Abstract
Ovarian steroids normally exert homeostatic negative feedback on GnRH release. During sustained exposure to elevated estradiol in the late follicular phase of the reproductive cycle, however, the feedback action of estradiol switches to positive, inducing a surge of GnRH release from the brain, which signals the pituitary LH surge that triggers ovulation. In rodents, this switch appears dependent on a circadian signal that times the surge to a specific time of day (e.g., late afternoon in nocturnal species). Although the precise nature of this daily signal and the mechanism of the switch from negative to positive feedback have remained elusive, work in the past decade has provided much insight into the role of circadian/diurnal and estradiol-dependent signals in GnRH/LH surge regulation and timing. Here we review the current knowledge of the neurobiology of the GnRH surge, in particular the actions of estradiol on GnRH neurons and their synaptic afferents, the regulation of GnRH neurons by fast synaptic transmission mediated by the neurotransmitters γ-aminobutyric acid and glutamate, and the host of excitatory and inhibitory neuromodulators including kisspeptin, vasoactive intestinal polypeptide, catecholamines, neurokinin B, and RFamide-related peptides, that appear essential for GnRH surge regulation, and ultimately ovulation and fertility.
A switch in estradiol feedback action on GnRH release from negative to positive induces a surge of GnRH release from the brain, which signals the pituitary LH surge that triggers ovulation. This review encompasses the current knowledge of the neurobiology of the GnRH surge. Particular focus is placed on the actions of estradiol on GnRH neurons and their synaptic inputs, the regulation of GnRH neurons by the neurotransmitters gamma-aminobutyric acid and glutamate, and various excitatory and inhibitory neuromodulators that appear essential for the surge.
I. Introduction
II. General Principles of Preovulatory and Estradiol-Induced GnRH and LH Surges
- III. In Vitro Studies of the Effects of In Vivo Estradiol and GnRH Surge Physiology
- A. Multiunit activity studies
- B. Direct recordings of individual GnRH neurons
- C. What can electrical recordings tell us about GnRH surge dynamics?
IV. Circadian Regulation of the GnRH/LH Surge and Ovulation
- V. Potential Points of Integration of Circadian and Estradiol Feedback Signals
- A. Suprachiasmatic nuclei
- B. Anteroventral periventricular area
- C. GnRH neurons
- VI. Regulation of GnRH and LH Surges by Fast GABAergic and Glutamatergic Synaptic Transmission
- A. Glutamate
- B. GABA
- C. Regulation of GnRH neuron activity by GABA and glutamate
- VII. Neuromodulatory Regulation of GnRH and LH Surges
- A. Kisspeptin
- B. Vasoactive intestinal polypeptide
- C. Vasopressin
- D. Catecholamines
- E. Nitric oxide
- F. Neurotensin
- G. GnRH
- H. Endogenous opioid peptides
- I. Neurokinin B
- J. Gonadotropin-inhibitory hormone/RFamide-related peptide-3
VIII. A Model for Neurobiological Regulation of the GnRH Surge by Fast Synaptic and Neuromodulatory Signals
IX. Conclusion
I. Introduction
GnRH neurons of the hypothalamus are responsible for the production and secretion of GnRH, and they form the final common pathway in the central regulation of fertility. The population of GnRH neurons extends caudally from the diagonal band of Broca, past the optic chiasm, and into the medial basal hypothalamus. In most mammals, the majority of GnRH neurons are found in the preoptic area and anterior hypothalamus; in higher primates, the caudal cells are predominant. Pulsatile GnRH release from axon terminals in the median eminence into the pituitary portal vasculature leads to synthesis and release of LH and FSH from the anterior pituitary. A pulsatile pattern of GnRH release is an absolute requirement for fertility because continuous GnRH administration down-regulates pituitary gonadotrope activity, leading to suppression of gonadotropin secretion and subsequent infertility (1). In female mammals, changing GnRH pulse frequencies preferentially stimulate LH or FSH release at specific times, thereby creating the appropriate hormone milieu for ovarian follicular development and driving the reproductive cycle (2,3,4). Higher GnRH pulse frequencies stimulate LH synthesis and release, whereas lower GnRH pulse frequencies favor FSH synthesis and release (3,5,6,7). Gonadotropins activate gametogenesis and steroid synthesis by the gonads, and these steroid hormones form both negative and positive feedback loops centrally to modulate GnRH neuron function and at the pituitary to regulate the response to GnRH.
At the end of the follicular phase (proestrus in rodents), when estradiol levels are highest, the response to it switches from negative to positive feedback through a mechanism that is still not well understood. The positive feedback action of estradiol initiates a large continuous increase in GnRH release (the GnRH surge) (8,9), which, along with an increase in gonadotrope responsiveness to GnRH (10,11,12,13), causes a surge in LH release from the pituitary, initiating ovulation. Preovulatory GnRH surges have been demonstrated in rats (14,15), sheep (2,9), monkeys (16), horses (17), and rabbits (18). This is one of the very rare nonhomeostatic feedback events in physiology.
Estradiol exerts potent feedback at both the neural and pituitary levels to regulate the hypothalamo-pituitary gonadal axis. For the purposes of this review, we will concentrate on the effects at the neural level to examine emerging knowledge of the neurobiology of the GnRH surge. Furthermore, we will focus on the mechanism of the surge in spontaneous ovulators, rather than species such as rabbits, in which mating induces the surge. In many species, estradiol feedback at the neural level interacts with signals relaying time-of-day information to correctly time the surge, and defects in circadian rhythms alter and in some cases prevent surge generation and fertility (19,20,21). Therefore, we will also examine the evidence for regulation of the GnRH/LH surge by the circadian timing system, and in particular how these signals may be integrated and transmitted to GnRH neurons to achieve proper regulation and timing of the surge. With respect to terminology, we will use the term “diurnal” in reference to effects that appear to depend on the time of day but for which a specific role of a circadian clock is not yet precisely defined, and “circadian” when discussing the circadian timing system.
With regard to the methodologies used in the studies discussed here, it is important to note that all techniques carry particular advantages and drawbacks. Ultimately, it will be important to incorporate a combination of whole-animal, cellular/electrophysiological, and molecular techniques to establish a full picture of the underlying mechanisms of a physiological phenomenon as complex as the GnRH/LH surge.
II. General Principles of Preovulatory and Estradiol-Induced GnRH and LH Surges
For the major part of the reproductive cycle, estradiol acts via negative feedback actions to reduce GnRH pulse amplitude (22,23,24,25), combined with negative actions on pituitary gonadotropes (26,27) to inhibit LH release. Circulating estradiol levels rise in the later stages of the follicular phase, and estradiol at these higher concentrations has a biphasic effect on GnRH secretion, with a marked suppression of pulsatile GnRH and LH secretion followed by induction of a robust high amplitude GnRH surge accompanying the LH surge (8,13). Despite the importance of the switch from estradiol negative to positive feedback action in regulating reproductive function, the underlying neurobiological mechanisms are not fully understood.
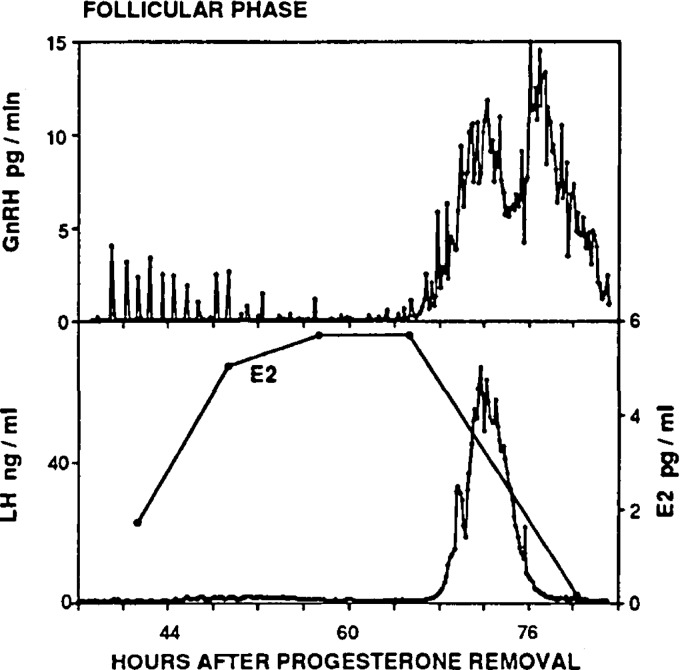
In larger animals such as sheep, numerous samples of pituitary portal blood can be drawn from conscious, normally behaving animals over a continuous period lasting several days, allowing the secretory pattern of GnRH and LH throughout the estrous cycle to be ascertained. Simultaneous measurements of GnRH in the pituitary portal blood and LH in the peripheral blood (2,8,9,28,29,30) demonstrated that a GnRH surge occurs as a response to the endogenous increase in circulating estradiol during the late follicular phase (Fig. 1). In some studies in sheep, the onsets of GnRH and LH surges were found to be coincident (9), whereas in others variable patterns of GnRH release were observed around the time of LH surge onset (31,32). In monkeys, by contrast, a delay of approximately 2.7 h was observed between the onsets of the GnRH and LH surges (33). The GnRH surge extends several hours beyond the termination of the LH surge, and GnRH stimulation throughout the LH surge is required for full LH surge progression (34). Thus, mechanisms downstream of GnRH neurons, such as desensitization of GnRH receptors on LH-producing gonadotropes, decreases in GnRH receptor expression (35,36,37), or depletion of pituitary LH stores (13), are likely responsible for determining LH surge duration.
Figure 1.
Measurement of GnRH, LH, and circulating estradiol (E2) in an individual ewe during the follicular phase demonstrates a preovulatory GnRH/LH surge. Note that the termination of the LH surge precedes that of the GnRH surge. [Data adapted from S. M. Moenter et al.: Endocrinology 129:1175–1182 (9). © 1991, The Endocrine Society].
In smaller species, such as mice, the difficulty of measuring GnRH release often necessitates the use of the LH surge as an assay of the central GnRH surge. This, however, brings the caveat that the ability to separate the positive feedback actions of estradiol at the pituitary (10,11,12,13,38) from those on GnRH neurons is compromised. In rats and mice, the LH surge occurs in the late afternoon of proestrus, leading to ovulation the following morning of estrus. In rats, a GnRH surge can be demonstrated to occur with similar timing (14), suggesting that the final central mechanism is similar in these species. Early surge-induction models in both rats and mice used protocols of ovariectomy and treatment with a basal level of estradiol for 1 wk (OVX+E), then a further injection of estradiol, and in some cases a subsequent injection of progesterone the following day (OVX+E+P) (39,40,41,42). The drawback of these approaches is that the levels of circulating hormones achieved with these injections were often supraphysiological, and the increased number of injections and manipulations necessarily increases the number of variables that must be accounted for in data interpretation.
For these reasons, another method of surge induction was developed from the observation that in some species the surge regularly occurs at a particular time of day. An influence of the circadian timing system on reproduction has been shown in many mammals, including humans. The LH surge in most women (43) and in the diurnal (i.e., predominantly active during daylight) rodent Arvicanthis niloticus (44) begins in the early morning. Similarly, in nocturnal rodents the LH surge begins in the early afternoon, preceding their active period (39). Furthermore, some experiments in sheep have suggested that LH surge onset is more prevalent at nighttime (45), although other studies suggest little to no time of day effect (46,47), possibly reflecting differences in surge induction methodology in studies utilizing this species.
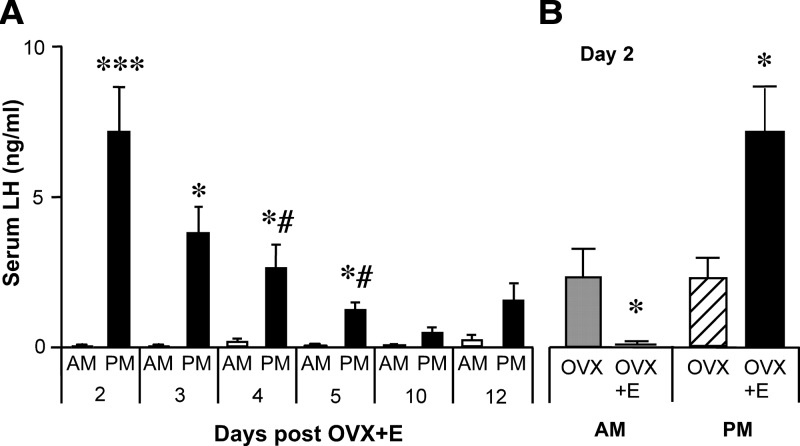
The specific timing of the surge and ovulation in rodents suggested the presence of a daily neural signal required for ovulation, which was first demonstrated in the seminal work of Everett and Sawyer (48). When neural activity in rats was temporarily blocked beginning 5 h before lights off on the day of proestrus using moderate barbiturate sedation, ovulation was delayed for 24 h. By contrast, there was no delay in ovulation when barbiturate administration was delayed until 3 h before lights off. This provided clear evidence that with barbiturate blockade during critical hours on the day of proestrus, the neural preovulatory signal (i.e., the GnRH surge) is postponed until similar hours the next day. This delay, however, is not simply an effect of the barbiturate treatment because that specific inhibitory action would only last a few hours. Rather, the 24-h periodicity was likely intrinsic to the neural component of the preovulatory surge mechanism. In subsequent studies, this 24-h periodicity was further revealed by treatment of OVX rats, hamsters, or mice with estradiol implants (OVX+E) that mimic the high constant levels of estradiol seen during proestrus; this induces repetitive daily LH surges that are appropriately timed to the late afternoon (49,50,51) (Fig. 2).
Figure 2.
Constant estradiol treatment of OVX mice induces daily LH surges. A, Serum LH levels (mean ± sem) sampled in OVX+E mice at 0700 h (open bars) or 1600 h (filled bars) (lights off at 1630 h). B, OVX mice show no diurnal difference in serum LH levels; estradiol administered from the time of OVX induces negative feedback in the morning and positive feedback in the evening on d 2 after surgery. *P < 0.05; ***P < 0.001; #P < 0.01 vs. d 2 p.m. [Data adapted from C. A. Christian et al.: Proc Natl Acad Sci USA 102:15682–15687 (49). © 2005, National Academy of Sciences, U.S.A.].
An important indication revealed by the study of daily surge rodent models is that estradiol interacts with the circadian system in generating the surge. Estradiol implants produce continuously high estradiol levels, yet the surge occurs only in the late afternoon. Elevated estradiol levels are thus required for conversion of the daily neural signal, postulated by Everett and Sawyer, to a surge response. Therefore, to form an integrated picture of the neurobiology of the GnRH surge, we will examine both the estradiol-dependent and circadian regulation of the surge and the ways these signals appear to be integrated, transmitted to, and received by GnRH neurons.
III. In Vitro Studies of the Effects of In Vivo Estradiol and GnRH Surge Physiology
Advances in neurobiological techniques over the last 20 yr have allowed for the exploration of estradiol effects on the function of GnRH and other neurons with increasing detail. In 1998, Herbison (52) published an excellent and extensive review on the studies of estrogen feedback to GnRH neurons up to that time, to which we refer interested readers. Here, we review the insights afforded by the use of neurophysiological methods, largely used in the last decade, to examine the mechanisms of in vivo estradiol feedback directly at the level of the GnRH neuron, with a focus on those studies that have particular relevance to GnRH surge regulation. A direct relationship between electrical activity and hormone release has been demonstrated in several neuroendocrine systems, such as magnocellular neurosecretory cells of the paraventricular (53,54) and supraoptic nuclei (55,56,57,58), as well as in immortalized GnRH neurons (59). Thus, an understanding of the electrophysiological properties of GnRH neurons and cellular changes that affect overall network activity is critical to elucidating the neurobiology of GnRH release and surge generation.
A. Multiunit activity studies
Before direct, targeted electrophysiological analyses of GnRH neuron function became technically feasible, several studies used the method of multiunit activity (MUA) recordings of synchronized hypothalamic electrical discharges to assess activity of unidentified neuronal populations, but with conflicting results. Episodic bursts of MUA in the preoptic area and/or medial basal hypothalamus were shown to be coincident with pulsatile LH secretion in ewes, monkeys, goats, and rats (60,61,62,63,64,65). Estradiol treatment that decreased LH release also decreased the amplitude and frequency of MUA volleys in rats (65), goats (64), and monkeys (66,67); similarly, estradiol treatment that increased LH release increased MUA in ewes (60). Paradoxically, MUA appeared to be diminished, if not completely abolished, during the LH surge in rats, goats, and monkeys (68,69,70). These results are difficult to interpret, however, given the heterogeneous nature of the cells being recorded, which likely includes both GnRH and non-GnRH cells; indeed, a recent report indicates that rhythmic MUA in the arcuate nucleus, which is largely devoid of GnRH neurons, also accompanies LH pulses in goats (71). Thus, it is difficult to differentiate GnRH neuron-specific activity from that of other cell populations. It is important to note that MUA volleys indicate synchronous activity of several neurons. Therefore, if GnRH neuronal activity becomes desynchronized during the surge, an increase in firing activity of individual neurons or an overall increase in network activity may not be detected using this methodology.
B. Direct recordings of individual GnRH neurons
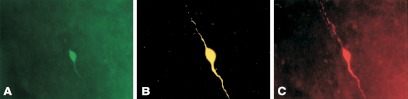
The advent of transgenic mouse lines that express genetic markers such as green fluorescent protein (GFP) (72,73,74) or β-galactosidase (75) under the control of the GnRH promoter has opened the door for direct electrophysiological analysis of GnRH neuron function, including examination of the effects of estradiol on these cells (Fig. 3). Long-term recordings of isolated GnRH neurons and GnRH neurons in brain slices show that their firing pattern is intrinsically episodic in nature (59,76,77), with episodes of increased firing rate punctuated by periods of quiescence of varying lengths. Several studies have examined the acute effect of estradiol on GnRH neurons from guinea pigs, monkeys, mice, or the GT1-7 neuronal cell line by directly applying estradiol to an in vitro preparation (78,79,80,81,82,83). Low concentrations of estradiol decrease GnRH neuron firing activity, whereas high physiological concentrations stimulate firing (83). In addition, rapid application of high physiological concentrations of estradiol can increase calcium currents in GnRH neurons (84). These studies are valuable for understanding nongenomic effects of estradiol on GnRH neurons, which can occur on a shorter time scale than changes dependent on classical or nonclassical genomic mechanisms. These nongenomic actions may indeed play a supporting role in mediating the biphasic effects of estradiol. During the reproductive cycle, however, sustained high levels of circulating estradiol are required to elicit a GnRH/LH surge, and animal models that lack classical genomic mechanisms of estrogen receptor signaling do not exhibit a GnRH/LH surge (85,86). Although the above findings with acute application of estradiol argue that any nonclassical mechanisms will not work against those induced by sustained elevations, we will concentrate on studies utilizing in vivo estradiol treatment combined with in vitro studies of GnRH physiology.
Figure 3.
Triple label of recorded GnRH neuron from a GnRH-GFP transgenic mouse. A, GFP signal used to target recording pipette to a GnRH neuron. B, Biocytin labeling of recorded cell detected with streptavidin-Cy3. C, Immunostaining for GnRH with Cy5-conjugated secondary antibody. [Data adapted from K. J. Suter et al.: Endocrinology 141:412–419 (73). © 2000, The Endocrine Society].
In the first studies examining the effects of in vivo estradiol on the electrophysiological properties of individual GnRH neurons, estradiol was administered to OVX mice in vivo for 5–9 d. This lowered LH levels, and GnRH neuron firing activity showed correlated decreases (87). In the same model, by contrast, estradiol increased the excitability of GnRH neurons, as exhibited in a decrease in both the threshold and latency for action potential generation via effects on intrinsic A-type potassium currents (88). These apparently contradictory results may in hindsight be due to the different times of day at which the studies were performed (morning in the former, late afternoon/evening in the latter). Nonetheless, these studies showed that at least some of the effects of an in vivo estradiol treatment persist in a brain slice preparation, and thus electrophysiological tools can be used to probe the effects of hormone feedback on the GnRH neuronal network. It should be noted that in any brain slice preparation some afferent inputs are inevitably lost, and thus the connections of the entire surge-generating mechanism will not be maintained. On the other hand, these types of preparations allow for direct functional examination of the surge mechanisms at the cellular level. Ultimately, the use and comparison of techniques employing differing levels of reductionism should yield a complete, integrated picture of the surge mechanism.
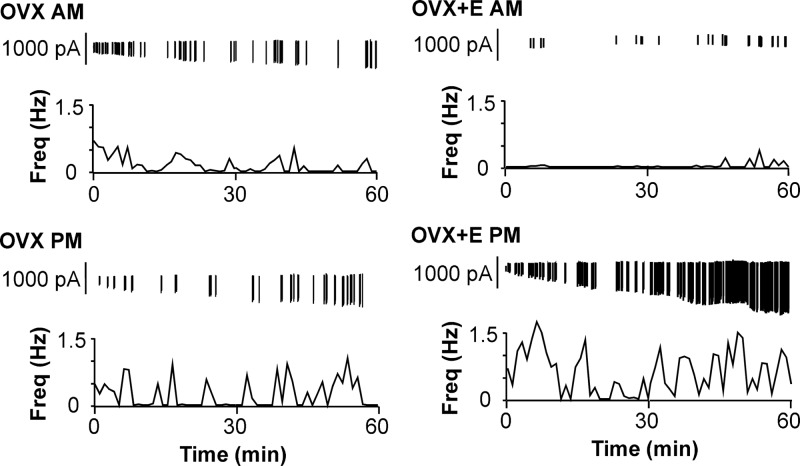
After the development of the daily LH surge mouse model, the effects of estradiol on GnRH neuron firing activity were reexamined in light of the clear time-of-day dependence on the effect of estradiol on LH levels. In this model, estradiol treatment induced marked diurnal variations in individual GnRH neuron firing rate and pattern that were consistent with the change in LH levels characteristic of the LH surge (49) (Fig. 4). Additionally, comparison of OVX and OVX+E cells in the morning and the evening indicated changes in firing activity associated with estradiol negative and positive feedback, respectively, which were also consistent with the observed changes in LH levels. Importantly, this demonstrates that major, possibly sufficient, portions of the surge generation mechanism persist in the brain slice preparation, opening the door for in vitro studies of the neurobiology of the surge. It is also possible that brain slice preparation does not “disturb” the progression of surge-associated changes because in vivo induction of the surge is largely complete by the time of brain slicing. For example, it has been demonstrated in sheep that estradiol does not need to be present for the entire presurge period to induce a GnRH surge (89). In addition, studies using the daily LH surge mouse model, recapitulating the classic Everett and Sawyer pentobarbital sedation work, showed that in vivo barbiturate sedation treatment of OVX+E mice administered at a specific time of day to block the LH surge also blocked the diurnal change in GnRH neuron firing activity (90). These findings further strengthened the correlation between changes in firing activity observed in vitro and changes in GnRH/LH release observed in vivo, and supported the hypothesis that there is a critical time of day during which the surge can be blocked, but after which the surge essentially becomes inevitable.
Figure 4.
GnRH neuron firing activity correlates with LH levels in the daily LH surge mouse model. Representative examples of firing patterns in GnRH neurons recorded extracellularly from OVX (left) or OVX+E (right) mice in the morning (top) or evening (bottom). Vertical lines at the top of each graph indicate timing of individual action currents recorded from an individual GnRH neuron. The frequency of action current firing is plotted in 1-min bins for each cell. Cells from OVX mice show no diurnal difference in firing properties, whereas cells from OVX+E mice show low levels of activity in the morning (negative feedback) and higher levels of activity in the evening (positive feedback). [Data adapted from C. A. Christian et al.: Proc Natl Acad Sci USA 102:15682–15687 (49). © 2005, National Academy of Sciences, U.S.A.].
Changes in firing pattern reflect an overall change in the activity of neurons. One major mechanism for changes in neuronal excitability is via alterations in the properties of intrinsic conductances. Studies of intrinsic currents in GnRH neurons in the daily surge OVX+E mouse model have revealed that some may change with time of day and others may not. For example, estradiol increases the amplitude of slow after-depolarization potentials and increases cellular excitability, independently of time of day (91). On the other hand, preliminary data indicate that currents mediated by voltage-gated calcium channels in GnRH neurons are smaller during negative feedback and larger during positive feedback, and these changes are estradiol-dependent (92). Studies reexamining potassium currents in GnRH neurons using the daily surge model are currently under way. Although preliminary, estradiol appears to slightly increase the A-type potassium current in the morning during negative feedback (93), the opposite of the effect seen in the previous study in which the recordings were done in the evening (88). Similarly, GnRH neurons from OVX+E-treated animals recorded during negative feedback show an increased response to diazoxide, which opens ATP-sensitive potassium channels (94). Because these channels are activated when glucose levels are low during metabolic inhibition, an estradiol-dependent enhancement of ATP-sensitive potassium channel conductances may reflect an integration of steroid and metabolic signals at the level of the GnRH neuron to further reduce GnRH neuron activity at times of low food availability. A recent study using a different surge induction protocol (OVX+E followed by a subsequent estradiol injection 6 d later) observed an estradiol-dependent increase in low voltage-activated T-type calcium currents in GnRH neurons that did not differ with time of day (95). Controlling for time of day as an experimental variable is thus critical to the determination of GnRH neuron conductances that are estradiol-sensitive and the delineation of those that may play a role in the biphasic effects of estradiol feedback.
C. What can electrical recordings tell us about GnRH surge dynamics?
The increased temporal resolution afforded by electrical recordings may allow us to resolve some questions regarding alterations in GnRH neuron function associated with the surge. Specifically, increased GnRH release as measured at the pituitary vasculature could be due to many cells firing simultaneously at a higher rate to secrete larger pulses of GnRH, and/or to network communication to desynchronize the firing of cells so that levels of GnRH in the portal blood are continuously elevated. Measurement of GnRH release dynamics during the surge in sheep has yielded conflicting results, with some indicating that the surge is built by a series of high frequency GnRH pulses (29,32), and others showing continuously elevated GnRH levels, suggesting a switch in the mode of GnRH release from episodic to continuous (96). In OVX+E rats, GnRH pulsatile release appears to continue through the surge, with larger amplitude pulses superimposed on a higher baseline level of release (97). In the daily surge mouse model, GnRH neurons recorded during positive feedback typically showed periods of low activity of variable duration, and many cells exhibited a bursting, episodic pattern of firing (49). Thus, GnRH neurons in brain slices do not exhibit an exclusively tonic or continuous mode of firing during the surge, but the increase in overall activity across the GnRH neuron population is likely sufficient to maintain a high level of GnRH release at the median eminence and available to stimulate LH release from the pituitary.
It remains unclear, however, if and how GnRH neurons fire in concert with each other during the surge and whether this relationship is altered, compared with other times of the reproductive cycle when pulsatile release of GnRH is the predominant pattern. It is also unknown whether GnRH neurons in the same brain slice show a synchronized or desynchronized pattern of firing. A decrease in MUA during the surge, as observed previously in monkeys and goats (68,69), could suggest a lack of synchronization among GnRH neurons because several cells must fire synchronously for a peak in MUA to be detected. One possibility for GnRH neuronal synchronization is via electrical coupling of cells through gap junctions. Cells of the immortalized GnRH neuron GT1-7 cell line exhibit coupling between cell bodies via gap junctions (98,99). Although GnRH neurons express mRNA for connexin-32 at the cell body, however, this was not detected in GnRH neurons adjacent to one another (100). Furthermore, such functional connections have not been observed to a high degree in native GnRH neurons (101). Thus, it is unlikely that gap junctions play a large role in GnRH neuron synchronization. Alternatively, GnRH neurons may synchronize with each other via dendro-dendritic bundled connections (102) or associations among nerve terminals in the median eminence (103,104,105). Another possible mode of inter-GnRH neuron communication is suggested by the estradiol-sensitive expression of glutamate receptors in GnRH nerve terminals in rats (106). GnRH neurons appear to express vesicular glutamate transporter-2 protein (107,108) and thus may release glutamate, which then acts on adjacent GnRH neuron terminals. The mechanisms by which GnRH neurons may synchronize and the regulation of such synchronization at various points in the reproductive cycle are extremely important questions for future studies of GnRH neuron physiology, and the utilization of both electrophysiological and anatomical methods together should help towards reaching this goal.
Another unanswered question is whether or not the same GnRH neurons participate in both surge and pulse modes of hormone release. One possibility is that the GnRH neuron population is subdivided into “pulse” cells and “surge” cells. For example, a subset of GnRH neurons located in the medial basal hypothalamus has been suggested to underlie pulsatile secretion in sheep (109). On the other hand, the medial basal hypothalamus has also been suggested as the site of estradiol action to induce the LH surge in the ewe, whereas the cells in the medial preoptic area may be the primary targets of negative feedback (110). With regard to a subpopulation that may underlie the surge in rodents, the firing rate of mouse GnRH neurons recorded during positive feedback in the daily surge model depends on the anatomical location of these cells. Specifically, medially located GnRH neurons show a significantly higher rate of firing than more lateral cells, suggesting that the medial subpopulation of GnRH neurons is primarily responsible for driving the GnRH surge (90). This finding is consistent with anatomical studies of expression of c-Fos, an immediate early gene product used as a marker of neuronal activity (111), during the surge in rats and sheep (112,113). Further studies are needed to determine whether the same cells mediate both pulsatile and surge secretion, to examine species differences in such functional divisions, and to understand the mechanisms by which hormonal and other signals differentially regulate specific subpopulations of GnRH neurons to achieve particular alterations in GnRH release at appropriate points of the reproductive cycle.
IV. Circadian Regulation of the GnRH/LH Surge and Ovulation
As outlined above, estradiol is an absolute requirement for surge generation in spontaneous ovulators, but in many species a daily signal times the surge to a specific time of day for optimal reproductive success. To understand the nature of a daily signal, it is necessary to examine the role of the circadian timing system in surge generation. When animals are housed under different light-dark cycles, the LH surge continues to occur near the time of activity onset (114). Changing the environmental lighting schedule alters the time of the LH surge in the same manner as circadian locomotor activity, indicating a constant phase relationship between circadian rhythms and the timing of the LH surge (115). When animals are held in constant dark or constant light conditions for a short time, the LH surge and ovulation continue to occur near the expected times of day in relation to the onset of activity (116,117,118). Together, these data suggest that the timing of the LH surge is tightly coupled to a fixed period in the environmental light-dark cycle, and the timing of the surge is likely controlled by a circadian clock mechanism.
Further evidence of a coupling of the circadian and LH surge mechanisms has been provided by studies exploiting the circadian phenomenon of “splitting” in hamsters, in which the activity portion of the daily rest-activity cycle loses its consolidation and splits so that two or more separate components can be observed within each 24-h period (119,120). In classic work by Swann and Turek (121), intact female hamsters were housed in constant light conditions and screened for the expression of split rhythms in their locomotor activity. Once animals exhibited split rhythms, they were OVX+E-treated in a daily surge paradigm. In this case, they showed two LH surges within one 24-h period, as measured 48 h after estradiol capsule implantation. Moreover, the timing of these surges was closely related to the beginning of each bout of activity, indicating that the same oscillatory clock mechanisms regulate circadian rhythms of LH release and locomotor activity.
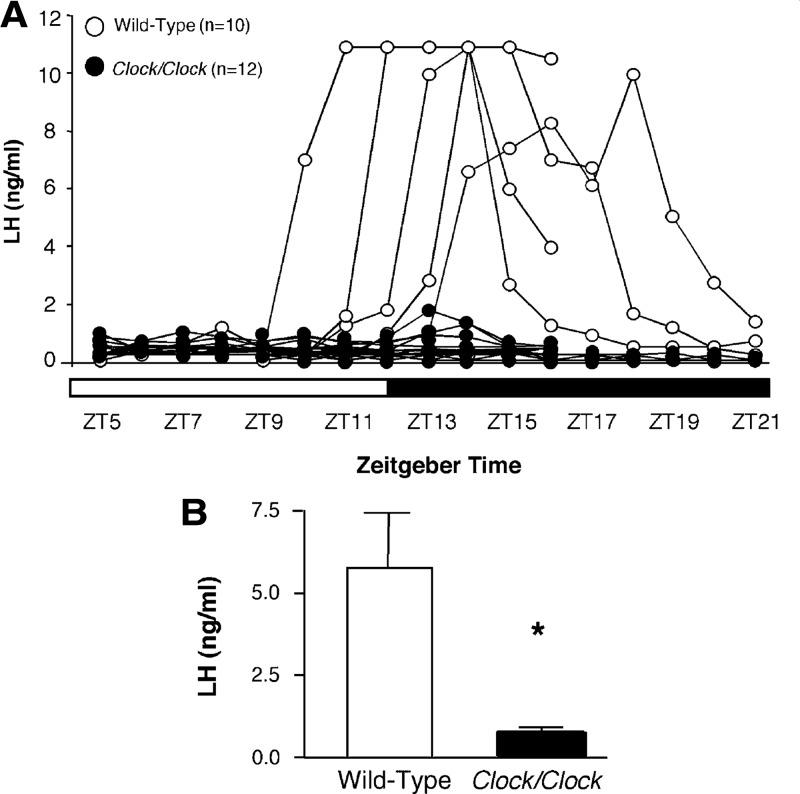
Mutations in the circadian system have provided additional insight into the role of the circadian system in regulating the LH surge. Daily LH surges in OVX+E tau mutant hamsters, which harbor disruptions to the enzyme casein kinase 1 ε (122), mirror the periodicity of their behavioral rhythms: homozygous mutants show a period of about 20.5 h, whereas wild-type hamsters exhibit a period of about 24 h (19). The Clock mutant mouse, which carries a 51-amino acid deletion in the transcriptional-activation domain of the CLOCK protein, exhibits extended, irregular estrous cycles and fails to have an LH surge both on the day of proestrus (in intact females) and in response to OVX+E treatment (20), suggesting a disruption of the timing and/or coordination of GnRH release on proestrus (Fig. 5). Therefore, it is clear that the timing of the LH surge mechanism in various rodent species is controlled by the intrinsic periodicity of the circadian system. Furthermore, surge generation thus appears dependent on both estradiol feedback and a properly functioning circadian timing system.
Figure 5.
Clock/Clock mutant mice fail to exhibit an LH surge on proestrus. A, Individual LH traces from wild-type (open circles) and Clock/Clock females (filled circles). White and black bars on x-axis represent times of lights on and lights off, respectively. B, Peak LH values obtained in wild-type and Clock/Clock female mice. *P < 0.01. [Data adapted from B. H. Miller et al.: Curr Biol 14:1367–1373 (20). © 2004, Elsevier].
V. Potential Points of Integration of Circadian and Estradiol Feedback Signals
A. Suprachiasmatic nuclei
Although the precise nature of the daily neural signal has yet to be determined, the most likely source of such a signal would be a central circadian clock. The site of the mammalian circadian pacemaker has been localized to the hypothalamic suprachiasmatic nuclei (SCN). The SCN are a pair of small structures each consisting of a cluster of neurons located in the anterior ventral hypothalamus, sitting on either side of the optic recess of the third ventricle. In rodents, the SCN contain approximately 10,000 neurons in each nucleus (123); these cells are not homogeneous and express a large number of different peptides and enzymes (124). Anterograde tract tracing studies have shown that SCN fibers project to the area of GnRH neurons (125), implying that a direct SCN-GnRH connection may exist, although specific functional evidence for a synaptic connection is lacking. Further studies have indicated possible reciprocal communication between the SCN and GnRH neurons (126,127,128). The SCN exhibit a diurnal rhythm of expression of cFos that begins to increase in an estradiol-independent manner approximately 1 h before surge-associated expression in GnRH neurons in rats (129), suggesting that this increase in SCN activity may reflect at least part of the daily signal.
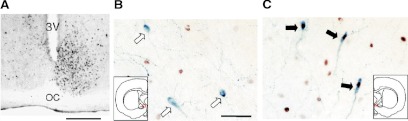
SCN lesions eliminate both the LH surge and ovulation (21), demonstrating the importance of an intact SCN for surge generation. SCN grafts capable of restoring locomotor rhythmicity do not restore LH surges in OVX+E hamsters (130), indicating that although the same clock may regulate both locomotor activity and surge timing, the clock regulates these two phenomena via distinct pathways. Specifically, a neural, rather than humoral (131), SCN output may be required for LH surge generation. Studies in behaviorally split hamsters have suggested that this is in fact the case. Behaviorally split hamsters show a dramatic left-right asymmetry in immunoreactivity to cFos in the SCN, indicating that the SCN are reorganized as two circadian pacemakers working in antiphase (132). In OVX+E split hamsters, the percentage of cells immunoreactive to both GnRH and cFos is approximately 5-fold higher on the side ipsilateral to the SCN half with the higher cFos immunoreactivity, which alternates from one half of the SCN to the other (133) (Fig. 6). Thus, each side of the SCN transmits information to ipsilateral GnRH neurons via a point-to-point neural mechanism.
Figure 6.
Asymmetric activation of GnRH neurons in hamsters exhibiting split circadian behavioral rhythms of activity. A, Coronal brain section through the SCN of a behaviorally split hamster stained for cFos immunoreactivity. 3V, Third ventricle; OC, optic chiasm. Scale bar, 500 μm. B, GnRH neurons (blue stain) on side of brain in which SCN does not show cFos activation do not express cFos (white arrows). Scale bar, 50 μm. C, GnRH neurons on side of brain in which SCN shows cFos expression coexpress cFos (brown stain, black arrows). [Data adapted from H. O. de la Iglesia et al.: J Neurosci 23:7412–7414 (133). © 2003, Society for Neuroscience].
Estradiol may influence the transmission of circadian signals to GnRH neurons by acting directly on the SCN, indirectly via synaptic afferents, or on downstream targets of SCN outputs. The mature SCN appear to express low levels of estrogen receptors (ERs), with ERβ expression predominant over ERα (134,135,136), indicating possible direct actions. ERα-expressing neurons project to the SCN (137), perhaps providing an indirect path of estradiol actions on SCN function. Estradiol-sensitive changes in gene expression, cellular properties, and circadian activity suggest a role for estradiol in regulating circadian rhythms. For example, estradiol alters expression of circadian genes, transcription factors, and gap junctions in the SCN (138,139,140,141,142) and can increase the responsiveness of SCN neuron firing rate to acetylcholine and serotonin (143). Estradiol also modulates the expression of the astrocytic marker glial fibrillary acid protein in peri-SCN regions, suggesting a role in glial modifications that may affect output of SCN-derived transmission (144). Estradiol treatment in OVX hamsters produces a phase advance of their wheel-running rhythm and shortens the free-running locomotor activity period. Moreover, in hamsters the onset of locomotor activity is earlier and closer to the time of lights off during late diestrus and proestrus than during estrus or early diestrus (145). OVX+E hamsters show a significantly reduced incidence of circadian splitting compared with OVX females (146). Thus, it appears that estradiol may act as a coupling agent to synchronize multiple circadian oscillators in the SCN, and possibly this effect could extend to GnRH neurons as well.
B. Anteroventral periventricular area
Neurons in the hypothalamic anteroventral periventricular area (AVPV), which are directly upstream of GnRH neurons and estrogen-responsive, also receive input from the SCN (147,148,149,150). The AVPV is therefore poised to play a pivotal role in integrating and transmitting inputs to GnRH neurons (Fig. 7). Furthermore, the AVPV is sexually dimorphic, with both cell number and projections from the AVPV to GnRH neurons being considerably stronger in females (151,152). The AVPV also receives inputs from regions such as the medial preoptic nucleus, the arcuate nucleus, and the posterior nucleus of the amygdala, which contain neurons that express estrogen and progesterone receptors (152), indicating that estradiol may act both directly on AVPV neurons and transsynaptically to modulate their function.
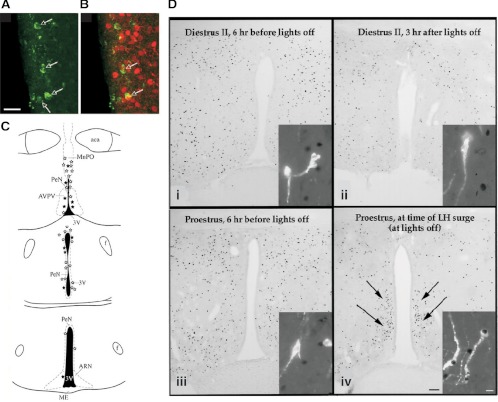
Figure 7.
Anatomical studies indicate that inputs important for surge generation are located in AVPV. A–C, GnRH neuron-specific viral tracing shows that afferents to GnRH neurons expressing ERα are located in AVPV. A, Neurons in AVPV express GFP immunoreactivity after Bartha virus (Ba2001) viral injection into rostral preoptic area in GnRH-Cre transgenic mice, which retrogradely labels afferent inputs. Scale bar, 15 μm. B, Dual labeling for GFP (green) and ERα (red) shows that GnRH neuron afferents in AVPV express ERα. C, Schematic coronal brain maps showing GFP-immunoreactive afferent cells that did not express ERα (open stars) and GFP+ERα-immunoreactive cells (filled stars). aca, Anterior commisure; ARN, arcuate nucleus; f, fornix; MnPO, medial preoptic nucleus; ME, median eminence, PeN, periventricular nucleus; 3V, third ventricle. D, Micrographs of cFos expression in the AVPV (brightfield images) and GnRH neurons (insets) before (Di–Diii) and during (Div) an LH surge. During diestrus and on proestrus before the LH surge, GnRH neurons do not exhibit cFos, and few AVPV nuclei are cFos-positive; but during the surge, both GnRH neurons and numerous AVPV cells express cFos. Scale bars, 100 μm (brightfield images) and 10 μm (fluorescence images). [Data in panels A–C adapted from T. M. Wintermantel et al.: Neuron 52:271–280 (150); 227 2006, Elsevier. Data in panel D adapted from W. W. Le et al.: Endocrinology 140:510–519 (157); © 1999, The Endocrine Society].
AVPV lesions block the LH surge in both intact and OVX+E+P rats (153,154,155,156). The AVPV exhibits increased cFos expression concomitantly with GnRH neurons at the time of the LH surge in rats (157,158) and in mice treated in the daily surge model (118). Unilateral lesions of the AVPV lead to the lack of cFos expression in GnRH neurons ipsilateral to the lesion during the LH surge (159). Additionally, when antiestrogens are administered through microcannulae into the AVPV, the occurrence of LH surges in OVX+E rats is significantly diminished (160). In rats, cAMP levels in the AVPV increase in the midafternoon on all days of the estrous cycle, which may be a reflection of transmission of the daily neural signal (161). Furthermore, intracerebroventricular injection of an adenylyl cyclase inhibitor blocks the LH surge in OVX+E rats, suggesting that the increased expression of cAMP is necessary for surge generation (161). The AVPV may therefore be an important point of integration of SCN and estradiol signals that are then transmitted to GnRH neurons to initiate the surge.
C. GnRH neurons
Circadian clock genes are present and rhythmically expressed in immortalized GnRH neurons (162,163,164), the pituitary (165), and the ovary (166,167). Because the SCN are required for the surge, it is unlikely that a GnRH neuron-expressed clock could itself underlie the daily neural signal. Rather, these genes may perhaps be involved in the reception and transduction of circadian inputs, for example via changes in neuronal excitability and expression of neurotransmitter receptors in GnRH neurons (164,168). A role for a clock mechanism endogenous to GnRH neurons is supported by a preliminary report demonstrating that treatment of immortalized GT1-7 GnRH neurons with an estradiol and progesterone hormone regimen designed to mimic the hormone changes observed during the estrous cycle induces increased GnRH release during the “proestrous” phase, but the same hormone treatment paradigm compressed to a 24-h duration suppresses GnRH release (169). This work using immortalized cells, however, should be confirmed using native GnRH neurons because it is unknown how the process of immortalization may affect gene expression in these cells. This caveat notwithstanding, these studies do suggest the interesting possibility that a GnRH neuron clock may function to measure the time of exposure to different hormone concentrations and modulate GnRH synthesis and release appropriate to cycle stage.
VI. Regulation of GnRH and LH Surges by Fast GABAergic and Glutamatergic Synaptic Transmission
Estradiol may act directly on GnRH neurons and/or indirectly to mediate its effects. GnRH neurons express mRNA for the β-isoform of the estrogen receptor (ERβ) (170). ERβ knockout mice, however, have normal LH levels (171), and disruptions to fertility such as reduced litter size appear to be due to effects on the ovary rather than at the level of the hypothalamus or pituitary (172). By contrast, a large body of evidence points to a necessary role for ERα in surge generation. ERα knockout mice are infertile (173) and do not exhibit either estradiol negative or positive feedback (85,150,173). Although ERα has been detected in immortalized GnRH neuronal cell lines (174,175) and primary cultures of GnRH neurons (176), neither protein nor mRNA for this receptor has been detected in native GnRH neurons (170,177). Likewise, estradiol-induced progesterone receptor expression is required for the surge (178), but progesterone receptors largely have not been found in GnRH neurons (179), with the exception of a small subpopulation of cells in the guinea pig (180). It should be noted, however, that the low levels of progesterone around the time of surge induction suggest that these receptors are activated in a manner independent of progesterone itself (178). In addition, mice with mutations in ERα that prevent the binding of the receptor to genomic estrogen response elements (“classical” signaling) (85) that are treated in the daily surge OVX+E mouse model do not exhibit the same changes in GnRH neuron firing activity seen in the wild-type (86). Together, these findings suggest a critical role for estradiol-sensitive afferents in surge regulation.
In this section, we will focus on synaptic communication mediated by postsynaptic ionotropic receptors, also known as fast synaptic transmission. Hypothalamic γ-aminobutyric acid (GABA) and glutamate cells express ERα (181,182,183,184); thus, GABAergic and glutamatergic fast synaptic transmission are likely candidates in mediating estradiol feedback to GnRH neurons critical for surge regulation. In addition, recent anatomical work suggests that AVPV neurons in female rats express vesicular transporters for both GABA and glutamate, and at the time of the LH surge the number of vesicles containing GABA transporters decreases, whereas the number of vesicles containing glutamate transporters increases (185). Thus, fast synaptic transmission mediated by GABA and/or glutamate is poised to play an important role in estradiol feedback to GnRH neurons.
A. Glutamate
GnRH neurons express ionotropic α-amino-3-hydro-5-methyl-4-isoxazole-propionic acid (AMPA) and N-methyl-d-aspartate (NMDA) glutamate receptors (72,186,187,188). Spontaneous postsynaptic currents (PSCs) mediated by AMPA receptors are expressed in GnRH neurons (189,190), and some GnRH neurons express spontaneous NMDA receptor-mediated currents (189). Intravenous injection of NMDA increases LH release in intact rhesus macaques (191). Early work measuring glutamate release associated with the LH surge in rats observed no difference in glutamate levels in the preoptic area in OVX+E animals (192), but subsequent studies have observed increases in glutamate release in the preoptic area during the LH surge in OVX+E and OVX+E+P models (193,194,195), and that these surge-associated changes are attenuated in middle-aged, reproductively senescent animals (196,197). Blockade of either NMDA or AMPA glutamate receptors blocks the LH surge in rats (198,199,200). In addition, the stimulation of GnRH or LH release by NMDA receptor agonists is augmented by estradiol treatment of OVX rats (201,202), whereas AMPA stimulates or inhibits LH release in OVX+E and OVX rats, respectively (200), suggesting that estradiol levels adjust responses mediated via these receptors.
To test the hypothesis that glutamatergic synaptic drive to GnRH neurons shows surge-associated changes, glutamatergic excitatory PSCs (EPSCs) were recorded in GnRH neurons using the daily LH surge mouse model (189). Approximately one third of GnRH neurons tested did not show EPSCs, at least as detectable at the cell body. Glutamate transmission appeared to be largely mediated by AMPA/kainate receptors, with a smaller percentage of cells exhibiting NMDA receptor-mediated EPSCs. This is consistent with electrophysiological analyses examining the selective response to AMPA or NMDA receptor activation, which showed that most GnRH neurons respond to AMPA but only about 20% respond to NMDA (72). It should be noted, however, that somatic recordings of EPSCs may miss currents elicited by receptors on distal dendrites (203) that are too small to be detected at the cell body. Comparison of EPSCs in cells from OVX+E vs. OVX mice showed an estradiol-dependent suppression of EPSC frequency and amplitude in the morning during negative feedback, but no difference during positive feedback. Blockade of action potential firing did not affect EPSC frequency, suggesting that these changes are activity-independent. Altered amplitude can be due to postsynaptic changes in receptor expression, composition, or phosphorylation, and/or presynaptic changes in the number of synaptic contacts, vesicle release probability, or vesicular content. The expression of AMPA receptor subunits in GnRH neurons appears to change during the surge (204), which may account at least in part for the observed changes in EPSC amplitude. This study therefore suggested that the primary role of estradiol-dependent changes in glutamate transmission to GnRH neurons may be in mediating negative feedback. These experiments, however, cannot exclude the possibility that estradiol increases glutamate transmission to distal receptors on GnRH neuron dendrites (203) or onto other critical afferent neurons during positive feedback. Such changes could potentially have a large impact on GnRH neuron activity because dendrites are a site of action potential initiation in GnRH neurons (205) and connections between GnRH neuron dendrites appear to be one form of communication between GnRH neurons (102).
The sources of glutamate input that mediate these changes in glutamate transmission remain to be determined. Several hypothalamic areas (including the preoptic area and ventromedial, dorsomedial, supraoptic, paraventricular, and arcuate nuclei) express type 2 vesicular glutamate transporters, an anatomic marker for glutamatergic cells (183,185,206). Although the overall number of AVPV-derived synaptic contacts does not change with time of day, the number of dual-phenotype contacts that express both vesicular GABA and glutamate transporters and (as well as type 2 vesicular glutamate transporters immunoreactivity within these terminals) increases around the onset of the LH surge (185). Furthermore, the dual-phenotype terminals are only detectable with estradiol treatment. These anatomical changes may reflect at least part of the suppression in glutamate transmission during negative feedback, as well as the switch from negative to positive feedback.
B. GABA
GnRH neurons express functional ionotropic GABAA receptors that are synaptically activated (72,207,208,209). GnRH neurons also express metabotropic GABAB receptors (79,210,211), but it remains unclear whether these receptors are activated by synaptically released GABA. GABA is often referred to as the primary inhibitory neurotransmitter in the brain (212), but the response of GnRH neurons to activation of GABAA receptors is controversial. Because of the apparent significance of GABA in surge regulation, we will review the evidence for excitatory and inhibitory actions of GABA in the GnRH system, with respect to both the pulse and surge modes of GnRH release.
1. Effects of in vivo GABA manipulations
Many studies examining neurotransmitter release in the whole animal have been performed to examine the effect of GABA on GnRH/LH release or to measure GABA levels in different reproductive states. GABA levels measured by microdialysis of the preoptic area are lower during the LH surge in both OVX+E rats (192,195,213,214) and OVX+E sheep (215). Injections of the GABAA receptor agonist muscimol intracerebroventricularly in OVX rats (216) or directly into the preoptic area in OVX rats (217) or OVX+E sheep (218) have been shown to suppress pulsatile LH release. Similarly, ip injections of muscimol in OVX+E+P rats (219) or infusions of GABA into the preoptic area of intact rats on proestrus (220) block the LH surge. Acute estradiol treatment of OVX rats that decreases pulsatile LH secretion also induces a rise in preoptic area GABA levels (221). Conversely, antagonism of GABAA receptors with bicuculline methiodide in the preoptic area also decreases pulsatile LH secretion in OVX rats (217) and OVX+E sheep (218). In intact rats, however, bicuculline was observed to have no effect on the LH surge in one study (220) but advanced the peak of the LH surge by 3 h in subsequent experiments (222). In middle-aged OVX+E+P rats, which show an attenuated LH surge, extracellular GABA levels in the preoptic area are increased, and bicuculline infusion in the preoptic area increases LH levels (196). Together, these studies suggested that GABA exerts an inhibitory effect on GnRH/LH release, although antagonism of endogenous GABA levels yielded conflicting data.
Although these state-of-the-art methods showed a clear significance for GABAergic signaling in GnRH neuron regulation, an important caveat to these approaches is that GnRH neurons are not the only cells in the preoptic area, and treatments and subsequent changes have the potential to alter the activity of many cells within the network. Thus, it is difficult to determine which cell type(s) either produce the change in transmitter levels or are affected by such changes. It is conceivable that lower GABA levels, as detected by these microdialysis studies, are required to diminish inhibition of upstream excitatory inputs on GnRH neurons. Similarly, infusion of GABA into the preoptic area may produce a suppression of GnRH/LH release via a potent inhibition of upstream excitatory inputs, or via a supraphysiological, prolonged activation of GABAA receptors that would strongly inhibit GnRH neurons (223), irrespective of the endogenous response to synaptic GABA transmission. Thus, caution is necessary when using in vivo whole animal studies to determine the effect of GABA at the single-cell level.
2. Studies on the effects of GABA in vitro
In addition to measurements of GABA release, assessments of mRNA levels for the GABA precursor glutamic acid decarboxylase (GAD) have also been used to investigate surge-associated changes in GABA dynamics but have yielded conflicting results. In an OVX+E+P surge induction paradigm in immature rats, progesterone was found to suppress GAD67 mRNA levels during surge onset and peak (224). Similarly, decreased GAD65 and GAD67 levels were observed in the organum vasculosum of the lamina terminalis and AVPV in the evening of proestrus during the peak of the LH surge in rats (225,226,227), and in the preoptic area during positive feedback in OVX+E guinea pigs (228). Lipopolysaccharide treatment, which inhibits the LH surge, increases immunoreactivity for GAD67 in the preoptic area (229). Other studies, however, observed increased GAD67 levels in the preoptic area at surge onset in proestrus rats (230). A recent study showing that GAD67 levels were decreased in the organum vasculosum of the lamina terminalis and rostral preoptic area during surge onset and peak, but increased in the caudal preoptic area during surge onset in rats (231), suggests that these discrepancies may be explained by the subdivision of the preoptic area investigated.
Recordings in the preoptic area of OVX+E guinea pigs indicated that the response of GABAergic neurons to the GABAB receptor agonist baclofen is decreased during estradiol negative feedback, suggesting that GABAergic neurons are less inhibited by GABA input at this time, and thus release more GABA onto their postsynaptic targets (228). From this study, however, it was not possible to determine whether these direct postsynaptic targets include GnRH neurons. Moreover, similar recordings were not performed during positive feedback in the same endocrine model, so it is unclear whether these changes were specific to the mode of estradiol feedback or a more general effect of estradiol treatment. These issues notwithstanding, these studies lent further evidence for a role for GABA in surge regulation, and the importance of determining the direct effect of GABA on GnRH neurons.
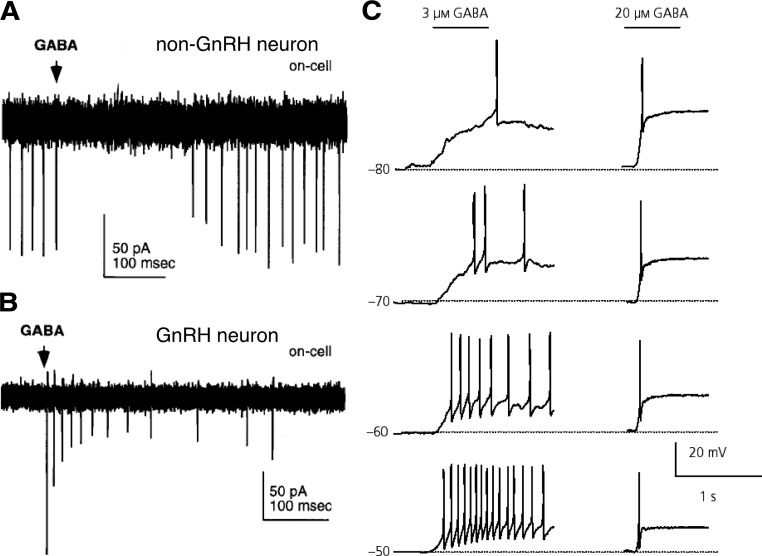
Recent electrophysiological studies in GnRH neurons of both rats and mice have suggested that activation of GABAA receptors via local, rapid GABA application can depolarize and potentially excite these cells (223,232,233) (Fig. 8). Measures made with gramicidin perforated patch recordings, which maintain the normal intracellular chloride milieu, indicate that GnRH neurons maintain elevated chloride levels in adulthood (223,233), similar to magnocellular neuroendocrine (234) and other neurons (235,236,237,238). This effect has been demonstrated across various developmental, reproductive, and circadian states and throughout the estrous cycle (90,223,233,239). GABA has also been shown to increase intracellular calcium in GnRH neurons in both prepubertal and adult mice (240,241). A report testing the effect of bath-applied GABA on GnRH neuron firing in mouse brain slices suggested that the response to GABA switches from excitation to inhibition at puberty (242). It should be noted, however, that although short puffs of GABA excite GnRH neurons, prolonged activation of GABAA receptors leads to a biphasic effect of excitation followed by inhibition, possibly due to a combination of rundown of the chloride gradient, receptor desensitization, and shunting inhibition (223). Blockade of GABA transmission to GnRH neurons in slices inhibits firing if glutamatergic drive was previously blocked (232) but increases firing if glutamate transmission is left intact (74,232). This suggests that the increased firing after GABA blockade may be due to disinhibition of upstream neurons rather than relief of a direct inhibitory effect of GABA on GnRH neurons. Indirect evidence for an excitatory effect of GABA on GnRH neurons is provided by studies showing that the frequency of GABA transmission and GnRH neuron responsiveness to GABA are positively correlated with GnRH neuron activity, LH release, and reproductive state (208,209,239,243,244,245,246).
Figure 8.
GABA can depolarize and induce firing activity in adult GnRH neurons in rats and mice but inhibits other neurons. A and B, Representative on-cell recordings, in which the internal milieu is not disturbed, of action currents in a non-GnRH (A) and a GnRH (B) neuron from GnRH-GFP mice. Rapid application of a puff of 1 mm GABA inhibits firing in non-GnRH hypothalamic neurons but elicits action current firing in GnRH neurons. C, GABA-induced depolarization and action potential generation in rat GnRH neurons recorded using gramicidin perforated patch-clamp in current-clamp mode. The membrane potentials as set by current injection are indicated to the left of each trace. GABA was puff-applied for 600 msec as indicated by horizontal bars above the traces; 3 μm GABA elicited a relatively slow depolarization and generated action potential(s), and 20 μm GABA rapidly depolarized the cell and generated a single action potential at all membrane potentials examined. [Data in panels A and B adapted from R. A. DeFazio et al.: Mol Endocrinol 16:2872–2891 (223); 227 2002, The Endocrine Society. Data in panel C adapted from C. Yin et al.: J Neuroendocrinol 20:566–575 (233). © 2008, Wiley-Blackwell].
Taken together, these data suggest that direct action of GABA on GnRH neurons can be excitatory. Whether this is a direct consequence of depolarization caused by activation of GABAA receptors or secondary to other events, such as activation of voltage-dependent channels, remains to be determined. It should be noted, however, that a depolarizing input can still produce a net inhibitory effect if the degree of depolarization is too small to reach the threshold for action potential generation. Such “shunting” inputs, via reductions in membrane resistance, decrease the excitability of the cell. Thus, it is possible that the degree of depolarization upon GABAA receptor activation is an important regulatory point for GnRH physiology. The drawback of these in vitro studies, of course, is that the entire system is not intact, and such disruptions could alter cellular and network properties.
Despite these limitations of brain slice preparations, such methods have allowed for the most direct measure of GABA transmission to GnRH neurons and the determination of changes in GABAergic synaptic drive that may play a role in estradiol feedback. Recordings of GABAergic PSCs in GnRH neurons using the daily LH surge OVX+E mouse model showed that the frequency of GABA transmission to GnRH neurons is directly correlated with estradiol negative and positive feedback (247) (Fig. 9). Elevated GABA transmission during the surge appeared to be targeted to approximately one third of GnRH neurons, and the anatomical distribution of cells showing elevated PSC frequency was similar to the pattern of cFos induction in GnRH neurons (112) and increased firing activity (90). Furthermore, all GnRH neurons tested showed GABAergic PSCs. Similarly to EPSCs, GABAergic spontaneous PSCs also exhibited changes in amplitude, with larger amplitude during positive feedback compared with negative feedback. Changes in GABAA receptor subunit composition with regard to the time of the surge have not yet been reported, but changes in subunit expression have been observed at different developmental stages (248,249,250), and thus a reorganization of GABAA receptor subunits around the time of the surge is possible. Therefore, in addition to the presynaptic effects of estradiol to increase GABAergic synaptic drive, there may also be postsynaptic changes to increase GnRH neuron responsiveness to GABA during the surge.
Figure 9.
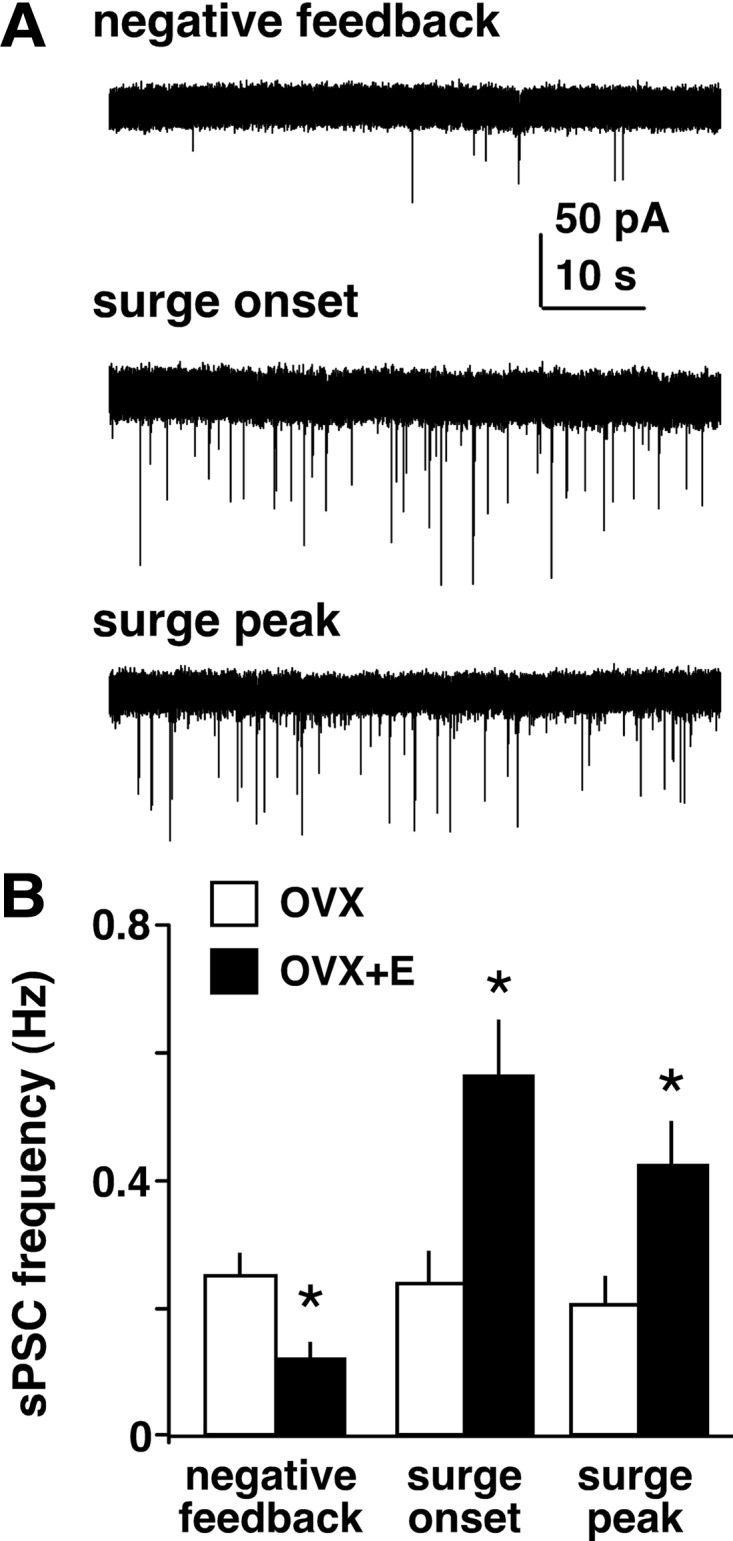
Estradiol decreases GABA transmission to GnRH neurons but increases it during the surge. A, Representative whole-cell voltage-clamp recordings of spontaneous GABAergic postsynaptic currents (sPSCs, downward deflections) in GnRH neurons from mice treated in the OVX+E daily surge model during negative feedback, surge onset, and surge peak. Recordings were performed in the presence of ionotropic glutamate receptor antagonists to isolate GABAergic currents. Note the increased frequency and amplitude of sPSCs during surge onset and peak compared with negative feedback. B, Mean ± sem of sPSC frequency in cells from OVX (open bars) and OVX+E (filled bars) mice during negative feedback, surge onset, and surge peak. *P < 0.05 vs. OVX. [Data adapted from C. A. Christian and S. M. Moenter: J Neurosci 27:1913–1921 (247). © 2007, Society for Neuroscience].
C. Regulation of GnRH neuron firing activity by GABA and glutamate
With regard to the question of whether or not the observed changes in neurotransmission do, in fact, affect the function of GnRH neurons during negative and positive feedback, the effect of ionotropic GABA and glutamate receptor blockade on GnRH neuron firing activity was examined (90). Blockade increased firing during negative feedback but decreased firing during positive feedback, essentially reversing some of the time-of-day-dependent changes in firing activity observed in control conditions. Thus, fast synaptic transmission mediated by GABA and glutamate plays a critical role in mediating both estradiol negative and positive feedback and, importantly, appears to control the timing of the switch in estradiol feedback action. These observations further emphasize the importance of GABA and glutamate transmission within the presynaptic network in the control of surge regulation. Although speculative, imbalances in hypothalamic network transmission may at least partially explain the increased incidence of anovulation in women with epilepsy (251,252), or they could be part of a possible relationship between mood disorders and infertility (253). Therefore, it is likely of clinical importance to assess the effects of epileptic states and antiepileptic treatments on neurotransmission within the GnRH neuron afferent network throughout the reproductive cycle, but particularly with regard to regulation of the GnRH surge.
Another interesting question is whether the same cells receive both increased GABA and glutamate transmission, or whether these inputs are differentially targeted to specific GnRH neuron subpopulations. Computer models suggest that AMPA and GABA conductances can interact in such a way that increased frequency of GABAergic inputs can drive increased firing activity even in the absence of changing AMPA conductances (254). This may explain the observation that during positive feedback in the daily surge mouse model the degree of glutamate transmission to GnRH neurons was not different between OVX and OVX+E animals, whereas GABAergic input was increased to approximately one third of GnRH neurons (189,247). Future work is needed to determine the precise roles and methods of synaptic integration in mediating estradiol feedback and other signals to GnRH neurons.
VII. Neuromodulatory Regulation of GnRH and LH Surges
Fast synaptic transmission is not the only way estradiol feedback and other signals can be conveyed transsynaptically to GnRH neurons. Indeed, roles for several neuromodulators in mediating both negative and positive feedback have been suggested. Furthermore, several cell populations contain not only classical transmitters such as GABA and/or glutamate in small clear vesicles, but also neuromodulator peptides in large dense-core vesicles (255,256). It is thus possible that changes in fast synaptic transmission, mediated by ionotropic receptors and thus detectable as discrete events with electrophysiological approaches, could reflect corresponding shifts in release of neuromodulators. In this section, we review the evidence for the major classes of neuromodulators studied.
A. Kisspeptin
Kisspeptin (257,258) is a potent activator of GnRH neuron activity and GnRH/LH release (259,260,261,262,263). Based on its highly excitatory properties, kisspeptin has been hypothesized to play a role in surge generation. Several comprehensive reviews on kisspeptin signaling and reproduction have been published recently (264,265,266,267,268); therefore, we will review only the most salient aspects of the relationship of kisspeptin signaling with the GnRH surge.
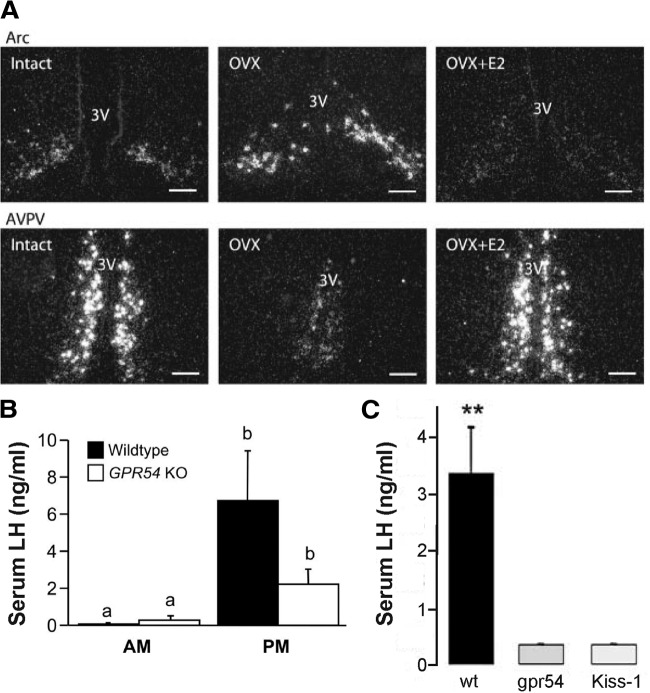
KiSS-1 mRNA is robustly expressed in the AVPV, arcuate nucleus, and periventricular nucleus. The high expression of kisspeptin in the AVPV suggests that this neuropeptide may play an important role in the integration and transmission of estradiol and circadian signals to GnRH neurons. Kisspeptin neurons express ERα and ERβ, and in the female mouse estradiol decreases KiSS-1 mRNA expression in the arcuate but increases it in the AVPV in an ERα-dependent manner (269) (Fig. 10). Furthermore, the increased KiSS-1 mRNA expression in AVPV depends on classical signaling mechanisms, whereas the inhibition in the arcuate is mediated via nonclassical signaling pathways (270). In rats, kisspeptin neurons of the AVPV exhibit increased cFos expression during the LH surge (271,272), and continuous infusion of an antibody to kisspeptin in the preoptic area can block the LH surge (271,273). Increased cFos and KiSS-1 mRNA expression in the AVPV at the time of the LH surge have also been observed in mice treated in the OVX+E daily surge model, even in constant dark conditions (118), suggesting that these changes are driven by the daily neural signal for the surge. In ewes, by contrast, it is the kisspeptin neurons of the arcuate nucleus that appear to play a role in surge generation (274,275). Knockout mice for the kisspeptin receptor G protein-coupled receptor 54 (GPR-54) can still mount an LH surge in response to an OVX+E daily surge induction paradigm (276). It should be noted that different lines of GPR-54 or kisspeptin-null mice fail to exhibit hormone-induced surges (277); it remains to be determined whether these differences are dependent on the mouse strain and/or surge induction paradigm.
Figure 10.
Estradiol regulates kisspeptin (KiSS-1) mRNA in the mouse AVPV and arcuate nucleus (Arc), and GPR54- and kisspeptin-null mice exhibit deficient LH surge generation. A, Dark-field micrographs showing KiSS-1 mRNA expression in the arcuate nucleus (top) and AVPV (bottom) in ovary-intact (left), OVX (middle), and OVX+E (right) mice. 3V, Third ventricle. Scale bars, 100 μm. B, Mean ± sem serum LH levels in wild-type and GPR54-null mice in the morning and evening. C, Mean ± sem serum LH levels in OVX+E+P mice in wild-type, GPR54-null, and kisspeptin-null mice. **P < 0.01. [Data in panel A adapted from J. T. Smith et al.: Endocrinology 146:3686–3692 (269); 227 2005, The Endocrine Society. Data in panel B adapted from H. M. Dungan et al.: J Neurosci 27:12088–12095 (276); 227 2007, Society for Neuroscience. Data in panel C adapted from J. Clarkson et al.: J Neurosci 28:8691–8697 (277); © 2008, Society for Neuroscience].
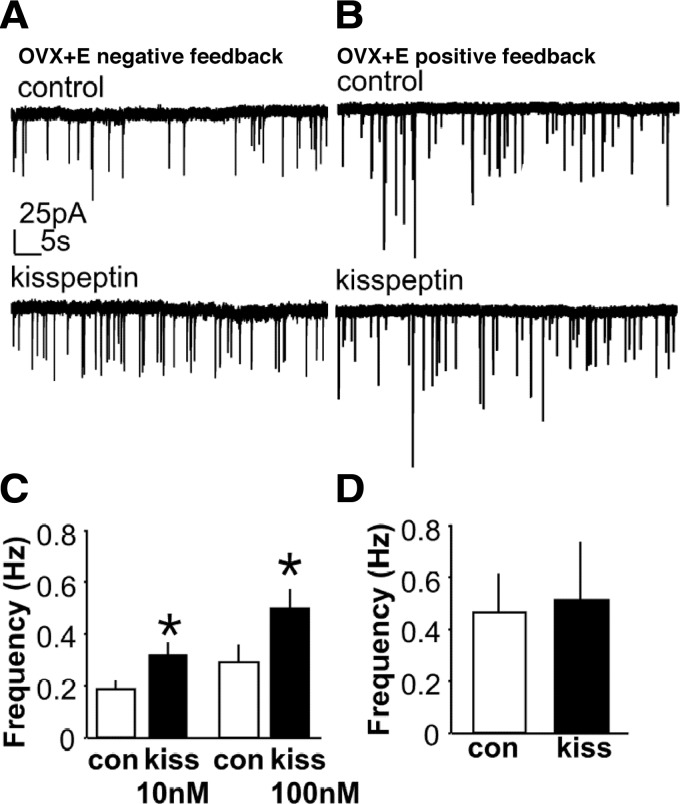
Kisspeptin appears to act both directly on GnRH neurons and via actions on synaptic afferents, and some of these modes of action are estradiol-sensitive. Blockade of ionotropic GABA and glutamate receptors reduces the firing response of GnRH neurons from OVX+E, but not OVX, mice to kisspeptin application (259), suggesting that estradiol treatment enables transsynaptic actions of kisspeptin. Accordingly, kisspeptin increases GABAergic and glutamatergic transmission to GnRH neurons recorded during negative feedback in the daily surge mouse model (278) but has no effect in cells from OVX mice. In addition, kisspeptin application does not induce a further increase in GABA transmission during positive feedback (278), suggesting that endogenous kisspeptin signaling at least partially drives the surge-associated increase in GABAergic synaptic drive (Fig. 11). Similarly, the increase in LH secretion induced by intracerebroventricular injection of kisspeptin is decreased by ovariectomy and increased after selective activation of ERα, but not ERβ (279). In addition, treatment of middle-aged rats, which show an attenuated LH surge using an OVX+E+P induction protocol, with kisspeptin infused into the preoptic area rescues the surge and alters extracellular levels of GABA and glutamate (280). Thus ERα-expressing afferents likely play a significant role in mediating kisspeptin actions on GnRH neurons.
Figure 11.
Kisspeptin increases GABAergic transmission to GnRH neurons during negative feedback but has no effect during positive feedback. A and B, Representative whole-cell voltage-clamp recordings of spontaneous GABAergic postsynaptic currents (sPSCs, downward deflections) in GnRH neurons from OVX+E mice during negative feedback (A) and positive feedback (B). C and D, Mean ± sem of sPSC frequency in cells from OVX+E mice during negative feedback (C) and positive feedback (D), with white bars showing control period and black bars showing kisspeptin treatment. Kisspeptin concentration in B and D = 10 nm. *P < 0.05. [Data adapted from J. Pielecka-Fortuna and S. M. Moenter: Endocrinology 151:291–300 (278). © 2010, The Endocrine Society].
In addition to synaptic mechanisms, kisspeptin appears to exert its long-lasting excitation of GnRH neurons via a phospholipase C-IP3R-calcium signaling cascade to effect closure of inwardly rectifying potassium channels and activation of cationic channels (108,281,282). Kisspeptin can also counteract the hyperpolarization induced by GABAB receptor activation with baclofen (211). This result is not unexpected given the strong GnRH neuron depolarization caused by kisspeptin, but the finding that baclofen can also reduce kisspeptin-induced excitation suggests that the degree of kisspeptin excitation can be modulated by other inputs.
All of these studies have used exogenous application of kisspeptin to investigate its effects. Important tools for future in vivo and in vitro studies are the recently developed kisspeptin antagonists (283), which will allow for examination of the roles of endogenous kisspeptin signals in regulating the surge and other aspects of GnRH neuron regulation. A recent report suggests that continuous intracerebroventricular infusion of a kisspeptin antagonist over 4 d can block the LH surge on proestrus in rats (284), but it remains unclear whether such a protocol of persistent antagonist treatment exerts these effects through direct blockade of the surge generation mechanism or via a more global disruption of the pattern of GnRH release over the estrous cycle.
B. Vasoactive intestinal polypeptide
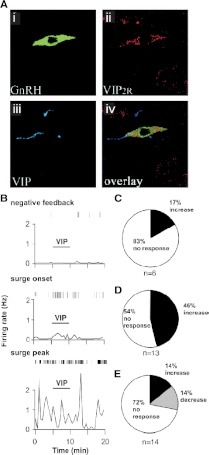
Vasoactive intestinal polypeptide (VIP) is synthesized by cells in the ventrolateral portion of the SCN that receives retinal light information (285,286) and is thus a prime candidate in mediating signals about the external light environment to SCN efferent targets. In rats, there is anatomical evidence for a VIPergic SCN-GnRH neuron connection (125,287), and VIP application or antagonism in the hypothalamus and preoptic area alters the LH surge (288,289,290). These previous studies, however, yielded conflicting results. In vivo antisense antagonism of VIP in the SCN disrupts diurnal rhythms of cFos expression in GnRH neurons in OVX+E rats (291), suggesting an excitatory role of VIP in surge generation. Similarly, measurements of GnRH neuron firing rate in the daily surge mouse model before and during VIP application demonstrated that VIP can excite a proportion of GnRH neurons that corresponds well to the percentage of GnRH neurons shown to express the VIP2 receptor in rats (292), and that this effect is dependent on estradiol treatment and only occurs at certain times of day (293) (Fig. 12). Furthermore, VIP receptor antagonism decreases GnRH neuron firing during the surge peak, suggesting that VIP-mediated excitation helps drive increased GnRH neuron activity during positive feedback.
Figure 12.
GnRH neurons express VIP2 receptors, and VIP excitation of GnRH neurons is time-of-day-dependent. A, Rat GnRH neurons express VIP2 receptors. Confocal micrograph showing a GnRH neuron (Ai) in the rostral preoptic area that expresses VIP2 receptor protein (Aii) and is in close apposition to VIP (Aiii). Aiv, Overlay of Ai–Aiii. B, Representative examples of firing patterns are shown for GnRH neurons from mice treated in the OVX+E daily surge model recorded extracellularly during negative feedback (top), surge onset (middle), and surge peak (bottom). Vertical lines at the top of each graph indicate timing of individual action currents recorded from an individual GnRH neuron. The frequency of action current firing is plotted in 30-sec bins for each cell. C–E, Rate of response for OVX+E cells recorded during negative feedback (C), surge onset (D), and surge peak (E). [Data in panel A adapted from M. J. Smith et al.: Endocrinology 141:4317–4320 (292); 227 2000, The Endocrine Society. Data in panels B–E adapted from C. A. Christian and S. M. Moenter: Endocrinology 149:3130–3136 (293); © 2008, The Endocrine Society].
Although some GnRH neurons appear to express VIP receptors, it remains unclear whether these effects are the result of direct effects on GnRH neurons on indirect signals mediated via upstream neurons or glial elements. A recent report suggests that one effect of VIP is to act on glial cells surrounding GnRH neurons to alter astrocyte morphometry in rats at the time of the surge (294). Interestingly, the degree of glial ensheathment of GnRH neurons decreases at the time of the LH surge (295), which would allow for a greater degree of synaptic connectivity. Further work is needed to clarify what pathways VIP may use to transmit convergence of estradiol and circadian signals to GnRH neurons.
C. Vasopressin
In addition to VIP, other SCN-derived neuromodulators likely play a role in surge regulation. Vasopressinergic neurons in the dorsomedial part of the SCN also project to the AVPV in rats (147,296), where mRNA for the vasopressin V1A receptor is expressed; V1A receptor mRNA has only rarely been found in GnRH neurons (297). Vasopressin may thus be a part of an indirect SCN-GnRH connection, although the possibility of a direct connection has not been tested functionally. Vasopressin induces an LH surge in both SCN-lesioned and SCN-intact OVX+E rats when given in the second half of the light period, indicating that it may act as a circadian signal during a specific time window to induce the surge (298,299). Vasopressin can also induce a surge in the Clock mutant mouse, suggesting that a defect in vasopressin release may at least partially underlie the lack of functional surges in this circadian mutant (300). It will be interesting to determine whether vasopressin, like VIP, can alter GnRH neuron activity and whether these effects show an estradiol or circadian dependence.
D. Catecholamines
The catecholamines norepinephrine and epinephrine have long been hypothesized to play a role in surge generation. Most adrenergic neurons in medullary brainstem regions exhibit increased expression of cFos around surge onset, and a smaller percentage exhibit ERα (301), indicating that at least some of these cells may be involved in mediating estradiol feedback signals. Measurements of circulating norepinephrine levels in women indicated an increase around the time of the LH surge (302). Infusion of norepinephrine into the third ventricle suppressed pulsatile LH release in OVX rats, but increased release in OVX+E+P rats (303), suggesting that estradiol modulates the response of GnRH/LH release to norepinephrine. A separate study, however, found increases in LH release after norepinephrine injection into the preoptic area in OVX rats (304). In an OVX+E daily surge rat model, norepinephrine turnover rates increase during the afternoon around the time of the surge in the medial preoptic area, SCN, and median eminence, suggesting increased norepinephrine release (305), which is consistent with measurements of catecholamine concentrations in the preoptic area in OVX+E rats (192) and in the median eminence of intact ewes (306). In OVX+E ewes, estradiol was shown to decrease levels of the membrane-bound conversion enzyme dopamine β hydroxylase in the preoptic area, suggesting increased release of norepinephrine-containing synaptic vesicles at the time of the surge (307). Increased norepinephrine concentrations after estradiol treatment were also observed in OVX rhesus macaques, along with increased expression of tyrosine hydroxylase mRNA in the locus coeruleus of the brainstem (308). Furthermore, dopamine β hydroxylase mRNA in the ventrolateral medulla and nucleus of the solitarii tract increase during the surge in OVX+E+P rats, and these changes are not observed in middle-aged animals that show a reduced surge response (309).
These previous studies largely suggest that catecholamines exert an excitatory effect on GnRH/LH release, at least in the presence of estradiol. A recent study, however, suggests that the direct effect of norepinephrine on GnRH neurons is hyperpolarizing throughout the estrous cycle and in OVX mice (310), although the effect of norepinephrine in an OVX+E treatment model was not investigated. Thus, future work is needed to determine how estradiol may modulate the GnRH neuron response to catecholamines, which of these effects may be direct on GnRH neurons, and which may involve other intermediaries.
E. Nitric oxide
The free radical gas nitric oxide (NO) is also produced in the SCN, which display a daily rhythm in expression of the NO synthase (NOS) enzyme and NO production in rats and hamsters (311,312). NOS, like GABA, colocalizes with VIP in the SCN (313), and is also highly expressed in the supraoptic and paraventricular nuclei and diagonal band of Broca in rats (314). Blockade of NO synthesis can block the LH surge in steroid-primed rats (315), and stimulation of NO release increases GnRH release from hypothalamic fragments containing the median eminence (315,316). Conversely, increasing NO release in the preoptic area inhibits GnRH neuron firing (317), suggesting possible differential regulation of GnRH release depending on the location of action along the GnRH neuron. Interestingly, daily rhythms of NO efflux in the medial preoptic area are generated independent of ovarian steroids, but the timing of peak NO efflux can be modulated by estradiol in rats (318). Estradiol also appears to modulate NOS synthesis in the rat median eminence, with increased expression on proestrus or after estradiol treatment (319), and promotes interaction between NOS and the NMDA receptor subunit NR2B in the preoptic area (320), with the highest level of complex formation around surge onset on proestrus (321). The role NO may play in transmitting time of day signals to GnRH neurons remains to be precisely determined.
F. Neurotensin
Neurotensin (NT) is a peptide synthesized in cells of the AVPV and medial preoptic area. Some AVPV cells coexpress immunoreactivity for both estradiol receptors and NT, and the degree of double-labeled cells is sexually dimorphic, with a higher number in females compared with males (322,323) and an increased number of NT mRNA-expressing cells in proestrous compared with diestrous rats (323). Assessments of the relationship between NT treatment and LH secretion have yielded conflicting reports. Some studies in OVX rats showed a decrease in LH levels with intracerebroventricular injection of NT (324,325), whereas others found an increase in LH release (304) or no effect (326). Regarding the effects of NT on the LH surge, injection of NT into the preoptic area amplifies the surge in OVX+E rats (326), whereas injection of an NT antiserum reduces the magnitude of the surge without affecting surge timing in OVX+E+P rats (327). Accordingly, push-pull measurements of NT release in the rat median eminence found that NT and GnRH levels significantly increased near the time of the surge rise in LH levels (328). Estradiol increases the level of NT mRNA expression in the preoptic area (329), and NT mRNA levels in the rostral medial preoptic area significantly increase before the LH surge on proestrus (330). A recent report in mice, however, suggests no effect of NT injected into the lateral ventricles on LH secretion (331).
NT may act directly on GnRH neurons because 50% of GnRH neurons in the mouse are apposed by NT-immunoreactive fibers (331), and up to 75% of GnRH neurons in rats and mice express mRNA and protein for NT receptors (330,331). In rats, the number of GnRH neurons exhibiting NT receptors was shown to peak on proestrus (330), whereas in the daily surge mouse model, no effect of time of day was observed on NT receptor expression (331). Whether these and other differences are based on species or methodology remains to be determined.
G. GnRH
GnRH itself may also regulate the function of GnRH neurons. GnRH administered intracerebroventricularly can alter plasma LH levels in a dose-dependent manner, with low doses of GnRH inhibiting LH release and high doses of GnRH having a stimulatory effect (332,333,334). Furthermore, iv injection of a GnRH receptor antagonist increases GnRH release in luteal phase ewes, when low frequency pulses of GnRH are predominant (335). Low doses of GnRH decrease GnRH neuron activity in mice, whereas high doses stimulate GnRH neuron firing (336), which would be consistent with a role for GnRH in forming an ultrashort-loop feedback circuit to decrease or increase GnRH neuron activity during negative and positive feedback, respectively. An intermediate dose of GnRH has been shown to hyperpolarize mouse GnRH neurons and activate the M-current, a subthreshold K+ current that lowers cellular excitability (337). In addition, low-dose GnRH decreases GABA transmission to GnRH neurons in male mice (338). It is unknown whether GnRH alters GABA transmission in females or whether this effect is altered by estradiol treatment, although GnRH has been reported to depolarize GnRH neurons in brain slices from female mice (339). Thus, although specific studies on the relationship of GnRH feedback on GnRH neurons and the surge are still needed, GnRH is poised to play a role in regulating GnRH neurons in different physiological states.
H. Endogenous opioid peptides
The endogenous opioid peptides exert largely inhibitory influences on GnRH/LH secretion and thus have been hypothesized to play a role in mediating negative feedback before the surge and in surge termination. β-Endorphin levels in the arcuate nucleus, preoptic area, and median eminence fluctuate during the rat estrous cycle, with the highest levels during diestrus and lowest on proestrus leading up to the LH surge (340). Accordingly, mRNA levels of the β-endorphin precursor proopiomelanocortin (POMC) in the rat rostral arcuate nucleus are low on proestrus just before surge onset, rise later during the surge, and remain high during estrus (341). Furthermore, estradiol treatment of OVX rats decreases POMC mRNA levels before surge onset, and progesterone treatment that advances the time of the LH surge also advances the time of the decrease in POMC mRNA expression. In addition, POMC mRNA levels subsequently increase during the surge with progesterone treatment, suggesting a biphasic effect of progesterone on β-endorphin neurons (342). A subsequent study, however, detected decreases in POMC primary transcript in the afternoon of both proestrus and estrus, suggesting that β-endorphin may be a component of the daily signal for surge generation (343).
In ewes, the possible role of β-endorphin in surge regulation is less clear. Concentrations of β-endorphin in the median eminence have been measured to be higher during the late follicular compared with early luteal phase (306) and correspondingly to show a progressive increase from the luteal to follicular phase (344). Moreover, infusion of β-endorphin into the posterior-lateral median eminence decreases both GnRH and LH release (344), and infusion of an opioid μ-receptor agonist into either the preoptic area or mediobasal hypothalamus delays LH surge onset (345). Measurements of POMC mRNA levels in the arcuate nucleus, however, either show no difference during the GnRH/LH surge compared with either the luteal phase or the portion of the follicular phase preceding the surge (346,347) or are increased at the peak of the LH surge in OVX+E ewes compared with OVX controls (346). It is important to bear in mind that a lack of change in mRNA levels does not rule out the possibility of downstream changes in synaptic release or postsynaptic response; similarly, a change in message levels does not mandate a change in neuromodulator release. β-Endorphin may play a role in progesterone feedback in this species because increased progesterone levels are associated with increased POMC mRNA expression (346). In addition, antagonists for endogenous opioid receptors increase LH pulse frequency and mean LH levels when injected into the medial basal hypothalamus or preoptic area of luteal phase ewes (348).
Furthermore, 90% of GnRH neurons in the medial basal hypothalamus and approximately 40–50% of GnRH neurons in other areas exhibit close appositions with varicosities containing another opioid peptide, dynorphin (348), although rat GnRH neurons do not appear to express receptors for dynorphin (349,350), suggesting that dynorphin acts via other intermediates to exert its effects. The AVPV contains four times as many cells expressing mRNA for the dynorphin precursor prodynorphin in female rats as in males, and this expression is increased by estradiol (351). In the mouse arcuate nucleus, by contrast, estradiol decreases dynorphin mRNA expression via a classical ERα signaling mechanism (270). Thus, similar to kisspeptin mRNA, the expression of dynorphin mRNA appears to be differentially regulated by estradiol in different cell types. Dynorphin appears to highly colocalize with kisspeptin and neurokinin B (NKB), which we will examine in the next section.
I. Neurokinin B
NKB is a member of the tachykinin peptide family that has been suggested to play a role in estradiol negative feedback. Mutations in the human TAC3 gene, which encodes NKB, or the TACR3 gene, which encodes the NKB receptor (NK3R), cause hypogonadotropic hypogonadism, indicating a critical role for NKB signaling in the regulation of reproduction (352,353). An early study examining the effect of intracerebroventricular injection of NKB in OVX mice observed no effect (354), although recent studies show that the NK3R agonist senktide decreases LH levels in OVX+E rats and OVX mice (355,356). NKB neurons are located in the arcuate nucleus in sheep (357,358,359,360,361), rats (362), and mice (356). Nearly all NKB neurons in the sheep arcuate nucleus express ERα, and females exhibit a dramatically higher number of NKB cells compared with males (357). Short-term (4 h) but not longer-term (8 to 26 h) estradiol treatment decreases NKB mRNA expression and the number of NKB-positive cells (357,358). GnRH neuron somata in ewes and rats do not appear to express NK3R, but NK3R has been detected in GnRH fibers, and GnRH neuron cell bodies have fibers expressing either NKB or NK3R in close proximity (357,361,362). NKB neurons themselves also express NK3R (361), suggesting an autoregulatory feedback mechanism or a mode of communication between NKB neurons in the arcuate nucleus.
In sheep, goats, and mice, a population of arcuate cells that coexpresses kisspeptin, dynorphin, and NKB has been identified (356,359,360,363,364). In mice, gene expression for kisspeptin, dynorphin, and NKB is decreased by estradiol treatment (356), consistent with the estradiol-induced decrease in NKB mRNA observed in sheep (358). Given the apparent inhibitory effect of NKB, however, it remains unclear how these decreases in expression relate to the negative feedback effects of estradiol. An interesting question for future studies is whether this population of cells is exclusively involved in regulating pulsatile GnRH secretion, or whether there is a role for dynorphin or NKB signaling in surge generation as well.
J. Gonadotropin-inhibitory hormone/ RFamide-related peptide-3
Gonadotropin-inhibitory hormone (GnIH) is an RFamide peptide like kisspeptin, but with dramatically different effects on GnRH and LH regulation. Because reviews on GnIH actions have been published recently (365,366,367), we will focus primarily on the studies indicating roles for GnIH in mediating estradiol feedback and surge regulation. GnIH was first identified in quail, with cell bodies located in the paraventricular nucleus and median eminence (368). GnIH derived its name from its ability to inhibit gonadotropin release from cultured quail pituitaries (368). The mammalian ortholog has since been identified as RFamide-related peptide-3 (RFRP-3), and studies in mammals use both the GnIH and RFRP-3 terminologies; here, we will use the terminology used by the respective papers. In rats, hamsters, and mice, GnIH-immunoreactive cell bodies have been localized to the dorsomedial hypothalamus, where they express ERα and project to form close appositions with GnRH neurons (369). Both intracerebroventricular and ip injections of GnIH in OVX hamsters rapidly suppressed LH secretion (369), suggesting both central and pituitary actions of GnIH. In rats, however, central vs. peripheral administration of RFRP-3 has yielded conflicting results. In one study, iv injection, but not intracerebroventricular administration, of RFRP-3 in OVX rats decreased LH release (370), but in another there was no effect of iv RFRP-3 on LH levels (371). Moreover, electrophysiological studies show that GnIH directly exerts a powerful inhibitory effect on GnRH neuron firing in mice via activation of an inwardly rectifying potassium conductance (372,373). The neural and pituitary actions of GnIH/RFRP-3, and any species differences therein, thus require further examination.
SCN-mediated relief of GnIH inhibition of GnRH neurons may be an important component of surge regulation. In hamsters, the SCN form ipsilateral projections with RFRP-3 cells, which show increased cFos expression when SCN activity is low and decreased expression during the LH surge, of interest because this is an opposite pattern to that associated with primarily excitatory neuromodulator neurons (374) (Fig. 13). Furthermore, OVX hamsters do not exhibit these differences, but estradiol-treated hamsters do, suggesting that these changes are dependent upon gonadal estradiol (374). It will be interesting to determine whether removal of GnIH inhibition is part of the daily signal for surge generation and whether these time-of-day-dependent changes in GnIH neuron function are present in other species.
Figure 13.
Activation of GnIH/RFRP cells is reduced during the LH surge in hamsters. A, Low-power micrograph of RFRP-immunoreactive cells expressing cFos in the dorsomedial hypothalamus on diestrus. B and C, RFRP-ir cells expressing cFos on proestrus during the trough (1800 h; B) and peak (2300 h; C) of expression. D, Mean percentages of RFRP-immunoreactive cells expressing cFos on diestrus and at various times on the day of proestrus. [Data adapted from E. M. Gibson et al.: Endocrinology 149:4958–4969 (374). © 2008, The Endocrine Society.]
VIII. A Model for Neurobiological Regulation of the GnRH Surge by Fast Synaptic and Neuromodulatory Signals
Given the importance of the surge for ovulation and reproductive success, it is likely that multiple redundant signals regulate changes in GnRH neuron activity associated with negative and positive feedback. We postulate a working model of the neurobiological mechanisms by which these signals are transmitted to GnRH neurons to regulate the GnRH surge (Fig. 14).
Figure 14.
Schematic working model of multiple signals involved in surge generation. The gray dotted box indicates portion of sagittal brain sketch magnified in schematic figure. For simplicity only, the substances with the most evidence for involvement in surge regulation are depicted; see text for full description of each neuromodulator. Excitatory inputs (green lines and text) arise from the AVPV, SCN, and medulla, mediated by kisspeptin (kiss), GABA, VIP, and catecholamines. Inhibitory inputs (red text) arising from the arcuate nucleus (ARC) and dorsomedial hypothalamus (DMH) mediated by endogenous opioid peptides (EOPs), NKB, and GnIH/RFRP-3 are reduced (dashed red line) during the surge. The SCN provides excitatory input to the AVPV and inhibitory input to the DMH at appropriate times of day to coordinate changes in downstream inputs to GnRH neurons. Sagittal brain sketch courtesy of Dr. Paul Heideman. (The College of William and Mary, Williamsburg, Virginia).
In this model, the primary signals for surge regulation arise from the AVPV, SCN, arcuate nucleus, dorsomedial hypothalamus, and medullary brainstem. To generate the surge, transmission of inhibitory signals, such as GnIH/RFRP-3, endogenous opioid peptides, and NKB, is reduced. Concomitantly, excitatory signals such as those mediated by GABA via GABAA receptors on GnRH neurons, kisspeptin, and VIP are increased to drive increased GnRH neuron firing activity, and thus increased GnRH release, during the surge.
IX. Conclusion
The work of the last decade has greatly expanded our knowledge of the neurobiological mechanisms of GnRH/LH surge generation, particularly with regard to the actions of estradiol on GnRH neurons and their synaptic inputs, and the identification of several neurotransmitters and neuromodulators that appear to play significant roles in surge regulation. In particular, the avenues opened up by the ability to use electrophysiological techniques to study these questions has already yielded much evidence that can be used to further understand the integrated neurobiological system of the surge. Nevertheless, many fundamental questions surrounding surge neurobiology remain. Principally, which of these changes are causal, and which are merely associative? What are the mechanisms of interactions between these various signals? How do GnRH neurons integrate these inputs to know when to pulse and when to surge? What network and intrinsic properties of GnRH neurons are altered to cause a massive surge in GnRH secretion? How are glial cells surrounding GnRH neurons and their afferents regulated during pulse vs. surge modes of release? What other factors influence GnRH neuron regulation in the periovulatory period? These and other questions will provide fascinating avenues for future research into the neurobiological mechanisms of surge generation and the neural control of ovulation.
Footnotes
This work was supported by National Institute of Child Health and Human Development/National Institutes of Health Grant R01 HD41469 and by National Institute of Neurological Disorders and Stroke National Research Service Award F31 NS53253 (to C.A.C.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 17, 2010
Abbreviations: AMPA, α-Amino-3-hydro-5-methyl-4-isoxazole-propionic acid; AVPV, anteroventral periventricular area; EPSC, excitatory PSC; ER, estrogen receptor; GABA, γ-aminobutyric acid; GAD, glutamic acid decarboxylase; GFP, green fluorescent protein; GnIH, gonadotropin-inhibitory hormone; GPR, G protein-coupled receptor; MUA, multiunit activity; NKB, neurokinin B; NK3R, NKB receptor; NMDA, N-methyl-d-aspartate; NO, nitric oxide; NOS, NO synthase; NT, neurotensin; OVX, ovariectomized or ovariectomy; OVX+E, ovariectomy plus estradiol treatment; OVX+E+P, ovariectomy plus estradiol plus progesterone treatment; POMC, proopiomelanocortin; PSC, postsynaptic current; RFRP-3, RFamide-related peptide-3; SCN, suprachiasmatic nuclei; VIP, vasoactive intestinal polypeptide.
References
- Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E 1978 Hypophysial responses to continuous and intermittent delivery of hypothalamic gonadotropin-releasing hormone. Science 202:631–633 [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Thomas GB, Yao B, Cummins JT 1987 GnRH secretion throughout the ovine estrous cycle. Neuroendocrinology 46:82–88 [DOI] [PubMed] [Google Scholar]
- Wildt L, Häusler A, Marshall G, Hutchison JS, Plant TM, Belchetz PE, Knobil E 1981 Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology 109:376–385 [DOI] [PubMed] [Google Scholar]
- Marshall JC, Griffin ML 1993 The role of changing pulse frequency in the regulation of ovulation. Hum Reprod 8:57–61 [DOI] [PubMed] [Google Scholar]
- Haisenleder DJ, Dalkin AC, Ortolano GA, Marshall JC, Shupnik MA 1991 A pulsatile gonadotropin-releasing hormone stimulus is required to increase transcription of the gonadotropin subunit genes: evidence for differential regulation of transcription by pulse frequency in vivo. Endocrinology 128:509–517 [DOI] [PubMed] [Google Scholar]
- Knobil E 1980 The neuroendocrine control of the menstrual cycle. Recent Prog Horm Res 36:53–88 [DOI] [PubMed] [Google Scholar]
- Dalkin AC, Haisenleder DJ, Ortolano GA, Ellis TR, Marshall JC 1989 The frequency of gonadotropin-releasing-hormone stimulation differentially regulates gonadotropin subunit messenger ribonucleic acid expression. Endocrinology 125:917–924 [DOI] [PubMed] [Google Scholar]
- Moenter SM, Caraty A, Karsch FJ 1990 The estradiol-induced surge of gonadotropin-releasing hormone in the ewe. Endocrinology 127:1375–1384 [DOI] [PubMed] [Google Scholar]
- Moenter SM, Caraty A, Locatelli A, Karsch FJ 1991 Pattern of gonadotropin-releasing hormone (GnRH) secretion leading up to ovulation in the ewe: existence of a preovulatory GnRH surge. Endocrinology 129:1175–1182 [DOI] [PubMed] [Google Scholar]
- Adams TE, Norman RL, Spies HG 1981 Gonadotropin-releasing hormone receptor binding and pituitary responsiveness in estradiol-primed monkeys. Science 213:1388–1390 [DOI] [PubMed] [Google Scholar]
- Aiyer MS, Fink G, Greig F 1974 Changes in the sensitivity of the pituitary gland to luteinizing hormone releasing factor during the oestrous cycle of the rat. J Endocrinol 60:47–64 [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Cummins JT, Crowder ME, Nett TM 1988 Pituitary receptors for gonadotropin-releasing hormone in relation to changes in pituitary and plasma gonadotropins in ovariectomized hypothalamo/pituitary-disconnected ewes. II. A marked rise in receptor number during the acute feedback effects of estradiol. Biol Reprod 39:349–354 [DOI] [PubMed] [Google Scholar]
- Crowder ME, Nett TM 1984 Pituitary content of gonadotropins and receptors for gonadotropin-releasing hormone (GnRH) and hypothalamic content of GnRH during the periovulatory period of the ewe. Endocrinology 114:234–239 [DOI] [PubMed] [Google Scholar]
- Sarkar DK, Chiappa SA, Fink G, Sherwood NM 1976 Gonadotropin-releasing hormone surge in pro-oestrous rats. Nature 264:461–463 [DOI] [PubMed] [Google Scholar]
- Park OK, Ramirez VD 1989 Spontaneous changes in LHRH release during the rat estrous cycle, as measured with repetitive push-pull perfusions of the pituitary gland in the same female rats. Neuroendocrinology 50:66–72 [DOI] [PubMed] [Google Scholar]
- Pau KY, Berria M, Hess DL, Spies HG 1993 Preovulatory gonadotropin-releasing hormone surge in ovarian-intact rhesus macaques. Endocrinology 133:1650–1656 [DOI] [PubMed] [Google Scholar]
- Irvine CH, Alexander SL 1994 The dynamics of gonadotrophin-releasing hormone, LH and FSH secretion during the spontaneous surge of the mare as revealed by intensive sampling of pituitary venous blood. J Endocrinol 140:283–295 [DOI] [PubMed] [Google Scholar]
- Tsou RC, Dailey RA, McLanahan CS, Parent AD, Tindall GT, Neill JD 1977 Luteinizing hormone releasing hormone (LHRH) levels in pituitary stalk plasma during the preovulatory gonadotropin surge of rabbits. Endocrinology 101:534–539 [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Stirland JA, Darrow JM, Menaker M, Loudon ASI 1999 Free running circadian rhythms of melatonin, luteinizing hormone, and cortisol in Syrian hamsters bearing the circadian tau mutation. Endocrinology 140:758–764 [DOI] [PubMed] [Google Scholar]
- Miller BH, Olson SL, Turek FW, Levine JE, Horton TH, Takahashi JS 2004 Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr Biol 14:1367–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Grant K, Raisman G 1977 Abnormalities in reproductive function associated with the destruction of the suprachiasmatic nuclei in female rats. Proc R Soc Lond B Biol Sci 198:279–296 [DOI] [PubMed] [Google Scholar]
- Caraty A, Locatelli A, Martin GB 1989 Biphasic response in the secretion of gonadotrophin-releasing hormone in ovariectomized ewes injected with oestradiol. J Endocrinol 123:375–382 [DOI] [PubMed] [Google Scholar]
- Sarkar DK, Fink G 1980 Luteinizing hormone releasing factor in pituitary stalk plasma from long-term ovariectomized rats: effects of steroids. J Endocrinol 86:511–524 [DOI] [PubMed] [Google Scholar]
- Chongthammakun S, Terasawa E 1993 Negative feedback effects of estrogen on luteinizing hormone-releasing hormone release occur in pubertal, but not prepubertal, ovariectomized female rhesus monkeys. Endocrinology 132:735–743 [DOI] [PubMed] [Google Scholar]
- Evans NP, Dahl GE, Glover BH, Karsch FJ 1994 Central regulation of pulsatile gonadotropin-releasing hormone (GnRH) secretion by estradiol during the period leading up to the preovulatory GnRH surge in the ewe. Endocrinology 134:1806–1811 [DOI] [PubMed] [Google Scholar]
- Shupnik MA, Gharib SD, Chin WW 1988 Estrogen suppresses rat gonadotropin gene transcription in vivo. Endocrinology 122:1842–1846 [DOI] [PubMed] [Google Scholar]
- Shupnik MA 1996 Gonadotropin gene modulation by steroids and gonadotropin-releasing hormone. Biol Reprod 54:279–286 [DOI] [PubMed] [Google Scholar]
- Moenter SM, Brand RM, Midgley AR, Karsch FJ 1992 Dynamics of gonadotropin-releasing hormone release during a pulse. Endocrinology 130:503–510 [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Cummins JT 1985 Increased gonadotropin-releasing hormone pulse frequency associated with estrogen-induced luteinizing hormone surges in ovariectomized ewes. Endocrinology 116:2376–2383 [DOI] [PubMed] [Google Scholar]
- Clarke IJ 1988 Gonadotrophin-releasing hormone secretion (GnRH) in anoestrous ewes and the induction of GnRH surges by oestrogen. J Endocrinol 117:355–360 [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Cummins JT, Jenkin M, Phillips DJ 1989 The oestrogen-induced surge of LH requires a ‘signal’ pattern of gonadotrophin-releasing hormone input to the pituitary gland in the ewe. J Endocrinol 122:127–134 [DOI] [PubMed] [Google Scholar]
- Clarke IJ 1993 Variable patterns of gonadotropin-releasing hormone secretion during the estrogen-induced luteinizing hormone surge in ovariectomized ewes. Endocrinology 133:1624–1632 [DOI] [PubMed] [Google Scholar]
- Xia L, Van Vugt D, Alston EJ, Luckhaus J, Ferin M 1992 A surge of gonadotropin-releasing hormone accompanies the estradiol-induced gonadotropin surge in the rhesus monkey. Endocrinology 131:2812–2820 [DOI] [PubMed] [Google Scholar]
- Evans NP, Dahl GE, Caraty A, Padmanabhan V, Thrun LA, Karsch FJ 1996 How much of the gonadotropin-releasing hormone (GnRH) surge is required for generation of the luteinizing hormone surge in the ewe? Duration of the endogenous GnRH signal. Endocrinology 137:4730–4737 [DOI] [PubMed] [Google Scholar]
- Brooks J, Taylor PL, Saunders PT, Eidne KA, Struthers WJ, McNeilly AS 1993 Cloning and sequencing of the sheep pituitary gonadotropin-releasing hormone receptor and changes in expression of its mRNA during the estrous cycle. Mol Cell Endocrinol 94:R23–R27 [DOI] [PubMed] [Google Scholar]
- Clayton RN, Solano AR, Garcia-Vela A, Dufau ML, Catt KJ 1980 Regulation of pituitary receptors for gonadotropin-releasing hormone during the rat estrous cycle. Endocrinology 107:699–706 [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Dalkin A, Yasin M, Haisenleder DJ, Marshall JC, Landefeld TD 1995 Are immediate early genes involved in gonadotropin-releasing hormone receptor gene regulation? Characterization of changes in GnRH receptor (GnRH-R), c-fos, and c-jun messenger ribonucleic acids during the ovine estrous cycle. Biol Reprod 53:263–269 [DOI] [PubMed] [Google Scholar]
- Kaynard AH, Malpaux B, Robinson JE, Wayne NL, Karsch FJ 1988 Importance of pituitary and neural actions of estradiol in induction of the luteinizing hormone surge in the ewe. Neuroendocrinology 48:296–303 [DOI] [PubMed] [Google Scholar]
- Bronson FH, Vom Saal FS 1979 Control of the preovulatory release of luteinizing hormone by steroids in the mouse. Endocrinology 104:1247–1255 [DOI] [PubMed] [Google Scholar]
- Bronson FH 1981 The regulation of luteinizing hormone secretion by estrogen: relationships among negative feedback, surge potential, and male stimulation in juvenile, peripubertal, and adult female mice. Endocrinology 108:506–516 [DOI] [PubMed] [Google Scholar]
- Hoffman GE, Lee WS, Attardi B, Yann V, Fitzsimmons MD 1990 Luteinizing hormone-releasing hormone neurons express c-fos antigen after steroid activation. Endocrinology 126:1736–1741 [DOI] [PubMed] [Google Scholar]
- Herbison AE, Porteous R, Pape JR, Mora JM, Hurst PR 2008 Gonadotropin-releasing hormone neuron requirements for puberty, ovulation, and fertility. Endocrinology 149:597–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerdelhué B, Brown S, Lenoir V, Queenan Jr JT, Jones GS, Scholler R, Jones Jr HW 2002 Timing of initiation of the preovulatory luteinizing hormone surge and its relationship with the circadian cortisol rhythm in the human. Neuroendocrinology 75:158–163 [DOI] [PubMed] [Google Scholar]
- Mahoney MM, Sisk C, Ross HE, Smale L 2004 Circadian regulation of gonadotropin-releasing hormone neurons and the preovulatory surge in luteinizing hormone in the diurnal rodent, Arvicanthis niloticus, and in a nocturnal rodent, Rattus norvegicus. Biol Reprod 70:1049–1054 [DOI] [PubMed] [Google Scholar]
- Fabre-Nys C, Martin GB, Cognié Y, Thiéry JC 1984 Onset of the preovulatory LH surge and of oestrus in intact ewes: night is a preferred period. Theriogenology 22:489–495 [DOI] [PubMed] [Google Scholar]
- Martin GB, Cognié Y, Schirar A, Nunes-Ribeiro A, Fabre-Nys C, Thiéry JC 1985 Diurnal variation in the response of anoestrous ewes to the ram effect. J Reprod Fertil 75:275–284 [DOI] [PubMed] [Google Scholar]
- Fabre-Nys C, Martin GB 1991 Roles of progesterone and oestradiol in determining the temporal sequency and quantitative expression of sexual receptivity and the preovulatory LH surge in the ewe. J Endocrinol 130:367–379 [DOI] [PubMed] [Google Scholar]
- Everett JW, Sawyer CH 1950 A 24-hour periodicity in the “LH-release apparatus” of female rats, disclosed by barbiturate sedation. Endocrinology 47:198–218 [DOI] [PubMed] [Google Scholar]
- Christian CA, Mobley JL, Moenter SM 2005 Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proc Natl Acad Sci USA 102:15682–15687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legan SJ, Karsch FJ 1975 A daily signal for the LH surge in the rat. Endocrinology 96:57–62 [DOI] [PubMed] [Google Scholar]
- Norman RL, Blake CA, Sawyer CH 1973 Estrogen-dependent 24-hour periodicity in pituitary LH release in the female hamster. Endocrinology 93:965–970 [DOI] [PubMed] [Google Scholar]
- Herbison AE 1998 Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev 19:302–330 [DOI] [PubMed] [Google Scholar]
- Wakerley JB, Lincoln DW 1973 The milk-ejection reflex of the rat: a 20- to 40-fold acceleration in the firing of paraventricular neurones during oxytocin release. J Endocrinol 57:477–493 [DOI] [PubMed] [Google Scholar]
- Poulain DA, Wakerley JB, Dyball RE 1977 Electrophysiological differentiation of oxytocin- and vasopressin-secreting neurones. Proc R Soc Lond B Biol Sci 196:367–384 [DOI] [PubMed] [Google Scholar]
- Lincoln DW, Wakerley JB 1974 Electrophysiological evidence for the activation of supraoptic neurones during the release of oxytocin. J Physiol 242:533–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimble MJ, Dyball RE 1977 Characterization of the responses of oxytocin- and vasopressin-secreting neurones in the supraoptic nucleus to osmotic stimulation. J Physiol 271:253–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton A, Dyball RE 1979 Phasic firing enhances vasopressin release from the rat neurohypophysis. J Physiol 290:433–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell RJ, Leng G 1981 Relative efficiency of neural firing patterns for vasopressin release in vitro. Neuroendocrinology 33:295–299 [DOI] [PubMed] [Google Scholar]
- Nunemaker CS, DeFazio RA, Geusz ME, Herzog ED, Pitts GR, Moenter SM 2001 Long-term recordings of networks of immortalized GnRH neurons reveal episodic patterns of electrical activity. J Neurophysiol 86:86–93 [DOI] [PubMed] [Google Scholar]
- Thiéry JC, Pelletier J 1981 Multiunit activity in the anterior median eminence and adjacent areas of the hypothalamus of the ewe in relation to LH secretion. Neuroendocrinology 32:217–224 [DOI] [PubMed] [Google Scholar]
- Wilson RC, Kesner JS, Kaufman JM, Uemura T, Akema T, Knobil E 1984 Central electrophysiologic correlates of pulsatile luteinizing hormone secretion in the rhesus monkey. Neuroendocrinology 39:256–260 [DOI] [PubMed] [Google Scholar]
- Cardenas H, Ordög T, O'Byrne KT, Knobil E 1993 Single unit components of the hypothalamic multiunit electrical activity associated with the central signal generator that directs the pulsatile secretion of gonadotropic hormones. Proc Natl Acad Sci USA 90:9630–9634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GB, Thiéry JC 1987 Hypothalamic multiunit activity and LH secretion in conscious sheep. Exp Brain Res 67:469–478 [DOI] [PubMed] [Google Scholar]
- Mori Y, Nishihara M, Tanaka T, Shimizu T, Yamaguchi M, Takeuchi Y, Hoshino K 1991 Chronic recording of electrophysiological manifestation of the hypothalamic gonadotropin-releasing hormone pulse generator activity in the goat. Neuroendocrinology 53:392–395 [DOI] [PubMed] [Google Scholar]
- Kato A, Hiruma H, Kimura F 1994 Acute estradiol modulation of electrical activity of the LHRH pulse generator in the ovariectomized rat: restoration by naloxone. Neuroendocrinology 59:426–431 [DOI] [PubMed] [Google Scholar]
- Kesner JS, Wilson RC, Kaufman JM, Hotchkiss J, Chen Y, Yamamoto H, Pardo RR, Knobil E 1987 Unexpected responses of the hypothalamic gonadotropin-releasing hormone “pulse generator” to physiological estradiol inputs in the absence of the ovary. Proc Natl Acad Sci USA 84:8745–8749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Byrne KT, Chen MD, Nishihara M, Williams CL, Thalabard JC, Hotchkiss J, Knobil E 1993 Ovarian control of gonadotropin hormone-releasing hormone pulse generator activity in the rhesus monkey: duration of the associated hypothalamic signal. Neuroendocrinology 57:588–592 [DOI] [PubMed] [Google Scholar]
- O'Byrne KT, Thalabard JC, Grosser PM, Wilson RC, Williams CL, Chen MD, Ladendorf D, Hotchkiss J, Knobil E 1991 Radiotelemetric monitoring of hypothalamic gonadotropin-releasing hormone pulse generator activity throughout the menstrual cycle of the rhesus monkey. Endocrinology 129:1207–1214 [DOI] [PubMed] [Google Scholar]
- Tanaka T, Mori Y, Hoshino K 1992 Hypothalamic GnRH pulse generator activity during the estradiol-induced LH surge in ovariectomized goats. Neuroendocrinology 56:641–645 [DOI] [PubMed] [Google Scholar]
- Nishihara M, Sano A, Kimura F 1994 Cessation of the electrical activity of gonadotropin-releasing hormone pulse generator during the steroid-induced surge of luteinizing hormone in the rat. Neuroendocrinology 59:513–519 [DOI] [PubMed] [Google Scholar]
- Ohkura S, Takase K, Matsuyama S, Mogi K, Ichimaru T, Wakabayashi Y, Uenoyama Y, Mori Y, Steiner RA, Tsukamura H, Maeda KI, Okamura H 2009 Gonadotrophin-releasing hormone pulse generator activity in the hypothalamus of the goat. J Neuroendocrinol 21:813–821 [DOI] [PubMed] [Google Scholar]
- Spergel DJ, Krüth U, Hanley DF, Sprengel R, Seeburg PH 1999 GABA- and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J Neurosci 19:2037–2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter KJ, Song WJ, Sampson TL, Wuarin JP, Saunders JT, Dudek FE, Moenter SM 2000 Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology 141:412–419 [DOI] [PubMed] [Google Scholar]
- Han SK, Todman MG, Herbison AE 2004 Endogenous GABA release inhibits the firing of adult gonadotropin-releasing hormone neurons. Endocrinology 145:495–499 [DOI] [PubMed] [Google Scholar]
- Skynner MJ, Slater R, Sim JA, Allen ND, Herbison AE 1999 Promoter transgenics reveal multiple gonadotropin-releasing hormone-I-expressing cell populations of different embryological origin in mouse brain. J Neurosci 19:5955–5966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehl-Kovarik MC, Pouliot WA, Halterman GL, Handa RJ, Dudek FE, Partin KM 2002 Episodic bursting activity and response to excitatory amino acids in acutely dissociated gonadotropin-releasing hormone neurons genetically targeted with green fluorescent protein. J Neurosci 22:2313–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunemaker CS, Straume M, DeFazio RA, Moenter SM 2003 Gonadotropin-releasing hormone neurons generate interacting rhythms in multiple time domains. Endocrinology 144:823–831 [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Ronnekleiv OK, Eskay RL 1984 Identification of estrogen-responsive LHRH neurons in the guinea pig hypothalamus. Brain Res Bull 12:399–407 [DOI] [PubMed] [Google Scholar]
- Lagrange AH, Rønnekleiv OK, Kelly MJ 1995 Estradiol-17 β and μ-opioid peptides rapidly hyperpolarize GnRH neurons: a cellular mechanism of negative feedback? Endocrinology 136:2341–2344 [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Rønnekleiv OK, Ibrahim N, Lagrange AH, Wagner EJ 2002 Estrogen modulation of K(+) channel activity in hypothalamic neurons involved in the control of the reproductive axis. Steroids 67:447–456 [DOI] [PubMed] [Google Scholar]
- Abe H, Terasawa E 2005 Firing pattern and rapid modulation of activity by estrogen in primate luteinizing hormone releasing hormone-1 neurons. Endocrinology 146:4312–4320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura I, Ui-Tei K, Saigo K, Ishii H, Sakuma Y, Kato M 2008 17β-estradiol at physiological concentrations augments Ca(2+)-activated K+ currents via estrogen receptor β in the gonadotropin-releasing hormone neuronal cell line GT1-7. Endocrinology 149:774–782 [DOI] [PubMed] [Google Scholar]
- Chu Z, Andrade J, Shupnik MA, Moenter SM 2009 Differential regulation of gonadotropin-releasing hormone neuron activity and membrane properties by acutely applied estradiol: dependence on dose and estrogen receptor subtype. J Neurosci 29:5616–5627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Moenter SM 17-estradiol rapidly potentiates voltage-gated calcium current in gonadotropin-releasing hormone (GnRH) neurons. Program of the 91st Annual Meeting of The Endocrine Society, Washington, DC, 2009 Abstract P2-265 [Google Scholar]
- Glidewell-Kenney C, Hurley LA, Pfaff L, Weiss J, Levine JE, Jameson JL 2007 Nonclassical estrogen receptor α signaling mediates negative feedback in the female mouse reproductive axis. Proc Natl Acad Sci USA 104:8173–8177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian CA, Glidewell-Kenney C, Jameson JL, Moenter SM 2008 Classical estrogen receptor α signaling mediates negative and positive feedback on gonadotropin-releasing hormone neuron firing. Endocrinology 149:5328–5334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunemaker CS, DeFazio RA, Moenter SM 2002 Estradiol-sensitive afferents modulate long-term episodic firing patterns of GnRH neurons. Endocrinology 143:2284–2292 [DOI] [PubMed] [Google Scholar]
- DeFazio RA, Moenter SM 2002 Estradiol feedback alters potassium currents and firing properties of gonadotropin-releasing hormone neurons. Mol Endocrinol 16:2255–2265 [DOI] [PubMed] [Google Scholar]
- Evans NP, Dahl GE, Padmanabhan V, Thrun LA, Karsch FJ 1997 Estradiol requirements for induction and maintenance of the gonadotropin-releasing hormone surge: implications for neuroendocrine processing of the estradiol signal. Endocrinology 138:5408–5414 [DOI] [PubMed] [Google Scholar]
- Christian CA, Moenter SM 2008 Critical roles for fast synaptic transmission in mediating estradiol negative and positive feedback in the neural control of ovulation. Endocrinology 149:5500–5508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Z, Moenter SM 2006 Physiologic regulation of a tetrodotoxin-sensitive sodium influx that mediates a slow after depolarization potential in gonadotropin-releasing hormone neurons: possible implications for the central regulation of fertility. J Neurosci 26:11961–11973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Moenter SM, Voltage-gated calcium channels of gonadotropin-releasing hormone (GnRH) neurons are regulated by estradiol in a diurnal manner during negative and positive feedback. Proc of 38th Annual Meeting of the Society for Neuroscience, Washington, DC, 2008 (Abstract 381.1) [Google Scholar]
- Pielecka-Fortuna J, Moenter SM, Kisspeptin reduces transient voltage-gated potassium currents in gonadotropin-releasing hormone (GnRH) neurons. Proc of 39th Annual Meeting of the Society for Neuroscience, Chicago, IL, 2009 (Abstract 665.15) [Google Scholar]
- Zhang C, Bosch MA, Levine JE, Rønnekleiv OK, Kelly MJ 2007 Gonadotropin-releasing hormone neurons express K(ATP) channels that are regulated by estrogen and responsive to glucose and metabolic inhibition. J Neurosci 27:10153–10164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Bosch MA, Rick EA, Kelly MJ, Rønnekleiv OK 2009 17β-Estradiol regulation of T-type calcium channels in gonadotropin-releasing hormone neurons. J Neurosci 29:10552–10562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moenter SM, Brand RC, Karsch FJ 1992 Dynamics of gonadotropin-releasing hormone (GnRH) secretion during the GnRH surge: insights into the mechanism of GnRH surge induction. Endocrinology 130:2978–2984 [DOI] [PubMed] [Google Scholar]
- Chappell PE, Levine JE 2000 Stimulation of gonadotropin-releasing hormone surges by estrogen. I. Role of hypothalamic progesterone receptors. Endocrinology 141:1477–1485 [DOI] [PubMed] [Google Scholar]
- Funabashi T, Suyama K, Uemura T, Hirose M, Hirahara F, Kimura F 2001 Immortalized gonadotropin-releasing hormone neurons (GT1-7 cells) exhibit synchronous bursts of action potentials. Neuroendocrinology 73:157– 165 [DOI] [PubMed] [Google Scholar]
- Vazquez-Martinez R, Shorte SL, Boockfor FR, Frawley LS 2001 Synchronized exocytotic bursts from gonadotropin-releasing hormone-expressing cells: dual control by intrinsic cellular pulsatility and gap junctional communication. Endocrinology 142:2095–2101 [DOI] [PubMed] [Google Scholar]
- Hosny S, Jennes L 1998 Identification of gap junctional connexin-32 mRNA and protein in gonadotropin-releasing hormone neurons of the female rat. Neuroendocrinology 67:101–108 [DOI] [PubMed] [Google Scholar]
- Suter KJ, Wuarin JP, Smith BN, Dudek FE, Moenter SM 2000 Whole-cell recordings from preoptic/hypothalamic slices reveal burst firing in gonadotropin-releasing hormone neurons identified with green fluorescent protein in transgenic mice. Endocrinology 141:3731–3736 [DOI] [PubMed] [Google Scholar]
- Campbell RE, Gaidamaka G, Han SK, Herbison AE 2009 Dendro-dendritic bundling and shared synapses between gonadotropin-releasing hormone neurons. Proc Natl Acad Sci USA 106:10835–10840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD 1993 Episodic gonadotropin-releasing hormone release from the rat isolated median eminence in vitro. Neuroendocrinology 58:511–518 [DOI] [PubMed] [Google Scholar]
- Purnelle G, Gérard A, Czajkowski V, Bourguignon JP 1997 Pulsatile secretion of gonadotropin-releasing hormone by rat hypothalamic explants without cell bodies of GnRH neurons. Neuroendocrinology 66:305–312 [DOI] [PubMed] [Google Scholar]
- Tsukahara S, Maekawa F, Tsukamura H, Hirunagi K, Maeda K 1999 Morphological characterization of relationship between gap junctions and gonadotropin-releasing hormone nerve terminals in the rat median eminence. Neurosci Lett 261:105–108 [DOI] [PubMed] [Google Scholar]
- Yin W, Mendenhall JM, Bratton SB, Oung T, Janssen WG, Morrison JH, Gore AC 2007 Novel localization of NMDA receptors within neuroendocrine gonadotropin-releasing hormone terminals. Exp Biol Med (Maywood) 232:662–673 [PubMed] [Google Scholar]
- Hrabovszky E, Turi GF, Kalló I, Liposits Z 2004 Expression of vesicular glutamate transporter-2 in gonadotropin-releasing hormone neurons of the adult male rat. Endocrinology 145:4018–4021 [DOI] [PubMed] [Google Scholar]
- Dumalska I, Wu M, Morozova E, Liu R, van den Pol A, Alreja M 2008 Excitatory effects of the puberty-initiating peptide kisspeptin and group 1 metabotropic glutamate receptor agonists differentiate two distinct subpopulations of gonadotropin-releasing hormone neurons. J Neurosci 28:8003–8013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukhliq R, Goodman RL, Berriman SJ, Adrian B, Lehman MN 1999 A subset of gonadotropin-releasing hormone neurons in the ovine medial basal hypothalamus is activated during increased pulsatile luteinizing hormone secretion. Endocrinology 140:5929–5936 [DOI] [PubMed] [Google Scholar]
- Caraty A, Fabre-Nys C, Delaleu B, Locatelli A, Bruneau G, Karsch FJ, Herbison A 1998 Evidence that the mediobasal hypothalamus is the primary site of action of estradiol in inducing the preovulatory gonadotropin releasing hormone surge in the ewe. Endocrinology 139:1752–1760 [DOI] [PubMed] [Google Scholar]
- Hoffman GE, Lyo D 2002 Anatomical markers of activity in neuroendocrine systems: are we all ‘fos-ed out’? J Neuroendocrinol 14:259–268 [DOI] [PubMed] [Google Scholar]
- Lee WS, Smith MS, Hoffman GE 1990 Luteinizing hormone-releasing hormone neurons express Fos protein during the proestrous surge of luteinizing hormone. Proc Natl Acad Sci USA 87:5163–5167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moenter SM, Karsch FJ, Lehman MN 1993 Fos expression during the estradiol-induced gonadotropin-releasing hormone (GnRH) surge of the ewe: induction in GnRH and other neurons. Endocrinology 133:896–903 [DOI] [PubMed] [Google Scholar]
- Moline ML, Albers HE, Todd RB, Moore-Ede MC 1981 Light-dark entrainment of proestrous LH surges and circadian locomotor activity in female hamsters. Horm Behav 15:451–458 [DOI] [PubMed] [Google Scholar]
- Moline ML, Albers HE 1988 Response of circadian locomotor activity and the proestrous luteinizing hormone surge to phase shifts of the light-dark cycle in the hamster. Physiol Behav 43:435–440 [DOI] [PubMed] [Google Scholar]
- Hoffmann JC 1967 Effects of light deprivation on the rat estrous cycle. Neuroendocrinology 2:1–10 [Google Scholar]
- McCormack CE, Sridaran R 1978 Timing of ovulation in rats during exposure to continuous light: evidence for a circadian rhythm of luteinizing hormone secretion. J Endocrinol 76:135–144 [DOI] [PubMed] [Google Scholar]
- Robertson JL, Clifton DK, de la Iglesia HO, Steiner RA, Kauffman AS 2009 Circadian regulation of Kiss1 neurons: implications for timing the preovulatory gonadotropin-releasing hormone/luteinizing hormone surge. Endocrinology 150:3664–3671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulos Z, Morin LP 1985 Entrainment of split circadian activity rhythms in hamsters. J Biol Rhythms 1:1–15 [DOI] [PubMed] [Google Scholar]
- Pickard GE, Turek FW 1982 Splitting of the circadian rhythm of activity is abolished by unilateral lesions of the suprachiasmatic nuclei. Science 215:1119–1121 [DOI] [PubMed] [Google Scholar]
- Swann JM, Turek FW 1985 Multiple circadian oscillators regulate the timing of behavioral and endocrine rhythms in female golden hamsters. Science 228:898–900 [DOI] [PubMed] [Google Scholar]
- Ralph MR, Menaker M 1988 A mutation of the circadian system in golden hamsters. Science 241:1225–1227 [DOI] [PubMed] [Google Scholar]
- LeSauter J, Silver R 1999 Localization of a suprachiasmatic nucleus subregion regulating locomotor rhythmicity. J Neurosci 19:5574–5585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Tsujimoto KL 1985 Neurotransmitters of the suprachiasmatic nucleus: immunocytochemical analysis of 25 neuronal antigens. Neuroscience 15:1049–1086 [DOI] [PubMed] [Google Scholar]
- Van der Beek EM, Horvath TL, Wiegant VM, Van den Hurk R, Buijs RM 1997 Evidence for a direct neuronal pathway from the suprachiasmatic nucleus to the gonadotropin-releasing hormone system: combined tracing and light and electron microscopic immunocytochemical studies. J Comp Neurol 384:569–579 [DOI] [PubMed] [Google Scholar]
- van der Beek EM, Wiegant VM, van Oudheusden HJ, van der Donk HA, van den Hurk R, Buijs RM 1997 Synaptic contacts between gonadotropin-releasing hormone-containing fibers and neurons in the suprachiasmatic nucleus and perichiasmatic area: an anatomical substrate for feedback regulation? Brain Res 755:101–111 [DOI] [PubMed] [Google Scholar]
- Boehm U, Zou Z, Buck LB 2005 Feedback loops link odor and pheromone signaling with reproduction. Cell 123:683–695 [DOI] [PubMed] [Google Scholar]
- Yoon H, Enquist LW, Dulac C 2005 Olfactory inputs to hypothalamic neurons controlling reproduction and fertility. Cell 123:669–682 [DOI] [PubMed] [Google Scholar]
- Tsukahara S 2006 Increased Fos immunoreactivity in suprachiasmatic nucleus before luteinizing hormone surge in estrogen-treated ovariectomized female rats. Neuroendocrinology 83:303–312 [DOI] [PubMed] [Google Scholar]
- Meyer-Bernstein EL, Jetton AE, Matsumoto SI, Markuns JF, Lehman MN, Bittman EL 1999 Effects of suprachiasmatic transplants on circadian rhythms of neuroendocrine function in golden hamsters. Endocrinology 140:207–218 [DOI] [PubMed] [Google Scholar]
- Silver R, LeSauter J, Tresco PA, Lehman MN 1996 A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature 382:810–813 [DOI] [PubMed] [Google Scholar]
- de la Iglesia HO, Meyer J, Carpino Jr A, Schwartz WJ 2000 Antiphase oscillation of the left and right suprachiasmatic nuclei. Science 290:799–801 [DOI] [PubMed] [Google Scholar]
- de la Iglesia HO, Meyer J, Schwartz WJ 2003 Lateralization of circadian pacemaker output: activation of left- and right-sided luteinizing hormone-releasing hormone neurons involves a neural rather than a humoral pathway. J Neurosci 23:7412–7414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW 1990 Distribution of androgen and oestrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol 294:76–95 [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I 1997 Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J Comp Neurol 388:507–525 [DOI] [PubMed] [Google Scholar]
- Vida B, Hrabovszky E, Kalamatianos T, Coen CW, Liposits Z, Kalló I 2008 Oestrogen receptor α and β immunoreactive cells in the suprachiasmatic nucleus of mice: distribution, sex differences and regulation by gonadal hormones. J Neuroendocrinol 20:1270–1277 [DOI] [PubMed] [Google Scholar]
- De La Iglesia HO, Blaustein JD, Bittman EL 1999 Oestrogen receptor-α-immunoreactive neurones project to the suprachiasmatic nucleus of the female Syrian hamster. J Neuroendocrinol 11:481–490 [DOI] [PubMed] [Google Scholar]
- Shinohara K, Funabashi T, Mitushima D, Kimura F 2000 Effects of estrogen on the expression of connexin32 and connexin43 mRNAs in the suprachiasmatic nucleus of female rats. Neurosci Lett 286:107–110 [DOI] [PubMed] [Google Scholar]
- Abizaid A, Mezei G, Horvath TL 2004 Estradiol enhances light-induced expression of transcription factors in the SCN. Brain Res 1010:35–44 [DOI] [PubMed] [Google Scholar]
- Nakamura TJ, Shinohara K, Funabashi T, Kimura F 2001 Effect of estrogen on the expression of Cry1 and Cry2 mRNAs in the suprachiasmatic nucleus of female rats. Neurosci Res 41:251–255 [DOI] [PubMed] [Google Scholar]
- Shinohara K, Funabashi T, Nakamura TJ, Kimura F 2001 Effects of estrogen and progesterone on the expression of connexin-36 mRNA in the suprachiasmatic nucleus of female rats. Neurosci Lett 309:37–40 [DOI] [PubMed] [Google Scholar]
- Peterfi Z, Churchill L, Hajdu I, Obal Jr F, Krueger JM, Parducz A 2004 Fos-immunoreactivity in the hypothalamus: dependency on the diurnal rhythm, sleep, gender, and estrogen. Neuroscience 124:695–707 [DOI] [PubMed] [Google Scholar]
- Kow LM, Pfaff DW 1984 Suprachiasmatic neurons in tissue slices from ovariectomized rats: electrophysiological and neuropharmacological characterization and the effects of estrogen treatment. Brain Res 297:275–286 [DOI] [PubMed] [Google Scholar]
- Fernandez-Galaz MC, Martinez Muñoz R, Villanua MA, Garcia-Segura LM 1999 Diurnal oscillation in glial fibrillary acidic protein in a perisuprachiasmatic area and its relationship to the luteinizing hormone surge in the female rat. Neuroendocrinology 70:368–376 [DOI] [PubMed] [Google Scholar]
- Morin LP, Fitzgerald KM, Zucker I 1977 Estradiol shortens the period of hamster circadian rhythms. Science 196:305–307 [DOI] [PubMed] [Google Scholar]
- Morin LP, Cummings LA 1982 Splitting of wheelrunning rhythms by castrated or steroid treated male and female hamsters. Physiol Behav 29:665–675 [DOI] [PubMed] [Google Scholar]
- Watson Jr RE, Langub Jr MC, Engle MG, Maley BE 1995 Estrogen-receptive neurons in the anteroventral periventricular nucleus are synaptic targets of the suprachiasmatic nucleus to the gonadotropin-releasing hormone system: combined tracing and light and electron microscopic immunocytochemical studies. Brain Res 689:254–264 [DOI] [PubMed] [Google Scholar]
- de la Iglesia HO, Blaustein JD, Bittman EL 1995 The suprachiasmatic area in the female hamster projects to neurons containing estrogen receptors and GnRH. Neuroreport 6:1715–1722 [DOI] [PubMed] [Google Scholar]
- Polston EK, Simerly RB 2006 Ontogeny of the projections from the anteroventral periventricular nucleus of the hypothalamus in the female rat. J Comp Neurol 495:122–132 [DOI] [PubMed] [Google Scholar]
- Wintermantel TM, Campbell RE, Porteous R, Bock D, Gröne HJ, Todman MG, Korach KS, Greiner E, Pérez CA, Schütz G, Herbison AE 2006 Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron 52:271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu GB, Simerly RB 1997 Projections of the sexually dimorphic anteroventral periventricular nucleus in the female rat. J Comp Neurol 384:142–164 [PubMed] [Google Scholar]
- Simerly RB 1998 Organization and regulation of sexually dimorphic neuroendocrine pathways. Behav Brain Res 92:195–203 [DOI] [PubMed] [Google Scholar]
- Wiegand SJ, Terasawa E 1982 Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology 34:395–404 [DOI] [PubMed] [Google Scholar]
- Wiegand SJ, Terasawa E, Bridson WE 1978 Persistent estrus and blockade of progesterone-induced LH release follows lesions which do not damage the suprachiasmatic nucleus. Endocrinology 102:1645–1648 [DOI] [PubMed] [Google Scholar]
- Wiegand SJ, Terasawa E, Bridson WE, Goy RW 1980 Effects of discrete lesions of preoptic and suprachiasmatic structures in the female rat. Alterations in the feedback regulation of gonadotropin secretion. Neuroendocrinology 31:147–157 [DOI] [PubMed] [Google Scholar]
- Ronnekleiv OK, Kelly MJ 1988 Plasma prolactin and luteinizing hormone profiles during the estrous cycle of the female rat: effects of surgically induced persistent estrus. Neuroendocrinology 47:133–141 [DOI] [PubMed] [Google Scholar]
- Le WW, Berghorn KA, Rassnick S, Hoffman GE 1999 Periventricular preoptic area neurons coactivated with luteinizing hormone (LH)-releasing hormone (LHRH) neurons at the time of the LH surge are LHRH afferents. Endocrinology 140:510–519 [DOI] [PubMed] [Google Scholar]
- Le WW, Attardi B, Berghorn KA, Blaustein J, Hoffman GE 1997 Progesterone blockade of a luteinizing hormone surge blocks luteinizing hormone-releasing hormone Fos activation and activation of its preoptic area afferents. Brain Res 778:272–280 [DOI] [PubMed] [Google Scholar]
- Le WW, Wise PM, Murphy AZ, Coolen LM, Hoffman GE 2001 Parallel declines in Fos activation of the medial anteroventral periventricular nucleus and LHRH neurons in middle-aged rats. Endocrinology 142:4976–4982 [DOI] [PubMed] [Google Scholar]
- Petersen SL, Barraclough CA 1989 Suppression of spontaneous LH surges in estrogen-treated ovariectomized rats by microimplants of antiestrogens into the preoptic brain. Brain Res 484:279–289 [DOI] [PubMed] [Google Scholar]
- Chappell PE, Lee J, Levine JE 2000 Stimulation of gonadotropin-releasing hormone surges by estrogen. II. Role of cyclic adenosine 3′5′-monophosphate. Endocrinology 141: 1486–1492 [DOI] [PubMed] [Google Scholar]
- Gillespie JM, Chan BP, Roy D, Cai F, Belsham DD 2003 Expression of circadian rhythm genes in gonadotropin-releasing hormone-secreting GT1-7 neurons. Endocrinology 144:5285–5292 [DOI] [PubMed] [Google Scholar]
- Chappell PE, White RS, Mellon PL 2003 Circadian gene expression regulates pulsatile gonadotropin-releasing hormone (GnRH) secretory patterns in the hypothalamic GnRH-secreting GT1-7 cell line. J Neurosci 23:11202–11213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansuy V, Risold PY, Glauser M, Fraichard A, Pralong FP 2009 Expression of the GABAA receptor associated protein Gec1 is circadian and dependent upon the cellular clock machinery in GnRH secreting GnV-3 cells. Mol Cell Endocrinol 307:68–76 [DOI] [PubMed] [Google Scholar]
- Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD 2002 Circadian rhythms in isolated brain regions. J Neurosci 22:350–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrenkrug J, Georg B, Hannibal J, Hindersson P, Gräs S 2006 Diurnal rhythmicity of the clock genes Per1 and Per2 in the rat ovary. Endocrinology 147:3769–3776 [DOI] [PubMed] [Google Scholar]
- Karman BN, Tischkau SA 2006 Circadian clock gene expression in the ovary: effects of luteinizing hormone. Biol Reprod 75:624–632 [DOI] [PubMed] [Google Scholar]
- Chappell PE, Goodall CP, Tonsfeldt KJ, White RS, Bredeweg E, Latham KL 2009 Modulation of gonadotrophin-releasing hormone secretion by an endogenous circadian clock. J Neuroendocrinol 21:339–345 [DOI] [PubMed] [Google Scholar]
- Latham KL, White RS, Goodall CP, Fitzgerald K, Mellon PL, Chappell PE, Steroids modulate transcript and protein levels of large-conductance Ca2+-activated potassium (BK) channels in GT1-7 cells, correlated with changes in GnRH secretion in vitro. Program of the 90th Annual Meeting of The Endocrine Society, San Francisco, CA, 2008 (Abstract P1-696) [Google Scholar]
- Hrabovszky E, Steinhauser A, Barabás K, Shughrue PJ, Petersen SL, Merchenthaler I, Liposits Z 2001 Estrogen receptor-β immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology 142:3261–3264 [DOI] [PubMed] [Google Scholar]
- Couse JF, Yates MM, Walker VR, Korach KS 2003 Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) null mice reveals hypergonadism and endocrine sex reversal in females lacking ERα but not ERβ. Mol Endocrinol 17:1039–1053 [DOI] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O 1998 Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc Natl Acad Sci USA 95:15677–15682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse JF, Korach KS 1999 Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev 20:358–417 [DOI] [PubMed] [Google Scholar]
- Roy D, Angelini NL, Belsham DD 1999 Estrogen directly represses gonadotropin-releasing hormone (GnRH) gene expression in estrogen receptor-α (ERα)- and ERβ-expressing GT1-7 GnRH neurons. Endocrinology 140:5045– 5053 [DOI] [PubMed] [Google Scholar]
- Radovick S, Ticknor CM, Nakayama Y, Notides AC, Rahman A, Weintraub BD, Cutler Jr GB, Wondisford FE 1991 Evidence for direct estrogen regulation of the human gonadotropin-releasing hormone gene. J Clin Invest 88:1649–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro CE, Saeed SA, Murdock C, Martinez-Fuentes AJ, Arora KK, Krsmanovic LZ, Catt KJ 2003 Regulation of cyclic adenosine 3′,5′-monophosphate signaling and pulsatile neurosecretion by Gi-coupled plasma membrane estrogen receptors in immortalized gonadotrophin-releasing hormone neurons. Mol Endocrinol 17:1792–1804 [DOI] [PubMed] [Google Scholar]
- Skynner MJ, Sim JA, Herbison AE 1999 Detection of estrogen receptor α and β messenger ribonucleic acids in adult gonadotropin-releasing hormone neurons. Endocrinology 140:5195–5201 [DOI] [PubMed] [Google Scholar]
- Chappell PE, Schneider JS, Kim P, Xu M, Lydon JP, O'Malley BW, Levine JE 1999 Absence of gonadotropin surges and gonadotropin-releasing hormone self-priming in ovariectomized (OVX), estrogen (E2)-treated, progesterone receptor knockout (PRKO) mice. Endocrinology 140:3653–3658 [DOI] [PubMed] [Google Scholar]
- Skinner DC, Caraty A, Allingham R 2001 Unmasking the progesterone receptor in the preoptic area and hypothalamus of the ewe: no colocalization with gonadotropin-releasing neurons. Endocrinology 142:573–579 [DOI] [PubMed] [Google Scholar]
- King JC, Tai DW, Hanna IK, Pfeiffer A, Haas P, Ronsheim PM, Mitchell SC, Turcotte JC, Blaustein JD 1995 A subgroup of LHRH neurons in guinea pigs with progestin receptors is centrally positioned within the total population of LHRH neurons. Neuroendocrinology 61:265–275 [DOI] [PubMed] [Google Scholar]
- Flügge G, Oertel WH, Wuttke W 1986 Evidence for estrogen-receptive GABAergic neurons in the preoptic/anterior hypothalamic area of the rat brain. Neuroendocrinology 43:1–5 [DOI] [PubMed] [Google Scholar]
- Leranth C, Shanabrough M, Naftolin F 1991 Estrogen induces ultrastructural changes in progesterone receptor-containing GABA neurons of the primate hypothalamus. Neuroendocrinology 54:571–579 [DOI] [PubMed] [Google Scholar]
- Eyigor O, Lin W, Jennes L 2004 Identification of neurones in the female rat hypothalamus that express oestrogen receptor-α and vesicular glutamate transporter-2. J Neuroendocrinol 16:26–31 [DOI] [PubMed] [Google Scholar]
- Pompolo S, Pereira A, Scott CJ, Fujiyma F, Clarke IJ 2003 Evidence for estrogenic regulation of gonadotropin-releasing hormone neurons by glutamatergic neurons in the ewe brain: an immunohistochemical study using an antibody against vesicular glutamate transporter-2. J Comp Neurol 465:136–144 [DOI] [PubMed] [Google Scholar]
- Ottem EN, Godwin JG, Krishnan S, Petersen SL 2004 Dual-phenotype GABA/glutamate neurons in adult preoptic area: sexual dimorphism and function. J Neurosci 24:8097–8105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottem EN, Godwin JG, Petersen SL 2002 Glutamatergic signaling through the N-methyl-D-aspartate receptor directly activates medial subpopulations of luteinizing hormone-releasing hormone (LHRH) neurons, but does not appear to mediate the effects of estradiol on LHRH gene expression. Endocrinology 143:4837–4845 [DOI] [PubMed] [Google Scholar]
- Brann DW, Zamorano PL, Chorich LP, Mahesh VB 1993 Steroid hormone effects on NMDA receptor binding and NMDA receptor mRNA levels in the hypothalamus and cerebral cortex of the adult rat. Neuroendocrinology 58:666–672 [DOI] [PubMed] [Google Scholar]
- Gore AC, Wu TJ, Rosenberg JJ, Roberts JL 1996 Gonadotropin-releasing hormone and NMDA receptor gene expression and colocalization change during puberty in female rats. J Neurosci 16:5281–5289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian CA, Pielecka-Fortuna J, Moenter SM 2009 Estradiol suppresses glutamatergic transmission to gonadotropin-releasing hormone neurons in a model of negative feedback in mice. Biol Reprod 80:1128–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter KJ 2004 Control of firing by small (S)-α-amino-3-hydroxy-5-methyl-isoxazolepropionic acid-like inputs in hypothalamic gonadotropin-releasing hormone (GnRH) neurons. Neuroscience 128:443–450 [DOI] [PubMed] [Google Scholar]
- Urbanski HF, Garyfallou VT, Kohama SG, Hess DL 1997 α-Adrenergic receptor antagonism and N-methyl-D-aspartate (NMDA) induced luteinizing hormone release in female rhesus macaques. Brain Res 744:96–104 [DOI] [PubMed] [Google Scholar]
- Demling J, Fuchs E, Baumert M, Wuttke W 1985 Preoptic catecholamine, GABA, and glutamate release in ovariectomized and ovariectomized estrogen-primed rats utilizing a push-pull cannula technique. Neuroendocrinology 41:212–218 [DOI] [PubMed] [Google Scholar]
- Jarry H, Hirsch B, Leonhardt S, Wuttke W 1992 Amino acid neurotransmitter release in the preoptic area of rats during the positive feedback actions of estradiol on LH release. Neuroendocrinology 56:133–140 [DOI] [PubMed] [Google Scholar]
- Ping L, Mahesh VB, Wiedmeier VT, Brann DW 1994 Release of glutamate and aspartate from the preoptic area during the progesterone-induced LH surge: in vivo microdialysis studies. Neuroendocrinology 59:318–324 [DOI] [PubMed] [Google Scholar]
- Jarry H, Leonhardt S, Schwarze T, Wuttke W 1995 Preoptic rather than mediobasal hypothalamic amino acid neurotransmitter release regulates GnRH secretion during the estrogen-induced LH surge in the ovariectomized rat. Neuroendocrinology 62:479–486 [DOI] [PubMed] [Google Scholar]
- Neal-Perry GS, Zeevalk GD, Shu J, Etgen AM 2008 Restoration of the luteinizing hormone surge in middle-aged female rats by altering the balance of GABA and glutamate transmission in the medial preoptic area. Biol Reprod 79:878–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal-Perry GS, Zeevalk GD, Santoro NF, Etgen AM 2005 Attenuation of preoptic area glutamate release correlates with reduced luteinizing hormone secretion in middle-aged female rats. Endocrinology 146:4331–4339 [DOI] [PubMed] [Google Scholar]
- López FJ, Donoso AO, Negro-Vilar A 1990 Endogenous excitatory amino acid neurotransmission regulates the estradiol-induced LH surge in ovariectomized rats. Endocrinology 126:1771–1773 [DOI] [PubMed] [Google Scholar]
- Brann DW, Mahesh VB 1991 Endogenous excitatory amino acid involvement in the preovulatory and steroid-induced surge of gonadotropins in the female rat. Endocrinology 128:1541–1547 [DOI] [PubMed] [Google Scholar]
- Ping L, Mahesh VB, Bhat GK, Brann DW 1997 Regulation of gonadotropin-releasing hormone and luteinizing hormone secretion by AMPA receptors. Evidence for a physiological role of AMPA receptors in the steroid-induced luteinizing hormone surge. Neuroendocrinology 66:246–253 [DOI] [PubMed] [Google Scholar]
- Arias P, Jarry H, Leonhardt S, Moguilevsky JA, Wuttke W 1993 Estradiol modulates the LH release response to N-methyl-D-aspartate in adult female rats: studies on hypothalamic luteinizing hormone-releasing hormone and neurotransmitter release. Neuroendocrinology 57:710–715 [DOI] [PubMed] [Google Scholar]
- Carbone S, Szwarcfarb B, Otero Losada ME, Moguilevsky JA 1992 Effects of ovarian steroids on the gonadotropin response to N-methyl-D-aspartate and on hypothalamic excitatory amino acid levels during sexual maturation in female rats. Endocrinology 130:1365–1370 [DOI] [PubMed] [Google Scholar]
- Roberts CB, Best JA, Suter KJ 2006 Dendritic processing of excitatory synaptic input in hypothalamic gonadotropin-releasing hormone neurons. Endocrinology 147:1545–1555 [DOI] [PubMed] [Google Scholar]
- Bailey JD, Centers A, Jennes L 2006 Expression of AMPA receptor subunits (GluR1-GluR4) in gonadotrophin-releasing hormone neurones of young and middle-aged persistently oestrous rats during the steroid-induced luteinising hormone surge. J Neuroendocrinol 18:1–12 [DOI] [PubMed] [Google Scholar]
- Roberts CB, Campbell RE, Herbison AE, Suter KJ 2008 Dendritic action potential initiation in hypothalamic gonadotropin releasing hormone (GnRH) neurons. Endocrinology 149:3355–3360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, McKinney K, Liu L, Lakhlani S, Jennes L 2003 Distribution of vesicular glutamate transporter-2 messenger ribonucleic acid and protein in the septum-hypothalamus of the rat. Endocrinology 144:662–670 [DOI] [PubMed] [Google Scholar]
- Sullivan SD, Moenter SM 2003 Neurosteroids alter γ-aminobutyric acid postsynaptic currents in gonadotropin-releasing hormone neurons: a possible mechanism for direct steroidal control. Endocrinology 144:4366–4375 [DOI] [PubMed] [Google Scholar]
- Sullivan SD, Moenter SM 2004 γ-Aminobutyric acid neurons integrate and rapidly transmit permissive and inhibitory metabolic cues to gonadotropin-releasing hormone neurons. Endocrinology 145:1194–1202 [DOI] [PubMed] [Google Scholar]
- Sullivan SD, Moenter SM 2004 Prenatal androgens alter GABAergic drive to gonadotropin-releasing hormone neurons: implications for a common fertility disorder. Proc Natl Acad Sci USA 101:7129–7134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwowska JH, Billings HJ, Goodman RL, Lehman MN 2006 Immunocytochemical colocalization of GABA-B receptor subunits in gonadotropin-releasing hormone neurons of the sheep. Neuroscience 141:311–319 [DOI] [PubMed] [Google Scholar]
- Zhang C, Bosch MA, Rønnekleiv OK, Kelly MJ 2009 γ-Aminobutyric acid B receptor mediated inhibition of gonadotropin-releasing hormone neurons is suppressed by kisspeptin-G protein-coupled receptor 54 signaling. Endocrinology 150:2388–2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decavel C, Van den Pol AN 1990 GABA: a dominant neurotransmitter in the hypothalamus. J Comp Neurol 302:1019–1037 [DOI] [PubMed] [Google Scholar]
- Tin-Tin-Win-Shwe, Mitsushima D, Shinohara K, Kimura F 2004 Sexual dimorphism of GABA release in the medial preoptic area and luteinizing hormone release in gonadectomized estrogen-primed rats. Neuroscience 127:243–250 [DOI] [PubMed] [Google Scholar]
- Jarry H, Perschl A, Wuttke W 1988 Further evidence that preoptic anterior hypothalamic GABAergic neurons are part of the GnRH pulse and surge generator. Acta Endocrinol 118:573–579 [DOI] [PubMed] [Google Scholar]
- Robinson JE, Kendrick KM, Lambart CE 1991 Changes in the release of γ-aminobutyric acid and catecholamines in the preoptic/septal area prior to and during the preovulatory surge of luteinizing hormone in the ewe. J Neuroendocrinol 3:393–399 [DOI] [PubMed] [Google Scholar]
- Leonhardt S, Seong JY, Kim K, Thorun Y, Wuttke W, Jarry H 1995 Activation of central GABAA-but not of GABAB-receptors rapidly reduces pituitary LH release and GnRH gene expression in the preoptic/anterior hypothalamic area of ovariectomized rats. Neuroendocrinology 61:655–662 [DOI] [PubMed] [Google Scholar]
- Herbison AE, Chapman C, Dyer RG 1991 Role of medial preoptic GABA neurones in regulating luteinising hormone secretion in the ovariectomized rat. Exp Brain Res 87:345–352 [DOI] [PubMed] [Google Scholar]
- Scott CJ, Clarke IJ 1993 Inhibition of luteinizing hormone secretion in ovariectomized ewes during the breeding season by γ-aminobutyric acid (GABA) is mediated by GABA-A receptors, but not GABA-B receptors. Endocrinology 132:1789–1796 [DOI] [PubMed] [Google Scholar]
- Adler BA, Crowley WR 1986 Evidence for γ-aminobutyric acid modulation of ovarian hormonal effects on luteinizing hormone secretion and hypothalamic catecholamine activity in the female rat. Endocrinology 118:91–97 [DOI] [PubMed] [Google Scholar]
- Herbison AE, Dyer RG 1991 Effect on luteinizing hormone secretion of GABA receptor modulation in the medial preoptic area at the time of proestrous luteinizing hormone surge. Neuroendocrinology 53:317–320 [DOI] [PubMed] [Google Scholar]
- Herbison AE, Heavens RP, Dye S, Dyer RG 1991 Acute action of oestrogen on medial preoptic γ-aminobutyric acid neurons: correlation with oestrogen negative feedback on luteinizing hormone secretion. J Neuroendocrinol 3:101–106 [DOI] [PubMed] [Google Scholar]
- Funabashi T, Jinnai K, Kimura F 1997 Bicuculline infusion advances the timing of Fos expression in LHRH neurons in the preoptic area of proestrous rats. Neuroreport 8:771–774 [DOI] [PubMed] [Google Scholar]
- DeFazio RA, Heger S, Ojeda SR, Moenter SM 2002 Activation of A-type γ-aminobutyric acid receptors excites gonadotropin-releasing hormone neurons. Mol Endocrinol 16:2872–2891 [DOI] [PubMed] [Google Scholar]
- Unda R, Brann DW, Mahesh VB 1995 Progesterone suppression of glutamic acid decarboxylase (GAD67) mRNA levels in the preoptic area: correlation to the luteinizing hormone surge. Neuroendocrinology 62:562–570 [DOI] [PubMed] [Google Scholar]
- Grattan DR, Rocca MS, Strauss KI, Sagrillo CA, Selmanoff M, McCarthy MM 1996 GABAergic neuronal activity and mRNA levels for both forms of glutamic acid decarboxylase (GAD65 and GAD67) are reduced in the diagonal band of Broca during the afternoon of proestrus. Brain Res 733:46–55 [DOI] [PubMed] [Google Scholar]
- Curran-Rauhut MA, Petersen SL 2002 Regulation of glutamic acid decarboxylase 65 and 67 gene expression by ovarian steroids: identification of two functionally distinct populations of GABA neurones in the preoptic area. J Neuroendocrinol 14:310–317 [DOI] [PubMed] [Google Scholar]
- Cashion AB, Smith MJ, Wise PM 2004 Glutamic acid decarboxylase (GAD67) gene expression in discrete regions of the rostral preoptic area change during the oestrous cycle and with age. J Neuroendocrinol 16:711–716 [DOI] [PubMed] [Google Scholar]
- Wagner EJ, Ronnekleiv OK, Bosch MA, Kelly MJ 2001 Estrogen biphasically modifies hypothalamic GABAergic function concomitantly with negative and positive control of luteinizing hormone release. J Neurosci 21:2085–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akema T, He D, Sugiyama H 2005 Lipopolysaccharide increases γ-aminobutyric acid synthesis in medial preoptic neurones in association with inhibition of steroid-induced luteinising hormone surge in female rats. J Neuroendocrinol 17:672–678 [DOI] [PubMed] [Google Scholar]
- Leonhardt S, Böning B, Luft H, Wuttke W, Jarry H 2000 Activation of gene expression of the γ-aminobutyric acid rather than the glutamatergic system in the preoptic area during the preovulatory gonadotropin surge of the rat. Neuroendocrinology 71:8–15 [DOI] [PubMed] [Google Scholar]
- Grove-Strawser D, Jimenez-Linan M, Rubin BS 2007 Middle-aged female rats lack the dynamic changes in GAD(67) mRNA levels observed in young females on the day of a luteinising hormone surge. J Neuroendocrinol 19:708–716 [DOI] [PubMed] [Google Scholar]
- Moenter SM, DeFazio RA 2005 Endogenous γ-aminobutyric acid can excite gonadotropin-releasing hormone neurons. Endocrinology 146:5374–5379 [DOI] [PubMed] [Google Scholar]
- Yin C, Ishii H, Tanaka N, Sakuma Y, Kato M 2008 Activation of A-type γ-amino butyric acid receptors excites gonadotrophin-releasing hormone neurones isolated from adult rats. J Neuroendocrinol 20:566–575 [DOI] [PubMed] [Google Scholar]
- Zhang SJ, Jackson MB 1993 GABA-activated chloride channels in secretory nerve endings. Science 259:531–534 [DOI] [PubMed] [Google Scholar]
- Sung KW, Kirby M, McDonald MP, Lovinger DM, Delpire E 2000 Abnormal GABAA receptor-mediated currents in dorsal root ganglion neurons isolated from Na-K-2Cl co-transporter null mice. J Neurosci 20:7531–7538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RF, Dacheux RF 1983 Intracellular chloride in retinal neurons: measurement and meaning. Vision Res 23:399–411 [DOI] [PubMed] [Google Scholar]
- Lowe G, Gold GH 1993 Nonlinear amplification by calcium-dependent chloride channels in olfactory receptor cells. Nature 366:283–286 [DOI] [PubMed] [Google Scholar]
- Marty A, Llano I 2005 Excitatory effects of GABA in established brain networks. Trends Neurosci 28:284–289 [DOI] [PubMed] [Google Scholar]
- Sullivan SD, DeFazio RA, Moenter SM 2003 Metabolic regulation of fertility through presynaptic and postsynaptic signaling to gonadotropin-releasing hormone neurons. J Neurosci 23:8578–8585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Sakuma Y, Kato M 2009 GABAA receptors mediate excitation in adult rat GnRH neurons. Biol Reprod 81:327–332 [DOI] [PubMed] [Google Scholar]
- Constantin S, Jasoni CL, Wadas B, Herbison AE 2010 γ-Aminobutyric acid and glutamate differentially regulate intracellular calcium concentrations in mouse gonadotropin-releasing hormone neurons. Endocrinology 151:262–270 [DOI] [PubMed] [Google Scholar]
- Han SK, Abraham IM, Herbison AE 2002 Effect of GABA on GnRH neurons switches from depolarization to hyperpolarization at puberty in the female mouse. Endocrinology 143:1459–1466 [DOI] [PubMed] [Google Scholar]
- Bilger M, Heger S, Brann DW, Paredes A, Ojeda SR 2001 A conditional tetracycline-regulated increase in γ amino butyric acid production near luteinizing hormone-releasing hormone nerve terminals disrupts estrous cyclicity in the rat. Endocrinology 142:2102–2114 [DOI] [PubMed] [Google Scholar]
- Sullivan SD, Moenter SM 2005 GABAergic integration of progesterone and androgen feedback to gonadotropin-releasing hormone neurons. Biol Reprod 72:33–41 [DOI] [PubMed] [Google Scholar]
- Chu Z, Moenter SM 2005 Endogenous activation of metabotropic glutamate receptors modulates GABAergic transmission to gonadotropin-releasing hormone neurons and alters their firing rate: a possible local feedback circuit. J Neurosci 25:5740–5749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielecka J, Quaynor SD, Moenter SM 2006 Androgens increase gonadotropin-releasing hormone neuron firing activity in females and interfere with progesterone negative feedback. Endocrinology 147:1474–1479 [DOI] [PubMed] [Google Scholar]
- Christian CA, Moenter SM 2007 Estradiol induces diurnal shifts in GABA transmission to gonadotropin-releasing hormone neurons to provide a neural signal for ovulation. J Neurosci 27:1913–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H, Shannon EM, Fritschy JM, Ojeda SR 1998 Several GABAA receptor subunits are expressed in LHRH neurons of juvenile female rats. Brain Res 780:218–229 [DOI] [PubMed] [Google Scholar]
- Sim JA, Skynner MJ, Pape JR, Herbison AE 2000 Late postnatal reorganization of GABA(A) receptor signalling in native GnRH neurons. Eur J Neurosci 12:3497–3504 [DOI] [PubMed] [Google Scholar]
- Temple JL, Wray S 2005 Developmental changes in GABA receptor subunit composition within the gonadotrophin-releasing hormone-1 neuronal system. J Neuroendocrinol 17:591–599 [DOI] [PubMed] [Google Scholar]
- Morrell MJ, Giudice L, Flynn KL, Seale CG, Paulson AJ, Doñe S, Flaster E, Ferin M, Sauer MV 2002 Predictors of ovulatory failure in women with epilepsy. Ann Neurol 52:704–711 [DOI] [PubMed] [Google Scholar]
- Morrell MJ 1999 Epilepsy in women: the science of why it is special. Neurology 53:S42–S48 [PubMed] [Google Scholar]
- Williams KE, Marsh WK, Rasgon NL 2007 Mood disorders and fertility in women: a critical review of the literature and implications for future research. Hum Reprod Update 13:607–616 [DOI] [PubMed] [Google Scholar]
- Roberts CB, Hemond P, Suter KJ 2008 Synaptic integration in hypothalamic gonadotropin-releasing hormone (GnRH) neurons. Neuroscience 154:1337–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfermann I 1991 Functional studies of cotransmission. Physiol Rev 71:683–732 [DOI] [PubMed] [Google Scholar]
- Nusbaum MP, Blitz DM, Swensen AM, Wood D, Marder E 2001 The roles of co-transmission in neural network modulation. Trends Neurosci 24:146–154 [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno Jr JS, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley Jr WF, Aparicio SA, Colledge WH 2003 The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, Ishibashi Y, Watanabe T, Asada M, Yamada T, Suenaga M, Kitada C, Usuki S, Kurokawa T, Onda H, Nishimura O, Fujino M 2001 Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 411:613–617 [DOI] [PubMed] [Google Scholar]
- Pielecka-Fortuna J, Chu Z, Moenter SM 2008 Kisspeptin acts directly and indirectly to increase GnRH neuron activity and its effects are modulated by estradiol. Endocrinology 149:1979–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE 2005 Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 25:11349–11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA 2005 Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA 102:1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA 2004 A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145:4073–4077 [DOI] [PubMed] [Google Scholar]
- Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, Ghatei MA, Bloom SR 2004 Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol 16:850–858 [DOI] [PubMed] [Google Scholar]
- Seminara SB 2005 Metastin and its G protein-coupled receptor, GPR54: critical pathway modulating GnRH secretion. Front Neuroendocrinol 26:131–138 [DOI] [PubMed] [Google Scholar]
- Dungan HM, Clifton DK, Steiner RA 2006 Minireview: kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology 147:1154–1158 [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Clifton DK, Steiner RA 2007 Emerging ideas about kisspeptin-GPR54 signaling in the neuroendocrine regulation of reproduction. Trends Neurosci 30:504–511 [DOI] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE 2009 Oestrogen, kisspeptin, GPR54 and the pre-ovulatory luteinising hormone surge. J Neuroendocrinol 21:305–311 [DOI] [PubMed] [Google Scholar]
- Oakley AE, Clifton DK, Steiner RA 2009 Kisspeptin signaling in the brain. Endocr Rev 30:713–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA 2005 Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146:3686–3692 [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Navarro VM, Zhao Z, Glidewell-Kenney C, Weiss J, Jameson JL, Clifton DK, Levine JE, Steiner RA 2009 Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. J Neurosci 29:9390–9395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K 2007 Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev 53:367–378 [DOI] [PubMed] [Google Scholar]
- Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA 2006 Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci 26:6687–6694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K 2005 Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology 146:4431–4436 [DOI] [PubMed] [Google Scholar]
- Estrada KM, Clay CM, Pompolo S, Smith JT, Clarke IJ 2006 Elevated KiSS-1 expression in the arcuate nucleus prior to the cyclic preovulatory gonadotropin-releasing hormone/luteinising hormone surge in the ewe suggests a stimulatory role for kisspeptin in oestrogen-positive feedback. J Neuroendocrinol 18:806–809 [DOI] [PubMed] [Google Scholar]
- Smith JT, Li Q, Pereira A, Clarke IJ 2009 Kisspeptin neurons in the ovine arcuate nucleus and preoptic area are involved in the preovulatory luteinizing hormone surge. Endocrinology 150:5530–5538 [DOI] [PubMed] [Google Scholar]
- Dungan HM, Gottsch ML, Zeng H, Gragerov A, Bergmann JE, Vassilatis DK, Clifton DK, Steiner RA 2007 The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. J Neurosci 27:12088–12095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE 2008 Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci 28:8691–8697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielecka-Fortuna J, Moenter SM 2010 Kisspeptin increases γ-aminobutyric acidergic and glutamatergic transmission directly to gonadotropin-releasing hormone neurons in an estradiol-dependent manner. Endocrinology 151:291–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roa J, Vigo E, Castellano JM, Gaytan F, Navarro VM, Aguilar E, Dijcks FA, Ederveen AG, Pinilla L, van Noort PI, Tena-Sempere M 2008 Opposite roles of estrogen receptor (ER)α and ERβ in the modulation of luteinizing hormone responses to kisspeptin in the female rat: implications for the generation of the preovulatory surge. Endocrinology 149:1627–1637 [DOI] [PubMed] [Google Scholar]
- Neal-Perry G, Lebesgue D, Lederman M, Shu J, Zeevalk GD, Etgen AM 2009 The excitatory peptide kisspeptin restores the luteinizing hormone surge and modulates amino acid neurotransmission in the medial preoptic area of middle-aged rats. Endocrinology 150:3699–3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Roepke TA, Kelly MJ, Rønnekleiv OK 2008 Kisspeptin depolarizes gonadotropin-releasing hormone neurons through activation of TRPC-like cationic channels. J Neurosci 28:4423–4434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Lee K, Herbison AE 2008 Kisspeptin excites gonadotropin-releasing hormone neurons through a phospholipase C/calcium-dependent pathway regulating multiple ion channels. Endocrinology 149:4605–4614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, Pineda R, Gottsch ML, Tena-Sempere M, Moenter SM, Terasawa E, Clarke IJ, Steiner RA, Millar RP 2009 Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci 29:3920–3929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda R, Garcia-Galiano D, Roseweir A, Romero M, Sanchez-Garrido MA, Ruiz-Pino F, Morgan K, Pinilla L, Millar RP, Tena-Sempere M 2010 Critical roles of kisspeptins in female puberty and preovulatory gonadotropin surges as revealed by a novel antagonist. Endocrinology 151:722–730 [DOI] [PubMed] [Google Scholar]
- Stephan FK, Berkley KJ, Moss RL 1981 Efferent connections of the rat suprachiasmatic nucleus. Neuroscience 6:2625–2641 [DOI] [PubMed] [Google Scholar]
- Tanaka M, Ichitani Y, Okamura H, Tanaka Y, Ibata Y 1993 The direct retinal projection to VIP neuronal elements in the rat SCN. Brain Res Bull 31:637–640 [DOI] [PubMed] [Google Scholar]
- Horvath TL, Cela V, van der Beek EM 1998 Gender-specific apposition between vasoactive intestinal peptide-containing axons and gonadotrophin-releasing hormone-producing neurons in the rat. Brain Res 795:277–281 [DOI] [PubMed] [Google Scholar]
- Weick RF, Stobie KM 1992 Vasoactive intestinal peptide inhibits the steroid-induced LH surge in the ovariectomized rat. J Endocrinol 133:433–437 [DOI] [PubMed] [Google Scholar]
- Harney JP, Scarbrough K, Rosewell KL, Wise PM 1996 In vivo antisense antagonism of vasoactive intestinal peptide in the suprachiasmatic nuclei causes aging-like changes in the estradiol-induced luteinizing hormone and prolactin surges. Endocrinology 137:3696–3701 [DOI] [PubMed] [Google Scholar]
- van der Beek EM, Swarts HJ, Wiegant VM 1999 Central administration of antiserum to vasoactive intestinal peptide delays and reduces luteinizing hormone and prolactin surges in ovariectomized, estrogen-treated rats. Neuroendocrinology 69:227–237 [DOI] [PubMed] [Google Scholar]
- Gerhold LM, Rosewell KL, Wise PM 2005 Suppression of vasoactive intestinal polypeptide in the suprachiasmatic nucleus leads to aging-like alterations in cAMP rhythms and activation of gonadotropin-releasing hormone neurons. J Neurosci 25:62–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MJ, Jiennes L, Wise PM 2000 Localization of the VIP2 receptor protein on GnRH neurons in the female rat. Endocrinology 141:4317–4320 [DOI] [PubMed] [Google Scholar]
- Christian CA, Moenter SM 2008 Vasoactive intestinal polypeptide can excite gonadotropin-releasing hormone neurons in a manner dependent on estradiol and gated by time of day. Endocrinology 149:3130–3136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhold LM, Wise PM 2006 Vasoactive intestinal polypeptide regulates dynamic changes in astrocyte morphometry: impact on gonadotropin-releasing hormone neurons. Endocrinology 147:2197–2202 [DOI] [PubMed] [Google Scholar]
- Cashion AB, Smith MJ, Wise PM 2003 The morphometry of astrocytes in the rostral preoptic area exhibits a diurnal rhythm on proestrus: relationship to the luteinizing hormone surge and effects of age. Endocrinology 144:274–280 [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW 1987 The distribution of neurotransmitter-specific cells and fibers in the anteroventral periventricular nucleus: implications for the control of gonadotropin secretion in the rat. Brain Res 400:11–34 [DOI] [PubMed] [Google Scholar]
- Kalamatianos T, Kalló I, Goubillon ML, Coen CW 2004 Cellular expression of V1A vasopressin receptor mRNA in the female rat preoptic area: effects of oestrogen. J Neuroendocrinol 16:525–533 [DOI] [PubMed] [Google Scholar]
- Palm IF, Van Der Beek EM, Wiegant VM, Buijs RM, Kalsbeek A 1999 Vasopressin induces a luteinizing hormone surge in ovariectomized, estradiol-treated rats with lesions of the suprachiasmatic nucleus. Neuroscience 93:659–666 [DOI] [PubMed] [Google Scholar]
- Palm IF, van der Beek EM, Wiegant VM, Buijs RM, Kalsbeek A 2001 The stimulatory effect of vasopressin on the luteinizing hormone surge in ovariectomized, estradiol-treated rats is time-dependent. Brain Res 901:109–116 [DOI] [PubMed] [Google Scholar]
- Miller BH, Olson SL, Levine JE, Turek FW, Horton TH, Takahashi JS 2006 Vasopressin regulation of the proestrous luteinizing hormone surge in wild-type and Clock mutant mice. Biol Reprod 75:778–784 [DOI] [PubMed] [Google Scholar]
- Lee EJ, Moore CT, Hosny S, Centers A, Jennes L 2000 Expression of estrogen receptor-α and c-Fos in adrenergic neurons of the female rat during the steroid-induced LH surge. Brain Res 875:56–65 [DOI] [PubMed] [Google Scholar]
- Rosner JM, Nagle CA, de Laborde NP, Pedroza E, Badano A, Figueroa Casas PR, Carril M 1976 Plasma levels of norepinephrine (NE) during the periovulatory period and after LH-RH stimulation in women. Am J Obstet Gynecol 124:567–572 [DOI] [PubMed] [Google Scholar]
- Gallo RV, Drouva SV 1979 Effect of intraventricular infusion of catecholamines on luteinizing hormone release in ovariectomized and ovariectomized, steroid-primed rats. Neuroendocrinology 29:149–162 [DOI] [PubMed] [Google Scholar]
- Ferris CF, Pan JX, Singer EA, Boyd ND, Carraway RE, Leeman SE 1984 Stimulation of luteinizing hormone release after stereotaxic microinjection of neurotensin into the medial preoptic area of rats. Neuroendocrinology 38:145–151 [DOI] [PubMed] [Google Scholar]
- Wise PM 1984 Estradiol-induced daily luteinizing hormone and prolactin surges in young and middle-aged rats: correlations with age-related changes in pituitary responsiveness and catecholamine turnover rats in microdissected brain areas. Endocrinology 115:801–809 [DOI] [PubMed] [Google Scholar]
- Domañski E, Chomicka LK, Ostrowska A, Gajewska A, Mateusiak K 1991 Release of luteinizing hormone-releasing hormone, β-endorphin and noradrenaline by the nucleus infundibularis/median eminence during periovulatory period in the sheep. Neuroendocrinology 54:151–158 [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Scott CJ, Pereira A, Rawson J 1999 Levels of dopamine β hydroxylase immunoreactivity in the preoptic hypothalamus of the ovariectomised ewe following injection of oestrogen: evidence for increased noradrenaline release around the time of the oestrogen-induced surge in luteinizing hormone. J Neuroendocrinol 11:503–512 [DOI] [PubMed] [Google Scholar]
- Pau KY, Hess DL, Kohama S, Bao J, Pau CY, Spies HG 2000 Oestrogen upregulates noradrenaline release in the mediobasal hypothalamus and tyrosine hydroxylase gene expression in the brainstem of ovariectomized rhesus macaques. J Neuroendocrinol 12:899–909 [DOI] [PubMed] [Google Scholar]
- Temel S, Lin W, Lakhlani S, Jennes L 2002 Expression of estrogen receptor-α and cFos in norepinephrine and epinephrine neurons of young and middle-aged rats during the steroid-induced luteinizing hormone surge. Endocrinology 143:3974–3983 [DOI] [PubMed] [Google Scholar]
- Han SK, Herbison AE 2008 Norepinephrine suppresses gonadotropin-releasing hormone neuron excitability in the adult mouse. Endocrinology 149:1129–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitome M, Shirakawa T, Oshima S, Nakamura W, Oguchi H 2001 Circadian rhythm of nitric oxide production in the dorsal region of the suprachiasmatic nucleus in rats. Neurosci Lett 303:161–164 [DOI] [PubMed] [Google Scholar]
- Ferreyra GA, Cammarota MP, Golombek DA 1998 Photic control of nitric oxide synthase activity in the hamster suprachiasmatic nuclei. Brain Res 797:190–196 [DOI] [PubMed] [Google Scholar]
- Reuss S, Decker K, Rösseler L, Layes E, Schollmayer A, Spessert R 1995 Nitric oxide synthase in the hypothalamic suprachiasmatic nucleus of rat: evidence from histochemistry, immunohistochemistry and Western blot; and colocalization with VIP. Brain Res 695:257–262 [DOI] [PubMed] [Google Scholar]
- Grossman AB, Rossmanith WG, Kabigting EB, Cadd G, Clifton D, Steiner RA 1994 The distribution of hypothalamic nitric oxide synthase mRNA in relation to gonadotrophin-releasing hormone neurons. J Endocrinol 140:R5–R8 [DOI] [PubMed] [Google Scholar]
- Bonavera JJ, Sahu A, Kalra PS, Kalra SP 1993 Evidence that nitric oxide may mediate the ovarian steroid-induced luteinizing hormone surge: involvement of excitatory amino acids. Endocrinology 133:2481–2487 [DOI] [PubMed] [Google Scholar]
- Prevot V, Croix D, Rialas CM, Poulain P, Fricchione GL, Stefano GB, Beauvillain JC 1999 Estradiol coupling to endothelial nitric oxide stimulates gonadotropin-releasing hormone release from rat median eminence via a membrane receptor. Endocrinology 140:652–659 [DOI] [PubMed] [Google Scholar]
- Clasadonte J, Poulain P, Beauvillain JC, Prevot V 2008 Activation of neuronal nitric oxide release inhibits spontaneous firing in adult gonadotropin-releasing hormone neurons: a possible local synchronizing signal. Endocrinology 149:587–596 [DOI] [PubMed] [Google Scholar]
- Pu S, Kalra PS, Kalra SP 1998 Ovarian steroid-independent diurnal rhythm in cyclic GMP/nitric oxide efflux in the medial preoptic area: possible role in preovulatory and ovarian steroid-induced LH surge. J Neuroendocrinol 10:617–625 [DOI] [PubMed] [Google Scholar]
- Knauf C, Ferreira S, Hamdane M, Mailliot C, Prevot V, Beauvillain JC, Croix D 2001 Variation of endothelial nitric oxide synthase synthesis in the median eminence during the rat estrous cycle: an additional argument for the implication of vascular blood vessel in the control of GnRH release. Endocrinology 142:4288–4294 [DOI] [PubMed] [Google Scholar]
- d'Anglemont de Tassigny X, Campagne C, Steculorum S, Prevot V 2009 Estradiol induces physical association of neuronal nitric oxide synthase with NMDA receptor and promotes nitric oxide formation via estrogen receptor activation in primary neuronal cultures. J Neurochem 109:214–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Anglemont de Tassigny X, Campagne C, Dehouck B, Leroy D, Holstein GR, Beauvillain JC, Buée-Scherrer V, Prevot V 2007 Coupling of neuronal nitric oxide synthase to NMDA receptors via postsynaptic density-95 depends on estrogen and contributes to the central control of adult female reproduction. J Neurosci 27:6103–6114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE, Theodosis DT 1992 Localization of oestrogen receptors in preoptic neurons containing neurotensin but not tyrosine hydroxylase, cholecystokinin or luteinizing hormone-releasing hormone in the male and female rat. Neuroscience 50:283–298 [DOI] [PubMed] [Google Scholar]
- Alexander MJ, Kiraly ZJ, Leeman SE 1991 Sexually dimorphic distribution of neurotensin/neuromedin N mRNA in the rat preoptic area. J Comp Neurol 311:84–96 [DOI] [PubMed] [Google Scholar]
- McCann SM, Vijayan E 1992 Control of anterior pituitary hormone secretion by neurotensin. Ann NY Acad Sci 668:287–297 [DOI] [PubMed] [Google Scholar]
- Motta M, Martini L 1981 Neurotensin inhibits LH release. Proc Soc Exp Biol Med 168:62–64 [DOI] [PubMed] [Google Scholar]
- Akema T, Praputpittaya C, Kimura F 1987 Effects of preoptic microinjection of neurotensin on luteinizing hormone secretion in unanesthetized ovariectomized rats with or without estrogen priming. Neuroendocrinology 46:345–349 [DOI] [PubMed] [Google Scholar]
- Alexander MJ, Mahoney PD, Ferris CF, Carraway RE, Leeman SE 1989 Evidence that neurotensin participates in the central regulation of the preovulatory surge of luteinizing hormone in the rat. Endocrinology 124:783–788 [DOI] [PubMed] [Google Scholar]
- Watanobe H, Takebe K 1993 In vivo release of neurotensin from the median eminence of ovariectomized estrogen-primed rats as estimated by push-pull perfusion: correlation with luteinizing hormone and prolactin surges. Neuroendocrinology 57:760–764 [DOI] [PubMed] [Google Scholar]
- Alexander MJ, Dobner PR, Miller MA, Bullock BP, Dorsa DM, Leeman SE 1989 Estrogen induces neurotensin/neuromedin N messenger ribonucleic acid in a preoptic nucleus essential for the preovulatory surge of luteinizing hormone in the rat. Endocrinology 125:2111–2117 [DOI] [PubMed] [Google Scholar]
- Smith MJ, Wise PM 2001 Neurotensin gene expression increases during proestrus in the rostral medial preoptic nucleus: potential for direct communication with gonadotropin-releasing hormone neurons. Endocrinology 142:3006–3013 [DOI] [PubMed] [Google Scholar]
- Dungan Lemko HM, Naderi R, Adjan V, Jennes L, Navarro VM, Clifton D, Steiner RA 2010 Interactions between neurotensin and GnRH neurons in the positive feedback control of GnRH/LH secretion in the mouse. Am J Physiol Endocrinol Metab 298:E80–E88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePaolo LV, King RA, Carrillo AJ 1987 In vivo and in vitro examination of an autoregulatory mechanism for luteinizing hormone-releasing hormone. Endocrinology 120: 272–279 [DOI] [PubMed] [Google Scholar]
- Valença MM, Johnston CA, Ching M, Negro-Vilar A 1987 Evidence for a negative ultrashort loop feedback mechanism operating on the luteinizing hormone-releasing hormone neuronal system. Endocrinology 121:2256–2259 [DOI] [PubMed] [Google Scholar]
- Bedran de Castro JC, Khorram O, McCann SM 1985 Possible negative ultra-short loop feedback of luteinizing hormone releasing hormone (LHRH) in the ovariectomized rat. Proc Soc Exp Biol Med 179:132–135 [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Evans NP, Dahl GE, McFadden KL, Mauger DT, Karsch FJ 1995 Evidence for short or ultrashort loop negative feedback of gonadotropin-releasing hormone secretion. Neuroendocrinology 62:248–258 [DOI] [PubMed] [Google Scholar]
- Xu C, Xu XZ, Nunemaker CS, Moenter SM 2004 Dose-dependent switch in response of gonadotropin-releasing hormone (GnRH) neurons to GnRH mediated through the type I GnRH receptor. Endocrinology 145:728–735 [DOI] [PubMed] [Google Scholar]
- Xu C, Roepke TA, Zhang C, Rønnekleiv OK, Kelly MJ 2008 Gonadotropin-releasing hormone (GnRH) activates the M-current in GnRH neurons: an autoregulatory negative feedback mechanism? Endocrinology 149:2459–2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Moenter SM 2009 GABAergic transmission to gonadotropin-releasing hormone (GnRH) neurons is regulated by GnRH in a concentration-dependent manner engaging multiple signaling pathways. J Neurosci 29:9809–9818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todman MG, Han SK, Herbison AE 2005 Profiling neurotransmitter expression in mouse gonadotropin-releasing hormone neurons using green fluorescent protein-promoter transgenics and microarrays. Neuroscience 132:703–712 [DOI] [PubMed] [Google Scholar]
- Kerdelhué B, Parnet P, Lenoir V, Schirar A, Gaudoux F, Levasseur MC, Palkovits M, Blacker C, Scholler R 1988 Interactions between 17 β-estradiol and the hypothalamo-pituitary β-endorphin system in the regulation of the cyclic LH secretion. J Steroid Biochem 30:161–168 [DOI] [PubMed] [Google Scholar]
- Bohler Jr HC, Tracer H, Merriam GR, Petersen SL 1991 Changes in proopiomelanocortin messenger ribonucleic acid levels in the rostral periarcuate region of the female rat during the estrous cycle. Endocrinology 128:1265–1269 [DOI] [PubMed] [Google Scholar]
- Petersen SL, Keller ML, Carder SA, McCrone S 1993 Differential effects of estrogen and progesterone on levels of POMC mRNA levels in the arcuate nucleus: relationship to the timing of LH surge release. J Neuroendocrinol 5:643–648 [DOI] [PubMed] [Google Scholar]
- Scarbrough K, Jakubowski M, Levin N, Wise PM, Roberts JL 1994 The effect of time of day on levels of hypothalamic proopiomelanocortin primary transcript, processing intermediate and messenger ribonucleic acid in proestrous and estrous rats. Endocrinology 134:555–561 [DOI] [PubMed] [Google Scholar]
- Conover CD, Kuljis RO, Rabii J, Advis JP 1993 β-Endorphin regulation of luteinizing hormone-releasing hormone release at the median eminence in ewes: immunocytochemical and physiological evidence. Neuroendocrinology 57:1182–1195 [DOI] [PubMed] [Google Scholar]
- Walsh JP, Clarke IJ 1998 Blockade of the oestrogen-induced luteinizing hormone surge in ovariectomized ewes by a highly selective opioid μ-receptor agonist: evidence for site of action. Neuroendocrinology 67:164–170 [DOI] [PubMed] [Google Scholar]
- Taylor JA, Goubillon ML, Broad KD, Robinson JE 2007 Steroid control of gonadotropin-releasing hormone secretion: associated changes in pro-opiomelanocortin and preproenkephalin messenger RNA expression in the ovine hypothalamus. Biol Reprod 76:524–531 [DOI] [PubMed] [Google Scholar]
- Walsh JP, Rao A, Simmons DM, Clarke IJ 1998 Proopiomelanocortin mRNA levels in ovine hypothalamus are not reduced at the time of the preovulatory luteinising hormone surge. J Neuroendocrinol 10:803–808 [DOI] [PubMed] [Google Scholar]
- Goodman RL, Coolen LM, Anderson GM, Hardy SL, Valent M, Connors JM, Fitzgerald ME, Lehman MN 2004 Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology 145:2959–2967 [DOI] [PubMed] [Google Scholar]
- Mitchell V, Prevot V, Jennes L, Aubert JP, Croix D, Beauvillain JC 1997 Presence of μ and κ opioid receptor mRNAs in galanin but not in GnRH neurons in the female rat. Neuroreport 8:3167–3172 [DOI] [PubMed] [Google Scholar]
- Sannella MI, Petersen SL 1997 Dual label in situ hybridization studies provide evidence that luteinizing hormone-releasing hormone neurons do not synthesize messenger ribonucleic acid for μ, κ, or δ opiate receptors. Endocrinology 138:1667–1672 [DOI] [PubMed] [Google Scholar]
- Simerly RB 1991 Prodynorphin and proenkephalin gene expression in the anteroventral periventricular nucleus of the rat: sexual differentiation and hormonal regulation. Mol Cell Neurosci 2:473–484 [DOI] [PubMed] [Google Scholar]
- Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O'Rahilly S, Semple RK 2009 TAC3 and TACR3 mutation in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet 41:354–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guran T, Tolhurst G, Bereket A, Rocha N, Porter K, Turan S, Gribble FM, Kotan LD, Akcay T, Atay Z, Canan H, Serin A, O'Rahilly S, Reimann F, Semple RK, Topaloglu AK 2009 Hypogonadotropic hypogonadism due to a novel missense mutation in the first extracellular loop of the neurokinin B receptor. J Clin Endocrinol Metab 94:3633–3639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu A, Kalra SP 1992 Effects of tachykinins on luteinizing hormone release in female rats: potent inhibitory action of neuropeptide K. Endocrinology 130:1571–1577 [DOI] [PubMed] [Google Scholar]
- Sandoval-Guzmán T, Rance NE 2004 Central injection of senktide, an NK3 receptor agonist, or neuropeptide Y inhibits LH secretion and induces different patterns of Fos expression in the rat hypothalamus. Brain Res 1026:307–312 [DOI] [PubMed] [Google Scholar]
- Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA 2009 Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 29:11859–11866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goubillon ML, Forsdike RA, Robinson JE, Ciofi P, Caraty A, Herbison AE 2000 Identification of neurokinin B-expressing neurons as an highly estrogen-receptive, sexually dimorphic cell group in the ovine arcuate nucleus. Endocrinology 141:4218–4225 [DOI] [PubMed] [Google Scholar]
- Pillon D, Caraty A, Fabre-Nys C, Bruneau G 2003 Short-term effect of oestradiol on neurokinin B mRNA expression in the infundibular nucleus of ewes. J Neuroendocrinol 15:749–753 [DOI] [PubMed] [Google Scholar]
- Foradori CD, Amstalden M, Goodman RL, Lehman MN 2006 Colocalisation of dynorphin a and neurokinin B immunoreactivity in the arcuate nucleus and median eminence of the sheep. J Neuroendocrinol 18:534–541 [DOI] [PubMed] [Google Scholar]
- Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ 2007 Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology 148:5752–5760 [DOI] [PubMed] [Google Scholar]
- Amstalden M, Coolen LM, Hemmerle AM, Billings HJ, Connors JM, Goodman RL, Lehman MN 2010 Neurokinin 3 receptor immunoreactivity in the septal region, preoptic area and hypothalamus of the female sheep: colocalisation in neurokinin B cells of the arcuate nucleus but not in gonadotrophin-releasing hormone neurones. J Neuroendocrinol 22:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE 2005 Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. J Comp Neurol 489:372–386 [DOI] [PubMed] [Google Scholar]
- Ohkura S, Wakabayashi Y, Nakada T, Murata K, Navarro VM, Mori Y, Tsukamura H, Maeda K-I, Steiner RA, Okamura H, Functional significance of dynorphin as a cotransmitter in kisspeptin neurons in the regulation of pulsatile GnRH secretion. Proc of 39th Annual Meeting of the Society for Neuroscience, Chicago, IL, 2009 (Abstract 665.9) [Google Scholar]
- Wakabayashi Y, Nakada T, Murata K, Ohkura S, Navarro VM, Mori Y, Tsukamura H, Maeda K-I, Steiner RA, Okamura H, Neurokinin B as a cotransmitter in kisspeptin neurons in the regulation of pulsatile GnRH secretion. Proc of 39th Annual Meeting of the Society for Neuroscience, Chicago, IL, 2009 (Abstract 665.10) [Google Scholar]
- Bentley GE, Ubuka T, McGuire NL, Calisi R, Perfito N, Kriegsfeld LJ, Wingfield JC, Tsutsui K 2009 Gonadotrophin-inhibitory hormone: a multifunctional neuropeptide. J Neuroendocrinol 21:276–281 [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Qi Y, Puspita Sari I, Smith JT 2009 Evidence that RF-amide related peptides are inhibitors of reproduction in mammals. Front Neuroendocrinol 30:371–378 [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ 2006 Driving reproduction: RFamide peptides behind the wheel. Horm Behav 50:655–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui K, Saigoh E, Ukena K, Teranishi H, Fujisawa Y, Kikuchi M, Ishii S, Sharp PJ 2000 A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem Biophys Res Commun 28:661–667 [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, Ukena K, Tsutsui K, Silver R 2006 Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci USA 103:2410–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Matsuzaki T, Iwasa T, Yasui T, Irahara M, Osugi T, Tsutsui K 2008 Hypophysiotropic role of RFamide-related peptide-3 in the inhibition of LH secretion in female rats. J Endocrinol 199:105–112 [DOI] [PubMed] [Google Scholar]
- Rizwan MZ, Porteous R, Herbison AE, Anderson GM 2009 Cells expressing RFamide-related peptide-1/3, the mammalian gonadotropin-inhibitory hormone orthologs, are not hypophysiotropic neuroendocrine neurons in the rat. Endocrinology 150:1413–1420 [DOI] [PubMed] [Google Scholar]
- Ducret E, Anderson GM, Herbison AE 2009 RFamide-related peptide-3, a mammalian gonadotropin-inhibitory hormone ortholog, regulates gonadotropin-releasing hormone neuron firing in the mouse. Endocrinology 150:2799–2804 [DOI] [PubMed] [Google Scholar]
- Wu M, Dumalska I, Morozova E, van den Pol AN, Alreja M 2009 Gonadotropin inhibitory hormone inhibits basal forebrain vGluT2-gonadotropin-releasing hormone neurons via a direct postsynaptic mechanism. J Physiol 587:1401–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EM, Humber SA, Jain S, Williams 3rd WP, Zhao S, Bentley GE, Tsutsui K, Kriegsfeld LJ 2008 Alterations in RFamide-related peptide expression are coordinated with the preovulatory luteinizing hormone surge. Endocrinology 149:4958–4969 [DOI] [PMC free article] [PubMed] [Google Scholar]