Abstract
The melanocortin-4 receptor (MC4R) was cloned in 1993 by degenerate PCR; however, its function was unknown. Subsequent studies suggest that the MC4R might be involved in regulating energy homeostasis. This hypothesis was confirmed in 1997 by a series of seminal studies in mice. In 1998, human genetic studies demonstrated that mutations in the MC4R gene can cause monogenic obesity. We now know that mutations in the MC4R are the most common monogenic form of obesity, with more than 150 distinct mutations reported thus far. This review will summarize the studies on the MC4R, from its cloning and tissue distribution to its physiological roles in regulating energy homeostasis, cachexia, cardiovascular function, glucose and lipid homeostasis, reproduction and sexual function, drug abuse, pain perception, brain inflammation, and anxiety. I will then review the studies on the pharmacology of the receptor, including ligand binding and receptor activation, signaling pathways, as well as its regulation. Finally, the pathophysiology of the MC4R in obesity pathogenesis will be reviewed. Functional studies of the mutant MC4Rs and the therapeutic implications, including small molecules in correcting binding and signaling defect, and their potential as pharmacological chaperones in rescuing intracellularly retained mutants, will be highlighted.
The aim of this review is to summarize the studies on the melanocortin-4 receptor (MC4R), from its cloning and tissue distribution, to its physiological roles in regulating energy homeostasis, cardiovascular function, glucose and lipid homeostasis, reproduction and sexual function, and others. The pharmacology of the receptor, including ligand binding and receptor activation, signaling pathways, as well as its regulation, is also summarized. Mutations in the MC4R are the most common monogenic form of obesity. Functional studies of the mutant MC4Rs and the therapeutic implications, including small molecules acting as pharmacological chaperones in rescuing intracellularly retained mutants, are highlighted.
I. Introduction
- II. Molecular Cloning and Localization of the Melanocortin-4 Receptor
- A. Molecular cloning
- B. Tissue distribution
- III. Physiology of the Melanocortin-4 Receptor
- A. Energy homeostasis
- B. Cachexia
- C. Cardiovascular function
- D. Glucose and lipid homeostasis
- E. Reproduction and sexual function
- F. Miscellaneous functions of the MC4R
- IV. Pharmacology of the Melanocortin-4 Receptor
- A. Ligand binding and receptor activation
- B. Signaling pathways
- C. Internalization, desensitization, and dimerization
- V. Pathophysiology of the Melanocortin-4 Receptor
- A. Naturally occurring MC4R mutations
- B. Molecular classification of the MC4R mutants
- C. Therapeutic implications
VI. Conclusions and Future Directions
I. Introduction
The melanocortin system consists of several agonists, two antagonists, and five receptors. The agonists, including α-MSH, β-MSH, γ-MSH, and ACTH, as well as some less studied peptides such as γ3-MSH and desacetyl-α-MSH, are all derived from tissue-specific posttranslational processing of a pre-prohormone, pro-opiomelanocortin (POMC) (1,2). In the anterior pituitary gland, POMC is processed into ACTH and other peptides by prohormone convertase (PC) 1. In lower vertebrates such as fish and amphibians, as well as during the fetal and infantile periods in humans, PC2 expressed in the pars intermedia also causes the release of MSH. In skin and hair follicles as well as in the brain (hypothalamus and brainstem), POMC is further processed by PC2 into MSH. POMC is an ancient gene, with expression in sea lamprey, the most ancient vertebrate, suggesting that it existed 700 million years ago (3,4).
The melanocortin system is unique in that two endogenous antagonists, Agouti and Agouti-related peptide (AgRP), exist. So far, no other endogenous antagonists have been identified in other G protein-coupled receptor (GPCR) systems. There are also several other ancillary proteins, such as mahogany and syndecan-3, that modulate receptor function by interacting with Agouti and AgRP (5).
Five melanocortin receptors (MCRs) mediate the diverse actions of these melanocortins. They are numbered MC1R to MC5R according to the sequence of their cloning. After the cloning of the MC1R (6,7) and MC2R (6), three additional MCRs, the MC3R, the MC4R, and the MC5R, were cloned. The MC1R is the classical MSH receptor expressed in skin and hair follicles that regulates pigmentation. The MC2R is the classical ACTH receptor expressed in the adrenal cortex that regulates adrenal steroidogenesis and cell proliferation. The MC3R and the MC4R are expressed primarily in the central nervous system, and are therefore referred to as the neural MCRs. The MC5R is expressed widely, especially in exocrine glands. Knockout experiments showed that the MC5R is involved in regulating exocrine gland secretions (8). For general reviews on the melanocortin system, the reader is referred to several excellent review articles (5,9,10,11,12).
Both MC3R and MC4R are involved in regulating energy homeostasis. The role of the MC3R in regulating food intake is controversial. Data from knockout animals revealed that Mc3r knockout results in increased feed efficiency and adiposity (13,14). Recently, it was shown that the MC3R “is required for entrainment to meal intake” (15). The MC4R regulates both food intake and energy expenditure and is the focus of this article.
II. Molecular Cloning and Localization of the Melanocortin-4 Receptor
A. Molecular cloning
The groups of Gantz and Cone (16,17) independently cloned the human MC4R (hMC4R) through degenerate PCR and homology screening. The human MC4R is an intronless gene with an open reading frame of 999 bp that encodes a protein of 332 amino acids. Alignment of MC4R with other MCRs showed that it has the highest homology with the MC3R, with 58% identity and 76% similarity. By fluorescent in situ hybridization, the MC4R gene was localized to chromosome 18q21.3 (16,18).
Since the cloning of hMC4R, the MC4R has been cloned from mouse, rat, hamster, guinea pig, dog, cat, fox, pig, sheep, cow, and several primates including marmoset, cynomolgus macaque, vervet monkey, and orangutan (see http://www.gpcr.org/7tm/classes/melanocortin-type-4/proteins/). It has also been cloned from several nonmammalian species including fish, chicken, and pigeon. The amino acid sequences between the different species are highly conserved (19,20). For example, there is 93% identity between rat and human MC4Rs and 87% identity between chicken and human MC4Rs (21). Even the zebrafish and spiny dogfish MC4Rs share 71% identity with hMC4R and more than 90% in the transmembrane domains (TMs) (22,23,24). Evolutionary analyses of MC4R sequences from many different species showed that the MC4R has been subject to high levels of continuous purifying selection with codon usage bias, leading to the unusually low levels of silent polymorphisms in humans (20).
The MC4R is a member of family A GPCRs with seven TMs connected by alternating extracellular loops (ELs) and intracellular loops. The N terminus is extracellular, and the C terminus is intracellular. The MC4R, like the other MCRs, has some unique features compared with other family A GPCRs (Fig. 1). For example, the highly conserved disulfide bond linking the top of TM3 and EL2 is missing in the MC4R, although there is an intraloop disulfide bond in EL3 (25). The intracellular loops and ELs are short, especially EL2, making MC4R one of the shortest members in the GPCR superfamily. Highly conserved Pro in TM5 and Asn in TM7 (in the NPxxY motif, N7.49) in family A GPCRs are substituted by a Met and an Asp, respectively, in the MC4R. In Fig. 1, the demarcations of the TMs for hMC4R are based on the crystal structure of rhodopsin (26). [According to the numbering scheme of Ballesteros and Weinstein (27), the most highly conserved residue in each TM is defined as residue 50, preceded by the helix number. Other residues in the same TM are numbered according to their relative position to the most conserved residue. For example, in TM7, Pro in the NPxxY motif is the most highly conserved, therefore numbered as 7.50. Asn is one residue before the Pro, therefore numbered as 7.49. This numbering scheme facilitates the comparison of results obtained from different members of family A GPCRs, and is used herein when appropriate.]
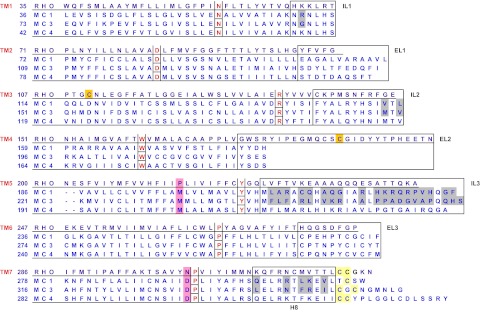
Figure 1.
Sequence alignment of bovine rhodopsin (RHO) with human MC1R, MC3R, and MC4R. The ELs and intracellular loops are boxed (note that the C terminus of rhodopsin is not complete). The divergent N termini are not shown here. The most conserved residues in each TM (50 residue) are in red and are boxed individually. The highly conserved Cys residues at the top of TM3 and EL2 in rhodopsin that form a disulfide bridge are highlighted in gold. The potential palmitoylation sites at the C termini are shaded in light yellow. In MCRs, TM5 is two residues shorter than rhodopsin; otherwise, EL2 would consist of only two residues, which is not likely. Note the following unique characteristics of the MCRs, including the absence of the highly conserved disulfide bridge between the EL2 and the top of TM3 (shaded in gold for rhodopsin). The intracellular loops and ELs of the MCRs are short, especially EL2, making MCRs some of the shortest members in the GPCR superfamily. Highly conserved Pro in TM5 and Asn in TM7 (in the NPxxY motif) in family A GPCRs are substituted by a Met and an Asp, respectively, in all MCRs (shaded in rose). In other family A GPCRs, this Asn is proposed to interact with the highly conserved Asp in TM2. In MCRs, Asp is present at both loci, unlikely to interact with each other. GenBank accession numbers for the genes are: bovine rhodopsin, NM_001014890; human MC1R, NM_002386; human MC3R, NM_019888; and human MC4R, NM_005912. IL, Intracellular loop.
There are four potential N-linked glycosylation sites (three at the N terminus: Asn3, Asn17, and Asn26; and one at EL1: Asn108). Although it is known that the MC4R is glycosylated, there are no reports on the experimental identification of the site(s) that is (are) indeed glycosylated. The exact functions of the glycosylation are also unknown. Two conserved Cys residues at the C terminus, Cys318 and Cys319, might serve as sites for palmitoylation anchoring the C terminus to the plasma membrane, forming a fourth intracellular loop. It is not known whether the MC4R is indeed palmitoylated or where this modification is located.
B. Tissue distribution
In the original cloning article of Gantz et al. (16), through Northern blotting of canine mRNAs, it was shown that the MC4R is primarily expressed in brain. By in situ hybridization in mouse brain sections, it was shown that the MC4R mRNA is localized in regions of the thalamus, hypothalamus, and hippocampus. Extensive labeling of the MC4R was found in CA1 and CA2 but not CA3 and CA4 regions of the hippocampus. The MC4R mRNA is also present in dentate gyrus, cortex, and amygdala (16).
Extensive localization studies on rat brain showed that the MC4R mRNA is widely expressed in adult rat brain, including cortex, thalamus, hypothalamus, brainstem, and spinal cord (17,28,29). In the hypothalamus, it is expressed highly in paraventricular nucleus (PVN), including both parvicellular and magnocellular neurons. A mouse line expressing green fluorescent protein under the control of the MC4R promoter showed similar distribution of green fluorescent protein as observed with in situ hybridization technique (30).
In addition to neurons, astrocytes were also reported to express the MC4R (31,32). Endogenous and synthetic peptide ligands as well as small molecule agonists can increase cAMP accumulation in rat astrocytes (31), consistent with earlier studies demonstrating that α-MSH and ACTH increase cAMP levels in astroglial cells (33,34) and melanocortins stimulate proliferation and induce morphological changes in cultured rat astrocytes (35). ACTH1–24 was also shown to down-regulate the expression of ciliary neurotrophic factor mRNA in rat astrocytes cultured in vitro (36). Very recently, it was suggested that functional MC4R is also expressed in human epidermal melanocytes that might contribute to melanogenesis (37).
Developmentally, it was shown that the MC4R mRNA is first expressed at embryonic day (E) 14 in diencephalon, telencephalon, lamina terminalis, and spinal trigeminal nucleus in the rat. By E19, the MC4R is widely expressed in many regions of the brain (38). The highest level of expression is in the autonomic nervous system. Similar data were obtained when radiolabeled [Nle4, D-Phe7]-MSH (NDP-MSH) was used as the probe (39). Although NDP-MSH also binds to the MC3R, the binding sites detected with radiolabeled NDP-MSH likely represent MC4R because the MC3R mRNA is only expressed postnatally, not during the fetal period (40). In addition, the MC4R mRNA is also expressed in several peripheral tissues during the fetal period, including the developing heart (E14), lung (E16), muscles involved in respiration such as diaphragm and intercostal muscle (E14), as well as other muscles (41). Although the MC4R mRNA cannot be detected in the adrenal gland, liver, pituitary gland, mesenteric fat, and spleen in the adult rat, it is expressed in heart, lung, kidney, and testis during the fetal period (41). The potential physiological functions of the MC4R in these organs remain to be elucidated. The expression of the MC4R (and its ontogeny) is different from that of the MC3R. The MC3R is expressed primarily within the hypothalamus, with limited expression in the thalamus and brainstem (42).
In nonmammalian species, the MC4R is also abundantly expressed in brain. In the spiny dogfish, it is not expressed in peripheral tissues such as eye, kidney, liver, heart, and muscle (23). However, in some other fish, the MC4R is also expressed in certain peripheral tissues, including eye, ovaries, and gastrointestinal tract in the zebrafish; ovary in the goldfish; and liver, ovary, and testis in the flounder (22,43,44). In the sea bass, in addition to the brain, the MC4R mRNA is expressed in retina and pituitary gland as well as liver, fat, testis, and white muscle at lower levels but not in spleen, gill, intestine, skin, red muscle, heart, and ovary (45). The physiological functions of the MC4Rs in these tissues are not known.
III. Physiology of the Melanocortin-4 Receptor
A. Energy homeostasis
1. Rodent studies
Earlier studies showed that intracerebroventricular (ICV) administration of α-MSH and ACTH decreases food intake in rats (46,47). However, “these studies did not receive the attention they deserved” (10) and the receptor mediating this effect was not known. A renaissance came after the molecular cloning of the MCRs. As mentioned earlier, the MC4R was first cloned by degenerate PCR and homology screening with unknown physiological functions. In 1997, several landmark studies were published that established the critical importance of the MC4R in regulating energy homeostasis.
Based on their studies demonstrating that Agouti, in addition to antagonizing the MC1R, is also an antagonist of the MC4R (48) and the fact that in Ay mice, the well-known obesity mouse model, Agouti is ubiquitously expressed including in the hypothalamus (49), Cone and colleagues (50) hypothesized that the obesity in Ay mice might be due to Agouti antagonizing a central MCR. They showed that ICV administration of MTII (melanotan II) inhibits the hyperphagia in four different mouse models, and this inhibition was blocked by coadministration of SHU9119 (50). [MTII is a superpotent and stable cyclic analog of α-MSH, and SHU9119 is a high-affinity cyclic antagonist of MC3R and MC4R (51).] Unlike leptin, which is not effective in common obesity due to leptin resistance, the effect of MC4R agonism on food intake and body weight is more pronounced in obese than in lean rats (52,53,54). Administration of SHU9119 alone increases feeding (50), even in diet-induced obesity, therefore exacerbating the obesity (55). These experiments supported their hypothesis suggesting that hypothalamic melanocortinergic neurons exert a tonic inhibition on feeding, and the disruption of this mechanism might be responsible for the obesity in Ay mice (50). Injection of these compounds directly into the PVN achieved even more potent alterations in food intake, suggesting that neurons in the PVN, which express very high levels of the MC4R (17), are primary sites of action in melanocortin regulation of feeding behavior (56). The regulation of food intake by these ligands is not due to aversive effects (57), although the MC4R is expressed in brainstem parabrachial neurons (29,58). The suppression of food intake by MTII is due to reduced meal duration and meal size (59,60).
The use of more specific MC4R antagonists such as HS014 and HS024 provided further supporting evidence that MC4R is important in regulating food intake. For example, ICV infusions of these antagonists stimulate feeding in satiated rats, and long-term infusion leads to increased food intake and body weight (61,62,63,64,65,66).
Recently, studies on an enzyme that inactivates α-MSH, prolylcarboxypeptidase, provided further supporting evidence of the importance of maintaining normal levels of active α-MSH in body weight regulation (67). Inhibition of enzyme activity (resulting in increased α-MSH levels) decreases food intake. Mice lacking this enzyme gene are leaner and shorter and are resistant to high-fat diet-induced obesity (67).
Another landmark study published in 1997 is the report of the Mc4r knockout mouse model that provided the definitive evidence that the MC4R is critical for regulating energy homeostasis in mice (68). The homozygous knockout mice have maturity-onset obesity, hyperphagia, increased linear growth, hyperinsulinemia, and hyperglycemia. The heterozygous mice have intermediate body weights compared with the wild-type (WT) littermates and homozygous mice, suggesting that there is a gene dosage effect (68). Mc4r knockout mice have delayed meal termination and reduced sensitivity to cholecystokinin (CCK) (69,70). The obesity in Mc4r knockout mice is exacerbated when the mice are fed a high-fat diet because, unlike the WT mice, which respond to an increase in the fat content of the diet by rapidly increasing diet-induced thermogenesis and by increasing physical activity, the Mc4r knockout mice are impaired in eliciting these responses (71), leading to decreased insulin sensitivity (72). In addition to hyperphagia, the Mc4r knockout mice also have decreased energy expenditure. Change in food intake accounts for 60% of the effect of the MC4R on energy balance. The other 40% is accounted for by changes in energy expenditure (73). In young mice with similar body weights, the Mc4r knockout mice consumed less oxygen than WT littermates, and pair feeding of the Mc4r knockout mice led to more weight gain than the WT mice (74) [however, one study suggested that in young Mc4r knockout mice that are not obese, hyperphagia but not hypometabolism contributes to the early onset in these mice (75)]. MTII has no effect on food intake and energy expenditure in Mc4r knockout mice but is fully active in Mc3r knockout mice (76,77). A recent elegant study of selective reactivation of MC4R expression in specific neurons showed that the MC4R expressed in the PVN and amygdala are involved in the regulation of food intake, whereas MC4R expressed in other neurons are involved in controlling energy expenditure (73). Another study using RNA interference knockdown in the PVN of adult rat also showed that rats with MC4R knockdown exhibited an increase in food intake and excessive body weight gain when exposed to a high-fat diet (78).
The discovery of AgRP in 1997 represents another important breakthrough. Several groups independently cloned the AgRP gene (79,80,81) as an analog of Agouti (Agouti signaling protein in humans). AgRP is an antagonist for the two neural MCRs, MC3R and MC4R. Subsequent studies showed that it is indeed an inverse agonist for human and rodent MC4Rs decreasing basal signaling of WT or constitutively active mutant receptors (82,83,84). AgRP mRNA levels are increased 8- to 10-fold in ob/ob and db/db mice (79,85). Transgenic overexpression of AgRP in mice results in obesity (79,80) but not yellow fur, different from Ay mice. Pharmacological studies showed that AgRP is orexigenic (reviewed in Ref. 86). ICV administration of AgRP increases food intake for at least 24 h and blocks the inhibitory effect of α-MSH (87). Bloom and colleagues (88) further investigated which hypothalamic areas known to express MC4R are involved in the AgRP regulation of feeding by inserting cannulae directly into discrete rat hypothalamic nuclei. They showed that the PVN, the dorsomedial nucleus, and the medial preoptic area were the areas with the greatest response to AgRP, whereas no changes in feeding were seen after the administration of AgRP into the arcuate nucleus (ARC) and lateral hypothalamic area (88). Wirth and Giraudo (89) showed that much lower doses of AgRP were needed to stimulate food intake when directly injected into the PVN, suggesting that the PVN is the primary site for AgRP regulation of food intake. AgRP also reverses leptin-induced inhibition of food intake and body weight in a dose-dependent manner (90). Although it was reported originally that Agrp knockout mice do not have defects in food intake, body weight, or susceptibility to diet-induced obesity (91) likely due to compensation by redundant genes, especially during development, a subsequent knockout study on mice with a different genetic background showed that 6-month-old homozygous Agrp knockout mice have reduced body weights, with increased metabolic rate and motor activity (92). Circulating thyroid hormones and brown adipose tissue (BAT) uncoupling protein (UCP) 1 expression are increased (92). Agrp knockout mice also have longer life span when fed with a high-fat diet (93). Furthermore, targeted postnatal destruction of AgRP/neuropeptide Y neurons results in hypophagia and weight loss, with both appetitive and consummatory behaviors affected, providing strong evidence of these neurons in maintaining energy homeostasis (94,95,96,97,98) (reviewed in Ref. 99). Finally, human genetic studies suggest that single nucleotide polymorphism (SNPs) in AgRP such as A67T might provide protection against obesity, associated with anorexia nervosa and leanness (100,101), although the exact mechanism for this protection remains to be investigated. Functional studies on the AgRP variant did not identify any defect in its interaction with the MC4R (102).
Another line of evidence supporting the melanocortin system in regulating energy homeostasis comes from the studies on mice transgenic for overexpression of syndecan-1. These mice are obese (103), similar to mice overexpressing AgRP. Although syndecan-1 is not normally expressed in the hypothalamus, it was suggested that syndecan-3, expressed in the hypothalamus, was the cause of obesity in transgenic mice with syndecan-1 expression in the hypothalamus. It acts by augmenting AgRP antagonism of α-MSH at the MC4R. Mice lacking syndecan-3 have reduced adipose mass compared with WT mice and are partially resistant to high-fat diet-induced obesity due to reduced food intake in males and increased energy expenditure in females relative to that of WT mice (104). These mice are also more sensitive to exogenously administered MTII, consistent with the hypothesis that in the absence of syndecan-3, MTII binds more efficiently to the MC4R and therefore is more efficacious (105).
MTII also increases energy expenditure as shown by increased oxygen consumption (53,106). The MC4R can directly regulate thermogenesis in BAT (reviewed in Ref. 107). ICV administration of MTII dose-dependently increases sympathetic nerve traffic to thermogenic BAT, and this effect is completely blocked by SHU9119 (108). The MC4R mediates the MTII action because MTII cannot induce UCP1gene expression in Mc4r knockout mice (74), and the renal sympathetic nerve activity is attenuated and abolished, respectively, in heterozygous and homozygous Mc4r knockout mice (109). SHU9119 also abolishes the stimulatory effect of leptin on UCP1 gene expression (110). MTII-induced thermogenic response is maintained in diet-induced obesity (111). MC4R blockade by AgRP or gene targeting leads to defect in high-fat diet-induced up-regulation of UCP1 in interscapular BAT (112). The Mc4r knockout mice, failing to up-regulate UCP1 expression when exposed to cold, are also defective in cold-induced thermogenesis (112). The neurons in the rostral raphe pallidus and its immediate vicinity are important in mediating MC4R action on thermogenesis (113). In summary, the MC4R regulates thermogenesis by activating both the sympathetic nervous system-BAT-UCP1 axis and the hypothalamic-pituitary-thyroid (HPT) axis (107).
Although α-MSH is used in most of the pharmacological experiments, there are several lines of evidence suggesting that β-MSH might also be an important endogenous ligand for the MC4R. β-MSH has higher affinity for the MC4R than α-MSH (114). β-MSH is present in hypothalamic nuclei classically associated with feeding (reviewed in Ref. 115). Pharmacologically, ICV administration of β-MSH or its analog inhibits food intake in rats and mice (116,117,118,119). Mutations in POMC that affect β-MSH cause obesity (120,121). All the evidence supports the hypothesis that β-MSH is also an endogenous agonist for the MC4R involved in regulating energy homeostasis.
In addition to the hypothalamus, the MC4R expressed in the brainstem is also involved in regulating energy homeostasis (122). The nucleus of the solitary tract, apart from the hypothalamus, is the other site of POMC expression in the central nervous system (123,124). The MC4R is expressed abundantly in caudal brainstem structures relevant to energy balance, with the dorsal motor nucleus of the vagus nerve having the highest MC4R expression in the brain (17). Administration of the MCR agonists and antagonists into the fourth ventricle modulates food intake and energy expenditure similar to the administration of these ligands into the third ventricle (125,126,127,128). Administration of MTII into the fourth ventricle also increases UCP1 mRNA in BAT to similar levels of rats administered with MTII into the third ventricle (129).
It is now well accepted that the MC4R in the hypothalamus is critical in mediating the effect of leptin (130) in regulating energy homeostasis. Leptin receptor is expressed in POMC neurons in the ARC (131), and leptin treatment increases POMC mRNA expression (132,133,134). Electrophysiological recordings showed that leptin increases the frequency of action potentials in the POMC neurons (135). Leptin receptor is also expressed in AgRP neurons in the ARC (136), and leptin treatment decreases AgRP mRNA in ob/ob (leptin-deficient) but not db/db (leptin receptor-deficient) mice (90). In ob/ob and db/db mice, hypothalamic AgRP mRNA expression is up-regulated (79,85). Fasting, with plasma leptin concentration decreased, results in up-regulation of AgRP mRNA (90,136,137). Finally, ICV administration of SHU9119 blocked the anorexigenic effect of leptin on food intake, whereas it has no effect on the anorexigenic effect of glucagon-like-peptide-1 (138). It should be pointed out that although the melanocortin system is the predominant mediator of leptin signaling, independent and additive effects of the two systems have also been reported (139,140), with other factors such as age, gender, and diet influencing the interactions between the two systems (141).
Taken together, in an oversimplified way, the response of the leptin-regulated melanocortin circuit to energy status can be summarized in the following way. When the animals are starved, leptin levels decrease, the activity of POMC neurons is decreased, and the activity of AgRP neurons is increased, resulting in decreased MC4R signaling. Increased food intake and decreased energy expenditure ensue. Conversely, when the animals are in the fed state, POMC neurons are activated, AgRP neurons are inhibited, and MC4R signaling is increased. Decreased food intake and increased energy expenditure ensue. Energy homeostasis is maintained in this elegant feedback system. Several outstanding reviews (142,143,144,145,146) have provided more extensive summaries on this topic.
The critical importance of leptin-regulated melanocortin circuit in energy homeostasis in rodents and humans is highlighted by the fact that defects in multiple molecules in this circuit cause obesity. The rodent models include ob/ob mice [deficient in leptin (130)], db/db mice (147) and Zucker fatty rats (148) (deficient in leptin receptor), Pomc knockout mice (149,150), fat/fat mice (deficient in carboxypeptidase E, an enzyme involved in POMC processing) (151), AgRP-overexpressing mice (79,80), and Mc4r knockout mice (68). Mutations in humans include leptin (152,153), leptin receptor (154), POMC (155), PC1 (156), and MC4R (see Section V.A), all resulting in monogenic obesity (reviewed in Refs. 157,158,159,160,161). Finally, loss of the transcription factor single-minded 1 (SIM1) in both mice and humans causes obesity (162,163) because the development of PVN, neurons expressing high levels of MC4R that are critically involved in MC4R regulation of food intake (but not energy expenditure) (73), is disrupted.
In addition to integrating the adipostatic signal of leptin, the MC4R is also involved in responding to acute signals regulating hunger and satiety, such as ghrelin, peptide YY3-36, and CCK, received primarily in the brainstem (reviewed in Refs. 99,122 and 164). For example, MC4R activation in the PVN is required for CCK-induced suppression of feeding, although MC4R in other brain regions (such as vagal afferents) as well as MC4R-independent mechanisms are also involved (69,70,165).
2. MC4R regulation of energy homeostasis in other mammals
In adult male rhesus monkeys, infusion of NDP-MSH into the lateral cerebral ventricle suppresses food intake dose-dependently, whereas infusion of AgRP stimulates food intake during the scheduled afternoon meal, suggesting that the central melanocortinergic system is a physiological regulator of energy balance in primates (166).
In pigs, Barb et al. (167) showed that ICV administration of NDP-MSH decreases food intake, but treatments with SHU9119 or AgRP fail to stimulate food intake, different from the results obtained in rodents (50,87,138) and sheep (168). In vitro, these ligands did act as antagonists at the WT porcine MC4R (pMC4R) (167). We also showed that pMC4R binds and responds to α-MSH and NDP-MSH similarly as hMC4R; it also binds to AgRP with similar affinity as hMC4R (169). It was suggested that the lack of response to the MC4R antagonists might be due to a mutation in some strains of pigs, D298N (170). Although earlier functional analyses suggested that D298N pMC4R binds to NDP-MSH normally, but is devoid of NDP-MSH-stimulated cAMP production (171), we showed that D298N pMC4R has normal binding and signaling to NDP-MSH; it also binds AgRP normally (169). Therefore, the reason for the lack of response to SHU9119 or AgRP is not known. The early study showed extremely low maximal response to NDP-MSH stimulation (171). Patten et al. (172) also reported that hMC4R D298N has normal response to NDP-MSH stimulation, similar to our data (169). In addition, of the genotype-phenotype association studies published since the original report (170), no consensus could be reached, with some studies supporting (173,174,175,176) and some studies disputing (177,178,179) the original association. Indeed, in a recent study, the opposite trend, i.e., higher average daily food intake for pigs with D298 (WT) than pigs homozygous for N298, was observed (180). Comparison of pigs homozygous for D298 or N298 showed that the MC4R genotype does not significantly affect gene expression, body weight, back fat depth, or any measured serum metabolite concentration (180).
In the sheep, leptin receptors are also expressed in POMC and neuropeptide Y/AgRP neurons (181). Fasting dramatically increases AgRP mRNA and protein levels (168,182,183). AgRP expression is also increased during lactation, when there is a negative energy balance and low leptin levels (184). However, change in POMC expression is more variable in different studies, perhaps suggesting that the POMC gene is less responsive to fasting in sheep, different from rodents (reviewed in Ref. 185). AgRP is a potent stimulator of food intake in both healthy and endotoxin-treated animals (168,185,186). These studies suggest that the MC4R is also a critical component of appetite regulation in sheep, a species that grazes instead of eating intermittently like rodents and other nonruminant species.
3. MC4R regulation of energy homeostasis in lower vertebrates
Recent studies showed that the mechanism of regulation of energy homeostasis by the MC4R is also operational in lower vertebrates. In chickens, administration of both α- and β-MSH suppresses food intake (187,188). Increased c-Fos activity (as a marker of neuronal activation) is observed in periventricular, paraventricular, and infundibular nuclei as well as the ventromedial hypothalamus but not the lateral hypothalamus (188). AgRP attenuates the anorexigenic effect of α-MSH in broiler chicks, and AgRP by itself increases food intake in layer-type chicks fed ad libitum but not in broiler chicks (189). It was suggested that the different orexigenic effect of AgRP and anorexigenic tone exerted by endogenous α-MSH might contribute to the difference in food intake between the two breeds (189). In another study, comparison between two lines of chicks showed that the line with low body weight has a lower threshold for the anorexigenic effect of central α-MSH than the line with high body weight (190). In the chick, the MC3R is not expressed in the brain, whereas the MC4R is (191). Therefore, these effects of α- and β-MSH are likely mediated by the MC4R.
In goldfish, ICV injection of NDP-MSH or MTII inhibits food intake, whereas the MC4R-specific antagonist HS024 increases food intake (43,192). These experiments suggested that the MC4R is exerting a tonic inhibitory effect on food intake. Similar results were obtained in rainbow trout (193). ICV injection of MTII decreases food intake, whereas ICV injection of HS024 and the MC3/4R antagonist SHU9119 increases food intake in rainbow trout (193).
The function of AgRP is also conserved in lower vertebrates. In birds (194) and fish (195,196), AgRP expression is restricted to discrete regions of the brain involved in sensing energy status of the organisms, homologous to the ARC of the mammals. Negative energy balance results in dramatically increased AgRP expression levels (195,196,197). Transgenic zebrafish overexpressing AgRP is obese with increased linear growth and adipocyte hypertrophy (198), similar to transgenic mice overexpressing AgRP.
4. Downstream mediators
Although it is well accepted that the activation of the MC4R results in decreased food intake and increased energy expenditure, the molecules mediating these effects are still unknown. Several candidates, including brain-derived neurotrophic factor (BDNF), CRH, TRH, melanin-concentrating hormone (MCH), and orexins, have been suggested to be involved.
MC4R signaling affects the expression of BDNF in the ventromedial hypothalamus (199). ICV injection of MTII reverses the decrease in BDNF mRNA expression in fasted mice. Impaired MC4R signaling such as in Ay or Mc4r knockout mice results in reduced BDNF mRNA expression in the ventromedial hypothalamus (199). In another study, a selective MC4R agonist, MK1, increases BDNF release from isolated rat hypothalami in vitro, and this effect is blocked by preincubation with SHU9119 (200). In vivo, ICV administration of an anti-BDNF antibody blocks the anorexigenic effect of peripherally administered MK1 (200). These data showed that BDNF release from the hypothalamus induced by MC4R activation is required for the MC4R effect on food intake (200), suggesting that BDNF is a downstream mediator of MC4R signaling in regulating energy balance. Similar observations were also made in the dorsal vagal complex (201).
Central administration of α-MSH results in up-regulation of CRH and TRH mRNA levels and down-regulation of orexin mRNA levels (202,203,204). Double-labeling in situ hybridization showed that a subpopulation of CRH neurons in the PVN also expresses the MC4R (205). Central administration of MTII to conscious and freely moving rats induces a rapid induction of CRH gene transcription in the PVN, accompanied by a rise in plasma corticosterone levels. The increase in plasma corticosterone levels induced by MTII is attenuated by the selective MC4R antagonist HS014 and nonselective CRH receptor antagonist α-helical-CRH9-41. Pretreatment with α-helical-CRH9-41 also abolishes half of the inhibitory effect of MTII on food intake (205). Similarly, in chicks, central administration of β-MSH significantly decreases food intake, accompanied by a significant up-regulation of CRH mRNA levels; CRH type 2 receptor antagonist α-helical-CRH blocks the anorexigenic effect of β-MSH (206). In goldfish, CRH is also suggested to mediate the anorexigenic effect of α-MSH (207). These results suggest that CRH may be involved in the anorexigenic action of MSH from fish to mammals.
POMC and AgRP neurons project to the hypophysiotropic TRH neurons in the PVN and are poised anatomically to regulate the HPT axis (202). It was suggested that these ARC neurons mediate the effects of leptin on the HPT axis. For example, during fasting, decreased release of α-MSH and increased secretion of AgRP result in decreased signaling at the MC4R, thereby inhibiting the HPT axis activity (reviewed in Ref. 208). Centrally administered α-MSH prevents the fasting-induced drop in TRH expression (202) and increases TSH levels (209). In vitro, α-MSH stimulates TRH release in hypothalamic slices that is blocked by AgRP (209). ICV administration of AgRP decreases circulating levels of TSH and thyroid hormone and inhibits proTRH (TRH prohormone) mRNA level in the PVN of WT (210,211) but not Mc4r knockout mice (212). About 70% of TRH neurons are innervated by AgRP but not by POMC (212), suggesting that AgRP acts as an inverse agonist rather than competitive antagonist in these neurons. MC4R activation can directly act on the TRH promoter through cAMP response element binding protein (213). In chicks, however, TRH might not be involved in the anorexigenic effect of β-MSH (206).
Several studies investigated whether MCH or orexin mediates MC4R action in the hypothalamus. Expression of MCH is markedly elevated in Pomc-null mice, suggesting that melanocortins negatively regulate MCH neuronal activity (150). POMC neurons send projections to the orexin neurons (214). One study showed that in several models of disrupted MC4R signaling, including Agouti overexpression in Ay mice and antagonism of the MC4R by AgRP or SHU9119, orexin expression is not changed, although MCH expression is significantly increased (204). However, in another study, it was shown that Pomc-null mice have increased orexin expression in the lateral hypothalamic area and this increase is not reversed by corticosterone (215). Central administration of α-MSH in these mice restores orexin expression back down to that of WT mice, suggesting that α-MSH inhibits orexin expression in Pomc-null mice (215). It was suggested that the discrepant results might be due to residual MC4R signaling in the earlier study vs. the complete absence of melanocortins in the latter study. AgRP was shown to primarily activate orexin (rather than MCH) expression in the lateral hypothalamic area.
Another potential mediator of MC4R action is SIM1. SIM1 is a transcription factor required for the development of the PVN (216). Heterozygous mutation in SIM1 is one cause of monogenic obesity in humans (163). The patient with SIM1 heterozygous mutation had hyperphagia but normal energy expenditure, presenting with early-onset severe obesity (163). Homozygous Sim1 knockout mice die shortly after birth (216). Heterozygous Sim1 knockout mice are viable but have similar phenotypes as the SIM1 haploinsufficient patient, with hyperphagia but normal energy expenditure, resulting in early-onset obesity, together with increased linear growth, hyperinsulinemia, and hyperleptinemia (162). MTII treatment increases hypothalamic Sim1 gene expression (217). Heterozygous Sim1 knockout mice, with similar numbers of PVN neurons as WT mice, have diminished anorectic response to MTII despite a normal increase in their energy expenditure (217). Transgenic mice that overexpress human SIM1 are resistant to diet-induced obesity on a high-fat diet through reduced food intake, although there is no change in energy expenditure (218). The SIM1 transgene also completely corrects the hyperphagia and partially corrects the obesity of Ay mice (the portion due to the hyperphagia; the portion due to energy expenditure could not be corrected) (218). When the MC4R is reactivated in the Sim1 neurons in the PVN in Mc4r knockout mice, hyperphagia is completely corrected (73). These data suggest that Sim1 or its transcriptional target mediates the MC4R action in controlling food intake but not energy expenditure (218).
The molecules listed above are some of the mediators likely involved in mediating the action of the MC4R in regulating energy homeostasis. To gain a better understanding of the complete gene sets mediating MC4R action on energy homeostasis, techniques such as laser capture microdissection and gene expression profiling by microarray are needed (219).
B. Cachexia
Cachexia, wasting of lean body mass due to cancer or infectious disease (such as AIDS), is frequently accompanied by anorexia. Patients with other chronic diseases, such as renal failure, heart failure, and rheumatoid arthritis, are also affected by cachexia. Cachexia is an important prognostic for morbidity and mortality with these chronic diseases. Current therapies for cachexia are not effective, and new therapeutic options are urgently needed.
During the past few years, the melanocortin system has been shown to be involved in the pathogenesis of anorexia and weight loss associated with cachexia (for reviews, see Refs. 220,221,222). Many chronic diseases cause increased levels of proinflammatory cytokines. Marks and colleagues (223) showed that the POMC neurons in the ARC express type I IL-1 receptor and respond to IL-1β stimulation with increased release of α-MSH. An intact MC4R is required for cachexia induced by lipopolysaccharide (LPS) administration (to induce inflammation, systemic administration of LPS is frequently used as a model for anorexia due to acute infection) (224) or tumor (225) or associated with uremia (226). MC4R blockade has been shown to block cytokine-induced anorexia (224,227,228,229). Exogenous α-MSH enhances LPS-induced anorexia (227). Further studies showed that ICV administration of AgRP or SHU9119 also protects against cancer-induced anorexia in both rats and mice (224,230) as well as uremic cachexia (231). The MC4R may also mediate changes in adaptive thermogenesis in cachexia (107).
MC4R antagonism by small molecule MC4R antagonists has been shown to alleviate cachexia in several models. Vos et al. (232) showed that ML00253764, [2-{2-[2-(5-bromo-2-methoxyphenyl)-ethyl]-3-fluorophenyl}-4,5-dihydro-1H-imidazolium hydrochloride], a small molecule MC4R inverse agonist, could reach the central nervous system after sc administration and reduce CT26 (colorectal) tumor-induced weight loss. This was confirmed in another study that grafted mice with Lewis lung carcinoma tumors (233). With another small molecule MC4R antagonist developed by Neurocrine Biosciences, NBI-12i, as well as other compounds, it was shown that cancer and uremic cachexia could be attenuated by ip administration of the compounds (234,235,236). Recently, it was shown that two small molecule MC4R antagonists can penetrate the blood-brain barrier when given orally and almost completely prevent weight loss and fat and muscle wasting induced by the C26 adenocarcinoma tumor (237).
In summary, MC4R antagonists, including inverse agonists, have potential as therapeutics for treating cachexia associated with many chronic illness and inflammatory states.
C. Cardiovascular function
The MC4R is expressed in the nucleus of the solitary tract, a region that is known to be important for regulating cardiovascular function. MC4R activation raises arterial pressure despite decreased food intake, whereas MC4R inhibition causes marked weight gain without raising arterial pressure, partially mediating leptin’s action on these parameters (238,239,240). For example, central blockade of the MC4R by chronic infusion of SHU9119 or AgRP decreases both mean arterial pressure (MAP) and heart rate (HR), although as expected, food intake and weight gain are increased markedly (241,242). Endogenous MC4R activity likely contributes to the elevated arterial pressure in spontaneously hypertensive rats (243). The effects of MC4R on MAP and HR are likely mediated by adrenergic activation (243,244,245), and the MC4R in PVN is likely to be involved in this regulation (246). MC4R agonism in the hindbrain can link sympathetic outflows to cardiac responses (127).
Although the ligands used in some of these studies could not differentiate whether the MC3R or the MC4R or both mediates these pressor effects, studies using knockout mice showed that the MC4R is likely the mediator. ICV administration of α-MSH to WT mice led to increases in both MAP and HR; however, the same treatment does not change these parameters in the Mc4r knockout mice, suggesting that the MC4R mediates the α-MSH action on MAP and HR (247). The Mc4r knockout mice are normotensive with no renal damage despite severe obesity, hyperinsulinemia, and hyperglycemia (247,248).
Clinical studies with MC4R-deficient subjects further extended these findings to humans (249). Although the patients with MC4R haploinsufficiency are obese (see Section V.A), the prevalence of hypertension is significantly lower in these patients than in control subjects with a similar degree of obesity. The blood pressure is also significantly lower than that in control subjects. MC4R agonism led to significant increases in both systolic and diastolic blood pressure (249).
Taken together, these data suggest that although MC4R agonism is a promising therapeutic approach for obesity treatment, there is doubt whether MC4R agonists would offer “a significant therapeutic advantage over currently used appetite suppressants such as sibutramine” (250). Hypertension might be an important adverse side effect for MC4R agonists. Close attention is warranted in any clinical trials.
D. Glucose and lipid homeostasis
In rodents, the MC4R also directly and acutely regulates glucose homeostasis and insulin sensitivity, independent of its effects on food intake and body weight (251,252,253,254,255,256,257,258). In young lean Mc4r knockout mice, plasma insulin level is already increased, and impaired insulin tolerance takes place before the onset of detectable hyperphagia or obesity (251). Central administration of MTII dose-dependently inhibits basal insulin release and improves insulin sensitivity in a number of animal models, including genetic and diet-induced obesity (251,256). MCR expressed in medial hypothalamic nuclei mediates the MTII-induced glucose uptake in peripheral tissues such as skeletal muscle and BAT (259). MC4R signaling in vagal efferents might also be involved (165). ICV administration of NDP-MSH also reduces serum insulin levels, and this effect is blocked by MC4R-specific blocker HS014 (258). In pair-fed animals, ICV administration of α-MSH or MTII markedly enhances the actions of insulin on both glucose uptake and production, whereas SHU9119 exerts opposite effects (252,254). Transgenic overexpression of α-MSH also leads to improved glucose metabolism in both genetic and diet-induced obese models (260,261). A recent study showed that the improvement in hyperinsulinemia after MCR agonist treatment is retained in Mc4r knockout mice, suggesting that other MCRs such as peripheral MC1R and/or MC3R might also be involved in affecting insulin sensitivity (257).
ICV administration of MTII increases skeletal muscle AMP-activated protein kinase activity, even in mice fed with a high-fat diet (262). AMP-activated protein kinase is a critical regulator of skeletal muscle fatty acid β-oxidation; therefore, these data suggest that there is a metabolic link between the hypothalamic melanocortin system and fatty acid mobilization (263). Pharmacological inhibition of MC4R in rats and Mc4r knockout in mice directly and potently promotes lipid uptake, triglyceride synthesis, and fat accumulation in white adipose tissue, whereas increased MCR signaling in the central nervous system triggers lipid mobilization, and these effects are independent of food intake (264). MTII administration increases fat utilization in rats as demonstrated by decreased respiratory quotient (53,265). Similarly, patients with MC4R deficiency also have increased respiratory quotient compared with obese controls (264). By shifting substrate utilization and nutrient partitioning but not elevating circulating triglyceride or free fatty acid levels, the MC4R agonists might be promising therapeutics not just for obesity but also for obesity-associated metabolic disorders such as ectopic lipid deposits and lipotoxicity (263). MC4R activation can also control dietary fat intake, with receptor activation resulting in decreased fat consumption (266) and receptor blockade by Agouti or AgRP or Pomc knockout promoting fat consumption (267,268,269), providing potential further therapeutic benefits.
E. Reproduction and sexual function
Several studies suggested that the MC4R is also involved in modulating reproductive function. Although the Mc4r knockout mice are fertile, reproductive aging is advanced in females. In a mouse line expressing a mutant MC4R with reduced function (I194F), decreased numbers of corpus luteum and increased cystic follicles are observed in aged females (270). Male Mc4r knockout mice have erectile dysfunction (271) that can be corrected by exercise (272), suggesting that the reproductive dysfunction might be due to obesity.
It is well known that leptin regulates both energy homeostasis and reproduction. MC4R mediates the effect of leptin on energy homeostasis (63,138). However, whether it also mediates the effect of leptin on reproduction is not well established. The majority of GnRH neurons are closely apposed by fibers expressing immunoreactive β-endorphin (hence α-MSH) (273). AgRP neurons project to the medial preoptic area, an area that also contains GnRH neurons (274). ICV administration of AgRP results in significant increases in plasma LH and FSH in rats and stimulates GnRH release from hypothalamic explants but has no direct effect on LH release from dispersed anterior pituitary cells. The MC4R is expressed in hypothalamic GT1-1 cells, and stimulation of these cells with NDP-MSH increases GnRH secretion (275,276). Ovariectomized female rats primed with estrogen and progesterone display characteristic LH and prolactin (PRL) surges that are completely abolished by starving. MC4R antagonists decrease the magnitude of LH and PRL surges in normally fed rats and significantly block the leptin stimulation of the hormonal surges in starved rats, suggesting that the MC4R might be an important mediator of the leptin stimulation of LH and PRL surges (277). AgRP abolishes LH and PRL surges in these female rats, whereas anti-AgRP antiserum can partially reinstate both LH and PRL surges (278). These results suggest that the melanocortin system is important for the hormonal surges in female rats. MTII but not γ-MSH (selective agonist for MC3R) restores PRL surge in starved rats, suggesting that the MC4R, not the MC3R, mediates the preovulatory surge in PRL (279). In rhesus monkey, central administration of AgRP inhibits pulsatile LH release, suggesting that AgRP may mediate the effect of a negative energy balance on the reproductive system by suppressing the GnRH pulse generator in primates (280). Further studies are needed to investigate what functions the MC4R have in the males.
In the late 1960s, it was shown that ACTH administered into the cerebral ventricles induces penile erections and ejaculation in male rabbits and beagle dogs (reviewed in Ref. 4). Recently, it was shown that the MC4R is expressed in penile tissues. Van der Ploeg et al. (271) showed that the MC4R mRNA is expressed in nerve fibers and mechanoreceptors in the glans of rat and human penis, and rat spinal cord and pelvic ganglion, the major autonomic relay center to the penis. They further showed that a highly selective nonpeptide MC4R agonist, THIQ ([(N-[(3R)-1,2,3,4-tetrahydroisoquinolinium-3-ylcarbonyl]-(1R)-1-(4-chlorobenzyl)-2-[4-cyclohexyl-4- (1H-1,2,4-triazol-1-ylmethyl)piperidin-1-yl]-2-oxoethylamine (1)]), augments erectile activity initiated by electrical stimulation of the cavernous nerve in WT but not Mc4r knockout mice. Copulatory behavior is enhanced by THIQ administration and is diminished in Mc4r knockout mice. Taken together, these data suggest that the MC4R modulates penile erectile function, likely through neuronal circuitry in spinal cord erectile centers and somatosensory afferent nerve terminals of the penis.
In male rats, MTII given ICV or intrathecally induces penile erection dose-dependently, and SHU9119 completely blocks this response (281). Intrathecal MTII administration induced almost twice as many erections as ICV administration. These results suggest that in addition to activation of MC4R in the brain, MTII also activates the MCRs in distal spinal cord (likely MC4R) to elicit erectile responses (281).
Several clinical studies have also shown that MTII and its derivative PT-141 can induce transient erections in men with erectile dysfunction (282,283) (reviewed in Refs. 284 and 285). PT-141 also selectively stimulates solicitational behaviors in the female rat, suggesting a potential of this compound in treating female sexual desire disorders (286). Increase in penile erection was also observed with another MC4R agonist, LY2112688, a synthetic peptide agonist with 100-fold higher affinity for the MC4R than the MC3R (249). A recent report showed that a MC4R selective agonist developed by Merck, MK-0493, does not induce erectile responses (287). Therefore, the structure of the particular agonist is likely important in determining whether erectile responses are elicited or not.
F. Miscellaneous functions of the MC4R
In addition to the melanocortins, the processing of POMC gives rise to β-endorphin, which activates the μ-opioid receptor. The MC4R and μ-opioid receptor have similar distributions in the spinal cord and the brain, providing the anatomical basis for potential interactions between the two receptors (reviewed in Ref. 288). For example, the MC4R mRNA is expressed in the striatum, nucleus accumbens, and periaqueductal gray, regions implicated in the behavioral effects of opiates (289). α-MSH antagonism of the morphine-induced analgesia, tolerance, and dependence, has been known for more than 25 yr (290). Chronic administration of morphine results in down-regulation of MC4R mRNA expression in the striatum, nucleus accumbens, and periaqueductal gray, but not in other brain regions such as the hypothalamus, frontal cortex, and substantia nigra (289). These data, together with previous studies demonstrating melanocortin antagonism of different functional effects of opiate treatments (reviewed in Ref. 291), led to the suggestion that this MC4R down-regulation might promote the development of opiate tolerance and dependence (289). Repeated (but not acute) administration of cocaine increases MC4R mRNA expression in the striatum, nucleus accumbens, and hippocampus, but not in the cerebral cortex (292,293). This up-regulation in MC4R mRNA comes with functional consequences, accompanied by increased behavioral responses to α-MSH infusion (292). Blockade of the MC4R in the nucleus accumbens blocks the reinforcing, incentive motivational, and locomotor-sensitizing effects of cocaine (293). MTII also potentiates amphetamine reward as measured by the lowering of the threshold for lateral hypothalamic self-stimulation (294).
Because ethanol drinking and food intake are both appetitive and consummatory behaviors, and both ethanol and food have rewarding properties, it was hypothesized that overlapping central pathways are involved with uncontrolled eating and excessive ethanol consumption (295). ICV administration of MTII reduces ethanol self-administration in both WT and Mc3r knockout mice, suggesting that the MC3R is not involved in mediating the effect of MTII; AgRP increases ethanol self-administration (296). The involvement of the MC4R was further confirmed by demonstrating that a highly selective MC4R agonist, cyclo(NH-CH2-CH2-CO-His-D-Phe-Arg-Trp-Glu)-NH2, also reduces ethanol self-administration (296). Administration of MTII also reduces ethanol intake and ethanol preference in alcohol-preferring rats, likely by modulating ethanol-induced changes in opioid peptide levels (297,298). Even peripheral administration of MTII can reduce ethanol intake in mice (299). α-MSH in discrete areas of the brain may also play a role in the antidepressant-like response of ethanol and depression induced by ethanol withdrawal (300).
The MC4R may also be involved in pain perception. It is expressed in dorsal root ganglia (DRG) and spinal cord, suggesting its potential involvement in presynaptic nociception (301). α-MSH or MTII administration results in increased sensitivity to pain (302,303). The intrathecal injection of synthetic MC4R antagonists and AgRP reduces mechanical allodynia in neuropathic rats (304,305). Pharmacological and genetic blockade of the MC4R increases the antinociceptive effects of morphine but does not change its potency for locomotor activity (288,306). Administration of HS014 (MC4R antagonist) with chronic morphine delays the development of tolerance and prevents withdrawal hyperalgesia (307). MC4R mRNA is induced after axonal injury in both hypoglossal motor neurons and DRG (308). In vitro treatment with α-MSH promotes neurite outgrowth in DRG neurons, and a MC4R-specific antagonist blocks this effect (308), similar to findings in Neuro2A cells (309). These results suggest that MC4R antagonists might be used for the treatment of chronic pain by improving the effectiveness of morphine (288,307).
Although the MC1R and the MC3R are the primary MCRs mediating the anti-inflammatory effect of melanocortins (reviewed in Refs. 310 and 311), there is also some evidence that the MC4R is involved in brain inflammation (reviewed in Ref. 312). Melanocortins inhibit the hypothalamic production of proinflammatory molecules such as nitric oxide and prostaglandins induced by IL-1β (313). Small molecule MC4R agonists were also shown to attenuate brain inflammation and promote survival (314,315). The MC4R also has a neuroprotective effect on cerebral ischemia (316,317) (reviewed in Ref. 318). Glial cells that express the MC4R might also be involved in mediating the antiinflammatory and neuroprotective effects of melanocortins (32,312). Treatment of astrocytes with α-MSH decreases the inducible nitric oxide synthase, and cycloxygenase-2 expression induced by LPS and interferon-γ therefore attenuates inflammation. The MC4R antagonist HS024 blocks the effects of α-MSH, suggesting that the MC4R mediates the α-MSH action (32).
The MC4R has also been shown to modulate anxiety behavior, e.g., that induced by IL-1β (319) or ethanol withdrawal (320), by modulating serotonin transmission (reviewed in Ref. 321). Chaki et al. (322) showed that a potent and selective MC4R antagonist they developed has anxiolytic- and antidepressant-like activities in various rodent models, suggesting that MC4R antagonists might be used for treating patients with stress-related disorders such as depression and/or anxiety. The MC4R also mediates the antipyretic actions of α-MSH (323). Although MC4R activation results in increased thermogenesis, it also suppresses fever (324). α-MSH, through the MC4R, can also correct the memory impairment caused by IL-1β treatment (325).
The potential for targeting the MC4R in treating drug addiction, alcohol abuse, anxiety, and depression remains to be further explored.
IV. Pharmacology of the Melanocortin-4 Receptor
A. Ligand binding and receptor activation
Of the four major endogenous melanocortins, the MC4R has the highest affinity for β-MSH, followed by α-MSH and ACTH, and then γ-MSH (β-MSH > α-MSH > ACTH > γ-MSH) (114). Both Agouti and AgRP are antagonists for the MC4R. The dogfish MC4R has similar pharmacological properties as the hMC4R; therefore, the ligand binding sites were established more than 450 million years ago (23).
Peptide agonists bind in a β-turn conformation that organizes the pharmacophore His-Phe-Arg-Trp in an optimal arrangement for binding and activation of the receptor (326). The N terminus and the ELs are not important for peptide agonist binding (327,328,329). Rather, the binding determinants are located in the TMs. Ionic and aromatic residues in the upper regions of the TMs are important for ligand binding and signaling. These residues can form salt bridge and aromatic-aromatic interactions with residues in the HFRW pharmacophore. Mutations of E100 (2.60) in TM2; D122 (3.25) and D126 (3.29) in TM3; and W258 (6.48), F261 (6.51), and H264 (6.54) in TM6 result in decreased binding affinity for NDP-MSH, suggesting that these residues are potential ligand interaction sites, with D122 interacting with Arg in the pharmacophore (330,331,332). Acidic residues were also found to be important for peptide agonist binding in the MC3R (333). Phe284 (7.35) in TM7 has been proposed to interact with Phe in the HFRW pharmacophore through hydrophobic interactions (334).
It is well known that γ-MSH has selectivity for MC3R over the MC4R (335). Chimera and mutagenesis experiments showed that Y268 (6.58) in TM6 of the MC4R is the primary determinant for the low affinity for a γ-MSH analog, [Nle (4)]Lys-γ2-MSH (336), whereas residues in TM6, including F267 (6.57), Y268, I269 (6.59), and S270 (6.60), are important in determining the selectivity of [Pro (5), DNal(2′) (8)]-γ-MSH (337).
A recent study used in silico mutagenesis to predict the functional relevance of mutating each residue in hMC4R to all other natural amino acids individually (338). It would be of great interest to experimentally verify the predictions that can potentially contribute significantly to improve our understanding of the MC4R pharmacology.
Another way to study the structure-function relationships of GPCRs is by performing detailed functional characterization of naturally occurring mutants. For example, functional studies of naturally occurring MC4R mutants (see Section V.B) showed that the binding defective mutants are clustered in TM2, EL1, and TM3 (339), implicating this domain as important for ligand binding, consistent with data from previous site-directed mutagenesis experiments (330,331).
Mutation in the most highly conserved Asp in TM2 to Asn, D90N (2.50), causes a loss-of-signaling despite normal ligand binding (Ref. 340 and our unpublished observations), consistent with previous site-directed mutagenesis experiments in other GPCRs (341,342,343). We and others recently showed that mutation of a fully conserved Ser in TM3, S136 (3.39) to Phe, also results in loss of G protein coupling/activation (344,345,346). To gain a better understanding of the role of this codon in receptor function, we generated seven additional mutants, including mutating S136 into hydrophobic amino acids such as Ala and Leu; polar amino acids such as Thr, Tyr, and Cys; and charged amino acids such as Asp and Arg. All seven mutants have normal binding and can signal in response to NDP-MSH stimulation (346). In another study, S136P is retained intracellularly with decreased maximal binding (345); therefore, the phenotype of mutants at S136 depends on the newly introduced residue.
We showed that a naturally occurring mutation in the MC3R, I183N (3.54), at the cytoplasmic end of TM3, causes loss of signaling with normal ligand binding (347). We then generated the corresponding mutation in hMC4R and showed that I151N is also defective in G protein coupling/activation (347).
AgRP has been shown to be not only a competitive antagonist but also an inverse agonist. This was first shown in WT human and constitutively active mouse MC4Rs (82,83). Recently, AgRP was also shown to be an inverse agonist for fish MC4R (45). There is also in vivo evidence supporting the inverse agonist activity of AgRP: AgRP can still regulate energy homeostasis in the absence of endogenous melanocortin agonists (348). Several studies addressed the binding of AgRP to the MC4R. Chimeras generated between the MC1R (which does not bind AgRP) and MC4R (which binds AgRP) (349) showed that the N terminus and EL1 are not involved in AgRP binding; EL2 and EL3 confer binding to AgRP (328). EL3 also confers selectivity for Agouti binding in the MC4R (350). Residues in TM2, TM3, and TM4 are also important for AgRP binding (331,351).
Recently, studies have also been performed on the ligand binding and signaling determinants for small molecule ligands. For example, THIQ was first described by researchers at Merck & Co. (352). Several groups tried to identify how THIQ binds to the MC4R (326,353,354). Using chimeras and site-directed mutagenesis, residues from several TMs, including TM3, TM6, and TM7, were found to be involved in ligand binding. By comparing the MC2R (which does not bind THIQ) and MC4R, both residues conserved between the two receptors (such as E100, D122, D126, F254, and W258), and residues that are not conserved between the two receptors (such as N123, I129, and S131 in TM3) were found to be important for THIQ binding (354). Therefore, THIQ shares some binding determinants with peptide agonists but also has unique binding determinants. Overlapping binding determinants for peptide and nonpeptide ligands were also shown with other analogs (355). Identification of the molecular determinants responsible for receptor binding and signaling of novel peptide and small molecule ligands are important for rational design of drugs targeting the MC4R for treating a variety of diseases.
One widely used method to study GPCR activation is to measure the constitutive activity of the mutant receptors (356). For the MC4Rs, earlier site-directed mutagenesis studies did not report any constitutive activity of the WT or any mutant receptors. The first constitutively active mutant MC4R, in fact, came from characterization of mutant MC4Rs identified from obese patients (357). In this study, L250Q was shown to have very robust constitutive activity. Although it is not compatible with the phenotype (increased activity at the MC4R is expected to result in a lean phenotype or perhaps even anorexia nervosa), it did provide a starting point for further mutagenesis experiments. Multiple mutagenesis together with homology modeling was used to study the functions of L250 in receptor activation (358). Several additional mutants, including S127L, H158R, and P230L, were also reported to cause constitutive activation (346,359,360,361). More detailed studies at these loci have not been reported.
In contrast to other GPCRs, such as LH receptor (362), C5a receptor (363), m1 muscarinic acetylcholine receptor (364), β2-adrenergic receptor (365), follitropin receptor (365), and TSH receptor (366,367), mutation of a highly conserved Leu in TM3 (3.43) in hMC4R does not cause constitutive activation (356). Rather, L140R has lower basal activity than WT MC4R, suggesting important differences in the role of L3.43 in maintaining the inactive conformation in the MC4R compared with the other GPCRs cited here (356).
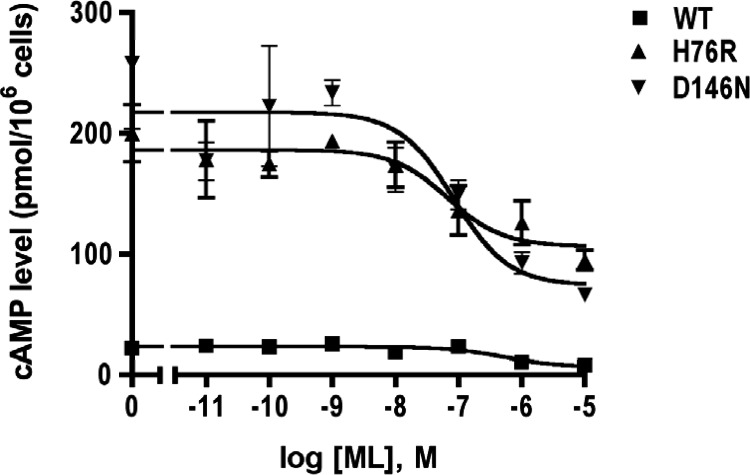
We recently showed that H76R and D146N also have high constitutive activities (368). As shown in Fig. 2, ML00253784 can partially decrease the basal signaling of the mutant and WT MC4Rs, proving that these mutants are indeed constitutively active.
Figure 2.
Constitutive activation of two naturally occurring MC4R mutations, H76R and D146N. The basal activities of H76R and D146N are 10- to 15-fold higher than the basal activity of WT MC4R. The small molecule inverse agonist ML00253764 partially decreases the basal activities of the two mutants and the WT MC4R. cAMP levels were measured with RIA (496).
It should be emphasized that for the MC4R, the study of the constitutive activity is not purely of academic interest. It also has potential clinical relevance. Loss of constitutive activity was suggested to be one mechanism for obesity pathogenesis caused by MC4R mutations (369). AgRP serving not just as a competitive antagonist bus also an inverse agonist highlights additionally the physiological relevance of MC4R constitutive activity in regulating energy balance (370,371).
B. Signaling pathways
The classical signaling pathway for the MC4R is by coupling to the heterotrimeric stimulatory G protein (Gs). Receptor activation leads to increased cAMP production, and consequently protein kinase A (PKA) activation. In HEK293 cells stably expressing MC4R, MC4R activation was also shown to increase intracellular calcium (372,373) that was sensitive to cholera toxin (372). In GT1-1 cells, a mouse hypothalamic cell line expressing MC4R endogenously, it was shown that MC4R can increase intracellular calcium through Gq/phospholipase C-dependent signaling pathway (374), although in GT1-7 cells (another mouse hypothalamic cell line closely related to GT1-1 cells expressing MC4R endogenously), no increase in intracellular calcium concentrations can be observed (375). By measuring GTPγS binding, it was shown that the MC4R can also couple to Gi/o proteins (375). In both heterologous cells expressing WT MC4R and GT1-7 cells, MC4R activation stimulates pertussis toxin-sensitive GTPγS binding, implicating coupling to Gi/o proteins (375). Interestingly, AgRP also stimulates GTPγS binding, suggesting that it can decrease cAMP levels by both antagonizing Gs activation and stimulating Gi/o activation (375). The physiological relevance of these signaling pathways has not been investigated in detail.
In addition to activation of the Gs-cAMP-PKA pathway, MC4R also activates the MAPK pathway, and in vivo experiments showed that the activation of ERK1/2 is necessary for MTII-induced suppression of food intake (376). In COS-1 cells expressing rodent MC4R, MTII induce ERK1/2 activation in time- and dose-dependent manners (377). ICV injection of MTII into rat hypothalamus showed that this ERK1/2 activation also happens in vivo (377). Vongs et al. (378) showed that in CHO cells stably expressing hMC4R, NDP-MSH also induces ERK1/2 activation in time- and dose-dependent manners, and this activation is abolished by SHU9119. The GT1-1 cell line that expresses MC4R endogenously also responds to NDP-MSH stimulation with increased ERK1/2 activation (276). In cerebral microvessels, MC4R activation of MAPK leads to potentiation of leptin signaling (379).
Similar to other GPCRs, the signaling pathway leading to ERK1/2 activation is cell-line dependent. In CHO cells expressing hMC4R, phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 block the ERK1/2 activation, whereas PKA inhibitor Rp-cAMPS cannot, suggesting that NDP-MSH-induced ERK1/2 activation is mediated by phosphatidylinositol 3-kinase (378). In GT1-1 cells, increased ERK1/2 activation is mediated by calcium and protein kinase C (276). In vivo, ERK1/2 activation induced by fourth ventricle administration is mediated by the cAMP-PKA pathway (376).
Mulholland and colleagues (380) recently reported that MC4R activation inhibits the c-Jun N-terminal kinase (JNK), therefore inhibiting Ser307 phosphorylation at the insulin receptor substrate-1. These effects were blocked by SHU9119. MC4R agonist augments insulin-stimulated AKT phosphorylation both in vitro and in vivo. Increased insulin-stimulated glucose uptake was observed in GT1-1 cells after NDP-MSH stimulation. These observations suggest that MC4R activation can interact with insulin signaling through modulating JNK activity (380). This is consistent with an earlier observation that central administration of MTII improves insulin tolerance in diet-induced obese rats (256).
In summary, MC4R can couple to all three major classes of G proteins, Gs, Gi/o, and Gq, changing second messengers such as cAMP and calcium and activating MAPK including ERK1/2 and JNK.
C. Internalization, desensitization, and dimerization
Chronic administration of MTII and other agonists has been shown to cause tachyphylaxis (257,381,382), suggesting that there is desensitization. In GT1-7 cells, ligand-induced desensitization was indeed observed (383). In heterologous cells expressing MC4R, it was shown that peptide agonist-activated receptor undergoes GPCR kinase 2-, β-arrestin-, and dynamin-dependent internalization through clathrin-coated pits (383,384). SHU9119 blocks NDP-MSH-stimulated internalization, whereas another MC4R antagonist, HS014, does not induce receptor internalization (384). Nonpeptide agonists induce less internalization (373). AgRP induces MC4R association with the β-arrestins and subsequent internalization (385). Therefore, AgRP not only acts as an inverse agonist antagonizing agonist action; by inducing internalization, AgRP also reduces the amount of MC4R molecules on the cell surface accessible to agonists (385). MC4R expressed heterologously in neuronal cell lines were shown to undergo constitutive endocytosis, also through clathrin-coated pits (386). Prolonged stimulation of the MC4R leads to its translocation from the endosome to the lysosome, likely destined for degradation (384). Very limited recycling of internalized MC4R was observed (384). Detailed studies on the phosphorylation, internalization, and desensitization remain to be performed.
Similar to other GPCRs, MCRs also form dimers or higher-ordered oligomers. Using bioluminescence resonance energy transfer, MC1R and MC3R expressed in Cos-7 cells were shown to dimerize constitutively (387). Coimmunoprecipitation also showed that MC1R dimerize (388). These dimers form early in the biosynthetic pathway, because intracellularly retained mutants also form dimers. In cotransfection experiments, the intracellularly retained mutants dimerize with WT receptors, thereby exerting dominant negative activity (388). Disulfide bond and noncovalent interactions are likely involved in MC1R dimerization (389).
For the MC4R, Biebermann et al. (340), using sandwich enzyme-linked immunosorbent assays and fluorescence resonance energy transfer techniques, showed that WT MC4R dimerize. Furthermore, they showed that a naturally occurring mutation, D90N, can heterodimerize with WT MC4R and exert dominant negative activity (340). Bioluminescence resonance energy transfer also showed that the MC4R dimerizes constitutively and is not affected by ligand binding (390). Several studies that functionally characterize the naturally occurring MC4R mutations showed that the intracellularly retained mutants do not have dominant negative activity (391,392,393). These studies are in sharp contrast to extensive studies in other GPCRs, where intracellularly retained mutant receptors heterodimerize with WT receptors, resulting in decreased cell surface expression and signaling of cotransfected WT receptors (see Refs. 394,395,396,397 for examples). Although there are a few skeptics (398), GPCR dimerization is now widely accepted and is thought to play an important role in biosynthesis and maturation of these receptors (399,400). The reason for the discordant observations in MC4R compared with other GPCRs remains to be elucidated (reviewed in Ref. 339).
Studies designed to identify the forces involved in dimerization showed that unlike the MC1R, mutations to the extracellular cysteine residues do not affect dimerization, suggesting that the TMs are involved in dimerization (401). The molecular determinants for dimerization and the functions of dimerization await further studies.
V. Pathophysiology of the Melanocortin-4 Receptor
A. Naturally occurring MC4R mutations
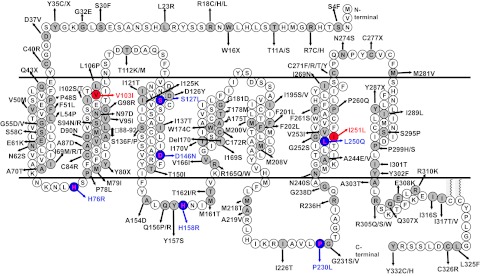
In 1998, the groups of Froguel and O’Rahilly (402,403) independently reported the first frameshift mutations in the MC4R gene associated with severe early-onset obesity. Since then, more than 150 distinct mutations have been identified from patient cohorts of different ethnic origins (25,340,344,345,357,359,360,361,392,393,404,405,406,407,408,409,410,411,412,413,414,415,416,417,418,419,420,421,422,423,424,425,426,427,428,429,430,431,432,433,434). These mutations include at least 122 missense mutations, two inframe deletion mutations, and seven nonsense mutations (Fig. 3), as well as dozens of frameshift mutations (not shown in Fig. 3). From Fig. 3, it is very clear that these mutations are scattered throughout the MC4R. A total of at least 105 residues are mutated, representing 32% of the receptor (reviewed in Ref. 435). Several earlier articles provided more detailed summaries on the clinical characteristics of the MC4R-deficient patients and biochemical phenotypes of the mutant proteins and can be consulted (339,413,436,437). I recently published a comprehensive review on this topic (435). The following is an abbreviated account.
Figure 3.
Naturally occurring missense, nonsense, and inframe deletion mutations of the MC4R identified from various patient cohorts. The two polymorphisms that confer protection from obesity are indicated with red filling and letters. The mutations that cause constitutive activation are indicated with blue filling and letters. References describing these mutations are listed in the text. [Reprinted, with minor modification, with permission from Y. X. Tao: Prog Mol Biol Transl Sci 88:173–204, 2009 (435). ©Elsevier 2009.]
Although it is well accepted that the level of MC4R gene expression is important for maintaining energy homeostasis (haploinsufficiency causes obesity), no mutations in the promoter region that regulate the MC4R expression have been identified. The polymorphisms identified so far do not cosegregate with obesity phenotype, and therefore their relevance to the pathogenesis of obesity is questionable (417,438,439). One report suggested that a polymorphism in the MC4R promoter might be related to physical activity phenotype: for the C-2745T variant, the T/T genotype is associated with the highest inactivity score (440). Several recent genome-wide association scans showed that SNPs near MC4R are associated with increased risk for obesity (441,442,443), although the mechanism for these SNPs to change MC4R expression has not been elucidated yet. Overall, it seems that defect in MC4R gene transcription is likely not a major cause of severe early-onset obesity.
The prevalence of MC4R mutations appears to vary depending on the ethnic origin, severity of obesity, and age of obesity onset. Up to 6% of early-onset morbidly obese patients in British Caucasians were found to have MC4R mutations (413). In other studies, lower prevalence was found: 0% in morbidly obese Belgian children and adults (444), 1.9% in obese German children and adolescents (359,432), 1.8% in obese Finnish children (360), 1.7% in obese European Caucasians (430), 4.6% in Pima Indians (417), and 2.3% in North American severely obese adults (445). Lower prevalence is observed in Asian and Mediterranean populations (435,446).
Although there are some subjects homozygous for MC4R mutations (392,408,413,416,447), the vast majority of the subjects are heterozygous. Because not all subjects with MC4R mutations are obese, O’Rahilly et al. (448) suggested that the most appropriate mechanism for the mode of inheritance is codominance with modulation of expression and penetrance of phenotype. Obesity is a multifactorial disease with interaction between genotype and environment. For example, a generational effect was observed in the penetrance of MC4R mutations in causing obesity, with younger generation having higher penetrance, likely due to the obesogenic environment of recent decades compared with decades surrounding World War II (430). Considering these facts, it was suggested that “major gene” or “oligogenic” instead of “monogenic” should be used to describe MC4R mutation and obesity (359,449). MC4R genotype also affects the extent of success of lifestyle intervention in achieving weight reduction. Children with decreased MC4R signaling, despite normal weight loss after lifestyle intervention, have much greater difficulty in maintaining weight loss (450).
The MC4R-deficient patients are hyperphagic. When they are offered a buffet meal, they have increased caloric intake (413). The hyperphagia decreases with increasing age. The degree of hyperphagia correlates with residual receptor activity. Mutations that result in a complete loss of signaling cause more severe obesity (413). Although earlier studies showed that MC4R mutations do not affect energy expenditure (413), different from mouse genetic studies (73,74), Baier and colleagues (431) recently showed that Pima Indians with two pathogenic MC4R mutations (R165Q and a frameshift mutation with A insertion at nucleotide 100) have decreased energy expenditure. Similarly, in Hispanic patients, MC4R variants are also associated with altered energy expenditure (434). In addition to obesity, MC4R mutation carriers were also reported to have increased longitudinal growth (413), similar to the Mc4r knockout mice (68). Patients homozygous for MC4R mutations are more severely obese than patients heterozygous for MC4R mutations (413). The effect of MC4R mutation on body mass index is more pronounced in females than in males: MC4R mutation carriers, compared with MC4R WT relatives, are 9.5 and 4 kg/m2 higher in body mass index than middle-aged women and men, respectively (430,449). The exact mechanism for this MC4R genotype-sex interaction is not clear at present. Sex steroids might be contributing factors.
Interestingly, several studies suggested that the MC4R is a double-edged sword that can either cause obesity or protect from obesity. Two MC4R polymorphisms, V103I and I251L, have been shown to confer resistance to obesity (highlighted in red in Fig. 3).
V103I is a common polymorphism with the minor allele appearing at a frequency of 2–4%. Rosmond et al. (451) first reported lower abdominal obesity in heterozygotes for V103I compared with homozygotes for V103. Hebebrand and colleagues (452) then expanded this observation to a larger cohort with meta-analysis, confirming the negative association of the I103 allele with obesity rate. A later meta-analysis consisting of 29,563 individuals (10,975 cases and 18,588 controls) showed that for the V103I polymorphism, the I103 allele had an 18% lower risk of obesity compared with the V103 allele (453). Compared with those homozygous for the WT allele, subjects heterozygous for V103I have significantly lower triglyceride levels, decreased waist circumference, decreased glycosylated hemoglobin, and increased high-density lipoprotein-cholesterol, decreasing the odds of having three or more components of the metabolic syndrome by 50% (454,455). Although earlier functional studies did not find any differences between the V103 and I103 MC4Rs in binding and signaling (constitutive and ligand-induced signaling) (357,391,404,452), recently it was shown that I103 is less sensitive to AgRP inhibition (456) that might result in weaker orexigenic signal conferring lower risk of obesity.
I251L confers even stronger protection from obesity than V103I (457), possibly due to its higher constitutive activity (456). It is negatively associated with childhood and adult severe obesity, with odds ratios ranging from 0.25 to 0.76, averaging 0.52. Together with V103I (average odds ratio, 0.80), these two variants may provide 2% protection of obesity, similar to the roughly 2% cause of obesity by pathogenic loss-of-function mutations in the MC4R (457).
Whether there is an association between MC4R mutations and binge-eating disorder is controversial. Most of the earlier studies did not report the eating behavior of the patients harboring MC4R mutations. Only a few cases of binge-eating patients with functionally relevant MC4R mutations were reported (458,459). In 2003, Branson et al. (460) suggested that all obese patients with MC4R mutations had binge-eating disorder. However, 75% of their subjects had MC4R polymorphisms V103I, T112M, and I251L. These variants have been shown by multiple independent groups to be functioning normally (357,392,404), and V103I and I251L are indeed protective for obesity (see above). We showed that the three novel variants they identified, T11A, F51L, and M200V, as well as T112M, have normal functions (461). These results raise serious doubts about the conclusion reached in the Branson et al. article (460). Subsequent clinical studies demonstrated that indeed, MC4R mutations are not associated with binge-eating disorder (421,462).
In summary, these studies unequivocally demonstrated that the MC4R is a critical regulator of energy homeostasis in humans. Mutations in the MC4R are the most common monogenic form of human obesity.
B. Molecular classification of the MC4R mutants
Identification of a MC4R variant from an obese patient does not necessarily prove that the mutation is the cause of obesity in the patient. Additional data, including cosegregation of the mutation with obesity in the family and absence of the variant in the ethnically matched controls, provide additional support for a causative role of the mutation in causing obesity (463). In addition, functional characterization of the mutant receptor in vitro is indispensable to prove a causative role (463).
The MC4R pharmacology can be studied in several aspects. Ligand binding assays can be done in live cells or membranes. Binding assays done in live cells are preferred because any binding measured represents cell surface binding. Binding assays with crude membrane preparations that include intracellular (such as endoplasmic reticulum or Golgi apparatus) membranes cannot distinguish between binding from cell surface receptors or intracellular receptors. It is not clear whether intracellular MC4Rs can bind ligand. However, other immature GPCRs (such as LH receptor) can bind ligand. The ligand of choice is iodinated NDP-MSH. Iodinated α-MSH can no longer bind the receptor; therefore, it cannot be used in receptor binding assays. The iodinated NDP-MSH needs to be purified by HPLC to isolate the monoiodinated NDP-MSH. Because of the special equipment needed, most laboratories use commercial iodinated NDP-MSH. However, the radioligand is extremely expensive; therefore, displacement experiments rather than saturation binding experiments are performed routinely.
Another property of the mutant receptor that needs to be measured is its signaling ability. Because MC4R activates Gs after ligand binding, resulting in increased intracellular cAMP levels, signaling can be measured by either direct assay of cAMP levels or indirect measurement of increased reporter gene expression driving by increased cAMP levels. These assays will reveal whether mutant receptors have either decreased or absent signaling in response to agonist stimulation. Signaling in the absence of ligand can be measured at the same time to determine the basal activity of the mutant receptor.
The third property of the mutant receptors is their cell surface expression, especially for mutants that are defective in ligand binding. Immunocytochemistry, flow cytometry, or ELISA can be used for this purpose. These data differentiate whether the defects in binding are due to defective cell surface expression or to defect in ligand binding per se. If no cell surface expression is observed, permeabilization will reveal whether intracellular staining can be observed, demonstrating whether the protein is produced but retained intracellularly or the mutant receptor expression level is impacted. Because transient transfection can overload the cell’s quality control system (see Ref. 397 for an example), resulting in an artificial intracellular retention of the receptors, cells stably transfected with the receptors are preferred for this purpose (435,464).
To catalog the numerous MC4R mutations, we were the first to propose a classification scheme for MC4R mutations (464). This scheme is based on the life cycle of the receptor and modeled after the classification of mutations in low-density lipoprotein receptor and cystic fibrosis transmembrane conductance regulator (465,466) (Fig. 4). [For a detailed listing of the mutants that belong to each class, the reader is referred to my recent review (435).]
Figure 4.
Molecular classification of naturally occurring MC4R mutations. See the text for detailed description. [Reprinted with permission from Y. X. Tao: Mol Cell Endocrinol 239:1–14, 2005 (339). ©Elsevier 2005.]
1. Class I: null mutations
Due to defective protein synthesis and/or accelerated protein degradation, receptor protein levels are decreased. Nonsense mutants might belong to this class, although this assumption needs to be verified experimentally. We recently showed that R7C, C84R, and W174C, with decreased total expression levels, belong to this class (346).
2. Class II: intracellularly retained mutants
The mutant receptors are produced but are retained intracellularly, most likely in the endoplasmic reticulum due to misfolding being detected by the cell’s quality control system. This class comprises the largest set of MC4R mutations reported to date.
3. Class III: binding defective mutants
These mutants are expressed on the cell surface but are defective in ligand binding per se, with decreased binding capacity and/or affinity. Signaling is frequently impaired secondary to the impairment in binding. Because AgRP is the natural antagonist of the MC4R, if the mutants are more sensitive to inhibition by AgRP, the mutants are functionally defective. However, very few studies measured the binding property to AgRP. I316S was shown to have altered relative affinities for α-MSH and AgRP (393), and V103I is less sensitive than WT MC4R to AgRP inhibition (456). Therefore, these mutants can be classified as a subclass within this class.
4. Class IV: signaling-defective mutants
These mutant MC4Rs are transported to the cell surface, bind ligand, but are defective in agonist-stimulated signaling with decreased efficacy and/or potency. Interestingly, although D90N cannot activate Gs, it can activate Gi/o (375). Whether the other signaling-defective mutants can also activate Gi/o remains to be investigated. The ability of naturally occurring mutants to activate MAPK has not been explored in detail either.
Vaisse and colleagues (369) suggested that decreased basal activities of some MC4R mutants might cause obesity. We showed that of nine mutants identified from patients with binge-eating disorder and nonobese or obese subjects, five mutants have decreased constitutive activities, whereas the other four mutants have normal basal activities (461). Further studies, for example vetting in transgenic animals, are needed to demonstrate unequivocally the physiological significance of the constitutive activity of MC4R (435). We suggest that mutants with defective basal signaling are also class IV mutants.
5. Class V: variants with unknown defect
These variants have normal cell surface expression, ligand binding, and signaling (basal and ligand-stimulated). Whether and how these variants cause energy imbalance and therefore obesity is unclear. It should be emphasized that in the in vitro expression system, there are spare receptors (339), whereas in vivo there is likely no spare receptor. In addition, different coupling to G proteins from in vivo situations and amplified potency and efficacy of agonists (31,467,468,469) are also possible with the in vitro expression system. Finally, in vivo, the MC4R is expressed in neurons. The data obtained with heterologous nonneuronal cells might not reveal any neuron-specific aspects of MC4R function (169,435). Therefore, caution is urged in interpretation of the in vitro data, especially when no overt functional defects are observed.
Since our initial proposal of the classification scheme described above, several other groups proposed modified or alternative classification schemes (345,436,437). The pros and cons of the different systems are compared in detail in Ref. 435.
One drawback of our classification scheme is that we did not include mutants that affect processes downstream of receptor activation, such as desensitization, internalization, and resensitization. Defects in these processes can also cause dysfunctional receptor. For example, a constitutively desensitized V2 vasopressin receptor mutant appeared at first as an apparent defect in signaling (470,471). Therefore, further refinement can be introduced.
In summary, it is important to analyze the functional properties when new variant MC4Rs are identified in obese subjects to more accurately determine whether the phenotype of the variant is indeed consistent with the clinical phenotype and to devise strategies for personalized medicine (see Section V.C). A classification system will be useful in cataloging the increasingly large array of MC4R mutations identified (435).
C. Therapeutic implications
Previously, several strategies for correcting the defects in naturally occurring mutations in GPCRs were summarized (472). Class I mutants due to nonsense mutations can potentially be corrected by aminoglycoside antibiotics by decreasing the codon-anticodon fidelity, resulting in read-through of the premature stop codon. Synthetic ligands can be tested for their abilities to correct class III and IV mutants, those mutants that are expressed on the cell surface but could not bind the endogenous ligands or respond to these ligands. Some of these synthetic ligands might bind to residues different from endogenous ligands and therefore activate some of the mutant receptors (339,473). Indeed, some synthetic MC4R ligands can stimulate various MC4R mutants that are impaired in responding to endogenous ligands (473).
Because class II mutants are most numerous, we have been interested in whether small molecule ligands that can pass the plasma and endoplasmic reticulum membranes might act as pharmacological chaperones correcting the folding defect of the mutant receptors. Some of the MC4R mutants have residual signaling activity. Approaches that result in their increased expression on the cell surface could potentially be of therapeutic value. Bouvier and colleagues pioneered this field with the V2 vasopressin receptor (474). Since then, similar results have been achieved in naturally occurring mutations in GnRH receptor (475,476), rhodopsin (477,478), and WT or laboratory-generated mutants in several other GPCRs (479,480,481,482,483,484,485). The clinical utility of pharmacological chaperones was shown by a recent clinical trial (486).
We showed that ML00253764 could rescue two MC4R mutants that are retained intracellularly, C84R and W174C (346). ML00253764, originally described by Vos et al. (232), has a molecular weight of only 377.3. We hypothesized that it might act as a pharmacological chaperone correcting the misfolding of intracellularly retained mutants. To test this hypothesis, cells stably expressing WT, C84R, or W174C MC4Rs, or transfected with the empty vector pcDNA3.1, were treated with 10−5m ML00253764 for 24 h. We then investigated cell surface expression of the receptors with confocal microscopy qualitatively and flow cytometry quantitatively. The cell surface expression of both mutants is increased to about 35% of that of the untreated WT MC4R (346). Even the cell surface expression of the WT MC4R is increased to approximately 152% after 24-h treatment with ML00253764, suggesting that WT MC4R maturation is not very efficient. To investigate whether the rescued mutant receptors are functional, the cells were stimulated with NDP-MSH. Both mutant receptors have increased cAMP production, up to 80% of the signaling generated by the untreated WT MC4R (346). We have expanded this observation to six additional naturally occurring mutants. As shown in Fig. 5, all six mutants are rescued to the cell surface to different extents. We have also obtained similar data showing that another small molecule MC4R inverse agonist also acts as a pharmacological chaperone (our unpublished observations). These data suggest that small molecule MC4R ligands can indeed act as pharmacological chaperones assisting the trafficking of intracellularly retained mutant receptors, and many of these rescued mutant receptors are capable of eliciting significant signaling. They could potentially be used to treat patients harboring these MC4R mutations. Because they can also increase the cell surface expression of the WT MC4R, they might also be used to treat common obesity for patients that do not have MC4R mutations.
Figure 5.
Pharmacological rescue of six intracellularly retained MC4R mutants by ML00253764. Human embryonic kidney 293 cells stably transfected with WT or mutant MC4Rs or empty vector pcDNA3.1 (as a negative control) were incubated with 10 μm ML00253764 for 24 h at 37 C. Cells were then stained with anti-myc monoclonal antibody to visualize the c-myc epitope at the N terminus of the MC4R by confocal microscopy. Detailed procedures are described in Ref. 346.
VI. Conclusions and Future Directions
Tremendous progress has been made on the MC4R since 1993 when it was cloned. Diverse approaches, including anatomical localization, pharmacological administration, gene targeting, and the continuous development of novel peptide and small molecule agonists and antagonists as new tools, led to the elucidation of numerous functions for the MC4R, including energy homeostasis, cachexia, cardiovascular function, glucose and lipid homeostasis, reproduction and sexual function, drug addiction, pain perception, and mood. Parallel clinical genetic studies demonstrate the pivotal importance of this gene in obesity pathogenesis. Ligands targeting the MC4R can potentially be used to treat obesity (12,99,487), cachexia (221,222), metabolic syndrome, sexual dysfunction, neuropathic pain, drug addiction, and mood disorders such as depression and anxiety.
There are several important questions that remain to be addressed.
What are the downstream mediators mediating the MC4R action in regulating the different physiological processes listed above? Do the same molecules mediate the MC4R action in the different processes, or more likely, do different molecules mediate different processes regulated by the MC4R? Proteomic analysis will likely generate novel leads.
A very exciting development in GPCR research during the past 2–3 yr is the series of novel crystal structures revealed, including inactive and active structures of GPCRs (488,489,490,491,492,493,494) (reviewed in Ref. 495). These reports represent a major breakthrough since the report of rhodopsin crystal structure in 2000 (26). A crystal structure, either inactive ligand-free form or complexed with ligands, would be warmly welcomed by scientists working on the pharmacology of the MC4R. The MC4R has constitutive activity and is extremely hydrophobic, characteristics likely hindering its purification and crystallization. Inverse agonists can be used to stabilize the receptor during purification and crystallization.
Functional studies showed that the majority of the dysfunctional MC4R mutants are retained intracellularly. Therefore, intracellular trafficking is a very important regulatory point for MC4R function. How the MC4R trafficking is regulated, for example, the molecular chaperones involved in MC4R folding, remains largely unknown.
Although numerous MC4R mutations were identified in humans, there are no reports of introducing these mutations into mice to develop mouse models. These mouse models will be important in further dissection of the pathophysiology as well as in preclinical testing of ligands that are found to be able to correct the defect in cells such as the pharmacological chaperones we identified.
Numerous laboratories, especially those in pharmaceutical companies, performed extensive studies trying to discover novel small molecule ligands for the MC4R. These studies are not reviewed herein. Although preclinical trials have shown that some of these MC4R compounds are effective, they have not reached clinical use yet. Side effects, including nausea, yawning and stretching, sexual arousal and penile erections, as well as cardiovascular complications such as hypertension, are partly to blame. Additional considerations are selectivity for the MC4R and tachyphylaxis. It has been pointed out that the MC4R can ameliorate other comorbidities such as lipid and glucose dyshomeostasis, in addition to the treatment of obesity, therefore offering great potential in treating metabolic syndrome (12,99). Further research in this rapidly expanding field offers great promise.
Acknowledgments
I apologize to those scientists whose important works are not cited due to my oversight and space constraint. I thank Dr. Zhen-Chuan Fan and Dr. Shu-Xiu Wang for contributing to some of the studies summarized here.
Footnotes
My studies on the MC4R were supported by American Heart Association (0265236Z), National Institutes of Health (R15DK077213), Diabetes Action Research and Education Foundation, Morris Animal Foundation, Alabama Agricultural Experiment Station, and intramural support from Auburn University, including Animal Health and Disease Research Program, Biogrant Program, Interdisciplinary Research Program, and startup funds for new faculty.
Disclosure Summary: The author has nothing to disclose.
First Published Online February 26, 2010
Abbreviations: AgRP, Agouti-related peptide; ARC, arcuate nucleus; BAT, brown adipose tissue; BDNF, brain-derived neurotrophic factor; CCK, cholecystokinin; DRG, dorsal root ganglia; E, embryonic day; EL, extracellular loop; GPCR, G protein-coupled receptor; Gs, stimulatory G protein; hMC4R, human MC4R; HPT, hypothalamic-pituitary-thyroid; HR, heart rate; ICV, intracerebroventricular; JNK, c-Jun N-terminal kinase; LPS, lipopolysaccharide; MAP, mean arterial pressure; MCH, melanin-concentrating hormone; MCR, melanocortin receptor; MTII, melanotan II; NDP-MSH, [Nle4, D-Phe7]-α-MSH; PC, prohormone convertase; PKA, protein kinase A; pMC4R, porcine MC4R; POMC, pro-opiomelanocortin; PRL, prolactin; PVN, paraventricular nucleus; Sim1, single-minded 1; SNP, single nucleotide polymorphism; TM, transmembrane domain; UCP, uncoupling protein; WT, wild type.
References
- Smith AI, Funder JW 1988 Proopiomelanocortin processing in the pituitary, central nervous system, and peripheral tissues. Endocr Rev 9:159–179 [DOI] [PubMed] [Google Scholar]
- Bertagna X 1994 Proopiomelanocortin-derived peptides. Endocrinol Metab Clin North Am 23:467–485 [PubMed] [Google Scholar]
- Heinig JA, Keeley FW, Robson P, Sower SA, Youson JH 1995 The appearance of proopiomelanocortin early in vertebrate evolution: cloning and sequencing of POMC from a Lamprey pituitary cDNA library. Gen Comp Endocrinol 99:137–144 [DOI] [PubMed] [Google Scholar]
- Bertolini A, Tacchi R, Vergoni AV 2009 Brain effects of melanocortins. Pharmacol Res 59:13–47 [DOI] [PubMed] [Google Scholar]
- Gantz I, Fong TM 2003 The melanocortin system. Am J Physiol Endocrinol Metab 284:E468–E474 [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Robbins LS, Mortrud MT, Cone RD 1992 The cloning of a family of genes that encode the melanocortin receptors. Science 257:1248–1251 [DOI] [PubMed] [Google Scholar]
- Chhajlani V, Wikberg JE 1992 Molecular cloning and expression of the human melanocyte stimulating hormone receptor cDNA. FEBS Lett 309:417–420 [DOI] [PubMed] [Google Scholar]
- Chen W, Kelly MA, Opitz-Araya X, Thomas RE, Low MJ, Cone RD 1997 Exocrine gland dysfunction in MC5-R-deficient mice: evidence for coordinated regulation of exocrine gland function by melanocortin peptides. Cell 91:789–798 [DOI] [PubMed] [Google Scholar]
- Cone RD, Lu D, Koppula S, Vage DI, Klungland H, Boston B, Chen W, Orth DN, Pouton C, Kesterson RA 1996 The melanocortin receptors: agonists, antagonists, and the hormonal control of pigmentation. Recent Prog Horm Res 51:287–317; discussion 318 [PubMed] [Google Scholar]
- Schiöth HB 2001 The physiological role of melanocortin receptors. Vitam Horm 63:195–232 [DOI] [PubMed] [Google Scholar]
- Cone RD 2006 Studies on the physiological functions of the melanocortin system. Endocr Rev 27:736–749 [DOI] [PubMed] [Google Scholar]
- Wikberg JE, Mutulis F 2008 Targeting melanocortin receptors: an approach to treat weight disorders and sexual dysfunction. Nat Rev Drug Discov 7:307–323 [DOI] [PubMed] [Google Scholar]
- Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, Rosenblum CI, Vongs A, Feng Y, Cao L, Metzger JM, Strack AM, Camacho RE, Mellin TN, Nunes CN, Min W, Fisher J, Gopal-Truter S, MacIntyre DE, Chen HY, Van der Ploeg LH 2000 Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet 26:97–102 [DOI] [PubMed] [Google Scholar]
- Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, Baetscher M, Cone RD 2000 A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology 141:3518–3521 [DOI] [PubMed] [Google Scholar]
- Sutton GM, Perez-Tilve D, Nogueiras R, Fang J, Kim JK, Cone RD, Gimble JM, Tschöp MH, Butler AA 2008 The melanocortin-3 receptor is required for entrainment to meal intake. J Neurosci 28:12946–12955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz I, Miwa H, Konda Y, Shimoto Y, Tashiro T, Watson SJ, DelValle J, Yamada T 1993 Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. J Biol Chem 268:15174–15179 [PubMed] [Google Scholar]
- Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD 1994 Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol 8:1298–1308 [DOI] [PubMed] [Google Scholar]
- Magenis RE, Smith L, Nadeau JH, Johnson KR, Mountjoy KG, Cone RD 1994 Mapping of the ACTH, MSH, and neural (MC3 and MC4) melanocortin receptors in the mouse and human. Mamm Genome 5:503–508 [DOI] [PubMed] [Google Scholar]
- Stäubert C, Tarnow P, Brumm H, Pitra C, Gudermann T, Grüters A, Schöneberg T, Biebermann H, Römpler H 2007 Evolutionary aspects in evaluating mutations in the melanocortin 4 receptor. Endocrinology 148:4642–4648 [DOI] [PubMed] [Google Scholar]
- Hughes DA, Hinney A, Brumm H, Wermter AK, Biebermann H, Hebebrand J, Stoneking M 2009 Increased constraints on MC4R during primate and human evolution. Hum Genet 124:633–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone RD 2000 The melanocortin-4 receptor. In: Cone RD, ed. The melanocortin receptors. Totowa, NJ: Humana Press; 405–447 [Google Scholar]
- Ringholm A, Fredriksson R, Poliakova N, Yan YL, Postlethwait JH, Larhammar D, Schiöth HB 2002 One melanocortin 4 and two melanocortin 5 receptors from zebrafish show remarkable conservation in structure and pharmacology. J Neurochem 82:6–18 [DOI] [PubMed] [Google Scholar]
- Ringholm A, Klovins J, Fredriksson R, Poliakova N, Larson ET, Kukkonen JP, Larhammar D, Schiöth HB 2003 Presence of melanocortin (MC4) receptor in spiny dogfish suggests an ancient vertebrate origin of central melanocortin system. Eur J Biochem 270:213–221 [DOI] [PubMed] [Google Scholar]
- Schiöth HB, Lagerström MC, Watanobe H, Jonsson L, Vergoni AV, Ringholm A, Skarphedinsson JO, Skuladottir GV, Klovins J, Fredriksson R 2003 Functional role, structure, and evolution of the melanocortin-4 receptor. Ann NY Acad Sci 994:74–83 [DOI] [PubMed] [Google Scholar]
- Tarnow P, Schoneberg T, Krude H, Gruters A, Biebermann H 2003 Mutationally induced disulfide bond formation within the third extracellular loop causes melanocortin 4 receptor inactivation in patients with obesity. J Biol Chem 278:48666–48673 [DOI] [PubMed] [Google Scholar]
- Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M 2000 Crystal structure of rhodopsin: a G protein-coupled receptor. Science 289:739–745 [DOI] [PubMed] [Google Scholar]
- Ballesteros JA, Weinstein H 1995 Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosci 25:366–428 [Google Scholar]
- van der Kraan M, Tatro JB, Entwistle ML, Brakkee JH, Burbach JP, Adan RA, Gispen WH 1999 Expression of melanocortin receptors and pro-opiomelanocortin in the rat spinal cord in relation to neurotrophic effects of melanocortins. Brain Res Mol Brain Res 63:276–286 [DOI] [PubMed] [Google Scholar]
- Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK 2003 Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol 457:213–235 [DOI] [PubMed] [Google Scholar]
- Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM, Friedman JM, Elmquist JK 2003 Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. J Neurosci 23:7143–7154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkirk JV, Nottebaum LM, Lee J, Yang W, Foster AC, Lechner SM 2007 Identification of differential melanocortin 4 receptor agonist profiles at natively expressed receptors in rat cortical astrocytes and recombinantly expressed receptors in human embryonic kidney cells. Neuropharmacology 52:459–466 [DOI] [PubMed] [Google Scholar]
- Caruso C, Durand D, Schiöth HB, Rey R, Seilicovich A, Lasaga M 2007 Activation of melanocortin 4 receptors reduces the inflammatory response and prevents apoptosis induced by lipopolysaccharide and interferon-γ in astrocytes. Endocrinology 148:4918–4926 [DOI] [PubMed] [Google Scholar]
- van Calker D, Löffler F, Hamprecht B 1983 Corticotropin peptides and melanotropins elevate the level of adenosine 3`:5`-cyclic monophosphate in cultured murine brain cells. J Neurochem 40:418–427 [DOI] [PubMed] [Google Scholar]
- Evans T, McCarthy KD, Harden TK 1984 Regulation of cyclic AMP accumulation by peptide hormone receptors in immunocytochemically defined astroglial cells. J Neurochem 43:131–138 [DOI] [PubMed] [Google Scholar]
- Zohar M, Salomon Y 1992 Melanocortins stimulate proliferation and induce morphological changes in cultured rat astrocytes by distinct transducing mechanisms. Brain Res 576:49–58 [DOI] [PubMed] [Google Scholar]
- Kokubo M, Asai K, Yamamoto N, Aoyama M, Morikawa M, Togari H, Wada Y, Kato T 2002 ACTH1-24 down-regulates expression of ciliary neurotrophic factor mRNA in cultured rat astrocyte. Pediatr Res 52:950–957 [DOI] [PubMed] [Google Scholar]
- Spencer JD, Schallreuter KU 2009 Regulation of pigmentation in human epidermal melanocytes by functional high-affinity β-melanocyte-stimulating hormone/melanocortin-4 receptor signaling. Endocrinology 150:1250–1258 [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Wild JM 1998 Melanocortin-4 receptor mRNA expression in the developing autonomic and central nervous systems. Brain Res Dev Brain Res 107:309–314 [DOI] [PubMed] [Google Scholar]
- Lichtensteiger W, Hanimann B, Siegrist W, Eberle AN 1996 Region- and stage-specific patterns of melanocortin receptor ontogeny in rat central nervous system, cranial nerve ganglia and sympathetic ganglia. Brain Res Dev Brain Res 91:93–110 [DOI] [PubMed] [Google Scholar]
- Kistler-Heer V, Lauber ME, Lichtensteiger W 1998 Different developmental patterns of melanocortin MC3 and MC4 receptor mRNA: predominance of Mc4 in fetal rat nervous system. J Neuroendocrinol 10:133–146 [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Jenny Wu CS, Dumont LM, Wild JM 2003 Melanocortin-4 receptor messenger ribonucleic acid expression in rat cardiorespiratory, musculoskeletal, and integumentary systems. Endocrinology 144:5488–5496 [DOI] [PubMed] [Google Scholar]
- Roselli-Rehfuss L, Mountjoy KG, Robbins LS, Mortrud MT, Low MJ, Tatro JB, Entwistle ML, Simerly RB, Cone RD 1993 Identification of a receptor for γ melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proc Natl Acad Sci USA 90:8856–8860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdá-Reverter JM, Ringholm A, Schiöth HB, Peter RE 2003 Molecular cloning, pharmacological characterization, and brain mapping of the melanocortin 4 receptor in the goldfish: involvement in the control of food intake. Endocrinology 144:2336–2349 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Tsuchiya K, Yamanome T, Schiöth HB, Kawauchi H, Takahashi A 2008 Food deprivation increases the expression of melanocortin-4 receptor in the liver of barfin flounder, Verasper moseri. Gen Comp Endocrinol 155:280–287 [DOI] [PubMed] [Google Scholar]
- Sánchez E, Rubio VC, Thompson D, Metz J, Flik G, Millhauser GL, Cerdá-Reverter JM 2009 Phosphodiesterase inhibitor-dependent inverse agonism of agouti-related protein on melanocortin 4 receptor in sea bass (Dicentrarchus labrax). Am J Physiol Regul Integr Comp Physiol 296:R1293–R1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggioli R, Vergoni AV, Bertolini A 1986 ACTH-(1-24) and α-MSH antagonize feeding behavior stimulated by κ opiate agonists. Peptides 7:843–848 [DOI] [PubMed] [Google Scholar]
- Vergoni AV, Poggioli R, Bertolini A 1986 Corticotropin inhibits food intake in rats. Neuropeptides 7:153–158 [DOI] [PubMed] [Google Scholar]
- Lu D, Willard D, Patel IR, Kadwell S, Overton L, Kost T, Luther M, Chen W, Woychik RP, Wilkison WO, Cone RD 1994 Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature 371:799–802 [DOI] [PubMed] [Google Scholar]
- Yen TT, Gill AM, Frigeri LG, Barsh GS, Wolff GL 1994 Obesity, diabetes, and neoplasia in yellow A(vy)/− mice: ectopic expression of the agouti gene. FASEB J 8:479–488 [DOI] [PubMed] [Google Scholar]
- Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD 1997 Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 385:165–168 [DOI] [PubMed] [Google Scholar]
- Hruby VJ, Lu D, Sharma SD, Castrucci AL, Kesterson RA, al-Obeidi FA, Hadley ME, Cone RD 1995 Cyclic lactam α-melanotropin analogues of Ac-Nle4-cyclo[Asp5, D-Phe7, Lys10] α-melanocyte-stimulating hormone-(4-10)-NH2 with bulky aromatic amino acids at position 7 show high antagonist potency and selectivity at specific melanocortin receptors. J Med Chem 38:3454–3461 [DOI] [PubMed] [Google Scholar]
- Hansen MJ, Ball MJ, Morris MJ 2001 Enhanced inhibitory feeding response to α-melanocyte stimulating hormone in the diet-induced obese rat. Brain Res 892:130–137 [DOI] [PubMed] [Google Scholar]
- Hwa JJ, Ghibaudi L, Gao J, Parker EM 2001 Central melanocortin system modulates energy intake and expenditure of obese and lean Zucker rats. Am J Physiol Regul Integr Comp Physiol 281:R444–R451 [DOI] [PubMed] [Google Scholar]
- Cettour-Rose P, Rohner-Jeanrenaud F 2002 The leptin-like effects of 3-d peripheral administration of a melanocortin agonist are more marked in genetically obese Zucker (fa/fa) than in lean rats. Endocrinology 143:2277–2283 [DOI] [PubMed] [Google Scholar]
- Hagan MM, Rushing PA, Schwartz MW, Yagaloff KA, Burn P, Woods SC, Seeley RJ 1999 Role of the CNS melanocortin system in the response to overfeeding. J Neurosci 19:2362–2367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudo SQ, Billington CJ, Levine AS 1998 Feeding effects of hypothalamic injection of melanocortin 4 receptor ligands. Brain Res 809:302–306 [DOI] [PubMed] [Google Scholar]
- Wirth MM, Olszewski PK, Yu C, Levine AS, Giraudo SQ 2001 Paraventricular hypothalamic α-melanocyte-stimulating hormone and MTII reduce feeding without causing aversive effects. Peptides 22:129–134 [DOI] [PubMed] [Google Scholar]
- Paues J, Mackerlova L, Blomqvist A 2006 Expression of melanocortin-4 receptor by rat parabrachial neurons responsive to immune and aversive stimuli. Neuroscience 141:287–297 [DOI] [PubMed] [Google Scholar]
- Azzara AV, Sokolnicki JP, Schwartz GJ 2002 Central melanocortin receptor agonist reduces spontaneous and scheduled meal size but does not augment duodenal preload-induced feeding inhibition. Physiol Behav 77:411–416 [DOI] [PubMed] [Google Scholar]
- Williams DL, Grill HJ, Weiss SM, Baird JP, Kaplan JM 2002 Behavioral processes underlying the intake suppressive effects of melanocortin 3/4 receptor activation in the rat. Psychopharmacology (Berl) 161:47–53 [DOI] [PubMed] [Google Scholar]
- Schiöth HB, Mutulis F, Muceniece R, Prusis P, Wikberg JE 1998 Discovery of novel melanocortin 4 receptor selective MSH analogues. Br J Pharmacol 124:75–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kask A, Rägo L, Mutulis F, Pähkla R, Wikberg JE, Schiöth HB 1998 Selective antagonist for the melanocortin 4 receptor (HS014) increases food intake in free-feeding rats. Biochem Biophys Res Commun 245:90–93 [DOI] [PubMed] [Google Scholar]
- Kask A, Rägo L, Wikberg JE, Schiöth HB 1998 Evidence for involvement of the melanocortin MC4 receptor in the effects of leptin on food intake and body weight. Eur J Pharmacol 360:15–19 [DOI] [PubMed] [Google Scholar]
- Kask A, Mutulis F, Muceniece R, Pähkla R, Mutule I, Wikberg JE, Rägo L, Schiöth HB 1998 Discovery of a novel superpotent and selective melanocortin-4 receptor antagonist (HS024): evaluation in vitro and in vivo. Endocrinology 139:5006–5014 [DOI] [PubMed] [Google Scholar]
- Skuladottir GV, Jonsson L, Skarphedinsson JO, Mutulis F, Muceniece R, Raine A, Mutule I, Helgason J, Prusis P, Wikberg JE, Schiöth HB 1999 Long term orexigenic effect of a novel melanocortin 4 receptor selective antagonist. Br J Pharmacol 126:27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergoni AV, Bertolini A, Guidetti G, Karefilakis V, Filaferro M, Wikberg JE, Schiöth HB 2000 Chronic melanocortin 4 receptor blockage causes obesity without influencing sexual behavior in male rats. J Endocrinol 166:419–426 [DOI] [PubMed] [Google Scholar]
- Wallingford N, Perroud B, Gao Q, Coppola A, Gyengesi E, Liu ZW, Gao XB, Diament A, Haus KA, Shariat-Madar Z, Mahdi F, Wardlaw SL, Schmaier AH, Warden CH, Diano S 2009 Prolylcarboxypeptidase regulates food intake by inactivating α-MSH in rodents. J Clin Invest 119:2291–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F 1997 Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88:131–141 [DOI] [PubMed] [Google Scholar]
- Fan W, Ellacott KL, Halatchev IG, Takahashi K, Yu P, Cone RD 2004 Cholecystokinin-mediated suppression of feeding involves the brainstem melanocortin system. Nat Neurosci 7:335–336 [DOI] [PubMed] [Google Scholar]
- Blevins JE, Morton GJ, Williams DL, Caldwell DW, Bastian LS, Wisse BE, Schwartz MW, Baskin DG 2009 Forebrain melanocortin signaling enhances the hindbrain satiety response to CCK-8. Am J Physiol Regul Integr Comp Physiol 296:R476–R484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AA, Marks DL, Fan W, Kuhn CM, Bartolome M, Cone RD 2001 Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nat Neurosci 4:605–611 [DOI] [PubMed] [Google Scholar]
- Sutton GM, Trevaskis JL, Hulver MW, McMillan RP, Markward NJ, Babin MJ, Meyer EA, Butler AA 2006 Diet-genotype interactions in the development of the obese, insulin-resistant phenotype of C57BL/6J mice lacking melanocortin-3 or -4 receptors. Endocrinology 147:2183–2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB 2005 Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123:493–505 [DOI] [PubMed] [Google Scholar]
- Ste Marie L, Miura GI, Marsh DJ, Yagaloff K, Palmiter RD 2000 A metabolic defect promotes obesity in mice lacking melanocortin-4 receptors. Proc Natl Acad Sci USA 97: 12339–12344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weide K, Christ N, Moar KM, Arens J, Hinney A, Mercer JG, Eiden S, Schmidt I 2003 Hyperphagia, not hypometabolism, causes early onset obesity in melanocortin-4 receptor knockout mice. Physiol Genomics 13:47–56 [DOI] [PubMed] [Google Scholar]
- Marsh DJ, Hollopeter G, Huszar D, Laufer R, Yagaloff KA, Fisher SL, Burn P, Palmiter RD 1999 Response of melanocortin-4 receptor-deficient mice to anorectic and orexigenic peptides. Nat Genet 21:119–122 [DOI] [PubMed] [Google Scholar]
- Chen AS, Metzger JM, Trumbauer ME, Guan XM, Yu H, Frazier EG, Marsh DJ, Forrest MJ, Gopal-Truter S, Fisher J, Camacho RE, Strack AM, Mellin TN, MacIntyre DE, Chen HY, Van der Ploeg LH 2000 Role of the melanocortin-4 receptor in metabolic rate and food intake in mice. Transgenic Res 9:145–154 [DOI] [PubMed] [Google Scholar]
- Garza JC, Kim CS, Liu J, Zhang W, Lu XY 2008 Adeno-associated virus-mediated knockdown of melanocortin-4 receptor in the paraventricular nucleus of the hypothalamus promotes high-fat diet-induced hyperphagia and obesity. J Endocrinol 197:471–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS 1997 Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 278:135–138 [DOI] [PubMed] [Google Scholar]
- Graham M, Shutter JR, Sarmiento U, Sarosi I, Stark KL 1997 Overexpression of Agrt leads to obesity in transgenic mice. Nat Genet 17:273–274 [DOI] [PubMed] [Google Scholar]
- Fong TM, Mao C, MacNeil T, Kalyani R, Smith T, Weinberg D, Tota MR, Van der Ploeg LH 1997 ART (protein product of agouti-related transcript) as an antagonist of MC-3 and MC-4 receptors. Biochem Biophys Res Commun 237:629–631 [DOI] [PubMed] [Google Scholar]
- Nijenhuis WA, Oosterom J, Adan RA 2001 AgRP(83-132) acts as an inverse agonist on the human melanocortin-4 receptor. Mol Endocrinol 15:164–171 [DOI] [PubMed] [Google Scholar]
- Haskell-Luevano C, Monck EK 2001 Agouti-related protein functions as an inverse agonist at a constitutively active brain melanocortin-4 receptor. Regul Pept 99:1–7 [DOI] [PubMed] [Google Scholar]
- Chai BX, Neubig RR, Millhauser GL, Thompson DA, Jackson PJ, Barsh GS, Dickinson CJ, Li JY, Lai YM, Gantz I 2003 Inverse agonist activity of agouti and agouti-related protein. Peptides 24:603–609 [DOI] [PubMed] [Google Scholar]
- Shutter JR, Graham M, Kinsey AC, Scully S, Lüthy R, Stark KL 1997 Hypothalamic expression of ART, a novel gene related to agouti, is up-regulated in obese and diabetic mutant mice. Genes Dev 11:593–602 [DOI] [PubMed] [Google Scholar]
- Ilnytska O, Argyropoulos G 2008 The role of the agouti-related protein in energy balance regulation. Cell Mol Life Sci 65:2721–2731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M, Kim MS, Morgan DG, Small CJ, Edwards CM, Sunter D, Abusnana S, Goldstone AP, Russell SH, Stanley SA, Smith DM, Yagaloff K, Ghatei MA, Bloom SR 1998 A C-terminal fragment of agouti-related protein increases feeding and antagonizes the effect of α-melanocyte stimulating hormone in vivo. Endocrinology 139:4428–4431 [DOI] [PubMed] [Google Scholar]
- Kim MS, Rossi M, Abusnana S, Sunter D, Morgan DG, Small CJ, Edwards CM, Heath MM, Stanley SA, Seal LJ, Bhatti JR, Smith DM, Ghatei MA, Bloom SR 2000 Hypothalamic localization of the feeding effect of agouti-related peptide and α-melanocyte-stimulating hormone. Diabetes 49:177–182 [DOI] [PubMed] [Google Scholar]
- Wirth MM, Giraudo SQ 2000 Agouti-related protein in the hypothalamic paraventricular nucleus: effect on feeding. Peptides 21:1369–1375 [DOI] [PubMed] [Google Scholar]
- Ebihara K, Ogawa Y, Katsuura G, Numata Y, Masuzaki H, Satoh N, Tamaki M, Yoshioka T, Hayase M, Matsuoka N, Aizawa-Abe M, Yoshimasa Y, Nakao K 1999 Involvement of agouti-related protein, an endogenous antagonist of hypothalamic melanocortin receptor, in leptin action. Diabetes 48:2028–2033 [DOI] [PubMed] [Google Scholar]
- Qian S, Chen H, Weingarth D, Trumbauer ME, Novi DE, Guan X, Yu H, Shen Z, Feng Y, Frazier E, Chen A, Camacho RE, Shearman LP, Gopal-Truter S, MacNeil DJ, Van der Ploeg LH, Marsh DJ 2002 Neither agouti-related protein nor neuropeptide Y is critically required for the regulation of energy homeostasis in mice. Mol Cell Biol 22:5027–5035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortley KE, Anderson KD, Yasenchak J, Murphy A, Valenzuela D, Diano S, Yancopoulos GD, Wiegand SJ, Sleeman MW 2005 Agouti-related protein-deficient mice display an age-related lean phenotype. Cell Metab 2:421–427 [DOI] [PubMed] [Google Scholar]
- Redmann Jr SM, Argyropoulos G 2006 AgRP-deficiency could lead to increased lifespan. Biochem Biophys Res Commun 351:860–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luquet S, Perez FA, Hnasko TS, Palmiter RD 2005 NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 310:683–685 [DOI] [PubMed] [Google Scholar]
- Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, Plum L, Balthasar N, Hampel B, Waisman A, Barsh GS, Horvath TL, Brüning JC 2005 Agouti-related peptide-expressing neurons are mandatory for feeding. Nat Neurosci 8:1289–1291 [DOI] [PubMed] [Google Scholar]
- Xu AW, Kaelin CB, Morton GJ, Ogimoto K, Stanhope K, Graham J, Baskin DG, Havel P, Schwartz MW, Barsh GS 2005 Effects of hypothalamic neurodegeneration on energy balance. PLoS Biol 3:e415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewick GA, Gardiner JV, Dhillo WS, Kent AS, White NE, Webster Z, Ghatei MA, Bloom SR 2005 Post-embryonic ablation of AgRP neurons in mice leads to a lean, hypophagic phenotype. FASEB J 19:1680–1682 [DOI] [PubMed] [Google Scholar]
- Wu Q, Howell MP, Cowley MA, Palmiter RD 2008 Starvation after AgRP neuron ablation is independent of melanocortin signaling. Proc Natl Acad Sci USA 105:2687–2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfield AS, Lam DD, Marston OJ, Przydzial MJ, Heisler LK 2009 Role of central melanocortin pathways in energy homeostasis. Trends Endocrinol Metab 20:203–215 [DOI] [PubMed] [Google Scholar]
- Vink T, Hinney A, van Elburg AA, van Goozen SH, Sandkuijl LA, Sinke RJ, Herpertz-Dahlmann BM, Hebebrand J, Remschmidt H, van Engeland H, Adan RA 2001 Association between an agouti-related protein gene polymorphism and anorexia nervosa. Mol Psychiatry 6:325–328 [DOI] [PubMed] [Google Scholar]
- Marks DL, Boucher N, Lanouette CM, Pérusse L, Brookhart G, Comuzzie AG, Chagnon YC, Cone RD 2004 Ala67Thr polymorphism in the agouti-related peptide gene is associated with inherited leanness in humans. Am J Med Genet A 126A:267–271 [DOI] [PubMed] [Google Scholar]
- de Rijke CE, Jackson PJ, Garner KM, van Rozen RJ, Douglas NR, Kas MJ, Millhauser GL, Adan RA 2005 Functional analysis of the Ala67Thr polymorphism in agouti related protein associated with anorexia nervosa and leanness. Biochem Pharmacol 70:308–316 [DOI] [PubMed] [Google Scholar]
- Reizes O, Lincecum J, Wang Z, Goldberger O, Huang L, Kaksonen M, Ahima R, Hinkes MT, Barsh GS, Rauvala H, Bernfield M 2001 Transgenic expression of syndecan-1 uncovers a physiological control of feeding behavior by syndecan-3. Cell 106:105–116 [DOI] [PubMed] [Google Scholar]
- Strader AD, Reizes O, Woods SC, Benoit SC, Seeley RJ 2004 Mice lacking the syndecan-3 gene are resistant to diet-induced obesity. J Clin Invest 114:1354–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizes O, Benoit SC, Strader AD, Clegg DJ, Akunuru S, Seeley RJ 2003 Syndecan-3 modulates food intake by interacting with the melanocortin/AgRP pathway. Ann NY Acad Sci 994:66–73 [DOI] [PubMed] [Google Scholar]
- Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD 1999 Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron 24:155–163 [DOI] [PubMed] [Google Scholar]
- Fan W, Voss-Andreae A, Cao WH, Morrison SF 2005 Regulation of thermogenesis by the central melanocortin system. Peptides 26:1800–1813 [DOI] [PubMed] [Google Scholar]
- Haynes WG, Morgan DA, Djalali A, Sivitz WI, Mark AL 1999 Interactions between the melanocortin system and leptin in control of sympathetic nerve traffic. Hypertension 33:542–547 [DOI] [PubMed] [Google Scholar]
- Rahmouni K, Haynes WG, Morgan DA, Mark AL 2003 Role of melanocortin-4 receptors in mediating renal sympathoactivation to leptin and insulin. J Neurosci 23:5998–6004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh N, Ogawa Y, Katsuura G, Numata Y, Masuzaki H, Yoshimasa Y, Nakao K 1998 Satiety effect and sympathetic activation of leptin are mediated by hypothalamic melanocortin system. Neurosci Lett 249:107–110 [DOI] [PubMed] [Google Scholar]
- Li G, Zhang Y, Wilsey JT, Scarpace PJ 2005 Hypothalamic pro-opiomelanocortin gene delivery ameliorates obesity and glucose intolerance in aged rats. Diabetologia 48:2376–2385 [DOI] [PubMed] [Google Scholar]
- Voss-Andreae A, Murphy JG, Ellacott KL, Stuart RC, Nillni EA, Cone RD, Fan W 2007 Role of the central melanocortin circuitry in adaptive thermogenesis of brown adipose tissue. Endocrinology 148:1550–1560 [DOI] [PubMed] [Google Scholar]
- Fan W, Morrison SF, Cao WH, Yu P 2007 Thermogenesis activated by central melanocortin signaling is dependent on neurons in the rostral raphe pallidus (rRPa) area. Brain Res 1179:61–69 [DOI] [PubMed] [Google Scholar]
- Schiöth HB, Muceniece R, Wikberg JE 1996 Characterisation of the melanocortin 4 receptor by radioligand binding. Pharmacol Toxicol 79:161–165 [DOI] [PubMed] [Google Scholar]
- Harrold JA, Williams G 2006 Melanocortin-4 receptors, β-MSH and leptin: key elements in the satiety pathway. Peptides 27:365–371 [DOI] [PubMed] [Google Scholar]
- Kask A, Rägo L, Wikberg JE, Schiöth HB 2000 Differential effects of melanocortin peptides on ingestive behaviour in rats: evidence against the involvement of MC(3) receptor in the regulation of food intake. Neurosci Lett 283:1–4 [DOI] [PubMed] [Google Scholar]
- Abbott CR, Rossi M, Kim M, AlAhmed SH, Taylor GM, Ghatei MA, Smith DM, Bloom SR 2000 Investigation of the melanocyte stimulating hormones on food intake. Lack of evidence to support a role for the melanocortin-3-receptor. Brain Res 869:203–210 [DOI] [PubMed] [Google Scholar]
- Hsiung HM, Hertel J, Zhang XY, Smith DP, Smiley DL, Heiman ML, Yang DD, Husain S, Mayer JP, Zhang L, Mo H, Yan LZ 2005 A novel and selective β-melanocyte-stimulating hormone-derived peptide agonist for melanocortin 4 receptor potently decreased food intake and body weight gain in diet-induced obese rats. Endocrinology 146:5257–5266 [DOI] [PubMed] [Google Scholar]
- Tung YC, Piper SJ, Yeung D, O'Rahilly S, Coll AP 2006 A comparative study of the central effects of specific proopiomelancortin (POMC)-derived melanocortin peptides on food intake and body weight in POMC null mice. Endocrinology 147:5940–5947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biebermann H, Castañeda TR, van Landeghem F, von Deimling A, Escher F, Brabant G, Hebebrand J, Hinney A, Tschöp MH, Grüters A, Krude H 2006 A role for β-melanocyte-stimulating hormone in human body-weight regulation. Cell Metab 3:141–146 [DOI] [PubMed] [Google Scholar]
- Lee YS, Challis BG, Thompson DA, Yeo GS, Keogh JM, Madonna ME, Wraight V, Sims M, Vatin V, Meyre D, Shield J, Burren C, Ibrahim Z, Cheetham T, Swift P, Blackwood A, Hung CC, Wareham NJ, Froguel P, Millhauser GL, O'Rahilly S, Farooqi IS 2006 A POMC variant implicates β-melanocyte-stimulating hormone in the control of human energy balance. Cell Metab 3:135–140 [DOI] [PubMed] [Google Scholar]
- Cone RD 2005 Anatomy and regulation of the central melanocortin system. Nat Neurosci 8:571–578 [DOI] [PubMed] [Google Scholar]
- Palkovits M, Mezey E, Eskay RL 1987 Pro-opiomelanocortin-derived peptides (ACTH/β-endorphin/α-MSH) in brainstem baroreceptor areas of the rat. Brain Res 436:323–338 [DOI] [PubMed] [Google Scholar]
- Bronstein DM, Schafer MK, Watson SJ, Akil H 1992 Evidence that β-endorphin is synthesized in cells in the nucleus tractus solitarius: detection of POMC mRNA. Brain Res 587:269–275 [DOI] [PubMed] [Google Scholar]
- Grill HJ, Ginsberg AB, Seeley RJ, Kaplan JM 1998 Brainstem application of melanocortin receptor ligands produces long-lasting effects on feeding and body weight. J Neurosci 18:10128–10135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KS, Gentry RM, Rowland NE 1998 Central injection in rats of α-melanocyte-stimulating hormone analog: effects on food intake and brain Fos. Regul Pept 78:89–94 [DOI] [PubMed] [Google Scholar]
- Skibicka KP, Grill HJ 2008 Energetic responses are triggered by caudal brainstem melanocortin receptor stimulation and mediated by local sympathetic effector circuits. Endocrinology 149:3605–3616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibicka KP, Grill HJ 2009 Hindbrain leptin stimulation induces anorexia and hyperthermia mediated by hindbrain melanocortin receptors. Endocrinology 150:1705–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL, Bowers RR, Bartness TJ, Kaplan JM, Grill HJ 2003 Brainstem melanocortin 3/4 receptor stimulation increases uncoupling protein gene expression in brown fat. Endocrinology 144:4692–4697 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM 1994 Positional cloning of the mouse obese gene and its human homologue. Nature 372:425–432 [DOI] [PubMed] [Google Scholar]
- Cheung CC, Clifton DK, Steiner RA 1997 Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology 138:4489–4492 [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ, Woods SC, Weigle DS, Campfield LA, Burn P, Baskin DG 1997 Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes 46:2119–2123 [DOI] [PubMed] [Google Scholar]
- Thornton JE, Cheung CC, Clifton DK, Steiner RA 1997 Regulation of hypothalamic proopiomelanocortin mRNA by leptin in ob/ob mice. Endocrinology 138:5063–5066 [DOI] [PubMed] [Google Scholar]
- Mizuno TM, Kleopoulos SP, Bergen HT, Roberts JL, Priest CA, Mobbs CV 1998 Hypothalamic pro-opiomelanocortin mRNA is reduced by fasting and [corrected] in ob/ob and db/db mice, but is stimulated by leptin. Diabetes [Erratum (1998) 47):696] 47:294–297 [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD, Low MJ 2001 Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411:480–484 [DOI] [PubMed] [Google Scholar]
- Hahn TM, Breininger JF, Baskin DG, Schwartz MW 1998 Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci 1:271–272 [DOI] [PubMed] [Google Scholar]
- Mizuno TM, Mobbs CV 1999 Hypothalamic agouti-related protein messenger ribonucleic acid is inhibited by leptin and stimulated by fasting. Endocrinology 140:814–817 [DOI] [PubMed] [Google Scholar]
- Seeley RJ, Yagaloff KA, Fisher SL, Burn P, Thiele TE, van Dijk G, Baskin DG, Schwartz MW 1997 Melanocortin receptors in leptin effects. Nature 390:349 [DOI] [PubMed] [Google Scholar]
- Boston BA, Blaydon KM, Varnerin J, Cone RD 1997 Independent and additive effects of central POMC and leptin pathways on murine obesity. Science 278:1641–1644 [DOI] [PubMed] [Google Scholar]
- Trevaskis JL, Butler AA 2005 Double leptin and melanocortin-4 receptor gene mutations have an additive effect on fat mass and are associated with reduced effects of leptin on weight loss and food intake. Endocrinology 146:4257–4265 [DOI] [PubMed] [Google Scholar]
- Trevaskis JL, Meyer EA, Galgani JE, Butler AA 2008 Counterintuitive effects of double-heterozygous null melanocortin-4 receptor and leptin genes on diet-induced obesity and insulin resistance in C57BL/6J mice. Endocrinology 149:174–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmquist JK, Elias CF, Saper CB 1999 From lesions to leptin: hypothalamic control of food intake and body weight. Neuron 22:221–232 [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte Jr D, Seeley RJ, Baskin DG 2000 Central nervous system control of food intake. Nature 404:661–671 [DOI] [PubMed] [Google Scholar]
- Spiegelman BM, Flier JS 2001 Obesity and the regulation of energy balance. Cell 104:531–543 [DOI] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW 2006 Central nervous system control of food intake and body weight. Nature 443:289–295 [DOI] [PubMed] [Google Scholar]
- Coll AP, Farooqi IS, O'Rahilly S 2007 The hormonal control of food intake. Cell 129:251–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP 1996 Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 84:491–495 [DOI] [PubMed] [Google Scholar]
- Phillips MS, Liu Q, Hammond HA, Dugan V, Hey PJ, Caskey CJ, Hess JF 1996 Leptin receptor missense mutation in the fatty Zucker rat. Nat Genet 13:18–19 [DOI] [PubMed] [Google Scholar]
- Yaswen L, Diehl N, Brennan MB, Hochgeschwender U 1999 Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med 5:1066–1070 [DOI] [PubMed] [Google Scholar]
- Challis BG, Coll AP, Yeo GS, Pinnock SB, Dickson SL, Thresher RR, Dixon J, Zahn D, Rochford JJ, White A, Oliver RL, Millington G, Aparicio SA, Colledge WH, Russ AP, Carlton MB, O'Rahilly S 2004 Mice lacking pro-opiomelanocortin are sensitive to high-fat feeding but respond normally to the acute anorectic effects of peptide-YY(3-36). Proc Natl Acad Sci USA 101:4695–4700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naggert JK, Fricker LD, Varlamov O, Nishina PM, Rouille Y, Steiner DF, Carroll RJ, Paigen BJ, Leiter EH 1995 Hyperproinsulinaemia in obese fat/fat mice associated with a carboxypeptidase E mutation which reduces enzyme activity. Nat Genet 10:135–142 [DOI] [PubMed] [Google Scholar]
- Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, Cheetham CH, Earley AR, Barnett AH, Prins JB, O'Rahilly S 1997 Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 387:903–908 [DOI] [PubMed] [Google Scholar]
- Strobel A, Issad T, Camoin L, Ozata M, Strosberg AD 1998 A leptin missense mutation associated with hypogonadism and morbid obesity. Nat Genet 18:213–215 [DOI] [PubMed] [Google Scholar]
- Clément K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte JM, Basdevant A, Bougnères P, Lebouc Y, Froguel P, Guy-Grand B 1998 A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature 392:398–401 [DOI] [PubMed] [Google Scholar]
- Krude H, Biebermann H, Luck W, Horn R, Brabant G, Grüters A 1998 Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet 19:155–157 [DOI] [PubMed] [Google Scholar]
- Jackson RS, Creemers JW, Ohagi S, Raffin-Sanson ML, Sanders L, Montague CT, Hutton JC, O'Rahilly S 1997 Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat Genet 16:303–306 [DOI] [PubMed] [Google Scholar]
- Barsh GS, Farooqi IS, O'Rahilly S 2000 Genetics of body-weight regulation. Nature 404:644–651 [DOI] [PubMed] [Google Scholar]
- Coll AP, Farooqi IS, Challis BG, Yeo GS, O'Rahilly S 2004 Proopiomelanocortin and energy balance: insights from human and murine genetics. J Clin Endocrinol Metab 89:2557–2562 [DOI] [PubMed] [Google Scholar]
- Bell CG, Walley AJ, Froguel P 2005 The genetics of human obesity. Nat Rev Genet 6:221–234 [DOI] [PubMed] [Google Scholar]
- Farooqi IS, O'Rahilly S 2008 Mutations in ligands and receptors of the leptin-melanocortin pathway that lead to obesity. Nat Clin Pract Endocrinol Metab 4:569–577 [DOI] [PubMed] [Google Scholar]
- Walley AJ, Asher JE, Froguel P 2009 The genetic contribution to non-syndromic human obesity. Nat Rev Genet 10:431–442 [DOI] [PubMed] [Google Scholar]
- Michaud JL, Boucher F, Melnyk A, Gauthier F, Goshu E, Lévy E, Mitchell GA, Himms-Hagen J, Fan CM 2001 Sim1 haploinsufficiency causes hyperphagia, obesity and reduction of the paraventricular nucleus of the hypothalamus. Hum Mol Genet 10:1465–1473 [DOI] [PubMed] [Google Scholar]
- Holder Jr JL, Butte NF, Zinn AR 2000 Profound obesity associated with a balanced translocation that disrupts the SIM1 gene. Hum Mol Genet 9:101–108 [DOI] [PubMed] [Google Scholar]
- Ellacott KL, Cone RD 2004 The central melanocortin system and the integration of short- and long-term regulators of energy homeostasis. Recent Prog Horm Res 59:395–408 [DOI] [PubMed] [Google Scholar]
- Gautron L, Lee C, Funahashi H, Friedman J, Lee S, Elmquist J 2010 Melanocortin-4 receptor expression in a vago-vagal circuitry involved in postprandial functions. J Comp Neurol 518:6–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koegler FH, Grove KL, Schiffmacher A, Smith MS, Cameron JL 2001 Central melanocortin receptors mediate changes in food intake in the rhesus macaque. Endocrinology 142: 2586–2592 [DOI] [PubMed] [Google Scholar]
- Barb CR, Robertson AS, Barrett JB, Kraeling RR, Houseknecht KL 2004 The role of melanocortin-3 and -4 receptor in regulating appetite, energy homeostasis and neuroendocrine function in the pig. J Endocrinol 181:39–52 [DOI] [PubMed] [Google Scholar]
- Wagner CG, McMahon CD, Marks DL, Daniel JA, Steele B, Sartin JL 2004 A role for agouti-related protein in appetite regulation in a species with continuous nutrient delivery. Neuroendocrinology 80:210–218 [DOI] [PubMed] [Google Scholar]
- Fan ZC, Sartin JL, Tao YX 2008 Pharmacological analyses of two naturally occurring porcine melanocortin-4 receptor mutations in domestic pigs. Domest Anim Endocrinol 34:383–390 [DOI] [PubMed] [Google Scholar]
- Kim KS, Larsen N, Short T, Plastow G, Rothschild MF 2000 A missense variant of the porcine melanocortin-4 receptor (MC4R) gene is associated with fatness, growth, and feed intake traits. Mamm Genome 11:131–135 [DOI] [PubMed] [Google Scholar]
- Kim KS, Reecy JM, Hsu WH, Anderson LL, Rothschild MF 2004 Functional and phylogenetic analyses of a melanocortin-4 receptor mutation in domestic pigs. Domest Anim Endocrinol 26:75–86 [DOI] [PubMed] [Google Scholar]
- Patten CS, Daniels D, Suzuki A, Fluharty SJ, Yee DK 2007 Structural and signaling requirements of the human melanocortin 4 receptor for MAP kinase activation. Regul Pept 142:111–122 [DOI] [PubMed] [Google Scholar]
- Liu GL, Jiang SW, Xiong YZ, Zheng R, Qu YC 2002 [Molecular screening of MC4R gene and association with fat traits in pig resource family]. Yi Chuan Xue Bao 29:497–501 [PubMed] [Google Scholar]
- Hernández-Sánchez J, Visscher P, Plastow G, Haley C 2003 Candidate gene analysis for quantitative traits using the transmission disequilibrium test: the example of the melanocortin 4-receptor in pigs. Genetics 164:637–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston RD, Cameron ND, Rance KA 2004 A melanocortin-4 receptor (MC4R) polymorphism is associated with performance traits in divergently selected Large White pig populations. Anim Genet 35:386–390 [DOI] [PubMed] [Google Scholar]
- Meidtner K, Wermter AK, Hinney A, Remschmidt H, Hebebrand J, Fries R 2006 Association of the melanocortin 4 receptor with feed intake and daily gain in F2 Mangalitsa × Pietrain pigs. Anim Genet 37:245–247 [DOI] [PubMed] [Google Scholar]
- Park HB, Carlborg O, Marklund S, Andersson L 2002 Melanocortin-4 receptor (MC4R) genotypes have no major effect on fatness in a Large White × Wild Boar intercross. Anim Genet 33:155–157 [DOI] [PubMed] [Google Scholar]
- Andersson L 2003 Melanocortin receptor variants with phenotypic effects in horse, pig, and chicken. Ann NY Acad Sci 994:313–318 [DOI] [PubMed] [Google Scholar]
- Stachowiak M, Szydlowski M, Obarzanek-Fojt M, Switonski M 2006 An effect of a missense mutation in the porcine melanocortin-4 receptor (MC4R) gene on production traits in Polish pig breeds is doubtful. Anim Genet 37:55–57 [DOI] [PubMed] [Google Scholar]
- Lkhagvadorj S, Qu L, Cai W, Couture OP, Barb CR, Hausman GJ, Nettleton D, Anderson LL, Dekkers JC, Tuggle CK 2009 Microarray gene expression profiles of fasting induced changes in liver and adipose tissues of pigs expressing the melanocortin-4 receptor D298N variant. Physiol Genomics 38:98–111 [DOI] [PubMed] [Google Scholar]
- Iqbal J, Pompolo S, Dumont LM, Wu CS, Mountjoy KG, Henry BA, Clarke IJ 2001 Long-term alterations in body weight do not affect the expression of melanocortin receptor-3 and -4 mRNA in the ovine hypothalamus. Neuroscience 105:931–940 [DOI] [PubMed] [Google Scholar]
- Henry BA, Rao A, Ikenasio BA, Mountjoy KG, Tilbrook AJ, Clarke IJ 2001 Differential expression of cocaine- and amphetamine-regulated transcript and agouti related-protein in chronically food-restricted sheep. Brain Res 918:40–50 [DOI] [PubMed] [Google Scholar]
- Archer ZA, Findlay PA, McMillen SR, Rhind SM, Adam CL 2004 Effects of nutritional status and gonadal steroids on expression of appetite-regulatory genes in the hypothalamic arcuate nucleus of sheep. J Endocrinol 182:409–419 [DOI] [PubMed] [Google Scholar]
- Sorensen A, Adam CL, Findlay PA, Marie M, Thomas L, Travers MT, Vernon RG 2002 Leptin secretion and hypothalamic neuropeptide and receptor gene expression in sheep. Am J Physiol Regul Integr Comp Physiol 282:R1227–R1235 [DOI] [PubMed] [Google Scholar]
- Sartin JL, Wagner CG, Marks DL, Daniel JA, McMahon CD, Obese FY, Partridge C 2005 Melanocortin-4 receptor in sheep: a potential site for therapeutic intervention in disease models. Domest Anim Endocrinol 29:446–455 [DOI] [PubMed] [Google Scholar]
- Sartin JL, Marks DL, McMahon CD, Daniel JA, Levasseur P, Wagner CG, Whitlock BK, Steele BP 2008 Central role of the melanocortin-4 receptors in appetite regulation after endotoxin. J Anim Sci 86:2557–2567 [DOI] [PubMed] [Google Scholar]
- Kawakami S, Bungo T, Ando R, Ohgushi A, Shimojo M, Masuda Y, Furuse M 2000 Central administration of α-melanocyte stimulating hormone inhibits fasting- and neuropeptide Y-induced feeding in neonatal chicks. Eur J Pharmacol 398:361–364 [DOI] [PubMed] [Google Scholar]
- Smith ML, Prall B, Nandar W, Cline MA 2008 β-Melanocyte-stimulating hormone potently reduces appetite via the hypothalamus in chicks. J Neuroendocrinol 20:220–226 [DOI] [PubMed] [Google Scholar]
- Tachibana T, Sugahara K, Ohgushi A, Ando R, Kawakami S, Yoshimatsu T, Furuse M 2001 Intracerebroventricular injection of agouti-related protein attenuates the anorexigenic effect of α-melanocyte stimulating hormone in neonatal chicks. Neurosci Lett 305:131–134 [DOI] [PubMed] [Google Scholar]
- Cline MA, Nandar W, Bowden C, Hein PP, Denbow DM, Siegel PB 2008 Differential feeding responses to central α-melanocyte stimulating hormone in genetically low and high body weight selected lines of chickens. Life Sci 83:208–213 [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Takahashi S 1999 A possible involvement of melanocortin 3 receptor in the regulation of adrenal gland function in the chicken. Biochim Biophys Acta 1448:512–518 [DOI] [PubMed] [Google Scholar]
- Cerdá-Reverter JM, Schiöth HB, Peter RE 2003 The central melanocortin system regulates food intake in goldfish. Regul Pept 115:101–113 [DOI] [PubMed] [Google Scholar]
- Schjolden J, Schiöth HB, Larhammar D, Winberg S, Larson ET 2009 Melanocortin peptides affect the motivation to feed in rainbow trout (Oncorhynchus mykiss). Gen Comp Endocrinol 160:134–138 [DOI] [PubMed] [Google Scholar]
- Boswell T, Li Q, Takeuchi S 2002 Neurons expressing neuropeptide Y mRNA in the infundibular hypothalamus of Japanese quail are activated by fasting and co-express agouti-related protein mRNA. Brain Res Mol Brain Res 100:31–42 [DOI] [PubMed] [Google Scholar]
- Cerdá-Reverter JM, Peter RE 2003 Endogenous melanocortin antagonist in fish: structure, brain mapping, and regulation by fasting of the goldfish agouti-related protein gene. Endocrinology 144:4552–4561 [DOI] [PubMed] [Google Scholar]
- Song Y, Golling G, Thacker TL, Cone RD 2003 Agouti-related protein (AGRP) is conserved and regulated by metabolic state in the zebrafish, Danio rerio. Endocrine 22:257–265 [DOI] [PubMed] [Google Scholar]
- Phillips-Singh D, Li Q, Takeuchi S, Ohkubo T, Sharp PJ, Boswell T 2003 Fasting differentially regulates expression of agouti-related peptide, pro-opiomelanocortin, prepro-orexin, and vasoactive intestinal polypeptide mRNAs in the hypothalamus of Japanese quail. Cell Tissue Res 313:217–225 [DOI] [PubMed] [Google Scholar]
- Song Y, Cone RD 2007 Creation of a genetic model of obesity in a teleost. FASEB J 21:2042–2049 [DOI] [PubMed] [Google Scholar]
- Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, Tecott LH, Reichardt LF 2003 Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci 6:736–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson JR, Peter JC, Lecourt AC, Barde YA, Hofbauer KG 2007 Melanocortin-4 receptor activation stimulates hypothalamic brain-derived neurotrophic factor release to regulate food intake, body temperature and cardiovascular function. J Neuroendocrinol 19:974–982 [DOI] [PubMed] [Google Scholar]
- Bariohay B, Roux J, Tardivel C, Trouslard J, Jean A, Lebrun B 2009 Brain-derived neurotrophic factor/tropomyosin-related kinase receptor type B signaling is a downstream effector of the brainstem melanocortin system in food intake control. Endocrinology 150:2646–2653 [DOI] [PubMed] [Google Scholar]
- Fekete C, Légrádi G, Mihály E, Huang QH, Tatro JB, Rand WM, Emerson CH, Lechan RM 2000 α-Melanocyte-stimulating hormone is contained in nerve terminals innervating thyrotropin-releasing hormone-synthesizing neurons in the hypothalamic paraventricular nucleus and prevents fasting-induced suppression of prothyrotropin-releasing hormone gene expression. J Neurosci 20:1550–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete C, Légrádi G, Mihály E, Tatro JB, Rand WM, Lechan RM 2000 α-Melanocyte stimulating hormone prevents fasting-induced suppression of corticotropin-releasing hormone gene expression in the rat hypothalamic paraventricular nucleus. Neurosci Lett 289:152–156 [DOI] [PubMed] [Google Scholar]
- Hanada R, Nakazato M, Matsukura S, Murakami N, Yoshimatsu H, Sakata T 2000 Differential regulation of melanin-concentrating hormone and orexin genes in the agouti-related protein/melanocortin-4 receptor system. Biochem Biophys Res Commun 268:88–91 [DOI] [PubMed] [Google Scholar]
- Lu XY, Barsh GS, Akil H, Watson SJ 2003 Interaction between α-melanocyte-stimulating hormone and corticotropin-releasing hormone in the regulation of feeding and hypothalamo-pituitary-adrenal responses. J Neurosci 23:7863–7872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamisoyama H, Honda K, Saneyasu T, Sugahara K, Hasegawa S 2009 Corticotropin-releasing factor is a downstream mediator of the β-melanocyte-stimulating hormone-induced anorexigenic pathway in chicks. Neurosci Lett 458:102–105 [DOI] [PubMed] [Google Scholar]
- Matsuda K, Kojima K, Shimakura S, Wada K, Maruyama K, Uchiyama M, Kikuyama S, Shioda S 2008 Corticotropin-releasing hormone mediates α-melanocyte-stimulating hormone-induced anorexigenic action in goldfish. Peptides 29:1930–1936 [DOI] [PubMed] [Google Scholar]
- Lechan RM, Fekete C 2006 Role of melanocortin signaling in the regulation of the hypothalamic-pituitary-thyroid (HPT) axis. Peptides 27:310–325 [DOI] [PubMed] [Google Scholar]
- Kim MS, Small CJ, Stanley SA, Morgan DG, Seal LJ, Kong WM, Edwards CM, Abusnana S, Sunter D, Ghatei MA, Bloom SR 2000 The central melanocortin system affects the hypothalamo-pituitary thyroid axis and may mediate the effect of leptin. J Clin Invest 105:1005–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small CJ, Kim MS, Stanley SA, Mitchell JR, Murphy K, Morgan DG, Ghatei MA, Bloom SR 2001 Effects of chronic central nervous system administration of agouti-related protein in pair-fed animals. Diabetes 50:248–254 [DOI] [PubMed] [Google Scholar]
- Fekete C, Sarkar S, Rand WM, Harney JW, Emerson CH, Bianco AC, Lechan RM 2002 Agouti-related protein (AGRP) has a central inhibitory action on the hypothalamic-pituitary-thyroid (HPT) axis; comparisons between the effect of AGRP and neuropeptide Y on energy homeostasis and the HPT axis. Endocrinology 143:3846–3853 [DOI] [PubMed] [Google Scholar]
- Fekete C, Marks DL, Sarkar S, Emerson CH, Rand WM, Cone RD, Lechan RM 2004 Effect of agouti-related protein in regulation of the hypothalamic-pituitary-thyroid axis in the melanocortin 4 receptor knockout mouse. Endocrinology 145:4816–4821 [DOI] [PubMed] [Google Scholar]
- Harris M, Aschkenasi C, Elias CF, Chandrankunnel A, Nillni EA, Bjøorbaek C, Elmquist JK, Flier JS, Hollenberg AN 2001 Transcriptional regulation of the thyrotropin-releasing hormone gene by leptin and melanocortin signaling. J Clin Invest 107:111–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias CF, Saper CB, Maratos-Flier E, Tritos NA, Lee C, Kelly J, Tatro JB, Hoffman GE, Ollmann MM, Barsh GS, Sakurai T, Yanagisawa M, Elmquist JK 1998 Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol 402:442–459 [PubMed] [Google Scholar]
- López M, Lage R, Tung YC, Challis BG, Varela L, Virtue S, O'Rahilly S, Vidal-Puig A, Diéguez C, Coll AP 2007 Orexin expression is regulated by α-melanocyte-stimulating hormone. J Neuroendocrinol 19:703–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud JL, Rosenquist T, May NR, Fan CM 1998 Development of neuroendocrine lineages requires the bHLH-PAS transcription factor SIM1. Genes Dev 12:3264–3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kublaoui BM, Holder Jr JL, Gemelli T, Zinn AR 2006 Sim1 haploinsufficiency impairs melanocortin-mediated anorexia and activation of paraventricular nucleus neurons. Mol Endocrinol 20:2483–2492 [DOI] [PubMed] [Google Scholar]
- Kublaoui BM, Holder Jr JL, Tolson KP, Gemelli T, Zinn AR 2006 SIM1 overexpression partially rescues agouti yellow and diet-induced obesity by normalizing food intake. Endocrinology 147:4542–4549 [DOI] [PubMed] [Google Scholar]
- Segal JP, Stallings NR, Lee CE, Zhao L, Socci N, Viale A, Harris TM, Soares MB, Childs G, Elmquist JK, Parker KL, Friedman JM 2005 Use of laser-capture microdissection for the identification of marker genes for the ventromedial hypothalamic nucleus. J Neurosci 25:4181–4188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks DL, Cone RD 2001 Central melanocortins and the regulation of weight during acute and chronic disease. Recent Prog Horm Res 56:359–375 [DOI] [PubMed] [Google Scholar]
- Deboer MD, Marks DL 2006 Cachexia: lessons from melanocortin antagonism. Trends Endocrinol Metab 17:199–204 [DOI] [PubMed] [Google Scholar]
- DeBoer MD, Marks DL 2006 Therapy insight: use of melanocortin antagonists in the treatment of cachexia in chronic disease. Nat Clin Pract Endocrinol Metab 2:459–466 [DOI] [PubMed] [Google Scholar]
- Scarlett JM, Jobst EE, Enriori PJ, Bowe DD, Batra AK, Grant WF, Cowley MA, Marks DL 2007 Regulation of central melanocortin signaling by interleukin-1β. Endocrinology 148:4217–4225 [DOI] [PubMed] [Google Scholar]
- Marks DL, Ling N, Cone RD 2001 Role of the central melanocortin system in cachexia. Cancer Res 61:1432–1438 [PubMed] [Google Scholar]
- Marks DL, Butler AA, Turner R, Brookhart G, Cone RD 2003 Differential role of melanocortin receptor subtypes in cachexia. Endocrinology 144:1513–1523 [DOI] [PubMed] [Google Scholar]
- Cheung W, Yu PX, Little BM, Cone RD, Marks DL, Mak RH 2005 Role of leptin and melanocortin signaling in uremia-associated cachexia. J Clin Invest 115:1659–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang QH, Hruby VJ, Tatro JB 1999 Role of central melanocortins in endotoxin-induced anorexia. Am J Physiol 276:R864–R871 [DOI] [PubMed] [Google Scholar]
- Lawrence CB, Rothwell NJ 2001 Anorexic but not pyrogenic actions of interleukin-1 are modulated by central melanocortin-3/4 receptors in the rat. J Neuroendocrinol 13:490–495 [DOI] [PubMed] [Google Scholar]
- Joppa MA, Ling N, Chen C, Gogas KR, Foster AC, Markison S 2005 Central administration of peptide and small molecule MC4 receptor antagonists induce hyperphagia in mice and attenuate cytokine-induced anorexia. Peptides 26:2294–2301 [DOI] [PubMed] [Google Scholar]
- Wisse BE, Frayo RS, Schwartz MW, Cummings DE 2001 Reversal of cancer anorexia by blockade of central melanocortin receptors in rats. Endocrinology 142:3292–3301 [DOI] [PubMed] [Google Scholar]
- Cheung WW, Rosengren S, Boyle DL, Mak RH 2008 Modulation of melanocortin signaling ameliorates uremic cachexia. Kidney Int 74:180–186 [DOI] [PubMed] [Google Scholar]
- Vos TJ, Caracoti A, Che JL, Dai M, Farrer CA, Forsyth NE, Drabic SV, Horlick RA, Lamppu D, Yowe DL, Balani S, Li P, Zeng H, Joseph IB, Rodriguez LE, Maguire MP, Patane MA, Claiborne CF 2004 Identification of 2-[2-[2-(5-bromo-2-methoxyphenyl)-ethyl]-3-fluorophenyl]-4,5-dihydro-1H-imidazole (ML00253764), a small molecule melanocortin 4 receptor antagonist that effectively reduces tumor-induced weight loss in a mouse model. J Med Chem 47:1602–1604 [DOI] [PubMed] [Google Scholar]
- Nicholson JR, Kohler G, Schaerer F, Senn C, Weyermann P, Hofbauer KG 2006 Peripheral administration of a melanocortin 4-receptor inverse agonist prevents loss of lean body mass in tumor-bearing mice. J Pharmacol Exp Ther 317:771–777 [DOI] [PubMed] [Google Scholar]
- Markison S, Foster AC, Chen C, Brookhart GB, Hesse A, Hoare SR, Fleck BA, Brown BT, Marks DL 2005 The regulation of feeding and metabolic rate and the prevention of murine cancer cachexia with a small-molecule melanocortin-4 receptor antagonist. Endocrinology 146:2766–2773 [DOI] [PubMed] [Google Scholar]
- Cheung WW, Kuo HJ, Markison S, Chen C, Foster AC, Marks DL, Mak RH 2007 Peripheral administration of the melanocortin-4 receptor antagonist NBI-12i ameliorates uremia-associated cachexia in mice. J Am Soc Nephrol 18:2517–2524 [DOI] [PubMed] [Google Scholar]
- Chen C, Tucci FC, Jiang W, Tran JA, Fleck BA, Hoare SR, Wen J, Chen T, Johns M, Markison S, Foster AC, Marinkovic D, Chen CW, Arellano M, Harman J, Saunders J, Bozigian H, Marks D 2008 Pharmacological and pharmacokinetic characterization of 2-piperazine-α-isopropyl benzylamine derivatives as melanocortin-4 receptor antagonists. Bioorg Med Chem 16:5606–5618 [DOI] [PubMed] [Google Scholar]
- Weyermann P, Dallmann R, Magyar J, Anklin C, Hufschmid M, Dubach-Powell J, Courdier-Fruh I, Henneböhle M, Nordhoff S, Mondadori C 2009 Orally available selective melanocortin-4 receptor antagonists stimulate food intake and reduce cancer-induced cachexia in mice. PLoS One 4:e4774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo JJ, Silva AA, Hall JE 2003 Hypothalamic melanocortin receptors and chronic regulation of arterial pressure and renal function. Hypertension 41:768–774 [DOI] [PubMed] [Google Scholar]
- Silva AA, Kuo JJ, Tallam LS, Liu J, Hall JE 2006 Does obesity induce resistance to the long-term cardiovascular and metabolic actions of melanocortin 3/4 receptor activation? Hypertension 47:259–264 [DOI] [PubMed] [Google Scholar]
- da Silva AA, do Carmo JM, Freeman JN, Tallam LS, Hall JE 2009 A functional melanocortin system may be required for chronic CNS-mediated antidiabetic and cardiovascular actions of leptin. Diabetes 58:1749–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallam LS, Kuo JJ, da Silva AA, Hall JE 2004 Cardiovascular, renal, and metabolic responses to chronic central administration of agouti-related peptide. Hypertension 44:853–858 [DOI] [PubMed] [Google Scholar]
- da Silva AA, Kuo JJ, Hall JE 2004 Role of hypothalamic melanocortin 3/4-receptors in mediating chronic cardiovascular, renal, and metabolic actions of leptin. Hypertension 43:1312–1317 [DOI] [PubMed] [Google Scholar]
- da Silva AA, do Carmo JM, Kanyicska B, Dubinion J, Brandon E, Hall JE 2008 Endogenous melanocortin system activity contributes to the elevated arterial pressure in spontaneously hypertensive rats. Hypertension 51:884–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura K, Tsuchihashi T, Abe I, Iida M 2002 Central α-melanocyte-stimulating hormone acts at melanocortin-4 receptor to activate sympathetic nervous system in conscious rabbits. Brain Res 948:145–148 [DOI] [PubMed] [Google Scholar]
- Kuo JJ, da Silva AA, Tallam LS, Hall JE 2004 Role of adrenergic activity in pressor responses to chronic melanocortin receptor activation. Hypertension 43:370–375 [DOI] [PubMed] [Google Scholar]
- Balthasar N 2009 Feeding signals to the hungry mind. Exp Physiol 94:857–866 [DOI] [PubMed] [Google Scholar]
- Ni XP, Butler AA, Cone RD, Humphreys MH 2006 Central receptors mediating the cardiovascular actions of melanocyte stimulating hormones. J Hypertens 24:2239–2246 [DOI] [PubMed] [Google Scholar]
- do Carmo JM, Tallam LS, Roberts JV, Brandon EL, Biglane J, da Silva AA, Hall JE 2009 Impact of obesity on renal structure and function in the presence and absence of hypertension: evidence from melanocortin-4 receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol 297:R803–R812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Cameron GS, Astruc B, Mayer JP, Brage S, See TC, Lomas DJ, O'Rahilly S, Farooqi IS 2009 Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med 360:44–52 [DOI] [PubMed] [Google Scholar]
- Nordheim U, Nicholson JR, Dokladny K, Dunant P, Hofbauer KG 2006 Cardiovascular responses to melanocortin 4-receptor stimulation in conscious unrestrained normotensive rats. Peptides 27:438–443 [DOI] [PubMed] [Google Scholar]
- Fan W, Dinulescu DM, Butler AA, Zhou J, Marks DL, Cone RD 2000 The central melanocortin system can directly regulate serum insulin levels. Endocrinology 141:3072–3079 [DOI] [PubMed] [Google Scholar]
- Obici S, Feng Z, Tan J, Liu L, Karkanias G, Rossetti L 2001 Central melanocortin receptors regulate insulin action. J Clin Invest 108:1079–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banno R, Arima H, Sato I, Hayashi M, Goto M, Sugimura Y, Murase T, Oiso Y 2004 The melanocortin agonist melanotan II increases insulin sensitivity in OLETF rats. Peptides 25:1279–1286 [DOI] [PubMed] [Google Scholar]
- Heijboer AC, van den Hoek AM, Pijl H, Voshol PJ, Havekes LM, Romijn JA, Corssmit EP 2005 Intracerebroventricular administration of melanotan II increases insulin sensitivity of glucose disposal in mice. Diabetologia 48:1621–1626 [DOI] [PubMed] [Google Scholar]
- Zhou L, Sutton GM, Rochford JJ, Semple RK, Lam DD, Oksanen LJ, Thornton-Jones ZD, Clifton PG, Yueh CY, Evans ML, McCrimmon RJ, Elmquist JK, Butler AA, Heisler LK 2007 Serotonin 2C receptor agonists improve type 2 diabetes via melanocortin-4 receptor signaling pathways. Cell Metab 6:398–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banno R, Arima H, Hayashi M, Goto M, Watanabe M, Sato I, Ozaki N, Nagasaki H, Ozaki N, Oiso Y 2007 Central administration of melanocortin agonist increased insulin sensitivity in diet-induced obese rats. FEBS Lett 581:1131–1136 [DOI] [PubMed] [Google Scholar]
- Kumar KG, Sutton GM, Dong JZ, Roubert P, Plas P, Halem HA, Culler MD, Yang H, Dixit VD, Butler AA 2009 Analysis of the therapeutic functions of novel melanocortin receptor agonists in MC3R- and MC4R-deficient C57BL/6J mice. Peptides 30:1892–1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour M, White D, Wernette C, Dennis J, Tao YX, Collins R, Parker L, Morrison E 2010 Pancreatic neuronal melanocortin-4 receptor modulates serum insulin levels independent of leptin receptor. Endocrine 37:220–230 [DOI] [PubMed] [Google Scholar]
- Toda C, Shiuchi T, Lee S, Yamato-Esaki M, Fujino Y, Suzuki A, Okamoto S, Minokoshi Y 2009 Distinct effects of leptin and a melanocortin receptor agonist injected into medial hypothalamic nuclei on glucose uptake in peripheral tissues. Diabetes 58:2757–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno TM, Kelley KA, Pasinetti GM, Roberts JL, Mobbs CV 2003 Transgenic neuronal expression of proopiomelanocortin attenuates hyperphagic response to fasting and reverses metabolic impairments in leptin-deficient obese mice. Diabetes 52:2675–2683 [DOI] [PubMed] [Google Scholar]
- Lee M, Kim A, Chua Jr SC, Obici S, Wardlaw SL 2007 Transgenic MSH overexpression attenuates the metabolic effects of a high-fat diet. Am J Physiol Endocrinol Metab 293:E121–E131 [DOI] [PubMed] [Google Scholar]
- Tanaka T, Masuzaki H, Yasue S, Ebihara K, Shiuchi T, Ishii T, Arai N, Hirata M, Yamamoto H, Hayashi T, Hosoda K, Minokoshi Y, Nakao K 2007 Central melanocortin signaling restores skeletal muscle AMP-activated protein kinase phosphorylation in mice fed a high-fat diet. Cell Metab 5:395–402 [DOI] [PubMed] [Google Scholar]
- Masuzaki H, Tanaka T, Ebihara K, Hosoda K, Nakao K 2009 Hypothalamic melanocortin signaling and leptin resistance—perspective of therapeutic application for obesity-diabetes syndrome. Peptides 30:1383–1386 [DOI] [PubMed] [Google Scholar]
- Nogueiras R, Wiedmer P, Perez-Tilve D, Veyrat-Durebex C, Keogh JM, Sutton GM, Pfluger PT, Castaneda TR, Neschen S, Hofmann SM, Howles PN, Morgan DA, Benoit SC, Szanto I, Schrott B, Schürmann A, Joost HG, Hammond C, Hui DY, Woods SC, Rahmouni K, Butler AA, Farooqi IS, O'Rahilly S, Rohner-Jeanrenaud F, Tschöp MH 2007 The central melanocortin system directly controls peripheral lipid metabolism. J Clin Invest 117:3475–3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Zhang Y, Wilsey JT, Scarpace PJ 2004 Unabated anorexic and enhanced thermogenic responses to melanotan II in diet-induced obese rats despite reduced melanocortin 3 and 4 receptor expression. J Endocrinol 182:123–132 [DOI] [PubMed] [Google Scholar]
- Samama P, Rumennik L, Grippo JF 2003 The melanocortin receptor MCR4 controls fat consumption. Regul Pept 113:85–88 [DOI] [PubMed] [Google Scholar]
- Koegler FH, Schaffhauser RO, Mynatt RL, York DA, Bray GA 1999 Macronutrient diet intake of the lethal yellow agouti (Ay/a) mouse. Physiol Behav 67:809–812 [DOI] [PubMed] [Google Scholar]
- Hagan MM, Rushing PA, Benoit SC, Woods SC, Seeley RJ 2001 Opioid receptor involvement in the effect of AgRP-(83-132) on food intake and food selection. Am J Physiol Regul Integr Comp Physiol 280:R814–R821 [DOI] [PubMed] [Google Scholar]
- Tung YC, Rimmington D, O'Rahilly S, Coll AP 2007 Pro-opiomelanocortin modulates the thermogenic and physical activity responses to high-fat feeding and markedly influences dietary fat preference. Endocrinology 148:5331–5338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrock M, Schulz A, Merkwitz C, Schöneberg T, Spanel-Borowski K, Ricken A 2009 Reduction in corpora lutea number in obese melanocortin-4-receptor-deficient mice. Reprod Biol Endocrinol 7:24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Ploeg LH, Martin WJ, Howard AD, Nargund RP, Austin CP, Guan X, Drisko J, Cashen D, Sebhat I, Patchett AA, Figueroa DJ, DiLella AG, Connolly BM, Weinberg DH, Tan CP, Palyha OC, Pong SS, MacNeil T, Rosenblum C, Vongs A, Tang R, Yu H, Sailer AW, Fong TM, Huang C, Tota MR, Chang RS, Stearns R, Tamvakopoulos C, Christ G, Drazen DL, Spar BD, Nelson RJ, MacIntyre DE 2002 A role for the melanocortin 4 receptor in sexual function. Proc Natl Acad Sci USA 99:11381–11386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani BG, Xiang Z, Moore MC, Mandel RJ, Haskell- Luevano C 2005 Voluntary exercise delays monogenetic obesity and overcomes reproductive dysfunction of the melanocortin-4 receptor knockout mouse. Biochem Biophys Res Commun 326:638–644 [DOI] [PubMed] [Google Scholar]
- Ward DR, Dear FM, Ward IA, Anderson SI, Spergel DJ, Smith PA, Ebling FJ 2009 Innervation of gonadotropin-releasing hormone neurons by peptidergic neurons conveying circadian or energy balance information in the mouse. PLoS One 4:e5322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley SA, Small CJ, Kim MS, Heath MM, Seal LJ, Russell SH, Ghatei MA, Bloom SR 1999 Agouti related peptide (Agrp) stimulates the hypothalamo pituitary gonadal axis in vivo, in vitro in male rats. Endocrinology 140:5459–5462 [DOI] [PubMed] [Google Scholar]
- Khong K, Kurtz SE, Sykes RL, Cone RD 2001 Expression of functional melanocortin-4 receptor in the hypothalamic GT1-1 cell line. Neuroendocrinology 74:193–201 [DOI] [PubMed] [Google Scholar]
- Chai B, Li JY, Zhang W, Newman E, Ammori J, Mulholland MW 2006 Melanocortin-4 receptor-mediated inhibition of apoptosis in immortalized hypothalamic neurons via mitogen-activated protein kinase. Peptides 27:2846–2857 [DOI] [PubMed] [Google Scholar]
- Watanobe H, Schiöth HB, Wikberg JE, Suda T 1999 The melanocortin 4 receptor mediates leptin stimulation of luteinizing hormone and prolactin surges in steroid-primed ovariectomized rats. Biochem Biophys Res Commun 257:860–864 [DOI] [PubMed] [Google Scholar]
- Schioth HB, Kakizaki Y, Kohsaka A, Suda T, Watanobe H 2001 Agouti-related peptide prevents steroid-induced luteinizing hormone and prolactin surges in female rats. Neuroreport 12:687–690 [DOI] [PubMed] [Google Scholar]
- Watanobe H, Yoneda M, Kakizaki Y, Kohsaka A, Suda T, Schiöth HB 2001 Further evidence for a significant participation of the melanocortin 4 receptor in the preovulatory prolactin surge in the rat. Brain Res Bull 54:521–525 [DOI] [PubMed] [Google Scholar]
- Vulliémoz NR, Xiao E, Xia-Zhang L, Wardlaw SL, Ferin M 2005 Central infusion of agouti-related peptide suppresses pulsatile luteinizing hormone release in the ovariectomized rhesus monkey. Endocrinology 146:784–789 [DOI] [PubMed] [Google Scholar]
- Wessells H, Hruby VJ, Hackett J, Han G, Balse-Srinivasan P, Vanderah TW 2003 Ac-Nle-c[Asp-His-DPhe-Arg-Trp-Lys]-NH2 induces penile erection via brain and spinal melanocortin receptors. Neuroscience 118:755–762 [DOI] [PubMed] [Google Scholar]
- Dorr RT, Lines R, Levine N, Brooks C, Xiang L, Hruby VJ, Hadley ME 1996 Evaluation of melanotan-II, a superpotent cyclic melanotropic peptide in a pilot phase-I clinical study. Life Sci 58:1777–1784 [DOI] [PubMed] [Google Scholar]
- Wessells H, Gralnek D, Dorr R, Hruby VJ, Hadley ME, Levine N 2000 Effect of an α-melanocyte stimulating hormone analog on penile erection and sexual desire in men with organic erectile dysfunction. Urology 56:641–646 [DOI] [PubMed] [Google Scholar]
- Hadley ME, Dorr RT 2006 Melanocortin peptide therapeutics: historical milestones, clinical studies and commercialization. Peptides 27:921–930 [DOI] [PubMed] [Google Scholar]
- King SH, Mayorov AV, Balse-Srinivasan P, Hruby VJ, Vanderah TW, Wessells H 2007 Melanocortin receptors, melanotropic peptides and penile erection. Curr Top Med Chem 7:1098–1106 [PMC free article] [PubMed] [Google Scholar]
- Pfaus JG, Shadiack A, Van Soest T, Tse M, Molinoff P 2004 Selective facilitation of sexual solicitation in the female rat by a melanocortin receptor agonist. Proc Natl Acad Sci USA 101:10201–10204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna R, Wong P, Stevens C, De Lepeleire I, Van Dyck K, Rosen RC, Gendrano 3rd IN, Peeters M, Wagner JA, Herman GA 2008 Lack of erectogenic activity of a novel selective melanocortin-4 receptor agonist in a clinical experimental model. J Clin Pharmacol 48:1237–1241 [DOI] [PubMed] [Google Scholar]
- Ercil NE, Galici R, Kesterson RA 2005 HS014, a selective melanocortin-4 (MC4) receptor antagonist, modulates the behavioral effects of morphine in mice. Psychopharmacology (Berl) 180:279–285 [DOI] [PubMed] [Google Scholar]
- Alvaro JD, Tatro JB, Quillan JM, Fogliano M, Eisenhard M, Lerner MR, Nestler EJ, Duman RS 1996 Morphine down-regulates melanocortin-4 receptor expression in brain regions that mediate opiate addiction. Mol Pharmacol 50:583–591 [PubMed] [Google Scholar]
- Contreras PC, Takemori AE 1984 Antagonism of morphine-induced analgesia, tolerance and dependence by α-melanocyte-stimulating hormone. J Pharmacol Exp Ther 229:21–26 [PubMed] [Google Scholar]
- Alvaro JD, Tatro JB, Duman RS 1997 Melanocortins and opiate addiction. Life Sci 61:1–9 [DOI] [PubMed] [Google Scholar]
- Alvaro JD, Taylor JR, Duman RS 2003 Molecular and behavioral interactions between central melanocortins and cocaine. J Pharmacol Exp Ther 304:391–399 [DOI] [PubMed] [Google Scholar]
- Hsu R, Taylor JR, Newton SS, Alvaro JD, Haile C, Han G, Hruby VJ, Nestler EJ, Duman RS 2005 Blockade of melanocortin transmission inhibits cocaine reward. Eur J Neurosci 21:2233–2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Kim GY, Carr KD 2002 The melanocortin receptor agonist MTII augments the rewarding effect of amphetamine in ad-libitum-fed and food-restricted rats. Psychopharmacology (Berl) 161:77–85 [DOI] [PubMed] [Google Scholar]
- Thiele TE, Navarro M, Sparta DR, Fee JR, Knapp DJ, Cubero I 2003 Alcoholism and obesity: overlapping neuropeptide pathways? Neuropeptides 37:321–337 [DOI] [PubMed] [Google Scholar]
- Navarro M, Cubero I, Chen AS, Chen HY, Knapp DJ, Breese GR, Marsh DJ, Thiele TE 2005 Effects of melanocortin receptor activation and blockade on ethanol intake: a possible role for the melanocortin-4 receptor. Alcohol Clin Exp Res 29:949–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploj K, Roman E, Kask A, Hyytiä P, Schiöth HB, Wikberg JE, Nylander I 2002 Effects of melanocortin receptor ligands on ethanol intake and opioid peptide levels in alcohol-preferring AA rats. Brain Res Bull 59:97–104 [DOI] [PubMed] [Google Scholar]
- Polidori C, Geary N, Massi M 2006 Effect of the melanocortin receptor stimulation or inhibition on ethanol intake in alcohol-preferring rats. Peptides 27:144–149 [DOI] [PubMed] [Google Scholar]
- Navarro M, Cubero I, Knapp DJ, Thiele TE 2003 MTII-induced reduction of voluntary ethanol drinking is blocked by pretreatment with AgRP-(83-132). Neuropeptides 37:338–344 [DOI] [PubMed] [Google Scholar]
- Kokare DM, Singru PS, Dandekar MP, Chopde CT, Subhedar NK 2008 Involvement of α-melanocyte stimulating hormone (α-MSH) in differential ethanol exposure and withdrawal related depression in rat: neuroanatomical-behavioral correlates. Brain Res 1216:53–67 [DOI] [PubMed] [Google Scholar]
- Beltramo M, Campanella M, Tarozzo G, Fredduzzi S, Corradini L, Forlani A, Bertorelli R, Reggiani A 2003 Gene expression profiling of melanocortin system in neuropathic rats supports a role in nociception. Brain Res Mol Brain Res 118:111–118 [DOI] [PubMed] [Google Scholar]
- Sandman CA, Kastin AJ 1981 Intraventricular administration of MSH induces hyperalgesia in rats. Peptides 2:231–233 [DOI] [PubMed] [Google Scholar]
- Starowicz K, Przewlocki R, Gispen WH, Przewlocka B 2002 Modulation of melanocortin-induced changes in spinal nociception by μ-opioid receptor agonist and antagonist in neuropathic rats. Neuroreport 13:2447–2452 [DOI] [PubMed] [Google Scholar]
- Vrinten DH, Gispen WH, Groen GJ, Adan RA 2000 Antagonism of the melanocortin system reduces cold and mechanical allodynia in mononeuropathic rats. J Neurosci 20:8131–8137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertorelli R, Fredduzzi S, Tarozzo G, Campanella M, Grundy R, Beltramo M, Reggiani A 2005 Endogenous and exogenous melanocortin antagonists induce anti-allodynic effects in a model of rat neuropathic pain. Behav Brain Res 157:55–62 [DOI] [PubMed] [Google Scholar]
- Starowicz K, Sieja A, Bilecki W, Obara I, Przewlocka B 2003 The effect of morphine on MC4 and CRF receptor mRNAs in the rat amygdala and attenuation of tolerance after their blockade. Brain Res 990:113–119 [DOI] [PubMed] [Google Scholar]
- Kalange AS, Kokare DM, Singru PS, Upadhya MA, Chopde CT, Subhedar NK 2007 Central administration of selective melanocortin 4 receptor antagonist HS014 prevents morphine tolerance and withdrawal hyperalgesia. Brain Res 1181:10–20 [DOI] [PubMed] [Google Scholar]
- Tanabe K, Gamo K, Aoki S, Wada K, Kiyama H 2007 Melanocortin receptor 4 is induced in nerve-injured motor and sensory neurons of mouse. J Neurochem 101:1145–1152 [DOI] [PubMed] [Google Scholar]
- Adan RA, van der Kraan M, Doornbos RP, Bär PR, Burbach JP, Gispen WH 1996 Melanocortin receptors mediate α-MSH-induced stimulation of neurite outgrowth in neuro 2A cells. Brain Res Mol Brain Res 36:37–44 [DOI] [PubMed] [Google Scholar]
- Catania A, Gatti S, Colombo G, Lipton JM 2004 Targeting melanocortin receptors as a novel strategy to control inflammation. Pharmacol Rev 56:1–29 [DOI] [PubMed] [Google Scholar]
- Getting SJ 2006 Targeting melanocortin receptors as potential novel therapeutics. Pharmacol Ther 111:1–15 [DOI] [PubMed] [Google Scholar]
- Lasaga M, Debeljuk L, Durand D, Scimonelli TN, Caruso C 2008 Role of α-melanocyte stimulating hormone and melanocortin 4 receptor in brain inflammation. Peptides 29:1825–1835 [DOI] [PubMed] [Google Scholar]
- Cragnolini AB, Caruso C, Lasaga M, Scimonelli TN 2006 α-MSH and γ-MSH modulate early release of hypothalamic PGE2 and NO induced by IL-1β differently. Neurosci Lett 409:168–172 [DOI] [PubMed] [Google Scholar]
- Giuliani D, Mioni C, Bazzani C, Zaffe D, Botticelli AR, Capolongo S, Sabba A, Galantucci M, Iannone A, Grieco P, Novellino E, Colombo G, Tomasi A, Catania A, Guarini S 2007 Selective melanocortin MC4 receptor agonists reverse haemorrhagic shock and prevent multiple organ damage. Br J Pharmacol 150:595–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muceniece R, Zvejniece L, Vilskersts R, Liepinsh E, Baumane L, Kalvinsh I, Wikberg JE, Dambrova M 2007 Functional evaluation of THIQ, a melanocortin 4 receptor agonist, in models of food intake and inflammation. Basic Clin Pharmacol Toxicol 101:416–420 [DOI] [PubMed] [Google Scholar]
- Giuliani D, Mioni C, Altavilla D, Leone S, Bazzani C, Minutoli L, Bitto A, Cainazzo MM, Marini H, Zaffe D, Botticelli AR, Pizzala R, Savio M, Necchi D, Schiöth HB, Bertolini A, Squadrito F, Guarini S 2006 Both early and delayed treatment with melanocortin 4 receptor-stimulating melanocortins produces neuroprotection in cerebral ischemia. Endocrinology 147:1126–1135 [DOI] [PubMed] [Google Scholar]
- Giuliani D, Leone S, Mioni C, Bazzani C, Zaffe D, Botticelli AR, Altavilla D, Galantucci M, Minutoli L, Bitto A, Squadrito F, Guarini S 2006 Broad therapeutic treatment window of [Nle(4), D-Phe(7)]α-melanocyte-stimulating hormone for long-lasting protection against ischemic stroke, in Mongolian gerbils. Eur J Pharmacol 538:48–56 [DOI] [PubMed] [Google Scholar]
- Catania A 2008 Neuroprotective actions of melanocortins: a therapeutic opportunity. Trends Neurosci 31:353–360 [DOI] [PubMed] [Google Scholar]
- Cragnolini AB, Schiöth HB, Scimonelli TN 2006 Anxiety-like behavior induced by IL-1β is modulated by α-MSH through central melanocortin-4 receptors. Peptides 27:1451–1456 [DOI] [PubMed] [Google Scholar]
- Kokare DM, Chopde CT, Subhedar NK 2006 Participation of α-melanocyte stimulating hormone in ethanol-induced anxiolysis and withdrawal anxiety in rats. Neuropharmacology 51:536–545 [DOI] [PubMed] [Google Scholar]
- Chaki S, Okuyama S 2005 Involvement of melanocortin-4 receptor in anxiety and depression. Peptides 26:1952–1964 [DOI] [PubMed] [Google Scholar]
- Chaki S, Hirota S, Funakoshi T, Suzuki Y, Suetake S, Okubo T, Ishii T, Nakazato A, Okuyama S 2003 Anxiolytic-like and antidepressant-like activities of MCL0129 (1-[(S)-2-(4-fluorophenyl)-2-(4-isopropylpiperadin-1-yl)ethyl]-4-[4-(2-met hoxynaphthalen-1-yl)butyl]piperazine), a novel and potent nonpeptide antagonist of the melanocortin-4 receptor. J Pharmacol Exp Ther 304:818–826 [DOI] [PubMed] [Google Scholar]
- Sinha PS, Schiöth HB, Tatro JB 2004 Roles of the melanocortin-4 receptor in antipyretic and hyperthermic actions of centrally administered α-MSH. Brain Res 1001:150–158 [DOI] [PubMed] [Google Scholar]
- Glyn JR, Lipton JM 1981 Hypothermic and antipyretic effects of centrally administered ACTH (1-24) and α-melanotropin. Peptides 2:177–187 [DOI] [PubMed] [Google Scholar]
- Gonzalez PV, Schiöth HB, Lasaga M, Scimonelli TN 2009 Memory impairment induced by IL-1β is reversed by α-MSH through central melanocortin-4 receptors. Brain Behav Immun 23:817–822 [DOI] [PubMed] [Google Scholar]
- Hogan K, Peluso S, Gould S, Parsons I, Ryan D, Wu L, Visiers I 2006 Mapping the binding site of melanocortin 4 receptor agonists: a hydrophobic pocket formed by I3.28(125), I3.32(129), and I7.42(291) is critical for receptor activation. J Med Chem 49:911–922 [DOI] [PubMed] [Google Scholar]
- Schiöth HB, Petersson S, Muceniece R, Szardenings M, Wikberg JE 1997 Deletions of the N-terminal regions of the human melanocortin receptors. FEBS Lett 410:223–228 [DOI] [PubMed] [Google Scholar]
- Yang YK, Dickinson CJ, Zeng Q, Li JY, Thompson DA, Gantz I 1999 Contribution of melanocortin receptor exoloops to agouti-related protein binding. J Biol Chem 274:14100–14106 [DOI] [PubMed] [Google Scholar]
- Yang Y, Chen M, Lai Y, Gantz I, Georgeson KE, Harmon CM 2002 Molecular determinants of human melanocortin-4 receptor responsible for antagonist SHU9119 selective activity. J Biol Chem 277:20328–20335 [DOI] [PubMed] [Google Scholar]
- Yang YK, Fong TM, Dickinson CJ, Mao C, Li JY, Tota MR, Mosley R, Van Der Ploeg LH, Gantz I 2000 Molecular determinants of ligand binding to the human melanocortin-4 receptor. Biochemistry 39:14900–14911 [DOI] [PubMed] [Google Scholar]
- Haskell-Luevano C, Cone RD, Monck EK, Wan YP 2001 Structure activity studies of the melanocortin-4 receptor by in vitro mutagenesis: identification of agouti-related protein (AGRP), melanocortin agonist and synthetic peptide antagonist interaction determinants. Biochemistry 40:6164–6179 [DOI] [PubMed] [Google Scholar]
- Chen M, Cai M, Aprahamian CJ, Georgeson KE, Hruby V, Harmon CM, Yang Y 2007 Contribution of the conserved amino acids of the melanocortin-4 receptor in D-[Nle4,Phe7]-α-melanocyte-stimulating hormone binding and signaling. J Biol Chem 282:21712–21719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SX, Fan ZC, Tao YX 2008 Functions of acidic transmembrane residues in human melanocortin-3 receptor binding and activation. Biochem Pharmacol 76:520–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickolls SA, Cismowski MI, Wang X, Wolff M, Conlon PJ, Maki RA 2003 Molecular determinants of melanocortin 4 receptor ligand binding and MC4/MC3 receptor selectivity. J Pharmacol Exp Ther 304:1217–1227 [DOI] [PubMed] [Google Scholar]
- Adan RA, Cone RD, Burbach JP, Gispen WH 1994 Differential effects of melanocortin peptides on neural melanocortin receptors. Mol Pharmacol 46:1182–1190 [PubMed] [Google Scholar]
- Oosterom J, Nijenhuis WA, Schaaper WM, Slootstra J, Meloen RH, Gispen WH, Burbach JP, Adan RA 1999 Conformation of the core sequence in melanocortin peptides directs selectivity for the melanocortin MC3 and MC4 receptors. J Biol Chem 274:16853–16860 [DOI] [PubMed] [Google Scholar]
- Chen M, Cai M, McPherson D, Hruby V, Harmon CM, Yang Y 2009 Contribution of the transmembrane domain 6 of melanocortin-4 receptor to peptide [Pro5, DNal (2`)8]-γ-MSH selectivity. Biochem Pharmacol 77:114–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg Y, Overton J, Vaisse C, Leibel RL, Rost B 2009 In silico mutagenesis: a case study of the melanocortin 4 receptor. FASEB J 23:3059–3069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao YX 2005 Molecular mechanisms of the neural melanocortin receptor dysfunction in severe early onset obesity. Mol Cell Endocrinol 239:1–14 [DOI] [PubMed] [Google Scholar]
- Biebermann H, Krude H, Elsner A, Chubanov V, Gudermann T, Grüters A 2003 Autosomal-dominant mode of inheritance of a melanocortin-4 receptor mutation in a patient with severe early-onset obesity is due to a dominant-negative effect caused by receptor dimerization. Diabetes 52:2984–2988 [DOI] [PubMed] [Google Scholar]
- Ceresa BP, Limbird LE 1994 Mutation of an aspartate residue highly conserved among G-protein-coupled receptors results in nonreciprocal disruption of α2-adrenergic receptor-G-protein interactions. J Biol Chem 269:29557–29564 [PubMed] [Google Scholar]
- Tao Q, Abood ME 1998 Mutation of a highly conserved aspartate residue in the second transmembrane domain of the cannabinoid receptors, CB1 and CB2, disrupts G-protein coupling. J Pharmacol Exp Ther 285:651–658 [PubMed] [Google Scholar]
- Roche JP, Bounds S, Brown S, Mackie K 1999 A mutation in the second transmembrane region of the CB1 receptor selectively disrupts G protein signaling and prevents receptor internalization. Mol Pharmacol 56:611–618 [DOI] [PubMed] [Google Scholar]
- Rettenbacher E, Tarnow P, Brumm H, Prayer D, Wermter AK, Hebebrand J, Biebermann H, Hinney A, Widhalm K 2007 A novel non-synonymous mutation in the melanocortin-4 receptor gene (MC4R) in a 2-year-old Austrian girl with extreme obesity. Exp Clin Endocrinol Diabetes 115:7–12 [DOI] [PubMed] [Google Scholar]
- Tan K, Pogozheva ID, Yeo GS, Hadaschik D, Keogh JM, Haskell-Leuvano C, O'Rahilly S, Mosberg HI, Farooqi IS 2009 Functional characterization and structural modeling of obesity associated mutations in the melanocortin 4 receptor. Endocrinology 150:114–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan ZC, Tao YX 2009 Functional characterization and pharmacological rescue of melanocortin-4 receptor mutations identified from obese patients. J Cell Mol Med 13:3268–3282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao YX, Segaloff DL 2004 Functional characterization of melanocortin-3 receptor variants identify a loss-of-function mutation involving an amino acid critical for G protein-coupled receptor activation. J Clin Endocrinol Metab 89:3936–3942 [DOI] [PubMed] [Google Scholar]
- Tolle V, Low MJ 2008 In vivo evidence for inverse agonism of agouti-related peptide in the central nervous system of proopiomelanocortin-deficient mice. Diabetes 57:86–94 [DOI] [PubMed] [Google Scholar]
- Yang YK, Ollmann MM, Wilson BD, Dickinson C, Yamada T, Barsh GS, Gantz I 1997 Effects of recombinant agouti-signaling protein on melanocortin action. Mol Endocrinol 11:274–280 [DOI] [PubMed] [Google Scholar]
- Oosterom J, Garner KM, den Dekker WK, Nijenhuis WA, Gispen WH, Burbach JP, Barsh GS, Adan RA 2001 Common requirements for melanocortin-4 receptor selectivity of structurally unrelated melanocortin agonist and endogenous antagonist, agouti protein. J Biol Chem 276:931–936 [DOI] [PubMed] [Google Scholar]
- Yang Y, Chen M, Lai Y, Gantz I, Yagmurlu A, Georgeson KE, Harmon CM 2003 Molecular determination of agouti-related protein binding to human melanocortin-4 receptor. Mol Pharmacol 64:94–103 [DOI] [PubMed] [Google Scholar]
- Sebhat IK, Martin WJ, Ye Z, Barakat K, Mosley RT, Johnston DB, Bakshi R, Palucki B, Weinberg DH, MacNeil T, Kalyani RN, Tang R, Stearns RA, Miller RR, Tamvakopoulos C, Strack AM, McGowan E, Cashen DE, Drisko JE, Hom GJ, Howard AD, MacIntyre DE, van der Ploeg LH, Patchett AA, Nargund RP 2002 Design and pharmacology of N-[(3R)-1,2,3,4-tetrahydroisoquinolinium-3-ylcarbonyl]-(1R)-1-(4-chlorobenzyl)-2-[4-cyclohexyl-4-(1H-1,2,4-triazol-1-ylmethyl)piperidin-1-yl]-2-oxoethylamine(1), a potent, selective, melanocortin subtype-4 receptor agonist. J Med Chem 45:4589–4593 [DOI] [PubMed] [Google Scholar]
- Pogozheva ID, Chai BX, Lomize AL, Fong TM, Weinberg DH, Nargund RP, Mulholland MW, Gantz I, Mosberg HI 2005 Interactions of human melanocortin 4 receptor with nonpeptide and peptide agonists. Biochemistry 44:11329–11341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Cai M, Chen M, Qu H, McPherson D, Hruby V, Harmon CM 2009 Key amino acid residues in the melanocortin-4 receptor for nonpeptide THIQ specific binding and signaling. Regul Pept 155:46–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleck BA, Chen C, Yang W, Huntley R, Markison S, Nickolls SA, Foster AC, Hoare SR 2005 Molecular interactions of nonpeptide agonists and antagonists with the melanocortin-4 receptor. Biochemistry 44:14494–14508 [DOI] [PubMed] [Google Scholar]
- Tao YX 2008 Constitutive activation of G protein-coupled receptors and diseases: insights into mechanism of activation and therapeutics. Pharmacol Ther 120:129–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisse C, Clement K, Durand E, Hercberg S, Guy-Grand B, Froguel P 2000 Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. J Clin Invest 106:253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proneth B, Xiang Z, Pogozheva ID, Litherland SA, Gorbatyuk OS, Shaw AM, Millard WJ, Mosberg HI, Haskell-Luevano C 2006 Molecular mechanism of the constitutive activation of the L250Q human melanocortin-4 receptor polymorphism. Chem Biol Drug Des 67:215–229 [DOI] [PubMed] [Google Scholar]
- Hinney A, Hohmann S, Geller F, Vogel C, Hess C, Wermter AK, Brokamp B, Goldschmidt H, Siegfried W, Remschmidt H, Schäfer H, Gudermann T, Hebebrand J 2003 Melanocortin-4 receptor gene: case-control study and transmission disequilibrium test confirm that functionally relevant mutations are compatible with a major gene effect for extreme obesity. J Clin Endocrinol Metab 88:4258–4267 [DOI] [PubMed] [Google Scholar]
- Valli-Jaakola K, Lipsanen-Nyman M, Oksanen L, Hollenberg AN, Kontula K, Bjørbaek C, Schalin-Jäntti C 2004 Identification and characterization of melanocortin-4 receptor gene mutations in morbidly obese Finnish children and adults. J Clin Endocrinol Metab 89:940–945 [DOI] [PubMed] [Google Scholar]
- Hinney A, Bettecken T, Tarnow P, Brumm H, Reichwald K, Lichtner P, Scherag A, Nguyen TT, Schlumberger P, Rief W, Vollmert C, Illig T, Wichmann HE, Schäfer H, Platzer M, Biebermann H, Meitinger T, Hebebrand J 2006 Prevalence, spectrum, and functional characterization of melanocortin-4 receptor gene mutations in a representative population-based sample and obese adults from Germany. J Clin Endocrinol Metab 91:1761–1769 [DOI] [PubMed] [Google Scholar]
- Latronico AC, Abell AN, Arnhold IJ, Liu X, Lins TS, Brito VN, Billerbeck AE, Segaloff DL, Mendonca BB 1998 A unique constitutively activating mutation in the third transmembrane helix of the luteinizing hormone receptor causes sporadic male gonadotropin independent precocious puberty. J Clin Endocrinol Metab 83:2435–2440 [DOI] [PubMed] [Google Scholar]
- Baranski TJ, Herzmark P, Lichtarge O, Gerber BO, Trueheart J, Meng EC, Iiri T, Sheikh SP, Bourne HR 1999 C5a receptor activation: genetic identification of critical residues in four transmembrane helices. J Biol Chem 274:15757–15765 [DOI] [PubMed] [Google Scholar]
- Lu ZL, Hulme EC 1999 The functional topography of transmembrane domain 3 of the m1 muscarinic acetylcholine receptor, revealed by scanning mutagenesis. J Biol Chem 274:7309–7315 [DOI] [PubMed] [Google Scholar]
- Tao YX, Abell AN, Liu X, Nakamura K, Segaloff DL 2000 Constitutive activation of G protein-coupled receptors as a result of selective substitution of a conserved leucine residue in transmembrane helix III. Mol Endocrinol 14:1272–1282 [DOI] [PubMed] [Google Scholar]
- Kosugi S, Hai N, Okamoto H, Sugawa H, Mori T 2000 A novel activating mutation in the thyrotropin receptor gene in an autonomously functioning thyroid nodule developed by a Japanese patient. Eur J Endocrinol 143:471–477 [DOI] [PubMed] [Google Scholar]
- Trülzsch B, Krohn K, Wonerow P, Chey S, Holzapfel HP, Ackermann F, Führer D, Paschke R 2001 Detection of thyroid-stimulating hormone receptor and Gsα mutations: in 75 toxic thyroid nodules by denaturing gradient gel electrophoresis. J Mol Med 78:684–691 [DOI] [PubMed] [Google Scholar]
- Wang SX, Saunders D, Williams JN, Tao YX, Functional characterization of fifteen novel melanocortin-4 receptor mutations identified from obese patients. Program of the 91st Annual Meeting of The Endocrine Society, Washington, DC, 2009 (Abstract P3-379) [Google Scholar]
- Srinivasan S, Lubrano-Berthelier C, Govaerts C, Picard F, Santiago P, Conklin BR, Vaisse C 2004 Constitutive activity of the melanocortin-4 receptor is maintained by its N-terminal domain and plays a role in energy homeostasis in humans. J Clin Invest 114:1158–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adan RA, Kas MJ 2003 Inverse agonism gains weight. Trends Pharmacol Sci 24:315–321 [DOI] [PubMed] [Google Scholar]
- Adan RA 2006 Constitutive receptor activity series: endogenous inverse agonists and constitutive receptor activity in the melanocortin system. Trends Pharmacol Sci 27:183–186 [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Kong PL, Taylor JA, Willard DH, Wilkison WO 2001 Melanocortin receptor-mediated mobilization of intracellular free calcium in HEK293 cells. Physiol Genomics 5:11–19 [DOI] [PubMed] [Google Scholar]
- Nickolls SA, Fleck B, Hoare SR, Maki RA 2005 Functional selectivity of melanocortin 4 receptor peptide and nonpeptide agonists: evidence for ligand-specific conformational states. J Pharmacol Exp Ther 313:1281–1288 [DOI] [PubMed] [Google Scholar]
- Newman EA, Chai BX, Zhang W, Li JY, Ammori JB, Mulholland MW 2006 Activation of the melanocortin-4 receptor mobilizes intracellular free calcium in immortalized hypothalamic neurons. J Surg Res 132:201–207 [DOI] [PubMed] [Google Scholar]
- Büch TR, Heling D, Damm E, Gudermann T, Breit A 2009 Pertussis toxin sensitive signalling of melanocortin-4 receptors in hypothalamic GT1–7 cells defines AGRP as a biased agonist. J Biol Chem 284:26411–26420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton GM, Duos B, Patterson LM, Berthoud HR 2005 Melanocortinergic modulation of cholecystokinin-induced suppression of feeding through extracellular signal-regulated kinase signaling in rat solitary nucleus. Endocrinology 146:3739–3747 [DOI] [PubMed] [Google Scholar]
- Daniels D, Patten CS, Roth JD, Yee DK, Fluharty SJ 2003 Melanocortin receptor signaling through mitogen-activated protein kinase in vitro and in rat hypothalamus. Brain Res 986:1–11 [DOI] [PubMed] [Google Scholar]
- Vongs A, Lynn NM, Rosenblum CI 2004 Activation of MAP kinase by MC4-R through PI3 kinase. Regul Pept 120:113–118 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wu X, He Y, Kastin AJ, Hsuchou H, Rosenblum CI, Pan W 2009 Melanocortin potentiates leptin-induced STAT3 signaling via MAPK pathway. J Neurochem 110:390–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai B, Li JY, Zhang W, Wang H, Mulholland MW 2009 Melanocortin-4 receptor activation inhibits c-Jun N-terminal kinase activity and promotes insulin signaling. Peptides 30:1098–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierroz DD, Ziotopoulou M, Ungsunan L, Moschos S, Flier JS, Mantzoros CS 2002 Effects of acute and chronic administration of the melanocortin agonist MTII in mice with diet-induced obesity. Diabetes 51:1337–1345 [DOI] [PubMed] [Google Scholar]
- Jonsson L, Skarphedinsson JO, Skuladottir GV, Watanobe H, Schiöth HB 2002 Food conversion is transiently affected during 4-week chronic administration of melanocortin agonist and antagonist in rats. J Endocrinol 173:517–523 [DOI] [PubMed] [Google Scholar]
- Shinyama H, Masuzaki H, Fang H, Flier JS 2003 Regulation of melanocortin-4 receptor signaling: agonist-mediated desensitization and internalization. Endocrinology 144:1301–1314 [DOI] [PubMed] [Google Scholar]
- Gao Z, Lei D, Welch J, Le K, Lin J, Leng S, Duhl D 2003 Agonist-dependent internalization of the human melanocortin-4 receptors in human embryonic kidney 293 cells. J Pharmacol Exp Ther 307:870–877 [DOI] [PubMed] [Google Scholar]
- Breit A, Wolff K, Kalwa H, Jarry H, Büch T, Gudermann T 2006 The natural inverse agonist agouti-related protein induces arrestin-mediated endocytosis of melanocortin-3 and -4 receptors. J Biol Chem 281:37447–37456 [DOI] [PubMed] [Google Scholar]
- Mohammad S, Baldini G, Granell S, Narducci P, Martelli AM, Baldini G 2007 Constitutive traffic of melanocortin-4 receptor in neuro2A cells and immortalized hypothalamic neurons. J Biol Chem 282:4963–4974 [DOI] [PubMed] [Google Scholar]
- Mandrika I, Petrovska R, Wikberg J 2005 Melanocortin receptors form constitutive homo- and heterodimers. Biochem Biophys Res Commun 326:349–354 [DOI] [PubMed] [Google Scholar]
- Sánchez-Laorden BL, Sánchez-Más J, Martínez-Alonso E, Martínez-Menárguez JA, García-Borrón JC, Jiménez- Cervantes C 2006 Dimerization of the human melanocortin 1 receptor: functional consequences and dominant-negative effects. J Invest Dermatol 126:172–181 [DOI] [PubMed] [Google Scholar]
- Zanna PT, Sánchez-Laorden BL, Pérez-Oliva AB, Turpín MC, Herraiz C, Jiménez-Cervantes C, García-Borrón JC 2008 Mechanism of dimerization of the human melanocortin 1 receptor. Biochem Biophys Res Commun 368:211–216 [DOI] [PubMed] [Google Scholar]
- Nickolls SA, Maki RA 2006 Dimerization of the melanocortin 4 receptor: a study using bioluminescence resonance energy transfer. Peptides 27:380–387 [DOI] [PubMed] [Google Scholar]
- Ho G, MacKenzie RG 1999 Functional characterization of mutations in melanocortin-4 receptor associated with human obesity. J Biol Chem 274:35816–35822 [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Yeo GS, Keogh JM, Aminian S, Jebb SA, Butler G, Cheetham T, O'Rahilly S 2000 Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J Clin Invest 106:271–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo GS, Lank EJ, Farooqi IS, Keogh J, Challis BG, O'Rahilly S 2003 Mutations in the human melanocortin-4 receptor gene associated with severe familial obesity disrupts receptor function through multiple molecular mechanisms. Hum Mol Genet 12:561–574 [DOI] [PubMed] [Google Scholar]
- Colley NJ, Cassill JA, Baker EK, Zuker CS 1995 Defective intracellular transport is the molecular basis of rhodopsin-dependent dominant retinal degeneration. Proc Natl Acad Sci USA 92:3070–3074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkirane M, Jin DY, Chun RF, Koup RA, Jeang KT 1997 Mechanism of transdominant inhibition of CCR5-mediated HIV-1 infection by ccr5Δ32. J Biol Chem 272:30603–30606 [DOI] [PubMed] [Google Scholar]
- Overton MC, Blumer KJ 2000 G-protein-coupled receptors function as oligomers in vivo. Curr Biol 10:341–344 [DOI] [PubMed] [Google Scholar]
- Tao YX, Johnson NB, Segaloff DL 2004 Constitutive and agonist-dependent self-association of the cell surface human lutropin receptor. J Biol Chem 279:5904–5914 [DOI] [PubMed] [Google Scholar]
- Chabre M, Deterre P, Antonny B 2009 The apparent cooperativity of some GPCRs does not necessarily imply dimerization. Trends Pharmacol Sci 30:182–187 [DOI] [PubMed] [Google Scholar]
- Terrillon S, Bouvier M 2004 Roles of G-protein-coupled receptor dimerization. EMBO Rep 5:30–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulenger S, Marullo S, Bouvier M 2005 Emerging role of homo- and heterodimerization in G-protein-coupled receptor biosynthesis and maturation. Trends Pharmacol Sci 26:131–137 [DOI] [PubMed] [Google Scholar]
- Elsner A, Tarnow P, Schaefer M, Ambrugger P, Krude H, Grüters A, Biebermann H 2006 MC4R oligomerizes independently of extracellular cysteine residues. Peptides 27:372–379 [DOI] [PubMed] [Google Scholar]
- Vaisse C, Clement K, Guy-Grand B, Froguel P 1998 A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat Genet 20:113–114 [DOI] [PubMed] [Google Scholar]
- Yeo GS, Farooqi IS, Aminian S, Halsall DJ, Stanhope RG, O'Rahilly S 1998 A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat Genet 20:111–112 [DOI] [PubMed] [Google Scholar]
- Gu W, Tu Z, Kleyn PW, Kissebah A, Duprat L, Lee J, Chin W, Maruti S, Deng N, Fisher SL, Franco LS, Burn P, Yagaloff KA, Nathan J, Heymsfield S, Albu J, Pi-Sunyer FX, Allison DB 1999 Identification and functional analysis of novel human melanocortin-4 receptor variants. Diabetes 48:635–639 [DOI] [PubMed] [Google Scholar]
- Hinney A, Schmidt A, Nottebom K, Heibült O, Becker I, Ziegler A, Gerber G, Sina M, Görg T, Mayer H, Siegfried W, Fichter M, Remschmidt H, Hebebrand J 1999 Several mutations in the melanocortin-4 receptor gene including a nonsense and a frameshift mutation associated with dominantly inherited obesity in humans. J Clin Endocrinol Metab 84:1483–1486 [DOI] [PubMed] [Google Scholar]
- Dubern B, Clément K, Pelloux V, Froguel P, Girardet JP, Guy-Grand B, Tounian P 2001 Mutational analysis of melanocortin-4 receptor, agouti-related protein, and α-melanocyte-stimulating hormone genes in severely obese children. J Pediatr 139:204–209 [DOI] [PubMed] [Google Scholar]
- Mergen M, Mergen H, Ozata M, Oner R, Oner C 2001 A novel melanocortin 4 receptor (MC4R) gene mutation associated with morbid obesity. J Clin Endocrinol Metab 86:3448–3451 [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Ogawa Y, Shintani M, Ebihara K, Shimodahira M, Iwakura T, Hino M, Ishihara T, Ikekubo K, Kurahachi H, Nakao K 2002 A novel homozygous missense mutation of melanocortin-4 receptor (MC4R) in a Japanese woman with severe obesity. Diabetes 51:243–246 [DOI] [PubMed] [Google Scholar]
- Miraglia Del Giudice E, Cirillo G, Nigro V, Santoro N, D'Urso L, Raimondo P, Cozzolino D, Scafato D, Perrone L 2002 Low frequency of melanocortin-4 receptor (MC4R) mutations in a Mediterranean population with early-onset obesity. Int J Obes Relat Metab Disord 26:647–651 [DOI] [PubMed] [Google Scholar]
- Jacobson P, Ukkola O, Rankinen T, Snyder EE, Leon AS, Rao DC, Skinner JS, Wilmore JH, Lönn L, Cowan Jr GS, Sjöström L, Bouchard C 2002 Melanocortin 4 receptor sequence variations are seldom a cause of human obesity: the Swedish Obese Subjects, the HERITAGE Family Study, and a Memphis cohort. J Clin Endocrinol Metab 87:4442–4446 [DOI] [PubMed] [Google Scholar]
- Marti A, Corbalán MS, Forga L, Martinez JA, Hinney A, Hebebrand J 2003 A novel nonsense mutation in the melanocortin-4 receptor associated with obesity in a Spanish population. Int J Obes Relat Metab Disord 27:385–388 [DOI] [PubMed] [Google Scholar]
- Lubrano-Berthelier C, Durand E, Dubern B, Shapiro A, Dazin P, Weill J, Ferron C, Froguel P, Vaisse C 2003 Intracellular retention is a common characteristic of childhood obesity-associated MC4R mutations. Hum Mol Genet 12:145–153 [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O'Rahilly S 2003 Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med 348:1085–1095 [DOI] [PubMed] [Google Scholar]
- Donohoue PA, Tao YX, Collins M, Yeo GS, O'Rahilly S, Segaloff DL 2003 Deletion of codons 88–92 of the melanocortin-4 receptor gene: a novel deleterious mutation in an obese female. J Clin Endocrinol Metab 88:5841–5845 [DOI] [PubMed] [Google Scholar]
- Santini F, Maffei M, Ceccarini G, Pelosini C, Scartabelli G, Rosellini V, Chiellini C, Marsili A, Lisi S, Tonacchera M, Agretti P, Chiovato L, Mammoli C, Vitti P, Pinchera A 2004 Genetic screening for melanocortin-4 receptor mutations in a cohort of Italian obese patients: description and functional characterization of a novel mutation. J Clin Endocrinol Metab 89:904–908 [DOI] [PubMed] [Google Scholar]
- Lubrano-Berthelier C, Le Stunff C, Bougnères P, Vaisse C 2004 A homozygous null mutation delineates the role of the melanocortin-4 receptor in humans. J Clin Endocrinol Metab 89:2028–2032 [DOI] [PubMed] [Google Scholar]
- Ma L, Tataranni PA, Bogardus C, Baier LJ 2004 Melanocortin 4 receptor gene variation is associated with severe obesity in Pima Indians. Diabetes 53:2696–2699 [DOI] [PubMed] [Google Scholar]
- Larsen LH, Echwald SM, Sørensen TI, Andersen T, Wulff BS, Pedersen O 2005 Prevalence of mutations and functional analyses of melanocortin 4 receptor variants identified among 750 men with juvenile-onset obesity. J Clin Endocrinol Metab 90:219–224 [DOI] [PubMed] [Google Scholar]
- Buono P, Pasanisi F, Nardelli C, Ieno L, Capone S, Liguori R, Finelli C, Oriani G, Contaldo F, Sacchetti L 2005 Six novel mutations in the proopiomelanocortin and melanocortin receptor 4 genes in severely obese adults living in southern Italy. Clin Chem 51:1358–1364 [DOI] [PubMed] [Google Scholar]
- Shao XY, Jia WP, Cai SB, Fang QC, Zhang R, Lu JX, Xiang KS 2005 Cloning and functional analysis of melanocortin 4 receptor mutation gene F261S. Zhonghua Yi Xue Za Zhi 85:366–369 [PubMed] [Google Scholar]
- Lubrano-Berthelier C, Dubern B, Lacorte JM, Picard F, Shapiro A, Zhang S, Bertrais S, Hercberg S, Basdevant A, Clement K, Vaisse C 2006 Melanocortin 4 receptor mutations in a large cohort of severely obese adults: prevalence, functional classification, genotype-phenotype relationship, and lack of association with binge eating. J Clin Endocrinol Metab 91:1811–1818 [DOI] [PubMed] [Google Scholar]
- Rong R, Tao YX, Cheung BM, Xu A, Cheung GC, Lam KS 2006 Identification and functional characterization of three novel human melanocortin-4 receptor gene variants in an obese Chinese population. Clin Endocrinol (Oxf) 65:198–205 [DOI] [PubMed] [Google Scholar]
- Wang CL, Liang L, Wang HJ, Fu JF, Hebebrand J, Hinney A 2006 Several mutations in the melanocortin 4 receptor gene are associated with obesity in Chinese children and adolescents. J Endocrinol Invest 29:894–898 [DOI] [PubMed] [Google Scholar]
- Alharbi KK, Spanakis E, Tan K, Smith MJ, Aldahmesh MA, O'Dell SD, Sayer AA, Lawlor DA, Ebrahim S, Davey Smith G, O'Rahilly S, Farooqi S, Cooper C, Phillips DI, Day IN 2007 Prevalence and functionality of paucimorphic and private MC4R mutations in a large, unselected European British population, scanned by meltMADGE. Hum Mutat 28:294–302 [DOI] [PubMed] [Google Scholar]
- Ahituv N, Kavaslar N, Schackwitz W, Ustaszewska A, Martin J, Hebert S, Doelle H, Ersoy B, Kryukov G, Schmidt S, Yosef N, Ruppin E, Sharan R, Vaisse C, Sunyaev S, Dent R, Cohen J, McPherson R, Pennacchio LA 2007 Medical sequencing at the extremes of human body mass. Am J Hum Genet 80:779–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa MC, Azcona C, Biebermann H, Brumm H, Razquin C, Wermter AK, Martínez JA, Hebebrand J, Hinney A, Moreno-Aliaga MJ, Marti A, Patiño A, Chueca M, Oyarzabal M, Pelach R 2007 A novel mutation Thr162Arg of the melanocortin 4 receptor gene in a Spanish children and adolescent population. Clin Endocrinol (Oxf) 66:652–658 [DOI] [PubMed] [Google Scholar]
- Reinehr T, Hinney A, de Sousa G, Austrup F, Hebebrand J, Andler W 2007 Definable somatic disorders in overweight children and adolescents. J Pediatr 150:618–622, 622.e1–e5 [DOI] [PubMed] [Google Scholar]
- Hainerová I, Larsen LH, Holst B, Finková M, Hainer V, Lebl J, Hansen T, Pedersen O 2007 Melanocortin 4 receptor mutations in obese Czech children: studies of prevalence, phenotype development, weight reduction response, and functional analysis. J Clin Endocrinol Metab 92:3689–3696 [DOI] [PubMed] [Google Scholar]
- Lee YS, Poh LK, Kek BL, Loke KY 2008 Novel melanocortin 4 receptor gene mutations in severely obese children. Clin Endocrinol (Oxf) 68:529–535 [DOI] [PubMed] [Google Scholar]
- Stutzmann F, Tan K, Vatin V, Dina C, Jouret B, Tichet J, Balkau B, Potoczna N, Horber F, O'Rahilly S, Farooqi IS, Froguel P, Meyre D 2008 Prevalence of melanocortin-4 receptor deficiency in Europeans and their age-dependent penetrance in multigenerational pedigrees. Diabetes 57:2511–2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakoff J, Ma L, Kobes S, Knowler WC, Hanson RL, Bogardus C, Baier LJ 2008 Lower metabolic rate in individuals heterozygous for either a frameshift or a functional missense MC4R variant. Diabetes 57:3267–3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth CL, Ludwig M, Woelfle J, Fan ZC, Brumm H, Biebermann H, Tao YX 2009 A novel melanocortin-4 receptor gene mutation in a female patient with severe childhood obesity. Endocrine 36:52–59 [DOI] [PubMed] [Google Scholar]
- Wangensteen T, Kolsgaard ML, Mattingsdal M, Joner G, Tonstad S, Undlien D, Retterstol L 2009 Mutations in the melanocortin 4 receptor (MC4R) gene in obese patients in Norway. Exp Clin Endocrinol Diabetes 117:266–273 [DOI] [PubMed] [Google Scholar]
- Cole SA, Butte NF, Voruganti VS, Cai G, Haack K, Kent Jr JW, Blangero J, Comuzzie AG, McPherson JD, Gibbs RA 2010 Evidence that multiple genetic variants of MC4R play a functional role in the regulation of energy expenditure and appetite in Hispanic children. Am J Clin Nutr 91:191–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao YX 2009 Mutations in melanocortin-4 receptor and human obesity. Prog Mol Biol Transl Sci 88:173–204 [DOI] [PubMed] [Google Scholar]
- Govaerts C, Srinivasan S, Shapiro A, Zhang S, Picard F, Clement K, Lubrano-Berthelier C, Vaisse C 2005 Obesity-associated mutations in the melanocortin 4 receptor provide novel insights into its function. Peptides 26:1909–1919 [DOI] [PubMed] [Google Scholar]
- MacKenzie RG 2006 Obesity-associated mutations in the human melanocortin-4 receptor gene. Peptides 27:395–403 [DOI] [PubMed] [Google Scholar]
- Lubrano-Berthelier C, Cavazos M, Le Stunff C, Haas K, Shapiro A, Zhang S, Bougneres P, Vaisse C 2003 The human MC4R promoter: characterization and role in obesity. Diabetes 52:2996–3000 [DOI] [PubMed] [Google Scholar]
- Valli-Jaakola K, Palvimo JJ, Lipsanen-Nyman M, Salomaa V, Peltonen L, Kontula K, Schalin-Jäntti C 2006 A two-base deletion-439delGC in the melanocortin-4 receptor promoter associated with early-onset obesity. Horm Res 66:61–69 [DOI] [PubMed] [Google Scholar]
- Loos RJ, Rankinen T, Tremblay A, Pérusse L, Chagnon Y, Bouchard C 2005 Melanocortin-4 receptor gene and physical activity in the Quebec Family Study. Int J Obes (Lond) 29:420–428 [DOI] [PubMed] [Google Scholar]
- Chambers JC, Elliott P, Zabaneh D, Zhang W, Li Y, Froguel P, Balding D, Scott J, Kooner JS 2008 Common genetic variation near MC4R is associated with waist circumference and insulin resistance. Nat Genet 40:716–718 [DOI] [PubMed] [Google Scholar]
- Loos RJ, Lindgren CM, Li S, Wheeler E, Zhao JH, Prokopenko I, Inouye M, Freathy RM, Attwood AP, Beckmann JS, Berndt SI, Jacobs KB, Chanock SJ, Hayes RB, Bergmann S, Bennett AJ, Bingham SA, Bochud M, Brown M, Cauchi S, Connell JM, Cooper C, Smith GD, Day I, Dina C, De S, Dermitzakis ET, Doney AS, Elliott KS, Elliott P, Evans DM, Sadaf Farooqi I, Froguel P, Ghori J, Groves CJ, Gwilliam R, Hadley D, Hall AS, Hattersley AT, Hebebrand J, Heid IM, Lamina C, Gieger C, Illig T, Meitinger T, Wichmann HE, Herrera B, Hinney A, Hunt SE, Jarvelin MR, Johnson T, Jolley JD, Karpe F, Keniry A, Khaw KT, Luben RN, Mangino M, Marchini J, McArdle WL, McGinnis R, Meyre D, Munroe PB, Morris AD, Ness AR, Neville MJ, Nica AC, Ong KK, O'Rahilly S, Owen KR, Palmer CN, Papadakis K, Potter S, Pouta A, Qi L, Randall JC, Rayner NW, Ring SM, Sandhu MS, Scherag A, Sims MA, Song K, Soranzo N, Speliotes EK, Syddall HE, Teichmann SA, Timpson NJ, Tobias JH, Uda M, Vogel CI, Wallace C, Waterworth DM, Weedon MN, Willer CJ, Wraight, Yuan X, Zeggini E, Hirschhorn JN, Strachan DP, Ouwehand WH, Caulfield MJ, Samani NJ, Frayling TM, Vollenweider P, Waeber G, Mooser V, Deloukas P, McCarthy MI, Wareham NJ, Barroso I, Jacobs KB, Chanock SJ, Hayes RB, Lamina C, Gieger C, Illig T, Meitinger T, Wichmann HE, Kraft P, Hankinson SE, Hunter DJ, Hu FB, Lyon HN, Voight BF, Ridderstrale M, Groop L, Scheet P, Sanna S, Abecasis GR, Albai G, Nagaraja R, Schlessinger D, Jackson AU, Tuomilehto J, Collins FS, Boehnke M, Mohlke KL 2008 Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet 40:768–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L, Kraft P, Hunter DJ, Hu FB 2008 The common obesity variant near MC4R gene is associated with higher intakes of total energy and dietary fat, weight change and diabetes risk in women. Hum Mol Genet 17:3502–3508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckers S, Mertens I, Peeters A, Van Gaal L, Van Hul W 2006 Screening for melanocortin-4 receptor mutations in a cohort of Belgian morbidly obese adults and children. Int J Obes (Lond) 30:221–225 [DOI] [PubMed] [Google Scholar]
- Calton MA, Ersoy BA, Zhang S, Kane JP, Malloy MJ, Pullinger CR, Bromberg Y, Pennacchio LA, Dent R, McPherson R, Ahituv N, Vaisse C 2009 Association of functionally significant melanocortin-4 but not melanocortin-3 receptor mutations with severe adult obesity in a large North American case-control study. Hum Mol Genet 18:1140–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kublaoui BM, Zinn AR 2006 Editorial: MC4R mutations—weight before screening! J Clin Endocrinol Metab 91:1671–1672 [DOI] [PubMed] [Google Scholar]
- Dubern B, Bisbis S, Talbaoui H, Le Beyec J, Tounian P, Lacorte JM, Clément K 2007 Homozygous null mutation of the melanocortin-4 receptor and severe early-onset obesity. J Pediatr 150:613–617, 617.e1 [DOI] [PubMed] [Google Scholar]
- O'Rahilly S, Farooqi IS, Yeo GS, Challis BG 2003 Minireview: human obesity—lessons from monogenic disorders. Endocrinology 144:3757–3764 [DOI] [PubMed] [Google Scholar]
- Dempfle A, Hinney A, Heinzel-Gutenbrunner M, Raab M, Geller F, Gudermann T, Schäfer H, Hebebrand J 2004 Large quantitative effect of melanocortin-4 receptor gene mutations on body mass index. J Med Genet 41:795–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinehr T, Hebebrand J, Friedel S, Toschke AM, Brumm H, Biebermann H, Hinney A 2009 Lifestyle intervention in obese children with variations in the melanocortin 4 receptor gene. Obesity (Silver Spring) 17:382–389 [DOI] [PubMed] [Google Scholar]
- Rosmond R, Chagnon M, Bouchard C, Björntorp P 2001 A missense mutation in the human melanocortin-4 receptor gene in relation to abdominal obesity and salivary cortisol. Diabetologia 44:1335–1338 [DOI] [PubMed] [Google Scholar]
- Geller F, Reichwald K, Dempfle A, Illig T, Vollmert C, Herpertz S, Siffert W, Platzer M, Hess C, Gudermann T, Biebermann H, Wichmann HE, Schäfer H, Hinney A, Hebebrand J 2004 Melanocortin-4 receptor gene variant I103 is negatively associated with obesity. Am J Hum Genet 74:572–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EH, Wareham NJ, Farooqi S, Hinney A, Hebebrand J, Scherag A, O'rahilly S, Barroso I, Sandhu MS 2007 The V103I polymorphism of the MC4R gene and obesity: population based studies and meta-analysis of 29,563 individuals. Int J Obes (Lond) 31:1437–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brönner G, Sattler AM, Hinney A, Soufi M, Geller F, Schäfer H, Maisch B, Hebebrand J, Schaefer JR 2006 The 103I variant of the melanocortin 4 receptor is associated with low serum triglyceride levels. J Clin Endocrinol Metab 91:535–538 [DOI] [PubMed] [Google Scholar]
- Heid IM, Vollmert C, Kronenberg F, Huth C, Ankerst DP, Luchner A, Hinney A, Brönner G, Wichmann HE, Illig T, Döring A, Hebebrand J 2008 Association of the MC4R V103I polymorphism with the metabolic syndrome: the KORA Study. Obesity (Silver Spring) 16:369–376 [DOI] [PubMed] [Google Scholar]
- Xiang Z, Litherland SA, Sorensen NB, Proneth B, Wood MS, Shaw AM, Millard WJ, Haskell-Luevano C 2006 Pharmacological characterization of 40 human melanocortin-4 receptor polymorphisms with the endogenous proopiomelanocortin-derived agonists and the agouti-related protein (AGRP) antagonist. Biochemistry 45:7277–7288 [DOI] [PubMed] [Google Scholar]
- Stutzmann F, Vatin V, Cauchi S, Morandi A, Jouret B, Landt O, Tounian P, Levy-Marchal C, Buzzetti R, Pinelli L, Balkau B, Horber F, Bougnères P, Froguel P, Meyre D 2007 Non-synonymous polymorphisms in melanocortin-4 receptor protect against obesity: the two facets of a Janus obesity gene. Hum Mol Genet 16:1837–1844 [DOI] [PubMed] [Google Scholar]
- Sina M, Hinney A, Ziegler A, Neupert T, Mayer H, Siegfried W, Blum WF, Remschmidt H, Hebebrand J 1999 Phenotypes in three pedigrees with autosomal dominant obesity caused by haploinsufficiency mutations in the melanocortin-4 receptor gene. Am J Hum Genet 65:1501–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebebrand J, Fichter M, Gerber G, Görg T, Hermann H, Geller F, Schäfer H, Remschmidt H, Hinney A 2002 Genetic predisposition to obesity in bulimia nervosa: a mutation screen of the melanocortin-4 receptor gene. Mol Psychiatry 7:647–651 [DOI] [PubMed] [Google Scholar]
- Branson R, Potoczna N, Kral JG, Lentes KU, Hoehe MR, Horber FF 2003 Binge eating as a major phenotype of melanocortin 4 receptor gene mutations. N Engl J Med 348:1096–1103 [DOI] [PubMed] [Google Scholar]
- Tao YX, Segaloff DL 2005 Functional analyses of melanocortin-4 receptor mutations identified from patients with binge eating disorder and nonobese or obese subjects. J Clin Endocrinol Metab 90:5632–5638 [DOI] [PubMed] [Google Scholar]
- Hebebrand J, Geller F, Dempfle A, Heinzel-Gutenbrunner M, Raab M, Gerber G, Wermter AK, Horro FF, Blundell J, Schäfer H, Remschmidt H, Herpertz S, Hinney A 2004 Binge-eating episodes are not characteristic of carriers of melanocortin-4 receptor gene mutations. Mol Psychiatry 9:796–800 [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Yeo GS, O'Rahilly S 2003 Binge eating as a phenotype of melanocortin 4 receptor gene mutations. N Engl J Med 349:606–609; author reply 606–609 [PubMed] [Google Scholar]
- Tao YX, Segaloff DL 2003 Functional characterization of melanocortin-4 receptor mutations associated with childhood obesity. Endocrinology 144:4544–4551 [DOI] [PubMed] [Google Scholar]
- Hobbs HH, Russell DW, Brown MS, Goldstein JL 1990 The LDL receptor locus in familial hypercholesterolemia: mutational analysis of a membrane protein. Annu Rev Genet 24:133–170 [DOI] [PubMed] [Google Scholar]
- Welsh MJ, Smith AE 1993 Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell 73:1251–1254 [DOI] [PubMed] [Google Scholar]
- Hermans E, Challiss RA, Nahorski SR 1999 Effects of varying the expression level of recombinant human mGlu1α receptors on the pharmacological properties of agonists and antagonists. Br J Pharmacol 126:873–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkirk JV, Price GW, Nahorski SR, Challiss RA 2001 Cell type-specific differences in the coupling of recombinant mGlu1α receptors to endogenous G protein sub-populations. Neuropharmacology 40:645–656 [DOI] [PubMed] [Google Scholar]
- Gao XB, van den Pol AN 2002 Melanin-concentrating hormone depresses L-, N-, and P/Q-type voltage-dependent calcium channels in rat lateral hypothalamic neurons. J Physiol 542:273–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal W, Antaramian A, Gilbert S, Birnbaumer M 1993 Nephrogenic diabetes insipidus. A V2 vasopressin receptor unable to stimulate adenylyl cyclase. J Biol Chem 268:13030–13033 [PubMed] [Google Scholar]
- Barak LS, Oakley RH, Laporte SA, Caron MG 2001 Constitutive arrestin-mediated desensitization of a human vasopressin receptor mutant associated with nephrogenic diabetes insipidus. Proc Natl Acad Sci USA 98:93–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao YX 2006 Inactivating mutations of G protein-coupled receptors and diseases: structure-function insights and therapeutic implications. Pharmacol Ther 111:949–973 [DOI] [PubMed] [Google Scholar]
- Xiang Z, Pogozheva ID, Sorenson NB, Wilczynski AM, Holder JR, Litherland SA, Millard WJ, Mosberg HI, Haskell-Luevano C 2007 Peptide and small molecules rescue the functional activity and agonist potency of dysfunctional human melanocortin-4 receptor polymorphisms. Biochemistry 46:8273–8287 [DOI] [PubMed] [Google Scholar]
- Morello JP, Salahpour A, Laperriere A, Bernier V, Arthus MF, Lonergan M, Petaja-Repo U, Angers S, Morin D, Bichet DG, Bouvier M 2000 Pharmacological chaperones rescue cell-surface expression and function of misfolded V2 vasopressin receptor mutants. J Clin Invest 105:887–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janovick JA, Maya-Nunez G, Conn PM 2002 Rescue of hypogonadotropic hypogonadism-causing and manufactured GnRH receptor mutants by a specific protein-folding template: misrouted proteins as a novel disease etiology and therapeutic target. J Clin Endocrinol Metab 87:3255–3262 [DOI] [PubMed] [Google Scholar]
- Janovick JA, Goulet M, Bush E, Greer J, Wettlaufer DG, Conn PM 2003 Structure-activity relations of successful pharmacologic chaperones for rescue of naturally occurring and manufactured mutants of the gonadotropin-releasing hormone receptor. J Pharmacol Exp Ther 305:608–614 [DOI] [PubMed] [Google Scholar]
- Noorwez SM, Kuksa V, Imanishi Y, Zhu L, Filipek S, Palczewski K, Kaushal S 2003 Pharmacological chaperone-mediated in vivo folding and stabilization of the P23H-opsin mutant associated with autosomal dominant retinitis pigmentosa. J Biol Chem 278:14442–14450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorwez SM, Malhotra R, McDowell JH, Smith KA, Krebs MP, Kaushal S 2004 Retinoids assist the cellular folding of the autosomal dominant retinitis pigmentosa opsin mutant P23H. J Biol Chem 279:16278–16284 [DOI] [PubMed] [Google Scholar]
- Petäjä-Repo UE, Hogue M, Bhalla S, Laperrière A, Morello JP, Bouvier M 2002 Ligands act as pharmacological chaperones and increase the efficiency of δ opioid receptor maturation. EMBO J 21:1628–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaipatikul V, Erickson-Herbrandson LJ, Loh HH, Law PY 2003 Rescuing the traffic-deficient mutants of rat μ-opioid receptors with hydrophobic ligands. Mol Pharmacol 64:32–41 [DOI] [PubMed] [Google Scholar]
- Fan J, Perry SJ, Gao Y, Schwarz DA, Maki RA 2005 A point mutation in the human melanin concentrating hormone receptor 1 reveals an important domain for cellular trafficking. Mol Endocrinol 19:2579–2590 [DOI] [PubMed] [Google Scholar]
- Van Craenenbroeck K, Clark SD, Cox MJ, Oak JN, Liu F, Van Tol HH 2005 Folding efficiency is rate-limiting in dopamine D4 receptor biogenesis. J Biol Chem 280:19350–19357 [DOI] [PubMed] [Google Scholar]
- Robert J, Auzan C, Ventura MA, Clauser E 2005 Mechanisms of cell-surface rerouting of an endoplasmic reticulum-retained mutant of the vasopressin V1b/V3 receptor by a pharmacological chaperone. J Biol Chem 280:42198–42206 [DOI] [PubMed] [Google Scholar]
- Hawtin SR 2006 Pharmacological chaperone activity of SR49059 to functionally recover misfolded mutations of the vasopressin V1a receptor. J Biol Chem 281:14604–14614 [DOI] [PubMed] [Google Scholar]
- Fortin JP, Dziadulewicz EK, Gera L, Marceau F 2006 A nonpeptide antagonist reveals a highly glycosylated state of the rabbit kinin B1 receptor. Mol Pharmacol 69:1146–1157 [DOI] [PubMed] [Google Scholar]
- Bernier V, Morello JP, Zarruk A, Debrand N, Salahpour A, Lonergan M, Arthus MF, Laperrière A, Brouard R, Bouvier M, Bichet DG 2006 Pharmacologic chaperones as a potential treatment for X-linked nephrogenic diabetes insipidus. J Am Soc Nephrol 17:232–243 [DOI] [PubMed] [Google Scholar]
- Markison S, Foster AC 2005 Targeting melanocortin receptors for the treatment of obesity. Drug Discov Today 3:569–576 [Google Scholar]
- Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, Burghammer M, Ratnala VR, Sanishvili R, Fischetti RF, Schertler GF, Weis WI, Kobilka BK 2007 Crystal structure of the human β2 adrenergic G-protein-coupled receptor. Nature 450:383–387 [DOI] [PubMed] [Google Scholar]
- Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, Stevens RC 2007 High-resolution crystal structure of an engineered human β2-adrenergic G protein-coupled receptor. Science 318:1258–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum DM, Cherezov V, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Yao XJ, Weis WI, Stevens RC, Kobilka BK 2007 GPCR engineering yields high-resolution structural insights into β2-adrenergic receptor function. Science 318:1266–1273 [DOI] [PubMed] [Google Scholar]
- Jaakola VP, Griffith MT, Hanson MA, Cherezov V, Chien EY, Lane JR, Ijzerman AP, Stevens RC 2008 The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science 322:1211–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Scheerer P, Hofmann KP, Choe HW, Ernst OP 2008 Crystal structure of the ligand-free G-protein-coupled receptor opsin. Nature 454:183–187 [DOI] [PubMed] [Google Scholar]
- Warne T, Serrano-Vega MJ, Baker JG, Moukhametzianov R, Edwards PC, Henderson R, Leslie AG, Tate CG, Schertler GF 2008 Structure of a β1-adrenergic G-protein-coupled receptor. Nature 454:486–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheerer P, Park JH, Hildebrand PW, Kim YJ, Krauss N, Choe HW, Hofmann KP, Ernst OP 2008 Crystal structure of opsin in its G-protein-interacting conformation. Nature 455:497–502 [DOI] [PubMed] [Google Scholar]
- Rosenbaum DM, Rasmussen SG, Kobilka BK 2009 The structure and function of G-protein-coupled receptors. Nature 459:356–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan ZC, Sartin JL, Tao YX 2008 Molecular cloning and pharmacological characterization of porcine melanocortin-3 receptor. J Endocrinol 196:139–148 [DOI] [PubMed] [Google Scholar]