Abstract
Before a bolus is pushed into the pharynx, oral sensory processing is critical for planning movements of the subsequent pharyngeal swallow, including hyoid bone and laryngeal (hyo-laryngeal) kinematics. However, oral and pharyngeal sensory processing for hyo-laryngeal kinematics is not fully understood. In 11 healthy adults, we examined changes in kinematics with sensory adaptation, sensitivity shifting, with oropharyngeal swallows vs. pharyngeal swallows (no oral processing), and with various bolus volumes and tastes. Only pharyngeal swallows showed sensory adaptation (gradual changes in kinematics with repeated exposure to the same bolus). Conversely, only oropharyngeal swallows distinguished volume differences, whereas pharyngeal swallows did not. No taste effects were observed for either swallow type. The hyo-laryngeal kinematics were very similar between oropharyngeal swallows and pharyngeal swallows with a comparable bolus. Sensitivity shifting (changing sensory threshold for a small bolus when it immediately follows several very large boluses) was not observed in pharyngeal or oropharyngeal swallowing. These findings indicate that once oral sensory processing has set a motor program for a specific kind of bolus (i.e., 5 ml water), hyo-laryngeal movements are already highly standardized and optimized, showing no shifting or adaptation regardless of repeated exposure (sensory adaptation) or previous sensory experiences (sensitivity shifting). Also, the oral cavity is highly specialized for differentiating certain properties of a bolus (volume) that might require a specific motor plan to ensure swallowing safety, whereas the pharyngeal cavity does not make the same distinctions. Pharyngeal sensory processing might not be able to adjust motor plans created by the oral cavity once the swallow has already been triggered.
Keywords: sensory adaptation, deglutition, hyoid bone, larynx
oropharyngeal swallowing relies heavily on sensory information provided by the bolus (32). Reduction of sensory information, such as topical anesthesia and multiple dry swallows, delays swallowing initiation (18, 19, 33). Conversely, heightened sensory information (i.e., sour bolus) can accelerate swallowing initiation in dysphagic patients (17) and modify swallowing in healthy adults (9, 13). Thus the initiation of pharyngeal swallowing and its subsequent cascade of events are both regulated by sensory input. Many agree that sensory-motor processing in the oral cavity is responsible for preparing a bolus that the pharynx can safely manage while providing sensory input about the impending bolus (6). Yet the extent to which the pharyngeal cavity processes sensory information that affects swallowing kinematics is not completely understood. This could be important if an error occurs once the bolus is already transferred from the oral cavity to the pharyngeal cavity, possibly requiring the pharynx to modify its motor plan.
Irrepressible pharyngeal swallows (or reflexively elicited pharyngeal swallows) have been investigated. Shaker et al. (31) compared oropharyngeal swallowing to isolated pharyngeal swallowing (bolus delivered directly to the pharynx) and found no differences in bolus flow or movements of the velum, hyoid bone, or larynx with water swallowing. Pouderoux et al. (27) also elicited isolated pharyngeal swallows with varied temperatures and tastes and found no changes in the latency to swallow onset. These experiments suggest that the control of swallowing elicitation (investigator or participant), the location of elicitation (oral vs. pharyngeal), and the bolus properties (temperature, taste) have no bearing on the subsequent cascade of swallowing events in healthy adults.
However, before such conclusions are accepted, it is necessary to compare oropharyngeal swallowing with isolated pharyngeal swallowing, considering other kinematics such as range of motion and timing as well as other bolus properties (i.e., volume or taste). Also, repeated bolus presentation to the oral or pharyngeal regions might further differentiate swallowing kinematics, as in the case of sensory adaptation or sensitivity shifting (discussed below).
When a bolus is delivered to the oral cavity and swallowing kinematics modulate with different bolus properties (i.e., volume), the assumption is that ongoing sensory input from the oral sensory receptors is updating the motor response on a bolus-by-bolus basis. Although Pouderoux et al. (27) found no differences after altering taste or temperature with pharyngeal delivery, it is not completely clear whether other bolus properties can modify swallowing kinematics with pharyngeal bolus delivery. Also, there is even less information about the effects of a repeated bolus on swallowing kinematics when delivered to the mouth or to the pharynx. Gradual changes due to redundant bolus exposures could mean that the motor plan is being steadily modified to reach optimal swallowing functioning.
Sensory adaptation occurs when sensory receptors alter their sensitivity to a repeated stimulus and is represented as a gradual loss or gain in response to that stimulus (20). Sensory motor changes due to adaptation are often implicit (3) and may occur secondary to pain (22) or in the presence of a perturbation to reduce an error (29). In the oropharynx, one's perception of oropharyngeal sensation also adapts (7, 34, 35), yet it is not clear whether oropharyngeal sensation can manifest as changes in swallowing kinematics.
Sensitivity shifting, another way of testing sensory adaptation, occurs when there is a change in the ability to discriminate a particular stimulus (20). Sensitivity shifts have been found in the auditory, olfactory, gustatory, and tactile systems (8, 15, 21, 30) as well as in sensory motor systems for locomotion (1). A well-known example of sensitivity shifting is a temporary threshold shift (TTS or auditory fatigue) where a temporary hearing loss occurs after exposure to loud noise. Sensitivity shifting might be important for swallowing because it suggests that repeated exposure to only one type of bolus for a fixed amount of time (i.e., very large bolus) might alter sensitivity, and possibly swallowing kinematics, to a new type of bolus (i.e., very small bolus) that is presented immediately after.
The goal of this study was to answer four questions using hyoid bone and laryngeal kinematics as the outcome measure: 1) Does sensory adaptation occur after repeated exposure to a fixed stimulus over several swallows? 2) Does sensitivity shifting occur with a small bolus that immediately follows several very large boluses? 3) Does the location of delivery (oral vs. pharyngeal) alter hyoid or laryngeal kinematics? 4) Are volume or taste effects evident within both oral and pharyngeal delivery locations?
We hypothesized that both sensory adaptation and sensitivity shifting in hyo-laryngeal kinematics would be observed. We predicted that hyo-laryngeal movements during swallowing would not differ by location of delivery [as in Shaker et al. (31)], but that only volume and taste comparisons within the oral cavity would show significant volume or taste effects.
The results of these experiments are important to the design of rehabilitation strategies. Patients with sensory-motor deficits that lead to dysphagia might have differences in sensory adaptation or might require different types of sensory information to elicit a swallow response. Furthermore, our findings will emphasize the importance of sensory processing in the oral cavity, which can be accessed for treatment, on pharyngeal swallowing.
MATERIALS AND METHODS
The institutional review board of the Johns Hopkins School of Medicine approved this study. All participants provided written informed consent prior to participating in this study and were screened for conditions that might affect their swallowing or performance in this study, such as previous neurological, psychiatric, gastrointestinal conditions, or disorders of the mouth or neck.
General procedures.
Eleven healthy volunteers (6 women; mean age 26 ± 6 yr) swallowed water 72 times while seated during videofluoroscopy to image hyoid and laryngeal movements during swallowing. Videofluoroscopy is a radiographic technique that allows imaging and analysis of swallowing kinematics. We obtained full resolution fluoroscopic digital images in real time (30 frames/s) in the lateral plane. The image intensifier was focused on the oral cavity, the posterior pharyngeal wall, and just below the upper esophageal sphincter in the lateral plane. A time stamp was superimposed onto images as a unique identifier of each frame. All data collected were digital and synchronized using Chart software 7 Pro (ADInstruments).
Condition categories are divided into location of delivery (oral vs. pharyngeal), taste (sour bolus: citric acid USP 0.65 g/100 ml distilled water, odorless), and volume. Water swallowing was chosen rather than barium sulfate to avoid constipation, as this protocol requires consuming more than 550 ml of thin liquid. The 0.65 g/100 ml citric acid concentration was chosen for a sour bolus because it was associated with gradual increases in blood oxygen level-dependent signal (BOLD signal) in the primary sensory cortex and inferior parietal cortex in healthy adults (10). Room temperature distilled water was manually delivered either to the oral or pharyngeal cavity via a syringe (BD brand: 1 ml, 5 ml, 30 ml) that was connected to a flexible, plastic 33-in. tube (diameter 2 mm). For oral delivery, the plastic tube was positioned just inside the lips aimed in the posterior direction. For pharyngeal delivery the tube was housed in a custom mouthpiece using dental impression material and terminated near the posterior pharyngeal wall. Each custom mouthpiece was created with dental impression material (Extrude MVP, Kerr MFG) and adhered to the hard palate and top molars to maintain the position of the tube for pharyngeal bolus delivery. Wet, unformed dental impression material was placed in an upper-palate dental tray that adhered to the hard palate. While wet, before it was placed in each participant's mouth, the tube was pressed into the dental impression material (end facing posteriorly), and then the dental tray was affixed to the palate to form and harden. The drying process lasted 2 min.
Pharyngeal boluses were rapidly delivered (∼1 s) with the goal of eliciting an irrepressible pharyngeal swallow response, as previously described (31). Orally delivered boluses were slowly infused (∼4 s) and participants were expected to wait for a visual cue to begin swallowing. The visual cue provided information about the volume, taste of the upcoming bolus and the “go” cue to swallow (oral only). For oral swallows, participants were instructed not to swallow before the visual cue and any inadvertent preswallow was excluded from the analysis. A bolus was in their mouths for ∼2 s before the “go” cue.
Study design.
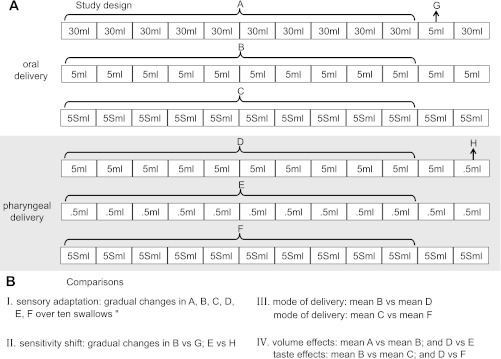
There were six swallowing series evenly divided by delivery location (Fig. 1A). For oral delivery, the three series included: 1) eleven 30 ml water swallows plus one 5 ml swallow (incongruent trial); 2) twelve 5 ml water swallows, and 3) twelve 5 ml sour swallows. For pharyngeal delivery, the three series included: 1) eleven 5 ml water swallows plus one 0.5 ml swallow (incongruent trial); 2) twelve 0.5 ml water swallows, and; 3) twelve 5 ml sour swallows (Fig. 1A). The intertrial interval within each series was 15 s. The order was randomized, first by delivery location and second within delivery location. Whenever a water series followed a sour bolus series, participants flushed their oral cavities with water to avoid taste contamination on water trials. Incongruent trials did not occur at the same time within a series to minimize prediction by the participant.
Fig. 1.
Study design (A) and all comparisons (B). Sequences are randomized first by bolus delivery location, second by sequence within the location.
Data analysis, comparisons, and statistics.
The investigators performing the kinematic analyses were blinded to volume, taste, and trial order. All videofluoroscopic recordings were digitized using Peak Motus (ViconPeak, Centennial, CO) version 9 for kinematic analysis. We measured range of motion and timing of the hyoid bone and larynx by digitizing the trajectory of the hyoid bone and larynx for each swallow (no hyoid bone reaction time for oral swallows, caveat described below) (Fig. 2).
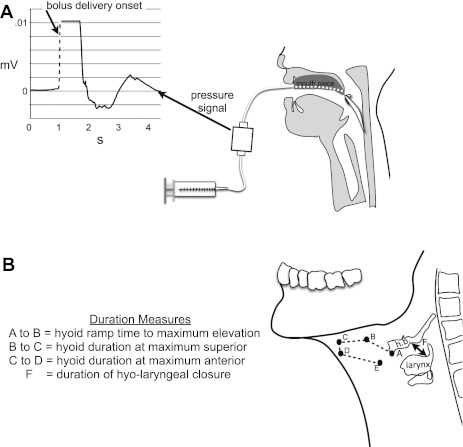
Fig. 2.
A: schematic of hyoid bone reaction time for pharyngeal delivery swallows. Dotted line indicates onset of bolus delivery (time one) used to derive swallowing reaction times for pharyngeal delivery swallows. B: schematic of definition of all duration measures of the hyoid bone and larynx movement made for all swallows. Points A through E show the full trajectory of the hyoid bone that was marked. hb, hyoid bone; F, the time that the laryngeal vestibule is closed (time from first contact between arytenoids and base of epiglottis until last contact); HX, hyoid anterior displacement; HY, hyoid superior displacement; LY, laryngeal superior displacement.
Range of motion.
To measure the change in position of the larynx, we extracted values for the superior/posterior aspect of the subglottal air column (y-axis) and the hyoid bone was tracked with its anterior/inferior most point (x- and y-axes). Therefore, we had three range of motion measures, which were horizontal (HX) and vertical (HY) hyoid movements and laryngeal vertical movements (LY). HX, HY, and LY were determined by subtracting the maximum extent of movement values from the average starting point for each swallow. The fifth cervical vertebra was also used to adjust for any whole body movement during swallowing and a radio-opaque sphere was used to calibrate the digitized images into millimeters (11).
Temporal/duration measures.
We considered five temporal measures of the hyoid bone and larynx (Fig. 2). They included hyoid bone reaction time (time between bolus delivery and onset of hyoid bone movement)(Fig. 2A), hyoid bone ramp time (time from hyoid bone movement onset until first max peak elevation)(A to B in Fig. 2B), duration of time of hyoid bone at peak elevation and at anterior positions (B to C and C to D in Fig. 2B), and duration of laryngeal closure (F in Fig. 2B) (16, 28). For reaction times of pharyngeal delivery, we tracked the bolus delivery with a pressure transducer (ADInstruments) that was positioned between the syringe and the mouthpiece (Fig. 2A), which provided a clear, objective signal of bolus delivery as used in Pouderoux et al. (27). The rise in signal from the pressure transducer indicating bolus delivery onset (time one) (Fig. 2A, dotted line) was subtracted from the time of onset of hyoid bone movement (time two) to derive swallowing reaction times for pharyngeal delivery swallows. The signal from the pressure transducer was synchronized with the videofluoroscopic images. We could not measure hyoid bone reaction time in the oral delivery swallows because it is necessary to visualize the location of the bolus (16) (we used water with videofluoroscopy) and it is not possible to determine the location of the water bolus with videofluoroscopy.
For each of the 8 measures (i.e., HX, reaction time), all swallows (72 total) were scaled using a range from 0 (lowest mm value) to 100 (highest mm value) individually for each participant. This was needed to adjust for individual differences in range of hyo-laryngeal movement during swallowing that might exacerbate the variance, thus our data are presented in percent range.
Four different comparisons are made in this study (Fig. 1B). (1) To answer the question: Does sensory adaptation occur after repeated exposures to a fixed stimulus over several swallows? We examined gradual increases or decreases in hyo-laryngeal kinematics over ten swallows within each of the six series (comparison I in Fig. 1B). (2) To test whether sensitivity shifts occur with a small bolus that immediately follows several very large boluses, the incongruent trials were compared with the average of their corresponding series. That means that the 5 ml swallow toward the end of the 30 ml oral delivery series was compared with the average of first ten swallows of the 5 ml oral delivery series. The same comparison was made within the pharyngeal delivery swallows where the 0.5 ml swallow at the end of the 5 ml swallow series was compared with the average of the first ten swallows in the 0.5 ml pharyngeal swallow series. These comparisons tested whether the experience of swallowing a series of large boluses altered sensitivity to the small bolus that immediately followed (comparison II in Fig. 1B). (3) To answer the question: Does location of delivery (oral vs. pharyngeal) alter hyoid or laryngeal kinematics? We compared the mean of the first ten 5 ml water bolus swallows between oral delivery and pharyngeal delivery trials and the mean of the first ten 5 ml sour water bolus swallows between oral delivery and pharyngeal delivery trials (comparison III in Fig. 1B). (4) Finally, to test whether volume or taste effects are evident within oral delivery or within pharyngeal delivery swallows, we compared the mean of the first ten 30 ml to 5 ml (oral) and 0.5 ml to 5 ml (pharyngeal) swallows for volume, and the 5 ml sour and 5 ml water trials were also compared within oral and pharyngeal delivery locations independently (comparison IV in Fig. 1B).
Just before each of the 72 swallows (during the 15-s intertrial interval), a visual cue was provided about the upcoming bolus, indicating the volume of the bolus (0.5 ml, 5 ml, or 30 ml) and whether it was water or sour water. However, both incongruent trials were intentionally mislabeled, so that the visual cue for the 5 ml incongruent trial in the 30 ml oral delivery series was labeled “30 ml water” and the 0.5 ml incongruent trial in the 5 ml pharyngeal delivery series was labeled “5 ml water”. This was necessary to ensure that the participant was not expecting a change in the bolus type.
Statistical analysis.
Statistical testing was done using separate repeated-measures mixed model multi-factorial ANOVA (repeated variable: subject; fixed variable: condition, i.e., delivery location, volume, taste) for all measures, including Bonferroni test for multiple comparisons. Post hoc testing compared the incongruent trials with its corresponding means using an ANOVA (2 levels). Sensory adaptation over ten trials was tested with a least squares regression analysis. To test inter-rater and intra-rater reliability of all measurements we computed intraclass correlation coefficients (ICC) on 10% of the data (5). The ICC represents the proportion of total variation (between-subject variability and measurement variability) that may be attributed to between-subject variability. Values near 1 suggest nearly all variability is essentially biological, whereas values near 0 indicate that variability is primarily a result of measurement problems. All statistical analyses were completed with SYSTAT version 13.
RESULTS
All participants were able to complete the study without adverse events. Although aspiration could not be objectively confirmed in this study, we recorded signs of aspiration for each swallow (throat clears and coughing) and observed very few incidences based on delivery location (oral 1%, pharyngeal 2%) or for the incongruent trials (<1%). The inter-rater reliability was very good or excellent (< 0.829) for all measures except hyoid duration at maximum anterior and superior positions, which were moderate (Table 1).The kinematics results for range of motion (mm) and temporal measures (ms) can be viewed in Table 2.
Table 1.
Measurement inter-rater and intra-rater reliability for range of motion and timing measures
| Measure | Intra-Rater | Inter-Rater |
|---|---|---|
| Range of motion | ||
| HY | 0.930 | 0.967 |
| LY | 0.958 | 0.898 |
| HX | 0.972 | 0.948 |
| Timing | ||
| Hyoid at max anterior | 0.576 | 0.543 |
| Hyoid at max superior | 0.672 | 0.654 |
| Hyoid ramp time | 0.971 | 0.989 |
| Hyo-laryngeal closure | 0.829 | 0.971 |
| Hyoid reaction time | 0.991 | 0.999 |
HY, hyoid vertical movement; LY, laryngeal vertical movement; HX, hyoid horizontal movement).
Table 2.
Kinematic results for each measure by condition, for incongruent trials, and sensory adaptation means across 10 swallows (only two measures with significant results)
| Condition Means | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Condition | HY | SE | LY | SE | HX | SE | DOHME | SE | DOHMA | SE | DTMLE | SE | LVC | SE | RT | SE |
| 5 ml oral | 12.90 | 1.41 | 26.71 | 2.71 | 12.51 | 0.98 | 89.73 | 13.09 | 96.00 | 6.92 | 136.64 | 27.14 | 410.30 | 33.49 | N/A | N/A |
| 30 ml oral | 18.10 | 1.44 | 27.25 | 2.71 | 14.65 | 0.99 | 96.94 | 13.04 | 98.88 | 6.60 | 290.79 | 24.97 | 524.82 | 34.92 | N/A | N/A |
| 5 ml sour oral | 12.89 | 1.42 | 26.04 | 2.73 | 13.13 | 0.92 | 97.71 | 12.96 | 98.40 | 6.23 | 184.19 | 26.88 | 431.73 | 35.40 | N/A | N/A |
| 0.5 ml pharyngeal | 14.49 | 1.44 | 25.37 | 2.50 | 11.60 | 1.24 | 83.45 | 11.48 | 91.09 | 7.14 | 110.73 | 23.05 | 375.55 | 29.21 | 1153.64 | 407.24 |
| 5 ml pharyngeal | 13.08 | 1.44 | 24.49 | 2.49 | 11.23 | 1.20 | 80.45 | 7.68 | 86.73 | 7.74 | 169.45 | 24.82 | 457.36 | 29.98 | 994.91 | 415.68 |
| 5 ml sour pharyngeal | 14.15 | 1.44 | 24.59 | 2.48 | 12.92 | 1.07 | 90.00 | 7.10 | 88.58 | 7.74 | 192.67 | 28.09 | 504.55 | 39.40 | 999.91 | 402.24 |
| Incongruent Trials | ||||||||||||||||
| 5 ml oral | 16.07 | 2.71 | 24.96 | 2.92 | 12.99 | 0.93 | 78.00 | 13.56 | 120.00 | 18.97 | 105.00 | 27.29 | 399.00 | 31.95 | N/A | N/A |
| 0.5 ml pharyngeal | 15.56 | 2.72 | 24.79 | 2.91 | 9.78 | 0.89 | 90.00 | 18.97 | 87.00 | 17.92 | 84.00 | 27.13 | 400.00 | 31.45 | 1119.00 | 322.82 |
| Sensory adaptation | ||||||||||||||||
| HX swallows 1-10 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||||||
| 5 ml pharyngeal | 10.73 | 9.52 | 10.77 | 10.83 | 12.24 | 11.49 | 11.42 | 11.76 | 11.56 | 11.95 | ||||||
| Error | 1.41 | 1.64 | 1.10 | 1.00 | 1.28 | 1.11 | 1.69 | 1.17 | 1.09 | 0.84 | ||||||
| LY swallows 1-10 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||||||
| 5 ml sour water | 26.78 | 25.73 | 24.20 | 25.66 | 24.39 | 22.70 | 25.86 | 23.82 | 23.96 | 23.65 | ||||||
| Error | 3.04 | 3.33 | 4.01 | 3.14 | 2.47 | 2.88 | 3.81 | 3.35 | 3.21 | 3.10 | ||||||
HY, hyoid elevation (mm); LY, laryngeal elevation (mm); HX, hyoid anterior movement (mm); DOHME, duration of max hyoid elevation (ms); DOHMA, duration of max hyoid anterior movement (ms); DTMLE, duration until max hyoid elevation (ms); LVC, laryngeal vestibule closure (ms); RT, hyoid bone reaction time (m; pharyngeal swallows only); SE, standard error of the mean. Sensory adaptation means only show those measures with significant changes over time.
Does sensory adaptation occur after repeated exposure to a fixed stimulus over several swallows?
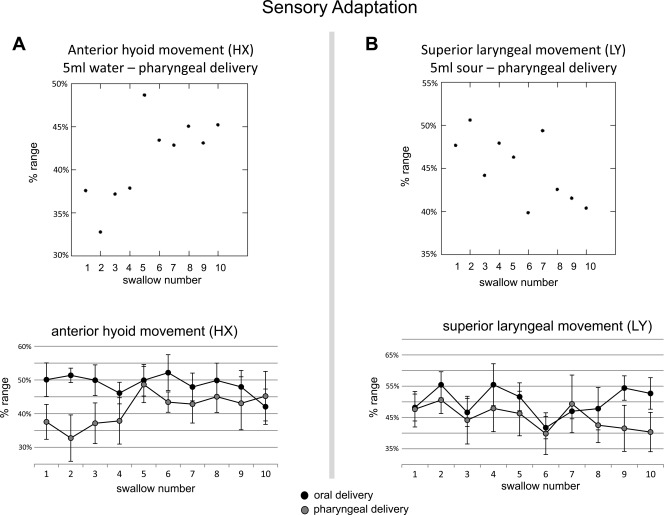
Evidence of sensory adaptation was only found with pharyngeal delivery. Pharyngeal delivery of 5 ml water over ten consecutive swallows revealed increasingly more anterior hyoid movement (P = 0.018; r = 0.464)(Fig. 3A). Pharyngeal delivery of 5 ml sour liquid resulted in increasingly less superior laryngeal movement over ten consecutive swallows (P = 0.037; r = 0.367) (Fig. 3B). Unlike pharyngeal delivery, oral delivery showed little to no linear change over time (P ≤ 0.054).
Fig. 3.
Sensory adaptation results. A (top): scatter plot of mean anterior hyoid movement (HX) over ten 5 ml water swallows for pharyngeal delivery (r = 0.464) showing increasingly anterior hyoid movement over time. A (bottom): line graph contrasting mean anterior hyoid movement (HX) over ten 5 ml water swallows between oral delivery (●) and pharyngeal delivery ( ). B (top): scatter plot of mean superiorlaryngeal movement (LY) over ten 5 ml sour water swallows for pharyngeal delivery (r = 0.367) showing diminishing superiorlaryngeal movement over time. B (bottom): line graph contrasting mean superiorlaryngeal movement (LY) over ten 5 ml sour water swallows between oral delivery (●) and pharyngeal delivery (
). B (top): scatter plot of mean superiorlaryngeal movement (LY) over ten 5 ml sour water swallows for pharyngeal delivery (r = 0.367) showing diminishing superiorlaryngeal movement over time. B (bottom): line graph contrasting mean superiorlaryngeal movement (LY) over ten 5 ml sour water swallows between oral delivery (●) and pharyngeal delivery ( ).
).
Do sensitivity shifts occur with a small bolus that immediately follows several very large boluses?
Sensitivity shifts were not found in pharyngeal or oropharyngeal swallowing (P ≤ 0.18).
Does the location of delivery (oral vs. pharyngeal) alter hyoid or laryngeal kinematics?
There were no differences in hyoid or laryngeal kinematics when oral delivery and pharyngeal delivery swallowing were compared (P ≤ 0.191).
Are volume or taste effects evident within oral or within pharyngeal delivery swallows?
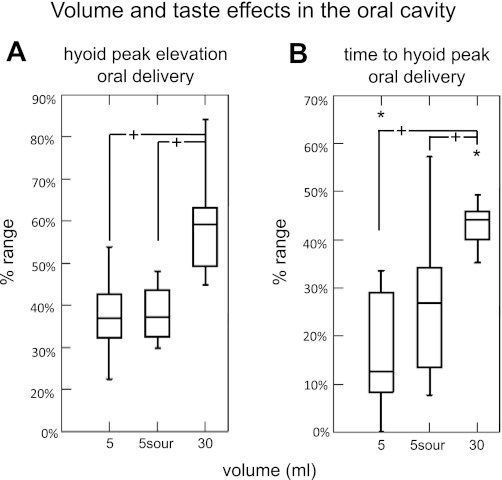
Volume comparisons for oral delivery revealed significantly more hyoid elevation for the 30 ml swallow compared with the 5 ml swallow (P = 0.001)(Fig. 4A). Hyoid bone time to peak elevation (ramp time) was significantly longer for 30 ml swallows compared with 5 ml swallows (P = 0.017; Fig. 4B). No taste effects were found with oral delivery for any measure. No volume or taste effects were found with pharyngeal delivery swallows (P ≤ 0.149).
Fig. 4.
Volume and taste effects for oral delivery. A: box plots of mean hyoid superior movement showing significantly more elevation with 30 ml swallows, but no differences for sour swallows. B: box plots of mean time to hyoid peak position showing significantly longer duration with 30 ml swallows, but no differences for sour swallows. +Statistically significant. *Outlier data point.
DISCUSSION
Our goal was to investigate the role of sensation on hyo-laryngeal swallowing kinematics in a novel way by manipulating the bolus and its presentation in an experimental setting. We have evidence that swallowing kinematics during the pharyngeal phase does adapt over several trials, but is not susceptible to sensory shifts in healthy young adults. Our data further highlight the role of oral sensory processing and volitional posterior propulsion by contrasting them with comparable conditions that were delivered directly to the pharynx.
Sensory adaptation.
Only isolated pharyngeal swallows showed gradual increases or decreases with repeated stimuli over time. Oral delivery of a bolus resulted in highly regular hyo-laryngeal kinematics. Therefore, the oral cavity likely integrates multiple forms of sensory information about a bolus and standardizes the motor plan for pharyngeal events at the most optimal level possible, yielding a more stable kinematic outcome over several trials. On the other hand, pharyngeal bolus delivery is novel and might have involved gradual, implicit learning over repeated exposures in an attempt to attain the most optimal outcome. Participants were likely learning and adjusting with isolated pharyngeal swallows. Furthermore, with pharyngeal bolus delivery, the pharynx is responsible for both sensory input and constriction in a very short period of time to achieve a safe swallow.
It is notable that only range of motion measures showed significant, gradual changes for sensory adaptation, whereas timing measures did not. The kinematic measures that changed gradually were hyoid anterior range of motion, which increased with repeated 5 ml water swallows and extent of laryngeal elevation, which decreased in duration with repeated 5 ml sour water swallows. No changes were observed with 0.5 ml overtime, possibly because it was a manageable volume throughout the series. Future studies should investigate whether a more challenging oral delivery swallow (i.e., 40 ml bolus) changes over time, under the assumption that initial trials will be less optimal.
In an fMRI study, we previously showed that the cortex gradually modifies its processing of a series of ten, oropharyngeal 5 ml water swallows, demonstrated by increasing signal (sensitization) bilaterally in the anterior cingulate cortex (10). The anterior cingulate cortex is commonly involved in cortical processing of swallowing and swallowing-related activities (12, 23). Although our hyo-laryngeal kinematic analysis did not reveal sensory adaptation in oropharyngeal swallows, changing cortical processing of this sensory-motor behavior is evident and warrants further examination.
Sensitivity shifting.
The kinematics of a small bolus were not significantly different after a series of very large boluses. Although oral and pharyngeal sensory processing integrates a vast array of diverse sensory receptors, they were not influenced by prior experiences and might process each bolus on a case-by-case basis.
Humans eat a diet with a wide variety of tastes, bite sizes, viscosities, and temperatures, often in one meal. So, this case-by-case processing might be a safety function. For example, multiple small boluses followed by a very large one might result in an error in sensory-motor integration that could cause aspiration during a poorly planned swallow. It is possible that we did not observe sensitivity shifting because we chose to change the bolus volume (instead of another property such as temperature, for instance) or because we suddenly decreased the volume, rather than increasing it. Future studies are warranted to investigate this phenomenon fully.
Location of delivery, volume, and taste effects.
Our data support the findings published by Shaker et al. (31). We found that hyoid and laryngeal kinematics in both oral and pharyngeal bolus delivery were very similar. For volume and taste effects on kinematics, only the oropharyngeal swallows showed differences for 30 vs. 5 ml boluses (6-fold difference), whereas a 10-fold difference with pharyngeal delivery of water (0.5 vs. 5 ml) did not reveal significant kinematic differences. The volume differences in oropharyngeal swallows show that 30 ml swallowing requires greater extent of hyoid elevation and, thus, longer durations to reach that peak. Overall, we did not find taste effects in the oral or pharyngeal delivery swallows. Pouderoux et al. (27) also reported no taste differences in latency to swallow onset with quinine, citric acid (approximately USP 0.63 g/100 ml distilled water), or glucose tastants in isolated pharyngeal swallows, nor did they report temperature effects. Their sour bolus concentration was very similar to ours. Although previous studies have reported differences in submental electromyography recordings with sour swallowing (2, 4, 14, 25, 26), we are not aware of any investigation that sought to examine hyo-laryngeal kinematics with a tastant. In a previous functional neuroimaging study we compared ten 5 ml sour boluses with ten 5 ml water boluses using the same sour bolus concentration. Sour swallows were associated with significantly more BOLD response in the right supplementary motor area (involved in motor planning) in healthy adults (10). Therefore, it is possible that oropharyngeal swallowing of citric acid at our chosen concentration (USP 0.65 g/100 ml distilled water) does involve differences in sensory-motor processing that might not be observed in hyoid or laryngeal swallowing kinematics. Thus the hyo-laryngeal kinematics in isolated pharyngeal swallows are not affected by the way that swallowing is elicited, whether investigator or participant controlled, oral vs. pharyngeal, or specific to the bolus properties such as volume, taste, or temperature.
Limitations.
As in Shaker et al. (31) and Pouderoux et al. (27), we have elicited a pharyngeal swallow reflexively, which requires directly delivering a bolus to the pharynx either transnasally or through the posterior oral cavity. Both methods are somewhat invasive, requiring perfusion tubing, and might have altered sensory processing for swallowing. Also, swallowing involves a great deal of individual variability, including hyo-laryngeal kinematics (24), thus broad interpretations about swallowing kinematics across healthy adults should be interpreted with caution. However, the sample size that we have was sufficient to find significant differences as well as enough power to overcome individual variability.
Conclusions.
Future studies will be needed to test other forms of stimulation in different populations to determine whether the findings in this study are specific to our experimental procedures. For instance, pharyngeal activity might yield different results than hyo-laryngeal kinematics. Nonetheless, we show that when sensory receptors in the pharyngeal cavity are solely responsible for eliciting the pharyngeal response, they gradually modify hyoid and laryngeal kinematics over repeated exposures to the same stimulus (sensory adaptation). With oral delivery, conversely, sensory adaptation was not observed. This dissimilarity between the two locations of delivery suggests that the motor plan for the pharyngeal swallow is set at the time of posterior oral propulsion in oropharyngeal swallowing (based on oral sensory processing). This might explain why patients might not be able to correct errors in the pharyngeal swallow (i.e., hyo-laryngeal movements) while the pharyngeal swallow is ongoing, even if part of the bolus has already entered the airway during swallowing. It also highlights the importance of accurate oral sensory processing of the oral cavity, which is encouraging for swallowing rehabilitation, as the oral cavity is more accessible for intervention than the pharynx.
GRANTS
This study was funded by the National Institutes of Health, NIDCD 1K23DC010776-01, 2009-2014.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: I.A.H. conception and design of research; I.A.H., A.L., H.C., and A.S. performed experiments; I.A.H., A.L., H.C., and A.S. analyzed data; I.A.H. and R.Z.G. interpreted results of experiments; I.A.H. prepared figures; I.A.H. drafted manuscript; I.A.H. and R.Z.G. edited and revised manuscript; I.A.H. and R.Z.G. approved final version of manuscript.
REFERENCES
- 1. Bunday KL, Reynolds RF, Kaski D, Rao M, Salman S, Bronstein AM. The effect of trial number on the emergence of the “broken escalator” locomotor after effect. Exp Brain Res 174: 270–278, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Chee C, Arshad S, Singh S, Mistry S, Hamdy S. The influence of chemical gustatory stimuli and oral anaesthesia on healthy human pharyngeal swallowing. Chem Senses 30: 393–400, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Cressman EK, Salomonczyk D, Henriques DY. Visuomotor adaptation and proprioceptive recalibration in older adults. Exp Brain Res 205: 533–544, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Ding R, Logemann JA, Larson CR, Rademaker AW. The effects of taste and consistency on swallow physiology in younger and older healthy individuals: a surface electromyographic study. J Speech Lang Hear Res 46: 977–989, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Fleiss JL. The Design and Analysis of Clinical Experiments. New York: Wiley, 1999, p. 1–11 [Google Scholar]
- 6. Foster KD, Grigor JM, Cheong JN, Yoo MJ, Bronlund JE, Morgenstern MP. The role of oral processing in dynamic sensory perception. J Food Sci 76: R49–61, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Fucci D, Petrosino L, Harris D, Randolph-Tyler E, Wagner S. Lingual vibrotactile threshold shift during magnitude-estimation scaling: effects on magnitude-estimation responses and scaling behavior. Percept Psychophys 46: 275–278, 1989 [DOI] [PubMed] [Google Scholar]
- 8. Gagnon P, Mergler D, Lapare S. Olfactory adaptation, threshold shift and recovery at low levels of exposure to methyl isobutyl ketone (MIBK). Neurotoxicology 15: 637–642, 1994 [PubMed] [Google Scholar]
- 9. Hoffman MR, Ciucci MR, Mielens JD, Jiang JJ, McCulloch TM. Pharyngeal swallow adaptations to bolus volume measured with high-resolution manometry. Laryngoscope 120: 2367–2373, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Humbert IA, Joel S. Tactile, gustatory, and visual biofeedback stimuli modulate neural substrates of deglutition. Neuroimage 59: 1485–1490, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Humbert IA, Poletto CJ, Saxon KG, Kearney PR, Crujido L, Wright-Harp W, Payne J, Jeffries N, Sonies BC, Ludlow CL. The effect of surface electrical stimulation on hyolaryngeal movement in normal individuals at rest and during swallowing. J Appl Physiol 101: 1657–1663, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Humbert IA, Robbins J. Normal swallowing and functional magnetic resonance imaging: a systematic review. Dysphagia 22: 266–275, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jacob P, Kahrilas PJ, Logemann JA, Shah V, Ha T. Upper esophageal sphincter opening and modulation during swallowing. Gastroenterology 97: 1469–1478, 1989 [DOI] [PubMed] [Google Scholar]
- 14. Leow LP, Huckabee ML, Sharma S, Tooley TP. The influence of taste on swallowing apnea, oral preparation time, and duration and amplitude of submental muscle contraction. Chem Senses 32: 119–128, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Leung YY, Bensmaia SJ, Hsiao SS, Johnson KO. Time-course of vibratory adaptation and recovery in cutaneous mechanoreceptive afferents. J Neurophysiol 94: 3037–3045, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lof GL, Robbins J. Test-retest variability in normal swallowing. Dysphagia 4: 236–242, 1990 [DOI] [PubMed] [Google Scholar]
- 17. Logemann JA, Pauloski BR, Colangelo L, Lazarus C, Fujiu M, Kahrilas PJ. Effects of a sour bolus on oropharyngeal swallowing measures in patients with neurogenic dysphagia. J Speech Hear Res 38: 556–563, 1995 [DOI] [PubMed] [Google Scholar]
- 18. Mansson I, Sandberg N. Oro-pharyngeal sensitivity and elicitation of swallowing in man. Acta Otolaryngol (Stockh) 79: 140–145, 1975 [DOI] [PubMed] [Google Scholar]
- 19. Mansson I, Sandberg N. Salivary stimulus and swallowing reflex in man. Acta Otolaryngol (Stockh) 79: 445–450, 1975 [DOI] [PubMed] [Google Scholar]
- 20. McBurney DH, Balaban CD. A heuristic model of sensory adaptation. Atten Percept Psychophys 71: 1941–1961, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Melnick W. Human temporary threshold shift (TTS) and damage risk. J Acoust Soc Am 90: 147–154, 1991 [DOI] [PubMed] [Google Scholar]
- 22. Michelotti A, Farella M, Martina R. Sensory and motor changes of the human jaw muscles during induced orthodontic pain. Eur J Orthod 21: 397–404, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Michou E, Hamdy S. Cortical input in control of swallowing. Curr Opin Otolaryngol Head Neck Surg 17: 166–171, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Molfenter SM, Steele CM. Physiological variability in the deglutition literature: hyoid and laryngeal kinematics. Dysphagia 26: 67–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Palmer PM, McCulloch TM, Jaffe D, Neel AT. Effects of a sour bolus on the intramuscular electromyographic (EMG) activity of muscles in the submental region. Dysphagia 20: 210–217, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Pelletier CA, Dhanaraj GE. The effect of taste and palatability on lingual swallowing pressure. Dysphagia 21: 121–128, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Pouderoux P, Logemann JA, Kahrilas PJ. Pharyngeal swallowing elicited by fluid infusion: role of volition and vallecular containment. Am J Physiol Gastrointest Liver Physiol 270: G347–G354, 1996 [DOI] [PubMed] [Google Scholar]
- 28. Robbins J, Hamilton JW, Lof GL, Kempster GB. Oropharyngeal swallowing in normal adults of different ages. Gastroenterology 103: 823–829, 1992 [DOI] [PubMed] [Google Scholar]
- 29. Rochet-Capellan A, Ostry DJ. Simultaneous acquisition of multiple auditory-motor transformations in speech. J Neurosci 31: 2657–2662, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schifferstein HN. Sweetness suppression in fructose/citric acid mixtures: a study of contextual effects. Percept Psychophys 56: 227–237, 1994 [DOI] [PubMed] [Google Scholar]
- 31. Shaker R, Ren J, Zamir Z, Sarna A, Liu J, Sui Z. Effect of aging, position, and temperature on the threshold volume triggering pharyngeal swallows. Gastroenterology 107: 396–402, 1994 [DOI] [PubMed] [Google Scholar]
- 32. Steele CM, Miller AJ. Sensory input pathways and mechanisms in swallowing: a review. Dysphagia 25: 323–333, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Teismann IK, Steinstraeter O, Stoeckigt K, Suntrup S, Wollbrink A, Pantev C, Dziewas R. Functional oropharyngeal sensory disruption interferes with the cortical control of swallowing. BMC Neurosci 8: 62, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weinstein S. Effects of local anesthetics on tactile sensitivity thresholds for cutaneous and mucous membranes. J Invest Dermatol 69: 136–145, 1977 [DOI] [PubMed] [Google Scholar]
- 35. Yamagiwa M, Fukuo H, Sakakura Y, Miyoshi Y. Sensation elicited by mechanical stimuli to the oropharyngeal mucosa. Auris Nasus Larynx 11: 37–42, 1984 [DOI] [PubMed] [Google Scholar]