Abstract
Direct transmission of avian influenza viruses to mammals has become an increasingly investigated topic during the past decade; however, isolates that have been primarily investigated are typically ones originating from human or poultry outbreaks. Currently there is minimal comparative information on the behavior of the innumerable viruses that exist in the natural wild bird host. We have previously demonstrated the capacity of numerous North American avian influenza viruses isolated from wild birds to infect and induce lesions in the respiratory tract of mice. In this study, two isolates from shorebirds that were previously examined in mice (H1N9 and H6N1 subtypes) are further examined through experimental inoculations in the ferret with analysis of viral shedding, histopathology, and antigen localization via immunohistochemistry to elucidate pathogenicity and transmission of these viruses. Using sequence analysis and glycan binding analysis, we show that these avian viruses have the typical avian influenza binding pattern, with affinity for cell glycoproteins/glycolipids having terminal sialic acid (SA) residues with α 2,3 linkage [Neu5Ac(α2,3)Gal]. Despite the lack of α2,6 linked SA binding, these AIVs productively infected both the upper and lower respiratory tract of ferrets, resulting in nasal viral shedding and pulmonary lesions with minimal morbidity. Moreover, we show that one of the viruses is able to transmit to ferrets via direct contact, despite its binding affinity for α 2,3 linked SA residues. These results demonstrate that avian influenza viruses, which are endemic in aquatic birds, can potentially infect humans and other mammals without adaptation. Finally this work highlights the need for additional study of the wild bird subset of influenza viruses in regard to surveillance, transmission, and potential for reassortment, as they have zoonotic potential.
Introduction
The host and virulence range for avian influenza viruses (AIV) continues to surprise, with numerous cases of direct transmission from birds to mammals that result in a range of disease including pneumonia, conjunctivitis, and occasionally systemic disease [1]–[3]. Although transmission of AIV to humans resulting in disease has been limited to poultry adapted viruses, there is evidence of both direct transmission of AIV to other mammalian species [3]–[5] and experimental evidence that numerous AIV hemagglutinin (HA) subtypes can infect mammals [5]–[10]. However, there remains a great void of knowledge regarding the capacity of AIV to infect mammals, especially related to AIVs from the wild bird reservoir. Human AIV infections have been limited to the H5, H7, and H9 subtypes [2], [11]–[14] and these viruses are of concern because they have a pandemic potential if they become highly transmissible in the human population. Recent studies have demonstrated the high compatibility of avian and human influenza reassortants in vitro and in vivo and generation of viable reassortants in vivo in ferrets, further raising the concern of the natural generation of a pandemic strain [15]–[18]. Examining the capacity of a spectrum of wild bird AIVs to infect mammals is necessary to complete our understanding of AIV host range restrictions and to better define potential risks of mammalian infection and viral reassortment.
We have previously screened wild bird AIVs in a mouse model and demonstrated their varying capacity to replicate in the lung of mice, with some isolates exhibiting robust pulmonary replication regardless of HA subtype and causing mild clinical disease [19]. Ferrets are a better model for influenza infection and transmission in humans as they are naturally susceptible to the virus and have a similar distribution of sialic acid glycans in the respiratory tract; they have also been used for numerous studies of AIV that have resulted in human disease [20]–[22]. In this study, two wild bird AIVs (H1N9 and H6N1 subtypes) that exhibited robust pulmonary replication in mice were further studied in a ferret model to better assess pathogenesis and transmission capacity in mammals.
Viral contributors to host range restriction and virulence of AIVs in mammals have been demonstrated to be a multifactorial. The interaction between the major viral glycoprotein, the hemagglutinin (HA) and the host cell sialic acid receptors is considered critical for establishing an influenza infection, and species specific binding restrictions have been identified. Influenza viruses of avian origin preferentially bind terminal sialic acids with a α2,3 linkage located in cells in the gastrointestinal tract of birds and on the ciliated cells and type II pneumocyte in the human respiratory tract [23]–[28]. Conversely, human influenza viruses exhibit preferential binding to terminal sialic acids with a α2,6 SA linkage located most prominently on non-ciliated cells of the human upper respiratory tract (nasopharynx and trachea) [23], [27], [29]–[32]. It is thought that the receptor specificity of influenza viruses is a large component of host restriction; where in some AIV cases (H5, H7, and H9 subtypes), the viruses are able to infect and cause disease in humans yet exhibit poor human to human transmission [14], [33]–[35].
The amino acid residues contributing to α2,3 versus α2,6 SA binding specificity have been described for some viruses and mutation analysis has shown some of these residues to be directly involved with altering viral receptor specificity. In human H3 strains, amino acids Leu226 and Ser228 (H3 numbering) results in α2,6 SA binding, where avian strains that preferentially bind α2,3 SA receptors exhibit a Gln226 and Gly228 amino acid sequence [26], [27], [32], [36]. Amino acid residues 138, 190, 194, and 225 (H3 numbering) have also been shown to be differentially conserved in avian and human influenza viruses [32].
Here we examine the potential for infection and transmission of an H1N9 (A/Ruddy Turnstone/DE/1171/02abbreviated H1N9) and an H6N1 (A/Ruddy Turnstone/DE/892/02abbreviated H6N1) AIV. Using sequence, in vitro binding, and glycan microarray analysis we determined the receptor specificity of these viruses. Using the ferret model, the most representative animal model of human influenza virus infection, we performed in vivo assessment of the potential for infection, replication, and transmission of these viruses in mammals. Despite a dominant α2,3 (avian) binding specificity we demonstrate that both of these viruses replicate in both the upper and lower respiratory tract of ferrets, inducing pulmonary lesions, but resulting in little morbidity. Moreover, we demonstrate that one of these viruses (H1N9) is able to transmit via direct contact, despite its dominant avian α2,3 SA binding preference. These findings support the recent findings of others [37] and taken together demonstrate that AIV circulating in wild bird populations can transmit to mammals, albeit not always causing clinical disease, and that transmissibility of AIV to mammals is not restricted to specific subtypes (i.e. H5N1 or H7). Further investigation of these circulating AIVs is warranted to better understand their zoonotic potential.
Results
Wild bird influenza viruses replicated in ferrets but exhibited low virulence
Infection in H1N9 and H6N1 inoculated ferrets and in H1N9 contact ferrets was demonstrated with presence of virus in nasal washes and seroconversion despite minimal clinical signs (Table 1). Three ferrets infected with H6N1 had transient, mild weight loss that was most prominent day 1 pi (Table 1), but two of these ferrets took an additional two to six days to return to pre-infection weight. In contrast, six out of seven ferrets infected with H1N9 had mild weight loss most prominent at day 1 post inoculation (pi) (Table 1) in which it took up to four days to return to pre-infection weight. The average temperature of H1N9 and H6N1 inoculated ferrets was most elevated on day 1 pi (Table 1). Direct contact H1N9 ferrets had elevated temperatures that did correlate with shedding of virus in nasal washes, while direct contact H6N1 ferrets, which did not become infected, had rare, inconsistent temperature elevations. Sneezing was not observed in any group of ferrets and all ferrets remained bright and alert through the duration of the study. There were no statistically significant differences in the total or differential leukocyte parameters for group by day interactions. However, mean lymphocytes (mean+/−standard error) decreased in both H6N1 (3.50+/−0.06 pre inoculation to 3.26+/−0.06 day 1 pi) and H1N9 (3.76+/−0.06 pre inoculation to 3.61+/−0.09 day 1 pi) inoculated groups compared to the allantoic inoculated group (3.46+/−0.05 pre inoculation to 3.45+/−0.06 day 1 pi) for day 1 pi, and then increased for the H1N9 (4.06+/−0.16 day 7 pi) inoculated group compared to the allantoic inoculated group (3.67+/−0.11 day 7 pi) and remained elevated through day 18 pi (Table S1).
Table 1. Morbidity, seroconversion, and respiratory viral replication of ferrets inoculated with wild bird avian influenza viruses H1N9 and H6N1.
| Inoculated animals | |||||||
| Clinical parameters | Virus shedding | Seroconversion | |||||
| Virus | Weight loss (max %, average %) | Range temperature increasea | Sneezing | Virus detection in nasal wash (peak log10 TCID50/mL)b | Average TCID50/g virus in lung (day p.i.)c | Number with seroconversion (HI titers) | Number with seroconversion (MN titers) |
| H6N1 | 3/7 (6.3, 5.8) | 0.1–1.6 | 0/3 | 7/7 (5.2) | Not detected (3,7) | 3/3 (1∶160, 1∶160, 1∶160) | 3/3 (1∶2560, 1∶1280, 1∶640) |
| H1N9 | 6/7 (12.8, 4.5) | 0.1–1.7 | 0/3 | 7/7 (5.8) | 5.1 (2); Not detected (3,7) | 3/3 (1∶40, 1∶40, 1∶40) | 3/3 (1∶320, 1∶640, 1∶320) |
| Allantoic fluid | 0/3 | 0.1–2.4d | 0/3 | 0/3 | NDe | 0/3f | ND |
Temperature is in degrees Celsius.
Limit of detection for nasal wash 1.5 log10 TCID50/mL.
Limit of detection for lung day 7 pi both viruses and day 3 pi for H6N1 is 1.3 TCID50/g; for days 2 and 3 pi for H1N9 is 1.0 TCID50/g.
One control ferret potentially had an unrelated infection, but remained influenza seronegative.
Not done.
Tested against both H6N1 and H1N9.
Both H1N9 and H6N1 wild bird influenza viruses replicated in the upper respiratory tract of ferrets
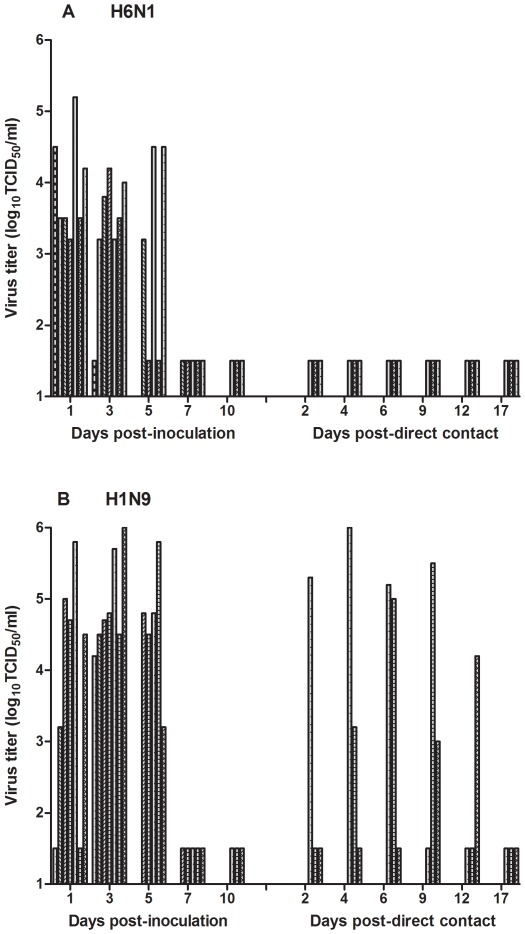
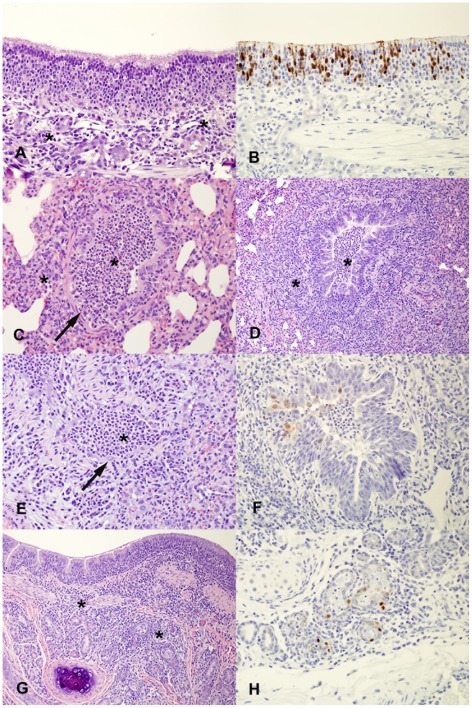
Both H1N9 and H6N1 demonstrated replication in the upper respiratory tract of ferrets, evident by nasal washes and immunohistochemistry (IHC). Although both H1N9 and H6N1 replicated in the nasal cavity, H1N9 replication was more robust, reaching consistently higher titers than H6N1 for days 3 and 5 pi with shedding occurring consistently longer (Figure 1A, B and Table 1). Lesions of influenza infection were present in the nasal turbinates of ferrets infected with H1N9 and H6N1 (Figure 2A), however, only a single ferret in the H6N1 group (day 3 pi) had lesions while nasal turbinates of all four ferrets infected with H1N9 had lesions (both days 3 and 7 pi). This correlates with the viral titers obtained from nasal washes of these ferrets, as viral shedding quickly declined over a short period of time in this group with some ferrets shedding virus only up to day 3 pi (Figure 1). Lesions in the nasal turbinates included infiltration of the submucosa with mild to moderate numbers of lymphocytes with fewer plasma cells and occasional submucosal edema on both days 3 and 7 pi, with an increased amount of inflammation on day 7 pi for H1N9 (Figure 2A). The presence of influenza viral antigen was confirmed by abundant strong intranuclear and frequent intracytoplasmic staining on IHC for the nucleoprotein (NP) of influenza A in the nasal turbinates epithelium (Figure 2B). Positive staining occurred on day 3 pi in the nasal turbinates of ferrets that had lesions and was not present by day 7 pi for both viruses.
Figure 1. Nasal shedding and direct contact transmission of wild bird influenza viruses in ferrets.
Seven ferrets were intranasally inoculated with 5×105 PFUs of either H6N1 (A) or H1N9 (B) and nasal washes were collected and titered on MDCK cells (days post-inoculation portion of graph). Three naïve ferrets were paired with three of the inoculated ferrets 24 hours post inoculation for each virus group (days post-direct contact portion); nasal washes were collected titered on MDCK cells. Both H6N1 and H1N9 demonstrated replication in the upper respiratory tract of the ferrets, however, viral shedding was consistently greater in magnitude and duration for H1N9. H1N9 demonstrated direct contact transmission, but H6N1 did not transmit to direct contact ferrets.
Figure 2. Histopathologic lesions and influenza antigen localization in ferrets inoculated with wild bird influenza viruses.
A) Nasal turbinates of ferrets inoculated with H6N1 or H1N9 demonstrated moderate submucosal inflammation (asterisk) (H1N9, d3pi). B) There is widespread strong intranuclear and some intracytoplasmic positive immunoreactivity for the nucleoprotein of influenza A on immunohistochemistry in ferrets inoculated with H6N1 or H1N9 on day 3 pi (H1N9, d3pi). C) Epithelial damage in the lung was early for ferrets inoculated with H1N9, with necrosis in the bronchioles (arrow) and inflammation (asterisks) within and around bronchioles on day 2 pi. D and E) There was evidence of early repair with regeneration of bronchiolar epithelium (arrow) and persistence of inflammation (asterisks) on day 3 pi for ferrets inoculated with H6N1 and H1N9 (H1N9, d3pi). The arrow highlights the stretched and plump bronchiolar epithelial cells, indicating regeneration. F) Presence of influenza antigen was confirmed in ferrets inoculated with H6N1 and H1N9 by strong positive intranuclear staining of bronchiolar epithelial cells with immunohistochemistry on day 3 pi (H6N1, d3pi). G) Inflammation around larger airways in the lung was also present in ferrets inoculated with H6N1 and H1N9, with prominent periglandular bronchial inflammation (asterisk) (H6N1, d7pi). H) There was strong positive intranuclear staining for the nucleoprotein of influenza in the peribronchial glandular epithelial cells on immunohistochemistry on day 3 pi in ferrets inoculated with H6N1 and H1N9 (H1N9, d3pi).
Both H1N9 and H6N1 wild bird influenza viruses replicated in the lower respiratory tract of ferrets
There was evidence of replication of both H1N9 and H6N1 in the lung of inoculated ferrets, with similar histopathologic progression of lesions for both viruses. Influenza was detected in the lung for both viruses for all ferrets day 3 pi using virus isolation in ECEs, but was not isolated for day 7 pi from the lung for either group of ferrets (Table 2). For both viruses, histopathologic change in the lung on day 3 pi was characterized by a small amount of mucus admixed with neutrophils and macrophages within the lumens of bronchi and large bronchioles with peribronchial inflammation, primarily lymphocytes, that surrounded and occasionally infiltrated peribronchial glands (Figure 2G). There was positive intranuclear immunoreactivity against the NP of influenza A in small numbers of bronchiolar cells in the lungs of ferrets infected with H1N9 and H6N1 on day 3 pi (Figure 2F). No influenza antigen was present via IHC in the lung of ferrets infected with either of the two viruses on day 7 pi, supporting that virus was cleared from the lung by that time point. Rare peribronchiolar glands contained cellular debris and necrosis of the glandular epithelium, with presence of influenza antigen in the epithelial cells demonstrated via IHC on day 3 pi (Figure 2H). Smaller bronchioles were frequently filled with neutrophils, macrophages, and cellular debris and lined by a mixture of ectatic and very plump epithelial cells, indicative of early repair after previous epithelial damage (Figure 2D, E). Smaller bronchioles still had intraluminal inflammatory exudate and epithelial regeneration on day 7 pi. Peribronchiolar alveoli of affected bronchioles were occasionally filled by macrophages, but notably, there were minimal alveolar changes. Some pulmonary vessels had small perivascular cuffs composed primarily of lymphocytes. On both day 3 and day 7 pi for both viruses, the caudal lung lobes were more affected in severity and extent than the cranial lung lobes. Importantly, only segmental areas of the lung were affected in ferrets inoculated with either virus, with some lung lobes in individual ferrets having no histopathologic lesions at all. There was evidence of viral replication in the trachea, with rare neutrophils present in the epithelium for both H1N9 and H6N1 and rare positive intranuclear epithelial immunoreactivity for the NP of influenza for one H1N9 inoculated ferret. Lung in the three inoculated ferrets from repeat transmission studies was examined microscopically at day 21 pi; these tissues had no significant lesions, indicating that complete resolution of pulmonary damage and inflammation had occurred by this time.
Table 2. Presence of influenza virus in ferret organs by virus isolation in ECEs for ferrets infected with wild bird avian influenza viruses H6N1 and H1N9.
| Virus | Days post inoculation | Lung | Rectal swab | Intestine | Olfactory bulb | Liver | Spleen |
| H6N1 | 3 | 2/2 | 2/7 | 0/2 | 1/2 | 1/2 | 0/2 |
| 5 | NDa | 1/5 | ND | ND | ND | ND | |
| 7 | 0/2 | 0/5 | ND | ND | ND | ND | |
| H1N9 | 3 | 2/2 | 1/7 | 0/2 | 0/2 | 2/2 | 1/2 |
| 5 | ND | 1/5 | ND | ND | ND | ND | |
| 7 | 0/2 | 1/5 | ND | ND | ND | ND |
For each organ listed, the number of animals with a positive isolation of virus out of the number of animals tested is shown.
Not done for this sample.
As there was evidence of pulmonary damage with regeneration, we suspected that viral replication in the lung had already peaked by day 3 pi. A titer could not be obtained for the lung via TCID50 assay for day 3 pi for either virus (limit of detection = 1.3 or 1.0 log10 TCID50/g) despite positive virus isolation. Therefore, two additional ferrets were inoculated with H1N9 and lung examined at day 2 pi. In the day 2 pi ferret lung, a virus titer was obtained using a 1∶3 dilution scheme (Table 1). Histopathologic changes in the lung on day 2 pi also confirmed suspicion of early viral replication, damage, and clearance, as the affected bronchiolar epithelium had necrosis and sloughing without indication of the regeneration that was observed on day 3 pi (Figure 2C). Positive intranuclear immunoreactivity against the NP of influenza A was also observed in small numbers of bronchiolar cells in the lungs for day 2 pi.
Extra-respiratory detection of wild bird influenza viruses in ferrets
Few changes in other organs were observed on histopathology. There was mild to moderate gross enlargement of tracheobronchial lymph nodes with microscopic lesions of moderate follicular hyperplasia as a result of the antigenic stimulation in all ferrets (both H1N9 and H6N1, days 3 and 7 pi). Perivascular cuffs of lymphocytes were present in the olfactory nerves and olfactory bulbs of the brain for one of the two ferrets infected with H6N1 on day 7 pi and one of the two ferrets infected with H1N9 on day 7 pi, although no influenza antigen was detected via immunohistochemistry on day 3 pi in the olfactory bulb for any of the ferrets. However, these lesions in combination with positive virus isolation on olfactory bulb in one of the ferrets infected with H6N1 on day 3 pi, are supportive of probable direct extension of virus from the infected nasal epithelium, as has been shown in numerous experimental intranasal influenza inoculations (Table 2) [6], [21], [38]. Virus isolation was also performed on several other organs with sporadic positive results for rectal swabs, liver, and spleen, despite the absence of histopathologic lesions in these organs (Table 2).
Wild bird H1N9 influenza virus transmitted via contact between ferrets
Interestingly, H1N9 exhibited contact transmission between ferrets consistently in all three ferret pairs, but H6N1 did not directly transmit (Figure 1). All three direct contact ferrets that became infected with H1N9 had similar peak viral titers (average peak of 5.2 log10 TCID50 for transmission ferrets and average peak of 5.4 log10 TCID50 for inoculated ferrets) with similar length of shedding time (5 days for both groups) in the nasal wash compared to inoculated ferrets, although the time point of transmission varied greatly between pairs. Transmission variability may be somewhat explained by the varied time point of peak virus in the inoculated ferrets, which matched the pattern of transmission to the paired direct contact ferret (e.g. the later the peak virus in the inoculated ferret, the later the paired contact ferret had indication of transmission). Again, in ferrets that were infected via direct contact transmission, ferret health including clinical signs and morbidity parameters (temperature/weight loss) were minimally affected (Table 1). Direct contact transmission of H1N9 was repeated with a second study, and subsequently confirmed by two out of three direct contact ferrets becoming infected (data not shown).
Wild bird influenza viruses exhibited typical AIV receptor specificity
Effective transmission by the H1N9 virus raised questions regarding potential mechanisms for transmission. Segment four, encoding the HA gene was sequenced for each virus to examine receptor specificity as compared to HA sequences defined in the literature. Both H6N1 and H1N9 viruses contained glutamic acid (E) and glycine (G) at positions 190 and 225 of the HA, in contrast to human influenza strains, A/North Carolina/1/1918 and A/Pennsylvania/08/2008, that contains aspartic acid (D) at both 190 and 225 position (Table 3) that is associated with to α2,6 linked sialic acid receptor specificity [39], [40]. The glutamine (Q) at position 226 and glycine (G) at position 228 of the HA are characteristic of avian strains but have been also been identified in human strains and have been shown to influence α2,3 linked sialic acid receptor specificity. The H1N9 and H6N1 viruses contained the HA amino acid residues most commonly present in avian influenza strains that exhibit α2,3 linked sialic acid specificity.
Table 3. Comparison of critical amino acids involved in receptor specificity of influenza hemagglutinin.
| Hemagglutinin Amino Acidsa | ||||||||
| Virus | Isolate ID | HA Subtype | Host | 190 | 225 | 226 | 228 | Accession No. |
| A/Ruddy Turnstone/DE/1171/2002 | H1N9 | H1 | Avian | E | G | Q | G | CY116631 |
| A/Ruddy Turnstone/DE/892/2002 | H6N1 | H6 | Avian | E | G | Q | G | CY116633 |
| A/Duck/Alberta/35/1976 | H1 | Avian | E | G | Q | G | AF091309 | |
| A/South Carolina/1/1918 | H1 | Human | D | D | Q | G | AF117241 | |
| A/New Caledonia/20/1999 | H1 | Human | N | D | Q | G | AB304818 | |
| A/Pennsylvania/08/2008 | H1 | Human | D | D | Q | G | FJ549047 | |
| A/California/04/2009 | H1 | Human | D | D | Q | G | FJ966082 | |
Hemagglutinin residues using H3 numbering.
To functionally assess the sialic acid receptor specificity of H1N9 and H6N1, we examined the erythrocyte binding of the avian influenza strains. Both viruses were able to agglutinate equine erythrocytes to a 512 HAU/ml titer (Table 4), while the human influenza strains (A/New Caledonia/20/1999 and A/California/04/2009) generated no detectable titer. Red blood cells (RBCs) from most species express both α2,3 and α2,6 linked sialic acids, but equine erythrocytes are unique in that they exhibit predominantly α2,3 linked SA receptors. Both H6N1 and H1N9 agglutinated guinea pig and turkey RBCs to similar levels as compared to a human influenza. Turkey erythrocytes contain a mixture of α2,3 and α2,6 linked sialic acid linked receptors and guinea pig RBCs express largely α2,6 linked sialic acids with lower levels of α2,3 linked sialic acid receptors [41]. While the mixed α2,3 and α2,6 linkages on these RBCs precludes determination of definitive α2,6 linked sialic acid binding, the lack of clear changes in binding to erythrocytes expressing predominantly α2,6 linked sialic acids suggests limited binding to these glycans.
Table 4. Agglutination of erythrocytes from different animal species by human and avian influenza viruses.
| Hemagglutination Titersa | ||||||
| Virus | Isolate ID | HA Subtype | Host | Turkey | Equine | Guinea Pig |
| A/Ruddy Turnstone/DE/1171/2002 | H1N9 | H1 | Avian | 1024 | 512 | 512 |
| A/Ruddy Turnstone/DE/892/2002 | H6N1 | H6 | Avian | 1024 | 512 | 256 |
| A/New Caledonia/20/1999 | H1 | Human | 1024 | 0 | 256 | |
| A/California/04/2009 | H1 | Human | 64 | 0 | 16 | |
| A/Pennsylvania/08/2008 | H1 | Human | 128 | 0 | 32 | |
Hemagglutination titers are provide as the reciprocal of the highest virus dilution generating agglutination.
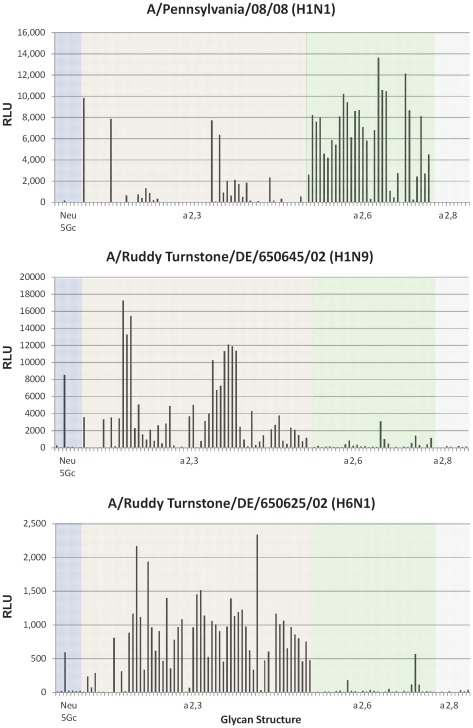
To further define the receptor specificity of the viruses, glycan microarrays were utilized to determine the precise sialyl-oligosaccharide binding profile for H1N9 and H6N1 as compared to a previously defined seasonal H1N1 human influenza virus, A/Pennsylvania/08/2008 [40]. Purified and fluorescently labeled viruses were submitted to Core H of the Consortium for Functional Glycomics and binding was assessed against 511 glycans (Table S2). The binding motif for all three viruses shows that the H1N9 and H6N1 predominantly bind oligosaccharides that contain N-acetylneuraminic acid α2,3 moieties with little binding observed for the α2,6 and α2,8 linked sialic acid containing glycans (Fig. 3A,B). There was limited N-glyconeuraminic acid (NeuGc) recognition for the H1N9 and H6N1 with only the α2,3 NeuGc glycans demonstrated binding. The NeuGc moieties are the predominant sialic acid species on equine erythrocytes, where the H1N9 and H6N1 α2,3 NeuGc SA binding preference corroborates the horse erythrocyte agglutination data (Table 4) [42]. In contrast, the human control strain (A/Pennsylvania/08/2008) demonstrated the predicted α2,6 linked sialic acid receptor binding properties (Fig. 3C) with minor α2,3 linked sialic acid binding, which is common among human influenza viruses. This uncharacteristic α2,3 linked sialic acid receptor specificity is often the result of fucosylated and sulfated modifications to the oligosaccahrides enhancing suboptimal sialic acid receptor recognition, which, as previously shown, appears to be the case for the human control strain (A/Pennsylvania/08/2008) [40].
Figure 3. Glycan binding analysis of wild bird avian or human influenza viruses.
Influenza viruses were propagated in Madin-Darby kidney cells, purified on a 25% sucrose cushion by ultracentrifugation, and labeled with Alexa488 before being applied to the microarray. The data was organized based on Neu5GC, α2,3 SA, α2,6 SA and α2,8 SA glycan structures and represented by different color schemes. Glycan microarray binding analysis was performed by Core H of the Consortium for Functional Glycomics. A) A/Ruddy Turnstone/DE/1171/02 (H1N9), B) A/Ruddy Turnstone/DE/892/02 (H6N1), C) A/Pennsylvania/08/2008 (H1N1).
Discussion
It has been demonstrated that some low pathogenic H6, H7, and H9 AIV subtypes have the ability to replicate in ferrets; however studies that have demonstrated this examined primarily poultry adapted isolates and have limited scope in examining the full pathogenesis of these infections [6], [7], [10], [43]. Van Hoven et al [43] demonstrated that a two H1N1 AIVs isolated a ducks and a mallard could infect ferrets and replicate to high titers in the upper respiratory tract of ferrets. We have previously shown that current circulating North American wild bird LPAIVs do have a capacity to infect and replicate in mammals using a mouse model of infection, although they caused little or no disease [19]. More recently, Nam et al [37] identified an H6N5 isolated from a fecal sample of an aquatic bird in Korea, A/AB/Kor/CN5/09 (H6N5), that infected mice, replicated to high titer and caused mortality without adaptation. Interestingly, this virus also disseminated to extrapulmonary tissues. From the previous work we selected two viruses of distinct subtype for further study in the ferret model, which most accurately emulated human infection and transmission of influenza virus [44]–[46]. We have expanded upon previous work by demonstrating that subtypes of “lesser concern” isolated from migratory shore birds (i.e. Ruddy Turnstone) can also directly infect and replicate in mammals, even with the potential for direct contact mammal to mammal transmission (Table 1, Figure 1). In the study by Nam et al the A/AB/Kor/CN5/09 (H6N5) virus also replicated to high titers in the upper respiratory tract of ferrets and caused middle disease. Similar to our findings with the H1N9 virus, the H6N5 virus showed limited transmission to contact naïve ferrets [37]. Interestingly, the PB1 segment of the H6N5 virus was similar to and possibly derived from the H5N1 highly pathogenic avian influenza viruses circulating in Asia. In contrast, comparison of the sequences from the AIVs described here demonstrates that the segments are all of LPAIV origin, unrelated to HPAI viruses (data not shown). The H6N5 virus was isolated from feces, and so the species of origin is unknown. It would be interesting to identify the potential reservoirs of this virus through live bird surveillance.
Our viruses were passaged in embryonated chicken eggs, which may lend to some adaptive mutations; however, we kept serial passages low to minimize alteration of the original viruses. Interestingly, these viruses replicated to relatively high titers in the upper respiratory tract of the ferret and induced lesions in both the upper and lower respiratory tracts, but minimal disease was observed clinically with complete resolution of pulmonary lesions (Table 1, Figures 1–2). Importantly, lesions and antigen localization in the lung indicate there was no alveolar replication of the virus. Alveolar localization of influenza with alveolar lesions has been associated with increased virulence [47]. Additionally, the results of virus titration and histopathology comparing days 2 and 3 pi is supportive that infection was rapid, minimal, and had rapid clearance in the lower respiratory tract. These observations may have played a role in the minimal induction of disease for these two viruses in the ferret. A study that examined a variety of AIVs of the H6 subtype not only demonstrated replication with variable morbidity in ferrets, but also showed no correlation between the ability to infect the ferrets and the source of the virus (e.g. wild bird vs. poultry) [7]. This study provides additional indication that the source of virus may not be as important of a factor in transmission to mammals, regardless of subtype, although many other factors would play a role in natural transmission including host interactions and amount of virus shed.
Avian influenza virus infections and H7 infectious in particular have presented as conjunctivitis [2]. While this route of administration is understudied in ferrets, Belser et al [48] recently explored infection of ferrets with human influenza viruses and avian influenza viruses isolated from human infections. Both human and avian-origin influenza viruses, including subtypes H1, H3, H5, and H7, and HPAI and LPAI types could infect via the ocular route and spread to the pulmonary tract. Interestingly, the H7 influenzas transmitted to naïve contact ferrets [48]. We did not explore ocular infection in this study; however in light of the demonstrated infection and transmission with H7 influenza viruses, this should be an area of future study.
Pulmonary replication of these two AIVs was rapid in onset and rapid to resolve regardless of magnitude of virus and pulmonary lesions. This rapid transient infection may be related to inoculation methods, which place a large dose of virus in an anesthetized ferret where it becomes inhaled deep into the respiratory tract. This confounds risk assessment, as natural exposure would be through a fomite or aerosol droplet from another infected individual. Early pulmonary viral titers and histopathology have not been examined in transmission ferrets in other transmission experiments or in this study. This would be an interesting component to evaluate to aid in determining the pulmonary replication capacity of these AIVs in a more realistic transmission setting.
Few studies have examined hematologic parameters in influenza infected ferrets, but investigation into this is worthy as it may be a good measure of clinical disease [49]. Examining clinical pathologic findings of such a small sample size can be difficult, given the marked variation between individuals. Nonetheless, there was a trend in both groups H6N1 and H1N9 compared to the allantoic inoculated group of a decrease in lymphocytes on day 1 pi that resolved by day 3 pi for individual animals. Lymphopenia is well established to occur in the very early stages of viral infections. Indeed, it has been demonstrated that experimental ferret infections with HPAI H5N1 viruses result in a profound lymphopenia days 3 and 5 pi [50]. Also, ferrets infected with a variety of H1N1 influenza viruses had a decrease in lymphocytes days 3 and 7 pi [49]. For H1N9 inoculated animals, lymphocytes were increased between days 7 pi through 18 pi for many individuals. Lymphocytosis has not been described in ferrets experimentally infected with influenza, although this finding seems logical, given that chronic antigenic stimulation can induce increases in lymphocytes resulting in peripheral lymphocytosis.
Infectivity of AIVs in mammals and humans is thought to be reliant on the viral hemagglutinin binding sialic acid (SA) residues on host cells, and differences in binding between mammalian versus avian influenza viruses are suggested to be partially responsible for host specificity and localization of infection [24], [51]. It is thought that the avian influenza preference for binding α2,3 linked SA receptors compared to the human influenza preference for binding α2,6 linked SA receptors provides somewhat of a barrier to transmission in ferrets and humans due to the paucity of α2,3 linked SA in the upper respiratory tract. However, there is a presence of α2,3 linked SA in the lower respiratory tract of both these species, and it has been proposed that if enough influenza can be deposited in the lower respiratory tract, pulmonary infection will predominate in these species [47]. We did observe infection and replication in the lung of these ferrets for both H6N1 and H1N9 that supports viral attachment in the lung (bronchioles, but not alveoli), however, we also observed robust upper respiratory tract infections that were more productive with higher viral titers present than compared to the lung despite the alpha α2,3 SA binding preference (Table 1, Figures 1–2).We and others typically observe that ferrets infected with human influenza viruses have viral replication restricted to the upper respiratory tract [52]–[54]. The absence of pulmonary infection is confirmed by negative virus isolation, absence of lesions on histopathology, and absence of viral antigen on IHC. Very mild pulmonary infections have been demonstrated in other laboratories with human influenza viruses in experimental ferret infections, however severe pneumonia is only associated with highly virulent human influenza viruses or highly pathogenic avian influenza viruses [38], [47], [55]. In this study, neither H6N1 nor H1N9 virus appeared to infect alveolar epithelial cells (Figure 2) yet, both viruses did infect mouse and feline alveolar epithelial cells in other in vivo experimental trials in our laboratory (submitted and [19]). Other experiments have demonstrated replication of AIVs in the upper and lower respiratory tract of ferrets with some viruses having higher replication in the nasal turbinates and others with higher replication in the lung [6], [7], [21]. Clearly, cellular tropism in an influenza infected host is complex, and while the cellular SA ligand for HA binding is certainly an important component, additional mechanisms are likely at work.
In our study, the H1N9 subtype AIV exhibited efficient direct contact transmission between ferrets. Aerosol transmission was not explored in this study due to lack of a validated aerosol transmission model in our facilities, however, it would be an interesting next step. Many experiments have established the importance of receptor binding in influenza transmission, demonstrating limited or no contact or aerosol transmission in viruses that have a binding preference for α2,3 linked SA over α2,6 linked SA [10], [43], [56], [57]. Both the H1N9 and H6N1 viruses have avian specific α 2,3 linked SA receptor binding as shown in the erythrocyte binding assays. There are no previously defined HA amino acid residues that would suggest an altered receptor specificity and this assumption is supported by the glycan microarray analysis, where H1N9 dominantly bound glycans having α2,3 SA linkages. However, there may be other unidentified amino acids in the HA or other viral gene segments that mediated the efficient direct transmission of the H1N9 in ferrets, where other H1N1 viruses failed to efficiently transmit via contact [43]. Direct contact H6N1 ferrets did not become infected and did not seroconvert (Figure 1). Perhaps this is due to lower levels of viral shedding for a shorter period of time in H6N1 infected ferrets as compared to H1N9 infected ferrets.
The viral polymerase has also been suggested to potentially have a role in efficient avian to mammalian transmission, replication, and localization of viral infection based upon differences in temperature for optimal replication, tissue/species tropism for replication, rate of replication, and effect on efficiency of viral nuclear transport [43], [58]–[64]. Sequence analysis of H1N9 PB2 found avian specific Glu627 and Asp701 residues (GenBank Accession ACY79819; data not shown), suggesting that there may be other genetic features contributing to the robust upper respiratory replication and transmission of this H1N9 virus in ferrets.
Our study, in combination with additional studies of AIV infections in ferrets, indicates that there is a capacity for wild bird AIVs, subtype notwithstanding, to directly infect mammals with minimal clinical signs. The results support the potential for direct interspecies transmission or formation of a viable AIV reassortant. Although we have demonstrated the low virulence and rapid clearance of these AIVs, possibilities for reassortment in susceptible wild and domestic mammalian species make these species of particular interest and worth further investigation. Furthermore, the variable magnitude of seroconversion despite productive influenza infection could make surveillance and monitoring for mammalian infection with AIVs difficult. Together, these studies support the need for expanded analysis of influenza viruses from their reservoir species as understanding of the mechanisms of infection and transmission is incomplete and subsequent risk assessment imperfect.
Materials and Methods
Ethics statement
These studies were conducted in strict accordance with guidelines approved by the Institutional Animal Care and Use Committee and supported through the Office of Animal Care and use of the University of Georgia, following guidelines established by AAALAC International (Association for Assessment and Accreditation of Laboratory Animal Care; Accreditation Date: 3/2/2011), licensed by the USDA (USDA #57-R-005), and maintaining an Assurance of Compliance with the U.S. Department of Health and Human Services (PHS Assurance #A3437-01). The protocols utilized for these studies were approved by the Institutional Animal Care and Use Committees of the University of Georgia and the Centers for Disease Control and Prevention.
Viruses
Avian influenza viruses used were cloacal swab isolates from wild birds in the United States acquired from Southeastern Cooperative Wildlife Disease Study (Athens, GA). Viruses were isolated from cloacal swabs in 9 day old embryonated chicken eggs (ECE) at 37°C for 72 hours and then minimally passaged (3 or fewer passages) in ECEs. Select virus isolates were screened in a previous study in BALB/c mice [19]. Two viruses that exhibited efficient pulmonary replication and induced pulmonary lesions in BALB/c mice were selected for in vivo studies in ferrets: A/Ruddy Turnstone/DE/892/02 (H6N1 subtype; NCBI Taxonomy ID: 680602; abbreviated H6N1) and A/Ruddy Turnstone/DE/1171/02 (H1N9 subtype; NCBI Taxonomy ID: 680596; abbreviated H1N9). The original low passage isolates, once selected by screening methods, were grown once more in 9 to 10 day old ECE to generate a stock of the virus. Stock viruses were aliquoted and stored at −80°C until use. Stock virus titers were determined by plaque assay on MDCK cells.
Sequencing
Total viral RNA was extracted from AIV infected allantoic fluid using the RNeasy kit (QIAGEN, Inc., Valencia, CA) according to the manufacturer's protocol. One-step RT-PCR was performed on viral RNA using a universal primer set (Uni12/Inf-1 5′-GGGGGGAGCAAAAGCAGG-3′ and Uni13/Inf-1 5′-CGGGTTATTAGTAGAAACAAGG-3′) as previously described [65]. All 8 segments were generated, the HA segment was excised and gel purified using the QIAquick Gel Extraction kit (QIAGEN, Inc., Valencia, CA). The HA was sequenced using BigDye Terminator v3.1 Cycle Sequencing kit (Applied Biosystems) with subtype specific primers (primer sequences available upon request). Some sequences for the H1N9 and H6N1 viruses were already available on GenBank. The accession numbers are GU050646.1, GU050647.1, GU050624.1, GU050623.1, GU050622.1, GU050620.1, GU050619.1, GU050618.1, and GU050621.1 (A/ruddy turnstone/Delaware/892/2002 segment 1 and segment 2, and A/ruddy turnstone/Delaware/1171/2002 segments, 1, 2, 3, 5, 6, 7, and 8 respectively). HA sequences are available at GenBank. Segments sequenced for this study and submitted to GenBank include A/ruddy turnstone/Delaware/892/2002 segments 3, 4, 6, and 8 and A/ruddy turnstone/Delaware/1171/2002 segment 4 (GenBank Accession numbers CY116632, CY116633, CY116634, CY116633, and CY116631, respectively).
Erythrocyte binding assays
Fresh turkey, guinea pig, and equine erythrocytes were thoroughly washed with 1× PBS and resuspended to 1% v/v in 1× PBS/.5%BSA. A standard hemagglutination assay was performed for each AIV isolate against all types of erythrocytes, and appearance of agglutination was scored after a 60-min incubation period.
Glycan microarray binding
Viruses were cultured at an MOI of 0.01 on MDCKs for 72 hours in 1× Minimal Essential Medium supplemented with 1 µg/ml TPCK [L-(tosylamido-2-pheyl) ethyl chloromethyl ketone]-treated trypsin (Worthington Biochemical Corporation, Lakewood, NJ). The viral supernatant (10 ml) was collected and centrifuged at 5,000 RPM for 5 minutes to remove cell debris before viral purification. Virus in the culture supernatant was purified on a 25% sucrose cushion and resuspended in 1× PBS with 1 mM EDTA. Briefly, each virus was purified through a 25% sucrose gradient by high speed centrifugation at 28,000 rpm at 4°C for 3 hours. The purified viruses were resuspended in1XPBS with 1 mM EDTA on ice for 4 hours and stored at −80°C. Viral titers were determined by standard plaque assay on MDCK cells. Approximately 107 PFU of each purified strain were labeled with 25 µg of Alexa488 dye in 1 M NaHCO3 (pH 9) for 1 hour. To remove residual dye, each sample was dialyzed in a 7000 MWCO Slide-A-Lyzer MINI dialysis cassette (Thermo Scientific) against PBS with 1 mM EDTA overnight. The labeled viruses were analyzed via glycan microarray by the Core H of the Consortium of Functional Glycomics (www.functionalglycomics.org), where 70 µl of labeled virus was added to glycan microarray slide and incubated at 4°C for 1 hour. Each microarray was scanned by Perkin-Elmer ProScanAray that detected SA binding peaks designated as relative fluorescent units (RFUs).
Ferrets
Castrated male Fitch ferrets (Triple F Farms, Sayre, PA), 3 months old and seronegative to circulating human H1N1 and H3N2 influenza viruses, were used for the study. Ferrets were housed in a BSL2 facility in HEPA filtered isolator caging (Allentown, Allentown, NJ). A subcutaneous temperature transponder (BMDS, Seaford, DE) was implanted in each ferret for identification and temperature measurement.
Infection and direct transmission study
Seven ferrets were inoculated per virus (four for tissue examination and three for transmission study) and three additional naive ferrets were used to assay direct contact transmission. In the contact trials, one direct contact ferret was housed with one inoculated ferret as paired cage mates. An additional three ferrets were mock infected with allantoic fluid in PBS for negative controls for nasal washes, serology, and complete blood counts (CBC). Inoculated ferrets were lightly anesthetized with isoflurane and intranasally inoculated with 5×105 PFU in 500 µL of sterile PBS (250 µL of per nostril) with either H6N1 or H1N9. Direct contact ferrets were placed with inoculated ferrets twenty-four hours post inoculation. An additional study was performed with H1N9 as previously described to confirm direct contact transmission, using three inoculated and three direct contact ferrets. Temperatures monitored for four days to establish baseline then temperature, weights, and complete blood counts were monitored in inoculated ferrets on days 1, 3, 5, 7, 10, 13, 18, and 21 pi and in direct contact ferrets on days 2, 4, 6, 9, 12, 17, and 20 post contact (pc). Nasal washes were sampled from ferrets on days 1, 3, 5, 7, 10 pi or days 2, 4, 6, 9, 12, and 17 pc to monitor for viral infection. For nasal washes, ferrets were anesthetized with 4 mg ketamine via intramuscular injection and 1 mL of sterile PBS with penicillin (4000 U/ml) (Calbiochem, Gibbstown, NJ), streptomycin (800 µg/ml) (Sigma, St. Louis, MO), polymyxin B (400 U/ml) (MP Biochmemicals, LLC, Solon, OH), and gentamicin (100 µg/ml) (Gibco, Carlsbad, CA) was introduced into the nostrils to induce sneezing and collected in specimen cups. For repeat study ferrets, temperature, weights, and nasal washes were performed for inoculated ferrets and transmission ferrets on days 1, 3, 5, 7, 9, 11, 13 and 15 pi and on days 2, 4, 6, 8, 10, 12, and 14 pc. Repeat study ferrets were humanely euthanized at day 21 pi (day 20 pc) and samples of lung from all ferrets were fixed in neutral buffered formalin for histopathology.
Determination of viral titers
Nasal washes were immediately tested via real time RT-PCR to aid in determining days for sample collection. Briefly, viral RNA was extracted from nasal wash by using RNeasy mini kit (QIAGEN, Inc., Valencia, CA) and the Qiagen one-step RT-PCR kit was used for RRT-PCR with a Stratagene MX300P/3005P thermocyler and Mx Pro QPCR software (La Jolla, CA). Reaction mixture and PCR cycling protocol is available upon request. An influenza virus matrix gene specific primer and probe set were used as follows: primer M+25, sequence AGA TGA GTC TTC TAA CCG AGG TCG; primer M-124, sequence TGC AAA AAC ATC TTC AAG TCT CTG; and probe M+64, sequence FAM-TCA GGC CCC CTC AAA GCC GA-TAMRA [66] (Biosearch Technologies, Novato, CA).
Four ferrets per virus were humanely euthanized (two on day 3 pi and two on day 7 pi per virus) and lung, nasal turbinate, liver, spleen, and olfactory bulb were sampled under sterile conditions and frozen at −80°C for virus isolation. Based on histopathology and viral titers from the lung, we suspected early pulmonary infection with rapid clearance, therefore an additional two ferrets were intranasally inoculated as previously described with H1N9 and humanely euthanized at day 2 pi with fresh lung collected to examine the earlier time point. Tissues were later homogenized in 1 mL PBS with antibiotics, clarified by centrifugation, and 100 µL of clarified homogenate was inoculated into 9 to 10 day old ECEs for virus isolation (4 eggs per sample, 72 hour incubation). Nasal washes and clarified lung homogenate were titrated in MDCK cells with serial 1∶10 or 1∶3 dilutions with a 1.5 log10TCID50/mL (nasal wash) and 1. 3 to 1.0 log10 TCID50/gram (lung).
Serology and hematology
At day 21 pi (day 20 pc), blood was collected and seroconversion was determined via hemagluttination inhibition (HI) and microneutralization (MN) assays. All blood samples were analyzed the same day as sample collection. Complete blood counts were performed using VetScan analyzer (Abaxis, Union City, CA). Leukocyte counts were log transformed and statistically analyzed using repeated measures ANOVA (Stata version 11.0) to examine differences between viral groups and days pi. Degrees of freedom for F-tests of repeated measures factors were adjusted using the Greenhouse-Geisser estimate of epsilon to correct for any departures from the sphericity assumption. All testing assumed a two-sided alternative hypothesis and P-values<0.05 were considered significant.
Histopathology and immunohistochemistry
Lung (cranial and caudal lobes), trachea, tracheobronchial lymph node, esophagus, heart, spleen, liver, stomach, small intestine, large intestine, pancreas, mesenteric lymph node, kidneys, adrenal gland, bladder, brain, and nasal turbinates were collected on days 3 and 7 pi from inoculated ferrets (two ferrets per virus per day, the same ferrets as described for virus isolation in fresh tissues). The additional two ferrets that were intranasally inoculated with H1N9 and humanely euthanized at day 2 pi had the same set of tissues collected for histopathology and immunohistochemistry. All tissues were preserved in 10% neutral buffered formalin. Tissues were routinely processed, embedded and stained with hematoxylin and eosin. Immunohistochemical staining was performed on lung, trachea, and nasal turbinates for all ferrets. Immunohistochemistry was performed using a commercially available goat polyclonal antibody to the nucleoprotein of influenza A virus at a 1∶10,000 dilution (Biodesign International, Sako, Maine). Tissues were deparaffinized and blocked with a commercial protein blocking agent (Dako Cytomation, Carpinteria, CA) and a linked strepavidin-biotin immunoperoxidase system was used for immunolabeling. The reaction was visualized with 3, 3′-diaminobenzidine substrate (Dako Cytomation, Carpinteria, CA).
Supporting Information
Total and differential leukocyte counts for ferrets inoculated with wild bird avian influenza viruses H1N9 and H6N1.
(DOCX)
Raw Glycan Microarray Data.
(DOCX)
Acknowledgments
The authors wish to thank the Animal Resources personnel at the College of Veterinary Medicine, University of Georgia, for excellent animal husbandry. We would also like to thank histotechnicians in the Veterinary Pathology Department at University of Georgia for their assistance, especially Abbie Butler for her outstanding immunohistochemistry support. Additional thanks go to Taiana Costa, Deb Carter and Jon Gabbard for superior assistance with animal work and Cheryl Jones for excellent technical support at the College of Veterinary Medicine, University of Georgia. The authors would also like to acknowledge The Consortium for Functional Glycomics for support of the glycan array analysis.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by Centers for Disease Control (CDC) grant 5U19Cl00040102 and National Institutes of Health contract HHSN266200700006C. The CDC provided CDC approval of animal protocols for this study and reviewed this manuscript prior to submission for publication. Otherwise, the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Beigel JH, Farrar J, Han AM, Hayden FG, Hyer R, et al. Avian influenza A (H5N1) infection in humans. The New England Journal Of Medicine. 2005;353:1374–1385. doi: 10.1056/NEJMra052211. [DOI] [PubMed] [Google Scholar]
- 2.Belser JA, Bridges CB, Katz JM, Tumpey TM. Past, present, and possible future human infection with influenza virus A subtype H7. Emerging Infectious Diseases. 2009;15:859–865. doi: 10.3201/eid1506.090072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klopfleisch R, Wolf PU, Uhl W, Gerst S, Harder T, et al. Distribution of lesions and antigen of highly pathogenic avian influenza virus A/Swan/Germany/R65/06 (H5N1) in domestic cats after presumptive infection by wild birds. Veterinary Pathology. 2007;44:261–268. doi: 10.1354/vp.44-3-261. [DOI] [PubMed] [Google Scholar]
- 4.Keawcharoen J, Oraveerakul K, Kuiken T, Fouchier RA, Amonsin A, et al. Avian influenza H5N1 in tigers and leopards. Emerging Infectious Diseases. 2004;10:2189–2191. doi: 10.3201/eid1012.040759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song D, Kang B, Lee C, Jung K, Ha G, et al. Transmission of avian influenza virus (H3N2) to dogs. Emerging Infectious Diseases. 2008;14:741–746. doi: 10.3201/eid1405.071471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belser JA, Lu X, Maines TR, Smith C, Li Y, et al. Pathogenesis of avian influenza (H7) virus infection in mice and ferrets: enhanced virulence of Eurasian H7N7 viruses isolated from humans. Journal Of Virology. 2007;81:11139–11147. doi: 10.1128/JVI.01235-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gillim-Ross L, Santos C, Chen Z, Aspelund A, Yang CF, et al. Avian influenza H6 viruses productively infect and cause illness in mice and ferrets. Journal Of Virology. 2008;82:10854–10863. doi: 10.1128/JVI.01206-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hinshaw VS, Webster RG, Easterday BC, Bean WJ Replication of avian influenza A viruses in mammals. Infection and Immunity. 1981;34:354–361. doi: 10.1128/iai.34.2.354-361.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joseph T, McAuliffe J, Lu B, Jin H, Kemble G, et al. Evaluation of replication and pathogenicity of avian influenza a H7 subtype viruses in a mouse model. Journal Of Virology. 2007;81:10558–10566. doi: 10.1128/JVI.00970-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wan H, Sorrell EM, Song H, Hossain MJ, Ramirez-Nieto G, et al. Replication and transmission of H9N2 influenza viruses in ferrets: evaluation of pandemic potential. PLoS One. 2008;3:e2923. doi: 10.1371/journal.pone.0002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butt KM, Smith GJ, Chen H, Zhang LJ, Leung YH, et al. Human infection with an avian H9N2 influenza A virus in Hong Kong in 2003. Journal Of Clinical Microbiology. 2005;43:5760–5767. doi: 10.1128/JCM.43.11.5760-5767.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fouchier RA, Schneeberger PM, Rozendaal FW, Broekman JM, Kemink SA, et al. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proceedings Of The National Academy Of Sciences Of The United States Of America. 2004;101:1356–1361. doi: 10.1073/pnas.0308352100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo Y, Li J, Cheng X. Discovery of men infected by avian influenza A (H9N2) virus. Chinese Journal of Experimental and Clinical Virology. 1999;13:105–108. [PubMed] [Google Scholar]
- 14.Koopmans M, Wilbrink B, Conyn M, Natrop G, van der Nat H, et al. Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in the Netherlands. Lancet. 2004;363:587–593. doi: 10.1016/S0140-6736(04)15589-X. [DOI] [PubMed] [Google Scholar]
- 15.Octaviani CP, Ozawa M, Yamada S, Goto H, Kawaoka Y. High level of genetic compatibility between swine-origin H1N1 and highly pathogenic avian H5N1 influenza viruses. Journal Of Virology. 2010;84:10918–10922. doi: 10.1128/JVI.01140-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Y, Qin K, Wang J, Pu J, Tang Q, et al. High genetic compatibility and increased pathogenicity of reassortants derived from avian H9N2 and pandemic H1N1/2009 influenza viruses. Proceedings Of The National Academy Of Sciences Of The United States Of America. 2011;108:4164–4169. doi: 10.1073/pnas.1019109108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen LM, Davis CT, Zhou H, Cox NJ, Donis RO. Genetic compatibility and virulence of reassortants derived from contemporary avian H5N1 and human H3N2 influenza A viruses. PLoS Pathogens. 2008;4:e1000072. doi: 10.1371/journal.ppat.1000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson S, Van Hoeven N, Chen LM, Maines TR, Cox NJ, et al. Reassortment between avian H5N1 and human H3N2 influenza viruses in ferrets: a public health risk assessment. Journal Of Virology. 2009;83:8131–8140. doi: 10.1128/JVI.00534-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Driskell EA, Jones CA, Stallknecht DE, Howerth EW, Tompkins SM. Avian influenza virus isolates from wild birds replicate and cause disease in a mouse model of infection. Virology. 2010;399:280–289. doi: 10.1016/j.virol.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 20.van Riel D, Munster VJ, de Wit E, Rimmelzwaan GF, Fouchier RA, et al. H5N1 virus attachment to lower respiratory tract. Science (New York, N Y) 2006;312:399. doi: 10.1126/science.1125548. [DOI] [PubMed] [Google Scholar]
- 21.Maines TR, Lu XH, Erb SM, Edwards L, Guarner J, et al. Avian influenza (H5N1) viruses isolated from humans in Asia in 2004 exhibit increased virulence in mammals. Journal Of Virology. 2005;79:11788–11800. doi: 10.1128/JVI.79.18.11788-11800.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yen HL, Lipatov AS, Ilyushina NA, Govorkova EA, Franks J, et al. Inefficient transmission of H5N1 influenza viruses in a ferret contact model. Journal Of Virology. 2007;81:6890–6898. doi: 10.1128/JVI.00170-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson CI, Barclay WS, Zambon MC, Pickles RJ. Infection of human airway epithelium by human and avian strains of influenza a virus. Journal Of Virology. 2006;80:8060–8068. doi: 10.1128/JVI.00384-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shinya K, Ebina M, Yamada S, Ono M, Kasai N, et al. Avian flu: influenza virus receptors in the human airway. Nature. 2006;440:435–436. doi: 10.1038/440435a. [DOI] [PubMed] [Google Scholar]
- 25.Matrosovich MN, Matrosovich TY, Gray T, Roberts NA, Klenk HD. Human and avian influenza viruses target different cell types in cultures of human airway epithelium. Proceedings Of The National Academy Of Sciences Of The United States Of America. 2004;101:4620–4624. doi: 10.1073/pnas.0308001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Connor RJ, Kawaoka Y, Webster RG, Paulson JC. Receptor specificity in human, avian, and equine H2 and H3 influenza virus isolates. Virology. 1994;205:17–23. doi: 10.1006/viro.1994.1615. [DOI] [PubMed] [Google Scholar]
- 27.Rogers GN, Paulson JC. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology. 1983;127:361–373. doi: 10.1016/0042-6822(83)90150-2. [DOI] [PubMed] [Google Scholar]
- 28.Ito T, Suzuki Y, Mitnaul L, Vines A, Kida H, et al. Receptor specificity of influenza A viruses correlates with the agglutination of erythrocytes from different animal species. Virology. 1997;227:493–499. doi: 10.1006/viro.1996.8323. [DOI] [PubMed] [Google Scholar]
- 29.Nicholls JM, Chan MC, Chan WY, Wong HK, Cheung CY, et al. Tropism of avian influenza A (H5N1) in the upper and lower respiratory tract. Nature Medicine. 2007;13:147–149. doi: 10.1038/nm1529. [DOI] [PubMed] [Google Scholar]
- 30.Nicholls JM, Bourne AJ, Chen H, Guan Y, Peiris JS. Sialic acid receptor detection in the human respiratory tract: evidence for widespread distribution of potential binding sites for human and avian influenza viruses. Respiratory research. 2007;8:73. doi: 10.1186/1465-9921-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skehel JJ, Wiley DC. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annual Review of Biochemistry. 2000;69:531–569. doi: 10.1146/annurev.biochem.69.1.531. [DOI] [PubMed] [Google Scholar]
- 32.Matrosovich MN, Gambaryan AS, Teneberg S, Piskarev VE, Yamnikova SS, et al. Avian influenza A viruses differ from human viruses by recognition of sialyloligosaccharides and gangliosides and by a higher conservation of the HA receptor-binding site. Virology. 1997;233:224–234. doi: 10.1006/viro.1997.8580. [DOI] [PubMed] [Google Scholar]
- 33.Kemink SA, Fouchier RA, Rozendaal FW, Broekman JM, Koopmans M, et al. [A fatal infection due to avian influenza-A (H7N7) virus and adjustment of the preventive measures]. Nederlands Tijdschrift voor Geneeskunde. 2004;148:2190–2194. [PubMed] [Google Scholar]
- 34.Uyeki TM, Chong YH, Katz JM, Lim W, Ho YY, et al. Lack of evidence for human-to-human transmission of avian influenza A (H9N2) viruses in Hong Kong, China 1999. Emerging Infectious Diseases. 2002;8:154–159. doi: 10.3201/eid0802.010148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Claas EC, de Jong JC, van Beek R, Rimmelzwaan GF, Osterhaus AD. Human influenza virus A/HongKong/156/97 (H5N1) infection. Vaccine. 1998;16:977–978. doi: 10.1016/s0264-410x(98)00005-x. [DOI] [PubMed] [Google Scholar]
- 36.Vines A, Wells K, Matrosovich M, Castrucci MR, Ito T, et al. The role of influenza A virus hemagglutinin residues 226 and 228 in receptor specificity and host range restriction. Journal Of Virology. 1998;72:7626–7631. doi: 10.1128/jvi.72.9.7626-7631.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nam JH, Kim EH, Song D, Choi YK, Kim JK, et al. Emergence of mammalian species-infectious and-pathogenic avian influenza H6N5 virus with no evidence of adaptation. Journal Of Virology. 2011;85:13271–13277. doi: 10.1128/JVI.05038-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Govorkova EA, Rehg JE, Krauss S, Yen HL, Guan Y, et al. Lethality to ferrets of H5N1 influenza viruses isolated from humans and poultry in 2004. Journal Of Virology. 2005;79:2191–2198. doi: 10.1128/JVI.79.4.2191-2198.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glaser L, Stevens J, Zamarin D, Wilson IA, Garcia-Sastre A, et al. A single amino acid substitution in 1918 influenza virus hemagglutinin changes receptor binding specificity. Journal Of Virology. 2005;79:11533–11536. doi: 10.1128/JVI.79.17.11533-11536.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bradley KC, Jones CA, Tompkins SM, Tripp RA, Russell RJ, et al. Comparison of the receptor binding properties of contemporary swine isolates and early human pandemic H1N1 isolates (Novel 2009 H1N1). Virology. 2011;413:169–182. doi: 10.1016/j.virol.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 41.Medeiros R, Escriou N, Naffakh N, Manuguerra JC, van der Werf S. Hemagglutinin residues of recent human A(H3N2) influenza viruses that contribute to the inability to agglutinate chicken erythrocytes. Virology. 2001;289:74–85. doi: 10.1006/viro.2001.1121. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki Y, Matsunaga M, Matsumoto M. N-Acetylneuraminyllactosylceramide, GM3-NeuAc, a new influenza A virus receptor which mediates the adsorption-fusion process of viral infection. Binding specificity of influenza virus A/Aichi/2/68 (H3N2) to membrane-associated GM3 with different molecular species of sialic acid. J Biol Chem. 1985;260:1362–1365. [PubMed] [Google Scholar]
- 43.Van Hoeven N, Pappas C, Belser JA, Maines TR, Zeng H, et al. Human HA and polymerase subunit PB2 proteins confer transmission of an avian influenza virus through the air. Proceedings Of The National Academy Of Sciences Of The United States Of America. 2009;106:3366–3371. doi: 10.1073/pnas.0813172106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Belser JA, Szretter KJ, Katz JM, Tumpey TM. Use of animal models to understand the pandemic potential of highly pathogenic avian influenza viruses. Advances in virus research. 2009;73:55–97. doi: 10.1016/S0065-3527(09)73002-7. [DOI] [PubMed] [Google Scholar]
- 45.Tripp RA, Tompkins SM. Animal models for evaluation of influenza vaccines. Current topics in microbiology and immunology. 2009;333:397–412. doi: 10.1007/978-3-540-92165-3_19. [DOI] [PubMed] [Google Scholar]
- 46.O'Donnell CD, Subbarao K. The contribution of animal models to the understanding of the host range and virulence of influenza A viruses. Microbes and infection/Institut Pasteur. 2011;13:502–515. doi: 10.1016/j.micinf.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van den Brand JM, Stittelaar KJ, van Amerongen G, Rimmelzwaan GF, Simon J, et al. Severity of pneumonia due to new H1N1 influenza virus in ferrets is intermediate between that due to seasonal H1N1 virus and highly pathogenic avian influenza H5N1 virus. The Journal Of Infectious Diseases. 2010;201:993–999. doi: 10.1086/651132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Belser JA, Gustin KM, Maines TR, Pantin-Jackwood MJ, Katz JM, et al. Influenza virus respiratory infection and transmission following ocular inoculation in ferrets. PLoS Pathogens. 2012;8:e1002569. doi: 10.1371/journal.ppat.1002569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Belser JA, Gustin KM, Maines TR, Blau DM, Zaki SR, et al. Pathogenesis and transmission of triple-reassortant swine H1N1 influenza viruses isolated before the 2009 H1N1 pandemic. Journal Of Virology. 2011;85:1563–1572. doi: 10.1128/JVI.02231-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zitzow LA, Rowe T, Morken T, Shieh WJ, Zaki S, et al. Pathogenesis of avian influenza A (H5N1) viruses in ferrets. Journal Of Virology. 2002;76:4420–4429. doi: 10.1128/JVI.76.9.4420-4429.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Riel D, Munster VJ, de Wit E, Rimmelzwaan GF, Fouchier RA, et al. Human and avian influenza viruses target different cells in the lower respiratory tract of humans and other mammals. The American Journal Of Pathology. 2007;171:1215–1223. doi: 10.2353/ajpath.2007.070248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rowe T, Leon AJ, Crevar CJ, Carter DM, Xu L, et al. Modeling host responses in ferrets during A/California/07/2009 influenza infection. Virology. 2010;401:257–265. doi: 10.1016/j.virol.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sweet C, Bird RA, Coates DM, Overton HA, Smith H. Recent H1N1 viruses (A/USSR/90/77, A/Fiji/15899/83, A/Firenze/13/83) replicate poorly in ferret bronchial epithelium. Brief report. Archives Of Virology. 1985;85:305–311. doi: 10.1007/BF01314239. [DOI] [PubMed] [Google Scholar]
- 54.Smith JH, Nagy T, Driskell E, Brooks P, Tompkins SM, et al. Comparative Pathology in Ferrets Infected with H1N1 Influenza A Viruses Isolated from Different Hosts. Journal Of Virology. 2011;85:7572–7581. doi: 10.1128/JVI.00512-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McBrayer A, Camp JV, Tapp R, Yamshchikov V, Grimes S, et al. Course of seasonal influenza A/Brisbane/59/07 H1N1 infection in the ferret. Virology journal. 2010;7:149. doi: 10.1186/1743-422X-7-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tumpey TM, Maines TR, Van Hoeven N, Glaser L, Solorzano A, et al. A two-amino acid change in the hemagglutinin of the 1918 influenza virus abolishes transmission. Science (New York, N Y) 2007;315:655–659. doi: 10.1126/science.1136212. [DOI] [PubMed] [Google Scholar]
- 57.Belser JA, Blixt O, Chen LM, Pappas C, Maines TR, et al. Contemporary North American influenza H7 viruses possess human receptor specificity: Implications for virus transmissibility. Proceedings Of The National Academy Of Sciences Of The United States Of America. 2008;105:7558–7563. doi: 10.1073/pnas.0801259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Hoeven N, Belser JA, Szretter KJ, Zeng H, Staeheli P, et al. Pathogenesis of 1918 pandemic and H5N1 influenza virus infections in a guinea pig model: antiviral potential of exogenous alpha interferon to reduce virus shedding. Journal Of Virology. 2009;83:2851–2861. doi: 10.1128/JVI.02174-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hatta M, Hatta Y, Kim JH, Watanabe S, Shinya K, et al. Growth of H5N1 influenza A viruses in the upper respiratory tracts of mice. PLoS Pathogens. 2007;3:1374–1379. doi: 10.1371/journal.ppat.0030133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Steel J, Lowen AC, Mubareka S, Palese P. Transmission of influenza virus in a mammalian host is increased by PB2 amino acids 627K or 627E/701N. PLoS Pathogens. 2009;5:e1000252. doi: 10.1371/journal.ppat.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao Y, Zhang Y, Shinya K, Deng G, Jiang Y, et al. Identification of amino acids in HA and PB2 critical for the transmission of H5N1 avian influenza viruses in a mammalian host. PLoS Pathogens. 2009;5:e1000709. doi: 10.1371/journal.ppat.1000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shinya K, Hamm S, Hatta M, Ito H, Ito T, et al. PB2 amino acid at position 627 affects replicative efficiency, but not cell tropism, of Hong Kong H5N1 influenza A viruses in mice. Virology. 2004;320:258–266. doi: 10.1016/j.virol.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 63.Subbarao EK, London W, Murphy BR. A single amino acid in the PB2 gene of influenza A virus is a determinant of host range. Journal Of Virology. 1993;67:1761–1764. doi: 10.1128/jvi.67.4.1761-1764.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gabriel G, Herwig A, Klenk HD. Interaction of polymerase subunit PB2 and NP with importin alpha1 is a determinant of host range of influenza A virus. PLoS Pathogens. 2008;4:e11. doi: 10.1371/journal.ppat.0040011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou B, Donnelly ME, Scholes DT, St George K, Hatta M, et al. Single-reaction genomic amplification accelerates sequencing and vaccine production for classical and Swine origin human influenza a viruses. Journal Of Virology. 2009;83:10309–10313. doi: 10.1128/JVI.01109-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spackman E, Senne DA, Myers TJ, Bulaga LL, Garber LP, et al. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. Journal Of Clinical Microbiology. 2002;40:3256–3260. doi: 10.1128/JCM.40.9.3256-3260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Total and differential leukocyte counts for ferrets inoculated with wild bird avian influenza viruses H1N9 and H6N1.
(DOCX)
Raw Glycan Microarray Data.
(DOCX)