Abstract
Carboxypeptidase E (CPE) or carboxypeptidase H was first discovered in 1982 as an enkephalin-convertase that cleaved a C-terminal basic residue from enkephalin precursors to generate enkephalin. Since then, CPE has been shown to be a multifunctional protein that subserves many essential nonenzymatic roles in the endocrine and nervous systems. Here, we review the phylogeny, structure, and function of CPE in hormone and neuropeptide sorting and vesicle transport for secretion, alternative splicing of the CPE transcript, and single nucleotide polymorphisms in humans. With this and the analysis of mutant and knockout mice, the data collectively support important roles for CPE in the modulation of metabolic and glucose homeostasis, bone remodeling, obesity, fertility, neuroprotection, stress, sexual behavior, mood and emotional responses, learning, and memory. Recently, a splice variant form of CPE has been found to be an inducer of tumor growth and metastasis and a prognostic biomarker for metastasis in endocrine and nonendocrine tumors.
-
Introduction to Functions of CPE
Discovery of CPE as a prohormone-processing enzyme
Role of CPE in prohormone sorting, vesicle transport, and secretion
Biomedical implications of CPE in physiological function and disease
-
The CPE Gene, Biosynthesis, Protein Structure, and Distribution
Phylogenic analysis of CPE
Structure, biosynthesis, and intracellular trafficking of CPE
Distribution of CPE in embryonic and adult tissues
Biochemical and enzymatic properties of CPE
-
CPE in Prohormone Sorting and Vesicle Transport
CPE function as a prohormone sorting receptor for the regulated pathway
CPE mediates post-Golgi hormone vesicle transport
CPE mediates synaptic vesicle localization to nerve terminal preactive zone
-
CPE Action in the Endocrine System—Insights from Mouse Models
Diabetes in CPE-deficient mice
Obesity in CPE-deficient mice
Bone metabolism in CPE-deficient mice
Infertility and poor sexual performance in CPE-deficient mice
-
CPE in Neural Function and Behavior
Aberrant neurotransmission and dendritic architecture in CPE KO mice
Role of CPE in stress and neuroprotection
Regulation of mood and emotional responses in CPE mice
Deficits in learning and memory in CPE KO mice
-
CPE and Cancer in Humans
Splice isoform of CPE (CPE-ΔN) promotes tumor growth and metastasis
CPE/CPE-ΔN as a diagnostic and prognostic biomarker for (neuro)endocrine and nonendocrine cancers
-
Human CPE Genetic Mutations and Disease
Single nucleotide polymorphisms in human CPE associated with disease
CPE-associated human disease
Conclusions
Future Directions
I. Introduction to Functions of CPE
Although discovered in 1982, carboxypeptidase E (CPE) has remained a molecule of keen interest to many investigators. In addition to its carboxypeptidase activity, numerous studies over the last 1.5 decades have indicated that CPE is a multifunctional protein that plays many nonenzymatic roles in the endocrine and nervous systems. This review presents a comprehensive look at CPE from structure to function and disease, with a focus on new roles that this unique protein plays in many physiological systems.
A. Discovery of CPE as a prohormone-processing enzyme
Processing of propeptides often begins with the endoproteolytic cleavage at paired or sometimes at single basic amino acid residues. Since the discovery of the prohormone convertases (PC enzymes), which cleave prohormones between or on the carboxyl side of pairs of basic residues (1), it became clear that another enzyme was needed to remove the basic residues from the intermediates to produce the mature bioactive hormone. CPE, which was first identified as enkephalin convertase (2, 3), was subsequently found to be the enzyme responsible for cleaving the C-terminally extended basic residues from peptide intermediates in endocrine cells and neuropeptides in peptidergic neurons. CPE differed from other carboxypeptidases in that its optimal pH was in the acidic range, consistent with its localization to acidic compartments of the trans Golgi network (TGN) and to dense core secretory granules of endocrine cells and peptidergic vesicles of neurons where processing occurs. CPE is localized primarily to endocrine tissues and to specific areas of the central nervous system. The importance of CPE as a processing enzyme was further realized when a mutation in the Cpe gene was found in the Cpefat/fat mouse that presented with severe obesity, diabetes, and infertility. Studies on the structure, biosynthesis, forms, tissue distribution, and enzymatic properties of CPE are discussed in Sections II.A to II.D.
B. Role of CPE in prohormone sorting, vesicle transport, and secretion
Prohormones/propeptides are synthesized in the rough endoplasmic reticulum (RER) and then inserted into the RER cisternae. From there the precursors are transported in vesicles to the Golgi apparatus where they are sorted at the TGN into budding granules along with their processing enzymes such as the PCs and CPE, as well as other cargo proteins such as the granins [for review, see Bartolomucci et al. (4)]. The precursors are then processed within the secretory granules en route to the storage and release sites at the periphery of the cell. Intracellular transport of proteins to various organelles has been found to be mediated by sorting signal motifs and respective receptors. For example, the KDEL signal motif and KDEL receptors mediate the retention of endoplasmic reticulum (ER) resident proteins in the ER (5). The search for sorting motifs for targeting prohormones/propeptides at the TGN into the granules of the regulated pathway has been challenging. The identification of sorting signal motifs on these precursors and evidence supporting a role for a membrane form of CPE as a sorting receptor are discussed in Section III.A.
Prohormone-containing granules that have budded off from the TGN are transported to the plasma membrane via microtubule- and actin-based transport systems. In Section III.B, the role of a transmembrane form of CPE in facilitating the post-Golgi transport of these granules to the cell periphery is discussed. The cytoplasmic tail of the transmembrane form of CPE appears to interact with microtubule motors, actin, and other cytoskeletal proteins to enable granules to be transported via the regulated secretory pathway (RSP) to the plasma membrane for exocytosis.
Recently, the transmembrane form of CPE was also found in synaptic vesicles (SV) in a subset of hypothalamic neurons. The localization of these vesicles to the active zone of the synapse for neurotransmitter release was shown to be dependent on the interaction of the CPE cytoplasmic tail with cytoskeletal proteins. The significance of CPE in facilitating SV localization to the synapse is discussed in Section III.C.
C. Biomedical implications of CPE in physiological function and disease
Much has been learned about the physiological functions and disease states caused by the lack of CPE from Cpefat/fat (6) and knockout (KO) mice (7). In Sections IV and V, insights gained from using these mouse models are discussed. These include revealing the role of CPE in obesity, bone remodeling, diabetes, reproduction, neuroprotection, mood, and emotional responses.
More recently, a splice variant form of CPE was discovered that had activity in promoting the growth and migration of cancer cells (8). Unlike wild-type (WT) full-length CPE, this splice isoform (i.e., CPE-ΔN) lacks the signal peptide at the N terminus that normally directs it into the secretory pathway. It exists in the cytoplasm and in metastatic tumor cells. CPE-ΔN moves into the nucleus and functions to activate metastatic and antiapoptotic genes, thus inducing or promoting tumor metastasis. Use of CPE-ΔN as a biomarker for diagnosing and predicting future metastasis in several types of endocrine and epithelial cancers is discussed in Section VI.
Several single nucleotide polymorphisms (SNP) occurring in the CPE gene have been found in humans. Some result in the loss of enzymatic activity as identified in type II diabetic patients, whereas others lead to unstable molecules that are misfolded and degraded in the ER (see Section VII).
We conclude the review with a summary of the new nonenzymatic roles for CPE, its splice isoform CPE-ΔN, and discuss the mechanisms by which CPE controls functions in health and disease, and the potential use of CPE as a therapeutic drug target (Section IX).
II. The CPE Gene, Biosynthesis, Protein Structure, and Distribution
A. Phylogenic analysis of CPE
CPE falls into the peptidase M14-like superfamily of enzymes (9). The M14 family of metallocarboxypeptidases is a group of zinc-binding carboxypeptidases that hydrolyze single, C-terminal amino acids from polypeptide chains and have a recognition site for the free C-terminal carboxyl group, a key determinant of specificity. Based on substrate specificity, CPE is classified into carboxypeptidase B-like (CPB-like) enzymes because it only cleaves the basic residues lysine or arginine. Various metallocarboxypeptidase proteins containing a Zn-carboxypeptidase domain have been highly conserved from bacteria to mammals (10, 11). To date, 23 genes encoding Zn-carboxypeptidase domain proteins have been identified in the human genome (10). Comparison of the sequence of CPE with carboxypeptidase A (A1, A2, A3, A4, A5 and A6) and carboxypeptidase B (B1 and B2) show a 21% identity at the protein level with 55% and 37% sequence coverage, respectively. Carboxypeptidase O is 22% identical at the protein level with just 32% sequence coverage. Additionally, carboxypeptidase M is 46% identical at the protein level with 82% sequence coverage, and carboxypeptidase N1 is 51% identical at the protein level with 86% sequence coverage. Interestingly, carboxypeptidae D has multiple Zn-carboxypeptidase domains and is 50% identical at the protein level with 86% sequence coverage. There is very little conservation outside the Zn-carboxypeptidase domain between CPE and CPA/B proteins, suggesting that these proteins diverged very early during evolution.
The orthologous protein sequences of the Cpe gene have been identified in many species, covering a phylogenetic distance from invertebrate Protostomia (Ecdysozoa, nematodes) to vertebrate Deuterostomia. The phylogenetic relationships, as estimated from amino acid sequence similarities, are shown in a cladogram tree (Fig. 1). According to this tree, the CPE sequences of Caenorhabditis elegans (nematode) were found at separate branches from those of the other species. CPE has been biochemically characterized only in a few species so far. It was first cloned from bovine adrenal chromaffin granules (12) and has been well studied in mouse (Mus musculus) (6) and in rat (Rattus norvegicus) (13–15). Human CPE was first characterized by Manser et al. (16). CPE was also studied in chicken (Gallus gallus), thymus (17), and in nematode (C. elegans) (18). Subsequently, by blast search and data mining, CPE was also found to exist in genome sequences of the chimpanzee (Pan troglodytes) (19), macaque (Macaca fascicularis) (20), boar (Sus scrofa) (21), clawed frog (Xenopus tropicalis) (22), sea-slug (Aplysia californica) (23), and zebrafish (Danio rerio) (24) (Table 1). The remarkable degree of similarity at the protein level of CPE across the different phyla suggests its functional importance early in evolution.
Figure 1.
Phylogenetic analysis of CPE protein. The phylogenetic tree was built using 476 representative amino acids using Phylogeny.fr platform (www.phylogeny.fr/version2_cgi/phylogeny.cgi) and determined by the program Gblocks (328), which eliminates poorly aligned positions and divergent regions (removes alignment noise) after sequence alignment using multiple sequence comparison by log-expectation (MUSCLE) (329). Bootstrap values above 50% (0.5) are shown. Conserved position for at least half the number of sequences is represented by +1.
Table 1.
Different species with a conserved CPE gene
| Species | Nucleotide sequence accession no. | Nucleotide identities (%) | Protein |
Chromosome | |
|---|---|---|---|---|---|
| Identities (%) | Similarities (%) | ||||
| Homo sapien (man) | NM_001873 | 100 | 100 | 100 | 4q32.3 |
| Pan troglodytes (common chimpanzee) | NM_001098559 | 99 | 99 | 99 | 4 |
| Macaca fascicularis (crab-eating macaque) | AB169871 | 98 | 99 | 99 | 4 |
| Bos taurus (cow) | NM_173903.3 | 88 | 93 | 96 | 17 |
| Sus scrofa (boar) | NM_001097439.1 | 87 | 92 | 95 | 8 |
| Mus musculus (mouse) | NM_013494.3 | 88 | 97 | 98 | 8, 32.6 cM |
| Rattus norvegicus (rat) | NM_013128.1 | 88 | 96 | 98 | 16p13 |
| Gallus gallus (chicken) | CR388992.1 | 85 | 94 | 97 | 4 |
| Danio rerio (zebrafish) | NM_214810.1 | 53 | 83 | 94 | 1 |
| Xenopus laevis (African clawed frog) | NM_001127813.1 | 78 | 83 | 92 | |
| Aplysia californica (sea slug) | NM_001204485 | 11 | 47 | 65 | |
| Caenorhabditis elegans (nematode) | NM_069534.5 | 9 | 44 | 60 | 4 |
All the species except Macaca fascicularis and Gallus gallus have annotated gene entry in the NCBI database. The percentage identity and similarities were compared with human mRNA and protein. Chromosome loci for the Cpe gene have not been assigned for Xenopus laevis and Aplysia californica. cM, Centimorgan.
Alternative splicing of CPE transcripts
In mammals, the CPE gene contains nine exons (Fig. 2) (13–16). In humans, two alternatively spliced transcripts of CPE mRNA have been found, one of which encodes a truncated protein lacking a partial N-terminal region (CPE-ΔN) due to alternative splicing of the first exon (Fig. 2) (8). CPE-ΔN is alternatively spliced with noncanonical alternative 3′ and 5′ splice sites, which remove 98 nucleotides of the first exon. This type of noncanonical splice site is more inclined to occur at a 5′ untranslated GC-rich region (25–28) and is common in certain cancers (29, 30). Interestingly, the spliced region also contains G-quadruplex-like elements (31); these elements are thought to modulate gene expression at the translational level by forming stable RNA hairpin secondary structures in the 5′ untranslated region (UTR) of mRNA inhibiting the process of translation (32). Splicing of such elements found in the CPE-ΔN transcript could result in a translational “advantage” during tumor metastasis (see Section VI). The second splice variant CPE mRNA transcript is derived from alternative splicing at the 3′ donor site of exon 6 and the 5′ donor site of exon 7 (Fig. 2, variant 1). This splicing event results in an 18-amino acid deletion within the area of the active site of the CPE protein, rendering this protein enzymatically inactive. This variant has a signal peptide and is therefore translocated into the RER cisternae and the secretory pathway. It is likely secreted into the extracellular space and may function as a ligand or signaling molecule. The evidence for these splice variants was derived from expressed sequence tag (EST) database searches. Interestingly, all these variants were found in human brain tissue, suggesting that the brain contains cells that are active in alternative splicing of CPE (33, 34).
Figure 2.
Schematic representation of the CPE gene and alternatively spliced variants. Rectangular boxes denote the exons (1–9). Dark solid boxes are the UTR, and light and textured boxes are the coding exons. Alternative splice sites to yield hCPE-ΔN and variant 1 transcripts are indicated with arrows on the exons. Stop codons are also indicated with arrows and introns as solid lines.
B. Structure, biosynthesis, and intracellular trafficking of CPE
A schematic of the primary structure of the CPE protein is shown in Fig. 3A. It has a signal peptide directing the protein into the RER cisternae, a catalytic domain, and a highly acidic C-terminal domain. The three-dimensional structure of CPE (Fig. 3B) has been modeled based on the crystal structure of carboxypeptidase D, an enzyme homologous to CPE (35). The model (Fig. 3B) indicates several functional domains of CPE: the enzymatic active site showing the zinc (cofactor) binding site; a prohormone sorting signal binding site (see Section III.A), an amphipathic α-helical transmembrane domain, and the cytoplasmic tail that interacts with microtubule proteins for vesicle transport (see Section III.B).
Figure 3.
Schematic diagram and molecular model of the CPE protein. A, The preproCPE protein is 476 amino acids in length and contains a signal peptide (SP) that directs it into the ER. After cleavage of the SP, the proCPE (57 kDa) is trafficked through the Golgi and sorted into the granules of the RSP via interaction of its C-terminal amphipathic α-helical domain with cholesterol-sphingolipid-rich microdomains in the TGN. The Pro region (Pro) is processed within a post-Golgi compartment to generate the mature full-length membrane-bound CPE (55 kDa). Within the granules, the C terminus of this membrane-associated CPE can be cleaved presumably by a PC at a paired-basic residue cleavage site to generate a soluble form of mature CPE (53 kDa). B, The molecular model of CPE was based on the crystal structure of CPD. The red areas indicate the common overlapping homologous sequences shared between the two proteins, demonstrating a high degree of structural similarity. Unique to CPE are the two basic residues, Arg255 and Lys260, that were demonstrated to interact with the acidic residue-based prohormone sorting-signal found in POMC, proinsulin, and proBDNF. CPE also contains a unique C-terminal sequence that forms an amphipathic α-helix under acidic conditions. This C-terminal region is involved in tight membrane association and for a subset of CPE molecules can traverse the lipid bilayer, resulting in a small cytoplasmic tail that interacts with cytoplasmic proteins such as Arf6 and dynactin. The green ball represents the zinc atom in the active site. Lollipop symbols indicate the asparagine-linked glycosylation sites.
CPE is a 476-amino acid protein synthesized as a precursor with a 25-amino acid signal peptide that directs proCPE into the cisternae of the RER and is then removed. The proCPE is transported from the ER through the Golgi complex to the granules of the RSP where the 17-amino acid “pro” region is removed after a penta-arginine sequence (RRRRR42) (Fig. 3A), to generate the mature protein (CPE43–476) (36). Processing of the pro region is required neither for enzymatic activity because proCPE is enzymatically active as a carboxypeptidase (37) nor for intracellular trafficking of CPE (38). The CPE protein is glycosylated at two N-linked glycosylation consensus sites, Asn139 and Asn390. Under mildly acidic conditions and increasing calcium concentrations similar to that of the TGN, CPE has been shown to aggregate in vitro (39) with granule cargo proteins (40), suggesting that this occurs in vivo as a mechanism of condensation and sorting to the RSP. Binding of potential prohormone cargo to CPE via a prohormone sorting signal, such as that found in proopiomelanocortin (POMC) (41), could also occur in this compartment (42) through interaction of its prohormone binding site, composed of the amino acids Arg255 and Lys260 on CPE (43). In addition, the carboxyl terminus of CPE forms an amphipathic α-helix under acidic conditions and is involved in binding tightly to cellular membranes (44, 45). This binding step is important for its trafficking from the TGN to the granules of the RSP (46, 47). Binding of the carboxyl terminus of CPE with cholesterol-sphingolipid-rich domains (lipid-rafts) in the TGN membrane has been demonstrated (48), allowing it to act as a sorting receptor for prohormones (see Section III.A). A subpopulation of CPE molecules appears to have a transmembrane topology (49). CPE together with bound prohormones are packaged into immature granules budding from the TGN. Indeed, analysis of secretory granule membranes from bovine pituitary indicates that it is highly enriched with cholesterol, consistent with the notion that granules are budded from lipid raft domains of the TGN (50). Within these granules, some of the CPE molecules are further processed (51) at Arg455-Lys456 (Fig. 3A) to yield a soluble form (molecular mass, ∼50 kDa) (51), which is enzymatically more active than the membrane-associated form (molecular mass, ∼53 kDa) (52). Soluble CPE then functions to cleave the C-terminal extended basic residues from the peptide hormone intermediates liberated by PC1 and PC2 in the granules. Subsequent to granule exocytosis, membrane CPE can recycle from the plasma membrane through the early endosomes and back to the TGN where it gets reused (53, 54).
Much debate has surrounded whether the membrane form of CPE does and can assume a transmembrane topology in the granules membrane. For instance, the primary structure of CPE at the C terminus that associates with the membrane does not contain a typical transmembrane domain as predicted from modeling programs (45) and as shown in biochemical experiments (45, 46, 51) that involved carbonate extraction studies (45) and secretion experiments (46). Additionally, the C-terminal membrane binding domain is highly acidic (Fig. 3A) and theoretically does not favor localization in a membrane lipid bilayer. However, studies on the insertion of a CPE C-terminal peptide (last 22 amino acids) into model membranes have indicated that it can shallowly embed in a lipid bilayer by itself at an acidic pH (49). Also, evidence from cell biological experiments strongly support the existence of a cytoplasmic tail in CPE that interacts with various cytoplasmic molecules such as dynactin to mediate granule transport in neuroendocrine cells (see Section III.B). In addition, after granule exocytosis, the C-terminus cytoplasmic tail of CPE has been shown to interact with Arf6, a small cytoplasmic GTPase, to mediate recycling of CPE from the plasma membrane back to the TGN for reuse (53). Furthermore, in another study, it was demonstrated that Arf6-dependent recycling of CPE mediated the endocytosis of the eosinophil cationic protein, a CPE-interacting protein (54). All these studies indicate the existence of a cytoplasmic tail and a transmembrane orientation of some CPE.
There are two possibilities by which CPE could achieve a transmembrane orientation. One is the insertion of the C terminus of CPE, under acidic conditions, through the TGN membrane with the help of a chaperone protein that could shield the acidic charges of the CPE C terminus in the lipid bilayer, allowing it to penetrate the membrane. As an example of this kind of mechanism, the diphtheria toxin α-subunit only partially penetrates artificial lipid bilayers by itself but is able to move across the membrane upon introduction of globule-like proteins as a chaperone (55). In addition, whereas it is known that C-terminal tail-anchored proteins (e.g., cytochrome b5) are transmembrane proteins, the mechanism by which large domains of polypeptide are translocated across the phospholipid bilayer in an unassisted manner is still not fully understood because it is independent of the Sec61 translocon (56, 57). Of particular interest is the finding that CPE specifically interacts with Wolframin (58), an ER resident protein of unknown function, but may be involved in protein folding and intracellular transport. Mutations in the WFS1 gene cause Wolfram syndrome—a syndrome characterized by diabetes insipidus, childhood-onset diabetes mellitus, optic atrophy, and deafness. Additional supporting evidence for a functional partnership between CPE and Wolframin derives from the observation that both CPE and Wolframin are co-up-regulated in the amygdala of male rats subjected to cat odor (fear response) (59). These observations raise the possibility that Wolframin may assist CPE in its folding and/or trafficking as a chaperone through the ER to allow CPE to function efficiently downstream. Another report has noted the interdependence of CPE and phogrin, a receptor tyrosine phosphatase-like protein found in mature secretory granules of AtT20 cells (60). Under the mildly acidic environment of the TGN (61), CPE can interact with the N-terminal luminal domain of phogrin. Additionally, small interfering RNA (siRNA) silencing of CPE or phogrin reduces sorting of phogrin and CPE, respectively, into granules. Hence, the interaction of CPE with phogrin in the TGN may, in part, facilitate and stabilize a nonclassical transmembrane domain of CPE in that compartment.
The second possibility is that CPE does not fully enter the ER cisternae during synthesis because the C terminus is retained by an interacting protein on the outside of the organelle, leaving a cytoplasmic tail exposed (62). Understanding how CPE assumes a transmembrane orientation awaits further studies.
C. Distribution of CPE in embryonic and adult tissues
After the initial identification and characterization of CPE (2, 3), antisera specific for CPE became available. Initial studies were performed by immunohistochemistry (IHC) (63, 64), autoradiography, and binding studies using tritiated guanidinoethylmercaptosuccinic acid (GEMSA), a potent inhibitor of CPE (65–69). The IHC studies in rats showed a general localization of CPE in neuropeptide-rich areas of the brain and endocrine tissues such as the median eminence, supraoptic nucleus, paraventricular nucleus, and suprachiasmatic nucleus of the hypothalamus; the neural, intermediate, and selected cells in the anterior lobe of the pituitary; and the bovine adrenal medulla. Staining was also evident in the pyramidal neurons of the hippocampus, dentate gyrus, and amygdala. Using radiolabeled GEMSA, staining was reported in the rat epithelial cells of the stomach, colon, oviduct, and the acinar cells of the submandibular gland as well as the pancreatic islets of Langerhans (70) and the adrenal medulla (67). Staining in the rat heart atrial tissue (71, 72) also suggests a role for CPE in the physiology of atrial natriuretic factor in this organ. Other IHC studies have reported the expression of CPE in somatostatin-producing cells in rat brain (15); the gastrin cells and progenitor gastrin-somatostatin cells of the antropyloric mucosa of the gut in rats (73); and in different areas of the lung as part of an opioid network involved in respiratory function in humans (74, 75).
Subsequent to cloning of bovine and rat Cpe cDNA (12, 76), oligonucleotide probes were used for Northern blot and in situ hybridization. The expression patterns of CPE have been studied extensively in the rat brain, during embryonic development (77), and in the adult (78–80). In the adult brain, Cpe mRNA is highly expressed in pyramidal neurons of the hippocampus, amygdala, supraoptic nucleus, paraventricular nucleus, and ependymal cells of the lateral ventricle. Other areas of the brain include the piriform and entorhinal cortex, cerebellar cortex, thalamus, medial geniculate, and lateral septal nuclei. High expression is also found in the anterior and intermediate lobes of the pituitary, the adrenal medulla (77, 79), and pancreatic islets. With the characterization of fat cells as an endocrine tissue (81), CPE has also been found in sc and visceral fat (82), although its role in this tissue is unclear at this time.
During development, Cpe mRNA is first seen at embryonic day (E) 10, specifically in the diencephalon and spinal cord in rats (77). Because PC1 and PC2 are not expressed at this time nor is there a defined endocrine system, the role that CPE plays in these tissues at this developmental stage is unknown. It is possible that other PC-like enzymes not identified here are present; or furin, a ubiquitously expressed PC found in the TGN involved in processing constitutively secreted proproteins, could function upstream of CPE to provide proprotein intermediates as substrates for CPE. However, the furin expression pattern at this stage does not overlap with that of CPE. By E12, CPE expression is seen more extensively throughout the embryo, specifically in the nervous system in areas such as the neuroepithelium, peripheral ganglia, mesenchymal cells around the midgut mesentery, and the epithelia of the branchial arch. At E13, whereas the expression of PC1 and PC2 transcripts is restricted to the developing nervous system, high levels of CPE expression are seen throughout the embryo that overlap with PC1 and PC2 expression in addition to that for furin. There have been many other characterizations of CPE in specific cells/tissues under different experimental paradigms that can be found in Table 2.
Table 2.
Literature summary of the identification or expression of CPE in various tissue and cell systems
| Tissue/cell | Experimental context | Experimental procedure | Ref. |
|---|---|---|---|
| Mouse cDNA library | Screen for caspase substrates | Cleavage of expressed proteins | 337 |
| Corpus luteum | Changes during luteal phase | Gene array, qRT-PCR | 338 |
| Dorsal root ganglia | Effect of monensin | Activity | 339 |
| Eye ciliary body | Ciliary epithelium as a neuroepithelium | Expression and subtractive libraries | 203 |
| H4-II-E-C3 hepatoma | Angiotensin II processing | RT-PCR | 340 |
| Rat CNS | Compare CPE and CPD | ISH, IHC | 341 |
| Breast cancer cells | Vasopressin processing | qRT-PCR, Western blot | 342 |
| Pituitary neurointermediate lobe | Regulation of expression | Northern blot | 343 |
| Testicular and epididymal transcriptomes | Analysis of epididymal segments | cDNA microarray | 344 |
| Bovine DNA | SNP analysis for meat quality | RFPL sequencing | 345 |
| Gastric enterochromaffin-like cells | Components of vesicle release | IHC | 251 |
| Antropyloric mucosa | Gastrin localization | IHC | 73 |
| Nervous system/mouse model of MS | Development of disease state | Oligonucleotide microarrays | 325 |
| RPE/choroid | Age-related changes in genes | cDNA microarray, RT-PCR | 204 |
| Intestine 407, human fetal epithelial cells | Overexpression of NeuroD | cRNA microarray | 346 |
| C. elegans neuromuscular junction | Impaired acetylcholine release | Paralysis assay | 18 |
| Pancreatic β-cells | Palmitate-induced apoptosis | Proteomics, Western blot | 238 |
| Brain | Global ischemia | Western blot | 231 |
| Lungs | CPE fat/fat mouse | Response to ozone | 347 |
| Airway responsiveness in mice | Effect of ozone inhalation | Compare CPE fat/fat mice to WT | 348 |
| Olfactory bulb, amygdala | Rat exposure to cat odor | Differential gene expression | 59 |
| Synaptic vesicles and PC12 cells | Characterization | EM, TIRF, subcellular organelles | 142 |
| Lung epithelium | Effect of smoke inhalation | cDNA microarray | 349 |
| Seminal plasma | Identification of proteins | LC-MS/MS | 350 |
| Immunocytes | Inflammation/pain | IHC | 351 |
| Rats | Alcohol effects on behavior | CPE activity | 352 |
| Multiple cancer tissues | Review | Database mining | 247 |
| Cultured lens tissue | Calcium influx by ionophore | 2D-PAGE/MS | 353 |
| Small-cell carcinoma | Peptide processing | qRT-PCR, Western blot | 253 |
| N/A | PI3K-mTOR pathway analysis | Yeast-2-hybrid, interactome mapping | 354 |
| Prefrontal cortex of piglets | Social isolation stress | Microarray, qRT-PCR | 235 |
| Cat visual cortex | Young vs. adult cats | Subtractive hybridization | 355 |
| Avian pancreas | Embryonic development | IHC | 356 |
| Placenta, umbilical cord | Compare CPD and CPE | IHC | 357 |
| Ovary | FSH-responsive cells | cDNA microarray | 358 |
| Neural complex | Analysis in chordates | EST sequencing, ISH | 359 |
| Placenta | Stages of gestation | ISH | 360 |
| Retinal tissue/cells | Processing of NPY | Activity, ICC, Western blot | 361 |
| RGC-5 retinal cells | Ischemia | Western blot, activity | 362 |
| Cultured astrocytes and neurons | Secretion and tissue analysis | CPE activity and Northern blots | 363 |
| Basophilic mast cells/Jurkat cells | Secretory vesicles | ICC | 364 |
| Chicken thymus | Colocalization with CgA | RT-PCR, Western blot, IHC | 17 |
| Brain | Ischemia | ISH, ICC, Western blot | 232 |
| Retinal photoreceptors | CPE KO and fat/fat mouse | IHC | 141 |
Table includes published literature where CPE expression was identified and/or characterized in a wide variety of tissues and cell systems. The experimental context and technique of the characterization of CPE is annotated. Some citations made in the text may be duplicated here. CNS, Central nervous system; ISH, in situ hybridization; qRT-PCR, quantitative real time PCR; ICC, immunocytochemistry; LC/MS, liquid chromatography/mass spectroscopy; EM, electron microscopy; 2D-PAGE, two-dimensional PAGE; N/A, not applicable; MS, mass spectroscopy; PI3K-mTOR, phosphatidylinositol 3-kinase-mammalian target of rapamycin.
D. Biochemical and enzymatic properties of CPE
CPE is a Zn++ metallocarboxypeptidase that cleaves the carboxy-terminal arginine (Arg) or lysine (Lys) residues from protein substrates; however, it can also cleave histidine poorly under acidic conditions. CPE prefers Arg over Lys residues with KM values at approximately 50–100 μm and approximately 200 μm, respectively, for Met-enkephalin extended peptides (2). CPE has an optimum activity at pH 5–6 with the Vmax declining sharply at pH below 5.0 due to a single ionizing group in the active site (83). Multiple ionizing groups in the active site at pH above 7.0 also have deleterious effects on activity such that CPE becomes inactive at pH 7.4. Aside from pH requirements, CPE utilizes zinc as the coordinating metal in its active site to mediate peptide hydrolysis (84). Cobalt, another divalent transition-state metal, can stimulate enzymatic activity (2). Although calcium can bind CPE (85) and this binding decreases the thermostability of the enzyme (85), it exerts no effects on the aggregation of the soluble CPE (39). CPE is a metallocarboxypeptidase; therefore, chelating agents, such as 1,10-o-phenanthroline, are effective inhibitors. Several thiol-directed inhibitors are also highly effective, such as p-chloromercuriphenylsulfonate and HgCl2 (86). In addition, mimetic substrates designed as active site-directed inhibitors, e.g., GEMSA, with a Ki of approximately 8 nm (87), are used as potent inhibitors.
The importance of CPE in peptide hormone and neuropeptide processing has become especially evident with the CPE-deficient mice. Specifically, using a peptidomics approach comparing WT and Cpefat/fat mice, new potential substrates of CPE have been identified (88, 89). Indeed, identification of proSAAS, an endogenous inhibitor of PC1/3, was made by this approach (90), as was the discovery of the involvement of CPE in the processing of hemopressins (91)—hemoglobin peptides found in the brain that bind cannabinoid CB1 receptors (92). Other studies include WT and Cpefat/fat mice comparisons of peptides in the prefrontal cortex, pituitary, and brain (93–95). Additional effects of food deprivation and exercise on hypothalamic peptides have been investigated (96). Peptidomic analyses have also been applied to hypothalamus and hypothalamic responses to chronic morphine (97) and cocaine treatments (98) to study the possible role of neuropeptides in drug addiction.
III. CPE in Prohormone Sorting and Vesicle Transport
Endocrine and neuroendocrine cells have two different secretory pathways: constitutive and regulated. The constitutive secretory pathway (CSP) supports continuous protein secretion, independent of stimulation (99). In the CSP, small secretory vesicles formed at the TGN are constantly transported to and fused to the plasma membrane without storage. Because CSP proteins are continuously secreted, this pathway is driven primarily by the biosynthesis of secretory proteins at the ER (100). The CSP provides membrane proteins, such as receptors (101), to the plasma membrane, as well as various secretory proteins (102, 103), for maintenance of cell survival, differentiation, and growth. Although the CSP is present in all cells, the RSP is unique to endocrine and exocrine cells and neurons. Hormones and neuropeptides that are critical for mediating various endocrine functions, neurotransmission, and neuronal plasticity (104–107) are secreted via the RSP.
RSP proteins such as prohormones and proneuropeptides are synthesized at the RER, inserted into the RER cisternae, and then transported to the Golgi complex. At the TGN, the prohormones and their processing enzymes are sorted away from constitutively secreted and lysosomal proteins and are packaged into specialized budding vesicles called immature granules destined for regulated secretion. The newly synthesized hormone-containing immature granules are then transported from the post-Golgi network to storage sites in the proximity of secretion sites at the plasma membrane while undergoing maturation that includes acidification of the granule, processing and condensation of cargo proteins, as well as removal of constitutive proteins via the clathrin-dependent constitutive-like secretory pathway (108, 109) to give rise to the mature granule or large dense-core vesicle (LDCV). Upon stimulation of endocrine cells or neurons, the mature granules dock at the plasma membrane and undergo exocytosis to release their contents (Fig. 4). The mechanisms by which CPE mediates prohormone sorting at the TGN to the RSP, peptide processing, and granule transport to the release sites are reviewed below.
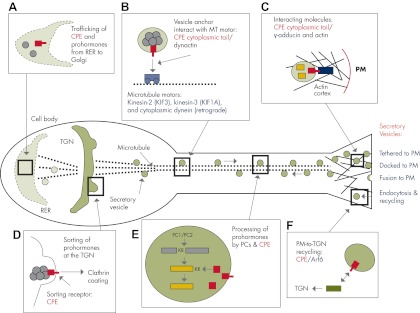
Figure 4.
Trafficking of CPE in the RSP of (neuro)endocrine cells. A, Newly synthesized CPE (red) and prohormones (gray balls) in RER move from the RER to the Golgi complex via a microtubule-based vesicle transport. Thick dotted lines represent microtubules. B, Within the TGN (pH = 6.0–6.5), the amphipathic region of C-terminal CPE forms an α-helix structure that embeds into lipid raft domains in the TGN. In a subpopulation of CPE molecules, this C-terminus domain penetrates through the lipid-raft-rich domains of the TGN membrane to form a cytoplasmic tail. Prohormones aggregate and bind to membrane CPE (sorting receptor) at the TGN and are then sorted into the RSP. C, Budded RSP vesicles containing prohormones bound to CPE recruit dynactin, an anchor for microtubules and microtubule motors, via the CPE cytoplasmic tail. Kinesin-2 and kinesin-3 mediate anterograde vesicle transport toward the secretion sites in the neurite terminals, whereas cytoplasmic dynein mediates retrograde transport toward the cell body. MT, Microtubules. D, During Golgi-to-plasma membrane (PM) transport, proprotein convertases 1 and 2 (PC1 and PC2) cleave prohormones between or on the carboxyl side of paired-basic residues, usually lysine (K) and arginine (R). CPE then cleaves off the extended basic residue(s) from the C terminus to generate mature neuropeptides/hormones. E, At the proximity of the plasma membrane in endocrine cells and at the presynaptic terminal for neurons, respectively, the CPE cytoplasmic tail can interact with γ-adducin, an actin cortex-interacting molecule. The interaction localizes/transports vesicles containing CPE and mature neuropeptides/hormones to the preactive zone beneath the plasma membrane, which is required for the activity-dependent secretion of neuropeptides and hormones. F, After exocytosis of hormones and neuropeptides, the transmembrane CPE is endocytosed and recycled back to the TGN via the interaction of its cytoplasmic tail with Arf6. PM, Plasma membrane.
A. CPE function as a prohormone sorting receptor for the regulated pathway
The search for the mechanisms involved in the sorting of prohormones at the TGN into vesicles of the RSP has been difficult and challenging. Several primary sequence domains, as well as regions representing loop structures stabilized by disulfide bridges, have been proposed as motifs for sorting various prohormones to the RSP (110–112). These have been reviewed elsewhere (110). However, the mechanism by which these domains mediate sorting is unclear. Nevertheless, it has also been proposed that prohormones are passively sorted into the RSP by aggregation that segregates them from other proteins (113–116). It is clear that whereas aggregation is important as a concentration step, it is insufficient to sort prohormones to the RSP [for review, see Dikeakos and Reudelhuber (117)]. Interaction of a specific domain of the prohormone with the TGN membrane seems to be necessary for sorting to occur. Molecular modeling studies have identified a three-dimensional consensus sorting motif that is comprised of two acidic amino acid residues located a specific distance apart from each other (12–15 Å) and two hydrophobic residues (5–7 Å apart) exposed on the surface of the molecule. This motif has been identified for POMC, proinsulin, and proenkephalin that facilitate their sorting into the RSP of endocrine cells (41, 110, 118, 119). The same conformation-dependent sorting motif has been subsequently identified in the structure of brain-derived neurotrophic factor (BDNF) and shown to be necessary for sorting this molecule to the RSP (120).
Molecular modeling studies of CPE have indicated a binding domain on CPE that contains two basic amino acid residues (Arg255 and Lys260; see Fig. 3) with the appropriate molecular distance from each other that allows docking with the two acidic residues in the sorting motif of POMC, proinsulin, proenkephalin, and pro-BDNF (119–121). Binding studies further demonstrated a specific interaction of membrane CPE with N-POMC1–26, a peptide containing the POMC sorting motif, with a KD of 6 μm.
Evidence in support of membrane CPE as a sorting/retention receptor came from using antisense or RNA interference technology to down-regulate CPE expression in model cell lines, as well as by using CPE KO and Cpefat/fat mice that show diminished regulated secretion and mis-sorting of proinsulin (118), POMC (41), and BDNF (120) to the CSP in the absence of CPE. These early studies were considered controversial because another study using pancreatic β-cells from the Cpefat/fat mouse revealed no significant differences in the regulated secretion of insulin vs. the normal mouse, suggesting that there is no defect in sorting insulin to the RSP in the absence of CPE (122). However, it was subsequently found that up to approximately 45% of mutant CPE escaped degradation within 2 h of expression and was found in the secretory vesicles in the pancreatic β-cell line derived from the Cpefat/fat mice (123), compared with the apparent complete degradation in the anterior and intermediate pituitary cells used in the initial studies by others (6, 124). The significant amount of mutant CPE present in the pancreatic β-cells may be sufficient to function as a sorting/retention receptor, thus explaining the differences in results. More recently, Hosaka et al. (125), using anterior pituitary cells from the Cpefat/fat mice, found that there was a significant increase in the amounts of the secretory granule protein, secretogranin III, in these cells that could bind and sort POMC at the TGN in these mice. This effect could in part compensate for the lack of CPE, leading to some, although diminished, regulated secretion of POMC/ACTH compared with WT mice. More importantly, they also demonstrated increased constitutive secretion of POMC/ACTH from the anterior pituitary cells of these mice, supporting a role for CPE in sorting POMC to the RSP (125). Thus, in the analysis of prohormone sorting, it is necessary to consider not only stimulated secretion, but also constitutive secretion, as well as possible effects on synthesis and degradation of the prohormone in the experimental condition, especially after suppression of CPE expression, to fully follow the routing and fate of the prohormone molecules. However, a recent report studying the secretion behavior of newly synthesized ACTH in AtT20 cells concluded that CPE did not play a role in POMC sorting in these cells (126). Unfortunately, the significantly elevated levels of constitutively secreted POMC, observed in this experiment when CPE expression was acutely reduced by siRNA silencing, were not addressed adequately. The amount of newly synthesized POMC was similar between scrambled and CPE siRNA-treated cells; hence, the elevated level of newly synthesized POMC in the medium was not due to elevated expression. This observation suggests that normal trafficking of POMC is grossly perturbed in the absence of CPE. Hence, CPE appears to be involved in POMC trafficking in AtT20 cells despite the production and stimulated secretion of ACTH.
B. CPE mediates post-Golgi hormone vesicle transport
The precursors of hormones and neuropeptides are packaged at the TGN into immature granules, which become mature granules or LDCV as they are transported to the secretion sites for activity-dependent secretion in endocrine cells and neurons (Fig. 4). To reach the secretion sites at the plasma membrane of endocrine cells or at the nerve terminals of neurons, LDCV use microtubule-dependent transport systems mediated by kinesins (127–133). Final movement of LDCV to just beneath the plasma membrane or active zone for release involves an actin-myosin-based mechanism (134–136). Unused LDCV in the transiting pool can be trafficked back to the cell body (137) by the retrograde microtubule motor complex, cytoplasmic dynein (138) for either reuse or degradation by the endosome/lysosome system.
Support for the role of CPE in vesicle transport comes from a number of correlative reports. Enhanced expression of CPE leads to increased trafficking of the dopamine transporter to the presynaptic membrane at the axonal terminal of dopaminergic neurons, thereby enhancing dopamine uptake (139). The neural cell adhesion molecule, contactin-associated protein 2 (Caspr2), directly interacts with CPE in the Golgi complex within the cell body of rat cortical neurons. This interaction increases transport of Caspr2 to apical dendrites (140). In C. elegans, the homolog of CPE (egl-21) has been shown to be involved in the release of acetylcholine at the neuromuscular junction (18), whereas studies with CPE KO and Cpefat/fat mice have suggested that transport of SV from the cell body at the inner segment of the retina to the nerve terminals at the outer plexiform layer is defective (141). More direct evidence comes from live cell-imaging studies, which showed that overexpression of the cytoplasmic tail (∼10 amino acids; see Fig. 3) of CPE directly reduced real-time movements of POMC/ACTH LDCV containing CPE tagged with green fluorescent protein (GFP; CPE-GFP) (133). The final movement of LDCV through the actin cortex just beneath the plasma membrane may also involve interaction of the CPE tail with actin-associated proteins, such as γ-adducin (142). Total internal reflection fluorescence (TIRF) microscopy studies suggest that localization of synaptic-like microvesicles (SLMV) to the plasma membrane (within <200 nm) in PC12 cells also involves the CPE tail (142).
To understand the mechanism of CPE involvement in granule transport, glutathione-S-transferase pull-down, copelleting, and coimmunoprecipitation experiments with AtT20 cell cytosol were carried out (133). These studies showed that the tail bound to a complex containing the anterograde motors, kinesin-2 and kinesin-3, as well as the retrograde motor, cytoplasmic dynein (138), and the dynactin complex (143); kinesin-1 was not involved (Fig. 5). Kinesin-2 consisting of KIF3A, KIF3B, and KAP moves along microtubules at speeds of 0.3–0.5 μm/sec. Kinesin-3, also known as KIF1A, is the fastest motor (∼1 μm/sec) and has also been reported by others to mediate anterograde transport of CPE (Egl-21)-containing peptidergic vesicles to the neuromuscular junction in C. elegans (18). Thus, kinesin-2, kinesin-3, and cytoplasmic dynein are associated with the CPE cytoplasmic tail via dynactin (144). Indeed, endogenous dynactin significantly colocalized with POMC/ACTH vesicles along the processes of AtT20 cells.
Figure 5.
Schematic diagram of the interaction of CPE to microtubule motors. The cytoplasmic tail of transmembrane CPE in secretory peptidergic granules recruits dynactin that associates with and confers processivity to KIF3A (kinesin 2) and KIF1A (kinesin 3). Kinesin 2 that consists of two motor proteins, KIF3A and KIF3B, and a cargo binder, KAP3, is known to bind dynactin directly. Kinesin 3 is a fast-moving (>1 μm/sec) plus-end microtubule-based motor and forms a homodimer. Kinesin 2 and kinesin 3 simultaneously bind dynactin and microtubules to mediate delivery of these vesicles to the release site for activity-dependent secretion of hormones and neuropeptides in (neuro)endocrine cells. Cytoplasmic dynein, a minus end-directed motor complex, also binds dynactin and mediates return of secretory granules from the end of the process back to the cell body under nonstimulated conditions.
Thus, the current studies indicate that the CPE cytoplasmic tails on LDCV bind dynactin that, in turn, recruits a motor complex of kinesin-2, kinesin-3, and cytoplasmic dynein. The recruitment is required for rapid processive movement of POMC/ACTH granules toward and along the processes of anterior pituitary cells for delivery to the release site for secretion (see model in Figs. 4 and 5), as well as for retrograde transport. Similar studies on BDNF vesicle transport in hippocampal neurons (132) also revealed involvement of the CPE cytoplasmic tail in the recruitment of dynactin for anterograde transport of BDNF for activity-dependent release and retrograde transport of these vesicles to the cell body, presumably for degradation to maintain homeostasis of the number of granules/vesicles at the storage depot in the proximity of the secretion sites (137).
C. CPE mediates synaptic vesicle localization to nerve terminal preactive zone
The presynaptic terminals of peptidergic/neuroendocrine neurons in the hypothalamus contain both synaptic and peptidergic vesicles, although SV predominate (142, 145, 146). Likewise, endocrine cells, such as chromaffin cells, have SLMV and LDCV beneath the plasma membrane (147–150). For SV and LDCV, there are different groups of vesicles that have been characterized on the basis of their sensitivity to extracellular stimuli: the reserve pool, the slow-response pool, and the readily releasable pool (151–154). For SV, approximately 80% at the terminal belong to the reserve pool that responds to stimulation very slowly (within minutes). The slow-response pool (approximately 19%) secretes its contents more acutely (within a few seconds). The reserve and slow-response pools are mixed and are held within the presynaptic bouton and at the proximity of the plasma membrane of neuroendocrine and endocrine cells, respectively, by actin-based tethering. The readily releasable pool (approximately 1%) of vesicles is docked to the presynaptic and plasma membrane at the active zone and responds immediately to stimulation. After stimulation, vesicular membrane proteins are endocytosed to form empty SV or SLMV, which are then refilled with neurotransmitters by vesicle-associated transporters, such as vesicular glutamate transporters and vesicular acetylcholine transporters (155, 156). Some vesicles formed by endocytosis are recycled back to late endosomes and lysosomes at the cell body for degradation to prevent overpopulation of vesicles at the nerve terminal and at the plasma membrane of (neuro)endocrine cells (157, 158).
Recently, transmembrane CPE has been found in SV in hypothalamic peptidergic neurons, but not in SV in the rest of the brain (142). Recruitment of transmembrane CPE into SV membranes is most likely achieved by recycling of the presynaptic membranes containing CPE deposited by LDCV after fusion, after stimulation of these neurons. In the hypothalamus of CPE KO mice, electron microscopy revealed a significant (∼3-fold) reduction of docked SV within the preactive zone (between 0 and 100 nm from the presynaptic membrane aligned with the postsynaptic density) in presynaptic boutons, compared with that in WT mice. Notably, the pool of SV that was absent within the 0- to 100-nm zone was found above 300 nm in the CPE KO mice. Consistent with impaired localization of SV at the proximity (<100 nm) of the presynaptic membrane from CPE KO mice, stimulated glutamate release from hypothalamic neurons in these mutants was decreased compared with WT mice (142). These data suggest that the pool of hypothalamic SV containing the cytoplasmic tail of CPE may interact with cytoplasmic proteins to mediate retention of SV within the less than 100-nm preactive zone. Although it is difficult to demonstrate this point in hypothalamic neurons, studies using TIRF microscopy on the neuroendocrine chromaffin cell line, PC12, were carried out to investigate this further (142). PC12 cells express CPE and contain LDCV as well as SV counterparts called “synaptic-like microvesicles” (SLMV). It was found that the average intensity of synaptophysin-red fluorescent protein containing SLMV in the TIRF zone was decreased approximately 2-fold when GFP-CPE cytoplasmic tail (C-terminal 15 amino acids acting as a dominant negative) was overexpressed compared with cells overexpressing GFP alone. Hence, excess CPE cytoplasmic tail peptides interfered with retention of SLMV within the TIRF zone, similar to the observation of the lack of SV within the preactive zone (<100 nm) in the hypothalamus of CPE KO mice, suggesting a common mechanism.
Defective glutamate-mediated neurotransmission has also been reported in the photoreceptors of CPE KO and Cpefat/fat mice. Electroretinograms showed reduced glutamate-mediated b-wave activity and decreased number of SV per synapse in the photoreceptors, likely due to impairment of SV transport and glutamate secretion in these CPE-deficient mice (141). Collectively, these studies indicate a critical role for the CPE cytoplasmic tail both in SV localization and in tethering to the active zone at the nerve terminal in some neurons to mediate neurotransmitter secretion.
IV. CPE Action in the Endocrine System—Insights from Mouse Models
One of the CPE animal models arose from a spontaneous autosomal recessive mutation identified in a colony of mice at Jackson Laboratories (159). Because the mice were observed to be obese, diabetic, and infertile, they were termed fat/fat mice. Gene mapping studies identified the fat mutation to be on chromosome 8, near the locus for Cpe. Subsequently, the mutation was localized to the Cpe gene and was termed Cpefat/fat. The mutation gave rise to a Ser202Pro amino acid change in the mature CPE protein (6) that rendered the protein unstable and subject to degradation (6, 160). When expressed in a baculovirus expression system, the CPE(Ser202Pro) mutant was shown to lack enzymatic activity and to be deficient in trafficking through the RSP, and it failed to be secreted when expressed in AtT20 cells but was degraded in the ER (161). Similar degradation and lack of secretion was reported in immortalized pancreatic β-cells (NIT3) from the Cpefat/fat mouse (162). Despite these findings, another study demonstrated that a portion (∼45%) of the newly synthesized CPE(Ser202Pro) protein escaped degradation in the ER, was colocalized in mature β-granules, and was secreted in a regulated manner from the NIT3 cells of the Cpefat/fat mouse (123). These latter findings demonstrated that whereas the Cpefat/fat mice were defective in CPE enzymatic activity, they were not completely devoid of CPE protein in all tissues. To clarify this point, a KO mouse was generated with deletion of exons 4 and 5 in Cpe (7). The CPE KO mice showed a complete absence of CPE and shared many of the phenotypic characteristics of the Cpefat/fat mice. In Sections IV.A to IV.C, insights into the role of CPE in diabetes, obesity, bone remodeling, and infertility gained from these two CPE-deficient mouse models will be discussed. Studies on these models highlight the effects of CPE mutations in humans that can lead to CPE deficiency (see Section VII).
A. Diabetes in CPE-deficient mice
The CPE KO and Cpefat/fat mice develop diabetes. Because of their different genetic backgrounds (i.e., C57BKS for Cpefat/fat and C57BKS/SV129 for the CPE KO mouse), slight differences in the progression of the disease were observed (6, 7, 163). However, in general, these mutants had higher glucose levels at 8–10 wk of age, which increased significantly soon after to peak at 17–20 wk. High glucose levels were maintained for approximately 2 months, after which levels began to decrease, suggestive of a reversible diabetic phenotype. The Cpefat/fat females failed to develop the severe hyperglycemia of the Cpefat/fat males (163). By comparison, CPE KO females not only developed hyperglycemia but also were more severely glucose intolerant than CPE KO males at similar ages. In addition, older females became insulin-resistant, whereas the males were less affected (7). The reversal of the diabetic phenotype seen for the male and female CPE KO mice and the Cpefat/fat males represents an interesting observation. Concomitant with the development of hyperglycemia, fasting levels of plasma insulin-like immunoreactivity increased in parallel and were composed primarily of proinsulin (6, 7). The plasma levels of proinsulin in both animal models were exceptionally high (up to 100 ng/ml in the CPE KO mice), and it reached a plateau in the KO mice at approximately 30 wk. This is within the age range when the hyperglycemia began to revert toward normal and suggests that the excessive levels of circulating proinsulin may have contributed to this reversal. This is not unexpected because proinsulin has insulin signaling activity, but at approximately 1% of that for mature insulin (164). The maintenance of elevated fasting glucose in the older CPE KO females (∼1 yr old) was likely due to the insulin resistance as demonstrated in the fat cells of these mice (7).
Hyperproinsulinemia is a phenotype of the CPE-deficient animals, and this condition suggests a processing and/or secretory defect of proinsulin from the pancreatic islets. Indeed, IHC specifically for proinsulin in Cpefat/fat islets showed significantly elevated staining in the pancreatic β-cells (163), and studies on isolated pancreatic islets showed stimulated secretion of primarily proinsulin (122). These findings were confirmed by transmission electron microscopy demonstrating the presence of granules without the characteristic electron-dense core seen in mature β-granules. Instead, granules were filled with an electron-lucent material consistent with noncrystallized proinsulin (6, 165).
B. Obesity in CPE-deficient mice
Besides being diabetic, the CPE KO and Cpefat/fat mice are also obese (7, 163). The CPE KO mice are born as runts; by approximately 4 wk of age, they begin to gain weight, so by 8 wk they are heavier than their WT littermates. This weight gain continues into adulthood where at approximately 1 yr, both male and female CPE KO mice are two to three times heavier than their WT or heterozygote littermates. The weight gain is due almost exclusively to increased fat deposition, where approximately 40% and approximately 54% of the weight is contributed by fat in male and female CPE KO mice, respectively. Their obesity phenotype appears to be more severe than those of the Cpefat/fat mice with weights reaching 70–80 g in the former. The onset of obesity in the CPE KO and Cpefat/fat mice appears to be due to increased consumption of food (7, 166), although younger Cpefat/fat have been reported to consume similar amounts of food as the WT controls (163). Additionally, the CPE KO mice have a decreased basal metabolic rate, reduced utilization of lipids for energy, and reduced spontaneous activity (7), all of which contribute to the obesity phenotype.
Eating and satiety are governed by multiple signals that involve the activation of both peripheral and central pathways, including leptin (167), cholecystokinin (CCK) (168), and glucagon-like peptide 1 (169) (Fig. 6). Leptin is a 16-kDa protein secreted from white fat cells in response to insulin (170) and is a key regulator of eating behavior (171). It activates receptors in the arcuate nucleus and ventromedial hypothalamus, which in turn signal hypothalamic POMC and cocaine- and amphetamine-regulated transcript (CART) neurons to generate α-MSH and mature CART. These latter peptides are strong anorexigenic peptides liberated by proteolytic processing of POMC and proCART, respectively (172, 173). At the same time, leptin also represses the signals from neuropeptide Y (NPY) and agouti-related peptide (AGRP) neurons that promote feeding. Although leptin levels in the CPE KO and Cpefat/fat mice are elevated, they are not exceptionally high (7). It should be emphasized that a certain degree of leptin resistance occurs, resulting in the lack of signaling to the hypothalamic neurons involved in food satiety (163).
Figure 6.
Summary of peptides involved in eating behavior. The control of eating behavior is complex and includes peptide signaling from peripheral and central sources. This is a short list of peptides that play a role in controlling this behavior. CART, α-MSH, insulin, glucagon-like peptide 1 (GLP-1), CCK8, and leptin all reduce eating behavior, indicated by the minus sign, whereas others like NPY, AGRP, and ghrelin stimulate eating, indicated by the plus sign. Peptides requiring CPE enzymatic activity are listed on the left, whereas those that do not require it are listed on the right. The down and up arrows indicate levels of the peptides in the CPE KO or CPEfat/fat mice as reduced or increased, respectively, compared to WT control mice. ?, Not determined.
Recently, it has been shown that FOXO1, a transcription factor involved in the regulation of food intake, negatively controls the expression of CPE in hypothalamic POMC neurons (174). Ablation of FOXO1 specifically in hypothalamic POMC neurons results both in an increase in CPE and α-MSH expression, reducing food intake (174). Furthermore, in diet-induced obesity where CPE is normally decreased, ablation of FOXO1 in POMC neurons protected the animals from weight gain due to sustained expression of CPE and levels of α-MSH. Hence, CPE and its ability to generate α-MSH in these hypothalamic neurons plays a pivotal role in the regulation of food intake. It is not surprising therefore that in the hypothalamus of the CPE KO mice, the levels of mature α-MSH are reduced by approximately 94% compared with WT littermates (124). Parenthetically, these findings help to explain the CPE KO hyperphagic behavior where not only is leptin signaling blunted but also POMC processing to α-MSH is depressed. Other peptides involved in feeding behavior that have been analyzed from CPE KO or Cpefat/fat mice include CART, NPY, and CCK (Fig. 6). Although overall levels of CART and NPY immunoreactivity in CPE KO hypothalamus are generally similar to WT controls, in both cases there is a marked lack of the mature bioactive peptides (124), suggesting the presence of precursor and intermediate forms of the peptides in this tissue. Reduced levels of CART peptide in humans with CART mutations (175, 176) and in the CART KO mice (177, 178) lead to an obesity phenotype. Hence, obesity is expected in the CPE KO mice. On the other hand, NPY is a powerful orexigenic peptide that stimulates feeding (179). Absence of mature NPY in the hypothalamus should result in reduced feeding and weight loss, as reported in the NPY KO mice (180). However, because obesity is seen in the CPE KO mice, it would appear that NPY is upstream of CART signaling. An additional contributor to control of feeding may be CCK8, a central peptide involved in food intake. Levels of CCK8 in the brains of Cpefat/fat mice were reduced by 74–90% with a concomitant increase in the precursor, CCK-Gly-Arg-Arg (181, 182).
Although the effect of CPE activity on feeding behavior is a prominent factor in the development of obesity, additional evidence suggests a role also for CPE in adipose tissue. For instance, CPE mRNA is abundant in sc and mesenteric fat (183). Interestingly, expression of CPE is at least 15-fold higher in visceral compared with sc fat along with thrombospondin-1 (82). Parenthetically, thrombospondin-1 is an extracellular matrix glycoprotein involved in forming multiprotein complexes with structural macromolecules that can interact with growth factors, cytokines, and proteases (184, 185). Because adipocytes do not contain a RSP (186) and because major peptide hormones (adiponectin, leptin, or resistin) produced by fat cells do not require processing by PC or CPE, the co-up-regulated expression of CPE with thrombospondin-1 suggests a functional role for CPE in this process that is distinct from peptide processing.
C. Bone metabolism in CPE-deficient mice
The regulation of bone metabolism is a complex balance between bone formation by osteoblasts and bone resorption by osteoclasts that dictate bone density (187, 188) (Fig. 7). In both male and female CPE KO mice, bone mineral density (BMD) is lower than WT littermates (124). In addition, levels of osteocalcin, a marker of osteoblast activity, and carboxy-terminal collagen crosslinks, a marker of osteoclast activity, were elevated, indicating that bone turnover was increased in the CPE KO mice (124). Osteocalcin is a peptide hormone produced by osteoblasts that acts on the pancreas to release insulin in addition to acting on fat cells to produce adiponectin that increases their sensitivity to insulin (189). The relationship of these two peptides to each other reflects the close connection between bone physiology and glucose homeostasis (Fig. 7).
Figure 7.
Schematic diagram showing the interplay of molecules involved in bone homeostasis. Peptides from the hypothalamus (CART, NPY, α-MSH), adipocytes (leptin) and osteoblasts (osteocalcin), and the sympathetic nervous system contribute to the regulation of bone remodeling. The plus sign indicates signaling in favor of osteoclastogenesis, and the minus sign indicates signaling that prevents it. a, Osteocalcin is released from osteoblasts and activates the pancreatic β-cell to release insulin and the adipocyte to release adiponectin (189, 330). b, ACTH plays a role in osteoblast proliferation (331, 332). c, Insulin acts on the adipocyte to release leptin (170). d, Adiponectin affects metabolic processes including insulin sensitivity (333). e, Leptin activates the hypothalamus to express the anorexigenic peptides, α-MSH and CART, and decrease the orexigenic peptides, NPY and AGRP, as well as to stimulate the sympathetic nervous system (334, 335). f, MC4R signaling is involved in bone metabolism, presumably through elevation of CART expression (191). g, Osteoprotegerin (OPG) regulates osteoblast differentiation (336). h, NPY plays a central role in bone remodeling (180). i, The sympathetic nervous system regulates bone remodeling (187). *, Reduced CART levels are associated with poor BMD and are downstream of α-MSH and NPY's role in bone metabolism (124).
The increased bone turnover in the CPE KO mice indicated a dysregulation in the peptides involved in governing this process, such as CART. Analysis of CART levels in the serum (7) and hypothalamus (124) of the CPE KO mice showed a virtual absence of the mature bioactive form. Thus, the inhibitory function of CART in bone resorption is absent in the CPE KO mice, which leads to a lower BMD. It is interesting to note that the lack of NPY and α-MSH, as mentioned above in the CPE KO hypothalamus, may be expected to increase the BMD, as it does in NPY KO mice (180) and NPY receptor Y2 KO mice (190), as well as in melanocortin 4 receptor (MC4R) KO mice (191). However, because BMD does not increase in the CPE KO mice, it appears that the involvement of CART in bone remodeling is downstream of NPY and α-MSH. Indeed, the increased bone mass associated with the MC4R KO mice has been attributed to increased CART expression because removing one allele of the Cart gene from mice heterozygous or homozygous for MC4R inactivation normalized bone parameters without changing energy metabolism (191).
A more direct role for CPE in bone was recently suggested for longitudinal bone growth. Microarray analysis of genes expressed in the perichondral and reserve growth plate zones identified Cpe as a highly expressed gene in both zones, although its role in this system is unknown (192). It is interesting to note that prohormones and/or proneuropeptides, in addition to the proteins involved in their processing, were not noted as highly or differentially expressed in this study, suggesting a dissociation between the enzyme function of CPE as a carboxypeptidase and the possibility of its functioning in an alternate capacity. In situ hybridization of Cpe mRNA during development also demonstrated a specific signal in the cartilage primordium of the ribs, suggesting involvement in rib formation during development (77).
D. Infertility and poor sexual performance in CPE-deficient mice
The obesity and diabetes in Cpefat/fat and CPE KO mice develop after puberty, whereas the infertility has been reported anecdotally (7, 159, 163). Because obesity in mice can often affect reproductive performance (193), the infertility of Cpefat/fat mice could be attributed to their obesity. Systematic investigations revealed that the fertilities of the WT and Cpe+/fat males and females were similar at approximately 90% (7, 194). However, before weight gain, only approximately 5% of the homozygous mutant matings from WT and Cpefat/fat mice became pregnant. By comparison, when Cpefat/fat males were mated with Cpe+/fat females at 45–50 d of age, fertilities were below 45%, and they declined dramatically as obesity developed. Fertilities of the CPE KO mice were even lower. Nevertheless, litter sizes were similar among all genotypes of WT and Cpefat/fat mice.
Successful reproduction requires coordination and feedback among GnRH, the gonadotropins, and sex steroids in the hypothalamic-pituitary-gonadal axis. Of these hormones, only GnRH undergoes proteolytic processing, and it has been proposed that CPE is involved in this process (see Refs. 195 and 196). An examination of GnRH-like immunoreactivity from Cpefat/fat and CPE KO hypothalami revealed that levels were reduced by up to 78% compared with their respective WT controls (7, 194). Combined HPLC and RIA analyses found that the molar percentages of the C-terminally extended GnRH intermediates were increased by 3- to 284-fold in male Cpefat/fat hypothalami compared with their WT or Cpe+/fat controls (194). Moreover, levels of pro-GnRH were increased by approximately 2-fold. Additionally, the molar percentages of GnRH-[Gly11], [hydroxy-Pro8]GnRH, and fully processed GnRH were decreased by 2- to 4-fold, whereas the [Gln1]GnRH intermediates were enhanced in the Cpefat/fat males. Although the effect on pro-GnRH processing was expected because PC2 activity was reduced in Cpefat/fat mice (160), the effects on the other processing steps were unanticipated.
Pituitary and gonadal functions were also assessed in Cpefat/fat males (194). Although concentrations of basal LH and FSH were similar among genotypes across age, levels were reduced in older Cpefat/fat mice. Interestingly, in vitro anterior pituitary responses to synthetic GnRH were enhanced in Cpefat/fat pituitary cultures, whereas LH and FSH responses to Ca2+ ionophore were not distinguished by genotype. Hence, it appears that the GnRH receptor is up-regulated in the Cpefat/fat pituitary, possibly due to decreased or altered release of GnRH.
Serum testosterone contents were similar among genotypes at 50 and 90 d of age but were decreased in Cpefat/fat males at 200 d (194). Nonetheless, these latter concentrations were within the normal range for mice (197). Sperm counts and sperm motility were also depressed in the older mice.
Although the initial reduction in fertility in young Cpefat/fat and CPE KO males was associated with the defect in hypothalamic GnRH processing, the rapid decline after this age appeared to be due to other factors. For instance, vaginal plugs were readily observed when young Cpefat/fat males were bred with heterozygous or WT females; they were rarely seen at older ages (194). These findings suggested that sexual behavior could be abnormal. Because sexual behavior in rodents is highly dependent upon intact olfaction (198), this sense was tested in Cpefat/fat males at 90 d of age when fertility rates were rapidly declining. Olfaction was normal, and penile erections were evident when Cpefat/fat males were paired with females. Thus, olfaction and physiological responses were intact. When an ovariectomized estrogen-progesterone-primed female was paired with either a WT or Cpefat/fat male, both animals interacted with the female. Although Cpefat/fat males spent more time with females than the WT controls, the latencies for full-mounting behavior were prolonged for Cpefat/fat males, and their responses were either incomplete or inappropriate. Importantly, no instances of intromission or ejaculation were observed. Thus, whereas the Cpefat/fat males show a high degree of sociability, their sexual behavior is aberrant. Because the Cpe mutation may affect the processing of many neuropeptides, future research should focus on identifying which peptides may be controlling sexual behavior, as well as what signaling pathways may be perturbed in the Cpefat/fat males.
V. CPE in Neural Function and Behavior
CPE KO mice exhibit a number of behavioral anomalies, including deficient learning and memory (7, 199) and abnormal mood and emotional responses. These behavioral deficiencies are discussed in Sections V.C and V.D. As described above (Sections II.D and III), CPE is important in processing neuropeptides, sorting neuropeptides to the RSP, and transporting peptidergic vesicles to the membrane to facilitate peptidergic neurotransmission. Moreover, localization of SV to the preactive zone for exocytosis of classical neurotransmitters in hypothalamic neurons is also dependent on CPE. In Section V.A, we review an additional role of CPE in modeling the cytoarchitecture of neurons with respect to dendritic growth and pruning and the dendritic spine formation that can impact synaptogenesis and neural function.
Recent studies suggest that CPE plays an important role in neuroprotection during stress as reviewed in Section V.B. Absence of CPE in CPE KO mice leads to degeneration of hippocampal neurons during stress. Conversely, CPE expression in the brain is increased during different types of stress, possibly to protect neurons from degeneration. Indeed, ex vivo experiments in cultured neurons indicate that CPE is a neuroprotective protein (199, 200).
A. Aberrant neurotransmission and dendritic architecture in CPE KO mice
Abnormal neurotransmission has been reported in Cpefat/fat and CPE KO mice specifically in the retina, hippocampus, and hypothalamus (141, 142, 199). The retina is one of the tissues with the highest expression of CPE in the mouse and rat (141, 201, 202). CPE is also highly expressed in the human ocular ciliary body (203) and the mouse retinal pigmented epithelia (RPE)/choroid in the eye (204). Immunohistochemical staining has localized CPE to photoreceptor synaptic terminals. With age, CPE KO and Cpefat/fat mice showed progressively reduced electroretinography response sensitivity, decreased b-wave amplitude, and delayed implicit time with age, whereas maintaining a normal a-wave amplitude and normal morphology. This is indicative of a defect in synaptic transmission from rod and cone photoreceptors to bipolar cells. Electron microscopy of retinas from Cpefat/fat mice has revealed significantly reduced spherule size, but normal synaptic ribbons and SV density, implicating a reduction in total number of vesicles per synapse in the photoreceptors of these mutants. This reduction in SV at the synaptic bouton (spherule) corroborates well with findings in peptidergic neurons in the hypothalamus that the CPE cytoplasmic tails on the SV are required for interaction with molecules to mediate localization of these vesicles to the preactive zone of the synapse for neurotransmitter release (Section III.C). In addition to impairment in glutamate release from CPE KO hypothalamic neurons (Section III.C), it has been reported that a mutation in the Cpe gene in C. elegans results in diminished acetylcholine release at the neuromuscular junctions (18). Together, these findings support an important role for CPE in facilitating localization of SV to the preactive zone and their tethering to the presynaptic membrane (see Section III.C).
The dendritic architecture and formation of specific types of dendritic spines also plays a central role in modulating neurotransmission (205). The pattern of dendritic growth and branching critically determines neuronal function (206). During brain development, neurons generally form elaborate dendritic arborizations that receive signals from axons. This stimulation is followed by modeling of the dendritic tree by pruning in the distal portion of the axon and in dendritic branches by retraction, degeneration, and dendritic shedding. Pruning is an essential part of the maintenance of the neuronal network (207).
Studies on 6-wk-old CPE KO mice revealed that the dendritic arborization in pyramidal layer V of cerebral cortex and hippocampal CA1 neurons was more complex than in WT controls; more dendrites were observed just proximal to the soma and with more branch points (208). Additionally, there were age-dependent changes in arborization in cerebral cortical neurons that differed between the genotypes. Between 6 and 14 wk of age, WT cortex showed a significant decrease in dendritic arborization. Such a decrease is generally attributed to dendritic pruning, resulting in fewer dendritic arbors (209–212). In contrast, in CPE KO cortex, similar amounts of dendritic arborization were seen at 6 and 14 wk. These findings indicate that lack of CPE gives rise to aberrant patterns of dendritic growth, as well as inhibition of dendritic pruning in cerebral cortical and hippocampal neurons (199). Currently, the mechanism of CPE modulation of dendritic patterning is poorly understood.
Various reports have indicated that transcriptional factors such as Hamlet and Spineless in Drosophila (213), and Neurogenin 2 and Dlx homeobox in mammals (214–216) play a role in controlling dendritic arborization. Additionally, extracellular signaling molecules such as neurotrophins and their receptors have been implicated in mediating axonal pruning (207, 217–221). Recently, nitric oxide synthase 1 adaptor protein (NOS1AP), previously linked to schizophrenia in regulating dendrite morphology (222–226), has been shown to play a role in dendritic outgrowth through a CPE-mediated pathway in cultured hippocampal neurons (227). Overexpression and knockdown experiments showed a direct relationship between NOS1AP and dendritic number and branching in developing hippocampal neurons. Subsequently, yeast two-hybrid, coimmunoprecipitation and glutathione-S-transferase pull-down experiments identified CPE as a specific interacting protein with NOS1AP. Furthermore, short hairpin RNA knockdown of CPE in these neurons attenuated the reduction of dendrite branching elicited by the overexpression of NOS1AP, demonstrating that NOS1AP functions through CPE and that these two molecules operating together play a pivotal role in maintaining proper dendritic morphology and function of the nervous system (227).
The spines on the surface of dendrites are necessary for synapse formation. Dendritic spine numbers and structure change with synaptic activity and development (228–230). For example, the size of the head and length of the neck of the spine reflect its function. Spines with a larger head and a longer neck (M-type) will generally reflect more activity and show greater plasticity, as opposed to those that are small and stubby (D-type).
Studies on 14-wk-old CPE KO mice showed that the total number of dendritic spines on the basilar tree of cortical layer V and CA1 pyramidal neurons was not significantly different between CPE KO and WT neurons (199). Additionally, no differences in the percentage of M and N spines within the internal segments and the terminal tips of spines were observed in the CPE KO and WT animals. The number of D-type spines, however, which are characterized as not fully functional, was significantly higher in terminal tips of the cortical and hippocampal neurons of the CPE KO compared with the same neurons in the WT mice. An increase in the percentage of D-type spines in the CPE KO mice would lead to deficits in synaptogenesis, and this is exemplified by the long term potentiation (LTP) impairment in CA1 neurons observed in these mutants (199). Indeed, the aberrant pattern of dendritic arborization and spine morphology likely also contributes to the neuropsychiatric deficits, such as poor memory and learning, and other abnormal behaviors observed in the CPE KO mice (see Sections V.C and V.D).
B. Role of CPE in stress and neuroprotection
Several studies have shown that CPE expression in the brain is modulated during different types of stress. Jin et al. (231) showed that after transient global ischemia, there was a greater and more sustained increase in CPE expression in CA3 hippocampus, which correlated with survival of those neurons. By contrast, neurons in the CA1 region showed a more transient increase in CPE and were more vulnerable to degeneration. Subsequently, Zhou et al. (232) reported that after focal cerebral ischemia, there was an increase in CPE mRNA and an accumulation of proCPE in ischemic cortex compared with controls. Furthermore, in Cpefat/fat mice, a sublethal episode of focal cerebral ischemia led to severe cell death in the ischemic cortex compared with WT mice. These findings suggest that after ischemic stress, neurons in the hippocampus and cortex up-regulate CPE expression, which is correlated with neuronal survival. However, the Cpefat/fat mouse neurons undergo apoptosis after ischemic stress, suggesting that CPE may have a neuroprotective role.
Additional studies on the CPE KO mouse have provided more evidence that the lack of CPE results in hippocampal neuronal degeneration under stressful conditions. Initially at 6 wk of age, the CA3 pyramidal neurons of the CPE KO hippocampus were shown to be completely degenerated (199). Subsequently, the degeneration was correlated with weaning (maternal separation) at 3 wk of age combined with the stress caused by ear-tagging and tail-clipping. Delaying weaning, ear-tagging, and tail-clipping by 1 wk shifted the CA3 degeneration by 1 wk. Maternal separation alone at wk 3 only caused partial degeneration of the CA3 region. Thus, the complete CA3 degeneration in CPE KO mice is likely attributed to stress caused by maternal separation, ear-tagging, and tail-clipping (233).
Another form of stress is that imparted by restraining the animal. In one such study on C57BL/6 mice, acute restraint stress for 1 h resulted in decreased expression of the CPE mRNA in the hippocampus (234). Stress by social isolation of nonweaned piglets for 15 min also resulted in a decrease in CPE mRNA expression in the frontal lobe (235). However, in contrast to these acute restraint and short-term social isolation stresses, mild chronic restraint stress for 1 h/d for 7 d led to an increase in expression of the CPE mRNA and protein in the hippocampus. This regulation of CPE in vivo appears to be mediated by glucocorticoids, because treatment of rat hippocampal neurons in culture with dexamethasone produces an up-regulation of CPE mRNA—a result that is consistent with a bioinformatic search showing a glucocorticoid regulatory element in the promoter of the Cpe gene. Also, for fear response stress, a small but significant up-regulation of CPE mRNA in the amygdala of male rats subjected to cat (predator) odor was observed that correlated with avoidance and anxiety-like behaviors (59).
All these studies indicate that CPE is modulated during different kinds of stress, and those stressors such as ischemia, ear-tagging, tail-clipping, fear, as well as chronic restraint stress result in up-regulation of CPE in the cortex, amygdala, and hippocampus. Hence, in mice lacking CPE, these stressors appear to result in neuronal degeneration in vivo, at least in the context of the hippocampus and cortex of the CPE KO and Cpefat/fat mice, respectively, and suggest that CPE may play a significant role in protecting neurons from a stressor. This is further indicated by ex vivo studies showing that cultured cerebellar neurons from Cpe+/− mice exhibited more cell death when exposed to low K+ to induce apoptosis compared with neurons from control animals (200). Additionally, studies on cultured rat hippocampal neurons have demonstrated that oxidative stress-induced apoptosis by H2O2 treatment was prevented by overexpression of CPE (199). Moreover, overexpression of an enzymatically inactive mutant form of the CPE with a substitution of glutamate residue 300 acid to glutamine (236) was also effective in preventing H2O2-induced apoptosis, indicating that the neuroprotective action does not depend on an active enzyme (200).
The above examples suggest that the presence of CPE in a system is beneficial, and conversely its lack appears detrimental. It is therefore interesting to speculate whether CPE expression levels change with age, and if so, does it associate with disease. One such disease that has been studied is age-related macular degeneration that results in abnormal changes in the RPE and choroid and subsequent loss of RPE cells. In a study screening for changes in gene expression in the complex of RPE, Bruch's membrane, and choroid during aging, it was reported that 20-month-old mice showed a decrease in CPE mRNA compared with 2-month-old mice (204). Interestingly, CPE was only one of two genes down-regulated with age compared with 148 that were up-regulated. Further study of the potential role of CPE in age-related macular degeneration would be expected to shed light on the mechanism and etiology of this disease.
Because neurodegeneration is a hallmark of many age-related neurodegenerative diseases such as Alzheimer's and Parkinson's diseases, it was not surprising to discover a human SNP in the CPE gene that was found in an Alzheimer's patient (see Section VII). This mutation in the CPE gene leads to neuronal degeneration when transfected into cultured hippocampal neurons (237). Given the apparent neuroprotective role of CPE, these observations would suggest that a decrease in CPE expression during aging or a specific mutation of the gene may contribute to neurodegenerative diseases such as macular degeneration and Alzheimer's disease seen in the aging human population.
In line with a role of CPE in cell survival/apoptosis, another observation involving CPE in apoptosis has been described for pancreatic β-cells (238, 239). It is known that elevated fatty acids reduce β-cell function and survival, linking hyperlipidemia to type 2 diabetes mellitus (T2DM) in obese patients. With palmitate treatment, human islets and MIN6 β-cells showed reduced CPE protein levels within 2 h of treatment, which preceded an ER stress response and ultimately cell death. The mechanism by which CPE is involved in this process is not fully understood. However, CPE translocation to the lysosome for degradation has been implicated. To address the issue of the specificity of palmitate action through CPE, short hairpin RNA was used to specifically reduce CPE by approximately 30%. This resulted in an increased ER stress response and increased apoptosis in islets. Conversely, overexpression of CPE partially rescued the β-cells from the palmitate-induced ER stress and apoptosis. Indeed, islets from Cpefat/fat mice with low levels of CPE showed increased apoptosis in vivo and in vitro. Thus, in this system, CPE appears to have a protective function against palmitate-induced apoptosis in β-cells.
C. Regulation of mood and emotional responses in CPE mice
In studies with the Cpefat/fat males, it appears that their reproductive dysfunction is due primarily to abnormalities in sexual behavior (194). Because anxiety and depression are comorbid with abnormal sexual behavior in humans (see Ref. 240), Cpefat/fat mice were evaluated for appearance of these responses at 2 months, just before the onset of obesity at 3 months, and after obesity was evident at 5 months of age (241). At 2 months of age, anxiety-like responses in the zero maze were similar between WT and Cpefat/fat mice, indicating that the mutants were not anxious. However, by 3 and 5 months of age, these behaviors were evident in both male and female Cpefat/fat animals, behaviors that could be alleviated by the anxiolytic drug, diazepam. Because selective serotonin reuptake inhibitors can be used to treat anxiety disorders in humans (242), behaviors were tested with fluoxetine. The anxiety-like responses of the Cpefat/fat mice were normalized also with this drug. The responses to both drugs appear specific for reducing anxiety in the zero maze because diazepam increased locomotor activity in the open field, whereas fluoxetine reduced it for both genotypes. These findings indicate that anxiety-like responses are not apparent in young Cpefat/fat mice but emerge before the onset of obesity. Notably, the anxiety-like behaviors are responsive to both diazepam and fluoxetine.
Because anxiety and depression may be comorbid in humans (243), Cpefat/fat mice were evaluated for depression. Responses in the forced swim and tail suspension tests were analyzed at 2, 3, or 5 months of age. Relative to WT controls, mutants showed increased immobility times in both tests at all ages examined. Anhedonic responses to sucrose were reduced also in Cpefat/fat mice. Hence, the mutants appear to display depressive-like behaviors. Interestingly, the depressive-like behaviors responded to acute treatment with a tricyclic antidepressant (imipramine) and a selective norepinephrine reuptake inhibitor (reboxetine), whereas the selective serotonin reuptake inhibitor (fluoxetine) or the atypical antidepressant (bupropion) was ineffective. However, when fluoxetine or bupropion was given for 14 consecutive days, the depressive-like behaviors were alleviated. Together, these findings indicate that Cpefat/fat mice display depressive-like behaviors, and their responses can be normalized to those of WT controls with acute administration of imipramine or reboxetine or by 14 d of treatment with fluoxetine or bupropion.
The Cpefat/fat model has a number of unique attributes for the study of anxiety and depression. First, the depressive-like behaviors appear to be preexisting in these mutants from an early age, whereas the anxiety-like responses emerge at a later time. This distinction is important because there is considerable controversy as to whether anxiety and depression are coexistent or whether one precedes the other. Second, the differential responses of anxiety- and depressive-like behaviors to fluoxetine are unique. Because acute fluoxetine treatment was successful in rescuing the anxiety-like behaviors whereas leaving the depressive-like responses intact, it appears that the underlying serotonergic mechanisms are different. The Cpefat/fat mice are one of the first animal models to show this differentiation of serotonergic functions, and, as such, they should serve as a useful model to separate the serotonergic mechanisms that underlie anxiety from those that control depression. Finally, alleviation of depression in humans often requires prolonged therapy with antidepressants. The forced swim and tail suspension tests are used as acute screens for antidepressant action. Most animal models of depression respond to acute administration of antidepressants in these tests. Because at least 2 wk of therapy with fluoxetine or bupropion were required to alleviate the depressive-like behaviors in the Cpefat/fat mice, these mutants may serve as an important model for human depression. An examination of this differential time-scale of response may reveal new mechanisms that are required for antidepressant efficacy.
D. Deficits in learning and memory in CPE KO mice
In previous work, it had been reported that after transient global ischemia, CPE expression was up-regulated and sustained in CA3 hippocampus, but transient in the CA1 region (231). These findings suggested that it might be important to examine hippocampal function in the CPE KO mice. The morphological and electrophysiological abnormalities have already been presented (Sections V.A and V.B). The behavioral functions of these mice were evaluated in tests of episodic and spatial memory, which require an intact hippocampus (see Refs. 244–246). In the novel object recognition task, individual WT and CPE KO mice were initially allowed to interact with two identical objects (199). The mice were then returned to their home-cage, and recognition memory was examined 20 min and 24 h later in a two-choice test where the now familiar and a novel object were presented. During both the 20-min and 24-h tests, WT mice showed marked preferences for the novel object. By comparison, CPE KO mice failed to demonstrate preference for either object during these times. Because no genotypic differences were observed in total object interaction time, these findings indicate that episodic short- and long-term recognition memory is impaired in the CPE KO animals. Similar conclusions were obtained in another test of episodic memory where the social transmission of food preference task was used (199).
The CPE KO mice were also evaluated for spatial memory in the Morris water maze (199). WT and CPE KO mice were first tested on the visible-platform version of the task. In this test, mice must learn to swim to a visible platform to escape the water. Because no genotype differences were observed on this task, general sensory and motor functions appear intact in both genotypes. On the hidden-platform version, mice must use and remember external cues in their environment to guide them to the submerged platform. Over the first 3 d of acquisition training, swim latencies and swim distances to locate the platform were prolonged in the CPE KO mice compared with those of the WT controls. Due to these deficiencies and because visible-platform performance was normal, it appears that spatial memory is impaired in the CPE KO mice.
The learning and memory processes of the WT and CPE KO mice were assessed when the animals were approximately 2–3 months old, at an age when the body weights of the mice were just beginning to diverge. As such, the deficiencies in learning and memory of the CPE KO mice cannot be attributed to performance issues that may be influenced later by obesity. It is noteworthy that aside from deficits in hippocampally based learning and memory processes, the CPE KO mice were deficient also in hippocampal LTP (199). Morphological analysis of the hippocampus from the CPE KO mice revealed that the pyramidal neurons in the CA3 region had degenerated by 6 wk of age, providing an explanation for the hippocampal deficits observed in these mutant mice (199).
VI. CPE and Cancer in Humans
WT and the splice variant, CPE-ΔN, mRNA (see Section II.A) are expressed in neuroendocrine tumors such as pheochromocytomas (8). In epithelial-derived tumors, such as hepatocellular carcinoma (HCC) and colorectal carcinoma, only the splice variant is expressed (8). CPE-ΔN has been shown to induce proliferation and invasion in various tumor cell lines and to promote growth and metastasis of tumors in orthotopic models of nude mice. In Section VI.A, the mechanism of action of CPE-ΔN in promoting growth and metastasis will be discussed. Additionally, microarray studies of biopsies from many types of tumors, including those from Ewing sarcoma and cervical and kidney cancers, have suggested that metastatic tumors are correlated with elevated levels of CPE mRNA compared with that in normal tissue or benign tumor [for review, see Murthy et al. (247)], suggesting that CPE could be a biomarker for diagnosing metastatic disease. Although in those studies it is unclear whether the CPE mRNA detected is the WT and/or CPE-ΔN mRNA, recent clinical studies on HCC and pheochromocytoma indicate that CPE-ΔN is a potentially excellent biomarker for diagnosing and predicting future metastatic disease (8). This will be discussed in Section VI.B. The link of CPE to cancer is perhaps not surprising because it is located on chromosome 4q32.2, a locus that shows loss of heterozygosity in human glioblastoma (248).
A. Splice isoform of CPE (CPE-ΔN) promotes tumor growth and metastasis
Expression of the splice isoform of CPE (see Section II.A), CPE-ΔN, was found in different cell lines derived from liver, colon, breast, and head and neck cancers (8). Moreover, the expression of the CPE-ΔN mRNA was significantly elevated in highly metastatic vs. low metastatic isogenic cell lines from the same type of cancer. Furthermore, these epithelial-derived cancers did not express the WT-CPE. When expressed in low metastatic HCC cell lines, CPE-ΔN induced proliferation and invasion, and immunocytochemical studies revealed that CPE-ΔN was localized to the nucleus in highly metastatic HCC cells (8). These findings indicate that CPE-ΔN may act in the nucleus, and subsequent coimmunoprecipitation experiments have confirmed an interaction of CPE-ΔN with histone deacetylase 1 and 2 (8). CPE-ΔN mRNA when transfected into HCC cells increased the expression of the “neural precursor cell expressed developmentally down-regulated” gene (NEDD 9) (8), a metastatic gene (249), in a histone deacetylase 1 and 2 activity-dependent manner (8). Thus, these results indicate that CPE-ΔN drives invasion in tumor cells ex vivo and metastasis in vivo. The latter point was confirmed by two separate experiments. First, when highly metastatic HCC cells were treated with siRNA to down-regulate CPE-ΔN and then injected into the flank of nude mice, little tumor growth or metastasis occurred. Similarly, subsequent transplantation of the siRNA CPE-ΔN-treated tumor into the liver in nude mice also did not exhibit tumor growth or metastasis, compared with HCC cells treated with scrambled siRNA. These studies demonstrate that CPE-ΔN induces tumorigenesis and metastasis through up-regulation of the expression of nedd9 gene.
B. CPE/CPE-ΔN as a diagnostic and prognostic biomarker for (neuro)endocrine and nonendocrine cancers
CPE protein and/or mRNA have been detected in (neuro)endocrine tumors such as insulinomas, pituitary tumors, neoplastic enterochromaffin-like cells, pheochromocytomas (PHEO)/paragangliomas (PGL) (247, 250–252), small-cell lung carcinomas (253, 254), and other cancerous cells infiltrating the lung in lung cancer patients (75). The abundance of CPE expression in these tumors is not surprising because CPE is required for biosynthesis of neuropeptides that serve as autocrine growth factors in these tumors (255). In a drug-resistant form of the small-cell lung carcinoma cell line (NCI H82), expression of both the 55- and approximately 50-kDa forms of CPE have been reported (253). In other endocrine tumors, significantly high levels of CPE expression were detected in some pituitary adenomas compared with normal pituitary. An oligonucleotide array analysis showed that CPE is differentially expressed in four subtypes of adenomas (256). Prolactin-secreting pituitary adenomas showed no difference from normal pituitary in CPE expression, but in ACTH-secreting pituitary adenomas, expression was lower. However, CPE mRNA expression in GH-secreting pituitary adenomas was significantly higher than normal pituitary. In contrast, no CPE immunoreactivity was detected in another study specific to nonfunctioning pituitary macroadenoma (257). In endocrine tumors where protein analysis was carried out, WT-CPE protein was detected. In other studies where no CPE protein data were presented, the CPE mRNA detected in neuroendocrine tumors is likely to be that encoding the WT-CPE protein. However, because these studies predate the discovery of CPE-ΔN, it is not known whether (neuro)endocrine tumors also express CPE-ΔN. By comparison, CPE-ΔN mRNA has been investigated and detected in PHEO/PGL (8).
WT-CPE has been proposed as a prognostic biomarker in neuroendocrine tumors. Quantitative immunostaining of CPE has shown that elevated CPE expression is a statistically significant predictor of good prognosis in pulmonary neuroendocrine tumors such as typical carcinoids, small-cell lung cancers, and large-cell neuroendocrine carcinomas (254). However, it is unclear at this time how elevated levels of WT-CPE indicate good prognosis in these pulmonary carcinomas. The use of WT-CPE as a potential prognostic biomarker awaits further investigation.
PHEO and PGL are rare neuroendocrine tumors derived from chromaffin cells. PHEO are derived from chromaffin cells in the adrenal medulla, and closely related tumors of extraadrenal sympathetic and parasympathetic paraganglia are classified as PGL (258). PHEO and PGL occur most often during young-adult to mid-adult life with a slightly higher prevalence in females than males (259). Practically all PHEO and sympathetic PGL produce catecholamines (norepinephrine, epinephrine, or dopamine), but only about 70–80% of these tumors secrete these transmitters (260). Nevertheless, almost all catecholamine-producing PHEO and PGL metabolize the catecholamines to produce metanephrine, normetanephrine, or methoxytyramine (261–264). Thus, whereas catecholamine excess reflects various clinical presentations of these tumors, metanephrine and methoxytyramine excess are used in the biochemical diagnosis of these tumors (see Refs. 265–270, 365). Because catecholamine excess causes nonspecific symptoms (hypertension, tachycardia, anxiety, nervousness, sweating, etc.), approximately 50% of these patients are not diagnosed sufficiently early and often visit a physician too late, i.e., when a tumor is large or, in about 10% of patients, when metastasis has occurred (259, 271–275). Parenthetically, larger and extraadrenal tumors have a higher incidence of becoming metastatic (276–279).
Most PHEO and PGL are sporadic; however, familial PHEO and PGL are found in more than one third of patients (280–284). At the present time, 10 PHEO or PGL susceptibility genes have been identified. Five of them represent major susceptibility genes (found in ∼90% of PHEO and PGL) and include the von Hippel-Lindau (VHL) gene; the RET protooncogene, which causes multiple endocrine neoplasia type 2 (MEN2); the neurofibromatosis type I (NF1) gene, associated with von Recklinghausen's disease; and the genes encoding succinate dehydrogenase subunits D (SDHD) and B (SDHB), associated with familial nonsyndromic PHEO or PGL (for review, see Ref. 285). Minor susceptibility genes consist of the genes encoding SDHA and SDHC, which cause head and neck PGL or PHEO and PGL, respectively; SDHA2; TMEM127; and the recently discovered MAX gene (284, 286–291).
In contrast to small-cell lung carcinoma, WT-CPE levels in these PHEO/PGL showed no obvious correlation with survival (S. R. K. Murthy, Y. P. Loh, and K. Pacak, unpublished data). However, in a study of 14 PHEO/PGL patients having SDHB/D or MEN2 mutations, high copy numbers of CPE-ΔN mRNA have been correlated with poor prognosis (8). SDHB patients diagnosed with metastatic tumors at the time of surgery showed high CPE-ΔN mRNA copy numbers in their resected tumors that were 5- to 10-fold greater than those in benign tumors. In two SDHB patients that were diagnosed as having a benign tumor at the time of surgery, one had a low CPE-ΔN mRNA copy number and was disease-free 2.4 yr after the surgery, whereas the other with a high copy number developed metastatic disease within 2 yr. Similarly, in a group of MEN2 patients, all but one, who was diagnosed with benign tumors at the time of surgery, had low CPE-ΔN mRNA copy numbers and were disease-free for at least 6 yr after surgery. The MEN2 patient with high CPE-ΔN mRNA copy number developed recurrence within 8 yr. Another MEN2 patient who had a recurrent PHEO at the time of surgery and showed a high CPE-ΔN mRNA copy number developed recurrence within 4 yr. Although the number of patients in this study was small, the findings are very promising because no other predictive biomarker exists for these types of neuroendocrine tumors. Moreover, the use of CPE-ΔN as a biomarker for predicting future metastasis was superior to pathological analysis. Studies with more patients will further validate CPE-ΔN mRNA as a biomarker for diagnosis and predicting future metastatic disease in PHEO/PGL.
CPE-ΔN expression has also been analyzed in patients with HCC and colorectal cancer. In a cohort of 180 patients with HCC, the ratio of CPE-ΔN mRNA or protein in tumor vs. normal surrounding tissue (T/N) predicted future recurrence (intrahepatic metastasis) with a sensitivity of 92% and a specificity of 76%. More importantly, stage 2 HCC patients who were given good prognosis but had a high T/N ratio developed recurrence within 2 yr, indicating again the value of CPE-ΔN as a biomarker for predicting future metastasis independent of pathological staging. High T/N (>2) values of CPE-ΔN were correlated with poor prognosis.
In a study of 68 colorectal cancer patients, 93.5% of those that did not have metastatic disease had tumor CPE-ΔN mRNA T/N ratios of 2 or less, whereas 86.5% of the patients that had lymph node or distant metastasis within 1 yr after surgery had tumor CPE-ΔN mRNA T/N ratios above 2 (8). These findings show that CPE-ΔN mRNA is also a good diagnostic biomarker for metastasis for colorectal cancer. Additionally, human oligodendrogliomas and astrocytomas also showed expression of CPE mRNA higher than in normal brain. Biopsies from patients with cervical cancer also indicated higher levels of CPE mRNA than in normal cervix (247). However, it has not been determined whether the mRNA in these cancers represents WT-CPE or CPE-ΔN mRNA. Future studies will indicate whether CPE or CPE-ΔN might also be a diagnostic and prognostic biomarker for metastasis in these types of tumors.
VII. Human CPE Genetic Mutations and Disease
Several mutations in the CPE gene have been identified and associated with diabetes and Alzheimer's disease (see Section VII.A). In addition, there are diseases such as hyperproinsulinemia and autoimmune diseases linked to CPE that are briefly reviewed in Section VII.B.
A. Single nucleotide polymorphisms in human CPE associated with disease
Transition or transversion of nucleotides in the genomes among individuals is referred to as SNP. There are three types of SNP. Nonsynonymous SNP lead to changes in the encoded amino acid, whereas synonymous SNP are silent and do not change the amino acid coding (292–295). The third type of SNP is present in the noncoding region, where they may influence promoter activity (gene expression) (296–298), mRNA conformation (i.e., stability) (299–303), subcellular localization of mRNA and/or proteins (304–307), and microRNA binding (308, 309).
The human CPE gene is located on chromosome 4 at 4q32.3 (310). Its coding region spans approximately 120 kb and consists of nine exons. The CPE gene is often associated with T2DM. Elevated levels in proinsulin and/or the molar ratio of proinsulin to insulin are one of the features of T2DM, which indicates the contribution of some nonfunctional proinsulin-processing enzymes. Recently, several SNP have been identified in the CPE gene. The first study of SNP in the human CPE gene was by Utsunomiya et al. (311) (Table 3, items 1–3). In this study, they analyzed SNP in the promoter and entire coding region in the CPE gene in 269 Japanese subjects with non-insulin-dependent diabetes mellitus, 28 nondiabetic obese subjects, and 104 nonobese and nondiabetic controls. Their analysis revealed three nucleotide changes: a G-to-T substitution at nucleotide −53 (relative to start of transcription); a G-to-A substitution at nucleotide −144 in the promoter region; and a silent G-to-A substitution in codon 219 in the coding region. However, none of these SNP correlated with non-insulin-dependent diabetes mellitus or obesity.
Table 3.
Various SNP in human CPE gene
| Item no. | Chr. 4 position | mRNA position | dbSNP rs cluster id | Heterozygosity | Type of SNP | A inserts | Protein residue | Amino acid position | Enzymatic function | Disease associated | PMID reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 166416762 | 939 | Missense | G-to-A | Asp-to-Asn | 219 | Active | None | 9662053 | ||
| 2 | 166415972 | 144 | 5′ UTR | G-to-A | NA | NA | Active | None | 9662053 | ||
| 3 | 166415881 | 53 | 5′ UTR | G-to-T | NA | NA | Active | None | 9662053 | ||
| 4 | 166415969 | 132 | 5′ UTR | G-to-C | NA | NA | Active | None | 11462236 | ||
| 5 | 166416905 | 1081 | Missense | C-to-T | His-to-His | 267 | Active | None | 11462236 | ||
| 6 | 166416952 | 1128 | Missense | C-to-T | Arg-to-Trp | 283 | Less active | Increased risk of diabetes | 11462236 | ||
| 7 | 166417373 | 1549 | Missense | C-to-T | Ala-to-Val | 423 | Active | None | 11462236 | ||
| 8 | 166403424 | 980 | rs34516004 | 0.147 | Missense | T-to-C | Trp-to-Arg | 235 | Not active | ? | Unpublished data of the authors |
| 9 | 166403445 | 1001 | rs77809551 | 0.24 | Missense | G-to-T | Val-to-Leu | 242 | ? | ? | |
| 10 | 166405670 | 1164 | rs116683740 | Missense | A-to-G | Asn-to-Ser | 296 | ? | ? | ||
| 11 | 166416807 | 1587 | rs75109518 | Missense | T-to-G | Val-to-Gly | 437 | ? | ? | ||
| 12 | 166416318 | 495, 515, 523 | Insert | A inserts | 9 new amino acids | Amino acids change from 72 to 80 | Not active | Possible Alzheimer's disease | EST evidence, unpublished data of the authors | ||
| 166416338 | |||||||||||
| 166416346 |
SNP database (dbSNP) rs numbers are assigned to items 8 to 11; other SNP in the table are mined from the literature. QQ mutant (item 12) has been mined from EST database base (dbEST). SNP item 8 falls in the zinc peptidase domain of CPE protein and was found to be enzymatically inactive. Chr., Chromosome; NA, not applicable; ?, unknown.
In another study conducted on a cohort of 272 Ashkenazi Jews with T2DM (312), four novel SNP in the transcript region and one at the promoter region of the CPE gene were identified (Table 3, items 4–7). At codon position 283, a C-to-T transition led to a nonsynonymous Arg-to-Trp (R283W) change at the protein level. The Arg283 residue is within the Zn-carboxypeptidase domain of the CPE protein and is conserved among CPE orthologs and most metallocarboxypeptidases that are enzymatically active, suggesting that this residue may contribute to the catalytic process. Indeed, the R283W CPE protein had a narrower pH optimum and was much less active at pH 6.0 to 6.5, indicating that the R283W CPE variant would be substantially less active than WT-CPE in vivo. In addition, the R283W CPE variant was less stable at higher temperatures than the WT enzyme. Of the T2DM cohort of Ashkenazi pedigrees, four specific families were found to segregate with the R283W mutation. Risk of early-onset T2DM was higher in patients within these four families who inherited one copy of this variant. Furthermore, the homozygous mutant is predicted to have a higher risk for the development of hyperproinsulinemia and diabetes.
A CPE SNP, rs34516004 (Table 3, item 8), has recently been analyzed (S. R. K. Murthy, unpublished data). This SNP consists of a T-to-C substitution that leads to a nonsynonymous Trp (W)-to-Arg (R) (W235R) change, and when expressed and assayed, this mutant was shown to be enzymatically inactive (N. X. Cawley, S. R. K. Murthy, and Y. P. Loh, unpublished data). This W235R mutant was also found in the AGI_ASP population, which is composed of African-Americans and Caucasians, with a heterozygosity value of 0.147, and as with the R283W mutation, people carrying this W235R mutation in the homozygous state could have higher risk of hyperproinsulinemia and diabetes.
An undocumented mutant was found in three EST from the Helix Research Institute, Japan, in Alzheimer's cortex, kidney tumor, and thalamus tissues (Table 3, item 12). This mutation contains three nucleotide insertions (A and/or C) that result in the substitution of amino acids after the prodomain of the CPE protein. The new amino acids in all three cases contain a common core sequence with two adjacent glutamine (Q) residues, hence termed QQ mutants. The mutation results in nine new amino acids replacing eight original amino acids at the N terminus in the mutant protein compared with the WT-CPE. The new amino acids have a combined isoelectric point that is two units lower than the WT sequence it replaces. The physicochemical properties of this mutant protein are predicted to be compromised due to the critical location of the mutation, i.e., at the start of the first β-pleated sheet, which would be important in the structural integrity of the mutant protein. A complete analysis of this mutant is currently in progress (N. X. Cawley, Y. Cheng, T. Yanik, C. Liu, S. Young, S. R. K. Murthy, A. Papazian and Y. P. Loh, manuscript in preparation).
Studies from several groups have screened Chinese patients with symptoms of coronary atherosclerosis (313–315). In these studies, a number of point mutations were found in the CPE gene, specifically exons 4 and 5, that when present were genetically linked to an increased severity of the disease.
B. CPE-associated human disease
Familial hyperproinsulinemia (FH) is a human disease that results from specific mutations in the proinsulin gene that cause substantial increases in the levels of circulating proinsulin (316). One site where the mutations occur results in Arg65 of the proinsulin being changed to His, Pro, or Leu. Because Arg65 is part of the C peptide-A chain junction processing site, changes at this site result in poorer processing of the proinsulin to insulin. Another mutation occurs at His10Asp on the B-chain of insulin. This His10Asp proinsulin mutant protein has been shown to be secreted through the CSP at a much higher rate compared with WT proinsulin (317, 318). Hence, trafficking of the mutant proinsulin is impaired. Recall that CPE can bind to prohormone sorting signals that aid trafficking to the granules of the RSP. One such signal was described in (pro)insulin. Thus, proinsulin can bind to CPE (42). Mutation of this sorting signal in proinsulin resulted in impaired sorting of the mutant insulin to the RSP. Furthermore, some of the human mutants found in FH that were expressed in cells bound poorly to CPE in binding assays (121). These and other results suggest that in addition to the lack of processing the Arg65 mutants and the inability of the His10Asp mutant to hexamerize to form a higher order sorting signal, reduced binding of these mutants to CPE may play a role in their increased constitutive secretion and thereby contributes to FH.
Several other diseased states have been found to have elevated CPE levels. Insulinomas are primary tumors of insulin-producing cells of the islets, and they contain the complement of expected processing enzymes for this cell type (250). Insulinomas can lead to excessive insulin secretion and organic hypoglycemia. Analysis of insulinomas has shown that expression of CPE and the Gsα signaling subunit are often elevated (319). Thus, enhanced regulated secretion of insulin in the presence of elevated CPE may contribute to the hyperinsulinemia. Some support for this idea comes from cell culture experiments where overexpression of CPE in proinsulin-secreting cells has been shown to exert a marginal, but significant, increase in regulated insulin secretion (320).
The presence of CPE autoantibodies in the circulation has been studied extensively in cohorts of Chinese patients that exhibit latent autoimmune diabetes in adults. Here, there appears to be increased diagnostic value of analyzing CPE antibodies in this condition compared with the analysis of antibodies to glutamic acid decarboxylase (321) or SOX13 (322, 323). In an effort to identify latent autoimmune diabetes in adults within a phenotypic T2DM cohort, the prevalence of CPE antibodies was found to be 4.8%, suggesting that analysis of CPE antibodies may allow discrimination of a more latent subset of the adult-onset autoimmune diabetes compared with patients with antibody-negative T2DM (324).
Experimental autoimmune encephalomyelitis (EAE) is a mouse model for multiple sclerosis because it is a polygenic chronic inflammatory demyelinating disease of the nervous system. Oligonucleotide microarray analysis of inflamed spinal cord from EAE mice found that expression of Cpe was decreased by 1.5- to 4.1-fold as the severity of the disease progressed (325). Another study identifying trait loci for causes of EAE mapped one such loci to Cpe on chromosome 8 (326). Although presently it is unclear how CPE may be linked to this phenotype, the recent findings that Caspr2 and CPE can interact (140) suggests that CPE may be involved indirectly in myelination because contactin is implicated in neurite outgrowth, axon fasciculation, and myelin formation.
Finally, a deletion of both cathepsin B and L in mice results in early-onset neurodegeneration, a phenotype reminiscent of neuronal ceroid lipofuscinoses in humans (327). Interestingly, of the 19 proteins significantly increased, four were increased over 10-fold: Rab14, the Delta/Notch-like epidermal growth factor-related receptor, calcyon, and CPE. IHC showed swollen axons in the early developmental stages of the double KO mice, leading the investigators to suggest a role of cathepsin B and L in the recycling process during axon growth and synapse formation. Presently, it is unclear how CPE could compensate in this process.
VIII. Conclusions
In this review, we have emphasized new functions for an “old” protein, CPE. This protein has been well recognized as a peptide-processing enzyme for at least three decades. However, over the past 10 yr, evidence has been accumulating that CPE may serve additional roles as an interacting molecule or as mRNA that is highly expressed or suppressed in a variety of paradigms and diseases. In the past, much of the CPE data from screens were ignored due to a lack of understanding of why or how a prohormone-processing enzyme like CPE could be involved in cancer metastasis or interact with molecules found in the nucleus or cytoplasm when it is localized within secretory vesicles. Nevertheless, it has always been intriguing that CPE should be present at 10-fold greater amounts than the other prohormone-processing enzymes such as PC1 and PC2 in endocrine tissues and is far in excess of what is needed for the enzymatic reactions. Additionally, CPE is expressed in embryos before the development of the peptide hormone endocrine system, hinting at a trophic rather than an enzymatic role for CPE—perhaps in cell proliferation and/or differentiation. The present review has attempted to highlight the many studies on CPE and to emphasize some of its newer roles. These new roles include a number of features. 1) CPE appears to function at the cell-biological level as a sorting receptor for directing prohormones into the RSP and in the transport of peptidergic vescicles to their release sites. These attributes are mediated by different domains of the protein (cytoplasmic tail) that are not involved in its enzymatic activity. 2) CPE also seems to exert antiapoptotic/protective effects against stress in endocrine cells and neurons, as well as contributing to synaptic transmission and dendritic pruning. 3) New insights have been gained using the CPE KO and Cpefat/fat mice. These functional roles include bone remodeling and effects on behavior that involve mood, learning, and memory. 4) The identification of the splice isoform, CPE-ΔN, has opened up new avenues of investigation, especially with regard to its possible role in tumorigenesis and metastatic disease. Importantly, CPE-ΔN also seems to have high potential as a prognostic biomarker for predicting future metastasis of some endocrine and nonendocrine tumors. 5) Finally, SNP in the human CPE gene have been linked to endocrinological and neurological diseases such as diabetes and Alzheimer's disease. In conclusion, CPE is a multifunctional protein that can subserve enzymatic as well as many nonenzymatic roles during development and in the adult endocrine and nervous systems. Mutations in the CPE gene are associated with a variety of disease states, and expression of its splice variant CPE-ΔN in tumors promotes metastatic disease in humans.
IX. Future Directions
Although the mechanisms of action of CPE in peptide processing are well-known and those mediating prohormone sorting and vesicle transport for secretion and neurotransmission are becoming better understood, considerable work lies ahead in understanding the mechanisms underlying the roles of CPE in four major areas: 1) cell protection and survival; 2) embryonic development; 3) neuropsychiatric disorders; and 4) tumor metastasis. The present review of the literature suggests that CPE promotes endocrine and neuronal cell survival in adults and may influence cell growth and proliferation in developing cells in young animals and in embryos. Thus, it is possible that secreted CPE might serve as an autocrine/paracrine trophic factor to activate different signaling pathways that trigger transcription of specific genes to mediate these effects. This possibility needs to be explored in detail in the future. Some examples include how secreted CPE may protect endocrine cells and neurons from cell death under conditions of palmitate-induced ER stress in β-cells, as well as under oxidative stress and amyloid protein-induced toxicity. In embryonic development, the expression pattern of the CPE splice variants, especially CPE-ΔN, should be investigated because molecules expressed in tumors often are expressed only during embryogenesis and have a role in proliferation and differentiation. Moreover, CPE-ΔN has been shown to activate gene transcription, and therefore overexpression and analysis of all its down-stream target genes in different cell types should provide new insights into its mechanism of action. Generating and studying mouse models bearing SNP of human CPE will also likely provide a better understanding of how these mutations cause disease states. Additionally, identification of both endogenous and environmental factors that can induce and regulate CPE expression and the splicing of the CPE gene to generate CPE-ΔN will greatly facilitate the understanding of how CPE can mediate abnormal behavioral responses and cause cancer and metastatic disease, respectively. Continued clinical studies on various endocrine and nonendocrine tumors should further validate CPE-ΔN as a promising multitumor prognostic biomarker for predicting future metastasis. One can envisage that future experiments will further reveal novel mechanisms of action for CPE and CPE-ΔN, especially as a prime regulator of many metabolic and endocrine functions, potentially as a survival and differentiating factor for neurons and endocrine cells, as well as a novel therapeutic target for cancer and degenerative diseases.
Acknowledgments
We thank Dr. Harold Gainer (National Institute of Neurological and Disorders and Stroke) for his comments and critical reading of the manuscript.
Research in the authors' laboratories was supported in part by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (to N.X.C., S.R.K.M., and K.P.), and NICHD K22 and American Recovery and Reinvestment Act grants (to J.J.P.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AGRP
- Agouti-related peptide
- BDNF
- brain-derived neurotrophic factor
- BMD
- bone mineral density
- CART
- cocaine- and amphetamine-regulated transcript
- Caspr2
- contactin-associated protein 2
- CCK
- cholecystokinin
- CPE
- carboxypeptidase E
- CPE-ΔN
- spliced variant of CPE without its N-terminus
- CSP
- constitutive secretory pathway
- E
- embryonic day
- EAE
- experimental autoimmune encephalomyelitis
- ER
- endoplasmic reticulum
- EST
- expressed sequence tag
- FH
- familial hyperproinsulinemia
- GEMSA
- guanidinoethylmercaptosuccinic acid
- GFP
- green fluorescent protein
- HCC
- hepatocellular carcinoma
- IHC
- immunohistochemistry
- KO
- knockout
- LDCV
- large dense-core vesicle
- LTP
- long term potentiation
- MC4R
- melanocortin 4 receptor
- MEN2
- multiple endocrine neoplasia type 2
- NOS1AP
- nitric oxide synthase 1 adaptor protein
- NPY
- neuropeptide Y
- PC
- prohormone convertase
- PGL
- paraganglioma
- PHEO
- pheochromocytoma
- POMC
- proopiomelanocortin
- RER
- rough ER
- RPE
- retinal pigmented epithelium
- RSP
- regulated secretory pathway
- SDHD
- succinate dehydrogenase subunit D
- siRNA
- small interfering RNA
- SLMV
- synaptic-like microvesicle
- SNP
- single nucleotide polymorphism
- SV
- synaptic vesicle
- T2DM
- type 2 diabetes mellitus
- TGN
- trans Golgi network
- TIRF
- total internal reflection fluorescence
- T/N
- tumor vs. normal surrounding tissue
- UTR
- untranslated region
- WT
- wild-type.
References
- 1. Steiner DF. 1998. The proprotein convertases. Curr Opin Chem Biol 2:31–39 [DOI] [PubMed] [Google Scholar]
- 2. Fricker LD, Snyder SH. 1982. Enkephalin convertase: purification and characterization of a specific enkephalin-synthesizing carboxypeptidase localized to adrenal chromaffin granules. Proc Natl Acad Sci USA 79:3886–3890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hook VY, Eiden LE, Brownstein MJ. 1982. A carboxypeptidase processing enzyme for enkephalin precursors. Nature 295:341–342 [DOI] [PubMed] [Google Scholar]
- 4. Bartolomucci A, Possenti R, Mahata SK, Fischer-Colbrie R, Loh YP, Salton SRK. 2011. The extended granin family: structure, function and biomedical implications. Endocr Rev 32:755–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lewis MJ, Pelham HR. 1990. A human homologue of the yeast HDEL receptor. Nature 348:162–163 [DOI] [PubMed] [Google Scholar]
- 6. Naggert JK, Fricker LD, Varlamov O, Nishina PM, Rouille Y, Steiner DF, Carroll RJ, Paigen BJ, Leiter EH. 1995. Hyperproinsulinaemia in obese fat/fat mice associated with a carboxypeptidase E mutation which reduces enzyme activity. Nat Genet 10:135–142 [DOI] [PubMed] [Google Scholar]
- 7. Cawley NX, Zhou J, Hill JM, Abebe D, Romboz S, Yanik T, Rodriguiz RM, Wetsel WC, Loh YP. 2004. The carboxypeptidase E knockout mouse exhibits endocrinological and behavioral deficits. Endocrinology 145:5807–5819 [DOI] [PubMed] [Google Scholar]
- 8. Lee TK, Murthy SR, Cawley NX, Dhanvantari S, Hewitt SM, Lou H, Lau T, Ma S, Huynh T, Wesley RA, Ng IO, Pacak K, Poon RT, Loh YP. 2011. An N-terminal truncated carboxypeptidase E splice isoform induces tumor growth and is a biomarker for predicting future metastasis in human cancers. J Clin Invest 121:880–892 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9. Reznik SE, Fricker LD. 2001. Carboxypeptidases from A to Z: implications in embryonic development and Wnt binding. Cell Mol Life Sci 58:1790–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rawlings ND, Barrett AJ. 1995. Evolutionary families of metallopeptidases. Methods Enzymol 248:183–228 [DOI] [PubMed] [Google Scholar]
- 11. Osterman AL, Grishin NV, Smulevitch SV, Matz MV, Zagnitko OP, Revina LP, Stepanov VM. 1992. Primary structure of carboxypeptidase T: delineation of functionally relevant features in Zn-carboxypeptidase family. J Protein Chem 11:561–570 [DOI] [PubMed] [Google Scholar]
- 12. Fricker LD, Evans CJ, Esch FS, Herbert E. 1986. Cloning and sequence analysis of cDNA for bovine carboxypeptidase E. Nature 323:461–464 [DOI] [PubMed] [Google Scholar]
- 13. Rodríguez C, Brayton KA, Brownstein M, Dixon JE. 1989. Rat preprocarboxypeptidase H. Cloning, characterization, and sequence of the cDNA and regulation of the mRNA by corticotropin-releasing factor. J Biol Chem 264:5988–5995 [PubMed] [Google Scholar]
- 14. Jung YK, Kunczt CJ, Pearson RK, Dixon JE, Fricker LD. 1991. Structural characterization of the rat carboxypeptidase-E gene. Mol Endocrinol 5:1257–1268 [DOI] [PubMed] [Google Scholar]
- 15. Billova S, Galanopoulou AS, Seidah NG, Qiu X, Kumar U. 2007. Immunohistochemical expression and colocalization of somatostatin, carboxypeptidase-E and prohormone convertases 1 and 2 in rat brain. Neuroscience 147:403–418 [DOI] [PubMed] [Google Scholar]
- 16. Manser E, Fernandez D, Loo L, Goh PY, Monfries C, Hall C, Lim L. 1990. Human carboxypeptidase E. Isolation and characterization of the cDNA, sequence conservation, expression and processing in vitro. Biochem J 267:517–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang X, Zhu J, Loh YP, Berghman LR. 2009. Carboxypeptidase E, an essential element of the regulated secretory pathway, is expressed and partially co-localized with chromogranin A in chicken thymus. Cell Tissue Res 337:371–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jacob TC, Kaplan JM. 2003. The EGL-21 carboxypeptidase E facilitates acetylcholine release at Caenorhabditis elegans neuromuscular junctions. J Neurosci 23:2122–2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sakate R, Suto Y, Imanishi T, Tanoue T, Hida M, Hayasaka I, Kusuda J, Gojobori T, Hashimoto K, Hirai M. 2007. Mapping of chimpanzee full-length cDNAs onto the human genome unveils large potential divergence of the transcriptome. Gene 399:1–10 [DOI] [PubMed] [Google Scholar]
- 20. Osada N, Hirata M, Tanuma R, Kusuda J, Hida M, Suzuki Y, Sugano S, Gojobori T, Shen CK, Wu CI, Hashimoto K. 2005. Substitution rate and structural divergence of 5′UTR evolution: comparative analysis between human and cynomolgus monkey cDNAs. Mol Biol Evol 22:1976–1982 [DOI] [PubMed] [Google Scholar]
- 21. Cargill EJ, Baskin LC, Pomp D. 1998. Rapid communication: localization of the porcine carboxypeptidase-E gene by linkage analysis further extends the region of synteny between human chromosome 4 and porcine chromosome 8. J Anim Sci 76:2211–2212 [DOI] [PubMed] [Google Scholar]
- 22. Klein SL, Strausberg RL, Wagner L, Pontius J, Clifton SW, Richardson P. 2002. Genetic and genomic tools for Xenopus research: the NIH Xenopus initiative. Dev Dyn 225:384–391 [DOI] [PubMed] [Google Scholar]
- 23. Fan X, Nagle GT. 1996. Molecular cloning of Aplysia neuronal cDNAs that encode carboxypeptidases related to mammalian prohormone processing enzymes. DNA Cell Biol 15:937–945 [DOI] [PubMed] [Google Scholar]
- 24. Vihtelic TS, Fadool JM, Gao J, Thornton KA, Hyde DR, Wistow G. 2005. Expressed sequence tag analysis of zebrafish eye tissues for NEIBank. Mol Vis 11:1083–1100 [PubMed] [Google Scholar]
- 25. Heinrich B, Zhang Z, Raitskin O, Hiller M, Benderska N, Hartmann AM, Bracco L, Elliott D, Ben-Ari S, Soreq H, Sperling J, Sperling R, Stamm S. 2009. Heterogeneous nuclear ribonucleoprotein G regulates splice site selection by binding to CC(A/C)-rich regions in pre-mRNA. J Biol Chem 284:14303–14315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pickrell JK, Pai AA, Gilad Y, Pritchard JK. 2010. Noisy splicing drives mRNA isoform diversity in human cells. PLoS Genet 6:e1001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tsai KW, Chan WC, Hsu CN, Lin WC. 2010. Sequence features involved in the mechanism of 3′ splice junction wobbling. BMC Mol Biol 11:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang J, Kuo CC, Chen L. 2011. GC content around splice sites affects splicing through pre-mRNA secondary structures. BMC Genomics 12:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Karambataki M, Malousi A, Maglaveras N, Kouidou S. 2010. Synonymous polymorphisms at splicing regulatory sites are associated with CpGs in neurodegenerative disease-related genes. Neuromolecular Med 12:260–269 [DOI] [PubMed] [Google Scholar]
- 30. Palaniswamy R, Teglund S, Lauth M, Zaphiropoulos PG, Shimokawa T. 2010. Genetic variations regulate alternative splicing in the 5′ untranslated regions of the mouse glioma-associated oncogene 1, Gli1. BMC Mol Biol 11:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kumari S, Bugaut A, Huppert JL, Balasubramanian S. 2007. An RNA G-quadruplex in the 5′ UTR of the NRAS proto-oncogene modulates translation. Nat Chem Biol 3:218–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kozak M. 1991. Structural features in eukaryotic mRNAs that modulate the initiation of translation. J Biol Chem 266:19867–19870 [PubMed] [Google Scholar]
- 33. Clark TA, Schweitzer AC, Chen TX, Staples MK, Lu G, Wang H, Williams A, Blume JE. 2007. Discovery of tissue-specific exons using comprehensive human exon microarrays. Genome Biol 8:R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Castle JC, Zhang C, Shah JK, Kulkarni AV, Kalsotra A, Cooper TA, Johnson JM. 2008. Expression of 24,426 human alternative splicing events and predicted cis regulation in 48 tissues and cell lines. Nat Genet 40:1416–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ishikawa T, Murakami K, Kido Y, Ohnishi S, Yazaki Y, Harada F, Kuroki K. 1998. Cloning, functional expression, and chromosomal localization of the human and mouse gp180-carboxypeptidase D-like enzyme. Gene 215:361–370 [DOI] [PubMed] [Google Scholar]
- 36. Song L, Fricker L. 1995. Processing of procarboxypeptidase E into carboxypeptidase E occurs in secretory vesicles. J Neurochem 65:444–453 [DOI] [PubMed] [Google Scholar]
- 37. Parkinson D. 1990. Two soluble forms of bovine carboxypeptidase H have different NH2-terminal sequences. J Biol Chem 265:17101–17105 [PubMed] [Google Scholar]
- 38. Song L, Fricker LD. 1997. The pro region is not required for the expression or intracellular routeing of carboxypeptidase E. Biochem J 323:265–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Song L, Fricker LD. 1995. Calcium- and pH-dependent aggregation of carboxypeptidase E. J Biol Chem 270:7963–7967 [DOI] [PubMed] [Google Scholar]
- 40. Rindler MJ. 1998. Carboxypeptidase E, a peripheral membrane protein implicated in the targeting of hormones to secretory granules, co-aggregates with granule content proteins at acidic pH. J Biol Chem 273:31180–31185 [DOI] [PubMed] [Google Scholar]
- 41. Cool DR, Normant E, Shen F, Chen HC, Pannell L, Zhang Y, Loh YP. 1997. Carboxypeptidase E is a regulated secretory pathway sorting receptor: genetic obliteration leads to endocrine disorders in Cpe(fat) mice. Cell 88:73–83 [DOI] [PubMed] [Google Scholar]
- 42. Cool DR, Loh YP. 1998. Carboxypeptidase E is a sorting receptor for prohormones: binding and kinetic studies. Mol Cell Endocrinol 139:7–13 [DOI] [PubMed] [Google Scholar]
- 43. Zhang CF, Snell CR, Loh YP. 1999. Identification of a novel prohormone sorting signal-binding site on carboxypeptidase E, a regulated secretory pathway-sorting receptor. Mol Endocrinol 13:527–536 [DOI] [PubMed] [Google Scholar]
- 44. Fricker LD. 1988. Activation and membrane binding of carboxypeptidase E. J Cell Biochem 38:279–289 [DOI] [PubMed] [Google Scholar]
- 45. Fricker LD, Das B, Angeletti RH. 1990. Identification of the pH-dependent membrane anchor of carboxypeptidase E (EC 3.4.17.10). J Biol Chem 265:2476–2482 [PubMed] [Google Scholar]
- 46. Mitra A, Song L, Fricker LD. 1994. The C-terminal region of carboxypeptidase E is involved in membrane binding and intracellular routing in AtT-20 cells. J Biol Chem 269:19876–19881 [PubMed] [Google Scholar]
- 47. Zhang CF, Dhanvantari S, Lou H, Loh YP. 2003. Sorting of carboxypeptidase E to the regulated secretory pathway requires interaction of its transmembrane domain with lipid rafts. Biochem J 369:453–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dhanvantari S, Loh YP. 2000. Lipid raft association of carboxypeptidase E is necessary for its function as a regulated secretory pathway sorting receptor. J Biol Chem 275:29887–29893 [DOI] [PubMed] [Google Scholar]
- 49. Dhanvantari S, Arnaoutova I, Snell CR, Steinbach PJ, Hammond K, Caputo GA, London E, Loh YP. 2002. Carboxypeptidase E, a prohormone sorting receptor, is anchored to secretory granules via a C-terminal transmembrane insertion. Biochemistry 41:52–60 [DOI] [PubMed] [Google Scholar]
- 50. Klemm RW, Ejsing CS, Surma MA, Kaiser HJ, Gerl MJ, Sampaio JL, de Robillard Q, Ferguson C, Proszynski TJ, Shevchenko A, Simons K. 2009. Segregation of sphingolipids and sterols during formation of secretory vesicles at the trans-Golgi network. J Cell Biol 185:601–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fricker LD, Devi L. 1993. Posttranslational processing of carboxypeptidase E, a neuropeptide-processing enzyme, in AtT-20 cells and bovine pituitary secretory granules. J Neurochem 61:1404–1415 [DOI] [PubMed] [Google Scholar]
- 52. Hook VY. 1985. Differential distribution of carboxypeptidase-processing enzyme activity and immunoreactivity in membrane and soluble components of chromaffin granules. J Neurochem 45:987–989 [DOI] [PubMed] [Google Scholar]
- 53. Arnaoutova I, Jackson CL, Al-Awar OS, Donaldson JG, Loh YP. 2003. Recycling of raft-associated prohormone sorting receptor carboxypeptidase E requires interaction with ARF6. Mol Biol Cell 14:4448–4457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wu CM, Chang HT, Chang MD. 2004. Membrane-bound carboxypeptidase E facilitates the entry of eosinophil cationic protein into neuroendocrine cells. Biochem J 382:841–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ren J, Kachel K, Kim H, Malenbaum SE, Collier RJ, London E. 1999. Interaction of diphtheria toxin T domain with molten globule-like proteins and its implications for translocation. Science 284:955–957 [DOI] [PubMed] [Google Scholar]
- 56. Brambillasca S, Yabal M, Soffientini P, Stefanovic S, Makarow M, Hegde RS, Borgese N. 2005. Transmembrane topogenesis of a tail-anchored protein is modulated by membrane lipid composition. EMBO J 24:2533–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Brambillasca S, Yabal M, Makarow M, Borgese N. 2006. Unassisted translocation of large polypeptide domains across phospholipid bilayers. J Cell Biol 175:767–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Luuk H, Toots U, Koks S, Vasar E. 2005. N-terminal domain of Wolframin interacts with carboxypeptidase E. Eur Neuropsychopharmacol 15:S5–S6 [Google Scholar]
- 59. Kõks S, Luuk H, Nelovkov A, Areda T, Vasar E. 2004. A screen for genes induced in the amygdaloid area during cat odor exposure. Genes Brain Behav 3:80–89 [DOI] [PubMed] [Google Scholar]
- 60. Saito N, Takeuchi T, Kawano A, Hosaka M, Hou N, Torii S. 2011. Luminal interaction of phogrin with carboxypeptidase E for effective targeting to secretory granules. Traffic 12:499–506 [DOI] [PubMed] [Google Scholar]
- 61. Machen TE, Leigh MJ, Taylor C, Kimura T, Asano S, Moore HP. 2003. pH of TGN and recycling endosomes of H+/K+-ATPase-transfected HEK-293 cells: implications for pH regulation in the secretory pathway. Am J Physiol Cell Physiol 285:C205–C214 [DOI] [PubMed] [Google Scholar]
- 62. De Matteis MA, Luini A. 2008. Exiting the Golgi complex. Nat Rev Mol Cell Biol 9:273–284 [DOI] [PubMed] [Google Scholar]
- 63. Hook VY, Mezey E, Fricker LD, Pruss RM, Siegel RE, Brownstein MJ. 1985. Immunochemical characterization of carboxypeptidase B-like peptide-hormone-processing enzyme. Proc Natl Acad Sci USA 82:4745–4749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lynch DR, Braas KM, Hutton JC, Snyder SH. 1990. Carboxypeptidase E (CPE): immunocytochemical localization in the rat central nervous system and pituitary gland. J Neurosci 10:1592–1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lynch DR, Strittmatter SM, Snyder SH. 1984. Enkephalin convertase localization by [3H]guanidinoethylmercaptosuccinic acid autoradiography: selective association with enkephalin-containing neurons. Proc Natl Acad Sci USA 81:6543–6547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Strittmatter SM, Lynch DR, Snyder SH. 1984. [3H]guanidinoethylmercaptosuccinic acid binding to tissue homogenates. Selective labeling of enkephalin convertase. J Biol Chem 259:11812–11817 [PubMed] [Google Scholar]
- 67. Strittmatter SM, Lynch DR, De Souza EB, Snyder SH. 1985. Enkephalin convertase demonstrated in the pituitary and adrenal gland by [3H]guanidinoethylmercaptosuccinic acid autoradiography: dehydration decreases neurohypophyseal levels. Endocrinology 117:1667–1674 [DOI] [PubMed] [Google Scholar]
- 68. Lynch DR, Strittmatter SM, Venable JC, Snyder SH. 1986. Enkephalin convertase: localization to specific neuronal pathways. J Neurosci 6:1662–1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Strittmatter SM, Lynch DR, Snyder SH. 1986. Differential ontogeny of rat brain peptidases: prenatal expression of enkephalin convertase and postnatal development of angiotensin-converting enzyme. Brain Res 394:207–215 [DOI] [PubMed] [Google Scholar]
- 70. Lynch DR, Strittmatter SM, Venable JC, Snyder SH. 1987. Enkephalin convertase in the gastrointestinal tract and associated organs characterized and localized with [3H]guanidinoethylmercaptosuccinic acid. Endocrinology 121:116–126 [DOI] [PubMed] [Google Scholar]
- 71. Lynch DR, Venable JC, Snyder SH. 1988. Enkephalin convertase in the heart: similar disposition to atrial natriuretic factor. Endocrinology 122:2683–2691 [DOI] [PubMed] [Google Scholar]
- 72. Lynch DR, Venable JC, Strittmatter SM, Snyder SH. 1988. Enkephalin convertase: characterization and localization using [3H]guanidinoethylmercaptosuccinic acid. Biochimie 70:57–64 [DOI] [PubMed] [Google Scholar]
- 73. Hougaard DM, Larsson LI. 2004. Carboxypeptidase E in rat antropyloric mucosa: distribution in progenitor and mature endocrine cell types. Histochem Cell Biol 121:55–61 [DOI] [PubMed] [Google Scholar]
- 74. Krajnik M, Schäfer M, Sobanski P, Kowalewski J, Bloch-Boguslawska E, Zylicz Z, Mousa SA. 2010. Enkephalin, its precursor, processing enzymes, and receptor as part of a local opioid network throughout the respiratory system of lung cancer patients. Hum Pathol 41:632–642 [DOI] [PubMed] [Google Scholar]
- 75. Mousa SA, Krajnik M, Sobanski P, Kowalewski J, Bloch-Boguslawska E, Zylicz Z, Schäfer M. 2010. Dynorphin expression, processing and receptors in the alveolar macrophages, cancer cells and bronchial epithelium of lung cancer patients. Histol Histopathol 25:755–764 [DOI] [PubMed] [Google Scholar]
- 76. Fricker LD, Adelman JP, Douglass J, Thompson RC, von Strandmann RP, Hutton J. 1989. Isolation and sequence analysis of cDNA for rat carboxypeptidase E [EC 3.4.17.10], a neuropeptide processing enzyme. Mol Endocrinol 3:666–673 [DOI] [PubMed] [Google Scholar]
- 77. Zheng M, Streck RD, Scott RE, Seidah NG, Pintar JE. 1994. The developmental expression in rat of proteases furin, PC1, PC2, and carboxypeptidase E: implications for early maturation of proteolytic processing capacity. J Neurosci 14:4656–4673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Birch NP, Rodriguez C, Dixon JE, Mezey E. 1990. Distribution of carboxypeptidase H messenger RNA in rat brain using in situ hybridization histochemistry: implications for neuropeptide biosynthesis. Brain Res Mol Brain Res 7:53–59 [DOI] [PubMed] [Google Scholar]
- 79. MacCumber MW, Snyder SH, Ross CA. 1990. Carboxypeptidase E (enkephalin convertase): mRNA distribution in rat brain by in situ hybridization. J Neurosci 10:2850–2860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Schäfer MK, Day R, Cullinan WE, Chrétien M, Seidah NG, Watson SJ. 1993. Gene expression of prohormone and proprotein convertases in the rat CNS: a comparative in situ hybridization analysis. J Neurosci 13:1258–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kershaw EE, Flier JS. 2004. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 89:2548–2556 [DOI] [PubMed] [Google Scholar]
- 82. Ramis JM, Franssen-van Hal NL, Kramer E, Llado I, Bouillaud F, Palou A, Keijer J. 2002. Carboxypeptidase E and thrombospondin-1 are differently expressed in subcutaneous and visceral fat of obese subjects. Cell Mol Life Sci 59:1960–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Greene D, Das B, Fricker LD. 1992. Regulation of carboxypeptidase E. Effect of pH, temperature and Co2+ on kinetic parameters of substrate hydrolysis. Biochem J 285:613–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Fricker LD. 2004. Carboxypeptidase E. In: Barrett AJ, Rawlings ND, Woessner JF, eds. Handbook of proteolytic enzymes. 2nd ed London: Academic Press; 840–844 [Google Scholar]
- 85. Nalamachu SR, Song L, Fricker LD. 1994. Regulation of carboxypeptidase E. Effect of Ca2+ on enzyme activity and stability. J Biol Chem 269:11192–11195 [PubMed] [Google Scholar]
- 86. Song L, Fricker LD. 1995. Purification and characterization of carboxypeptidase D, a novel carboxypeptidase E-like enzyme, from bovine pituitary. J Biol Chem 270:25007–25013 [DOI] [PubMed] [Google Scholar]
- 87. Fricker LD, Plummer TH, Jr, Snyder SH. 1983. Enkephalin convertase: potent, selective, and irreversible inhibitors. Biochem Biophys Res Commun 111:994–1000 [DOI] [PubMed] [Google Scholar]
- 88. Bures EJ, Courchesne PL, Douglass J, Chen K, Davis MT, Jones MD, McGinley MD, Robinson JH, Spahr CS, Sun J, Wahl RC, Patterson SD. 2001. Identification of incompletely processed potential carboxypeptidase E substrates from CpEfat/CpEfat mice. Proteomics 1:79–92 [DOI] [PubMed] [Google Scholar]
- 89. Fricker LD. 2007. Neuropeptidomics to study peptide processing in animal models of obesity. Endocrinology 148:4185–4190 [DOI] [PubMed] [Google Scholar]
- 90. Fricker LD, McKinzie AA, Sun J, Curran E, Qian Y, Yan L, Patterson SD, Courchesne PL, Richards B, Levin N, Mzhavia N, Devi LA, Douglass J. 2000. Identification and characterization of proSAAS, a granin-like neuroendocrine peptide precursor that inhibits prohormone processing. J Neurosci 20:639–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gelman JS, Sironi J, Castro LM, Ferro ES, Fricker LD. 2010. Hemopressins and other hemoglobin-derived peptides in mouse brain: comparison between brain, blood, and heart peptidome and regulation in Cpefat/fat mice. J Neurochem 113:871–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Gomes I, Grushko JS, Golebiewska U, Hoogendoorn S, Gupta A, Heimann AS, Ferro ES, Scarlata S, Fricker LD, Devi LA. 2009. Novel endogenous peptide agonists of cannabinoid receptors. FASEB J 23:3020–3029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Che FY, Yan L, Li H, Mzhavia N, Devi LA, Fricker LD. 2001. Identification of peptides from brain and pituitary of Cpe(fat)/Cpe(fat) mice. Proc Natl Acad Sci USA 98:9971–9976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Che FY, Biswas R, Fricker LD. 2005. Relative quantitation of peptides in wild-type and Cpe(fat/fat) mouse pituitary using stable isotopic tags and mass spectrometry. J Mass Spectrom 40:227–237 [DOI] [PubMed] [Google Scholar]
- 95. Che FY, Fricker LD. 2005. Quantitative peptidomics of mouse pituitary: comparison of different stable isotopic tags. J Mass Spectrom 40:238–249 [DOI] [PubMed] [Google Scholar]
- 96. Che FY, Yuan Q, Kalinina E, Fricker LD. 2005. Peptidomics of Cpe fat/fat mouse hypothalamus: effect of food deprivation and exercise on peptide levels. J Biol Chem 280:4451–4461 [DOI] [PubMed] [Google Scholar]
- 97. Décaillot FM, Che FY, Fricker LD, Devi LA. 2006. Peptidomics of Cpefat/fat mouse hypothalamus and striatum: effect of chronic morphine administration. J Mol Neurosci 28:277–284 [DOI] [PubMed] [Google Scholar]
- 98. Che FY, Vathy I, Fricker LD. 2006. Quantitative peptidomics in mice: effect of cocaine treatment. J Mol Neurosci 28:265–275 [DOI] [PubMed] [Google Scholar]
- 99. Kelly RB. 1985. Pathways of protein secretion in eukaryotes. Science 230:25–32 [DOI] [PubMed] [Google Scholar]
- 100. Wieland FT, Gleason ML, Serafini TA, Rothman JE. 1987. The rate of bulk flow from the endoplasmic reticulum to the cell surface. Cell 50:289–300 [DOI] [PubMed] [Google Scholar]
- 101. von Zastrow M, Link R, Daunt D, Barsh G, Kobilka B. 1993. Subtype-specific differences in the intracellular sorting of G protein-coupled receptors. J Biol Chem 268:763–766 [PubMed] [Google Scholar]
- 102. Kelly RB, Buckley KM, Burgess TL, Carlson SS, Caroni P, Hooper JE, Katzen A, Moore HP, Pfeffer SR, Schroer TA. 1983. Membrane traffic in neurons and peptide-secreting cells. Cold Spring Harb Symp Quant Biol 48:697–705 [DOI] [PubMed] [Google Scholar]
- 103. Green R, Shields D. 1984. Somatostatin discriminates between the intracellular pathways of secretory and membrane proteins. J Cell Biol 99:97–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Guillemin R, Vargo T, Rossier J, Minick S, Ling N, Rivier C, Vale W, Bloom F. 1977. β-Endorphin and adrenocorticotropin are selected concomitantly by the pituitary gland. Science 197:1367–1369 [DOI] [PubMed] [Google Scholar]
- 105. Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. 2000. Central nervous system control of food intake. Nature 404:661–671 [DOI] [PubMed] [Google Scholar]
- 106. Thoenen H. 2000. Neurotrophins and activity-dependent plasticity. Prog Brain Res 128:183–191 [DOI] [PubMed] [Google Scholar]
- 107. Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. 2003. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112:257–269 [DOI] [PubMed] [Google Scholar]
- 108. Arvan P, Kuliawat R, Prabakaran D, Zavacki AM, Elahi D, Wang S, Pilkey D. 1991. Protein discharge from immature secretory granules displays both regulated and constitutive characteristics. J Biol Chem 266:14171–14174 [PubMed] [Google Scholar]
- 109. Kuliawat R, Arvan P. 1992. Protein targeting via the “constitutive-like” secretory pathway in isolated pancreatic islets: passive sorting in the immature granule compartment. J Cell Biol 118:521–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Loh YP, Snell CR, Cool DR. 1997. Receptor-mediated targeting of hormones to secretory granules: role of carboxypeptidase E. Trends Endocrinol Metab 8:130–137 [DOI] [PubMed] [Google Scholar]
- 111. Van Horssen AM, Van Kuppeveld FJ, Martens GJ. 1998. Manipulation of disulfide bonds differentially affects the intracellular transport, sorting, and processing of neuroendocrine secretory proteins. J Neurochem 71:402–409 [DOI] [PubMed] [Google Scholar]
- 112. Mulcahy LR, Barker AJ, Nillni EA. 2006. Disruption of disulfide bond formation alters the trafficking of prothyrotropin releasing hormone (proTRH)-derived peptides. Regul Pept 133:123–133 [DOI] [PubMed] [Google Scholar]
- 113. Gerdes HH, Rosa P, Phillips E, Baeuerle PA, Frank R, Argos P, Huttner WB. 1989. The primary structure of human secretogranin II, a widespread tyrosine-sulfated secretory granule protein that exhibits low pH- and calcium-induced aggregation. J Biol Chem 264:12009–12015 [PubMed] [Google Scholar]
- 114. Chanat E, Huttner WB. 1991. Milieu-induced, selective aggregation of regulated secretory proteins in the trans-Golgi network. J Cell Biol 115:1505–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Bauerfeind R, Huttner WB. 1993. Biogenesis of constitutive secretory vesicles, secretory granules and synaptic vesicles. Curr Opin Cell Biol 5:628–635 [DOI] [PubMed] [Google Scholar]
- 116. Canaff L, Brechler V, Reudelhuber TL, Thibault G. 1996. Secretory granule targeting of atrial natriuretic peptide correlates with its calcium-mediated aggregation. Proc Natl Acad Sci USA 93:9483–9487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Dikeakos JD, Reudelhuber TL. 2007. Sending proteins to dense core secretory granules: still a lot to sort out. J Cell Biol 177:191–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Normant E, Loh YP. 1998. Depletion of carboxypeptidase E, a regulated secretory pathway sorting receptor, causes misrouting and constitutive secretion of proinsulin and proenkephalin, but not chromogranin A. Endocrinology 139:2137–2145 [DOI] [PubMed] [Google Scholar]
- 119. Loh YP, Maldonado A, Zhang C, Tam WH, Cawley N. 2002. Mechanism of sorting proopiomelanocortin and proenkephalin to the regulated secretory pathway of neuroendocrine cells. Ann NY Acad Sci 971:416–425 [DOI] [PubMed] [Google Scholar]
- 120. Lou H, Kim SK, Zaitsev E, Snell CR, Lu B, Loh YP. 2005. Sorting and activity-dependent secretion of BDNF require interaction of a specific motif with the sorting receptor carboxypeptidase E. Neuron 45:245–255 [DOI] [PubMed] [Google Scholar]
- 121. Dhanvantari S, Shen FS, Adams T, Snell CR, Zhang C, Mackin RB, Morris SJ, Loh YP. 2003. Disruption of a receptor-mediated mechanism for intracellular sorting of proinsulin in familial hyperproinsulinemia. Mol Endocrinol 17:1856–1867 [DOI] [PubMed] [Google Scholar]
- 122. Irminger JC, Verchere CB, Meyer K, Halban PA. 1997. Proinsulin targeting to the regulated pathway is not impaired in carboxypeptidase E-deficient Cpefat/Cpefat mice. J Biol Chem 272:27532–27534 [DOI] [PubMed] [Google Scholar]
- 123. Cawley NX, Rodriguez YM, Maldonado A, Loh YP. 2003. Trafficking of mutant carboxypeptidase E to secretory granules in a β-cell line derived from Cpe(fat)/Cpe(fat) mice. Endocrinology 144:292–298 [DOI] [PubMed] [Google Scholar]
- 124. Cawley NX, Yanik T, Woronowicz A, Chang W, Marini JC, Loh YP. 2010. Obese carboxypeptidase E knockout mice exhibit multiple defects in peptide hormone processing contributing to low bone mineral density. Am J Physiol Endocrinol Metab 299:E189–E197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Hosaka M, Watanabe T, Sakai Y, Kato T, Takeuchi T. 2005. Interaction between secretogranin III and carboxypeptidase E facilitates prohormone sorting within secretory granules. J Cell Sci 118:4785–4795 [DOI] [PubMed] [Google Scholar]
- 126. Kemppainen RJ, Behrend EN. 2010. Acute inhibition of carboxypeptidase E expression in AtT-20 cells does not affect regulated secretion of ACTH. Regul Pept 165:174–179 [DOI] [PubMed] [Google Scholar]
- 127. Hall DH, Hedgecock EM. 1991. Kinesin-related gene unc-104 is required for axonal transport of synaptic vesicles in C. elegans. Cell 65:837–847 [DOI] [PubMed] [Google Scholar]
- 128. Otsuka AJ, Jeyaprakash A, García-Añoveros J, Tang LZ, Fisk G, Hartshorne T, Franco R, Born T. 1991. The C. elegans unc-104 gene encodes a putative kinesin heavy chain-like protein. Neuron 6:113–122 [DOI] [PubMed] [Google Scholar]
- 129. Hamm-Alvarez SF, Da Costa S, Yang T, Wei X, Gierow JP, Mircheff AK. 1997. Cholinergic stimulation of lacrimal acinar cells promotes redistribution of membrane-associated kinesin and the secretory protein, β-hexosaminidase, and increases kinesin motor activity. Exp Eye Res 64:141–156 [DOI] [PubMed] [Google Scholar]
- 130. Senda T, Yu W. 1999. Kinesin cross-bridges between neurosecretory granules and microtubules in the mouse neurohypophysis. Neurosci Lett 262:69–71 [DOI] [PubMed] [Google Scholar]
- 131. Varadi A, Tsuboi T, Johnson-Cadwell LI, Allan VJ, Rutter GA. 2003. Kinesin I and cytoplasmic dynein orchestrate glucose-stimulated insulin-containing vesicle movements in clonal MIN6 β-cells. Biochem Biophys Res Commun 311:272–282 [DOI] [PubMed] [Google Scholar]
- 132. Park JJ, Cawley NX, Loh YP. 2008. A bi-directional carboxypeptidase E-driven transport mechanism controls BDNF vesicle homeostasis in hippocampal neurons. Mol Cell Neurosci 39:63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Park JJ, Cawley NX, Loh YP. 2008. Carboxypeptidase E cytoplasmic tail-driven vesicle transport is key for activity-dependent secretion of peptide hormones. Mol Endocrinol 22:989–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Rosé SD, Lejen T, Casaletti L, Larson RE, Pene TD, Trifaró JM. 2003. Myosins II and V in chromaffin cells: myosin V is a chromaffin vesicle molecular motor involved in secretion. J Neurochem 85:287–298 [DOI] [PubMed] [Google Scholar]
- 135. Rudolf R, Kögel T, Kuznetsov SA, Salm T, Schlicker O, Hellwig A, Hammer JA, 3rd, Gerdes HH. 2003. Myosin Va facilitates the distribution of secretory granules in the F-actin rich cortex of PC12 cells. J Cell Sci 116:1339–1348 [DOI] [PubMed] [Google Scholar]
- 136. Eichler TW, Kögel T, Bukoreshtliev NV, Gerdes HH. 2006. The role of myosin Va in secretory granule trafficking and exocytosis. Biochem Soc Trans 34:671–674 [DOI] [PubMed] [Google Scholar]
- 137. Shakiryanova D, Tully A, Levitan ES. 2006. Activity-dependent synaptic capture of transiting peptidergic vesicles. Nat Neurosci 9:896–900 [DOI] [PubMed] [Google Scholar]
- 138. Goldstein LS, Yang Z. 2000. Microtubule-based transport systems in neurons: the roles of kinesins and dyneins. Annu Rev Neurosci 23:39–71 [DOI] [PubMed] [Google Scholar]
- 139. Zhang H, Li S, Wang M, Vukusic B, Pristupa ZB, Liu F. 2009. Regulation of dopamine transporter activity by carboxypeptidase E. Mol Brain 2:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Oiso S, Takeda Y, Futagawa T, Miura T, Kuchiiwa S, Nishida K, Ikeda R, Kariyazono H, Watanabe K, Yamada K. 2009. Contactin-associated protein (Caspr) 2 interacts with carboxypeptidase E in the CNS. J Neurochem 109:158–167 [DOI] [PubMed] [Google Scholar]
- 141. Zhu X, Wu K, Rife L, Cawley NX, Brown B, Adams T, Teofilo K, Lillo C, Williams DS, Loh YP, Craft CM. 2005. Carboxypeptidase E is required for normal synaptic transmission from photoreceptors to the inner retina. J Neurochem 95:1351–1362 [DOI] [PubMed] [Google Scholar]
- 142. Lou H, Park JJ, Cawley NX, Sarcon A, Sun L, Adams T, Loh YP. 2010. Carboxypeptidase E cytoplasmic tail mediates localization of synaptic vesicles to the pre-active zone in hypothalamic pre-synaptic terminals. J Neurochem 114:886–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Berezuk MA, Schroer TA. 2007. Dynactin enhances the processivity of kinesin-2. Traffic 8:124–129 [DOI] [PubMed] [Google Scholar]
- 144. Park JJ, Loh YP. 2008. How peptide hormone vesicles are transported to the secretion site for exocytosis. Mol Endocrinol 22:2583–2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Klyachko VA, Jackson MB. 2002. Capacitance steps and fusion pores of small and large-dense-core vesicles in nerve terminals. Nature 418:89–92 [DOI] [PubMed] [Google Scholar]
- 146. Zhang Z, Bhalla A, Dean C, Chapman ER, Jackson MB. 2009. Synaptotagmin IV: a multifunctional regulator of peptidergic nerve terminals. Nat Neurosci 12:163–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Weihe E, Tao-Cheng JH, Schäfer MK, Erickson JD, Eiden LE. 1996. Visualization of the vesicular acetylcholine transporter in cholinergic nerve terminals and its targeting to a specific population of small synaptic vesicles. Proc Natl Acad Sci USA 93:3547–3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Liu Y, Edwards RH. 1997. Differential localization of vesicular acetylcholine and monoamine transporters in PC12 cells but not CHO cells. J Cell Biol 139:907–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Krantz DE, Waites C, Oorschot V, Liu Y, Wilson RI, Tan PK, Klumperman J, Edwards RH. 2000. A phosphorylation site regulates sorting of the vesicular acetylcholine transporter to dense core vesicles. J Cell Biol 149:379–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Yao J, Erickson JD, Hersh LB. 2004. Protein kinase A affects trafficking of the vesicular monoamine transporters in PC12 cells. Traffic 5:1006–1016 [DOI] [PubMed] [Google Scholar]
- 151. Rizzoli SO, Betz WJ. 2005. Synaptic vesicle pools. Nat Rev Neurosci 6:57–69 [DOI] [PubMed] [Google Scholar]
- 152. Ludwig M, Leng G. 2006. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci 7:126–136 [DOI] [PubMed] [Google Scholar]
- 153. Hou JC, Min L, Pessin JE. 2009. Insulin granule biogenesis, trafficking and exocytosis. Vitam Horm 80:473–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Liu Y, Schirra C, Edelmann L, Matti U, Rhee J, Hof D, Bruns D, Brose N, Rieger H, Stevens DR, Rettig J. 2010. Two distinct secretory vesicle-priming steps in adrenal chromaffin cells. J Cell Biol 190:1067–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Eiden LE. 1998. The cholinergic gene locus. J Neurochem 70:2227–2240 [DOI] [PubMed] [Google Scholar]
- 156. Ferguson SM, Savchenko V, Apparsundaram S, Zwick M, Wright J, Heilman CJ, Yi H, Levey AI, Blakely RD. 2003. Vesicular localization and activity-dependent trafficking of presynaptic choline transporters. J Neurosci 23:9697–9709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Cai Q, Lu L, Tian JH, Zhu YB, Qiao H, Sheng ZH. 2010. Snapin-regulated late endosomal transport is critical for efficient autophagy-lysosomal function in neurons. Neuron 68:73–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Lorenzen A, Samosh J, Vandewark K, Anborgh PH, Seah C, Magalhaes AC, Cregan SP, Ferguson SS, Pasternak SH. 2010. Rapid and direct transport of cell surface APP to the lysosome defines a novel selective pathway. Mol Brain 3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Coleman DL, Eicher EM. 1990. Fat (fat) and tubby (tub): two autosomal recessive mutations causing obesity syndromes in the mouse. J Hered 81:424–427 [DOI] [PubMed] [Google Scholar]
- 160. Berman Y, Mzhavia N, Polonskaia A, Devi LA. 2001. Impaired prohormone convertases in Cpe(fat)/Cpe(fat) mice. J Biol Chem 276:1466–1473 [DOI] [PubMed] [Google Scholar]
- 161. Varlamov O, Leiter EH, Fricker L. 1996. Induced and spontaneous mutations at Ser202 of carboxypeptidase E. Effect on enzyme expression, activity, and intracellular routing. J Biol Chem 271:13981–13986 [DOI] [PubMed] [Google Scholar]
- 162. Varlamov O, Fricker LD, Furukawa H, Steiner DF, Langley SH, Leiter EH. 1997. β-Cell lines derived from transgenic Cpe(fat)/Cpe(fat) mice are defective in carboxypeptidase E and proinsulin processing. Endocrinology 138:4883–4892 [DOI] [PubMed] [Google Scholar]
- 163. Leiter EH, Kintner J, Flurkey K, Beamer WG, Naggert JK. 1999. Physiologic and endocrinologic characterization of male sex-biased diabetes in C57BLKS/J mice congenic for the fat mutation at the carboxypeptidease E locus. Endocrine 10:57–66 [DOI] [PubMed] [Google Scholar]
- 164. Prager R, Schernthaner G. 1982. Receptor binding properties of human insulin (recombinant DNA) and human proinsulin and their interaction at the receptor site. Diabetes Care 5(Suppl 2):104–106 [DOI] [PubMed] [Google Scholar]
- 165. Michael DJ, Geng X, Cawley NX, Loh YP, Rhodes CJ, Drain P, Chow RH. 2004. Fluorescent cargo proteins in pancreatic β-cells: design determines secretion kinetics at exocytosis. Biophys J 87:L03–L05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Yuan Q, Fontenele-Neto JD, Fricker LD. 2004. Effect of voluntary exercise on genetically obese Cpefat/fat mice: quantitative proteomics of serum. Obes Res 12:1179–1188 [DOI] [PubMed] [Google Scholar]
- 167. Myers MG, Cowley MA, Münzberg H. 2008. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol 70:537–556 [DOI] [PubMed] [Google Scholar]
- 168. Moran TH, Ameglio PJ, Schwartz GJ, McHugh PR. 1992. Blockade of type A, not type B, CCK receptors attenuates satiety actions of exogenous and endogenous CCK. Am J Physiol 262:R46–R50 [DOI] [PubMed] [Google Scholar]
- 169. Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR. 1996. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 379:69–72 [DOI] [PubMed] [Google Scholar]
- 170. Cammisotto PG, Bukowiecki LJ. 2002. Mechanisms of leptin secretion from white adipocytes. Am J Physiol Cell Physiol 283:C244–C250 [DOI] [PubMed] [Google Scholar]
- 171. Elmquist JK, Maratos-Flier E, Saper CB, Flier JS. 1998. Unraveling the central nervous system pathways underlying responses to leptin. Nat Neurosci 1:445–450 [DOI] [PubMed] [Google Scholar]
- 172. Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, Wulff BS, Clausen JT, Jensen PB, Madsen OD, Vrang N, Larsen PJ, Hastrup S. 1998. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature 393:72–76 [DOI] [PubMed] [Google Scholar]
- 173. Wardlaw SL. 2011. Hypothalamic proopiomelanocortin processing and the regulation of energy balance. Eur J Pharmacol 660:213–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Plum L, Lin HV, Dutia R, Tanaka J, Aizawa KS, Matsumoto M, Kim AJ, Cawley NX, Paik JH, Loh YP, DePinho RA, Wardlaw SL, Accili D. 2009. The obesity susceptibility gene Cpe links FoxO1 signaling in hypothalamic pro-opiomelanocortin neurons with regulation of food intake. Nat Med 15:1195–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. del Giudice EM, Santoro N, Cirillo G, D'Urso L, Di Toro R, Perrone L. 2001. Mutational screening of the CART gene in obese children: identifying a mutation (Leu34Phe) associated with reduced resting energy expenditure and cosegregating with obesity phenotype in a large family. Diabetes 50:2157–2160 [DOI] [PubMed] [Google Scholar]
- 176. Yanik T, Dominguez G, Kuhar MJ, Del Giudice EM, Loh YP. 2006. The Leu34Phe ProCART mutation leads to cocaine- and amphetamine-regulated transcript (CART) deficiency: a possible cause for obesity in humans. Endocrinology 147:39–43 [DOI] [PubMed] [Google Scholar]
- 177. Asnicar MA, Smith DP, Yang DD, Heiman ML, Fox N, Chen YF, Hsiung HM, Köster A. 2001. Absence of cocaine- and amphetamine-regulated transcript results in obesity in mice fed a high caloric diet. Endocrinology 142:4394–4400 [DOI] [PubMed] [Google Scholar]
- 178. Wierup N, Richards WG, Bannon AW, Kuhar MJ, Ahrén B, Sundler F. 2005. CART knock out mice have impaired insulin secretion and glucose intolerance, altered β cell morphology and increased body weight. Regul Pept 129:203–211 [DOI] [PubMed] [Google Scholar]
- 179. Stanley BG, Kyrkouli SE, Lampert S, Leibowitz SF. 1986. Neuropeptide Y chronically injected into the hypothalamus: a powerful neurochemical inducer of hyperphagia and obesity. Peptides 7:1189–1192 [DOI] [PubMed] [Google Scholar]
- 180. Baldock PA, Lee NJ, Driessler F, Lin S, Allison S, Stehrer B, Lin EJ, Zhang L, Enriquez RF, Wong IP, McDonald MM, During M, Pierroz DD, Slack K, Shi YC, Yulyaningsih E, Aljanova A, Little DG, Ferrari SL, Sainsbury A, Eisman JA, Herzog H. 2009. Neuropeptide Y knockout mice reveal a central role of NPY in the coordination of bone mass to body weight. PLoS One 4:e8415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Lacourse KA, Friis-Hansen L, Samuelson LC, Rehfeld JF. 1998. Altered processing of procholecystokinin in carboxypeptidase E-deficient fat mice: differential synthesis in neurons and endocrine cells. FEBS Lett 436:61–66 [DOI] [PubMed] [Google Scholar]
- 182. Wang W, Cain BM, Beinfeld MC. 1998. Adult carboxypeptidase E-deficient fat/fat mice have a near-total depletion of brain CCK 8 accompanied by a massive accumulation of glycine and arginine extended CCK: identification of CCK 8 Gly as the immediate precursor of CCK 8 in rodent brain. Endocrine 9:329–332 [DOI] [PubMed] [Google Scholar]
- 183. Maeda K, Okubo K, Shimomura I, Mizuno K, Matsuzawa Y, Matsubara K. 1997. Analysis of an expression profile of genes in the human adipose tissue. Gene 190:227–235 [DOI] [PubMed] [Google Scholar]
- 184. Lawler J. 2000. The functions of thrombospondin-1 and-2. Curr Opin Cell Biol 12:634–640 [DOI] [PubMed] [Google Scholar]
- 185. Chen H, Herndon ME, Lawler J. 2000. The cell biology of thrombospondin-1. Matrix Biol 19:597–614 [DOI] [PubMed] [Google Scholar]
- 186. Cammisotto PG, Bukowiecki LJ, Deshaies Y, Bendayan M. 2006. Leptin biosynthetic pathway in white adipocytes. Biochem Cell Biol 84:207–214 [DOI] [PubMed] [Google Scholar]
- 187. Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. 2002. Leptin regulates bone formation via the sympathetic nervous system. Cell 111:305–317 [DOI] [PubMed] [Google Scholar]
- 188. Elefteriou F. 2008. Regulation of bone remodeling by the central and peripheral nervous system. Arch Biochem Biophys 473:231–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Ferron M, Hinoi E, Karsenty G, Ducy P. 2008. Osteocalcin differentially regulates β cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci USA 105:5266–5270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Baldock PA, Sainsbury A, Couzens M, Enriquez RF, Thomas GP, Gardiner EM, Herzog H. 2002. Hypothalamic Y2 receptors regulate bone formation. J Clin Invest 109:915–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Ahn JD, Dubern B, Lubrano-Berthelier C, Clement K, Karsenty G. 2006. Cart overexpression is the only identifiable cause of high bone mass in melanocortin 4 receptor deficiency. Endocrinology 147:3196–3202 [DOI] [PubMed] [Google Scholar]
- 192. Zhang M, Pritchard MR, Middleton FA, Horton JA, Damron TA. 2008. Microarray analysis of perichondral and reserve growth plate zones identifies differential gene expressions and signal pathways. Bone 43:511–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Bray GA, York DA. 1971. Genetically transmitted obesity in rodents. Physiol Rev 51:598–646 [DOI] [PubMed] [Google Scholar]
- 194. Srinivasan S, Bunch DO, Feng Y, Rodriguiz RM, Li M, Ravenell RL, Luo GX, Arimura A, Fricker LD, Eddy EM, Wetsel WC. 2004. Deficits in reproduction and pro-gonadotropin-releasing hormone processing in male Cpefat mice. Endocrinology 145:2023–2034 [DOI] [PubMed] [Google Scholar]
- 195. Wetsel WC, Mellon PL, Weiner RI, Negro-Vilar A. 1991. Metabolism of pro-luteinizing hormone-releasing hormone in immortalized hypothalamic neurons. Endocrinology 129:1584–1595 [DOI] [PubMed] [Google Scholar]
- 196. Wetsel WC, Liposits Z, Seidah NG, Collins S. 1995. Expression of candidate pro-GnRH processing enzymes in rat hypothalamus and an immortalized hypothalamic neuronal cell line. Neuroendocrinology 62:166–177 [DOI] [PubMed] [Google Scholar]
- 197. Beamer WG, Wilson MC, Leiter EH. 1983. Endocrinology. In: Foster HL, Small JD, Fox JG, eds. The mouse in biomedical research. Vol III Normative biology, immunology, and husbandry New York: Academic Press; 166–245 [Google Scholar]
- 198. Vandenbergh JG. 1994. Pheromones and mammalian reproduction. In: Knobil E, Neill J, eds. The physiology of reproduction. New York: Raven Press; 343–359 [Google Scholar]
- 199. Woronowicz A, Koshimizu H, Chang SY, Cawley NX, Hill JM, Rodriguiz RM, Abebe D, Dorfman C, Senatorov V, Zhou A, Xiong ZG, Wetsel WC, Loh YP. 2008. Absence of carboxypeptidase E leads to adult hippocampal neuronal degeneration and memory deficits. Hippocampus 18:1051–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Koshimizu H, Senatorov V, Loh YP, Gozes I. 2009. Neuroprotective protein and carboxypeptidase E. J Mol Neurosci 39:1–8 [DOI] [PubMed] [Google Scholar]
- 201. Nickells RW, Schlamp CL, Newton AC, Williams DS. 1993. Gene expression of the neuropeptide-processing enzyme carboxypeptidase E in rat photoreceptor cells. J Neurochem 61:1891–1900 [DOI] [PubMed] [Google Scholar]
- 202. Schlamp CL, Nickells RW. 1996. Light and dark cause a shift in the spatial expression of a neuropeptide-processing enzyme in the rat retina. J Neurosci 16:2164–2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Coca-Prados M, Escribano J, Ortego J. 1999. Differential gene expression in the human ciliary epithelium. Prog Retin Eye Res 18:403–429 [DOI] [PubMed] [Google Scholar]
- 204. Ida H, Boylan SA, Weigel AL, Hjelmeland LM. 2003. Age-related changes in the transcriptional profile of mouse RPE/choroid. Physiol Genomics 15:258–262 [DOI] [PubMed] [Google Scholar]
- 205. Schaefer AT, Larkum ME, Sakmann B, Roth A. 2003. Coincidence detection in pyramidal neurons is tuned by their dendritic branching pattern. J Neurophysiol 89:3143–3154 [DOI] [PubMed] [Google Scholar]
- 206. Jan YN, Jan LY. 2003. The control of dendrite development. Neuron 40:229–242 [DOI] [PubMed] [Google Scholar]
- 207. Singh KK, Park KJ, Hong EJ, Kramer BM, Greenberg ME, Kaplan DR, Miller FD. 2008. Developmental axon pruning mediated by BDNF-p75NTR-dependent axon degeneration. Nat Neurosci 11:649–658 [DOI] [PubMed] [Google Scholar]
- 208. Woronowicz A, Cawley NX, Chang SY, Koshimizu H, Phillips AW, Xiong ZG, Loh YP. 2010. Carboxypeptidase E knockout mice exhibit abnormal dendritic arborization and spine morphology in central nervous system neurons. J Neurosci Res 88:64–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Pokorný J, Trojan S. 1983. Chronic changes in the receptive field of the pyramidal cells of the rat hippocampus after intermittent postnatal hypoxia. Physiol Bohemoslov 32:393–402 [PubMed] [Google Scholar]
- 210. Pokorný J, Trojan S. 1986. The development of hippocampal structure and how it is influenced by hypoxia. Acta Univ Carol Med Monogr 113:1–79 [PubMed] [Google Scholar]
- 211. Groc L, Petanjek Z, Gustafsson B, Ben-Ari Y, Hanse E, Khazipov R. 2002. In vivo blockade of neural activity alters dendritic development of neonatal CA1 pyramidal cells. Eur J Neurosci 16:1931–1938 [DOI] [PubMed] [Google Scholar]
- 212. Silva-Gómez AB, Rojas D, Juárez I, Flores G. 2003. Decreased dendritic spine density on prefrontal cortical and hippocampal pyramidal neurons in postweaning social isolation rats. Brain Res 983:128–136 [DOI] [PubMed] [Google Scholar]
- 213. Parrish JZ, Emoto K, Kim MD, Jan YN. 2007. Mechanisms that regulate establishment, maintenance, and remodeling of dendritic fields. Annu Rev Neurosci 30:399–423 [DOI] [PubMed] [Google Scholar]
- 214. Cobos I, Calcagnotto ME, Vilaythong AJ, Thwin MT, Noebels JL, Baraban SC, Rubenstein JL. 2005. Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nat Neurosci 8:1059–1068 [DOI] [PubMed] [Google Scholar]
- 215. Hand R, Bortone D, Mattar P, Nguyen L, Heng JI, Guerrier S, Boutt E, Peters E, Barnes AP, Parras C, Schuurmans C, Guillemot F, Polleux F. 2005. Phosphorylation of neurogenin 2 specifies the migration properties and the dendritic morphology of pyramidal neurons in the neocortex. Neuron 48:45–62 [DOI] [PubMed] [Google Scholar]
- 216. Cobos I, Borello U, Rubenstein JL. 2007. Dlx transcription factors promote migration through repression of axon and dendrite growth. Neuron 54:873–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Lee KF, Bachman K, Landis S, Jaenisch R. 1994. Dependence on p75 for innervation of some sympathetic targets. Science 263:1447–1449 [DOI] [PubMed] [Google Scholar]
- 218. Kohn J, Aloyz RS, Toma JG, Haak-Frendscho M, Miller FD. 1999. Functionally antagonistic interactions between the TrkA and p75 neurotrophin receptors regulate sympathetic neuron growth and target innervation. J Neurosci 19:5393–5408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Singh KK, Miller FD. 2005. Activity regulates positive and negative neurotrophin-derived signals to determine axon competition. Neuron 45:837–845 [DOI] [PubMed] [Google Scholar]
- 220. Jaworski J, Sheng M. 2006. The growing role of mTOR in neuronal development and plasticity. Mol Neurobiol 34:205–219 [DOI] [PubMed] [Google Scholar]
- 221. Johnson EM, Craig ET, Yeh HH. 2007. TrkB is necessary for pruning at the climbing fibre-Purkinje cell synapse in the developing murine cerebellum. J Physiol 582:629–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Brzustowicz LM, Simone J, Mohseni P, Hayter JE, Hodgkinson KA, Chow EW, Bassett AS. 2004. Linkage disequilibrium mapping of schizophrenia susceptibility to the CAPON region of chromosome 1q22. Am J Hum Genet 74:1057–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Xu B, Wratten N, Charych EI, Buyske S, Firestein BL, Brzustowicz LM. 2005. Increased expression in dorsolateral prefrontal cortex of CAPON in schizophrenia and bipolar disorder. PLoS Med 2:e263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Zheng Y, Li H, Qin W, Chen W, Duan Y, Xiao Y, Li C, Zhang J, Li X, Feng G, He L. 2005. Association of the carboxyl-terminal PDZ ligand of neuronal nitric oxide synthase gene with schizophrenia in the Chinese Han population. Biochem Biophys Res Commun 328:809–815 [DOI] [PubMed] [Google Scholar]
- 225. Kremeyer B, García J, Kymäläinen H, Wratten N, Restrepo G, Palacio C, Miranda AL, López C, Restrepo M, Bedoya G, Brzustowicz LM, Ospina-Duque J, Arbeláez MP, Ruiz-Linares A. 2009. Evidence for a role of the NOS1AP (CAPON) gene in schizophrenia and its clinical dimensions: an association study in a South American population isolate. Hum Hered 67:163–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Wratten NS, Memoli H, Huang Y, Dulencin AM, Matteson PG, Cornacchia MA, Azaro MA, Messenger J, Hayter JE, Bassett AS, Buyske S, Millonig JH, Vieland VJ, Brzustowicz LM. 2009. Identification of a schizophrenia-associated functional noncoding variant in NOS1AP. Am J Psychiatry 166:434–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Carrel D, Du Y, Komlos D, Hadzimichalis NM, Kwon M, Wang B, Brzustowicz LM, Firestein BL. 2009. NOS1AP regulates dendrite patterning of hippocampal neurons through a carboxypeptidase E-mediated pathway. J Neurosci 29:8248–8258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Matsuzaki M. 2007. Factors critical for the plasticity of dendritic spines and memory storage. Neurosci Res 57:1–9 [DOI] [PubMed] [Google Scholar]
- 229. Bourne JN, Harris KM. 2008. Balancing structure and function at hippocampal dendritic spines. Annu Rev Neurosci 31:47–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. De Roo M, Klauser P, Garcia PM, Poglia L, Muller D. 2008. Spine dynamics and synapse remodeling during LTP and memory processes. Prog Brain Res 169:199–207 [DOI] [PubMed] [Google Scholar]
- 231. Jin K, Graham SH, Nagayama T, Goldsmith PC, Greenberg DA, Zhou A, Simon RP. 2001. Altered expression of the neuropeptide-processing enzyme carboxypeptidase E in the rat brain after global ischemia. J Cereb Blood Flow Metab 21:1422–1429 [DOI] [PubMed] [Google Scholar]
- 232. Zhou A, Minami M, Zhu X, Bae S, Minthorne J, Lan J, Xiong ZG, Simon RP. 2004. Altered biosynthesis of neuropeptide processing enzyme carboxypeptidase E after brain ischemia: molecular mechanism and implication. J Cereb Blood Flow Metab 24:612–622 [DOI] [PubMed] [Google Scholar]
- 233. Cawley NX, Woronowicz A, Koshimizu H, Gainer H, Loh YP. 2010. Carboxypeptidase E is neuroprotective against epileptic-like seizures in mouse hippocampal neurons. San Diego: Society for Neuroscience [Google Scholar]
- 234. Murthy SR, Thouennon E, Li W-S, Cheng Y, Bhupatkar J, Cawley NX, Lane M, Merchenthaler I, Loy YP. 2011. Glucocorticoid-induced neuroprotection of hippocampal neurons in stressed mice is mediated by carboxypeptidase E. J Neurosci (submitted) [Google Scholar]
- 235. Thouennon E, Murthy SRK, Li W, Bhupatkar J, Lane M, Merchenthaler I, Loh YP. Stress up-regulates the expression of the neuroprotective protein CPE, via the glucocorticoid pathway. Program of the 41st Annual Meeting of the Society for Neuroscience, Washington D.C., 2011 (Abstract 821.14/VV42) [Google Scholar]
- 236. Qian Y, Varlamov O, Fricker LD. 1999. Glu300 of rat carboxypeptidase E is essential for enzymatic activity but not substrate binding or routing to the regulated secretory pathway. J Biol Chem 274:11582–11586 [DOI] [PubMed] [Google Scholar]
- 237. Cheng Y, Cawley NX, Yanik T, Liu C, Murthy S, Loh Y. Carboxypeptidase E in neuroprotection: links to neurodegeneration and Alzheimer's disease. Program of 41st Annual Meeting of the Society for Neuroscience, Washington, D.C., 2011 (Abstract 48.01/O11) [Google Scholar]
- 238. Jeffrey KD, Alejandro EU, Luciani DS, Kalynyak TB, Hu X, Li H, Lin Y, Townsend RR, Polonsky KS, Johnson JD. 2008. Carboxypeptidase E mediates palmitate-induced β-cell ER stress and apoptosis. Proc Natl Acad Sci USA 105:8452–8457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Johnson JD. 2009. Proteomic identification of carboxypeptidase E connects lipid-induced β-cell apoptosis and dysfunction in type 2 diabetes. Cell Cycle 8:38–42 [DOI] [PubMed] [Google Scholar]
- 240. Dobkin RD, Leiblum SR, Rosen RC, Menza M, Marin H. 2006. Depression and sexual functioning in minority women: current status and future directions. J Sex Marital Ther 32:23–36 [DOI] [PubMed] [Google Scholar]
- 241. Wetsel WC, Rodriguiz RM, Wilkins JJ, Creson T, Biswas R, Berezniuk I, Fricker AD, Fricker LD. Emergence of anxiety-like behavior in depressive-like Cpefat/fat mice. Forty-seventh Annual Meeting of the American College of Neuropsychopharmacology, Scottsdale, AZ, 2008, (Poster 81) [Google Scholar]
- 242. Tollefson GD, Rosenbaum JF. 1998. Selective serotonin reuptake inhibitors. In: Schatzberg AF, Nemeroff CB, ed. Textbook of psychopharmacology. 2nd ed Washington, DC: American Psychiatric Press, Inc.; 219–237 [Google Scholar]
- 243. Zimmerman M, Chelminski I, McDermut W. 2002. Major depressive disorder and axis I diagnostic comorbidity. J Clin Psychiatry 63:187–193 [DOI] [PubMed] [Google Scholar]
- 244. Morris RG, Schenk F, Tweedie F, Jarrard LE. 1990. Ibotenate lesions of hippocampus and/or subiculum: dissociating components of allocentric spatial learning. Eur J Neurosci 2:1016–1028 [DOI] [PubMed] [Google Scholar]
- 245. Kogan JH, Frankland PW, Silva AJ. 2000. Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus 10:47–56 [DOI] [PubMed] [Google Scholar]
- 246. Dere E, Huston JP, De Souza Silva MA. 2007. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci Biobehav Rev 31:673–704 [DOI] [PubMed] [Google Scholar]
- 247. Murthy SR, Pacak K, Loh YP. 2010. Carboxypeptidase E: elevated expression correlated with tumor growth and metastasis in pheochromocytomas and other cancers. Cell Mol Neurobiol 30:1377–1381 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 248. Weber RG, Rieger J, Naumann U, Lichter P, Weller M. 2001. Chromosomal imbalances associated with response to chemotherapy and cytotoxic cytokines in human malignant glioma cell lines. Int J Cancer 91:213–218 [DOI] [PubMed] [Google Scholar]
- 249. Kim M, Gans JD, Nogueira C, Wang A, Paik JH, Feng B, Brennan C, Hahn WC, Cordon-Cardo C, Wagner SN, Flotte TJ, Duncan LM, Granter SR, Chin L. 2006. Comparative oncogenomics identifies NEDD9 as a melanoma metastasis gene. Cell 125:1269–1281 [DOI] [PubMed] [Google Scholar]
- 250. Azzoni C, D'Adda T, Tamburrano G, Coscelli C, Madsen OD, Scopsi L, Bordi C. 1998. Functioning human insulinomas. An immunohistochemical analysis of intracellular insulin processing. Virchows Arch 433:495–504 [DOI] [PubMed] [Google Scholar]
- 251. Höcker M, John M, Anagnostopoulos J, Buhr HJ, Solimena M, Gasnier B, Henry JP, Wang TC, Wiedenmann B. 1999. Molecular dissection of regulated secretory pathways in human gastric enterochromaffin-like cells: an immunohistochemical analysis. Histochem Cell Biol 112:205–214 [DOI] [PubMed] [Google Scholar]
- 252. Fan X, Olson SJ, Blevins LS, Allen GS, Johnson MD. 2002. Immunohistochemical localization of carboxypeptidases D, E, and Z in pituitary adenomas and normal human pituitary. J Histochem Cytochem 50:1509–1516 [DOI] [PubMed] [Google Scholar]
- 253. North WG, Du J. 1998. Key peptide processing enzymes are expressed by a variant form of small-cell carcinoma of the lung. Peptides 19:1743–1747 [DOI] [PubMed] [Google Scholar]
- 254. He P, Varticovski L, Bowman ED, Fukuoka J, Welsh JA, Miura K, Jen J, Gabrielson E, Brambilla E, Travis WD, Harris CC. 2004. Identification of carboxypeptidase E and γ-glutamyl hydrolase as biomarkers for pulmonary neuroendocrine tumors by cDNA microarray. Hum Pathol 35:1196–1209 [DOI] [PubMed] [Google Scholar]
- 255. Rozengurt E. 2002. Neuropeptides as growth factors for normal and cancerous cells. Trends Endocrinol Metab 13:128–134 [DOI] [PubMed] [Google Scholar]
- 256. Morris DG, Musat M, Czirják S, Hanzély Z, Lillington DM, Korbonits M, Grossman AB. 2005. Differential gene expression in pituitary adenomas by oligonucleotide array analysis. Eur J Endocrinol 153:143–151 [DOI] [PubMed] [Google Scholar]
- 257. Tatsumi KI, Tanaka S, Takano T, Tahara S, Murakami Y, Takao T, Hashimoto K, Kato Y, Teramoto A, Amino N. 2003. Frequent appearance of autoantibodies against prohormone convertase 1/3 and neuroendocrine protein 7B2 in patients with nonfunctioning pituitary macroadenoma. Endocrine 22:335–340 [DOI] [PubMed] [Google Scholar]
- 258. DeLellis RA, Lloyd RV, Heitz PU, Eng C. 2004. Tumours of endocrine organs. Lyon, France: IARC Press [Google Scholar]
- 259. Manger WM, Eisenhofer G. 2004. Pheochromocytoma: diagnosis and management update. Curr Hypertens Rep 6:477–484 [DOI] [PubMed] [Google Scholar]
- 260. Pacak K, Lenders JWM, Eisenhofer G. 2007. Pheochromocytoma: diagnosis, localization and treatment. Malden, MA: Blackwell Publishing [Google Scholar]
- 261. Eisenhofer G, Pecorella W, Pacak K, Hooper D, Kopin IJ, Goldstein DS. 1994. The neuronal and extraneuronal origins of plasma 3-methoxy-4-hydroxyphenylglycol in rats. J Auton Nerv Syst 50:93–107 [DOI] [PubMed] [Google Scholar]
- 262. Eisenhofer G, Keiser H, Friberg P, Mezey E, Huynh TT, Hiremagalur B, Ellingson T, Duddempudi S, Eijsbouts A, Lenders JW. 1998. Plasma metanephrines are markers of pheochromocytoma produced by catechol-O-methyltransferase within tumors. J Clin Endocrinol Metab 83:2175–2185 [DOI] [PubMed] [Google Scholar]
- 263. van Duinen N, Steenvoorden D, Kema IP, Jansen JC, Vriends AH, Bayley JP, Smit JW, Romijn JA, Corssmit EP. 2010. Increased urinary excretion of 3-methoxytyramine in patients with head and neck paragangliomas. J Clin Endocrinol Metab 95:209–214 [DOI] [PubMed] [Google Scholar]
- 264. Eisenhofer G, Lenders JW, Timmers H, Mannelli M, Grebe SK, Hofbauer LC, Bornstein SR, Tiebel O, Adams K, Bratslavsky G, Linehan WM, Pacak K. 2011. Measurements of plasma methoxytyramine, normetanephrine, and metanephrine as discriminators of different hereditary forms of pheochromocytoma. Clin Chem 57:411–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Eisenhofer G, Lenders JW, Linehan WM, Walther MM, Goldstein DS, Keiser HR. 1999. Plasma normetanephrine and metanephrine for detecting pheochromocytoma in von Hippel-Lindau disease and multiple endocrine neoplasia type 2. N Engl J Med 340:1872–1879 [DOI] [PubMed] [Google Scholar]
- 266. Lenders JW, Pacak K, Walther MM, Linehan WM, Mannelli M, Friberg P, Keiser HR, Goldstein DS, Eisenhofer G. 2002. Biochemical diagnosis of pheochromocytoma: which test is best? JAMA 287:1427–1434 [DOI] [PubMed] [Google Scholar]
- 267. Sawka AM, Jaeschke R, Singh RJ, Young WF., Jr 2003. A comparison of biochemical tests for pheochromocytoma: measurement of fractionated plasma metanephrines compared with the combination of 24-hour urinary metanephrines and catecholamines. J Clin Endocrinol Metab 88:553–558 [DOI] [PubMed] [Google Scholar]
- 268. Sawka AM, Gafni A, Thabane L, Young WF., Jr 2004. The economic implications of three biochemical screening algorithms for pheochromocytoma. J Clin Endocrinol Metab 89:2859–2866 [DOI] [PubMed] [Google Scholar]
- 269. Unger N, Pitt C, Schmidt IL, Walz MK, Schmid KW, Philipp T, Mann K, Petersenn S. 2006. Diagnostic value of various biochemical parameters for the diagnosis of pheochromocytoma in patients with adrenal mass. Eur J Endocrinol 154:409–417 [DOI] [PubMed] [Google Scholar]
- 270. Grouzmann E, Drouard-Troalen L, Baudin E, Plouin PF, Muller B, Grand D, Buclin T. 2010. Diagnostic accuracy of free and total metanephrines in plasma and fractionated metanephrines in urine of patients with pheochromocytoma. Eur J Endocrinol 162:951–960 [DOI] [PubMed] [Google Scholar]
- 271. Sutton MG, Sheps SG, Lie JT. 1981. Prevalence of clinically unsuspected pheochromocytoma. Review of a 50-year autopsy series. Mayo Clin Proc 56:354–360 [PubMed] [Google Scholar]
- 272. McNeil AR, Blok BH, Koelmeyer TD, Burke MP, Hilton JM. 2000. Phaeochromocytomas discovered during coronial autopsies in Sydney, Melbourne and Auckland. Aust N Z J Med 30:648–652 [DOI] [PubMed] [Google Scholar]
- 273. Amar L, Baudin E, Burnichon N, Peyrard S, Silvera S, Bertherat J, Bertagna X, Schlumberger M, Jeunemaitre X, Gimenez-Roqueplo AP, Plouin PF. 2007. Succinate dehydrogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. J Clin Endocrinol Metab 92:3822–3828 [DOI] [PubMed] [Google Scholar]
- 274. Young WF., Jr 2007. Clinical practice. The incidentally discovered adrenal mass. N Engl J Med 356:601–610 [DOI] [PubMed] [Google Scholar]
- 275. Cascón A, Pita G, Burnichon N, Landa I, López-Jiménez E, Montero-Conde C, Leskelä S, Leandro-García LJ, Letón R, Rodríguez-Antona C, Díaz JA, López-Vidriero E, González-Neira A, Velasco A, Matias-Guiu X, Gimenez-Roqueplo AP, Robledo M. 2009. Genetics of pheochromocytoma and paraganglioma in Spanish patients. J Clin Endocrinol Metab 94:1701–1705 [DOI] [PubMed] [Google Scholar]
- 276. O'Riordain DS, Young WF, Jr, Grant CS, Carney JA, van Heerden JA. 1996. Clinical spectrum and outcome of functional extraadrenal paraganglioma. World J Surg 20:916–921; discussion 922 [DOI] [PubMed] [Google Scholar]
- 277. Mannelli M, Ianni L, Cilotti A, Conti A. 1999. Pheochromocytoma in Italy: a multicentric retrospective study. Eur J Endocrinol 141:619–624 [DOI] [PubMed] [Google Scholar]
- 278. Park J, Song C, Park M, Yoo S, Park SJ, Hong S, Hong B, Kim CS, Ahn H. 2011. Predictive characteristics of malignant pheochromocytoma. Korean J Urol 52:241–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Zelinka T, Musil Z, Dušková J, Burton D, Merino MJ, Milosevic D, Widimský J, Jr, Pacak K. 2011. Metastatic pheochromocytoma: does the size and age matter? Eur J Clin Invest 41:1121–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Neumann HP, Bausch B, McWhinney SR, Bender BU, Gimm O, Franke G, Schipper J, Klisch J, Altehoefer C, Zerres K, Januszewicz A, Eng C, Smith WM, Munk R, Manz T, Glaesker S, Apel TW, Treier M, Reineke M, Walz MK, Hoang-Vu C, Brauckhoff M, Klein-Franke A, Klose P, Schmidt H, Maier-Woelfle M, Peçzkowska M, Szmigielski C, Eng C. 2002. Germ-line mutations in nonsyndromic pheochromocytoma. N Engl J Med 346:1459–1466 [DOI] [PubMed] [Google Scholar]
- 281. Benn DE, Gimenez-Roqueplo AP, Reilly JR, Bertherat J, Burgess J, Byth K, Croxson M, Dahia PL, Elston M, Gimm O, Henley D, Herman P, Murday V, Niccoli-Sire P, Pasieka JL, Rohmer V, Tucker K, Jeunemaitre X, Marsh DJ, Plouin PF, Robinson BG. 2006. Clinical presentation and penetrance of pheochromocytoma/paraganglioma syndromes. J Clin Endocrinol Metab 91:827–836 [DOI] [PubMed] [Google Scholar]
- 282. Plouin PF, Gimenez-Roqueplo AP. 2006. The genetic basis of pheochromocytoma: who to screen and how? Nat Clin Pract Endocrinol Metab 2:60–61 [DOI] [PubMed] [Google Scholar]
- 283. Petri BJ, van Eijck CH, de Herder WW, Wagner A, de Krijger RR. 2009. Phaeochromocytomas and sympathetic paragangliomas. Br J Surg 96:1381–1392 [DOI] [PubMed] [Google Scholar]
- 284. Bayley JP, Kunst HP, Cascon A, Sampietro ML, Gaal J, Korpershoek E, Hinojar-Gutierrez A, Timmers HJ, Hoefsloot LH, Hermsen MA, Suárez C, Hussain AK, Vriends AH, Hes FJ, Jansen JC, Tops CM, Corssmit EP, de Knijff P, Lenders JW, Cremers CW, Devilee P, Dinjens WN, de Krijger RR, Robledo M. 2010. SDHAF2 mutations in familial and sporadic paraganglioma and phaeochromocytoma. Lancet Oncol 11:366–372 [DOI] [PubMed] [Google Scholar]
- 285. Lenders JW, Eisenhofer G, Mannelli M, Pacak K. 2005. Phaeochromocytoma. Lancet 366:665–675 [DOI] [PubMed] [Google Scholar]
- 286. Müller U, Troidl C, Niemann S. 2005. SDHC mutations in hereditary paraganglioma/pheochromocytoma. Fam Cancer 4:9–12 [DOI] [PubMed] [Google Scholar]
- 287. Schiavi F, Savvoukidis T, Trabalzini F, Grego F, Piazza M, Amistà P, Demattè S, Del Piano A, Cecchini ME, Erlic Z, De Lazzari P, Mantero F, Opocher G. 2006. Paraganglioma syndrome: SDHB, SDHC, and SDHD mutations in head and neck paragangliomas. Ann NY Acad Sci 1073:190–197 [DOI] [PubMed] [Google Scholar]
- 288. Burnichon N, Brière JJ, Libé R, Vescovo L, Rivière J, Tissier F, Jouanno E, Jeunemaitre X, Bénit P, Tzagoloff A, Rustin P, Bertherat J, Favier J, Gimenez-Roqueplo AP. 2010. SDHA is a tumor suppressor gene causing paraganglioma. Hum Mol Genet 19:3011–3020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Qin Y, Yao L, King EE, Buddavarapu K, Lenci RE, Chocron ES, Lechleiter JD, Sass M, Aronin N, Schiavi F, Boaretto F, Opocher G, Toledo RA, Toledo SP, Stiles C, Aguiar RC, Dahia PL. 2010. Germline mutations in TMEM127 confer susceptibility to pheochromocytoma. Nat Genet 42:229–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Yao L, Schiavi F, Cascon A, Qin Y, Inglada-Pérez L, King EE, Toledo RA, Ercolino T, Rapizzi E, Ricketts CJ, Mori L, Giacchè M, Mendola A, Taschin E, Boaretto F, Loli P, Iacobone M, Rossi GP, Biondi B, Lima-Junior JV, Kater CE, Bex M, Vikkula M, Grossman AB, Gruber SB, Barontini M, Persu A, Castellano M, Toledo SP, Maher ER, Mannelli M, Opocher G, Robledo M, Dahia PL. 2010. Spectrum and prevalence of FP/TMEM127 gene mutations in pheochromocytomas and paragangliomas. JAMA 304:2611–2619 [DOI] [PubMed] [Google Scholar]
- 291. Neumann HP, Sullivan M, Winter A, Malinoc A, Hoffmann MM, Boedeker CC, Bertz H, Walz MK, Moeller LC, Schmid KW, Eng C. 2011. Germline mutations of the TMEM127 gene in patients with paraganglioma of head and neck and extraadrenal abdominal sites. J Clin Endocrinol Metab 96:E1279–E1282 [DOI] [PubMed] [Google Scholar]
- 292. Krawczak M, Reiss J, Cooper DN. 1992. The mutational spectrum of single base-pair substitutions in mRNA splice junctions of human genes: causes and consequences. Hum Genet 90:41–54 [DOI] [PubMed] [Google Scholar]
- 293. Martin N, Boomsma D, Machin G. 1997. A twin-pronged attack on complex traits. Nat Genet 17:387–3929398838 [Google Scholar]
- 294. Halushka MK, Fan JB, Bentley K, Hsie L, Shen N, Weder A, Cooper R, Lipshutz R, Chakravarti A. 1999. Patterns of single-nucleotide polymorphisms in candidate genes for blood-pressure homeostasis. Nat Genet 22:239–247 [DOI] [PubMed] [Google Scholar]
- 295. Lohrer HD, Tangen U. 2000. Investigations into the molecular effects of single nucleotide polymorphism. Pathobiology 68:283–290 [DOI] [PubMed] [Google Scholar]
- 296. LeVan TD, Bloom JW, Bailey TJ, Karp CL, Halonen M, Martinez FD, Vercelli D. 2001. A common single nucleotide polymorphism in the CD14 promoter decreases the affinity of Sp protein binding and enhances transcriptional activity. J Immunol 167:5838–5844 [DOI] [PubMed] [Google Scholar]
- 297. Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, Fingerlin TE, Zhang W, Gudmundsson G, Groshong SD, Evans CM, Garantziotis S, Adler KB, Dickey BF, du Bois RM, Yang IV, Herron A, Kervitsky D, Talbert JL, Markin C, Park J, Crews AL, Slifer SH, Auerbach S, Roy MG, Lin J, Hennessy CE, Schwarz MI, Schwartz DA. 2011. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med 364:1503–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Sonuga-Barke EJ, Kumsta R, Schlotz W, Lasky-Su J, Marco R, Miranda A, Mulas F, Oades RD, Banaschewski T, Mueller U, Andreou P, Christiansen H, Gabriels I, Uebel H, Kuntsi J, Franke B, Buitelaar J, Ebstein R, Gill M, Anney R, Roeyers H, Rothenberger A, Sergeant J, Steinhausen HC, Asherson P, Faraone SV. 2011. A functional variant of the serotonin transporter gene (SLC6A4) moderates impulsive choice in attention deficit/hyperactivity disorder boys and siblings. Biol Psychiatry 70:230–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Fritsche LG, Loenhardt T, Janssen A, Fisher SA, Rivera A, Keilhauer CN, Weber BH. 2008. Age-related macular degeneration is associated with an unstable ARMS2 (LOC387715) mRNA. Nat Genet 40:892–896 [DOI] [PubMed] [Google Scholar]
- 300. Magyar I, Colman D, Arnold E, Baumgartner D, Bottani A, Fokstuen S, Addor MC, Berger W, Carrel T, Steinmann B, Mátyás G. 2009. Quantitative sequence analysis of FBN1 premature termination codons provides evidence for incomplete NMD in leukocytes. Hum Mutat 30:1355–1364 [DOI] [PubMed] [Google Scholar]
- 301. Pointon JJ, Lok CY, Shearman JD, Suckling RJ, Rochette J, Merryweather-Clarke AT, Robson KJ. 2009. A novel HFE mutation (c.del478) results in nonsense-mediated decay of the mutant transcript in a hemochromatosis patient. Blood Cells Mol Dis 43:194–198 [DOI] [PubMed] [Google Scholar]
- 302. Shin JA, Chang HS, Park SM, Jang AS, Park SW, Park JS, Uh ST, Il Lim G, Rhim T, Kim MK, Choi IS, Chung IY, Park BL, Shin HD, Park CS. 2009. Genetic effect of CysLTR2 polymorphisms on its mRNA synthesis and stabilization. BMC Med Genet 10:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Hoffmann M, Schirmer MA, Tzvetkov MV, Kreuz M, Ziepert M, Wojnowski L, Kube D, Pfreundschuh M, Trümper L, Loeffler M, Brockmöller J. 2010. A functional polymorphism in the NAD(P)H oxidase subunit CYBA is related to gene expression, enzyme activity, and outcome in non-Hodgkin lymphoma. Cancer Res 70:2328–2338 [DOI] [PubMed] [Google Scholar]
- 304. Garcia-Rostan G, Tallini G, Herrero A, D'Aquila TG, Carcangiu ML, Rimm DL. 1999. Frequent mutation and nuclear localization of β-catenin in anaplastic thyroid carcinoma. Cancer Res 59:1811–1815 [PubMed] [Google Scholar]
- 305. Kammerer S, Burns-Hamuro LL, Ma Y, Hamon SC, Canaves JM, Shi MM, Nelson MR, Sing CF, Cantor CR, Taylor SS, Braun A. 2003. Amino acid variant in the kinase binding domain of dual-specific A kinase-anchoring protein 2: a disease susceptibility polymorphism. Proc Natl Acad Sci USA 100:4066–4071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Bohren KM, Nadkarni V, Song JH, Gabbay KH, Owerbach D. 2004. A M55V polymorphism in a novel SUMO gene (SUMO-4) differentially activates heat shock transcription factors and is associated with susceptibility to type I diabetes mellitus. J Biol Chem 279:27233–27238 [DOI] [PubMed] [Google Scholar]
- 307. Leskelä TT, Markkanen PM, Alahuhta IA, Tuusa JT, Petäjä-Repo UE. 2009. Phe27Cys polymorphism alters the maturation and subcellular localization of the human δ opioid receptor. Traffic 10:116–129 [DOI] [PubMed] [Google Scholar]
- 308. Chen K, Song F, Calin GA, Wei Q, Hao X, Zhang W. 2008. Polymorphisms in microRNA targets: a gold mine for molecular epidemiology. Carcinogenesis 29:1306–1311 [DOI] [PubMed] [Google Scholar]
- 309. Saunders MA, Liang H, Li WH. 2007. Human polymorphism at microRNAs and microRNA target sites. Proc Natl Acad Sci USA 104:3300–3305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Hall C, Manser E, Spurr NK, Lim L. 1993. Assignment of the human carboxypeptidase E (CPE) gene to chromosome 4. Genomics 15:461–463 [DOI] [PubMed] [Google Scholar]
- 311. Utsunomiya N, Ohagi S, Sanke T, Tatsuta H, Hanabusa T, Nanjo K. 1998. Organization of the human carboxypeptidase E gene and molecular scanning for mutations in Japanese subjects with NIDDM or obesity. Diabetologia 41:701–705 [DOI] [PubMed] [Google Scholar]
- 312. Chen H, Jawahar S, Qian Y, Duong Q, Chan G, Parker A, Meyer JM, Moore KJ, Chayen S, Gross DJ, Glaser B, Permutt MA, Fricker LD. 2001. Missense polymorphism in the human carboxypeptidase E gene alters enzymatic activity. Hum Mutat 18:120–131 [DOI] [PubMed] [Google Scholar]
- 313. Jia EZ, Wang J, Yang ZJ, Zhu TB, Wang LS, Chen B, Cao KJ, Huang J, Ma WZ. 2008. Molecular scanning of the human carboxypeptidase E gene for mutations in Chinese subjects with coronary atherosclerosis. Mol Cell Biochem 307:31–39 [DOI] [PubMed] [Google Scholar]
- 314. Wang J, Zhang Y, Yang ZJ, Zhu TB, Wang LS, Chen B, Cao KJ, Huang J, Ma WZ, Jia EZ. 2008. Association of human carboxypeptidase E exon5 gene polymorphisms with angiographical characteristics of coronary atherosclerosis in a Chinese population. Acta Pharmacol Sin 29:736–744 [DOI] [PubMed] [Google Scholar]
- 315. Jia EZ, Wang J, Yang ZJ, Zhu TB, Wang LS, Wang H, Li CJ, Chen B, Cao KJ, Huang J, Ma WZ. 2009. Association of the mutation for the human carboxypeptidase E gene exon 4 with the severity of coronary artery atherosclerosis. Mol Biol Rep 36:245–254 [DOI] [PubMed] [Google Scholar]
- 316. Gabbay KH, DeLuca K, Fisher JN, Jr, Mako ME, Rubenstein AH. 1976. Familial hyperproinsulinemia. An autosomal dominant defect. N Engl J Med 294:911–915 [DOI] [PubMed] [Google Scholar]
- 317. Chan SJ, Seino S, Gruppuso PA, Schwartz R, Steiner DF. 1987. A mutation in the B chain coding region is associated with impaired proinsulin conversion in a family with hyperproinsulinemia. Proc Natl Acad Sci USA 84:2194–2197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Carroll RJ, Hammer RE, Chan SJ, Swift HH, Rubenstein AH, Steiner DF. 1988. A mutant human proinsulin is secreted from islets of Langerhans in increased amounts via an unregulated pathway. Proc Natl Acad Sci USA 85:8943–8947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Wang XC, Xu SY, Wu XY, Song HD, Mao YF, Fan HY, Yu F, Mou B, Gu YY, Xu LQ, Zhou XO, Chen Z, Chen JL, Hu RM. 2004. Gene expression profiling in human insulinoma tissue: genes involved in the insulin secretion pathway and cloning of novel full-length cDNAs. Endocr Relat Cancer 11:295–303 [DOI] [PubMed] [Google Scholar]
- 320. Polastri L, Galbiati F, Folli F, Davalli AM. 2002. Effects of carboxypeptidase E overexpression on insulin mRNA levels, regulated insulin secretion, and proinsulin processing of pituitary GH3 cells transfected with a furin-cleavable human proinsulin cDNA. Cell Transplant 11:803–811 [PubMed] [Google Scholar]
- 321. Zhou ZG, Yang L, Huang G. 2003. Diagnostic value of carboxypeptidase-H autoantibodies in detecting latent autoimmune diabetes in adults. Hunan Yi Ke Da Xue Xue Bao 28:549–552 [PubMed] [Google Scholar]
- 322. Li LR, Zhou ZG, Huang G, Yang L, Li X, Chen XY, He L. 2005. [Diagnostic role of SOX13 antibody in latent autoimmune diabetes of adults]. Zhonghua Yi Xue Za Zhi 85:235–239 [PubMed] [Google Scholar]
- 323. Zhang D, Zhou Z, Li L, Weng J, Huang G, Jing P, Zhang C, Peng J, Xiu L. 2006. Islet autoimmunity and genetic mutations in Chinese subjects initially thought to have type 1B diabetes. Diabet Med 23:67–71 [DOI] [PubMed] [Google Scholar]
- 324. Yang L, Zhou ZG, Tan SZ, Huang G, Jin P, Yan X, Li X, Peng H, Hagopian W. 2008. Carboxypeptidase-H autoantibodies differentiate a more latent subset of autoimmune diabetes from phenotypic type 2 diabetes among Chinese adults. Ann NY Acad Sci 1150:263–266 [DOI] [PubMed] [Google Scholar]
- 325. Ibrahim SM, Mix E, Böttcher T, Koczan D, Gold R, Rolfs A, Thiesen HJ. 2001. Gene expression profiling of the nervous system in murine experimental autoimmune encephalomyelitis. Brain 124:1927–1938 [DOI] [PubMed] [Google Scholar]
- 326. Mazón Peláez I, Vogler S, Strauss U, Wernhoff P, Pahnke J, Brockmann G, Moch H, Thiesen HJ, Rolfs A, Ibrahim SM. 2005. Identification of quantitative trait loci controlling cortical motor evoked potentials in experimental autoimmune encephalomyelitis: correlation with incidence, onset and severity of disease. Hum Mol Genet 14:1977–1989 [DOI] [PubMed] [Google Scholar]
- 327. Stahl S, Reinders Y, Asan E, Mothes W, Conzelmann E, Sickmann A, Felbor U. 2007. Proteomic analysis of cathepsin B- and L-deficient mouse brain lysosomes. Biochim Biophys Acta 1774:1237–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Castresana J. 2000. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol 17:540–552 [DOI] [PubMed] [Google Scholar]
- 329. Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Lee NK, Karsenty G. 2008. Reciprocal regulation of bone and energy metabolism. Trends Endocrinol Metab 19:161–166 [DOI] [PubMed] [Google Scholar]
- 331. Evans JF, Niu QT, Canas JA, Shen CL, Aloia JF, Yeh JK. 2004. ACTH enhances chondrogenesis in multipotential progenitor cells and matrix production in chondrocytes. Bone 35:96–107 [DOI] [PubMed] [Google Scholar]
- 332. Zhong Q, Sridhar S, Ruan L, Ding KH, Xie D, Insogna K, Kang B, Xu J, Bollag RJ, Isales CM. 2005. Multiple melanocortin receptors are expressed in bone cells. Bone 36:820–831 [DOI] [PubMed] [Google Scholar]
- 333. Fu Y, Luo N, Klein RL, Garvey WT. 2005. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J Lipid Res 46:1369–1379 [DOI] [PubMed] [Google Scholar]
- 334. Elefteriou F, Takeda S, Ebihara K, Magre J, Patano N, Kim CA, Ogawa Y, Liu X, Ware SM, Craigen WJ, Robert JJ, Vinson C, Nakao K, Capeau J, Karsenty G. 2004. Serum leptin level is a regulator of bone mass. Proc Natl Acad Sci USA 101:3258–3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G. 2005. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 434:514–520 [DOI] [PubMed] [Google Scholar]
- 336. Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. 1998. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93:165–176 [DOI] [PubMed] [Google Scholar]
- 337. Bae S, Ha TS, Yoon Y, Lee J, Cha HJ, Yoo H, Choe TB, Li S, Sohn I, Kim JY, Kim CS, Jin HO, Lee HC, Park IC, Kim CS, Jin YW, Ahn SK. 2008. Genome-wide screening and identification of novel proteolytic cleavage targets of caspase-8 and -10 in vitro. Int J Mol Med 21:381–386 [PubMed] [Google Scholar]
- 338. Bogan RL, Murphy MJ, Stouffer RL, Hennebold JD. 2008. Systematic determination of differential gene expression in the primate corpus luteum during the luteal phase of the menstrual cycle. Mol Endocrinol 22:1260–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Chikuma T, Inomata Y, Tsuchida K, Hojo H, Kato T. 2002. Effect of monensin on the levels of tachykinins and their processing enzyme activity in rat dorsal root ganglia. Neurosci Lett 326:89–92 [DOI] [PubMed] [Google Scholar]
- 340. Cook JL, Giardina JF, Zhang Z, Re RN. 2002. Intracellular angiotensin II increases the long isoform of PDGF mRNA in rat hepatoma cells. J Mol Cell Cardiol 34:1525–1537 [DOI] [PubMed] [Google Scholar]
- 341. Dong W, Fricker LD, Day R. 1999. Carboxypeptidase D is a potential candidate to carry out redundant processing functions of carboxypeptidase E based on comparative distribution studies in the rat central nervous system. Neuroscience 89:1301–1317 [DOI] [PubMed] [Google Scholar]
- 342. Du J, Keegan BP, North WG. 2001. Key peptide processing enzymes are expressed by breast cancer cells. Cancer Lett 165:211–218 [DOI] [PubMed] [Google Scholar]
- 343. Grigoriants O, Devi L, Fricker LD. 1993. Dopamine antagonist haloperidol increases carboxypeptidase E mRNA in rat neurointermediate pituitary but not in various other rat tissues. Brain Res Mol Brain Res 19:161–164 [DOI] [PubMed] [Google Scholar]
- 344. Guyonnet B, Marot G, Dacheux JL, Mercat MJ, Schwob S, Jaffrézic F, Gatti JL. 2009. The adult boar testicular and epididymal transcriptomes. BMC Genomics 10:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Haegeman A, Williams JL, Law A, Van Zeveren A, Peelman LJ. 2003. Mapping and SNP analysis of bovine candidate genes for meat and carcass quality. Anim Genet 34:349–353 [DOI] [PubMed] [Google Scholar]
- 346. Ishizuka N, Minami K, Okumachi A, Okuno M, Seino S. 2007. Induction by NeuroD of the components required for regulated exocytosis. Biochem Biophys Res Commun 354:271–277 [DOI] [PubMed] [Google Scholar]
- 347. Johnston RA, Theman TA, Shore SA. 2006. Augmented responses to ozone in obese carboxypeptidase E-deficient mice. Am J Physiol Regul Integr Comp Physiol 290:R126–R133 [DOI] [PubMed] [Google Scholar]
- 348. Johnston RA, Zhu M, Hernandez CB, Williams ES, Shore SA. 2010. Onset of obesity in carboxypeptidase E-deficient mice and effect on airway responsiveness and pulmonary responses to ozone. J Appl Physiol 108:1812–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Maresh JG, Xu H, Jiang N, Gairola CG, Shohet RV. 2005. Tobacco smoke dysregulates endothelial vasoregulatory transcripts in vivo. Physiol Genomics 21:308–313 [DOI] [PubMed] [Google Scholar]
- 350. Marquínez AC, Andreetta AM, González N, Wolfenstein-Todel C, Scacciati de Cerezo JM. 2003. Identification of gp17 glycoprotein and characterization of prostatic acid phosphatase (PAP) and carboxypeptidase E (CPE) fragments in a human seminal plasma fraction interacting with concanavalin A. J Protein Chem 22:423–429 [DOI] [PubMed] [Google Scholar]
- 351. Mousa SA, Shakibaei M, Sitte N, Schäfer M, Stein C. 2004. Subcellular pathways of β-endorphin synthesis, processing, and release from immunocytes in inflammatory pain. Endocrinology 145:1331–1341 [DOI] [PubMed] [Google Scholar]
- 352. Mukhina ES, Saldaev DA, Vernigora AN, Gengin MT. 2005. [The effect of ethanol consumption by dams on the offspring locomotion in the open field test and carboxypeptidase activities in the rat brain and adrenal medulla]. Ross Fiziol Zh Im I M Sechenova 91:239–245 [PubMed] [Google Scholar]
- 353. Nakajima E, David LL, Riviere MA, Azuma M, Shearer TR. 2009. Human and monkey lenses cultured with calcium ionophore form αB-crystallin lacking the C-terminal lysine, a prominent feature of some human cataracts. Invest Ophthalmol Vis Sci 50:5828–5836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Pilot-Storck F, Chopin E, Rual JF, Baudot A, Dobrokhotov P, Robinson-Rechavi M, Brun C, Cusick ME, Hill DE, Schaeffer L, Vidal M, Goillot E. 2010. Interactome mapping of the phosphatidylinositol 3-kinase-mammalian target of rapamycin pathway identifies deformed epidermal autoregulatory factor-1 as a new glycogen synthase kinase-3 interactor. Mol Cell Proteomics 9:1578–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Prasad SS, Cynader MS. 1994. Identification of cDNA clones expressed selectively during the critical period for visual cortex development by subtractive hybridization. Brain Res 639:73–84 [DOI] [PubMed] [Google Scholar]
- 356. Rawdon BB, Larsson LI. 2000. Development of hormonal peptides and processing enzymes in the embryonic avian pancreas with special reference to co-localisation. Histochem Cell Biol 114:105–112 [DOI] [PubMed] [Google Scholar]
- 357. Reznik SE, Salafia CM, Lage JM, Fricker LD. 1998. Immunohistochemical localization of carboxypeptidases E and D in the human placenta and umbilical cord. J Histochem Cytochem 46:1359–1368 [DOI] [PubMed] [Google Scholar]
- 358. Sasson R, Dantes A, Tajima K, Amsterdam A. 2003. Novel genes modulated by FSH in normal and immortalized FSH-responsive cells: new insights into the mechanism of FSH action. FASEB J 17:1256–1266 [DOI] [PubMed] [Google Scholar]
- 359. Sekiguchi T, Kawashima T, Satou Y, Satoh N. 2007. Further EST analysis of endocrine genes that are preferentially expressed in the neural complex of Ciona intestinalis: receptor and enzyme genes associated with endocrine system in the neural complex. Gen Comp Endocrinol 150:233–245 [DOI] [PubMed] [Google Scholar]
- 360. Singh U, Yu Y, Kalinina E, Konno T, Sun T, Ohta H, Wakayama T, Soares MJ, Hemberger M, Fundele RH. 2006. Carboxypeptidase E in the mouse placenta. Differentiation 74:648–660 [DOI] [PubMed] [Google Scholar]
- 361. Tang SS, Zhang JH, Liu HX, Li HZ. 2009. PC2/CPE-mediated pro-protein processing in tumor cells and its differentiated cells or tissues. Mol Cell Endocrinol 303:43–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Tang SS, Zhang JH, Liu HX, Zhou D, Qi R. 2009. Pro-protein convertase-2/carboxypeptidase-E mediated neuropeptide processing of RGC-5 cell after in vitro ischemia. Neurosci Bull 25:7–14 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 363. Vilijn MH, Das B, Kessler JA, Fricker LD. 1989. Cultured astrocytes and neurons synthesize and secrete carboxypeptidase E, a neuropeptide-processing enzyme. J Neurochem 53:1487–1493 [DOI] [PubMed] [Google Scholar]
- 364. Wang X, Huynh H, Gjörloff-Wingren A, Monosov E, Stridsberg M, Fukuda M, Mustelin T. 2002. Enlargement of secretory vesicles by protein tyrosine phosphatase PTP-MEG2 in rat basophilic leukemia mast cells and Jurkat T cells. J Immunol 168:4612–4619 [DOI] [PubMed] [Google Scholar]
- 365. Eisenhofer G, Lenders JW, Siegert G, Bornstein SR, Friberg P, Milosevic D, Mannelli M, Linehan WM, Adams K, Timmers HJ, Pacak K. Plasma methoxytyramine: A novel biomarker of metastatic pheochromocytoma and paraganglioma in relation to established risk factors of tumour size, location and SDHB mutation status. Eur J Cancer Oct 28, 2011. 10.1016/j.ejca.2011.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]