Abstract
Sex differences in the biology of different organ systems and the influence of sex hormones in modulating health and disease are increasingly relevant in clinical and research areas. Although work has focused on sex differences and sex hormones in cardiovascular, musculoskeletal, and neuronal systems, there is now increasing clinical evidence for sex differences in incidence, morbidity, and mortality of lung diseases including allergic diseases (such as asthma), chronic obstructive pulmonary disease, pulmonary fibrosis, lung cancer, as well as pulmonary hypertension. Whether such differences are inherent and/or whether sex steroids play a role in modulating these differences is currently under investigation. The purpose of this review is to define sex differences in lung structure/function under normal and specific disease states, with exploration of whether and how sex hormone signaling mechanisms may explain these clinical observations. Focusing on adult age groups, the review addresses the following: 1) inherent sex differences in lung anatomy and physiology; 2) the importance of certain time points in life such as puberty, pregnancy, menopause, and aging; 3) expression and signaling of sex steroid receptors under normal vs. disease states; 4) potential interplay between different sex steroids; 5) the question of whether sex steroids are beneficial or detrimental to the lung; and 6) the potential use of sex steroid signaling as biomarkers and therapeutic avenues in lung diseases. The importance of focusing on sex differences and sex steroids in the lung lies in the increasing incidence of lung diseases in women and the need to address lung diseases across the life span.
Introduction
-
Sex Differences in Lung Structure and Function
Measurement of lung structure and function
Historical studies
Sex differences in prenatal and early postnatal lung
Sex differences in puberty and beyond
-
Sex Differences in Lung Diseases
Asthma
Atopy and allergic rhinitis
COPD and lung cancer
Fibrotic diseases
Pulmonary hypertension
Other conditions
-
Sex Steroids in Lung Physiology and Pathophysiology
Sex steroid signaling
Upper and lower airways
Lung parenchyma
Pulmonary vasculature
Immune cells and function
Clinical Implications of Sex Differences and Sex Steroid Signaling
Future Directions
Conclusions
I. Introduction
Sex differences in health and disease as clinical and research issues have long been topics of interest, especially in cardiovascular structure and function (1–5), metabolism (6–9), and cognition (10–15). Despite such recognition, in 2001, the Institute of Medicine (IOM) published an enlightening report (“Exploring Biological Contributions to Human Health—Does Sex Matter?”; http://www.nap.edu/openbook.php?isbn=0309072816), which highlighted the importance of sex as a biological variable (rather than an observational feature) with recommendations for including sex as a factor in clinical practice norms as well as a topic of bench and clinical research. These recommendations are reiterated in an article in the journal Nature (497). Although the IOM report focused on sex differences in several organ systems (other than the reproductive system), there was surprisingly little attention paid to the respiratory system, i.e., the upper and lower conducting airways and the lung parenchyma.
Inherent sex differences in the lung are apparent from early in life and throughout the human life span. Both clinical and basic research studies have examined sex differences in lung structure and function in both health and disease. Although some studies have focused on the likely role of hormones in sex differences, others have explored inherent physiological differences as well as sociocultural factors. There is also considerable epidemiological evidence for a role for sex in the incidence, susceptibility and severity of a variety of lung diseases. Here, the healthcare and financial burden of lung disease is certainly not trivial (∼35 million persons with chronic lung disease and 350,000 deaths in the United States alone in 2006; American Lung Association 2006 lung disease data), and it highlights the importance of recognizing and studying sex differences in lung anatomy and physiology. As an example, Fig. 1 compares the average number of publications per year that focus on “sex” and “heart” (or other terms relating to the cardiovascular system) to “sex” and “lung” (or other terms relating to the respiratory system). Although research regarding influence of sex on lung diseases is not as well studied as in the cardiovascular realm, there appears to be a steady increase from year to year. Accordingly, the major goal of this review is to highlight this growth in research relating to sex and the lung. We will largely address research on the adult lung, where most of the past work has focused, with brief backgrounds on prenatal, early postnatal, and prepubertal sex differences in lung anatomy/physiology and the influence of sex steroids. The reader will be referred to excellent and detailed reviews by other investigators on these topics.
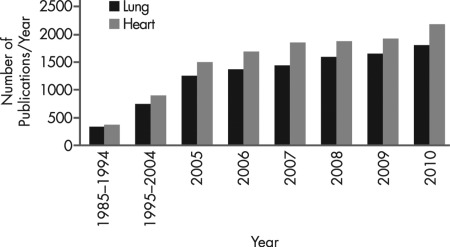
Figure 1.
Publications relating to sex and the lung. A PubMed search was conducted to compare the number of articles published per year related to sex differences in the lung vs. heart. Search terms (title, abstract, and keywords) used were “sex” or “gender” along with “heart, cardiac, vascular (not pulmonary)” to define the broad term “heart” represented in the figure. The search was repeated with “sex” or “gender” and search terms “respiratory, lung, airway, and pulmonary,” which comprise the term “lung.” Searches were conducted annually from 2005 to 2010 (for recent trends), and decade searches 1985–1994 and 1995–2004 (with average numbers of articles per year presented). Over the last two decades, there has been a substantial increase in the number of publications related to sex and lung, with tremendous increases in recent years. However, these numbers remain substantially smaller than those involving sex and heart. Nonetheless, the encouraging upward trend in studies illustrates the recognition that visibility of and research on sex differences in the lung needs to be enhanced. Within this subset is the additional recognition of the role of sex steroids in lung physiology and pathophysiology.
This review briefly introduces modern techniques and parameters for clinical and bench-research assessment of lung structure and function (defining common terms and abbreviations; Section II). With a brief historical perspective, we will describe what is currently known regarding inherent lung structure and function in the two sexes (focusing largely on the postnatal time period), and highlighting key life events involving large hormonal changes such as puberty, pregnancy, and menopause. Subsequently, clinical data on sex differences in specific lung diseases will be summarized (Section III), setting the stage to address the roles of inherent sex differences, as well as sex steroids in lung health and disease. We have focused on diseases where sex differences have been recognized, with the understanding that absence of sex difference does not necessarily rule out a role for sex steroids influencing a particular disease. However, considering the limited data, we felt it important to at least highlight diseases where sex steroid signaling could be implicated. Furthermore, these diseases span different cellular and extracellular components of the lung, and thus provide a rationale for dividing the subsequent section (Section IV) along these lines. Here, we discuss controversies not only in the epidemiology, but also in the potential role of sex steroids as being beneficial vs. detrimental. Nonetheless, Sections II and III will establish the importance of researching the lung from a sex-specific perspective. Section IV will then systematically examine what is known regarding expression and signaling of steroid receptors in lung components, introducing in vitro cellular studies, animal work, as well as examination of human samples. The impact of sex differences and steroid signaling on specific lung components in the disease process will be discussed with the understanding that many lung diseases involve multiple cell types. Finally, we will provide commentaries on the importance of sex differences and sex steroids in lung health and disease and identify areas where more basic and clinical research is needed (Section V). Given the increasing recognition of the importance of sex steroids in the lung, it is only a matter of time before modulation of their signaling is considered a viable therapeutic option. Furthermore, if sex differences in specific diseases are related to expression or function of steroid signaling pathways, then perhaps these pathways could serve as markers of disease risk, severity, and/or outcome. These issues will be briefly discussed (Section VI).
II. Sex Differences in Lung Structure and Function
A. Measurement of lung structure and function
Evaluation of lung structure and function is an important aspect of pulmonary research and medicine. Clinically and in the human-based laboratory setting, several methods are commonly used: spirometry, measurement of lung volumes, and quantification of diffusing capacity. Spirometry is the most common pulmonary function test for measurement of volume and air flow rate during inhalation and exhalation. Pneumotachographs generated via spirometry are used to assess clinical conditions such as obstructive pulmonary diseases [e.g., asthma, chronic obstructive pulmonary disease (COPD), and restrictive diseases, e.g., pulmonary fibrosis (PF), respiratory muscle weakness]. Two important measurements in spirometry are forced expiratory volume in 1 sec (FEV1) and forced vital capacity (FVC). The ratio of FEV1 to FVC is a useful parameter for distinguishing obstructive vs. restrictive lung diseases. In general, decreased FEV1/FVC suggests an obstructive condition, whereas a normal or even increased ratio with substantial reduction in FVC is seen as a restrictive problem (16–18). Flow-volume loops, which include forced inspiratory and expiratory maneuvers, are useful in determining static and dynamic obstructions to airflow. Here, larger, less compliant airways with rigid structures such as cartilage contribute significantly to static resistance to airflow, whereas smaller more compliant airways within the lung can dynamically vary in resistance. Other parameters such as forced expiratory flow (FEF) are calculated using flow-volume loops, and these additional parameters are used to differentiate between effort-dependent and effort-independent expiratory airflow. Contractility or reactivity of the airways is tested standardly using the methacholine challenge wherein increasing concentrations of this bronchoconstrictor agonist are provided by nebulization, and changes in resistance to positive pressure inspiratory and expiratory airflow are measured. Concurrently, bronchodilator testing is useful in determining the reversibility of airway obstruction in diseases such as asthma or COPD. Measurement of maximal inspiratory and expiratory pressures is performed to determine causes of decreased vital capacity (VC) or muscle strength. Measurement of lung volumes complements spirometry. Common measurements include total lung capacity (TLC), functional residual capacity (FRC), and residual volume, which are used to distinguish between disease types. Measurement of diffusing capacity for carbon monoxide (DLCO) assesses gas exchange. Considering the long-standing testing and use of these measurement techniques and parameters, scales for males and females of different age groups are available, allowing for comparisons between studies and equally effective detection of disease conditions in both sexes.
Within the bench laboratory setting, many of the tests and parameters used in humans can also be applied to a range of test animals. For example, a commonly used technique is the noninvasive unrestrained (or restrained) whole body plethysmography where animals are enclosed in rigid chambers and their breathing patterns, tidal volumes, and other parameters are measured using pneumotachometers. A useful derived parameter commonly used to report resistance to inspiratory/expiratory airflow is enhanced pause (Penh) (19, 20), with the caveat that there is disagreement regarding the physiological variables that are represented by Penh (some investigators believe that Penh may reflect sensory nervous activity or airway irritability rather than actual changes in airway resistance). A second, and complementary but invasive technique to measure lung function is by direct measurement of resistance and compliance using an endotracheal tube or tracheostomy in an anesthetized (sometimes paralyzed) animal. Both the noninvasive and invasive approaches are amenable to the methacholine challenge test with bronchodilator response. In small animal studies, a common procedure after invasive or noninvasive measurement of airway mechanics is the collection of bronchoalveolar cells and fluid using lavage, which can then be analyzed for relative amounts of immune cells (neutrophils vs. macrophages vs. eosinophils) or cytokines and other inflammation mediators.
At the tissue and cell levels, gross structures can be evaluated in lungs that are inflated at standardized pressures [e.g., 25 mm Hg in the mouse (21)] and fixed (e.g., formalin or paraformaldehyde) or rapidly frozen. Changes in airway or pulmonary vascular structures are typically determined in sections processed via immunohistochemistry, hematoxylin-eosin staining, or other histological procedures to identify specific cell types. Here, standards have been introduced for measurement of airway epithelial vs. smooth muscle dimensions, and alveolar branching and septation, thus allowing comparison across studies (21). Immunostaining allows for semiquantitative evaluation of expression and localization of proteins within different parts of the lung (22). Many studies use gel electrophoresis or PCR of whole lung samples to determine changes in protein or mRNA. However, the heterogeneity in cell type and cell-specific differences in expression of the same protein within the lung frequently limit interpretation. Other techniques such as fluorescence immunohistochemistry followed by digital imaging (e.g., confocal microscopy) can be used to provide semiquantitative estimation of expression and localization of proteins within specific parts of the lung (22). A relatively new technique that may be helpful is laser capture microdissection (23, 24) wherein specific areas (e.g., epithelium vs. smooth muscle vs. endothelium) or even single cells can be excised from lung sections using a laser and then analyzed for mRNA or protein (23, 24).
B. Historical studies
In the mid 19th century, a study by Hutchinson (25) identified sex differences in breathing mechanics using basic spirometry. In the few women studied, breathing maneuvers equivalent to VC measurements were attributed to rib muscle contractions in women, compared to a diaphragm-based effort in men. Rib-based breathing in women was attributed to accommodation for impaired diaphragmatic function in the pregnant woman. In contrast, Ellis (26) found that VC in women, when corrected for height, was smaller, attributing this to presumed lower metabolic rate of women and thus less need for larger breaths. In perhaps the first systematic study of hormonal effects on respiratory parameters (although not the primary goal of the study), Ott (27) performed daily measurements of VC, respiratory muscle force, and other characteristics in healthy women over several months and menstrual cycles. A complex index representing “functional energy” was found to be at approximately 50% midcycle, peaking to 75% before menstruation, and then ebbing substantially after menstrual bleeding. These early studies are described in more detail in a recent review by Becklake and Kauffmann (28).
C. Sex differences in prenatal and early postnatal lung
The lung is one of the few organs that continue to develop after birth, up to 2–4 yr of age in humans (28–30), with continued growth and changes in lung complexity well into adolescence (28, 29, 31). Sex differences in lung development and maturation have been observed as early as 16–24 wk gestation (32, 33). For example, the number of bronchi is fewer in female fetuses compared with males, but female fetuses mature faster (34). Later in gestation, surfactant is produced earlier by female fetuses and is thought to help maintain patency of small airways (35, 36). Neonatal females have higher expiratory flow rates compared with male neonates when corrected for size (37), a comparison that remains true throughout their life span (see Section II.D). Table 1 summarizes sex differences in lung anatomy and physiology over the course of prenatal lung development and postnatal growth into adulthood. The reader is referred to other in-depth reviews on this fascinating topic (28, 30, 38–42). In addition to inherent sex differences in the lung in utero, maternal and/or fetal sex steroids can also have effects. The reader is referred to excellent in-depth reviews on the complex role of steroids in the developing lung (28, 39, 43, 44).
Table 1.
Influence of sex steroids on the lung at different life stages in humans
| Age/time frame | Lung anatomy and physiology | Observed sex difference | Refs. |
|---|---|---|---|
| 16–26 wk gestational age (canalicular phase of lung development) | Fetal breathing movements; bronchial lumens enlarge; vascularization of lung tissue. Respiratory bronchioles and alveolar ducts develop from terminal bronchioles (wk 24). | Females exhibit mouth movements earlier than males. | 32 |
| 26–36 wk gestational age (saccular phase of lung development) | Establishment of blood-air interface (respiratory epithelium with type I and II alveolar cells); surfactant production; terminal sacs form and expand; overall increase in lung volume. | Females produce surfactant earlier than males. | 35, 36 |
| 36 wk (first postnatal week) | Alveoli form and multiply; increased FEF and decreased airway resistance. | Females have smaller lungs and lower specific airway resistance than males. | 37, 41, 42 |
| Up to 1 yr of age | Lung grows linearly with age and airway resistance increases rapidly; alveolar multiplication continues. | Females have higher FEF rates than males. | 32, 37, 38, 42 |
| Prepubertal childhood years (1–10 yr) | Alveolar multiplication continues (up to 2 yr); lung growth is dysanaptic. | Females have larger airways in relation to lung size and lower specific resistance than males. | 30, 34, 491 |
| Female lungs are smaller overall as compared to males. | 37, 42 | ||
| Large airways grow proportionately to lung in females, this growth lags in males. Smaller airways grow faster in females as compared to males. | 48 | ||
| Adolescence, puberty (10–18 yr) | Lung growth increases with age (FVC and TLC). | Female growth velocity for FVC peaks sooner than males. Specific airway resistance decreases in females up to 18 yr but not in males. Higher effort independent flows in women than men. | 37, 42, 52, 492 |
| Adults (20–70 yr) | Peak expiratory flow, FEV1, and transpulmonary pressure decrease with age; decrease in lung elastic recoil, vital capacity, chest wall compliance; increased residual volume and FRC; overall increase in airway conductance with age. | Males have higher PEF than women; higher airway conductance in males than females; no observed differences in elastic recoil or chest wall compliance. | 56, 57, 87, 493, 494 |
| Pregnancy (8–36 wk) | No changes in FVC, FEV1, VC, airway resistance, or lung compliance throughout term (12 wk to 4 months postpartum). | 53, 54 | |
| Pregnancy (third trimester) | TLC and FRC decreased at 36 wk gestation; increase in inspiratory capacity in third trimester; increase in specific airway conductance (returns to normal 5 wk postpartum); 50% reduction in total pulmonary resistance. | 53, 54 |
PEF, Peak expiratory flow.
The clinical relevance of intrinsic and steroid-induced sex differences in lung development lies in the greater susceptibility of premature male infants to respiratory distress syndrome (RDS; Section III.F) (36, 45), presumably due to decreased surfactant production and larger lung size compared with the premature female infant. Premature male infants are also more susceptible to bronchopulmonary dysplasia (BPD; see Section III.F). Furthermore, early establishment of differences in lung structure and function may underlie or influence the course of diseases such as asthma in childhood and in adults (see Section III.A).
Early postnatal lung development and maturity occurs predominantly via exponential increases in number and size of alveoli (29, 46). At birth, the female lung is smaller than that of the male, with fewer respiratory bronchioles (30, 47), but not the number of alveoli per unit area. Accordingly, the total number of alveoli and surface area are actually greater in males throughout childhood.
D. Sex differences in puberty and beyond
During childhood and adolescence, female airways and lung parenchyma grow proportionally, whereas in males airway growth lags behind the growth of the lung, thereby exhibiting disproportionately fewer alveoli for the number of airways in boys (43, 48, 49), a concept called dysanapsis (for details, see Refs. 28, 40, 47, 50, and 51). Briefly, dysanapsis suggests that airway length, not diameter, determines FEF rates. Thus, larger lungs (e.g., male lungs) would have longer conducting airways and would thus be at a disadvantage during expiration [reflected by a higher dysanapsis index as determined by Mead (50)]. This concept has been confirmed by several lines of evidence based in lung morphometry, physiological measurements, and clinical evaluations (28, 40, 47, 51). The clinical and functional significance lies in methods to normalize lung function for differences in lung size, especially in the prepubertal period when growth rates for boys and girls differ substantially.
The proportional growth of the airways and lungs in females results in lower specific airway resistance and facilitates larger FEF rates (37). However, when somatic growth has ceased, VC, TLC, and peak flows are larger in males compared with females of equal height, although females still have larger expiratory flow rates (28, 52). Assuming that the original finding of Hutchinson (25) (Section II.B) of greater diaphragmatic contribution in men is valid, the contribution of respiratory muscles could be a confounding factor. During adolescence, males generate higher respiratory pressures than females at all lung volumes, which is attributed to the influence of testosterone and the changing shape of the thorax and respiratory muscles during puberty (52). However, such effects cannot compensate for the higher specific airway resistance in men.
Sex differences in lung size of neonates and prepubertal children, normalized for height, are maintained through the greater pubertal growth spurt of girls (41, 47). However, the greater effort-independent expiratory flow rates observed in prepubertal girls are less obvious in young women where the ratio of residual volume to TLC is increased (42) such that expiratory flow rates normalized to TLC are comparable between the sexes. Age-related increases in FVC occur more in men in their early postpubertal years, partly due to the greater respiratory pressures generated by males via a male sex steroid effect (52). Nonetheless, sex differences in the dysanaptic growth of the airways vs. lungs are maintained such that in adulthood, FEV1/FVC is higher in women (52), which should place them at an advantage in terms of respiratory mechanics and airflow. The importance of these differences lies in sex differences in diseases such as asthma in the prepubertal period and changes in incidence and severity that occurs after puberty (discussed in Section III.A).
Although sex differences in lung function noted in the postpubertal period are maintained during adulthood, pregnancy marks a unique situation in women where anatomical changes of the uterus affect the diaphragm and thorax, while resulting physiological changes also take place. TLC and FRC both decrease in the third trimester, due in part to fetal orientation compressing the abdominal contents into the thorax (53, 54). Despite these changes, FVC, FEV1, or VC are not decreased due in part to increases in inspiratory capacity and a 50% decrease in pulmonary resistance (54). The relevance of these changes lies in the modulation of asthma symptoms and severity during pregnancy, although such anatomical and functional changes alone are not entirely contributory (see Section III.A).
As adults age, independent of disease pathology, there is a loss of lung function. Lung elastic recoil and elastic recoil of the large airways decreases, along with a decrease in alveolar air volume with age, whereas the relative amount of connective tissue in the lungs increases (55, 56). These anatomical changes lead to a decrease in maximal expiratory flow rate for both men and women; however, the rate of decrease in lung elastic recoil and maximal expiratory flow rates tends to be greater in men compared with women (57, 58). Additionally, women are older at the onset of these decreases in lung function. The observed sex difference in declining lung function may be a result of smaller overall airways of women having a beneficial effect on lung mechanics or a negative aftermath of dysanaptic growth in boys from the prepubertal period. Thus, throughout adulthood, the female lung should be situated to function better than the male lung. However, this simplistic expectation does not appear to hold true, as discussed in Section III.A with reference to diseases such as asthma.
III. Sex Differences in Lung Diseases
As discussed in Sections II.C and II.D, from infancy through adulthood, intrinsic sex differences in lung anatomy and physiology are present. Although such intrinsic differences could contribute to the pathophysiology of lung disease of prematurity (e.g., RDS and BPD; Section III.F), lung diseases of children and adults are modulated by socioeconomic, cultural, environmental, and behavioral factors. Regardless, clear sex differences in the presentation of diseases such as asthma are apparent throughout the human life span. Before puberty, more boys than girls are diagnosed with asthma, whereas the opposite is true after the onset of puberty during the reproductive years in females. Additionally, there is a staggering increase in the number of females that are being diagnosed with COPD, whereas the number of men being diagnosed has stabilized. Although much of this change in COPD statistics may be a result of increased or maintained numbers of female smokers (compared with a decrease in the number of male smokers), there are data suggesting that women may also be more susceptible to cigarette smoke, additionally contributing to this sex disparity in COPD as well as lung cancer. Overall, this collection of epidemiological data underscores sex differences in the lung and raises the issue of what role inherent differences in lung structure vs. sex hormones play in modulating lung health and disease.
In Section III, we will summarize the current state of clinical knowledge on sex differences in several important lung diseases (especially in adults), providing a basis for considering both intrinsic differences in lung structure and function in males vs. females as well as the potential contribution of sex steroids. Here, it is important to emphasize that it is not always possible to clearly separate intrinsic vs. sex steroid-induced differences; indeed, in the adult lung, it is likely that there is a complex and incompletely understood interaction between these two aspects. Furthermore, considering the multifactorial nature of most lung diseases and the involvement of multiple cell types, differing sex steroid effects on specific cell types in the lung make the issue even more complex. Therefore, in Section III, we focus on aspects of lung diseases that are likely due to intrinsic sex differences in lung structure or function, as well as aspects likely involving the overall effect of sex steroids (where known). In Section IV, we proceed to dissect out the potential contribution of sex steroid effects on specific lung cells contributing to the diseases of Section III.
A. Asthma
Asthma is an inflammatory disease of the airways that is likely multifactorial in origin and involves both intrinsic and environmental factors. Hallmark symptoms of asthma include exaggerated airway narrowing in response to endogenous bronchoconstrictors [e.g., acetylcholine (ACh)] as well as environmental stimuli (e.g., pollen, cigarette smoke), resulting in expiratory airflow limitation and accompanying dyspnea and wheezing (59–65). Despite substantially improved understanding of the pathogenesis of asthma, as well as development and implantation of a large variety of drugs targeting different mechanisms, there continues to be a global increase in the prevalence, morbidity, and even mortality of asthma over the past few decades (∼300 million people worldwide; World Health Organization, Asthma fact sheet no. 307), with the prevalence and severity of pediatric asthma increasing at even higher rates compared with adults or the overall population (66–68). There is now epidemiological evidence for sex differences in asthma and related diseases that may be due to biological susceptibility, age-related changes in the hormonal milieu, environmental exposures, as well as healthcare and socioeconomic factors (69, 70).
Although a number of factors can result in asthma, early events in life may be contributory. With the rates of preterm birth rising, but survival increasing, respiratory morbidity of children born prematurely is high, with poor respiratory and pulmonary function persisting through adulthood. Children with classical BPD (typically born before 1990) who may now be adults demonstrate both structural and functional lung problems (71–77). For example, preterm birth at less than 26 wk is associated with reduced expiratory flow, increased respiratory symptoms, and medication use at 6 yr of age and, as recently demonstrated, to 11 yr of age (prepubertal) (78, 79). Although the use of prenatal steroids and surfactant have reduced the incidence of RDS and classical BPD, a newer form of BPD involving alveolar and vascular dysmorphogenesis still occurs (see Section III.F). It appears that both populations (classical and new BPD) may be at the same risk for childhood respiratory problems including asthma. The reader is referred to several recent reviews on prenatal, perinatal, and early postnatal causes of childhood and adult asthma (71, 80–86).
Regardless of gestational age at birth, in prepubertal children, male airway growth lags behind that of female airway growth, suggesting an inherent risk factor for greater asthma in male children (43, 49, 87). During childhood, more boys than girls are diagnosed with asthma. A confounding factor may be the relative underdiagnosis and undertreatment of girls with asthma (88, 89). Although large-scale systematic studies have not been performed to determine the reasons underlying the underdiagnosis of asthmatic girls, lower activity levels compared with age-matched boys (and thus less frequent triggering of asthma exacerbations) may be contributory. Furthermore, a single study in Swiss children (90) suggested a sex-based bias in recognition of symptoms in girls by healthcare providers, unless the severity and reporting of symptoms was comparable to that of boys [colloquially referred to as Yentl syndrome in the context of coronary artery disease (91)]. Nonetheless, it is likely that asthma symptoms are indeed greater in boys. The disease presents in infancy within the first few years of life. Male gender is a major risk factor for the development of asthma at this early age (92, 93) and the ratio of boys to girls is approximately 2:1 before the age of 5 yr (94, 95), with the risk of chronic asthma being 4-fold greater for boys until the age of 14. Here, it is important to emphasize that both male sex (biological factors) and male gender (socio-constructs relating to outdoor play and indoor pet exposure) are factors for the development of asthma. Thus, sex vs. gender as factors may be difficult to separate in this instance. Nonetheless, these epidemiological data are consistent with the concept of structural dysanapsis and suggest that, in the prepubertal setting of low sex steroid levels, inherent structural differences may be more important to sex differences in asthma in childhood.
Male predominance in asthma persists until the onset of puberty (ages 10–14) when the number of boys and girls with this disease is approximately equal (88, 96). Interestingly, puberty results in a switch in the sex distribution of asthma. After puberty, during the reproductive years, approximately twice as many women as men have asthma (93, 97, 98). The switch in sex ratio for asthma at the time of puberty may be partially attributable to pubertal growth patterns such that female airway and lung sizes are smaller than for males. However, size (i.e., inherent structural differences) alone is not a sufficient explanation, and there is now clear evidence that sex hormones influence asthma development and severity. Girls who undergo early menarche (before the age of 12), have twice the risk of developing asthma after puberty compared with women in whom menarche occurs later (99, 100). The underlying mechanisms for this increased risk or incidence have not been examined. However, as discussed in Section IV, the pleiotropic effects of female sex steroids (particularly estrogens) on airway cells may be contributory.
In adult women, the cyclical variations in sex steroid levels with the menstrual cycle may also influence asthma symptoms. However, the temporal correlation between asthma symptoms and steroid levels does not provide a simple answer to whether estrogen and/or progesterone improve or worsen asthma. Approximately 40% of women with asthma will experience premenstrual asthma, i.e., changes in severity at specific times during their menstrual cycle (101–103). These changes have been documented to occur in the luteal and late-luteal phase of the menstrual cycle when there are large fluctuations in the levels of progesterone and estrogen. However, it should be noted that if anything, worsening of symptoms usually occurs when estrogen levels are on the downswing, suggesting that estrogens may be normally protective for asthma symptoms. Of course, an alternative explanation is that elevated estrogen levels in the preceding days (i.e., midcycle) may manifest as worsening of symptoms after considerable delay, which would make estrogens detrimental to airway function (see Section IV). Regardless of the underlying mechanisms, changes in symptoms often lead to increased bronchodilator use and increased hospitalization (93, 101, 104). Some women with mild to moderate asthma, but not with severe asthma, have found relief of premenstrual asthma exacerbations with the use of oral contraceptives (OC) which suppress large fluctuations in circulating hormones (105, 106).
These effects of exogenous estrogens modulating premenstrual asthma negate a simple explanation of beneficial vs. detrimental effects of sex steroids on asthma, underscoring the multifactorial nature of such diseases and a potentially complex short- or long-term interplay between sex steroids and underlying pathophysiological mechanisms. Some studies suggest that cyclical variations in asthma severity are partially attributable to abnormal or absent up-regulation of β2-adrenoceptors in circulating lymphocytes of menstruating asthmatic women during the late luteal phase; however, increased responsiveness of lymphocytes to cAMP may compensate for the lack of receptor up-regulation in these women (107). Furthermore, exhaled nitric oxide (eNO) levels (a marker of inflammation) increase in nonasthmatic women (108) during the luteal phase, suggesting that premenstrual asthma involves increased airway inflammation (102, 109, 110). A number of other mechanisms have been proposed including altered airway contractility, progesterone effects on bronchodilation, and altered immune function. Current knowledge on these important topics is presented in greater detail in Section IV.
Women with asthma face another challenge of sex steroid influence with pregnancy. Asthma is the most frequent respiratory disease experienced by pregnant women [as many as 8% of all pregnant women have asthma (111, 112)]. Estrogen and progesterone concentrations rise steadily during pregnancy and reach peak levels during the third trimester (93). Despite the number of pregnant women with a diagnosis of asthma, only approximately 10% experience acute asthmatic exacerbations during pregnancy as compared with the near 40% of women experiencing premenstrual worsening (94, 113). It has been reported that approximately one third of women with asthma show an improvement of symptoms in their third trimester, whereas another third demonstrate worsening of their asthma, and a final third show no changes (114, 115). Here, women with severe asthma predominantly show worsening of asthma symptoms during pregnancy compared with women with mild to moderate asthma (115, 116). It is interesting that in both premenstrual variations and during pregnancy, severely asthmatic women appear to have worsening of symptoms, are less responsive to bronchodilator therapy than mild to moderate asthmatic women, and do not benefit from OC in alleviating premenstrual exacerbations (106). Whether female sex steroids contribute to this recalcitrant situation, or whether severe asthma is a different entity altogether (117–119) remains to be determined. An interesting caveat here is the limited data suggesting that the sex of the fetus can influence severity of maternal asthma symptoms. Androgens have antiinflammatory properties, and thus male fetuses may protect women prone to asthma exacerbations compared with female fetuses (93, 112). However, other studies have found that the sex of the fetus has no effect on maternal asthma severity (120). Separately, progesterone is known to be a respiratory stimulant and may have bronchodilatory properties (121, 122) (see Section IV). However, the lack of consistent alleviation in asthma symptoms across pregnant women with preexisting asthma suggests the need to explore other mechanisms that could be contributory.
After the shift toward increased incidence and severity of asthma in women after puberty, the next major change occurs at menopause. Menopause is generally characterized by a hormonal shift consisting of high levels of FSH and LH and low levels of estrogen and progesterone (123, 124). The sex ratio for asthma development decreases around the age of menopause and approaches unity, indicating no sex difference in asthma presentation in this age range. Studies have shown that the peak in adult asthma exacerbations in women with preexisting disease occurs at approximately 50 yr of age, the median for onset of menopause (125, 126). In women with preexisting asthma, menopause generally leads to more frequent exacerbations (suggesting that in premenopausal years sex steroids may have been protective); however, there is a slight decrease in the number of reported asthma-related hospital admissions in women more than 50 yr old as compared with women 20–50 yr of age (124). Furthermore, postmenopausal women have a decreased risk of developing de novo asthma compared with age-matched, premenopausal women (127). Although these data would again suggest a role for sex steroids on changes in asthma exacerbations and severity at the time of menopause, as with pregnancy, a simple deleterious vs. protective role for female sex steroids cannot be easily assigned.
Occurrence of asthma after menopause is generally very severe in nature and requires aggressive therapy including high doses of oral corticosteroids (128). Postmenopausal asthma also generally presents without a family history and without atopy, although chronic sinusitis is often observed in conjunction with exacerbations (129). In postmenopausal women with asthma, low serum levels of FSH and LH with high levels of 17β-estradiol were detected, compared with nonasthmatic postmenopausal women (130) as well as age-matched women with preexisting disease (131).
Menopausal hormone therapy (MHT) in women [used by up to 60% of postmenopausal women (132)] has confounding effects on asthma development and airway disease (133). In general, use of MHT, specifically estrogen, increases the risk of developing asthma, as well as increasing asthma symptoms, in a dose-dependent manner (127, 134). One of the largest studies, the U.S. Nurses Health Study (1995–2004), prospectively examined MHT use and postmenopausal asthma onset. In this study, estrogen and estrogen-progesterone combination posed similar risks to the development of asthma, but not COPD (135). A similar, more recent study, although corroborative for the estrogen MHT effects, found no increased risk for new asthma onset in the combined estrogen-progesterone group (134). Taken together, these data suggest that increasing levels of estrogen, either endogenously or exogenously, can increase asthma symptoms and the risk of asthma onset.
The above discussion largely focused on age-related changes in asthma in women. However, it is important to recognize that incidence/severity of asthma in men can also change, albeit not to the same extent. Indeed, in men, severity of asthma is relatively stable from puberty until later in life (e.g., >50 yr) when decreasing serum testosterone levels may contribute to an increase in asthma (136, 137). Taken in combination with the reversal in male:female ratio for asthma at puberty, these data would suggest that testosterone may be beneficial in asthma. On the other hand, a recent study found no correlation between hypogonadism in aged men and asthma (138). Although much research on asthma has focused on the effects of female sex steroids, little has been reported on the effects of exogenous androgens in alleviating asthmatic symptoms. In a small study, testosterone (10 mg/d for 5 d) was found to improve symptoms in 88% of women suffering from premenstrual asthma (139). Female patients suffering from status asthmaticus found rapid, acute relief (within 20 min) with a combined testosterone-gonadotropin injection (12.5 mg, 500 IU, respectively) (139). Although these limited data are from relatively old studies, they do suggest that increased androgen levels may be beneficial in both males and females. Further work is needed to determine the underlying mechanisms and the potential for androgen therapy as an acute alternative therapeutic option in cases of severe asthma. In Section IV, we discuss current understanding of androgen effects on different cellular components of the lung that may contribute to diseases such as asthma.
B. Atopy and allergic rhinitis
Atopy is defined as the tendency to exhibit an adverse IgE response to specific allergens or to show increased responsiveness, measured in wheal size, to skin prick tests (140) (with the caveat that skin reactivity does not always correlate with elevated IgE levels). The relevance of atopy lies in the evidence that skin prick tests correlate with allergic rhinitis, whereas elevated IgE levels are a predictor for allergic asthma (141, 142). Furthermore, allergic sensitization to food can be an underrecognized risk factor for asthma (143).
In general, atopy is more prevalent in prepubertal boys compared with girls, as are asthma and allergic rhinitis (140, 144). This sex difference may be due in part to differences in environmental exposure between girls and boys, reflecting outdoor and recreational activity patterns (145). However, even after puberty, when there is a sex reversal in the prevalence of asthma (Section III.A), men continue to exhibit a similar, if not greater, prevalence of atopy compared with women (146, 147). In contrast, some reports suggest that more women present with atopy after puberty when compared with men (148). This discrepancy in the prevalence of atopy may be attributable to differences in population selection criteria between studies, or to menstrual status of women included in the later study. There is a notable decrease in atopy with age for both males and females, but this is most pronounced in women with preexisting disease after the onset of menopause (149).
At birth, both boys and girls have relatively similar levels of cord blood IgE. However, serum IgE levels rise more rapidly in boys at an early age, contributing to the increased incidence of prepubescent atopy (150). Conversely, women show a sharp decline in serum IgE levels during puberty (141). Males continue to have higher IgE levels than women for every decade (141), although these levels decline steadily in both men and women with age (151). Although males and females with allergic asthma have higher IgE levels than nonasthmatics, male asthmatics have consistently higher IgE levels compared with females (149, 150). Interestingly, women who use OC have significantly higher IgE levels than nonusers, albeit significantly less than those detected in males (149). Women in the periovulatory phase (cycle d 10–20) also show significantly lower IgE levels than women in other phases of the menstrual cycle (141). Increased IgE levels are associated with increased risk of eczema, which carries a female predominance in adults (152) and correlates strongly with the incidence of asthma and allergic rhinitis (153). Accordingly, these patterns in atopy and IgE may reflect sex differences in allergic diseases such as rhinitis and provide clues to underlying mechanisms not only for rhinitis but for asthma as well.
Allergic airway inflammation is marked by increases in eNO and may be a surrogate measure for airway eosinophilia (154). eNO has been found to be generally greater in men compared with women, in both normal subjects and those with atopy (155), corresponding to lower eosinophil counts in women with allergies (156). However, estrogen enhances adhesion of eosinophils to nasal mucosa vasculature as well as eosinophil degranulation, whereas testosterone attenuates this response (157). Additionally, there is a stronger correlation of airway eosinophil count and asthma in women than in men, suggesting that women may be generally more sensitive than men to a given level of eosinophilia (149).
It is thought that estrogens are involved in the production of cytokines as well as triggering T helper 2 (Th2)-dominant immune response (158, 159) (see Section IV.E for more detailed discussion). Progesterone may also produce Th2-dominant cytokines including IL-4 (160). Estrogen and progesterone are both implicated in degranulation of eosinophils (161). In contrast, testosterone has an opposing effect on the immune system (162, 163). It is well-recognized that a majority of immune diseases exhibit a female predominance; however, the role of sex steroids in the incidence and number of exacerbations in atopy and other diseases is still unclear. Regardless, the above, albeit complex, summary highlights the need for recognition and identification of sex-specific factors in atopy and allergic diseases relevant to the airways and lungs.
From the discussion above, it appears likely that in addition to inherent sex differences, sex steroids play a modulatory role in a range of allergic airway diseases. The role of sex steroids has been examined in animal models of asthma (44, 164–168). However, data from these studies are not entirely consistent with the clinical data in humans. Female mice are more susceptible to airway disease (e.g., induced using ovalbumin sensitization and challenge) compared to males (169), but they display higher IgE levels, greater bronchiolar inflammation, and relative resistance to glucocorticoid (166, 170, 171). In these animal models, androgens appear to have a protective role, with estrogens being actually proinflammatory. For example, castration in males exacerbates disease (171), whereas ovariectomy or estrogen receptor (ER) blockade in females alleviates it (reversed by exogenous estrogens) (172). However, in contrast to the expected bronchodilatory effects, exogenous progesterone worsens allergic airway disease in mice (173). An important aspect of these findings that only complicates interpretation is the seemingly opposing effect of estrogens on airway inflammation vs. airway reactivity (the two elements of diseases such as asthma). In contrast to the findings of enhanced inflammation, estrogens appear to protect against airway hyperresponsiveness (AHR) (174, 175) with the absence of estrogens (ovariectomy) or ER signaling (knockout mouse) resulting in lower responsiveness to bronchoconstrictor stimuli (methacholine challenge). In rats, estradiol blunts ACh-induced bronchoconstriction, potentially via enhanced epithelial acetylcholinesterase activity or enhanced nitric oxide (NO) signaling (176). ER seem to be involved here because mice lacking ER show spontaneous AHR, with increased neuronally derived ACh (168). Conversely, AHR is greater in male mice but may be due to androgen effects on neuronally mediated airway reflexes rather than direct effects on the airway itself (177). Overall, these animal data verify a role for sex steroids in asthma as observed in humans. However, the discrepancy between airway inflammation vs. responsiveness suggests that comparison of animal vs. human data, acute vs. chronic alterations in sex steroid levels or action, and the parameters being measured (reactivity vs. inflammation) should be done with caution. Furthermore, it is important to consider the differential effects of sex steroids on specific cell types (e.g., epithelium vs. smooth muscle) and species differences therein that could contribute to discrepant results in the overall effects of steroids on airway function. These issues are discussed in more depth in Section IV.
C. COPD and lung cancer
Despite the recognized harm of both active smoking and passive exposure (secondhand smoke), diseases caused by environmental tobacco smoke exposure are on the rise worldwide. In this regard, secondhand smoke can affect all age groups from the fetus (via placental transfer of toxic components in cigarette smoke) through the aging adult. In utero exposure to cigarette smoke toxins due to maternal smoking carries independent risk for reduced postnatal lung function (178–181) as well as continued respiratory problems such as asthma, wheezing, and respiratory infections in young children (182). Whether sex differences exist in the effect of antenatal cigarette smoke exposure is not clear. However, if cigarette smoke impairs bronchiolar development early in gestation, this will be limiting to airway performance because the number of bronchi are set by birth (unlike postnatal growth of alveoli; Table 1). Considering sex differences in the number of bronchi and the concept of dysanapsis (Section II.D), males may be particularly susceptible. Indeed, maternal smoking during pregnancy, which is associated with significantly reduced FRC and expiratory flow rates in the newborn, has greater effects on male infants (180). Such reduced expiratory flow rates can persist throughout childhood and even early adulthood (183). Thus, it is likely that early exposure to cigarette smoke may set the stage for lifelong lung disease, especially in males. However, in the absence of longitudinal cohort studies in animals or in humans from early postnatal development through senescence, a definite link between fetal exposure to cigarette smoke components and COPD has not been established. Here, it would be of interest to explore sex differences in the progression toward COPD in offspring of mothers who smoked (or were exposed to secondhand smoke) during pregnancy or during the postnatal period.
COPD is a disease characterized by symptoms including progressive dyspnea upon exertion that does not reverse with bronchodilator therapy and is mostly unresponsive to steroids (184–186). It involves emphysematous destruction of lung parenchyma and narrowing of the airways (187). COPD has long been thought of as a disease of the male smoker. However, there has been an alarming rise in the number of women diagnosed with COPD every year compared with men (185, 188), with the number of deaths from COPD being higher in women than in men in both the United States and Canada for the first time (185, 189). Social and environmental factors are likely contributors in this change in diagnosis rate. Women are smoking more than ever, whereas more men are quitting smoking. Approximately 25% of the cigarettes sold in the United States were sold to women in the 1980s, and this number has stayed relatively stable; however, the number of adolescent females who are smoking is increasing steadily (188, 190, 191). Furthermore, physicians are becoming increasingly comfortable in diagnosing COPD in women, whereas previously women were commonly given the diagnosis of asthma, even when decreased lung function did not resolve with bronchodilator therapy (192).
Although socioeconomic and cultural factors may partially explain the sex difference in COPD at present, women may have an inherently higher susceptibility to cigarette smoke than men (193–195). Women typically develop symptoms of the disease at a younger age than men and also have a substantially smaller pack-year smoking history when compared to men. When normalized for pack-years smoked, the rate of lung function decline in women is faster than that of men, with women losing as much as 10 ml/pack-year and men losing 8 ml/pack year (193, 196). In this regard, it is interesting that whereas the female infant displays less susceptibility to maternal smoking (180), the adult female displays greater susceptibility. Here, it is not known whether female sex steroids play a role in modulating susceptibility, or alternatively whether male sex steroids are protective.
In addition to smokers with COPD, women are the majority in a subsection of COPD cases involving nonsmokers or never-smokers (excluding those with α-1 antitrypsin deficiency, a genetic risk factor for early development of COPD). For example, one study found that nearly 80% of those with early-onset COPD were nonsmoking females (197). This corresponded with another study which found that more than 85% of nonsmoking COPD cases were women (198). Because nonsmokers represent only 12% of all COPD cases, these data suggest that women have a higher predisposition to the disease. An alternative hypothesis here is that these data represent more secondhand smoke exposure in women with male partners who smoke.
Women have a greater risk of being hospitalized for COPD-related reasons compared with men. This could be due, in part, to an increased perception of symptoms and a greater tendency to seek healthcare treatment than men (199). Regardless, there was no increased risk of death for women during their hospital stay. Conversely, recent studies suggest that once discharged, women have better outcomes than men when on long-term oxygen therapy (200). Additionally, in patients with COPD who have successfully quit smoking, women had a 2.5-fold greater improvement in lung function compared with men who quit (201).
A diagnosis of COPD encompasses both emphysema and chronic bronchitis with airway narrowing. There exists a sex bias in the type of COPD diagnosed even when normalized for smoking history and severity of disease. Men typically have more emphysematous deterioration of the lung, whereas women tend to have more reactive airways and more pronounced airway narrowing (202, 203). Analysis of computed tomography data from the National Emphysema Treatment Trial showed statistically less emphysema in women despite similar FEV1 compared with their male cohorts. Although MHT could have varying effects on airway function, hormone replacement has been reported to be associated with higher FEV1 in elderly women (204). Interestingly, this may explain why there is a greater bronchodilator response in female, but not male, smoking relatives of early-onset COPD probands.
Overall, the above clinical evidence indicates a clear difference between men and women in the presentation and diagnosis of COPD. Sex, hormonal state, and environmental factors, which include the extent and intensity of cigarette smoke exposure, may all play a role in the observed differences, but much more research is necessary to elucidate the mechanisms underlying these differences. Furthermore, it is important to consider these mechanisms in the context of lung cancer, which can be a devastating consequence of cigarette smoke exposure.
Lung cancer is the leading cause of cancer-related death in both men and women in the United States (205). Sex differences in both clinical and pathological outcomes of patients with lung cancer have been examined extensively (205–210). Lung cancer kills more women each year than breast, uterine, and ovarian cancer combined (211, 212). Although the incidence of lung cancer in men is declining, the incidence in women is continuing to rise, and the observed gender gap in this disease is narrowing (212). The increased incidence in women is partially attributable to increased smoking habits in women, which peaked in 1960 (213). Although the current smoking rate for men is still significantly higher than in women (22.3 vs. 17.4%), women tend to smoke fewer pack-years than men but are diagnosed at an earlier age. Taken together, these data would suggest that women have a higher susceptibility to cigarette smoke (as is also suggested by the COPD statistics) (214–217). Independent of smoking status, however, nonsmokers diagnosed with lung cancer are approximately three times more likely to be female, suggesting an additional hormonal component (210, 216). Fortunately, women diagnosed with lung cancer have better prognoses and 5-yr survival than men at all stages and subtypes of disease including small cell lung cancer, which characteristically carries a poor prognosis (205, 217, 218).
Non-small cell lung cancer (NSCLC; includes adenocarcinoma, squamous cell carcinoma, and large cell carcinoma) is the leading lung cancer histology, accounting for approximately 85% of cases. Among smokers, men have more squamous cell carcinoma, whereas women have more adenocarcinoma (218, 219). When considering nonsmokers, adenocarcinoma is the predominant histology, with women forming the majority of presenters with this disease (206, 217).
The above clinical and epidemiological data would suggest that sex hormones may contribute to lung cancer. However, data on estrogen, progesterone, and MHT prevent clear conclusions from being drawn. Menstrual and reproductive factors related to lung cancer risk were evaluated in multiple studies (220–222). The Shanghai Women's Health Study evaluated the risk of lung cancer in never-smoking females and found reduced risk with increased number of births, later age of menopause, and a longer reproductive period (i.e., greater hormonal exposure). These data were contradicted by others noting that early onset menopause (age 40 yr or younger) increased the risk of adenocarcinoma in women (221), and that longer reproductive periods, defined as early-age menarche and late-age onset of menopause, increased the risk of lung cancer in Japanese never-smokers (222). Furthermore, limited data relating specifically to MHT are mixed, showing increased lung cancer risk, no effect, or decreased lung cancer risk in MHT users (209, 223–226). For NSCLC, women diagnosed at older ages have better survival (227), and higher circulating estrogen levels in general correlate with shorter survival (228). These results suggest that menopausal women not on MHT should have better survival rates, as evidenced by one study (226). Conflicting reports suggest that MHT alone does not alter lung cancer risk in women but increases the risk if women use MHT and continue to smoke, whereas others have found MHT to be protective against lung cancer in female smokers (209). Finally, one of the largest trials evaluating MHT in the United States was the Women's Health Initiative. Lung cancer was not a primary outcome of the study; however, post hoc analysis revealed that there was no significant increase in lung cancer incidence in women receiving hormone therapy.
Overall, the above seemingly conflicting and complex data suggest that the dose, timing, and duration of sex steroids (e.g., number of births, MHT) vs. those of cigarette smoke exposure (e.g., total pack-years, smoking cessation during pregnancy/lactation) are modulating factors in susceptibility and course of lung cancer in women. Although it is not clear how sex steroids influence responses to inhaled tobacco smoke, tumor biology and growth, or response to therapies, it is known that ER are present in normal lung tissue and lung cancer tumors. In fact, ERβ-positive lung tumors are a positive predictor for survival, whereas patients with ERα-positive tumors have decreased survival rates (229, 230). Given this and other sex differences in incidence and survival in lung cancer, further research exploring the role of sex steroids in lung cancer therapies should be pursued.
D. Fibrotic diseases
PF is a progressive disease characterized by inflammation and scarring of the lungs, it affects approximately 5 million persons worldwide, and it is more common in men than women (231). This restrictive lung disease is characterized by progressive decline in FVC and FEV1 as well as a reduction in TLC. The incidence of PF ranges from 1.4:1 to 2.1:1 (male:female) (231, 232). Environmental factors including cigarette smoking increase the odds ratio of developing PF by 2-fold. Median survival is 2–5 yr from diagnosis, and the only viable treatment option is lung transplantation. Mortality rates from PF have been reported to be higher in men compared with women in the United States from 1992–2003 (233). Interestingly, the mortality rates are increasing more rapidly in women than in men, suggesting that the observed sex differences in PF would be abolished or even reversed in the near future (233).
Sex differences in interstitial lung diseases such as PF have been studied in animal models. Cigarette smoke exposure leads to greater emphysematous changes of alveoli of female mice (234). In bleomycin models of PF, higher mortality and greater fibrosis (collagen deposition, cytokine expression) have been observed in female rats compared with males: effects diminished by ovariectomy, but exacerbated by estrogen (235). However, the opposite sex difference has been observed in mouse models of bleomycin injury. The role of sex steroids in these models is not entirely clear. In vitro, estrogens are known to enhance release of profibrotic factors in lung fibroblasts (see Section IV.C). A single study has further suggested that airway fibrosis is controlled by both relaxin and estrogen with the latter having a protective effect in the absence of relaxin (236). In vitro, data regarding development and progression of PF are lacking, and many studies on sex steroid effects on fibroblasts and extracellular matrix proteins have not been lung specific. Given that mortality rates are increasing more rapidly in women, detailed investigation on the relationships between sex hormones and fibrosis is warranted.
E. Pulmonary hypertension
Pulmonary hypertension (PH) is a relatively rare disease that affects approximately one or two people per million, with approximately 300 new cases every year in the United States. However, PH leads to more than 15,000 deaths and more than 250,000 hospital visits per year (237, 238). PH is characterized by mean pulmonary arterial pressure of at least 25 mm Hg, increased vascular proliferation and remodeling, intimal fibrosis, plexiform lesions, right ventricular hypertrophy, and ultimately right heart failure (237, 239, 240). There is female predominance of both idiopathic and familial (heritable) PH, with female:male prevalence ratio from 2:1 to as high as 4:1 (239–241). Additionally, PH generally presents in women in their 30s, approximately 10 yr earlier than in men (242, 243). With the observed female predominance in all types of PH—idiopathic, familial, pulmonary arterial hypertension (PAH), and portopulmonary hypertension—the influence of gender and sex steroids has been a focus of ongoing research and has been reviewed recently (239).
PAH occurs in two distinct age groups of women: those of childbearing age generally in their 30s, and postmenopausal women (240, 244). Early PAH was at first attributed to the now well-recognized adverse effects of appetite suppressants (including aminorex fumarate and fenfluramine) in young women (245, 246); however, despite the removal of such harmful agents from the market, the increased prevalence in women has persisted, suggesting a possible hormonal link. Here, OC, specific to younger women, may be a risk factor (247); however, some epidemiological data do not support this idea (246). The use of MHT in postmenopausal females suggests a protective effect from development of PH (248). Experimental evidence, as well as data from systemic hypertension research, suggests that estrogens have a protective effect on pulmonary vasculature; however, this does not appear to be the case in the predominantly female patient population (240). Several autoimmune diseases associated with the development of PH such as lupus, scleroderma, and rheumatoid arthritis (238, 249) have a female predominance. Estrogen and progesterone have been shown to exacerbate immune responses, whereas androgens are protective (250–252). Given that the majority of PH presents without preexisting autoimmune disorders, the correlation between these diseases, although interesting, does not fully explain the observed sex differences in PH.
Although the role of estrogens in PH is recognized clinically, the direct effects of estrogen and its metabolites on the pulmonary vasculature are still under investigation. Animal data and traditional models of PH show that estrogens have protective effects, yet women are consistently diagnosed more often than men. The role of estrogens and the development of PH have been reviewed extensively (240, 253, 254). Briefly, estrogen is known to stimulate both NO and prostacyclin production in the pulmonary vasculature (255, 256). NO is one of the most effective endogenous vasodilators. Furthermore, continuous iv infusion of epoprostenol has been shown to have beneficial effects in patients with PH, increasing both short- and long-term survival (239). Additionally, estrogen down-regulates gene expression of endothelin-1, which is a potent vasoconstrictor and vascular smooth muscle mitogen (257). Preliminary results employing endothelin-1 receptor antagonist, sitaxsentan, suggest beneficial effects in patients with PH (239). 2-Methoxy estradiol (2ME), an estrogen metabolite, has been shown to inhibit endothelin-1 and stimulate prostacyclin production in vasculature (253). Estrogens themselves have also been shown to be mitogenic (endothelial and vascular smooth muscle cells) and prothrombotic. The development of in situ thromboses is a hallmark of idiopathic PH and may be a result of the hormonal milieu in women who develop this disease. Additionally, effects of estrogen on endothelial growth and dysfunction are unclear; however, data suggest that estrogens negatively regulate endothelial cell plexiform lesion development. Taken together, the above data suggest that sex steroids likely contribute (either positively or negatively) to sex differences in PH pathogenesis, progression, and response to therapy. It is also clear, however, that it is not simply estrogen that makes women more susceptible. Further research into the underlying mechanisms, the roles of progesterone in women, and perhaps a protective role in men is necessary. Here, the differential effects of sex steroids on different cell types of the pulmonary vasculature (endothelium vs. smooth muscle) are also important. Section IV.D explores current understanding of these effects in more detail. Finally, the development of animal models more representative of the human disease will also be essential in discovering novel therapies for the treatment of PH.
F. Other conditions
1. RDS
Advances in neonatal intensive care have greatly increased survival in babies as young as 24 wk gestation. Considering the time course of fetal lung development, the premature infant is at great disadvantage from a respiratory and survival perspective. Indeed, many infants weighing less than 1000 g at birth have RDS (hyaline membrane disease) with initial hypoxia and the need for supplemental oxygen, as well as requiring intubation and mechanical ventilation (258). Male fetuses are at higher risk for premature birth (∼55% of premature infants are boys) and for developing RDS (relative risk, ∼1.6) (36, 259–261). Administration of antenatal corticosteroids to mothers at risk for premature delivery reduces RDS more in female premature infants (262, 263) (however, see recent meta-analysis in Ref. 264 suggesting equal benefit of corticosteroids in males and females). Earlier surfactant production in the female fetus, compared with the males, may play a role in the maintenance of airway patency (28).
2. Bronchopulmonary dysplasia
A chronic disease of the immature lung (265), BPD occurs in more than 20% of the approximately 50,000 premature births in the United States of less than 1500 g weight (266, 267). BPD is a risk factor for childhood asthma and other respiratory illnesses that may persist into adulthood. There is now considerable evidence that preterm male infants are also at higher risk for BPD (268, 269). Although the mechanisms underlying BPD are still under investigation, they include RDS itself, inflammation, exposure to increased oxygen concentration, and (if applicable) barotrauma resulting from mechanical ventilation. In this regard, the levels of TGFβ, known to be involved in tissue repair, are higher in tracheal aspirates of preterm male infants who went on to develop BPD than in preterm males who did not develop BPD (268). The fetus is exposed to high levels of estradiol and progesterone, which decreases by several orders of magnitude after parturition. Premature birth deprives the newborn of these female sex hormones at an earlier stage of lung development. Accordingly, there has been recent interest in the replacement of estrogen and/or progesterone in extremely premature infants who are at risk for BPD (270–272), although the results are not conclusive. Other mechanisms such as sex steroid modulation of NO may also play a role in regulation of airway and vascular tone and remain to be examined.
3. Cystic fibrosis (CF)
Although many of the diseases described above suggest a role for sex steroids, the contribution of inherent sex differences is exemplified by CF, a systemic disease caused by a mutation in the CF transmembrane conductance regulator gene that manifests in airway destruction from repeated infections and impaired mucus clearance. There is no sex-linked difference in the incidence of CF (273); however females aged 1–20 yr have a significantly higher mortality (274, 275) and reduced life span (276) compared with males. In CF, girls have lower FEV1 than males, contrary to what is found in healthy children and adolescents (273, 275). Anatomically, the smaller lungs of females in combination with impaired mucus clearance may explain increased risk of infection in females, another predictor of mortality (275, 276). Interestingly, the relative risk of death for females remains roughly constant at 1.6 throughout childhood, adolescence, and young adulthood, suggesting that hormonal influences at puberty may not be contributory (275, 276). However, recent studies have reported cyclical decreases in lung function in female patients with CF, which may increase their susceptibility to bacterial infection when estrogen levels are low (277). Alternatively, periovulatory (peak) concentrations of estradiol can decrease uridine triphosphate-induced chloride secretion, which may contribute to impaired mucus clearance (278).
IV. Sex Steroids in Lung Physiology and Pathophysiology
A. Sex steroid signaling
The synthesis pathway for sex steroids is thoroughly established (for recent reviews, see Refs. 279 and 280). Both gonadally derived and locally produced sex steroids (281–283) determine the eventual concentration systemically and in various tissues. In this regard, studies evaluating the effects of sex steroids in the lung report values in a variety of formats that range from exogenous molar concentrations to endogenous serum levels, which makes drawing comparisons between studies somewhat difficult. Table 2 provides normal ranges of testosterone, estradiol, and progesterone for men and women (nonpregnant, pregnant, and postmenopausal) in both nanograms per milliliter and molarity and is derived from a number of established sources (284–286).
Table 2.
Serum sex steroid values in humans
| Males | Nonpregnant females |
Pregnant females | Menopausal females | |||
|---|---|---|---|---|---|---|
| Follicular | Preovulatory | Luteal | ||||
| Testosterone | 2–15 ng/ml | 200–800 pg/ml | 200–800 pg/ml | 200–800 pg/ml | 1–1.4 ng/ml | 200–800 pg/ml |
| 6–50 nm | 0.7–2.5 nm | 0.7–2.5 nm | 0.7–2.5 nm | 3.5–5 nm | 0.7–2.5 nm | |
| Estradiol | 15–50 pg/ml | 20–100 pg/ml | 150–400 pg/ml | 60–200 pg/ml | 1–40 ng/ml | 10–30 pg/ml |
| 50–200 pm | 80–500 pm | 0.5–1.5 nm | 0.2–0.8 nm | 1–150 nm | 40–120 pm | |
| Progesterone | 250–900 pg/ml | 0.3–1.2 ng/ml | 0.7–2.5 ng/ml | 1–18 ng/ml | 9–300 ng/ml | <0.2–1.1 ng/ml |
| 0.8–2.8 nm | 0.3–1.5 nm | 2–10 nm | 10–60 nm | 25–1000 nm | 0.6–3.5 nm | |
An important aspect in terms of research on sex steroid effects in the lung or experimental use of sex steroids is the need to approximate physiological serum levels of hormones when assessing the contribution of sex steroid signaling to disease pathogenesis or amelioration. Hormone levels vary greatly in women after puberty, but are more stable in men. Hormonal changes with the menstrual cycle, changes with pregnancy, and menopause can correlate to changes in disease state and symptoms in a variety of lung disease (see text).
Both genomic (11, 287–292) and nongenomic (289–295) aspects of sex steroid signaling (albeit in tissues other than the lung) have been extensively reviewed. Classical sex steroid receptors include two ER (ERα and ERβ), two progesterone receptors (PR-A and PR-B), and the androgen receptor (AR). As members of the superfamily of nuclear receptors (296, 297), these receptors bind their respective hormones, resulting in receptor translocation from cytoplasm to nucleus, where homodimerization and in some cases heterodimerization can occur. Classically, ERα is a better transcriptional activator than ERβ, with the suggestion that ERβ antagonizes ERα (298, 299). Nuclear heterodimers can form between ERα and ERβ, and ERα with AR (287). For progesterone, PR-B is the main activator of gene transcription, whereas PR-A acts as a repressor of PR-B and ER transcription (290, 300). The AR binds both testosterone and the more active metabolite, 5α-dihydrotestosterone (DHT) (301, 302).
Rapid sex steroid signaling events involve membrane-localized steroid receptors, either full-length or truncated, as well as G protein-coupled receptors (GPCR) that are capable of binding sex steroids [e.g., the GPCR30 or GPER that is estrogen-sensitive (303, 304)]. Nongenomic effects of steroids have been reviewed extensively by several authors, and we refer readers to these reviews for more detailed explanations (289–295). A major aspect of nongenomic signaling of sex steroids is modulation of intracellular calcium ([Ca2+]i) (253, 292, 305–307). Modulation of [Ca2+]i can involve increasing Ca2+ via the phospholipase C-diacylglycerol-inositol trisphosphate signaling cascade (305) or more commonly reducing Ca2+ by inhibiting a variety of influx mechanisms such as L-type Ca2+ channels (306, 308), K+ channels (309), or chloride currents (310, 311).
Downstream effectors of GPCR including cyclic nucleotides, protein kinase C, protein kinase A, and protein kinase G, are also modulated by sex steroids (312, 313). Steroid receptor activation can also induce a myriad of intracellular signaling pathways including MAPK, tyrosine kinases, and lipid kinases. In turn, activation of these pathways can alter subsequent steroid receptor activation, including ligand-independent activation or direct phosphorylation of these receptors by MAPK (314–316). Estradiol has been shown to activate ERK1/2, p38, and JNK pathways leading to both c-Jun and c-Fos gene transcription (290, 291, 317, 318). Thus, rapid, nongenomic actions of estrogens can indeed exhibit genomic downstream effects as well. PR-B exhibits cross talk with ER whereby it primes ER to activate the Src-Ras-ERK pathways (319). Additionally, PR can activate p42 MAPK and phosphatidylinositol-3-kinase in Xenopus oocytes (315). In vascular endothelial cells, ERα activation leads to phosphatidylinositol-3-kinase-Akt-eNOS activation producing the vasodilator NO (291, 320). Activation of AR involves the c-Src, Raf-1, and ERK-2 pathways leading to downstream involvement of MAPK (321, 322).
In addition to signaling intermediates, the action of steroid receptors (especially genomic effects) can be further modulated by a large number of coregulators that fine-tune enhancement (coactivator) or suppression (corepressor) of steroid-responsive gene expression. In the absence of ligand, the receptors are associated with heat shock proteins and other chaperones that prevent unwanted activity. With ligand binding, receptor activation, and nuclear translocation, binding to hormone-responsive elements of target genes results in recruitment of coactivators to help up-regulate transcription. In contrast, corepressors interact with steroid receptors in the absence of hormone or in the presence of antihormone. Thus, coregulators can greatly influence sex steroid function. A number of coactivators and corepressors have been identified to date, although the tissue-specific distribution, regulation, and function of such coregulators are still under investigation. Accordingly, more detailed review of this important, but emerging topic is beyond the scope of this article. The reader is referred to a few recent reviews in this area (323–326).
The above brief description of sex steroid signaling highlights the fact that depending on the cell type, steroid concentration, relative receptor expression, coregulators, and the relative importance of these signaling pathways, sex steroids have the potential for wide-ranging and complex modulation of cellular function. Cross talk between receptor signaling pathways may allow for even more nuanced regulation. These issues have not been specifically examined in lung or the cells therein but are likely to be present in this organ as well.
B. Upper and lower airways
1. Nasal epithelium
The majority of the work to date (albeit limited) on sex steroid signaling in nasal epithelium has largely been in the context of diseases such as rhinitis. From these data, a role for sex steroids (particularly estrogens) in nasal epithelium can probably be assumed. The relevance of such a role lies in the modulation of nasal epithelial function during specific phases of adulthood, such as pregnancy where sex steroid levels can change substantially.
ER and PR are present in the nasal mucosa. Interestingly, although AR mRNA has been detected, no protein has been found in human turbinate samples (327). Accordingly, it is likely that the female sex steroids are the ones of relevance in the nasal epithelium. ER and PR expression are substantially up-regulated in the presence of increased circulating levels of estrogen and progesterone, for example as occurs with pregnancy (328). Histological changes consistent with increased edema and mucus secretion in the nasal epithelium of women have been reported to occur with different phases of the menstrual cycle (329–331). These changes may underlie altered rhinitis symptoms in women during the menstrual cycle and with pregnancy. However, the relationship between protein expression and function is less clear. For example, only ERβ (but not ERα or PR isoforms) is expressed in the nasal mucosa of both males and females (332). Some studies suggest that ERβ may actually serve to inhibit action of other steroid receptors (294, 333), and thus estrogen effects on nasal epithelium may occur only in the presence of up-regulated ERα expression (e.g., during pregnancy). Interestingly, progesterone has little to no effect on the nasal mucosa (334). These data would suggest that estrogen rather than progesterone is likely involved in rhinitis exacerbations in women; however, functional studies on sex steroid signaling in allergic rhinitis are few.
Some studies suggest that innate allergy and hypersensitivity to endogenous sex steroids or OC explain changes in rhinitis symptoms in women (335, 336). In one study, nasopharynx-nostril pressure gradients were used to assess nasal mucosal reactivity. Approximately 33% of women who suffered from allergic rhinitis and were also taking OC had a positive nasal response to topical administration of OC. There was no response in control groups, which consisted of males without atopy, females without atopy on OC, or females with atopy not on OC (336). Although there was a positive correlation of rhinitis symptoms in a subset of allergic women on OC in both studies, a large percentage of the cohort in Ref. 336 were unreactive to the sex hormone stimulation per se. However, older studies have reported thickening of nasal mucosa in a variety of mammals subsequent to exogenous estrogen treatment (334, 337). Although actual ER isoform expression was not examined in these studies, these data suggest a more complex influence of sex hormones on allergic rhinitis than a simple increase in mucus production by the epithelium (157, 338, 339). For example, in nasal biopsies from pregnant women suffering from nasal symptoms, there was an up-regulation of cholinergic nerve activity around nasal blood vessels (338). Estrogen also increased the density of muscarinic receptors in nasal mucosa of pregnant guinea pigs (339). Histamine receptor mRNA in cultured human nasal epithelial cells and human mucosal microvascular endothelial cells was increased by estrogen and progesterone (but not testosterone) (340). Histamine is released in response to allergen stimuli, and hyperreactivity of nasal mucosa to histamine is a hallmark of allergic rhinitis.
The clinical and limited basic research data suggest that in the nasal epithelium, a number of mechanisms can be modulated, largely by estrogens. In addition to effects on mucus production, estrogen may affect cholinergic and histaminergic signaling in the nasal passageways, resulting in altered blood flow and mucosal thickening. The relevance of these findings lies in identifying targets (epithelium vs. vasculature vs. innervation) to alleviate nasal symptoms during the menstrual cycle or pregnancy and provide relief to rhinitis sufferers. Furthermore, studies are needed to determine whether mechanisms underlying rhinitis exacerbation in males are similar and involve sex steroid signaling due to local conversion of testosterone. Finally, with the understanding that rhinitis and similar diseases involve a large inflammatory component, it will be important to establish the effect of sex steroids on immune cell function and inflammatory signaling within the epithelium.
2. Bronchial epithelium
The bronchial epithelium serves as a barrier within the airway, an early recipient of and responder to external stimuli including pathogens, allergens, and pollutants, and as a modulator of airway tone. Bronchial epithelial inflammation is a key factor in the pathogenesis of asthma, bronchitis, and a host of other “lung” diseases. Lung cancers (e.g., squamous cell carcinomas, NSCLC) are derived from bronchial epithelial cells (BEC). Accordingly, understanding sex differences in BEC structure/function or the effects of sex steroids on BEC is important. However, few studies have examined the effect of sex steroids on BEC.
In immortalized airway epithelial lines, both ERα (341, 342) and ERβ (342) were present, with ERβ being the predominant isoform. In a recent study, we found that freshly isolated human bronchial epithelial tissue, as well as single BEC express abundant ERα and ERβ in fairly comparable and abundant quantities (343). Whether PR or AR is expressed in BEC is not known, although expression is likely. Regardless, it is possible that as with nasal epithelium, only ER are of relevance in the bronchial epithelium.
The relevance of sex steroid signaling in BEC lies, at least in part, in the potential regulation of NO in the airway. Studies in vascular endothelium have already established that estrogens can facilitate dissociation of endothelial NO synthase (eNOS) from caveolae of the plasma membrane, resulting in activation of the NO pathway and potentiation of vasodilation (344). Akin to endothelium, NO produced by the bronchial epithelium can be a potent bronchodilator (345, 346). Furthermore, with inflammation, eNO is used as an indicator of airway inflammation (110, 154), although the source of such NO may actually be inducible NO synthase (iNOS) (347), with BEC being the predominant source. In an immortalized BEC line H441, estradiol has been shown to acutely increase the conversion of [3H]L-arginine to [3H]L-citrulline through eNOS activation, an effect inhibited by the ER antagonist fulvestrant (ICI 182,780) (341). In a recent study, we found that both ERα and ERβ activation [achieved using receptor-specific ligands (R,R)-5,11-diethyl-5,6,11,12-tetrahydro-2,8-chrysenediol ((R,R)-THC), diaryl-propionitrile] acutely (within minutes) increases NO production in nonimmortalized, enzymatically dissociated human BEC (343). Such effects are accompanied by eNOS phosphorylation. Furthermore, in human bronchial rings from females, physiologically relevant concentrations of estrogens (<10 nm) produce potent bronchodilation, which is substantially blunted by epithelial denudation. These limited data suggest that, similar to endothelium, estrogens are capable of inducing NO in bronchial epithelium via nongenomic mechanisms, thus potentially modulating bronchodilation.
In humans, eNO fluctuates in menstruating women compared with women on OC such that eNO decreased when estrogen levels were high, whereas increased progesterone levels correlated with increased eNO. Additionally, women on OC did not exhibit fluctuations in eNO (110). Other studies on the relationship of menstrual status and eNO have yielded conflicting results (108, 109, 348, 349). eNO is thought to affect activity of iNOS and inflammatory cells in the lung. No work to date has examined the effect of sex steroids on iNOS regulation in BEC. Nonetheless, menstrual fluctuations in eNO suggest the contribution of both genomic (considering the baseline levels of estrogens or progesterone) and possibly nongenomic (during specific points of the hormonal cycle) mechanisms.
In addition to altered production of NO, BEC growth and proliferation are effected in diseases such as bronchitis and asthma. In other tissues, estrogens, progesterone, and testosterone are all known to modulate cell proliferation (291, 350, 351). There is currently limited information on the effect of sex steroids on BEC proliferation. Effects of estrogen on proliferation of immortalized BEC were conflicting (342). Estradiol increased proliferation in three of five cell lines; however, only two of the three lines exhibited ER activation. To date, no work on the effects of androgens or progesterone on BEC has been reported.
Finally, sex steroid signaling in bronchial epithelium may also be important in CF. Although the role of sex steroids is not well established in CF, cyclical decreases in lung function in female CF patients (277) and estradiol effects on uridine triphosphate-induced chloride secretion (278) suggest that susceptibility to infection and impaired mucus clearance may both be modulated by estrogens. Considering the importance of the airway epithelium, these are certainly topics that should be pursued from a perspective of understanding sex steroid signaling in the airway, as well as for development of new avenues for drug therapy targeting airway diseases.
3. Alveolar epithelium
Sex steroid signaling in alveolar epithelium is relevant across the life span. Sex steroids, specifically androgens, affect the maturation of alveolar type II epithelial cells and the subsequent production of surfactant in male fetuses (352–354). Changes in the histopathology of type II cells in adults show a sex difference, with men developing more emphysematic COPD than women (202, 203) and with women having a greater predominance of lung adenocarcinoma (217–219, 355). The presence of ERα and ERβ as well as PR and AR has been reported in the alveolar epithelial layer of normal lung biopsies as well as in tumors (355–357) and immortalized alveolar epithelial cell lines including NCI-A549 and NCI-H23 (355, 357, 358). Furthermore, sex steroid enzymes aromatase and 17β-hydroxysteroid dehydrogenase have also been detected (358, 359). Thus, the potential exists for a range of sex steroid effects within the airway epithelium. However, as with nasal and bronchial epithelium, studies to date have largely focused on these effects in the context of disease.
Studies on the actions of sex steroid in emphysema are lacking. Early research in rats suggested that administration of progesterone or medroxyprogesterone is able to reverse emphysematic changes in lung air spaces (360, 361). A similar effect of progesterone was reported in an experimental model of papain-induced emphysema (362, 363). Interestingly, administration of progesterone to patients suffering from emphysema reduced hypercapnia and improved symptoms (364); however, mechanisms underlying such effects were not studied, and it is not entirely clear whether there is actual improvement in lung structure. Furthermore, it is not known whether these effects can be attributed to signaling in alveolar epithelium per se. Nonetheless, these limited data would suggest that female sex hormones may be protective against the development of emphysema, although much more work on this topic is warranted before clinical implementation of sex steroids in emphysematic COPD.
Estrogen has been implicated in the predominance of NSCLC in females as well in the progression of tumors. Women are generally younger than men and develop adenocarcinoma with less pack-year smoking history than men. It is thought that female sex hormones play a role in these observations, especially given the well-documented effect of estrogen on ER-positive breast cancer progression. Although the selective ER modulator tamoxifen has proven efficacy in preventing breast cancer proliferation, it is suspected to promote lung tumor growth (365). Indeed, estradiol-induced enhancement of NSCLC proliferation was reported in the NCI-H23 cell line, an effect attenuated with small interfering RNA directed against ERα or ERβ or the pharmacological ER antagonist fulvestrant (ICI 182,780) (366). The combination of epidermal growth factor (EGF) and estradiol increased p42/p44 MAPK activity in NCI-H23 cells above EGF or estradiol alone. Functional interactions between ER and EGF receptor (EGFR) were found in lung cancer cells (367). EGFR activation subsequently activates ER independent of ligand binding (366). Accordingly, a combination of fulvestrant and the EGFR kinase inhibitor erlotinib has been shown to prevent xenograft tumor growth better than either intervention alone (366). This correlates with the clinical observation that patients with tumors overexpressing EGFR and ERα have poorer outcomes (368). Additionally, favorable outcomes are negatively correlated to serum estrogen levels (228). In addition to gonadally derived estrogens, local production may also be relevant in the case of lung cancers. Aromatase, the enzyme that converts testosterone to estrogen, was found in more than 85% of NSCLC from male and female patients (369), and aromatase expression negatively correlates with long-term survival in postmenopausal women (370). Overall, if anything, these data suggest a detrimental role for estrogens in the alveolar epithelium when it comes to cancer, which appears to be in sharp contrast to a potentially beneficial role in COPD or even in the bronchial epithelium. This only emphasizes the need for more careful examination of sex steroid signaling within specific lung cell types and exploration of targeted delivery of drugs to modulate these effects within cells of interest.
Contrary to the pro-proliferative effects of estrogen, progesterone inhibits proliferation in a dose-dependent manner in NSCLC cell lines and reduces tumor volume in nude mice (355). Nearly 50% of NSCLC patient specimens stain positive for PR (371), which predicts better outcomes. Local progesterone synthesis (via 3β hydroxysteroid dehydrogenase) may also be important (372, 373). Thus, in women, the relative amounts of circulating and local levels, of estrogen vs. progesterone may be important determinants of tumor progression and patient prognosis. However, much more work is needed to understand the potential interaction between ER and PR signaling within tumors and the relative influence of estrogens vs. progesterone on these receptors, especially in women.
Androgen effects in lung cancer have been barely studied. Androgens promote proliferation in AR-positive small cell lung cancer cells (374). AR are present in lung cancer tissue samples and the NCI-A549 cell line, with testosterone up-regulating AR in these cells (357). AR expression is usually low in the NCI-A549 cell line but is significantly up-regulated with the androgen DHT, which works with EGF via p38 MAPK to enhance cellular growth (375). In addition to proliferative effects, testosterone may also up-regulate genes such as CYP1A1 (357), which encodes proteins that convert estradiol to more potent metabolites (253), catalyze elements of cigarette smoke and other carcinogens, and have been associated with increased susceptibility to bronchogenic carcinoma (376). These limited data generally suggest a deleterious effect of androgens in lung cancer. Although much more work is needed here, it is important to consider whether local aromatase-induced conversion of testosterone to estrogen could potentiate cancer cell proliferation, especially via interactions with the EGFR.
In addition to lung cancer, there has been considerable recent interest in the role of sex steroids in acute lung injury. A beneficial and protective role for estrogens, but a potentially detrimental role for progesterone, has been reported in cardiac and renal ischemia/reperfusion injury (377–380) in both animals and humans. These data set the stage for examining the impact of sex steroids on acute lung injury, although to date such effects have been examined only in animal experiments. In general, it appears that male sex and androgens are both detrimental to AHR and inflammation after aspiration of bacterial lipopolysaccharide (LPS) (167). Indeed, even in females, testosterone worsens the inflammatory response. Furthermore, even in lung injury secondary to injury in other organs (e.g., to the gut), androgens are detrimental whereas estrogens are protective (381–383). Consistent with a protective role, administration of exogenous estrogen to previously ovariectomized mice reverses LPS-induced lung injury (384), potentially through modulation of cytokine profiles and cell adhesion molecules (384), as well as modulation of cellular apoptosis (385). Furthermore, estrogens, acting via ERβ may modulate iNOS and thus reduce the level of inflammation (386). These exciting data provide a basis for work in humans to explore both sex differences in the response to (and recovery from) acute lung injury and the potential protective role of estrogens.
4. Airway smooth muscle (ASM)
Research regarding the role of sex steroids in modulation of ASM is rapidly progressing given clinically observed sex differences in asthma and airway-predominant COPD. Both increased AHR to cholinergic agents, and ASM hypertrophy and hyperplasia (increased ASM mass) contribute to the pathogenesis of asthma (43, 387). Additionally, increased ASM mass and decreasing lumen diameter are hallmarks of chronic bronchitis and COPD (388). An important caveat here is that considering the multifactorial nature of diseases such as asthma or COPD, sex differences in airway structure and function may reflect a net effect of sex steroids on multiple cell types within the airway. We will focus on BEC vs. ASM vs. immune cells in the current review. However, it is important to recognize the potential role of other mechanisms including airway innervation that may regulate airway irritability (e.g., in the presence of environmental triggers) as well as airway tone (by release of bronchoconstrictors such as ACh vs. bronchodilators such as NO). Another consideration is the contribution of genomic vs. nongenomic mechanisms. Here, we will review what is known on the direct role of sex steroids on ASM reactivity and proliferation.
The general consensus from in vitro work, although limited, is that estrogens are bronchodilatory. However, attention should be paid to the concentrations being used as well as whether acute or chronic effects are being considered. An early study (122) on the relatively acute (60 min) effects of estradiol, testosterone, and progesterone reported potentiation of isoprenaline-induced relaxation in isolated pig bronchus. Here, supraphysiological concentrations of estradiol were found to be the strongest potentiator of the bronchodilatory response to isoprenaline (122). Another set of studies found that chronic (21 d), low doses of estradiol (10 μg/kg) increased the concentration of inhaled ACh necessary to double airway resistance in ovariectomized rats (176) (an epithelium-dependent effect), whereas rats receiving high-dose estradiol (100 μg/kg) had increased responsiveness to ACh (389) (an epithelium-independent effect). Although species differences may explain the discrepancies between these studies, the acute exposure in one study (122) vs. chronic exposure in the others (176, 389) should be noted.
Studies on estradiol-induced relaxation of tracheal strips from rabbits have been consistent with the rat studies but have implicated a different mechanism involving direct effects on ASM (390, 391). For example, 100 μm estradiol has been found to relax rabbit tracheal strips preconstricted with ACh, an effect not reduced by the NO synthase (NOS) inhibitor NG-nitro-L-Arginine or removal of the epithelium (391). Although the estradiol concentration in this study was substantially higher than the physiological range, estradiol effects were attributed (at least in part) to prostaglandin synthesis and cGMP modulation of ASM tone (391). This is significant because both cGMP and estrogens can influence mechanisms such as Ca2+ influx channels, and thus estrogens can potentiate the effects of epithelially derived NO. Separately, prostaglandins can modulate cAMP levels, which may provide an additional avenue for estrogens to potentiate bronchodilation.
The mechanisms by which estrogens directly influence ASM contractility (i.e., not via epithelially derived NO or innervation) have not been systematically examined. As with other cell types, estrogens could modulate membrane potential (e.g., via K+ channels) as well as other Ca2+ regulatory mechanisms. In the mouse, it has been reported that estrogens enhance activity of Ca2+ activated K+ channels, thus lowering membrane potential and indirectly reducing Ca2+ (174). In human ASM cells, physiological concentrations of estrogens as low as 100 pm substantially decrease Ca2+ responses to agonists such as ACh (392). These effects appear to predominantly involve ERα, with only a minor contribution of ERβ despite relatively equivalent expression of both receptors in human ASM. These effects also appear to involve inhibition of L-type channels as well as store-operated calcium channels, which are important in regulating [Ca2+]i. Regulation of [Ca2+]i in ASM involves both calcium influx and calcium release from intracellular stores (392–394). Estrogens do not appear to have a significant effect on [Ca2+]i stores in human ASM, whereas in human BEC, we recently found that the same concentrations of estrogens can induce sarcoplasmic reticulum Ca2+ release via inositol trisphosphate receptor channels (343). Overall, these limited data suggest that a major mechanism by which estrogens can produce bronchodilation is by reduction of [Ca2+]i in ASM in a nongenomic fashion. Genomic effects of estrogens on Ca2+ regulation in ASM have not been examined but could potentially involve altered expression of Ca2+ regulatory proteins or intracellular signaling mechanisms that may indirectly modulate both Ca2+ and the contractile apparatus of ASM.
A potential, but obvious reason for sex differences in airway reactivity may be differences in ER expression of ASM derived from male vs. female humans and/or animals. In pilot studies, we have found that ASM derived from male vs. female patients express full-length ERα and ERβ to comparable extents (E. A. Townsend, M. A. Thompson, C. M. Pabelick, and Y. S. Prakash, unpublished observations), whereas others have found ER expression in lungs from both male and female mice (395). Accordingly, the potential exists for ER activation in both males and females. In our previous study on calcium regulation, we examined ASM cells derived only from female patients (392). Therefore, sex differences in airway reactivity at the cellular or whole animal level may involve differences in the activation of signaling pathways downstream of the ER and should be examined systematically.
Although the in vitro work in tracheal or bronchial rings is consistent with the idea of estrogen-induced bronchodilation, in vivo studies in mice on sex differences in asthma are less clear. A potential problem here is that it is difficult to isolate the effects of sex steroid on ASM alone in the setting of elevated presence and activity of inflammatory cells and cytokines, as well as steroid effects on other airway elements (especially epithelium or airway innervation). Several murine models of allergic asthma exhibit sex differences in airway responsiveness vs. airway inflammation, but the data are conflicting (44, 167, 169, 171, 174, 175, 177). For example, male C57BL/6 mice show more AHR than females, indicating a protective effect of estrogen (167). However, an inherent sex difference in AHR does not necessarily suggest a constrictive or dilatory effect of sex steroids on ASM. Furthermore, female mice actually exhibit more airway inflammation (166, 170). Again, it is not clear whether estrogen and/or progesterone are actually involved. Importantly, these studies highlight the importance of distinguishing between AHR vs. inflammation. In these models, ovalbumin, LPS, or tobacco smoke was used to induce an allergic phenotype resulting in subsequent AHR.
Focusing on AHR, estradiol substantially blunts carbachol-induced airway constriction via the NO-cGMP-PKG pathway, resulting in increased activation of Ca2+-activated potassium channels (174). This effect may be sex-specific because only male mice exhibited methacholine-AHR, and administration of estrogen to males attenuated this AHR (175). Female mice lacking the ERα receptor display enhanced airway responsiveness to methacholine, thought to be related to M2 muscarinic receptor dysfunction (168). Furthermore, in response to electrical field stimulation (a common technique used to identify the contribution of neurally derived signals), tracheal rings from these knockout mice released more ACh from airway innervation compared with their wild-type controls. These limited data suggest at least partial involvement of ER signaling within airway nerves as well as NO in sex differences in AHR. Interestingly, these animal studies would suggest a lack of direct effect of estrogens on ASM, which appears to be in sharp contrast to the in vitro work (especially in human cells). Several reasons may underlie this discrepancy, including species differences in estrogen action on ASM; the interaction between ASM and other cells including epithelium, immune, and nerves in vivo that is absent in vitro; and the lack of current in vitro data on how estrogens affect ASM in the presence of inflammation. Further work is required to determine the true importance of ASM in mediating estrogen effects in the airway, especially in the presence of inflammation.
The effects of progesterone on ASM contractility have been less studied. Progesterone has been reported to potentiate the relaxant properties of isoprenaline in isolated, constricted pig bronchial rings (122). Here, 40 μm progesterone had a greater potentiating effect than testosterone, but it was less effective than estradiol. Again, the extremely high concentration of progesterone should be noted (Table 2). Another study reported that progesterone and 5β-pregnenolone prevented agonist-induced contraction of guinea pig trachea, with a greater effect of progesterone (396). These effects were proposed to involve direct inhibition of Ca2+ entry but not via γ-aminobutyric acidA-receptors (396).
Overall, the limited in vitro data suggest that, similar to estrogens, progesterone has a bronchodilatory effect. However, further study is required to determine whether such effects are maintained in the presence of inflammation and can thus be used as a potentially new avenue to target AHR. However, in male mice sensitized with ovalbumin, progesterone actually exacerbates AHR (173), but only in sensitized animals. Direct effects of progesterone on ASM are hard to assess in this context because only male mice were used without confirmation of PR expression. Additionally, there was the confounding presence of inflammation in sensitized mice (173).
The effects of oxytocin on airway responsiveness were recently reported (397). Oxytocin was found to increase cytosolic Ca2+ in human ASM cells and promote force generation and airway narrowing in murine tracheal rings and precision cut lung slices (397). Progesterone is known to inhibit oxytocin binding to the oxytocin receptor in uterine smooth muscle to maintain quiescence during pregnancy (398). As such, progesterone may prevent oxytocin-induced airway narrowing through competitive binding. Whether such an effect also contributes to airway tone remains to be determined.
The nongenomic effects of androgens on ASM have been examined recently (399, 400). Physiological concentrations of testosterone have been found to relax preconstricted rabbit tracheal smooth muscle in a dose-dependent manner (399). Removal of the epithelium attenuates the testosterone response; however, inhibition of AR with flutamide does not alter the relaxant effects of testosterone. This group also used BSA-conjugated, membrane-impermeant testosterone and saw similar relaxant effects in epithelium-intact strips, but not upon removal of the epithelium (399). Similarly, in male guinea pig and bovine tracheal rings and strips, DHT produces substantial relaxation (400). These relaxations required pharmacological concentrations of the androgen, and the effects were epithelium independent, in contrast to the work of Kouloumenta et al. (399). In agreement with previous studies, however, AR inhibition with flutamide did not affect the DHT-induced relaxation. These relaxant effects were attributed to voltage-gated Ca2+ channel antagonism (400). Again, these limited in vitro data do not provide any clear indication as to whether ASM is actually involved in mediating androgen effects on bronchodilation and stress the need for further examination of this topic. Here, as with progesterone, the in vivo animal data are confounding. As stated earlier, male C57BL/6 mice are more hyperresponsive than female mice (167) and appear to involve testosterone-mediated vagal effects on the airway. Castrated male mice have lower airway responsiveness to methacholine than intact males, similar to that of female mice. Exogenous testosterone therapy to castrated males or females increased responsiveness. Furthermore, bilateral cervical vagotomy abrogated methacholine responsiveness in intact male, but not female mice (177). These data suggest that airway responsiveness is controlled through different physiological mechanisms in male vs. female mice. Another study indicated that male mice treated with LPS developed more airway responsiveness than female mice (167). This sex difference may be due to the effects of LPS on Th2 inflammatory cytokines (401) and their role in allergic airway disease (discussed in Section IV.E). Again, direct effects of testosterone or androgens on ASM per se are difficult to determine in these in vivo models and warrant further work.
In addition to altered ASM Ca2+, increased smooth muscle mass and hyperplasia can also lead to increased contraction and force generation. In this regard, ASM hyperplasia is an important aspect of airway remodeling in diseases such as asthma. Here, work regarding sex steroid effects on ASM proliferation is lacking. Although some cues may be taken from steroid effects on vascular smooth muscle, other effects have been proven to differ between the airway and vasculature, and further investigation on this topic specifically in the airway is warranted. Pretreatment of ASM with physiological concentrations of testosterone, estradiol, and progesterone (1 nm–1 μm) has been reported to have no effect on thrombin-induced cell proliferation (402). In this study, EGF was also used to stimulate ASM proliferation, but sex steroid effects in combination with this mitogen were not evaluated. Given the potentiating effect of estrogen and EGF signaling in alveolar epithelium, these effects in smooth muscle should be considered further. Furthermore, the testosterone and estrogen precursor dehydroepiandrosterone (DHEA) significantly and in a dose-dependent manner inhibited rat tracheal smooth muscle proliferation in response to fetal bovine serum or platelet-derived growth factor. This was attributed to the prevention of DNA binding with activator protein-1 (403). The effects of DHEA metabolites were not examined in this study. Because DHEA is a precursor to sex steroids, this strengthens the argument for examining sex steroid effects on ASM proliferation. A very recent study has indeed found that physiological concentrations of both testosterone and 17β-estradiol substantially enhance proliferation of rabbit ASM cells to comparable levels (404). The contrast in the data that sex steroids potentially induce bronchodilation (at least acutely) but with chronic exposure can worsen airway remodeling by enhancing cell proliferation should be noted, especially for future work in determining whether sex steroids (especially estrogens) are actually beneficial or detrimental to airway function. This question is only further complicated by the role of inflammation in airway diseases and sex steroid effects on the immune system (Section IV.E).
C. Lung parenchyma
Although PF is more common in males, mortality is actually higher in women, suggesting a need to investigate sex hormone effects on collagen and fibrotic deposition as well as enzymes responsible for the breakdown of the extracellular matrix, including matrix metalloproteinases (MMPs). Bleomycin treatment is the most common rodent model of PF. It induces similar histology in rodents as in humans developing PF after treatment with this chemotherapeutic drug in a dose-dependent fashion (405). One study (235) reported higher mortality with greater lung collagen deposition in female rats, an effect blunted substantially by ovariectomy, but enhanced by estradiol replacement. Furthermore, a positive correlation between estradiol dose and TGF-β mRNA suggested a possible mechanism for the observed increase in fibrosis (235). However, another study (406) found that male mice had a tendency toward greater fibrosis adjacent to the airways compared with female mice, demonstrating decreased lung function with increased static lung compliance. This decline in lung function subsequent to bleomycin treatment closely approximates that observed in humans. Castration of male mice protected them from decline in lung function; however, replacement with DHT exacerbated the decline. These data suggest that androgens are detrimental in bleomycin-induced lung fibrosis (406). Conversely, a protective effect for estrogens has been reported in rats where ovariectomy was found to exacerbate PF and ASM thickening, effects reversed by 2ME replacement (407). Taken together, these animal data indicate that female sex hormones and metabolites may be beneficial in retarding fibroblast growth and progression of pulmonary fibrosis. This has been verified by the few studies performed in lung fibroblasts in vitro where growth of human lung fibroblasts decreased upon exposure to 2ME (407), consistent with the in vivo data. Early studies in fetal lung fibroblasts showed that high concentrations of testosterone, estrogen, and progesterone (5 μg/ml) all decrease cell density in culture (408). In lung myofibroblasts, incubation with 20 nm estradiol inhibited proliferation via a nongenomic, rapid estradiol effect involving phosphorylation of the Raf1-ERK-MAPK pathway (409). Additionally, peroxisome proliferator-activated receptor-γ activation inhibited lung fibroblast proliferation in a bleomycin model of PF (410). DHEA, the testosterone and estradiol precursor, may be an agonist for peroxisome proliferator-activated receptor-γ. Thus, the presence of enzymes that metabolize this steroid in lung cells would greatly influence the effect of sex steroids on lung fibroblast proliferation. This concept remains to be verified.
Increased secretion of collagen, fibronectin, laminin, and other profibrotic proteins is not the sole mechanism responsible for development of PF. MMPs degrade collagen and extracellular matrix components, and changes in the activity of these endopeptidases can lead to various pathologies. Decreased MMP activity is implicated in preventing carcinoma metastasis and decreasing tumor angiogenesis, while exacerbating lung fibrosis. As such, effects of sex steroid on MMPs are equally important in balancing proper synthesis and degradation of fibrotic proteins in the lung. Again, data in a variety of tissues are conflicting regarding sex steroid effects on MMPs (and are not reviewed here), with almost no data relating to the lung. This represents an important aspect of sex steroid signaling in the lung that is ripe for research exploration, with the potential for modifying the process of diseases such as asthma and PF. Here, particular attention should be paid to MMP-2 and MMP-9, which are thought to be highly relevant to lung disease.
D. Pulmonary vasculature
Potential beneficial effects of estrogens on the systemic cardiovascular system with regard to hypertension would suggest a protective contribution of female sex steroids against the development or progression of PH. Indeed, in experimental animal models of PH, estradiol attenuates or inhibits development of the disease. However, this protective nature is not realized clinically, thus forming the basis for an “estrogen paradox” in PH. Furthermore, compared with the systemic vasculature, less is known on the role of other steroids (progesterone, testosterone, and DHEA) in the development or modulation of PH. Several excellent reviews regarding sex steroids in the pulmonary vasculature cover these topics (239, 240, 253, 256, 411). Here, we briefly summarize what is known, and not known, and highlight the need for further consideration of sex steroid signaling in regard to PH.
Estrogen has been the most extensively studied sex hormone in regard to the pulmonary vasculature, and most studies show a vasodilatory effect. The acute vasodilatory and protective effects of estradiol are present in both hypoxia- and monocrotaline-induced PH models (253). Female rats and pigs develop less severe PH when exposed to hypoxia compared with males (412, 413). Additionally, ovariectomy exacerbates hypoxia-induced PH, whereas estradiol replacement reverses this exacerbation (412, 414). In isolated rings of mouse pulmonary arteries, estradiol relaxes preconstricted vessels from both males and females (415). Overall, these data indicate a protective role for estradiol in attenuating hypoxic pulmonary vasoconstriction (416). In monocrotaline-induced models of PH, female rats develop less severe disease than either male rats or ovariectomized female rats (417). Estradiol treatment in both males and ovariectomized female rats attenuates PH and approximates that of intact females (418, 419). These data would also be consistent with a protective role for estrogens. However, in other animal models of PH, estrogens were not found to be beneficial in preventing disease development, with female animals developing more severe PH than males animals (420, 421). These latter models employ occlusion and serotonin receptor overexpression in the development of PH. The occlusive model more closely approximates the human condition compared to hypoxia and monocrotaline-induced PH, whereas overexpression of serotonin may approximate endothelial dysfunction. The discrepancies in estrogen effects in these different models cannot be explained currently.
Estrogenic effects on the pulmonary vasculature are regulated in part by endothelial production of NO, which is mediated through both ERα and ERβ (197, 256). This production appears to be in contrast to estradiol effects in the systemic circulation, where ERα is primarily involved (422, 423). In addition to rapid activation of eNOS in pulmonary endothelium, estrogen stimulates transcriptional up-regulation of NOS (424), along with down-regulation of the potent vasoconstrictor and smooth muscle mitogen endothelin-1 (257). Additionally, estrogen stimulates release of prostacyclin and also modulates vasodilatory enzymes, cyclooxygenase-1 and -2 (255, 425).
In addition to the endothelium, estradiol can directly affect vascular smooth muscle. Estradiol has been shown to acutely relax endothelium-denuded vascular smooth muscle from coronary arteries by opening the hyperpolarizing BKCa via a cGMP-dependent mechanism (312, 426). Other studies have shown estrogen effects on the voltage-sensitive L-type calcium channels (427, 428). Estrogen also inhibits the RhoA/Rho-kinase pathway (429), wherein RhoA translocation to the membrane induces smooth muscle contraction and proliferation, and thus inhibition of this pathway should enhance relaxation. Although these effects of estrogens have been extensively demonstrated in the systemic vasculature, far fewer studies have reported similar effects in the pulmonary circuit (430–432). Here, it is important to emphasize that despite overlap between vascular smooth muscle of systemic vs. pulmonary arteries in mechanisms of [Ca2+]i and force regulation, their relative contribution and thus the overall effect of estrogens are different. Furthermore, regulatory mechanisms can differ substantially between pulmonary arteries of different species. Accordingly, it is important to determine the mechanisms of estrogen action in pulmonary arterial smooth muscle of species and the disease models of interest.
Prolonged exposure to estradiol can be pro-proliferative or proapoptotic, depending on concentration, and is tissue-specific. Estradiol stimulates proliferation of pulmonary artery smooth muscle cells, measured by thymidine incorporation, in a dose-dependent manner (418). However, others have shown that estradiol prevents proliferation of vascular smooth muscle cells derived from the systemic circulation, possibly by inhibiting the Rho-kinase cascade. This effect has yet to be demonstrated in the pulmonary system (429). Additionally, estrogen can regulate serum levels of vascular endothelial growth factor, thereby indirectly affecting cell proliferation. These studies suggest that even if estrogens have a beneficial effect on pulmonary vascular tone, more chronic effects of estrogens may be detrimental in changing vascular structure.
Effects of progesterone on the pulmonary vasculature have not been studied as extensively as estrogen. Progesterone receptors are present in pulmonary endothelium of patients with PH (433); however, their function has not been examined. In isolated rat pulmonary arteries, progesterone was the most potent hormone vasodilator when compared with testosterone and estrogen in both male and female rats (434). The vasodilatory properties of progesterone have been found to involve inhibition of both voltage-gated and receptor-operated Ca2+ channels (435). In addition, treatment with the NOS inhibitor NG-nitro-L-Arginine reduced progesterone-induced dilation, implying an endothelial-dependent NO mechanism (435). Progesterone has been found to blunt PH and right ventricular hypertrophy in monocrotaline-induced PH of ovariectomized female rats (436). In systemic vasculature, progesterone inhibits both endothelial cell and vascular smooth muscle cell proliferation (437–439). Whether a similar alleviating effect of chronic progesterone treatment occurs in the pulmonary vasculature is not known. Nonetheless, one study in patients with PH suggests efficacy of progesterone therapy in improving the PH phenotype where patients with more severe disease had lower serum progesterone levels and treatment with progesterone reduced pulmonary artery pressures (440). Considering the fact that estrogens and progesterone are simultaneously present in women, much more research is needed to understand the interactions between these sex steroids vis-à-vis pulmonary artery structure and function. This may be of particular relevance in conditions such as women patients with preexisting PH who become pregnant and are at high risk of exacerbated PH during and after pregnancy.
As with progesterone, testosterone has been largely understudied in the context of PH. Treatment of rat pulmonary arteries with testosterone results in an acute vasodilation more potent than estradiol in arteries of both male and female rats (434). Testosterone effects do not appear to involve prostaglandins or NO, in contrast to female sex steroids, and also do not involve the AR (441). However, similar to progesterone, testosterone inhibits both voltage-gated and receptor operated Ca2+ channels (441). Interestingly, testosterone effects are more pronounced in the human systemic circulation. An in vitro study of human pulmonary and mesenteric arteries showed that testosterone was only half as effective in producing vasodilation of pulmonary vessels as in systemic vasculature (442), whereas an in vivo study showed no vasodilatory effects of testosterone in the pulmonary circulation (443). DHEA, which has the highest circulating levels of all steroids, can produce vasodilation in a hypoxic model of PH in male rats (444). These vasodilatory properties have been proposed to involve BKCa channels (445) as well as the up-regulation of soluble guanylate cyclase (446). DHEA has been found to stimulate endothelial proliferation in bovine aortic endothelial cells (447). Because proliferation of endothelial cells and the formation of plexiform lesions exacerbate PH, the effects of DHEA on cellular proliferation as well as relaxation need further investigation. Additionally, previous studies were conducted solely in male animals, but the combination of DHEA with estrogen and progesterone should be considered, given the female predominance of PH and the fact that DHEA is a precursor to both testosterone and estrogen.
E. Immune cells and function
Allergic airway diseases involve a substantial inflammatory component with key players such as dendritic cells (DCs), CD4+ lymphocytes, regulatory T cells, and B lymphocytes. The complex interactions between these elements (along with an increasingly recognized contribution of structural cells within the lung) results in an overall increase in the level of Th2 cytokines such as IL-4, IL-5, and IL-13, leading to increased IgE levels (which may further enhance mast cell activation), eosinophilia, and AHR.
The classic thinking was that cytokines produced from respective cells of the T helper 1 (Th1) vs. Th2 response reciprocally inhibit each other, and disease manifestations represent a Th1/Th2 imbalance; however, as our understanding of interactions between immune cells and other components has improved, this concept has proven far more complex. Nonetheless, based on the idea of differential immune regulation, several studies have examined the relationship between autoimmune and allergic diseases in predominantly female cohorts, hypothesizing that individuals suffering from autoimmune diseases should be less susceptible to allergic diseases and vice versa. These studies have shown both positive (448, 449) and inverse (450–452) correlations between autoimmune and allergic diseases. Nonetheless, the overwhelming female predominance in autoimmune diseases such as systemic lupus erythematosus, rheumatoid arthritis, and multiple sclerosis and a higher female:male ratio for allergic asthma in adults suggest that sex hormones likely play a role in modulating immunological inflammation. The reader is referred to several comprehensive reviews regarding sex hormones, inflammation, and various immune cells (295, 453) for more detailed discussions. The following discussion is limited to exploring the role of immune cells relevant to lung diseases (where data are available) and the effects of sex steroids. Figure 2 schematically summarizes current knowledge of sex steroid effects on specific types of immune cells, and illustrates the likely overall complex effect of sex steroids on immune function. An important caveat, as described elegantly by Straub (453) is that sex steroid effects on immune cell and function is dependent on concentration, timing, duration, and context of exposure, which is difficult to control and/or evaluate in most clinical settings as well as in many experimental protocols.
Figure 2.
Sex steroid effects on immune cells. Many lung diseases involve a substantial inflammatory component with key players such as DC and monocytes/macrophages that are particularly important in the initial response to antigens, CD4+ lymphocytes, regulatory T cells (Tregs), B lymphocytes, and other immune cells. In diseases such as asthma, mast cells, CD4+ T lymphocytes, and eosinophils are particularly important. Interactions between antigen-presenting cells and naive CD4 T lymphocytes induces the generation of polarized T lymphocytes characterized at Th1 (predominantly INF-γ secreting) or Th2 (predominantly IL-4 secreting); Th2 polarized cells subsequently have additional downstream effects on B-cell antibody secretion that may further enhance mast cell activation. The effects of estrogen (E), progesterone (P), or testosterone (T) on different types of immune cells have been examined to varying and incomplete extents (see Section IV.E), mostly in the context of autoimmune diseases. This figure schematically summarizes current knowledge of sex steroid effects on specific types of immune cells that are particularly important in lung diseases and illustrates the likely overall complex effect of sex steroids on immune function. An important caveat not represented here (see Ref. 453) is that sex steroid effects on immune cell and function is dependent on concentration, timing and duration, and context of exposure.
1. T cells
The ability of immune cells to bind estrogens and androgens has been known for several decades. Both peripheral blood mononuclear cells (PBMCs) and thymic cells bind estradiol, whereas in humans, only thymic cells have been found to express binding sites for androgens. Although estrogens do modulate PBMC function, the underlying mechanisms are not clear. A recent study showed intracellular expression of both ERα (albeit truncated ERα-46 being abundant) and ERβ in all types of lymphocytes including peripheral natural killer (NK) cells (454), with estrogen activation of intracellular signaling cascades. Although these limited data suggest that estrogens can modulate T cells, whether such effects are detrimental or alleviating for lung disease is not entirely clear.
The potential relevance of estrogen effects on T cells lies in exacerbation of asthma during the luteal phase and during pregnancy, when the high estrogen levels shift the female immune system toward a Th2-type response (455). Increased levels of IL-4 and IL-10 secretion (Th2-specific cytokines) occur during pregnancy and in in vitro experiments involving CD4+ T cells from humans (453). Additionally, TNFα (Th1-specific cytokine) secretion is attenuated at higher physiological concentrations of these estrogens (∼40–400 nm) (456) [although another Th1 cytokine, interferon (IFN)γ is actually up-regulated]. These effects appear to be mediated via ERα (453, 457). Relevant to allergic airway disease, in NK cells, estrogens can promote IFNγ, which can up-regulate iNOS and cyclooxygenase-2, subsequently contributing to inflammation.
Progesterone effects on immune cells similar to those of estrogen have been documented, although the presence of PR in various immune cells is not clear (295, 458–461). Progesterone inhibits generation of Th1 type cells in both humans and mice (160, 462) and induces both IL-4 (160) and IL-10 (463) cytokine production while antagonizing nuclear factor-κB activation, preventing TNFα action (464). Accordingly, in both pregnant and nonpregnant women, progesterone and estrogen may synergize, or act additively, to alter the Th1/Th2 balance and thus contribute to asthma exacerbation and its sequelae. A potential confounder is the relative concentration of either sex steroid, as well as their additional effects on resident airway cells such as epithelium. There are currently no data on these topics, but they should clearly be a focus for future research in suppressing airway inflammation, especially in the pregnant asthmatic.
In contrast to female sex steroids, androgens favor a Th1 immune response. Testosterone increases IFNγ secretion in CD4+ T cells (251, 465, 466). Lymphocytes from males stimulated with phytohemagglutinin show higher secretion of Th1 cytokines IFNγ and IL-2 (163). DHEA, precursor to estrogen and testosterone, circulates as DHEA 3β-sulfate and is higher in males than females. Data regarding DHEA effects on Th1/Th2 cytokine balance are contradictory, stemming in part from the fact that rodents (where many studies have been conducted) do not secrete DHEA from the gonads or the adrenals. Human T cells treated with DHEA followed by mitogens or immunogens secrete more IL-2 (Th1) (466, 467), a finding also reported for mice (468). DHEA attenuates allergic airway inflammation in mice, which may involve Th1 up-regulation by DHEA (469). Interestingly, male patients with asthma and atopic dermatitis have lower circulating levels of DHEA than healthy controls. PBMC isolated from asthmatics and treated with DHEA reduced both Th1 and Th2 cytokine responses compared with controls (470). These studies, however, were conducted primarily in males due to limited enrollment of women with AHR in the study (470).
Overall, these data, although not entirely consistent, suggest that female sex steroids may tilt the immune response toward Th2, whereas male sex steroids either tilt the response to Th1 or suppress inflammation, but more attention needs to be paid to the sex of the cells being studied and the sex ratio of patient populations involved in the trials. These differences are very important in understanding sex differences in allergic airway diseases as well as their exacerbation in the setting of altered hormonal status such as pregnancy, menopause, and even aging.
2. Dendritic cells
DCs are initiators of the immune response, and they induce T-cell responses to antigens and other signals after migration to lymphoid tissue. Additionally, macrophages residing in the lung may activate DC migration and maturation. Macrophages implicated in allergic asthma promote Th2 immune responses and are referred to as alternatively activated macrophages. Female mice sensitized with ovalbumin show increased numbers of migrating myeloid DC as well as lung macrophages compared with males (471). DC have been shown to express PR-A and both ERs, whereas macrophages derived from different regions express all steroid receptors (295). However, there are currently no data on how female sex steroids may modulate DC activation, migration, or their triggering of the immune response in lung diseases. In studies not involving lung, estrogen has been shown to promote functional DC formation from bone marrow precursors, which is blunted by ER modulators such as tamoxifen (472), and to promote DC stimulation of T cells (471). In human DCs, DHEA enhances expression of Th1 markers (473) and induces maturity of DCs, leading to Th1 immune response. These very limited data suggest that sex steroids modulate the very early steps in allergic airway disease. However, their role in prepubertal asthma (in both males and females) as well as at puberty, where the male:female ratio switches (or women develop asthma de novo), remains to be determined. It would also be of relevance to determine whether sex steroid modulation of DC function underlies sex differences in asthma.
3. Eosinophils
Eosinophilia is a hallmark of allergic asthma. Increased eosinophilia has been observed in ovalbumin sensitized BALB/c female mice compared to males, an effect prevented by depletion of female sex hormones before sensitization (474). Eosinophils are known to bind estradiol (475, 476); however, no PR or AR expression has been reported. In response to estrogen, eosinophils degranulate and increase in adhesiveness (157, 295, 476). Despite lacking binding sites, progesterone has been shown to increase eosinophilia-related AHR in ovalbumin-sensitized BALB/c mice (173). This may be attributed to the conversion of progesterone to estrogen. These limited data would suggest that female sex steroid-induced eosinophilia, in combination with steroid effects on T cells, would help to exacerbate the inflammatory process in favor of allergic asthma. Whether an opposing role for androgens is present in males is not known.
4. B cells
Activated Th2 cells influence B-cell activation, leading to increased serum levels of IgE. Here, female sex steroids may be important because testosterone stimulates mast cell degranulation (477); however, this effect appears to be indirect. It is well-documented that women have higher serum antibody concentrations compared to men. Furthermore, IgE binds mast cells causing degranulation and the release of histamine, IL-4, and IL-13. IgE levels in women have been shown to vary with hormonal status (141), which may play a part in premenstrual asthma exacerbations. Mast cells express both PR subtypes A and B as well as ERα and ERβ, but do not express AR. Progesterone inhibits mast cell migration and proliferation and histamine release (295, 478, 479). Conversely, estrogen stimulates degranulation and increases histamine secretion in primed mast cells. This is a case where progesterone antagonizes the effects of estrogen in immune response. Furthermore, depending on the relative effects of estrogen vs. progesterone on T cells, eosinophils vs. B cells, the overall inflammatory response may vary, as may occur in premenstrual asthma or in pregnancy. There is currently no information examining these latter issues.
V. Clinical Implications of Sex Differences and Sex Steroid Signaling
In Sections II, III and IV, we presented an overview of some of the current knowledge on sex differences in lung structure and function across the life span in health and disease and the potential contribution of sex steroids to such differences. Although much remains to be established regarding the complex roles of sex steroids in the body in general, it is clear that sex steroid signaling in lung tissues should be a major focus of bench and clinical research. Basic understanding of the mechanisms of sex differences might not yet be complete, but the physiological importance of sex differences in lung structure and function have already been incorporated to a certain extent into clinical medicine via the use of sex-specific values for lung function corrected for size and age (i.e., predicted values for common variables in clinical lung function tests). Unfortunately, the correction of these variables for age and size somewhat minimizes the intrinsic sex differences that exist in the lung at any given age, although the need for such corrections is understandable for the purpose of simplifying clinical interpretations (e.g., comparing the growth of boys vs. girls in the setting of different activity levels, nutrition, and racial characteristics). An important caveat is that the statistical models used to produce these parameters, while well-fitting in the midrange of age and size, can deviate from the actual situation because they do not always consider the variations in lung parameters at the onset of puberty and in the timeframes of somatic vs. lung growth, or the nonlinear relationship between the two (i.e., dysanapsis). Furthermore, models in adults also work well in the midrange of age and size (e.g., height), but not necessarily at the extremes. These caveats result in overestimation of predicted lung function at the higher extremes of age but underestimation in the young. Finally, a majority of the models do not consider race or ethnic origin in developing the nomograms. Nonetheless, such nomograms have helped incorporate sex differences in lung function in clinical medicine and form a platform to examine variations across individuals as well as changes with disease. Here, in terms of sex differences, studies are needed to include factors such as smoking histories (especially in adolescents, where the female airway may be more susceptible), reproductive history, family history (including genetic factors), and individual variations. Furthermore, age and physical characteristic limits of the predictive models and the need for their update are particularly relevant today where human life span has been substantially extended, and there is a growing worldwide problem of obesity along with its associated comorbidities.
Factors that contribute to decreased or impaired lung structure and function early in development can contribute to respiratory diseases throughout the life span. Accordingly, by virtue of their effects on fetal and postnatal lung development, sex steroids can contribute to fetal, childhood, pubertal, and adult lung in terms of development, growth, and aging (see Section III.F for respiratory diseases of the newborn, Section III.A for asthma, and Section III.B for potential contribution to COPD). Alterations in sex steroid levels can influence lung structural development with significant postnatal consequences, manifested by diseases such as RDS and BPD early in childhood (Section III.F), and asthma in childhood and beyond (Section III.A). Here, the effect of in utero and childhood tobacco smoke exposure may be significant. Clinical interventions to minimize maternal smoking during pregnancy and postpartum should be encouraged. Indeed, cigarette smoking is undoubtedly detrimental to the lung at all ages. This is only further emphasized by the limited, yet worrisome, data that there may a link between in utero exposure to cigarette smoke components and adult diseases such as COPD (Section III.B). And even here, males and females appear to differ in susceptibility, as suggested by sex differences in age-related changes in lung function and the incidence and outcomes of diseases such as COPD and lung cancer. In this regard, the Lung Health Study (195) demonstrated the importance of early interventions in smoking to potentially reverse the declines in flow rates, even in patients with COPD, with substantially greater effectiveness in women compared with men [albeit with some data suggesting greater difficulty for women in abstaining from smoking (480)]. Considering the ongoing problem of smoking in women (especially adolescents) worldwide, studies should focus on abstinence and smoking cessation programs in the school and social environments.
One clinically important area where sex differences and/or sex steroid effects are highly relevant is allergic inflammation of the upper and lower airways. As discussed in Section III.B, atopy can correlate with allergic rhinitis and asthma. Here, the greater incidence of atopy, allergic rhinitis, and asthma in prepubertal boys (140, 144) may be intrinsic or due in part to differences in environmental exposure between girls and boys reflecting outdoor and recreational activity patterns (145). However, after puberty, the complex effects of sex steroids come into play, such that some studies find that the prevalence of atopy can remain higher in adult men compared with women (146), whereas others find a switch in this pattern (148) and further changes with menopause (149) that are consistent with the peripubertal and perimenopausal changes in male:female ratio for asthma. Based on animal data, these patterns are consistent with a protective role for androgens but a proinflammatory effect of estrogens. However, it is important to recognize that interpretation is complicated by the opposing effect of estrogens on inflammation per se (e.g., see Section IV.E) vs. bronchoconstriction and bronchodilation (see discussion on bronchial epithelium and ASM in Section IV.B), the two elements of diseases such as asthma. These dual effects may be particularly important in women during periods of large and/or sustained changes in sex steroid levels, as exemplified by changes in IgE levels with OC use (Section III.B) and by changes in asthma symptoms during the menstrual cycle (premenstrual asthma) and during pregnancy (Section III.A). Furthermore, estrogens and progesterone seem to sometimes produce similar effects (e.g., eosinophil degranulation relevant to rhinitis) and could thus synergize in their overall effect, whereas in some instances the two sex steroids can have opposing effects (as in cell proliferation; Section IV.B). Accordingly, depending on their relative levels, receptor expression, and the context of exposure, interactions between these steroids may help explain premenstrual asthma and changes during pregnancy. Individual genetic and physiological variations in the asthma phenotype, responsiveness to sex steroids, and ongoing medications (e.g., steroids or β-adrenoceptor agonists) may further color the overall symptomatology. Finally, sex steroid effects on airway cells may be further modulated by the presence and extent of inflammation. These complex issues are yet to be systematically examined. Here, the clinical realm may be ideal due to the possibility of long-term monitoring and thus at least a correlative examination of sex steroids and airway diseases.
The clinical relevance of sex steroids also lies in several other lung diseases that show a female predominance, especially PH. Although PH is multifactorial in origin, its incidence early in women and its exacerbation with pregnancy suggest both sex differences and a role for female sex steroids. Clearly, understanding how these two aspects contribute to PH will be critical to management strategies, including informing patients regarding the risks of pregnancy.
In contrast to diseases such as asthma in adults and PH with a female predominance, fibrotic lung diseases such as PF have been considered more common in men (231). However, the protective vs. detrimental roles of male or female sex steroids in such diseases are not all clear (Section III.D). Given that mortality rates for PF are increasing more rapidly in women (233), further investigation on sex hormones and fibrosis is warranted.
The themes of sex differences and the role of sex steroids raise the question of whether (and how) our understanding of sex steroids can be used to clinically and physiologically develop biomarkers to diagnose, monitor, and perhaps treat lung diseases. Measurement of circulating hormonal levels would certainly be one option, with the obvious caveat that corrections will need to be made for age, comorbidities, and in the case of women of childbearing age menstrual or pregnancy status. Furthermore, circulating levels may not necessarily reflect those at the site of action, where local tissue hormone production may also play a role. Accordingly, it may be important to test for enzymes such as aromatase, especially in the lung, where locally derived hormones may be cell-specific. In addition to hormone levels, receptor expression and functionality within the tissue of interest (e.g., bronchial epithelium, smooth muscle) will be key, especially considering the variety of receptors and the myriad of pathways they can activate. Finally, it will be important to determine whether genetic variations and polymorphisms of receptors (or downstream signaling pathways) play a role in sex differences in disease presentation and progression. For example, there is currently no single nucleotide polymorphism of ER associated with asthma, but an association with ERα gene variants has been reported (481). In NSCLC, ERβ, aromatase, and EGFR expression may be predictive of survival (229, 230). These multimodal clinical testing approaches are currently available but have not been widely or systematically implemented for lung diseases.
The therapeutic potential of sex steroids in lung disease has been barely examined. Here, a simplistic approach of administering any of these steroids is not feasible due to their pleiotropic effects. Administration of estradiol and progesterone to neonates has been attempted for treatment of BPD but was found to be without benefit (270). Male sex is a risk factor for sepsis and organ failure after trauma where lung injury is a major complication, whereas estradiol is protective for women (379, 380, 383, 482–486). In animal studies, estrogen administration to males or ovariectomized females prevents hemorrhagic shock-induced sepsis and organ failure and also promotes wound healing (483, 487–489). Here, estrogen bolsters the immune response, which is depressed after trauma, leading to improved survival (490). These estrogen effects are observed acutely, with estrogen supplementation given 1 h before experimental injury providing protection (377, 453). Whether a similar therapy can be used in humans has never been tested, and the underlying mechanisms of estrogen protection are unknown. However, these initial studies underline growing interest in the use or inhibition of sex steroids in the clinical arena.
VI. Future Directions
There are intrinsic differences in the lungs of males vs. females; however, there is no doubt that right from the fetal stage, sex steroids (be they locally produced or circulating) have a profound influence on lung development and etiology of respiratory diseases. These influences change in extent, target, and effectiveness over the life span. Current understanding of sex hormone contribution to lung disease is summarized in Table 3; sex steroid influences on individual cells in the lung are schematically represented in Fig. 3. Although much remains to be established regarding sex steroid signaling, it is clear that their role in lung tissues should be a major focus of bench and clinical research. Here, the goal should be to better understand the mechanisms by which sex steroids (acting either genomically or nongenomically) modulate the structure and function of individual cell types within the lung under normal conditions and in the presence of other factors that contribute to lung diseases (e.g., inflammation, infection, fibrosis). In many situations, estrogen and progesterone may interact to either enhance or suppress the other's action. A specific example of such cooperative vs. opposing interactions between sex steroids is their effects on airway cells, relevant to asthma. As schematically illustrated in Fig. 4 using current (but limited) knowledge in this area, effects of individual sex steroids on one cell type may be detrimental to airway structure/function (e.g., estrogen effects on inflammation or epithelial and ASM proliferation), whereas the same steroid could help alleviate symptoms via its effects on another cell type (e.g., reducing [Ca2+]i in ASM cells and enhancing bronchodilation). Complicating these effects is the sometimes additive effect of other sex steroids (e.g., progesterone enhancement of inflammation; Fig. 4; also see Fig. 2). This has particular relevance to lung diseases in women where cyclical variations in the levels of these steroids as well as rapid and large changes with pregnancy may explain aspects of premenstrual asthma and pregnancy-associated exacerbations. Furthermore, such interactions may also be important in the pulmonary vasculature in the development of PH and in the interstitium relevant to PF. Even in males, age-related change in the relative levels of testosterone vs. estradiol may contribute individually or via interactions to preexisting lung diseases.
Table 3.
Sex differences and sex steroid effects in lung diseases
| Disease | Clinical sex differencea | Hormone effects |
Ref. | |||
|---|---|---|---|---|---|---|
| Clinical |
Experimental |
|||||
| Alleviates | Exacerbates | Alleviates | Exacerbates | |||
| Allergic rhinitis | Boys > girls | 140, 495 | ||||
| Women1 ≠ men, controversial | T (Th1 shift) | E2 (Th2 shift), P4? | 146–148 | |||
| Asthma | Boys > girls | T (dysanapsis) | 92–94, 100 | |||
| Women > men | T P4 | E2 (Th2 shift) | E2, P4 | T | 43, 93, 98, 99, 101, 102, 122, 174, 175, 177, 396 | |
| COPD | 185, 188 | |||||
| Airway | Women > men | E2? | 203 | |||
| Emphysema | Men > women | P4 | 202, 360, 364 | |||
| PH | Women > men | E2, P4 | E2, P4 | T | 237, 238, 241–243, 256, 412, 414, 434, 441, 496 | |
| PF | Men > women | E2, T | E2, T | 231, 232, 235, 406 | ||
| CF | Boys≈girlsb and men≈women | E2 | 273–275, 277, 278 | |||
| Lung cancer | ||||||
| Adenocarcinoma | Women > men | P4 | T, E2 | 228, 355, 356, 375 | ||
| Squamous cell | Men > women | |||||
E2, Estradiol; P4, progesterone; T, testosterone; ?, clinical data is limited but suggestive.
Refers to what is observed clinically in human patients and to distinguish this difference from animal models of disease using male vs. female specimens, because they do not always correspond with what is observed clinically.
Although CF incidence shows little sex difference, girls are usually at higher risk for infection and mortality.
Figure 3.
Summary of sex steroid effects on individual cell types of the lung. Many cell types are involved in the pathogenesis of lung disease. Therefore, individual effects of sex steroids must be integrated to achieve a better understanding of sex hormone effects in the manifestation, exacerbation, or alleviation of these diseases as a whole. Androgens, estrogen, and progesterone can have different effects at the cellular level that are concentration-, dose-, and time-dependent (which are described in the text). In general, progesterone and estrogen can either enhance or counterbalance their mutual effects in the female, whereas androgens generally have opposing effects, compared with estrogen, in the male.
Figure 4.
Sex steroid effects in asthma. The complex effects of individual sex steroids on specific cell types of the lung, as well as cooperative vs. opposing effects of different sex steroids within a cell type, are illustrated in the case of asthma (with the caveat that current knowledge of sex steroid effects in asthma is limited). Asthma represents an inflammation-driven response of the airway to environmental or intrinsic allergens, pollutants, and other factors such as cigarette smoke. In response, the airway undergoes structural changes represented by epithelial thickening with increased mucous production, ASM proliferation, and hyperreactivity, as well as remodeling of the extracellular matrix. Sex steroids, especially estrogens, may on the one hand have detrimental effects on airway structure/function by enhancing inflammation or epithelial and ASM proliferation, while also alleviating symptoms via reducing [Ca2+]i in ASM and thus enhancing bronchodilation. Complicating these effects is the sometimes additive, sometimes opposing, effect of other sex steroids. For example, progesterone may also enhance Th2 inflammation and cell proliferation but further bronchodilation. On the other hand, estrogen and progesterone have opposing effects on eNO. In general, testosterone has effects opposite to those of female sex steroids (but note the enhancement of ASM proliferation).
Basic research will help clarify the potential contribution of sex steroids to lung diseases in men vs. women and form the basis for the potential development of clinical therapies that target sex steroid signaling either in a stimulatory or an inhibitory fashion. Conversely, more clinical work will help drive the basic research to improve on current therapies and development of new ones. These areas of research present an exciting and timely opportunity to improve, enhance, and individualize the health care provided to women and men at different times in their life span.
In terms of mechanistic understanding of sex differences in lung structure or function, a critical stumbling block is identifying the relative importance of genomic vs. nongenomic sex steroid signaling in lung tissue. Certainly, in the area of cancers or cardiovascular disease, it is the myriad of genomic effects of sex steroids, especially estrogens, that are considered important, with the rapid aspects of nongenomic effects being essentially of intellectual curiosity (with the understanding that nongenomic effects could lead to eventual changes at the nuclear level). With regard to the lung, we believe that both aspects of sex steroid signaling may be important, as is the case in the vasculature. Rapid, nongenomic effects may serve to modulate airway or vascular tone, alter the profile of immune cell-derived factors such as cytokines or chemokines, and by virtue of triggering a host of signaling pathways (e.g. calcium, MAPK, cAMP response element-binding) set the stage of further genomic effects. Genomic effects may occur on a background of sex steroid concentrations in both males and females, with variations in steroid levels (e.g., menstrual cycle, pregnancy, menopause) leading to initial nongenomic effects as well as enhancement of ongoing genomic effects. In this regard, responsiveness of different components of the lung (epithelium vs. smooth muscle vs. immune cells, for example) may differ, depending on the receptor profile, as well as modulating factors such as inflammation. These complex issues remain to be well-examined.
With the development of rapid automated assay systems, it may be possible to design and implement biomarker assays for circulating and tissue hormone levels as well as receptor expression (or even their variants) and downstream signaling pathways. Responsiveness of cells and tissues from individuals to sex steroids, either alone or in combination, may then be examined to tailor therapies.
The lung is a unique organ for drug delivery, in that inhalation of pharmacological agents or other drug delivery systems can help achieve high local levels, but not necessarily high systemic levels. Accordingly, targeted delivery of agents that modulate sex steroid signaling to specific cell types is an attractive option. With advances in elucidating the genomic and nongenomic mechanisms of sex steroids on lung cell physiology, possible therapeutics involving these avenues are entirely possible, including modulation of eNOS-induced vaso- and bronchodilation by estrogen, immune response Th1/Th2 switching by androgens, calcium-induced smooth muscle relaxation via progesterone and testosterone, and inhibition of proliferation via ER antagonists or progesterone therapy. Indeed, modulation of estrogen signaling is being explored for PH and lung cancer (208, 217, 239, 253) where therapies involving predominantly estrogen signaling have examined the role of this hormone in aberrant cellular proliferation.
The use of OC in attenuating premenstrual asthma exacerbations has had varying results, but it highlights the potential of sex steroid pathways as an alternative therapeutic pathway beyond standard approaches such as glucocorticoids and β-adrenoceptor agonists. For example, in ongoing studies in human ASM, we have found that estrogens can potentiate β-adrenoceptor signaling in terms of reducing [Ca2+]i, thus facilitating bronchodilation (E. A. Townsend and Y. S. Prakash, unpublished observations). Although these preliminary explorations suggest a novel approach to bronchodilation, these data need to be verified, and a number of hurdles still need to be overcome before the potential for use in humans is even considered. Obviously, targeting of cells such as bronchial and alveolar epithelium, innervation, smooth muscle and pulmonary vasculature requires different approaches. Furthermore, due to the somewhat ubiquitous expression of sex steroid receptors within the lung, targeting of specific cell types relevant to a disease is not straightforward. However, novel technologies such as nanoparticles and promoter-driven expression systems may be helpful. Another important caveat is the complex interactions between sex steroids within a cell type (illustrated in Fig. 2 for immune cells, Fig. 3 across different cell types, and Fig. 4 as such interactions relate to asthma), as well as interactions with factors such as inflammatory mediators or infectious agents, which may limit efficacy of therapeutic agents or result in unintended effects. Furthermore, from studies in the cardiovascular system, it is clear that the effects of estrogens can vary by individual due to genetic variations in receptor structure/function, downstream signaling, and metabolism of the drug. Finally, studies in humans and animals on the vascular effects of estrogens suggest that timing of hormone treatment is important to achieve preventive and treatment goals. All of these important, complex issues remain to be examined in the lung.
VII. Conclusions
There is increasing evidence that sex differences in the lung exist at every level from intracellular signaling through whole organ structure and function. Sex differences occur not only under normal conditions, but also in a variety of lung diseases. Furthermore, sex differences arise as a result of intrinsic differences as well as complex effects of sex steroids. Modulation of lung structure and function by sex steroids appears to be not just a feature of the postpubertal period, but also occurs even during lung development and continues throughout the life span, albeit in different, complex, and incompletely understood ways. Here, the type of sex steroid, concentration, duration of exposure, and a myriad of factors appear to be involved in determining the overall effect on lung structure or function. Although much remains to be established regarding intrinsic sex differences or the effects of sex steroids, what is now clear is that, consistent with the 2001 IOM report on the importance of sex in human health (“Exploring Biological Contributions to Human Health-Does Sex Matter?” http://www.nap.edu/openbook.php?isbn=0309072816), sex is clearly a biological variable rather than an observational feature to consider both in bench and clinical research, as well as in medical practice. Here, the study and implementation of sex differences in the respiratory system clearly lags behind current knowledge in other nongonadal organ systems such as the heart, the systemic vasculature, the brain, and the endocrine system. However, there is much hope that the increasing attention to this topic, as evidenced by the numbers of publications in this area over the past 10 yr, will bring about a change in the research and clinical culture as it relates to lung disease.
Acknowledgments
This work was supported in part by the Mayo Graduate School (to E.A.T.), National Institutes of Health Grants HL088029 and HL056470 (to Y.S.P.), the Flight Attendants Medical Research Institute (to Y.S.P.), and the Mayo Clinic Departments of Physiology and Biomedical Engineering (to V.M.M. and Y.S.P.), Anesthesiology (to Y.S.P.), and Surgery (to V.M.M.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACh
- Acetylcholine
- AHR
- airway hyperresponsiveness
- AR
- androgen receptor
- ASM
- airway smooth muscle
- BEC
- bronchial epithelial cell
- BPD
- bronchopulmonary dysplasia
- [Ca2+]i
- intracellular calcium
- CF
- cystic fibrosis
- COPD
- chronic obstructive pulmonary disease
- DC
- dendritic cell
- DHEA
- dehydroepiandrosterone
- DHT
- 5α-dihydrotestosterone
- DLCO
- diffusing capacity for carbon monoxide
- EGF
- epidermal growth factor
- EGFR
- EGF receptor
- eNO
- exhaled NO
- eNOS
- endothelial NOS
- ER
- estrogen receptor
- FEF
- forced expiratory flow
- FEV1
- forced expiratory volume in 1 sec
- FRC
- functional residual capacity
- FVC
- forced VC
- GPCR
- G protein-coupled receptor
- IFN
- interferon
- iNOS
- inducible NOS
- LPS
- lipopolysaccharide
- 2ME
- 2-methoxy estradiol
- MHT
- menopausal hormone therapy
- MMP
- matrix metalloproteinase
- NK
- natural killer
- NO
- nitric oxide
- NOS
- NO synthase
- NSCLC
- non-small cell lung cancer
- OC
- oral contraceptives
- PAH
- pulmonary arterial hypertension
- PBMC
- peripheral blood mononuclear cell
- Penh
- enhanced pause
- PF
- pulmonary fibrosis
- PH
- pulmonary hypertension
- PR
- progesterone receptor
- RDS
- respiratory distress syndrome
- Th1
- T helper 1
- Th2
- T helper 2
- TLC
- total lung capacity
- VC
- vital capacity.
References
- 1. Pérez-López FR, Larrad-Mur L, Kallen A, Chedraui P, Taylor HS. 2010. Gender differences in cardiovascular disease: hormonal and biochemical influences. Reprod Sci 17:511–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Konhilas JP. 2010. What we know and do not know about sex and cardiac disease. J Biomed Biotechnol 2010:562051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arain FA, Kuniyoshi FH, Abdalrhim AD, Miller VM. 2009. Sex/gender medicine. The biological basis for personalized care in cardiovascular medicine. Circ J 73:1774–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miller VM. 2010. Sex-based differences in vascular function. Womens Health (Lond Engl) 6:737–752 [DOI] [PubMed] [Google Scholar]
- 5. Leuzzi C, Sangiorgi GM, Modena MG. 2010. Gender-specific aspects in the clinical presentation of cardiovascular disease. Fundam Clin Pharmacol 24:711–717 [DOI] [PubMed] [Google Scholar]
- 6. Bigos KL, Pollock BG, Stankevich BA, Bies RR. 2009. Sex differences in the pharmacokinetics and pharmacodynamics of antidepressants: an updated review. Gend Med 6:522–543 [DOI] [PubMed] [Google Scholar]
- 7. Beierle I, Meibohm B, Derendorf H. 1999. Gender differences in pharmacokinetics and pharmacodynamics. Int J Clin Pharmacol Ther 37:529–547 [PubMed] [Google Scholar]
- 8. Wang X, Magkos F, Mittendorfer B. 2011. Sex differences in lipid and lipoprotein metabolism: it's not just about sex hormones. J Clin Endocrinol Metab 96:885–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Greenhill C. 2011. Metabolism: sex differences in fatty liver disease. Nat Rev Endocrinol 7:313. [DOI] [PubMed] [Google Scholar]
- 10. McEwen BS. 2010. Stress, sex, and neural adaptation to a changing environment: mechanisms of neuronal remodeling. Ann NY Acad Sci 1204(Suppl):E38–E59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gillies GE, McArthur S. 2010. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol Rev 62:155–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Manson JE. 2008. Prenatal exposure to sex steroid hormones and behavioral/cognitive outcomes. Metabolism 57(Suppl 2):S16–S21 [DOI] [PubMed] [Google Scholar]
- 13. Hines M. 2010. Sex-related variation in human behavior and the brain. Trends Cogn Sci 14:448–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reddy DS. 2010. Neurosteroids: endogenous role in the human brain and therapeutic potentials. In: Ivanka S. ed. Progress in brain research. New York: Elsevier; 113–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Janicki SC, Schupf N. 2010. Hormonal influences on cognition and risk for Alzheimer's disease. Curr Neurol Neurosci Rep 10:359–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fry DL, Hyatt RE. 1960. Pulmonary mechanics. A unified analysis of the relationship between pressure, volume and gasflow in the lungs of normal and diseased human subjects. Am J Med 29:672–689 [DOI] [PubMed] [Google Scholar]
- 17. Milic-Emili J, Koulouris NG, D'Angelo E. 1999. Spirometry and flow-volume loops. Eur Respir Mon 12:20–32 [Google Scholar]
- 18. Hayes D, Jr, Kraman SS. 2009. The physiologic basis of spirometry. Respir Care 54:1717–1726 [PubMed] [Google Scholar]
- 19. Lomask M. 2006. Further exploration of the Penh parameter. Exp Toxicol Pathol 57(Suppl 2):13–20 [DOI] [PubMed] [Google Scholar]
- 20. Hoymann HG. 2007. Invasive and noninvasive lung function measurements in rodents. J Pharmacol Toxicol Methods 55:16–26 [DOI] [PubMed] [Google Scholar]
- 21. Hsia CC, Hyde DM, Ochs M, Weibel ER. 2010. An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med 181:394–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seaborn T, Khan PA, Cloutier M, Maltais F, Piedboeuf B. 2007. Short-term response to tracheal occlusion during perinatal lung development in mice. Exp Lung Res 33:441–457 [DOI] [PubMed] [Google Scholar]
- 23. Espina V, Heiby M, Pierobon M, Liotta LA. 2007. Laser capture microdissection technology. Expert Rev Mol Diagn 7:647–657 [DOI] [PubMed] [Google Scholar]
- 24. Espina V, Wulfkuhle JD, Calvert VS, VanMeter A, Zhou W, Coukos G, Geho DH, Petricoin EF, 3rd, Liotta LA. 2006. Laser-capture microdissection. Nat Protoc 1:586–603 [DOI] [PubMed] [Google Scholar]
- 25. Hutchinson J. 1846. On the capacity of the lungs, and on the respiratory functions, with a view of establishing a precise and easy method of detecting disease by the spirometer. Med Chir Trans 29:137–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ellis H. 1894. Man and woman: a study of human secondary sexual characters. New York: Charles Scribner's Sons [Google Scholar]
- 27. Ott O. 1890. Les lois de la periodicite de la fonction physiologique dans l'organisme feminin. Nouvelles archives d'obstetrique et de gynecologie 502–506 [Google Scholar]
- 28. Becklake MR, Kauffmann F. 1999. Gender differences in airway behaviour over the human life span. Thorax 54:1119–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dezateux C, Stocks J. 1997. Lung development and early origins of childhood respiratory illness. Br Med Bull 53:40–57 [DOI] [PubMed] [Google Scholar]
- 30. Thurlbeck WM. 1975. Postnatal growth and development of the lung. Am Rev Respir Dis 111:803–844 [DOI] [PubMed] [Google Scholar]
- 31. Thurlbeck WM, Angus GE. 1975. Growth and aging of the normal human lung. Chest 67:3S–6S [DOI] [PubMed] [Google Scholar]
- 32. Hepper PG, Shannon EA, Dornan JC. 1997. Sex differences in fetal mouth movements. Lancet 350:1820. [DOI] [PubMed] [Google Scholar]
- 33. Boddy K, Dawes GS. 1975. Fetal breathing. Br Med Bull 31:3–7 [DOI] [PubMed] [Google Scholar]
- 34. Thurlbeck WM. 1982. The pathology of small airways in chronic airflow limitation. Eur J Respir Dis Suppl 121:9–18 [PubMed] [Google Scholar]
- 35. Fleisher B, Kulovich MV, Hallman M, Gluck L. 1985. Lung profile: sex differences in normal pregnancy. Obstet Gynecol 66:327–330 [PubMed] [Google Scholar]
- 36. Torday JS, Nielsen HC. 1987. The sex difference in fetal lung surfactant production. Exp Lung Res 12:1–19 [DOI] [PubMed] [Google Scholar]
- 37. Doershuk CF, Fisher BJ, Matthews LW. 1974. Specific airway resistance from the perinatal period into adulthood. Alterations in childhood pulmonary disease. Am Rev Respir Dis 109:452–457 [DOI] [PubMed] [Google Scholar]
- 38. Langston C, Kida K, Reed M, Thurlbeck WM. 1984. Human lung growth in late gestation and in the neonate. Am Rev Respir Dis 129:607–613 [PubMed] [Google Scholar]
- 39. Seaborn T, Simard M, Provost PR, Piedboeuf B, Tremblay Y. 2010. Sex hormone metabolism in lung development and maturation. Trends Endocrinol Metab 21:729–738 [DOI] [PubMed] [Google Scholar]
- 40. Schwartz J, Katz SA, Fegley RW, Tockman MS. 1988. Sex and race differences in the development of lung function. Am Rev Respir Dis 138:1415–1421 [DOI] [PubMed] [Google Scholar]
- 41. Merkus PJ, ten Have-Opbroek AA, Quanjer PH. 1996. Human lung growth: a review. Pediatr Pulmonol 21:383–397 [DOI] [PubMed] [Google Scholar]
- 42. Hibbert M, Lannigan A, Raven J, Landau L, Phelan P. 1995. Gender differences in lung growth. Pediatr Pulmonol 19:129–134 [DOI] [PubMed] [Google Scholar]
- 43. Carey MA, Card JW, Voltz JW, Arbes SJ, Jr, Germolec DR, Korach KS, Zeldin DC. 2007. It's all about sex: gender, lung development and lung disease. Trends Endocrinol Metab 18:308–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Carey MA, Card JW, Voltz JW, Germolec DR, Korach KS, Zeldin DC. 2007. The impact of sex and sex hormones on lung physiology and disease: lessons from animal studies. Am J Physiol Lung Cell Mol Physiol 293:L272–L278 [DOI] [PubMed] [Google Scholar]
- 45. Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S. 2006. Births: final data for 2004. Natl Vital Stat Rep 55:1–101 [PubMed] [Google Scholar]
- 46. Stocks J, Quanjer PH. 1995. Reference values for residual volume, functional residual capacity and total lung capacity. ATS workshop on lung volume measurements. Official Statement of The European Respiratory Society. Eur Respir J 8:492–506 [DOI] [PubMed] [Google Scholar]
- 47. Thurlbeck WM. 1982. Postnatal human lung growth. Thorax 37:564–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pagtakhan RD, Bjelland JC, Landau LI, Loughlin G, Kaltenborn W, Seeley G, Taussig LM. 1984. Sex differences in growth patterns of the airways and lung parenchyma in children. J Appl Physiol 56:1204–1210 [DOI] [PubMed] [Google Scholar]
- 49. Hoffstein V. 1986. Relationship between lung volume, maximal expiratory flow, forced expiratory volume in one second, and tracheal area in normal men and women. Am Rev Respir Dis 134:956–961 [DOI] [PubMed] [Google Scholar]
- 50. Mead J. 1980. Dysanapsis in normal lungs assessed by the relationship between maximal flow, static recoil, and vital capacity. Am Rev Respir Dis 121:339–342 [DOI] [PubMed] [Google Scholar]
- 51. Taussig LM, Cota K, Kaltenborn W. 1981. Different mechanical properties of the lung in boys and girls. Am Rev Respir Dis 123:640–643 [DOI] [PubMed] [Google Scholar]
- 52. Schrader PC, Quanjer PH, Olievier IC. 1988. Respiratory muscle force and ventilatory function in adolescents. Eur Respir J 1:368–375 [PubMed] [Google Scholar]
- 53. García-Río F, Pino-García JM, Serrano S, Racionero MA, Terreros-Caro JG, Alvarez-Sala R, Villasante C, Villamor J. 1997. Comparison of helium dilution and plethysmographic lung volumes in pregnant women. Eur Respir J 10:2371–2375 [DOI] [PubMed] [Google Scholar]
- 54. Gee JB, Packer BS, Millen JE, Robin ED. 1967. Pulmonary mechanics during pregnancy. J Clin Invest 46:945–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Turner JM, Mead J, Wohl ME. 1968. Elasticity of human lungs in relation to age. J Appl Physiol 25:664–671 [DOI] [PubMed] [Google Scholar]
- 56. Gibson GJ, Pride NB, O'cain C, Quagliato R. 1976. Sex and age differences in pulmonary mechanics in normal nonsmoking subjects. J Appl Physiol 41:20–25 [DOI] [PubMed] [Google Scholar]
- 57. Pelzer AM, Thomson ML. 1966. Effect of age, sex, stature, and smoking habits on human airway conductance. J Appl Physiol 21:469–476 [DOI] [PubMed] [Google Scholar]
- 58. Brody JS. 1985. Cell-to-cell interactions in lung development. Pediatr Pulmonol 1:S42–S48 [PubMed] [Google Scholar]
- 59. James A. 2005. Remodelling of airway smooth muscle in asthma: what sort do you have? Clin Exp Allergy 35:703–707 [DOI] [PubMed] [Google Scholar]
- 60. Hirota S, Helli PB, Catalli A, Chew A, Janssen LJ. 2005. Airway smooth muscle excitation-contraction coupling and airway hyperresponsiveness. Can J Physiol Pharmacol 83:725–732 [DOI] [PubMed] [Google Scholar]
- 61. Joubert P, Hamid Q. 2005. Role of airway smooth muscle in airway remodeling. J Allergy Clin Immunol 116:713–716 [DOI] [PubMed] [Google Scholar]
- 62. Lazaar AL, Panettieri RA., Jr 2005. Airway smooth muscle: a modulator of airway remodeling in asthma. J Allergy Clin Immunol 116:488–495; quiz 496 [DOI] [PubMed] [Google Scholar]
- 63. Lukacs NW, Hogaboam CM, Kunkel SL. 2005. Chemokines and their receptors in chronic pulmonary disease. Curr Drug Targets Inflamm Allergy 4:313–317 [DOI] [PubMed] [Google Scholar]
- 64. Frieri M. 2005. New concepts in asthma pathophysiology. Curr Allergy Asthma Rep 5:339–340 [DOI] [PubMed] [Google Scholar]
- 65. Frieri M. 2005. Inflammatory issues in allergic rhinitis and asthma. Allergy Asthma Proc 26:163–169 [PubMed] [Google Scholar]
- 66. Visness CM, London SJ, Daniels JL, Kaufman JS, Yeatts KB, Siega-Riz AM, Calatroni A, Zeldin DC. 2010. Association of childhood obesity with atopic and nonatopic asthma: results from the National Health and Nutrition Examination Survey 1999–2006. J Asthma 47:822–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Michelson PH, Williams LW, Benjamin DK, Barnato AE. 2009. Obesity, inflammation, and asthma severity in childhood: data from the National Health and Nutrition Examination Survey 2001–2004. Ann Allergy Asthma Immunol 103:381–385 [DOI] [PubMed] [Google Scholar]
- 68. Joseph SP, Borrell LN, Shapiro A. 2010. Self-reported lifetime asthma and nativity status in U.S. children and adolescents: results from the National Health and Nutrition Examination Survey 1999–2004. J Health Care Poor Underserved 21:125–139 [DOI] [PubMed] [Google Scholar]
- 69. McHugh MK, Symanski E, Pompeii LA, Delclos GL. 2009. Prevalence of asthma among adult females and males in the United States: results from the National Health and Nutrition Examination Survey (NHANES), 2001–2004. J Asthma 46:759–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. McHugh MK, Symanski E, Pompeii LA, Delclos GL. 2010. Prevalence of asthma by industry and occupation in the U.S. working population. Am J Ind Med 53:463–475 [DOI] [PubMed] [Google Scholar]
- 71. Kwinta P, Pietrzyk JJ. 2010. Preterm birth and respiratory disease in later life. Expert Rev Respir Med 4:593–604 [DOI] [PubMed] [Google Scholar]
- 72. Greenough A. 2008. Long-term pulmonary outcome in the preterm infant. Neonatology 93:324–327 [DOI] [PubMed] [Google Scholar]
- 73. Nickerson BG. 1985. Bronchopulmonary dysplasia. Chronic pulmonary disease following neonatal respiratory failure. Chest 87:528–535 [DOI] [PubMed] [Google Scholar]
- 74. Doyle LW, Anderson PJ. 2009. Long-term outcomes of bronchopulmonary dysplasia. Semin Fetal Neonatal Med 14:391–395 [DOI] [PubMed] [Google Scholar]
- 75. Bhandari A, Panitch HB. 2006. Pulmonary outcomes in bronchopulmonary dysplasia. Semin Perinatol 30:219–226 [DOI] [PubMed] [Google Scholar]
- 76. Bhutani VK, Abbasi S. 1992. Long-term pulmonary consequences in survivors with bronchopulmonary dysplasia. Clin Perinatol 19:649–671 [PubMed] [Google Scholar]
- 77. Maritz GS, Morley CJ, Harding R. 2005. Early developmental origins of impaired lung structure and function. Early Hum Dev 81:763–771 [DOI] [PubMed] [Google Scholar]
- 78. Kirkby J, Welsh L, Lum S, Fawke J, Rowell V, Thomas S, Marlow N, Stocks J. 2008. The EPICure study: comparison of pediatric spirometry in community and laboratory settings. Pediatr Pulmonol 43:1233–1241 [DOI] [PubMed] [Google Scholar]
- 79. Fawke J, Lum S, Kirkby J, Hennessy E, Marlow N, Rowell V, Thomas S, Stocks J. 2010. Lung function and respiratory symptoms at 11 years in children born extremely preterm: the EPICure study. Am J Respir Crit Care Med 182:237–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sly PD. 2011. The early origins of asthma: who is really at risk? Curr Opin Allergy Clin Immunol 11:24–28 [DOI] [PubMed] [Google Scholar]
- 81. Brown MA, Halonen M. 1999. Perinatal events in the development of asthma. Curr Opin Pulm Med 5:4–9 [DOI] [PubMed] [Google Scholar]
- 82. Pole JD, Mustard CA, To T, Beyene J, Allen AC. 2009. Antenatal steroid therapy for fetal lung maturation: is there an association with childhood asthma? J Asthma 46:47–52 [DOI] [PubMed] [Google Scholar]
- 83. Kumar R. 2008. Prenatal factors and the development of asthma. Curr Opin Pediatr 20:682–687 [DOI] [PubMed] [Google Scholar]
- 84. Prescott SL, Clifton V. 2009. Asthma and pregnancy: emerging evidence of epigenetic interactions in utero. Curr Opin Allergy Clin Immunol 9:417–426 [DOI] [PubMed] [Google Scholar]
- 85. Saglani S, Bush A. 2007. The early-life origins of asthma. Curr Opin Allergy Clin Immunol 7:83–90 [DOI] [PubMed] [Google Scholar]
- 86. Le Souëf PN. 2005. Can asthma be predicted from an early age? Curr Opin Allergy Clin Immunol 5:71–75 [DOI] [PubMed] [Google Scholar]
- 87. Boezen HM, Jansen DF, Postma DS. 2004. Sex and gender differences in lung development and their clinical significance. Clin Chest Med 25:237–245 [DOI] [PubMed] [Google Scholar]
- 88. Sennhauser FH, Kühni CE. 1995. Prevalence of respiratory symptoms in Swiss children: is bronchial asthma really more prevalent in boys? Pediatr Pulmonol 19:161–166 [DOI] [PubMed] [Google Scholar]
- 89. Siersted HC, Boldsen J, Hansen HS, Mostgaard G, Hyldebrandt N. 1998. Population based study of risk factors for underdiagnosis of asthma in adolescence: Odense Schoolchild Study. BMJ 316:651–655; discussion 655–656 [PMC free article] [PubMed] [Google Scholar]
- 90. KühniCE, Sennhauser FH. 1995. The Yentl syndrome in childhood asthma: risk factors for undertreatment in Swiss children. Pediatr Pulmonol 19:156–160 [DOI] [PubMed] [Google Scholar]
- 91. Orth-Gomer K. 2000. New light on the Yentl syndrome. Eur Heart J 21:874–875 [DOI] [PubMed] [Google Scholar]
- 92. Myers TR. 2000. Pediatric asthma epidemiology: incidence, morbidity, and mortality. Respir Care Clin N Am 6:1–14 [DOI] [PubMed] [Google Scholar]
- 93. Melgert BN, Ray A, Hylkema MN, Timens W, Postma DS. 2007. Are there reasons why adult asthma is more common in females? Curr Allergy Asthma Rep 7:143–150 [DOI] [PubMed] [Google Scholar]
- 94. Caracta CF. 2003. Gender differences in pulmonary disease. Mt Sinai J Med 70:215–224 [PubMed] [Google Scholar]
- 95. Bjornson CL, Mitchell I. 2000. Gender differences in asthma in childhood and adolescence. J Gend Specif Med 3:57–61 [PubMed] [Google Scholar]
- 96. Vink NM, Postma DS, Schouten JP, Rosmalen JG, Boezen HM. 2010. Gender differences in asthma development and remission during transition through puberty: the TRacking Adolescents' Individual Lives Survey (TRAILS) study. J Allergy Clin Immunol 126:498–504 e1–e6 [DOI] [PubMed] [Google Scholar]
- 97. Redline S, Gold D. 1994. Challenges in interpreting gender differences in asthma. Am J Respir Crit Care Med 150:1219–1221 [DOI] [PubMed] [Google Scholar]
- 98. Schatz M, Camargo CA., Jr 2003. The relationship of sex to asthma prevalence, health care utilization, and medications in a large managed care organization. Ann Allergy Asthma Immunol 91:553–558 [DOI] [PubMed] [Google Scholar]
- 99. Salam MT, Wenten M, Gilliland FD. 2006. Endogenous and exogenous sex steroid hormones and asthma and wheeze in young women. J Allergy Clin Immunol 117:1001–1007 [DOI] [PubMed] [Google Scholar]
- 100. Postma DS. 2007. Gender differences in asthma development and progression. Gend Med 4(Suppl B):S133–S146 [DOI] [PubMed] [Google Scholar]
- 101. Vrieze A, Postma DS, Kerstjens HA. 2003. Perimenstrual asthma: a syndrome without known cause or cure. J Allergy Clin Immunol 112:271–282 [DOI] [PubMed] [Google Scholar]
- 102. Chhabra SK. 2005. Premenstrual asthma. Indian J Chest Dis Allied Sci 47:109–116 [PubMed] [Google Scholar]
- 103. Farha S, Asosingh K, Laskowski D, Hammel J, Dweik RA, Wiedemann HP, Erzurum SC. 2009. Effects of the menstrual cycle on lung function variables in women with asthma. Am J Respir Crit Care Med 180:304–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Gibbs CJ, Coutts II, Lock R, Finnegan OC, White RJ. 1984. Premenstrual exacerbation of asthma. Thorax 39:833–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Dratva J, Schindler C, Curjuric I, Stolz D, Macsali F, Gomez FR, Zemp E. 2010. Perimenstrual increase in bronchial hyperreactivity in premenopausal women: results from the population-based SAPALDIA 2 cohort. J Allergy Clin Immunol 125:823–829 [DOI] [PubMed] [Google Scholar]
- 106. Forbes L, Jarvis D, Burney P. 1999. Do hormonal contraceptives influence asthma severity? Eur Respir J 14:1028–1033 [DOI] [PubMed] [Google Scholar]
- 107. Tan KS, McFarlane LC, Lipworth BJ. 1997. Loss of normal cyclical β2 adrenoceptor regulation and increased premenstrual responsiveness to adenosine monophosphate in stable female asthmatic patients. Thorax 52:608–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kharitonov SA, Logan-Sinclair RB, Busset CM, Shinebourne EA. 1994. Peak expiratory nitric oxide differences in men and women: relation to the menstrual cycle. Br Heart J 72:243–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Oguzulgen IK, Turktas H, Erbas D. 2002. Airway inflammation in premenstrual asthma. J Asthma 39:517–522 [DOI] [PubMed] [Google Scholar]
- 110. Mandhane PJ, Hanna SE, Inman MD, Duncan JM, Greene JM, Wang HY, Sears MR. 2009. Changes in exhaled nitric oxide related to estrogen and progesterone during the menstrual cycle. Chest 136:1301–1307 [DOI] [PubMed] [Google Scholar]
- 111. Schatz M, Dombrowski MP. 2009. Clinical practice. Asthma in pregnancy. N Engl J Med 360:1862–1869 [DOI] [PubMed] [Google Scholar]
- 112. Kwon HL, Triche EW, Belanger K, Bracken MB. 2006. The epidemiology of asthma during pregnancy: prevalence, diagnosis, and symptoms. Immunol Allergy Clin North Am 26:29–62 [DOI] [PubMed] [Google Scholar]
- 113. Pereira A, Krieger BP. 2004. Pulmonary complications of pregnancy. Clin Chest Med 25:299–310 [DOI] [PubMed] [Google Scholar]
- 114. Schatz M, Dombrowski MP, Wise R, Thom EA, Landon M, Mabie W, Newman RB, Hauth JC, Lindheimer M, Caritis SN, Leveno KJ, Meis P, Miodovnik M, Wapner RJ, Paul RH, Varner MW, O'sullivan MJ, Thurnau GR, Conway D, McNellis D. 2003. Asthma morbidity during pregnancy can be predicted by severity classification. J Allergy Clin Immunol 112:283–288 [DOI] [PubMed] [Google Scholar]
- 115. Juniper EF, Daniel EE, Roberts RS, Kline PA, Hargreave FE, Newhouse MT. 1989. Improvement in airway responsiveness and asthma severity during pregnancy. A prospective study. Am Rev Respir Dis 140:924–931 [DOI] [PubMed] [Google Scholar]
- 116. Schatz M, Harden K, Forsythe A, Chilingar L, Hoffman C, Sperling W, Zeiger RS. 1988. The course of asthma during pregnancy, post partum, and with successive pregnancies: a prospective analysis. J Allergy Clin Immunol 81:509–517 [PubMed] [Google Scholar]
- 117. Hermosa JL, Sánchez CB, Rubio MC, Mínguez MM, Walther JL. 2010. Factors associated with the control of severe asthma. J Asthma 47:124–130 [DOI] [PubMed] [Google Scholar]
- 118. Wenzel SE. 2005. Severe asthma in adults. Exp Lung Res 31(Suppl 1):22. [PubMed] [Google Scholar]
- 119. Louis R. 2009. Severe asthma: how can we differentiate phenotypes? Swiss Med Wkly 139:274–277 [DOI] [PubMed] [Google Scholar]
- 120. Firoozi F, Ducharme FM, Lemière C, Beauchesne MF, Perreault S, Forget A, Blais L. 2009. Effect of fetal gender on maternal asthma exacerbations in pregnant asthmatic women. Respir Med 103:144–151 [DOI] [PubMed] [Google Scholar]
- 121. Slatkovska L, Jensen D, Davies GA, Wolfe LA. 2006. Phasic menstrual cycle effects on the control of breathing in healthy women. Respir Physiol Neurobiol 154:379–388 [DOI] [PubMed] [Google Scholar]
- 122. Foster PS, Goldie RG, Paterson JW. 1983. Effect of steroids on β-adrenoceptor-mediated relaxation of pig bronchus. Br J Pharmacol 78:441–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Kohler PC, Trump DL. 1986. Ectopic hormone syndromes. Cancer Invest 4:543–554 [DOI] [PubMed] [Google Scholar]
- 124. Balzano G, Fuschillo S, Melillo G, Bonini S. 2001. Asthma and sex hormones. Allergy 56:13–20 [DOI] [PubMed] [Google Scholar]
- 125. Skobeloff EM, Spivey WH, St Clair SS, Schoffstall JM. 1992. The influence of age and sex on asthma admissions. JAMA 268:3437–3440 [PubMed] [Google Scholar]
- 126. Bonner JR. 1984. The epidemiology and natural history of asthma. Clin Chest Med 5:557–565 [PubMed] [Google Scholar]
- 127. Troisi RJ, Speizer FE, Willett WC, Trichopoulos D, Rosner B. 1995. Menopause, postmenopausal estrogen preparations, and the risk of adult-onset asthma. A prospective cohort study. Am J Respir Crit Care Med 152:1183–1188 [DOI] [PubMed] [Google Scholar]
- 128. Balzano G, Fuschillo S, De Angelis E, Gaudiosi C, Mancini A, Caputi M. 2007. Persistent airway inflammation and high exacerbation rate in asthma that starts at menopause. Monaldi Arch Chest Dis 67:135–141 [DOI] [PubMed] [Google Scholar]
- 129. Bellia V, Augugliaro G. 2007. Asthma and menopause. Monaldi Arch Chest Dis 67:125–127 [DOI] [PubMed] [Google Scholar]
- 130. Della Torre F, Cassani L, Segale M. 1988. Asma ed orticaria nell'anziano: ruolo degli ormoni ipofiso-gonadici in menopausa. Rassegna Geriatrica 24:165–171 [Google Scholar]
- 131. Farina F, Colombi S, Cantone R, Pastore M, Centanni S, Galimberti M. 1986. [Study of hypophyseal and gonadal hormones and cases of postmenopausal occurrence of bronchial asthma]. Minerva Med 77:243–247 [PubMed] [Google Scholar]
- 132. Ballard K. 2002. Women's use of hormone replacement therapy for disease prevention; results of a community survey. Br J Gen Pract 52:835–837 [PMC free article] [PubMed] [Google Scholar]
- 133. Hepburn MJ, Dooley DP, Morris MJ. 2001. The effects of estrogen replacement therapy on airway function in postmenopausal, asthmatic women. Arch Intern Med 161:2717–2720 [DOI] [PubMed] [Google Scholar]
- 134. Romieu I, Fabre A, Fournier A, Kauffmann F, Varraso R, Mesrine S, Leynaert B, Clavel-Chapelon F. 2010. Postmenopausal hormone therapy and asthma onset in the E3N cohort. Thorax 65:292–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Barr RG, Camargo CA., Jr 2004. Hormone replacement therapy and obstructive airway diseases. Treat Respir Med 3:1–7 [DOI] [PubMed] [Google Scholar]
- 136. Canguven O, Albayrak S. 2011. Do low testosterone levels contribute to the pathogenesis of asthma? Med Hypotheses 76:585–588 [DOI] [PubMed] [Google Scholar]
- 137. Zannolli R, Morgese G. 1997. Does puberty interfere with asthma? Med Hypotheses 48:27–32 [DOI] [PubMed] [Google Scholar]
- 138. Guay A, Seftel AD, Traish A. 2010. Hypogonadism in men with erectile dysfunction may be related to a host of chronic illnesses. Int J Impot Res 22:9–19 [DOI] [PubMed] [Google Scholar]
- 139. Wulfsohn NL, Politzer WM, Henrico JS. 1964. Testosterone therapy in bronchial asthma. S Afr Med J 38:170–172 [PubMed] [Google Scholar]
- 140. Sears MR, Burrows B, Flannery EM, Herbison GP, Holdaway MD. 1993. Atopy in childhood. I. Gender and allergen related risks for development of hay fever and asthma. Clin Exp Allergy 23:941–948 [DOI] [PubMed] [Google Scholar]
- 141. Vellutini M, Viegi G, Parrini D, Pedreschi M, Baldacci S, Modena P, Biavati P, Simoni M, Carrozzi L, Giuntini C. 1997. Serum immunoglobulins E are related to menstrual cycle. Eur J Epidemiol 13:931–935 [DOI] [PubMed] [Google Scholar]
- 142. Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG. 1989. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med 320:271–277 [DOI] [PubMed] [Google Scholar]
- 143. Liu AH, Jaramillo R, Sicherer SH, Wood RA, Bock SA, Burks AW, Massing M, Cohn RD, Zeldin DC. 2010. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol 126:798–806 e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Bertelsen RJ, Carlsen KC, Carlsen KH, Granum B, Doekes G, Håland G, Mowinckel P, Løvik M. 2010. Childhood asthma and early life exposure to indoor allergens, endotoxin and β(1,3)-glucans. Clin Exp Allergy 40:307–316 [DOI] [PubMed] [Google Scholar]
- 145. Bousquet J, Fokkens W, Burney P, Durham SR, Bachert C, Akdis CA, Canonica GW, Dahlen SE, Zuberbier T, Bieber T, Bonini S, Bousquet PJ, Brozek JL, Cardell LO, Crameri R, Custovic A, Demoly P, van Wijk RG, Gjomarkaj M, Holland C, Howarth P, Humbert M, Johnston SL, Kauffmann F, Kowalski ML, Lambrecht B, Lehmann S, Leynaert B, Lodrup-Carlsen K, Mullol J, Niggemann B, Nizankowska-Mogilnicka E, Papadopoulos N, Passalacqua G, Schünemann HJ, Simon HU, Todo-Bom A, Toskala E, Valenta R, Wickman M, Zock JP. 2008. Important research questions in allergy and related diseases: nonallergic rhinitis: a GA2LEN paper. Allergy 63:842–853 [DOI] [PubMed] [Google Scholar]
- 146. Senthilselvan A, Rennie D, Chénard L, Burch LH, Babiuk L, Schwartz DA, Dosman JA. 2008. Association of polymorphisms of toll-like receptor 4 with a reduced prevalence of hay fever and atopy. Ann Allergy Asthma Immunol 100:463–468 [DOI] [PubMed] [Google Scholar]
- 147. PausJenssen ES, Cockcroft DW. 2003. Sex differences in asthma, atopy, and airway hyperresponsiveness in a university population. Ann Allergy Asthma Immunol 91:34–37 [DOI] [PubMed] [Google Scholar]
- 148. Fagan JK, Scheff PA, Hryhorczuk D, Ramakrishnan V, Ross M, Persky V. 2001. Prevalence of asthma and other allergic diseases in an adolescent population: association with gender and race. Ann Allergy Asthma Immunol 86:177–184 [DOI] [PubMed] [Google Scholar]
- 149. Siroux V, Curt F, Oryszczyn MP, Maccario J, Kauffmann F. 2004. Role of gender and hormone-related events on IgE, atopy, and eosinophils in the Epidemiological Study on the Genetics and Environment of Asthma, bronchial hyperresponsiveness and atopy. J Allergy Clin Immunol 114:491–498 [DOI] [PubMed] [Google Scholar]
- 150. Bottema RW, Reijmerink NE, Koppelman GH, Kerkhof M, Postma DS. 2005. Phenotype definition, age, and gender in the genetics of asthma and atopy. Immunol Allergy Clin North Am 25:621–639 [DOI] [PubMed] [Google Scholar]
- 151. Barbee RA, Halonen M, Lebowitz M, Burrows B. 1981. Distribution of IgE in a community population sample: correlations with age, sex, and allergen skin test reactivity. J Allergy Clin Immunol 68:106–111 [DOI] [PubMed] [Google Scholar]
- 152. Harrop J, Chinn S, Verlato G, Olivieri M, Norbäck D, Wjst M, Janson C, Zock JP, Leynaert B, Gislason D, Ponzio M, Villani S, Carosso A, Svanes C, Heinrich J, Jarvis D. 2007. Eczema, atopy and allergen exposure in adults: a population-based study. Clin Exp Allergy 37:526–535 [DOI] [PubMed] [Google Scholar]
- 153. Anderson HR, Pottier AC, Strachan DP. 1992. Asthma from birth to age 23: incidence and relation to prior and concurrent atopic disease. Thorax 47:537–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Jatakanon A, Lim S, Kharitonov SA, Chung KF, Barnes PJ. 1998. Correlation between exhaled nitric oxide, sputum eosinophils, and methacholine responsiveness in patients with mild asthma. Thorax 53:91–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Taylor DR, Mandhane P, Greene JM, Hancox RJ, Filsell S, McLachlan CR, Williamson AJ, Cowan JO, Smith AD, Sears MR. 2007. Factors affecting exhaled nitric oxide measurements: the effect of sex. Respir Res 8:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Barrenäs F, Andersson B, Cardell LO, Langston M, Mobini R, Perkins A, Soini J, Ståhl A, Benson M. 2008. Gender differences in inflammatory proteins and pathways in seasonal allergic rhinitis. Cytokine 42:325–329 [DOI] [PubMed] [Google Scholar]
- 157. Hamano N, Terada N, Maesako K, Numata T, Konno A. 1998. Effect of sex hormones on eosinophilic inflammation in nasal mucosa. Allergy Asthma Proc 19:263–269 [DOI] [PubMed] [Google Scholar]
- 158. Sekigawa I, Naito T, Hira K, Mitsuishi K, Ogasawara H, Hashimoto H, Ogawa H. 2004. Possible mechanisms of gender bias in SLE: a new hypothesis involving a comparison of SLE with atopy. Lupus 13:217–222 [DOI] [PubMed] [Google Scholar]
- 159. Talal N. 1992. Sjogren's syndrome: historical overview and clinical spectrum of disease. Rheum Dis Clin North Am 18:507–515 [PubMed] [Google Scholar]
- 160. Piccinni MP, Giudizi MG, Biagiotti R, Beloni L, Giannarini L, Sampognaro S, Parronchi P, Manetti R, Annunziato F, Livi C. 1995. Progesterone favors the development of human T helper cells producing Th2-type cytokines and promotes both IL-4 production and membrane CD30 expression in established Th1 cell clones. J Immunol 155:128–133 [PubMed] [Google Scholar]
- 161. Hamano N, Terada N, Maesako K, Hohki G, Ito T, Yamashita T, Konno A. 1998. Effect of female hormones on the production of IL-4 and IL-13 from peripheral blood mononuclear cells. Acta Otolaryngol Suppl 537:27–31 [PubMed] [Google Scholar]
- 162. Araneo BA, Dowell T, Diegel M, Daynes RA. 1991. Dihydrotestosterone exerts a depressive influence on the production of interleukin-4 (IL-4), IL-5, and γ-interferon, but not IL-2 by activated murine T cells. Blood 78:688–699 [PubMed] [Google Scholar]
- 163. Girón-González JA, Moral FJ, Elvira J, García-Gil D, Guerrero F, Gavilán I, Escobar L. 2000. Consistent production of a higher TH1:TH2 cytokine ratio by stimulated T cells in men compared with women. Eur J Endocrinol 143:31–36 [DOI] [PubMed] [Google Scholar]
- 164. Allen JE, Bischof RJ, Sucie Chang HY, Hirota JA, Hirst SJ, Inman MD, Mitzner W, Sutherland TE. 2009. Animal models of airway inflammation and airway smooth muscle remodelling in asthma. Pulm Pharmacol Ther 22:455–465 [DOI] [PubMed] [Google Scholar]
- 165. Chang HY, Mitzner W. 2007. Sex differences in mouse models of asthma. Can J Physiol Pharmacol 85:1226–1235 [DOI] [PubMed] [Google Scholar]
- 166. Corteling R, Trifilieff A. 2004. Gender comparison in a murine model of allergen-driven airway inflammation and the response to budesonide treatment. BMC Pharmacol 4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Card JW, Carey MA, Bradbury JA, DeGraff LM, Morgan DL, Moorman MP, Flake GP, Zeldin DC. 2006. Gender differences in murine airway responsiveness and lipopolysaccharide-induced inflammation. J Immunol 177:621–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Carey MA, Card JW, Bradbury JA, Moorman MP, Haykal-Coates N, Gavett SH, Graves JP, Walker VR, Flake GP, Voltz JW, Zhu D, Jacobs ER, Dakhama A, Larsen GL, Loader JE, Gelfand EW, Germolec DR, Korach KS, Zeldin DC. 2007. Spontaneous airway hyperresponsiveness in estrogen receptor-α-deficient mice. Am J Respir Crit Care Med 175:126–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Melgert BN, Postma DS, Kuipers I, Geerlings M, Luinge MA, van der Strate BW, Kerstjens HA, Timens W, Hylkema MN. 2005. Female mice are more susceptible to the development of allergic airway inflammation than male mice. Clin Exp Allergy 35:1496–1503 [DOI] [PubMed] [Google Scholar]
- 170. Seymour BW, Friebertshauser KE, Peake JL, Pinkerton KE, Coffman RL, Gershwin LJ. 2002. Gender differences in the allergic response of mice neonatally exposed to environmental tobacco smoke. Dev Immunol 9:47–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Hayashi T, Adachi Y, Hasegawa K, Morimoto M. 2003. Less sensitivity for late airway inflammation in males than females in BALB/c mice. Scand J Immunol 57:562–567 [DOI] [PubMed] [Google Scholar]
- 172. Ligeiro de Oliveira AP, Oliveira-Filho RM, da Silva ZL, Borelli P, Tavares de Lima W. 2004. Regulation of allergic lung inflammation in rats: interaction between estradiol and corticosterone. Neuroimmunomodulation 11:20–27 [DOI] [PubMed] [Google Scholar]
- 173. Hellings PW, Vandekerckhove P, Claeys R, Billen J, Kasran A, Ceuppens JL. 2003. Progesterone increases airway eosinophilia and hyper-responsiveness in a murine model of allergic asthma. Clin Exp Allergy 33:1457–1463 [DOI] [PubMed] [Google Scholar]
- 174. Dimitropoulou C, White RE, Ownby DR, Catravas JD. 2005. Estrogen reduces carbachol-induced constriction of asthmatic airways by stimulating large-conductance voltage and calcium-dependent potassium channels. Am J Respir Cell Mol Biol 32:239–247 [DOI] [PubMed] [Google Scholar]
- 175. Matsubara S, Swasey CH, Loader JE, Dakhama A, Joetham A, Ohnishi H, Balhorn A, Miyahara N, Takeda K, Gelfand EW. 2008. Estrogen determines sex differences in airway responsiveness after allergen exposure. Am J Respir Cell Mol Biol 38:501–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Degano B, Prévost MC, Berger P, Molimard M, Pontier S, Rami J, Escamilla R. 2001. Estradiol decreases the acetylcholine-elicited airway reactivity in ovariectomized rats through an increase in epithelial acetylcholinesterase activity. Am J Respir Crit Care Med 164:1849–1854 [DOI] [PubMed] [Google Scholar]
- 177. Card JW, Voltz JW, Ferguson CD, Carey MA, DeGraff LM, Peddada SD, Morgan DL, Zeldin DC. 2007. Male sex hormones promote vagally mediated reflex airway responsiveness to cholinergic stimulation. Am J Physiol Lung Cell Mol Physiol 292:L908–L914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Moshammer H, Neuberger M. 2006. Smoking in pregnancy. Environ Health Perspect 114:A150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Moshammer H, Hoek G, Luttmann-Gibson H, Neuberger MA, Antova T, Gehring U, Hruba F, Pattenden S, Rudnai P, Slachtova H, Zlotkowska R, Fletcher T. 2006. Parental smoking and lung function in children: an international study. Am J Respir Crit Care Med 173:1255–1263 [DOI] [PubMed] [Google Scholar]
- 180. Young S, Sherrill DL, Arnott J, Diepeveen D, LeSouëf PN, Landau LI. 2000. Parental factors affecting respiratory function during the first year of life. Pediatr Pulmonol 29:331–340 [DOI] [PubMed] [Google Scholar]
- 181. Gilliland FD, Li YF, Peters JM. 2001. Effects of maternal smoking during pregnancy and environmental tobacco smoke on asthma and wheezing in children. Am J Respir Crit Care Med 163:429–436 [DOI] [PubMed] [Google Scholar]
- 182. Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. 1995. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med 332:133–138 [DOI] [PubMed] [Google Scholar]
- 183. Li YF, Gilliland FD, Berhane K, McConnell R, Gauderman WJ, Rappaport EB, Peters JM. 2000. Effects of in utero and environmental tobacco smoke exposure on lung function in boys and girls with and without asthma. Am J Respir Crit Care Med 162:2097–2104 [DOI] [PubMed] [Google Scholar]
- 184. Celli BR. 1995. Pathophysiology of chronic obstructive pulmonary disease. Chest Surg Clin N Am 5:623–634 [PubMed] [Google Scholar]
- 185. Chapman KR. 2004. Chronic obstructive pulmonary disease: are women more susceptible than men? Clin Chest Med 25:331–341 [DOI] [PubMed] [Google Scholar]
- 186. Pauwels R. 2001. Global initiative for chronic obstructive lung diseases (GOLD): time to act. Eur Respir J 18:901–902 [PubMed] [Google Scholar]
- 187. Davis RM, Novotny TE. 1989. The epidemiology of cigarette smoking and its impact on chronic obstructive pulmonary disease. Am Rev Respir Dis 140:S82–S84 [DOI] [PubMed] [Google Scholar]
- 188. Varkey AB. 2004. Chronic obstructive pulmonary disease in women: exploring gender differences. Curr Opin Pulm Med 10:98–103 [DOI] [PubMed] [Google Scholar]
- 189. Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. 2002. Chronic obstructive pulmonary disease surveillance–United States, 1971–2000. MMWR Surveill Summ 51:1–16 [PubMed] [Google Scholar]
- 190. Gritz ER. 1984. Cigarette smoking by adolescent females: implications for health and behavior. Women Health 9:103–115 [DOI] [PubMed] [Google Scholar]
- 191. Gold DR, Wang X, Wypij D, Speizer FE, Ware JH, Dockery DW. 1996. Effects of cigarette smoking on lung function in adolescent boys and girls. N Engl J Med 335:931–937 [DOI] [PubMed] [Google Scholar]
- 192. Chapman KR, Tashkin DP, Pye DJ. 2001. Gender bias in the diagnosis of COPD. Chest 119:1691–1695 [DOI] [PubMed] [Google Scholar]
- 193. Prescott E, Osler M, Andersen PK, Hein HO, Borch-Johnsen K, Lange P, Schnohr P, Vestbo J. 1998. Mortality in women and men in relation to smoking. Int J Epidemiol 27:27–32 [DOI] [PubMed] [Google Scholar]
- 194. Carter R, Nicotra B, Huber G. 1994. Differing effects of airway obstruction on physical work capacity and ventilation in men and women with COPD. Chest 106:1730–1739 [DOI] [PubMed] [Google Scholar]
- 195. Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, Conway WA, Jr, Enright PL, Kanner RE, O'Hara P. 1994. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA 272:1497–1505 [PubMed] [Google Scholar]
- 196. Gan WQ, Man SF, Postma DS, Camp P, Sin DD. 2006. Female smokers beyond the perimenopausal period are at increased risk of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Respir Res 7:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Silverman EK, Weiss ST, Drazen JM, Chapman HA, Carey V, Campbell EJ, Denish P, Silverman RA, Celedon JC, Reilly JJ, Ginns LC, Speizer FE. 2000. Gender-related differences in severe, early-onset chronic obstructive pulmonary disease. Am J Respir Crit Care Med 162:2152–2158 [DOI] [PubMed] [Google Scholar]
- 198. Birring SS, Brightling CE, Bradding P, Entwisle JJ, Vara DD, Grigg J, Wardlaw AJ, Pavord ID. 2002. Clinical, radiologic, and induced sputum features of chronic obstructive pulmonary disease in nonsmokers: a descriptive study. Am J Respir Crit Care Med 166:1078–1083 [DOI] [PubMed] [Google Scholar]
- 199. Prescott E, Lange P, Vestbo J. 1997. Effect of gender on hospital admissions for asthma and prevalence of self-reported asthma: a prospective study based on a sample of the general population. Copenhagen City Heart Study Group. Thorax 52:287–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Miyamoto K, Aida A, Nishimura M, Aiba M, Kira S, Kawakami Y. 1995. Gender effect on prognosis of patients receiving long-term home oxygen therapy. The Respiratory Failure Research Group in Japan. Am J Respir Crit Care Med 152:972–976 [DOI] [PubMed] [Google Scholar]
- 201. Connett JE, Murray RP, Buist AS, Wise RA, Bailey WC, Lindgren PG, Owens GR. 2003. Changes in smoking status affect women more than men: results of the Lung Health Study. Am J Epidemiol 157:973–979 [DOI] [PubMed] [Google Scholar]
- 202. Camp PG, Coxson HO, Levy RD, Pillai SG, Anderson W, Vestbo J, Kennedy SM, Silverman EK, Lomas DA, Paré PD. 2009. Sex differences in emphysema and airway disease in smokers. Chest 136:1480–1488 [DOI] [PubMed] [Google Scholar]
- 203. Martinez FJ, Curtis JL, Sciurba F, Mumford J, Giardino ND, Weinmann G, Kazerooni E, Murray S, Criner GJ, Sin DD, Hogg J, Ries AL, Han M, Fishman AP, Make B, Hoffman EA, Mohsenifar Z, Wise R. 2007. Sex differences in severe pulmonary emphysema. Am J Respir Crit Care Med 176:243–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Carlson CL, Cushman M, Enright PL, Cauley JA, Newman AB. 2001. Hormone replacement therapy is associated with higher FEV1 in elderly women. Am J Respir Crit Care Med 163:423–428 [DOI] [PubMed] [Google Scholar]
- 205. Shafer D, Albain K. 2009. Lung cancer outcomes in women. Semin Oncol 36:532–541 [DOI] [PubMed] [Google Scholar]
- 206. Egleston BL, Meireles SI, Flieder DB, Clapper ML. 2009. Population-based trends in lung cancer incidence in women. Semin Oncol 36:506–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Ramchandran K, Patel JD. 2009. Sex differences in susceptibility to carcinogens. Semin Oncol 36:516–523 [DOI] [PubMed] [Google Scholar]
- 208. Siegfried JM, Hershberger PA, Stabile LP. 2009. Estrogen receptor signaling in lung cancer. Semin Oncol 36:524–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Chlebowski RT. 2009. Menopausal hormone therapy, hormone receptor status, and lung cancer in women. Semin Oncol 36:566–571 [DOI] [PubMed] [Google Scholar]
- 210. Harichand-Herdt S, Ramalingam SS. 2009. Gender-associated differences in lung cancer: clinical characteristics and treatment outcomes in women. Semin Oncol 36:572–580 [DOI] [PubMed] [Google Scholar]
- 211. Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. 2003. Cancer statistics, 2003. CA Cancer J Clin 53:5–26 [DOI] [PubMed] [Google Scholar]
- 212. Rivera MP, Stover DE. 2004. Gender and lung cancer. Clin Chest Med 25:391–400 [DOI] [PubMed] [Google Scholar]
- 213. Fiore MC, Novotny TE, Pierce JP, Hatziandreu EJ, Patel KM, Davis RM. 1989. Trends in cigarette smoking in the United States. The changing influence of gender and race. JAMA 261:49–55 [PubMed] [Google Scholar]
- 214. Henschke CI, Yip R, Miettinen OS. 2006. Women's susceptibility to tobacco carcinogens and survival after diagnosis of lung cancer. JAMA 296:180–184 [DOI] [PubMed] [Google Scholar]
- 215. Risch HA, Howe GR, Jain M, Burch JD, Holowaty EJ, Miller AB. 1993. Are female smokers at higher risk for lung cancer than male smokers? A case-control analysis by histologic type. Am J Epidemiol 138:281–293 [DOI] [PubMed] [Google Scholar]
- 216. Kabat GC. 1996. Aspects of the epidemiology of lung cancer in smokers and nonsmokers in the United States. Lung Cancer 15:1–20 [DOI] [PubMed] [Google Scholar]
- 217. Belani CP, Marts S, Schiller J, Socinski MA. 2007. Women and lung cancer: epidemiology, tumor biology, and emerging trends in clinical research. Lung Cancer 55:15–23 [DOI] [PubMed] [Google Scholar]
- 218. Ferguson MK, Wang J, Hoffman PC, Haraf DJ, Olak J, Masters GA, Vokes EE. 2000. Sex-associated differences in survival of patients undergoing resection for lung cancer. Ann Thorac Surg 69:245–249; discussion 249–250 [DOI] [PubMed] [Google Scholar]
- 219. Siegfried JM. 2001. Women and lung cancer: does oestrogen play a role? Lancet Oncol 2:506–513 [DOI] [PubMed] [Google Scholar]
- 220. Weiss JM, Lacey JV, Jr, Shu XO, Ji BT, Hou L, Yang G, Li H, Rothman N, Blair A, Gao YT, Chow WH, Zheng W. 2008. Menstrual and reproductive factors in association with lung cancer in female lifetime nonsmokers. Am J Epidemiol 168:1319–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Taioli E, Wynder EL. 1994. Re: Endocrine factors and adenocarcinoma of the lung in women. J Natl Cancer Inst 86:869–870 [DOI] [PubMed] [Google Scholar]
- 222. Liu Y, Inoue M, Sobue T, Tsugane S. 2005. Reproductive factors, hormone use and the risk of lung cancer among middle-aged never-smoking Japanese women: a large-scale population-based cohort study. Int J Cancer 117:662–666 [DOI] [PubMed] [Google Scholar]
- 223. Schabath MB, Wu X, Vassilopoulou-Sellin R, Vaporciyan AA, Spitz MR. 2004. Hormone replacement therapy and lung cancer risk: a case-control analysis. Clin Cancer Res 10:113–123 [DOI] [PubMed] [Google Scholar]
- 224. Baik CS, Strauss GM, Speizer FE, Feskanich D. 2010. Reproductive factors, hormone use, and risk for lung cancer in postmenopausal women, the Nurses' Health Study. Cancer Epidemiol Biomarkers Prev 19:2525–2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Ramnath N, Menezes RJ, Loewen G, Dua P, Eid F, Alkhaddo J, Paganelli G, Natarajan N, Reid ME. 2007. Hormone replacement therapy as a risk factor for non-small cell lung cancer: results of a case-control study. Oncology 73:305–310 [DOI] [PubMed] [Google Scholar]
- 226. Ganti AK, Sahmoun AE, Panwalkar AW, Tendulkar KK, Potti A. 2006. Hormone replacement therapy is associated with decreased survival in women with lung cancer. J Clin Oncol 24:59–63 [DOI] [PubMed] [Google Scholar]
- 227. Albain KS, Crowley JJ, LeBlanc M, Livingston RB. 1991. Survival determinants in extensive-stage non-small-cell lung cancer: the Southwest Oncology Group experience. J Clin Oncol 9:1618–1626 [DOI] [PubMed] [Google Scholar]
- 228. Albain KS, Belani CP, Bonomi P, O'Byrne KJ, Schiller JH, Socinski M. 2006. PIONEER: a phase III randomized trial of paclitaxel poliglumex versus paclitaxel in chemotherapy-naive women with advanced-stage non-small-cell lung cancer and performance status of 2. Clin Lung Cancer 7:417–419 [DOI] [PubMed] [Google Scholar]
- 229. Sun HB, Zheng Y, Ou W, Fang Q, Li P, Ye X, Zhang BB, Yang H, Wang SY. 2011. Association between hormone receptor expression and epidermal growth factor receptor mutation in patients operated on for non-small cell lung cancer. Ann Thorac Surg 91:1562–1567 [DOI] [PubMed] [Google Scholar]
- 230. Mah V, Marquez D, Alavi M, Maresh EL, Zhang L, Yoon N, Horvath S, Bagryanova L, Fishbein MC, Chia D, Pietras R, Goodglick L. 19. April 2011 Expression levels of estrogen receptor β in conjunction with aromatase predict survival in non-small cell lung cancer. Lung Cancer doi:10.1016/j.lungcan.2011.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Meltzer EB, Noble PW. 2008. Idiopathic pulmonary fibrosis. Orphanet J Rare Dis 3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Gribbin J, Hubbard RB, Le Jeune I, Smith CJ, West J, Tata LJ. 2006. Incidence and mortality of idiopathic pulmonary fibrosis and sarcoidosis in the UK. Thorax 61:980–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Olson AL, Swigris JJ, Lezotte DC, Norris JM, Wilson CG, Brown KK. 2007. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med 176:277–284 [DOI] [PubMed] [Google Scholar]
- 234. March TH, Wilder JA, Esparza DC, Cossey PY, Blair LF, Herrera LK, McDonald JD, Campen MJ, Mauderly JL, Seagrave J. 2006. Modulators of cigarette smoke-induced pulmonary emphysema in A/J mice. Toxicol Sci 92:545–559 [DOI] [PubMed] [Google Scholar]
- 235. Gharaee-Kermani M, Hatano K, Nozaki Y, Phan SH. 2005. Gender-based differences in bleomycin-induced pulmonary fibrosis. Am J Pathol 166:1593–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Lekgabe ED, Royce SG, Hewitson TD, Tang ML, Zhao C, Moore XL, Tregear GW, Bathgate RA, Du XJ, Samuel CS. 2006. The effects of relaxin and estrogen deficiency on collagen deposition and hypertrophy of nonreproductive organs. Endocrinology 147:5575–5583 [DOI] [PubMed] [Google Scholar]
- 237. Runo JR, Loyd JE. 2003. Primary pulmonary hypertension. Lancet 361:1533–1544 [DOI] [PubMed] [Google Scholar]
- 238. Robles AM, Shure D. 2004. Gender issues in pulmonary vascular disease. Clin Chest Med 25:373–377 [DOI] [PubMed] [Google Scholar]
- 239. Pugh ME, Hemnes AR. 2010. Development of pulmonary arterial hypertension in women: interplay of sex hormones and pulmonary vascular disease. Womens Health (Lond Engl) 6:285–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Sakao S, Tanabe N, Tatsumi K. 2010. The estrogen paradox in pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 299:L435–L438 [DOI] [PubMed] [Google Scholar]
- 241. Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, Benza RL, Liou TG, Turner M, Giles S, Feldkircher K, Miller DP, McGoon MD. 2010. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest 137:376–387 [DOI] [PubMed] [Google Scholar]
- 242. Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E, Simonneau G. 2006. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 173:1023–1030 [DOI] [PubMed] [Google Scholar]
- 243. Rich S, Dantzker DR, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Koerner SK, Levy PC, Reid LM, Vreim CE, Williams GW. 1987. Primary pulmonary hypertension. A national prospective study. Ann Intern Med 107:216–223 [DOI] [PubMed] [Google Scholar]
- 244. Taraseviciute A, Voelkel NF. 2006. Severe pulmonary hypertension in postmenopausal obese women. Eur J Med Res 11:198–202 [PubMed] [Google Scholar]
- 245. Kay JM, Smith P, Heath D. 1971. Aminorex and the pulmonary circulation. Thorax 26:262–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Abenhaim L, Moride Y, Brenot F, Rich S, Benichou J, Kurz X, Higenbottam T, Oakley C, Wouters E, Aubier M, Simonneau G, Bégaud B. 1996. Appetite-suppressant drugs and the risk of primary pulmonary hypertension. International Primary Pulmonary Hypertension Study Group. N Engl J Med 335:609–616 [DOI] [PubMed] [Google Scholar]
- 247. Masi AT. 1976. Editorial: pulmonary hypertension and oral contraceptive usage. Chest 69:451–453 [DOI] [PubMed] [Google Scholar]
- 248. Beretta L, Caronni M, Origgi L, Ponti A, Santaniello A, Scorza R. 2006. Hormone replacement therapy may prevent the development of isolated pulmonary hypertension in patients with systemic sclerosis and limited cutaneous involvement. Scand J Rheumatol 35:468–471 [DOI] [PubMed] [Google Scholar]
- 249. Opravil M, Pechère M, Speich R, Joller-Jemelka HI, Jenni R, Russi EW, Hirschel B, Lüthy R. 1997. HIV-associated primary pulmonary hypertension. A case control study. Swiss HIV Cohort Study. Am J Respir Crit Care Med 155:990–995 [DOI] [PubMed] [Google Scholar]
- 250. Cutolo M, Capellino S, Sulli A, Serioli B, Secchi ME, Villaggio B, Straub RH. 2006. Estrogens and autoimmune diseases. Ann NY Acad Sci 1089:538–547 [DOI] [PubMed] [Google Scholar]
- 251. Martin JT. 2000. Sexual dimorphism in immune function: the role of prenatal exposure to androgens and estrogens. Eur J Pharmacol 405:251–261 [DOI] [PubMed] [Google Scholar]
- 252. Rubtsov AV, Rubtsova K, Kappler JW, Marrack P. 2010. Genetic and hormonal factors in female-biased autoimmunity. Autoimmun Rev 9:494–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Tofovic SP. 2010. Estrogens and development of pulmonary hypertension: interaction of estradiol metabolism and pulmonary vascular disease. J Cardiovasc Pharmacol 56:696–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Smith AM, Jones RD, Channer KS. 2006. The influence of sex hormones on pulmonary vascular reactivity: possible vasodilator therapies for the treatment of pulmonary hypertension. Curr Vasc Pharmacol 4:9–15 [DOI] [PubMed] [Google Scholar]
- 255. Sherman TS, Chambliss KL, Gibson LL, Pace MC, Mendelsohn ME, Pfister SL, Shaul PW. 2002. Estrogen acutely activates prostacyclin synthesis in ovine fetal pulmonary artery endothelium. Am J Respir Cell Mol Biol 26:610–616 [DOI] [PubMed] [Google Scholar]
- 256. Lahm T, Crisostomo PR, Markel TA, Wang M, Weil BR, Novotny NM, Meldrum DR. 2008. The effects of estrogen on pulmonary artery vasoreactivity and hypoxic pulmonary vasoconstriction: potential new clinical implications for an old hormone. Crit Care Med 36:2174–2183 [DOI] [PubMed] [Google Scholar]
- 257. Earley S, Resta TC. 2002. Estradiol attenuates hypoxia-induced pulmonary endothelin-1 gene expression. Am J Physiol Lung Cell Mol Physiol 283:L86–L93 [DOI] [PubMed] [Google Scholar]
- 258. Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, Saugstad OD, Simeoni U, Speer CP, Halliday HL. 2010. European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants—2010 update. Neonatology 97:402–417 [DOI] [PubMed] [Google Scholar]
- 259. Farrell PM, Avery ME. 1975. Hyaline membrane disease. Am Rev Respir Dis 111:657–688 [DOI] [PubMed] [Google Scholar]
- 260. Ingemarsson I. 2003. Gender aspects of preterm birth. BJOG 110(Suppl 20):34–38 [DOI] [PubMed] [Google Scholar]
- 261. Khoury MJ, Marks JS, McCarthy BJ, Zaro SM. 1985. Factors affecting the sex differential in neonatal mortality: the role of respiratory distress syndrome. Am J Obstet Gynecol 151:777–782 [DOI] [PubMed] [Google Scholar]
- 262. Heljić S, Maksić H, Kalkan I, Krdalić B. 2009. The effects of antenatal corticosteroids and surfactant replacement on neonatal respiratory distress syndrome. Bosn J Basic Med Sci 9:225–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Jobe AH, Bancalari E. 2001. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 163:1723–1729 [DOI] [PubMed] [Google Scholar]
- 264. Roberge S, Lacasse Y, Tapp S, Tremblay Y, Kari A, Liu J, Fekih M, Qublan HS, Amorim MM, Bujold E. 2011. Role of fetal sex in the outcome of antenatal glucocorticoid treatment to prevent respiratory distress syndrome: systematic review and meta-analysis. J Obstet Gynaecol Can 33:216–226 [DOI] [PubMed] [Google Scholar]
- 265. Northway WH, Jr, Rosan RC, Porter DY. 1967. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med 276:357–368 [DOI] [PubMed] [Google Scholar]
- 266. Fanaroff AA, Hack M, Walsh MC. 2003. The NICHD neonatal research network: changes in practice and outcomes during the first 15 years. Semin Perinatol 27:281–287 [DOI] [PubMed] [Google Scholar]
- 267. Bhandari A, Bhandari V. 2009. Pitfalls, problems, and progress in bronchopulmonary dysplasia. Pediatrics 123:1562–1573 [DOI] [PubMed] [Google Scholar]
- 268. Popova AP, Bozyk PD, Bentley JK, Linn MJ, Goldsmith AM, Schumacher RE, Weiner GM, Filbrun AG, Hershenson MB. 2010. Isolation of tracheal aspirate mesenchymal stromal cells predicts bronchopulmonary dysplasia. Pediatrics 126:e1127–e1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Gortner L, Misselwitz B, Milligan D, Zeitlin J, Kollée L, Boerch K, Agostino R, Van Reempts P, Chabernaud JL, Bréart G, Papiernik E, Jarreau PH, Carrapato M, Gadzinowski J, Draper E. 2011. Rates of bronchopulmonary dysplasia in very preterm neonates in Europe: results from the MOSAIC cohort. Neonatology 99:112–117 [DOI] [PubMed] [Google Scholar]
- 270. Trotter A, Maier L, Kron M, Pohlandt F. 2007. Effect of oestradiol and progesterone replacement on bronchopulmonary dysplasia in extremely preterm infants. Arch Dis Child Fetal Neonatal Ed 92:F94–F98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Thome UH, Bischoff A, Maier L, Pohlandt F, Trotter A. 2006. Amiloride-sensitive nasal potential difference is not changed by estradiol and progesterone replacement but relates to BPD or death in a randomized trial on preterm infants. Pediatr Res 60:619–623 [DOI] [PubMed] [Google Scholar]
- 272. McCurnin DC, Pierce RA, Willis BC, Chang LY, Yoder BA, Yuhanna IS, Ballard PL, Clyman RI, Waleh N, Maniscalco W, Crapo JD, Grubb PH, Shaul PW. 2009. Postnatal estradiol up-regulates lung nitric oxide synthases and improves lung function in bronchopulmonary dysplasia. Am J Respir Crit Care Med 179:492–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Gurwitz D, Corey M, Francis PW, Crozier D, Levison H. 1979. Perspectives in cystic fibrosis. Pediatr Clin North Am 26:603–615 [DOI] [PubMed] [Google Scholar]
- 274. Davis PB. 1999. The gender gap in cystic fibrosis survival. J Gend Specif Med 2:47–51 [PubMed] [Google Scholar]
- 275. Rosenfeld M, Davis R, FitzSimmons S, Pepe M, Ramsey B. 1997. Gender gap in cystic fibrosis mortality. Am J Epidemiol 145:794–803 [DOI] [PubMed] [Google Scholar]
- 276. Demko CA, Byard PJ, Davis PB. 1995. Gender differences in cystic fibrosis: Pseudomonas aeruginosa infection. J Clin Epidemiol 48:1041–1049 [DOI] [PubMed] [Google Scholar]
- 277. Johannesson M, Lúdvíksdóttir D, Janson C. 2000. Lung function changes in relation to menstrual cycle in females with cystic fibrosis. Respir Med 94:1043–1046 [DOI] [PubMed] [Google Scholar]
- 278. Coakley RD, Sun H, Clunes LA, Rasmussen JE, Stackhouse JR, Okada SF, Fricks I, Young SL, Tarran R. 2008. 17β-Estradiol inhibits Ca2+-dependent homeostasis of airway surface liquid volume in human cystic fibrosis airway epithelia. J Clin Invest 118:4025–4035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Ghayee HK, Auchus RJ. 2007. Basic concepts and recent developments in human steroid hormone biosynthesis. Rev Endocr Metab Disord 8:289–300 [DOI] [PubMed] [Google Scholar]
- 280. Payne AH, Hales DB. 2004. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev 25:947–970 [DOI] [PubMed] [Google Scholar]
- 281. Luu-The V, Labrie F. 2010. The intracrine sex steroid biosynthesis pathways. Prog Brain Res 181:177–192 [DOI] [PubMed] [Google Scholar]
- 282. Labrie F, Simard J, Luu-The V, Trudel C, Martel C, Labrie C, Zhao HF, Rheaume E, Couet J, Breton N. 1991. Expression of 3β-hydroxysteroid dehydrogenase/Δdelta 5-Δ4 isomerase (3β-HSD) and 17β-hydroxysteroid dehydrogenase (17 β-HSD) in adipose tissue. Int J Obes 15(Suppl 2):91–99 [PubMed] [Google Scholar]
- 283. Labrie F. 2003. Extragonadal synthesis of sex steroids: intracrinology. Ann Endocrinol (Paris) 64:95–107 [PubMed] [Google Scholar]
- 284. Elmlinger MW, Kühnel W, Ranke MB. 2002. Reference ranges for serum concentrations of lutropin (LH), follitropin (FSH), estradiol (E2), prolactin, progesterone, sex hormone-binding globulin (SHBG), dehydroepiandrosterone sulfate (DHEAS), cortisol and ferritin in neonates, children and young adults. Clin Chem Lab Med 40:1151–1160 [DOI] [PubMed] [Google Scholar]
- 285. Cummings SR, Browner WS, Bauer D, Stone K, Ensrud K, Jamal S, Ettinger B. 1998. Endogenous hormones and the risk of hip and vertebral fractures among older women. Study of Osteoporotic Fractures Research Group. N Engl J Med 339:733–738 [DOI] [PubMed] [Google Scholar]
- 286. Lippe BM, LaFranchi SH, Lavin N, Parlow A, Coyotupa J, Kaplan SA. 1974. Serum 17-α-hydroxyprogesterone, progesterone, estradiol, and testosterone in the diagnosis and management of congenital adrenal hyperplasia. J Pediatr 85:782–787 [DOI] [PubMed] [Google Scholar]
- 287. Bennett NC, Gardiner RA, Hooper JD, Johnson DW, Gobe GC. 2010. Molecular cell biology of androgen receptor signalling. Int J Biochem Cell Biol 42:813–827 [DOI] [PubMed] [Google Scholar]
- 288. Lamont KR, Tindall DJ. 2010. Androgen regulation of gene expression. Adv Cancer Res 107:137–162 [DOI] [PubMed] [Google Scholar]
- 289. Foradori CD, Weiser MJ, Handa RJ. 2008. Non-genomic actions of androgens. Front Neuroendocrinol 29:169–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Edwards DP. 2005. Regulation of signal transduction pathways by estrogen and progesterone. Annu Rev Physiol 67:335–376 [DOI] [PubMed] [Google Scholar]
- 291. Simoncini T, Genazzani AR. 2003. Non-genomic actions of sex steroid hormones. Eur J Endocrinol 148:281–292 [DOI] [PubMed] [Google Scholar]
- 292. Miller VM, Duckles SP. 2008. Vascular actions of estrogens: functional implications. Pharmacol Rev 60:210–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Papadopoulou N, Papakonstanti EA, Kallergi G, Alevizopoulos K, Stournaras C. 2009. Membrane androgen receptor activation in prostate and breast tumor cells: molecular signaling and clinical impact. IUBMB Life 61:56–61 [DOI] [PubMed] [Google Scholar]
- 294. Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Ström A, Treuter E, Warner M, Gustafsson JA. 2007. Estrogen receptors: how do they signal and what are their targets. Physiol Rev 87:905–931 [DOI] [PubMed] [Google Scholar]
- 295. Gilliver SC. 2010. Sex steroids as inflammatory regulators. J Steroid Biochem Mol Biol 120:105–115 [DOI] [PubMed] [Google Scholar]
- 296. Beato M, Klug J. 2000. Steroid hormone receptors: an update. Hum Reprod Update 6:225–236 [DOI] [PubMed] [Google Scholar]
- 297. McKenna NJ, O'Malley BW. 2002. Minireview: nuclear receptor coactivators—an update. Endocrinology 143:2461–2465 [DOI] [PubMed] [Google Scholar]
- 298. Zhang Z, Teng CT. 2000. Estrogen receptor-related receptor α 1 interacts with coactivator and constitutively activates the estrogen response elements of the human lactoferrin gene. J Biol Chem 275:20837–20846 [DOI] [PubMed] [Google Scholar]
- 299. Hall JM, McDonnell DP. 1999. The estrogen receptor β-isoform (ERβ) of the human estrogen receptor modulates ERα transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology 140:5566–5578 [DOI] [PubMed] [Google Scholar]
- 300. Giangrande PH, McDonnell DP. 1999. The A and B isoforms of the human progesterone receptor: two functionally different transcription factors encoded by a single gene. Recent Prog Horm Res 54:291–313; discussion 313–314 [PubMed] [Google Scholar]
- 301. Lee YF, Shyr CR, Thin TH, Lin WJ, Chang C. 1999. Convergence of two repressors through heterodimer formation of androgen receptor and testicular orphan receptor-4: a unique signaling pathway in the steroid receptor superfamily. Proc Natl Acad Sci USA 96:14724–14729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Zhou ZX, Wong CI, Sar M, Wilson EM. 1994. The androgen receptor: an overview. Recent Prog Horm Res 49:249–274 [DOI] [PubMed] [Google Scholar]
- 303. Filardo EJ, Quinn JA, Frackelton AR, Jr, Bland KI. 2002. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol 16:70–84 [DOI] [PubMed] [Google Scholar]
- 304. Thomas P, Pang Y, Filardo EJ, Dong J. 2005. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 146:624–632 [DOI] [PubMed] [Google Scholar]
- 305. Le Mellay V, Grosse B, Lieberherr M. 1997. Phospholipase C β and membrane action of calcitriol and estradiol. J Biol Chem 272:11902–11907 [DOI] [PubMed] [Google Scholar]
- 306. Grosse B, Kachkache M, Le Mellay V, Lieberherr M. 2000. Membrane signalling and progesterone in female and male osteoblasts. I. Involvement of intracellular Ca(2+), inositol trisphosphate, and diacylglycerol, but not cAMP. J Cell Biochem 79:334–345 [PubMed] [Google Scholar]
- 307. Benten WP, Lieberherr M, Stamm O, Wrehlke C, Guo Z, Wunderlich F. 1999. Testosterone signaling through internalizable surface receptors in androgen receptor-free macrophages. Mol Biol Cell 10:3113–3123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Lieberherr M, Grosse B. 1994. Androgens increase intracellular calcium concentration and inositol 1,4,5-trisphosphate and diacylglycerol formation via a pertussis toxin-sensitive G-protein. J Biol Chem 269:7217–7223 [PubMed] [Google Scholar]
- 309. Valverde MA, Rojas P, Amigo J, Cosmelli D, Orio P, Bahamonde MI, Mann GE, Vergara C, Latorre R. 1999. Acute activation of Maxi-K channels (hSlo) by estradiol binding to the β subunit. Science 285:1929–1931 [DOI] [PubMed] [Google Scholar]
- 310. Lambert JJ, Belelli D, Peden DR, Vardy AW, Peters JA. 2003. Neurosteroid modulation of GABAA receptors. Prog Neurobiol 71:67–80 [DOI] [PubMed] [Google Scholar]
- 311. Frye CA, Gardiner SG. 1996. Progestins can have a membrane-mediated action in rat midbrain for facilitation of sexual receptivity. Horm Behav 30:682–691 [DOI] [PubMed] [Google Scholar]
- 312. White MM, Zamudio S, Stevens T, Tyler R, Lindenfeld J, Leslie K, Moore LG. 1995. Estrogen, progesterone, and vascular reactivity: potential cellular mechanisms. Endocr Rev 16:739–751 [DOI] [PubMed] [Google Scholar]
- 313. Harrison DA, Carr DW, Meizel S. 2000. Involvement of protein kinase A and A kinase anchoring protein in the progesterone-initiated human sperm acrosome reaction. Biol Reprod 62:811–820 [DOI] [PubMed] [Google Scholar]
- 314. Nazareth LV, Weigel NL. 1996. Activation of the human androgen receptor through a protein kinase A signaling pathway. J Biol Chem 271:19900–19907 [DOI] [PubMed] [Google Scholar]
- 315. Bagowski CP, Myers JW, Ferrell JE., Jr 2001. The classical progesterone receptor associates with p42 MAPK and is involved in phosphatidylinositol 3-kinase signaling in Xenopus oocytes. J Biol Chem 276:37708–37714 [DOI] [PubMed] [Google Scholar]
- 316. Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, Metzger D, Chambon P. 1995. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science 270:1491–1494 [DOI] [PubMed] [Google Scholar]
- 317. Endoh H, Sasaki H, Maruyama K, Takeyama K, Waga I, Shimizu T, Kato S, Kawashima H. 1997. Rapid activation of MAP kinase by estrogen in the bone cell line. Biochem Biophys Res Commun 235:99–102 [DOI] [PubMed] [Google Scholar]
- 318. Pearson G, Barry P, Timmins C, Stickley J, Hocking M. 2001. Changes in the profile of paediatric intensive care associated with centralisation. Intensive Care Med 27:1670–1673 [DOI] [PubMed] [Google Scholar]
- 319. Migliaccio A, Piccolo D, Castoria G, Di Domenico M, Bilancio A, Lombardi M, Gong W, Beato M, Auricchio F. 1998. Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen receptor. EMBO J 17:2008–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Simoncini T, Genazzani AR, Liao JK. 2002. Nongenomic mechanisms of endothelial nitric oxide synthase activation by the selective estrogen receptor modulator raloxifene. Circulation 105:1368–1373 [DOI] [PubMed] [Google Scholar]
- 321. Kousteni S, Bellido T, Plotkin LI, O'Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC. 2001. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell 104:719–730 [PubMed] [Google Scholar]
- 322. Migliaccio A, Castoria G, Di Domenico M, de Falco A, Bilancio A, Lombardi M, Barone MV, Ametrano D, Zannini MS, Abbondanza C, Auricchio F. 2000. Steroid-induced androgen receptor-oestradiol receptor β-Src complex triggers prostate cancer cell proliferation. EMBO J 19:5406–5417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Heinlein CA, Chang C. 2002. Androgen receptor (AR) coregulators: an overview. Endocr Rev 23:175–200 [DOI] [PubMed] [Google Scholar]
- 324. Thakur MK, Paramanik V. 2009. Role of steroid hormone coregulators in health and disease. Horm Res 71:194–200 [DOI] [PubMed] [Google Scholar]
- 325. Tremblay GB, Giguère V. 2002. Coregulators of estrogen receptor action. Crit Rev Eukaryot Gene Expr 12:1–22 [DOI] [PubMed] [Google Scholar]
- 326. Smith CL, O'Malley BW. 2004. Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr Rev 25:45–71 [DOI] [PubMed] [Google Scholar]
- 327. Shirasaki H, Watanabe K, Kanaizumi E, Konno N, Sato J, Narita S, Himi T. 2004. Expression and localization of steroid receptors in human nasal mucosa. Acta Otolaryngol 124:958–963 [DOI] [PubMed] [Google Scholar]
- 328. Tulchinsky D, Hobel CJ, Yeager E, Marshall JR. 1972. Plasma estrone, estradiol, estriol, progesterone, and 17-hydroxyprogesterone in human pregnancy. I. Normal pregnancy. Am J Obstet Gynecol 112:1095–1100 [DOI] [PubMed] [Google Scholar]
- 329. Haeggström A, Ostberg B, Stjerna P, Graf P, Hallén H. 2000. Nasal mucosal swelling and reactivity during a menstrual cycle. ORL J Otorhinolaryngol Relat Spec 62:39–42 [DOI] [PubMed] [Google Scholar]
- 330. Paulsson B, Gredmark T, Burian P, Bende M. 1997. Nasal mucosal congestion during the menstrual cycle. J Laryngol Otol 111:337–339 [DOI] [PubMed] [Google Scholar]
- 331. Ellegård E, Karlsson G. 1994. Nasal congestion during the menstrual cycle. Clin Otolaryngol Allied Sci 19:400–403 [DOI] [PubMed] [Google Scholar]
- 332. Philpott CM, Wild DC, Wolstensholme CR, Murty GE. 2008. The presence of ovarian hormone receptors in the nasal mucosa and their relationship to nasal symptoms. Rhinology 46:221–225 [PubMed] [Google Scholar]
- 333. Levin ER. 2001. Cell localization, physiology, and nongenomic actions of estrogen receptors. J Appl Physiol 91:1860–1867 [DOI] [PubMed] [Google Scholar]
- 334. Taylor M. 1961. An experimental study of the influence of the endocrine system on the nasal respiratory mucosa. J Laryngol Otol 75:972–977 [DOI] [PubMed] [Google Scholar]
- 335. Zondek B, Bromberg YM. 1947. Mechanism of endocrine allergy; interpretation of principal phenomena observed in hypersensitivity to the endogenous hormones. Acta Med Orient 6:1–8 [PubMed] [Google Scholar]
- 336. Pelikan Z. 1978. Possible immediate hypersensitivity reaction of the nasal mucosa to oral contraceptives. Ann Allergy 40:211–219 [PubMed] [Google Scholar]
- 337. Mortimer H, Wright RP, Collip JB. 1936. The effect of the administration of oestrogenic hormones on the nasal mucosa of the monkey (Macaca mulatta). Can Med Assoc J 35:503–513 [PMC free article] [PubMed] [Google Scholar]
- 338. Toppozada H, Michaels L, Toppozada M, El-Ghazzawi I, Talaat M, Elwany S. 1982. The human respiratory nasal mucosa in pregnancy. An electron microscopic and histochemical study. J Laryngol Otol 96:613–626 [DOI] [PubMed] [Google Scholar]
- 339. Konno A, Terada N, Okamoto Y. 1986. Effects of female hormones on the muscarinic and α 1-adrenergic receptors of the nasal mucosa. An experimental study in guinea pigs. ORL J Otorhinolaryngol Relat Spec 48:45–51 [DOI] [PubMed] [Google Scholar]
- 340. Hamano N, Terada N, Maesako K, Ikeda T, Fukuda S, Wakita J, Yamashita T, Konno A. 1998. Expression of histamine receptors in nasal epithelial cells and endothelial cells—the effects of sex hormones. Int Arch Allergy Immunol 115:220–227 [DOI] [PubMed] [Google Scholar]
- 341. Kirsch EA, Yuhanna IS, Chen Z, German Z, Sherman TS, Shaul PW. 1999. Estrogen acutely stimulates endothelial nitric oxide synthase in H441 human airway epithelial cells. Am J Respir Cell Mol Biol 20:658–666 [DOI] [PubMed] [Google Scholar]
- 342. Ivanova MM, Mazhawidza W, Dougherty SM, Klinge CM. 2010. Sex differences in estrogen receptor subcellular location and activity in lung adenocarcinoma cells. Am J Respir Cell Mol Biol 42:320–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Townsend EA, Meuchel LW, Thompson MA, Pabelick CM, Prakash YS. 22. September 2011 Estrogen increases nitric oxide production in human bronchial epithelium. J Pharmacol Exp Ther doi:10.1124/jpet.111.184416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Sud N, Wiseman DA, Black SM. 2010. Caveolin 1 is required for the activation of endothelial nitric oxide synthase in response to 17β-estradiol. Mol Endocrinol 24:1637–1649 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 345. Hamad AM, Clayton A, Islam B, Knox AJ. 2003. Guanylyl cyclases, nitric oxide, natriuretic peptides, and airway smooth muscle function. Am J Physiol Lung Cell Mol Physiol 285:L973–L983 [DOI] [PubMed] [Google Scholar]
- 346. Folkerts G, Nijkamp FP. 2006. Nitric oxide in asthma therapy. Curr Pharm Des 12:3221–3232 [DOI] [PubMed] [Google Scholar]
- 347. Batra J, Pratap Singh T, Mabalirajan U, Sinha A, Prasad R, Ghosh B. 2007. Association of inducible nitric oxide synthase with asthma severity, total serum immunoglobulin E and blood eosinophil levels. Thorax 62:16–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Morris NH, Sooranna SR, Steer PJ, Warren JB. 1996. The effect of the menstrual cycle on exhaled nitric oxide and urinary nitrate concentration. Eur J Clin Invest 26:481–484 [DOI] [PubMed] [Google Scholar]
- 349. Jilma B, Kastner J, Mensik C, Vondrovec B, Hildebrandt J, Krejcy K, Wagner OF, Eichler HG. 1996. Sex differences in concentrations of exhaled nitric oxide and plasma nitrate. Life Sci 58:469–476 [DOI] [PubMed] [Google Scholar]
- 350. Vihko P, Herrala A, Härkönen P, Isomaa V, Kaija H, Kurkela R, Pulkka A. 2006. Control of cell proliferation by steroids: the role of 17HSDs. Mol Cell Endocrinol 248:141–148 [DOI] [PubMed] [Google Scholar]
- 351. Folkerd EJ, Dowsett M. 2010. Influence of sex hormones on cancer progression. J Clin Oncol 28:4038–4044 [DOI] [PubMed] [Google Scholar]
- 352. Dammann CE, Ramadurai SM, McCants DD, Pham LD, Nielsen HC. 2000. Androgen regulation of signaling pathways in late fetal mouse lung development. Endocrinology 141:2923–2929 [DOI] [PubMed] [Google Scholar]
- 353. Provost PR, Simard M, Tremblay Y. 2004. A link between lung androgen metabolism and the emergence of mature epithelial type II cells. Am J Respir Crit Care Med 170:296–305 [DOI] [PubMed] [Google Scholar]
- 354. Nielsen HC. 1985. Androgen receptors influence the production of pulmonary surfactant in the testicular feminization mouse fetus. J Clin Invest 76:177–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Ishibashi H, Suzuki T, Suzuki S, Niikawa H, Lu L, Miki Y, Moriya T, Hayashi S, Handa M, Kondo T, Sasano H. 2005. Progesterone receptor in non-small cell lung cancer—a potent prognostic factor and possible target for endocrine therapy. Cancer Res 65:6450–6458 [DOI] [PubMed] [Google Scholar]
- 356. Márquez-Garbán DC, Chen HW, Fishbein MC, Goodglick L, Pietras RJ. 2007. Estrogen receptor signaling pathways in human non-small cell lung cancer. Steroids 72:135–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Mikkonen L, Pihlajamaa P, Sahu B, Zhang FP, Jänne OA. 2010. Androgen receptor and androgen-dependent gene expression in lung. Mol Cell Endocrinol 317:14–24 [DOI] [PubMed] [Google Scholar]
- 358. Márquez-Garbán DC, Chen HW, Goodglick L, Fishbein MC, Pietras RJ. 2009. Targeting aromatase and estrogen signaling in human non-small cell lung cancer. Ann NY Acad Sci 1155:194–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Plante J, Simard M, Rantakari P, Côté M, Provost PR, Poutanen M, Tremblay Y. 2009. Epithelial cells are the major site of hydroxysteroid (17β) dehydrogenase 2 and androgen receptor expression in fetal mouse lungs during the period overlapping the surge of surfactant. J Steroid Biochem Mol Biol 117:139–145 [DOI] [PubMed] [Google Scholar]
- 360. Ino T, Aviado DM. 1971. Cardiopulmonary effects of progestational agents in emphysematous rats. Chest 59:659–666 [DOI] [PubMed] [Google Scholar]
- 361. Ito H, Aviado DM. 1968. Prevention of pulmonary emphysema in rats by progesterone. J Pharmacol Exp Ther 161:197–204 [PubMed] [Google Scholar]
- 362. Giles RE, Finkel MP, Williams JC, Winbury MM. 1974. The therapeutic effect of progesterone in papain-induced emphysema. Proc Soc Exp Biol Med 147:489–493 [DOI] [PubMed] [Google Scholar]
- 363. Giles RE, Williams JC, Finkel MP, Winbury MM. 1973. Progesterone antagonism of papain emphysema: role of sex, estrogens and serum antitrypsin. Proc Soc Exp Biol Med 144:487–491 [DOI] [PubMed] [Google Scholar]
- 364. Tyler JM. 1960. The effect of progesterone on the respiration of patients with emphysema and hypercapnia. J Clin Invest 39:34–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Hershberger PA, Vasquez AC, Kanterewicz B, Land S, Siegfried JM, Nichols M. 2005. Regulation of endogenous gene expression in human non-small cell lung cancer cells by estrogen receptor ligands. Cancer Res 65:1598–1605 [DOI] [PubMed] [Google Scholar]
- 366. Márquez DC, Lee J, Lin T, Pietras RJ. 2001. Epidermal growth factor receptor and tyrosine phosphorylation of estrogen receptor. Endocrine 16:73–81 [DOI] [PubMed] [Google Scholar]
- 367. Stabile LP, Lyker JS, Gubish CT, Zhang W, Grandis JR, Siegfried JM. 2005. Combined targeting of the estrogen receptor and the epidermal growth factor receptor in non-small cell lung cancer shows enhanced antiproliferative effects. Cancer Res 65:1459–1470 [DOI] [PubMed] [Google Scholar]
- 368. Kawai H, Ishii A, Washiya K, Konno T, Kon H, Yamaya C, Ono I, Ogawa J. 2005. Combined overexpression of EGFR and estrogen receptor α correlates with a poor outcome in lung cancer. Anticancer Res 25:4693–4698 [PubMed] [Google Scholar]
- 369. Weinberg OK, Marquez-Garban DC, Fishbein MC, Goodglick L, Garban HJ, Dubinett SM, Pietras RJ. 2005. Aromatase inhibitors in human lung cancer therapy. Cancer Res 65:11287–11291 [DOI] [PubMed] [Google Scholar]
- 370. Mah V, Seligson DB, Li A, Márquez DC, Wistuba II, Elshimali Y, Fishbein MC, Chia D, Pietras RJ, Goodglick L. 2007. Aromatase expression predicts survival in women with early-stage non small cell lung cancer. Cancer Res 67:10484–10490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Su JM, Hsu HK, Chang H, Lin SL, Chang HC, Huang MS, Tseng HH. 1996. Expression of estrogen and progesterone receptors in non-small-cell lung cancer: immunohistochemical study. Anticancer Res 16:3803–3806 [PubMed] [Google Scholar]
- 372. Nishizawa Y, Yamasaki M, Katayama H, Amakata Y, Fushiki S, Nishizawa Y. 2007. Establishment of a progesterone-sensitive cell line from human lung cancer. Oncol Rep 18:685–690 [PubMed] [Google Scholar]
- 373. Verstuyf A, Sobis H, Vandeputte M. 1989. Morphological and immunological characteristics of a rat choriocarcinoma. Int J Cancer 44:879–884 [DOI] [PubMed] [Google Scholar]
- 374. Maasberg M, Rotsch M, Jaques G, Enderle-Schmidt U, Weehle R, Havemann K. 1989. Androgen receptors, androgen-dependent proliferation, and 5 α-reductase activity of small-cell lung cancer cell lines. Int J Cancer 43:685–691 [DOI] [PubMed] [Google Scholar]
- 375. Recchia AG, Musti AM, Lanzino M, Panno ML, Turano E, Zumpano R, Belfiore A, Andò S, Maggiolini M. 2009. A cross-talk between the androgen receptor and the epidermal growth factor receptor leads to p38MAPK-dependent activation of mTOR and cyclin D1 expression in prostate and lung cancer cells. Int J Biochem Cell Biol 41:603–614 [DOI] [PubMed] [Google Scholar]
- 376. Kellermann G, Shaw CR, Luyten-Kellerman M. 1973. Aryl hydrocarbon hydroxylase inducibility and bronchogenic carcinoma. N Engl J Med 289:934–937 [DOI] [PubMed] [Google Scholar]
- 377. Bullard MK, Bir N, Kwan R, Cureton E, Knudson P, Harken A. 2010. Women rule. Surgery 147:134–137 [DOI] [PubMed] [Google Scholar]
- 378. Wang M, Crisostomo PR, Markel T, Wang Y, Lillemoe KD, Meldrum DR. 2008. Estrogen receptor β mediates acute myocardial protection following ischemia. Surgery 144:233–238 [DOI] [PubMed] [Google Scholar]
- 379. Choudhry MA, Schwacha MG, Hubbard WJ, Kerby JD, Rue LW, Bland KI, Chaudry IH. 2005. Gender differences in acute response to trauma-hemorrhage. Shock 24(Suppl 1):101–106 [DOI] [PubMed] [Google Scholar]
- 380. Raju R, Chaudry IH. 2008. Sex steroids/receptor antagonist: their use as adjuncts after trauma-hemorrhage for improving immune/cardiovascular responses and for decreasing mortality from subsequent sepsis. Anesth Analg 107:159–166 [DOI] [PubMed] [Google Scholar]
- 381. Ananthakrishnan P, Cohen DB, Xu DZ, Lu Q, Feketeova E, Deitch EA. 2005. Sex hormones modulate distant organ injury in both a trauma/hemorrhagic shock model and a burn model. Surgery 137:56–65 [DOI] [PubMed] [Google Scholar]
- 382. Caruso JM, Deitch EA, Xu DZ, Lu Q, Dayal SD. 2003. Gut injury and gut-induced lung injury after trauma hemorrhagic shock is gender and estrus cycle specific in the rat. J Trauma 55:531–539 [DOI] [PubMed] [Google Scholar]
- 383. Caruso JM, Xu DZ, Lu Q, Dayal SD, Deitch EA. 2001. The female gender protects against pulmonary injury after trauma hemorrhagic shock. Surg Infect (Larchmt) 2:231–240 [DOI] [PubMed] [Google Scholar]
- 384. Speyer CL, Rancilio NJ, McClintock SD, Crawford JD, Gao H, Sarma JV, Ward PA. 2005. Regulatory effects of estrogen on acute lung inflammation in mice. Am J Physiol Cell Physiol 288:C881–C890 [DOI] [PubMed] [Google Scholar]
- 385. Tesfaigzi Y, Rudolph K, Fischer MJ, Conn CA. 2001. Bcl-2 mediates sex-specific differences in recovery of mice from LPS-induced signs of sickness independent of IL-6. J Appl Physiol 91:2182–2189 [DOI] [PubMed] [Google Scholar]
- 386. Yu HP, Hsieh YC, Suzuki T, Shimizu T, Choudhry MA, Schwacha MG, Chaudry IH. 2006. Salutary effects of estrogen receptor-β agonist on lung injury after trauma-hemorrhage. Am J Physiol Lung Cell Mol Physiol 290:L1004–L1009 [DOI] [PubMed] [Google Scholar]
- 387. Ebina M, Takahashi T, Chiba T, Motomiya M. 1993. Cellular hypertrophy and hyperplasia of airway smooth muscles underlying bronchial asthma. A 3-D morphometric study. Am Rev Respir Dis 148:720–726 [DOI] [PubMed] [Google Scholar]
- 388. Camp PG, Appleton J, Reid WD. 2000. Quality of life after pulmonary rehabilitation: assessing change using quantitative and qualitative methods. Phys Ther 80:986–995 [PubMed] [Google Scholar]
- 389. Degano B, Mourlanette P, Valmary S, Pontier S, Prévost MC, Escamilla R. 2003. Differential effects of low and high-dose estradiol on airway reactivity in ovariectomized rats. Respir Physiol Neurobiol 138:265–274 [DOI] [PubMed] [Google Scholar]
- 390. Kouloumenta V, Hatziefthimiou A, Paraskeva E, Gourgoulianis K, Molyvdas PA. 2007. Sexual dimorphism in airway responsiveness to sex hormones in rabbits. Am J Physiol Lung Cell Mol Physiol 293:L516. [DOI] [PubMed] [Google Scholar]
- 391. Pang JJ, Xu XB, Li HF, Zhang XY, Zheng TZ, Qu SY. 2002. Inhibition of β-estradiol on trachea smooth muscle contraction in vitro and in vivo. Acta Pharmacol Sin 23:273–277 [PubMed] [Google Scholar]
- 392. Townsend EA, Thompson MA, Pabelick CM, Prakash YS. 2010. Rapid effects of estrogen on intracellular Ca2+ regulation in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 298:L521–L530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393. Prakash YS, Thompson MA, Vaa B, Matabdin I, Peterson TE, He T, Pabelick CM. 2007. Caveolins and intracellular calcium regulation in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 293:L1118–L1126 [DOI] [PubMed] [Google Scholar]
- 394. Ay B, Prakash YS, Pabelick CM, Sieck GC. 2004. Store-operated Ca2+ entry in porcine airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 286:L909–L917 [DOI] [PubMed] [Google Scholar]
- 395. Jia S, Zhang X, He DZ, Segal M, Berro A, Gerson TG, Wang Z, Casale TB. 3. June 2011 Expression and function of a novel variant of estrogen receptor-ER-α36 in mouse airway. Am J Respir Cell Mol Biol doi: 10.1165/rcmb.2010–0268OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396. Perusquía M, Hernández R, Montaño LM, Villalón CM, Campos MG. 1997. Inhibitory effect of sex steroids on guinea-pig airway smooth muscle contractions. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 118:5–10 [DOI] [PubMed] [Google Scholar]
- 397. Amrani Y, Syed F, Huang C, Li K, Liu V, Jain D, Keslacy S, Sims MW, Baidouri H, Cooper PR, Zhao H, Siddiqui S, Brightling CE, Griswold D, Li L, Panettieri RA., Jr 2010. Expression and activation of the oxytocin receptor in airway smooth muscle cells: regulation by TNFα and IL-13. Respir Res 11:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398. Grazzini E, Guillon G, Mouillac B, Zingg HH. 1998. Inhibition of oxytocin receptor function by direct binding of progesterone. Nature 392:509–512 [DOI] [PubMed] [Google Scholar]
- 399. Kouloumenta V, Hatziefthimiou A, Paraskeva E, Gourgoulianis K, Molyvdas PA. 2006. Non-genomic effect of testosterone on airway smooth muscle. Br J Pharmacol 149:1083–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400. Bordallo J, de Boto MJ, Meana C, Velasco L, Bordallo C, Suárez L, Cantabrana B, Sánchez M. 2008. Modulatory role of endogenous androgens on airway smooth muscle tone in isolated guinea-pig and bovine trachea; involvement of β2-adrenoceptors, the polyamine system and external calcium. Eur J Pharmacol 601:154–162 [DOI] [PubMed] [Google Scholar]
- 401. Rodríguez D, Keller AC, Faquim-Mauro EL, de Macedo MS, Cunha FQ, Lefort J, Vargaftig BB, Russo M. 2003. Bacterial lipopolysaccharide signaling through Toll-like receptor 4 suppresses asthma-like responses via nitric oxide synthase 2 activity. J Immunol 171:1001–1008 [DOI] [PubMed] [Google Scholar]
- 402. Stewart AG, Fernandes D, Tomlinson PR. 1995. The effect of glucocorticoids on proliferation of human cultured airway smooth muscle. Br J Pharmacol 116:3219–3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403. Dashtaki R, Whorton AR, Murphy TM, Chitano P, Reed W, Kennedy TP. 1998. Dehydroepiandrosterone and analogs inhibit DNA binding of AP-1 and airway smooth muscle proliferation. J Pharmacol Exp Ther 285:876–883 [PubMed] [Google Scholar]
- 404. Stamatiou R, Paraskeva E, Papagianni M, Molyvdas PA, Hatziefthimiou A. 2011. The mitogenic effect of testosterone and 17β-estradiol on airway smooth muscle cells. Steroids 76:400–408 [DOI] [PubMed] [Google Scholar]
- 405. Kawai K, Akaza H. 2003. Bleomycin-induced pulmonary toxicity in chemotherapy for testicular cancer. Expert Opin Drug Saf 2:587–596 [DOI] [PubMed] [Google Scholar]
- 406. Voltz JW, Card JW, Carey MA, Degraff LM, Ferguson CD, Flake GP, Bonner JC, Korach KS, Zeldin DC. 2008. Male sex hormones exacerbate lung function impairment after bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol 39:45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407. Tofovic SP, Zhang X, Jackson EK, Zhu H, Petrusevska G. 2009. 2-Methoxyestradiol attenuates bleomycin-induced pulmonary hypertension and fibrosis in estrogen-deficient rats. Vascul Pharmacol 51:190–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408. Kondo H, Kasuga H, Noumura T. 1983. Effects of various steroids on in vitro lifespan and cell growth of human fetal lung fibroblasts (WI-38). Mech Ageing Dev 21:335–344 [DOI] [PubMed] [Google Scholar]
- 409. Flores-Delgado G, Bringas P, Buckley S, Anderson KD, Warburton D. 2001. Nongenomic estrogen action in human lung myofibroblasts. Biochem Biophys Res Commun 283:661–667 [DOI] [PubMed] [Google Scholar]
- 410. Milam JE, Keshamouni VG, Phan SH, Hu B, Gangireddy SR, Hogaboam CM, Standiford TJ, Thannickal VJ, Reddy RC. 2008. PPAR-γ agonists inhibit profibrotic phenotypes in human lung fibroblasts and bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 294:L891–L901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411. Smith AM, Bennett RT, Jones TH, Cowen ME, Channer KS, Jones RD. 2008. Characterization of the vasodilatory action of testosterone in the human pulmonary circulation. Vasc Health Risk Manag 4:1459–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412. Rabinovitch M, Gamble WJ, Miettinen OS, Reid L. 1981. Age and sex influence on pulmonary hypertension of chronic hypoxia and on recovery. Am J Physiol 240:H62–H72 [DOI] [PubMed] [Google Scholar]
- 413. McMurtry IF, Frith CH, Will DH. 1973. Cardiopulmonary responses of male and female swine to simulated high altitude. J Appl Physiol 35:459–462 [DOI] [PubMed] [Google Scholar]
- 414. Resta TC, Kanagy NL, Walker BR. 2001. Estradiol-induced attenuation of pulmonary hypertension is not associated with altered eNOS expression. Am J Physiol Lung Cell Mol Physiol 280:L88–L97 [DOI] [PubMed] [Google Scholar]
- 415. Lahm T, Patel KM, Crisostomo PR, Markel TA, Wang M, Herring C, Meldrum DR. 2007. Endogenous estrogen attenuates pulmonary artery vasoreactivity and acute hypoxic pulmonary vasoconstriction: the effects of sex and menstrual cycle. Am J Physiol Endocrinol Metab 293:E865–E871 [DOI] [PubMed] [Google Scholar]
- 416. Wetzel RC, Zacur HA, Sylvester JT. 1984. Effect of puberty and estradiol on hypoxic vasomotor response in isolated sheep lungs. J Appl Physiol 56:1199–1203 [DOI] [PubMed] [Google Scholar]
- 417. Kiyatake K, Kakusaka I, Kasahara Y, Qi TM, Suzuki A, Nakano K, Kaneko N, Kitada M, Kuriyama T. 1994. [Relationship between the converting ability of liver microsomes and monocrotaline-induced pulmonary hypertension in male, female and castrated male rats]. Nihon Kyobu Shikkan Gakkai Zasshi 32:125–129 [PubMed] [Google Scholar]
- 418. Farhat MY, Chen MF, Bhatti T, Iqbal A, Cathapermal S, Ramwell PW. 1993. Protection by oestradiol against the development of cardiovascular changes associated with monocrotaline pulmonary hypertension in rats. Br J Pharmacol 110:719–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419. Ahn BH, Park HK, Cho HG, Lee HA, Lee YM, Yang EK, Lee WJ. 2003. Estrogen and enalapril attenuate the development of right ventricular hypertrophy induced by monocrotaline in ovariectomized rats. J Korean Med Sci 18:641–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420. Tofovic S, Rafikova O. 2009. Preventive and therapeutic effects of 2-methoxyestradiol, but not estradiol, in severe occlusive pulmonary arterial hypertension in female rats. Am J Respir Crit Care Med 179:A1802 [Google Scholar]
- 421. Dempsie Y, Nilsen M, Loughlin L, MacLean MR. 2009. The effects of gender on the development of pulmonary arterial hypertension in mice over-expressing the serotonin transporter. Am J Respir Crit Care Med 179:A1809 [Google Scholar]
- 422. Bolego C, Cignarella A, Sanvito P, Pelosi V, Pellegatta F, Puglisi L, Pinna C. 2005. The acute estrogenic dilation of rat aorta is mediated solely by selective estrogen receptor-α agonists and is abolished by estrogen deprivation. J Pharmacol Exp Ther 313:1203–1208 [DOI] [PubMed] [Google Scholar]
- 423. Douglas G, Cruz MN, Poston L, Gustafsson JA, Kublickiene K. 2008. Functional characterization and sex differences in small mesenteric arteries of the estrogen receptor-β knockout mouse. Am J Physiol Regul Integr Comp Physiol 294:R112–R120 [DOI] [PubMed] [Google Scholar]
- 424. Tostes RC, Nigro D, Fortes ZB, Carvalho MH. 2003. Effects of estrogen on the vascular system. Braz J Med Biol Res 36:1143–1158 [DOI] [PubMed] [Google Scholar]
- 425. Akarasereenont P, Techatraisak K, Thaworn A, Chotewuttakorn S. 2000. The induction of cyclooxygenase-2 by 17β-estradiol in endothelial cells is mediated through protein kinase C. Inflamm Res 49:460–465 [DOI] [PubMed] [Google Scholar]
- 426. Prakash YS, Togaibayeva AA, Kannan MS, Miller VM, Fitzpatrick LA, Sieck GC. 1999. Estrogen increases Ca2+ efflux from female porcine coronary arterial smooth muscle. Am J Physiol 276:H926–H934 [DOI] [PubMed] [Google Scholar]
- 427. Kitazawa T, Hamada E, Kitazawa K, Gaznabi AK. 1997. Non-genomic mechanism of 17 β-oestradiol-induced inhibition of contraction in mammalian vascular smooth muscle. J Physiol 499:497–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428. Nakajima T, Kitazawa T, Hamada E, Hazama H, Omata M, Kurachi Y. 1995. 17β-Estradiol inhibits the voltage-dependent L-type Ca2+ currents in aortic smooth muscle cells. Eur J Pharmacol 294:625–635 [DOI] [PubMed] [Google Scholar]
- 429. Shimokawa H, Takeshita A. 2005. Rho-kinase is an important therapeutic target in cardiovascular medicine. Arterioscler Thromb Vasc Biol 25:1767–1775 [DOI] [PubMed] [Google Scholar]
- 430. Abe K, Shimokawa H, Morikawa K, Uwatoku T, Oi K, Matsumoto Y, Hattori T, Nakashima Y, Kaibuchi K, Sueishi K, Takeshit A. 2004. Long-term treatment with a Rho-kinase inhibitor improves monocrotaline-induced fatal pulmonary hypertension in rats. Circ Res 94:385–393 [DOI] [PubMed] [Google Scholar]
- 431. Nagaoka T, Fagan KA, Gebb SA, Morris KG, Suzuki T, Shimokawa H, McMurtry IF, Oka M. 2005. Inhaled Rho kinase inhibitors are potent and selective vasodilators in rat pulmonary hypertension. Am J Respir Crit Care Med 171:494–499 [DOI] [PubMed] [Google Scholar]
- 432. Abe K, Morikawa K, Hizume T, Uwatoku T, Oi K, Seto M, Ikegaki I, Asano T, Kaibuchi K, Shimokawa H. 2005. Prostacyclin does not inhibit rho-kinase: an implication for the treatment of pulmonary hypertension. J Cardiovasc Pharmacol 45:120–124 [DOI] [PubMed] [Google Scholar]
- 433. Barberis MC, Veronese S, Bauer D, De Juli E, Harari S. 1995. Immunocytochemical detection of progesterone receptors. A study in a patient with primary pulmonary hypertension. Chest 107:869–872 [DOI] [PubMed] [Google Scholar]
- 434. English KM, Jones RD, Jones TH, Morice AH, Channer KS. 2001. Gender differences in the vasomotor effects of different steroid hormones in rat pulmonary and coronary arteries. Horm Metab Res 33:645–652 [DOI] [PubMed] [Google Scholar]
- 435. Li HF, Zheng TZ, Li W, Qu SY, Zhang CL. 2001. Effect of progesterone on the contractile response of isolated pulmonary artery in rabbits. Can J Physiol Pharmacol 79:545–550 [PubMed] [Google Scholar]
- 436. Tofovic PS, Zhang X, Petrusevska G. 2009. Progesterone inhibits vascular remodeling and attenuates monocrotaline-induced pulmonary hypertension in estrogen-deficient rats. Prilozi 30:25–44 [PubMed] [Google Scholar]
- 437. Vázquez F, Rodríguez-Manzaneque JC, Lydon JP, Edwards DP, O'Malley BW, Iruela-Arispe ML. 1999. Progesterone regulates proliferation of endothelial cells. J Biol Chem 274:2185–2192 [DOI] [PubMed] [Google Scholar]
- 438. Morey AK, Pedram A, Razandi M, Prins BA, Hu RM, Biesiada E, Levin ER. 1997. Estrogen and progesterone inhibit vascular smooth muscle proliferation. Endocrinology 138:3330–3339 [DOI] [PubMed] [Google Scholar]
- 439. Lee WS, Liu CW, Juan SH, Liang YC, Ho PY, Lee YH. 2003. Molecular mechanism of progesterone-induced antiproliferation in rat aortic smooth muscle cells. Endocrinology 144:2785–2790 [DOI] [PubMed] [Google Scholar]
- 440. Pribylova NN, Goloshchapov OA, Parygina EI. 1984. [Significance of progesterone in the pathogenesis and treatment of pulmonary hypertension and cardiopulmonary failure]. Ter Arkh 56:94–97 [PubMed] [Google Scholar]
- 441. Jones RD, English KM, Pugh PJ, Morice AH, Jones TH, Channer KS. 2002. Pulmonary vasodilatory action of testosterone: evidence of a calcium antagonistic action. J Cardiovasc Pharmacol 39:814–823 [DOI] [PubMed] [Google Scholar]
- 442. Rowell KO, Hall J, Pugh PJ, Jones TH, Channer KS, Jones RD. 2009. Testosterone acts as an efficacious vasodilator in isolated human pulmonary arteries and veins: evidence for a biphasic effect at physiological and supra-physiological concentrations. J Endocrinol Invest 32:718–723 [DOI] [PubMed] [Google Scholar]
- 443. Pugh PJ, Jones TH, Channer KS. 2003. Acute haemodynamic effects of testosterone in men with chronic heart failure. Eur Heart J 24:909–915 [DOI] [PubMed] [Google Scholar]
- 444. Bonnet S, Dumas-de-La-Roque E, Bégueret H, Marthan R, Fayon M, Dos Santos P, Savineau JP, Baulieu EE. 2003. Dehydroepiandrosterone (DHEA) prevents and reverses chronic hypoxic pulmonary hypertension. Proc Natl Acad Sci USA 100:9488–9493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445. Farrukh IS, Peng W, Orlinska U, Hoidal JR. 1998. Effect of dehydroepiandrosterone on hypoxic pulmonary vasoconstriction: a Ca(2+)-activated K(+)-channel opener. Am J Physiol 274:L186–L195 [DOI] [PubMed] [Google Scholar]
- 446. Oka M, Karoor V, Homma N, Nagaoka T, Sakao E, Golembeski SM, Limbird J, Imamura M, Gebb SA, Fagan KA, McMurtry IF. 2007. Dehydroepiandrosterone upregulates soluble guanylate cyclase and inhibits hypoxic pulmonary hypertension. Cardiovasc Res 74:377–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447. Liu D, Iruthayanathan M, Homan LL, Wang Y, Yang L, Wang Y, Dillon JS. 2008. Dehydroepiandrosterone stimulates endothelial proliferation and angiogenesis through extracellular signal-regulated kinase 1/2-mediated mechanisms. Endocrinology 149:889–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448. Edwards LJ, Constantinescu CS. 2004. A prospective study of conditions associated with multiple sclerosis in a cohort of 658 consecutive outpatients attending a multiple sclerosis clinic. Mult Scler 10:575–581 [DOI] [PubMed] [Google Scholar]
- 449. Provenzano G, Donato G, Brai G, Rinaldi F. 2002. Prevalence of allergic respiratory diseases in patients with RA. Ann Rheum Dis 61:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450. Rudwaleit M, Andermann B, Alten R, Sörensen H, Listing J, Zink A, Sieper J, Braun J. 2002. Atopic disorders in ankylosing spondylitis and rheumatoid arthritis. Ann Rheum Dis 61:968–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451. Bergamaschi R, Villani S, Crabbio M, Ponzio M, Romani A, Verri A, Bargiggia V, Cosi V. 2009. Inverse relationship between multiple sclerosis and allergic respiratory diseases. Neurol Sci 30:115–118 [DOI] [PubMed] [Google Scholar]
- 452. Tremlett HL, Evans J, Wiles CM, Luscombe DK. 2002. Asthma and multiple sclerosis: an inverse association in a case-control general practice population. QJM 95:753–756 [DOI] [PubMed] [Google Scholar]
- 453. Straub RH. 2007. The complex role of estrogens in inflammation. Endocr Rev 28:521–574 [DOI] [PubMed] [Google Scholar]
- 454. Pierdominici M, Maselli A, Colasanti T, Giammarioli AM, Delunardo F, Vacirca D, Sanchez M, Giovannetti A, Malorni W, Ortona E. 2010. Estrogen receptor profiles in human peripheral blood lymphocytes. Immunol Lett 132:79–85 [DOI] [PubMed] [Google Scholar]
- 455. Soldan SS, Alvarez Retuerto AI, Sicotte NL, Voskuhl RR. 2003. Immune modulation in multiple sclerosis patients treated with the pregnancy hormone estriol. J Immunol 171:6267–6274 [DOI] [PubMed] [Google Scholar]
- 456. Cenci S, Weitzmann MN, Roggia C, Namba N, Novack D, Woodring J, Pacifici R. 2000. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-α. J Clin Invest 106:1229–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457. Lambert KC, Curran EM, Judy BM, Milligan GN, Lubahn DB, Estes DM. 2005. Estrogen receptor α (ERα) deficiency in macrophages results in increased stimulation of CD4+ T cells while 17β-estradiol acts through ERα to increase IL-4 and GATA-3 expression in CD4+ T cells independent of antigen presentation. J Immunol 175:5716–5723 [DOI] [PubMed] [Google Scholar]
- 458. Butts CL, Shukair SA, Duncan KM, Harris CW, Belyavskaya E, Sternberg EM. 2007. Evaluation of steroid hormone receptor protein expression in intact cells using flow cytometry. Nucl Recept Signal 5:e007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459. Chiu L, Nishimura M, Ishii Y, Nieda M, Maeshima M, Takedani Y, Shibata Y, Tadokoro K, Juji T. 1996. Enhancement of the expression of progesterone receptor on progesterone-treated lymphocytes after immunotherapy in unexplained recurrent spontaneous abortion. Am J Reprod Immunol 35:552–557 [DOI] [PubMed] [Google Scholar]
- 460. Mansour I, Reznikoff-Etievant MF, Netter A. 1994. No evidence for the expression of the progesterone receptor on peripheral blood lymphocytes during pregnancy. Hum Reprod 9:1546–1549 [DOI] [PubMed] [Google Scholar]
- 461. Schust DJ, Anderson DJ, Hill JA. 1996. Progesterone-induced immunosuppression is not mediated through the progesterone receptor. Hum Reprod 11:980–985 [DOI] [PubMed] [Google Scholar]
- 462. Miyaura H, Iwata M. 2002. Direct and indirect inhibition of Th1 development by progesterone and glucocorticoids. J Immunol 168:1087–1094 [DOI] [PubMed] [Google Scholar]
- 463. Huck B, Steck T, Habersack M, Dietl J, Kämmerer U. 2005. Pregnancy associated hormones modulate the cytokine production but not the phenotype of PBMC-derived human dendritic cells. Eur J Obstet Gynecol Reprod Biol 122:85–94 [DOI] [PubMed] [Google Scholar]
- 464. Hardy DB, Janowski BA, Corey DR, Mendelson CR. 2006. Progesterone receptor plays a major antiinflammatory role in human myometrial cells by antagonism of nuclear factor-κB activation of cyclooxygenase 2 expression. Mol Endocrinol 20:2724–2733 [DOI] [PubMed] [Google Scholar]
- 465. Huber SA, Kupperman J, Newell MK. 1999. Hormonal regulation of CD4(+) T-cell responses in coxsackievirus B3-induced myocarditis in mice. J Virol 73:4689–4695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466. Namazi MR. 2009. The Th1-promoting effects of dehydroepiandrosterone can provide an explanation for the stronger Th1-immune response of women. Iran J Allergy Asthma Immunol 8:65–69 [PubMed] [Google Scholar]
- 467. Suzuki T, Suzuki N, Daynes RA, Engleman EG. 1991. Dehydroepiandrosterone enhances IL2 production and cytotoxic effector function of human T cells. Clin Immunol Immunopathol 61:202–211 [DOI] [PubMed] [Google Scholar]
- 468. Araghi-Niknam M, Zhang Z, Jiang S, Call O, Eskelson CD, Watson RR. 1997. Cytokine dysregulation and increased oxidation is prevented by dehydroepiandrosterone in mice infected with murine leukemia retrovirus. Proc Soc Exp Biol Med 216:386–391 [DOI] [PubMed] [Google Scholar]
- 469. Yu CK, Liu YH, Chen CL. 2002. Dehydroepiandrosterone attenuates allergic airway inflammation in Dermatophagoides farinae-sensitized mice. J Microbiol Immunol Infect 35:199–202 [PubMed] [Google Scholar]
- 470. Choi IS, Cui Y, Koh YA, Lee HC, Cho YB, Won YH. 2008. Effects of dehydroepiandrosterone on Th2 cytokine production in peripheral blood mononuclear cells from asthmatics. Korean J Intern Med 23:176–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471. Melgert BN, Oriss TB, Qi Z, Dixon-McCarthy B, Geerlings M, Hylkema MN, Ray A. 2010. Macrophages: regulators of sex differences in asthma? Am J Respir Cell Mol Biol 42:595–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472. Paharkova-Vatchkova V, Maldonado R, Kovats S. 2004. Estrogen preferentially promotes the differentiation of CD11c+ CD11b(intermediate) dendritic cells from bone marrow precursors. J Immunol 172:1426–1436 [DOI] [PubMed] [Google Scholar]
- 473. Canning MO, Grotenhuis K, de Wit HJ, Drexhage HA. 2000. Opposing effects of dehydroepiandrosterone and dexamethasone on the generation of monocyte-derived dendritic cells. Eur J Endocrinol 143:687–695 [DOI] [PubMed] [Google Scholar]
- 474. Riffo-Vasquez Y, Ligeiro de Oliveira AP, Page CP, Spina D, Tavares-de-Lima W. 2007. Role of sex hormones in allergic inflammation in mice. Clin Exp Allergy 37:459–470 [DOI] [PubMed] [Google Scholar]
- 475. Klebanoff SJ. 1977. Estrogen binding by leukocytes during phagocytosis. J Exp Med 145:983–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476. Katayama ML, Federico MH, Brentani RR, Brentani MM. 1998. Eosinophil accumulation in rat uterus following estradiol administration is modulated by laminin and its integrin receptors. Cell Adhes Commun 5:409–424 [DOI] [PubMed] [Google Scholar]
- 477. Mayo JC, Sáinz RM, Antolín I, Uría H, Menéndez-Peláez A, Rodríguez C. 1997. Androgen-dependent mast cell degranulation in the Harderian gland of female Syrian hamsters: in vivo and organ culture evidence. Anat Embryol (Berl) 196:133–140 [DOI] [PubMed] [Google Scholar]
- 478. Belot MP, Abdennebi-Najar L, Gaudin F, Lieberherr M, Godot V, Taïeb J, Emilie D, Machelon V. 2007. Progesterone reduces the migration of mast cells toward the chemokine stromal cell-derived factor-1/CXCL12 with an accompanying decrease in CXCR4 receptors. Am J Physiol Endocrinol Metab 292:E1410–E1417 [DOI] [PubMed] [Google Scholar]
- 479. Vasiadi M, Kempuraj D, Boucher W, Kalogeromitros D, Theoharides TC. 2006. Progesterone inhibits mast cell secretion. Int J Immunopathol Pharmacol 19:787–794 [DOI] [PubMed] [Google Scholar]
- 480. Gritz ER, Nielsen IR, Brooks LA. 1996. Smoking cessation and gender: the influence of physiological, psychological, and behavioral factors. J Am Med Womens Assoc 51:35–42 [PubMed] [Google Scholar]
- 481. Dijkstra A, Howard TD, Vonk JM, Ampleford EJ, Lange LA, Bleecker ER, Meyers DA, Postma DS. 2006. Estrogen receptor 1 polymorphisms are associated with airway hyperresponsiveness and lung function decline, particularly in female subjects with asthma. J Allergy Clin Immunol 117:604–611 [DOI] [PubMed] [Google Scholar]
- 482. Oberholzer A, Keel M, Zellweger R, Steckholzer U, Trentz O, Ertel W. 2000. Incidence of septic complications and multiple organ failure in severely injured patients is sex specific. J Trauma 48:932–937 [DOI] [PubMed] [Google Scholar]
- 483. Angele MK, Schwacha MG, Ayala A, Chaudry IH. 2000. Effect of gender and sex hormones on immune responses following shock. Shock 14:81–90 [DOI] [PubMed] [Google Scholar]
- 484. Kher A, Wang M, Tsai BM, Pitcher JM, Greenbaum ES, Nagy RD, Patel KM, Wairiuko GM, Markel TA, Meldrum DR. 2005. Sex differences in the myocardial inflammatory response to acute injury. Shock 23:1–10 [DOI] [PubMed] [Google Scholar]
- 485. Deitch EA, Livingston DH, Lavery RF, Monaghan SF, Bongu A, Machiedo GW. 2007. Hormonally active women tolerate shock-trauma better than do men: a prospective study of over 4000 trauma patients. Ann Surg 246:447–453; discussion 453–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486. Offner PJ, Moore EE, Biffl WL. 1999. Male gender is a risk factor for major infections after surgery. Arch Surg 134:935–938; discussion 938–940 [DOI] [PubMed] [Google Scholar]
- 487. Ashcroft GS, Greenwell-Wild T, Horan MA, Wahl SM, Ferguson MW. 1999. Topical estrogen accelerates cutaneous wound healing in aged humans associated with an altered inflammatory response. Am J Pathol 155:1137–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488. Kovacs EJ, Messingham KA, Gregory MS. 2002. Estrogen regulation of immune responses after injury. Mol Cell Endocrinol 193:129–135 [DOI] [PubMed] [Google Scholar]
- 489. Jarrar D, Wang P, Knöferl MW, Kuebler JF, Cioffi WG, Bland KI, Chaudry IH. 2000. Insight into the mechanism by which estradiol improves organ functions after trauma-hemorrhage. Surgery 128:246–252 [DOI] [PubMed] [Google Scholar]
- 490. Yu HP, Chaudry IH. 2009. The role of estrogen and receptor agonists in maintaining organ function after trauma-hemorrhage. Shock 31:227–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491. Martin TR, Castile RG, Fredberg JJ, Wohl ME, Mead J. 1987. Airway size is related to sex but not lung size in normal adults. J Appl Physiol 63:2042–2047 [DOI] [PubMed] [Google Scholar]
- 492. Wang X, Dockery DW, Wypij D, Gold DR, Speizer FE, Ware JH, Ferris BG., Jr 1993. Pulmonary function growth velocity in children 6 to 18 years of age. Am Rev Respir Dis 148:1502–1508 [DOI] [PubMed] [Google Scholar]
- 493. Harms CA. 2006. Does gender affect pulmonary function and exercise capacity? Respir Physiol Neurobiol 151:124–131 [DOI] [PubMed] [Google Scholar]
- 494. Pride NB. 2005. Ageing and changes in lung mechanics. Eur Respir J 26:563–565 [DOI] [PubMed] [Google Scholar]
- 495. Bertelsen RJ, Instanes C, Granum B, Lødrup Carlsen KC, Hetland G, Carlsen KH, Mowinckel P, Løvik M. 2010. Gender differences in indoor allergen exposure and association with current rhinitis. Clin Exp Allergy 40:1388–1397 [DOI] [PubMed] [Google Scholar]
- 496. Loyd JE, Butler MG, Foroud TM, Conneally PM, Phillips JA, 3rd, Newman JH. 1995. Genetic anticipation and abnormal gender ratio at birth in familial primary pulmonary hypertension. Am J Respir Crit Care Med 152:93–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497. 2010. Putting gender on the agenda. Nature 465:665. [DOI] [PubMed] [Google Scholar]