Abstract
Radiation exposure of the thyroid at a young age is a recognized risk factor for the development of differentiated thyroid cancer lasting for four decades and probably for a lifetime after exposure. Medical radiation exposure, however, occurs frequently, including among the pediatric population, which is especially sensitive to the effects of radiation. In the past, the treatment of benign medical conditions with external radiation represented the most significant thyroid radiation exposures. Today, diagnostic medical radiation represents the largest source of man-made radiation exposure. Radiation exposure related to the use of computerized tomography is rising exponentially, particularly in the pediatric population. There is direct epidemiological evidence of a small but significant increased risk of cancer at radiation doses equivalent to computerized tomography doses used today.
Paralleling the increasing use of medical radiation is an increase in the incidence of papillary thyroid cancer. At present, it is unclear how much of this increase is related to increased detection of subclinical disease from the increased utilization of ultrasonography and fine-needle aspiration, how much is due to a true increase in thyroid cancer, and how much, if any, can be ascribed to medical radiation exposure. Fortunately, the amount of radiation exposure from medical sources can be reduced. In this article we review the sources of thyroid radiation exposure, radiation risks to the thyroid gland, strategies for reducing radiation exposure to the thyroid, and ways that endocrinologists can participate in this effort. Finally, we provide some suggestions for future research directions.
Paralleling the increase in the incidence of papillary thyroid cancer is an increasing use of medical radiation. At present it is unclear to what extent, if any, these two are related. This review summarizes the current sources of thyroid radiation exposure, radiation risks to the thyroid gland, strategies for reducing radiation exposure to the thyroid, and ways that endocrinologists can participate in this effort.
- I. Introduction
- A. Trends in thyroid cancer
- B. Units of radiation measurement
- C. Sources of radiation exposure
- D. Effect of age on radiation sensitivity
- II. Exposure of the Thyroid Gland from Medical X-Rays
- A. Conventional x-rays
- B. CT scans
- C. Fluoroscopy
- D. Nuclear medicine procedures
- E. Dental x-rays
- F. Radiation treatment
III. In Utero Exposure of the Thyroid Gland
IV. Thyroid Cancer Risk Associated with Low-Dose Radiation Exposure
- V. Practice and Public Health
- A. Screening for thyroid cancer
- B. Regulations and guidelines
- VI. Research Directions
- A. Determination of risks at lower doses and with longer follow-up times
- B. Methods to ameliorate the risks
- C. Identification of radiation-related thyroid cancers, i.e., radiation “signatures”
- D. Increased susceptibility to the effects of radiation
VII. Conclusions
I. Introduction
In this review we discuss trends in radiation exposures to the thyroid from diagnostic imaging and treatment and the potential risks to the thyroid from them. We limit ourselves largely to medical, as opposed to occupational, radiation because medical exposure is increasing rapidly and is more controllable than other sources. The main focus of the review is on childhood exposure because the sensitivity of the thyroid gland to radiation is significantly greater at young ages (1).
The main concern is radiation-induced thyroid cancer because radiation is the most important modifiable cause. Although it is not entirely clear how much radiation is contributing to the increasing incidence of thyroid cancer, it makes sense to reduce radiation exposure, especially of children. To better understand the relationship between radiation and thyroid cancer incidence, in the first sections of this review we describe the trends in thyroid cancer incidence, review the basic nomenclature used to explain radiation dosing, and discuss current medical radiation exposures, with special attention to the pediatric population. The last sections summarize some of the public health guidelines and initiatives that deal with radiation protection and the directions of future research in this area.
A. Trends in thyroid cancer
Thyroid cancer rates come from many sources, and therefore the time periods covered vary, but the upward trend in incidence has been observed in many developed countries. Data from developing countries are limited.
The worldwide age-standardized incidence of thyroid cancer during the 2–5 yr before 2002 was estimated to be 3.3 and 1.3 per 100,000 for women and men, respectively (International Agency for Research on Cancer, http://www-dep.iarc.fr). In more developed regions, the incidence is much higher; in the United States during 2002–2006, it was 14.2 and 4.9 per 100,000 for women and men, respectively (International Agency for Research on Cancer, http://www-dep.iarc.fr) (3). Thyroid cancer comprises about 2.0% of all malignancies in women and 0.62% in men, and it is one of a small number of cancers for which the incidence is increasing (International Agency for Research on Cancer, http://www-dep.iarc.fr). In the United States between 1980 and 1997, it increased by 2.4% per year, and between 1997 and 2006 it increased by 6.4% per year (3). Among women, thyroid cancer is the cancer that is rising the most rapidly. Projections for 2009 in the United States are that about 37,200 people (10,000 men and 27,200 women) will be diagnosed, and 1,630 will die of thyroid cancer (3). For a person born in the United States today, the lifetime risk of developing thyroid cancer is 1 in 119 based on 2004–2006 data (3). It is estimated that about 410,000 people currently living in the United States have been diagnosed with thyroid cancer at some time (3).
It is probable that much of the increased incidence of thyroid cancer is due to a greater rate of detection of “subclinical” disease fostered by the popularity and improvements in thyroid ultrasonography and fine-needle aspiration (FNA) of the thyroid, leading to the detection of small papillary cancers that otherwise would not become clinically evident. This hypothesis is supported by evidence that papillary thyroid cancer accounts for essentially all of the increase and that the average size of papillary thyroid cancers at detection is becoming smaller, with up to 87% of the increase attributable to cancers <2 cm in size (4,5). However, large papillary cancers have also increased, with those larger than 5.0 cm more than doubling among white women along with increases in tumors that had spread within the neck and to distant sites (6,7).
Radiation-related cases may be contributing to the increasing incidence because it is the papillary form of thyroid cancer that is most closely associated with radiation exposure. The extreme radiosensitivity of the thyroid is evident from the fact that the slope of the dose-response curve for thyroid cancer is as great, or greater, than many other radiation-related malignancies (8). Although external radiation therapy for benign conditions largely ended in the early 1960s, people can be exposed to radiation from nuclear accidents (as at Chernobyl), selected occupations (e.g., radiologists), diagnostic examinations [especially computerized tomography (CT)], and radiation therapy for malignant conditions. CT involves much larger radiation doses compared with conventional x-ray imaging procedures. Although direct epidemiological data are not yet available, statistical risk models based on data from survivors of atomic bomb radiation exposure have estimated a small but significant increase in the overall risk of cancer associated with the radiation exposure from CT scans (9,10). Even a small increased risk, when applied to a large number of individuals, could have significant public health implications.
B. Units of radiation measurement
An understanding of the basic terminology pertaining to radiation dose measurement is imperative. The absorbed dose is the radiation dose absorbed by a specific organ or tissue per unit of mass. It is expressed in the Système International (SI) units of Gray (Gy) and is often measured or calculated using anthropomorphic or mathematical phantoms. The “equivalent” dose is derived from the absorbed dose using factors that take into account the different types of radiation and the rates with which they are delivered. For x-rays, the absorbed dose and equivalent dose are the same. The equivalent dose is measured in rem or sieverts (Sv).
“Effective” doses are calculated from organ doses and are a method of representing whole body doses. They are also measured in sieverts. Effective doses are calculated using the International Commission on Radiological Protection (ICRP) guidelines and are used to compare radiation effects, such as the risk of cancer from different sources of exposure (11).
Radiation effects are divided into deterministic effects and stochastic effects. The frequency and severity of deterministic effects rise with increasing dose after a threshold dose is reached. Deterministic effects include skin reddening and infertility at estimated thresholds of about 2.5 and 6 Gy, respectively (11). Typically, diagnostic radiological procedures do not reach the doses at which these effects occur. In contrast, stochastic effects do not have a threshold at which they begin to occur (11). Rather, the chance of occurrence, but not severity, increases with increasing lifetime accumulation of radiation exposure. Carcinogenesis is a stochastic effect. Major national and international organizations responsible for evaluating radiation risks agree that there is probably no safe lower dose radiation “threshold” for inducing cancer. For the purpose of public health decisions, they generally use a “linear nonthreshold” model that assumes the probability of incurring radiation-related cancer increases proportionately to any given increment in dose.
The expression of radiation-related measurements has changed over time, but currently most organizations and publications use SI units, i.e., gray, sievert, and becquerel for absorbed dose, effective dose, and activity, respectively. Table 1 shows the relationships between commonly used units.
Table 1.
Units related to radiation safety used by the National Council on Radiation Protection and Measurements
| Absorbed dose |
| SI units: 1 gray (Gy) = 1000 mGy |
| Old units: 1 rad = 10 mGy |
| Effective dose |
| SI units: 1 sievert (Sv) = 100 rem; 1 rem = 10 mSv |
| Old units: 1 rad = 1 rem (for x-rays) |
| Amounts of radioactivity |
| 1 megabequerel (MBq; 106Bq) = 0.027 mCi |
| 1 mCi = 37 MBq |
| 30 mCi = 1111 MBq |
Radiation epidemiologists relate risk to dose. In addition to relative risk (RR), they often express risks in terms of excess RR (ERR), which is RR − 1. In other words, when the risk is doubled, RR = 2 and ERR = 1. ERR is usually considered statistically significant when the 95% confidence interval (CI) does not include zero.
C. Sources of radiation exposure
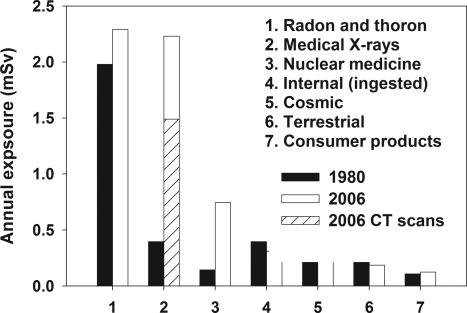
The average exposure from background radiation in the United States is about 3.0 mSv/yr. Most of the background radiation comes from radon and thoron (12). In the 1980s in the United States, the average total exposure, including background radiation, was 3.6 mSv/yr, with only about 15% (0.54 Sv) coming from man-made sources (13,14). By 2006, the average total exposure had increased nearly 2-fold to 6.2 mSv/yr, with the fraction from man-made sources increasing to about 50% (3 mSv/yr) (12,14). A breakdown of radiation sources, comparing 1980 to 2006, is given in Fig. 1 (12). Between 1980 and 2006, the average exposure to medical x-rays increased more than 5-fold, from an estimated 0.4 to 2.2 mSv. The fraction of man-made sources in 2006 (3 mSv) due to CT imaging increased dramatically to 48% (1.5 mSv), whereas the fraction from nuclear medicine, mostly for cardiac imaging, rose to 24% (0.74 mSv) (12,14).
Figure 1.
Sources of radiation exposure in the United States, comparing 1980 and 2006.
D. Effect of age on radiation sensitivity
Epidemiological studies of radiation-exposed populations have demonstrated a much greater sensitivity to radiation in children compared with adults. Even doses as small as 50–100 mGy have been associated with an increased risk of thyroid malignancy in children, with a linear dose-response up to about 10–20 Gy when the risk begins to level off (1,15,16). Of note, the excess risk persists for at least four decades after exposure (1,17).
Children develop radiation-related cancer more often than adults for a number of reasons. Their tissues are growing and cells are dividing more rapidly, making them more prone to the mutagenic effects of ionizing radiation. Effective doses from CT scans that are not modified for pediatric patients are higher in children because of their smaller organs and masses compared with adults (18,19). The carcinogenic potential of radiation appears to continue throughout life, and children have a long life expectancy during which time radiation-induced cancers may be expressed. As a result, the adverse effects of radiation are much greater in children than in adults. For all solid tumors, the RR decreases by 17% for each 10-yr increase in age at exposure, and for thyroid cancer the corresponding decrease is 56% (11).
A pooled analysis of studies evaluating thyroid cancer risk after radiation exposure to the head and neck of children showed a strong inverse relationship between age at exposure and the risk of thyroid cancer. By age 15, the risk of thyroid cancer diminished to the point where it was not statistically significant (1). In the Childhood Cancer Survivors Study of over 14,000 patients, patients treated with radiation before age 10 had a higher risk of developing thyroid cancer compared with those older than age 10 (16). A small, significant risk for thyroid cancer has been detected among female, but not male, survivors of the atomic bomb who were older than 20 yr at the time of the explosion (20).
II. Exposure of the Thyroid Gland from Medical X-Rays
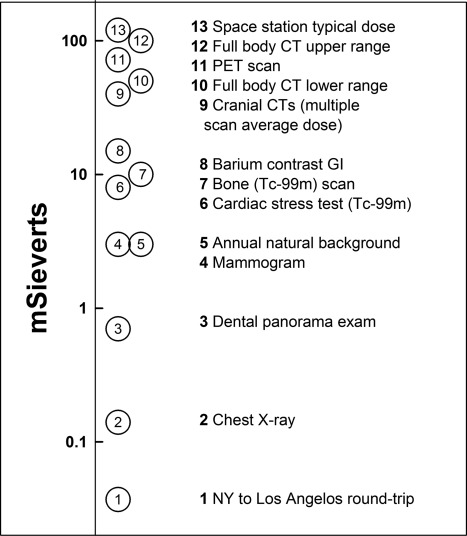
As mentioned in Section I, subsection C, medical radiation is currently the largest source of man-made radiation exposure. It has been estimated that the cancer risk attributable to diagnostic radiation ranges from 0.6 and 0.9% in the United Kingdom and the United States, respectively, to 3.8% in Japan (21,22). Figure 2 shows typical doses from common radiological procedures.
Figure 2.
Magnitude of radiation exposure from various sources. [Adapted from N. F. Metting: http://lowdose.energy.gov/imagegallery.aspx (32).] For cranial CT, the dose takes into account the multiple scans performed for the typical patient. GI, Gastrointestinal; PET, positron emission tomography.
A. Conventional x-rays
In general, conventional x-rays result in relatively low levels of thyroid radiation exposure (Fig. 1). However, due to the small size of newborns, conventional radiographs performed in the neonatal intensive care unit are associated with high radiation exposure of nonrelevant body regions. In one study, 95% of chest x-rays and 45% of abdominal x-rays included the neck, thus exposing the thyroid to potentially unnecessary radiation (23). In addition, up to 20% of all radiographs needed to be repeated because the radiographs did not include the intended body tissues, further increasing radiation exposure of infants. However, even during prolonged and complicated neonatal intensive care unit stays, the cumulative effective dose from conventional diagnostic x-rays is relatively low, between 0.04 and 0.54 mSv in one study (24).
B. CT scans
This is the area that is currently receiving the most attention. First we review issues related to CT in general and then focus on the thyroid gland.
1. General cancer risks associated with diagnostic CT
Much of the concern about thyroid radiation exposure results from the escalating use of CT scans in children (9,26). Since its introduction in 1970, CT has become the gold standard imaging study for the diagnosis of many disease entities (27,28). In 2006 in the United States, 67 million CTs were performed, including 4–7 million on children (14,29). The use of pediatric CTs has risen 8-fold since 1980, i.e., an increase of about 10% per year, even faster than in adults (30). In the United Kingdom and elsewhere, there has been a similar trend (31).
Radiation doses from CT scanning are considerably larger than those from conventional radiological studies. A CT scan of the abdomen and chest obtained in 15 sec can impart up to 100 mSv of radiation, which is about 1000 times the dose from a conventional chest x-ray (about 0.1 mSv) (32). In the United States, CT scans account for only about 15% of all radiological diagnostic procedures performed, yet they result in more than half of the radiation dose to patients from these procedures (14,33). Data from the United Kingdom also demonstrate the disproportionate contribution of CT scans to exposure; they account for only 7% of all radiological procedures but contribute 47% to the collective diagnostic radiation dose (31). Advances in CT technology have permitted faster imaging and potentially lower doses, but the imaging of more body regions per scan and the selection of a greater number of slices per scan have counterbalanced this.
The typical organ dose from a CT is 10–20 mGy; for example, the stomach receives 10 mGy from an adult abdominal CT (9). As a result, increased attention has been focused on the potential for radiation exposure from CT leading to the development of cancers, including thyroid cancer. A study evaluating CT use in a cohort of 31,462 adults seen at a tertiary referral center found very high rates of recurrent CT imaging, with 33% of patients having undergone more than five CT examinations and 5% of patients having undergone at least 22 CT examinations over a 22-yr period (35). Alarmingly, 15% of the cohort had accrued cumulative effective doses in excess of 100 mSv. There is epidemiological evidence, particularly from atomic bomb survivors, of a small yet significant increase in the risk of cancer mortality at about 35 mSv (36,37). This is in the range of the typical organ doses from two to three CT scans. Based on CT use in the United States from 1991 to 1996, it has been estimated that the attributable risk for cancer through age 75 yr due to exposure from CT scans is 0.9% (22). Given the current use of CT technology, this estimate will be getting higher.
Brenner et al. (38) estimated that for a 1 yr old, the estimated lifetime cancer mortality risk attributable to radiation exposure was 0.18 and 0.07% for a single abdominal or head CT scan, respectively. On a population basis, they estimated that in the United States there would be an increase of 0.35% over the background rate in lifetime cancer mortality related to the radiation exposure from 1 yr of CT scans in children less than 15 yr old (38). This estimate is equivalent to a lifetime cancer mortality risk attributable to radiation from an abdominal CT in a 1 yr old of about 1 in 550 and about 1 in 1500 for a head CT (38). They went on to estimate that 500 future excess cancer deaths would result from 600,000 pediatric CT studies. A similar analysis from Israel based on the current annual pediatric CT use projected an increase in the lifetime risk of cancer mortality of 0.29% (39).
In summary, CT is a powerful tool in the practice of pediatric medicine, and the benefits to an individual generally outweigh the risks. Currently, there are no empirical data quantifying cancer risks associated with CT; however, risk models based on extrapolation from the atomic bomb survivors cohort predict small but meaningful risks. Thus, the use of CT should be based on a proper understanding of its risks and benefits and when used correctly, the benefits of CT outweigh the potential risks. The primary public health issue is the increasingly large pediatric population exposed to these small risks (29).
2. Thyroid-specific risks related to diagnostic CT
Over one third of all CT scans are performed in the region of the head and neck (30). A survey of physicians of the American Society of Emergency Radiology revealed that 61% of respondents included the thyroid gland as part of the coincident CT studies of the cervical spine and the chest in trauma protocols (40). There is increasing concern regarding radiation exposure to the thyroid gland from pediatric CT scans. Over a 10-yr span, from 1996 to 2005, at one large academic institution the fraction of multiregion CT scans in children increased from 4.9% to 7.8%, likely increasing the radiation dose to the thyroid (41). If multiphase CT examinations are performed, the dose is increased even more (29,42). Additionally, the use of iodinated contrast with CT increases the radiation absorbed by the thyroid by up to 35% (43).
A study evaluating patient and organ doses in pediatric and adult chest examinations demonstrated that for each CT scan the thyroid doses depended on the scanner and protocol used and for children and adults were up to 21 and 20 mGy, respectively (44). For CT torso protocols in children, the thyroid doses were from about 10 to 21 mGy, corresponding to ages from newborn to 10 yr (45). Notably, the dose could be decreased by about 60% in a 10 yr old by using automatic exposure control. In a cohort of 80 cystic fibrosis patients managed in a French regional referral center with a total follow-up of 1231 patient years, each CT scan resulted in a mean dose of 3.5 mGy (range, 0.3–19.5) to the thyroid (46). Because the mean lifetime number of CT scans per patient was 3.2 (range, 0–13) scans, the average lifetime thyroid dose was about 10 mGy per patient, but in some cases it was substantially higher.
Common indications in children often result in the performance of cervical spine and chest CTs at the same time. A recent survey has disclosed that in many instances there is an overlap of the radiation fields, including the thyroid in the overlapped area (40). Avoiding or reducing this overlap and the use of contrast when it is not necessary are ways to reduce thyroid exposure.
Baker and Bhatti (47) present arguments, predominantly based on temporal patterns, that the dose burden to the thyroid from CT exams of the thorax, head, and neck and the increasing number of CT scans has contributed to the increasing incidence of thyroid cancer. Mazonakis et al. (48) studied radiation doses and their associated risk for thyroid cancer induction associated with common head and neck CT examinations performed during childhood. They found that during CT of the neck, the thyroid gland is exposed to 15.2–52 mGy, and they projected that this would increase the cases of thyroid malignancies by up to 390 per million exposed people. Scattered radiation during CTs of the head would be associated with an increase of 4 to 65 thyroid cancer cases per million people. Berrington de González et al. (49) estimated that there would be 1200 excess future cases of thyroid cancer resulting from the CT scans performed in the United States in 2007.
C. Fluoroscopy
Interventional fluoroscopy is an increasingly valuable tool for diagnosis and guiding treatment; however, radiation doses to the patient can be substantial. CT fluoroscopy used as imaging guidance generates a radiation dose of about 10 times the dose of conventional CT (50). Interventional fluoroscopy is widely used in pediatric cardiology, urology, and neurointerventional procedures, and as it has become more complex, patient doses have risen. Pediatric procedures requiring fluoroscopy are typically more time consuming than adult procedures, thus exposing a child to higher cumulative radiation doses.
The number and extent of neurointerventional procedures being performed in clinical practice is growing rapidly, including among children (51,52). Fluoroscopy is also used with mounting frequency in pediatric cardiology for both diagnostic and therapeutic purposes. The Spanish Society of Cardiology reported a 13% increase in pediatric procedures from 2000 to 2004 (53,54). The radiation exposure associated with fluoroscopically guided cardiac resynchronization device procedures in adults is considerable, especially in complicated and prolonged procedures (55,56). Based on an analysis of doses received by 137 pediatric patients who underwent diagnostic catheterizations or therapeutic procedures, it was estimated that the probability of a fatal cancer attributable to each fluoroscopically-guided cardiac procedure would be 0.07% (54). During pediatric cardiac catheterization, in one study, the dose measured at the surface of the skin above the thyroid was between 0.2 and 0.6 R (roentgen, in this setting about equivalent to a rad), depending on fluoroscopy settings (57). The thyroid dose would depend on age and length of the procedure (58).
Micturating cystourethrography (MCU) accounts for 30–50% of all fluoroscopic examinations in children (59,60). MCU is regarded as the best test detecting and grading vesicoureteric reflux and evaluating urethral or bladder abnormalities (59). Because the mean dose to the thyroid during MCU is very low (0.006 mGy), the calculated risk of developing radiation-related thyroid cancer from MCU was less than 1 per million exposed patients (59).
Methods of reducing thyroid exposure from fluoroscopic examinations are available. Shortt et al. (61) reported that the high thyroid dose exposure received during neurointerventional procedures requiring fluoroscopy could be reduced by half by simply shielding of the thyroid (unshielded, 8.29 mSv; shielded, 4.09 mSv).
D. Nuclear medicine procedures
The number of diagnostic nuclear medicine procedures being performed in the United States has increased from 3.5 million in 1973 to 7.5 million in 1982 and 17.2 million in 2005 (62). The estimated average radiation dose per person from diagnostic nuclear medicine examinations increased by 550% between 1982 and 2005 (62). By far the largest contributor to this trend is cardiac imaging, which, fortunately, is rarely performed in children (62).
Despite the large increase in diagnostic nuclear medicine procedures, thyroid radiation exposure from them has gone down. There has been a dramatic decrease in thyroid imaging, from 13.1% of all nuclear medicine diagnostic examinations in 1973 to less than 1% in 2005, and there has been a shift away from 131I to other isotopes (62,63). For a 40-pound, 5-yr-old child, assuming a 15% uptake, a thyroid scan with 0.1–0.3 MBq/kg 123I-iodide exposes the thyroid to 18–55 mGy, compared with 500-2000 mGy using 0.025–0.1 MBq/kg 131I-iodide (64). Using 1–5 MBq/kg 99mTc-pertechnetate, the upper large intestine is exposed to 3.8–19 mGy, a dose larger than the thyroid receives (64). No significant increase in the risk of thyroid cancer has been found after the use of 131I for diagnostic purposes in children (65,66,67); however, because the number of children studied has been very limited, the statistical power was not adequate to detect small risks.
E. Dental x-rays
Radiation exposure to children via routine intraoral dental x-rays is of potential concern given the anatomic position of the thyroid. For the U.S. population in general, dental x-rays account for 2.5% of the effective dose received from conventional radiographs and fluoroscopies (12). Orthodontic therapy is highly prevalent in children and adolescents, and in the United States it has increased from 1 million patients treated in 1992 to 1.6 million in 2006 (68).
At the University of Washington in Seattle, the thyroid dose received during orthodontic care decreased from 7 mGy before 1992 to 2.8 mGy afterward (69). A study evaluating orthodontic treatment and radiation exposure demonstrated that the use of a smaller field of irradiation combined with specified collimator dimensions compared with what is normally used led to a statistically significant reduction in the absorbed radiation dose to the thyroid gland of around 30% (70).
There has been an increasing use of preoperative CT imaging of the lower third molars and, according to Ohman et al. (71), in an adult, this exposes the thyroid to 1.5 mGy, about 15–60 times more than other imaging modalities. They do not provide data for children. In adults, a single jaw or panoramic radiograph results in very low exposure to the thyroid, 0.06 and 0.07 mGy, respectively (72). The American Dental Association recommends the use of shielding to reduce thyroid radiation exposure, and these recommendations appear to be partly successful (73). Results of surveys indicate that rates of thyroid collar use to reduce thyroid radiation exposure exceeds 48% in dental practice (74).
F. Radiation treatment
Because survival from the majority of childhood cancers is relatively high and is continuing to improve, with a 5-yr survival of about 79% in the United States during 1995–2000, treatment-related second cancers are becoming a disturbing problem (75). In many studies, the use of radiotherapy for the treatment of malignancies involving the head and neck and for Hodgkin’s disease, especially among children, has been associated with an increase in the development of secondary malignancies, including those of the thyroid (16,76,77,78,79,80,81,82,83). One indication of the magnitude of the risk is that 7.5% of all secondary malignancies identified in a cohort of childhood cancer survivors in 58 hospitals in Germany, Austria, and Switzerland were thyroid cancers (84). Most persuasively, observations from the Late Effects Study Group and the Childhood Cancer Survivor Study established dose-response relationships and large estimated odds ratio for the development of thyroid cancer after high-dose therapy in childhood (for the Childhood Cancer Survivor Study, odds ratio, 9.8; 95% confidence interval, 3.2–34.8) (16). Although exposure to high-dose external radiation leads to an increased risk of secondary thyroid cancer, it is uncertain whether the risk decreases at the highest doses. The Childhood Cancer Survivor Study suggested a fall-off of risk at doses greater than 30 Gy (16). It is also unclear how long the risk persists, but it appears to continue for decades (1,67,76,85,86).
Childhood leukemia patients often received prophylactic cranial irradiation because it significantly reduces the occurrence of leukemic relapses in the central nervous system. However, an increased risk of thyroid cancer has been recognized in survivors receiving this therapy. In one case series of 142 survivors of childhood malignancy, cranial radiation therapy for hematological malignancies was found to be a significant risk factor for the development of thyroid cancer (80). Thyroid doses from prophylactic cranial irradiation vary according to whether gamma rays of 60Co or 6 MV photon beams are used and the angle of administration. In adults and children, the ranges are 0.12 to 0.22 Gy and 0.23 to 0.32 Gy, respectively (87).
In recent decades, there has been an increase in the use of several types of radiation treatment, such as high-dose gamma-knife and stereotactic therapy for the treatment of benign central nervous system tumors such as acoustic neuromas, meningiomas, pituitary adenomas, and hemangioblastomas. To date, no data have been published linking them to thyroid cancer (88).
Cancer is not the only thyroid-related disease caused by high-dose radiation (89). There is a substantial risk of developing hypothyroidism, where the underlying mechanism is felt to be cellular death. This is now usually detected by TSH screening. Benign thyroid nodules also develop as a result of radiation exposure, the risk increasing with increasing dose (90). As discussed in Section V, subsection A, these benign nodules complicate the decision about whether to use ultrasound for routine follow-up.
The sequelae of the external radiation treatments once used for benign conditions such as “enlarged thymus” or “enlarged tonsils” are well known and have been reviewed elsewhere (67). Although these have been abandoned, there remain a few benign conditions for which external radiation is still employed (Table 2) (91). One of the conditions on the list that is not rare in children is craniopharyngioma. Also, based on the paucity of reports of cancer after its use, it has been suggested that radiation can be used as an adjunct to surgery in the treatment of keloids, including in children (92).
Table 2.
Benign conditions for which external radiotherapy may be used, according to Jha et al. (91)
| I. Tumors |
| Ameloblastomas |
| Craniopharyngiomas |
| Desmoid tumors |
| Meningiomas |
| Keratoacanthomas |
| Pituitary adenomas |
| II. Dermatological conditions |
| Keloids |
| Plantar warts |
| Skin hemangiomas |
| III. Other benign conditions |
| Aneurysmal bone cysts |
| Arteriovenous malformations |
| Exophthalmos |
| Gynecomastia |
| Hepatic cavernous hemangiomas |
| Ocular pseudotumors |
| Ovarian castration |
| Peyronie’s disease |
| Pterygiums |
| Vertebral hemangiomas |
It should be noted that radiotherapy is rarely the primary mode of therapy.
The results regarding cancer incidence or cancer-related death are somewhat inconsistent after the therapeutic use of 131I for adults with hyperthyroidism (93,94,95,96,97,98,99). Small increases in thyroid cancer (96,97), total cancer incidence, or mortality have been observed in some studies, but no clear dose-response was demonstrated (93,94,98,99).
III. In Utero Exposure of the Thyroid Gland
The Oxford Survey of Childhood Cancer was one of the first epidemiological studies to look at the effect of low-dose radiation in humans (100). The study found that in utero exposure to one or two x-rays, the equivalent of 10–20 mSv, in a pregnant woman increased the incidence of cancer in the offspring by age 10 by about 50%. This was the first definitive human evidence linking x-ray to the development of cancer. The in utero effects of radiation have been confirmed—such exposure among atomic bomb survivors resulted in a dose-response associated increase in solid cancers (101).
Accidental fetal exposure occurred as a result of the Chernobyl nuclear accident. A screening study of thyroid cancer prevalence among individuals exposed in utero to iodine isotopes, principally 131I, from the Chernobyl fallout suggested that in utero exposure to radioiodines may have increased the risk of thyroid carcinoma approximately 20 yr later (102). The mean thyroid dose was 72 mGy, with a range of 0–3230 mGy. Although the excess odds ratio in this study was quite large, the number of cases was small and the estimate was not statistically significant.
Accidental medical exposure is possible when 131I is given to a woman with an unrecognized pregnancy. The whole body dose to the fetus from maternal 131I is greatest when 131I is given at 2 months fetal age, is dependent on how much is taken up by the mother’s thyroid, and has been estimated to be 1.8–3.1 mGy/mCi (103). The dose specifically to the fetal thyroid is much larger and is greatest when the 131I is given at 6 month gestation, estimated to be between 4.8 and 44 Gy/mCi (104). The carcinogenic potential of such exposure has not been established (105).
Currently, it is only in extreme situations that a fetus is exposed to medical radiation. At the earliest time of gestation, maternal CT scanning for renal stones, appendicitis, and pulmonary embolism results in doses of approximately 10, 16, and 0.3 mGy, respectively (106). For a fetus at 3 month gestation, the corresponding doses would be 5.5, 30, and 0.6 mGy (106).
IV. Thyroid Cancer Risk Associated with Low-Dose Radiation Exposure
Although the shape of the dose-response relationship below the level of epidemiological observation is unknown, most scientific and regulatory organizations consider it unlikely that there is a threshold for radiation-induced cancer (11,13,107,108).
It is still relevant to ask what is the lowest level at which effects have been demonstrated. Much of the quantitative information on radiation-related cancer risk comes from studies of atomic bomb survivors, and these data are often used to project radiation-related risks for other populations (13). Specifically, there is a significant increase in the incidence of cancer among atomic bomb survivors exposed to doses between 5 and 150 mSv (mean dose, 40 mSv) (37,109,110). Although the atomic bomb survivors received acute radiation exposure, a recent study of 400,000 radiation workers in the nuclear industry who were chronically exposed to an average dose of 19.4 mSv showed a significant association between radiation dose and all cancer (5233 cases) mortality (111,112). There were only 17 thyroid cancer cases, and only lung cancer (1447 cases) showed a dose-response relationship.
From the 1930s to the 1960s, radiation therapy was used for the treatment of a variety of benign conditions The most complete description of the relationship between radiation dose and thyroid cancer is a study pooling data from seven studies, five of which were cohort studies. An elevated risk of thyroid cancer was observed at doses as small as 100 mGy (1). More recently, an updated analysis of thyroid cancer after irradiation for tinea capitis in Israel showed a significantly elevated risk at levels near 50 mGy (113). As seen in Fig. 3 of the review by Brenner and Hall (26), similar thyroid doses can be reached when an infant receives multiple head CT scans.
The pooled analysis also demonstrated a strong inverse relationship between age at exposure and the risk of thyroid cancer development; however, above age 15 the risk was no longer statistically significant. The ERR of thyroid cancer was 7.7/Gy for irradiation before age 15 (1). For a patient who received 1 Gy to the thyroid, the estimated attributable risk of developing thyroid cancer (the proportion of thyroid cancer that occurred due to radiation exposure) was 88% (1). The radiation effect was somewhat greater in women, but not significantly so.
Whether radiation-related thyroid cancer has the same clinical behavior as sporadic thyroid cancers is not known with certainty. Radiation-related thyroid cancers are frequently multifocal and have a different spectrum of somatic molecular mutations. Although most studies find that these features do not affect their behavior, other studies suggest a more aggressive course (67,114,115,116,117). The Chernobyl-related thyroid cancer cases in children are of interest because they presented at a particularly early age, had short latency periods, and had features associated with aggressive behaviors, namely extrathyroidal extension and frequent lymph node metastases (118). Possible explanations for the apparent aggressive behavior of the Chernobyl-related thyroid cancers include their younger age at diagnosis and the presence of iodine deficiency in some areas near Chernobyl, which may have promoted more rapid progression of cancers.
V. Practice and Public Health
A. Screening for thyroid cancer
For asymptomatic people, the only way to adequately screen for thyroid nodules and thyroid cancer is by imaging, usually with ultrasound. Palpation, even by an experienced examiner, has low sensitivity. Imaging by other modalities is neither more sensitive nor more specific and is more costly. The question of when the benefits of screening outweigh the risks is a difficult one, in part because it has not been tested in a prospective trial.
The presence of thyroid nodules in cancer survivors and other patients exposed to moderate to high dose ionizing radiation are of particular concern because of the risk of malignancy, leading some to propose periodic ultrasound surveillance (119). The rationale for screening is stronger as radiation dose increases and age at exposure decreases (89). The goal of early detection of thyroid nodules is to decrease the morbidity and mortality of radiation-related thyroid cancers. Although ultrasound increases the detection of thyroid cancer, it also results in surgery for some cancers that may never progress and for some benign nodules that cannot be adequately evaluated by FNA (120).
A review in 1989 suggested that after an exposure of 2–5 Gy, new nodules developed at a rate of about 2% annually, reaching a peak at 15–25 yr (121). Similarly, in a retrospective case series of 142 survivors of childhood malignancy, there was a statistically significant increase (odds ratio, 1.2; 95% CI, 1.1–1.3) in the risk of an abnormal ultrasound result with each year after original diagnosis (80). The prevalence of thyroid nodules approached 70% during follow-up. Although a palpable thyroid nodule was the only significant predictor of malignancy, the authors recommended following radiation-treated patients with annual evaluation of the thyroid by physical examination, ultrasound, thyroid function tests, and thyroglobulin levels (80). Crom et al. (119) recommended a baseline ultrasound evaluation within 1 yr of completion of radiotherapy for childhood cancer and screening every 2–3 yr for this high-risk population.
Schneider et al. (67,89,122) recommended that patients who have received external irradiation to the head and neck region as children be evaluated for thyroid nodule and cancer development, hyperparathyroidism, salivary gland tumors, and neural tumors. For those where the risk is thought to be high, they recommend thyroid ultrasound. Because thyroid cancers typically grow slowly, they recommended repeating an ultrasound examination every 12–24 months in patients with small nodules that otherwise are not suspicious, more often in those with larger or suspicious nodules, and every 3–5 yr in patients with no evident nodules.
The Children’s Oncology Group, in its guidelines for survivors of childhood cancer, recommends annual evaluations of the thyroid gland (123). To evaluate function, they suggest TSH and free T4 annually, more often during rapid growth. With respect to neoplasia, they recommend annual palpation, followed by ultrasound and other tests if a palpable nodule is present. As mentioned earlier in this section, others advocate routine imaging.
The American Thyroid Association, in their guidelines for the management of thyroid nodules, suggests that FNA should be performed for smaller nodules than usual when there is a history of radiation exposure (124). A study by Hatipoglu et al. (125) demonstrated that for a given nodule, the sensitivity and specificity of FNA in irradiated patients is similar to that reported for the general population. This was borne out by a similar study in a prospectively followed cohort exposed by the Chernobyl accident (126). However, malignancy in smaller nodules in other areas of the thyroid cannot be excluded (125,127).
B. Regulations and guidelines
Several organizations issue periodic scholarly reports on the medical effects of radiation (Table 3). The most current expert opinions regarding radiation exposure derive from the BEIR (Biological Effects of Ionizing Radiation) VII, phase 2 report of the National Academy of Sciences and ICRP reports 103 and 105 (11,13,128). BEIR VII evaluates and employs data from atomic bomb survivor studies and from medical and occupational radiation studies (13). It provides risk estimates for the general U.S. population. It supports the linear no-threshold risk model for low-dose x-ray exposures (13). According to the report, a single effective dose of 10 mSv to an adult population results in a 1 in 1000 lifetime risk of developing an associated solid cancer or leukemia. From the same dose of radiation, the risk is 10–15 times greater in a 1-yr-old child than a 50-yr-old adult (13).
Table 3.
Organizations (listed alphabetically) that issue scholarly reports on the effects of ionizing radiation
| Organization | Mission statement | Latest thyroid cancer-related publication(s) |
|---|---|---|
| The International Commission on Radiological Protection (ICRP) | ″The ICRP is an Independent Registered Charity, established to advance for the public benefit the science of radiological protection, in particular by providing recommendations and guidance on all aspects of protection against ionising radiation.″ | 2007 Recommendations of the ICRP. Publication no. 103 (11) 2007 Radiological protection in medicine. ICRP Publication no. 105 (128) |
| ″[The ICRP] is an Independent Registered Charity (a ″not-for-profit organisation’) in the United Kingdom; and currently has its small Scientific Secretariat in Canada.″ | ||
| The National Council on Radiation Protection and Measurements (NCRP) | ″The NCRP seeks to formulate and widely disseminate information, guidance and recommendations on radiation protection and measurements which represent the consensus of leading scientific thinking. The Council is always on the alert for areas in which the development and publication of NCRP materials can make an important contribution to the public interest.″ | 2009 Risks to the thyroid from ionizing radiation. Report no. 159. Bethesda, MD; NCRP (17) |
| ″The Council’s mission also encompasses the responsibility to facilitate and stimulate cooperation among organizations concerned with the scientific and related aspects of radiation protection and measurements.″ | ||
| ″It should be noted that while the [Congressional] Charter recognizes the importance and the national character of the NCRP, it does not make the Council a governmental body; it is a private corporation.″ | ||
| The National Research Council: Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation | ″The National Research Council was organized by the National Academy of Sciences in 1916 to associate the broad community of science and technology with the Academy’s purposes of furthering knowledge and advising the federal government. Functioning in accordance with general policies determined by the Academy, the Council has become the principal operating agency of both the National Academy of Sciences and the National Academy of Engineering in providing services to the government, the public, and the scientific and engineering communities. The Council is administered jointly by both Academies and the Institute of Medicine.″ | 2006 Health risks from exposure to low levels of ionizing radiation. BEIR VII Phase 2. Washington, DC: National Academy Press (13) |
| The United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) | ″UNSCEAR was established by the General Assembly of the United Nations in 1955. Its mandate in the United Nations system is to assess and report levels and effects of exposure to ionizing radiation. Governments and organizations throughout the world rely on the Committee’s estimates as the scientific basis for evaluating radiation risk and for establishing protective measures.″ | 2006 UNSCEAR Report: effects of ionizing radiation. Vol 1. New York: United Nations (108) |
From the organization’s web site as of November, 2009.
In the 1970s, the ALARA (As Low As Reasonably Achievable) principle regarding radiation exposure was enunciated. It is based on the nonthreshold linear model which, as described in Section I, subsection B, presumes that any amount of radiation exposure can increase the chance of negative biological effects such as cancer, and that the probability of the occurrence of such a negative effect increases with cumulative lifetime doses (128). The risks related to pediatric CT scans led the Society for Pediatric Radiology in 2001 to convene an ALARA conference, and at about the same time the U.S. Food and Drug Administration issued a Public Health Notification including the warning that “children less than 10 yr of age are several times more sensitive to radiation than middle-aged adults” (33,129,130). The amount of radiation exposure to the pediatric population can be reduced by performing CT scans only when necessary, adjusting exposure parameters for pediatric CTs, setting scan resolution to the lowest level necessary, and minimizing the use of multiple scans obtained during different phases of contrast enhancement (multiphase exams) (129,131,132). Without loss of quality, age- and/or weight-related adjustments to the tube current of CT scanners or using automatic current modulation techniques can reduce exposure in pediatric CT examinations (133,134,135). Additionally, innocent bystander tissues such as the thyroid gland should be protected with appropriate lead shielding during all CT examinations. The risk to selected organs can be lowered by a factor of two with appropriate shielding (136).
A policy document issued jointly by the National Cancer Institute and the Society for Pediatric Radiology concluded that a small radiation exposure dose from CT to the pediatric population represents “a public health concern” (29). Besides endorsing the concept that there is no low-dose threshold for inducing cancer, they call for strategies to “minimize CT dose, disseminate relevant information, and clarify the relationship between CT radiation and cancer risk.” Recently, 13 organizations comprising the Alliance for Radiation Safety in Pediatric Imaging are attempting to minimize pediatric exposure to radiation with an “Image Gently Campaign” (137).
Although there was a doubling of the number of CT scans from 1998 to 2003, a study found that referring physicians don’t appreciate and do not explain to patients the radiation exposure involved (138). Of concern is the inappropriate use of CT scans. In a retrospective study in one hospital in Finland, based on the guidelines of the European Commission, 3–77% of diagnostic CTs, depending on anatomic site, were found to be unjustified (139). Many physicians appear to be ill-informed about the risks of diagnostic examinations utilizing radiation (138,140,141). A study of physician awareness of radiation risks demonstrated that only 47% of radiologists and less than 10% of the other physicians surveyed believed CT examinations might increase a patient’s cancer risk (138). There is a clear need to educate the medical profession and the general public about the risks regarding CT imaging.
An anonymous survey of members of the American Pediatric Surgical Association (APSA) revealed that only half of them believed that one abdominal/pelvic CT increased the lifetime risk of cancer, and more than 75% of them underestimated CT scan’s radiation dose compared with a conventional x-ray (142). To increase awareness of radiation exposure and risks associated with CT scans, the National Cancer Institute and the Society of Pediatric Radiology published guidelines and recommendations for clinicians and the general public (29), and the APSA published a review of the medical literature.
The European Union and the United Kingdom each regulate safety precautions for medical radiation (143,144). There are no corresponding regulations in the United States, but the American College of Radiology has published a “white paper” on the subject (145). These are general statements of radiation safety principles and have no specific measures related to protecting the thyroid gland.
VI. Research Directions
A. Determination of risks at lower doses and with longer follow-up times
There is a need for follow-up studies of large cohorts to determine the risks at lower doses and over longer times spans (36). The question of risks at lower doses pertains particularly to CT scans, especially for children, and to other forms of low-dose radiation exposure. A status report of several ongoing large studies, including the follow-up of some cohorts cited in this review, was recently published (146). Large studies of cancer incidence and mortality associated with CT scans in young patients are planned or are under way in the United States, the United Kingdom, Canada, Australia, Europe, and Israel. Despite their planned size, they will be limited in their ability to quantify site-specific risks, especially of sites such as the thyroid where mortality is rare and ascertainment is highly dependent on diagnostic methods.
B. Methods to ameliorate the risks
The efforts at minimizing the risk of ionizing medical radiation, particularly associated with CT, are summarized in Section V, subsection B, but new innovative methods need to be developed and evaluated. For example, a tertiary referral hospital in Boston is modifying its electronic medical record system to track how often a patient receives radiation (35). This should enable doctors to calculate the cumulative dose a patient has been exposed to in the past.
C. Identification of radiation-related thyroid cancers, i.e., radiation “signatures”
Perhaps the greatest barrier to studying radiation-induced thyroid cancer at low levels of exposure is the fact that it is not possible to distinguish a case caused by radiation from one that is not. By studying somatic mutations in thyroid cancers, attempts are ongoing to overcome this problem. However, it is not yet clear that radiation and nonradiation cases can be distinguished by this approach.
A distinctive feature of radiation-related cases is the high frequency of RET proto-oncogene rearrangements. Two studies using model systems, one normal human thyroid tissue transplanted to severe combined immunodeficient mice and the other fetal human thyroid cells transfected with SV40 (simian virus 40), have been used to demonstrate the sensitivity of the RET gene to radiation (148,149). The preferred recombination with the H4 gene to form PTC1 is likely a result of the fact that the two genes are in proximity to each other within the nucleus of the thyroid cell (150). In Japanese survivors of the atomic bombs, RET rearrangements have been correlated to the dose of radiation exposure, strongly supporting the idea that RET activation is not only age related, but also radiation related (151). A similar correlation was not found in Russians exposed to radiation from the Chernobyl accident, perhaps due to limited sample size (152).
Because RET rearrangements are, by themselves, insufficient to identify radiation-induced cases of thyroid cancer, efforts were made to see whether identifying the specific breakpoints would be informative (153,154,155). The most extensive of these studies, involving 26 cases of RET rearranged, post-Chernobyl cases, found evidence for topoisomerase I sites near each breakpoint and the use of nonhomologous DNA end joining to repair radiation-induced DNA damage (155). However, this has not as yet been replicated and would probably not be sufficiently specific to identify radiation-induced cases.
Most recently, efforts have been made to apply genome-wide expression studies using microarray analysis (156,157,158,159). Two promising studies report expression patterns that might distinguish radiation and sporadic cases, but the findings do not appear to overlap (157,159). These studies are complicated by the fact that patterns may arise from factors other than radiation exposure. For one, iodine deficiency and ethnic patterns related to Chernobyl cases could affect expression patterns (160). Also, as discussed in a recent review of the subject, radiation susceptibility factors may also show up in expression array patterns (157).
In summary, at the present time it is not possible to identify radiation-induced cases unequivocally. It is not clear whether this goal will be achieved with advancing technologies. However, even presumptive identifications, making it more or less likely that a case is related to radiation, might augment epidemiological studies. Specifically, if the increased incidence of thyroid cancer is related to low-dose exposure from diagnostic radiation, one might expect an accompanying proportionate increase in cases with somatic recombination events.
D. Increased susceptibility to the effects of radiation
That there are a few, uncommon syndromes associated with markedly increased radiation susceptibility is well known (161). It is less clear whether there are more subtle variations in susceptibility in the general population. With respect to thyroid cancer, one cohort study that included a large number of sibling groups was unable to detect patterns that would indicate a genetic basis for susceptibility (162). Recently, there have been intriguing findings suggesting that there are germ-line factors associated with an increased risk of developing thyroid cancer (163,164). So far, these have not been linked to susceptibility radiation-induced thyroid cancer. However, one study in people exposed to radioactive fallout from nuclear tests in Kazakhstan has found evidence of genetic factors associated with thyroid nodules, and one of them, XRCC1, appears to interact with radiation exposure as a risk factor (165).
Understanding these factors might lead to enhanced protection, or at least enhanced subsequent screening, for susceptible people. In the longer term, it may also lead to ways of reducing the risks of radiation exposure.
VII. Conclusions
Radiation therapy for the treatment of benign conditions of the head and neck area carries a checkered history, having resulted in a substantial increased risk for thyroid, parotid, parathyroid, and central nervous system tumors (67,166). This legacy emphasizes the need for continued research on radiation risks and for strategies to minimize radiation exposures. The literature on potential cancer risks related to radiation exposure from diagnostic CT scanning, currently the largest man-made radiation exposure, continues to evolve. The implications for public health may be substantial because even a small increased cancer risk associated with CT use, if applied to a large population, can result in many, potentially avoidable cases.
Although there is an increasing awareness for the potential cancer risks from radiation exposure, especially from CT, a strong need for educating the physicians who order the tests remains. Knowing the potential risks of radiation exposure is important for promoting the proper use of CT imaging for children. Radiation exposures related to imaging need to be optimized to reduce exposure. The risks need to be weighed against the anticipated patient benefit from the diagnostic information obtainable from the scan, even while there are uncertainties regarding the risks at the low doses encountered in diagnostic CT examinations (36,107,167). It is prudent to act on the assumption that such risks are real and assume, as do most national and international radiation protection and regulatory agencies, that they are proportional to dose (147).
As exemplified by the ALARA principle, it is recognized that the population should not be exposed to more radiation than is necessary, and diagnostic modalities that do not involve ionizing radiation should always be considered (25,34). Attention should be paid to the total number of CTs performed on an individual because the cumulative doses of radiation over time may reach meaningful doses (2). The realization that the estimated lifetime radiation risks for children undergoing CT are not negligible may stimulate more careful calibration of the CT exposure settings for pediatric patients.
Physicians and health authorities should work together to minimize the medical radiation exposure of pediatric patients by encouraging responsible use of CT and other radiological procedures (Table 4) (39). Endocrinologists have a special role in pointing out radiation risks related to the thyroid. They should be aware of the current sources of radiation exposure so they can be active participants in encouraging policies and procedures that implement strategies to reduce radiation doses to the thyroid.
Table 4.
Methods of reducing thyroid radiation exposure in children
| Use fields and instrument adjustments specific for children. |
| Avoid contrast when it is not necessary, especially when the thyroid will be exposed. |
| Avoid overlapping fields, if possible, especially when cervical spine and chest CTs are performed together. |
| Protect the thyroid from exposure with shielding, when possible. |
| Institute policies, both procedural and educational, among radiologists and Radiology Departments to institute the above. |
| Increase the awareness of practicing pediatricians about the risks and benefits of radiological procedures and how to reduce the former without compromising the latter. |
| Institute methods to explain the risk and benefits in an accurate and understandable way. |
Footnotes
E.R. is supported by the intramural research program of the National Institutes of Health, and the research of A.B.S. has been supported by a grant from the National Cancer Institute (CA 21815).
Current address for B.S.: Section of Mineral Metabolism, University of Texas Southwestern Medical Center at Dallas, Dallas, Texas 75390.
Disclosure Summary: A.B.S. has served as an expert witness in cases related to radiation and thyroid cancer. B.S. and E.R. have nothing to declare.
First Published Online July 21, 2010
Abbreviations: CI, Confidence interval; CT, computerized tomography (scan); ERR, excess RR; FNA, fine-needle aspiration; RR, relative risk.
References
- Ron E, Lubin JH, Shore RE, Mabuchi K, Modan B, Pottern LM, Schneider AB, Tucker MA, Boice Jr JD 1995 Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiat Res 141:259–277 [PubMed] [Google Scholar]
- Sadetzki S 2007 Excess lifetime cancer mortality risk attributed to radiation exposure from pediatric computed tomography scan. Isr Med Assoc J 9:607–609 [PubMed] [Google Scholar]
- Horner MJ, Ries LAG, Krapcho M, Neyman N, Aminou R, Howlader N, Altekruse SF, Feuer EJ, Huang L, Mariotto A, Miller BA, Lewis DR, Eisner MP, Stinchcomb DG, Edwards BK 2009 SEER cancer statistics review, 1975–2006. Bethesda, MD: National Cancer Institute (http://seer.cancer.gov/csr/1975_2006, based on November 2008 SEER data submission, posted to the SEER web site, 2009) [Google Scholar]
- Trimboli P, Ulisse S, Graziano FM, Marzullo A, Ruggieri M, Calvanese A, Piccirilli F, Cavaliere R, Fumarola A, D'Armiento M 2006 Trend in thyroid carcinoma size, age at diagnosis, and histology in a retrospective study of 500 cases diagnosed over 20 years. Thyroid 16:1151–1155 [DOI] [PubMed] [Google Scholar]
- Davies L, Welch HG 2006 Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 295:2164–2167 [DOI] [PubMed] [Google Scholar]
- Enewold L, Zhu K, Ron E, Marrogi AJ, Stojadinovic A, Peoples GE, Devesa SS 2009 Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev 18:784–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AY, Jemal A, Ward EM 2009 Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer 115:3801–3807 [DOI] [PubMed] [Google Scholar]
- UNSCEAR (United Nations Scientific Committee on the Effects of Radiation) 2000 Sources and effects of ionizing radiation. Vol 2. New York: United Nations [Google Scholar]
- Hall EJ, Brenner DJ 2008 Cancer risks from diagnostic radiology. Br J Radiol 81:362–378 [DOI] [PubMed] [Google Scholar]
- Chodick G, Kim KP, Shwarz M, Horev G, Shalev V, Ron E 2009 Radiation risks from pediatric computed tomography scanning. Pediatr Endocrinol Rev 7:109–116 [PMC free article] [PubMed] [Google Scholar]
- International Commission on Radiological Protection 2007 Recommendations of the ICRP. Publication no. 103. Ann ICRP 37:1–332 [DOI] [PubMed] [Google Scholar]
- 2009 Ionizing radiation exposure of the population of the United States. Report no. 160. Bethesda, MD: National Council on Radiation Protection and Measurement [Google Scholar]
- National Research Council 2006 Health risks from exposure to low levels of ionizing radiation: BEIR VII Phase 2. Washington, DC: National Academy Press [PubMed] [Google Scholar]
- Mettler Jr FA, Thomadsen BR, Bhargavan M, Gilley DB, Gray JE, Lipoti JA, McCrohan J, Yoshizumi TT, Mahesh M 2008 Medical radiation exposure in the U.S. in 2006: preliminary results. Health Phys 95:502–507 [DOI] [PubMed] [Google Scholar]
- Ron E, Modan B, Preston D, Alfandary E, Stovall M, Boice Jr JD 1989 Thyroid neoplasia following low-dose radiation in childhood. Radiat Res 120:516–531 [PubMed] [Google Scholar]
- Sigurdson AJ, Ronckers CM, Mertens AC, Stovall M, Smith SA, Liu Y, Berkow RL, Hammond S, Neglia JP, Meadows AT, Sklar CA, Robison LL, Inskip PD 2005 Primary thyroid cancer after a first tumour in childhood (the Childhood Cancer Survivor Study): a nested case-control study. Lancet 365:2014–2023 [DOI] [PubMed] [Google Scholar]
- 2009 Risks to the thyroid from ionizing radiation. Report no. 159. Bethesda, MD: National Council on Radiation Protection and Measurement [Google Scholar]
- Huda W, Atherton JV, Ware DE, Cumming WA 1997 An approach for the estimation of effective radiation dose at CT in pediatric patients. Radiology 203:417–422 [DOI] [PubMed] [Google Scholar]
- Huda W 2002 Dose and image quality in CT. Pediatr Radiol 32:709–713; discussion 751–754 [DOI] [PubMed] [Google Scholar]
- Richardson DB 2009 Exposure to ionizing radiation in adulthood and thyroid cancer incidence. Epidemiology 20:181–187 [DOI] [PubMed] [Google Scholar]
- Ron E 2003 Cancer risks from medical radiation. Health Phys 85:47–59 [DOI] [PubMed] [Google Scholar]
- Berrington de González A, Darby S 2004 Risk of cancer from diagnostic x-rays: estimates for the UK and 14 other countries. Lancet 363:345–351 [DOI] [PubMed] [Google Scholar]
- Bader D, Datz H, Bartal G, Juster AA, Marks K, Smolkin T, Zangen S, Kugelman A, Hoffmann C, Shani G, Ben-Shlomo A, Margaliot M, Sadetzki S 2007 Unintentional exposure of neonates to conventional radiography in the Neonatal Intensive Care Units. J Perinatol 27:579–585 [DOI] [PubMed] [Google Scholar]
- Sutton PM, Arthur RJ, Taylor C, Stringer MD 1998 Ionising radiation from diagnostic x rays in very low birthweight babies. Arch Dis Child Fetal Neonatal Ed 78:F227–F229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis CE, Slovis TL 2005 The ALARA concept in pediatric CR and DR: dose reduction in pediatric radiographic exams—a white paper conference. AJR Am J Roentgenol 184:373–374 [DOI] [PubMed] [Google Scholar]
- Brenner DJ, Hall EJ 2007 Computed tomography—an increasing source of radiation exposure. N Engl J Med 357:2277–2284 [DOI] [PubMed] [Google Scholar]
- Petrik V, Apok V, Britton JA, Bell BA, Papadopoulos MC 2006 Godfrey Hounsfield and the dawn of computed tomography. Neurosurgery 58:780–787 [DOI] [PubMed] [Google Scholar]
- Semelka RC, Armao DM, Elias Jr J, Huda W 2007 Imaging strategies to reduce the risk of radiation in CT studies, including selective substitution with MRI. J Magn Reson Imaging 25:900–909 [DOI] [PubMed] [Google Scholar]
- 2008 Radiation risks and pediatric computed tomography (CT): a guide for health care providers. National Cancer Institute, Society for Pediatric Radiology http://www.cancer.gov/cancertopics/causes/radiation-risks-pediatric-CT (accessed July 3, 2010) [Google Scholar]
- Mettler Jr FA, Wiest PW, Locken JA, Kelsey CA 2000 CT scanning: patterns of use and dose. J Radiol Prot 20:353–359 [DOI] [PubMed] [Google Scholar]
- Hart D, Wall BF 2004 UK population dose from medical x-ray examinations. Eur J Radiol 50:285–291 [DOI] [PubMed] [Google Scholar]
- Metting NF 2010 Ionizing radiation dose ranges. http://lowdose.energy.gov/imagegallery.aspx (accessed July 3, 2010) [Google Scholar]
- Linton OW, Mettler Jr FA 2003 National conference on dose reduction in CT, with an emphasis on pediatric patients. AJR Am J Roentgenol 181:321–329 [DOI] [PubMed] [Google Scholar]
- Slovis TL, Berdon WE 2002 Perfect is the enemy of the very good. Pediatr Radiol 32:217–218 [DOI] [PubMed] [Google Scholar]
- Sodickson A, Baeyens PF, Andriole KP, Prevedello LM, Nawfel RD, Hanson R, Khorasani R 2009 Recurrent CT, cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults. Radiology 251:175–184 [DOI] [PubMed] [Google Scholar]
- Brenner DJ, Doll R, Goodhead DT, Hall EJ, Land CE, Little JB, Lubin JH, Preston DL, Preston RJ, Puskin JS, Ron E, Sachs RK, Samet JM, Setlow RB, Zaider M 2003 Cancer risks attributable to low doses of ionizing radiation: assessing what we really know. Proc Natl Acad Sci USA 100:13761–13766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston DL, Ron E, Tokuoka S, Funamoto S, Nishi N, Soda M, Mabuchi K, Kodama K 2007 Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res 168:1–64 [DOI] [PubMed] [Google Scholar]
- Brenner D, Elliston C, Hall E, Berdon W 2001 Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol 176:289–296 [DOI] [PubMed] [Google Scholar]
- Chodick G, Ronckers CM, Shalev V, Ron E 2007 Excess lifetime cancer mortality risk attributable to radiation exposure from computed tomography examinations in children. Isr Med Assoc J 9:584–587 [PubMed] [Google Scholar]
- Baker SR, Hsieh YH, Maldjian PD, Scanlan MT 2009 Inadvertent thyroid irradiation in protocol-driven trauma CT: a survey of hospital ERs. Emerg Radiol 16:203–207 [DOI] [PubMed] [Google Scholar]
- Dang PA, Kalra MK, Sistrom CL, Boland GW, Dreyer KJ, Thrall JH 2008 Are multi-region CT scans too many, too often? A 10-year audit in a large practice. Proc 94th Annual Meeting of the Radiological Society of North America, Chicago, Illinois, 2008 (Abstract) [Google Scholar]
- Paterson A, Frush DP, Donnelly LF 2001 Helical CT of the body: are settings adjusted for pediatric patients? AJR Am J Roentgenol 176:297–301 [DOI] [PubMed] [Google Scholar]
- Dawson P, Punwani S 2009 The thyroid dose burden in medical imaging: a re-examination. Eur J Radiol 69:74–79 [DOI] [PubMed] [Google Scholar]
- Fujii K, Aoyama T, Koyama S, Kawaura C 2007 Comparative evaluation of organ and effective doses for paediatric patients with those for adults in chest and abdominal CT examinations. Br J Radiol 80:657–667 [DOI] [PubMed] [Google Scholar]
- Brisse HJ, Robilliard M, Savignoni A, Pierrat N, Gaboriaud G, De Rycke Y, Neuenschwander S, Aubert B, Rosenwald JC 2009 Assessment of organ absorbed doses and estimation of effective doses from pediatric anthropomorphic phantom measurements for multi-detector row CT with and without automatic exposure control. Health Phys 97:303–314 [DOI] [PubMed] [Google Scholar]
- Donadieu J, Roudier C, Saguintaah M, Maccia C, Chiron R 2007 Estimation of the radiation dose from thoracic CT scans in a cystic fibrosis population. Chest 132:1233–1238 [DOI] [PubMed] [Google Scholar]
- Baker SR, Bhatti WA 2006 The thyroid cancer epidemic: is it the dark side of the CT revolution? Eur J Radiol 60:67–69 [DOI] [PubMed] [Google Scholar]
- Mazonakis M, Tzedakis A, Damilakis J, Gourtsoyiannis N 2007 Thyroid dose from common head and neck CT examinations in children: is there an excess risk for thyroid cancer induction? Eur Radiol 17:1352–1357 [DOI] [PubMed] [Google Scholar]
- Berrington de González A, Mahesh M, Kim KP, Bhargavan M, Lewis R, Mettler F, Land C 2009 Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med 169:2071–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman SG, Tuncali K, Adams DF, Nawfel RD, Zou KH, Judy PF 1999 CT fluoroscopy-guided abdominal interventions: techniques, results, and radiation exposure. Radiology 212:673–681 [DOI] [PubMed] [Google Scholar]
- terBrugge KG 1999 Neurointerventional procedures in the pediatric age group. Childs Nerv Syst 15:751–754 [DOI] [PubMed] [Google Scholar]
- Gnanalingham KK, Apostolopoulos V, Barazi S, O'Neill K 2006 The impact of the international subarachnoid aneurysm trial (ISAT) on the management of aneurysmal subarachnoid haemorrhage in a neurosurgical unit in the UK. Clin Neurol Neurosurg 108:117–123 [DOI] [PubMed] [Google Scholar]
- López-Palop R, Moreu J, Fernández-Vázquez F, Hernández Antolín R 2004 Spanish registry of cardiac catheterization and coronary interventions. Thirteenth official report of the working group on cardiac catheterization and Interventional Cardiology of the Spanish Society of Cardiology (1990–2003). Rev Esp Cardiol 57:1076–1089 [PubMed] [Google Scholar]
- Martinez LC, Vano E, Gutierrez F, Rodriguez C, Gilarranz R, Manzanas MJ 2007 Patient doses from fluoroscopically guided cardiac procedures in pediatrics. Phys Med Biol 52:4749–4759 [DOI] [PubMed] [Google Scholar]
- Valentin J 2000 Avoidance of radiation injuries from medical interventional procedures. Ann ICRP 30:7–67 [DOI] [PubMed] [Google Scholar]
- Perisinakis K, Theocharopoulos N, Damilakis J, Manios E, Vardas P, Gourtsoyiannis N 2005 Fluoroscopically guided implantation of modern cardiac resynchronization devices: radiation burden to the patient and associated risks. J Am Coll Cardiol 46:2335–2339 [DOI] [PubMed] [Google Scholar]
- Campbell RM, Strieper MJ, Frias PA, Jeager G, Balfour G, Costello L, Sullivan KM 2005 Quantifying and minimizing radiation exposure during pediatric cardiac catheterization. Pediatr Cardiol 26:29–33 [DOI] [PubMed] [Google Scholar]
- Wu JR, Huang TY, Wu DK, Hsu PC, Weng PS 1991 Radiation exposure of pediatric patients and physicians during cardiac catheterization and balloon pulmonary valvuloplasty. Am J Cardiol 68:221–225 [DOI] [PubMed] [Google Scholar]
- Sulieman A, Theodorou K, Vlychou M, Topaltzikis T, Kanavou D, Fezoulidis I, Kappas C 2007 Radiation dose measurement and risk estimation for paediatric patients undergoing micturating cystourethrography. Br J Radiol 80:731–737 [DOI] [PubMed] [Google Scholar]
- Schneider K, Krüger-Stollfuss I, Ernst G, Kohn MM 2001 Paediatric fluoroscopy—a survey of children’s hospitals in Europe. I. Staffing, frequency of fluoroscopic procedures and investigation technique. Pediatr Radiol 31:238–246 [DOI] [PubMed] [Google Scholar]
- Shortt CP, Fanning NF, Malone L, Thornton J, Brennan P, Lee MJ 2007 Thyroid dose during neurointerventional procedures: does lead shielding reduce the dose? Cardiovasc Intervent Radiol 30:922–927 [DOI] [PubMed] [Google Scholar]
- Mettler Jr FA, Bhargavan M, Thomadsen BR, Gilley DB, Lipoti JA, Mahesh M, McCrohan J, Yoshizumi TT 2008 Nuclear medicine exposure in the United States, 2005–2007: preliminary results. Semin Nucl Med 38:384–391 [DOI] [PubMed] [Google Scholar]
- Rosenberg RD, Mettler Jr FA, Moseley Jr RD, Parker T, Williams AG, Christie JH, Kelsey CA 1985 Thyroid radiation absorbed dose from diagnostic procedures in U.S. population. Radiology 156:183–185 [DOI] [PubMed] [Google Scholar]
- Becker DV, Charkes ND, Hurley JR, McDougall IR Price DC, Royal HD, Sarkar SD, Dworkin HJ 1999 Society of Nuclear Medicine procedure guidelines for thyroid scintigraphy. Version 2. http://interactive.snm.org/index.cfm?PageID=772 (accessed October 16, 2009) [Google Scholar]
- Hahn K, Schnell-Inderst P, Grosche B, Holm LE 2001 Thyroid cancer after diagnostic administration of iodine-131 in childhood. Radiat Res 156:61–70 [DOI] [PubMed] [Google Scholar]
- Dickman PW, Holm LE, Lundell G, Boice Jr JD, Hall P 2003 Thyroid cancer risk after thyroid examination with I-131: a population-based cohort study in Sweden. Int J Cancer 106:580–587 [DOI] [PubMed] [Google Scholar]
- Schneider AB, Sarne DH 2005 Long-term risks for thyroid cancer and other neoplasms after exposure to radiation. Nat Clin Pract Endocrinol Metab 1:82–91 [DOI] [PubMed] [Google Scholar]
- Bollen AM, Cunha-Cruz J, Hujoel PP 2007 Secular trends in preadult orthodontic care in the United States: 1942–2002. Am J Orthod Dentofacial Orthop 132:579–585 [DOI] [PubMed] [Google Scholar]
- Hujoel P, Hollender L, Bollen AM, Young JD, McGee M, Grosso A 2008 Head-and-neck organ doses from an episode of orthodontic care. Am J Orthod Dentofacial Orthop 133:210–217 [DOI] [PubMed] [Google Scholar]
- Svenson B, Sjöholm B, Jonsson B 2004 Reduction of absorbed doses to the thyroid gland in orthodontic treatment planning by reducing the area of irradiation. Swed Dent J 28:137–147 [PubMed] [Google Scholar]
- Ohman A, Kull L, Andersson J, Flygare L 2008 Radiation doses in examination of lower third molars with computed tomography and conventional radiography. Dentomaxillofac Radiol 37:445–452 [DOI] [PubMed] [Google Scholar]
- Ekestubbe A, Thilander-Klang A, Lith A, Gröndahl HG 2004 Effective and organ doses from scanography and zonography: a comparison with periapical radiography. Dentomaxillofac Radiol 33:87–92 [DOI] [PubMed] [Google Scholar]
- 2001 An update on radiographic practices: information and recommendations. ADA Council on Scientific Affairs. J Am Dent Assoc 132:234–238 [DOI] [PubMed] [Google Scholar]
- Geist JR, Katz JO 2002 Radiation dose-reduction techniques in North American dental schools. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 93:496–505 [DOI] [PubMed] [Google Scholar]
- Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ 2005 Cancer statistics, 2005. CA Cancer J Clin 55:10–30 [DOI] [PubMed] [Google Scholar]
- Soberman N, Leonidas JC, Cherrick I, Schiff R, Karayalcin G 1991 Sonographic abnormalities of the thyroid gland in longterm survivors of Hodgkin disease. Pediatr Radiol 21:250–253 [DOI] [PubMed] [Google Scholar]
- Tucker MA, Jones PH, Boice Jr JD, Robison LL, Stone BJ, Stovall M, Jenkin RD, Lubin JH, Baum ES, Siegel SE 1991 Therapeutic radiation at a young age is linked to secondary thyroid cancer. The Late Effects Study Group. Cancer Res 51:2885–2888 [PubMed] [Google Scholar]
- Green DM, Hyland A, Barcos MP, Reynolds JA, Lee RJ, Hall BC, Zevon MA 2000 Second malignant neoplasms after treatment for Hodgkin’s disease in childhood or adolescence. J Clin Oncol 18:1492–1499 [DOI] [PubMed] [Google Scholar]
- Inskip PD 2001 Thyroid cancer after radiotherapy for childhood cancer. Med Pediatr Oncol 36:568–573 [DOI] [PubMed] [Google Scholar]
- Somerville HM, Steinbeck KS, Stevens G, Delbridge LW, Lam AH, Stevens MM 2002 Thyroid neoplasia following irradiation in adolescent and young adult survivors of childhood cancer. Med J Australia 176:584–587 [DOI] [PubMed] [Google Scholar]
- Bhatia S, Yasui Y, Robison LL, Birch JM, Bogue MK, Diller L, DeLaat C, Fossati-Bellani F, Morgan E, Oberlin O, Reaman G, Ruymann FB, Tersak J, Meadows AT 2003 High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin’s disease: report from the Late Effects Study Group. J Clin Oncol 21:4386–4394 [DOI] [PubMed] [Google Scholar]
- Mohanti BK, Bansal M 2005 Late sequelae of radiotherapy in adults. Support Care Cancer 13:775–780 [DOI] [PubMed] [Google Scholar]
- Abedin S, Gupta V, Choudhury PS, Kapoor G, Dewan AK 2007 Thyroid carcinoma as second malignant neoplasm after Hodgkin’s lymphoma. Indian J Med Paed Oncol 28:39–41 [Google Scholar]
- Black P, Straaten A, Gutjahr P 1998 Secondary thyroid carcinoma after treatment for childhood cancer. Med Pediat Oncol 31:91–95 [DOI] [PubMed] [Google Scholar]
- Stewart RR, David CL, Eftekhari F, Ried HL, Fuller LM, Fornage BD 1989 Thyroid gland: US in patients with Hodgkin disease treated with radiation therapy in childhood. Radiology 172:159–163 [DOI] [PubMed] [Google Scholar]
- Shore RE 1992 Issues and epidemiological evidence regarding radiation-induced thyroid cancer. Radiat Res 131:98–111 [PubMed] [Google Scholar]
- Acun H, Kemikler G, Karadeniz A 2007 Dosimetric analysis of thyroid doses from total cranial irradiation. Radiat Prot Dosimetry 123:498–504 [DOI] [PubMed] [Google Scholar]
- Evans DG, Birch JM, Ramsden RT, Sharif S, Baser ME 2006 Malignant transformation and new primary tumours after therapeutic radiation for benign disease: substantial risks in certain tumour prone syndromes. J Med Genet 43:289–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarne DH, Schneider A 2008 Radiation-induced thyroid tumors. In: Hay ID, Wass JAH, eds. Clinical endocrine oncology. 2nd ed. Malden, MA: Blackwell Publishing; 166–171 [Google Scholar]
- Ding SH, Pledge SD, Harrison BJ, Peck RJ, Bull MJ, Holland P, Weetman A, Hancock BW 2002 The incidence of thyroid abnormalities in adults irradiated for lymphoma. Int J Oncol 20:1065–1069 [DOI] [PubMed] [Google Scholar]
- Jha AK, Prasiko R, Mod H, Chaurasia PP, Srivastava R 2008 Radiotherapy for benign diseases. JNMA J Nepal Med Assoc 47:151–155 [PubMed] [Google Scholar]
- Ogawa R, Yoshitatsu S, Yoshida K, Miyashita T 2009 Is radiation therapy for keloids acceptable? The risk of radiation-induced carcinogenesis. Plast Reconstr Surg 124:1196–1201 [DOI] [PubMed] [Google Scholar]
- Holm LE, Hall P, Wiklund K, Lundell G, Berg G, Bjelkengren G, Cederquist E, Ericsson UB, Hallquist A, Larsson LG 1991 Cancer risk after iodine-131 therapy for hyperthyroidism. J Natl Cancer Inst 83:1072–1077 [DOI] [PubMed] [Google Scholar]
- Hall P, Berg G, Bjelkengren G, Boice Jr JD, Ericsson UB, Hallquist A, Lidberg M, Lundell G, Tennvall J, Wiklund K 1992 Cancer mortality after iodine-131 therapy for hyperthyroidism. Int J Cancer 50:886–890 [DOI] [PubMed] [Google Scholar]
- Franklyn JA, Maisonneuve P, Sheppard MC, Betteridge J, Boyle P 1998 Mortality after the treatment of hyperthyroidism with radioactive iodine. N Engl J Med 338:712–718 [DOI] [PubMed] [Google Scholar]
- Ron E, Doody MM, Becker DV, Brill AB, Curtis RE, Goldman MB, Harris 3rd BS, Hoffman DA, McConahey WM, Maxon HR, Preston-Martin S, Warshauer ME, Wong FL, Boice Jr JD 1998 Cancer mortality following treatment for adult hyperthyroidism. JAMA 280:347–355 [DOI] [PubMed] [Google Scholar]
- Franklyn JA, Maisonneuve P, Sheppard M, Betteridge J, Boyle P 1999 Cancer incidence and mortality after radioiodine treatment for hyperthyroidism: a population-based cohort study. Lancet 353:2111–2115 [DOI] [PubMed] [Google Scholar]
- Metso S, Auvinen A, Huhtala H, Salmi J, Oksala H, Jaatinen P 2007 Increased cancer incidence after radioiodine treatment for hyperthyroidism. Cancer 109:1972–1979 [DOI] [PubMed] [Google Scholar]
- Metso S, Jaatinen P, Huhtala H, Auvinen A, Oksala H, Salmi J 2007 Increased cardiovascular and cancer mortality after radioiodine treatment for hyperthyroidism. J Clin Endocrinol Metab 92:2190–2196 [DOI] [PubMed] [Google Scholar]
- Stewart A, Webb J, Hewwitt D 1958 A survey of childhood malignancies. Br Med J 1:1495–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston DL, Cullings H, Suyama A, Funamoto S, Nishi N, Soda M, Mabuchi K, Kodama K, Kasagi F, Shore RE 2008 Solid cancer incidence in atomic bomb survivors exposed in utero or as young children. J Natl Cancer Inst 100:428–436 [DOI] [PubMed] [Google Scholar]
- Hatch M, Brenner A, Bogdanova T, Derevyanko A, Kuptsova N, Likhtarev I, Bouville A, Tereshchenko V, Kovgan L, Shpak V, Ostroumova E, Greenebaum E, Zablotska L, Ron E, Tronko M 2009 A screening study of thyroid cancer and other thyroid diseases among individuals exposed in utero to iodine-131 from Chernobyl fallout. J Clin Endocrinol Metab 94:899–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabin MG, Watson EE, Marcus CS, Salk RD 1991 Radiation dosimetry for the adult female and fetus from iodine-131 administration in hyperthyroidism. J Nucl Med 32:808–813 [PubMed] [Google Scholar]
- Berg GE, Nyström EH, Jacobsson L, Lindberg S, Lindstedt RG, Mattsson S, Niklasson CA, Norén AH, Westphal OG 1998 Radioiodine treatment of hyperthyroidism in a pregnant woman. J Nucl Med 39:357–361 [PubMed] [Google Scholar]
- Lloyd RD, Tripp DA, Kerber RA 1996 Limits of fetal thyroid risk from radioiodine exposure. Health Phys 70:559–562 [DOI] [PubMed] [Google Scholar]
- Hurwitz LM, Yoshizumi T, Reiman RE, Goodman PC, Paulson EK, Frush DP, Toncheva G, Nguyen G, Barnes L 2006 Radiation dose to the fetus from body MDCT during early gestation. AJR Am J Roentgenol 186:871–876 [DOI] [PubMed] [Google Scholar]
- 2001 Evaluation of the linear-nonthreshold dose-response model for ionizing radiation. Report no. 136. Bethesda, MD: National Council on Radiation Protection and Measurement [Google Scholar]
- UNSCEAR (United Nations Scientific Committee on the Effects of Radiation) 2008 UNSCEAR 2006 Report: effects of ionizing radiation. Vol 1. New York: United Nations [Google Scholar]
- Pierce DA, Preston DL 2000 Radiation-related cancer risks at low doses among atomic bomb survivors. Radiat Res 154:178–186 [DOI] [PubMed] [Google Scholar]
- Preston DL, Shimizu Y, Pierce DA, Suyama A, Mabuchi K 2003 Studies of mortality of atomic bomb survivors. Report 13: Solid cancer and noncancer disease mortality: 1950–1997. Radiat Res 160:381–407 [DOI] [PubMed] [Google Scholar]
- Cardis E, Vrijheid M, Blettner M, Gilbert E, Hakama M, Hill C, Howe G, Kaldor J, Muirhead CR, Schubauer-Berigan M, Yoshimura T, Bermann F, Cowper G, Fix J, Hacker C, Heinmiller B, Marshall M, Thierry-Chef I, Utterback D, Ahn YO, Amoros E, Ashmore P, Auvinen A, Bae JM, Solano JB, Biau A, Combalot E, Deboodt P, Diez Sacristan A, Eklof M, Engels H, Engholm G, Gulis G, Habib R, Holan K, Hyvonen H, Kerekes A, Kurtinaitis J, Malker H, Martuzzi M, Mastauskas A, Monnet A, Moser M, Pearce MS, Richardson DB, Rodriguez-Artalejo F, Rogel A, Tardy H, Telle-Lamberton M, Turai I, Usel M, Veress K 2005 Risk of cancer after low doses of ionising radiation: retrospective cohort study in 15 countries. BMJ 331:77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardis E, Vrijheid M, Blettner M, Gilbert E, Hakama M, Hill C, Howe G, Kaldor J, Muirhead CR, Schubauer-Berigan M, Yoshimura T, Bermann F, Cowper G, Fix J, Hacker C, Heinmiller B, Marshall M, Thierry-Chef I, Utterback D, Ahn YO, Amoros E, Ashmore P, Auvinen A, Bae JM, Bernar J, Biau A, Combalot E, Deboodt P, Diez Sacristan A, Eklöf M, Engels H, Engholm G, Gulis G, Habib RR, Holan K, Hyvonen H, Kerekes A, Kurtinaitis J, Malker H, Martuzzi M, Mastauskas A, Monnet A, Moser M, Pearce MS, Richardson DB, Rodriguez-Artalejo F, Rogel A, Tardy H, Telle-Lamberton M, Turai I, Usel M, Veress K 2007 The 15-Country Collaborative Study of Cancer Risk among Radiation Workers in the Nuclear Industry: estimates of radiation-related cancer risks. Radiat Res 167:396–416 [DOI] [PubMed] [Google Scholar]
- Sadetzki S, Chetrit A, Lubina A, Stovall M, Novikov I 2006 Risk of thyroid cancer after childhood exposure to ionizing radiation for tinea capitis. J Clin Endocrinol Metab 91:4798–4804 [DOI] [PubMed] [Google Scholar]
- Schneider AB, Recant W, Pinsky SM, Ryo UY, Bekerman C, Shore-Freedman E 1986 Radiation-induced thyroid carcinoma: clinical course and results of therapy in 296 patients. Ann Intern Med 105:405–412 [DOI] [PubMed] [Google Scholar]
- Sarne D, Schneider AB 1996 External radiation and thyroid neoplasia. Endocrinol Metab Clin North Am 25:181–195 [DOI] [PubMed] [Google Scholar]
- Naing S, Collins BJ, Schneider AB 2009 Clinical behavior of radiation-induced thyroid cancer: factors related to recurrence. Thyroid 19:479–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaberg RM, Eski S, Freeman JL 2009 Influence of previous radiation exposure on pathologic features and clinical outcome in patients with thyroid cancer. Arch Otolaryngol Head Neck Surg 135:355–359 [DOI] [PubMed] [Google Scholar]
- Pacini F, Vorontsova T, Demidchik EP, Molinaro E, Agate L, Romei C, Shavrova E, Cherstvoy ED, Ivashkevitch Y, Kuchinskaya E, Schlumberger M, Ronga G, Filesi M, Pinchera A 1997 Post-Chernobyl thyroid carcinoma in Belarus children and adolescents: comparison with naturally occurring thyroid carcinoma in Italy and France. J Clin Endocrinol Metab 82:3563–3569 [DOI] [PubMed] [Google Scholar]
- Crom DB, Kaste SC, Tubergen DG, Greenwald CA, Sharp GB, Hudson MM 1997 Ultrasonography for thyroid screening after head and neck irradiation in childhood cancer survivors. Med Pediatr Oncol 28:15–21 [DOI] [PubMed] [Google Scholar]
- Eden K, Mahon S, Helfand M 2001 Screening high-risk populations for thyroid cancer. Med Pediatr Oncol 36:583–591 [DOI] [PubMed] [Google Scholar]
- DeGroot LJ 1989 Diagnostic approach and management of patients exposed to irradiation to the thyroid. J Clin Endocrinol Metab 69:925–928 [DOI] [PubMed] [Google Scholar]
- Mihailescu DV, Collins BJ, Wilbur A, Malkin J, Schneider AB 2005 Ultrasound-detected thyroid nodules in radiation-exposed patients: changes over time. Thyroid 15:127–133 [DOI] [PubMed] [Google Scholar]
- Landier W 2008 Long-term follow-up guidelines for survivors of childhood, adolescent and young adult cancers. Version 3.0. Arcadia, CA: Children’s Oncology Group [Google Scholar]
- Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Sherman SI, Tuttle RM 2006 Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 16:109–142 [DOI] [PubMed] [Google Scholar]
- Hatipoglu BA, Gierlowski T, Shore-Freedman E, Recant W, Schneider AB 2000 Fine-needle aspiration of thyroid nodules in radiation-exposed patients. Thyroid 10:63–69 [DOI] [PubMed] [Google Scholar]
- Bozhok Y, Greenebaum E, Bogdanova TI, McConnell RJ, Zelinskaya A, Brenner AV, Zurnadzhy LY, Zablotska L, Tronko MD, Hatch M 2009 NA cohort study of thyroid cancer and other thyroid diseases after the Chernobyl accident: cytohistopathologic correlation and accuracy of fine-needle aspiration biopsy in nodules detected during the first screening in Ukraine (1998–2000). Cancer Cytopathol 117:73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi S, Perrier ND, Ituarte PH, Treseler PA, Siperstein AE, Duh QY, Greenspan FS, Clark OH 2003 Accuracy of fine-needle aspiration cytology in patients with radiation-induced thyroid neoplasms. Brit J Surg 90:755–758 [DOI] [PubMed] [Google Scholar]
- 2007 Radiological protection in medicine. International Commission on Radiological Protection Publication 105. Ann ICRP 37:1–63 [DOI] [PubMed] [Google Scholar]
- Feigel Jr DW 2002 FDA public health notification: reducing radiation risk from computed tomography for pediatric and small adult patients. Pediatr Radiol 32:314–316 [DOI] [PubMed] [Google Scholar]
- Slovis TL 2002 Introduction to seminar in radiation dose reduction. Pediatr Radiol 32:707–708; discussion 751–754 [DOI] [PubMed] [Google Scholar]
- Catalano C, Francone M, Ascarelli A, Mangia M, Iacucci I, Passariello R 2007 Optimizing radiation dose and image quality. Eur Radiol 17(Suppl 6):F26–F32 [DOI] [PubMed] [Google Scholar]
- McCollough CH, Primak AN, Braun N, Kofler J, Yu L, Christner J 2009 Strategies for reducing radiation dose in CT. Radiol Clin North Am 47:27–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody DD, Moxley DM, Krugh KT, O'Daniel JC, Wagner LK, Eftekhari F 2004 Strategies for formulating appropriate MDCT techniques when imaging the chest, abdomen, and pelvis in pediatric patients. AJR Am J Roentgenol 182:849–859 [DOI] [PubMed] [Google Scholar]
- McLean D, Malitz N, Lewis S 2003 Survey of effective dose levels from typical paediatric CT protocols. Australas Radiol 47:135–142 [DOI] [PubMed] [Google Scholar]
- Greess H, Lutze J, Nömayr A, Wolf H, Hothorn T, Kalender WA, Bautz W 2004 Dose reduction in subsecond multislice spiral CT examination of children by online tube current modulation. Eur Radiol 14:995–999 [DOI] [PubMed] [Google Scholar]
- Kase KR, Svensson GK, Wolbarst AB, Marks MA 1983 Measurements of dose from secondary radiation outside a treatment field. Int J Radiat Oncol Biol Phys 9:1177–1183 [DOI] [PubMed] [Google Scholar]
- Goske MJ, Applegate KE, Boylan J, Butler PF, Callahan MJ, Coley BD, Farley S, Frush DP, Hernanz-Schulman M, Jaramillo D, Johnson ND, Kaste SC, Morrison G, Strauss KJ, Tuggle N 2008 The Image Gently campaign: working together to change practice. AJR Am J Roentgenol 190:273–274 [DOI] [PubMed] [Google Scholar]
- Lee CI, Haims AH, Monico EP, Brink JA, Forman HP 2004 Diagnostic CT scans: assessment of patient, physician, and radiologist awareness of radiation dose and possible risks. Radiology 231:393–398 [DOI] [PubMed] [Google Scholar]
- Oikarinen H, Meriläinen S, Pääkkö E, Karttunen A, Nieminen MT, Tervonen O 2009 Unjustified CT examinations in young patients. Eur Radiol 19:1161–1165 [DOI] [PubMed] [Google Scholar]
- Finestone A, Schlesinger T, Amir H, Richter E, Milgrom C 2003 Do physicians correctly estimate radiation risks from medical imaging? Arch Environ Health 58:59–61 [DOI] [PubMed] [Google Scholar]
- Shiralkar S, Rennie A, Snow M, Galland RB, Lewis MH, Gower-Thomas K 2003 Doctors’ knowledge of radiation exposure: questionnaire study. BMJ 327:371–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice HE, Frush DP, Harker MJ, Farmer D, Waldhausen JH 2007 Peer assessment of pediatric surgeons for potential risks of radiation exposure from computed tomography scans. J Pediatr Surg 42:1157–1164 [DOI] [PubMed] [Google Scholar]
- 2000 The ionising radiation (medical exposure) regulations. Statutory Instrument 2000, no. 1059. London: Her Majesty’s Stationery Office [Google Scholar]
- 1997 Health protection of individuals against the dangers of ionizing radiation in relation to medical exposure. EU Directive 1997/43/Euratom. Official journal no. L180:0022–0027. Brussels, Belgium [Google Scholar]
- Amis Jr ES, Butler PF, Applegate KE, Birnbaum SB, Brateman LF, Hevezi JM, Mettler FA, Morin RL, Pentecost MJ, Smith GG, Strauss KJ, Zeman RK 2007 American College of Radiology white paper on radiation dose in medicine. J Am Coll Radiol 4:272–284 [DOI] [PubMed] [Google Scholar]
- Hall EJ, Metting N, Puskin J, Ron E 2009 Low dose radiation epidemiology: what can it tell us? Radiat Res 172:134–138 [DOI] [PubMed] [Google Scholar]
- 1996 Instruction concerning risks from occupational radiation exposure. Regulatory Guide 8.29. Washington, DC: U.S. Nuclear Regulatory Commission [Google Scholar]
- Mizuno T, Iwamoto KS, Kyoizumi S, Nagamura H, Shinohara T, Koyama K, Seyama T, Hamatani K 2000 Preferential induction of RET/PTC1 rearrangement by x-ray irradiation. Oncogene 19:438–443 [DOI] [PubMed] [Google Scholar]
- Volpato CB, Martínez-Alfaro M, Corvi R, Gabus C, Sauvaigo S, Ferrari P, Bonora E, De Grandi A, Romeo G 2008 Enhanced sensitivity of the RET proto-oncogene to ionizing radiation in vitro. Cancer Res 68:8986–8992 [DOI] [PubMed] [Google Scholar]
- Nikiforova MN, Stringer JR, Blough R, Medvedovic M, Fagin JA, Nikiforov YE 2000 Proximity of chromosomal loci that participate in radiation-induced rearrangements in human cells. Science 290:138–141 [DOI] [PubMed] [Google Scholar]
- Hamatani K, Eguchi H, Ito R, Mukai M, Takahashi K, Taga M, Imai K, Cologne J, Soda M, Arihiro K, Fujihara M, Abe K, Hayashi T, Nakashima M, Sekine I, Yasui W, Hayashi Y, Nakachi K 2008 RET/PTC rearrangements preferentially occurred in papillary thyroid cancer among atomic bomb survivors exposed to high radiation dose. Cancer Res 68:7176–7182 [DOI] [PubMed] [Google Scholar]
- Tuttle RM, Lukes Y, Onstad L, Lushnikov E, Abrosimov A, Troshin V, Tsyb A, Davis S, Kopecky KJ, Francis G 2008 ret/PTC activation is not associated with individual radiation dose estimates in a pilot study of neoplastic thyroid nodules arising in Russian children and adults exposed to Chernobyl fallout. Thyroid 18:839–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongarzone I, Butti MG, Fugazzola L, Pacini F, Pinchera A, Vorontsova TV, Demidchik EP, Pierotti MA 1997 Comparison of the breakpoint regions of ELE1 and RET genes involved in the generation of RET/PTC3 oncogene in sporadic and in radiation-associated papillary thyroid carcinomas. Genomics 42:252–259 [DOI] [PubMed] [Google Scholar]
- Nikiforov YE, Koshoffer A, Nikiforova M, Stringer J, Fagin JA 1999 Chromosomal breakpoint positions suggest a direct role for radiation in inducing illegitimate recombination between the ELE1 and RET genes in radiation-induced thyroid carcinomas. Oncogene 18:6330–6334 [DOI] [PubMed] [Google Scholar]
- Klugbauer S, Pfeiffer P, Gassenhuber H, Beimfohr C, Rabes HM 2001 RET rearrangements in radiation-induced papillary thyroid carcinomas: high prevalence of topoisomerase I sites at breakpoints and microhomology-mediated end joining in ELE1 and RET chimeric genes. Genomics 73:149–160 [DOI] [PubMed] [Google Scholar]
- Detours V, Wattel S, Venet D, Hutsebaut N, Bogdanova T, Tronko MD, Dumont JE, Franc B, Thomas G, Maenhaut C 2005 Absence of a specific radiation signature in post-Chernobyl thyroid cancers. Br J Cancer 92:1545–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detours V, Delys L, Libert F, Weiss Solís D, Bogdanova T, Dumont JE, Franc B, Thomas G, Maenhaut C 2007 Genome-wide gene expression profiling suggests distinct radiation susceptibilities in sporadic and post-Chernobyl papillary thyroid cancers. Br J Cancer 97:818–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detours V, Versteyhe S, Dumont JE, Maenhaut C 2008 Gene expression profiles of post-Chernobyl thyroid cancers. Curr Opin Endocrinol Diabetes Obes 15:440–445 [DOI] [PubMed] [Google Scholar]
- Port M, Boltze C, Wang Y, Röper B, Meineke V, Abend M 2007 A radiation-induced gene signature distinguishes post-Chernobyl from sporadic papillary thyroid cancers. Radiat Res 168:639–649 [DOI] [PubMed] [Google Scholar]
- Williams ED, Abrosimov A, Bogdanova T, Demidchik EP, Ito M, LiVolsi V, Lushnikov E, Rosai J, Tronko MD, Tsyb AF, Vowler SL, Thomas GA 2008 Morphologic characteristics of Chernobyl-related childhood papillary thyroid carcinomas are independent of radiation exposure but vary with iodine intake. Thyroid 18:847–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan JM 2008 Genetic susceptibility to radiogenic cancer in humans. Health Phys 95:677–686 [DOI] [PubMed] [Google Scholar]
- Mihailescu D, Shore-Freedman E, Mukani S, Lubin J, Ron E, Schneider AB 2002 Multiple neoplasms in an irradiated cohort: pattern of occurrence and relationship to thyroid cancer outcome. J Clin Endocrinol Metab 87:3236–3241 [DOI] [PubMed] [Google Scholar]
- Adjadj E, Schlumberger M, de Vathaire F 2009 Germ-line DNA polymorphisms and susceptibility to differentiated thyroid cancer. Lancet Oncol 10:181–190 [DOI] [PubMed] [Google Scholar]
- Ho T, Li G, Lu J, Zhao C, Wei Q, Sturgis EM 2009 Association of XRCC1 polymorphisms and risk of differentiated thyroid carcinoma: a case-control analysis. Thyroid 19:129–135 [DOI] [PubMed] [Google Scholar]
- Sigurdson AJ, Land CE, Bhatti P, Pineda M, Brenner A, Carr Z, Gusev BI, Zhumadilov Z, Simon SL, Bouville A, Rutter JL, Ron E, Struewing JP 2009 Thyroid nodules, polymorphic variants in DNA repair and RET-related genes, and interaction with ionizing radiation exposure from nuclear tests in Kazakhstan. Radiat Res 171:77–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider AB, Ron E 2003 Radiation and thyroid cancer: lessons from a half century of study. In: Braverman LE, ed. Contemporary endocrinology: diseases of the thyroid. 2nd ed. Totowa, NJ: Humana Press, Inc.; 239–262 [Google Scholar]
- 1997 Uncertainties in fatal cancer risk estimates used in radiation protection. Report no. 126. Bethesda, MD: National Council on Radiation Protection and Measurement [Google Scholar]