. Panel (F) to (I) are located adjacent to each other on the same slide. (Original magnification is 4x for panel C, 10x for panel H, 20x for panel A, D, and G, 60x for panel B, C, E, and I)
Normal gastroesophageal junction
The superficial layers of the squamous epithelium were negative, whereas the deeper layers of squamous epithelium had patchy positive immunoreactivity (Figure 1F). In general, the esophageal glands were negative but weak positive immunoreactivitiy was noted in occasional glandular epithelial cells with atrophic changes (Figure 1F and 1G). Contents in the lumen of the glands were positive in some of the glands. Strong AKR1C3 immunoreactivity was detected in the gastric type epithelium at the gastroesophageal junction. The superficially located epithelium tended to be stronger in immunoreactivity than those located in the deeper parts of the glands (Figure 1H and 1I). In the deeper part of the gastric glands, occasional negative glandular epithelial cells among strongly positive epithelial cells were identified (Figure 1I).
Squamous cell carcinoma of lung
Of the 18 cases of squamous cell carcinoma of the lung, 12 (66.6%) showed 100% AKR1C3 positive immunoreactivity and, all except one, demonstrated strong immunoreactivity (Figure 2A). Another 3 cases (16.6%) showed substantial amounts of immunoreactivity (> 25%) and at least medium intensity (Figure 2B); and the remaining 3 tumors (16.6%), 1 case exhibited either small amount of positive immunoreactivity (< 5%), 1 case expressed weak immunoreactivity (Figure 2C), and 1 case is completely negative. Detailed AKR1C3 immunoreactivity is described in Table 1.
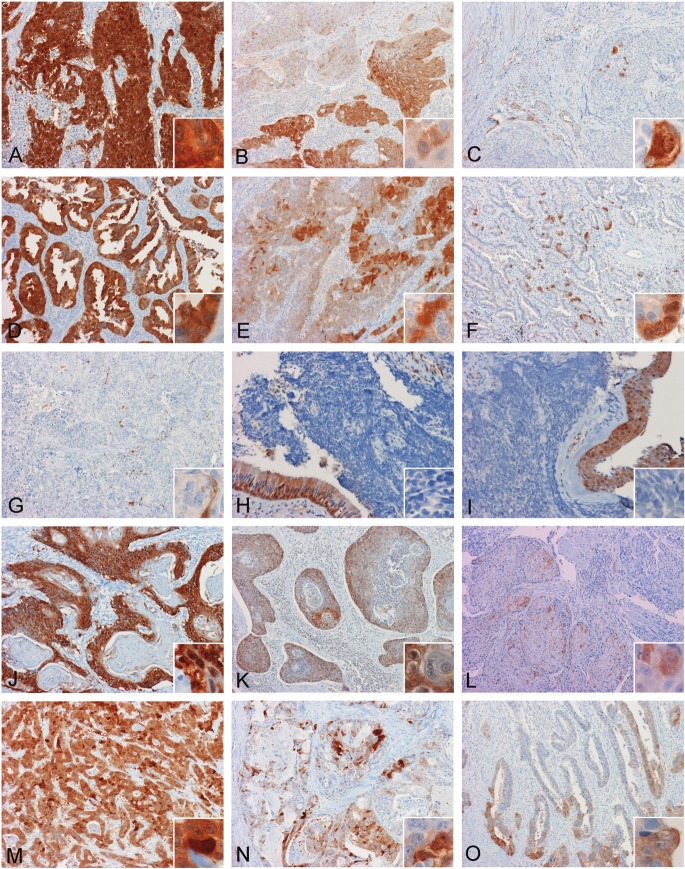
Figure 2.
Expression of AKR1C3 in tumors. (A, B, C) are three different cases of squamous cell carcinoma and (D, E, F) are three different cases of adenocarcinocarcinomas arising in lung with diffuse, widespread, and focal positive immunoreactivity respectively. Note that in the less extensively stained cases, strongly positive tumor cells can be found immediately adjacent to negatively stained cells. (G, H, I) are three different cases of small cell carcinoma of lung. Note that all of these tumors are negative. The scant positive immunoreactivity in (G) represents positive endothelial cells (inset in G). The overlying residual positive bronchial epithelium (insets in H and I) serves as positive internal control. (A, B, C) are three different cases of squamous cell carcinoma and (D, E, F) are adenocarcinocarcinomas arising at the gastroesophageal junction with diffuse, widespread, and focal positive immunoreactivity respectively. Note that in the less extensively stained cases, strongly positive tumor cells are often found immediately adjacent to negative cells. (Original magnification is 10x for panel A to F and J to O, 20x for panel G to I, and 60x for all insets)
Adenocarcinoma of lung
Of the 14 cases of adenocarcinoma of the lung, strong and 100% immunoreactivity was demonstrated in 4 cases (28.6%) (Figure 2D). Focal immunoreactivity (positivity > 5% but ≤ 25%) medium to strong positive immunoreactivity was demonstrated in 7 cases (50%) (Figure 2E). The remaining 3 cases exhibited various degrees of AKR1C3 immunoreactivity: 1 case with > 5% but ≤ 25% weak positivity, 1 case with 5% or less positivity but the immunoreactivity was strong (Figure 2F), and 1 case was completely negative (Table 1).
Small cell carcinoma of lung
All 15 cases of small cell carcinoma were completely negative in AKR1C3 immunoreactivity (Table 1). However, positive immunoreactivity was detected in the entrapped blood vessels (Figure 2G) and residual respiratory type mucosa (Figure 2G, 2H, and 2I).
Squamous cell carcinoma of gastroesophageal junction
Among the 15 cases of squamous cell carcinoma arising in the gastroesophageal junction (Table 1), 4 cases (26.7%) showed 100% medium to strong immunoreactivity (Figure 2J). Substantial amount of positive immunoreactivity (> 25%) and medium intensity were observed in 6 cases (40%) (Figure 2K). One case (6.7%) had small amount (< 5%) but strong immunoreactivity; and another case (6.7%) had small amount (< 5%) of weak immunoreactivity (Figure 2L). The remaining 3 cases (20%) were completely negative.
Adenocarcinoma of gastroesophageal junction
In the 15 cases of adenocarcinoma of the gastroesophageal junction, 9 of the cases (60%) showed 100% strong to medium positive immunoreactivity (Figure 2M). Another 5 cases (33.3%) demonstrated substantial positive immunoreactivity (> 25%) with medium to strong intensity (Figure 2N). One of these cases (6.7%) showed at least 25% positivity but weak immunoreactivity (Figure 2O). There was no completely negative case (Table 1).
Discussion
As per our immunohistochemical investigation on spatial and cancer-specific distributions of AKR1C3, we demonstrated positive immunoreactivity on bronchial epithelium but not bronchial glands or type II pneumocytes. AKR1C3 was extensively and strongly expressed in columnar epithelium at the gastroesophageal junction. In contrast, expression was patchy and limited to the deeper layers of squamous cell mucosa. Stromal cells were negative. Similar to our earlier observations in other types of tissues [5,12,19], AKR1C3 positive immunoreactivity was observed in endothelial cells.
AKR1C3 was extensively expressed in both adenocarcinoma and squamous cell carcinoma arising in the lung and gastroesophageal junction but was absent in small cell carcinoma. All of the 15 cases (100%) of small cell carcinoma were negative. Positive immunoreactivity was demonstrated in 13 out of the 14 cases (92.8%) of adenocarcinoma and 17 out of the 18 cases (94.4%) of squamous cell carcinoma arising from the lung; and all 15 cases (100%) of adenocarcinoma arising from the gastroesophageal junction. Although positive immunoreactivity is only limited in normal gastroesophageal squamous cell mucosa, positivity was present in 12 out of the 15 cases (80%) of squamous cell carcinoma arising from the gastroesophageal junction.
Differentiation of small cell carcinoma from NSCLC carries significant clinical implications because of the differences in treatment and prognosis. The bulk of NSCLC is composed of squamous cell carcinoma and adenocarcinoma. As most carcinomas of the lung are diagnosed by small biopsies, histologic diagnosis could be challenging in some cases. Immunohistochemical confirmation is a common practice in these challenging cases. Currently, a panel of molecular markers is used and this differentiation includes molecules specific for neuroendocrine phenotypes such as synaptophysin and chromogranin [26], squamous cell differentiation such as p63 protein [26] and cytokeratin 5/6 [26], and adenocarcinoma such as thyroid transcription-1 and Napsin A [27]. In addition, mucicarmine stain can be helpful in identifying adenocarcinoma by demonstrating mucin in adenocarcinomas that produce mucin. AKR1C3 can be used in conjunction with the existing markers in differentiating small cell carcinoma from NSCLC.
Tobacco smoke and air pollutants contain many kinds of PAHs including benz[a]anthracene (BA) and benzo[a]pyrene (BaP). These compounds require activation to electrophilic metabolites to exert their carcinogenic or mutagenic effects. AKR1C3 may play an important role in BA and BaP carcinogenesis via oxidative DNA damage by catalyzing NAD(P)+-linked oxidation of dihydrodiols of aromatic hydrocarbons to corresponding catechols [28]. The DNA damage caused through the production of reactive metabolites of PAH involve DNA covalent binding to form stable or depurinating adducts, formation of apurinic sites, and oxidative damage [29]. Levels of AKR1C isoenzyme expression are negatively correlated with the formation of BaP DNA adducts in lung cancer cell lines [30], but positively correlated with oxidative stress [31]. Based on the AKR1C3-mediated PAH metabolism, elevated expression of AKR1C3 in NSCLC suggests that AKR1C3-mediated pathway may contribute to PAH activation in NSCLC. Although elevated AKR1C isoforms expression has been positively correlated with higher incidence of early tumor recurrence, distant metastasis, and poor prognosis of patients with advanced NSCLC [24], pathological roles of AKR1C3-mediated PAH activation in NSCLC development and progression require further study.
One study suggests that AKR1C3 modulates risks for lung cancer due to exposure to PAH; and subjects with AKR1C3-Gln/Gln genotype have a higher risk of lung cancer [32]. A high level of AKR1C isozymes expression has also been shown in A549 cells, a human lung adenocarcinoma cell line [33]. Thus, a mechanism of AKR1C-mediated carcinogenesis in the lung has been proposed. PAH and reactive oxygen species (ROS) induce the expression of AKR1C isozymes. While AKR1C isozymes provides a defense mechanism against the harmful effects of ROS, the increased level of AKR1C isozymes can divert PAH trans-dihydrodiols from P450 peroxidases or CYP superfamily proteins to the deleterious o-quinones which would cause covalent and oxidative DNA damage if they are not eliminated promptly.
Our results of widespread AKR1C3 expression in NSCLC are consistent with the observation of DDs overexpression in NSCLC by Hsu et al. [24]. In their study, elevated expression of AKR1C transcripts was detected in primary NSCLC by mRNA differential display and confirmed by in situ hybridization using a fluorescence-labeled AKR1C antisense probe; but expression of these mRNA species in normal bronchial epithelium was not described. In a separate study, the expression of DDs was found to be higher in squamous cell carcinoma as determined by immunohistochemical staining using a mouse polyclonal antibody raised against recombinant AKR1C1 [25]. Due to a high nucleotide sequence identity and high amino acids similarities among AKR1C isoforms, in situ hybridization or polyclonal antibody against AKR1C1 cannot be sufficient to identify isoform-specific tissue distribution, but identify all AKR1C isoforms. The monoclonal antibody used in the study has AKR1C3 monospecificity, and does not cross-react with other identified AKR1C isoforms [4].
In addition to PAH metabolism, AKR1C iszymes could be involved in drug detoxification. The expression levels of AKR1C transcripts are positively correlated with the cells’ resistance to cisplatin, adriamycin and radiotherapy in multiple human lung adenocarcinoma cell lines [34], and resistance to daunorubicin in the human stomach carcinoma cell line EPG85-257 [35]. It has also been demonstrated that elevated AKR1C isozymes expression leads to the induction of resistance to platinum-based drugs in human ovarian, lung, cervical, and germ cell tumor cell lines [36,37]; and forced expression of AKR1C3 in human ovarian cells confers a cisplatin resistant phenotype with concomitant generation of ROS [38]. In contrast to keratinocytes, fibroblast, and basal cell carcinoma cell lines that have undetectable or low levels of AKR1C, the human epidermoid carcinoma A431 cells constitutively express high levels of AKR1C isozymes. Targeted suppression of AKR1C expression in A431 cells using small interfering RNA technique significantly increased sensitivity to UVB-induced apoptosis and cytotoxicity of chemotherapeutic agent bleomycin [39]. Elevated expression of AKR1C is also identified in ethacrynic acid-induced drug-resistant human colon cancer HT29 cells using mRNA differential display [40]. The impact of AKR1C3 on drug resistance of lung cancer cells, however, remains to be clarified as to whether this is a mechanism of drug inactivation and detoxification.
Epidemiological studies indicate that there is a gender difference in the susceptibility to tobacco and environmental carcinogens. This gender difference is suspected to result in a higher risk for lung cancer incidence among women. However, molecular mechanisms underlying this sexual dimorphism remain unclear. Estrogen metabolism may contribute to lung cancer in non-smoking women. The ERβ regulates lung development, in particular, alveolar formation and surfactant homeostasis, and has been shown to correlate with the expression of Phase I/II carcinogen-metabolizing enzymes [41,42]. In lung tumors, overexpression of ERβ has been observed, and this difference is significantly more common in tumors from nonsmokers (53.5%) than in smokers (36.6%). Among nonsmokers, higher ERβ expression was reported significantly more frequently in female patients (58.3%) than in male patients [43]. In addition, levels of PR transcripts were found to be significantly less in cancerous as compared to nonmalignant lung tissue. Using immunohistochemistry, expression of PR was observed in the nucleus and extra nuclear compartments in the majority of human tumor specimens examined. Combinations of estrogen and progestin administration promote secretion of vascular endothelial growth factor (VEGF) by tumor cells and support tumor-associated angiogenesis in vitro [44]. Furthermore, simultaneous administration of estradiol and progestin increased the numbers of putative tumor stem/progenitor cells. Thus, ER- and/or PR-targeted therapies have been proposed as new approaches to manage NSCLC. It has also been reported that there is an increased incidence of lung cancer associated with increasing duration of estrogen plus progestin use, but no association with duration of unopposed estrogen use [45]. AKR1C3 is involved in estrogen and progesterone metabolism and is capable of regulating the occupancy of the ER and PR. The study of AKR1C3-regulated estrogen and progesterone levels, as well as ER and PR trans-activation in the development and progression of lung cancer may delineate important pathways involved in hormone metabolism and lung cancer.
In conclusion, AKR1C3 immunoreactivity was specifically detected in squamous cell carcinoma and adenocarcinoma of the lung and gastroesophageal junction but not in small cell carcinoma of the lung. Thus, AKR1C3 and other AKR1C isozymes might be used as adjunct markers to differentiate small cell carcinoma from adenocarcinoma and squamous cell carcinoma which constitute the bulk of NSCLC. AKR1C3 has multiple substrates including steroids, PG, PAH, and cancer chemotherapeutic drugs. Preliminary evidence proposes that AKR1C3 may contribute to the defense of ROS in carcinogenesis but its significance in carcinogenesis remain unclear. The widespread expression of AKR1C3 in columnar epithelium of both the bronchial epithelium and the gastrointestinal junction suggest that it may not only play a role as a chemical barrier to xenobiotics but may also be involved in lung carcinogen metabolism. These biological and pathological properties of the multifunctional enzyme are worthwhile for future investigations.
Acknowledgement
We would like to express our most sincere thanks to the technical assistance of Ms. Kelly Smith, Jeanne Frazer, Angela Graff, Crystal Glass, Linda Freeman, and other staff of the histology laboratory of OU Medical Center. We also thank the technical assistance of Mr. Howard Doughty of the Department of Pathology, University of Oklahoma Health Sciences Center. This project is supported in part by an internal grant awarded to KMF and PM and a scholarship awarded to VLM. The project was also supported by P30 ES013508 awarded to TMP.
Abbreviations
- 3α-diol
5α-androstane-3α, 17β-diol
- AKR
alde-keto reductase
- DD
dihydrodiol dehydrogenase
- ER
estrogen receptor
- HSD
hydroxysteroid dehydrogenase
- NSCLC
non-small cell lung carcinoma
- PAH
polycyclic aromatic hydrocarbon
- PG
prostaglandin
- PR
progesterone receptor
- ROS
reactive oxygen species
References
- 1.Jez JM, Flynn TG, Penning TM. A new nomenclature for the aldo-keto reductase superfamily. Biochem Pharmacol. 1997;54:639–647. doi: 10.1016/s0006-2952(97)84253-0. [DOI] [PubMed] [Google Scholar]
- 2.Khanna M, Qin KN, Wang RW, Cheng KC. Substrate specificity, gene structure, and tissue-specific distribution of multiple human 3a-hydroxysteroid dehydrogenases. J Biol Chem. 1995;270:20162–20168. doi: 10.1074/jbc.270.34.20162. [DOI] [PubMed] [Google Scholar]
- 3.Lin HK, Jez JM, Schlegel BP, Peehl DM, Pachter JA, Penning TM. Expression and characterization of recombinant type 2 3a-hydroxysteroid dehydrogenase (HSD) from human prostate: demonstration of bifunctional 3a/17b-HSD activity and cellular distribution. Mol Endocrinol. 1997;11:1971–1984. doi: 10.1210/mend.11.13.0026. [DOI] [PubMed] [Google Scholar]
- 4.Lin HK, Steckelbroeck S, Fung KM, Jones AN, Penning TM. Characterization of a monoclonal antibody for human aldo-keto reductase AKR1C3 (type 2 3a-hydroxysteroid dehydrogenase/type 5 17b-hydroxysteroid dehydrogenase); immunohistochemical detection in breast and prostate. Steroids. 2004;69:795–801. doi: 10.1016/j.steroids.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Fung KM, Samara ENS, Wong C, Metwalli A, Krlin R, Bane B, Liu CZ, Yang JT, Pitha JT, Culkin DJ, Kropp BP, Penning TM, Lin HK. Increased expression of type 2 3a-hydroxysteroid dehydrogenase/type 5 17b-hydroxysteroid dehydrogenase (AKR1C3) and its relationship with androgen receptor in prostate carcinoma. Endocr Relat Cancer. 2006;13:169–180. doi: 10.1677/erc.1.01048. [DOI] [PubMed] [Google Scholar]
- 6.Penning TM, Burczynski ME, Jez JM, Hung CF, Lin HK, Ma H, Moore M, Palackal N, Ratnam K. Human 3a-hydroxysteroid dehydrogenase isoforms (AKR1C1-AKR1C4) of the aldo-keto reductase superfamily: functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem J. 2000;351:67–77. doi: 10.1042/0264-6021:3510067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuura K, Shiraishi H, Hara A, Sato K, Deyashiki Y, Ninomiya M, Sakai S. Identification of a principal mRNA species for human 3a-hydroxysteroid dehydrogenase isoform (AKR1C3) that exhibits high prostaglandin D2 11-ketoreductase activity. J Biochem (Tokyo) 1998;124:940–946. doi: 10.1093/oxfordjournals.jbchem.a022211. [DOI] [PubMed] [Google Scholar]
- 8.Rizner TL, Lin HK, Peehl DM, Steckelbroeck S, Bauman DR, Penning TM. Human type 3 3a-hydroxysteroid dehydrogenase (aldo-keto reductase 1C2) and androgen metabolism in prostate cells. Endocrinology. 2003;144:2922–2932. doi: 10.1210/en.2002-0032. [DOI] [PubMed] [Google Scholar]
- 9.Byrns MC, Duan L, Lee SH, Blair IA, Penning TM. Aldo-keto reductase 1C3 expression in MCF-7 cells reveals roles in steroid hormone and prostaglandin metabolism that may explain its over-expression in breast cancer. J Steroid Biochem Mol Biol. 2010;118:177–187. doi: 10.1016/j.jsbmb.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Penning TM, Steckelbroeck S, Bauman DR, Miller MW, Jin Y, Peehl DM, Fung KM, Lin HK. Aldo-keto reductase (AKR) 1C3: Role in prostate disease and the development of specific inhibitors. Mol Cell Endocrinol. 2006;248:182–191. doi: 10.1016/j.mce.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Rizner TL, Smuc T, Rupreht R, Sinkovec J, Penning TM. AKR1C1 and AKR1C3 may determine progesterone and estrogen ratios in endometrial cancer. Mol Cell Endocrinol. 2006;248:126–135. doi: 10.1016/j.mce.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Zakharov V, Lin HK, Azzarello J, McMeekin S, Moore KN, Penning TM, Fung KM. Suppressed expression of type 2 3a/type 5 17b-hydroxysteroid dehydrogenase (AKR1C3) in endometrial hyperplasia and carcinoma. Int J Clin Exp Pathol. 2010;3:608–617. [PMC free article] [PubMed] [Google Scholar]
- 13.Ashley RA, Yu Z, Fung KM, Frimberger D, Kropp BP, Penning TM, Lin HK. Developmental evaluation of aldo-keto reductase 1C3 expression in the cryptorchid testis. Urology. 2010;76:67–72. doi: 10.1016/j.urology.2009.09.046. [DOI] [PubMed] [Google Scholar]
- 14.Lewis MJ, Wiebe JP, Heathcote JG. Expression of progesterone metabolizing enzyme genes (AKR1C1, AKR1C2, AKR1C3, SRD5A1, SRD5A2) is altered in human breast carcinoma. BMC Cancer. 2004;4:27. doi: 10.1186/1471-2407-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito K, Utsunomiya H, Suzuki T, Saitou S, Akahira J, Okamura K, Yaegashi N, Sasano H. 17b-hydroxysteroid dehydrogenases in human endometrium and its disorders. Mol Cell Endocrinol. 2006;248:136–140. doi: 10.1016/j.mce.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura Y, Suzuki T, Nakabayashi M, Endoh M, Sakamoto K, Mikami Y, Moriya T, Ito A, Takahashi S, Yamada S, Arai Y, Sasano H. In situ androgen producing enzymes in human prostate cancer. Endocr Relat Cancer. 2005;12:101–107. doi: 10.1677/erc.1.00914. [DOI] [PubMed] [Google Scholar]
- 17.Stanbrough M, Bubley G, Ross K, Golub TR, Rubin MA, Penning TM, Febbo PG, Balk SP. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 18.Wako K, Kawasaki T, Yamana K, Suzuki K, Jiang S, Umezu H, Nishiyama T, Takahashi K, Hamakubo T, Kodama T, Naito M. Expression of androgen receptor through androgen-converting enzymes is associated with biological aggressiveness in prostate cancer. J Clin Pathol. 2008;61:448–454. doi: 10.1136/jcp.2007.050906. [DOI] [PubMed] [Google Scholar]
- 19.Azzarello J, Fung KM, Lin HK. Tissue distribution of human AKR1C3 and rat homolog in adult genitourinary system. J Histochem Cytochem. 2008;56:853–861. doi: 10.1369/jhc.2008.951384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park AL, Lin HK, Yang Q, Sing CW, Fan M, Mapstone TB, Gross NL, Gumerlock MK, Martin MD, Rabb CH, Fung KM. Differential expression of type 2 3a/type 5 17b-hydroxysteroid dehydrogenase (AKR1C3) in tumors of the central nervous system. Int J Clin Exp Pathol. 2010;3:743–754. [PMC free article] [PubMed] [Google Scholar]
- 21.Azzarello JT, Lin HK, Gherezghiher A, Zakharov V, Yu Z, Kropp BP, Culkin DJ, Penning TM, Fung KM. Expression of AKR1C3 in renal cell carcinoma, papillary urothelial carcinoma, and Wilms' tumor. Int J Clin Exp Pathol. 2009;3:147–155. [PMC free article] [PubMed] [Google Scholar]
- 22.Penning TM, Burczynski ME, Hung CF, McCoull KD, Palackal NT, Tsuruda LS. Dihydrodiol dehydrogenases and polycyclic aromatic hydrocarbon activation: generation of reactive and redox active o-quinones. Chem Res Toxicol. 1999;12:1–18. doi: 10.1021/tx980143n. [DOI] [PubMed] [Google Scholar]
- 23.Smithgall TE, Harvey RG, Penning TM. Regio- and stereospecificity of homogeneous 3a-hydroxysteroid-dihydrodiol dehydrogenase for trans-dihydrodiol metabolites of polycyclic aromatic hydrocarbons. J Biol Chem. 1986;261:6184–6191. [PubMed] [Google Scholar]
- 24.Hsu NY, Ho HC, Chow KC, Lin TY, Shih CS, Wang LS, Tsai CM. Overexpression of dihydrodiol dehydrogenase as a prognostic marker of non-small cell lung cancer. Cancer Res. 2001;61:2727–2731. [PubMed] [Google Scholar]
- 25.Chen CY, Hsu CP, Hsu NY, Shih CS, Lin TY, Chow KC. Expression of dihydrodiol dehydrogenase in the resected stage I non-small cell lung cancer. Oncol Rep. 2002;9:515–519. [PubMed] [Google Scholar]
- 26.Travis WD. Update on small cell carcinoma and its differentiation from squamous cell carcinoma and other non-small cell carcinomas. Mod Pathol. 2012;25(Suppl 1):S18–30. doi: 10.1038/modpathol.2011.150. [DOI] [PubMed] [Google Scholar]
- 27.Turner BM, Cagle PT, Sainz IM, Fukuoka J, Shen SS, Jagirdar J. Napsin a, a new marker for lung adenocarcinoma, is complementary and more sensitive and specific than thyroid transcription factor 1 in the differential diagnosis of primary pulmonary carcinoma: evaluation of 1674 cases by tissue microarray. Arch Pathol Lab Med. 2012;136:163–171. doi: 10.5858/arpa.2011-0320-OA. [DOI] [PubMed] [Google Scholar]
- 28.Seike K, Murata M, Hirakawa K, Deyashiki Y, Kawanishi S. Oxidative DNA damage induced by benz[a] anthracene dihydrodiols in the presence of dihydrodiol dehydrogenase. Chem Res Toxicol. 2004;17:1445–1451. doi: 10.1021/tx0498814. [DOI] [PubMed] [Google Scholar]
- 29.Xue W, Warshawsky D. Metabolic activation of polycyclic and heterocyclic aromatic hydrocarbons and DNA damage: a review. Toxicol Appl Pharmacol. 2005;206:73–93. doi: 10.1016/j.taap.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Cheng YW, Tsai HJ, Wu JY, Hsu YF, Chen CY, Hao NJ, Lee H. A possible role for dihydrodiol dehydrogenase in the formation of benzo[a] pyrene-DNA adducts in lung cancer cells and tumor tissues. Environ Mol Mutagen. 2007;48:14–21. doi: 10.1002/em.20270. [DOI] [PubMed] [Google Scholar]
- 31.Burczynski ME, Lin HK, Penning TM. Isoform-specific induction of a human aldo-keto reductase by polycyclic aromatic hydrocarbons (PAHs), electrophiles, and oxidative stress: implications for the alternative pathway of PAH activation catalyzed by human dihydrodiol dehydrogenase. Cancer Res. 1999;59:607–614. [PubMed] [Google Scholar]
- 32.Lan Q, Mumford JL, Shen M, Demarini DM, Bonner MR, He X, Yeager M, Welch R, Chanock S, Tian L, Chapman RS, Zheng T, Keohavong P, Caporaso N, Rothman N. Oxidative damage-related genes AKR1C3 and OGG1 modulate risks for lung cancer due to exposure to PAH-rich coal combustion emissions. Carcinogenesis. 2004;25:2177–2181. doi: 10.1093/carcin/bgh240. [DOI] [PubMed] [Google Scholar]
- 33.Palackal NT, Lee SH, Harvey RG, Blair IA, Penning TM. Activation of polycyclic aromatic hydrocarbon trans-dihydrodiol proximate carcinogens by human aldo-keto reductase (AKR1C) enzymes and their functional overexpression in human lung carcinoma (A549) cells. J Biol Chem. 2002;277:24799–24808. doi: 10.1074/jbc.M112424200. [DOI] [PubMed] [Google Scholar]
- 34.Hung JJ, Chow KC, Wang HW, Wang LS. Expression of dihydrodiol dehydrogenase and resistance to chemotherapy and radiotherapy in adenocarcinoma cells of lung. Anticancer Res. 2006;26:2949–2955. [PubMed] [Google Scholar]
- 35.Ax W, Soldan M, Koch L, Maser E. Development of daunorubicin resistance in tumour cells by induction of carbonyl reduction. Biochem Pharmacol. 2000;59:293–300. doi: 10.1016/s0006-2952(99)00322-6. [DOI] [PubMed] [Google Scholar]
- 36.Deng HB, Adikari M, Parekh HK, Simpkins H. Ubiquitous induction of resistance to platinum drugs in human ovarian, cervical, germ-cell and lung carcinoma tumor cells overexpressing isoforms 1 and 2 of dihydrodiol dehydrogenase. Cancer Chemother Pharmacol. 2004;54:301–307. doi: 10.1007/s00280-004-0815-0. [DOI] [PubMed] [Google Scholar]
- 37.Deng HB, Parekh HK, Chow KC, Simpkins H. Increased expression of dihydrodiol dehydrogenase induces resistance to cisplatin in human ovarian carcinoma cells. J Biol Chem. 2002;277:15035–15043. doi: 10.1074/jbc.M112028200. [DOI] [PubMed] [Google Scholar]
- 38.Chen J, Adikari M, Pallai R, Parekh HK, Simpkins H. Dihydrodiol dehydrogenases regulate the generation of reactive oxygen species and the development of cisplatin resistance in human ovarian carcinoma cells. Cancer Chemother Pharmacol. 2008;61:979–987. doi: 10.1007/s00280-007-0554-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chow KC, Lu MP, Wu MT. Expression of dihydrodiol dehydrogenase plays important roles in apoptosis- and drug-resistance of A431 squamous cell carcinoma. J Dermatol Sci. 2006;41:205–212. doi: 10.1016/j.jdermsci.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Tew KD, O'Brien M, Laing NM, Shen H. Coordinate changes in expression of protective genes in drug-resistant cells. Chem Biol Interact. 1998;111-112:199–211. doi: 10.1016/s0009-2797(97)00162-2. [DOI] [PubMed] [Google Scholar]
- 41.Patrone C, Cassel TN, Pettersson K, Piao YS, Cheng G, Ciana P, Maggi A, Warner M, Gustafsson JA, Nord M. Regulation of postnatal lung development and homeostasis by estrogen receptor b. Mol Cell Biol. 2003;23:8542–8552. doi: 10.1128/MCB.23.23.8542-8552.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spivack SD, Hurteau GJ, Fasco MJ, Kaminsky LS. Phase I and II carcinogen metabolism gene expression in human lung tissue and tumors. Clin Cancer Res. 2003;9:6002–6011. [PubMed] [Google Scholar]
- 43.Wu CT, Chang YL, Shih JY, Lee YC. The significance of estrogen receptor b in 301 surgically treated non-small cell lung cancers. J Thorac Cardiovasc Surg. 2005;130:979–986. doi: 10.1016/j.jtcvs.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 44.Marquez-Garban DC, Mah V, Alavi M, Maresh EL, Chen HW, Bagryanova L, Horvath S, Chia D, Garon E, Goodglick L, Pietras RJ. Progesterone and estrogen receptor expression and activity in human non-small cell lung cancer. Steroids. 2011;76:910–920. doi: 10.1016/j.steroids.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Slatore CG, Chien JW, Au DH, Satia JA, White E. Lung cancer and hormone replacement therapy: association in the vitamins and lifestyle study. J. Clin. Oncol. 2010;28:1540–1546. doi: 10.1200/JCO.2009.25.9739. [DOI] [PMC free article] [PubMed] [Google Scholar]