Abstract
Our previous work has demonstrated that cyclosporin A (CsA) up-regulates but CD82 down-regulates the invasiveness of human trophoblasts. In the present study, we further investigated whether CsA can modulate the trophoblasts invasion through regulating the expression of CD82 in decidual stromal cells (DSCs). A co-culture model was established to investigate the effect of CsA on trophoblasts invasiveness. In-cell Western was performed to evaluate the expression of CD82, p53, β-catenin and the phosphorylation level of NF-κB p50 in DSCs. The secretion of CXCL12 of trophoblasts and DSCs was determined by enzyme-linked immunosorbent assay (ELISA). We found that CsA could not directly change the expression of CD82 in DSCs, but the CsA-treated trophoblasts significantly enhanced CD82 expression, NF-κB p50 phosphorylation and p53 expression, and decreased β-catenin expression in DSCs, and these effects could be abolished by anti-CXCL12 or CXCR4 neutralizing antibody. In addition, the invasiveness of trophoblast cells was markedly decreased after blocking CXCR4 of trophoblasts. Interestingly, when DSCs were pretreated with anti-CXCR4 neutralizing antibody, the invasiveness of trophoblast cells was enhanced in the coculture unit, and blocking CXCR4 on DSCs could reverse the decrease of trophoblasts invasiveness induced by CD82. Moreover, CsA further amplified these effects mediated by CXCL12 and CD82. Our results suggest that CsA not only promotes the trophoblasts invasiveness through stimulating the secretion of CXCL12, but also limits the invasiveness of trophoblasts by indirectly up-regulating the expression CD82. Therefore, CsA may contribute to the appropriate invasiveness of trophoblasts via strengthening the crosstalk between trophoblasts and DSCs.
Keywords: CsA, CD82, trophoblasts, invasiveness, DSCs, CXCL12
Introduction
A typical feature of placentation is the trophoblasts with high degree invasion into the maternal decidua during the first trimester gestation [1]. The first-trimester trophoblast cells proliferate, migrate and invade into the decidua and decidual vasculature in order to nourish the developing fetus that is similar to tumor [2]. Either insufficient invasion or inadequate proliferation can contribute to pregnancy-induced hypertension or pre-eclampsia, fetal intrauterine restriction, spontaneous pregnancy loss [3-9]. However, as opposed to malignant invasion, the trophoblast invasion is strictly limited in healthy pregnancy, and regulated by the cross-talk of paracrine and autocrine factors between the trophoblast cells and DSCs at the maternal-fetal interface [10]. DSCs secrete a lot of cytokines and express proteins, such as tissue inhibitor of metalloproteinases 1 (TIMP1) and tumor metastasis suppressor CD82 [11,12], which can control the invasiveness of the trophoblast cells.
CD82 plays an important role in inhibiting cancer cell motility, invasion, and metastasis, and thus inhibits the formation of tumor metastasis without affecting tumor growth. More and more discovery has linked the transcription regulation of CD82 to NF-κB p50 [13,14], p53, β-catenin [15-17], and so on. Besides NF-κB, other transcription factors in interleukin-1 (IL-1) and TNF signaling pathways may also regulate CD82 transcription since IL-1 and TNF induce CD82 gene expression [18]. Our previous work has demonstrated that the trophoblast cell-derived CXCL12 not only increases the invasiveness in an autocrine manner through binding the receptor CXCR4, but also controls the excessive invasion of trophoblasts through promoting CD82 expression on DSCs in a paracrine manner, which maintains a physiological balance of human trophoblasts invasiveness via the dialogue between trophoblasts and DSCs [11].
Cyclosporin A (CsA) is a powerful immunosuppressive that has been widely used to prevent from organ rejection, and to treat certain autoimmune diseases [19,20]. With further studies, it has been found that CsA not only inhibits the activation of T lymphocytes through inactivating the calcineurin/calmodulin/nuclear factor of activated T cells (NF-AT) signaling pathway which is important to the transcriptional activation of IL-2 [21-23], but also influences functions of other immuno-competent cells, including natural killer cells [24], macrophages [25] as well as dendritic cells [26,27]. We have proved that CsA can promote proliferation and invasion of human first-trimester trophoblast cells, and increase fetal viability in abortion-prone matings to that of normal pregnant matings in mice [28-30]. Moreover, CsA increases invasiveness in vitro of the first-trimester human trophoblast cells via the mitogen activated protein kinase (MAPK) pathway [31].
Therefore, the present study was undertaken to investigate whether CsA could regulate the invasiveness of trophoblasts in implantation and placentation through regulating the expression of CD82 on DSC, and further clarify the mechanism of this regulation process.
Materials and methods
Tissue collection, and cell isolation and culture
All procedures involving participants in this study were approved by the Human Research Ethics Committee of Obstetrics and Gynecology Hospital, Fudan University, and all subjects completed an informed consent to collect tissue samples.
Decidual tissues and placental tissues were from selective termination of the first-trimester pregnancy (gestational age, 6-8 weeks) for no medical reason. The tissues from the first-trimester pregnancy were put immediately into ice-cold Dulbecco’s modified Eagle’s medium (DMEM high D-glucose; Gibco Grand Island, NY, USA), transported to the laboratory within 30 min after surgery, and washed with Hank’s balanced salt solution for isolation of DSCs and trophoblast cells.
The DSCs and trophoblasts were isolated according to our previous procedures [11,12,31]. These methods supplied >98% vimentin-positive and cytokeratin-negative DSCs and 95% purity of trophoblast cells, respectively.
Treatment with CsA, anti-CXCL12 and or anti-CXCR4 neutralizing antibody
The primary DSCs in 96-well plate were directly incubated with CsA (0.1uM and 1uM), or incubated with CsA-treated trophoblasts supernatant for 48h with no CsA treatment as control, and then some culture unit was also added anti-CXCL12 neutralizing antibody (2-50ug/ml) (R&D Systems, Abingdon, UK), or anti-CXCR4 neutralizing antibody (0.8-20ug/ml) (R&D Systems, Abingdon, UK) for 48h, with mouse anti-human IgG isotype as control. Then we used in-cell western to detect the expression level of CD82 in DSCs.
Moreover, we treated DSCs with trophoblasts supernatant or CsA(1uM)-treated trophoblasts supernatant, and DSCs in the latter group were also incubated with anti-CXCL12 neutralizing antibody (50ug/ml) or anti-CXCR4 neutralizing antibody (4ug/ml) for 48h, with mouse antihuman IgG isotype as control. Subsequently, in-cell western was performed to analyze the expression of p53, β-catenin and the phosphorylation of NF-κB p50.
In-cell western
According to the description by Egorina [32] and our previous procedure [11,33], we used a newly set-up assay called in-cell Western to determine the in-cell protein level of CD82, p53, β-catenin, and the phosphorylation of NF-κB p50. The procedure was as follows: DSCs in 96-well plate were immediately fixed with 4% paraformaldehyde for 20min at room temperature. After washing with 0.1% Triton, these cells were blocked by 150ul of LI-COR Odyssey Blocking Buffer (LI-COR Biosciences, Lincoln, Nebraska, USA) for 90min at room temperature, and then incubated with mouse anti-human CD82 (20ug/ ml, Santa Cruz Biotechnology, USA), mouse antihuman phospho-NF-κB p50 (1:50, Santa Cruz Biotechnology, USA), rabbit anti-human p53 (1:50, Santa Cruz Biotechnology, USA) or mouse anti-human β-catenin (1:50, BD Biosciences, USA) antibodies with rabbit anti-human actin (1:80, Santa Cruz Biotechnology, USA)/rabbit anti-human NF-κB p50 (1:50, Santa Cruz Biotechnology, USA)/mouse anti-human GAPDH (for p53 group) (1:80, Santa Cruz Biotechnology, USA) antibody as control. After overnight treatment at 4°C, the wells were incubated with corresponding second IRDyeTM700DX-conjugated affinity purified (red fluorescence) anti-mouse antibody and IRDyeTM800DX-conjugated affinity purified (green fluorescence) anti-rabbit antibody, fluorescence antibodies recommended by the manufacturer (Rockland, Inc, Gibertsvile, PA, USA). This procedure was carried out in the dark. Images of the target gene were obtained by using the Odyssey Infrared Imaging System (LI-COR Biosciences German version of Ltd.). The expression level of the correspondent molecules was calculated as the ratio of the intensity of target proteins to actin, GAPDH (for p53 group) or total signal molecules (such as total NF-κB p50). The experiments were carried out in triplicate, and repeated three times.
Enzyme-linked immunosorbent assay for determination of CXCL12
In order to evaluate the secretion level of CXCL12, DSCs alone (1×105cells/well), trophoblasts alone (1×105cells/well) or the coculture of these two cells (the proportion of DSCs and trophoblast cells was 1:1) in 24-well plates were treated with 0.1uM or 1uM CsA for 48h, and then the culture supernatants were harvested, centrifuged to remove cellular debris, and stored at -80°C until being assayed by enzyme-linked immunosorbent assay (ELISA) for CXCL12 determination. The CXCL12 assay (R&D Systems, Abingdon, UK) sensitivity is 1 pg/ml.
CD82 silence in DSCs
For siRNA transfection, according to the description by our previous method [11,33], the primary DSCs were seeded in 96-well plates. When cells had reached confluency, medium was changed to OPTIMEM (Invitrogen, USA). The short interfering RNA (siRNA) oligonucleotides targeting CD82 (set of three oligonucleotides; Stealth Select RNAi; Invitrogen) and LipofectamineTM 2000 (Invitrogen, USA) were mixed in OPTIMEM, and then added to the cells at room temperature with nontargeting siRNA oligonucleotides as negative control. After 6h incubation, the cells were incubated in DMEM for further 72h in 5% CO2 at 37°C, and the gene knockdown was confirmed by in-cell Western [11].
Matrigel invasion assay
The invasion of trophoblast cells across matrigel was evaluated objectively in invasion chamber based on our previous procedure [12]. Briefly, the cells inserts (8μm pore size, 6.5mm diameter, Corning, USA) coated with 15-25ul matrigel were placed in a 24-well plate. The CD82-silenced DSCs (1×105cells/well) in the lower chamber and/or trophoblast cells (1×105cells/well) in the upper chamber were pretreated with or without anti-CXCR4 neutralizing antibody (4ug/ml) for 4h, then was formed an indirect coculture unit and treated with or without CsA (1uM). The cells were then incubated at 37°C for 48h. The inserts were removed, washed in PBS and the non-invading cells together with the matrigel were removed from the upper surface of the filter by wiping with a cotton bud. The inserts were then fixed in methanol for 10min at room temperature and stained with hematoxylin. The result was observed under Olympus BX51+DP70 microscope (Olympus, Tokyo, Japan). The cells migrated to the lower surfaces were counted in full fields at a magnification of × 200. Each experiment was carried out in triplicate, and repeated three times.
Statistics
All values are shown as the mean±SD. One-way ANOVA analysis of variance was used to detect the difference of the CD82, p53, β-catenin expression, and the phosphorylation of NF-κB p50 in DSCs, the secretion of CXCL12, and invasion of primary trophoblasts. Differences were considered as statistically significant at P<0.05.
Results
CsA indirectly up-regulates CD82 expression in DSCs through educating trophoblasts
In order to investigate whether CsA can regulate the expression of CD82, we treated primary DSCs with CsA, CsA-treated or vehicle-treated trophoblasts supernatant for 48h, and then the CD82 expression in DSCs was detected by in-cell Western. As depicted in Figure 1, trophoblasts supernatant up-regulated the expression of CD82 in DSCs (P<0.05) (Figure 1B), the CsA-treated trophoblasts supernatant could further increase the expression of CD82 in DSCs, especially at the concentration of 1uM (P<0.01) (Figure 1B). However, there is no significant difference in CD82 expression of DSCs between direct treatment with or without CsA groups (P>0.05), (Figure 1A). These results above suggest that CsA may indirectly promote CD82 expression in DSCs through stimulating the secretion of soluble molecules derived from trophoblasts.
Figure 1.
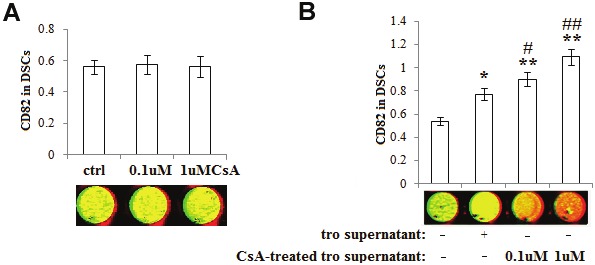
CsA indirectly up-regulates CD82 expression in DSCs through educating trophoblasts. After treatment with CsA (A), vehicle-pretreated or CsA-treated trophoblasts supernatant (B), the expression of CD82 in DSCs was detected by in-cell Western. Here CD82 (red); actin (green). Results were highly reproducible in three independent experiments. Herein tro supernatant: vehicle-treated trophoblasts supernatant; CsA-treated tro supernatant: CsA-treated trophoblasts supernatant. Error bars depict the standard error of the mean. *P<0.05, **P<0.01 compared to the control, #P<0.05, ##P<0.01 compared to tro supernatant treatment group.
CsA increases CD82 expression in DSCs through stimulating CXCL12 secretion of trophoblasts
Our previous studies have established that trophoblast-derived CXCL12 can boost the expression of CD82 and facilitate the interaction between DSCs and trophoblasts [12,34]. In addition, CsA can modulate the invasiveness of primary trophoblasts [31]. We speculated that CsA indirectly regulate CD82 expression in DSCs by stimulating the secretion of CXCL12 of trophoblasts. It was found that CsA can obviously stimulate the CXCL12 production of trophoblasts alone and in the co-culture unit of trophoblasts and DSCs (P<0.05, P<0.01) (Figure 2A), but cannot change the CXCL12 level of DSCs alone (P>0.05) (Figure 2A).
Figure 2.
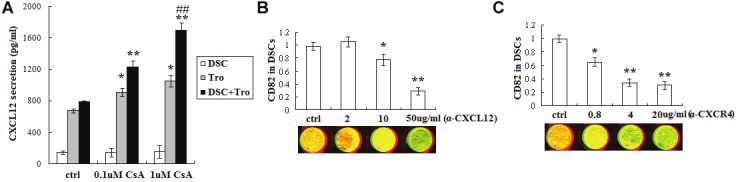
CsA increases CD82 expression in DSCs through stimulating CXCL12 secretion of trophoblasts. The secretion of CXCL12 of trophoblasts alone (1×105cell/well), DSCs alone (1×105cell/well) and these two cells co-culture unit was analyzed by ELISA (A). Thereafter, (B, C) in-cell Western was used to detect the expression of CD82 in DSCs which were treated with CsA(1uM)-pretreated trophoblasts supernatant and neutralizing antibody to CXCL12 (50ug/ml) or CXCR4 (4ug/ml) for 48h, and with vehicle as controls. Here CD82 (red); actin (green). Results were highly reproducible in three independent experiments. Error bars depict the standard error of the mean. *P<0.05, **P<0.01 compared to the control. ##P<0.01 compared to 0.1uM CsA treatment group.
Subsequently, to further analyze whether CsA (1uM) modulates the CD82 expression in DSCs through regulating the secretion of CXCL12 of trophoblasts, we treated DSCs with CsA-treated trophoblasts supernatant combined with antihuman CXCL12 or CXCR4 neutralizing antibody for 48h, and detected the expression of CD82 by in-cell Western. Data were presented in Figure 2B and Figure 2C that both anti-human CXCL12 (P<0.05, P<0.01) (Figure 2B) and CXCR4 (P<0.05, P<0.01) (Figure 2C) neutralizing antibodies can decrease the expression of CD82 in DSCs increased by the CsA-treated trophoblasts supernatant.
CsA activates NF-κB p50, increases p53 expression and decreases β-catenin expression in DSCs via promoting CXCL12 secretion of trophoblast cells
In order to probe the mechanism of CsA and CXCL12 on the CD82 regulation, we used in-cell western to evaluate the expression or phosphorylation levels of transcription factors (NF-κB p50, p53 and β-catenin) in DSCs, which were incubated with CsA-treated trophoblasts supernatant and anti-human CXCL12 or CXCR4 neutralizing antibody. As shown in Figure 3, the CsA-treated trophoblasts markedly increased NF-κB p50 phosphorylation and p53 expression, and decreased β-catenin expression in DSCs (P<0.05), and these effects could be inhibited by blocking CXCL12 or CXCR4 (P<0.05, P<0.01) (Figure 3).
Figure 3.
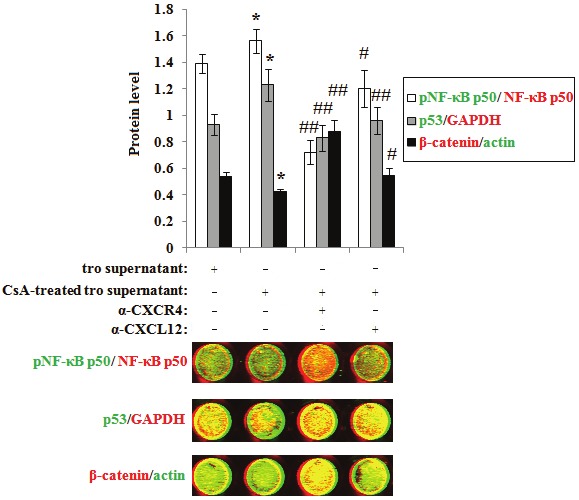
CsA activates NF-κB p50, increases p53 expression and decreases β-catenin expression in DSCs via promoting CXCL12 secretion of trophoblast cells. DSCs were incubated with CsA-treated trophoblasts supernatant and neutralizing antibody to CXCL12 or CXCR4 for 48h, and with vehicle as controls. Then in-cell Western was used to analyze the phosphorylation level of NF-κB p50, the expression of p53 and β-catenin. Here Phospho-NF-κB p50, GAPDH and β-catenin (red); NF-κB p50, p53 and actin (green). These pictures are representatives of three individual experiments. Error bars depict the standard error of the mean. *P<0.05, **P<0.01 compared to tro supernatant treatment group, #P<0.05, ##P<0.01 compared to CsA-treated tro supernatant treatment group.
CsA regulates the trophoblasts invasiveness through improving the cross-talk between DSCs and trophoblasts via CXCL12 and CD82
Matrigel invasion assay was performed to explore whether CsA can modulate trophoblasts invasiveness through regulating the secretion of CXCL12 of trophoblasts and the expression of CD82 in DSCs. As shown, CD82 silence or/and anti-CXCR4 treatment to DSCs increased trophoblasts invasiveness (P<0.05, P<0.01) (Figure 4). On the contrary, the invasiveness of trophoblasts was decreased significantly if the trophoblast cells were pretreated by neutralizing antibody to CXCR4 (P<0.01) (Figure 4). Moreover, CsA increased trophoblasts invasion in the co-culture of these two cells, and enhanced the effect of CXCL12 and CD82 on the trophoblasts invasiveness (P<0.05, P<0.01) (Figure 4).
Figure 4.
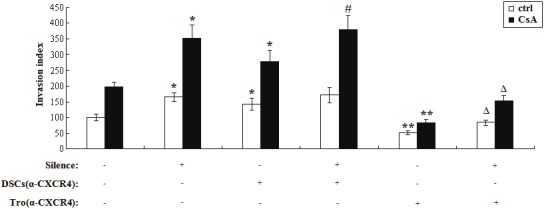
CsA regulates the trophoblasts invasiveness through improving the cross-talk between DSCs and trophoblasts via CXCL12 and CD82. The CD82-silenced DSCs (1×105cells/well) in the lower and or trophoblast cells (1×105cells/well) in the upper chamber were pretreated with or without anti-CXCR4 neutralizing antibody (4ug/ml) for 4h, then was formed a indirect co-culture unit and treated with or without CsA (1uM). Subsequently, Matrigel invasion assay was used to detect the invasiveness of trophoblasts in co-culture unit. Silence: CD82 was knocked down. DSCs (α-CXCR4): DSCs were pretreated with anti-CXCR4 neutralizing antibody; Tro (α-CXCR4): Trophoblasts were pretreated with anti-CXCR4 neutralizing antibody. Results were highly reproducible in three independent experiments. Error bars depict the standard error of the mean. *P<0.05, **P<0.01 compared to nontargeting siRNA control. #P<0.05 compared to nontargeting siRNA control and anti-CXCR4 neutralizing antibody pretreated DSCs. ΔP<0.05 compared to compared to nontargeting siRNA control and anti-CXCR4 neutralizing antibody pretreated trophoblasts.
Discussion
As a key component of human placenta, trophoblasts are the only embryo-derived cells which interact with the maternal derived cells directly. Trophoblasts proliferation and invasion are a series of tightly controlled processes that are pivotal to implantation and placentation. Excessive and insufficient trophoblasts growth and invasion are associated with some pregnancy complications, such as fetal growth restriction, pre-eclampsia and early pregnancy loss [35,36]. The homeostasis of trophoblasts invasiveness plays an important role in the normal pregnancy.
Our previous studies have indicated that first-trimester human trophoblasts co-expressed CXCL16 and CXCR6 [37], and chemokine CXCL12 and CXCR4 [34,12], which induce their proliferation and invasion in an autocrine manner. Moreover, first-trimester human DSCs co-expressed CCL2 and CCR2 and secreted CCL2 spontaneously [38], which also stimulate the proliferation and invasiveness of trophoblasts in a paracrine manner. Conversely, DSCs-derived CD82 controls the invasion of trophoblasts through inactivating MAPK/Erk1/2 signal pathway [11]. Interestingly, CXCL12 can prevent from the excessive invasion of trophoblasts through up-regulating the CD82 expression in DSCs in a paracrine manner [12]. Therefore, there might be a complicated chemokine network at the materno-fetal interface. Trophoblasts or DSCs not only modulate their own biological functions via their respective chemokines/chemokine receptors, but also interact with each other through chemokines secretion, through which trophoblasts and DSCs build a multiple connection, and participate in the complex materno-fetal dialogue. The normal cross-talk between trophoblasts and DSCs via CXCL12 and CD82 interaction maybe involved in maintaining the appropriate invasiveness of trophoblasts, which is benefit to normal pregnancy.
CsA is an immunosuppressant which is used for preventing from allograft rejection. It has been demonstrated that CsA promotes the invasiveness of trophoblasts by increasing the expression of MMP9 and MMP2 in vitro [31]. However, it remains unclear that whether CsA can modulate the invasiveness of trophoblasts by regulating CD82 expression at the maternal-fetal interface. In this study, we have found that CsA cannot directly change CD82 expression, but indirectly increases the expression of CD82 in DSCs through educating trophoblasts. These results indicate that CsA may coordinate trophoblasts and DSCs through promoting the CXCL12 production of trophoblasts and further indirectly increasing the CD82 expression of DSCs, which may contribute to the balance of trophoblasts invasiveness.
The loss of CD82 expression in invasive and metastatic cancer is due to a complex, epigenetic mechanism that probably involves transcription factors such as NF-κB, p53, and β-catenin [37]. Moreover, IL-1β [14,39-40] and TNF [14,39] are also involved in regulating the expression of CD82. It has been reported that CXCL12 can activate NF-κB [41], which plays an important role in enhancing CD82 transcription. The present study has further shown that the increase of CXCL12 secretion from CsA-educated trophoblasts might promote CD82 expression in DSCs through activating NF-κB p50, increasing the expression of p53 and decreasing the expression of β-catenin. However, we still need to further research to clarify whether the regulatory effect of CXCL12 on CD82 expression in DSCs is associated with IL-1β and TNF-α.
To better understand the role of CsA in the cross-talk between trophoblasts and DSCs, we established a co-culture model. As we predicted, trophoblasts-secreted CXCL12 increases, but DSCs-derived CD82 decreases the trophoblasts invasiveness. Moreover, CsA can amplify the directly stimulatory and indirectly inhibitory effect of CXCL12 on the invasiveness of trophoblasts at the maternal-fetal interface.
The appropriate invasion of human trophoblast cells is a critical step in establishment and maintenance of normal pregnancy. Taking into account these findings above, it may be proposed that CsA can directly promote trophoblasts invasiveness by stimulating CXCL12 secretion in an autocrine manner; on the other hand, CsA may restrict the excessive invasion of trophoblasts via indirectly increasing CXCL12-regulated CD82 expression in DSCs in a paracrine manner. These integral effects will be benefit to maintain the balance of trophoblast invasiveness. Therefore, the dialogue of trophoblasts and DSCs mediated by CXCL12 and CD82 is strengthened by CsA, which may facilitate the establishment and maintenance of the early pregnancy. As an important issue, the effect of CsA on pregnancy following transplantation is still in research now. Although there is a relatively high rate of premature and low birth weight infants, female transplant recipients can safely undergo pregnancy [42,43]. Especially, most of these recipients were treated with CsA as a traditional immunosuppressant, thus the fetuses were exposed to a high concentration of CsA during the whole pregnancy. However, our study in vivo found that administration of CsA (1 mg/kg) at day 4 of gestation (the window of implantation) reduced embryo resorption and improved pregnant outcome in the CBA/J×DBA/2 abortion-prone matings [44]. Therefore, a low dose of CsA only in the window of implantation can provide a rational therapy for some pregnancy complications. Of course, we still need more studies to evaluate the safety and long-term consequences of this drug application.
Funding
This study was supported by National Basic Research Program of China (2006CB944007) to DJL; Major International Joint Research Project of National Natural Science Foundation of China (NSFC) (30910103909) to DJL; National and Shanghai Leading Academic Discipline Project (211XK22) to DJL; Program for Outstanding Medical Academic Leader of Shanghai to DJL; NSFC 31101064 to MQL; Research Program of Shanghai Health Bureau to MQL (2011Y080); Ministry of Education Research Fund for Doctoral Program to MQL (20110071120092).
References
- 1.Aplin JD. Implantation, trophoblast differentiation and haemochorial placentation: mechanistic evidence in vivo and in vitro. J Cell Sci. 1991;99:681–682. doi: 10.1242/jcs.99.4.681. [DOI] [PubMed] [Google Scholar]
- 2.Fitzgerald JS, Poehlmann TG, Schleussner E, Markert UR. Trophoblast invasion: the role of intracellular cytokine signalling via signal transducer and activator of transcription 3 (STAT3) Hum Reprod Update. 2008;14:335–344. doi: 10.1093/humupd/dmn010. [DOI] [PubMed] [Google Scholar]
- 3.Lyall F, Bulmer JN, Duffie E, Cousins F, Theriault A, Robson SC. Human trophoblast invasion and spiral artery transformation: the role of PECAM-1 in normal pregnancy, pre-eclampsia, and fetal growth restriction. Am J Pathol. 2001;158:1713–1721. doi: 10.1016/S0002-9440(10)64127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olivares EG, Munoz R, Tejerizo G, Montes MJ, Gomez-Molina F, Abadia-Molina AC. Decidual lymphocytes of human spontaneous abortions induce apoptosis but not necrosis in JEG-3 extravillous trophoblast cells. Biol Reprod. 2002;67:1211–1217. doi: 10.1095/biolreprod67.4.1211. [DOI] [PubMed] [Google Scholar]
- 5.Crocker IP, Cooper S, Ong SC, Baker PN. Differences in apoptotic susceptibility of cytotrophoblasts and syncytiotrophoblasts in normal pregnancy to those complicated with preeclampsia and intrauterine growth restriction. Am J Pathol. 2003;162:637–643. doi: 10.1016/S0002-9440(10)63857-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greer IA. Thrombophilia: implications for pregnancy outcome. Thromb Res. 2003;109:73–81. doi: 10.1016/s0049-3848(03)00095-1. [DOI] [PubMed] [Google Scholar]
- 7.Bose P, Black S, Kadyrov M, Weissenborn U, Neulen J, Regan L, Huppertz B. Heparin and aspirin attenuate placental apoptosis in vitro: implications for early pregnancy failure. Am J Obstet Gynecol. 2005;192:23–30. doi: 10.1016/j.ajog.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 8.Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod. 2003;69:1–7. doi: 10.1095/biolreprod.102.014977. [DOI] [PubMed] [Google Scholar]
- 9.Bose P, Kadyrov M, Goldin R, Hahn S, Backos M, Regan L, Huppertz B. Aberrations of early trophoblast differentiation predispose to pregnancy failure: lessons from the antiphospholipid syndrome. Placenta. 2006;27:869–875. doi: 10.1016/j.placenta.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Elsebeth SR, Eliezer S. Human trophoblast function during the implantation process. Reprod Biol Endocrinol. 2005;3:56. doi: 10.1186/1477-7827-3-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li MQ, Hou XF, Shao J, Tang CL, Li DJ. The DSCs-expressed CD82 controls the invasiveness of trophoblast cells via integrinβ1/MAPK/MAPK3/1 signaling pathway in human first-trimester pregnancy. Biol Reprod. 2010;82:968–979. doi: 10.1095/biolreprod.109.080739. [DOI] [PubMed] [Google Scholar]
- 12.Li MQ, Tang CL, Du MR, Fan DX, Zhao HB, Xu B, Li DJ. CXCL12 controls over-invasion of trophoblasts via up-regulating CD82 expression in DSCs at maternal-fetal interface of human early pregnancy in a paracrine manner. Int J Clin Exp Pathol. 2011;4:276–286. [PMC free article] [PubMed] [Google Scholar]
- 13.Telese F, Bruni P, Donizetti A, Gianni D, D’Ambrosio C, Scaloni A, Zambrano N, Rosenfeld MG, Russo T. Transcription regulation by the adaptor protein Fe65 and the nucleosome assembly factor SET. Eur Mol Biol Org Rep. 2005;6:77–82. doi: 10.1038/sj.embor.7400309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baek SH, Ohgi KA, Rose DW, Koo EH, Glass CK, Rosenfeld MG. Exchange of N-CoR corepressor and Tip60 coactivator complexes links gene expression by NF-kappaB and beta-amyloid precursor protein. Cell. 2002;110:55–67. doi: 10.1016/s0092-8674(02)00809-7. [DOI] [PubMed] [Google Scholar]
- 15.Mashimo T, Watabe M, Hirota S, Hosobe S, Miura K, Tegtmeyer PJ, Rinker-Shaeffer CW, Watabe K. The expression of the KAI1 gene, a tumor metastasis suppressor, is directly activated by p53. PNAS. 1998;95:11307–11311. doi: 10.1073/pnas.95.19.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duriez C, Falette N, Cortes U, Moyret-Lalle C, Puisieux A. Absence of p53-dependent induction of the metastatic suppressor KAI1 gene after DNA damage. Oncogene. 2000;19:2461–2464. doi: 10.1038/sj.onc.1203580. [DOI] [PubMed] [Google Scholar]
- 17.Marreiros A, Dudgeon K, Dao V, Grimm MO, Czolij R, Crossley M. KAI1 promoter activity is dependent on p53, junB and AP2: evidence for a possible mechanism underlying loss of KAI1 expression in cancer cells. Oncogene. 2005;24:637–649. doi: 10.1038/sj.onc.1208216. [DOI] [PubMed] [Google Scholar]
- 18.Kim JH, Kim B, Cai L, Choi HJ, Ohgi KA, Tran C, Chen C, Chung CH, Huber O, Rose DW, Sawyers CL, Rosenfeld MG, Baek SH. Transcriptional regulation of a metastasis suppressor gene by Tip60 and beta-catenin complexes. Nature. 2005;434:921–926. doi: 10.1038/nature03452. [DOI] [PubMed] [Google Scholar]
- 19.Sketris I, Yatscoff R, Keown P, Canafax DM, First MR, Holt DW, Schroeder TJ, Wright M. Optimizing the use of cyclosporine in renal transplantation. Clin Biochem. 1995;28:195–211. doi: 10.1016/0009-9120(95)91341-y. [DOI] [PubMed] [Google Scholar]
- 20.Tugwell P, Bombardier C, Gent M, Bennett KJ, Bensen WG, Carette S, Chalmers A, Esdaile JM, Klinkhoff AV, Kraag GR. Low-dose cyclosporin versus placebo in patients with rheumatoid arthritis. Lancet. 1990;335:1051–1055. doi: 10.1016/0140-6736(90)92630-z. [DOI] [PubMed] [Google Scholar]
- 21.Emmel EA, Werweij CL, Durand DB, Higgins KM, Lacy E, Crabtree GR. Cyclosporin A specifically inhibits function of nuclear proteins involved in T cell activation. Science. 1989;246:1617–1620. doi: 10.1126/science.2595372. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Sakane T, Tsunematsu T. The effects of FK-506 and cyclosporin A on the proliferation of PHA-stimulated T cells in response to IL-2, IL-4 or IL-6. Int Arch Allergy Immunol. 1992;98:293–298. doi: 10.1159/000236201. [DOI] [PubMed] [Google Scholar]
- 23.Nelson PA, Akselband Y, Kawamura A, Su M, Tung RD, Rich DH, Kishore V, Rosborough SL, DeCenzo MT, Livingston DJ, Harding MW. Immunosuppressive activity of [MeBm2t] 1-, D-diaminobutyryl-8-, and D-diaminopropyl-8-cyclosporin analogues correlates with inhibition of calcineurin phosphatase activity. J Immunol. 1993;150:2139–2147. [PubMed] [Google Scholar]
- 24.Poggi A, Zocchi MR. Cyclosporin A regulates human NK cell apoptosis induced by soluble HLA-I or by target cells. Autoimmun Rev. 2005;4:532–536. doi: 10.1016/j.autrev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 25.Wasowska BA, Zheng XX, Strom TB, Kupieck-Weglinski JW. Adjunctive rapamycin and CsA treatment inhibits monocyte/macrophage associated cytokines/chemokines in sensitized cardiac graft recipients. Transplantation. 2001;71:1179–1183. doi: 10.1097/00007890-200104270-00029. [DOI] [PubMed] [Google Scholar]
- 26.Sauma D, Fierro A, Mora JR, Lennon-Dumenil AM, Bono MR, Rosemblatt M, Morales J. Cyclosporine preconditions dendritic cells during differentiation and reduces IL-2 and IL-12 production following activation: a potential tolerogenic effect. Transplant Proc. 2003;35:2515–2517. doi: 10.1016/j.transproceed.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 27.Chen T, Guo J, Yang M, Han C, Zhang M, Chen W, Liu Q, Wang J, Cao X. Cyclosporin A impairs dendritic cell migration by regulating chemokine receptor expression and inhibiting cyclooxygenase-2 expression. Blood. 2004;103:413–421. doi: 10.1182/blood-2003-07-2412. [DOI] [PubMed] [Google Scholar]
- 28.Yan FT, Li DJ, Sun XX, Zhu Y, Wang MY, Meng Y, Yu J. Effect of cyclosporin A on the growth of human first-trimester cytotrophoblasts in vitro. Zhonghua Fu Chan Ke Za Zhi. 2002;37:74–76. [PubMed] [Google Scholar]
- 29.Du MR, Dong L, Zhou WH, Yan FT, Li DJ. Cyclosporin A improves pregnancy outcome by promoting functions of trophoblasts and inducing maternal tolerance to the allogeneic fetus in abortion-prone matings in the mouse. Biol Reprod. 2007;76:906–914. doi: 10.1095/biolreprod.106.056648. [DOI] [PubMed] [Google Scholar]
- 30.Du MR, Zhou WH, Yan FT, Zhu XY, He YY, Yang JY, Li DJ. Cyclosporin A induces titin expression via MAPK/ERK signaling and improves proliferative and invasive potential of human trophoblast cells. Hum Reprod. 2007;22:2528–2537. doi: 10.1093/humrep/dem222. [DOI] [PubMed] [Google Scholar]
- 31.Zhou WH, Du MR, Dong L, Zhu XY, Yang JY, He YY, Li DJ. Cyclosporin A improves expression of Matrix Metalloproteinase 9 and 2 and invasiveness in vitro of the first-trimester human trophoblast cells via the mitogen-activated protein kinase pathway. Hum Reprod. 2007;22:2743–2750. doi: 10.1093/humrep/dem097. [DOI] [PubMed] [Google Scholar]
- 32.Egorina EM, Sovershaev MA, Osterud B. In-cell Western assay: a new approach to visualize tissue factor in human monocytes. J Thromb Haemost. 2006;4:614–620. doi: 10.1111/j.1538-7836.2005.01781.x. [DOI] [PubMed] [Google Scholar]
- 33.Li MQ, Hou XF, Lv SJ, Meng YH, Wang XQ, Tang CL, Li DJ. CD82 gene suppression in endometrial stromal cells leads to increase of the cell invasiveness in the endometriotic milieu. J Mol Endocrinol. 2011;47:195–208. doi: 10.1530/JME-10-0165. [DOI] [PubMed] [Google Scholar]
- 34.Zhou WH, Du MR, Dong L, Yu J, Li DJ. Chemokine CXCL12 promotes the cross-talk between trophoblasts and decidual stromal in human first-trimester pregnancy. Hum Reprod. 2008;23:2669–2679. doi: 10.1093/humrep/den308. [DOI] [PubMed] [Google Scholar]
- 35.Hustin J, Jauniaux E, Schaaps JP. Histological study of the materno-embryonic interface in spontaneous abortion. Placenta. 1990;11:477–486. doi: 10.1016/s0143-4004(05)80193-6. [DOI] [PubMed] [Google Scholar]
- 36.Sheppard BL, Bonnar J. Uteroplacental hemostasis in intrauterine fetal growth retardation. Semin Thromb Hemost. 1999;25:443–446. doi: 10.1055/s-2007-994947. [DOI] [PubMed] [Google Scholar]
- 37.Huang Y, Zhu XY, Du MR, Wu X, Wang MY, Li DJ. Chemokine CXCL16, a scavenger receptor, induces proliferation and invasion of first-trimester human trophoblast cells in an autocrine manner. Hum Reprod. 2006;21:1083–1091. doi: 10.1093/humrep/dei436. [DOI] [PubMed] [Google Scholar]
- 38.He YY, Du MR, Guo PF, He XJ, Zhou WH, Zhu XY, Li DJ. Regulation of C-C motif chemokine ligand2 and its receptor in human decidual stromal cells by pregnancy-associated hormones in early gestation. Hum Reprod. 2007;22:2733–2742. doi: 10.1093/humrep/dem208. [DOI] [PubMed] [Google Scholar]
- 39.Gellersen B, Briese J, Obernsororfer M, Redlin K, Samalecos A, Richter DU, Loning T, Schulte HM, Bamberger AM. Expression of the metastasis suppresspor KAI1 in decidual cells at the human maternal-fetal interface: Regulation and functional implications. Am J Pathol. 2007;170:126–139. doi: 10.2353/ajpath.2007.060175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huber AV, Saleh L, Bauer S, Husslein P, Knofler M. TNFa-Mediated Induction of PAI-1 Restricts Invasion of HTR-8/SVneo Trophoblast Cells. Placenta. 2006;27:127–136. doi: 10.1016/j.placenta.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 41.Ganju RK, Brubaker SA, Meyer J, Dutt P, Yang YM, Qin SX, Newman W, Groopman JE. The alpha-chemokine, stromal cell-derived fator-1 alpha, binds to the transmembrane G-proteincoupled CXCR-4 receptor and activates multiple signal transduction pathways. J Biol Chem. 1998;273:23169–23175. doi: 10.1074/jbc.273.36.23169. [DOI] [PubMed] [Google Scholar]
- 42.Coscia LA, Constantinescu S, Moritz MJ, Frank AM, Ramirez CB, Maley WR, Doria C, McGrory CH, Armenti VT. Report from the National Transplantation Pregnancy registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl. 2005:69–83. [PubMed] [Google Scholar]
- 43.Christopher V, Al-Chalabi T, Richardson PD, Muiesan P, Rela M, Heaton ND, O'Grady JG, Heneghan MA. Pregnancy outcome after liver transplantation: a single-center experience of 71 pregnancies in 45 recipients. Liver Transpl. 2006;12:1138–1143. doi: 10.1002/lt.20810. [DOI] [PubMed] [Google Scholar]
- 44.Zhou WH, Dong L, Du MR, Zhu XY, Li DJ. Cyclosporin A improves murine pregnancy outcome in abortion-prone matings: involvement of CD80/86 and CD28/CTLA-4. Reproduction. 2008;135:385–395. doi: 10.1530/REP-07-0063. [DOI] [PubMed] [Google Scholar]


