Abstract
Background aims
Tumor antigen-specific cytotoxic T lymphocytes (CTL) have been used in the treatment of human cancer, including leukemia. Several studies have established PR1 peptide, an HLA-A2.1-restricted peptide derived from proteinase 3 (P3), as a human leukemia-associated antigen. PR1-specific CTL elicited in vitro from healthy donors have been shown to lyse P3-expressing AML cells from patients. We investigated whether PR1-CTL can be adoptively transferred into NOD/SCID mice to eliminate human leukemia cells.
Methods
PR1-CTL were generated in bulk culture from peripheral blood mononuclear cells (PBMC) stimulated with autologous dendritic cells. Human acute myeloid leukemia (AML) patient samples were injected and engrafted in murine bone marrow at 2 weeks post-transfer.
Results
Following adoptive transfer, bone marrow aspirate from mice that received AML alone had 72–88% blasts in a hypercellular marrow, whereas mice that received AML plus PR1-CTL co-infusion had normal hematopoietic elements and only 3–18% blasts in a hypocellular marrow. The PR1-CTL persisted in the bone marrow and liver and maintained a CD45RA− CD28+ effector phenotype.
Conclusions
We found that adoptive transfer of PR1-CTL generated in vitro is associated with reduced AML cells in NOD/SCID mice. PR1-CTL can migrate to the sites of disease and maintain their capacity to kill the AML cells. The surface phenotype of PR1-CTL was consistent with their trafficking pattern in both vascular and end-organ tissues.
Keywords: acute myeloid leukemia, adoptive therapy, cytotoxic T lymphocytes, NOD/SCID, PR1
Introduction
Adoptive immunotherapy using antigen-specific cytotoxic T lymphocytes (CTL) has achieved some success in the treatment of leukemia. Recent advances in peptide/major histocompatibility complex (MHC) tetramer technology allow us to measure directly T-cell mediated antigen-specific responses (1). Several approaches are being evaluated to deliver antigen-specific therapy to augment the graft-versus-leukemia (GvL) effect without causing graft-versus-host disease (GvHD) in allogeneic hematopoietic stem cell transplantation (allo-HCT), such as adoptive immunotherapy with T cells primed against tumor and immunization of patients with tumor antigens (2,3). Because most patients receive allo-HCT from MHC-matched donors, two types of antigens have been exploited as targets to separate anti-leukemia from anti-host reactions: (i) hematopoietic tissue minor histocompatibility antigens (mHA), such as HA-1 and HA-2 (4–6); and (ii) leukemia-associated antigens, such as Wilms’ tumor-suppressor and proteinase 3 (7–12).
PR1 is a nine-amino acid HLA-A2-restricted peptide (VLQELNVTV) derived from two azurophil granule proteins, proteinase 3 and neutrophil elastase, both leukemia-associated self-antigens that are aberrantly expressed in myeloid lineage-derived leukemias such as chronic myelogenous leukemia (CML), acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) (9–10). CTL specific for PR1 (PR1-CTL), are capable of killing leukemia cells and may contribute to the elimination of CML and AML (11–14). Moreover, the presence of PR1-CTL in patients has been shown to be associated with GvL activity after allo-HCT (13–16). PR1-CTL that occur naturally or are induced in CML patients mediate anti-leukemia function (12,14,15). It is conceivable that the PR1-CTL elicited and expanded in vitro can be transferred adoptively into stem cell transplant recipients to enhance GvL and reduce GvHD associated with allo-HCT. We have used the well-established NOD/SCID mouse as a model to determine whether adoptively transferred PR1-CTL can eliminate AML cells that are co-transferred into the mice from human leukemia bone marrow samples (17–19). We found PR1-CTL can migrate to the sites of disease and are associated with a reduced leukemia burden in NOD/SCID mice.
Methods
PR1-CTL production
PR1-CTL were expanded in bulk culture using a method described previously, with some modification (9). Autologous dendritic cells (DC) were generated from a HLA-A2.1+ healthy donor. Briefly, adherent monocytes from a normal donor were stimulated for 7 days with a combination of cytokines granulocyte–macrophage colony-stimulating factor (GM-CSF) (500 IU/mL) and interferon (INF)-α (1000 IU/mL). The activated DC were collected, and a fraction of the cells was analyzed by fluorescence-activated cell sorter (FACS) for CD80 and CD14 expression. In the presence of interleukin (IL)-2 (20 IU/ml), DC were pulsed with PR1, PR2 or pp65 peptides. PR2 (RLFPDFFTRV) is another HLA-A2-restricted peptide derived from proteinase 3, but PR2-CTL are incapable of killing leukemia cells (9). Peripheral blood mononuclear cells (PBMC) were stimulated weekly with peptide-pulsed autologous DC for 3–4 weeks. A fraction of PR1-CTL was harvested and tested for specific lysis using a CTL cytotoxicity assay as described previously (9). The remaining CTL from bulk culture were purified with a sorter using antibodies (Ab) to deplete CD4, B and natural killer (NK) cells simultaneously.
NOD/SCID AML xenograft model
NOD/SCID-HLA-A2.1 mice were kindly provided by Dr Leonard Shultz from the Jackson Laboratory (Bar Harbor, ME, USA) and housed at the MD Anderson Cancer Center (Houston, TX, USA) according to the IACUC-approved protocol. Human AML bone marrow samples were obtained from patients at the MD Anderson Cancer Center according to an IRB-approved protocol. AML cells from two HLA-A2.1+ patients (UPN1 and UPN2) were transferred intravenously into irradiated (200 cGy) NOD/SCID mice either alone or mixed with CTL. Animals were killed 2 weeks post-transfer, and tissues were harvested for flow cytometry (FACS) and immunohistochemistry (IHC) analysis. Human CD45 Ab and mouse CD45.2 Ab were used to identify cells of human origin. The differential cell counts were performed on cytospin preparations and smears prepared from bone marrow. Formalin-fixed paraffin-embedded sections of tissue organs harvested were stained with hematoxylin and eosin stain.
Results and discussion
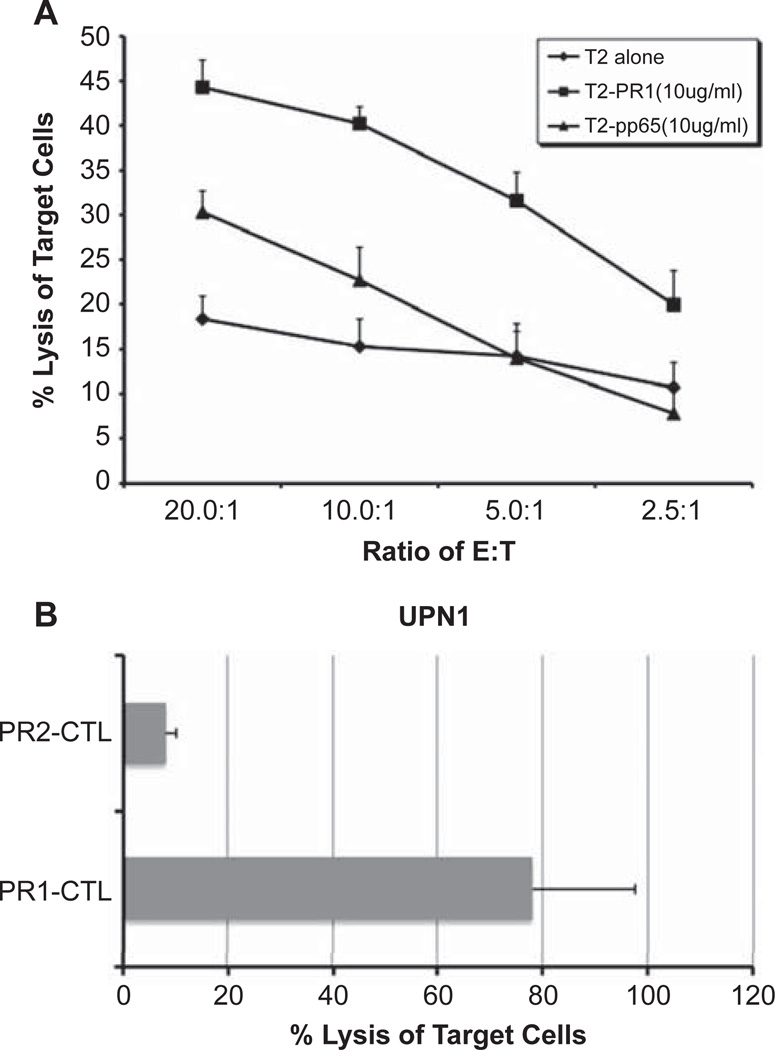
PR1-CTL elicited in vitro from healthy donors were shown to lyse P3-expressing AML and CML from patients (1–4). We examined whether these PR1-CTL can be adoptively transferred to NOD/SCID mice to eliminate the leukemia cells as a pre-clinical model for PR1-CTL immunotherapy for AML patients. We generated and expanded PR1-CTL from a HLA-A2.1+ healthy donor. Autologous DC were isolated and pulsed with PR1 peptide. PBMC were stimulated with the peptide-loaded DC every week and the bulk culture was harvested after 4 weeks. A fraction of CTL culture was analyzed for the presence of PR1-CTL by FACS and specific lysis using a cytotoxicity assay (Figure 1). As shown in Figure 1A, the PR1-CTL culture was incubated with peptide-pulsed T2 cells to demonstrate the percentage of specific lysis of PR1 peptide-loaded target cells. Only bulk culture of CTL with detectable PR1/tetramer+ CD8 cells and specific cytoxicity for PR1 peptide-loaded T2 target cells was used for subsequent experiments. The same methods were used to generate PR2-CTL and pp65-CTL as controls.
Figure 1.
Characterization of pre-transplant PR1-CTL. CTL elicited with PR1 from PBMC were characterized for cytotoxicity. (A) PR1-CTL were incubated for 4 h at 37°C with PR1 or pp65 peptide-pulsed T2 cells at the indicated peptide concentrations at an effector:target (E:T) ratio of 10:1, and the percentage specific lysis determined. Three replicate wells were used for each dilution of effector cells. (B) PR1-CTL and PR2-CTL were elicited simultaneously from the same donor in parallel. CTL were incubated for 4 h at 37°C with AML cells from UPN1 patient at an E:T ratio of 10:1 and percentage specific lysis determined. Results are the means and SD from three replicate wells.
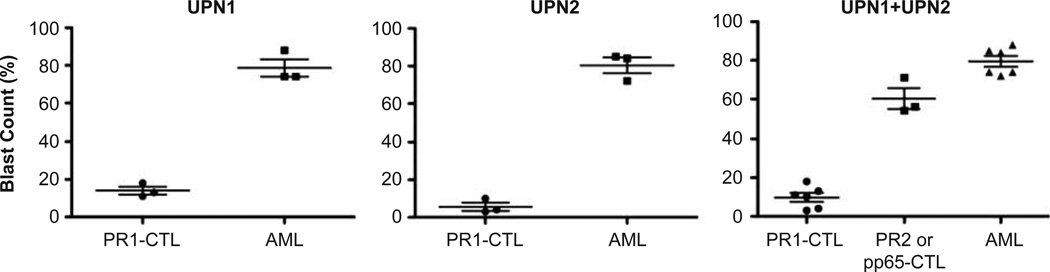
The NOD/SCID-HLA-A2.1 mice were used for the engraftment of AML cells. We selected two HLA-A2.1+ AML patient samples (UPN1 and UPN2; Table I) that were able to engraft successfully in bone marrow at various doses (107, 106, 105) 2 weeks post-transfer. The dose of 1 × 106 AML cells from both patients was used in subsequent experiments. Mice received AML alone or AML plus PR1-CTL, PR2-CTL or pp65-CTL co-infusion after irradiation. Blood was collected 3 and 7 days post-transfer with detectable circulating AML and CTL (data not shown). Mice were killed at 2 weeks post-transfer and tissues were collected for analysis.
Table I.
Patient characteristics and AML blast counts in NOD/SCID bone marrow. Two AML patient samples (UPN1 and UPN2) were used in the six sets of experiments. CTL (PR1, PR2 or pp65) were elicited from the same healthy donor in each set of experiments summarized. The percentage of blast counts in the bone marrow and the number of CD8+ CTL co-infused (in parentheses) are shown.
| Blast count in mouse bone marrow post-transfer (%) and number of CD8+ CTL infused | |||||
|---|---|---|---|---|---|
| Patient characteristics | Experiment | PR1-CTL | PR2-CTL | pp65-CTL | AML |
| UPN1 | 1 | 18 (7 × 105) | 71 (4 × 105) | ND | 88 |
| AML-M5, refractory disease | 2 | 13 (4 × 105) | ND | ND | 74 |
| 64% blasts | 3 | 11 (5 × 105) | ND | ND | 74 |
| UPN2 | 1 | 10 (3 × 105) | ND | 56 (6 × 105) | 85 |
| AML-M2, refractory relapse disease | 2 | 4 (5 × 105) | ND | 54 (7 × 105) | 84 |
| 98% blasts | 3 | 3 (7 × 105) | ND | ND | 72 |
ND, no data.
AML blasts were significantly decreased in the bone marrow of mice that received cotransferred PR1-CTL compared with those that received AML cells only (Table I). For each patient, three sets of experiments were performed. CTL were elicited from the same healthy donor in each set of experiments and a total of six different donors was used in the six sets of experiments summarized. The CTL were purified from bulk culture using a cell sorter to deplete CD4, B and NK cells. A total of about 3–7 × 105 purified CTL was collected from the cell sort per bulk culture and used in each single mouse experiment. In the first set of experiments using UPN1 (experiment 1), the mice receiving PR1-CTL co-transfer had a reduced blast count in the bone marrow compared with mice receiving AML alone, 18% versus 88%, respectively. In addition, mice receiving PR1-CTL generated from two other healthy donors (experiments 2 and 3) were shown to have reduced bone marrow blast counts, 13% versus 11%, respectively, in comparison with mice receiving AML alone (74%). To determine whether the reduction of blast count was contributed by alloreactivity of the healthy donor against UPN1, we simultaneously elicited PR2-CTL from the same donor in parallel and successfully generated PR2-CTL in experiment 1. Mice receiving PR2-CTL had 71% blasts in the bone marrow, suggesting specificity of PR1-CTL against AML from UPN1. This was consistent with the cytotoxicity assay (Figure 1B), which demonstrated specific lysis of AML cells by PR1-CTL but not PR2-CTL culture in vitro. Similar results were obtained using UPN2, where the bone marrow of mice receiving PR1-CTL had significantly reduced blast counts (10%, 4% and 3%) compared with mice receiving AML alone (85%, 84% and 72%). We also successfully elicited pp65-CTL from two out of the three donors in parallel as controls, and mice receiving these pp65-CTL had blast counts of 56% and 54%, respectively. As UPN2 was a cytomegalovirus (CMV) seropositive patient, the reduction of blasts in mice with pp65-CTL co-transfer was most probably contributed either by pp65-CTL against CMV+ AML cells or alloreactivity. As shown in Figure 2, the reduction of AML blast count in PR1-CTL-treated versus AML-alone mice was statistically significant in both UPN1 (P = 0.0002) and UPN2 (P < 0.0001) groups. To compare PR1-CTL, control CTL (PR2- or pp65-CTL) and AML-alone groups, we used an ANOVA test and found a statistically significant difference (P < 0.0001). In addition, the Tukey range test was used to determine which of the groups were significantly different and showed significance among all three groups (P < 0.05). Thus, regardless of the possible alloreactivity demonstrated by the control CTL (PR2- or pp65-CTL) in these experimental settings, the specificity of PR1-CTL against AML from both UPN1 and UPN2 was statistically significant.
Figure 2.
Statistical analysis of AML blast counts in NOD/SCID bone marrow. Comparisons between PR1-CTL-treated and AML-alone samples were performed using a two-tailed t-test. Significant differences were noted in both UPN1 (P = 0.0002) and UPN2 (P < 0.0001) groups. An anova test showed significance (P < 0.0001) between PR1-CTL-treated, PR2- or pp65-CTL-treated and AML-alone groups, and the Tukey range test showed significance among all three groups (P < 0.05).
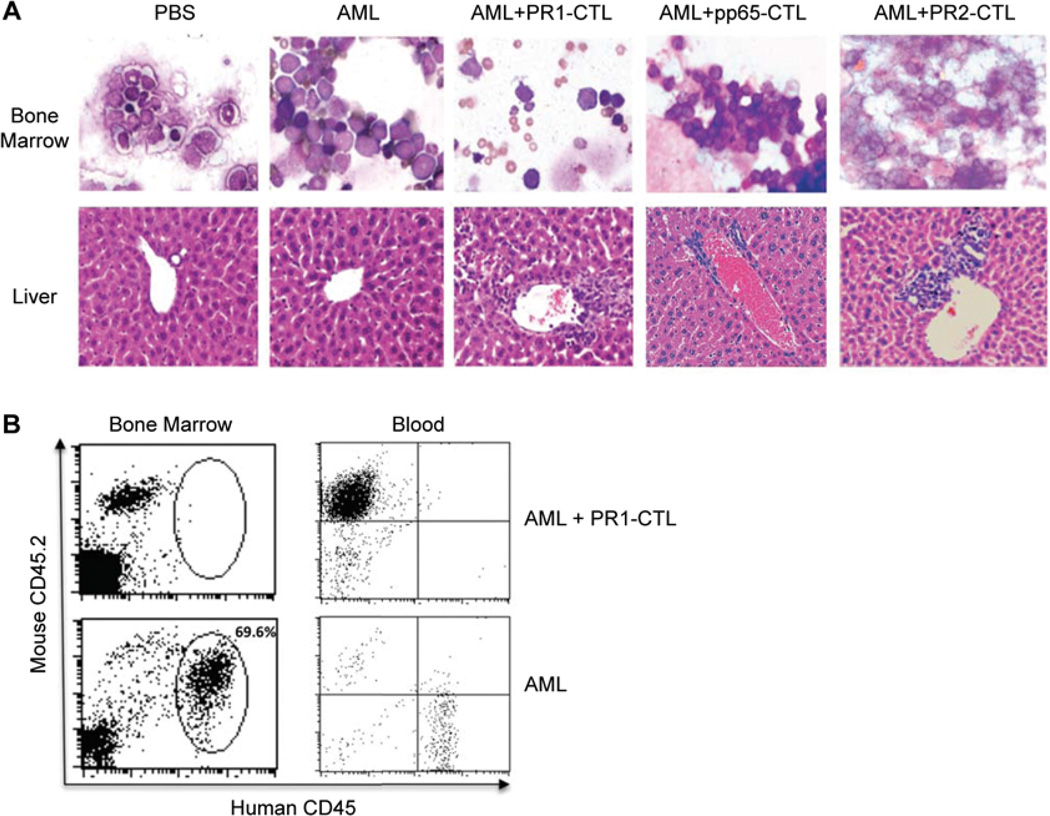
As shown in the upper panel of Figure 3A, the bone marrow smear from mice receiving AML alone, AML plus pp65-CTL or AML plus PR2-CTL had hypercellular bone marrow infiltrated with leukemic blasts. Mice that received AML plus PR1-CTL had reduced cellularity and increased lymphocytes with maturing hematopoietic elements in comparison with the hypocellullar marrow from mice only receiving phosphate-buffered saline (PBS). Although leukemic cells were detectable in the bone marrow and blood of mice receiving AML and co-transferred PR1-CTL, the number of leukemic cells was significantly lower than in the mice receiving AML alone (Figure 3B). Thus PR1-CTL can rapidly reduce the AML burden in NOD/SCID mice.
Figure 3.
PR1-CTL reduce AML burden in NOD/SCID mice. (A) Giemsa stain of bone marrow (upper panel) and hematoxylin and eosin stain of liver (lower panel). The panels are representative slides from the mice 2 weeks post-transfer of PBS, AML alone, AML plus PR1-CTL, AML plus pp65-CTL or AML plus PR2-CTL. (B) FACS of representative bone marrow and blood from the mice 2 weeks post-transfer of AML plus PR1-CTL (upper panel) and AML alone (lower panel). Human CD45 Ab and mouse CD45.2 Ab were used to identify cells of human origin. The percentage of gated human cells (69.6%) in the bone marrow of AML alone is displayed.
PR1-CTL also infiltrated extrameduallary organs such as the spleen and liver, by both FACS analysis and histologic examination. As shown in the lower panel of Figure 3A, intrasinuosidal and periportal collections of lymphocytes and leukemic blasts were seen in the mice receiving AML plus PR1-CTL. The blasts were associated with vascular damage, and PR1-CTL infiltrated these same regions. The PR1-CTL generated in vitro were predominantly CD45RA− CD28+ with an effector phenotype (data not shown).
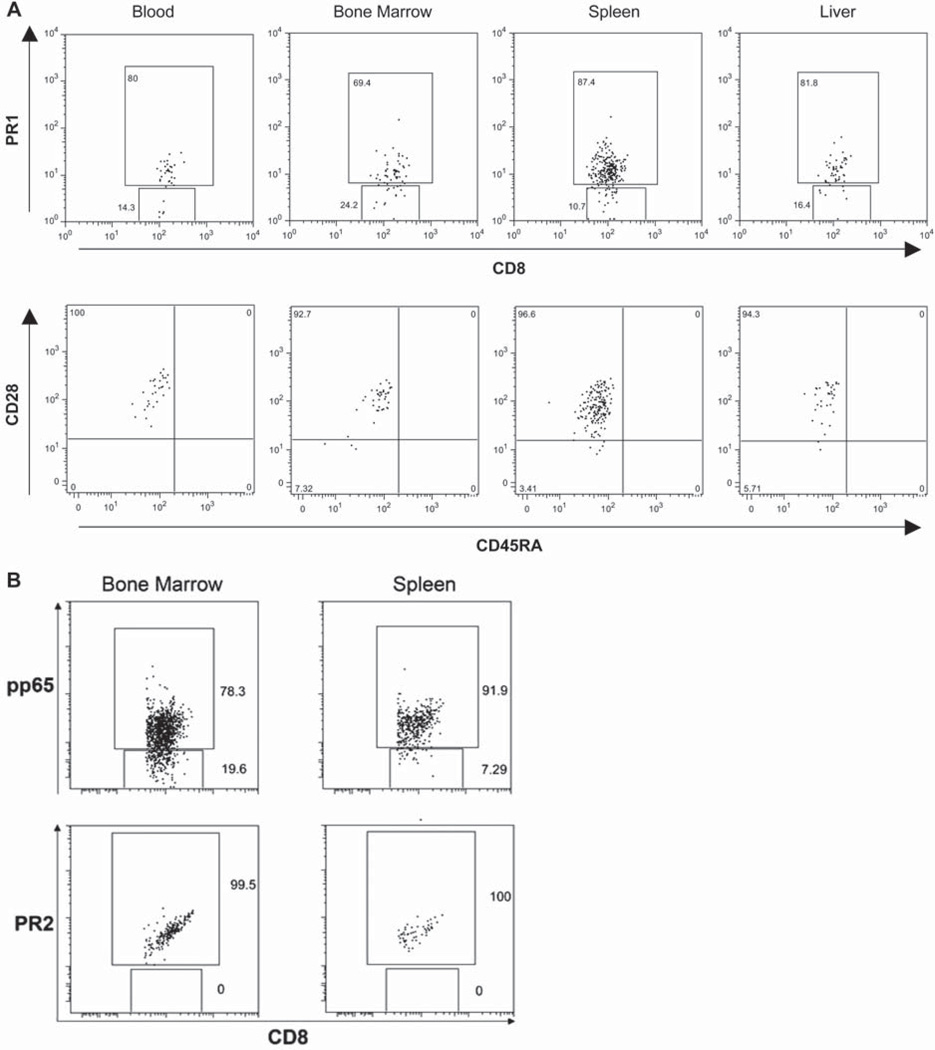
Two weeks post-transfer, PR1-CTL migrated to the secondary lymphoid organs, including spleen, peripheral and mesenteric lymph nodes, and were circulating in extrameduallary spaces such as liver. Using multicolor FACS, we examined the surface phenotype of these cells in blood, bone marrow, spleen and liver. As shown in Figure 4A (upper panel), the majority of CD8+ T cells in the bone marrow, spleen and liver were positive for the PR1/tetramer. These PR1-CTL maintained an effector phenotype of CD45RA− CD28+ (lower panel of Figure 4A), similar to the pre-infusion CTL. We also examined the control mice that were co-infused with pp65-CTL or PR2-CTL with AML, and found CD8+/tetramer+ CTL in the bone marrow and spleen (Figure 4B).
Figure 4.
Post-transplant CTL surface phenotype. (A) Cells from representative mice post-transplant with AML plus PR1-CTL were stained with Ab for the surface phenotype of CTL in blood, bone marrow, spleen and liver. The upper panel of each sample displays the percentage of cells positive for human CD8 and PR1 tetramer. The surface marker of these cells, including CD45RA and CD28, are displayed on the lower panel of each sample. (B) Cells from the bone marrow and spleen of mice post-transplant with AML plus pp65-CTL or PR2-CTL were stained with human CD8 and tetramer. Each sample displays the percentage of cells positive for CD8 and pp65 or PR2 tetramer.
To date, tumor immunotherapies such as vaccines and adoptive cell transfers have produced very few objective clinical responses. This is due, in part, to our historically limited understanding of fundamental immunobiology. The antigen-specific CTL, which are potent at killing leukemia cells, are either selectively deleted by the host leukemia cells or have compromised specific cytolytic function (14). Thus the sustained immune responses against leukemia depend upon the function of these antigen-specific CD8+ T cells. We have demonstrated that co-infusion of PR1-CTL elicited from bulk culture is associated with reduced leukemia burden in the AML xenograft model. PR1-CTL not only traffic to the sites of disease such as bone marrow and liver, but also home to secondary lymphoid organs.
The reduction of AML observed in the control mice suggests that there might be some alloreactivity in pp65-CTL. However, in comparison with the significant reduction of AML (3–18%) in PR1-CTL treated mice, the AML reduction in control mice (54–71% AML) is relatively minor (72–88% in AML alone). In addition, we performed a test (Tukey’s HSD) to compare each treatment. All treatments were significantly different from one another: AML was different to the PR1-CTL treatment (P < 0.001); the control was different to the PR1-CTL treatment (P < 0.001); AML was different to the control (P < 0.005). Although there are potential limitations of engraftment of human leukemia-initiating cells in NOD/SCID animals, this well-established model has been used successfully to demonstrate other human antigen-specific CTL in eliminating leukemia cells (19). Our results warrant a clinical trial using adoptively transferred PR1-CTL into AML patients to enhance GvL activity after allo-HCT.
Footnotes
Declaration of interest: we have no conflict of interests.
References
- 1.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 2.Appelbaum FR. Haematopoietic cell transplantation as immunotherapy. Nature. 2001;411:385–389. doi: 10.1038/35077251. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 4.Goulmy E. Human minor histocompatibility antigens: new concepts for marrow transplantation and adoptive immunotherapy. Immunol Rev. 1997;157:125–140. doi: 10.1111/j.1600-065x.1997.tb00978.x. [DOI] [PubMed] [Google Scholar]
- 5.den Haan JM, Meadows LM, Wang W, Pool J, Blokland E, Bishop TL, et al. The minor histocompatibility antigen HA-1: a diallelic gene with a single amino acid polymorphism. Science. 1998;279:1054–1057. doi: 10.1126/science.279.5353.1054. [DOI] [PubMed] [Google Scholar]
- 6.Mutis T, Verdijk R, Schrama E, Esendam B, Brand A, Goulmy E. Feasibility of immunotherapy of relapsed leukemia with ex vivo-generated cytotoxic T lymphocytes specific for hematopoietic system-restricted minor histocompatibility antigens. Blood. 1999;93:2336–2341. [PubMed] [Google Scholar]
- 7.Inoue K, Ogawa H, Sonoda Y, Kimura T, Sakabe H, Oka Y, et al. Aberrant overexpression of the Wilms tumor gene (WT1) in human leukemia. Blood. 1997;89:1405–1412. [PubMed] [Google Scholar]
- 8.Bergmann L, Miething C, Maurer U, Brieger J, Karakas T, Weidmann E, et al. High levels of Wilms’ tumor gene (wt1) mRNA in acute myeloid leukemias are associated with a worse long-term outcome. Blood. 1997;90:1217–1225. [PubMed] [Google Scholar]
- 9.Molldrem J, Dermime S, Parker K, Jiang YZ, Mavroudis D, Hensel N, et al. Targeted T-cell therapy for human leukemia: cytotoxic T lymphocytes specific for a peptide derived from proteinase 3 preferentially lyse human myeloid leukemia cells. Blood. 1996;88:2450–2457. [PubMed] [Google Scholar]
- 10.Molldrem JJ, Clave E, Jiang YZ, Mavroudis D, Raptis A, Hensel N, et al. Cytotoxic T lymphocytes specific for a nonpolymorphic proteinase 3 peptide preferentially inhibit chronic myeloid leukemia colony-forming units. Blood. 1997;90:2529–2534. [PubMed] [Google Scholar]
- 11.Molldrem JJ, Lee PP, Wang C, Champlin RE, Davis MM. A PR1-human leukocyte antigen-A2 tetramer can be used to isolate low-frequency cytotoxic T lymphocytes from healthy donors that selectively lyse chronic myelogenous leukemia. Cancer Res. 1999;59:2675–2681. [PubMed] [Google Scholar]
- 12.Molldrem JJ, Lee PP, Wang C, Felio K, Kantarjian HM, Champlin RE, et al. Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous leukemia. Nat Med. 2000;6:1018–1023. doi: 10.1038/79526. [DOI] [PubMed] [Google Scholar]
- 13.Rezvani K, Grube M, Brenchley JM, Sconocchia G, Fujiwara H, Price DA, et al. Functional leukemia-associated antigen-specific memory CD8+ T cells exist in healthy individuals and in patients with chronic myelogenous leukemia before and after stem cell transplantation. Blood. 2003;102:2892–2900. doi: 10.1182/blood-2003-01-0150. [DOI] [PubMed] [Google Scholar]
- 14.Molldrem JJ, Lee PP, Kant S, Wieder E, Jiang W, Lu S, et al. Chronic myelogenous leukemia shapes host immunity by selective deletion of high-avidity leukemiaspecific T cells. J Clin Invest. 2003;111:639–647. doi: 10.1172/JCI16398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rezvani K, Price DA, Brenchley JM, Kilical Y, Gostick E, Sconocchia G, et al. Transfer of PR1-specific T-cell clones from donor to recipient by stem cell transplantation and association with GvL activity. Cytotherapy. 2007;9:245–251. doi: 10.1080/14653240701218524. [DOI] [PubMed] [Google Scholar]
- 16.Gannage M, Abel M, Michallet AS, Delluc S, Lambert M, Giraudier S, et al. Ex vivo characterization of multiepitopic tumor-specific CD8 T cells in patients with chronic myeloid leukemia: implications for vaccine development and adoptive cellular immunotherapy. J Immunol. 2005;174:8210–8218. doi: 10.4049/jimmunol.174.12.8210. [DOI] [PubMed] [Google Scholar]
- 17.Hope KJ, Jin L, Dick JE. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat Immunol. 2004;5:738–743. doi: 10.1038/ni1080. [DOI] [PubMed] [Google Scholar]
- 18.Greiner DL, Hesselton RA, Shultz LD. SCID mouse models of human stem cell engraftment. Stem Cells. 1998;16:166–177. doi: 10.1002/stem.160166. [DOI] [PubMed] [Google Scholar]
- 19.Bonnet D, Warren EH, Greenberg PD, Dick JE, Riddell SR. CD8(+) minor histocompatibility antigen-specific cytotoxic T lymphocyte clones eliminate human acute myeloid leukemia stem cells. Proc Natl Acad Sci USA. 1999;96:8639–8644. doi: 10.1073/pnas.96.15.8639. [DOI] [PMC free article] [PubMed] [Google Scholar]