Abstract
In addition to dysregulated JAK/STAT signaling, activation of the AKT/mTOR pathway occurs in myelofibrosis, a myeloproliferative neoplasm with no approved therapies. We conducted a phase 1/2 study with everolimus, an mTOR inhibitor, in 39 high- or intermediate-risk primary or postpolycythemia vera/postessential thrombocythemia myelofibrosis subjects. Responses were evaluated in 30 patients of phase 2. No dose-limiting toxicity was observed in phase 1 up to 10 mg/d. When this dose was used in phase 2, grade ≥ 3 toxicities were infrequent; the commonest toxicity was grade 1-2 stomatitis. Rapid and sustained splenomegaly reduction of > 50% and > 30% occurred in 20% and 44% of subjects, respectively. A total of 69% and 80% experienced complete resolution of systemic symptoms and pruritus. Response in leukocytosis, anemia, and thrombocytosis occurred in 15%-25%. Clinical responses were not associated with reduced JAK2V617F burden, circulating CD34+ cells, or cytokine levels, whereas CCDN1 mRNA and phospho-p70S6K level, known targets of mTOR, and WT1 mRNA were identified as possible biomarkers associated with response. Response rate was 60% when European Network for Myelofibrosis criteria were used (8 major, 7 moderate, 3 minor responses) or 23% when IWG-MRT criteria (1 partial response, 6 clinical improvements) were used. These results provide proof-of-concept that targeting mTOR pathway in myelofibrosis may be clinically relevant.
Introduction
Myelofibrosis (MF) is a BCR/ABL1-negative chronic myeloproliferative neoplasm (MPN). It can occur as a primary disease (primary MF; PMF) or as the evolution of a previous polycythemia vera (PPV-MF) or essential thrombocythemia (PET-MF).1 The disease manifests with various abnormalities of leukocyte and platelet count, anemia, splenomegaly, constitutional symptoms (night sweats, weight loss, fever), pruritus, and thrombosis.2 Survival ranges from < 2 years to > 15 years, depending on the different risk categories that are accurately identified by the international prognostic score system (IPSS) developed by the International Working Group for Myelofibrosis Research and Treatment (IWG-MRT).3,4 No curative treatment exists apart from allogeneic stem cell transplantation, which is offered to selected, intermediate- or high-risk patients.5
MF is characterized by mutations in JAK2 (V617F) and MPL (W515) in approximately 60% and 8% of cases, respectively; other less-common mutations in TET2, ASXL1, IDH1/IDH2, CBL, LNK, IKZF1, and EZH2 have been reported.6–8 A prominent feature of the pathogenesis of MF, as well as of MPN in general, is the direct or indirect activation of the JAK/STAT pathway by mutated proteins,9 providing the rationale for the use of JAK2 inhibitors.10 However, dysregulated activation of the PI3K/Akt and ERK downstream pathways in MPN cells has also been described,11–13 and we recently reported that RAD001, a specific inhibitor of mammalian target of rapamycin (mTOR) signaling, prevented proliferation of MPN cell lines and primary cells14 (C. Bogani, manuscript submitted, June 2011).
The PI3K/AKT/mTOR pathway is frequently activated in human cancers and plays a critical role in cell growth, proliferation, survival, apoptosis, autophagy, as well as angiogenesis.15,16 A variety of signals, including hormones, growth factors, and nutrients, directly or indirectly lead to Akt and mTOR activation via PI3K. mTOR activity is associated with 2 multiprotein complexes, mTORC1 and mTORC2. mTORC1 contains raptor protein and intervenes in the regulation of mRNA transcription through phosphorylation of the ribosomal S6 kinase (p70S6K) and the eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1). The function of mTORC2, which contains rictor protein, is still poorly understood; however, it phosphorylates Akt at Ser473, protein kinase C α, and the serum glucocorticoid-regulated kinase 1, contributing to actin regulation, cytoskeleton formation, and cell survival. Rapamycin (sirolimus) is the founding member of a family of potent inhibitors of PI3K/AKT/mTOR pathway, collectively known as rapalogs. Initially used as immunosuppressants, temsirolimus and everolimus (RAD001) have been shown to yield survival benefits in patients with renal-cell carcinoma and mantle-cell lymphoma, reinforcing interest in this class of compounds as anticancer drugs.17 We report here the results of a phase 1/2 trial with everolimus in patients with primary and postpolycythemia vera/postessential thrombocythemia MF.
Methods
Trial design
The study was registered at ANZCTR number 12608000614392 (http://www.anzctr.org.au/trial_view.aspx?ID=83290) as a multicenter phase 1/2 trial aimed at evaluating the safety and efficacy of single-agent everolimus in MF. Phase 1 was designed to determine the maximum tolerated dose (MTD) determined by dose-limiting toxicity of everolimus in 3 dose-escalating cohorts at 5.0, 7.5, and 10.0 mg daily for 3 months, with 3 patients initially enrolled in each cohort (a “3 + 3” escalation rule; supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). On definition of the MTD, phase 2 was initiated for assessment of efficacy of everolimus according to a Simon 2-stage design, with 16 and 14 subjects in portion 1 and 2, respectively. Duration of treatment in phase 2 was 4 months. The protocol was reviewed and approved by the Istituto Superiore di Sanità and by the Ethics Committee of participating centers in Florence, Pavia, and Bergamo, Italy. Patients were informed and provided their written consent in accordance with the Declaration of Helsinki. For the full protocol of this trial, see https://heart.negrisud.it/rad001/login.php.
Patients
Adult subjects with a diagnosis of PMF or PPV/PET-MF according to the World Health Organization18 and IWG-MRT19 criteria who had not received previous treatment and were in need of treatment or were already treated but required further treatment because of persistent disease were all considered eligible for enrolment in the study. Requirement of treatment was defined as a disease falling into the intermediate- or high-risk category according to Lille scoring system (supplemental Table 1)20 or, if in the low-risk category, having a splenomegaly > 10 cm below the left costal margin (LCM). A BM biopsy, performed at the time of enrolment unless available in the previous 6 months, was required to confirm diagnosis and exclude an excess of blast cells. Additional eligibility and exclusion criteria are listed in supplemental Table 2. We used the Lille score for patient enrolment because it represented the most used scoring system at the time the trial was designed. However, to facilitate comparison with recently published trials, a posthoc analysis of risk category patient disposition according to the subsequently developed IPSS score (supplemental Table 3) was also performed.
Assessment of toxicity and efficacy
Safety was assessed monthly in both phase 1 and 2 of the study, whereas response was measured at the end of treatment. Grading of toxicity was performed by use of the National Cancer Institute Common Terminology Criteria for Adverse Events v3. Evaluation of spleen size was performed by accurate measurement of the farthest distance between spleen tip and LCM at the mammillary line by the use of a ruler; such measurement was performed independently by 2 investigators at baseline and at the end of treatment. According to the protocol design, the rate of clinical response was measured with the European Network for Myelofibrosis (EUMNET) response criteria (supplemental Table 4)21; however, a posthoc analysis with the IWG-MRT criteria (supplemental Table 5)22 was also performed to facilitate comparison with other recent trials. Furthermore, because at the time of trial design a validated patient self-assessment form was not available,23 response in constitutional symptoms (unexplained recurrent fever, a > 10% weight loss, drenching night sweats) and pruritus was not graded, but it was declared only in case the patients referred complete, durable resolution of the symptom(s).
Assessment of laboratory correlates
Several markers reported to be associated with the disease or representing known targets of everolimus were assessed. Measurement of JAK2V617F or MPLW515L/K allele burden was performed in granulocytes.24,25 Circulating CD34+ cells were enumerated according to the International Society of Hematotherapy and Graft Engineering criteria. Level of WT1 mRNA was measured by real-time quantitative PCR by the use of a commercially available assay (ProfileQuant Kit; Ipsogen), whereas cyclin D1 (CCDN1) or glut1 mRNA expression level was quantified in purified granulocytes by the use of predesigned assays from Applied Biosystem; the reference housekeeping gene was RNase-P.
For Western blot analysis of the phosphorylation status of p70S6K, peripheral blood cells were resuspended in RIPA lysis buffer (50mM Tris-HCl, pH 7.4; 150mM NaCl; 1% NP-40, 1mM EDTA) containing a proteinase inhibitor cocktail (Halt Protease Inhibitor Cocktail Kit; Pierce), and the extracted proteins were subjected to SDS-PAGE separation and Western blotting onto Immunoblot PVDF membrane (BioRad), according to standard protocols. Membranes were probed with primary antibody against phospho-p70S6K, total p70S6K (Cell Signaling Technology), and α-tubulin (Sigma-Aldrich), followed by HRP-conjugated anti-Ig antibody produced in rabbit (Sigma-Aldrich). Immunoreactive proteins were revealed with enhanced chemiluminescence with the Image Quant 350 apparatus (GE Healthcare). Quantification of plasma cytokines and inflammatory proteins was performed by the use of multiplexed immunoassays (human MAP panel v1.6) at Rules-Based Medicine Inc.
Statistical methods
Statistical testing of the efficacy hypothesis was performed only for the 30 patients included in phase 2. For the 9 patients included in phase 1, results about efficacy were merely described for each dose escalation cohort, without statistical testing, and they were not included in efficacy analysis. For phase 2, the efficacy analysis was designed on a total of 30 patients in a Simon 2-stage design to test the null hypothesis that the observed proportion of responses (complete, major, or moderate according to EUMNET criteria) was outside the range < 0.05 > 0.20 at an α level of 0.05 with power of 80%. Therefore, if 4 or more patients of 30 achieved a response, then the activity of the drug would be considered promising. Study analysis was intention to treat, with all patients who received at least 1 dose of RAD001 being eligible for analysis. In the per-protocol analysis, we included the 25 patients who completed the scheduled 4-month treatment period (83% of all subjects in phase 2). Safety data were reported as cumulative incidence of events. Responses were treated as categorical variables. Logistic regression and ordinal logistic regression were used to assess the association of a series of explanatory variables with response to treatment. All statistical tests were 2-sided. All data were collected through a web portal (https://heart.negrisud.it/rad001/login.php) and analyzed by the Data Coordinator core at Institute Mario Negri Sud, Chieti. All authors had access to the primary trial data.
Role of the funding source
The study was an investigator-promoted and -conducted independent trial that was financially supported by the Italian governmental drug agency (Agenzia Italiana per il Farmaco; AIFA) and in part by the nonprofit Associazione Italiana per la Ricerca sul Cancro. Novartis provided the drug free of cost but had no role in trial design and conduct, data collection, analysis, and interpretation or writing of the report. The corresponding author had full access to all of the data and the final responsibility for the decision to submit for publication.
Results
Patients characteristics
A total of 39 patients were enrolled; their clinical characteristics are reported in Table 1. Among the 30 subjects included in phase 2 for efficacy analysis, 16 had PMF, 8 PPV-MF, and 6 PET-MF. Twenty-one subjects (70%) had a JAK2V617F mutation, 2 had MPLW515L, and 1 had a MPLW515K mutation. The median duration of disease was 2.3 years (range, 0.1-20 years); 89% and 83% of the patients in phase 1 and 2, respectively, were unresponsive to at least 1 previous treatment, including cytotoxic drugs, erythropoiesis-stimulating agents, interferon, and immune modulators (details of the treatment history are provided in supplemental Table 6). The median value of palpable spleen size was 14 cm below the LCM; 83% of the patients had a spleen > 10 cm below the LCM. A total of 23% of the patients were considered transfusion-dependent, which was determined by a minimum requirement of 2 red cell transfusions per month in the previous 3 months. A total of 87% of the patients had constitutional symptoms, and 67 reported unexplained, intractable pruritus. Nine and twenty-one patients had Lille score 1 and 0, respectively; the latter group included most patients with large splenomegaly unresponsive to previous treatment(s). According to the IPSS criteria, 57%, 27%, and 16% of the patients were in high/intermediate-2, intermediate-1, and low-risk category, respectively.
Table 1.
Baseline characteristics of the patients
| Phase 1 | Phase 2 | |
|---|---|---|
| No. | 9 | 30 |
| Median age, y (range) | 59 (35-75) | 62 (37-79) |
| Sex (M/F) | 5/4 | 20/10 |
| PMF/PPV/PET | 7/2/0 | 16/8/6 |
| Median disease duration, mo (range) | 65 (7-124) | 27 (1-234) |
| Lille score, no. (%) | 7 (78) | 21 (70) |
| 0 | ||
| 1 | 2 (22) | 9 (30) |
| 2 | 0 (0) | 0 (0) |
| IWG-MRT, no. (%) | 3 (33.3) | 5 (16.7) |
| Low | ||
| Int-1 | 4 (44.4) | 8 (26.7) |
| Int-2 | 2 (22.2) | 9 (30) |
| High | 0 | 8 (26.6) |
| Median WBC × 109/L (range) | 12.2 (2.7-37.5) | 12.4 (4.1-50.3) |
| Median Hb g/L (range) | 126 (100-147) | 114 (75-173) |
| Median platelets × 109/L (range) | 250 (111-609) | 301 (99-1149) |
| Lactate dehydrogenase (mean ± SD) | 583.4 ± 169.8 | 977.4 ± 585.0 |
| JAK2V617F mutated, no. (%) | 7 (78) | 21 (70) |
| JAK2V617F allele burden (mean ± SD) | 55.4 ± 37.8 | 64.2 ± 20.8 |
| CD34+ cells, % (mean ± SD) | 0.24 ± 0.18 | 1.15 ± 1.73 |
| CD34+ cells × 106/L (mean ± SD) | 27.8 ± 34.4 | 96.8 ± 115.5 |
| Splenomegaly, cm from LCM (mean ± SD) | 14 (4-17) | 14 (2-25) |
| Previous therapy (%) | 8 (89) | 25 (83) |
| Transfusion dependent no. (%) | 2 (22.2) | 7 (23.3) |
| Pruritus, no. (%) | 4 (44.4) | 20 (67) |
| Constitutional symptoms no. (%) | 6 (66.7) | 26 (86.7) |
Int indicates intermediate; IWG-MRT, International Working Group for Myelofibrosis Research and Treatment; PET, previous essential thrombocythemia; PMF, primary myelofibrosis; and PPV, previous polycythemia vera.
Safety and adverse events
There was no dose-limiting toxicity in the 3 cohorts of patients enrolled in phase 1. Therefore, the 10 mg/d dose of everolimus was used as the MTD for expansion in phase 2. A total of 5 of 30 patients in phase 2 (17%) did not complete the scheduled 4-month treatment period. Reasons for permanent withdrawal were patient refusal in 1 case, physician's decision in 2 cases, and reversible grade 3 renal failure, considered not related to the drug, in 1 case. Finally, 1 patient with concomitant follicular lymphoma and chronic obstructive pulmonary disease developed grade 3 pneumonitis, which was considered as possibly related to the treatment; he died later because of multiorgan failure. Hematologic toxicity was relatively modest, mainly represented by decrease of hemoglobin that occurred in 8 patients (27%), 4 each of grade 2 and grade 3 anemia. A detailed analysis of the patients who developed anemia is presented in supplemental Table 7. Severe anemia improved (Hb > 80 g/L) at the end of treatment in all but 1 cases (Hb 75 g/L), suggesting that early toxicity on erythropoiesis could ameliorate during treatment; however, at a follow-up at 1-2 months after the end of treatment, baseline level of hemoglobin were recovered in all the 8 patients. Grade 2 neutropenia and thrombocytopenia occurred in 7% and 3% of the patients, respectively; they reverted at the end of treatment. Overall, nonhematologic effects of clinical relevance were infrequent, and mainly represented by grade1-2 stomatitis that occurred in 70% of the patients; only one grade 3 stomatitis was diagnosed (Table 2). Other commonest toxicities were transient elevation of cholesterol and triglyceride levels.
Table 2.
Nonhematological toxicities in patients included in phase 2
| Adverse event | All grade (%) | Grade > 3, % |
|---|---|---|
| Diarrhea | 6.7 | 0 |
| Fatigue | 16.9 | 0 |
| Musculoskeletal | 33.3 | 0 |
| Stomatitis | 70 | 3 |
| Skin disorders | 36.7 | 0 |
| Bleeding | 13.3 | 0 |
| Hypertriglyceridemia | 70 | 0 |
| Hypercholesterolemia | 23.3 | 0 |
| AST increase | 7 | 3 |
| ALT increase | 7 | 3 |
| Bronchitis | 3 | 0 |
| Pneumonitis | 7 | 7 |
| Urinary tract infection | 7 | 3 |
| Renal failure | 3 | 3 |
| Others | 20 | 0 |
ALT indicates alanine aminotransferase; and AST, aspartate aminotransferase.
Response
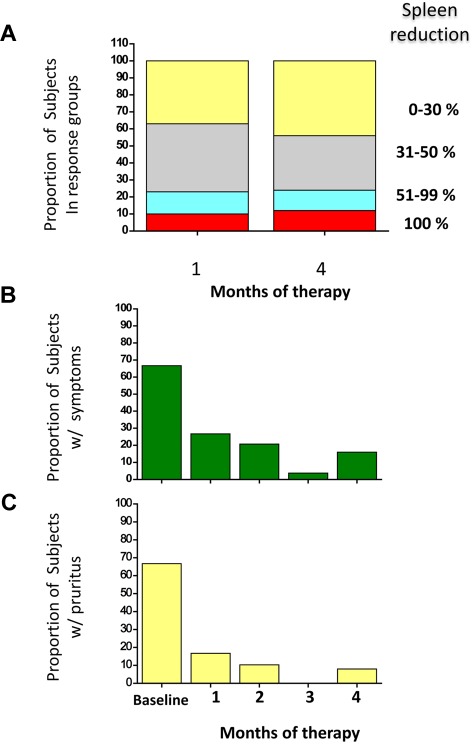
According to EUMNET criteria, in phase 1 we observed 2 moderate and 1 major response in the 5.0 mg/daily dose; 1 moderate and 2 “no response” in the 7.5 mg/daily; and 1 minor response and 2 “no response” in the 10 mg/daily dose. A detailed analysis of responses to treatment with everolimus in phase 2 is presented in Table 3.21 All the 30 patients were included in the intention-to-treat analysis; 25 (83%) were evaluated in the per-protocol analysis. The rate of responses were as follows: 25% for anemia, 15% for leukocytosis (complete response in 3 patients who normalized their leukocyte count), 25% for thrombocytosis (complete response in 5 patients), 44% for splenomegaly (2 patients had complete resolution of splenomegaly, and 11 had a partial response), whereas 69% and 80% of the patients reported complete resolution of constitutional symptoms and pruritus, respectively. Concerning responses in anemia, 5 patients achieved a partial response: 3 of them showed a decrease in transfusion requirement > 50%, whereas in 2 nontransfusion-dependent subjects the hemoglobin increased of a median of 2.5 g/dL. All responses occurred within 1 month of treatment, were maintained for the duration of treatment (Figure 1), and lasted > 3 months after trial termination.
Table 3.
Details of responses, according to EUMNET definitions, in patients included in Phase 2
| Response in | Intention to treat (n = 30)* | Per-protocol (n = 25) |
|---|---|---|
| Anemia | ||
| CR | 0 (0.0) | 0 (0.0) |
| PR | 5 (25.0) | 4 (23.5) |
| Pro | 0 (0.0) | 0 (0.0) |
| NR | 15 (75.0) | 13 (76.5) |
| Leukocyte count | ||
| CR | 3 (15.0) | 2 (11.8) |
| PR | 0 (0.0) | 0 (0.0) |
| NR | 17 (85.0) | 15 (88.2) |
| Platelet count | ||
| CR | 5 (25.0) | 4 (25.0) |
| PR | 0 (0.0) | 0 (0.0) |
| NR | 15 (75.0) | 12 (75.0) |
| Splenomegaly | ||
| CR | 2 (6.7) | 2 (8.0) |
| PR | 11 (36.7) | 10 (40.0) |
| Pro | 1 (3.1) | 0 (0.0) |
| NR | 16 (53.3) | 13 (52.0) |
| Constitutional symptoms | ||
| CR | 11 (68.7) | 11 (78.6) |
| Pro | 1 (6.2) | 1 (7.2) |
| NR | 4 (25.0) | 2 (14.3) |
| Pruritus | ||
| CR | 16 (80.0) | 14 (87.5) |
| NR | 4 (20.0) | 2 (12.5) |
Response in pruritus was declared only in subjects who reported CR of the symptom, such as for constitutional symptoms.
CR indicates complete response; EUMNET, European Network for Myelofibrosis;PR, partial response; Pro, progression; and NR, no response.
The number of patients evaluable for each of the end points differs depending on their baseline characteristics.
Figure 1.
Responses of splenomegaly, constitutional symptoms, and pruritus to RAD001 therapy. The proportion of patients who presented variable reduction of splenomegaly at 1 and 4 months of therapy, expressed as percent change from the baseline, is shown in panel A. The decrease of spleen enlargement was already maximal at 1 month, and it was maintained up to the end of treatment. Panel B and C represent the proportion of patients who presented constitutional symptoms (B) or pruritus (C) at baseline and their response to therapy at each month. Only patients who reported complete disappearance of night sweats, fever (B), or pruritus (C) were considered as responders.
According to the EUMNET criteria, 60% of patients were defined as responders; 8 patients presented major (27%), 7 moderate (23%), and 3 minor (10%) response (Table 4). Of these 12 were JAK2V617F-mutated (6 major, 5 moderate, 1 minor response), 4 were JAK2/MPL wild-type (1 major, 1 moderate, 2 minor response), whereas 2 MPLW515-mutated patients presented 1 major and 1moderate response, respectively.
Table 4.
Categorization of overall responses based on the EUMNET and IWG-MRT criteria
| Intention to treat (n = 30) | Per-protocol (n = 25) | |
|---|---|---|
| EUMNET criteria | ||
| Complete response | 0 (0.0) | 0 (0.0) |
| Major response | 8 (26.7) | 8 (32.0) |
| Moderate response | 7 (23.3) | 5 (20.0) |
| Minor response | 3 (10.0) | 2 (8.0) |
| No response | 12 (40.0) | 10 (40.0) |
| Progression | 0 (0.0) | 0 (0.0) |
| IWG-MRT criteria | ||
| Complete response | 0 (0.0) | 0 (0.0) |
| Partial remission | 1 (3.3) | 1 (4.0) |
| Clinical improvement | 6 (20.0) | 5 (20.0) |
| Progressive disease | 0 (0.0) | 0 (0.0) |
| Stable disease | 23 (76.7) | 19 (76.0) |
| Relapse | 0 (0.0) | 0 (0.0) |
EUMNET indicates European Network for Myelofibrosis; and IWG-MRT, International Working Group for Myelofibrosis Research and Treatment.
According to the IWG-MRT criteria, responses were obtained in 23% of the patients; clinical improvement was declared in 6 subjects (20%) and partial remission in one. Of these, 4 had a JAK2V617F mutation, 1 had a MPLW515 mutation, and 2 exhibited JAK2/MPL wild type. The remaining patients presented stable disease with no progression. Partial response was declared in a JAK2/MPL wild-type subject because of complete resolution of splenomegaly from a baseline of 5 cm from LCM and normalization of platelet count (304 × 109/L from 485 × 109/L at baseline). Clinical improvement was declared in 1 patient because of sustained hemoglobin increase from 75 g/L to 109 g/L and in 5 because of a response in splenomegaly. Of the 23 patients considered as having a “stable disease” per IWG-MRT criteria, 18 (78%) presented a median reduction of splenomegaly of 26% (8%-41%) from the baseline.
Effect on biologic markers
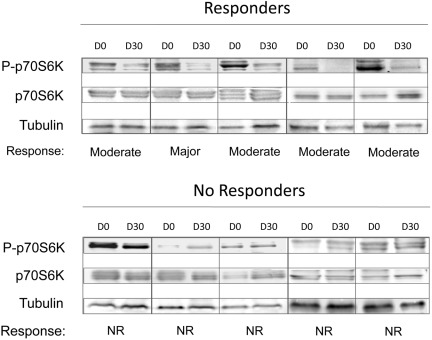
We measured the RNA level of CCDN1 and Glut-1, 2 genes known to be positively regulated by phosphorylated mTOR, in RNA purified from granulocytes at 1 month of treatment. We found that patients who had a response (according to EUMNET criteria) presented significantly lower CCDN1 mRNA level compared with nonresponders (55% ± 25% vs 195% ± 113% the baseline level in responders and nonresponders, respectively; P = .03; supplemental Figure 2). This figure was 60% ± 33% in responders versus 195% ± 289% in nonresponders defined according to the IWG-MRT criteria. However, there was no significant difference in the baseline expression level of CCDN1 between responders and no responders (supplemental Figure 3). At variance with CCDN1, Glut-1 mRNA expression level did not modify significantly compared with baseline (not shown). We also assessed, by Western blotting, the changes in phosphorylation status of the kinase p70S6K, a direct target of mTOR, using peripheral blood cells obtained from 5 patients each who showed, or did not show, a clinical response at the end of treatment (Figure 2). We found that responders displayed a uniform, although of various degrees, reduction in the level of p70S6K phosphorylation, unlike no responders in whom the level of phosphorylated p70S6K was steadily maintained (Figure 2).
Figure 2.
The phosphorylation status of the mTOR target, p70S6K, measured at 1 month of treatment, correlates with obtainment of clinical response. Proteins extracted from peripheral blood cells, collected at baseline (D0) and at 1 month of treatment (D30), were subjected to SDS-PAGE electrophoresis and Western blotting and probed with antibodies against the total and phosphorylated form of p70S6K; tubulin was used for loading normalization. Top, 5 patients who showed a major-to-moderate clinical response; bottom, 5 patients who were defined as nonresponders.
No significant change was observed in the number of circulating CD34+ cells during treatment (144 ± 206 106/L, 161 ± 248 106/L, 187 ± 249 106/L, and 151 ± 174 × 106/L at each month of treatment compared with 123 ± 88 × 106/L at baseline). Finally, the burden of V617F allele remained substantially stable over time: it was 62% ± 20%, 62% ± 17%, 59% ± 19%, and 62% ± 15% at each month versus 64% ± 21% at baseline. Five patients showed a decrease of 12% to 26% of V617F burden at the end of treatment that was not correlated with clinical response.
We then measured the expression levels of WT1 mRNA in granulocytes at baseline and at 1 month of treatment; WT1 mRNA is significantly overexpressed in patients with MF, and it was shown to correlate with high CD34+ cell count and (Lille) prognostic score.26 We found that the level of WT1 mRNA at 1 month of treatment resulted significantly lower in responders (WT1 mRNA copy number: 3458 ± 1692) compared with no responders (5579 ± 1448; P = .017). However, no difference was found in the WT1 mRNA copy number at baseline between subjects who were classified as responders compared with nonresponders (4318 ± 1952 and 4384 ± 2550, respectively).
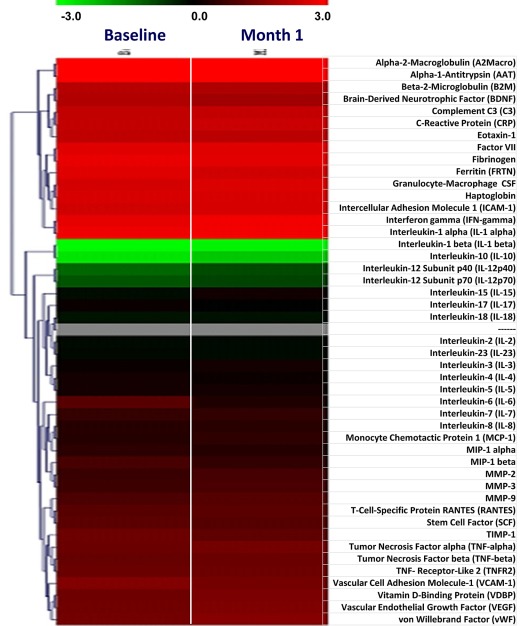
The level of 46 plasma proteins and inflammatory cytokines was measured at baseline and at 1 month of therapy (Figure 3). The baseline level of many inflammatory cytokines was markedly greater than the upper reference limit in control subjects, confirming recent reports27,28; however, we did not observe a normalization nor a significant down-regulation of most cytokines after treatment with everolimus, with the exception of a few, including IL-10, monocyte chemotactic protein-1, and macrophage inflammatory protein-1β that showed at least a 2-fold reduction compared with baseline (P < .05).
Figure 3.
Changes in plasma levels of selected cytokines and inflammatory markers in patients included in phase 2 of the study. Plasma levels of 46 cytokines and inflammatory proteins were evaluated in samples obtained at baseline and after 1 month of therapy. The HumanMAP Version 1.6 panel (Rules-Based Medicine), was used for this analysis. Results are shown as heat map, with each row indicating the plasma marker and the columns corresponding to individual patients. In the left part of the plot, corresponding to baseline samples, the green and red denote proteins that were present in the patients at lower or higher levels, respectively, relative to normal control subjects. In the columns on the right, corresponding to 1 month of treatment, red and green indicate the proteins whose levels increased or decreased, respectively, compared with the baseline level for the same patient.
Discussion
The pathogenesis of MPNs is mainly ascribed to an abnormal activation of the JAK2/STAT signaling because of mutations in JAK2 or other components of this pathway (such as MPL, Lnk, and possibly others). However, the activation of PI3K/Akt/mTOR signaling cascade also has been documented in JAK2V617F-mutated cells in vitro and in mice with a conditional JAK2V617F knockin allele.13 Inhibitors of the JAK/STAT or PI3K/Akt pathway caused comparable inhibition of spontaneous and erythropoietin-induced erythroid differentiation in cultures of PV progenitor cells.11 Increased phosphorylation of STAT5 and Akt was demonstrated by immunocytochemistry in the BM of patients with MPN.12 Finally, RAD001 prevented in vitro cytokine-stimulated and cytokine-independent growth of primary MPN patients' cells,14 overall providing a rationale for exploring the effectiveness of mTOR targeting in MPN treatment.
Small molecule inhibitors of JAK2 represent the current investigational scenario in the treatment of MF. However, it should be considered that currently no inhibitor specifically targets mutated JAK2, and the wild-type molecule is inhibited as well; furthermore, none of available molecules is a “pure” JAK2 inhibitor and most target other kinases in addition to JAK2. In a phase 2 study in patients with MF, the JAK1 and JAK2 inhibitor INCB18424 induced a substantial decrease of splenomegaly and caused rapid and durable control of constitutional symptoms and pruritus; myelosuppression was modest, mainly on erythroid cell lineage, and usually manageable with dose titration.27 Of note, only modest changes in JAK2V617F allele burden were documented in mutated patients, and the drug was similarly effective in those who lacked the mutation. Comparable clinical efficacy was described for the more JAK2 selective inhibitor TG10134829 and the JAK1/JAK2 inhibitor CYT387.30 However, TG101348 was found to exert more profound effects on the V617F allele burden, whereas preliminary results with CYT387 suggested activity in alleviating anemia.
The current study showed that the mTOR inhibitor everolimus displayed clinical activity in patients with MF with an acceptable safety profile. We documented that the drug was effective in ameliorating splenomegaly, systemic symptoms, and pruritus, thus reproducing most of the effects reported for JAK1 and JAK2 inhibitors. Myelosuppression was modest, and hematologic toxicity was mainly represented by grade 2/3 reversible decrease of hemoglobin; however, responses in anemia were conversely observed in 25% of anemic subjects, suggesting that such toxicity could be managed with dose titration. Similar to INCB18424 we documented irrelevant changes of JAK2V617F allele burden, although the short treatment period (4 months) could have masked any potential effect; at this regard, a decrease of allele burden in polycythemia vera patients receiving pegylated interferon became meaningful after 12 months. However, a median decrease of 62% of JAK2V617F allele burden was documented in 16 of 20 patients with MF after 6 months of treatment with TG101348.29
Reduction in splenomegaly was one critical determinant of the clinical response in this study. Changes in spleen enlargement were determined by accurate physical examination. A more precise evaluation of modifications in spleen volume caused by treatment could have been obtained by the use of imaging techniques, such as nuclear magnetic resonance (NMR); however, because of the investigator-initiated nature of the trial, this was not technically and economically feasible. However, it is worthwhile that in the phase 2 evaluation of the JAK1 and JAK2 inhibitor INC1842427 a direct comparison between clinical assessment of spleen size and NMR volumetric determination in 24 patients showed a very good parallel between the reduction in spleen volume detected by NMR and the reduction in spleen size detected by physical examination: after 6 months of therapy, the median reduction in spleen volume was 33% and the median reduction in spleen length was 52%.27
Similar to reports with the JAK1 and JAK2 inhibitors, the reduction of spleen enlargement in patients treated with everolimus occurred rapidly within the first month, without evidence of tumor cell lysis (no increase in lactate dehydrogenase or uric acid level) or progenitor cell migration (no change in CD34+ cell number in the circulation). At present, mechanism(s) for spleen shrinking are unknown and could involve either, or both, a selective targeting of clonal cells resident in the organ and/or changes in splenic vasculature. Finally, similar to the observations reported with JAK1 and JAK2 inhibitors, halting the treatment with everolimus was followed by a reenlargment of the spleen that occurred slowly during the course of a couple of months, without any illness (that could be attributed to a cytokine rebound), apart for the complaint of abdominal discomfort in patients with the largest spleen. This finding would suggest that continuous administration of everolimus is needed to obtain a maintained symptomatic control of the disease.
We have provided evidence that the clinical efficacy of everolimus was directly mirrored by changes in proteins that are involved in the activated mTOR pathway in patients with MF. p70S6K is a 70- to 85-KDa mitogen activated Ser/Thr protein kinase involved in the regulation of cell growth and G1 cell-cycle progression through phosphorylation of the S6 protein of the 40S ribosomal subunit. The activity of p70S6K is controlled by multiple phosphorylation events mediated by mTOR and other kinases as well. Phosphorylation of p70S6K (and of 4eBP1, another direct target of mTOR) results in enhanced transcription of target genes required for G1-S phase cell transition, including c-MYC, cyclin D1, and Glut-1. We found that changes in the expression level of cyclin D1 mRNA and the extent of phosphorylation of p70S6K at 1 month of treatment were both correlated with the occurrence of a clinical response, measured at the end of treatment. These observations suggest that both cyclin D1 and p70S6K can represent surrogate biomarkers of response.
Most of the clinical benefits reported in patients included in the phase 2 study with the JAK1 and JAK2 inhibitor INC18424 were attributed to an anti-JAK1 mediated, dramatic decrease of inflammatory cytokines,31 whose levels are greatly increased in most patients with MF. Here, on the contrary, the mechanism of action of everolimus was not apparently mediated by normalization of the inflammatory cytokine milieu; as a matter of fact, we found modest changes in circulating inflammatory proteins that were not meaningfully associated with clinical response. Of interest, similar findings have been reported with TG101348; however, although a more direct anticlonal activity was advocated in case of TG101348,29 the minimal changes of JAK2V617F allele burden observed in this everolimus trial are in favor of an alternative mechanism, yet to be clarified. Therefore, it is of interest that, in addition to the evidence that measurable mTOR inhibition was correlated with clinical response, we also observed a significant reduction of the level of WT1 mRNA that was considered as a marker of a more active disease.26
Two independent systems for evaluating response to treatment in MF, the EUMNET and the IWG-MRT, have been both developed in 2005 on the basis of expert consensus; they use different criteria and actually produced very different results in the analysis of this trial. To our knowledge, this is the first time they have been used comparatively in a clinical trial. We surmise that observed differences may be mainly attributable to the fact that the IWG-MRT system does not consider resolution of constitutional symptoms as a response end point unless in case of disease complete remission; definition of the latter requires BM histology remission and ideally corresponds to the concept of a “cure” that at present time can be achieved only with allogeneic stem cell transplantation. Thus, these 2 systems are hardly comparable. In particular, the IPSS largely disqualifies the improvement in constitutional symptoms that is one of the most notable effects of novel JAK1 and JAK2 inhibitors (or other novel drugs) and 1 distinctive advantage of these drugs over conventional therapies. Results of the current face-to-face comparison, within a single clinical trial, might finally suggest that both EUMNET and IWG-MRT response criteria systems should be critically revised to produce novel (hopefully, unified) response criteria that take into consideration the clinical improvements produced by novel drugs.
Notwithstanding the rate of responses evaluated with the IWG-MRT criteria in this single-dose everolimus trial was slightly lower than that reported with JAK1 and JAK2 inhibitors, the study provides proof-of-concept that targeting mTOR pathway in MF is clinically relevant and suggests the opportunity of further clinical experimentation with everolimus either as a single agent, perhaps with the use of different drug dosage and time schedules, or in combination with other novel molecules, such as histone deacethylase inhibitors,32 immunomodulators,33 and possibly others.
Supplementary Material
Acknowledgments
The study was supported by the Italian govern agency AIFA (Agenzia Italiana per il Farmaco), contract #FARM6AKR9W, and in part by Associazione Italiana per la Ricerca sul Cancro (Milano) “Special Program Molecular Clinical Oncology 5 × 1000” (#10005) to AGIMM (AIRC-Gruppo Italiano Malattie Mieloproliferative; a detailed description of the AGIMM project is available at http://www.progettoagimm.it).
Footnotes
Presented in part at the annual meeting of the American Society of Hematology, Orlando, FL, December 6, 2010.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: P.G. and A.M.V. were involved in the conception and design of the trial, patient enrollment, analysis and interpretation of the data, and writing the report; G.B., A.R., G.F., A.B., and T.B. contributed to trial design, enrollment of patients, and revised the manuscript; S.C. and S.P. provided scientific support; R.M., A.M., and E.M. contributed to design and implementation of data collection software, statistical design of the study, collected data, analyzed raw data, and performed statistical analysis; E.G., M.L.L., G.F., E.A., M.C.S., L.P., E.U., U.O., and A.G. enrolled patients and acted as reference physicians; N.B. performed Western blot analysis; L.T., F.B., and A.P. performed all laboratory analyses; and all authors have read, commented and approved the final version of the manuscript.
Conflict-of-interest disclosure: S.C. and S.P. are employees of Novartis Italia. The remaining authors declare no competing financial interests.
A complete list of AIRC-Gruppo Italiano Malattie Mieloproliferative investigators appears in the supplemental Appendix.
Correspondence: Alessandro M. Vannucchi, MD, Department of Medical and Surgical Care, Section of Hematology, University of Florence, Viale Morgagni 85, 50134 Florence, Italy; e-mail: amvannucchi@unifi.it.
References
- 1.Vannucchi AM, Guglielmelli P, Tefferi A. Advances in understanding and management of myeloproliferative neoplasms. CA Cancer J Clin. 2009;59(3):171–191. doi: 10.3322/caac.20009. [DOI] [PubMed] [Google Scholar]
- 2.Tefferi A. Myelofibrosis with myeloid metaplasia. N Engl J Med. 2000;342(17):1255–1265. doi: 10.1056/NEJM200004273421706. [DOI] [PubMed] [Google Scholar]
- 3.Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113(13):2895–2901. doi: 10.1182/blood-2008-07-170449. [DOI] [PubMed] [Google Scholar]
- 4.Passamonti F, Cervantes F, Vannucchi AM, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood. 2010;115:1703–1708. doi: 10.1182/blood-2009-09-245837. [DOI] [PubMed] [Google Scholar]
- 5.Tefferi A. How I treat myelofibrosis. Blood. 2011;117(13):3494–3504. doi: 10.1182/blood-2010-11-315614. [DOI] [PubMed] [Google Scholar]
- 6.Ernst T, Chase AJ, Score J, et al. Inactivating mutations of the histone methyltransferase gene EZH2 in myeloid disorders. Nat Genet. 2010;42(8):722–726. doi: 10.1038/ng.621. [DOI] [PubMed] [Google Scholar]
- 7.Tefferi A. Novel mutations and their functional and clinical relevance in myeloproliferative neoplasms: JAK2, MPL, TET2, ASXL1, CBL, IDH and IKZF1. Leukemia. 2010;24(6):1128–1138. doi: 10.1038/leu.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oh ST, Simonds EF, Jones C, et al. Novel mutations in the inhibitory adaptor protein LNK drive JAK-STAT signaling in patients with myeloproliferative neoplasms. Blood. 2010;116(6):988–992. doi: 10.1182/blood-2010-02-270108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levine RL, Pardanani A, Tefferi A, Gilliland DG. Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders. Nat Rev Cancer. 2007;7(9):673–683. doi: 10.1038/nrc2210. [DOI] [PubMed] [Google Scholar]
- 10.Geron I, Abrahamsson AE, Barroga CF, et al. Selective inhibition of JAK2-driven erythroid differentiation of polycythemia vera progenitors. Cancer Cell. 2008;13(4):321–330. doi: 10.1016/j.ccr.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 11.Ugo V, Marzac C, Teyssandier I, et al. Multiple signaling pathways are involved in erythropoietin-independent differentiation of erythroid progenitors in polycythemia vera. Exp Hematol. 2004;32(2):179–187. doi: 10.1016/j.exphem.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Grimwade LF, Happerfield L, Tristram C, et al. Phospho-STAT5 and phospho-Akt expression in chronic myeloproliferative neoplasms. Br J Haematol. 2009;147(4):495–506. doi: 10.1111/j.1365-2141.2009.07870.x. [DOI] [PubMed] [Google Scholar]
- 13.Akada H, Yan D, Zou H, Fiering S, Hutchison RE, Mohi MG. Conditional expression of heterozygous or homozygous Jak2V617F from its endogenous promoter induces a polycythemia vera-like disease. Blood. 2010;115(17):3589–3597. doi: 10.1182/blood-2009-04-215848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vannucchi AM, Bogani C, Bartalucci N, et al. The mTOR inhibitor, RAD001, inhibits the growth of cells from patients with myeloproliferative neoplasms. Blood. 2009;114:2914A. [Google Scholar]
- 15.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9(8):550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 16.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12(1):9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Albert S, Serova M, Dreyer C, Sablin M-P, Faivre S, Raymond E. New inhibitors of the mammalian target of rapamycin signaling pathway for cancer. Exp Opin Invest Drugs. 2010;19(8):919–930. doi: 10.1517/13543784.2010.499121. [DOI] [PubMed] [Google Scholar]
- 18.Tefferi A, Thiele J, Orazi A, et al. Proposals and rationale for revision of the World Health Organization diagnostic criteria for polycythemia vera, essential thrombocythemia, and primary myelofibrosis: recommendations from an ad hoc international expert panel. Blood. 2007;110(4):1092–1097. doi: 10.1182/blood-2007-04-083501. [DOI] [PubMed] [Google Scholar]
- 19.Barosi G, Mesa RA, Thiele J, et al. Proposed criteria for the diagnosis of post-polycythemia vera and post-essential thrombocythemia myelofibrosis: a consensus statement from the international working group for myelofibrosis research and treatment. Leukemia. 2008;22:437–438. doi: 10.1038/sj.leu.2404914. [DOI] [PubMed] [Google Scholar]
- 20.Dupriez B, Morel P, Demory JL, et al. Prognostic factors in agnogenic myeloid metaplasia: a report on 195 cases with a new scoring system. Blood. 1996;88(3):1013–1018. [PubMed] [Google Scholar]
- 21.Barosi G, Bordessoule D, Briere J, et al. Response criteria for myelofibrosis with myeloid metaplasia: results of an initiative of the European Myelofibrosis Network (EUMNET). Blood. 2005;106(8):2849–2853. doi: 10.1182/blood-2005-04-1520. [DOI] [PubMed] [Google Scholar]
- 22.Tefferi A, Barosi G, Mesa RA, et al. International Working Group (IWG) consensus criteria for treatment response in myelofibrosis with myeloid metaplasia, for the IWG for Myelofibrosis Research and Treatment (IWG-MRT). Blood. 2006;108(5):1497–1503. doi: 10.1182/blood-2006-03-009746. [DOI] [PubMed] [Google Scholar]
- 23.Scherber R, Dueck AC, Johansson P, et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood. 2011;118(2):401–408. doi: 10.1182/blood-2011-01-328955. [DOI] [PubMed] [Google Scholar]
- 24.Guglielmelli P, Barosi G, Specchia G, et al. Identification of patients with poorer survival in primary myelofibrosis based on the burden of JAK2V617F mutated allele. Blood. 2009;114(8):1477–1483. doi: 10.1182/blood-2009-04-216044. [DOI] [PubMed] [Google Scholar]
- 25.Pancrazzi A, Guglielmelli P, Ponziani V, et al. A sensitive detection method for MPLW515L or MPLW515K mutation in chronic myeloproliferative disorders with locked nucleic acid-modified probes and real-time polymerase chain reaction. J Mol Diagn. 2008;10(5):435–441. doi: 10.2353/jmoldx.2008.080015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guglielmelli P, Zini R, Bogani C, et al. Molecular profiling of CD34+ cells in idiopathic myelofibrosis identifies a set of disease-associated genes and reveals the clinical significance of Wilms' tumor gene 1 (WT1). Stem Cells. 2007;25(1):165–173. doi: 10.1634/stemcells.2006-0351. [DOI] [PubMed] [Google Scholar]
- 27.Verstovsek S, Kantarjian H, Mesa RA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363(12):1117–1127. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tefferi A, Vaidya R, Caramazza D, Finke C, Lasho T, Pardanani A. Circulating interleukin (IL)-8, IL-2R, IL-12, and IL-15 levels are independently prognostic in primary myelofibrosis: a comprehensive cytokine profiling study. J Clin Oncol. 2011;29(10):1356–1363. doi: 10.1200/JCO.2010.32.9490. [DOI] [PubMed] [Google Scholar]
- 29.Pardanani A, Gotlib JR, Jamieson C, et al. Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J Clin Oncol. 2011;29(7):789–796. doi: 10.1200/JCO.2010.32.8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pardanani A, George G, Lasho T, et al. A phase I/II study of CYT387, an oral JAK-1/2 inhibitor, in myelofibrosis: significant response rates in anemia, splenomegaly, and constitutional symptoms. Blood. 2010;116:460A. [Google Scholar]
- 31.Vannucchi AM. From palliation to targeted therapy in myelofibrosis. N Engl J Med. 2010;363(12):1180–1182. doi: 10.1056/NEJMe1005856. [DOI] [PubMed] [Google Scholar]
- 32.Rambaldi A, Dellacasa CM, Finazzi G, et al. A pilot study of the histone-deacetylase inhibitor givinostat in patients with JAK2V617F positive chronic myeloproliferative neoplasms. Br J Haematol. 2010;150(4):446–455. doi: 10.1111/j.1365-2141.2010.08266.x. [DOI] [PubMed] [Google Scholar]
- 33.Tefferi A, Verstovsek S, Barosi G, et al. Pomalidomide is active in the treatment of anemia associated with myelofibrosis. J Clin Oncol. 2009;27(27):4563–4569. doi: 10.1200/JCO.2008.21.7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.