Abstract
Biological, genetic, and clinical data provide compelling proof for N-type voltage-gated calcium channels (CaV2.2) as therapeutic targets for chronic pain. While decreasing channel function is ultimately anti-nociceptive, directly targeting the channel can lead to multiple adverse effects. Targeting regulators of channel activity may facilitate improved analgesic properties associated with channel block and afford a broader therapeutic window. Towards this end, we recently identified a short peptide, designated CBD3, derived from collapsin response mediator protein 2 (CRMP-2) that suppressed inflammatory and neuropathic hypersensitivity by inhibiting CRMP-2 binding to CaV2.2 [Brittain et al., Nature Medicine 17:822–829 (2011)]. Rodents administered CBD3 intraperitoneally, fused to the HIV TAT protein cell penetrating domain, exhibited antinociception lasting ~4 hours highlighting potential instability, limited oral bioavailability, and/or rapid elimination of peptide. This report focuses on improving upon the parental CBD3 peptide. Using SPOTScan analysis of synthetic versions of the parental CBD3 peptide, we identified peptides harboring single amino acid mutations that bound with greater affinity to CaV2.2. One such peptide, harboring a phenylalanine instead of glycine (G14F), was tested in rodent models of migraine and neuropathic pain. In vivo laser Doppler blood flowmetry measure of capsaicin-induced meningeal vascular responses related to headache pain was almost completely suppressed by dural application of the G14F peptide. The G14F mutant peptide, administered intraperitoneally, also exhibited greater antinociception in Stavudine (2'-3'-didehydro-2'-3'-dideoxythymidine (d4T)/Zerit®) model of AIDS therapy-induced peripheral neuropathy compared to the parent CBD3 peptide. These results demonstrate the patent translational value of small biologic drugs targeting CaV2.2 for management of clinical pain.
Keywords: N-type calcium channel, CRMP-2, Uncoupling peptide, Meningeal blood flow, Migraine model, d4T/Zerit/Stavudine, NTR, AIDS therapy-induced neuropathic pain, Chronic pain
1. Introduction
The management of severe chronic pain in patients is challenging as often the reason for the pain is not clear and effective pharmacological treatment is difficult to achieve with currently available drugs [1]. One recent success story for chronic neuropathic pain treatment is the FDA approval of a synthetic ω-conotoxin, Ziconotide (Prialt®), which serves to block a voltage-gated ion channel, known as the N-type voltage-gated calcium channel, (CaV2.2) [2]. By this mechanism, Ziconotide effectively reduces pain. However, ziconotide can only be used to treat a small subset of patients, has a narrow therapeutic window due to the numerous off-target effects within and external to the CNS.
N-type calcium channels are multiprotein complexes comprised of a pore-forming α-subunits and auxiliary α2/δ, β, and γ subunits [3]. CaV2.2 channels serve as the nidus for pain transduction as mice lacking CaV2.2 exhibit an increased threshold for pain [4], and expression of CaV2.2 is upregulated following a chronic constrictive nerve injury [5]. The importance of CaV2.2 in pain is further highlighted by the demonstration of a naturally occurring alternative splice form of CaV2.2 (i.e., exon 37a) in small-diameter nociceptors [6]; neurons with exon 37a containing CaV2.2 are critical for thermal hyperalgesia, and thermal and mechanical nociception [7]. Mechanistically, the presence of CaV2.2 in the presynaptic nerve terminal of primary afferent nociceptive neurons is thought to release excitatory neurotransmitters into the synapse present between nociceptive neurons of the peripheral nervous system and the dorsal horn of the spinal cord. In sum, by virtue of their ability to control the regulated release of neurotransmitters, the N-type Ca2+ channels are a prime target for the development of novel analgesics [2,8,9].
The purported pharmacological mechanism of action of Ziconotide involves tonic blockade of the closed state of the CaV2.2 channel which is likely to contribute to many of the drug's side effects. One manner to sidestep this issue is to design a state-dependent blocker of CaV2.2 which would preferentially inhibit the channel during higher frequencies of firing associated with pain compared to the slower frequencies associated with normal physiological function. Pharmaceutical companies have recently reported TROX-1 as a state-dependent, non-subtype selective CaV2 channel inhibitor [10,11].
We have advanced an alternative approach – targeting modulators of channel trafficking as a novel route of drug discovery for treatment of clinical pain. Precedence for such a strategy exists as the drug gabapentin (Neurontin®) likely targets the α2δ1 subunit of voltage-gated calcium channels by impairing its trafficking, resulting in reduced neurotransmitter and spinal sensitization [12]. Recently, we identified collapsin response mediator protein 2 (CRMP-2) as a novel modulator of CaV2.2 [13,14]. CRMP-2 is a cytosolic phosphoprotein originally identified as a mediator of semaphorin3A growth cone collapse [15]. CRMP-2 has been shown to regulate axon number and length [16] and neuronal polarity [17–19]. We determined that CRMP-2 interacts with CaV2.2 and that overexpression of CRMP-2 leads to increased surface expression of CaV2.2 and enhanced Ca2+ currents [13,14,20]. CRMP-2 overexpression also increases stimulated release of the excitatory neuropeptide calcitonin gene-related peptide (CGRP) from dorsal root ganglia (DRG) [14]. Furthermore, knockdown of CRMP-2 dramatically reduced Ca2+ currents and transmitter release [13,14]. These findings suggest that the biochemical interaction between CRMP-2 and CaV2.2 is required for proper channel trafficking and function.
Disruption of the interaction between CaV2.2 and CRMP2 by a short peptide (CBD3) corresponding to a 15 amino acid region of CRMP-2 produces a number of changes including pain signal transmission [21]. Fusing CBD3 to the HIV TAT protein resulted in a cell permeable protein, which reduced CaV2.2-mediated currents in vitro, decreased neuropeptide release from sensory neurons and inhibited excitatory synaptic transmission in dorsal horn neurons of the spinal cord [21]. Furthermore, CBD3 administration in vivo reduced hypersensitivity to tactile stimuli in a number of pain models, including a model of neuropathic pain induced by anti-retroviral drug treatment [21] and a chronic inflammatory pain model involving focal demyelination of the sciatic nerve [22]. In a battery of rodent behavioral tests, CBD3 was found to be mildly anxiolytic without affecting memory retrieval, sensorimotor function, or depression. Importantly, sympathetic activity was not affected by CBD3 [22]. In this report, we investigate the usefulness of a novel peptide derived from the parental CBD3 peptide in animal models of migraine and AIDS therapy-induced peripheral neuropathy. Our results point to the high translational value of small biologic drugs targeting CaV2.2 for the management of clinical pain.
2. Experimental Procedures
2.1 Peptides
TAT-CBD3 (YGRKKRRQRRRARSRLAELRGVPRGL; the transduction domain of the HIV TAT protein is indicated in the underlined text), TAT-CBD3-G14F (YGRKKRRQRRRARSRLAELRGVPRFL) and TATCBD3reverse (YGRKKRRQRRRLGRPVGRLEALRSRA; a reversed version of the CBD3 sequence with no homology to any known sequence) were synthesized by GenScript USA Inc. (Piscataway, NJ) and verified by mass spectroscopy (Department of Chemistry, IUSM) prior to use. Peptides were dissolved in saline prior to use.
2.2 Animals
Pathogen-free, adult male (for blood flow) or female (for pain studies) Sprague-Dawley rats (150–200 g; Harlan Laboratories, Madison, WI) were housed in temperature (23 ± 3°C) and light (12-hlight: 12-h dark cycle; lights on at 07:00 h) controlled rooms with standard rodent chow and water available ad libitum. Experiments were performed during the light cycle. These experiments were approved by the Institutional Animal Care and Use Committee of Indiana University/Purdue University in Indianapolis. All procedures were conducted in accordance with the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health and the ethical guidelines of the International Association for the Study of Pain. All animals were randomly assigned to either treatment or control groups.
2.3 Laser Doppler flowmetry
Six to nine male rats per group were anesthetized with ketamine/xylazine and the animal's body temperature was maintained at 37°C with a homeothermic blanket. For the measurement of meningeal blood flow, the animals head was fixed in a stereotaxic frame and a cranial window prepared [23] with the dura left intact. Dural blood flow was measured with a laser Doppler flowmeter (TSI, MN). A needle type probe was placed over a large branch of the middle meningeal artery (MMA), distant from visible cortical blood vessels and the cranial window kept moist with synthetic interstitial solution (SIF) consisting of: 135 mM NaCl, 5 mM KCl, 5 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, 10 mM D-glucose (pH 7.3). Blood flow was recorded on-line at a frequency of 1 Hz using Axoscope software (Axon Instruments, CA).
2.4 Drug administration
Drugs or peptides were diluted fresh daily in SIF. TAT-CBD3 G14F was administered to the dural surface (50 μl, 10 μM). Capsaicin was dissolved in SIF to 100 nM for nasal administration. To stimulate the nasal mucosa, 50 μl of capsaicin solution was applied over a 30 s period at a site 2 mm into the right nostril using a Pipetman pipette [24]. SIF or SIF containing 0.1% ethanol was applied to the dura or nasal mucosa as a control in all experiments 15 minutes prior to drug application and had no effect on meningeal blood flow.
2.5 Data collection and statistics
Data was collected at 1 Hz and binned by averaging 60 samples (1 minute intervals) for statistical analysis or 10 samples (10 s intervals) for graphical representation. Basal blood flow was determined as the mean flow rate measured during a 3 minute period prior to drug application and the effects of test compounds were calculated by comparing the peak response within three minutes of administration to the average blood flow in the three minutes proceeding administration. Changes in blood flow for each animal were calculated, averaged within treatment groups and expressed as percentage changes relative to the basal blood flow. Comparison of blood flow changes was performed using an unpaired tudent's t-test. Data values are presented as means ± SEM. The significance level for all tests was set at p < 0.05.
2.6 Immunohistochemistry of trigeminal ganglion neurons (TRGs)
Adult Sprague-Dawley rats were injected (i.p.) with 20 mg/kg FITC-TAT-CBD3 peptide. Rats were then euthanized 15 minutes later with CO2 and transcardially perfused with saline followed by 4% paraformaldehyde. Trigeminal root ganglia were immediately removed and post fixed for 24 hours. The tissues were immersed in 4% paraformaldehyde at 4°C for 24 hours and then in 15% sucrose buffer for 24 hours at 4°C. Sagittal sections of the TRG were serially cut at 14 mm onto SuperFrost Plus microscope slides (Fisher Scientific, Pittsburgh PA). At least 6 sections were obtained for immunocytochemical analysis per TRG. All sections were stained with Hoechst 33258 nuclear marker nuclear label (1:1000, 5 minutes; Invitrogen Corporation, Carlsbad, CA). The signal from labeled cells was captured with fluorescent microscopes fitted with a CoolSNAP HQ2 charge-coupled devise camera (Photometrics, Tucson, AZ). Cells were visualized at 10 × magnification using a Nikon Eclipse 90i upright microscope (Melville, NY) and at 20 × magnification using a Delta Vision Core (Applied Precision, Issaquah, WA) with an Olympus 1×71 microscope (Olympus America Inc., Center Valley, PA).
2.7 d4T model of peripheral neuropathy
Hyperalgesia and allodynia were established by a single injection (50 mg/kg) of the FDA approved antiretroviral drug 2′-3′-didehydro-2′-3′-dideoxythymidine ((d4T)/Zerit®, Sigma) given i.p. A single administration of d4T produced a significant bilateral decrease in paw withdrawal threshold to von Frey hair stimulation starting at post-injection day (PID) 3 through the last day of testing at PID42 [25].
The von Frey test was performed on the area of the hind paws as previously described [21,26]. Briefly, the rat was placed on a metal mesh floor and covered with a transparent plastic dome where the animal rested quietly after an initial few minutes of exploration. Animals were habituated to this testing apparatus for 15 minutes a day, two days prior to pre-injection behavioral testing. Following acclimation, each filament was applied to six spots spaced across the glabrous side of the hind paw; two distinct spots for the distribution of each nerve branch (saphenous, tibial and sural). Mechanical stimuli were applied with seven filaments, each differing in the bending force delivered (10, 20, 40, 60, 80, 100, and 120 mN), but each fitted a flat tip and a fixed diameter of 0.2 mm. The force equivalence of mN to grams is: 100 mN=10.197 grams. The filaments were tested in order of ascending force, with each filament delivered for 1 s in sequence from the 1st to the 6th spot alternately from one paw to the other. The interstimulus interval was 10–15 s. A cutoff value of 120 mN was used; animals that did not respond at 120 mN were assigned that value.
Measurements were taken on 3 successive days before rats were subjected to peptides. At least 6 rats were used per condition. Stimuli were applied randomly to left and right hind paws to determine the stimulus intensity threshold stiffness required to elicit a paw withdrawal response. The incidence of foot withdrawal was expressed as a percentage of six applications of each filament as a function of force. A Hill equation was fitted to the function (Origin version 6.0, Microcal Software) relating the percentage of indentations eliciting a withdrawal to the force of indentation. From this equation, the threshold force was obtained and defined as the force corresponding to a 50% withdrawal rate. A threshold that exhibits at least a −20 mN difference from the baseline threshold of testing in a given animal is representative of neuropathic pain [27].
Threshold values were statistically analyzed for each foot separately and the significance of differences between the average of at least two pre-injection tests and the mean obtained for each post-injection test. In all tests, baseline data were obtained for the d4T-treated and sham-treated groups before drug or vehicle administration. Within each treatment group, post-administration means were compared with the baseline values by repeated measures analyses of variance (RMANOVA) followed by post hoc pairwise comparisons (Student-Newman-Keuls Method). A probability level of p <0.05 indicates significance.
3. Results
CBD3, a peptide from CRMP-2. Over the last few years, we have described an interaction between CaV2.2 and tetrameric collapsin response mediator protein 2 (CRMP-2) that positively regulates channel function by increasing cell surface trafficking [13,14,20]. The interaction between CaV2.2 and CRMP2 can be disrupted by a short peptide (CBD3) corresponding to a 15 amino acid region of CRMP-2 (Figure 1A,C). Fusing CBD3 to a short region of the transduction domain of the HIV TAT protein resulted in a cell permeable protein, which reduced CaV2.2-mediated currents in vitro, decreased neuropeptide release from sensory neurons and inhibited excitatory synaptic transmission in dorsal horn neurons of the spinal cord [21]. Furthermore, CBD3 administration in vivo reduced pain behavior in a number of pain models, including a model of neuropathic pain induced by anti-retroviral drug treatment [21] and a chronic inflammatory pain model involving focal demyelination of the sciatic nerve [22]. In a battery of rodent behavioral tests, CBD3 was found to be mildly anxiolytic without affecting memory retrieval, sensorimotor function, or depression. Importantly, sympathetic activity was not affected by CBD3 [22]. Given a wide therapeutic window of efficacy of CBD3, we explored potential strategies of optimizing the parent peptide. One such approach, probing peptide arrays of systematic single site mutants of CBD3 coupled with assessment of binding to CaV2.2 using a far-Western technique, resulted in the discovery of several peptides with greater binding affinity to CaV2.2 [22]. Here, we explored the potential usefulness of one such CBD3 variant, G14F, in in vivo models of migraine and pain. The glycine residue is fully conserved between rodents and humans and also between CRMP-1, -3, and-4 (Figure 1B). Further, the region of the CBD3 is likely surface exposed and thus available for protein-protein interactions (Figure 1C).
Figure 1.
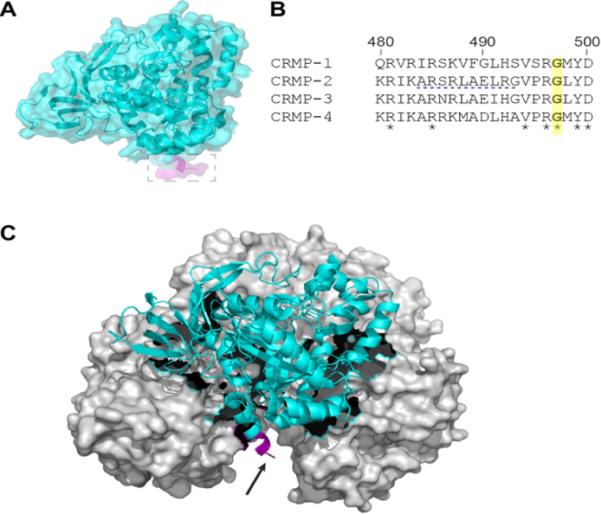
CRMP-2 structure illustrating location of partial CBD3 peptide. (A) Superimposed ribbon overlaid on top of surface representations of the three-dimensional structure of the CRMP-2 monomer (RCSB databank PDB code: 2GSE) [45]. The location of the 6 amino acids of the CBD3 peptide are illustrated in the boxed region. (B) Amino acid alignment of the region of CBD3 (amino acids 484–489) across CRMPs 1–4. Fully conserved residues are indicated by the asterisk. The purple dotted line represents the CBD3 peptide present in the crystal structure (boxed region in A). The glycine residue at the 14th position of the CBD3 peptide is indicated in yellow shading. (C) Tetrameric structure of CRMP-2 highlighting the location of the peptide (arrow). For clarity, only the CBD3 peptide emanating from the monomer shown in ribbon representation (in cyan) is illustrated. Crystallographic images were rendered using PyMol.
3.1 G14F blunts meningeal blood flow in an animal model of migraine
The dura mater is innervated by trigeminal capsaicin-sensitive peptidergic nociceptive afferent nerves which underlie meningeal vascular responses related to headache pain [28]. The role of calcitonin gene-related peptide (CGRP) released by sensory fibers in headache and chronic migraine has recently been highlighted by clinical trials demonstrating the efficacy of CGRP antagonists [29]. Since we have previously reported the involvement of calcium channels [30] and CRMP-2 [14] in CGRP release, we consequently tested the potential involvement of the CRMP-2 in vasodilatation in the rat dura. Capsaicin induced a rapid and robust increase in meningeal blood flow (Figure 2A) which returned toward baseline values within minutes. We have previously shown that dural application of TAT CBD3 prior to capsaicin significantly inhibited the capsaicin-induced blood flow changes [21]. Here, we tested if G14F variant of CBD3 was similarly effective. Changes in meningeal blood flow in response to 100 nM capsaicin were assayed using laser Doppler flowmetry as previously described [21,22,31]. The peptide was administered topically to the dura 15 min before nasal administration of capsaicin. G14F significantly reduced capsaicin evoked changes in blood flow (Cap, 35 ± 5% (n = 9) versus G14F, 7 ± 5% (n = 6); Figure 2B). Basal blood flow was not altered by the peptide compared to saline (Sal, −1 ± 2% (n = 9) versus G14F, −3 ± 2% (n = 6)). There was no effect of G14F administration alone on basal blood flow. We have previously demonstrated that a TAT scramble peptide and the synthetic interstitial fluid (control) do not alter basal blood flow or inhibit capsaicin-induced blood flow [21]. Because the activation of trigeminal sensory fibers by capsaicin elicits meningeal CGRP-dependent vasodilatation and is a readout of trigeminal activity, we confirmed that trigeminal ganglion neurons (TRGs) can be targeted by TAT-CBD3 (Figure 3).
Figure 2.
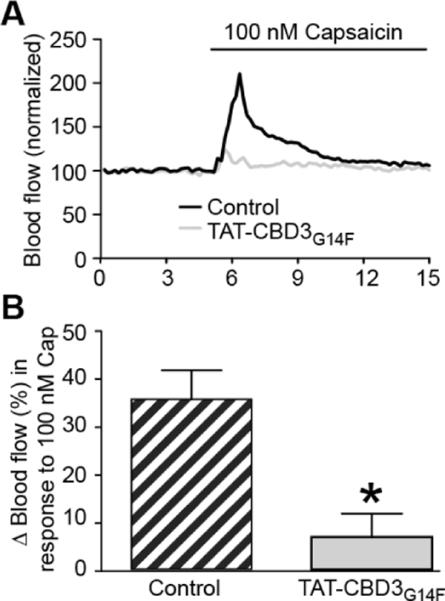
Effect of a mutant CDB3 peptide on capsaicin-stimulated meningeal blood flow. (A) Representative normalized traces of middle meningeal blood flow changes in response to nasally administered capsaicin (100 nM) in the absence (control, black trace) or presence of TAT-G14K peptide (grey trace). The peptide (30 μM) was administered to the dura 15 min before capsaicin administration. Laser Doppler flowmetry measurements were collected at 1 Hz and binned by averaging every 10 samples for graphical representation. The data from each rat were normalized to the first 3 min of basal data. (B) Summary of blood flow changes after nasal administration of capsaicin in the absence or presence of previous administration of TAT-CBD3 G14F to the dura. Changes in blood flow were calculated by comparing the average of 3 one minute intervals before capsaicin compared to the one minute interval after capsaicin. Bars indicate mean ± SEM with the n=6–9 rats tested per group. *p< 0.05 versus capsaicin alone (unpaired Student's t test).
Figure 3.
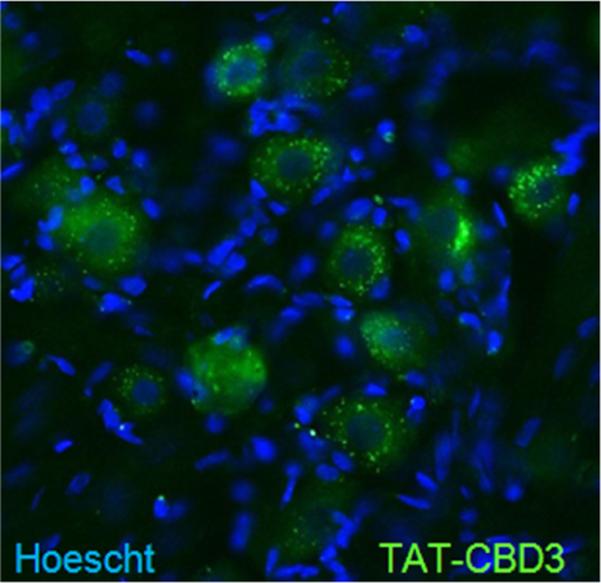
TRGs are targeted by CBD3. Representative example of FITC-TAT CBD3 fluorescence, 1 hr after a 20 mg/kg i.p. injection into a control rat, in sensory neurons of rat TRG. Nuclei of all cells stained with Hoescht dye.
3.2 G14F attenuates d4T-induced neuropathic pain behavior
Next, we examined the effects of the peptide on chronic nociceptive behavior in an animal model of AIDS therapy-induced painful neuropathy [25,32]. Nucleoside reverse transcriptase inhibitors (NRTIs), part of the highly active antiretroviral treatment (HAART) for AIDS includes drugs such as zidovudine (AZT), zalcitabine (ddC), didanosine (ddI) and stavudine (d4T) that produce side-effects including painful neuropathies. The ability of peptides to reverse tactile hypersensitivity was evaluated in rats seven days after a single injection of d4T. TAT-CBD3 alone had no effect on paw withdrawal threshold (PWT) (Figure 4). TAT-CBD3, but not TAT-reverse, caused an increase in PWT when administered intraperitoneally (i.p.) (Figure 4). In contrast, at the same i.p. dose of 10 mg/kg, TAT-CBD3-G14F produced a complete reversal of tactile hypersensitivity at 1 h after i.p. injection. Four hours after injection, TAT-CBD3 was no longer effective whereas TAT-CBD3-G14F-induced reversal of hypersensitivity had diminished by 50% but was still significantly better than control. By 24 hours, the effect of both peptides had subsided with no difference in hypersensitivity compared to d4T-treated rats (Figure 4).
Figure 4.
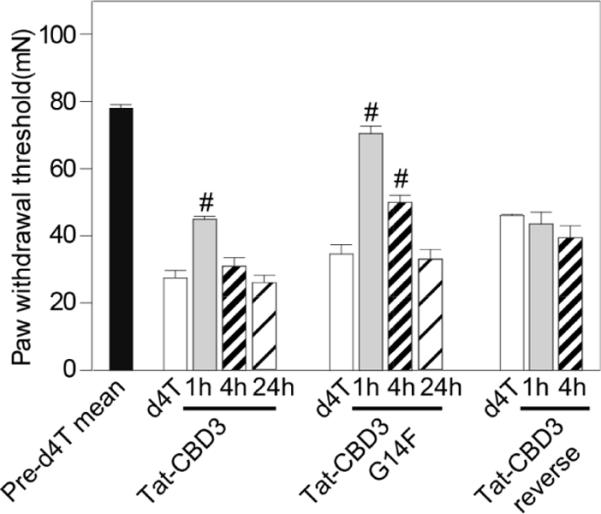
TAT-CBD3 G14F peptide reduces antiretroviral toxic neuropathic pain. Effect of TAT-CBD3 parent and mutant peptides on d4T-induced decreases in paw withdrawal threshold (PWT) in the rat at 1, 4 and 24 post injection. Animals subjected to a single d4T injection exhibited a decrease in PWT that was abolished by i.p. administration of TAT-CBD3 or TAT-CBD3G14F peptides on post-injection day 14 or later. Data represent means ± S.E.M.; #, p < 0.05 versus d4T-treated animals (n=6–8 each).
4. Discussion
In this report, we demonstrate that a variant of the CBD3 peptide, designed for the purpose of blocking the interaction between CRMP-2 and the calcium channel complex, is effective in blocking capsaicin-induced meningeal vasodilatation (as a model of headache pain) and in a NRTI-induced painful peripheral neuropathy model. These findings provide further proof of the importance of targeting voltage-gated calcium channels, particularly the N-type channel, for mitigation of pain.
Although targeting CaV2.2 has been convincingly shown to be beneficial in terms of pain, complete block of CaV2.2, however, is not without side-effects. As we have recently reported [21,22] and report in this paper, taking the alternative approach of targeting regulators of CaV2.2 is a promising strategy for developing pain therapeutics that may circumvent some of the psychiatric symptoms, cognitive impairment, and postural hypotension observed following Prialt usage, likely attributable to CaV2.2's importance in the central and sympathetic nervous systems [2]. N-type calcium channels are present in the presynaptic terminals of primary afferent sensory neurons, notably A-δ and C fibers [33], thus accounting for the actions of CBD3 peptides on the CNS. CaV2.2-containing neurons within the peripheral nervous system are responsible for relaying nociceptive signals primarily through their connections within the spinothalamic tract, in laminae I and II [2,9,34]. Calcium influx through these channels is the primary mechanism underlying substance P and CGRP release from these fibers [2,9,35].
Pharmacologic block of CaV2.2 not only reduces neurotransmitter release but may also decrease the excitability of the postsynaptic neurons within lamina I of the spinal cord [35]. We had previously shown that CRMP-2 modulates CGRP release from dorsal root ganglia [14]. Therefore, we tested the novel CBD3 variant in a model of meningeal vasodilatation in response to noxious stimuli [21]. In this model of `migraine', activation of trigeminal sensory fibers by noxious stimuli elicits meningeal vasodilatation which is CGRP-dependent [36–39] therefore measuring changes in meningeal vasodilation is an indirect method of measuring trigeminal activity. Efficacy of a compound in this model is highly predictive of success in clinical trials [40,41] therefore this model is widely used despite the fact that the association between meningeal vasodilatation and migraine pain is not well-understood [42,43]. We have previously used this model to determine the effects of environmental irritants on meningeal vasodilatation using Laser Doppler flowmetry [31]. G14F CBD3 was very effective at suppressing the capsaicin-elicited increase in meningeal blood flow, demonstrating its usefulness in attenuating acute pain signals.
The G14F CBD3 peptide was also successful in suppressing hypersensitivity in an animal model of HIV-treatment-induced peripheral neuropathy. Nucleoside reverse transcriptase inhibitors exacerbate the distal symmetrical peripheral neuropathy characterized by multi-organ pain, numbness, burning and/or dysaethesia observed in AIDS sufferers [44], resulting in discontinuation of use in up to 10% of the patients. This model of chronic pain employs the anti-retroviral treatment 2'-3'-didehydro-2'-3'-dideoxythymidine (d4T) to induce the small fiber dying back neuropathy that is seen in post-treatment AIDS patients [25]. Animals treated with d4T exhibit hyperalgesia and allodynia [25]. The effect of the novel G14F CBD3 peptide on chronic pain is likely attributed to its ability to block the functional interaction of CRMP2 and CaV2.2. It had been posited that the neuropathy within the d4T model is a result of decreased calcium buffering, as treatment with calcium buffering agents attenuated the d4T-induced neuropathy to a slight degree [25]. The efficacy of the G14F CBD3 peptide suggests that N-type voltage calcium channels on the presynaptic terminals of these small afferent sensory neurons are a key component of the d4T-induced neuropathy.
Chronic pain syndromes are a significant clinical problem because of the scope of individuals diagnosed with the disorders, the complexity of the conditions, the lack of understanding of the etiology of neuropathic pains, and the lack of adequate treatment modalities. As a result, the discovery of novel targets for the potential treatment of chronic pain is critically important. The low therapeutic index of the current N-type blockers propels the search for alternative methods of treatment. TAT CBD3 peptides, an effective uncoupler of CaV channels, represent a class of molecules with demonstrable efficacy in abolishing the pain hypersensitivity in an animal model of chronic neuropathic pain. Here, we propose that variants of the CBD3 peptide can be tailored to improve upon the parent peptide. Future studies will be aimed at translating the success of the CBD3 peptide(s) to an oral formulation that could be potentially useful in managing a broad spectrum of clinical pain conditions.
Acknowledgements
The authors thank colleagues at the Stark Neurosciences Research Institute (SNRI) for comments on the manuscript. This work was supported, in part, by grants from the Indiana Clinical and Translational Sciences Institute funded, in part by a Project Development Team Grant Number (RR025761) from the National Institutes of Health, National Center for Research Resources, Clinical and Translational Sciences Award, the Indiana State Department of Health – Spinal Cord and Brain Injury Fund (A70-9-079138 to R.K.), NIH Neurological Disorders and Stroke NINDS NS049136 NIDA DA026040 to F.A.W.), NIEHS (ES17430 to J.H.H.), the Indiana University Biomedical Committee – Research Support Funds (2286501 to R.K), a National Scientist Development from the American Heart Association (SDG5280023 to R.K.), and the Elwert Award in Medicine to R.K. R.K. is a shareholder of Sophia Therapeutics LLC.
References
- [1].Relieving pain in America: A blueprint for transforming prevention, care, education and research. The National Academies Press; 2011. Institute of medicine report from the committee on advancing pain research care and education. [PubMed] [Google Scholar]
- [2].Snutch TP. Targeting chronic and neuropathic pain: the N-type calcium channel comes of age. NeuroRx. 2005;2:662–670. doi: 10.1602/neurorx.2.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Catterall WA, Few AP. Calcium channel regulation and presynaptic plasticity. Neuron. 2008;59:882–901. doi: 10.1016/j.neuron.2008.09.005. [DOI] [PubMed] [Google Scholar]
- [4].Saegusa H, Kurihara T, Zong S, Kazuno A, Matsuda Y, Nonaka T, et al. Suppression of inflammatory and neuropathic pain symptoms in mice lacking the N-type Ca2+ channel. EMBO J. 2001;20:2349–2356. doi: 10.1093/emboj/20.10.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cizkova D, Marsala J, Lukacova N, Marsala M, Jergova S, Orendacova J, et al. Localization of N-type Ca2+ channels in the rat spinal cord following chronic constrictive nerve injury. Exp. Brain Res. 2002;147:456–463. doi: 10.1007/s00221-002-1217-3. [DOI] [PubMed] [Google Scholar]
- [6].Bell TJ, Thaler C, Castiglioni AJ, Helton TD, Lipscombe D. Cell-specific alternative splicing increases calcium channel current density in the pain pathway. Neuron. 2004;41:127–138. doi: 10.1016/s0896-6273(03)00801-8. [DOI] [PubMed] [Google Scholar]
- [7].Altier C, Dale CS, Kisilevsky AE, Chapman K, Castiglioni AJ, Matthews EA, et al. Differential role of N-type calcium channel splice isoforms in pain. J. Neurosci. 2007;27:6363–6373. doi: 10.1523/JNEUROSCI.0307-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zamponi GW, Feng ZP, Zhang L, Pajouhesh H, Ding Y, Belardetti F, et al. Scaffold-based design and synthesis of potent N-type calcium channel blockers. Bioorg. Med. Chem. Lett. 2009;19:6467–6472. doi: 10.1016/j.bmcl.2009.09.008. [DOI] [PubMed] [Google Scholar]
- [9].Zamponi GW, Lewis RJ, Todorovic SM, Arneric SP, Snutch TP. Role of voltage-gated calcium channels in ascending pain pathways. Brain Res. Rev. 2009;60:84–89. doi: 10.1016/j.brainresrev.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Swensen AM, Herrington J, Bugianesi RM, Dai G, Haedo RJ, Ratliff KS, et al. Characterization of the substituted N-triazole oxindole, TROX-1, a small molecule, state-dependent inhibitor of CaV2 calcium channels. Mol. Pharmacol. 2011 doi: 10.1124/mol.111.075226. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [11].Abbadie C, McManus OB, Sun SY, Bugianesi RM, Dai G, Haedo RJ, et al. Analgesic effects of a substituted N-triazole oxindole (TROX-1), a state-dependent, voltage-gated calcium channel 2 blocker. J. Pharmacol. Exp. Ther. 2010;334:545–555. doi: 10.1124/jpet.110.166363. [DOI] [PubMed] [Google Scholar]
- [12].Bauer CS, Nieto Rostro M., Rahman W, Tran-Van-Minh A, Ferron L, Douglas L, et al. The increased trafficking of the calcium channel subunit alpha2delta-1 to presynaptic terminals in neuropathic pain is inhibited by the alpha2delta ligand pregabalin. J. Neurosci. 2009;29:4076–4088. doi: 10.1523/JNEUROSCI.0356-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Brittain JM, Piekarz AD, Wang Y, Kondo T, Cummins TR, Khanna R. An atypical role for collapsin response mediator protein 2 (CRMP-2) in neurotransmitter release via interaction with presynaptic voltage-gated Ca2+ channels. J. Biol. Chem. 2009;284:31375–31390. doi: 10.1074/jbc.M109.009951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chi XX, Schmutzler BS, Brittain JM, Hingtgen CM, Nicol GD, Khanna R. Regulation of N-type voltage-gated calcium (CaV2.2) channels and transmitter release by collapsin response mediator protein-2 (CRMP-2) in sensory neurons. J. Cell. Sci. 2009;23:4351–4362. doi: 10.1242/jcs.053280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hensley K, Venkova K, Christov A, Gunning W, Park J. Collapsin response mediator protein-2: an emerging pathologic feature and therapeutic target for neurodisease indications. Mol. Neurobiol. 2011;43:180–191. doi: 10.1007/s12035-011-8166-4. [DOI] [PubMed] [Google Scholar]
- [16].Inagaki N, Chihara K, Arimura N, Ménager C, Kawano Y, Matsuo N, et al. CRMP-2 induces axons in cultured hippocampal neurons. Nat. Neurosci. 2001;4:781–782. doi: 10.1038/90476. [DOI] [PubMed] [Google Scholar]
- [17].Arimura N, Hattori A, Kimura T, Nakamuta S, Funahashi Y, Hirotsune S, et al. CRMP-2 directly binds to cytoplasmic dynein and interferes with its activity. J. Neurochem. 2009;111:380–390. doi: 10.1111/j.1471-4159.2009.06317.x. [DOI] [PubMed] [Google Scholar]
- [18].Morita T, Sobue K. Specification of neuronal polarity regulated by local translation of CRMP2 and Tau via the mTOR-p70S6K pathway. J. Biol. Chem. 2009;284:27734–27745. doi: 10.1074/jbc.M109.008177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yoshimura T, Kawano Y, Arimura N, Kawabata S, Kikuchi A, Kaibuchi K. GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell. 2005;120:137–149. doi: 10.1016/j.cell.2004.11.012. [DOI] [PubMed] [Google Scholar]
- [20].Wang Y, Brittain JM, Wilson SM, Khanna R. Emerging roles of collapsin response mediator proteins (CRMPs) as regulators of voltage-gated calcium channels and synaptic transmission. Commun. Integr. Biol. 2010;3:1–4. doi: 10.4161/cib.3.2.10620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Brittain JM, Duarte DB, Wilson SM, Zhu W, Ballard C, Johnson PL, et al. Suppression of inflammatory and neuropathic pain by uncoupling CRMP-2 from the presynaptic Ca(2+) channel complex. Nat. Med. 2011;17:822–829. doi: 10.1038/nm.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wilson SM, Brittain JM, Piekarz AD, Ballard CJ, Ripsch MS, Cummins TR, et al. Further insights into the antinociceptive potential of a peptide disrupting the N-type calcium channel-CRMP-2 signaling complex. Channels (Austin) 2011;5:449–456. doi: 10.4161/chan.5.5.17363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kurosawa M, Messlinger K, Pawlak M, Schmidt RF. Increase of meningeal blood flow after electrical stimulation of rat dura mater encephali: mediation by calcitonin gene-related peptide. Br. J. Pharmacol. 1995;114:1397–1402. doi: 10.1111/j.1476-5381.1995.tb13361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gottselig R, Messlinger K. Noxious chemical stimulation of rat facial mucosa increases intracranial blood flow through a trigemino-parasympathetic reflex--an experimental model for vascular dysfunctions in cluster headache. Cephalalgia. 2004;24:206–214. doi: 10.1111/j.1468-2982.2004.00649.x. [DOI] [PubMed] [Google Scholar]
- [25].Joseph EK, Chen X, Khasar SG, Levine JD. Novel mechanism of enhanced nociception in a model of AIDS therapy-induced painful peripheral neuropathy in the rat. Pain. 2004;107:147–158. doi: 10.1016/j.pain.2003.10.010. [DOI] [PubMed] [Google Scholar]
- [26].LaMotte RH, Friedman RM, Lu C, Khalsa PS, Srinivasan MA. Raised object on a planar surface stroked across the fingerpad: responses of cutaneous mechanoreceptors to shape and orientation. J. Neurophysiol. 1998;80:2446–2466. doi: 10.1152/jn.1998.80.5.2446. [DOI] [PubMed] [Google Scholar]
- [27].Ma C, Shu Y, Zheng Z, Chen Y, Yao H, Greenquist KW, et al. Similar electrophysiological changes in axotomized and neighboring intact dorsal root ganglion neurons. J. Neurophysiol. 2003;89:1588–1602. doi: 10.1152/jn.00855.2002. [DOI] [PubMed] [Google Scholar]
- [28].Goadsby PJ. Calcitonin gene-related peptide (CGRP) antagonists and migraine: is this a new era? Neurology. 2008;70:1300–1301. doi: 10.1212/01.wnl.0000309214.25038.fd. [DOI] [PubMed] [Google Scholar]
- [29].Olesen J, Diener HC, Husstedt IW, Goadsby PJ, Hall D, Meier U, et al. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N. Engl. J. Med. 2004;350:1104–1110. doi: 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- [30].Xiao Y, Richter JA, Hurley JH. Release of glutamate and CGRP from trigeminal ganglion neurons: Role of calcium channels and 5-HT1 receptor signaling. Mol. Pain. 2008;4:12. doi: 10.1186/1744-8069-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kunkler PE, Ballard CJ, Oxford GS, Hurley JH. TRPA1 receptors mediate environmental irritant-induced meningeal vasodilatation. Pain. 2011;152:38–44. doi: 10.1016/j.pain.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bhangoo SK, Ripsch MS, Buchanan DJ, Miller RJ, White FA. Increased chemokine signaling in a model of HIV1-associated peripheral neuropathy. Mol. Pain. 2009;5:48. doi: 10.1186/1744-8069-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Westenbroek RE, Hoskins L, Catterall WA. Localization of Ca2+ channel subtypes on rat spinal motor neurons, interneurons, and nerve terminals. J. Neurosci. 1998;18:6319–6330. doi: 10.1523/JNEUROSCI.18-16-06319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kerr LM, Filloux F, Olivera BM, Jackson H, Wamsley JK. Autoradiographic localization of calcium channels with [125I] omega-conotoxin in rat brain. Eur. J. Pharmacol. 1988;146:181–183. doi: 10.1016/0014-2999(88)90501-8. [DOI] [PubMed] [Google Scholar]
- [35].Heinke B, Balzer E, Sandkuhler J. Pre- and postsynaptic contributions of voltage-dependent Ca2+ channels to nociceptive transmission in rat spinal lamina I neurons. Eur. J. Neurosci. 2004;19:103–111. doi: 10.1046/j.1460-9568.2003.03083.x. [DOI] [PubMed] [Google Scholar]
- [36].Akerman S, Williamson DJ, Goadsby PJ. Voltage-dependent calcium channels are involved in neurogenic dural vasodilatation via a presynaptic transmitter release mechanism. Br. J. Pharmacol. 2003;140:558–566. doi: 10.1038/sj.bjp.0705456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Dux M, Santha P, Jancso G. Capsaicin-sensitive neurogenic sensory vasodilatation in the dura mater of the rat. J. Physiol. 2003;552:859–867. doi: 10.1113/jphysiol.2003.050633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zimmermann K, Reeh PW, Averbeck B. S+ -flurbiprofen but not 5-HT1 agonists suppress basal and stimulated CGRP and PGE2 release from isolated rat dura mater. Pain. 2003;103:313–320. doi: 10.1016/S0304-3959(02)00459-1. [DOI] [PubMed] [Google Scholar]
- [39].Peitl B, Petho G, Porszasz R, Nemeth J, Szolcsanyi J. Capsaicin-insensitive sensory-efferent meningeal vasodilatation evoked by electrical stimulation of trigeminal nerve fibres in the rat. Br. J. Pharmacol. 1999;127:457–467. doi: 10.1038/sj.bjp.0702561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Eikermann-Haerter K, Moskowitz MA. Animal models of migraine headache and aura. Curr. Opin. Neurol. 2008;21:294–300. doi: 10.1097/WCO.0b013e3282fc25de. [DOI] [PubMed] [Google Scholar]
- [41].Reuter U, Sanchez del RM, Moskowitz MA. Experimental models of migraine. Funct. Neurol. 2000;15(Suppl 3):9–18. [PubMed] [Google Scholar]
- [42].Panconesi A, Bartolozzi ML, Guidi L. Migraine pain: reflections against vasodilatation. J. Headache Pain. 2009;10:317–325. doi: 10.1007/s10194-009-0130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Strassman AM, Levy D. Response properties of dural nociceptors in relation to headache. J. Neurophysiol. 2006;95:1298–1306. doi: 10.1152/jn.01293.2005. [DOI] [PubMed] [Google Scholar]
- [44].Moyle GJ, Sadler M. Peripheral neuropathy with nucleoside antiretrovirals: risk factors, incidence and management. Drug Saf. 1998;19:481–494. doi: 10.2165/00002018-199819060-00005. [DOI] [PubMed] [Google Scholar]
- [45].Deo RC, Schmidt EF, Elhabazi A, Togashi H, Burley SK, Strittmatter SM. Structural bases for CRMP function in plexin-dependent semaphorin3A signaling. EMBO J. 2004;23:9–22. doi: 10.1038/sj.emboj.7600021. [DOI] [PMC free article] [PubMed] [Google Scholar]


