Abstract
Aggressive behavior is widely present throughout the animal kingdom and is crucial to ensure survival and reproduction. Aggressive actions serve to acquire territory, food, or mates and in defense against predators or rivals; while in some species these behaviors are involved in establishing a social hierarchy. Aggression is a complex behavior, influenced by a broad range of genetic and environmental factors. Recent studies in Drosophila provide insight into the genetic basis and control of aggression. The state of the art on aggression in Drosophila and the many opportunities provided by this model organism to unravel the genetic and neurobiological basis of aggression are reviewed.
Keywords: aggression, behavior, Drosophila, genetics, neurobiology
Introduction
Aggressive behavior is widely present throughout the animal kingdom and is crucial to ensure survival and reproduction. Aggressive actions are used to acquire territory, food, or mates and in defense of the individual or its progeny against predators or conspecific rivals. Additionally, in some species these behaviors are necessary to establish a social hierarchy. By contrast, excessive aggression implies risky and energy consuming acts, which can be evolutionary unfavorable.
Aggression is a complex behavior influenced by a broad range of genetic and environmental factors. Many of these factors, such as neurotransmitters, hormones, pheromones, sex and individual anatomical differences, have been studied in a variety of species.1-19 These studies, however, often reported inconclusive or contradictory results both within and between species. Examples of divergent results within species include the role of testosterone and cortisol in vertebrate aggression and the differential effects of neurotransmitters on aggression in Drosophila (see below for a detailed discussion).3 An example of a contradictory result between species is the opposite role for certain neurotransmitters between crustaceans and vertebrates.3,4,8,16,20 Overall, these observations illustrate the complex regulatory mechanisms underlying this behavior.
Recent studies in Drosophila provide insight into the genetic basis and control of complex behaviors, including aggression, and highlight the importance of interaction networks among many pleiotropic loci with relatively small effect sizes.11,21-25 Thanks to the availability of numerous genetic resources, the ease to perform genetic manipulations and the possibility to control environmental influences as well as the genetic background, Drosophila melanogaster offers the opportunity to integrate single gene molecular genetics and whole genome quantitative genetics and thus unravel the genetic complexity and the neurological basis of this behavior.
Aggression in Drosophila
Agonistic behavior in general was first defined by Scott and Fredericson in 1951 as a continuum of behaviors from threat to aggression to submission.26 The term aggression, however, has been much harder to define. In the context of social behaviors, aggression can be defined as species-specific behaviors associated with attack, and more broadly, threat.27,28 This definition covers the parameters used to study this behavior in Drosophila.
Aggressive behavior in Drosophila was first observed in 1915 by Sturtevant, who reported males to spread their wings, run at each other, and apparently butt heads when courting the same female.29 The first genetic study was the discovery of the involvement of the ebony gene in aggressive behavior.30 In recent years, the continuously expanding collection of genetic tools in Drosophila has made it increasingly possible to study the genetic and neurobiological basis of aggression.
Inter-male aggression in Drosophila has been well described ethologically and shown to consist of behavioral modules which include both threat and attack behaviors.31-35 In Drosophila melanogaster, these modules include: approaching, where one fly lowers his body and moves in the direction of the other; wing threats, where one fly quickly raises his wings toward its opponent; lunging, where one fly throws himself on his opponent; boxing, where both flies raise up on their hind legs and hit each other with their forelegs; tussling, where both flies tumble over each other; fencing or kicking; chasing and holding (see Table 1).35 These aggression modules can form a continuous level of low aggression that can escalate to modules with high aggression, but they can also happen as isolated events.14,35 Most of the fighting time is taken up by low intensity aggression such as fencing, while high intensity events such as lunging, boxing and tussling have been reported to be rare in different Drosophila species.35-37 Most research mainly focused on males because they display more aggression. However, female aggression has also been described in detail and shown to differ from male aggression both ethologically and motivationally. Part of the aggression behavioral repertoire is shared between males and females but some sex-specific behavioral modules occur (see Table 1).5 Boxing and tussling, for instance, have been reported to be male-specific. Furthermore, while males fight both over food and females, females fight mainly over food resources, especially if these resources include yeast. It has been suggested that female aggression in Drosophila melanogaster relates mostly to reproductive behaviors such as egg laying which is enhanced by yeast and its metabolites.5,38
Table 1. Modules of aggressive behavior in male and female Drosophila.
| Male | Female |
|---|---|
| Retreat | |
| Walking, flying or running away |
Walking or flying away |
| Approach/turn toward | |
| Walk toward the opponent while lowering body |
Turn/walk toward the opponent |
| Wing threat | |
| Raise both wings to a 45° angle toward opponent (> 1s) Flicks wings at 45° angle while facing away from opponent |
Raise one or both wings to a 45–90° angle toward opponent (< 1s) |
| Lunge | |
| Rear up on hind legs and collapse on opponent |
Rear up on hind legs and collapse on opponent |
| Shove | |
| Not observed |
Thrust the torso toward the opponent with both forelegs extended without recoil |
| Thrust with a wing threat | |
| Not observed |
Thrust and lift one or both wings to a 45–90° angle (< 1s) |
| Head butt | |
| Not observed |
Thrust the torso toward the opponent and strike the opponent with the head; usually followed by recoiling of the torso |
| Fencing | |
| Fencing (low): extend one leg and tap opponent’s leg Fencing (high): extend leg forward and push opponent facing each other |
Fencing (low): Extend leg and contact the opponent in a normal standing posture Fencing (high): Stand tall on the middle and rear legs and contact the opponent with the forelegs (can be combined with a wing threat < 1s) Fencing and feeding: Extend the middle or rear legs and contact the opponent while feeding Fencing threat: Extend the middle legs without contacting the opponent |
| Chasing | |
| Run after opponent |
Not observed |
| Holding | |
| Grasp opponent with forelegs and try to immobilize |
Not observed |
| Tussling | |
| Tumble over each other, sometimes leaving food surface |
Not observed |
| Boxing | |
| Rear both up on hind legs and strike the opponent with forelegs | Not observed |
Ethogram describing the observed modules of aggressive behavior in male and female Drosophila. The listed modules include threat, attack and retreat behaviors. Some of these modules are used by both sexes or show only subtle differences, while others are sex-specific.
Drosophila male aggressive behavioral modules have been used to analyze two closely related social behaviors, dominance and territoriality. Dominance can be defined as having a higher social status than other individuals of the group and often implies “priority acquired by past or present aggressive behavior.” However, in other species it was shown that aggression is not crucial to obtain and keep a dominant status. Dominance is a male-specific behavior that is stable over a certain amount of time.39-46 Dominance is studied by analyzing the males that win or lose subsequent fights and involves flies remembering their previous opponents over a certain amount of time.47,48
Territoriality can be defined as occupancy of a defended area that is used exclusively by the defending individual.39-41,49,50 Different studies have described the ecological circumstances under which Drosophila species exhibit territoriality and the evolutionary relevance of this behavior. In different Drosophila species, territoriality in a natural habitat has been shown to be closely linked with mating behavior. Males display territoriality in order to ensure the control over a resource for breeding and feeding. In highly territorial species, including the Hawaiian Drosophila heteroneura and Drosophila silvestris, this behavior even results in the aggregation of males in a lek in which these males defend their own leaf or part of rotten fruit.51-54 Drosophila melanogaster displays much less territorial behavior than its related Hawaiian species. The few observations of Drosophila melanogaster under field conditions show that defense of territory is rare and that, contrary to Drosophila pseudoobscura, fighting in Drosophila melanogaster might be unimportant to acquire a mating advantage in the field.54,55 The majority of the observations of melanogaster territoriality have been made in the lab.30,31,56-59 Under these conditions, Drosophila melanogaster territoriality has been described as a conditional strategy, where flies would only invest in the defense of an area under certain conditions where territoriality could lead to a mating advantage. These would include presence of females or males, attractive food, but also the occurrence of only a small number of other males and only a relatively small area of food to defend. It seems that only under these conditions, the territorial males obtain a mating advantage.60 Remarkably, in Drosophila silvestris, the highly territorial and aggressive Hawaiian species, mating success has not been linked with territoriality in a lab environment.61
These findings illustrate that although many characteristics of the behavioral repertoire of different insects have been reproduced in lab assays, the extrapolation to and the relevance of these data for a natural habitat need to be made with caution. A recent study of cricket behavior supports this notion. While dominant cricket males in the lab are capable of monopolizing access to females, in the wild these males seem to have many fewer mates than their subordinate opponents.62 A number of factors that affect the outcome of aggression assays with Drosophila melanogaster have been identified. The observation of aggressive behaviors in a lab setting shows that the different Drosophila species have the innate capability to fight, but also illustrates that there are clear effects of the environment on the initiation and sequence of aggressive behaviors. It has been reported that the size of the arena in which the experiment takes places can have a significant influence on the behavior of the flies. Smaller arenas have been described to cause an unconditioned reflex reaction which leads to increased activity or arousal.63 Furthermore, although these findings have not been analyzed further, an optimal arena size and shape have been proposed to induce aggression between Drosophila males while the lack of possibility for the losing male to escape can enhance aggression.15,35,63 These influences of the size of the available territory on social strategy have also been described in other species. The pupfish Cyprinodon variegates, for instance, establishes a territorial breeding system in large tanks while small tanks lead to a dominance hierarchy, in which one male controls most of the oviposition sites and mates with most females.64,65
How to study aggression in Drosophila
In general, three main setups have been described to analyze aggression in Drosophila (Fig. 1). All three assays allow the observation of the aggression behavioral modules in males and females. Depending on the setup dominance or territoriality can also be analyzed. The various published studies are further distinguished by additional variables at the social level, such as the number of flies tested in the arena, their social experience and the presence or absence of females, or at the level of different aggression modules being measured. In some cases the focus was on actions that are exerted by a single fly, while in others the focus was on dyadic behaviors.47,66 Some studies focus only on high intensity aggression modules which have been reported to constitute only a small portion of the fighting time but which lead to dominance. Other studies show the benefit of analyzing all aggressive modules to decipher the mechanisms underlying aggression. Different serotonin (5-HT) receptors, for instance, have been shown to alter different parts of the aggressive repertoire.14 Opposite effects on lunging, tussling and chasing compared with wing threats were also observed upon genetic manipulation of cholinergic neurons.67
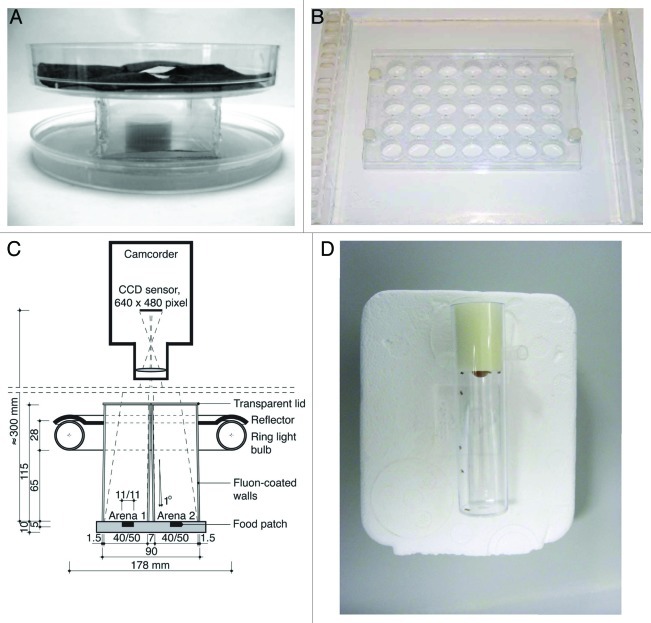
Figure 1. Different set-ups to study aggression in Drosophila. (A) Arena with a centrally placed food cup that can contain yeast paste or a virgin female, allowing the videotaping of aggressive behavior.68 (B) Set-up allowing the simultaneous recording of 35 arenas, usually during a 15 min period.72 (C) Scheme of a set-up compatible with CADABRA software allowing automated scoring of lunging, tussling, wing threats and chasing of two pairs of flies simultaneously. This set-up can be expanded to analyze eight pairs of flies simultaneously.67 (D) Set-up allowing the instant analysis of the aggressive encounters between groups of eight flies over a 2 min period.6,12,73
The first reported assays are variations on the same setup and make use of an arena with a centrally placed food cup to which yeast paste or a virgin female can be added.5,7,14,15,68-71 Whereas in the original assay six males were used, in a simpler variant, aggressive encounters between two flies are recorded, usually during a 20–30 min period. This setup is compatible with CADABRA software, an application which allows the automated scoring of lunging, tussling, wing threats and chasing of eight pairs of flies simultaneously.67 Next, an arena chamber was described that consists of a thick plexiglass plate with 35 evenly spaced cells, allowing the simultaneous recording of 35 arenas during a 15 min period.37,72 Each arena contains two flies, but no food or virgin is introduced. Finally, a very simple assay, first used to describe altered aggression in fruitless (fru) mutant males, was described that allows the analysis of the aggressive encounters between groups of eight flies over a two-minute period.6,12,33,73 This assay encompasses the instant scoring of the behavior, i.e., counting of all aggressive interactions among all eight males, without the need of analyzing recordings after the test period. This assay makes use of a standard food vial with a small drop of food.
Neurotransmitters and Aggression
Multiple neurotransmitters as well as the neuroactive peptide NPF have been shown to play a role in the processing of sensory information relevant to aggression and the generation of an appropriate aggressive response.1,7,9,13-15,66,74 The effects of these neurotransmitters are often ambiguous and are likely to depend on multiple factors including the receptor subtypes involved, the necessity of intermediate levels of the neurotransmitter or the spatio-temporal activation pattern of the pathways (Fig. 2). Furthermore, some of the observed discrepancies could be due to the different assays or arenas used, the differences in the genetic background of the flies, the different ways of scoring aggressive behavior and the use of flies with different social experience.
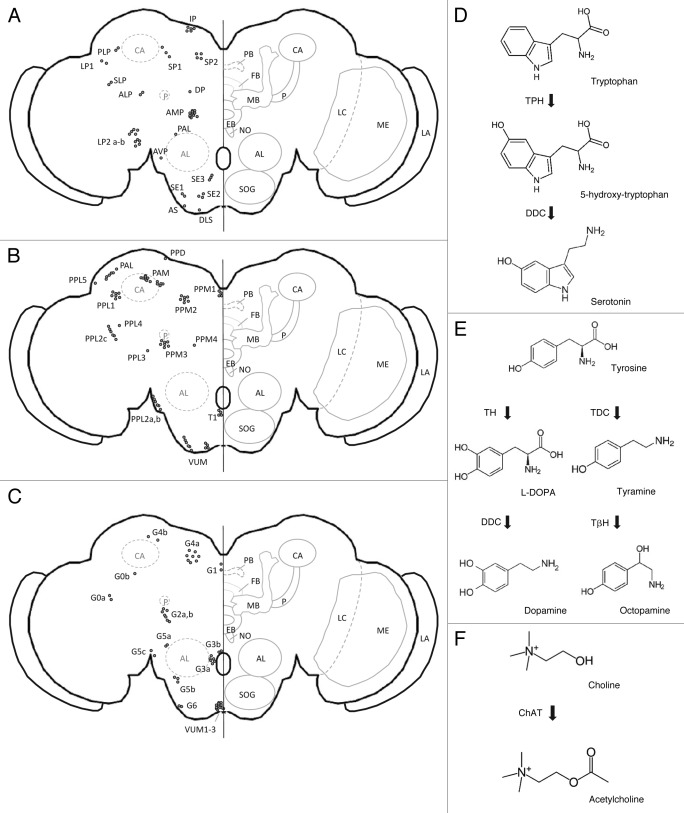
Figure 2. (A–C) Schematic representation of adult central brain cells reported to be serotonergic, dopaminergic or octopaminergic. Cholinergic cells were reported to be widespread throughout the entire brain making it difficult to present a schematic overview.140-143 PB, protocerebral bridge; FB, fan-shaped body; EB, ellipsoid body; NO, noduli; MB, mushroom bodies; P, mushroom body peduncle; CA, mushroom body calyx; AL, antennal lobe; SOG, subesophageal ganglion; LC, lobula complex; ME, medulla; LA, lamina. (A) Serotonergic cells. LLP1, posterior lateral protocerebrum; LP2a, between the medulla and the lateral protocerebrum; LP2b, between the medulla and the lateral protocerebrum; DP, dorsal protocerebrum; SP1, posterior to superior median protocerebrum; SP2, posterior median protocerebrum; IP, posterior inferior median protocerebrum; PLP, posterior lateral protocerebrum; SLP, superior lateral protocerebrum; AMP, anterior median protocerebrum; ALP, anterior lateral protocerebrum; AVP, anterior ventral protocerebrum; PAL, posterior to antennal lobe; DLS, dorsal lateral subesophageal ganglion; AS, anterior subesophageal ganglion; SE1, lateral subesophageal ganglion; SE2, anterior lateral subesophageal ganglion; SE3, most ventral subesophageal ganglion.144-146 (B) Dopaminergic cells. PAM, dorsomedial anterior protocerebral; PAL, dorsolateral anterior protocerebral; PPM, dorsomedial posterior protocerebral; PPL1, dorsolateral posterior protocerebral; PPL2, lateral posterior protocerebral; PPD, protocerebral posterial dorsal; T1, tritocerebrum; VUM, ventral unpaired medial neurons.76,147,148 (C) Octopaminergic cells (nomenclature according to Sinakevitch and Strausfeld, 2006; nomenclature between brackets according to Monastirioti et al., 1995). Cluster G0a, Cluster G0b (LP, lateral protocerebrum cell), Cluster G1 (DMC, dorsal medial cluster), Cluster G2a (~DAC, dorsal anterior cluster?), Cluster G2b (~DAC, dorsal anterior cluster?), Cluster G3a (AL, antennal lobe cluster), Cluster G3b, Cluster G4a (DPC, dorsal posterior cluster), Cluster G4b (DPC, dorsal posterior cluster), Cluster G5a, Cluster G5b,c, Cluster G6, VUM, Ventral unpaired median neurons 1–3 (SM, subesophageal medial). (D-F) Diagrams representing the biosynthetic pathways of acetylcholine and the monoaminergic neurotransmitters: serotonin, dopamine and octopamine. (D) Serotonin synthesis. TPH, Tryptophan hydroxylase; DDC, DOPA decarboxylase. (E) Dopamine/octopamine synthesis. TH, Tyrosine hydroxylase; TDC, Tyrosine decarboxylase; DDC, DOPA decarboxylase; TβH, Dopamine β hydroxylase (F) Acetylcholine synthesis. ChAT, Choline acetyltransferase
Octopamine-tyramine
In the context of aggressive behavior, tyramine (TA) and octopamine (OA) have been the most extensively studied. Tyramine-β-hydroxylase (TβH), the enzyme responsible for the conversion of TA to OA, was one of the first enzymes shown to be involved in aggression in Drosophila. TβHnM18 null mutants have undetectable levels of octopamine and a 10-fold increase in tyramine levels. TβHnM18 males display decreased aggressive behavior, reduced transitions to aggressive behavior and decreased lunging, while females show prolonged fighting latency and reduced head butting. The magnitude of the effect seems to depend on the genetic background of the flies.7,13,15,74Interestingly, TβHnM18 has also been associated with an increase in courtship behavior in socially naïve males, which could suggest a switch in behavioral choice between aggression or courtship.74 However, this change in behavior could not be replicated.13
The changes in aggression in TβH-null mutants can in principle be due either to the decreases in OA or to the increase in TA. Flies have two tyrosine decarboxylase genes that convert tyrosine to TA, the non-neuronally expressed Tdc1 and the neuronally expressed Tdc2. Male flies mutant for Tdc2 (Tdc2R054) have no detectable levels of TA or OA in the brain and are less aggressive compared with controls.15 Thus, it is the loss of OA that is responsible for the observed aggression phenotype.
The aggression modulating effect of OA has been further analyzed using pharmacological or genetic techniques. Pharmacological stimulation of OA signaling has been shown to enhance male aggression, but this effect is only present in socially experienced flies.13,15 Two explanations for this observation have been proposed. First, OA might be important in the regulation of social experience, hence resetting aggression after social experience. Second, it might be impossible to detect higher aggression levels in already highly aggressive socially naïve flies.13 However, it also cannot be excluded that the different results are due to experimental variations such as different arenas or differences in scored parameters.
Genetically induced changes in OA signaling were also shown to alter aggression. Ubiquitous TβH expression can induce elevated aggression in socially experienced wild-type flies but not in socially naïve flies.13 However, it does seem to partially rescue the aggression phenotype of socially naïve TβHnM18 null mutants.15
Neuronal activation of OA neurons in adults leads to the same phenotype of elevated aggression in socially experienced flies while acute silencing results in the opposite effect in socially naïve flies, indicating that the aggression phenotype in TβH mutants is not due to developmental defects.13,15 Activation of OA neurons in socially naïve flies, however, does not affect aggressive behavior.13,75
In the Drosophila adult brain, there are approximately 100 octopaminergic neurons. An important question is then whether it is only specific subsets of these neurons that are required for aggressive behavior. Expression of TβH selectively in the Tdc2 circuit rescues the aggression phenotypes in both male and female hypoaggressive TβH-null mutants, thereby demonstrating the neuronal requirement of OA in the phenotype.13 Further dissection of this circuit narrowed the relevant neurons down to a distinct subset of octopaminergic neurons in the subesophageal ganglion (SOG). In the SOG, a second subset of octopaminergic neurons in which OA colocalizes with FRU, another gene affecting aggression, have been proposed to play a role in the decision-making network that controls the shift between aggressive and courtship behavior.74,75
Serotonin
Initially, pharmacological studies reported that 5-HT does not have an influence on aggression in Drosophila.7 More recently, it has been shown that drug-induced increases of 5-HT and overexpression of Tryptophan hydroxylase (Trh), the rate limiting enzyme in 5-HT synthesis increases aggression.66 Consistent with this, selective activation of serotonergic neurons in the brain by means of Trh -gal4 results in increased aggression with flies that escalate fights faster and with increased intensity.9 By contrast, Dierick and Greenspan (2007) showed that pharmacological inhibition or silencing of 5-HT neurons has no effect on aggression. However, Alekseyenko et al. (2010) found that flies with acutely inhibited 5-HT neurons fight but do not escalate.9 Overall, these results indicate that the effects of 5-HT are complex. The different effects of 5-HT might depend on the differential regulation of the behavior by different receptor types.14 Drosophila expresses three types of 5-HT receptors; 5-HT2Dro, 5-HT1A-like and 5-HT7.14 Using specific pharmacological modulation of these receptors, Johnson et al. showed that 5-HT2 and 5-HT1A-like receptors differentially regulate aggression in Drosophila. While activation of 5-HT2 receptors decreases overall aggression, activation of 5-HT1A-like receptors induces the opposite effect.14 Both receptors influence different aspects of the behavior: 5-HT2 receptor manipulation primarily alters lunging and boxing, whereas 5-HT1A-like receptor manipulation primarily affects wing threats and fencing.14
Dopamine
Pharmacological alteration of DA levels in Drosophila suggests that the effects of DA on aggressive behavior are complex.7 Spatiotemporal inactivation of neurons expressing Dopa decarboxylase (Ddc), the enzyme responsible for the final common step in 5-HT and DA biosynthesis, eliminated mid- and high-level aggression.9 However, neither silencing serotonergic nor dopaminergic neurons individually mimics the phenotypes seen when both circuits are silenced simultaneously, suggesting that the interplay between both circuits is required for the regulation of aggression.9 Recently, it has been shown that there are at least eight types of dopaminergic neurons.76 This observation raises the possibility that there may be dopaminergic neuron subtype-specific effects on aggression.
Acetylcholine
In Drosophila, most sensory neurons and many central neurons are cholinergic. The effect of these neurons on aggressive behavior in Drosophila has not been extensively studied and the direct effects of alterations in neurotransmitter levels have not been investigated. Two independent publications report the effects of feminizing these neurons using Choline acetyltransferase (Cha)-gal4 and UAS-transformer and show that this leads to an increase in specific aggressive actions such as lunging, boxing and chasing, whereas others such as wing threats are reportedly decreased.1,67 The overall level of aggression, however, remains normal.1 Although alterations in genes of the sex determination hierarchy have been reported to induce female fighting patterns in males and vice versa, feminized Choline acetyltransferase (Cha)- gal4 ; UAS-transformer males show no changes in male fighting patterns. Instead, they show an increase in male-male courting behavior and an absence of male-female courting.1,33,48,67 The reported effects seem to be due to a developmental effect with the phenocritical period in late larval to early pupal stages.1 Given the large number of cholinergic neurons in the nervous system, it is unclear which subset(s) could be responsible for the observed behavioral changes and whether these differences in aggressive behavior relate to inappropriate sensory input arriving in the CNS or to changes within the CNS circuits themselves.1 It has been argued that male specific cholinergic neurons, which express the male forms of fruitless or doublesex and represent 10% of the total number of cholinergic neurons, could play a major role, but this has not been investigated further.1
Neuronal Circuits Involved in Aggressive Behavior
Integration of sensory input
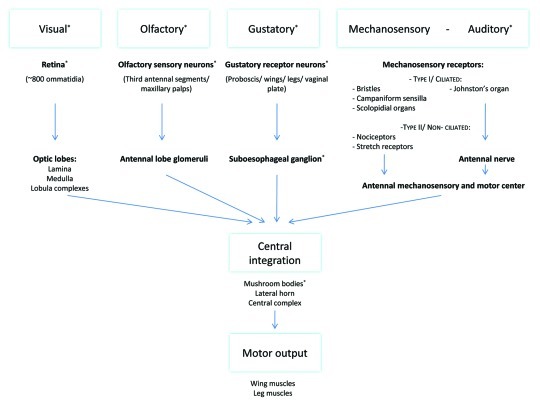
Aggressive behavior requires reception of sensory input followed by the interpretation and integration of these cues and the generation of an appropriate response. Differences in the development or function of the neuroanatomical structures mediating these processes can lead to the generation of an abnormal aggressive response to a certain cue (Fig. 3).
Figure 3. Scheme representing the flow of information provided by external stimuli to integration centers in the central brain. This central integration results in interpretation and integration of the different inputs and the generation of an appropriate response. The role of stimuli or neuronal structures marked with an asterisk (*) in aggression has been more closely analyzed. Visual information is received by the retinal ommatidia and travels through the different layers of the optic lobes, lamina, medulla and lobula complexes, to the central brain.149 The described higher integration centers of this visual information include the mushroom bodies, the central complex and different neurons in the lateral protocerebrum.97-99,150 Olfactory information is sensed by olfactory neurons expressing olfactory receptors located on the third antennal segments and the maxillary palps. Odorant cues travel via these neurons to the glomeruli in the antennal lobe from where projection neurons send this information to higher integration centers, including the mushroom bodies and the lateral horn.93,151-155 Gustatory signals are sensed by gustatory receptors expressing gustatory receptors located on the proboscis, wings, legs and vaginal plate. These neurons all project to the SOG. The SOG has been shown to project toward multiple other brain regions including the antennal lobe, the lateral horn and the mushroom bodies.96,155,156 Mechanosensory information is sensed by a variety of receptors, located all over the body, which can be subdivided into a ciliated and a non-ciliated group. Non-ciliated mechanosensory receptors include nociceptors and muscle and visceral stretch receptors. Ciliated mechanosensory receptors include: bristles responsible for touch perception, campaniform sensilla on wings and haltere providing info on flight parameters and chordotonal organs including scolopidial organs providing proprioceptive and gravireceptive information.157,158 The fly's largest chordotonal organ, Johnston’s organ, is located in the second antennal segment and represents the flies ear. Part of these neurons have been shown to innervate the antennal mechanosensory and motor center of the brain, a neuropil lateral to the SOG and the antennal lobes, further higher integration is mainly unknown.100,157,158
The generation of an aggressive response to external stimuli is influenced by many factors. One of these is the social history of the individual fly. Social experience influences aggression with socially naïve males being more aggressive.13,67,77,78 About 200 genes have been shown to be differentially expressed between socially naïve and experienced flies.79 Interestingly, one of these genes, Cyp6a20, is associated with pheromone sensing, suggesting that sensitivity to these pheromones provides a manner in which social experience modulates aggressive behavior.79 In addition, memory of previous fights seemed to induce alterations in the fighting intensity among familiar male opponents.47
Pheromone signaling is the best studied sensory input system in the context of aggressive behavior.10,77,80-82 Volatile pheromones are detected by the olfactory system, while non-volatile cuticular hydrocarbon pheromones signal via the gustatory system. Both have been reported to modulate aggressive behavior. The volatile pheromone cVA promotes aggression among males via Or67d expressing olfactory receptor neurons.10 This pheromone also seems to mediate the aggression suppressing effect of group housing via Or65a expressing olfactory receptor neurons.81
The non-volatile sex-specific cuticular hydrocarbons produced by the oenocytes, play an important role in sex recognition and thus the appropriate behavioral response of males toward other males or females.82 Masculinization of female oenocytes for instance elicits an aggressive response of males toward those females. The aggression promoting effect of cuticular hydrocarbons was further supported by the reduced levels of aggression between oenocyte- depleted males.80 One of the most prominent cuticular hydrocarbons, (z)-7-tricosene, plays an important role in this aggression regulatory mechanism. This pheromone acts in a hierarchical manner with cVA through the activation of the Gr32a gustatory receptor where (z)-7-tricosene is required for the aggression promoting effect of cVA, but not vice versa.80 Aside from pheromones, auditory and visual sensory systems have been reported in the context of aggression.15,83
Blind norpA and motion blind homozygous ninaE males perform significantly fewer lunges thereby implicating visual information in aggression.15 The white gene is another gene that has been investigated in the context of vision and aggression. Mutations in this gene lead to visual abnormalities, especially at high light intensities, but these flies are not blind. They have normal phototactive responses and show responses to light stimuli on ERG that are stronger due to the lack of light buffering by pigmentation.84,85 White mutants show different behavioral abnormalities, but these could also be due to effects of this ABC-transporter in central brain structures. Indeed, for some behaviors it has been described that the effects in central brain structures can be independent from the effects on eye pigmentation, possibly due to alterations in different monoamine levels in the brain.85-90 The effect on aggression of the w1118 allele, a null allele of white with a deletion at the 5′ of the gene that includes exon 1, has been examined in different assays by different groups. One report did not show a significant alteration in aggression in these mutants, whereas other groups showed a significant reduction in lunging as well as other high intensity fighting and an increase in fighting latency.9,13,15,91,92 Furthermore, an eye specific RNAi knockdown of the white gene using GMR-gal4 has also been shown to cause a significant reduction in lunging.15 However, the effect of w1118 on lunging does not seem to be solely due to the effects in the eye.15 Overexpression of white in the eye using GMR-gal4 in a w1118 mutant only partially rescues the lunging phenotype and white RNAi using various drivers expressed throughout the brain lead to significant decreases in this behavior.15 In summary, the possible role of the white gene in aggression seems complex and not fully understood.
Auditory signals have been proposed to play a role in the recognition of an opponent in Drosophila melanogaster males.83 Sound production during aggression seems to be a male specific trait.83 Males produce acoustic signals during aggressive encounters that differ from courtship sounds. These signals mainly occur upon tapping by an opponent and seem to be a recognition reaction by the tapped male when he finds out that his opponent is also a male, thus inducing an aggressive reaction. Further observations supporting the aggressive nature of these sounds are that they are capable of inducing retreat of the opponent and that they are only produced in certain situations. Retreat of the opponent, for instance, is solely accompanied by silent wing movements.
Neural circuits and aggression
Different central brain structures and neuronal populations play a role in the integration and interpretation of stimuli and in the generation of a behavioral response. However, only a subset of these structures has been more closely investigated in the context of aggressive behavior.
Olfactory information is transmitted along olfactory sensory neurons toward the glomeruli of the antennal lobe. In the antennal lobe, peripheral olfactory receptor neurons are interconnected by local interneurons involved in the local processing of olfactory information. This interconnectivity has been suggested to modulate the interplay between cVA signaling via the aggression promoting Or67d receptor and the aggression suppressing Or65a receptor.81 The ORNs also connect to projection neurons which forward information toward the mushroom bodies and the lateral horn. The latter mediates innate behavioral responses to odors.93,94 The projection neurons that transmit the information of the male-specific, aggression-mediating pheromone cVA toward the lateral horn are FRU positive and show sexual dimorphism, suggesting that these neurons may modulate aggressive behavior.95
Gustatory receptor information on the other hand is primarily transmitted via gustatory receptor neurons toward the subesophageal ganglion. How this information is further processed in the brain is less well understood, but subesophageal ganglion neurons project toward multiple regions in the brain, including the antennal lobe, the lateral horn and the mushroom bodies, which could present a way to relay gustatory and contact pheromone sensory data to different higher processing centers in the brain.74,96
Visual information passes through multiple well-studied layers in the optic lobes. The central integration of this information, however, is less well known. The mushroom bodies and the ellipsoid body as well as different neurons in the lateral protocerebrum have been implicated in certain forms of visual learning.97-99
Auditory information is transmitted by neurons of the Johnston’s organ that innervate the antennal mechanosensory and motor center of the brain, a neuropil lateral to the subesophageal ganglion and antennal lobes.100 It is unknown how this information is further integrated in the brain.
One of the main higher integration sites in the brain, the mushroom bodies, are involved in the integration of many of the aforementioned sensory cues. Mushroom bodies have been shown to play a key role in multiple behaviors such as olfactory information processing, memory formation, sleep, the higher control of locomotion, and the processing of visual context information.99,101-104The mushroom bodies have also been implicated in aggression.6,7,11,12 Blocking their synaptic output results in the abolishment of aggressive behavior.7 Many pleiotropic genes influencing aggression also function in mushroom body development suggesting a relation between brain development and aggressive behavior in adult flies.6,11,12 The length of the α lobes of the mushroom bodies has been correlated with aggressive behavior in viable P-element insertion mutants.11 The significance of this observation is unclear. However, a relationship between mushroom body volume and aggression has also been described in two paperwasp species, Polistes instabilis and Mischocyttarus mastigophorus, suggesting there could be conserved roles involving mushroom body structure and its plasticity in insect aggression.105,106
Finally, the fruitless (fru) circuit has been shown to play an important role in the regulation of sexually dimorphic responses to sensory cues. fru, known for its prominent role in male courtship behavior undergoes sex-specific splicing, and it is involved in the sex determination hierarchy. Alterations in splicing of this gene are sufficient to elicit female fighting patterns in males and vice versa.33,48 Subgroups of fru neurons are involved in the control of these sexually dimorphic patterns.107 Specific fru-positive octopaminergic neurons in the subesophageal ganglion, for example, have been implicated in the decision between aggressive or courtship behavior.75 The subesophageal ganglion plays a role in pheromone recognition in addition to its better-known function in taste processing. The role of the subesophageal ganglion in aggression is further supported by the presence therein of other, non-fru-positive, octopaminergic neurons that also modulate aggression.13
Due to the complexity of aggressive behavior and the vast connectivity pattern throughout the brain of the neuropils known to be involved in aggression, it is expected that many more neuronal populations will exert an influence on this behavior.
Pleiotropic Networks of Many Interacting Genes
In the 1990s, the first attempts to map human loci involved in complex behavioral traits using the available genome-wide markers identified a limited number of loci with a large effect size.108-112 However, novel high resolution mapping in model organisms and genome wide association studies in patients suffering from disorders of which aggression represents an endophenotype, revealed that the genetic effect sizes for common variation are a lot smaller than previously expected.109,113,114 These studies showed the involvement of a large number of genes and the potentially high importance of rare variants in the control of these complex behaviors.
In Drosophila, quantitative analyses demonstrated that investigating genes involved in biologically likely pathways only partly identify the genetic network and the neuronal populations that regulate this behavior.11,12,23,37,73,115 In effect, currently available evidence reveals that aggressive behavior in Drosophila is controlled by (a) complex genetic network(s) involving a large number of pleiotropic and epistatically interacting genes. The existence of such an elaborate network controlling aggression is not surprising given the genetic architecture of other complex traits in Drosophila and other organisms and given the number of neurobiological processes that have been shown to influence aggression in Drosophila itself.21,109,116-120 Furthermore, it also makes sense from an evolutionary and biological point of view. As previously discussed, complex behaviors rely on the perception and integration of many layers of information as well as the capability to execute the behavior. Thus they depend on a vast number of biological processes, each characterized by their own (sub)network of genes and each under different evolutionary pressures.
Two independent studies that investigated the effects of artificial selection for aggressive behavior on transcript abundance provided a first insight into the overall genetic network underlying this trait. Dierick et al. identified 80 differentially expressed transcripts, while Edwards et al. found 1,539 altered transcripts.37,73 Both experiments illustrate the possible role in aggression of transcripts involved in a wide variety of biological processes and molecular functions. A possible explanation for the difference in gene numbers between both groups is that genetic variation in the fly stocks (laboratory stock vs. recently derived from nature) that were used to initiate the selection experiments was different.
It is important to consider that transcriptional alterations do not provide information about the causality of the identified genes for the behavioral trait. However, for many of the transcripts it has been shown that these genes affect aggressive behavior. A screen of P[GT1] insertion lines, which was enriched for candidate aggression genes identified by the selection experiment of Edwards et al. (2006), showed direct effects on this behavior of mutations in 59 genes. Mutations in Cyp6a20, a gene identified by Dierick et al. directly alters aggression levels.37,121 Interestingly, this gene is involved in the regulation of behavioral differences between socially naïve and experienced flies.79
These studies also illustrate the extensive pleiotropy of the identified genes. Many of these genes show clear effects on other behaviors such as resistance to starvation stress, sleep and olfactory and locomotion behavior.21,116,120,122 Others play a role in the correct development of sensory bristles and neuropils, such as the mushroom bodies and the central complex.6,12,123 A detailed analysis of the neuralized gene revealed that alternative splicing can provide a molecular mechanism which forms the functional basis of the phenotypic pleiotropy.6
Analyses of the genetic networks underlying other complex behaviors such as olfactory avoidance behavior and startle induced locomotion showed that the corresponding genes often interact in a non-additive manner.21,116 Therefore, it is not unexpected that epistasis would also be present among the genes that form the genetic basis of aggression. A first indication came from the mapping of aggression QTLs which are characterized by epistasis.115 Furthermore, the analysis of the variation in aggressive behavior among 40 wild-derived inbred lines provided more evidence for the presence of non-additive interactions among the genes involved.23 Recently, the analysis of the epistatic interactions between a set of ten hyperaggressive P[GT1] insertion lines provided the first insights into the complex nature of these interactions and their widespread influences on transcript abundance.11
Further analysis of the transcriptional network underlying aggression in the 40 wild-derived inbred lines identified networks of coregulated genes that are involved in this behavior.23 In this analysis, the genetic network associated with natural variation in aggressive behavior was mapped by investigating the associations between aggression and quantitative trait transcripts (QTT) and single feature polymorphisms (SFP) and subsequently grouping the associated transcripts into genetically correlated modules. Two hundred sixty-six candidate aggression genes were identified. While mutations in some of the identified genes were shown to have a clear effect on aggression or have been shown to have an important role in this behavior, e.g., members of the Cytochrome P450 gene family, the identified genes showed only a small overlap with previously identified genes. However, they were part of nine distinct modules of genetically intercorrelated transcripts enriched for gene ontology categories previously implicated in aggressive behavior, such as neurodevelopment, visual perception and metabolic functions and male-biased transcripts.
The difference between the genes identified by the selection experiments, the QTL mapping and the transcriptional analysis of the inbred lines as well as the absence of some of the previously identified genes involved in aggressive behavior could be attributed to multiple factors.23,37,73,115 First of all, as these experiments, with exception of the QTL mapping, are all based on expression analyses, the candidate aggression genes would not be identified if they are not genetically variable at the transcript level or if their transcripts do not vary or are only expressed at low levels during the analyzed developmental stage.23,37,73 As the genome sequence of the 40 inbred lines will be available in the near future, it will be interesting to see whether polymorphisms at the DNA level linked with aggression will identify these previously known aggression genes. Furthermore, all of the described whole genome analyses, i.e., both selection experiments, the QTL mapping experiment and the transcript analysis of the 40 inbred lines, use different parental lines as a starting point.23,37,73,115 As we already pointed out, epistasis plays an important role in controlling behaviors, thus a different genetic context, resulting from the different parental lines could have a major influence. Furthermore, it could be that the genetic variation captured in each sample was different, as would happen, for example, if the genetic basis of natural variation in aggressive behavior involves many different rare alleles with small effects. Finally, the known candidate genes, such as fru, are often involved in other important processes. This could make them subject to strong purifying selection and thus might not allow functional variation.23
The transcript analyses of the 40 inbred lines provides the first attempt to generate an overview of transcriptional modules that control aggression and could form the starting point for a systems genetics analysis of natural variation in this behavior. Such an approach aims to integrate the different layers of biological information between DNA and observed phenotype, consisting of RNA, proteins and metabolites.124 In the near future, the availability of genome sequences of these 40 lines, and of in total 192 lines will give the opportunity to fill in another layer of this network, creating the possibility to directly link polymorphism to alterations in the coexpression network that result in behavioral changes.23,125
Perspectives
The genetic architecture of behaviors, such as aggression, is shaped by many interacting genes with pleiotropic effects. Quantitative genetic whole genome analyses enable more insight into the overall genetic networks and the molecular context that are at the basis of these behaviors. However, it is the combination of complex quantitative analyses with single gene molecular genetics that will allow the definition of the exact molecular functions of subsets of genes in this network.
The study of aggression in Drosophila using such integrative approach will lead to a better understanding of its genetic and neurobiological basis and the identification and characterization of neural networks that mediate or influence this behavior. We surmise that these insights will contribute to our understanding of the evolution of aggressive behavior and of the genetic basis of aggression in other species, including humans.
In Drosophila, territorial aggression has been observed in different species. Drosophila hawaiensis males, for instance, have been shown to vigorously defend their mating territory.126,127 Analysis of differences in the genetic background between closely related species could help to understand the evolutionary forces leading to divergent aggressive behavior. In addition, the analysis of sequence variation and its functional consequences for genes involved in aggressive behavior in Drosophila melanogaster represents another avenue to study the genetics and evolution of aggression in other species. Supporting this contention, many factors involved in the control of aggression seem to be conserved among insects. Experience and chemical cues influence this behavior, while neurotransmitters implicated in Drosophila aggression also mediate this behavior in ants and crickets.128-136 Furthermore, QTL mapping revealed, besides neurodevelopmental genes and GPCRs, the metabotropic GABA-B-R1 receptor as a candidate aggression gene in honey bees.137,138 Not only neurotransmitters and receptors appear to play comparable roles in insect aggression, but also the same brain structures mediate aggressive behavior. Similar to Drosophila, the mushroom bodies have been implicated in aggression in two paperwasp species, Polistes instabilis and Mischocyttarus mastigophorus.105,106 The role of mushroom bodies is also supported by the observation that agonistic behavior in crickets is accompanied by the induction of c-Fos/FRA-like expression in the mushroom body neuropil.139 It will be interesting and important to determine the extent to which the molecular networks underlying aggression are conserved between vertebrates and insects and whether this conservation is mainly situated at the gene level, with common functions in behavioral regulation between homologs, or at the systems level, where networks affecting shared biological processes lead to behavioral alterations.
Footnotes
Previously published online: www.landesbioscience.com/journals/fly/article/19249
References
- 1.Mundiyanapurath S, Chan YB, Leung AK, Kravitz EA. Feminizing cholinergic neurons in a male Drosophila nervous system enhances aggression. Fly (Austin) 2009;3:179–84. doi: 10.4161/fly.3.3.8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anholt RR, Mackay TF. Quantitative genetic analyses of complex behaviours in Drosophila. Nat Rev Genet. 2004;5:838–49. doi: 10.1038/nrg1472. [DOI] [PubMed] [Google Scholar]
- 3.Craig IW, Halton KE. Genetics of human aggressive behaviour. Hum Genet. 2009;126:101–13. doi: 10.1007/s00439-009-0695-9. [DOI] [PubMed] [Google Scholar]
- 4.Huber R, Orzeszyna M, Pokorny N, Kravitz EA. Biogenic amines and aggression: experimental approaches in crustaceans. Brain Behav Evol. 1997;50(Suppl 1):60–8. doi: 10.1159/000113355. [DOI] [PubMed] [Google Scholar]
- 5.Nilsen SP, Chan YB, Huber R, Kravitz EA. Gender-selective patterns of aggressive behavior in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2004;101:12342–7. doi: 10.1073/pnas.0404693101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rollmann SM, Zwarts L, Edwards AC, Yamamoto A, Callaerts P, Norga K, et al. Pleiotropic effects of Drosophila neuralized on complex behaviors and brain structure. Genetics. 2008;179:1327–36. doi: 10.1534/genetics.108.088435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baier A, Wittek B, Brembs B. Drosophila as a new model organism for the neurobiology of aggression? J Exp Biol. 2002;205:1233–40. doi: 10.1242/jeb.205.9.1233. [DOI] [PubMed] [Google Scholar]
- 8.Miczek KA, de Almeida RM, Kravitz EA, Rissman EF, de Boer SF, Raine A. Neurobiology of escalated aggression and violence. J Neurosci. 2007;27:11803–6. doi: 10.1523/JNEUROSCI.3500-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alekseyenko OV, Lee C, Kravitz EA. Targeted manipulation of serotonergic neurotransmission affects the escalation of aggression in adult male Drosophila melanogaster. PLoS One. 2010;5:e10806. doi: 10.1371/journal.pone.0010806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Anderson DJ. Identification of an aggression-promoting pheromone and its receptor neurons in Drosophila. Nature. 2010;463:227–31. doi: 10.1038/nature08678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zwarts L, Magwire MM, Carbone MA, Versteven M, Herteleer L, Anholt RR, et al. Complex genetic architecture of Drosophila aggressive behavior. Proc Natl Acad Sci U S A. 2011;108:17070–5. doi: 10.1073/pnas.1113877108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards AC, Zwarts L, Yamamoto A, Callaerts P, Mackay TF. Mutations in many genes affect aggressive behavior in Drosophila melanogaster. BMC Biol. 2009;7:29. doi: 10.1186/1741-7007-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou C, Rao Y, Rao Y. A subset of octopaminergic neurons are important for Drosophila aggression. Nat Neurosci. 2008;11:1059–67. doi: 10.1038/nn.2164. [DOI] [PubMed] [Google Scholar]
- 14.Johnson O, Becnel J, Nichols CD. Serotonin 5-HT(2) and 5-HT(1A)-like receptors differentially modulate aggressive behaviors in Drosophila melanogaster. Neuroscience. 2009;158:1292–300. doi: 10.1016/j.neuroscience.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoyer SC, Eckart A, Herrel A, Zars T, Fischer SA, Hardie SL, et al. Octopamine in male aggression of Drosophila. Curr Biol. 2008;18:159–67. doi: 10.1016/j.cub.2007.12.052. [DOI] [PubMed] [Google Scholar]
- 16.Popova NK. From gene to aggressive behavior: the role of brain serotonin. Neurosci Behav Physiol. 2008;38:471–5. doi: 10.1007/s11055-008-9004-7. [DOI] [PubMed] [Google Scholar]
- 17.Pinna G, Agis-Balboa RC, Pibiri F, Nelson M, Guidotti A, Costa E. Neurosteroid biosynthesis regulates sexually dimorphic fear and aggressive behavior in mice. Neurochem Res. 2008;33:1990–2007. doi: 10.1007/s11064-008-9718-5. [DOI] [PubMed] [Google Scholar]
- 18.Rillich J, Schildberger K, Stevenson PA. Octopamine and occupancy: an aminergic mechanism for intruder-resident aggression in crickets. Proc Biol Sci. 2011;278:1873–80. doi: 10.1098/rspb.2010.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miczek KA, Maxson SC, Fish EW, Faccidomo S. Aggressive behavioral phenotypes in mice. Behav Brain Res. 2001;125:167–81. doi: 10.1016/S0166-4328(01)00298-4. [DOI] [PubMed] [Google Scholar]
- 20.Huber R, Smith K, Delago A, Isaksson K, Kravitz EA. Serotonin and aggressive motivation in crustaceans: altering the decision to retreat. Proc Natl Acad Sci U S A. 1997;94:5939–42. doi: 10.1073/pnas.94.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto A, Zwarts L, Callaerts P, Norga K, Mackay TF, Anholt RR. Neurogenetic networks for startle-induced locomotion in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2008;105:12393–8. doi: 10.1073/pnas.0804889105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anholt RR, Dilda CL, Chang S, Fanara JJ, Kulkarni NH, Ganguly I, et al. The genetic architecture of odor-guided behavior in Drosophila: epistasis and the transcriptome. Nat Genet. 2003;35:180–4. doi: 10.1038/ng1240. [DOI] [PubMed] [Google Scholar]
- 23.Edwards AC, Ayroles JF, Stone EA, Carbone MA, Lyman RF, Mackay TF. A transcriptional network associated with natural variation in Drosophila aggressive behavior. Genome Biol. 2009;10:R76. doi: 10.1186/gb-2009-10-7-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harbison ST, Carbone MA, Ayroles JF, Stone EA, Lyman RF, Mackay TF. Co-regulated transcriptional networks contribute to natural genetic variation in Drosophila sleep. Nat Genet. 2009;41:371–5. doi: 10.1038/ng.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ayroles JF, Carbone MA, Stone EA, Jordan KW, Lyman RF, Magwire MM, et al. Systems genetics of complex traits in Drosophila melanogaster. Nat Genet. 2009;41:299–307. doi: 10.1038/ng.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scott JP, Fredericson E. The causes of fighting in mice and rats. Physiol Zool. 1951;24:273–309. [Google Scholar]
- 27.McGlone JJ. Agonistic behavior in food animals: review of research and techniques. J Anim Sci. 1986;62:1130–9. doi: 10.2527/jas1986.6241130x. [DOI] [PubMed] [Google Scholar]
- 28.Ewbank R, Meese GB. Aggressive behaviour in groups of domesticated pigs on removal and return of individuals. Anim Prod. 1971;13:685–93. doi: 10.1017/S0003356100000179. [DOI] [Google Scholar]
- 29.Sturtevant AH. Experiments on sex recognition and the problem of sexual selection in Drosophilia. J Anim Behav. 1915;5:351–66. doi: 10.1037/h0074109. [DOI] [Google Scholar]
- 30.Jacobs ME. Influence of light on mating of Drosophila Melanogaster. Ecology. 1960;41:182–8. doi: 10.2307/1931952. [DOI] [Google Scholar]
- 31.Dow MA, von Schilcher F. Aggression and mating success in Drosophila melanogaster. Nature. 1975;254:511–2. doi: 10.1038/254511a0. [DOI] [PubMed] [Google Scholar]
- 32.Jacobs ME. Influence of beta-alanine on mating and territorialism in Drosophila melanogaster. Behav Genet. 1978;8:487–502. doi: 10.1007/BF01067478. [DOI] [PubMed] [Google Scholar]
- 33.Lee G, Hall JC. A newly uncovered phenotype associated with the fruitless gene of Drosophila melanogaster: aggression-like head interactions between mutant males. Behav Genet. 2000;30:263–75. doi: 10.1023/A:1026541215546. [DOI] [PubMed] [Google Scholar]
- 34.Skrzipek KH, Kroner B, Hager H. Inter-male aggression in Drosophila melanogaster - Laboratory Study. Z Tierpsych - J Comp Ethol 1979; 43:107-20.
- 35.Chen S, Lee AY, Bowens NM, Huber R, Kravitz EA. Fighting fruit flies: a model system for the study of aggression. Proc Natl Acad Sci U S A. 2002;99:5664–8. doi: 10.1073/pnas.082102599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoffmann AA. A laboratory study of male territoriality in the sibling species Drosophila melanogaster and D. simulans. Anim Behav. 1987;35:807–18. doi: 10.1016/S0003-3472(87)80117-3. [DOI] [Google Scholar]
- 37.Dierick HA, Greenspan RJ. Molecular analysis of flies selected for aggressive behavior. Nat Genet. 2006;38:1023–31. doi: 10.1038/ng1864. [DOI] [PubMed] [Google Scholar]
- 38.Ueda A, Kidokoro Y. Aggressive behaviours of female Drosophila melanogaster are influenced by their social experience and food resources. Pysh Entomology. 2002;27:21–8. doi: 10.1046/j.1365-3032.2002.00262.x. [DOI] [Google Scholar]
- 39.Brown JL. The evolution of behavior. New York: W.W. Norton, 1975. [Google Scholar]
- 40.Fincke OM. Larval behaviour of a giant damselfly: territoriality or size-dependent dominance? Anim Behav. 1996;51:77–87. doi: 10.1006/anbe.1996.0007. [DOI] [Google Scholar]
- 41.Kaufmann JH. On the definitions and functions of dominance and territorality. Biol Rev Camb Philos Soc. 1983;58:1–20. doi: 10.1111/j.1469-185X.1983.tb00379.x. [DOI] [Google Scholar]
- 42.Fonberg E. Dominance and aggression. Int J Neurosci. 1988;41:201–13. doi: 10.3109/00207458808990726. [DOI] [PubMed] [Google Scholar]
- 43.Zagrodzka J, Fonberg E, Brudnias-Graczyk Z. Predatory dominance and aggressive display under imipramine treatment in cats. Acta Neurobiol Exp (Wars) 1985;45:137–49. [PubMed] [Google Scholar]
- 44.Fonberg E, Brudnias-Stepowska Z, Zagrodzka J. Various relations between the predatory dominance and aggressive behavior in pairs of cats. Aggress Behav. 1985;11:103–14. doi: 10.1002/1098-2337(1985)11:2<103::AID-AB2480110203>3.0.CO;2-R. [DOI] [Google Scholar]
- 45.King JA. Intra- and interspecific conflict of Mus and Peromyscus. Ecology. 1957;38:355–7. doi: 10.2307/1931697. [DOI] [Google Scholar]
- 46.Francis RC. On the relationship between aggression and social dominance. Ethology. 1988;78:223–37. doi: 10.1111/j.1439-0310.1988.tb00233.x. [DOI] [Google Scholar]
- 47.Yurkovic A, Wang O, Basu AC, Kravitz EA. Learning and memory associated with aggression in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2006;103:17519–24. doi: 10.1073/pnas.0608211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vrontou E, Nilsen SP, Demir E, Kravitz EA, Dickson BJ. fruitless regulates aggression and dominance in Drosophila. Nat Neurosci. 2006;9:1469–71. doi: 10.1038/nn1809. [DOI] [PubMed] [Google Scholar]
- 49.Morse DH. Behavioral mechanisms in ecology. Cambridge, Massachusetts: Harvard University Press, 1980. [Google Scholar]
- 50.Wittenberger JF. Animal social behavior. Boston, Massachusetts: Duxbury Press, 1981. [Google Scholar]
- 51.Hoffmann AA. Genetic Analysis of territoriality in Drosophila In: Boake CRB, ed. Quantitative genetic studies of behavioral evolution. Chicago: The University Of Chicago Press, 1994:188–205. [Google Scholar]
- 52.Spieth HT. Evolutionary implications of sexual behaviour in Drosophila. Evol Biol. 1968;2:157–93. [Google Scholar]
- 53.Shelly TE. Lek behaviour of Drosophila cnecopleura in Hawaii. Ecol Entomol. 1988;13:51–5. doi: 10.1111/j.1365-2311.1988.tb00332.x. [DOI] [Google Scholar]
- 54.Partridge L, Hoffman AA, Jones JS. Male size and mating succes in Drosophila melanogaster and D. pseudoobscura under field conditions. Anim Behav. 1987;35:468–76. doi: 10.1016/S0003-3472(87)80272-5. [DOI] [Google Scholar]
- 55.Taylor CE, Kekic V. Sexual selection in a natural population of Drosophila melanogaster. Evolution. 1988;42:197–9. doi: 10.2307/2409128. [DOI] [PubMed] [Google Scholar]
- 56.Hoffmann AA. Heritable variation for territorial success in two Drosophila melanogaster populations. Anim Behav. 1988;36:1180–9. doi: 10.1016/S0003-3472(88)80077-0. [DOI] [Google Scholar]
- 57.Hoffmann AA. A laboratory study of male territoriality in the sibling species Drosophila melanogaster and Drosophila simulans. Anim Behav. 1987;35:807–18. doi: 10.1016/S0003-3472(87)80117-3. [DOI] [Google Scholar]
- 58.Hoffmann AA. Geographic variation in the territorial success of Drosophila melanogaster males. Behav Genet. 1989;19:241–55. doi: 10.1007/BF01065908. [DOI] [PubMed] [Google Scholar]
- 59.Hoffmann AA, Cacoyianni Z. Selection for territoriality in Drosophila melanogaster - correlated responses in mating success and other fitness components. Anim Behav. 1989;38:23–34. doi: 10.1016/S0003-3472(89)80062-4. [DOI] [Google Scholar]
- 60.Hoffmann AA, Cacoyianni Z. Territoriality in Drosophila melanogaster as a conditional strategy. Anim Behav. 1990;40:526–37. doi: 10.1016/S0003-3472(05)80533-0. [DOI] [Google Scholar]
- 61.Boake CHR. Correlation between courtship success, aggressive success, and body size in a picturewinged fly, Drosophila silvestris. Ethology. 1989;80:318–29. doi: 10.1111/j.1439-0310.1989.tb00750.x. [DOI] [Google Scholar]
- 62.Rodri´guez-Muñoz R, Bretman A, Slate J, Walling CA, Tregenza T. Natural and sexual selection in a wild insect population. Science. 2010;328:1269–72. doi: 10.1126/science.1188102. [DOI] [PubMed] [Google Scholar]
- 63.Kamyshev NG, Smirnova GP, Kamysheva EA, Nikiforov ON, Parafenyuk IV, Ponomarenko VV. Plasticity of social behavior in Drosophila. Neurosci Behav Physiol. 2002;32:401–8. doi: 10.1023/A:1015832328023. [DOI] [PubMed] [Google Scholar]
- 64.Leiser JK, Itzkowitz M. Changing tactics: dominance, territoriality, and the responses of “primary” males to competition from conditional breeders in the variegated pupfish (Cyprinodon variegatus) Behav Processes. 2004;66:119–30. doi: 10.1016/j.beproc.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 65.Itzkowitz M. Interrelationships of dominance and territorial behaviour in the pupfish, Cyprinodon variegatus. Behav Processes. 1977;2:383–91. doi: 10.1016/0376-6357(77)90008-0. [DOI] [PubMed] [Google Scholar]
- 66.Dierick HA, Greenspan RJ. Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nat Genet. 2007;39:678–82. doi: 10.1038/ng2029. [DOI] [PubMed] [Google Scholar]
- 67.Dankert H, Wang L, Hoopfer ED, Anderson DJ, Perona P. Automated monitoring and analysis of social behavior in Drosophila. Nat Methods. 2009;6:297–303. doi: 10.1038/nmeth.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mundiyanapurath S, Certel S, Kravitz EA. Studying aggression in Drosophila (fruit flies) J Vis Exp. 2007;25:155. doi: 10.3791/155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ueda A, Wu CF. Effects of social isolation on neuromuscular excitability and aggressive behaviors in Drosophila: altered responses by Hk and gsts1, two mutations implicated in redox regulation. J Neurogenet. 2009;23:378–94. doi: 10.3109/01677060903063026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cabral LG, Foley BR, Nuzhdin SV. Does sex trade with violence among genotypes in Drosophila melanogaster? PLoS One. 2008;3:e1986. doi: 10.1371/journal.pone.0001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hoffman AA. Territorial encounters between Drosophila males of different sizes. Anim Behav. 1987;35:1899–901. doi: 10.1016/S0003-3472(87)80085-4. [DOI] [Google Scholar]
- 72.Dierick HA. A method for quantifying aggression in male Drosophila melanogaster. Nat Protoc. 2007;2:2712–8. doi: 10.1038/nprot.2007.404. [DOI] [PubMed] [Google Scholar]
- 73.Edwards AC, Rollmann SM, Morgan TJ, Mackay TF. Quantitative genomics of aggressive behavior in Drosophila melanogaster. PLoS Genet. 2006;2:e154. doi: 10.1371/journal.pgen.0020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Certel SJ, Savella MG, Schlegel DC, Kravitz EA. Modulation of Drosophila male behavioral choice. Proc Natl Acad Sci U S A. 2007;104:4706–11. doi: 10.1073/pnas.0700328104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Certel SJ, Leung A, Lin CY, Perez P, Chiang AS, Kravitz EA. Octopamine neuromodulatory effects on a social behavior decision-making network in Drosophila males. PLoS One. 2010;5:e13248. doi: 10.1371/journal.pone.0013248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mao Z, Davis RL. Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity. Front Neural Circuits. 2009;3:5. doi: 10.3389/neuro.04.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Svetec N, Ferveur JF. Social experience and pheromonal perception can change male-male interactions in Drosophila melanogaster. J Exp Biol. 2005;208:891–8. doi: 10.1242/jeb.01454. [DOI] [PubMed] [Google Scholar]
- 78.Hoffmann AA. The influence of age and experience with conspecifics on territorial behaviour in Drosophila melanogaster. J Insect Behav. 1990;3:1–12. doi: 10.1007/BF01049191. [DOI] [Google Scholar]
- 79.Wang L, Dankert H, Perona P, Anderson DJ. A common genetic target for environmental and heritable influences on aggressiveness in Drosophila. Proc Natl Acad Sci U S A. 2008;105:5657–63. doi: 10.1073/pnas.0801327105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang L, Han X, Mehren J, Hiroi M, Billeter JC, Miyamoto T, et al. Hierarchical chemosensory regulation of male-male social interactions in Drosophila. Nat Neurosci. 2011;14:757–62. doi: 10.1038/nn.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu W, Liang X, Gong J, Yang Z, Zhang YH, Zhang JX, et al. Social regulation of aggression by pheromonal activation of Or65a olfactory neurons in Drosophila. Nat Neurosci. 2011;14:896–902. doi: 10.1038/nn.2836. [DOI] [PubMed] [Google Scholar]
- 82.Fern´ndez MP, Chan YB, Yew JY, Billeter JC, Dreisewerd K, Levine JD, et al. Pheromonal and behavioral cues trigger male-to-female aggression in Drosophila. PLoS Biol. 2010;8:e1000541. doi: 10.1371/journal.pbio.1000541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jonsson T, Kravitz EA, Heinrich R. Sound production during agonistic behavior of male Drosophila melanogaster. Fly (Austin) 2011;5:29–38. doi: 10.4161/fly.5.1.13713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu CF, Wong F. Frequency characteristics in the visual system of Drosophila: genetic dissection of electroretinogram components. J Gen Physiol. 1977;69:705–24. doi: 10.1085/jgp.69.6.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Borycz J, Borycz JA, Kubo´w A, Lloyd V, Meinertzhagen IA. Drosophila ABC transporter mutants white, brown and scarlet have altered contents and distribution of biogenic amines in the brain. J Exp Biol. 2008;211:3454–66. doi: 10.1242/jeb.021162. [DOI] [PubMed] [Google Scholar]
- 86.Sitaraman D, Zars M, Laferriere H, Chen YC, Sable-Smith A, Kitamoto T, et al. Serotonin is necessary for place memory in Drosophila. Proc Natl Acad Sci U S A. 2008;105:5579–84. doi: 10.1073/pnas.0710168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Diegelmann S, Zars M, Zars T. Genetic dissociation of acquisition and memory strength in the heat-box spatial learning paradigm in Drosophila. Learn Mem. 2006;13:72–83. doi: 10.1101/lm.45506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hing AL, Carlson JR. Male-male courtship behavior induced by ectopic expression of the Drosophila white gene: role of sensory function and age. J Neurobiol. 1996;30:454–64. doi: 10.1002/(SICI)1097-4695(199608)30:4<454::AID-NEU2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 89.Nilsson EE, Asztalos Z, Lukacsovich T, Awano W, Usui-aoki K, Yamamoto D. Fruitless is in the regulatory pathway by which ectopic mini-white and transformer induce bisexual courtship in Drosophila. J Neurogenet. 2000;13:213–32. doi: 10.3109/01677060009084495. [DOI] [PubMed] [Google Scholar]
- 90.An X, Armstrong JD, Kaiser K, O'Dell KM. The effects of ectopic white and transformer expression on Drosophila courtship behavior. 227. J Neurogenet. 2000;14 doi: 10.3109/01677060009084500. [DOI] [PubMed] [Google Scholar]
- 91.Kurkulos M, Weinberg JM, Pepling ME, Mount SM. Polyadenylylation in copia requires unusually distant upstream sequences. Proc Natl Acad Sci U S A. 1991;88:3038–42. doi: 10.1073/pnas.88.8.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rabinow L, Birchler JA. A dosage-sensitive modifier of retrotransposon-induced alleles of the Drosophila white locus. EMBO J. 1989;8:879–89. doi: 10.1002/j.1460-2075.1989.tb03449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Benton R. Sensitivity and specificity in Drosophila pheromone perception. Trends Neurosci. 2007;30:512–9. doi: 10.1016/j.tins.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 94.Jefferis GS, Marin EC, Watts RJ, Luo L. Development of neuronal connectivity in Drosophila antennal lobes and mushroom bodies. Curr Opin Neurobiol. 2002;12:80–6. doi: 10.1016/S0959-4388(02)00293-3. [DOI] [PubMed] [Google Scholar]
- 95.Ruta V, Datta SR, Vasconcelos ML, Freeland J, Looger LL, Axel R. A dimorphic pheromone circuit in Drosophila from sensory input to descending output. Nature. 2010;468:686–90. doi: 10.1038/nature09554. [DOI] [PubMed] [Google Scholar]
- 96.Gerber B, Stocker RF, Tanimura T, Thum AS. Smelling, tasting, learning: Drosophila as a study case. Results Probl Cell Differ. 2009;47:139–85. doi: 10.1007/400_2008_9. [DOI] [PubMed] [Google Scholar]
- 97.Zars T. Spatial orientation in Drosophila. J Neurogenet. 2009;23:104–10. doi: 10.1080/01677060802441364. [DOI] [PubMed] [Google Scholar]
- 98.Joiner MA, Griffith LC. Visual input regulates circuit configuration in courtship conditioning of Drosophila melanogaster. Learn Mem. 2000;7:32–42. doi: 10.1101/lm.7.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu L, Wolf R, Ernst R, Heisenberg M. Context generalization in Drosophila visual learning requires the mushroom bodies. Nature. 1999;400:753–6. doi: 10.1038/22919. [DOI] [PubMed] [Google Scholar]
- 100.Murthy M. Unraveling the auditory system of Drosophila. Curr Opin Neurobiol. 2010;20:281–7. doi: 10.1016/j.conb.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 101.Heisenberg M. Mushroom body memoir: from maps to models. Nat Rev Neurosci. 2003;4:266–75. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- 102.Strauss R, Heisenberg M. A higher control center of locomotor behavior in the Drosophila brain. J Neurosci. 1993;13:1852–61. doi: 10.1523/JNEUROSCI.13-05-01852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sehgal A, Joiner W, Crocker A, Koh K, Sathyanarayanan S, Fang Y, et al. Molecular analysis of sleep: wake cycles in Drosophila. Cold Spring Harb Symp Quant Biol. 2007;72:557–64. doi: 10.1101/sqb.2007.72.018. [DOI] [PubMed] [Google Scholar]
- 104.Joiner WJ, Crocker A, White BH, Sehgal A. Sleep in Drosophila is regulated by adult mushroom bodies. Nature. 2006;441:757–60. doi: 10.1038/nature04811. [DOI] [PubMed] [Google Scholar]
- 105.Molina Y, O’Donnell S. Mushroom body volume is related to social aggression and ovary development in the paperwasp Polistes instabilis. Brain Behav Evol. 2007;70:137–44. doi: 10.1159/000102975. [DOI] [PubMed] [Google Scholar]
- 106.Molina Y, O’Donnell S. Age, sex, and dominance-related mushroom body plasticity in the paperwasp Mischocyttarus mastigophorus. Dev Neurobiol. 2008;68:950–9. doi: 10.1002/dneu.20633. [DOI] [PubMed] [Google Scholar]
- 107.Chan YB, Kravitz EA. Specific subgroups of FruM neurons control sexually dimorphic patterns of aggression in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2007;104:19577–82. doi: 10.1073/pnas.0709803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lander ES, Botstein D. Mapping mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics. 1989;121:185–99. doi: 10.1093/genetics/121.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Flint J, Mackay TF. Genetic architecture of quantitative traits in mice, flies, and humans. Genome Res. 2009;19:723–33. doi: 10.1101/gr.086660.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Belknap JK, Phillips TJ, O’Toole LA. Quantitative trait loci associated with brain weight in the BXD/Ty recombinant inbred mouse strains. Brain Res Bull. 1992;29:337–44. doi: 10.1016/0361-9230(92)90065-6. [DOI] [PubMed] [Google Scholar]
- 111.Johnson TE, DeFries JC, Markel PD. Mapping quantitative trait loci for behavioral traits in the mouse. Behav Genet. 1992;22:635–53. doi: 10.1007/BF01066635. [DOI] [PubMed] [Google Scholar]
- 112.Todd JA, Aitman TJ, Cornall RJ, Ghosh S, Hall JR, Hearne CM, et al. Genetic analysis of autoimmune type 1 diabetes mellitus in mice. Nature. 1991;351:542–7. doi: 10.1038/351542a0. [DOI] [PubMed] [Google Scholar]
- 113.Sullivan PF. The psychiatric GWAS consortium: big science comes to psychiatry. Neuron. 2010;68:182–6. doi: 10.1016/j.neuron.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wellcome Trust Case Control Consortium Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–78. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Edwards AC, Mackay TF. Quantitative trait loci for aggressive behavior in Drosophila melanogaster. Genetics. 2009;182:889–97. doi: 10.1534/genetics.109.101691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sambandan D, Yamamoto A, Fanara JJ, Mackay TF, Anholt RR. Dynamic genetic interactions determine odor-guided behavior in Drosophila melanogaster. Genetics. 2006;174:1349–63. doi: 10.1534/genetics.106.060574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Harbison ST, Carbone M, Ayroles JF, Stone EA, Lyman RF, Mackay TF. Co-regulated transcriptional networks contribute to natural genetic variation in Drosophila. Nat Genet. 2009;41:371–5. doi: 10.1038/ng.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Turri MG, Datta SR, DeFries J, Henderson ND, Flint J. QTL analysis identifies multiple behavioral dimensions in ethological tests of anxiety in laboratory mice. Curr Biol. 2001;11:725–34. doi: 10.1016/S0960-9822(01)00206-8. [DOI] [PubMed] [Google Scholar]
- 119.Legare ME, Bartlett FS, 2nd, Frankel WN. A major effect QTL determined by multiple genes in epileptic EL mice. Genome Res. 2000;10:42–8. [PMC free article] [PubMed] [Google Scholar]
- 120.Harbison ST, Yamamoto AH, Fanara JJ, Norga KK, Mackay TF. Quantitative trait loci affecting starvation resistance in Drosophila melanogaster. Genetics. 2004;166:1807–23. doi: 10.1534/genetics.166.4.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Robin C, Daborn PJ, Hoffmann AA. Fighting fly genes. Trends Genet. 2007;23:51–4. doi: 10.1016/j.tig.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 122.Harbison ST, Sehgal A. Quantitative genetic analysis of sleep in Drosophila melanogaster. Genetics. 2008;178:2341–60. doi: 10.1534/genetics.107.081232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Norga KK, Gurganus MC, Dilda CL, Yamamoto A, Lyman RF, Patel PH, et al. Quantitative analysis of bristle number in Drosophila mutants identifies genes involved in neural development. Curr Biol. 2003;13:1388–96. doi: 10.1016/S0960-9822(03)00546-3. [DOI] [PubMed] [Google Scholar]
- 124.Mackay TF, Stone EA, Ayroles JF. The genetics of quantitative traits: challenges and prospects. Nat Rev Genet. 2009;10:565–77. doi: 10.1038/nrg2612. [DOI] [PubMed] [Google Scholar]
- 125.Ledford H. Population genomics for fruitflies. Nature. 2008;453:1154–5. doi: 10.1038/4531154a. [DOI] [PubMed] [Google Scholar]
- 126.Spieth HT. Courtship behavior in Drosophila. Annu Rev Entomol. 1974;19:385–405. doi: 10.1146/annurev.en.19.010174.002125. [DOI] [PubMed] [Google Scholar]
- 127.Kaneshiro KYB. C.R.B. Sexual selection and speciation: issues raised by Hawaiian Drosophila. Trends Ecol Evol. 1987;27:207–12. doi: 10.1016/0169-5347(87)90022-X. [DOI] [PubMed] [Google Scholar]
- 128.Stevenson PA, Dyakonova V, Rillich J, Schildberger K. Octopamine and experience-dependent modulation of aggression in crickets. J Neurosci. 2005;25:1431–41. doi: 10.1523/JNEUROSCI.4258-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kostowski W, Tarchalska B, Wan´chowicz B. Brain catecholamines, spontaneous bioelectrical activity and aggressive behavior in ants (Formica rufa) Pharmacol Biochem Behav. 1975;3:337–42. doi: 10.1016/0091-3057(75)90040-4. [DOI] [PubMed] [Google Scholar]
- 130.Kostowski W, Tarchalska-Krynska B, Markowska L. Aggressive behavior and brain serotonin and catecholamines in ants (Formica rufa) Pharmacol Biochem Behav. 1975;3:717–9. doi: 10.1016/0091-3057(75)90200-2. [DOI] [PubMed] [Google Scholar]
- 131.Adamo SA, Linn CE, Hoy RR. The role of neurohormonal octopamine during ‘fight or flight’ behaviour in the field cricket Gryllus bimaculatus. J Exp Biol. 1995;198:1691–700. doi: 10.1242/jeb.198.8.1691. [DOI] [PubMed] [Google Scholar]
- 132.Rillich J, Schildberger K, Stevenson PA. Octopamine and occupancy: an aminergic mechanism for intruder-resident aggression in crickets. Proc Biol Sci. 2011;278:1873–80. doi: 10.1098/rspb.2010.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Guerrieri FJ, d’Ettorre P. The mandible opening response: quantifying aggression elicited by chemical cues in ants. J Exp Biol. 2008;211:1109–13. doi: 10.1242/jeb.008508. [DOI] [PubMed] [Google Scholar]
- 134.Yusuf AA, Pirk CW, Crewe RM, Njagi PG, Gordon I, Torto B. Nestmate recognition and the role of cuticular hydrocarbons in the African termite raiding ant Pachycondyla analis. J Chem Ecol. 2010;36:441–8. doi: 10.1007/s10886-010-9774-6. [DOI] [PubMed] [Google Scholar]
- 135.Hunt GJ. Flight and fight: a comparative view of the neurophysiology and genetics of honey bee defensive behavior. J Insect Physiol. 2007;53:399–410. doi: 10.1016/j.jinsphys.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Van Wilgenburg E, Cle´mencet J, Tsutsui ND. Experience influences aggressive behaviour in the Argentine ant. Biol Lett. 2010;6:152–5. doi: 10.1098/rsbl.2009.0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Arechavaleta-Velasco ME, Hunt GJ, Emore C. Quantitative trait loci that influence the expression of guarding and stinging behaviors of individual honey bees. Behav Genet. 2003;33:357–64. doi: 10.1023/A:1023458827643. [DOI] [PubMed] [Google Scholar]
- 138.Hunt GJ, Amdam GV, Schlipalius D, Emore C, Sardesai N, Williams CE, et al. Behavioral genomics of honeybee foraging and nest defense. Naturwissenschaften. 2007;94:247–67. doi: 10.1007/s00114-006-0183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ghosal K, Naples SP, Rabe AR, Killian KA. Agonistic behavior and electrical stimulation of the antennae induces Fos-like protein expression in the male cricket brain. Arch Insect Biochem Physiol. 2010;74:38–51. doi: 10.1002/arch.20360. [DOI] [PubMed] [Google Scholar]
- 140.Gorczyca MG, Hall JC. Immunohistochemical localization of choline acetyltransferase during development and in Chats mutants of Drosophila melanogaster. J Neurosci. 1987;7:1361–9. doi: 10.1523/JNEUROSCI.07-05-01361.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yasuyama K, Salvaterra PM. Localization of choline acetyltransferase-expressing neurons in Drosophila nervous system. Microsc Res Tech. 1999;45:65–79. doi: 10.1002/(SICI)1097-0029(19990415)45:2<65::AID-JEMT2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 142.Salvaterra PM, Kitamoto T. Drosophila cholinergic neurons and processes visualized with Gal4/UAS-GFP. Brain Res Gene Expr Patterns. 2001;1:73–82. doi: 10.1016/S1567-133X(01)00011-4. [DOI] [PubMed] [Google Scholar]
- 143.Kahsai L, Winther AM. Chemical neuroanatomy of the Drosophila central complex: distribution of multiple neuropeptides in relation to neurotransmitters. J Comp Neurol. 2011;519:290–315. doi: 10.1002/cne.22520. [DOI] [PubMed] [Google Scholar]
- 144.Bao X, Wang B, Zhang J, Yan T, Yang W, Jiao F, et al. Localization of serotonin/tryptophan-hydroxylase-immunoreactive cells in the brain and suboesophageal ganglion of Drosophila melanogaster. Cell Tissue Res. 2010;340:51–9. doi: 10.1007/s00441-010-0932-5. [DOI] [PubMed] [Google Scholar]
- 145.Blenau W, Thamm M. Distribution of serotonin (5-HT) and its receptors in the insect brain with focus on the mushroom bodies: lessons from Drosophila melanogaster and Apis mellifera. Arthropod Struct Dev. 2011;40:381–94. doi: 10.1016/j.asd.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 146.Monastirioti M. Biogenic amine systems in the fruit fly Drosophila melanogaster. Microsc Res Tech. 1999;45:106–21. doi: 10.1002/(SICI)1097-0029(19990415)45:2<106::AID-JEMT5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 147.Drobysheva D, Ameel K, Welch B, Ellison E, Chaichana K, Hoang B, et al. An optimized method for histological detection of dopaminergic neurons in Drosophila melanogaster. J Histochem Cytochem. 2008;56:1049–63. doi: 10.1369/jhc.2008.951137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nässel DR, Elekes K. Aminergic neurons in the brain of blowflies and Drosophila: dopamine- and tyrosine hydroxylase-immunoreactive neurons and their relationship with putative histaminergic neurons. Cell Tissue Res. 1992;267:147–67. doi: 10.1007/BF00318701. [DOI] [PubMed] [Google Scholar]
- 149.Bate M, Martinez Arias A. The development of Drosophila melanogaster Plainview, N.Y.: Cold Spring Harbor Laboratory Press, 1993. [Google Scholar]
- 150.Pan Y, Zhou Y, Guo C, Gong H, Gong Z, Liu L. Differential roles of the fan-shaped body and the ellipsoid body in Drosophila visual pattern memory. Learn Mem. 2009;16:289–95. doi: 10.1101/lm.1331809. [DOI] [PubMed] [Google Scholar]
- 151.Fiala A. Olfaction and olfactory learning in Drosophila: recent progress. Curr Opin Neurobiol. 2007;17:720–6. doi: 10.1016/j.conb.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 152.Carlson JR. Olfaction in Drosophila: from odor to behavior. Trends Genet. 1996;12:175–80. doi: 10.1016/0168-9525(96)10015-9. [DOI] [PubMed] [Google Scholar]
- 153.Masse NY, Turner GC, Jefferis GS. Olfactory information processing in Drosophila. Curr Biol. 2009;19:R700–13. doi: 10.1016/j.cub.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 154.Davis RL. Traces of Drosophila memory. Neuron. 2011;70:8–19. doi: 10.1016/j.neuron.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci. 2007;30:505–33. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- 156.Ebbs ML, Amrein H. Taste and pheromone perception in the fruit fly Drosophila melanogaster. Pflugers Arch. 2007;454:735–47. doi: 10.1007/s00424-007-0246-y. [DOI] [PubMed] [Google Scholar]
- 157.Lumpkin EA, Marshall KL, Nelson AM. The cell biology of touch. J Cell Biol. 2010;191:237–48. doi: 10.1083/jcb.201006074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kernan MJ. Mechanotransduction and auditory transduction in Drosophila. Pflugers Arch. 2007;454:703–20. doi: 10.1007/s00424-007-0263-x. [DOI] [PubMed] [Google Scholar]