Abstract
Mast cells are essential in allergic immune responses. Recent discoveries have revealed their direct participation in cardiovascular diseases and metabolic disorders. Although more sophisticated mechanisms are still unknown, data from animal studies suggest that mast cells act similarly to macrophages and other inflammatory cells and contribute to human diseases through cell–cell interactions and the release of proinflammatory cytokines, chemokines, and proteases to induce inflammatory cell recruitment, cell apoptosis, angiogenesis, and matrix protein remodeling. Reduced cardiovascular complications and improved metabolic symptoms in animals receiving over-the-counter antiallergy medications that stabilize mast cells open another era of mast cell biology and bring new hope to human patients suffering from these conditions.
Introduction
-
Mast Cell Activation
Overview
Mast cell activation pathways
-
Mast Cells in Atherosclerosis
Mast cells in atherosclerotic lesions
Role of mast cell proteases in atherosclerosis
Mast cell function in angiogenesis and apoptosis
Mast cells in experimental atherosclerosis
-
Distinct Role of Macrophages in Atherosclerosis
Macrophage types
Antiatherogenic and proatherogenic macrophages
Macrophage adhesion, migration, and proliferation
Macrophage apoptosis
Interaction of macrophages with other cell types
-
Mast Cells in AAA
Inflammatory cells in AAA
Mast cells in experimental AAA
Macrophages in AAA
-
Mast Cells in Obesity
Obesity as an inflammatory disease
Obesity and allergy
Possible interaction between mast cells and T cells
Role of macrophages in obesity
-
Relative Contribution of Mast Cells and Macrophages in Diabetes
Mast cells in diabetes
Macrophages in diabetes
-
Mast Cell–Macrophage Interactions in Cardiovascular and Metabolic Diseases
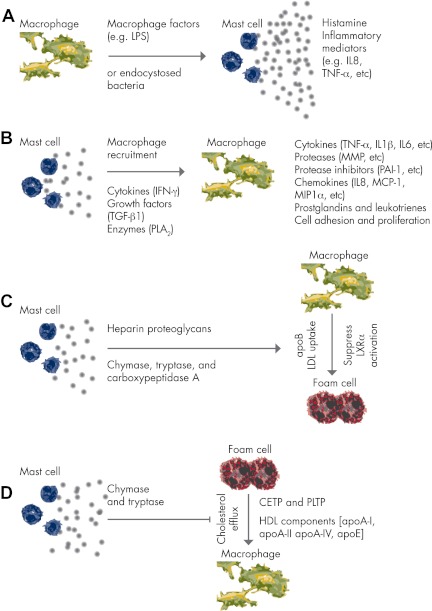
Macrophages activate mast cells
Mast cells activate macrophages
Role of mast cells in macrophage LDL uptake
Role of mast cells in macrophage cholesterol efflux
-
Clinical Implications
Mast cells as a therapeutic target
Macrophages as a therapeutic target
Conclusions
I. Introduction
Mast cells (MC) are inflammatory cells, but they are commonly regarded as “allergy” effectors because of their pathophysiological roles in IgE-mediated hypersensitivity reactions in the airways, skin, and gastrointestinal tract—common causes of asthma, allergic rhinitis, atopic dermatitis, and food allergy. These responses result mainly from the inflammatory mediators released from MC after allergen cross-linking of the cell surface allergen-specific IgE preoccupied receptor FcεRI. MC differ from other inflammatory cells in that they leave the bone marrow as CD34+CD117+CD13+FcεRI− pluripotent hematopoietic progenitors (1). They do not mature until they reach the target tissues, such as skin and various mucosal surfaces, where they acquire defined phenotypes (2). MC progenitors use integrins α4β1 and α4β7 for their initial interaction with intercellular adhesion molecule-1 (ICAM-1) from vascular endothelium (3), followed by interactions with cell surface chemokine receptors (e.g., CXCR2, CCR3, CXCR4, and CCR5) and targeting of tissue chemokines, such as eotaxin and stem cell factor (SCF) from stromal fibroblasts (4). Despite progress in understanding the recruitment of MC progenitors to the small intestine and inflamed lungs, little is known about the vasculature, adipose tissue, endocrine organs, or other connective tissues.
T cells (e.g., CD4+, CD8+, and NK-T) and macrophages (M1 and M2) have several subtypes with different surface receptors and functions. As we will discuss in Section IV.B), pathological stimuli may cause macrophage polarization by switching M2 to M1 phenotypes. MC behave similarly. The two types of human MC—those containing tryptase (MCT), and those containing both tryptase and chymase (MCTC)—have different protease profiles, and both convert under certain conditions (5). Rodent MC are classified based on their primary tissue distributions. Mucosal MC localize preferentially in the mucosa of the airways and gastrointestinal tract and express mouse MC protease (mMCP)-1 and -2. Connective tissue MC localize in the skin, synovium, peritoneum, and perivascular tissues and express mMCP-4, -5, -6, cathepsin G, and carboxypeptidase A. Like human MC, rodent MC phenotypes are reversible (6). One type of MC may outnumber another type in certain pathological conditions.
Studies have shown that both vascular complications and metabolic disorders are inflammatory diseases in which innate and adaptive immunities are essential (7). Because of their preferential locations lining the various surfaces of the body, such as the skin, airways, and intestines, MC are ideally positioned as the first responders, interacting with invading microorganisms and initiating immune responses (8). MC defend the host against parasites, bacteria, or viruses and are a general inducer of inflammation (9); they therefore have been implicated in promoting innate immunity. MC also contribute importantly to adaptive immunity. They constitutively express major histocompatibility complex (MHC)-I and increase MHC-II when stimulated with interferon (IFN)-γ, TNF-α, or endotoxin [lipopolysaccharide (LPS)] (10, 11). When an individual encounters an allergen and a low dose of a Toll-like receptor (TLR) activator [LPS or peptidoglycan (PGN)] from the environment, MC release TNF-α to activate other antigen-presenting cells (APC; such as dendritic cells) to take up the same antigens and express costimulatory molecules. This activation leads to T helper (Th) 2 response, resulting in allergen sensitization (12) and exacerbation of allergic disease. Upon activation, MC release the Th2 cytokines IL-4, IL-10, and IL-13 to polarize CD4+ T cells to become Th2 cells (13), which induce humoral immune responses to pathogens by producing cytokines that stimulate plasma cell antibody production. MC can also enhance T-cell proliferation through FcεR1-dependent and FcεR1-independent pathways (14). Although MC function in immunity has been thoroughly studied (15, 16), we have limited knowledge regarding vascular or metabolic diseases.
In this comprehensive review, we discuss the roles of MC in atherosclerosis, abdominal aortic aneurysm (AAA), obesity, and diabetes from histological studies to experimental models and from gene-deficient or cell-deficient animals to small molecule inhibitor-treated subjects. We also discuss the relative contributions of macrophages in these diseases because MC and macrophages regulate the progression of these common human diseases in similar and in different ways.
II. Mast Cell Activation
A. Overview
MC exert their physiological and pathological functions by releasing granule remnants of histamines, proteases, cytokines, chemokines, growth factors, proteoglycans, arachidonic acid metabolites, lipid mediator prostaglandins, and reactive oxygen and nitrogen species. These mediators can be grouped into preformed mediators that are rapidly released (within seconds to minutes) upon cell stimulation, newly synthesized mediators that are generated by cell activation, and cytokines and growth factors. Preformed MC mediators include mainly histamine, heparin, proteoglycans, serotonin, some cytokines (e.g., TNF-α), chemotactic factors, tryptase, chymase, carboxypeptidase A, and rennin (17), and newly synthesized mediators include arachidonic acid metabolites, prostaglandin-D2, leukotriene-C4, leukotriene-D4, platelet-activating factor, and free radicals (18). Most of these mediators have been implicated in the pathogenesis of vascular and metabolic diseases. As discussed in more detail in Sections III.C and IV.E, after activation, MC release vascular endothelial growth factor (VEGF), TNF-α, histamine, tryptase, IL-8, leukotrienes, and eotaxin to act as chemotactic signals, increasing endothelial cell (EC) adhesion molecule expression [selectin, integrin, ICAM, and vascular cell adhesion molecule (VCAM)] and stimulating leukocyte EC adhesion, rolling, and recruitment. For example, low neutrophil counts were detected in wounds of MC-null mice (19). In targeting tissues, MC use their chemokines, cytokines, and proteases to recruit more inflammatory cells, as well as to activate vascular cells or other inflammatory cells, promote angiogenesis, and stimulate cell apoptosis or proliferation.
B. Mast cell activation pathways
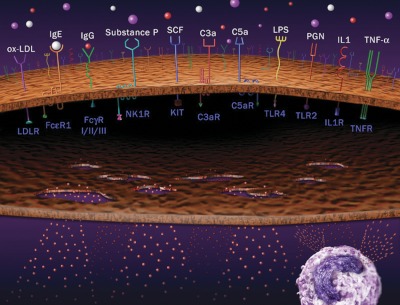
The most popular pathway for MC activation is via antigen-specific IgE-bound FcεRI, a multimeric receptor composed of α-, β-, and two disulfide-bound γ-chains that are expressed on MC and basophils (2, 20) (Fig. 1). Circulating IgE levels are high in patients with asthma and other allergic diseases, indicating active MC status under such conditions. IgE was shown to influence MC number, but not to affect MC progenitor recruitment to the airway, in IgE-deficient mice (21). Our unpublished observations demonstrated significantly higher serum IgE levels in patients with cardiovascular diseases, although exactly how much and to what level increased IgE contributes to MC activation in inflammatory tissues remains unknown.
Figure 1.
MC activation pathways. Various extracellular soluble ligands can bind to corresponding receptors on MC surfaces, followed by MC activation or degranulation as indicated. An active MC is shown on the lower right.
In addition to FcεRI, MC express many other cell receptors for their activation, including IgG-dependent Fcγ receptors (22, 23), MC growth factor SCF receptor KIT (i.e., CD117), anaphylatoxins C3a and C5a complement receptors, TLR, adenosine receptors, and cytokine receptors (24) (Fig. 1). Although there is only one form of MC FcεR1 (αβγ2), several types of FcγR occur in human and rodent MC. Human MC express FcγRI and FcγRII (25), and mouse MC express FcγRII and FcγRIII (26). Increased IgG levels in asthmatic patients may participate in MC activation via FcγR (27). Patients with coronary heart disease appear to have higher serum IgG levels than healthy subjects (28). The role of the Ig molecules IgE and IgG in MC activation and their association with the pathogenesis of vascular and metabolic diseases are important areas to explore.
KIT is a transmembrane receptor with tyrosine kinase activity that is expressed on MC and is the receptor for SCF (29), which is necessary for MC development and homeostasis (1, 30). All mature MC express high levels of KIT and FcεRI (2). Signals mediated by KIT at the time of FcεRI cross-linking enhance the magnitude of MC degranulation and cytokine production (31).
The complement system is important in innate immunity against bacteria and other pathogens, and MC-mediated innate immunity may associate with MC activation by complementary molecules. The anaphylatoxins C3a and C5a are by-products of complement activation, and they interact with the cell-surface receptors C3aR and C5aR on MC for activation (32, 33). C3aR and C5aR are seven-transmembrane Gαi-protein-coupled receptors. C3a and C5a may activate MC through two mechanisms—under low concentrations, C3a activates MC using C3aR; and under high concentrations, C3a activates MC with direct activation of Gαi. C3a and C5a may activate MC by enhancing cytokine and chemokine expression (9, 34) and causing chemotaxis of eosinophils, MC, and possibly macrophages and T cells. Deletion, pharmacological inhibition, or antibody neutralization of C3aR or C5aR attenuates airway hyperresponsiveness and inflammation (35–37). In human atherosclerotic lesions, T cells, EC, smooth-muscle cells (SMC), and MC all express C5aR or C3aR (38). C3a and C5a also may affect other inflammatory and vascular cell types.
TLR also associate closely with innate immunity (12, 39). For example, bacterial lipopeptide peptidoglycan activates TLR2, bacterial LPS activates TLR4, double-stranded RNA activates TLR3, and CpG motif in DNA activates TLR9. MC stimulation through TLR expresses IL-4, IL-6, IL-8, and TNF-α (40). As in SCF-KIT interactions, ligands to TLR2 and TLR4 act synergistically on signals mediated by FcεRI, resulting in reinforced cytokine production in MC (41). TLR4 stimulation with LPS increases the capacity of bone marrow-derived mouse MC (BMMC) to produce Th2 cytokines, leading to enhanced eosinophilic airway inflammation (12). In airway hypersensitivity in mice, antigen-induced eosinophilic inflammation in the lung is increased when mice are sensitized with allergen and LPS (12). This result is consistent with the synergistic effects of FcεRI and TLR4, which enhance MC production of proinflammatory TNF-α and IL-6 (38). When MC-null mice were reconstituted with BMMC from TLR4−/− mice and then sensitized with LPS and antigen, eosinophil infiltration failed to occur. In contrast, MC-deficient mice reconstituted with wild-type (WT) BMMC that were pretreated with LPS had increased eosinophilic lung infiltration upon antigen challenge. All such phenotypes were absent in MC-null mice repleted with LPS-treated TLR4−/− MC (2, 12).
In addition to these conventional receptor-mediated pathways, MC can be activated selectively by neighboring macrophages, T cells, cytokines, lipoproteins, or other endogenous peptides (Fig. 1). Both mucosal and connective-tissue MC are proximal to T cells and macrophages in the vasculature, as well as in adipose tissue (42, 43). IL-1 and TNF-α can induce MC secretion of cytokines in the absence of degranulation (without secretion of histamine and other granule components) (44, 45). CRH, a neuronal hormone produced by the paraventricular nucleus of the hypothalamus in response to stress, can activate MC and regulate VEGF release through a p38 pathway, resulting in enhanced vascular permeability (46). An important, similarly acting neuronal peptide, substance P, is released from the terminals of specific sensory nerves. It activates MC via neurokinin-1 receptor or directly interacts with G proteins in a receptor-independent manner, followed by the release of TNF-α and histamines to attract granulocyte infiltration to the site of inflammation (47, 48). Substance P also activates MC indirectly via TLR (49). Oxidized low-density lipoprotein (oxLDL) is an important component of vascular foam cells and autoimmune responses. An in vitro study demonstrated that interaction between oxLDL and LDL receptor induces MC expression of chemokine IL-8 (50). Intraarterial infusion of oxLDL in rats elicited MC degranulation and enhanced leukocyte adherence and emigration (51). Serine proteases, Ig light chains, and polybasic compounds also help trigger MC degranulation. Therefore, MC activation contains multiple mechanisms (Fig. 1).
Although all aforesaid MC activation pathways have been examined in cultured MC or in animal models of autoimmune diseases, asthma, or other allergic diseases (52), we have limited knowledge about which MC activation pathways are more important than others in cardiovascular or metabolic diseases. Among all activation pathways, only substance P has been examined in atherosclerosis (53). Substance P administration increased the number and activation of atherosclerosis lesion MC and intraplaque hemorrhage. Because MC share many activation mechanisms with macrophages and other inflammatory cells, testing individual MC activator in cardiovascular or metabolic diseases in vivo without confounding from other cells remains technically difficult. For example, substance P also activates neutrophils (54), which is essential in promoting atherosclerosis (55). oxLDL binding to TLR activates not only MC (50, 51), but also monocytes, macrophages (56), or dendritic cells (57). Altered pathogenesis of vascular diseases or metabolic disorders by simple interruption of oxLDL–TLR interaction may result in part from impaired MC activation. Therefore, although most of the ligands or receptors in Fig. 1 have been implicated in atherosclerosis, obesity, or diabetes, the relative contribution of each MC activation pathway to these cardiovascular and metabolic diseases remains untested.
III. Mast Cells in Atherosclerosis
A. Mast cells in atherosclerotic lesions
Increased serum IgE levels, eosinophilia, positive skin-prick tests, self-reported asthma, and enzymes that regulate leukotriene synthesis (5-lipoxygenase) predict a high risk for atherosclerosis, stroke, and myocardial infarction (MI). Constantinides (58, 59) first observed MC in atherosclerotic lesions more than half a century ago using metachromatic staining to detect MC in human and rabbit atherosclerotic lesions. Very few MC appear in normal coronary arteries, and only one fifth are activated. In contrast, many more MC are detected in fatty streaks and advanced atherosclerotic lesions (Fig. 2). In fatty streaks, MC appear underneath the subendothelium, where they release proteases (e.g., cathepsins) or inflammatory cytokines to induce neighboring cell protease expression (42), leading to vascular wall subendothelium disruption and further blood-borne leukocyte transmigration (60). MC-derived histamine is another vasoactive component that enhances vascular permeability to macromolecules. By releasing histamine, activated subendothelial coronary MC could disturb EC barriers, thereby enhancing lipoprotein permeability (61). When histamine acts on SMC in the media layer, the vascular tone in the segment is augmented, and coronary spasm may ensue (62), in addition to SMC proliferation (63). MC in advanced lesions also produce proteases (e.g., tryptase, cathepsins) or other mediators to alter the biology of surrounding cells (Fig. 2), enhancing tissue remodeling and plaque instability. More than 50% of these MC are activated with a gradient of granules as detected by metachromatic staining, particularly those in the shoulder regions (64–66), which are prone to erosion or rupture (65). MC clusters have been observed in ruptured areas of coronary atherosclerotic lesions from patients who have died of acute MI (67). In ruptured plaques, the activation of perivascular MC correlates with intraplaque hemorrhage, macrophage and EC apoptosis, vascular leakage, and CXCR2-mediated and very late antigen-4-mediated recruitment of leukocytes to plaques (68).
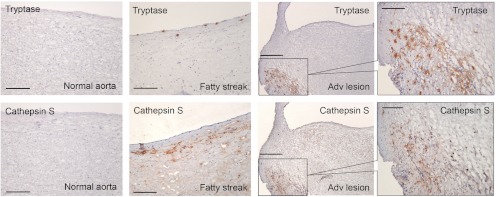
Figure 2.
MC and cathepsin S expression in human atherosclerotic lesions. Mouse antihuman tryptase monoclonal antibody (1:1500; Chemicon International, Temecula, CA) and rabbit antihuman cathepsin S polyclonal antibody (1:100) (60) detected tryptase-positive MC and cathepsin S expression on lumen endothelium and subendothelium compartment in human carotid artery fatty streak (100 μm), and both are colocalized in advanced atherosclerotic lesions (scale, 250 μm). Framed areas are shown enlarged in the right panels (scale, 100 μm). No cathepsin S or MC tryptase immunoreactivities were detected in normal aortas (scale, 100 μm). Frozen sections (6 μm) were used for immunostaining.
B. Role of mast cell proteases in atherosclerosis
MC proteases are one of the most important components of the secretory granules that contribute to atherosclerosis. Like macrophages, MC are rich in matrix metalloproteinases (MMP), cysteine protease cathepsins, and the serine proteases urokinase, plasmin, and cathepsin G. MC also contain their unique neutral serine proteases, chymases, and tryptases. Although we have not seen significant differences in serum chymase levels between patients with coronary artery disease (CAD) and healthy donors, serum tryptase levels are significantly higher in CAD patients than in those with normal angiography or without CAD (69, 70), suggesting their potential involvement in atherosclerosis. Treatment of human coronary arteries intraluminally with recombinant tryptase or chymase induced endothelial damage, characterized by disruption of EC adhesion followed by retraction and desquamation (71).
Chymase and tryptase functions in the vascular system include (but are not limited to) lipoprotein degradation, angiotensin-II (Ang-II) generation, proenzyme or procytokine activation, and matrix protein degradation. High-density lipoprotein (HDL)3 particles efficiently remove cholesterol from foam cells, but they lose this ability when MC are stimulated to degranulate (72). This occurs because both tryptase and chymase degrade the HDL3 components apolipoprotein (apo) E, apoA-I, and apoA-IV in plasma, in peritoneal fluid, and possibly in atherosclerotic lesions, rendering cholesterol-loaded macrophages (foam cells) unable to mediate cholesterol efflux (73). In addition to degrading HDL3 components and inhibiting foam-cell cholesterol efflux, chymases and tryptases promote macrophage LDL uptake. When MC were cocultured with rat or mouse macrophages and LDL, apoB-100 of LDL bound to heparin proteoglycan, which stabilizes tryptase active tetramers and protects chymase and cathepsin G from their natural inhibitors. This granule-bound complex is then proteolyzed by granule-bound chymase, leading to foam-cell formation (74). MC promotion of LDL uptake by macrophages has been tested in vivo. When MC were activated by ip administration of C48/80, a laboratory-use MC activator, LDL uptake rose by 7- to 24-fold (75). In contrast, the clinically used MC stabilizer cromolyn effectively blocked MC-dependent LDL uptake by macrophages (76).
Ang-II is a proinflammatory mediator that induces vascular SMC oxidative stress, EC nitric oxide (NO) signaling negative regulation, SMC and EC apoptosis, leukocyte recruitment, artery proteoglycan production, LDL retention, vascular SMC MMP expression, and intraplaque neovascularization and hemorrhage. MC chymase is important in generating Ang-II from Ang-I. High levels of Ang-II-forming activity and chymase expression have been demonstrated in human atherosclerotic lesions (77). Pericellular matrix protein degradation is another important function of chymase and tryptase. Chymase degrades fibronectin and vitronectin, with subsequent disruption of SMC and EC focal adhesion and Akt dephosphorylation, which are necessary for cell adhesion and survival, leading to arterial SMC and EC apoptosis (71, 78). Tryptase can similarly degrade pericellular fibronectin, vitronectin, and collagen type IV, which promote angiogenesis, tissue remodeling, and possibly vascular cell apoptosis (71, 79). Although the exact mechanism is not fully understood, MC chymase appears proangiogenic (80). BMMC from WT mice promoted microvessel sprouting in an in vitro aortic ring assay, but those from chymase-deficient mice did not (81). Through undefined mechanisms, both chymases and tryptases control vascular cell atherosclerosis-pertinent cathepsin expression (81). Chymase-mediated and tryptase-mediated bioactivation of proenzyme and latent cytokines also appears important to atherogenesis. Both chymase and tryptase generate active MMP-1, -2, -3, and -9 (82), all which have been implicated in plaque formation. Chymase also activates pro-IL-1β and generates TGF-β1 from matrix complex to regulate SMC differentiation, migration, proliferation, and matrix protein synthesis and secretion. In contrast, tryptase has been shown to induce chemokine [monocyte chemoattractant protein-1 (MCP-1), IL-8] and adhesion molecule (ICAM, VCAM, selectin) expression in human EC, which are essential for leukocyte homing. Detailed pathobiological activities of MC chymase and tryptase are thoroughly reviewed by Pejler et al. (83).
MC also produce cathepsin G, urokinase plasminogen activator, MMP, and cysteinyl cathepsins. Although detailed studies of these proteases from MC in atherosclerosis are currently limited, the same sets of proteases from other cell types have been well investigated. MC are rich reservoirs for cysteinyl cathepsins (84), which play critical roles in atherosclerosis by degrading vascular wall elastin and collagen, enhancing leukocyte recruitment, generating angiogenic factors, and promoting vascular cell apoptosis (85). MC secrete the inflammatory cytokines IL-6 and IFN-γ to induce vascular cell cathepsin expression (42). In fatty streaks, medial cathepsin S expression is localized near MC clusters, supporting the notion that MC-derived mediators may enhance nearby vascular cell cathepsin expression. In advanced lesions, cathepsin S and MC are colocalized, suggesting that some cathepsin S signals come from MC (Fig. 2).
C. Mast cell function in angiogenesis and apoptosis
Angiogenesis is an important hallmark of atherosclerosis and is essential to its pathogenesis. MC populate the connective tissue structure near vessels, positioned as key elements in processes like wound healing, tissue regeneration, and remodeling after injury, fibrosis, and angiogenesis. In human atherosclerotic lesions, MC are clustered next to the vasa vasorum in the adventitia (86), at 10 times as many as in the intima (87). As shown in Fig. 2, MC also appear in the superficial location next to the luminal endothelium or deep inside the intima near newly formed microvessels (88). MC associate with neovessel numbers formed within advanced atherosclerotic plaques. These observations suggest an interaction between MC and EC; indeed, many MC-derived mediators are angiogenic. MC in the human intima and adventitia express basic fibroblast growth factor and produce angiogenic histamine, leptin, VEGF, and angiopoietin-1. MC-derived heparin also contributes to neovascularization by stimulating EC migration. MC tryptase stimulates microvessel tube formation and enhances the growth of microvessel EC, whereas chymase promotes angiogenesis through the effects of Ang-II (80, 89).
MC induce vascular cell apoptosis. During atherogenesis, the first step may involve MC interaction with luminal EC, which increases EC adhesion molecule and chemokine expression (90), followed by endovascular adhesions of leukocytes (including MC) that may further induce EC apoptosis, leading to vulnerable EC detachment (71). MC-induced EC apoptosis can be mediated by TNF-α (91), which involves cytochrome C release from mitochondria to cytoplasm, and MC proteases to degrade subendothelium basement proteins, including fibronectin, vitronectin, nidogen, and VE-cadherin. MC also cause SMC death (78). Coculture of BMMC or degranulated cell supernatant from WT mice with aortic SMC induces SMC apoptosis. Under the same conditions, however, BMMC or cell lysates prepared from chymase-deficient (81) or tryptase-deficient (182) mice showed significantly low potency in inducing SMC apoptosis. Although detailed mechanisms remain unknown, MC-derived proteases may contribute to SMC apoptosis by catabolizing pericellular matrix protein (e.g., fibronectin) (69, 78) or via Ang-II generation (92). Figure 3 summarizes our current understanding of MC function in atherosclerosis.
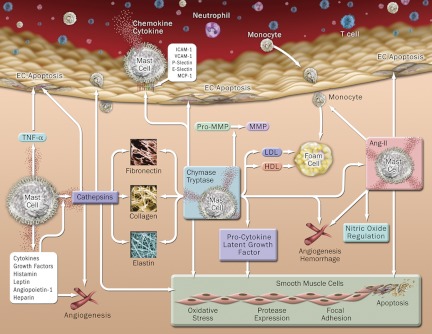
Figure 3.
MC functions in atherosclerosis. MC produce cytokines, chemokines, chymase, tryptase, cathepsins, and other inflammatory mediators to promote angiogenesis, vascular-cell apoptosis, blood-borne inflammatory cell adhesion and recruitment, foam-cell formation, lipid degradation, Ang-II generation, and activation of zymogen, procytokine, and latent growth factors.
D. Mast cells in experimental atherosclerosis
Animal models are important tools for testing MC functions in atherogenesis. Targeted activation of perivascular MC by dinitrofluorobenzene sensitization, followed by dinitrophenyl challenge in advanced atherosclerosis in apoE-deficient (Apoe−/−) mice, increased the incidence of intraplaque hemorrhage, macrophage apoptosis (via histamine and tryptase), vascular leakage, and CXCR2/very late antigen-4-mediated leukocyte recruitment to the plaque, whereas the MC stabilizer cromolyn prevented these adverse effects (68). These experiments established an essential role of MC activation in atherosclerosis. The same group recently proved the same concept using substance P to treat Apoe−/− mice (53). As anticipated, substance P enhanced the number and activation of adventitial MC and promoted intraplaque hemorrhage. Coadministration of neurokinin-1 receptor antagonist diminished these phenotypes. Chymase function in atherosclerosis has been confirmed in animals, likely due to the availability of its specific small molecule inhibitors, although not all observations were expected. In allergen-induced atherosclerosis in rats, intimal thickness correlated with chymase expression or MC homing (93). In diet-induced atherosclerosis in hamsters, chymase-selective inhibitor SUN-C8257 suppressed lipid aortic deposition but did not affect body weight, blood pressure, or serum levels of LDL cholesterol and Ang-II (94).
Our study established the direct participation of MC in atherogenesis by contrasting LDL receptor-deficient (Ldlr−/−) mice with the compound mutant Ldlr−/−KitW-sh/W-sh mice, from which we detected the absence of KIT+ MC, but no changes in the number or activity of lymphocytes, macrophages, neutrophils, or basophils in blood (42). Compared with those of Ldlr−/− mice, atheromata from Ldlr−/−KitW-sh/W-sh mice showed decreased lesion size, lipid deposition, T-cell contents, macrophage contents, cell proliferation, and apoptosis, but increased collagen content and fibrous cap formation. Using adoptive transfer of syngeneic BMMC to Ldlr−/−KitW-sh/W-sh mice, we demonstrated important roles of MC-derived IL-6 and IFN-γ in regulating vascular SMC and EC cysteinyl cathepsin expression (42). Using the same model, Lindstedt and colleagues (95) found reduced serum cholesterol, triglyceride, apoB particles, preβ-HDL, phospholipid transfer protein activity, and soluble ICAM in Ldlr−/−KitW-sh/W-sh mice.
IV. Distinct Role of Macrophages in Atherosclerosis
Macrophages and MC share many pathological activities during atherogenesis. Both types of cells migrate into intima as precursors (monocytes and MC precursors, respectively), where they differentiate or mature and become macrophages or foam cells and mature MC. After activation, these cells release a spectrum of inflammatory mediators to affect neighboring vascular and inflammatory cells. MC and macrophages produce similar types of mediators, such as inflammatory cytokines, chemokines, growth factors, proteases, reactive oxygen species (ROS), and NO. Both are nonprofessional APC and can mediate T-cell activation. Many MC activities therefore may apply to macrophages.
A. Macrophage types
After monocytes migrate into the endothelium, they differentiate into macrophages and dendritic cells (96). Macrophages in atherosclerotic lesions are of two types, M1 and M2, that express MCP-1 and mannose receptor (CD206), respectively (97). M1 macrophages, also called classically activated macrophages, require IFN-γ for initial activation and LPS and CpG-DNA or TNF-α and IL-1 for secondary activation (98–100). M1 macrophages release IL-6 and TNF-α to direct EC injury and express high MHC-II and costimulatory molecule CD80/86 to direct Th1 activation and IL-12 release (101). Unlike MC or M1 macrophages, M2 macrophages (also called alternatively activated macrophages) are antiinflammatory cells that are critical in the resolution of injury (98, 100, 102). Their maturation requires peroxisome proliferator-activated receptor-γ (PPAR-γ) (103, 104). M2 macrophages can be divided into three subgroups: M2a, M2b, and M2c (98). M2a are noncytotoxic cells activated by the Th2 cytokines IL-4 and IL-13 and participate in tissue repair and extracellular matrix (ECM) deposition (100). M2b cells are activated by LPS and release antiinflammatory IL-10, as well as the proinflammatory cytokines TNF-α, IL-6, and IL-1 (102, 105), while also promoting Th2-type adaptive immune responses with increased T-cell IL-4 production and B-cell IgG class switching (105). IL-10, TGF-β, and glucocorticoids activate M2c cells, which produce high levels of IL-10, limit inflammation by decreasing proinflammatory cytokines (98), and increase debris-cleaning activity.
B. Antiatherogenic and proatherogenic macrophages
Although both MC and macrophages are heterogeneous, current knowledge from in vitro and in vivo studies has not suggested an antiatherogenic role for MC in atherosclerosis. In addition to these distinct roles of macrophage apoptosis, apoptotic cell clearance (a process called efferocytosis), and necrotic core formation in atherogenesis, which we will discuss in Section IV.D, different types of macrophages contribute differently to atherogenesis. Th2 cytokine-induced M2 macrophage activation leads to antiatherogenic phenotypes (100). In atherosclerotic lesions, IL-10 receptor signaling induces antiinflammatory molecule suppressor of cytokine signaling 3, inhibits the nuclear factor-κB pathway (107), and enhances efferocytosis (108). When appropriately activated, M2 macrophages phagocytose cytotoxic lipoproteins, clear apoptotic bodies, secrete antiinflammatory cytokines, and synthesize matrix repair proteins that stabilize vulnerable plaques (109). In early stages, M2 macrophages scavenge cytotoxic lipoproteins and apoptotic cells to prevent accumulation and cytotoxicity. Efferocytosis occurs first in apoptotic neutrophils and then in apoptotic macrophages (110, 111). Efficient efferocytosis prevents secondary apoptosis and triggers an antiinflammatory response by inducing TGF-β, IL-10, and other antiinflammatory cytokines (112).
In the early to middle stage of atherogenesis, most macrophages ingest and metabolize lipoprotein-derived cholesterol. If engulfed cholesterol cannot exit, macrophages become foam cells, important sources of chemokines (MCP-1), cytokines (TNF, IL, and IFN), enzymes (cysteine, serine, and metalloproteases), growth factors (platelet-derived growth factor, VEGF, and IGF), bioactive lipids, and angiogenic factors, together with those from T cells that perpetuate the inflammatory process and promote EC and SMC migration from media to intima. Subsequent SMC proliferation in response to foam-cell mediators contributes to the enlarged fibro-fatty plaque and fibrous caps. Several cellular events contribute to vulnerable plaque formation, including the secretion of proinflammatory, procoagulant, and proteolytic molecules by macrophages; the death of macrophages, intimal SMC, and EC are important to plaque rupture (113). For example, Th1 cytokine IFN-γ or microbial agents activate M1 macrophages to express effector molecules (e.g., Fas-ligand and NO) that kill nearby cells, degrade ECM, and promote plaque rupture (114). Antigens or Ig molecules induce MC production of ROS (115–117). In turn, these ROS may induce MC chymase expression and indirectly participate in atherogenesis (118). Likewise, classically activated M1 macrophages in developing plaques also produce ROS, which cause cell death and lipoprotein oxidation (119). oxLDL engulfed by macrophages is digested and processed, and peptide antigens are presented to activate T cells for the adaptive immune system and cytotoxic Th1 immune response. Release of IFN-γ from Th1 cells amplifies inflammation and induces macrophage procoagulant tissue factor expression. Advanced plaque macrophages also release tissue factors as procoagulant and prothrombotic activities after plaque rupture (120). Therefore, macrophage depletion or suppression of macrophage migration provides resistance to atherosclerosis (121–124).
Due to the complex roles of macrophages in promoting and inhibiting progression of atherosclerotic plaques, studies employing simple inhibition of monocyte migration by disrupting chemokine/chemokine receptors that inhibit atherogenesis in experimental models (123–125) may not yield specific therapies for human diseases. Understanding the role of macrophages in regulating all stages of atherogenesis, therefore, is important to target this population of cells effectively for therapy.
C. Macrophage adhesion, migration, and proliferation
Early in atherosclerosis, monocytes/macrophages are recruited to the endothelium, aided by the endothelial expression of the adhesion molecules ICAM, VCAM, and ELAM (endothelial leukocyte adhesion molecule) and of P/E-selectins, which can be increased by smoking, hypertension, and diabetes and modulated by hemodynamic factors, arterial pressure, and sheer stress (114, 126). ICAM knockout mice are resistant to atherosclerosis (123), and mice with reduced VCAM have diminished atherosclerosis (127). MCP-1 or MCP-1 receptor CCR2 knockout animals had reduced atherosclerosis (124, 128). All of these mice, although manipulated differently, showed impaired macrophage accumulation in atherosclerotic lesions.
Macrophages in human or animal atherosclerotic lesions also proliferate (129–131), and this proliferation can be stimulated by oxLDL (132). oxLDL induces macrophage proliferation by increasing the expression of granulocyte-macrophage colony-stimulating factor via the protein kinase-C pathway, leading to phosphoinositide 3-kinase activation (133). Hyperglycemic Ldlr−/− mice fed a cholesterol-rich diet showed increased cholesterol levels concomitant with macrophage proliferation in atherosclerotic lesions (134, 135). Although a direct view of MC proliferation in atherosclerotic lesions is currently not available, increased expression of SCF, nerve growth factor, and T-cell cytokines in atherosclerotic lesions (136–138) may induce lesion MC proliferation (138–140).
D. Macrophage apoptosis
MC development, migration, and survival or apoptosis play detrimental roles in immediate hypersensitivity and chronic allergic reactions (1). Little is known, however, about the fate or apoptosis of MC in the setting of vascular diseases, likely because there are relatively fewer MC in the aortic wall compared with most other inflammatory cell types, and only a few groups have paid attention to this small population of cells. In contrast, macrophages are the most abundant inflammatory cells, and their apoptosis is probably one of the best-known phenotypes of human and animal atherosclerotic lesions. Alterations in macrophage apoptosis affect atherosclerotic lesion size and plaque progression (141–144).
The role of macrophage apoptosis in the pathogenesis of atherosclerosis can vary by stage. In early lesions, there is an inverse relationship between macrophage apoptosis and lesion size. AIM (apoptosis inhibitor of macrophages)-deficient Ldlr−/− mice have reduced atherosclerosis, and macrophages from these mice are more susceptible to oxLDL-induced apoptosis (143). Efferocytosis is efficient in early lesions, leading to fewer inflammatory cells and postnecrotic cells and more efferocyte-derived antiinflammatory mediators. Thus, macrophage apoptosis limits lesion cellularity and suppresses lesion progression.
Conversely, in advanced lesions, macrophage apoptosis promotes the development of the necrotic core—a key factor in plaque vulnerability and acute luminal thrombosis—and associates with plaque necrosis (110). As discussed in Section III.C, MC induce EC and SMC apoptosis via TNF-α, chymase, tryptase, and Ang-II (81, 91, 101, 146). Late-lesion macrophages release toxic oxygen and nitrogen radicals and cause apoptosis of surrounding cells via Fas-Fas ligand interaction (147). Proinflammatory mediators cause further apoptosis of SMC, EC, and leukocytes that accounts for much of the disruptive core of vulnerable plaques. Uncleared apoptotic cells shed plasma membrane microparticles that stimulate thrombosis (148). Macrophage efferocytosis is mediated mainly by M2 cells that suppress inflammation and engulf cellular debris (149). In advanced human atherosclerotic lesions, however, oxLDL binds to efferocytosis receptor Mertk (c-mer tyrosine kinase) (150), and inflammation induces its cleavage (151–153), leading to defective efferocytosis (109). Failure of efferocytosis further causes postapoptotic secondary necrosis and necrotic core formation (110, 154). The role of macrophages in atherosclerosis therefore depends on the stage of plaque development—activities likely unique to macrophages.
E. Interaction of macrophages with other cell types
Both MC and macrophages interact with virtually all atherosclerosis-pertinent cells, including T cells, EC, and SMC. MC are APC and activate T cells (155, 156), as well as release cytokines (42) or proteases (81) to induce EC protease expression and angiogenesis or to promote SMC apoptosis. Although macrophages also process and present antigens to activate T cells—particularly in the presence of high cholesterol, such as in atherosclerosis (157)—they also interact with other atherosclerosis-pertinent cells. We recently have shown that MC, macrophages, and neutrophils act similarly in inducing EC expression of ICAM-1, VCAM-1, P-selectin, and E-selectin (158), suggesting that these inflammatory cells act cooperatively in activating EC in the blood vessel wall and mediate their own adhesion, transmigration, and accumulation in the intima during atherogenesis.
Atherogenic lipids induce human coronary artery SMC chemokine (C-X3-C motif) ligand 1 (CX3CL1) expression, thereby increasing macrophage adhesion (159). In turn, macrophages induce EC and SMC apoptosis via Fas-Fas ligand interactions (114, 160, 161). Growth factors induce monocyte binding to SMC and retention in the endothelium in atherosclerosis (162). Coculture of monocytes with SMC induces monocyte CD36 expression and ERK1/2 phosphorylation, suggesting SMC-dependent local regulatory mechanisms for monocyte survival and differentiation in atherosclerosis (163). T cells interact with macrophages, causing further activation and regulation of growth and rupture of atherosclerotic plaques (164–166). Primary activation of T cells in secondary lymphoid tissues and secondary activation at lesion sites, at least in part by macrophages, leads to an amplified inflammatory response. Macrophages and T cells activate each other and cause further activation and increase of adhesion molecules, chemokines, scavenger receptors, and ECM-degrading proteases (167, 168).
V. Mast Cells in AAA
A. Inflammatory cells in AAA
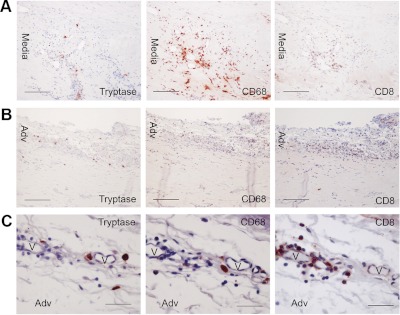
AAA and other advanced cardiovascular disorders—such as MI, stroke, and angina pectoris—share many pathophysiologies with atherosclerosis. Like atherosclerosis, the development of AAA involves extensive inflammatory cell infiltration, followed by cytokine, chemokine, and protease secretion, which recruit more inflammatory cells, induce vascular cell inflammation and apoptosis, catabolize the vascular ECM proteins (e.g., elastin and collagen), and promote angiogenesis. These infiltrates include mainly macrophages, neutrophils, T cells, and MC recruited from the lumen or the vasa vasorum. Macrophages appear in both the adventitia and the media in human AAA; MC also are abundant in these areas. In the outer media and adventitia of human AAA, MC number correlates positively with diameter (169). In thrombus-covered and thrombus-free human AAA lesions, most MC are located in the media and correlate with densities of neovascularization (170). In ruptured human AAA, MC are present in the adventitia and media and often colocalize with macrophages and T cells. The role of T cells in AAA is more complicated than we originally thought. CD4+ T-helper cells, Th1 and Th2 (171, 172), are predominant in human AAA and appear mainly in the adventitia. The absence of CD4+ T cells, or Th1 cytokines IFN-γ or TNF-α, protects CaCl2-induced AAA in mice (173, 174), but IFN-γ deficiency or signaling blockade leads to Ang-II-induced or allograft-induced AAA (175, 176). Differences in experimental models may result in these discrepancies regarding the functions of Th1 cells in AAA, and thus their exact role remains uncertain. In ruptured human AAA lesions, we detected clusters of CD8+ T cells, macrophages, and MC in the media (Fig. 4A) and the adventitia (Fig. 4B), particularly next to the vasa vasorum (Fig. 4C). These observations suggest that MC are as important—if not more so—as macrophages in AAA lesions, in T-cell activation, and in the secretion of proteases, cytokines, and chemokines via autocrine and paracrine mechanisms.
Figure 4.
Localization of MC, macrophages, and CD8+ T cells in ruptured human AAA lesions. Mouse antihuman tryptase monoclonal antibody (1:1500; Chemicon International), mouse antihuman CD68 monoclonal antibody (1:60; Dako, Glostrup, Denmark), and mouse antihuman CD8 monoclonal antibody (1:75; Abcam, Cambridge, MA) detected tryptase+ MC, CD68+ macrophages, and CD8+ T cells in ruptured human AAA media (A, scale, 100 μm), adventitia (B, scale, 100 μm), and vasa vasorum surrounding the adventitia (C, scale, 50 μm). Paraffin sections (5 μm) were used.
B. Mast cells in experimental AAA
Because MC function in AAA was recognized only recently, not many studies have been performed in this area (177). The first study was done in hamsters with aortic elastase perfusion-induced AAA, in which chymase inhibitor NK3201 (30 mg/kg · d, orally) reduced MC numbers and aortic diameters (77), suggesting a role of chymase in AAA. This hypothesis was proved further in dogs. Eight weeks after elastase perfusion, NK3201 at a much lower dose (1 mg/kg · d, orally) reduced Ang-II-forming and MMP-9 activities, as well as the aortic diameter, luminal area, and the number of chymase-positive cells and lesion neutrophils (178). Human AAA lesions contain not only high numbers of MC, but also many more activated MC than are found in atherosclerotic lesions or normal arteries—resulting in increased chymase and Ang-II-forming activities (46, 169) that initiate and promote AAA formation. As in atherosclerotic lesions, the neovascularized area in the media and adventitia correlates with the density of MC (Fig. 4C), with the densest MC and neovessels in thrombus-covered AAA samples (179). Real-time PCR analysis showed that VEGF, its receptor FLT1, VE-cadherin, CD31, tryptase, chymase, and cathepsin G were all significantly higher in AAA than in normal arteries, strongly suggesting increased angiogenesis levels in AAA lesions. Human AAA luminal surface showed CD42b+ platelets but lacked CD31/CD34 staining of EC, suggesting endothelial erosion of the AAA wall (179). Therefore, the role of MC in promoting angiogenesis and vascular-cell apoptosis within atherosclerosis may also apply to AAA pathogenesis.
MC-deficient KitW-sh/W-sh mice were used to test a direct role of MC in AAA formation (180). In elastase perfusion-induced and periaortic CaCl2 injury-induced AAA, all WT mice developed AAA lesions, but KitW-sh/W-sh mice were fully protected at any time point tested. Reduced AAA in KitW-sh/W-sh mice accompanied impaired internal elastic lamina degradation; decreased numbers of macrophages, CD3+ T lymphocytes, SMC, apoptotic cells, and CD31+ microvessels; and decreased levels of aortic tissue IL-6 and IFN-γ. Activation of MC in WT mice with C48/80 enhanced AAA growth, whereas MC stabilization with cromolyn diminished AAA formation, suggesting the importance of MC granule release to pathogenesis. In this study, we demonstrated that MC release proinflammatory IL-6 and IFN-γ to stimulate vascular wall elastinolytic cathepsin and MMP expression. Similar observations were made recently in periaortic CaCl2-induced AAA in rats; AAA formation accompanied MC and T-cell accumulation, MMP-9 activation, reduced elastin, and increased angiogenesis. All such phenotypes, however, disappeared in MC-deficient Ws/Ws rats (181). In this same study, MC stabilization with tranilast, another common MC stabilizer, attenuated AAA formation.
Both chymase and tryptase play essential roles in AAA formation. Recently, we demonstrated significant correlations of serum chymase and tryptase levels with AAA annual expansion rate in a Danish AAA patient follow-up study (81, 182). Interestingly, high serum tryptase levels significantly increased the risks of later surgical repair or death in this population (182). Both MC proteases were expressed highly in the media and adventitia of human AAA but were negligible in normal aortas. In elastase perfusion-induced AAA in mice, those mice lacking connective tissue chymase mMCP-4 (81) or tryptase mMCP-6 (182) developed significantly smaller AAA lesions than did WT mice. A mechanistic study suggested that both proteases contribute to vascular SMC apoptosis, inflammatory cell infiltration, and vascular cell elastinolytic cathepsin expression and activities; but only chymase, not tryptase, promoted angiogenesis both in vitro and in vivo in this mouse experimental AAA model, although further explanations are required for these distinct mechanisms.
C. Macrophages in AAA
Although only limited studies have shown the involvement of MC in AAA, much more evidence suggests the importance of macrophages in AAA pathogenesis. Macrophages greatly outnumber MC in human or animal AAA lesions, whereas normal aortas contain negligible numbers of macrophages. In elastase perfusion-induced AAA in rats, lesion chemokine MCP-1 and hematopoietic cytokine granulocyte-macrophage colony-stimulating factor levels correlate with macrophage densities in the media and adventitia. Human and animal AAA express increased levels of MC-specific chymase (81) and tryptase (182); similarly, human and animal AAA lesions contain macrophage-specific enzymes. In human AAA lesions, macrophage accumulation correlates with enhanced levels of their proteases, such as MMP-12, MMP-2, MMP-7, and MMP-9 (183). In the recent Lille Aneurysmal Study (184), investigators compared enzyme profiles in aortic lesions from AAA patients with those with peripheral artery occlusion (PAO) but without AAA, although both need surgical repair. Using transcriptomic array containing 137 protease and protease inhibitor genes; two-dimensional-differential in gel electrophoresis technology on proteome analysis of cytosolic proteins isolated from macrophages from the same patients; and antibody protein array containing 42 different proteins representing RNA and proteins found to be differentially modulated in macrophages from AAA and PAO patients, the investigators found that the expression of macrophage TIMP-3 (tissue inhibitor of MMP), ADAMTS5 (a disintegrin and metalloproteinases with thrombospondin motifs), and ADAMTS8 differed significantly in plasma from AAA compared with PAO patients. Therefore, macrophages in human AAA lesions may use these proteases and protease inhibitors to regulate AAA progression.
MC and macrophage adhesion, migration, and accumulation in the aortic wall are essential in AAA pathogenesis. We recently showed that inhibition of MC recruitment to the aortic wall effectively impaired Ang-II-induced AAA formation in Apoe−/− mice (G.-P. Shi, unpublished data). Multiple studies proved the same concept—that macrophage infiltration is essential to AAA formation. In elastase perfusion-induced AAA in rats, treatment with atorvastatin inhibited the expression of ICAM and MCP-1, suppressed macrophage recruitment into the aortic wall, and increased vascular wall collagen and elastin synthesis at 1 wk after perfusion, resulting in significantly reduced aneurysmal diameters at 4 wk after perfusion (185). Plasminogen is a protease bound to ECM. Upon conversion into plasmin, it degrades ECM, activates MMP, and promotes AAA formation (186), likely via increasing macrophage infiltration. In a periaortic CaCl2-induced AAA model, plasminogen-deficient Plg−/− mice displayed reduced macrophage infiltration and protected AAA formation. Intravenous administration of active MMP-9 reversed these phenotypes (187). In Ang-II-induced AAA in Apoe−/− mice, ultrasmall superparamagnetic iron oxide contrast agent allowed detection of real-time macrophage infiltration during AAA progression. Macrophages infiltrated in the shoulder and outer perianeurysm areas (188). As discussed in more detail in Section VI, in diet-induced obese (DIO) mice, white adipose tissue (WAT), but not brown adipose tissue (BAT), contains high levels of proinflammatory chemokine and chemokine receptor mRNA. In DIO mice infused with Ang-II, WAT surrounded the abdominal aortas, and BAT surrounded the thoracic aortas. Periaortic WAT explants from the abdominal aortas showed higher activity in promoting macrophage migration than did BAT explants from the thoracic aortas. In both DIO and ob/ob mice, Ang-II infusion greatly promoted macrophage infiltration to the periaortic and visceral adipose tissues and significantly increased AAA lesion size (189). When chemokine leukotriene B4 receptor BLT1 knockout mice were used in the same experimental model, leukocyte accumulation, lesion MMP-2 and MMP-9 expression, and AAA size were significantly reduced, compared with WT control mice (190). Thus, blockade of macrophage or MC migration can effectively control the progression of AAA, at least in experimental AAA (190).
VI. Mast Cells in Obesity
A. Obesity as an inflammatory disease
A growing body of evidence suggests a close association between the immune system, obesity, and vascular diseases (191). Recent studies have shown that obesity is an inflammatory disease, like atherosclerosis or AAA. In addition to adipogenesis (i.e., differentiation of preadipocytes into adipocytes), the development of obesity involves extensive angiogenesis, leukocyte infiltration, and ECM remodeling (191, 192). Most of these processes, defined as the key features of inflammation, are similar to those in vascular diseases. An analysis of the development of adipose tissue microvasculature indicated that angiogenesis often precedes adipogenesis (193), providing paths for leukocyte infiltration. Inflammatory macrophages, CD4+ and CD8+ T cells, regulatory T cells (Treg), and B cells have recently been detected in human or mouse WAT (194–196). These cells are rich sources of both proinflammatory and antiinflammatory cytokines, to which adipocytes respond for downstream regulation of inflammatory signaling cascade (191). Obesity and inflammation have been connected in the context of clinical weight loss studies. Weight loss caused decreases in circulating C-reactive protein, IL-6, TNF-α, ICAM-1, VCAM-1, and several other inflammatory mediators in patients from different age groups or with different body mass index (BMI) values (191, 197).
B. Obesity and allergy
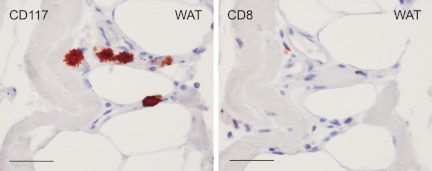
A Chinese idiom says, “Nine are asthmatic among 10 obese men.” Indeed, epidemiological surveys have shown that asthma is more prevalent in obese children and adults (198). Overweight and obesity are common among women with allergies (76%) (199), but MC function in obesity has never been recognized, probably because of the relatively low MC numbers in WAT. We recently found that serum tryptase levels were higher in obese subjects than in lean subjects. When we immunostained WAT sections for macrophages (HAM65) and MC (tryptase), we detected significantly more macrophages (2-fold) and MC (4-fold) in WAT from obese subjects than in those from lean subjects, although MC contents were only one third that of macrophages. Similar observations were made in mouse WAT. WAT from ob/ob mice contain high numbers of CD117+ MC and CD8+ T cells, and many of these cells cluster together (Fig. 5). This is particularly important, given that MC are APC and donate a large quantity of inflammatory mediator; both mechanisms may trigger T-cell activation, which regulates adipocyte inflammation and body weight gain (196, 200, 201).
Figure 5.
MC and CD8+ T cells in mouse WAT. CD117+ MC (left, rat antimouse CD117 monoclonal antibody, 1:40; eBioscience, San Diego, CA) and CD8+ T cells (right, rabbit antimouse CD8 monoclonal antibody, 1:300; Abcam) appear in the same locations in WAT from ob/ob mice. Scale, 50 μm.
MC participate directly in obesity. MC-deficient KitW-sh/W-sh and KitW/Wv mice or WT mice receiving MC stabilizer cromolyn or ketotifen demonstrated significantly reduced body weight gain, improved glucose intolerance and insulin sensitivity, and reduced adipose tissue inflammation with reduced leptin and insulin levels in circulation (43). Mechanistic studies suggest that MC contribute to obesity by affecting energy expenditure, adipose tissue angiogenesis, and preadipocyte differentiation. As discussed earlier, MC are reservoirs for inflammatory cytokines to stimulate vascular cells (42) and adipocytes (43) to release cysteinyl cathepsins—important proteases that can catabolize ECM protein fibronectin to promote adipogenesis, and degrade intracellular insulin receptor and glucose transporter (Glut)-4, leading to impaired insulin and glucose sensitivities (202, 203). MC can also release directly the same cathepsins along with their unique chymases and tryptases, all of which can regulate neovascularization and cell survival (204) that are essential for WAT growth (43) (Fig. 6).
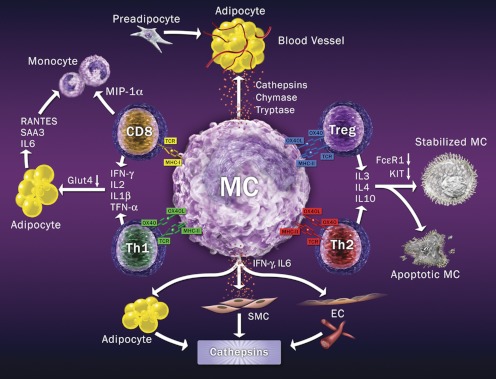
Figure 6.
MC and T cell interaction model. MC use MHC class-I to interact with T-cell receptor on CD8+ T cells, to stimulate inflammatory cytokines and monocyte chemokine MIP-1α expression. MC also activate Th1 cells via MHC class II and OX40L to stimulate Th1 cell inflammatory cytokine expression, which activates adipocytes. MC may activate Treg and Th2 cells using MHC class II and OX40L. Both Treg and Th2 may release IL-3, IL-4, and IL-10 to stabilize MC by decreasing MC FcεR1 and KIT expression or directly causing MC apoptosis. MC also produce proteases to promote angiogenesis and adipogenesis in adipose tissues or release inflammatory cytokines to induce vascular cell and adipocyte cathepsin expression, angiogenesis, and adipogenesis.
C. Possible interaction between mast cells and T cells
The most intriguing recent findings concern the role of T cells in obesity. Similar to observations regarding MC and CD8+ T cells in WAT from ob/ob mice (Fig. 5), WAT from obese mice contain more total T cells, CD8+ cells, and Th1 subtypes, but diminished CD4+Foxp3+ Treg and CD4+ Th2 cells. Both CD8+ and Th1 cells are rich sources of the inflammatory cytokines IL-2 and IFN-γ, which can trigger adipocyte inflammatory molecule production and lower Glut-4 expression (Fig. 6). Therefore, depletion of CD8+ T cells reduced macrophage infiltration, adipose tissue inflammation, and insulin resistance, whereas adoptive transfer of CD8+ T cells into CD8+-null mice reversed these effects (196). In contrast, reduced Treg cells are found in insulin-resistant obesity, along with increased fasting insulin and adipose tissue-derived inflammatory protein serum amyloid A3 in WAT (201). Anti-IL-2-mediated or anti-CD3-mediated increase of Treg in adipose tissues reduced serum glucose and insulin levels, enhanced glucose and insulin intolerance, and lowered body weight (200, 201). Th2 cells are also required for maintaining glucose homeostasis, but are compromised during obesity progression. Although T-cell-deficient Rag1-null mice developed obesity with high serum glucose and insulin levels and were glucose tolerant, CD4+ T cell (but not CD8+ T cell) adoptive transfer reversed these metabolic abnormalities (200). These observations raised the important question of whether cross talk occurs between MC and T cells in obesity. The model in Fig. 6 explains recent discoveries in this field. MC may act as central receivers, mediating the interactions between different T-cell subtypes. MC recruit Treg and CD4+ T cells (205), likely by releasing chemokines (e.g., lymphotactin, IL-16) and increasing EC adhesion molecule expression (206). Both cell adhesion and cognate MHC-II-mediated interactions between T cells and MC result in MC degranulation (206), thereby releasing more chemokines for T-cell infiltration.
MC may contribute to CD8+ cell-mediated obesity (196). As nonprofessional APC, MC express both MHC class-I and class-II molecules for antigen presentations, which may be induced by inflammatory cytokines or bacterial infections (207). Recent data suggest that MC induce antigen-specific CD8+ T-cell activation and proliferation by presenting antigens with MHC-I to induce CD8+ T-cell secretion of IL-1β, IL-2, IFN-γ, TNF-α, and macrophage inflammatory protein (MIP)-1α, a critical macrophage/monocyte chemoattractant (208). Adoptive transfer of antigen-pulsed MC induced MHC-I-dependent, antigen-specific CD8+ T-cell proliferation in mice (156), although such antigens in human or mouse adipose tissues remain elusive. CD8+ T cells use these inflammatory cytokines to stimulate adipocyte expression of IL-6, serum amyloid A-3, and chemokine regulated upon activation, normal T cell expressed and secreted with concomitant reduction of Glut-4 (201), leading to enhanced adipose tissue inflammation, impaired glucose uptake, and further leukocyte infiltration. CD8+ T-cell-derived MIP-1α may be used to recruit macrophages to adipose tissues (81)—a hypothesis consistent with the observation that CD8+ T-cell infiltration into adipose tissue precedes macrophage accumulation (196). Using MC-deficient KitW-sh/W-sh mice and MC stabilizers, we proved that MC appear ahead of macrophages in WAT and in the vascular wall (42, 43, 180); these findings agree with the model proposed in Fig. 6. MC may activate Th1 cells through a few mechanisms, such as MHC-II- mediated antigen presentation and OX40-OX40 ligand (OX40L) interaction (209). OX40 is a T-cell activator expressed on CD4+ T cells, CD8+ T cells, B cells, and vascular EC. Interaction of OX40 on Th1 cells with MC surface OX40L leads to Th1 cell proliferation and the secretion of inflammatory cytokines for subsequent stimulation of adipocytes, as in CD8+ T cells (Fig. 6).
In contrast to CD8+ and Th1 cells, both Treg and Th2 cells directly inhibit FcεR1-dependent MC degranulation by suppressing FcεR1 expression through cell–cell interaction between OX40 and OX40L in vivo (210) and in vitro (205) (Fig. 6). Consequently, coculture of MC with Treg or Th2 cells suppress IgE-mediated MC leukotriene C4 production (205). Depletion or inactivation of Treg cells enhances the anaphylactic response (210) and obesity (201). In addition to OX40-OX40L interaction, other pathways may also activate Th2 and Treg cells (such as MHC-II), enhancing production of Th2 cytokines (such as IL-3, IL-4, and IL-10) that suppress MC expression of KIT and FcεR1 (Fig. 1), thereby inactivating MC (Fig. 6). MC incubation, in combination with IL-3, IL-4, and IL-10, reduces KIT surface expression by 80% (211). IL-3 and IL-4 together reduce FcεR1 expression by 60%, resulting in an 80% reduction of IgE-mediated MC production of IL-4, IL-5, IL-6, and IL-13 (212). Extended culture of MC in the presence of IL-3, IL-4, and IL-10 leads to Fas-dependent MC apoptosis, with decreased expression of bcl-2 and bcl-xL (213). Treg cells (214), MC, and Th2 all produce IL-3, IL-4, and IL-10; therefore, Treg and Th2 may maintain MC homeostasis via these Th2 cytokines—a phenomenon that may occur in WAT in lean subjects.
D. Role of macrophages in obesity
Adipose tissue is a key endocrine organ and has a central role in obesity-associated dyslipidemia, insulin resistance, type 2 diabetes, and CAD (215). In adipose tissues, both adipocytes and cells in the stromal-vascular fraction (SVF), including T cells, macrophages, MC, neutrophils, and NK cells, release adipokines (IL-6, IL-8, IL-1β, MCP-1, leptin, and adiponectin) and influence other tissues, such as liver and muscle. SVF macrophages are the major source of adipose tissue TNF-α (216, 217). Adipose tissue macrophages were first detected in SVF preparation from WAT and BAT, in which SVF phagocytic and microbicidal activities, mainly from macrophages, were much greater in WAT than in BAT (218). Two independent groups then reported mouse WAT macrophage gene profiling and confirmed the presence of macrophages in mouse and human WAT SVF and correlation with BMI (194, 219). Like MC (43), macrophages are much more numerous in WAT from obese subjects than in that from lean subjects (220). A recent study demonstrated that in Pima Indians, abdominal sc adipose tissue macrophage content associates with serum total cholesterol, HDL, and triglyceride. Only HDL negatively associates with macrophages (221).
As in the vascular system, human and animal WAT macrophages also contain M1 and M2, but M2 is the main macrophage population in lean WAT from rodents (195) and humans (223). A high-fat diet (HFD) causes a switch from M2 to M1 in rodents by decreasing IL-10, heparin-binding lectin Ym1, and arginase, and increasing TNF-α and inducible NO synthase (224). Insulin resistance associates with an increased number of M1 and M1/M2 ratio in WAT (225). Weight reduction promotes the occurrence of M2 macrophages in WAT from obese patients (226, 227). Although M1 macrophages contain TNF-α, IL-6, and MCP-1 and contribute to the induction of insulin resistance, M2 macrophages express CD206, arginase-1, and IL-10; participate in tissue repair or remodeling (99, 100, 228); and restrain diet-induced WAT inflammation through the induction of Treg (200).
Macrophage adhesion, migration, and recruitment to WAT are similar to these processes in the aortic wall. Obesity is a chronic inflammation, with increased C-reactive protein, IL-6, IL-8, and TNF-α in obese patients and animals (230, 231). These inflammatory mediators may come from macrophages, T cells, MC, or neutrophils and mediate EC adhesion molecule expression (158)—which explains increased monocyte transmigration and accumulation in WAT in obese subjects (224, 232). After transmigration into WAT, monocytes differentiate into macrophages and act synergistically with adipocytes to amplify local inflammation (194) or produce more chemoattractants for further inflammatory cell recruitment (99, 219, 233). Most macrophages in WAT distribute around the necrotic adipocytes (234) and correlate with adipocyte size and death, suggesting that adipocyte death helps to mediate local macrophage infiltration (234). The adipokine leptin also exerts a mildly proinflammatory effect in inducing EC adhesion molecule expression, initiating macrophage infiltration, and activating macrophage phagocytosis and cytokine production (232).
The presence of different types of inflammatory cells in SVF raised the question of what triggers macrophage or MC infiltration into WAT. MCP-1 has been implicated in macrophage infiltration in WAT (236), but it is expressed in WAT before inflammatory macrophage markers (219), providing indirect evidence that cells other than macrophages produce MCP-1 and consequently drive macrophage infiltration. Several recent observations from in vivo experimental models proved this hypothesis. In experimental obesity in rodents, neutrophils, CD8+ T cells, and MC all appear in WAT before macrophages. In DIO mice, immunohistology analysis demonstrated the transient appearance of myeloperoxidase-positive neutrophils in WAT as early as 3 d after initiating HFD (237), suggesting that neutrophils participate in the initiation of the inflammatory cascade. In the same experimental model, CD8+ T-cell depletion using anti-CD8 antibody or in CD8a-deficient mice, SVF macrophage contents were significantly reduced. Reconstitution of CD8+ T cells greatly enhanced SVF macrophages (196), suggesting a role of CD8+ T cells in controlling macrophage infiltration into rodent WAT. MC also regulate macrophage migration and recruitment. In DIO mice, both macrophages and MC increased in WAT. MC deficiency (KitW-sh/W-sh) significantly reduced WAT macrophage contents. Although MC contents in WAT from cromolyn-treated DIO mice did not differ from that in untreated DIO control mice, macrophage numbers were significantly reduced after MC inactivation with cromolyn (43). Therefore, neutrophils, T cells, and MC all precede macrophages in rodent WAT, and possibly in human WAT as well.
MC and macrophages in obese subjects affect not only adipose tissue, but also other organs, such as liver and muscle. We have shown that KitW-sh/W-sh mice still had healthy livers without hepatic dyslipidemia (steatosis) after 3 to 6 months of HFD. MC stabilization with cromolyn yielded similar results. Although we did not examine whether deficiency or stabilization of MC affected liver macrophage accumulation, we did not detect much MC infiltration (43). Rats exposed to a 2-wk HFD or high-sucrose diet developed steatosis and hepatic insulin resistance. But these metabolic abnormalities did not occur in rats that received administration of gadolinium chloride (GdCl3; injection to the carotid artery) to deplete Kupffer cells (liver-specific macrophages). In a coculture experiment, Kupffer cells increased hepatocyte triglyceride accumulation and fatty acid esterification and decreased fatty acid oxidation and insulin responsiveness. Neutralizing antibody to TNF-α attenuated Kupffer cell-induced alteration of hepatocyte pathologies (238). These observations demonstrate that both MC and macrophages, and possibly other inflammatory cells, contribute to steatosis in obese subjects. Depletion or inactivation of these cells or blockade of their migration should benefit not only adipose tissue growth/expansion, but also its associated complications.
VII. Relative Contribution of Mast Cells and Macrophages in Diabetes
A. Mast cells in diabetes
Type 1 and type 2 diabetes mellitus (T1DM and T2DM) are the most common types of diabetes. Diet-induced obesity accompanies T2DM, as discussed earlier. Our data suggest that MC participate importantly in the pathogenesis of this type of diabetes. Glucose and insulin tolerance assays confirmed that deficiency or inhibition of MC improved these parameters, whereas diabetic mice had high serum insulin and glucose levels and high KIT-positive MC in WAT (43). Although not tested in our study, insulin may enhance allergen-induced MC FcεRI downstream signaling and degranulation (239). Serum tryptase levels appeared higher in T2DM patients than in patients without T2DM (G.-P. Shi unpublished observations), but a direct participation of MC in T1DM has never been tested, and findings of MC contents in T1DM animals have been controversial. Surprisingly, asthmatic patients had a low incidence of T1DM (240). Other allergic disorders, including atopic dermatitis and eczema, were also found mutually exclusive to T1DM. In patients with diabetic retinopathy, microvessel-enriched retinas contained no MC (241), although MC normally appear in microvessel-rich tissues.
MC contents in experimental T1DM vary by model. Alloxan, a toxic glucose analog, selectively destroys insulin-producing β-cells in the pancreas, leading to T1DM. In rats with alloxan-induced T1DM, IL-3 and MC in the small intestine (ileum fragment) or peritoneum were markedly reduced. These rats were clearly resistant to local and systemic allergic inflammatory responses and anaphylactoid reactions (242, 243). IL-3, SCF, and IL-9 are principal cytokines that regulate MC growth and differentiation (21). Changes in IL-3 content might contribute to MC depletion in diabetic rats. Treatment with insulin restored basal MC number and normal levels of IL-3 in the ileum of diabetic rats (244).
DRlyp/lyp rats develop spontaneous T1DM, including hyperglycemia, glycosuria, weight loss, and decreased plasma insulin. Unless insulin is administered, death follows shortly after onset due to severe hyperglycemia and ketoacidosis (245). In contrast to alloxan-induced T1DM, pancreatic lymph nodes in DRlyp/lyp rats contain more MC, and higher eotaxin and FcεR1 expression levels, than those in control rats (246). Although a direct role of MC in these T1DM rats is unknown, inactivation of MC delayed T1DM onset in DRlyp/lyp rats (247).
Streptozocin (STZ), a naturally occurring chemical, is particularly toxic to β-cells and induces T1DM. In rats with STZ-induced diabetes, T1DM developed with increased mesenteric vessel fibrosis and peritoneal MC (248), although MC from STZ-treated rats and control rats behaved similarly in histamine release (249). MC stabilization with tranilast reduced MC number and mesenteric vessel fibrosis, but when STZ was given to 2-d-old neonatal rats, they developed T2DM in adulthood with glucose tolerance and insulin resistance (250). Tranilast treatment in diabetic rats suppressed β-cell glucose uptake and glucose-induced insulin secretion in vivo and in vitro (251). Taking these results together, MC stabilization appears effective in improving both T1DM and T2DM, although mechanistic explanations for these observations are not yet available.
B. Macrophages in diabetes
Macrophage accumulation in diabetic tissues is a feature of T2DM associated with the development of diabetic complications (nephropathy, atherosclerosis, neuropathy, and retinopathy) and tissue damage. In human and experimental type 2 diabetic nephropathy, kidney macrophage accumulation associates with progression of renal injury and decline in renal function (252, 253). As in atherosclerotic plaques, AAA lesions, or WAT, inflammatory cell accumulation in diabetic organs, such as adipose tissue, pancreas, kidney, and liver, often occurs in pathogenesis. Although the presence of MC has not been closely examined, macrophages are prominent in these organs. Components of diabetic milieu (high glucose, advanced glycation end-products, and oxLDL) promote macrophage accumulation via induction of chemokine and adhesion molecule expression and macrophage activation within these diabetic tissues. Established T2DM further induces tissue expression of adhesion molecules, cytokines, and chemokines (253–255). In vitro studies showed that high glucose and advanced glycation end-products stimulate renal tubule cells to produce ICAM-1 and MCP-1 (254, 256). In db/db mice, deficiency of ICAM-1 or MCP-1 decreases kidney macrophage accumulation and diabetic renal injury (254, 256). In ob/ob mice, macrophages account for almost all kidney leukocyte accumulation, and their accrual correlates with the progression of diabetes (hyperglycemia, glycosylated hemoglobin) and the severity of kidney damage (histological lesions, renal function) (253). Peripheral neuropathy, a feature of human T2DM, associates with microvasculitis at the nerve site and local ischemic injury. Macrophages are prominent in these perivascular lesions and associate with nerve demyelination (257, 258). In peripheral retinopathy, macrophages are present in the epiretinal membrane (259). Macrophage-derived cytokines are often seen in vitreous samples (260), suggesting a role of macrophages in microvascular cell apoptosis, neovascularization, and fibrosis.
Most metabolic tissues contain a population of alternatively activated macrophages. As discussed, macrophages switch their phenotypes to classically activated cells in WAT that favors inflammation and insulin resistance (224). Macrophage transformations from alternatively activated into classically activated status not only change the cell inflammatory profile but also alter these pathobiological activities. Therefore, macrophages from T2DM and metabolic syndrome show decreased phagocytosis of microbes (261), reduced cholesterol efflux (262), decreased surface expression and tyrosine kinase activity of insulin receptor (263–265), and diminished insulin-stimulated signaling (263, 266, 267), but also show enhanced adhesion to the endothelium (268, 269), increased surface expression of CD36 and/or scavenger receptor-A (262, 263, 270–272), and binding and uptake of modified LDL and elevated cholesteryl ester formation (263). In HFD-fed mice, macrophages appear in islets before the onset of diabetes. These residential macrophages may belong to alternatively activated cells, and thus benefit islet function (273) and plasticity (274). But as the disease progresses, macrophages may become classically activated (275) and thus accelerate islet cell dysfunction and death. These activated macrophages become phagocytic to dead islet cells. Increased islet-derived inflammatory factors (IL-6, IL-8, and keratinocyte-derived cytokine) are produced and released by islets after exposure to T2DM (high glucose and/or nonesterified fatty acids) or HFD.
Spontaneously diabetic Torii (SDT) rats are used as a nonobese T2DM animal model. SDT rats develop hyperglycemia without obesity at 20 wk and manifest nephropathy and ocular complications, such as cataract and proliferative retinopathy (276). At 8–10 wk, these rats manifest microvascular abnormalities such as congestion and hemorrhage in pancreatic islets (277). Over time (4–10 wk), serum CD4+ and CD8+ T cells, monocytes, neutrophils, and NK cells increase consistently. At 9 wk, invasion of the pancreas by inflammatory cells leads to β-cell destruction (277). This inflammatory cell infiltration remains throughout the lifespan, along with high glucose levels in the blood (278). Blockade of macrophage accumulation and function could help prevent the development of T2DM and its complications.
Selective destruction of insulin-producing pancreatic cells via an immune-mediated process is characteristic of T1DM. Infiltration of inflammatory cells into the islets (insulitis) generally precedes β-cell destruction in human T1DM (278, 279). In nonobese diabetic mice (280) or multiple low-dose STZ-treated mice (281, 282), infiltrated cells cause β-cell damage or death either directly or via released cytokines or free radicals (281, 283). Macrophages from low-dose STZ-treated mice or after stimulation with LPS or Th1 cytokine express NO (284, 285), which mediates β-cell damage (285–289).
Macrophages can be depleted by systemic injection of liposomes containing clodronate (290). Islets from SDT rats show marked infiltration of CD68+ cells. Treatment with dichloromethylene diphosphonate-liposomes [a potent antimacrophage agent (291)] reduced islet inflammatory cell infiltration and serum concentrations of NO metabolites (NO2/NO3) in SDT rats and reduced macrophage infiltration into the pancreas (292). The PPAR-γ activator rosiglitazone, an insulin-sensitizing agent used in diabetic patients, reverses abnormal macrophage phenotypes with improved insulin signaling and decreased modified LDL binding in macrophages in ob/obLdlr−/− mice with T2DM (263). These observations suggest that promoting macrophage alternative activation (e.g., by activating PPAR-γ) (103, 104) or inhibiting classically activated macrophages can effectively control diabetes and associated tissue damage, at least in experimental models.
VIII. Mast Cell–Macrophage Interactions in Cardiovascular and Metabolic Diseases
The concept of MC functions in AAA and metabolic diseases is relatively new, compared with that in atherosclerosis. The direct participation of MC in AAA, obesity, and diabetes was established only recently (43, 180). In WAT, deficiency or pharmacological inactivation of MC inhibited macrophage accumulation (43), suggesting a role of MC in macrophage migration and accumulation to the site of inflammation, such as the arterial wall and WAT. These MC activities also may affect the recruitment of other inflammatory cells. None of these hypotheses, however, has been tested directly in vitro or in vivo. In atherosclerotic lesions, AAA lesions, and WAT, MC often locate anatomically together with macrophages (Fig. 4), suggesting their interaction either by direct cell–cell contact or via inflammatory mediators.
A. Macrophages activate mast cells
Atherosclerosis, AAA, obesity, and diabetes are all inflammatory diseases that share many pathological mechanisms with asthma and inflammatory lung diseases. Although scant evidence supports the activation of MC by macrophages in cardiovascular and metabolic diseases, macrophages seem to activate MC in airway allergic responses, and this may apply to cardiovascular and metabolic diseases. For example, human lung macrophages spontaneously secrete “macrophage factors” (Fig. 7A). By interacting with MC surface-bound IgE, these macrophage factors induce IgE-dependent histamine release (293) and possibly other inflammatory mediators from lung MC. As discussed, MC activations by C48/80 or substance P enhance AAA growth (180) or atherogenesis (53) in mice. Anti-IgE antibody-mediated or macrophage factor-mediated MC activation might yield the same results as from mice treated with C48/80 or substance P.
Figure 7.
MC–macrophage interaction. A, Macrophages release macrophage factors or endocytosed bacteria (e.g., C. pneumoniae) to activate MC. B, MC contribute to macrophage recruitment or release cytokines, growth factors, or enzymes to activate macrophages. C, MC release heparin proteoglycans or proteases to target apoB or nuclear receptor LXRα, thereby promoting macrophage LDL uptake and foam-cell formation. D, MC also release proteases to degrade the lipid transfer proteins CETP and PLTP, or to target directly HDL pre-β-migrating particles containing apoA-I, apoA-II, apoA-IV, and apoE, thereby inhibiting macrophage foam cell cholesterol efflux.
Macrophages also activate MC via exocytosed bacterium, such as Chlamydia pneumoniae and Aggregatibacter actinomycetemcomitans. A recent study demonstrated increased levels of C. pneumoniae antigens in human AAA lesions, throughout the tunica media to the adventitia (294), although serum levels of IgA or IgG against C. pneumoniae antigens were not significantly different between patients with or without AAA (295) and did not associate with AAA aortic expansion rate (296). DNA and antigens from C. pneumoniae have also been found in human atherosclerotic lesions (297). Infection of atherosclerosis-prone Apoe−/− mice with C. pneumoniae promotes atherogenesis (298). Blood levels of IgG against C. pneumoniae correlate with BMI and body fat percentage in young adults (299) and in women (300). Acute infection of obese C57BL/6 mice with this bacteria leads to insulin resistance and diabetes (301). Although the mechanisms by which C. pneumoniae contributes to AAA, atherosclerosis, obesity, and diabetes remain unknown, human monocyte-derived macrophages infected with C. pneumoniae induce proinflammatory responses in human peripheral blood-derived MC. MC cultured with C. pneumoniae-infected macrophages or conditioned media from these macrophages increased MC expression of IL-8 and TNF-α (302) (Fig. 7A). In Apoe−/− mice, infection with C. pneumoniae also increases MC activation in atherosclerotic lesions (302).
B. Mast cells activate macrophages
MC activate macrophages by releasing inflammatory cytokines, enzymes, growth factors, and heparin proteoglycans, although much of our knowledge about these processes was not generated from studies of cardiovascular or metabolic disease. In human synovial tissue explant culture, MC activation with rabbit antihuman IgE antibodies demonstrates much greater secretion of tissue histamine, TNF-α, and IL-1β, compared with those treated with nonspecific rabbit IgG. Immunostaining showed colocalization of increased cytokine expression to CD68+ macrophages (303), suggesting that MC directly act on macrophages and lead to macrophage cytokine expression. In a cell culture assay, conditioned media from the human leukemic MC line (HMC-1) increases MMP-9 activities from the human macrophage-like cell line U973, via MC-derived IFN-γ. Anti-IFN-γ antibody fully blocked such activities of HMC-1 conditioned media. MC stabilization with tranilast also abolishes this activity from HMC-1 conditioned media (181) (Fig. 7B).
MC also release phospholipase A2 (PLA2) to target macrophages. PLA2 is an enzyme that hydrolyzes the ester bond of phospholipids and generates free acids and lysophospholipids (304). Plasma levels of lipoprotein-associated PLA2 were significantly higher in patients with metabolic syndrome or carotid atherosclerosis than in control subjects (P < 0.05) and were especially higher in patients with three or four disorders of metabolic syndrome (P < 0.01) (305). Inhibition of lipoprotein-associated PLA2 with its inhibitor, darapladib—currently in phase-III trials for coronary heart disease by GlaxoSmithKline—reduces coronary atherosclerosis in diabetic and hypercholesterolemic pigs (306). MC, macrophages, and other blood-borne leukocytes express PLA2 (307, 308), but MC are a better source of PLA2 than are macrophages (309), whereas macrophages are a good target of PLA2. MC use PLA2 to activate macrophage expression of cytokines, chemokines, and MMP, cell adhesion and proliferation (308), and prostaglandin and leukotriene biosynthesis (309) (Fig. 7B).
MC granules contain chymase and latent TGF-β1; after activation, MC release these granules and the latent TGF-β1. After conversion into active TGF-β1 by chymase, TGF-β1 binds to type I and type II TGF-β serine/threonine kinase receptor TβRI and TβRII on macrophages, thereby inducing macrophage expression of plasminogen activator inhibitor type-1 (310). Selective stimulation of MC in vivo, by injecting 100 μg C48/80 in 1 ml of 1× PBS in rat peritoneal cavity, induces TGF-β1 production from peritoneal MC and TβRs and plasminogen activator inhibitor type-1 expression in peritoneal macrophages (310). All of these macrophage activities are essential to cardiovascular and metabolic diseases. MC in the arterial wall or in WAT also may use cytokines (e.g., IFN-γ), enzymes (e.g., PLA2), and growth factors (e.g., TGF-β1) to activate macrophages (Fig. 7B).
Foam-cell formation is one of the most important macrophage functions in atherogenesis. Intimal macrophages take up cholesterol when they ingest modified LDL, whereas HDL removes cholesterol from macrophages and carries it back to the circulation (311)—a process called reverse cholesterol transport or cholesterol efflux—which counteracts cholesterol accumulation and retards atherosclerosis (312). Cholesterol efflux via ATP-binding cassette (ABC) transporter ABCA1 requires direct or indirect binding of apoA-I or apoA-II (313, 314), and HDL phospholipids modulate the release of cellular cholesterol via ABC subfamily G member 1 (ABCG1) (315). MC contribute to macrophage foam-cell formation through at least two mechanisms—increasing extracellular LDL uptake, and decreasing intracellular cholesterol efflux—both of which require heparin proteoglycans and MC-specific proteases (chymase, tryptase, and carboxypeptidase A).
C. Role of mast cells in macrophage LDL uptake
After activation, MC degranulate and release heparin proteoglycan-containing granules. Soluble heparin proteoglycans interact with the apoB on LDL and form insoluble complexes with LDL (316) (Fig. 7C). Macrophages take up these complexes using the scavenger receptor of the acetyl-LDL receptor type. Adding heparin proteoglycans to cultured macrophages therefore increases LDL uptake by 45-fold (317, 318). When passively sensitized MC and macrophages from nonimmunized rat peritoneum were mixed together with IgE-containing serum, IgE-mediated MC activation increased macrophage [14C]sucrose-LDL uptake and LDL-derived cholesterol ester accumulation. MC inhibitor MY-1250, however, reduces MC histamine release and macrophage LDL uptake and esterification (76). Furthermore, passively sensitized and antigen-stimulated MC stimulate macrophage uptake and esterification of LDL in LDL-containing culture medium. The MC stabilizer cromolyn effectively blocks these MC activities. Mechanistically, both MY1250 and cromolyn prevent MC release of heparin proteoglycan (76).
MC also release chymase, carboxypeptidase A, and tryptase, which are tightly bound to the heparin proteoglycans of the granule remnants and degrade apoB-100 of the granule remnant-bound LDL (Fig. 7C). Although proteolytically inactive MC granules increase macrophage LDL uptake 10-fold, proteolytically active granules increase macrophage LDL uptake 50-fold (316)—suggesting an important role of MC-derived heparin proteoglycans and proteases in macrophage LDL uptake. Pharmacological prevention of MC degranulation reduces macrophage LDL uptake, whereas MC activation increases LDL uptake. When rats were immunized with ovalbumin (OVA) and adjuvant to provoke IgE and activate MC, coculture of peritoneal MC and macrophages from these rats showed OVA dose-dependent MC granule release and macrophage LDL uptake. In contrast, when only macrophages or both macrophages and MC from nonimmunized mice were used, antigen OVA had no effect on macrophage LDL uptake or MC granule release (319). In male Wistar rats receiving ip injection of [3H]cholesteryl linoleated-acetyl-LDL, intracellular radioactivity in peritoneal macrophages significantly increased after rats received MC activator C48/80 (72). These observations established an important role of MC activation and release of heparin proteoglycans and proteases (e.g., chymase and carboxypeptidase A) in macrophage LDL uptake and foam-cell formation.
Although whether tryptase contributes to macrophage LDL uptake during atherogenesis has not been tested, the addition of tryptase into THP-1-derived macrophages also induces intracellular lipid accumulation and total cholesterol level. Mechanistic studies suggest that tryptase suppresses the activation of lipid homeostasis essential nuclear receptor liver X receptor-α (LXRα) (320) (Fig. 7C), a process that can be blocked by tryptase inhibitor APC366 (321).
D. Role of mast cells in macrophage cholesterol efflux
Inhibition of macrophage cholesterol efflux is probably the best-studied mechanism of MC in controlling macrophage foam-cell formation. MC inhibit cholesterol efflux by releasing chymase and tryptase to target different lipid transfer proteins or directly to HDL components.
Two plasma lipid protein transfer proteins—phospholipid transfer protein (PLTP) and cholesteryl ester transfer protein (CETP)—remodel HDL and generate preβ-HDL particles (322) to promote cholesterol efflux from macrophage foam cells via ABCA1 and ABCG1 (323). CETP often forms a complex with HDL3 in the plasma (324), and it transfers cholesteryl esters, triglycerides, and phospholipids between circulating lipoproteins (325). MC chymase degrades CETP and reduces its cholesteryl ester transfer activities, although this cleavage is reduced when CETP is complexed with HDL3 (326). PLTP transfers phospholipids from liposome donor particles to acceptor HDL3 particles and facilitates pre-β-HDL formation. Chymase degrades 80-kDa PLTP into 70-, 52-, 48-, and 31-kDa fragments, thereby reducing PLTP pre-β-HDL formatting activity and inhibiting the high-affinity component of cholesterol efflux from macrophages (327) (Fig. 7D).
In addition to degrading these lipoprotein transfer proteins, MC chymase and tryptase also target HDL pre-β-migrating particles directly. In murine macrophage J774 cells, which express low levels of scavenger receptor-B1 and high levels of ABCA1, chymase degrades pre-β-migrating particles from HDL3 and reduces ABCA1-dependent cholesterol efflux (328). Specifically, chymase degrades the lipoprotein apoA (LpA) components in HDL3, and even those in the plasma. Adding proteolytically active MC granule remnants or purified human skin chymase to cholesterol-loaded human or mouse macrophages leads to degradation of pre-β1 LpA-I and LpA-IV, and reduction of apoA-I-induced high-affinity cholesterol efflux (329). Although plasma from WT mice or apoA-I knockout mice, which did not express apoA-IV but expressed normal levels of apoA-II and even higher levels of apoE, are equally potent in inducing foam-cell cholesterol efflux, activities in both plasmas were reduced after being treated with chymase. Addition of discoidal pre-β-migrating reconstituted HDL particles containing human apoA-I or apoA-II to chymase-treated apoA-I knockout plasma fully restored these activities (330), suggesting that both apoA-I and apoA-II are targets of MC chymase (Fig. 7D). Human serum and human aortic intimal fluid also showed defective cholesterol efflux from cultured macrophage foam cells, after these physiological fluids were treated with MC remnant chymase (331). Biochemical analysis demonstrated that chymase degrades apoA-I from these fluids (331). These MC chymase activities have been confirmed in mice. In C57BL/6 mice, ip administration of human apoA-I or chymase-treated apoA-I, together with [3H]cholesterol-labeled J774 macrophages, demonstrated that apoA-I—but not chymase-treated apoA-I—accelerated transfer of macrophage-derived [3H]cholesterol into the intestinal lumen. Stimulation of such reverse cholesterol transport by human apoA-I was blocked fully when C48/80 was also injected into the WT mice, but this effect was not seen in MC-deficient KitW-sh/W-sh mice (332). In a similar experiment, serum or peritoneal fluid from mice receiving ip injection of C48/80 showed reduced promotion of efficient cholesterol efflux from cultured mouse macrophages, due to degradation of serum or peritoneal cavity apoA-I, apoA-IV, and apoE in α-HDL and pre-β-HDL (242) (Fig. 7D).
MC tryptase acts similarly to chymase in degrading HDL. Incubation of purified HDL3 from human plasma with recombinant human tryptase demonstrated degradation of pre-β-HDL—preferentially the apoA-I and apoA-IV minor subfraction—thereby reducing cholesterol efflux in [3H]cholesterol-loaded macrophage foam cells. Proteoglycans isolated from human aortas enhance HDL3 degradation by tryptases (333). Tryptase in the arterial wall therefore may use matrix proteoglycans to degrade HDL3 and inhibit macrophage intracellular cholesterol efflux (Fig. 7D)—a hypothesis that merits further investigation. Figure 7 summarizes our current understanding of the interactions between MC and macrophages discussed in this Section, although we still lack direct evidence of most of these interactions in cardiovascular diseases or metabolic disorders.
IX. Clinical Implications
A. Mast cells as a therapeutic target
Although no allergy drugs have been used to treat patients with cardiovascular diseases or metabolic syndromes, these drugs—including antihistamines, chymase or tryptase inhibitors, and MC stabilizers—have a long history of application among patients with allergies and related medical conditions. Omalizumab, a Food and Drug Administration-approved humanized monoclonal antibody, binds to free IgE for the treatment of allergic diseases (334). R112 is the first inhibitor of the FcεR1 signaling molecule Syk used in clinical studies for allergies (335). The tryptase inhibitor APC2095 recently has been tested in the treatment of asthma and ulcerative colitis (336). Several oral chymase inhibitors (SUN-C8257, BCEAB, NK3201, and TEI-E548) are also available (337). The heparin antagonist protamine (used along with insulin to slow insulin action in diabetic patients) and Polybrene are also used to inhibit tryptase activity through dissociation of active tetrameric tryptase into inactive monomers (338). Many of these chymase inhibitors and tryptase inhibitors have been tested in the inhibition of AAA formation in animals.
MC stabilizers are probably the best-studied antiallergy drugs in vascular diseases and metabolic disorders, and many are used to treat patients with allergies. Drugs in this category include cromolyn (Gastrocrom), ketotifen (Zaditor), and tranilast (Rizaben). Cromolyn and ketotifen are used in pediatric allergic disorders. Tranilast is used in bronchial asthma, atopic dermatitis, and allergic conjunctivitis and has recently shown antiangiogenic activity via inhibition of chymase and TGF-β (248). Cromolyn (339) exerts its effect only on certain populations of MC, with limited effect on intestinal mucosal MC in rodents (340) and humans (341). These effects are particularly important because cromolyn may be used to inhibit MC in the connective tissues—such as those in the vasculature and WAT—without affecting the innate immunity of the mucosa against pathogens. In addition to its antiallergy effect, ketotifen can control chronic inflammation processes such as autoimmune encephalomyelitis and arthritis (342), if given sufficient time points.
Recent studies in animals suggest that MC stabilizers potentially can benefit patients with cardiovascular and metabolic diseases. As discussed, cromolyn prevented MC activation-induced intraplaque hemorrhage, macrophage apoptosis, and vascular leakage in atherosclerosis in Apoe−/− mice (68). We have used cromolyn to prevent aortic elastase-induced mouse AAA formation (180). Tranilast prevents periaortic CaCl2-induced AAA in mice (181). Our most recent data indicate that cromolyn and ketotifen are fully effective in preventing diet-induced obesity and diabetes in mice. Furthermore, we demonstrated that these MC stabilizers reversed preestablished obesity and diabetes (43); this result is particularly important for future clinical application. More comprehensive human trials are required to establish the applications of these MC drugs in patients.
B. Macrophages as a therapeutic target
The recognition of macrophages in adipose tissue and the study of their roles in metabolic diseases are relatively new concepts (194, 219) compared with those in cardiovascular diseases. Macrophages as a therapeutic target in vascular diseases recently have been reviewed (343). Animal models and human trials targeting macrophages focus on macrophage recruitment, cholesterol metabolism, cholesterol trafficking, inflammation, and ECM remodeling.
Macrophage recruitment is central to both metabolic and cardiovascular diseases. Vascular adhesion molecules, including ICAM-1, VCAM-1, P-selectin, and E-selectin, play key roles in macrophage adhesion and transmigration. Direct inhibition of these molecules or the enzymes responsible for their synthesis may effectively block macrophage accumulation in adipose tissues or in inflamed arterial walls. For example, VCAM-1 inhibitor succinobucol AGI-1067 (344) has been developed to block macrophage accumulation to the arterial wall for patients with acute coronary syndrome. The VCAM-1 inhibitor HUN-7293 (344) and the VCAM-1 synthase inhibitor CAM741 (345) also have been developed for clinical trials. Macrophage inflammation is another important target for drug development, at least in the cardiovascular system. Cyclooxygenase 1 inhibitors, such as aspirin, have been used to prevent atherothrombosis (346). The thromboxane prostanoid receptor antagonist S18886 (also called terutroban) may benefit atheroma patients (347), and the 5-lipoxygenase-activating protein inhibitors MK-886 and BAYx1005 reduce atherosclerosis in animal models (348, 349). Licofelone inhibits both 5-lipoxygenase and cyclooxygenase 2 and has antiplatelet activity (350). Macrophage-associated ECM remodeling had been focused on broad-spectrum MMP inhibitors to prevent atherosclerotic plaque expansion, but it was largely unsuccessful (351). More recent development has focused on cysteinyl cathepsin inhibitors. Although formal clinical trials targeting cardiovascular or metabolic diseases have not been announced, selective inhibitors for cathepsins S and K have been tested in clinical trials of patients with rheumatoid arthritis, psoriasis, and osteoporosis (352, 353). Such inhibitors may benefit patients with metabolic (202) and cardiovascular diseases (85).
Macrophage-associated cholesterol metabolism and trafficking are probably the main focus of drug development to target macrophages in cardiovascular diseases. LDL uptake by macrophages plays a central role in atherosclerosis. After uptake, LDL undergoes hydrolysis into fatty acids and free cholesterol that is further esterified into cholesterol esters by ACAT (acyl coenzyme A:cholesterol acyltransferase). The ACAT inhibitors eflucimibe (354), pactimibe, avasimibe (355), and K-604 (356) have been entered into atherosclerosis clinical trials at various stages of development. Cholesterol trafficking or efflux from macrophages has been an important area in both basic research and clinical prevention of human atherosclerosis. The LXR agonist TO-901317 prevents atherosclerosis by facilitating cholesterol efflux from macrophages, although it also increases hepatic and serum triglyceride levels (357). In the setting of clinical applications, the main drug discoveries in reducing circulatory LDL levels and lesion inflammation are the development of HDL mimicries, statin-series drugs, and PPAR activators.
HDL is the smallest lipoprotein particle that transports cholesterol to the liver and therefore is essential in removing cholesterol from macrophage foam cells, thereby playing a classically protective role toward atherosclerosis (358). The most abundant components in human HDL are apoA-I and apoA-II (359). Although pharmacological therapies such as fibrate and niacin increase HDL cholesterol levels (360, 361), fibrate does not impair overall mortality in atherosclerotic patients (360). Only niacin reduces atherosclerosis progression and cardiovascular events (362). Therefore, several classes of recombinant HDL (rHDL)—composed of apoA-I, lectin, and cholesterol—have effectively reduced lipid content in cultured vascular SMC (363) and atheroma progression or fatty streak content of established atherosclerotic lesions in rabbits (364, 365), and therefore have been developed for human trials. rHDL used in clinical trials include WT-apoA-I, apoA-I Milano, delipidated HDL, and apoA-I mimetic peptides. Wild-type apoA-I-containing rHDL formulations reduce restenosis after aortic injury, inhibit EC injury, diminish leukocyte infiltration, and promote endothelial progenitor cell-mediated endothelial repair in animal models. In human trials, rHDL CSL-111 (using human-derived apoA-I complexed with phosphatidylcholine) at a low-dose treatment (40 mg/kg) for 4 wk yielded significant improvement in coronary score (366). One dose of 80 mg/kg of CSL-111 led to a 63% reduction in atheroma lipid content, a 58% reduction in macrophage foam cell size, and a 43% reduction in intraatheroma VCAM expression (367). In T2DM patients, one dose of CSL-111 reduced EC expression of VCAM and ICAM and improved cholesterol efflux capacity by 35% (368). ApoA-I Milano-containing rHDL reduced lipid and macrophage content in atherosclerotic lesions and total atheroma volume in animal models (369–371). Two high doses (150 mg/kg) or 75 mg/kg at 4-wk intervals in rabbits demonstrated atherosclerosis regression, reduced lesion macrophage density, MCP-1 and cyclooxygenase-2 expression, and gelatinolytic activity (372, 373). In human trials, apoA-I Milano complexed with phospholipids (ETC-216) in a small cohort of patients with acute coronary syndrome, five weekly infusions at 15 mg/kg or 45 mg/kg reduced atheroma volume by 4.2% as determined by intravascular ultrasound (374). Delipidated endogenous HDL, in a small phase I/II trial, caused 28-fold increase of pre-β-HDL and statistically nonsignificant regression in coronary plaques, but plaque volume was reduced as in the apoA-I Milano trial (375). ApoA-I mimetic peptides were developed to avoid HDL source and price problems. In rabbits, apoA-I mimetic peptide 5A (20 mg/kg) reduced vascular inflammation and macrophage cholesterol efflux, EC ICAM and VCAM expression, neutrophil infiltration, and ROS enzyme production (376). Novartis APP018 peptide, although with low oral bioavailability, improved antiinflammatory activity and reduced aortic atherosclerosis in mice (377). In a phase I trial of CAD patients or those with coronary heart disease risk, APP018 treatment reduced inflammatory index of HDL (378). A clinical trial of another apoE-derived peptide, AT1–5261, has begun recently (Artery Therapeutics, Danville, CA) (379).
Statin treatment of atherosclerotic cardiovascular diseases reduced cardiovascular events by 20-40%, depending on the levels of LDL reduction, ABC transporters ABCA1 and ABCG1 in reducing macrophage foam-cell formation, and inflammation (380). For example, atorvastatin (10–80 mg/d) reduced total plasma cholesterol and LDL levels and decreased atherosclerotic lesion macrophage contents among hypercholesterolemic patients (381). Simvastatin (10 mg/d) and pitavastatin (2 mg/d) reduced plasma total cholesterol and LDL levels (382), and simvastatin demonstrated atherosclerotic plaque regression activity in human patients (383). In hypercholesterolemic patients, combined statin therapy (20 mg/d simvastatin, 40 mg/d pravastatin, or 10–20 mg/d atorvastatin) significantly reduced atheroma size, lesion contents of fibrous tissues, macrophage contents, neoangiogenesis, and hemorrhage, compared with an untreated hypercholesterolemic group (384). Similar observations were made in AAA patients. Atorvastatin (20 mg/d for 4 wk) reduced serum LDL by 40% and also reduced lesion JNK expression, dendritic cell and macrophage contents, and MMP expression (385).
Other nonstatin drugs and PPAR activators are also widely used in the clinic to lower plasma cholesterol levels. Ezetimibe, a potent cholesterol absorption inhibitor, reduces plasma cholesterol and LDL by approximately 50% (386, 387) and currently is in clinical trials (388, 389). PPAR activation (PPAR-α, PPAR-β, PPAR-δ) not only reduces macrophage recruitment by decreasing EC chemokine (MCP-1) and adhesion molecule (ICAM and VCAM) expression (390–392), but it also preferentially lowers lipid uptake and storage in macrophages. In patients with metabolic syndrome and elevated fasting triglyceride levels, 3 months of treatment with the PPAR-α agonist fenofibrate reduced blood monohydroxy fatty acids, MCP-1, MIP-1α, IL-1β, very LDL, and LDL levels, compared with placebo (393). PPAR-β/δ activation by the synthetic agonist GW50615 has been shown to promote apoA-I-specific cholesterol efflux and to induce ABCA1 gene expression in THP-1 macrophages, suggesting an enhancement of reverse cholesterol transport (394). PPAR-α and PPAR-γ activators enhance HDL receptor CLA-1/SR-BI expression (395). They also indirectly enhance ABCA1 transporter expression via up-regulating LXR-α expression, thereby promoting apoA-I-mediated cholesterol efflux from macrophages (396, 397). Expression of ABCG1 is also induced by PPAR-γ activation but is independent of LXR-α (398). As a consequence, PPAR-α and PPAR-γ clearly enhance the apoA-I-dependent and HDL-dependent cholesterol efflux in macrophages (396, 398, 399). PPAR-γ is the major actor in adipocyte differentiation, inducing lipid uptake and storage (400). PPAR-γ inhibits monocyte CCR2 expression, blocks MCP-1-mediated chemotaxis (401, 402), and enhances the alternative activation and differentiation of macrophages (97, 103). The PPAR-γ activators rosiglitazone and pioglitazone prevent Ang-II-induced vascular inflammation in rats (403). PPAR-α and PPAR-γ agonists in cardiovascular outcome trials have been summarized elsewhere (404).
In addition to these direct effects of PPAR activators or agonists, statins and angiotensin-converting enzyme (ACE) inhibitors also may affect atherosclerosis indirectly via PPAR activation. For example, simvastatin affects CCR2 gene expression and CCR2-dependent monocyte recruitment in response to MCP-1 in healthy men by acting in a PPAR-γ-dependent manner (405). Statins also activate PPAR-α and enhance its transrepression activity by inhibiting the PKC signaling pathway in mice (406). ACE inhibitors decrease atherosclerosis via a PPAR-independent pathway (407), but the ACE inhibitor enalapril up-regulates PPAR-α and PPAR-γ in mouse aortas and prevents Ang-II-induced expression of adhesion molecules and chemokines (408).
X. Conclusions
Recent evidence from studies in humans and animals demonstrates the important roles of MC and macrophages in cardiovascular and metabolic diseases. Through different mechanisms, MC and macrophages release inflammatory cytokines, chemokines, proteases, and other proinflammatory mediators to affect neighboring vascular cells (e.g., SMC and EC) and inflammatory cells (e.g., different types of T cells). Deficiency of inflammatory cytokines from MC demonstrated inhibitory effects in the pathogenesis of atherosclerosis, AAA, obesity, and diabetes. These include MC proinflammatory cytokines IL-6, IFN-γ, but not MC TNF-α (42, 43, 180). Whether similar inhibitory phenotypes may develop in cytokine-deficient macrophages remains untested. MC and macrophages are both rich in proteases. MC contain their unique chymases and tryptases that contribute to AAA formation by promoting inflammatory cell accumulation, neovascularization, and/or SMC apoptosis (81, 182). These MC proteases may apply similar mechanisms to atherosclerosis, obesity, and diabetes; this hypothesis merits further investigation. MC and macrophages both produce MMP and cysteinyl cathepsins (84, 106, 145, 409), which play essential roles in cardiovascular and metabolic diseases. Accumulation of MC and macrophages in inflamed arteries or WAT may associate with enhanced expression of MMP and cysteinyl cathepsins. Inhibition or genetic deficiency of these proteases has been proven effective in controlling cardiovascular and metabolic diseases (60, 145, 202, 203, 222, 229, 235). MC and macrophages are also APC that activate T cells in the aortic arteries and WAT, or in other endocrine organs, providing additional mechanisms to the pathogenesis of atherosclerosis, AAA, obesity, and diabetes. Although MC and macrophages may contribute to these human diseases through different mechanisms—for example, macrophages form foam cells and present in both antiinflammatory and proinflammatory subsets—the two cell types share many common activities during the pathogenesis of these cardiovascular and metabolic diseases. They may be equally important in the pathogenesis and as therapeutic targets.
Acknowledgments
We thank Dr. Galina K. Sukhova for assistance with the immunohistological analysis of human atherosclerotic and AAA lesions and murine adipose tissues; Dr. Robert W. Thompson of Washington University in St. Louis for providing the ruptured human AAA lesion sections; and Ms. Sara Karwacki for editorial assistance.
Studies cited in this article are supported by National Institutes of Health Grants HL60942, HL81090, and HL88547 (to G.-P.S.); and by an Established Investigator Award (0840118N) from the American Heart Association (to G.-P.S.).
Disclosure Summary: The authors disclose no conflict of interest.
Footnotes
- AAA
- Abdominal aortic aneurysm
- ABC
- ATP-binding cassette
- ACE
- angiotensin-converting enzyme
- Ang-II
- angiotensin-II
- APC
- antigen-presenting cell
- apo
- apolipoprotein
- BAT
- brown adipose tissue
- BMI
- body mass index
- BMMC
- bone marrow-derived mouse MC
- CAD
- coronary artery disease
- CETP
- cholesteryl ester transfer protein
- DIO
- diet-induced obese
- EC
- endothelial cell
- ECM
- extracellular matrix
- Glut
- glucose transporter
- HDL
- high-density lipoprotein
- HFD
- high-fat diet
- HMC-1
- human leukemic MC
- ICAM
- intercellular adhesion molecule
- IFN
- interferon
- LDL
- low-density lipoprotein
- LpA
- lipoprotein apoA
- LPS
- lipopolysaccharide
- LXRα
- liver X receptor-α
- MC
- mast cell
- MCP-1
- monocyte chemoattractant protein-1
- MHC
- major histocompatibility complex
- MI
- myocardial infarction
- MIP
- macrophage inflammatory protein
- mMCP
- mouse MC protease
- MMP
- matrix metalloproteinase
- NO
- nitric oxide
- OVA
- ovalbumin
- OX40L
- OX40 ligand
- oxLDL
- oxidized LDL
- PAO
- peripheral artery occlusion
- PGN
- peptidoglycan
- PLA2
- phospholipase A2
- PLTP
- phospholipid transfer protein
- PPAR-γ
- peroxisome proliferator-activated receptor-γ
- rHDL
- recombinant HDL
- ROS
- reactive oxygen species
- SCF
- stem cell factor
- SDT
- spontaneously diabetic Torii (rats)
- SMC
- smooth-muscle cell
- STZ
- streptozocin
- SVF
- stromal-vascular fraction
- T1DM
- type 1 diabetes mellitus
- T2DM
- type 2 diabetes mellitus
- Th
- T helper
- TLR
- Toll-like receptor
- Treg
- regulatory T cells
- VCAM
- vascular cell adhesion molecule
- VEGF
- vascular endothelial growth factor
- WAT
- white adipose tissue
- WT
- wild-type.
References
- 1. Okayama Y, Kawakami T. 2006. Development, migration, and survival of mast cells. Immunol Res 34:97–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. 2005. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol 23:749–786 [DOI] [PubMed] [Google Scholar]
- 3. Hallgren J, Jones TG, Abonia JP, Xing W, Humbles A, Austen KF, Gurish MF. 2007. Pulmonary CXCR2 regulates VCAM-1 and antigen-induced recruitment of mast cell progenitors. Proc Natl Acad Sci USA 104:20478–20483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haley KJ, Lilly CM, Yang JH, Feng Y, Kennedy SP, Turi TG, Thompson JF, Sukhova GH, Libby P, Lee RT. 2000. Overexpression of eotaxin and the CCR3 receptor in human atherosclerosis: using genomic technology to identify a potential novel pathway of vascular inflammation. Circulation 102:2185–2189 [DOI] [PubMed] [Google Scholar]
- 5. Irani AA, Schechter NM, Craig SS, DeBlois G, Schwartz LB. 1986. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci USA 83:4464–4468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kitamura Y. 1989. Heterogeneity of mast cells and phenotypic change between subpopulations. Annu Rev Immunol 7:59–76 [DOI] [PubMed] [Google Scholar]
- 7. Rocha VZ, Libby P. 2009. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol 6:399–409 [DOI] [PubMed] [Google Scholar]
- 8. Galli SJ, Maurer M, Lantz CS. 1999. Mast cells as sentinels of innate immunity. Curr Opin Immunol 11:53–59 [DOI] [PubMed] [Google Scholar]
- 9. Marshall JS. 2004. Mast-cell responses to pathogens. Nat Rev Immunol 4:787–799 [DOI] [PubMed] [Google Scholar]
- 10. Galli SJ, Nakae S, Tsai M. 2005. Mast cells in the development of adaptive immune responses. Nat Immunol 6:135–142 [DOI] [PubMed] [Google Scholar]
- 11. Grabbe J, Karau L, Welker P, Ziegler A, Henz BM. 1997. Induction of MHC class II antigen expression on human HMC-1 mast cells. J Dermatol Sci 16:67–73 [DOI] [PubMed] [Google Scholar]
- 12. Nigo YI, Yamashita M, Hirahara K, Shinnakasu R, Inami M, Kimura M, Hasegawa A, Kohno Y, Nakayama T. 2006. Regulation of allergic airway inflammation through Toll-like receptor 4-mediated modification of mast cell function. Proc Natl Acad Sci USA 103:2286–2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stelekati E, Orinska Z, Bulfone-Paus S. 2007. Mast cells in allergy: innate instructors of adaptive responses. Immunobiology 212:505–519 [DOI] [PubMed] [Google Scholar]
- 14. Nakae S, Suto H, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. 2005. Mast cells enhance T cell activation: importance of mast cell-derived TNF. Proc Natl Acad Sci USA 102:6467–6472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Galli SJ, Grimbaldeston M, Tsai M. 2008. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol 8:478–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abraham SN, St John AL. 2010. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol 10:440–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lundequist A, Pejler G. 2011. Biological implications of preformed mast cell mediators. Cell Mol Life Sci 68:965–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Raphael GD, Metcalfe DD. 1986. Mediators of airway inflammation. Eur J Respir Dis Suppl 147:44–56 [PubMed] [Google Scholar]
- 19. Egozi EI, Ferreira AM, Burns AL, Gamelli RL, Dipietro LA. 2003. Mast cells modulate the inflammatory but not the proliferative response in healing wounds. Wound Repair Regen 11:46–54 [DOI] [PubMed] [Google Scholar]
- 20. Blank U, Ra C, Miller L, White K, Metzger H, Kinet JP. 1989. Complete structure and expression in transfected cells of high affinity IgE receptor. Nature 337:187–189 [DOI] [PubMed] [Google Scholar]
- 21. Mathias CB, Freyschmidt EJ, Caplan B, Jones T, Poddighe D, Xing W, Harrison KL, Gurish MF, Oettgen HC. 2009. IgE influences the number and function of mature mast cells, but not progenitor recruitment in allergic pulmonary inflammation. J Immunol 182:2416–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nieuwenhuizen N, Herbert DR, Lopata AL, Brombacher F. 2007. CD4+ T cell-specific deletion of IL-4 receptor α prevents ovalbumin-induced anaphylaxis by an IFN-γ-dependent mechanism. J Immunol 179:2758–2765 [DOI] [PubMed] [Google Scholar]
- 23. Lappalainen J, Lindstedt KA, Oksjoki R, Kovanen PT. 2011. OxLDL-IgG immune complexes induce expression and secretion of proatherogenic cytokines by cultured human mast cells. Atherosclerosis 214:357–363 [DOI] [PubMed] [Google Scholar]
- 24. Hines C. 2002. The diverse effects of mast cell mediators. Clin Rev Allergy Immunol 22:149–160 [DOI] [PubMed] [Google Scholar]
- 25. Okayama Y, Kirshenbaum AS, Metcalfe DD. 2000. Expression of a functional high-affinity IgG receptor, Fc γ RI, on human mast cells: up-regulation by IFN-γ. J Immunol 164:4332–4339 [DOI] [PubMed] [Google Scholar]
- 26. Katz HR, Lobell RB. 1995. Expression and function of Fc γ R in mouse mast cells. Int Arch Allergy Immunol 107:76–78 [DOI] [PubMed] [Google Scholar]
- 27. Kitani S, Ito K, Miyamoto T. 1985. IgG, IgA, and IgM antibodies to mite in sera and sputa from asthmatic patients. Ann Allergy 55:612–620 [PubMed] [Google Scholar]
- 28. Kovanen PT, Mänttäri M, Palosuo T, Manninen V, Aho K. 1998. Prediction of myocardial infarction in dyslipidemic men by elevated levels of immunoglobulin classes A, E, and G, but not M. Arch Intern Med 158:1434–1439 [DOI] [PubMed] [Google Scholar]
- 29. Bischoff SC, Dahinden CA. 1992. c-kit Ligand: a unique potentiator of mediator release by human lung mast cells. J Exp Med 175:237–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tsai M, Takeishi T, Thompson H, Langley KE, Zsebo KM, Metcalfe DD, Geissler EN, Galli SJ. 1991. Induction of mast cell proliferation, maturation, and heparin synthesis by the rat c-kit ligand, stem cell factor. Proc Natl Acad Sci USA 88:6382–6386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hundley TR, Gilfillan AM, Tkaczyk C, Andrade MV, Metcalfe DD, Beaven MA. 2004. Kit and FcεRI mediate unique and convergent signals for release of inflammatory mediators from human mast cells. Blood 104:2410–2417 [DOI] [PubMed] [Google Scholar]
- 32. Hartmann K, Henz BM, Krüger-Krasagakes S, Köhl J, Burger R, Guhl S, Haase I, Lippert U, Zuberbier T. 1997. C3a and C5a stimulate chemotaxis of human mast cells. Blood 89:2863–2870 [PubMed] [Google Scholar]
- 33. Kildsgaard J, Hollmann TJ, Matthews KW, Bian K, Murad F, Wetsel RA. 2000. Cutting edge: targeted disruption of the C3a receptor gene demonstrates a novel protective anti-inflammatory role for C3a in endotoxin-shock. J Immunol 165:5406–5409 [DOI] [PubMed] [Google Scholar]
- 34. Venkatesha RT, Berla Thangam E, Zaidi AK, Ali H. 2005. Distinct regulation of C3a-induced MCP-1/CCL2 and RANTES/CCL5 production in human mast cells by extracellular signal regulated kinase and PI3 kinase. Mol Immunol 42:581–587 [DOI] [PubMed] [Google Scholar]
- 35. Humbles AA, Lu B, Nilsson CA, Lilly C, Israel E, Fujiwara Y, Gerard NP, Gerard C. 2000. A role for the C3a anaphylatoxin receptor in the effector phase of asthma. Nature 406:998–1001 [DOI] [PubMed] [Google Scholar]
- 36. Baelder R, Fuchs B, Bautsch W, Zwirner J, Köhl J, Hoymann HG, Glaab T, Erpenbeck V, Krug N, Braun A. 2005. Pharmacological targeting of anaphylatoxin receptors during the effector phase of allergic asthma suppresses airway hyperresponsiveness and airway inflammation. J Immunol 174:783–789 [DOI] [PubMed] [Google Scholar]
- 37. Taube C, Rha YH, Takeda K, Park JW, Joetham A, Balhorn A, Dakhama A, Giclas PC, Holers VM, Gelfand EW. 2003. Inhibition of complement activation decreases airway inflammation and hyperresponsiveness. Am J Respir Crit Care Med 168:1333–1341 [DOI] [PubMed] [Google Scholar]
- 38. Laine P, Pentikäinen MO, Würzner R, Penttilä A, Paavonen T, Meri S, Kovanen PT. 2002. Evidence for complement activation in ruptured coronary plaques in acute myocardial infarction. Am J Cardiol 90:404–408 [DOI] [PubMed] [Google Scholar]
- 39. Supajatura V, Ushio H, Nakao A, Okumura K, Ra C, Ogawa H. 2001. Protective roles of mast cells against enterobacterial infection are mediated by Toll-like receptor 4. J Immunol 167:2250–2256 [DOI] [PubMed] [Google Scholar]
- 40. Bachelet I, Levi-Schaffer F. 2007. Mast cells as effector cells: a co-stimulating question. Trends Immunol 28:360–365 [DOI] [PubMed] [Google Scholar]
- 41. Qiao H, Andrade MV, Lisboa FA, Morgan K, Beaven MA. 2006. FcεR1 and toll-like receptors mediate synergistic signals to markedly augment production of inflammatory cytokines in murine mast cells. Blood 107:610–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sun J, Sukhova GK, Wolters PJ, Yang M, Kitamoto S, Libby P, MacFarlane LA, Mallen-St Clair J, Shi GP. 2007. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med 13:719–724 [DOI] [PubMed] [Google Scholar]
- 43. Liu J, Divoux A, Sun J, Zhang J, Clément K, Glickman JN, Sukhova GK, Wolters PJ, Du J, Gorgun CZ, Doria A, Libby P, Blumberg RS, Kahn BB, Hotamisligil GS, Shi GP. 2009. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med 15:940–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kandere-Grzybowska K, Letourneau R, Kempuraj D, Donelan J, Poplawski S, Boucher W, Athanassiou A, Theoharides TC. 2003. IL-1 induces vesicular secretion of IL-6 without degranulation from human mast cells. J Immunol 171:4830–4836 [DOI] [PubMed] [Google Scholar]
- 45. Fischer M, Harvima IT, Carvalho RF, Möller C, Naukkarinen A, Enblad G, Nilsson G. 2006. Mast cell CD30 ligand is upregulated in cutaneous inflammation and mediates degranulation-independent chemokine secretion. J Clin Invest 116:2748–2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Donelan J, Boucher W, Papadopoulou N, Lytinas M, Papaliodis D, Dobner P, Theoharides TC. 2006. Corticotropin-releasing hormone induces skin vascular permeability through a neurotensin-dependent process. Proc Natl Acad Sci USA 103:7759–7764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Okayama Y, Ono Y, Nakazawa T, Church MK, Mori M. 1998. Human skin mast cells produce TNF-α by substance P. Int Arch Allergy Immunol 117(Suppl 1):48–51 [DOI] [PubMed] [Google Scholar]
- 48. Iwamoto I, Tomoe S, Tomioka H, Yoshida S. 1992. Substance P-induced granulocyte infiltration in mouse skin: the mast cell-dependent granulocyte infiltration by the N-terminal peptide is enhanced by the activation of vascular endothelial cells by the C-terminal peptide. Clin Exp Immunol 87:203–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kulka M, Tancowny BP, Schleimer RP. 2009. Substance P modulates Toll-like receptor-mediated activation of human mast cells. J Allergy Clin Immunol 123 Suppl:S195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kelley J, Hemontolor G, Younis W, Li C, Krishnaswamy G, Chi DS. 2006. Mast cell activation by lipoproteins. Methods Mol Biol 315:341–348 [DOI] [PubMed] [Google Scholar]
- 51. Liao L, Starzyk RM, Granger DN. 1997. Molecular determinants of oxidized low-density lipoprotein-induced leukocyte adhesion and microvascular dysfunction. Arterioscler Thromb Vasc Biol 17:437–444 [DOI] [PubMed] [Google Scholar]
- 52. Rivera J, Gilfillan AM. 2006. Molecular regulation of mast cell activation. J Allergy Clin Immunol 117:1214–1225; quiz 1226 [DOI] [PubMed] [Google Scholar]
- 53. Bot I, de Jager SC, Bot M, van Heiningen SH, de Groot P, Veldhuizen RW, van Berkel TJ, von der Thüsen JH, Biessen EA. 2010. The neuropeptide substance P mediates adventitial mast cell activation and induces intraplaque hemorrhage in advanced atherosclerosis. Circ Res 106:89–92 [DOI] [PubMed] [Google Scholar]
- 54. Wozniak A, McLennan G, Betts WH, Murphy GA, Scicchitano R. 1989. Activation of human neutrophils by substance P: effect on FMLP-stimulated oxidative and arachidonic acid metabolism and on antibody-dependent cell-mediated cytotoxicity. Immunology 68:359–364 [PMC free article] [PubMed] [Google Scholar]
- 55. Drechsler M, Megens RT, van Zandvoort M, Weber C, Soehnlein O. 2010. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation 122:1837–1845 [DOI] [PubMed] [Google Scholar]
- 56. Chávez-Sánchez L, Madrid-Miller A, Chávez-Rueda K, Legorreta-Haquet MV, Tesoro-Cruz E, Blanco-Favela F. 2010. Activation of TLR2 and TLR4 by minimally modified low-density lipoprotein in human macrophages and monocytes triggers the inflammatory response. Hum Immunol 71:737–744 [DOI] [PubMed] [Google Scholar]
- 57. Shamshiev AT, Ampenberger F, Ernst B, Rohrer L, Marsland BJ, Kopf M. 2007. Dyslipidemia inhibits Toll-like receptor-induced activation of CD8α-negative dendritic cells and protective Th1 type immunity. J Exp Med 204:441–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Constantinides P. 1953. Mast cells and susceptibility to experimental atherosclerosis. Science 117:505–506 [DOI] [PubMed] [Google Scholar]
- 59. Cairns A, Constantinides P. 1954. Mast cells in human atherosclerosis. Science 120:31–32 [DOI] [PubMed] [Google Scholar]
- 60. Sukhova GK, Zhang Y, Pan JH, Wada Y, Yamamoto T, Naito M, Kodama T, Tsimikas S, Witztum JL, Lu ML, Sakara Y, Chin MT, Libby P, Shi GP. 2003. Deficiency of cathepsin S reduces atherosclerosis in LDL receptor-deficient mice. J Clin Invest 111:897–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ma H, Kovanen PT. 1997. Degranulation of cutaneous mast cells induces transendothelial transport and local accumulation of plasma LDL in rat skin in vivo. J Lipid Res 38:1877–1887 [PubMed] [Google Scholar]
- 62. Kounis NG, Zavras GM. 1991. Histamine-induced coronary artery spasm: the concept of allergic angina. Br J Clin Pract 45:121–128 [PubMed] [Google Scholar]
- 63. Miyazawa N, Watanabe S, Matsuda A, Kondo K, Hashimoto H, Umemura K, Nakashima M. 1998. Role of histamine H1 and H2 receptor antagonists in the prevention of intimal thickening. Eur J Pharmacol 362:53–59 [DOI] [PubMed] [Google Scholar]
- 64. Kovanen PT. 2007. Mast cells: multipotent local effector cells in atherothrombosis. Immunol Rev 217:105–122 [DOI] [PubMed] [Google Scholar]
- 65. Kaartinen M, Penttilä A, Kovanen PT. 1994. Accumulation of activated mast cells in the shoulder region of human coronary atheroma, the predilection site of atheromatous rupture. Circulation 90:1669–1678 [DOI] [PubMed] [Google Scholar]
- 66. Kaartinen M, Penttilä A, Kovanen PT. 1996. Mast cells in rupture-prone areas of human coronary atheromas produce and store TNF-α. Circulation 94:2787–2792 [DOI] [PubMed] [Google Scholar]
- 67. Kovanen PT, Kaartinen M, Paavonen T. 1995. Infiltrates of activated mast cells at the site of coronary atheromatous erosion or rupture in myocardial infarction. Circulation 92:1084–1088 [DOI] [PubMed] [Google Scholar]
- 68. Bot I, de Jager SC, Zernecke A, Lindstedt KA, van Berkel TJ, Weber C, Biessen EA. 2007. Perivascular mast cells promote atherogenesis and induce plaque destabilization in apolipoprotein E-deficient mice. Circulation 115:2516–2525 [DOI] [PubMed] [Google Scholar]
- 69. Deliargyris EN, Upadhya B, Sane DC, Dehmer GJ, Pye J, Smith SC, Jr, Boucher WS, Theoharides TC. 2005. Mast cell tryptase: a new biomarker in patients with stable coronary artery disease. Atherosclerosis 178:381–386 [DOI] [PubMed] [Google Scholar]
- 70. Xiang M, Sun J, Lin Y, Zhang J, Chen H, Yang D, Wang J, Shi GP. 2011. Usefulness of serum tryptase level as an independent biomarker for coronary plaque instability in a Chinese population. Atherosclerosis 215:494–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mäyränpää MI, Heikkilä HM, Lindstedt KA, Walls AF, Kovanen PT. 2006. Desquamation of human coronary artery endothelium by human mast cell proteases: implications for plaque erosion. Coron Artery Dis 17:611–621 [DOI] [PubMed] [Google Scholar]
- 72. Lee-Rueckert M, Kovanen PT. 2006. Mast cell proteases: physiological tools to study functional significance of high density lipoproteins in the initiation of reverse cholesterol transport. Atherosclerosis 189:8–18 [DOI] [PubMed] [Google Scholar]
- 73. Lee M, Lindstedt LK, Kovanen PT. 1992. Mast cell-mediated inhibition of reverse cholesterol transport. Arterioscler Thromb 12:1329–1335 [DOI] [PubMed] [Google Scholar]
- 74. Oörni K, Pentikäinen MO, Ala-Korpela M, Kovanen PT. 2000. Aggregation, fusion, and vesicle formation of modified low density lipoprotein particles: molecular mechanisms and effects on matrix interactions. J Lipid Res 41:1703–1714 [PubMed] [Google Scholar]
- 75. Kokkonen JO. 1989. Stimulation of rat peritoneal mast cells enhances uptake of low density lipoproteins by rat peritoneal macrophages in vivo. Atherosclerosis 79:213–223 [DOI] [PubMed] [Google Scholar]
- 76. Ma H, Kovanen PT. 2000. Inhibition of mast cell-dependent conversion of cultured macrophages into foam cells with antiallergic drugs. Arterioscler Thromb Vasc Biol 20:E134–E142 [DOI] [PubMed] [Google Scholar]
- 77. Ihara M, Urata H, Kinoshita A, Suzumiya J, Sasaguri M, Kikuchi M, Ideishi M, Arakawa K. 1999. Increased chymase-dependent angiotensin II formation in human atherosclerotic aorta. Hypertension 33:1399–1405 [DOI] [PubMed] [Google Scholar]
- 78. Leskinen MJ, Lindstedt KA, Wang Y, Kovanen PT. 2003. Mast cell chymase induces smooth muscle cell apoptosis by a mechanism involving fibronectin degradation and disruption of focal adhesions. Arterioscler Thromb Vasc Biol 23:238–243 [DOI] [PubMed] [Google Scholar]
- 79. Meyer MC, Creer MH, McHowat J. 2005. Potential role for mast cell tryptase in recruitment of inflammatory cells to endothelium. Am J Physiol Cell Physiol 289:C1485–C1491 [DOI] [PubMed] [Google Scholar]
- 80. Muramatsu M, Katada J, Hayashi I, Majima M. 2000. Chymase as a proangiogenic factor. A possible involvement of chymase-angiotensin-dependent pathway in the hamster sponge angiogenesis model. J Biol Chem 275:5545–5552 [DOI] [PubMed] [Google Scholar]
- 81. Sun J, Zhang J, Lindholt JS, Sukhova GK, Liu J, He A, Abrink M, Pejler G, Stevens RL, Thompson RW, Ennis TL, Gurish MF, Libby P, Shi GP. 2009. Critical role of mast cell chymase in mouse abdominal aortic aneurysm formation. Circulation 120:973–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Johnson JL, Jackson CL, Angelini GD, George SJ. 1998. Activation of matrix-degrading metalloproteinases by mast cell proteases in atherosclerotic plaques. Arterioscler Thromb Vasc Biol 18:1707–1715 [DOI] [PubMed] [Google Scholar]
- 83. Pejler G, Abrink M, Ringvall M, Wernersson S. 2007. Mast cell proteases. Adv Immunol 95:167–255 [DOI] [PubMed] [Google Scholar]
- 84. Mallen-St Clair J, Shi GP, Sutherland RE, Chapman HA, Caughey GH, Wolters PJ. 2006. Cathepsins L and S are not required for activation of dipeptidyl peptidase I (cathepsin C) in mice. Biol Chem 387:1143–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Liu J, Sukhova GK, Sun JS, Xu WH, Libby P, Shi GP. 2004. Lysosomal cysteine proteases in atherosclerosis. Arterioscler Thromb Vasc Biol 24:1359–1366 [DOI] [PubMed] [Google Scholar]
- 86. Laine P, Kaartinen M, Penttilä A, Panula P, Paavonen T, Kovanen PT. 1999. Association between myocardial infarction and the mast cells in the adventitia of the infarct-related coronary artery. Circulation 99:361–369 [DOI] [PubMed] [Google Scholar]
- 87. Atkinson JB, Harlan CW, Harlan GC, Virmani R. 1994. The association of mast cells and atherosclerosis: a morphologic study of early atherosclerotic lesions in young people. Hum Pathol 25:154–159 [DOI] [PubMed] [Google Scholar]
- 88. Kaartinen M, Penttilä A, Kovanen PT. 1996. Mast cells accompany microvessels in human coronary atheromas: implications for intimal neovascularization and hemorrhage. Atherosclerosis 123:123–131 [DOI] [PubMed] [Google Scholar]
- 89. Blair RJ, Meng H, Marchese MJ, Ren S, Schwartz LB, Tonnesen MG, Gruber BL. 1997. Human mast cells stimulate vascular tube formation. Tryptase is a novel, potent angiogenic factor. J Clin Invest 99:2691–2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Meng H, Tonnesen MG, Marchese MJ, Clark RA, Bahou WF, Gruber BL. 1995. Mast cells are potent regulators of endothelial cell adhesion molecule ICAM-1 and VCAM-1 expression. J Cell Physiol 165:40–53 [DOI] [PubMed] [Google Scholar]
- 91. Lindstedt KA, Kovanen PT. 2004. Mast cells in vulnerable coronary plaques: potential mechanisms linking mast cell activation to plaque erosion and rupture. Curr Opin Lipidol 15:567–573 [DOI] [PubMed] [Google Scholar]
- 92. Tan NY, Li JM, Stocker R, Khachigian LM. 2009. Angiotensin II-inducible smooth muscle cell apoptosis involves the angiotensin II type 2 receptor, GATA-6 activation, and FasL-Fas engagement. Circ Res 105:422–430 [DOI] [PubMed] [Google Scholar]
- 93. Nishizono S, Kusaba M, Adan Y, Imaizumi K. 1999. Induction of atherosclerosis in Brown Norway rats by immunization with ovalbumin. Biosci Biotechnol Biochem 63:379–383 [DOI] [PubMed] [Google Scholar]
- 94. Uehara Y, Urata H, Ideishi M, Arakawa K, Saku K. 2002. Chymase inhibition suppresses high-cholesterol diet-induced lipid accumulation in the hamster aorta. Cardiovasc Res 55:870–876 [DOI] [PubMed] [Google Scholar]
- 95. Heikkilä HM, Trosien J, Metso J, Jauhiainen M, Pentikäinen MO, Kovanen PT, Lindstedt KA. 2010. Mast cells promote atherosclerosis by inducing both an atherogenic lipid profile and vascular inflammation. J Cell Biochem 109:615–623 [DOI] [PubMed] [Google Scholar]
- 96. Abedin M, Tintut Y, Demer LL. 2004. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol 24:1161–1170 [DOI] [PubMed] [Google Scholar]
- 97. Bouhlel MA, Derudas B, Rigamonti E, Dièvart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N, Staels B, Chinetti-Gbaguidi G. 2007. PPARγ activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab 6:137–143 [DOI] [PubMed] [Google Scholar]
- 98. Martinez FO, Sica A, Mantovani A, Locati M. 2008. Macrophage activation and polarization. Front Biosci 13:453–461 [DOI] [PubMed] [Google Scholar]
- 99. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. 2004. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25:677–686 [DOI] [PubMed] [Google Scholar]
- 100. Gordon S. 2003. Alternative activation of macrophages. Nat Rev Immunol 3:23–35 [DOI] [PubMed] [Google Scholar]
- 101. Wilson HM, Chettibi S, Jobin C, Walbaum D, Rees AJ, Kluth DC. 2005. Inhibition of macrophage nuclear factor-κB leads to a dominant anti-inflammatory phenotype that attenuates glomerular inflammation in vivo. Am J Pathol 167:27–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Martinez FO, Helming L, Gordon S. 2009. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol 27:451–483 [DOI] [PubMed] [Google Scholar]
- 103. Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW, Chawla A. 2007. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature 447:1116–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, Vats D, Morel CR, Goforth MH, Subramanian V, Mukundan L, Ferrante AW, Chawla A. 2008. Alternative M2 activation of Kupffer cells by PPARδ ameliorates obesity-induced insulin resistance. Cell Metab 7:496–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Anderson CF, Mosser DM. 2002. Cutting edge: biasing immune responses by directing antigen to macrophage Fc γ receptors. J Immunol 168:3697–3701 [DOI] [PubMed] [Google Scholar]
- 106. Chapman HA, Riese RJ, Shi GP. 1997. Emerging roles for cysteine proteases in human biology. Annu Rev Physiol 59:63–88 [DOI] [PubMed] [Google Scholar]
- 107. Mosser DM, Zhang X. 2008. Interleukin-10: new perspectives on an old cytokine. Immunol Rev 226:205–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lingnau M, Höflich C, Volk HD, Sabat R, Döcke WD. 2007. Interleukin-10 enhances the CD14-dependent phagocytosis of bacteria and apoptotic cells by human monocytes. Hum Immunol 68:730–738 [DOI] [PubMed] [Google Scholar]
- 109. Xu XH, Shah PK, Faure E, Equils O, Thomas L, Fishbein MC, Luthringer D, Xu XP, Rajavashisth TB, Yano J, Kaul S, Arditi M. 2001. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation 104:3103–3108 [DOI] [PubMed] [Google Scholar]
- 110. Tabas I. 2005. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol 25:2255–2264 [DOI] [PubMed] [Google Scholar]
- 111. Bhatia VK, Yun S, Leung V, Grimsditch DC, Benson GM, Botto MB, Boyle JJ, Haskard DO. 2007. Complement C1q reduces early atherosclerosis in low-density lipoprotein receptor-deficient mice. Am J Pathol 170:416–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Henson PM, Bratton DL, Fadok VA. 2001. Apoptotic cell removal. Curr Biol 11:R795–R805 [DOI] [PubMed] [Google Scholar]
- 113. Libby P, Geng YJ, Aikawa M, Schoenbeck U, Mach F, Clinton SK, Sukhova GK, Lee RT. 1996. Macrophages and atherosclerotic plaque stability. Curr Opin Lipidol 7:330–335 [DOI] [PubMed] [Google Scholar]
- 114. Boyle JJ. 2005. Macrophage activation in atherosclerosis: pathogenesis and pharmacology of plaque rupture. Curr Vasc Pharmacol 3:63–68 [DOI] [PubMed] [Google Scholar]
- 115. Kuehn HS, Swindle EJ, Kim MS, Beaven MA, Metcalfe DD, Gilfillan AM. 2008. The phosphoinositide 3-kinase-dependent activation of Btk is required for optimal eicosanoid production and generation of reactive oxygen species in antigen-stimulated mast cells. J Immunol 181:7706–7712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Sly LM, Kalesnikoff J, Lam V, Wong D, Song C, Omeis S, Chan K, Lee CW, Siraganian RP, Rivera J, Krystal G. 2008. IgE-induced mast cell survival requires the prolonged generation of reactive oxygen species. J Immunol 181:3850–3860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Swindle EJ, Coleman JW, DeLeo FR, Metcalfe DD. 2007. FcεRI- and Fcγ receptor-mediated production of reactive oxygen species by mast cells is lipoxygenase- and cyclooxygenase-dependent and NADPH oxidase-independent. J Immunol 179:7059–7071 [DOI] [PubMed] [Google Scholar]
- 118. Fujimi K, Uehara Y, Abe S, Kawamura A, Devarajan S, Miura S, Saku K, Urata H. 2010. Homocysteine-induced oxidative stress upregulates chymase in mouse mastocytoma cells. Hypertens Res 33:149–154 [DOI] [PubMed] [Google Scholar]
- 119. Sugiyama S, Okada Y, Sukhova GK, Virmani R, Heinecke JW, Libby P. 2001. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am J Pathol 158:879–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Croce K, Libby P. 2007. Intertwining of thrombosis and inflammation in atherosclerosis. Curr Opin Hematol 14:55–61 [DOI] [PubMed] [Google Scholar]
- 121. Danenberg HD, Fishbein I, Gao J, Mönkkönen J, Reich R, Gati I, Moerman E, Golomb G. 2002. Macrophage depletion by clodronate-containing liposomes reduces neointimal formation after balloon injury in rats and rabbits. Circulation 106:599–605 [DOI] [PubMed] [Google Scholar]
- 122. Stoneman V, Braganza D, Figg N, Mercer J, Lang R, Goddard M, Bennett M. 2007. Monocyte/macrophage suppression in CD11b diphtheria toxin receptor transgenic mice differentially affects atherogenesis and established plaques. Circ Res 100:884–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Collins RG, Velji R, Guevara NV, Hicks MJ, Chan L, Beaudet AL. 2000. P-Selectin or intercellular adhesion molecule (ICAM)-1 deficiency substantially protects against atherosclerosis in apolipoprotein E-deficient mice. J Exp Med 191:189–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. 1998. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell 2:275–281 [DOI] [PubMed] [Google Scholar]
- 125. Charo IF, Taubman MB. 2004. Chemokines in the pathogenesis of vascular disease. Circ Res 95:858–866 [DOI] [PubMed] [Google Scholar]
- 126. Davies PF. 2000. Spatial hemodynamics, the endothelium, and focal atherogenesis: a cell cycle link? Circ Res 86:114–116 [DOI] [PubMed] [Google Scholar]
- 127. Dansky HM, Barlow CB, Lominska C, Sikes JL, Kao C, Weinsaft J, Cybulsky MI, Smith JD. 2001. Adhesion of monocytes to arterial endothelium and initiation of atherosclerosis are critically dependent on vascular cell adhesion molecule-1 gene dosage. Arterioscler Thromb Vasc Biol 21:1662–1667 [DOI] [PubMed] [Google Scholar]
- 128. Boring L, Gosling J, Cleary M, Charo IF. 1998. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 394:894–897 [DOI] [PubMed] [Google Scholar]
- 129. Rosenfeld ME, Ross R. 1990. Macrophage and smooth muscle cell proliferation in atherosclerotic lesions of WHHL and comparably hypercholesterolemic fat-fed rabbits. Arteriosclerosis 10:680–687 [DOI] [PubMed] [Google Scholar]
- 130. Spagnoli LG, Orlandi A, Santeusanio G. 1991. Foam cells of the rabbit atherosclerotic plaque arrested in metaphase by colchicine show a macrophage phenotype. Atherosclerosis 88:87–92 [DOI] [PubMed] [Google Scholar]
- 131. Gordon D, Reidy MA, Benditt EP, Schwartz SM. 1990. Cell proliferation in human coronary arteries. Proc Natl Acad Sci USA 87:4600–4604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Yui S, Sasaki T, Miyazaki A, Horiuchi S, Yamazaki M. 1993. Induction of murine macrophage growth by modified LDLs. Arterioscler Thromb 13:331–337 [DOI] [PubMed] [Google Scholar]
- 133. Biwa T, Sakai M, Matsumura T, Kobori S, Kaneko K, Miyazaki A, Hakamata H, Horiuchi S, Shichiri M. 2000. Sites of action of protein kinase C and phosphatidylinositol 3-kinase are distinct in oxidized low density lipoprotein-induced macrophage proliferation. J Biol Chem 275:5810–5816 [DOI] [PubMed] [Google Scholar]
- 134. Lamharzi N, Renard CB, Kramer F, Pennathur S, Heinecke JW, Chait A, Bornfeldt KE. 2004. Hyperlipidemia in concert with hyperglycemia stimulates the proliferation of macrophages in atherosclerotic lesions: potential role of glucose-oxidized LDL. Diabetes 53:3217–3225 [DOI] [PubMed] [Google Scholar]
- 135. Senokuchi T, Matsumura T, Sakai M, Matsuo T, Yano M, Kiritoshi S, Sonoda K, Kukidome D, Nishikawa T, Araki E. 2004. Extracellular signal-regulated kinase and p38 mitogen-activated protein kinase mediate macrophage proliferation induced by oxidized low-density lipoprotein. Atherosclerosis 176:233–245 [DOI] [PubMed] [Google Scholar]
- 136. Miyamoto T, Sasaguri Y, Sasaguri T, Azakami S, Yasukawa H, Kato S, Arima N, Sugama K, Morimatsu M. 1997. Expression of stem cell factor in human aortic endothelial and smooth muscle cells. Atherosclerosis 129:207–213 [DOI] [PubMed] [Google Scholar]
- 137. Chaldakov GN, Stankulov IS, Fiore M, Ghenev PI, Aloe L. 2001. Nerve growth factor levels and mast cell distribution in human coronary atherosclerosis. Atherosclerosis 159:57–66 [DOI] [PubMed] [Google Scholar]
- 138. Nabel G, Galli SJ, Dvorak AM, Dvorak HF, Cantor H. 1981. Inducer T lymphocytes synthesize a factor that stimulates proliferation of cloned mast cells. Nature 291:332–334 [DOI] [PubMed] [Google Scholar]
- 139. Aloe L, Levi-Montalcini R. 1977. Mast cells increase in tissues of neonatal rats injected with the nerve growth factor. Brain Res 133:358–366 [DOI] [PubMed] [Google Scholar]
- 140. Mori A, Nakayama K, Suzuki J, Nikaido T, Isobe M, Fujii S. 1997. Analysis of stem cell factor for mast cell proliferation in the human myometrium. Mol Hum Reprod 3:411–418 [DOI] [PubMed] [Google Scholar]
- 141. Babaev VR, Chew JD, Ding L, Davis S, Breyer MD, Breyer RM, Oates JA, Fazio S, Linton MF. 2008. Macrophage EP4 deficiency increases apoptosis and suppresses early atherosclerosis. Cell Metab 8:492–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Liu J, Thewke DP, Su YR, Linton MF, Fazio S, Sinensky MS. 2005. Reduced macrophage apoptosis is associated with accelerated atherosclerosis in low-density lipoprotein receptor-null mice. Arterioscler Thromb Vasc Biol 25:174–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Arai S, Shelton JM, Chen M, Bradley MN, Castrillo A, Bookout AL, Mak PA, Edwards PA, Mangelsdorf DJ, Tontonoz P, Miyazaki T. 2005. A role for the apoptosis inhibitory factor AIM/Spα/Api6 in atherosclerosis development. Cell Metab 1:201–213 [DOI] [PubMed] [Google Scholar]
- 144. Wang BY, Ho HK, Lin PS, Schwarzacher SP, Pollman MJ, Gibbons GH, Tsao PS, Cooke JP. 1999. Regression of atherosclerosis: role of nitric oxide and apoptosis. Circulation 99:1236–1241 [DOI] [PubMed] [Google Scholar]
- 145. Newby AC. 2008. Metalloproteinase expression in monocytes and macrophages and its relationship to atherosclerotic plaque instability. Arterioscler Thromb Vasc Biol 28:2108–2114 [DOI] [PubMed] [Google Scholar]
- 146. Heikkilä HM, Lätti S, Leskinen MJ, Hakala JK, Kovanen PT, Lindstedt KA. 2008. Activated mast cells induce endothelial cell apoptosis by a combined action of chymase and tumor necrosis factor-α. Arterioscler Thromb Vasc Biol 28:309–314 [DOI] [PubMed] [Google Scholar]
- 147. Martinet W, Kockx MM. 2001. Apoptosis in atherosclerosis: focus on oxidized lipids and inflammation. Curr Opin Lipidol 12:535–541 [DOI] [PubMed] [Google Scholar]
- 148. Mallat Z, Hugel B, Ohan J, Lesèche G, Freyssinet JM, Tedgui A. 1999. Shed membrane microparticles with procoagulant potential in human atherosclerotic plaques: a role for apoptosis in plaque thrombogenicity. Circulation 99:348–353 [DOI] [PubMed] [Google Scholar]
- 149. Martinez FO, Helming L, Gordon S. 2009. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol 27:451–283 [DOI] [PubMed] [Google Scholar]
- 150. Chang MK, Bergmark C, Laurila A, Hörkkö S, Han KH, Friedman P, Dennis EA, Witztum JL. 1999. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition. Proc Natl Acad Sci USA 96:6353–6358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Thorp E, Cui D, Schrijvers DM, Kuriakose G, Tabas I. 2008. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of apoe−/− mice. Arterioscler Thromb Vasc Biol 28:1421–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Ait-Oufella H, Pouresmail V, Simon T, Blanc-Brude O, Kinugawa K, Merval R, Offenstadt G, Lesèche G, Cohen PL, Tedgui A, Mallat Z. 2008. Defective mer receptor tyrosine kinase signaling in bone marrow cells promotes apoptotic cell accumulation and accelerates atherosclerosis. Arterioscler Thromb Vasc Biol 28:1429–1431 [DOI] [PubMed] [Google Scholar]
- 153. Sather S, Kenyon KD, Lefkowitz JB, Liang X, Varnum BC, Henson PM, Graham DK. 2007. A soluble form of the Mer receptor tyrosine kinase inhibits macrophage clearance of apoptotic cells and platelet aggregation. Blood 109:1026–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Schrijvers DM, De Meyer GR, Herman AG, Martinet W. 2007. Phagocytosis in atherosclerosis: molecular mechanisms and implications for plaque progression and stability. Cardiovasc Res 73:470–480 [DOI] [PubMed] [Google Scholar]
- 155. Poncet P, Arock M, David B. 1999. MHC class II-dependent activation of CD4+ T cell hybridomas by human mast cells through superantigen presentation. J Leukoc Biol 66:105–112 [DOI] [PubMed] [Google Scholar]
- 156. Stelekati E, Bahri R, D'Orlando O, Orinska Z, Mittrücker HW, Langenhaun R, Glatzel M, Bollinger A, Paus R, Bulfone-Paus S. 2009. Mast cell-mediated antigen presentation regulates CD8+ T cell effector functions. Immunity 31:665–676 [DOI] [PubMed] [Google Scholar]
- 157. Hughes DA, Townsend PJ, Haslam PL. 1992. Enhancement of the antigen-presenting function of monocytes by cholesterol: possible relevance to inflammatory mechanisms in extrinsic allergic alveolitis and atherosclerosis. Clin Exp Immunol 87:279–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Zhang J, Alcaide P, Liu L, Sun J, He A, Luscinskas FW, Shi GP. 2011. Regulation of endothelial cell adhesion molecule expression by mast cells, macrophages, and neutrophils. PLoS One 6:e14525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Barlic J, Zhang Y, Murphy PM. 2007. Atherogenic lipids induce adhesion of human coronary artery smooth muscle cells to macrophages by up-regulating chemokine CX3CL1 on smooth muscle cells in a TNFα-NFκB-dependent manner. J Biol Chem 282:19167–19176 [DOI] [PubMed] [Google Scholar]
- 160. Boyle JJ, Bowyer DE, Weissberg PL, Bennett MR. 2001. Human blood-derived macrophages induce apoptosis in human plaque-derived vascular smooth muscle cells by Fas-ligand/Fas interactions. Arterioscler Thromb Vasc Biol 21:1402–1407 [DOI] [PubMed] [Google Scholar]
- 161. Boyle JJ, Weissberg PL, Bennett MR. 2002. Human macrophage-induced vascular smooth muscle cell apoptosis requires NO enhancement of Fas/Fas-L interactions. Arterioscler Thromb Vasc Biol 22:1624–1630 [DOI] [PubMed] [Google Scholar]
- 162. Cai Q, Lanting L, Natarajan R. 2004. Growth factors induce monocyte binding to vascular smooth muscle cells: implications for monocyte retention in atherosclerosis. Am J Physiol Cell Physiol 287:C707–C714 [DOI] [PubMed] [Google Scholar]
- 163. Cai Q, Lanting L, Natarajan R. 2004. Interaction of monocytes with vascular smooth muscle cells regulates monocyte survival and differentiation through distinct pathways. Arterioscler Thromb Vasc Biol 24:2263–2270 [DOI] [PubMed] [Google Scholar]
- 164. Daugherty A, Webb NR, Rateri DL, King VL. 2005. Thematic review series: the immune system and atherogenesis cytokine regulation of macrophage functions in atherogenesis. J Lipid Res 46:1812–1822 [DOI] [PubMed] [Google Scholar]
- 165. Raines EW, Ferri N. 2005. Thematic review series: the immune system and atherogenesis cytokines affecting endothelial and smooth muscle cells in vascular disease. J Lipid Res 46:1081–1092 [DOI] [PubMed] [Google Scholar]
- 166. Tedgui A, Mallat Z. 2006. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev 86:515–581 [DOI] [PubMed] [Google Scholar]
- 167. Libby P, Ridker PM, Maseri A. 2002. Inflammation and atherosclerosis. Circulation 105:1135–1143 [DOI] [PubMed] [Google Scholar]
- 168. Robertson AK, Hansson GK. 2006. T cells in atherogenesis: for better or for worse? Arterioscler Thromb Vasc Biol 26:2421–2432 [DOI] [PubMed] [Google Scholar]
- 169. Tsunemi K, Takai S, Nishimoto M, Yuda A, Hasegawa S, Sawada Y, Fukumoto H, Sasaki S, Miyazaki M. 2002. Possible roles of angiotensin II-forming enzymes, angiotensin converting enzyme and chymase-like enzyme, in the human aneurysmal aorta. Hypertens Res 25:817–822 [DOI] [PubMed] [Google Scholar]
- 170. Mäyränpää MI, Trosien JA, Fontaine V, Folkesson M, Kazi M, Eriksson P, Swedenborg J, Hedin U. 2009. Mast cells associate with neovessels in the media and adventitia of abdominal aortic aneurysms. J Vasc Surg 50:388–395; discussion 395–396 [DOI] [PubMed] [Google Scholar]
- 171. Galle C, Schandené L, Stordeur P, Peignois Y, Ferreira J, Wautrecht JC, Dereume JP, Goldman M. 2005. Predominance of type 1 CD4+ T cells in human abdominal aortic aneurysm. Clin Exp Immunol 142:519–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Schönbeck U, Sukhova GK, Gerdes N, Libby P. 2002. T(H)2 predominant immune responses prevail in human abdominal aortic aneurysm. Am J Pathol 161:499–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Xiong W, Zhao Y, Prall A, Greiner TC, Baxter BT. 2004. Key roles of CD4+ T cells and IFN-γ in the development of abdominal aortic aneurysms in a murine model. J Immunol 172:2607–2612 [DOI] [PubMed] [Google Scholar]
- 174. Xiong W, MacTaggart J, Knispel R, Worth J, Persidsky Y, Baxter BT. 2009. Blocking TNF-α attenuates aneurysm formation in a murine model. J Immunol 183:2741–2746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. King VL, Lin AY, Kristo F, Anderson TJ, Ahluwalia N, Hardy GJ, Owens AP, 3rd, Howatt DA, Shen D, Tager AM, Luster AD, Daugherty A, Gerszten RE. 2009. Interferon-γ and the interferon-inducible chemokine CXCL10 protect against aneurysm formation and rupture. Circulation 119:426–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Shimizu K, Shichiri M, Libby P, Lee RT, Mitchell RN. 2004. Th2-predominant inflammation and blockade of IFN-γ signaling induce aneurysms in allografted aortas. J Clin Invest 114:300–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Swedenborg J, Mäyränpää MI, Kovanen PT. 2011. Mast cells: important players in the orchestrated pathogenesis of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 31:734–740 [DOI] [PubMed] [Google Scholar]
- 178. Tsunemi K, Takai S, Nishimoto M, Jin D, Sakaguchi M, Muramatsu M, Yuda A, Sasaki S, Miyazaki M. 2004. A specific chymase inhibitor, 2-(5-formylamino-6-oxo-2-phenyl-1,6-dihydropyrimidine-1-yl)-N-[[3,4-dioxo-1-phenyl-7-(2-pyridyloxy)]-2-heptyl]acetamide (NK3201), suppresses development of abdominal aortic aneurysm in hamsters. J Pharmacol Exp Ther 309:879–883 [DOI] [PubMed] [Google Scholar]
- 179. Furubayashi K, Takai S, Jin D, Muramatsu M, Ibaraki T, Nishimoto M, Fukumoto H, Katsumata T, Miyazaki M. 2007. The significance of chymase in the progression of abdominal aortic aneurysms in dogs. Hypertens Res 30:349–357 [DOI] [PubMed] [Google Scholar]
- 180. Sun J, Sukhova GK, Yang M, Wolters PJ, MacFarlane LA, Libby P, Sun C, Zhang Y, Liu J, Ennis TL, Knispel R, Xiong W, Thompson RW, Baxter BT, Shi GP. 2007. Mast cells modulate the pathogenesis of elastase-induced abdominal aortic aneurysms in mice. J Clin Invest 117:3359–3368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Tsuruda T, Kato J, Hatakeyama K, Kojima K, Yano M, Yano Y, Nakamura K, Nakamura-Uchiyama F, Matsushima Y, Imamura T, Onitsuka T, Asada Y, Nawa Y, Eto T, Kitamura K. 2008. Adventitial mast cells contribute to pathogenesis in the progression of abdominal aortic aneurysm. Circ Res 102:1368–1377 [DOI] [PubMed] [Google Scholar]
- 182. Zhang J, Sun J, Lindholt JS, Sukhova GK, Sinnamon M, Stevens RL, Adachi R, Libby P, Thompson RW, Shi GP. 2011. Mast cell tryptase deficiency attenuates mouse abdominal aortic aneurysm formation. Circ Res 108:1316–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Curci JA, Liao S, Huffman MD, Shapiro SD, Thompson RW. 1998. Expression and localization of macrophage elastase (matrix metalloproteinase-12) in abdominal aortic aneurysms. J Clin Invest 102:1900–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Lamblin N, Ratajczak P, Hot D, Dubois E, Chwastyniak M, Beseme O, Drobecq H, Lemoine Y, Koussa M, Amouyel P, Pinet F. 2010. Profile of macrophages in human abdominal aortic aneurysms: a transcriptomic, proteomic, and antibody protein array study. J Proteome Res 9:3720–3729 [DOI] [PubMed] [Google Scholar]
- 185. Shiraya S, Miyake T, Aoki M, Yoshikazu F, Ohgi S, Nishimura M, Ogihara T, Morishita R. 2009. Inhibition of development of experimental aortic abdominal aneurysm in rat model by atorvastatin through inhibition of macrophage migration. Atherosclerosis 202:34–40 [DOI] [PubMed] [Google Scholar]
- 186. Carmeliet P, Moons L, Lijnen R, Baes M, Lemaître V, Tipping P, Drew A, Eeckhout Y, Shapiro S, Lupu F, Collen D. 1997. Urokinase-generated plasmin activates matrix metalloproteinases during aneurysm formation. Nat Genet 17:439–444 [DOI] [PubMed] [Google Scholar]
- 187. Gong Y, Hart E, Shchurin A, Hoover-Plow J. 2008. Inflammatory macrophage migration requires MMP-9 activation by plasminogen in mice. J Clin Invest 118:3012–3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Turner GH, Olzinski AR, Bernard RE, Aravindhan K, Boyle RJ, Newman MJ, Gardner SD, Willette RN, Gough PJ, Jucker BM. 2009. Assessment of macrophage infiltration in a murine model of abdominal aortic aneurysm. J Magn Reson Imaging 30:455–460 [DOI] [PubMed] [Google Scholar]
- 189. Police SB, Thatcher SE, Charnigo R, Daugherty A, Cassis LA. 2009. Obesity promotes inflammation in periaortic adipose tissue and angiotensin II-induced abdominal aortic aneurysm formation. Arterioscler Thromb Vasc Biol 29:1458–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Sho E, Sho M, Hoshina K, Kimura H, Nakahashi TK, Dalman RL. 2004. Hemodynamic forces regulate mural macrophage infiltration in experimental aortic aneurysms. Exp Mol Pathol 76:108–116 [DOI] [PubMed] [Google Scholar]
- 191. Berg AH, Scherer PE. 2005. Adipose tissue, inflammation, and cardiovascular disease. Circ Res 96:939–949 [DOI] [PubMed] [Google Scholar]
- 192. Cao Y. 2007. Angiogenesis modulates adipogenesis and obesity. J Clin Invest 117:2362–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Crandall DL, Hausman GJ, Kral JG. 1997. A review of the microcirculation of adipose tissue: anatomic, metabolic, and angiogenic perspectives. Microcirculation 4:211–232 [DOI] [PubMed] [Google Scholar]
- 194. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr 2003. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Wu H, Ghosh S, Perrard XD, Feng L, Garcia GE, Perrard JL, Sweeney JF, Peterson LE, Chan L, Smith CW, Ballantyne CM. 2007. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation 115:1029–1038 [DOI] [PubMed] [Google Scholar]
- 196. Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. 2009. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 15:914–920 [DOI] [PubMed] [Google Scholar]
- 197. Kopp HP, Kopp CW, Festa A, Krzyzanowska K, Kriwanek S, Minar E, Roka R, Schernthaner G. 2003. Impact of weight loss on inflammatory proteins and their association with the insulin resistance syndrome in morbidly obese patients. Arterioscler Thromb Vasc Biol 23:1042–1047 [DOI] [PubMed] [Google Scholar]
- 198. Weiss ST, Shore S. 2004. Obesity and asthma: directions for research. Am J Respir Crit Care Med 169:963–968 [DOI] [PubMed] [Google Scholar]
- 199. Ferencz V, Meszaros S, Csupor E, Toth E, Bors K, Falus A, Horvath C. 2006. Increased bone fracture prevalence in postmenopausal women suffering from pollen-allergy. Osteoporos Int 17:484–4891 [DOI] [PubMed] [Google Scholar]
- 200. Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, Maezawa Y, Drucker DJ, Engleman E, Winer D, Dosch HM. 2009. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med 15:921–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. 2009. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 15:930–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Yang M, Zhang Y, Pan J, Sun J, Liu J, Libby P, Sukhova GK, Doria A, Katunuma N, Peroni OD, Guerre-Millo M, Kahn BB, Clement K, Shi GP. 2007. Cathepsin L activity controls adipogenesis and glucose tolerance. Nat Cell Biol 9:970–977 [DOI] [PubMed] [Google Scholar]
- 203. Yang M, Sun J, Zhang T, Liu J, Zhang J, Shi MA, Darakhshan F, Guerre-Millo M, Clement K, Gelb BD, Dolgnov G, Shi GP. 2008. Deficiency and inhibition of cathepsin K reduce body weight gain and increase glucose metabolism in mice. Arterioscler Thromb Vasc Biol 28:2202–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Wang B, Sun J, Kitamoto S, Yang M, Grubb A, Chapman HA, Kalluri R, Shi GP. 2006. Cathepsin S controls angiogenesis and tumor growth via matrix-derived angiogenic factors. J Biol Chem 281:6020–6029 [DOI] [PubMed] [Google Scholar]
- 205. Kashyap M, Thornton AM, Norton SK, Barnstein B, Macey M, Brenzovich J, Shevach E, Leonard WJ, Ryan JJ. 2008. Cutting edge: CD4 T cell-mast cell interactions alter IgE receptor expression and signaling. J Immunol 180:2039–2043 [DOI] [PubMed] [Google Scholar]
- 206. Mekori YA, Metcalfe DD. 1999. Mast cell–T cell interactions. J Allergy Clin Immunol 104:517–523 [DOI] [PubMed] [Google Scholar]
- 207. Kambayashi T, Allenspach EJ, Chang JT, Zou T, Shoag JE, Reiner SL, Caton AJ, Koretzky GA. 2009. Inducible MHC class II expression by mast cells supports effector and regulatory T cell activation. J Immunol 182:4686–4695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. DiPietro LA, Burdick M, Low QE, Kunkel SL, Strieter RM. 1998. MIP-1α as a critical macrophage chemoattractant in murine wound repair. J Clin Invest 101:1693–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Kotani A, Hori T, Fujita T, Kambe N, Matsumura Y, Ishikawa T, Miyachi Y, Nagai K, Tanaka Y, Uchiyama T. 2007. Involvement of OX40 ligand+ mast cells in chronic GVHD after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 39:373–375 [DOI] [PubMed] [Google Scholar]
- 210. Gri G, Piconese S, Frossi B, Manfroi V, Merluzzi S, Tripodo C, Viola A, Odom S, Rivera J, Colombo MP, Pucillo CE. 2008. CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity 29:771–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Mirmonsef P, Shelburne CP, Fitzhugh Yeatman C, 2nd, Chong HJ, Ryan JJ. 1999. Inhibition of Kit expression by IL-4 and IL-10 in murine mast cells: role of STAT6 and phosphatidylinositol 3′-kinase. J Immunol 163:2530–2539 [PubMed] [Google Scholar]
- 212. Ryan JJ, DeSimone S, Klisch G, Shelburne C, McReynolds LJ, Han K, Kovacs R, Mirmonsef P, Huff TF. 1998. IL-4 inhibits mouse mast cell Fc εRI expression through a STAT6-dependent mechanism. J Immunol 161:6915–6923 [PubMed] [Google Scholar]
- 213. Yeatman CF, 2nd, Jacobs-Helber SM, Mirmonsef P, Gillespie SR, Bouton LA, Collins HA, Sawyer ST, Shelburne CP, Ryan JJ. 2000. Combined stimulation with the T helper cell type 2 cytokines interleukin (IL)-4 and IL-10 induces mouse mast cell apoptosis. J Exp Med 192:1093–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Baecher-Allan C, Wolf E, Hafler DA. 2006. MHC class II expression identifies functionally distinct human regulatory T cells. J Immunol 176:4622–4631 [DOI] [PubMed] [Google Scholar]
- 215. Gustafson B, Hammarstedt A, Andersson CX, Smith U. 2007. Inflamed adipose tissue: a culprit underlying the metabolic syndrome and atherosclerosis. Arterioscler Thromb Vasc Biol 27:2276–2283 [DOI] [PubMed] [Google Scholar]
- 216. Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S, Coppack SW. 1997. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-α, in vivo. J Clin Endocrinol Metab 82:4196–4200 [DOI] [PubMed] [Google Scholar]
- 217. Xu H, Uysal KT, Becherer JD, Arner P, Hotamisligil GS. 2002. Altered tumor necrosis factor-α (TNF-α) processing in adipocytes and increased expression of transmembrane TNF-α in obesity. Diabetes 51:1876–1883 [DOI] [PubMed] [Google Scholar]
- 218. Villena JA, Cousin B, Pénicaud L, Casteilla L. 2001. Adipose tissues display differential phagocytic and microbicidal activities depending on their localization. Int J Obes Relat Metab Disord 25:1275–1280 [DOI] [PubMed] [Google Scholar]
- 219. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. 2003. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112:1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. 2005. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 46:2347–2355 [DOI] [PubMed] [Google Scholar]
- 221. He J, Xu X, Francisco A, Ferrante A, Krakoff J. 2011. Markers of adipose tissue macrophage content are negatively associated with serum HDL-C concentrations. Atherosclerosis 215:243–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Kitamoto S, Sukhova GK, Sun J, Yang M, Libby P, Love V, Duramad P, Sun C, Zhang Y, Yang X, Peters C, Shi GP. 2007. Cathepsin L deficiency reduces diet-induced atherosclerosis in low-density lipoprotein receptor-knockout mice. Circulation 115:2065–2075 [DOI] [PubMed] [Google Scholar]
- 223. Zeyda M, Farmer D, Todoric J, Aszmann O, Speiser M, Györi G, Zlabinger GJ, Stulnig TM. 2007. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes (Lond) 31:1420–1428 [DOI] [PubMed] [Google Scholar]
- 224. Lumeng CN, Bodzin JL, Saltiel AR. 2007. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117:175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y, Tsuneyama K, Nagai Y, Takatsu K, Urakaze M, Kobayashi M, Tobe K. 2009. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes 58:2574–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Clément K, Viguerie N, Poitou C, Carette C, Pelloux V, Curat CA, Sicard A, Rome S, Benis A, Zucker JD, Vidal H, Laville M, Barsh GS, Basdevant A, Stich V, Cancello R, Langin D. 2004. Weight loss regulates inflammation-related genes in white adipose tissue of obese subjects. FASEB J 18:1657–1669 [DOI] [PubMed] [Google Scholar]
- 227. Aron-Wisnewsky J, Tordjman J, Poitou C, Darakhshan F, Hugol D, Basdevant A, Aissat A, Guerre-Millo M, Clément K. 2009. Human adipose tissue macrophages: m1 and m2 cell surface markers in subcutaneous and omental depots and after weight loss. J Clin Endocrinol Metab 94:4619–4623 [DOI] [PubMed] [Google Scholar]
- 228. Gordon S, Taylor PR. 2005. Monocyte and macrophage heterogeneity. Nat Rev Immunol 5:953–964 [DOI] [PubMed] [Google Scholar]
- 229. Lutgens E, Lutgens SP, Faber BC, Heeneman S, Gijbels MM, de Winther MP, Frederik P, van der Made I, Daugherty A, Sijbers AM, Fisher A, Long CJ, Saftig P, Black D, Daemen MJ, Cleutjens KB. 2006. Disruption of the cathepsin K gene reduces atherosclerosis progression and induces plaque fibrosis but accelerates macrophage foam cell formation. Circulation 113:98–107 [DOI] [PubMed] [Google Scholar]
- 230. Kahn SE, Zinman B, Haffner SM, O'Neill MC, Kravitz BG, Yu D, Freed MI, Herman WH, Holman RR, Jones NP, Lachin JM, Viberti GC; ADOPT Study Group 2006. Obesity is a major determinant of the association of C-reactive protein levels and the metabolic syndrome in type 2 diabetes. Diabetes 55:2357–2364 [DOI] [PubMed] [Google Scholar]
- 231. Kim CS, Park HS, Kawada T, Kim JH, Lim D, Hubbard NE, Kwon BS, Erickson KL, Yu R. 2006. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int J Obes (Lond) 30:1347–1355 [DOI] [PubMed] [Google Scholar]
- 232. Curat CA, Miranville A, Sengenès C, Diehl M, Tonus C, Busse R, Bouloumié A. 2004. From blood monocytes to adipose tissue-resident macrophages: induction of diapedesis by human mature adipocytes. Diabetes 53:1285–1292 [DOI] [PubMed] [Google Scholar]
- 233. Moraes RC, Blondet A, Birkenkamp-Demtroeder K, Tirard J, Orntoft TF, Gertler A, Durand P, Naville D, Bégeot M. 2003. Study of the alteration of gene expression in adipose tissue of diet-induced obese mice by microarray and reverse transcription-polymerase chain reaction analyses. Endocrinology 144:4773–4782 [DOI] [PubMed] [Google Scholar]
- 234. Suganami T, Nishida J, Ogawa Y. 2005. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor α. Arterioscler Thromb Vasc Biol 25:2062–2068 [DOI] [PubMed] [Google Scholar]
- 235. Lim CS, Shalhoub J, Gohel MS, Shepherd AC, Davies AH. 2010. Matrix metalloproteinases in vascular disease—a potential therapeutic target? Curr Vasc Pharmacol 8:75–85 [DOI] [PubMed] [Google Scholar]
- 236. Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. 2006. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 116:1494–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Elgazar-Carmon V, Rudich A, Hadad N, Levy R. 2008. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res 49:1894–1903 [DOI] [PubMed] [Google Scholar]
- 238. Huang W, Metlakunta A, Dedousis N, Zhang P, Sipula I, Dube JJ, Scott DK, O'Doherty RM. 2010. Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes 59:347–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Kettner A, Di Matteo M, Santoni A. 2010. Insulin potentiates FcεRI-mediated signaling in mouse bone marrow-derived mast cells. Mol Immunol 47:1039–1046 [DOI] [PubMed] [Google Scholar]
- 240. Huang SW. 1999. Asthma and diabetes. Lancet 354:515. [DOI] [PubMed] [Google Scholar]
- 241. Lopez R, Rand LI, Zetter BR. 1982. Absence of mast cells in diabetic retinopathy. Microvasc Res 24:87–93 [DOI] [PubMed] [Google Scholar]
- 242. Judström I, Jukkola H, Metso J, Jauhiainen M, Kovanen PT, Lee-Rueckert M. 2010. Mast cell-dependent proteolytic modification of HDL particles during anaphylactic shock in the mouse reduces their ability to induce cholesterol efflux from macrophage foam cells ex vivo. Atherosclerosis 208:148–154 [DOI] [PubMed] [Google Scholar]
- 243. Carvalho VF, Barreto EO, Cordeiro RS, Lagente V, Martins MA, e Silva PM. 2005. Mast cell changes in experimental diabetes: focus on attenuation of allergic events. Mem Inst Oswaldo Cruz 100(Suppl 1):121–125 [DOI] [PubMed] [Google Scholar]
- 244. Carvalho Vde F, Barreto Ede O, Farias-Filho FA, Gomes LH, Mendonça Lde L, Cordeiro RS, Martins MA, Rodrigues e Silva PM. 2009. Reduced expression of IL-3 mediates intestinal mast cell depletion in diabetic rats: role of insulin and glucocorticoid hormones. Int J Exp Pathol 90:148–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Mordes JP, Bortell R, Doukas J, Rigby M, Whalen B, Zipris D, Greiner DL, Rossini AA. 1996. The BB/Wor rat and the balance hypothesis of autoimmunity. Diabetes Metab Rev 12:103–109 [DOI] [PubMed] [Google Scholar]
- 246. Hessner MJ, Wang X, Meyer L, Geoffrey R, Jia S, Fuller J, Lernmark A, Ghosh S. 2004. Involvement of eotaxin, eosinophils, and pancreatic predisposition in development of type 1 diabetes mellitus in the BioBreeding rat. J Immunol 173:6993–7002 [DOI] [PubMed] [Google Scholar]
- 247. Geoffrey R, Jia S, Kwitek AE, Woodliff J, Ghosh S, Lernmark A, Wang X, Hessner MJ. 2006. Evidence of a functional role for mast cells in the development of type 1 diabetes mellitus in the BioBreeding rat. J Immunol 177:7275–7286 [DOI] [PubMed] [Google Scholar]
- 248. Jones SE, Gilbert RE, Kelly DJ. 2004. Tranilast reduces mesenteric vascular collagen deposition and chymase-positive mast cells in experimental diabetes. J Diabetes Complications 18:309–315 [DOI] [PubMed] [Google Scholar]
- 249. Casacó A, Carbajal D, García M. 1989. Bronchial asthma and diabetes mellitus. Experimental evidences of mutual exclusion. Allergol Immunopathol (Madr) 17:105–108 [PubMed] [Google Scholar]
- 250. Bonner-Weir S, Trent DF, Honey RN, Weir GC. 1981. Responses of neonatal rat islets to streptozotocin: limited B-cell regeneration and hyperglycemia. Diabetes 30:64–69 [DOI] [PubMed] [Google Scholar]
- 251. Ennis M, Trimble ER. 1991. Comparison of histamine release from peritoneal mast cells derived from diabetic and control rats. Agents Actions 33:23–25 [DOI] [PubMed] [Google Scholar]
- 252. Nguyen D, Ping F, Mu W, Hill P, Atkins RC, Chadban SJ. 2006. Macrophage accumulation in human progressive diabetic nephropathy. Nephrology 11:226–231 [DOI] [PubMed] [Google Scholar]
- 253. Chow F, Ozols E, Nikolic-Paterson DJ, Atkins RC, Tesch GH. 2004. Macrophages in mouse type 2 diabetic nephropathy: correlation with diabetic state and progressive renal injury. Kidney Int 65:116–128 [DOI] [PubMed] [Google Scholar]
- 254. Chow FY, Nikolic-Paterson DJ, Ozols E, Atkins RC, Tesch GH. 2005. Intercellular adhesion molecule-1 deficiency is protective against nephropathy in type 2 diabetic db/db mice. J Am Soc Nephrol 16:1711–1722 [DOI] [PubMed] [Google Scholar]
- 255. Tashimo A, Mitamura Y, Nagai S, Nakamura Y, Ohtsuka K, Mizue Y, Nishihira J. 2004. Aqueous levels of macrophage migration inhibitory factor and monocyte chemotactic protein-1 in patients with diabetic retinopathy. Diabet Med 21:1292–1297 [DOI] [PubMed] [Google Scholar]
- 256. Chow FY, Nikolic-Paterson DJ, Ma FY, Ozols E, Rollins BJ, Tesch GH. 2007. Monocyte chemoattractant protein-1-induced tissue inflammation is critical for the development of renal injury but not type 2 diabetes in obese db/db mice. Diabetologia 50:471–480 [DOI] [PubMed] [Google Scholar]
- 257. Pascoe MK, Low PA, Windebank AJ, Litchy WJ. 1997. Subacute diabetic proximal neuropathy. Mayo Clin Proc 72:1123–1132 [DOI] [PubMed] [Google Scholar]
- 258. Dyck PJ, Windebank AJ. 2002. Diabetic and nondiabetic lumbosacral radiculoplexus neuropathies: new insights into pathophysiology and treatment. Muscle Nerve 25:477–491 [DOI] [PubMed] [Google Scholar]
- 259. Tang S, Scheiffarth OF, Thurau SR, Wildner G. 1993. Cells of the immune system and their cytokines in epiretinal membranes and in the vitreous of patients with proliferative diabetic retinopathy. Ophthalmic Res 25:177–185 [DOI] [PubMed] [Google Scholar]
- 260. Demircan N, Safran BG, Soylu M, Ozcan AA, Sizmaz S. 2006. Determination of vitreous interleukin-1 (IL-1) and tumour necrosis factor (TNF) levels in proliferative diabetic retinopathy. Eye 20:1366–1369 [DOI] [PubMed] [Google Scholar]
- 261. Plotkin BJ, Paulson D, Chelich A, Jurak D, Cole J, Kasimos J, Burdick JR, Casteel N. 1996. Immune responsiveness in a rat model for type II diabetes (Zucker rat, fa/fa): susceptibility to Candida albicans infection and leucocyte function. J Med Microbiol 44:277–283 [DOI] [PubMed] [Google Scholar]
- 262. Mauldin JP, Srinivasan S, Mulya A, Gebre A, Parks JS, Daugherty A, Hedrick CC. 2006. Reduction in ABCG1 in type 2 diabetic mice increases macrophage foam cell formation. J Biol Chem 281:21216–21224 [DOI] [PubMed] [Google Scholar]
- 263. Liang CP, Han S, Okamoto H, Carnemolla R, Tabas I, Accili D, Tall AR. 2004. Increased CD36 protein as a response to defective insulin signaling in macrophages. J Clin Invest 113:764–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Comi RJ, Grunberger G, Gorden P. 1987. Relationship of insulin binding and insulin-stimulated tyrosine kinase activity is altered in type II diabetes. J Clin Invest 79:453–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Naidoo C, Jialal I, Dunn RD, Govender T, Joubert SM. 1987. Insulin binding to circulating monocytes and erythrocytes in patients with non-insulin-dependent diabetes in the young. Diabetes Res 4:35–38 [PubMed] [Google Scholar]
- 266. Han S, Liang CP, DeVries-Seimon T, Ranalletta M, Welch CL, Collins-Fletcher K, Accili D, Tabas I, Tall AR. 2006. Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell Metab 3:257–266 [DOI] [PubMed] [Google Scholar]
- 267. Hartman ME, O'Connor JC, Godbout JP, Minor KD, Mazzocco VR, Freund GG. 2004. Insulin receptor substrate-2-dependent interleukin-4 signaling in macrophages is impaired in two models of type 2 diabetes mellitus. J Biol Chem 279:28045–28050 [DOI] [PubMed] [Google Scholar]
- 268. Bouma G, Lam-Tse WK, Wierenga-Wolf AF, Drexhage HA, Versnel MA. 2004. Increased serum levels of MRP-8/14 in type 1 diabetes induce an increased expression of CD11b and an enhanced adhesion of circulating monocytes to fibronectin. Diabetes 53:1979–1986 [DOI] [PubMed] [Google Scholar]
- 269. Carantoni M, Abbasi F, Chu L, Chen YD, Reaven GM, Tsao PS, Varasteh B, Cooke JP. 1997. Adherence of mononuclear cells to endothelium in vitro is increased in patients with NIDDM. Diabetes Care 20:1462–1465 [DOI] [PubMed] [Google Scholar]
- 270. Griffin E, Re A, Hamel N, Fu C, Bush H, McCaffrey T, Asch AS. 2001. A link between diabetes and atherosclerosis: glucose regulates expression of CD36 at the level of translation. Nat Med 7:840–846 [DOI] [PubMed] [Google Scholar]
- 271. Sampson MJ, Davies IR, Braschi S, Ivory K, Hughes DA. 2003. Increased expression of a scavenger receptor (CD36) in monocytes from subjects with type 2 diabetes. Atherosclerosis 167:129–134 [DOI] [PubMed] [Google Scholar]
- 272. Fukuhara-Takaki K, Sakai M, Sakamoto Y, Takeya M, Horiuchi S. 2005. Expression of class A scavenger receptor is enhanced by high glucose in vitro and under diabetic conditions in vivo: one mechanism for an increased rate of atherosclerosis in diabetes. J Biol Chem 280:3355–3364 [DOI] [PubMed] [Google Scholar]
- 273. Ehses JA, Perren A, Eppler E, Ribaux P, Pospisilik JA, Maor-Cahn R, Gueripel X, Ellingsgaard H, Schneider MK, Biollaz G, Fontana A, Reinecke M, Homo-Delarche F, Donath MY. 2007. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes 56:2356–2370 [DOI] [PubMed] [Google Scholar]
- 274. Hess D, Li L, Martin M, Sakano S, Hill D, Strutt B, Thyssen S, Gray DA, Bhatia M. 2003. Bone marrow-derived stem cells initiate pancreatic regeneration. Nat Biotechnol 21:763–770 [DOI] [PubMed] [Google Scholar]
- 275. Weksler-Zangen S, Raz I, Lenzen S, Jörns A, Ehrenfeld S, Amir G, Oprescu A, Yagil Y, Yagil C, Zangen DH, Kaiser N. 2008. Impaired glucose-stimulated insulin secretion is coupled with exocrine pancreatic lesions in the Cohen diabetic rat. Diabetes 57:279–287 [DOI] [PubMed] [Google Scholar]
- 276. Shinohara M, Masuyama T, Shoda T, Takahashi T, Katsuda Y, Komeda K, Kuroki M, Kakehashi A, Kanazawa Y. 2000. A new spontaneously diabetic non-obese Torii rat strain with severe ocular complications. Int J Exp Diabetes Res 1:89–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Masuyama T, Komeda K, Hara A, Noda M, Shinohara M, Oikawa T, Kanazawa Y, Taniguchi K. 2004. Chronological characterization of diabetes development in male Spontaneously Diabetic Torii rats. Biochem Biophys Res Commun 314:870–877 [DOI] [PubMed] [Google Scholar]
- 278. Gepts W. 1965. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes 14:619–633 [DOI] [PubMed] [Google Scholar]
- 279. Gepts W, In't Veld PA. 1987. Islet morphologic changes. Diabetes Metab Rev 3:859–872 [DOI] [PubMed] [Google Scholar]
- 280. Jansen A, Homo-Delarche F, Hooijkaas H, Leenen PJ, Dardenne M, Drexhage HA. 1994. Immunohistochemical characterization of monocytes-macrophages and dendritic cells involved in the initiation of the insulitis and β-cell destruction in NOD mice. Diabetes 43:667–675 [DOI] [PubMed] [Google Scholar]
- 281. Mandrup-Poulsen T. 1996. The role of interleukin-1 in the pathogenesis of IDDM. Diabetologia 39:1005–1029 [DOI] [PubMed] [Google Scholar]
- 282. Zuccollo A, Navarro M, Catanzaro O. 1996. Effects of B1 and B2 kinin receptor antagonists in diabetic mice. Can J Physiol Pharmacol 74:586–589 [PubMed] [Google Scholar]
- 283. Rabinovitch A. 1998. An update on cytokines in the pathogenesis of insulin-dependent diabetes mellitus. Diabetes Metab Rev 14:129–151 [DOI] [PubMed] [Google Scholar]
- 284. Catanzaro OL, Dziubecki D, Labal E, Sirois P. 2010. Activation of peritoneal macrophages during the evolution of type 1 diabetes (insulitis) in streptozotocin-treated mice. Peptides 31:1884–1887 [DOI] [PubMed] [Google Scholar]
- 285. Chikano S, Sawada K, Shimoyama T, Kashiwamura SI, Sugihara A, Sekikawa K, Terada N, Nakanishi K, Okamura H. 2000. IL-18 and IL-12 induce intestinal inflammation and fatty liver in mice in an IFN-γ dependent manner. Gut 47:779–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Arnush M, Heitmeier MR, Scarim AL, Marino MH, Manning PT, Corbett JA. 1998. IL-1 produced and released endogenously within human islets inhibits β cell function. J Clin Invest 102:516–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Arnush M, Scarim AL, Heitmeier MR, Kelly CB, Corbett JA. 1998. Potential role of resident islet macrophage activation in the initiation of autoimmune diabetes. J Immunol 160:2684–2691 [PubMed] [Google Scholar]
- 288. Corbett JA, McDaniel ML. 1995. Intraislet release of interleukin 1 inhibits β cell function by inducing β cell expression of inducible nitric oxide synthase. J Exp Med 181:559–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Kröncke KD, Rodriguez ML, Kolb H, Kolb-Bachofen V. 1993. Cytotoxicity of activated rat macrophages against syngeneic islet cells is arginine-dependent, correlates with citrulline and nitrite concentrations and is identical to lysis by the nitric oxide donor nitroprusside. Diabetologia 36:17–24 [DOI] [PubMed] [Google Scholar]
- 290. Van Rooijen N, Sanders A. 1994. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods 174:83–93 [DOI] [PubMed] [Google Scholar]
- 291. van Rooijen N, van Nieuwmegen R. 1984. Elimination of phagocytic cells in the spleen after intravenous injection of liposome-encapsulated dichloromethylene diphosphonate. An enzyme-histochemical study. Cell Tissue Res 238:355–358 [DOI] [PubMed] [Google Scholar]
- 292. Inokuchi C, Ueda H, Hamaguchi T, Miyagawa J, Shinohara M, Okamura H, Namba M. 2009. Role of macrophages in the development of pancreatic islet injury in spontaneously diabetic torii rats. Exp Anim 58:383–394 [DOI] [PubMed] [Google Scholar]
- 293. Liu MC, Proud D, Lichtenstein LM, MacGlashan DW, Jr, Schleimer RP, Adkinson NF, Jr, Kagey-Sobotka A, Schulman ES, Plaut M. 1986. Human lung macrophage-derived histamine-releasing activity is due to IgE-dependent factors. J Immunol 136:2588–2595 [PubMed] [Google Scholar]
- 294. Xiong F, Zhao JC. 2011. Quantification and location of Chlamydia pneumoniae-specific antigen in the walls of abdominal aortic aneurysms. Ann Vasc Surg 25:256–263 [DOI] [PubMed] [Google Scholar]
- 295. Karlsson L, Björck M, Pärsson H, Wanhainen A. 2011. The association between serological markers for Chlamydophila pneumoniae and the development of abdominal aortic aneurysm. Ann Vasc Surg 25:322–326 [DOI] [PubMed] [Google Scholar]
- 296. Karlsson L, Gnarpe J, Bergqvist D, Lindbäck J, Pärsson H. 2009. The effect of azithromycin and Chlamydophilia pneumonia infection on expansion of small abdominal aortic aneurysms—a prospective randomized double-blind trial. J Vasc Surg 50:23–29 [DOI] [PubMed] [Google Scholar]
- 297. Mahony JB, Coombes BK. 2001. Chlamydia pneumoniae and atherosclerosis: does the evidence support a causal or contributory role? FEMS Microbiol Lett 197:1–9 [DOI] [PubMed] [Google Scholar]
- 298. Damy SB, Higuchi ML, Timenetsky J, Reis MM, Palomino SP, Ikegami RN, Santos FP, Osaka JT, Figueiredo LP. 2009. Mycoplasma pneumoniae and/or Chlamydophila pneumoniae inoculation causing different aggravations in cholesterol-induced atherosclerosis in apoE KO male mice. BMC Microbiol 9:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Rantala A, Lajunen T, Juvonen R, Bloigu A, Paldanius M, Silvennoinen-Kassinen S, Peitso A, Vainio O, Leinonen M, Saikku P. 2010. Chlamydia pneumoniae infection is associated with elevated body mass index in young men. Epidemiol Infect 138:1267–1273 [DOI] [PubMed] [Google Scholar]
- 300. Jaworowska A, Bazylak G. 2011. Chlamydophila pneumoniae antibodies may be independently associated with increased BMI and percentage of body fat among women. Int J Obes (Lond) 35:1225–1232 [DOI] [PubMed] [Google Scholar]
- 301. Wang C, Gao D, Kaltenboeck B. 2009. Acute Chlamydia pneumoniae reinfection accelerates the development of insulin resistance and diabetes in obese C57BL/6 mice. J Infect Dis 200:279–287 [DOI] [PubMed] [Google Scholar]
- 302. Oksaharju A, Lappalainen J, Tuomainen AM, Pussinen PJ, Puolakkainen M, Kovanen PT, Lindstedt KA. 2009. Pro-atherogenic lung and oral pathogens induce an inflammatory response in human and mouse mast cells. J Cell Mol Med 13:103–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Sandler C, Lindstedt KA, Joutsiniemi S, Lappalainen J, Juutilainen T, Kolah J, Kovanen PT, Eklund KK. 2007. Selective activation of mast cells in rheumatoid synovial tissue results in production of TNF-α, IL-1β and IL-1Ra. Inflamm Res 56:230–239 [DOI] [PubMed] [Google Scholar]
- 304. Schaloske RH, Dennis EA. 2006. The phospholipase A2 superfamily and its group numbering system. Biochim Biophys Acta 1761:1246–1259 [DOI] [PubMed] [Google Scholar]
- 305. Gong HP, Du YM, Zhong LN, Dong ZQ, Wang X, Mao YJ, Lu QH. 2011. Plasma lipoprotein-associated phospholipase A2 in patients with metabolic syndrome and carotid atherosclerosis. Lipids Health Dis 10:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Wilensky RL, Shi Y, Mohler ER, 3rd, Hamamdzic D, Burgert ME, Li J, Postle A, Fenning RS, Bollinger JG, Hoffman BE, Pelchovitz DJ, Yang J, Mirabile RC, Webb CL, Zhang L, Zhang P, Gelb MH, Walker MC, Zalewski A, Macphee CH. 2008. Inhibition of lipoprotein-associated phospholipase A2 reduces complex coronary atherosclerotic plaque development. Nat Med 14:1059–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Kudo I, Murakami M. 2002. Phospholipase A2 enzymes. Prostaglandins Other Lipid Mediat 68–69:3–58 [DOI] [PubMed] [Google Scholar]
- 308. Triggiani M, Granata F, Frattini A, Marone G. 2006. Activation of human inflammatory cells by secreted phospholipases A2. Biochim Biophys Acta 1761:1289–1300 [DOI] [PubMed] [Google Scholar]
- 309. Triggiani M, Giannattasio G, Calabrese C, Loffredo S, Granata F, Fiorello A, Santini M, Gelb MH, Marone G. 2009. Lung mast cells are a source of secreted phospholipases A2. J Allergy Clin Immunol 124:558–565, 565e1–e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Lindstedt KA, Wang Y, Shiota N, Saarinen J, Hyytiäinen M, Kokkonen JO, Keski-Oja J, Kovanen PT. 2001. Activation of paracrine TGF-β1 signaling upon stimulation and degranulation of rat serosal mast cells: a novel function for chymase. FASEB J 15:1377–1388 [DOI] [PubMed] [Google Scholar]
- 311. von Eckardstein A, Nofer JR, Assman G. 2011. High density lipoproteins and arteriosclerosis. Role of cholesterol efflux and reverse cholesterol transport. Arterioscler Thromb Vasc Biol 21:13–27 [DOI] [PubMed] [Google Scholar]
- 312. Cuchel M, Rader DJ. 2006. Macrophage reverse cholesterol transport: key to the regression of atherosclerosis? Circulation 113:2548–2555 [DOI] [PubMed] [Google Scholar]
- 313. Vedhachalam C, Duong PT, Nickel M, Nguyen D, Dhanasekaran P, Saito H, Rothblat GH, Lund-Katz S, Phillips MC. 2007. Mechanism of ATP-binding cassette transporter A1-mediated cellular lipid efflux to apolipoprotein A-I and formation of high density lipoprotein particles. J Biol Chem 282:25123–25130 [DOI] [PubMed] [Google Scholar]
- 314. Hassan HH, Denis M, Lee DY, Iatan I, Nyholt D, Ruel I, Krimbou L, Genest J. 2007. Identification of an ABCA1-dependent phospholipid-rich plasma membrane apolipoprotein A-I binding site for nascent HDL formation: implications for current models of HDL biogenesis. J Lipid Res 48:2428–2442 [DOI] [PubMed] [Google Scholar]
- 315. Yancey PG, Kawashiri MA, Moore R, Glick JM, Williams DL, Connelly MA, Rader DJ, Rothblat GH. 2004. In vivo modulation of HDL phospholipid has opposing effects on SR-BI- and ABCA1-mediated cholesterol efflux. J Lipid Res 45:337–346 [DOI] [PubMed] [Google Scholar]
- 316. Kokkonen JO, Kovanen PT. 1989. Proteolytic enzymes of mast cell granules degrade low density lipoproteins and promote their granule-mediated uptake by macrophages in vitro. J Biol Chem 264:10749–10755 [PubMed] [Google Scholar]
- 317. Lindstedt KA, Kokkonen JO, Kovanen PT. 1992. Soluble heparin proteoglycans released from stimulated mast cells induce uptake of low density lipoproteins by macrophages via scavenger receptor-mediated phagocytosis. J Lipid Res 33:65–75 [PubMed] [Google Scholar]
- 318. Kokkonen JO, Kovanen PT. 1987. Stimulation of mast cells leads to cholesterol accumulation in macrophages in vitro by a mast cell granule-mediated uptake of low density lipoprotein. Proc Natl Acad Sci USA 84:2287–2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Ma H, Kovanen PT. 1995. IgE-dependent generation of foam cells: an immune mechanism involving degranulation of sensitized mast cells with resultant uptake of LDL by macrophages. Arterioscler Thromb Vasc Biol 15:811–819 [DOI] [PubMed] [Google Scholar]
- 320. Barish GD, Evans RM. 2004. PPARs and LXRs: atherosclerosis goes nuclear. Trends Endocrinol Metab 15:158–165 [DOI] [PubMed] [Google Scholar]
- 321. Yeong P, Ning Y, Xu Y, Li X, Yin L. 2010. Tryptase promotes human monocyte-derived macrophage foam cell formation by suppressing LXRα activation. Biochim Biophys Acta 1801:567–576 [DOI] [PubMed] [Google Scholar]
- 322. Jiang XC, Zhou HW. 2006. Plasma lipid transfer proteins. Curr Opin Lipidol 17:302–308 [DOI] [PubMed] [Google Scholar]
- 323. Oram JF, Vaughan AM. 2006. ATP-Binding cassette cholesterol transporters and cardiovascular disease. Circ Res 99:1031–1043 [DOI] [PubMed] [Google Scholar]
- 324. Marcel YL, McPherson R, Hogue M, Czarnecka H, Zawadzki Z, Weech PK, Whitlock ME, Tall AR, Milne RW. 1990. Distribution and concentration of cholesteryl ester transfer protein in plasma of normolipemic subjects. J Clin Invest 85:10–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Tall AR. 1993. Plasma cholesteryl ester transfer protein. J Lipid Res 34:1255–1274 [PubMed] [Google Scholar]
- 326. Lee-Rueckert M, Vikstedt R, Metso J, Jauhiainen M, Kovanen PT. 2008. Association of cholesteryl ester transfer protein with HDL particles reduces its proteolytic inactivation by mast cell chymase. J Lipid Res 49:358–368 [DOI] [PubMed] [Google Scholar]
- 327. Lee M, Metso J, Jauhiainen M, Kovanen PT. 2003. Degradation of phospholipid transfer protein (PLTP) and PLTP-generated pre-β-high density lipoprotein by mast cell chymase impairs high affinity efflux of cholesterol from macrophage foam cells. J Biol Chem 278:13539–13545 [DOI] [PubMed] [Google Scholar]
- 328. Favari E, Lee M, Calabresi L, Franceschini G, Zimetti F, Bernini F, Kovanen PT. 2004. Depletion of pre-β-high density lipoprotein by human chymase impairs ATP-binding cassette transporter A1- but not scavenger receptor class B type I-mediated lipid efflux to high density lipoprotein. J Biol Chem 279:9930–9936 [DOI] [PubMed] [Google Scholar]
- 329. Lee M, von Eckardstein A, Lindstedt L, Assmann G, Kovanen PT. 1999. Depletion of pre β 1LpA1 and LpA4 particles by mast cell chymase reduces cholesterol efflux from macrophage foam cells induced by plasma. Arterioscler Thromb Vasc Biol 19:1066–1074 [DOI] [PubMed] [Google Scholar]
- 330. Lee M, Calabresi L, Chiesa G, Franceschini G, Kovanen PT. 2002. Mast cell chymase degrades apoE and apoA-II in apoA-I-knockout mouse plasma and reduces its ability to promote cellular cholesterol efflux. Arterioscler Thromb Vasc Biol 22:1475–1481 [DOI] [PubMed] [Google Scholar]
- 331. Lindstedt L, Lee M, Castro GR, Fruchart JC, Kovanen PT. 1996. Chymase in exocytosed rat mast cell granules effectively proteolyzes apolipoprotein AI-containing lipoproteins, so reducing the cholesterol efflux-inducing ability of serum and aortic intimal fluid. J Clin Invest 97:2174–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Lee-Rueckert M, Silvennoinen R, Rotllan N, Judström I, Blanco-Vaca F, Metso J, Jauhiainen M, Kovanen PT, Escola-Gil JC. 2011. Mast cell activation in vivo impairs the macrophage reverse cholesterol transport pathway in the mouse. Arterioscler Thromb Vasc Biol 31:520–527 [DOI] [PubMed] [Google Scholar]
- 333. Lee M, Sommerhoff CP, von Eckardstein A, Zettl F, Fritz H, Kovanen PT. 2002. Mast cell tryptase degrades HDL and blocks its function as an acceptor of cellular cholesterol. Arterioscler Thromb Vasc Biol 22:2086–2091 [DOI] [PubMed] [Google Scholar]
- 334. Djukanoviæ R, Wilson SJ, Kraft M, Jarjour NN, Steel M, Chung KF, Bao W, Fowler-Taylor A, Matthews J, Busse WW, Holgate ST, Fahy JV. 2004. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am J Respir Crit Care Med 170:583–593 [DOI] [PubMed] [Google Scholar]
- 335. Meltzer EO, Berkowitz RB, Grossbard EB. 2005. An intranasal Syk-kinase inhibitor (R112) improves the symptoms of seasonal allergic rhinitis in a park environment. J Allergy Clin Immunol 115:791–796 [DOI] [PubMed] [Google Scholar]
- 336. Tremaine WJ, Brzezinski A, Katz JA, Wolf DC, Fleming TJ, Mordenti J, Strenkoski-Nix LC, Kurth MC; AXYS Ulcerative Colitis Study Group 2002. Treatment of mildly to moderately active ulcerative colitis with a tryptase inhibitor (APC 2059): an open-label pilot study. Aliment Pharmacol Ther 16:407–413 [DOI] [PubMed] [Google Scholar]
- 337. Doggrell SA, Wanstall JC. 2004. Vascular chymase: pathophysiological role and therapeutic potential of inhibition. Cardiovasc Res 61:653–662 [DOI] [PubMed] [Google Scholar]
- 338. Hallgren J, Estrada S, Karlson U, Alving K, Pejler G. 2001. Heparin antagonists are potent inhibitors of mast cell tryptase. Biochemistry 40:7342–7349 [DOI] [PubMed] [Google Scholar]
- 339. Patalano F, Ruggieri F. 1989. Sodium cromoglycate: a review. Eur Respir J Suppl 6:556s–560s [PubMed] [Google Scholar]
- 340. Pearce FL, Befus AD, Gauldie J, Bienenstock J. 1982. Mucosal mast cells. II. Effects of anti-allergic compounds on histamine secretion by isolated intestinal mast cells. J Immunol 128:2481–2486 [PubMed] [Google Scholar]
- 341. Befus AD, Dyck N, Goodacre R, Bienenstock J. 1987. Mast cells from the human intestinal lamina propria. Isolation, histochemical subtypes, and functional characterization. J Immunol 138:2604–2610 [PubMed] [Google Scholar]
- 342. De Filippis D, D'Amico A, Iuvone T. 2008. Cannabinomimetic control of mast cell mediator release: new perspective in chronic inflammation. J Neuroendocrinol 20(Suppl 1):20–25 [DOI] [PubMed] [Google Scholar]
- 343. Saha P, Modarai B, Humphries J, Mattock K, Waltham M, Burnand KG, Smith A. 2009. The monocyte/macrophage as a therapeutic target in atherosclerosis. Curr Opin Pharmacol 9:109–118 [DOI] [PubMed] [Google Scholar]
- 344. Schreiner EP, Kern M, Steck A, Foster CA. 2004. Synthesis of ether analogues derived from HUN-7293 and evaluation as inhibitors of VCAM-1 expression. Bioorg Med Chem Lett 14:5003–5006 [DOI] [PubMed] [Google Scholar]
- 345. Besemer J, Harant H, Wang S, Oberhauser B, Marquardt K, Foster CA, Schreiner EP, de Vries JE, Dascher-Nadel C, Lindley IJ. 2005. Selective inhibition of cotranslational translocation of vascular cell adhesion molecule 1. Nature 436:290–293 [DOI] [PubMed] [Google Scholar]
- 346. Eikelboom JW, Hankey GJ. 2004. Failure of aspirin to prevent atherothrombosis: potential mechanisms and implications for clinical practice. Am J Cardiovasc Drugs 4:57–67 [DOI] [PubMed] [Google Scholar]
- 347. Cayatte AJ, Du Y, Oliver-Krasinski J, Lavielle G, Verbeuren TJ, Cohen RA. 2000. The thromboxane receptor antagonist S18886 but not aspirin inhibits atherogenesis in apo E-deficient mice: evidence that eicosanoids other than thromboxane contribute to atherosclerosis. Arterioscler Thromb Vasc Biol 20:1724–1728 [DOI] [PubMed] [Google Scholar]
- 348. Jawien J, Gajda M, Rudling M, Mateuszuk L, Olszanecki R, Guzik TJ, Cichocki T, Chlopicki S, Korbut R. 2006. Inhibition of five lipoxygenase activating protein (FLAP) by MK-886 decreases atherosclerosis in apoE/LDLR-double knockout mice. Eur J Clin Invest 36:141–146 [DOI] [PubMed] [Google Scholar]
- 349. Jawień J, Gajda M, Olszanecki R, Korbut R. 2007. BAY x 1005 attenuates atherosclerosis in apoE/LDLR-double knockout mice. J Physiol Pharmacol 58:583–588 [PubMed] [Google Scholar]
- 350. Vidal C, Gómez-Hernández A, Sánchez-Galán E, González A, Ortega L, Gómez-Gerique JA, Tuñón J, Egido J. 2007. Licofelone, a balanced inhibitor of cyclooxygenase and 5-lipoxygenase, reduces inflammation in a rabbit model of atherosclerosis. J Pharmacol Exp Ther 320:108–116 [DOI] [PubMed] [Google Scholar]
- 351. Newby AC. 2005. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol Rev 85:1–31 [DOI] [PubMed] [Google Scholar]
- 352. Palermo C, Joyce JA. 2008. Cysteine cathepsin proteases as pharmacological targets in cancer. Trends Pharmacol Sci 29:22–28 [DOI] [PubMed] [Google Scholar]
- 353. Lewiecki EM. 2009. Odanacatib, a cathepsin K inhibitor for the treatment of osteoporosis and other skeletal disorders associated with excessive bone remodeling. IDrugs 12:799–809 [PubMed] [Google Scholar]
- 354. Burnett JR. 2003. Eflucimibe. Pierre Fabre/Eli Lilly. Curr Opin Investig Drugs 4:347–351 [PubMed] [Google Scholar]
- 355. Pillarisetti S, Alexander CW, Saxena U. 2004. Atherosclerosis—new targets and therapeutics. Curr Med Chem Cardiovasc Hematol Agents 2:327–334 [DOI] [PubMed] [Google Scholar]
- 356. Ikenoya M, Yoshinaka Y, Kobayashi H, Kawamine K, Shibuya K, Sato F, Sawanobori K, Watanabe T, Miyazaki A. 2007. A selective ACAT-1 inhibitor, K-604, suppresses fatty streak lesions in fat-fed hamsters without affecting plasma cholesterol levels. Atherosclerosis 191:290–297 [DOI] [PubMed] [Google Scholar]
- 357. Tiwari RL, Singh V, Barthwal MK. 2008. Macrophages: an elusive yet emerging therapeutic target of atherosclerosis. Med Res Rev 28:483–544 [DOI] [PubMed] [Google Scholar]
- 358. Meurs I, Van Eck M, Van Berkel TJ. 2010. High-density lipoprotein: key molecule in cholesterol efflux and the prevention of atherosclerosis. Curr Pharm Des 16:1445–1467 [DOI] [PubMed] [Google Scholar]
- 359. Swaney JB. 1980. Characterization of the high-density lipoprotein and its major apoprotein from human, canine, bovine and chicken plasma. Biochim Biophys Acta 617:489–502 [DOI] [PubMed] [Google Scholar]
- 360. Benatar JR, Stewart RA. 2007. Is it time to stop treating dyslipidaemia with fibrates? N Z Med J 120:U2706. [PubMed] [Google Scholar]
- 361. Meyers CD, Kamanna VS, Kashyap ML. 2004. Niacin therapy in atherosclerosis. Curr Opin Lipidol 15:659–665 [DOI] [PubMed] [Google Scholar]
- 362. Drexel H. 2006. Reducing risk by raising HDL-cholesterol: the evidence. Eur Heart J Suppl 8(Suppl F):F23–F29 [Google Scholar]
- 363. Orekhov AN, Misharin AYu, Tertov VV, Khashimov KhA, Pokrovsky SN, Repin VS, Smirnov VN. 1984. Artificial HDL as an anti-atherosclerotic drug. Lancet 2:1149–1150 [DOI] [PubMed] [Google Scholar]
- 364. Badimon JJ, Badimon L, Galvez A, Dische R, Fuster V. 1989. High density lipoprotein plasma fractions inhibit aortic fatty streaks in cholesterol-fed rabbits. Lab Invest 60:455–461 [PubMed] [Google Scholar]
- 365. Badimon JJ, Badimon L, Fuster V. 1990. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J Clin Invest 85:1234–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. Tardif JC, Grégoire J, L'Allier PL, Ibrahim R, Lespérance J, Heinonen TM, Kouz S, Berry C, Basser R, Lavoie MA, Guertin MC, Rodés-Cabau J. 2007. Effect of rHDL on Atherosclerosis-Safety and Efficacy (ERASE) Investigators. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA 297:1675–1682 [DOI] [PubMed] [Google Scholar]
- 367. Shaw JA, Bobik A, Murphy A, Kanellakis P, Blombery P, Mukhamedova N, Woollard K, Lyon S, Sviridov D, Dart AM. 2008. Infusion of reconstituted high-density lipoprotein leads to acute changes in human atherosclerotic plaque. Circ Res 103:1084–1091 [DOI] [PubMed] [Google Scholar]
- 368. Patel S, Drew BG, Nakhla S, Duffy SJ, Murphy AJ, Barter PJ, Rye KA, Chin-Dusting J, Hoang A, Sviridov D, Celermajer DS, Kingwell BA. 2009. Reconstituted high-density lipoprotein increases plasma high-density lipoprotein anti-inflammatory properties and cholesterol efflux capacity in patients with type 2 diabetes. J Am Coll Cardiol 53:962–971 [DOI] [PubMed] [Google Scholar]
- 369. Shah PK, Nilsson J, Kaul S, Fishbein MC, Ageland H, Hamsten A, Johansson J, Karpe F, Cercek B. 1998. Effects of recombinant apolipoprotein A-I(Milano) on aortic atherosclerosis in apolipoprotein E-deficient mice. Circulation 97:780–785 [DOI] [PubMed] [Google Scholar]
- 370. Shah PK, Yano J, Reyes O, Chyu KY, Kaul S, Bisgaier CL, Drake S, Cercek B. 2001. High-dose recombinant apolipoprotein A-I(milano) mobilizes tissue cholesterol and rapidly reduces plaque lipid and macrophage content in apolipoprotein e-deficient mice. Potential implications for acute plaque stabilization. Circulation 103:3047–3350 [DOI] [PubMed] [Google Scholar]
- 371. Chiesa G, Monteggia E, Marchesi M, Lorenzon P, Laucello M, Lorusso V, Di Mario C, Karvouni E, Newton RS, Bisgaier CL, Franceschini G, Sirtori CR. 2002. Recombinant apolipoprotein A-I(Milano) infusion into rabbit carotid artery rapidly removes lipid from fatty streaks. Circ Res 90:974–980 [DOI] [PubMed] [Google Scholar]
- 372. Parolini C, Marchesi M, Lorenzon P, Castano M, Balconi E, Miragoli L, Chaabane L, Morisetti A, Lorusso V, Martin BJ, Bisgaier CL, Krause B, Newton RS, Sirtori CR, Chiesa G. 2008. Dose-related effects of repeated ETC-216 (recombinant apolipoprotein A-I Milano/1-palmitoyl-2-oleoyl phosphatidylcholine complexes) administrations on rabbit lipid-rich soft plaques: in vivo assessment by intravascular ultrasound and magnetic resonance imaging. J Am Coll Cardiol 51:1098–1103 [DOI] [PubMed] [Google Scholar]
- 373. Ibanez B, Vilahur G, Cimmino G, Speidl WS, Pinero A, Choi BG, Zafar MU, Santos-Gallego CG, Krause B, Badimon L, Fuster V, Badimon JJ. 2008. Rapid change in plaque size, composition, and molecular footprint after recombinant apolipoprotein A-I Milano (ETC-216) administration: magnetic resonance imaging study in an experimental model of atherosclerosis. J Am Coll Cardiol 51:1104–1109 [DOI] [PubMed] [Google Scholar]
- 374. Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, Eaton GM, Lauer MA, Sheldon WS, Grines CL, Halpern S, Crowe T, Blankenship JC, Kerensky R. 2003. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA 290:2292–2300 [DOI] [PubMed] [Google Scholar]
- 375. Waksman R, Torguson R, Kent KM, Pichard AD, Suddath WO, Satler LF, Martin BD, Perlman TJ, Maltais JA, Weissman NJ, Fitzgerald PJ, Brewer HB., Jr 2010. A first-in-man, randomized, placebo-controlled study to evaluate the safety and feasibility of autologous delipidated high-density lipoprotein plasma infusions in patients with acute coronary syndrome. J Am Coll Cardiol 55:2727–2735 [DOI] [PubMed] [Google Scholar]
- 376. Tabet F, Remaley AT, Segaliny AI, Millet J, Yan L, Nakhla S, Barter PJ, Rye KA, Lambert G. 2010. The 5A apolipoprotein A-I mimetic peptide displays antiinflammatory and antioxidant properties in vivo and in vitro. Arterioscler Thromb Vasc Biol 30:246–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377. Navab M, Anantharamaiah GM, Hama S, Garber DW, Chaddha M, Hough G, Lallone R, Fogelman AM. 2002. Oral administration of an Apo A-I mimetic peptide synthesized from D-amino acids dramatically reduces atherosclerosis in mice independent of plasma cholesterol. Circulation 105:290–292 [DOI] [PubMed] [Google Scholar]
- 378. Bloedon LT, Dunbar R, Duffy D, Pinell-Salles P, Norris R, DeGroot BJ, Movva R, Navab M, Fogelman AM, Rader DJ. 2008. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J Lipid Res 49:1344–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379. Datta G, White CR, Dashti N, Chaddha M, Palgunachari MN, Gupta H, Handattu SP, Garber DW, Anantharamaiah GM. 2010. Anti-inflammatory and recycling properties of an apolipoprotein mimetic peptide, Ac-hE18A-NH(2). Atherosclerosis 208:134–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Tall AR, Yvan-Charvet L, Terasaka N, Pagler T, Wang N. 2008. HDL, ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metab 7:365–375 [DOI] [PubMed] [Google Scholar]
- 381. Puato M, Faggin E, Rattazzi M, Zambon A, Cipollone F, Grego F, Ganassin L, Plebani M, Mezzetti A, Pauletto P. 2010. Atorvastatin reduces macrophage accumulation in atherosclerotic plaques: a comparison of a nonstatin-based regimen in patients undergoing carotid endarterectomy. Stroke 41:1163–1168 [DOI] [PubMed] [Google Scholar]
- 382. Nomura S, Shouzu A, Omoto S, Inami N, Shimazu T, Satoh D, Kajiura T, Yamada K, Urase F, Maeda Y, Iwasaka T. 2009. Effects of pitavastatin on monocyte chemoattractant protein-1 in hyperlipidemic patients. Blood Coagul Fibrinolysis 20:440–447 [DOI] [PubMed] [Google Scholar]
- 383. Corti R, Fuster V, Fayad ZA, Worthley SG, Helft G, Smith D, Weinberger J, Wentzel J, Mizsei G, Mercuri M, Badimon JJ. 2002. Lipid lowering by simvastatin induces regression of human atherosclerotic lesions: two years' follow-up by high-resolution noninvasive magnetic resonance imaging. Circulation 106:2884–2887 [DOI] [PubMed] [Google Scholar]
- 384. Pucci A, Sheiban I, Formato L, Celeste A, Brscic E, Moretti C, De Bernardi A, Alberti A, Bergamasco L, Trevi G, Fuster V. 2007. In vivo coronary plaque histology in patients with stable and acute coronary syndromes: relationships with hyperlipidemic status and statin treatment. Atherosclerosis 194:189–195 [DOI] [PubMed] [Google Scholar]
- 385. Kajimoto K, Miyauchi K, Kasai T, Shimada K, Kojima Y, Shimada A, Niinami H, Amano A, Daida H. 2009. Short-term 20-mg atorvastatin therapy reduces key inflammatory factors including c-Jun N-terminal kinase and dendritic cells and matrix metalloproteinase expression in human abdominal aortic aneurysmal wall. Atherosclerosis 206:505–511 [DOI] [PubMed] [Google Scholar]
- 386. Sudhop T, Lütjohann D, Kodal A, Igel M, Tribble DL, Shah S, Perevozskaya I, von Bergmann K. 2002. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation 106:1943–1948 [DOI] [PubMed] [Google Scholar]
- 387. Brown WV. 2003. Cholesterol absorption inhibitors: defining new options in lipid management. Clin Cardiol 26:259–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Cannon CP, Giugliano RP, Blazing MA, Harrington RA, Peterson JL, Sisk CM, Strony J, Musliner TA, McCabe CH, Veltri E, Braunwald E, Califf RM; IMPROVE-IT Investigators 2008. Rationale and design of IMPROVE-IT (IMProved Reduction of Outcomes: Vytorin Efficacy International Trial): comparison of ezetimbe/simvastatin versus simvastatin monotherapy on cardiovascular outcomes in patients with acute coronary syndromes. Am Heart J 156:826–832 [DOI] [PubMed] [Google Scholar]
- 389. Peto R, Emberson J, Landray M, Baigent C, Collins R, Clare R, Califf R. 2008. Analyses of cancer data from three ezetimibe trials. N Engl J Med 359:1357–1366 [DOI] [PubMed] [Google Scholar]
- 390. Marx N, Sukhova GK, Collins T, Libby P, Plutzky J. 1999. PPARα activators inhibit cytokine-induced vascular cell adhesion molecule-1 expression in human endothelial cells. Circulation 99:3125–3131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391. Xu X, Otsuki M, Saito H, Sumitani S, Yamamoto H, Asanuma N, Kouhara H, Kasayama S. 2001. PPARα and GR differentially down-regulate the expression of nuclear factor-κB-responsive genes in vascular endothelial cells. Endocrinology 142:3332–3339 [DOI] [PubMed] [Google Scholar]
- 392. Graham TL, Mookherjee C, Suckling KE, Palmer CN, Patel L. 2005. The PPARδ agonist GW0742X reduces atherosclerosis in LDLR(−/−) mice. Atherosclerosis 181:29–37 [DOI] [PubMed] [Google Scholar]
- 393. Rosenson RS, Huskin AL, Wolff DA, Helenowski IB, Rademaker AW. 2008. Fenofibrate reduces fasting and postprandial inflammatory responses among hypertriglyceridemia patients with the metabolic syndrome. Atherosclerosis 198:381–388 [DOI] [PubMed] [Google Scholar]
- 394. Oliver WR, Jr, Shenk JL, Snaith MR, Russell CS, Plunket KD, Bodkin NL, Lewis MC, Winegar DA, Sznaidman ML, Lambert MH, Xu HE, Sternbach DD, Kliewer SA, Hansen BC, Willson TM. 2001. A selective peroxisome proliferator-activated receptor δ agonist promotes reverse cholesterol transport. Proc Natl Acad Sci USA 98:5306–5311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395. Chinetti G, Gbaguidi FG, Griglio S, Mallat Z, Antonucci M, Poulain P, Chapman J, Fruchart JC, Tedgui A, Najib-Fruchart J, Staels B. 2000. CLA-1/SR-BI is expressed in atherosclerotic lesion macrophages and regulated by activators of peroxisome proliferator-activated receptors. Circulation 101:2411–2417 [DOI] [PubMed] [Google Scholar]
- 396. Chinetti G, Lestavel S, Bocher V, Remaley AT, Neve B, Torra IP, Teissier E, Minnich A, Jaye M, Duverger N, Brewer HB, Fruchart JC, Clavey V, Staels B. 2001. PPAR-α and PPAR-γ activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat Med 7:53–58 [DOI] [PubMed] [Google Scholar]
- 397. Chawla A, Boisvert WA, Lee CH, Laffitte BA, Barak Y, Joseph SB, Liao D, Nagy L, Edwards PA, Curtiss LK, Evans RM, Tontonoz P. 2001. A PPAR γ-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell 7:161–171 [DOI] [PubMed] [Google Scholar]
- 398. Akiyama TE, Sakai S, Lambert G, Nicol CJ, Matsusue K, Pimprale S, Lee YH, Ricote M, Glass CK, Brewer HB, Jr, Gonzalez FJ. 2002. Conditional disruption of the peroxisome proliferator-activated receptor γ gene in mice results in lowered expression of ABCA1, ABCG1, and apoE in macrophages and reduced cholesterol efflux. Mol Cell Biol 22:2607–2619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399. Li AC, Binder CJ, Gutierrez A, Brown KK, Plotkin CR, Pattison JW, Valledor AF, Davis RA, Willson TM, Witztum JL, Palinski W, Glass CK. 2004. Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARα, β/δ, and γ. J Clin Invest 114:1564–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400. Tontonoz P, Hu E, Spiegelman BM. 1994. Stimulation of adipogenesis in fibroblasts by PPAR γ 2, a lipid-activated transcription factor. Cell 79:1147–1156 [DOI] [PubMed] [Google Scholar]
- 401. Han KH, Chang MK, Boullier A, Green SR, Li A, Glass CK, Quehenberger O. 2000. Oxidized LDL reduces monocyte CCR2 expression through pathways involving peroxisome proliferator-activated receptor γ. J Clin Invest 106:793–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402. Chen Y, Green SR, Ho J, Li A, Almazan F, Quehenberger O. 2005. The mouse CCR2 gene is regulated by two promoters that are responsive to plasma cholesterol and peroxisome proliferator-activated receptor γ ligands. Biochem Biophys Res Commun 332:188–193 [DOI] [PubMed] [Google Scholar]
- 403. Diep QN, El Mabrouk M, Cohn JS, Endemann D, Amiri F, Virdis A, Neves MF, Schiffrin EL. 2002. Structure, endothelial function, cell growth, and inflammation in blood vessels of angiotensin II-infused rats: role of peroxisome proliferator-activated receptor-γ. Circulation 105:2296–2302 [DOI] [PubMed] [Google Scholar]
- 404. Bouhlel MA, Staels B, Chinetti-Gbaguidi G. 2008. Peroxisome proliferator-activated receptors—from active regulators of macrophage biology to pharmacological targets in the treatment of cardiovascular disease. J Intern Med 263:28–42 [DOI] [PubMed] [Google Scholar]
- 405. Han KH, Ryu J, Hong KH, Ko J, Pak YK, Kim JB, Park SW, Kim JJ. 2005. HMG-CoA reductase inhibition reduces monocyte CC chemokine receptor 2 expression and monocyte chemoattractant protein-1-mediated monocyte recruitment in vivo. Circulation 111:1439–1447 [DOI] [PubMed] [Google Scholar]
- 406. Paumelle R, Blanquart C, Briand O, Barbier O, Duhem C, Woerly G, Percevault F, Fruchart JC, Dombrowicz D, Glineur C, Staels B. 2006. Acute antiinflammatory properties of statins involve peroxisome proliferator-activated receptor-α via inhibition of the protein kinase C signaling pathway. Circ Res 98:361–369 [DOI] [PubMed] [Google Scholar]
- 407. Schölkens BA, Landgraf W. 2002. ACE inhibition and atherogenesis. Can J Physiol Pharmacol 80:354–359 [DOI] [PubMed] [Google Scholar]
- 408. da Cunha V, Tham DM, Martin-McNulty B, Deng G, Ho JJ, Wilson DW, Rutledge JC, Vergona R, Sullivan ME, Wang YX. 2005. Enalapril attenuates angiotensin II-induced atherosclerosis and vascular inflammation. Atherosclerosis 178:9–17 [DOI] [PubMed] [Google Scholar]
- 409. Edwards ST, Cruz AC, Donnelly S, Dazin PF, Schulman ES, Jones KD, Wolters PJ, Hoopes C, Dolganov GM, Fang KC. 2005. c-Kit immunophenotyping and metalloproteinase expression profiles of mast cells in interstitial lung diseases. J Pathol 206:279–290 [DOI] [PubMed] [Google Scholar]