Abstract
Alginate microgels with varied shapes, such as mushroom-like, hemi-spherical, red blood cell-like, and others, were generated by combining microfluidic and external ionic crosslinking methods. This novel method allows a continuous fine tuning of the microgel particles shape by simply varying the gelation conditions, e.g., viscosity of the gelation bath, collecting height, interfacial tension. The release behavior of iopamidol-loaded alginate microgel particles with varied morphologies shows significant differences. Our technique can also be extended to microgels formation from different anionic biopolymers, providing new opportunities to produce microgels with various anisotropic dimensions for the applications in drug delivery, optical devices, and in advanced materials formation.
INTRODUCTION
Microgels have attracted great attention in the past decade owing to their wide applications in drug delivery, adsorbents, and optical device.1, 2, 3, 4 Performance and application of the microgels generally depend upon their size and shape, just like many living micro-systems or organisms such as red blood cells, which possess a special shape for a specific application. In general, spherical microgels can be prepared by emulsion polymerization in order to minimize the interfacial free energy between the particles and the medium. Nonspherical microgels, however, are highly desirable due to their properties such as anisotropic responses to external force, large surface area, and building blocks for superstructures formation. Microfluidic technique provides a powerful platform suitable for the preparation of monodisperse spherical polymer microparticles, microcapsules, microgels, and so on.5, 6, 7, 8, 9, 10, 11, 12, 13, 14 Non-spherical microgel, such as rod-like, wedge-like, and disk-like microgels, can also be prepared via well-designed microfluidic channels or mask-photopolymerization.15, 16, 17, 18, 19, 20, 21, 22, 23
Nowadays, it still remains a challenge for preparing more complicated microgels with controllable shapes and built-in functionalities for novel applications, which mimic those of some living micro-creatures. Recently, Sarkar and his coworkers modeled the deformation of a viscoelastic drop falling through a Newtonian medium and pointed out that viscoelasticity can make an initially spherical drop deformed into an oblate shape with a dimple at the rear end.24 In this report, we demonstrate a facile, yet versatile strategy to prepare biomimetic alginate microgels with precisely controllable morphologies by means of a microfluidic-assisted procedure combining external ionic crosslinking. In comparison with the crosslinking occurred in the microchannels,21 this unique external ionic crosslinking (gelation bath) allows more experimental parameters for precisely controlling of the morphology, e.g., viscosity of the gelation bath, ratio of the oil/water in the gelation bath as well as the collecting height, excepting flow rate as shown in Fig. 1. These experimental parameters mainly involve the variation of interfacial tension and viscoelastic property of the droplets. In particular, these experimental parameters can be easily adjusted, resulting in more complicated morphologies of the microgels formed. Therefore, our strategy not only provides experimental evidence for the simulative results but also presents a new concept for the design and fabrication of non-spherical microgels.
Figure 1.
Schematic illustration of the experimental setup. Uniform water droplets containing 1.5 wt. % sodium alginate dispersed in n-decanol containing 5 wt. % Span 80 were generated in a microcapillary device. The droplets were then collected into a gelation bath which contained a double layer of two solutions: the upper n-decanol layer with 5 wt. % Span 80, and the bottom aqueous layer with 15 wt. % barium diacetate [Ba(Ac)2 or calcium diacetate, Ca(Ac)2], acting as a crosslinking agent. Glycerol was introduced to the bottom aqueous layer to regulate the viscosity of the fluid. Small amount of calcium chloride (CaCl2), acting as a pre-crosslinking agent, was added to the upper oil layer to form spherical or hemi-spherical microgels.
EXPERIMENTAL
Materials
Sodium alginate (SA), calcium chloride (CaCl2, purity: ≥93%), and sorbitane monooleate (Span80, purity: ≥99.0%) were obtained from Sigma–Aldrich. Polyoxyethylene (80) sorbitan monooleate (Tween 80, purity: ≥99.0%), barium acetate [Ba(Ac)2, purity: ≥99.0%], glycerol (purity: ≥99.0%), calcium acetate monohydrate (Ca(Ac)2.H2O, purity: ≥98.0%), and n-decanol (purity: ≥98.0%) were all obtained from Sinopharm Chemical Reagent Co. Ltd. All the chemicals were used without further purification.
Methods
Fabrication of microcapillary devices
To generate alginate aqueous solution–in–decanol emulsion droplets with well-defined sizes, we constructed microcapillary devices each consisting of a cylindrical injection and collection tube (1 mm O.D.) positioned inside a square glass capillary (1 mm I.D.), as illustrated in Fig. 1. The tip of the injection tube is tapered to an orifice of ∼50 μm by heating and pulling a cylindrical capillary to a sharp point (using a Narishige PC–10 micropipette puller) and breaking the tip to the desired opening diameter (with a Narishige MF–900 microforge). The tapered collecting tube was produced by axial heating of the end of a 580 μm inner diameter capillary tube using the micropipette puller. The heating conditions were adjusted to produce tubes with an inner diameter of ∼300 μm at the capillary tip. The size of the emulsion droplets could be easily controlled by increasing the shear force of the continuous fluid. This was accomplished by injecting the tip of the tapered injection tube into the cylindrical collecting tube, as shown in Fig. 1 and the inset optical microscope image shown in Fig. S1 (see supplementary material34).
Droplet preparation and collection
The disperse phase consisted of 1.5 wt. % aqueous sodium alginate solution while the continuous phase was n-decanol with 5 wt. % span 80 dispersed in it. In the gelation bath, the aqueous phase was a glycerol/water mixture (glycerol content: ranging from 0 to 70 wt. %) with 15 wt. % Ba(Ac)2. The oil phase was prepared by dissolving 15 wt. % CaCl2 in n-decanol solution with 5 wt. % Span 80 pre-dissolved. In general, flow rates of the continuous phase and disperse phase were 1800 and 30 (μl h−1), respectively. In both the microfluidics generation and gelation process, surfactants play an important role in regulating the interfacial tension of the oil/water system, and thus the deformability of the droplets.
In a typical microfluidic-assisted approach, droplets can be formed at the orifice of the channel; these droplets can then solidify to generate monodisperse microgel particles by physical or chemical crosslinking. The formed emulsion droplets were transferred into a 50 ml beaker containing the gelation bath through a silicone tubing (inner diameter: ∼500 μm), which could maintain smooth transition from the glass capillary to the silicone tubing. The emulsion droplets distance between each other was about ∼2 mm in the collecting silicone tubing, avoiding fusion of the droplets before crosslinking. The end of the collecting tubing was just inserted into the gelation bath. Each of the collecting beakers contained an 8 ml gelation bath, consisting of 15 wt. % Ba(Ac)2 (or Ca(Ac)2) and varied glycerol content (ranged from 0 to 70 wt. %) in aqueous solution. To test the collecting height effect on the microgel morphology, the end of the collecting tubing was contacted with the inner wall of the collecting beaker, leading to descending the droplets one by one rather than fusion into a large droplet.
To obtain the microgels with a dimple, the gelation bath should containing 50 wt. % glycerol while the height between the tip of the collecting tubing and the surface of the gelation bath should be higher than 15 mm. On the other hand, to prepare spherical microgels, the gelation bath was made up of double layers, where the upper layer contained 15 wt. % CaCl2 and 5 wt. % span 80 in n-decanol solution, while the bottom layer was an aqueous solution with 15 wt. % Ba(Ac)2. Moreover, the volume of the upper layer (∼15 ml) was much larger than that of the bottom layer (∼3 ml) to maintain the droplets’ spherical morphology during crosslinking. The end of the collecting tube, which was 5 mm higher than the surface layer of gelation bath, was also in contact with the inner wall of the beaker. The detailed experimental conditions for the microgels preparation are listed in the Tables S1 and S2 (see supplementary material34).
The microgel suspensions in the beaker were transferred to a 5 ml centrifugal tube and washed with deionized water, subsequently centrifuged at 4000 rpm, removing the supernatant. The above procedure was repeated 5 times to remove the free Ba2+ and/or Ca2+ from the solution. The resulting microgel particles were transferred into a 2 ml centrifugal tube for further characterization.
Release profile
Spherical, pear-like, and mushroom-like microgels were employed for the release studies. To study the encapsulation of hydrophilic species, 17 wt. % iopamidol as a model drug, was added to the initial sodium alginate aqueous solution. We note that the viscosity of the alginate aqueous solution increases upon the iopamidol addition. However, the shape of the microgels does not change much compared to that prepared in the same experimental setup without addition of iopamidol. Iopamidol is a common contrast agent, with a characteristic adsorption wavelength of 242 nm. Three types of the alginate microgel particles were prepared under the same flow rate of oil/water. Each initial emulsion droplet, and the corresponding microgel after crosslinking, would thus have same volume. The detailed procedure for preparing the microgels was the same as described above. Iopamidol was encapsulated into the microgels during the crosslinking process. In all cases, the same amount of the microgels was performed in the release study.
The resulting microgel samples were purified by the following procedure: the upper oil layer was removed, followed by the addition of 2 ml deionized water to dilute the aqueous layer. Subsequently, the aqueous solution was transferred into a 5 ml centrifugal tube, and centrifuged for 2 min with a speed of 4000 rpm. The supernatant fluid was removed. The above procedure was repeated 3 times. The concentrated microgel particles were transferred into dialysis tubing (DM27 EI9004, USA; cut off: 12 000–14 000). The dialysis tubing was pre-treated before use by boiling in a mixture of water/alcohol (1:1) for 30 min, followed by washing once with 0.01 mol/ml NaHCO3 and deionized water for 5 times, respectively. Dialysis was performed in a 100 ml beaker filled with 40 ml dionized water under stirring. Subsequently, 2.5 ml of the surrounding aqueous solution in the beaker was removed and placed into a quartz sample cell containing 3 ml deionized water for the UV–vis measurement. To visualize the release profile of the iopamidol-loaded microgels, the absorption was measured using UV–vis spectrophotometer (Beijing Rayleigh Analytical Instrument Co., China) at room temperature. Absorption spectra of the surrounding aqueous solution under 495 nm were recorded at same time interval (5 min) using deionized water as a reference. After each testing, the sample was poured back into the beaker to maintain the same solution volume. It was assumed that the iopamidol released completely when the ultraviolet absorbance became constant.
Characterization
The formation of emulsion droplets and microgel particles after rinsing was monitored by using an inverted optical microscope (Olympus IX71) in bright-field or phase-contrast modes. A drop of an aqueous dispersion containing microgel particles was placed on a clean silicon wafer and followed by freeze drying. The morphology of the freeze-dried microgel particles was investigated by environmental scanning electron microscope (E-SEM) (Quanta 200). The accelerating voltage was 10 kV. Interfacial tensions were measured using a pendent drops tensiometer (JC2000C1, Shanghai Zhongchen Digital Technol. Instrument Ltd. Co., China), which relies on the deformation of millimeter-sized droplets due to gravity.
The viscosities of the dispersed phase and collecting solutions were measured using a Brookfield R/S plus Rheometer. In a typical experiment, 22 ml of the solution were poured into the outer barrel of the rheometer, while the inner rotating bowl’s rotation was driven by a motor on the rheometer. The inner rotating bowl supplied the shearing force for the solution to be tested.
RESULTS AND DISCUSSION
In our generation strategy, uniform emulsion droplets containing sodium alginate can be formed at the microcapillary channel (see Fig. 1 and Fig. S1, supplementary material34); these droplets can then be solidified to generate microgels particles in the gelation bath with crosslinking agent. As shown in Fig. 2, several types of alginate microgels, such as hemi-spherical, mushroom-like, disk-like, red blood cell-like, among others were prepared by adjusting the flow rate of the microfluidics and the gelation conditions (see Table S1 and Fig. S2 in supplementary material34). We note that microfluidics technique has been used to prepare non-spherical microgels in the previous reports. For example, Zhao and the coworkers prepared microgels in the microfluidic channels by fusing the different water droplets containing CaCl2 and sodium alginate, respectively.21 Non-spherical microgels, such as disk-like or plug-like microgels can be obtained by adjusting the flow rates. In their strategy, crosslinking of sodium alginate occurred in the microfluidics channel, microgels with limited morphologies can thus be obtained by changing only the flow rates.
Figure 2.
Alginate microgel particles with varied morphologies obtained by accurately controlling the preparation conditions, which are listed in Table S1 (see supplementary material34).
In the current procedure, besides flow rate, a unique external ionic crosslinking (gelation bath) was designed, allowing more experimental parameters for precisely controlling of the morphologies, e.g., viscosity of the gelation bath, ratio of the oil/water in the gelation bath as well as the collecting height. These experimental parameters involve the variation of interfacial tension and viscoelastic property of the droplets. In particular, these experimental parameters can be easily adjusted, resulting in more complicated morphologies of the microgels formed, such as hemi-spherical, mushroom-like, red blood cell-like, and among others. Typically, hemi-spherical microgels were prepared by manipulating the height ratio of the oil/aqueous layer in the gelation bath to 16/6 (mm). In this case, the pre-crosslinking reaction of the droplets in the oil layer with CaCl2 does not make them completely solidified. Thus, when the droplets fell through the O/W interface, the effect of an impact force led to the formation of a flat bottom of the droplet while the upper half of the droplets retained a spherical shape. To obtain a preferable impact force, the flow rate of the continuous phase over the dispersed phase was 2100/30 (μl h−1). More interestingly, the shapes of the microgels can be maintained after freeze drying, as displayed in Fig. 3.
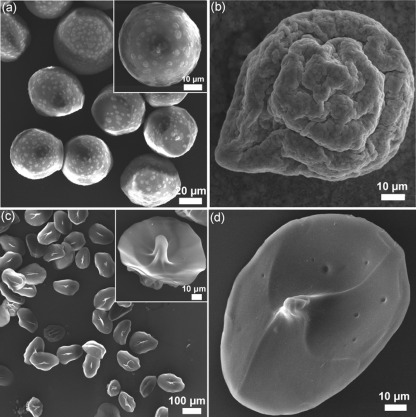
Figure 3.
SEM images of the freeze-dried microgel particles which were prepared from alginate microgels with varied shapes: (a) spherical microgels, corresponded to Fig. 4a, (b) tailed microgels, corresponded to Fig. 2b, 2c, 2d mushroom-like microgels, corresponded to Figs. 2b, 2c, respectively. It is clear that the shape of the microgel particles was maintained during the sample preparation and the analysis.
Similarly, other types of alginate microgels can be generated by optimizing experimental parameters (see Fig. S2 and Table S2 in supplementary material34). Red blood cell-like microgel can only be obtained in a gelation bath containing high concentration of glycerol (∼70 wt. %) whereas preferable height (h) between the tip of the collecting tube and the surface of the gelation bath had to be ca. −6 mm. Generally, a viscoelastic droplet moving in a Newtonian media would induce the formation of an inward dimple.24, 25, 26, 27 In this case, the droplet would appear an inward cusp when the droplet’s transient interfacial tension cannot balance the droplet’s viscoelastic stress.28 In this work, the red blood cell-like microgels can be solidified quickly once the inward cusp is formed due to the high concentration of crosslinking agent.
A key advantage of our procedure for preparing microgels is that the morphology of the alginate microgels can be tuned continuously by simply changing height h, as well as the viscosity of the gelation bath by varying the content of glycerol, as shown in Fig. 4. Although tailed microgels have been previously encountered under some special conditions,29, 30 the reproducibility, dispersity, and tunability of the tailed microgels still remains poor. Emulsion droplets would adopt prolate shapes inside the collecting tubing due to confinement effect of the tubing when ratio of the inner diameter of the tubing to that of the droplet is ∼2 (the ratio in our case was ∼1.9).26 After leaving the collecting tubing, the prolate droplets attempt to recover a spherical shape in order to minimize the interfacial free energy (Fig. 4a).31 The initial prolate droplets (right before entering the aqueous layer of the gelation bath) can be easily tuned by adjusting h. In the case of a low h (∼0 mm), prolate droplets quickly enter the Newtonian gelation bath (see Figures S3 and S4 in supplementary material34). Thus, the shape of the prolate droplets will be maintained and the tail will grow up gradually (Figs. 4b, 4c), owing to the interplay between the viscous and elastic forces during the subsequent gelation process.32 A further increase of glycerol concentration will lead to the deformation of spherical caps and the formation of mushroom-like microgels (see Fig. S5 in supplementary material34). In the case of high h (∼15 mm), the tailed droplet takes ∼2 s to enter into the gelation bath. The droplets will become oblate due to the interplay between interfacial tension and gravity during the falling process26 and evolve a dimple-like shape (Fig. 4d) during the subsequent gelation process.31
Figure 4.
Morphologies of alginate microgel can be continuously controlled by changing the viscosities and interfacial tension of the gelation bath. Detailed preparation conditions are summarized [see Table S2 in supplementary material34].
Interestingly, the aspect ratio (A/R) will decrease gradually with a further increase of the glycerol concentration (above 10 wt. %) as shown in Fig. 5, reflecting the effect of the varying viscous force on the shape of the microgel particles. The drag force caused by the competition between the viscous and the elastic stresses leads to an increase of the tail length upon increasing the glycerol concentration from 0 to 10 wt. %. The forces acting on the droplets can be classified into two types: (1) One will prevent the deformation of the droplet such as interfacial tension, while (2) the other will induce deformation of the droplet, for example, by a viscous force. The formation mechanism of such alginate microgel particles is still under active investigation but it likely reflects the interplay between these two kinds of forces. When the glycerol concentration in the collecting solution was high enough (about 50 wt. %), the droplets can hardly penetrate the interfacial membrane and will spread on the interface, resulting in an oblate cap. As the droplet falls through the gelation bath, various forces are applied to the surface of the droplet, including gravity, buoyancy, interfacial tension, and a viscous force.
Figure 5.
The plot shows the relationship between the aspect ratio [A/R, defined as the maximum length parallel to the tail (A) over the maximum length perpendicular to the tail (R)] of the tailed microgels and the glycerol concentration in the gelation bath. The microgels were prepared from an aqueous 1.5 wt. % SA solution with a flow rate of 1800/30 (O/W) μl h−1; in gelation bath, the upper oil layer consisting of n-decanol dispersed with 5 wt. % span 80 while the aqueous bottom layer consists of an aqueous solution containing 15 wt. % Ba(Ac)2 and varying glycerol concentration. The insets are the representative optical microscopy images for the selected points. Error bars represent the standard deviation.
Furthermore, different gelation parameters were employed to tune the microgel shapes. For instance, an inward dimple-like alginate microgel particle can be obtained by adding the water-soluble surfactant Tween 80 into the dispersed phase, thereby reducing the interfacial tension significantly (see Fig. S5 in supplementary material34). It was found that the dimple gradually grow from small to bowl-like (Figs. 4d, 4e) when the flow rate ratios of continuous and dispersed phase were 1800/30 and 2600/30 (μl h−1), respectively. Also, extended bowl-like particles can be obtained (Fig. 4f). In this process, the interfacial tension of the droplet was reduced due to the addition of surfactant, making the droplet deform more easily. The droplet surrounded by the oil phase would experience an inertial spreading because the droplets’ normal stress cannot be balanced by the interfacial tension.33 The formation mechanism of such alginate microgel particles is still under active investigation but it likely reflects the interplay between these two kinds of forces.
As a proof-of-concept, iopamidol as a model drug was encapsulated in the prepared microgels. As shown in Fig. 6, the microgels with a different shape display different release kinetics. The release equilibrium of iopamidol was reached within about 100 min from the spherical microgel, whereas a much shorter time (∼50 min) from the mushroom-like microgel. Obviously, the release of the spherical microgels, which is proportional to the square root of time, was controlled by Fickian diffusion, while the release profile of the mushroom-like microgels displays a rapid initial (apparent burst) release and followed by a sustained release over a 100 min period. The release kinetics curve for the pear-like microgels was between that of spherical and mushroom-like microgels. The different release rates can be attributed to shape variation (mainly surface area), the crosslinking degree and uniformity of the particles. Specifically, spherical microgel particles showed the slowest release rate, presumably owing to the fact that spherical particles possess the smallest interfacial area. Although uniformity of the crosslinking in the microgels is difficult to assess, we can hypothesize that the crosslinking among the three kinds of microgels might be non-uniform due to their different effects of sedimentation in the gelation bath, resulting in different degrees of crosslinking in different parts of the particle. For example, fast diffusion of iopamidol in the mushroom-like microgels may occur in the areas with a lower degree of crosslinking, while low diffusion will occur in the parts with a higher degree of crosslinking. Thus, the release profile of the mushroom-like microgels shows a rapid initial release of iopamidol and is followed by a sustained release.
Figure 6.
Release behavior of iopamidol-loaded alginate microgel with varied morphologies which were prepared through the procedure as described above.
CONCLUSIONS
In summary, alginate microgel with varied morphologies can be prepared via a microfluidic device and an external ionic crosslinking method. The morphology of the microgel can be finely tuned continuously by simply varying the gelation conditions. The release behavior of iopamidol-loaded alginate microgel particles with varied morphologies shows significant differences. Our technique can also be extended to microgels formation from different anionic biopolymers, providing new opportunities to produce biomimatic microgels with various anisotropic dimensions for the applications in drug delivery, optical devices, and in advanced materials formation.
ACKNOWLEDGMENTS
We gratefully acknowledge funding for this work provided by the National Natural Science Foundation of China (51073062 and 21004025) and the National Basic Research Program of China (973 Program, 2012CB812500). We also thank HUST Analytical and Testing Center for the SEM measurements.
References
- Das M., Zhang H., and Kumacheva E., Annu. Rev. Mater. Res. 36, 117 (2006). 10.1146/annurev.matsci.36.011205.123513 [DOI] [Google Scholar]
- Zhang H., Tumarkin E., Sullan R. M. A., Walker G. C., and Kumacheva E., Macromol. Rapid Commun. 28, 527 (2007). 10.1002/marc.200600776 [DOI] [Google Scholar]
- Panda P., Ali S., Lo E., Chung B. G., Hatton T. A., Khademhosseini A., and Doyle P. S., Lab Chip 8, 1056 (2008). 10.1039/b804234a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiddes L. K., Young E. W. K., Kumacheva E., and Wheeler A. R., Lab Chip 7, 863 (2007). 10.1039/b703297h [DOI] [PubMed] [Google Scholar]
- Xu S. Q., Nie Z. H., Seo M., Lewis P., Kumacheva E., Stone H. A., Garstecki P., Weibel D. B., Gitlin I., and Whitesides G. M., Angew. Chem., Int. Ed. 44, 3799 (2005). 10.1002/anie.200462226 [DOI] [PubMed] [Google Scholar]
- Shah R. K., Kim J. W., Agresti J. J., Weitz D. A., and Chu L. Y., Soft Matter 4, 2303 (2008). 10.1039/b808653m [DOI] [Google Scholar]
- Zhu J. and Hayward R. C., Angew. Chem., Int. Ed. 47, 2113 (2008). 10.1002/anie.200704863 [DOI] [PubMed] [Google Scholar]
- Tan W. H. and Takeuchi S., Adv. Mater. 19, 2696 (2007). 10.1002/adma.200700433 [DOI] [Google Scholar]
- Zhang H., Tumarkin E., Peerani R., Nie Z., Sullan R. M. A., Walker G. C., and Kumacheva E., J. Am. Chem. Soc. 128, 12205 (2006). 10.1021/ja0635682 [DOI] [PubMed] [Google Scholar]
- Kanai T., Lee D., Shum H. C., and Weitz D. A., Small 6, 807 (2010). 10.1002/smll.200902314 [DOI] [PubMed] [Google Scholar]
- De Geest B. G., Urbanski J. P., Thorsen T., Demeester J., and De Smedt S. C., Langmuir 21, 10275 (2005). 10.1021/la051527y [DOI] [PubMed] [Google Scholar]
- Thiele J. and Seiffert S., Lab Chip 11, 3188 (2011). 10.1039/c1lc20242a [DOI] [PubMed] [Google Scholar]
- Li W., Greener J., Voicu D., and Kumacheva E., Lab Chip 9, 2715 (2009). 10.1039/b906626h [DOI] [PubMed] [Google Scholar]
- Wong E. H. M., Rondeau E., Schuetz P., and Cooper-White J., Lab Chip 9, 2582 (2009). 10.1039/b903774h [DOI] [PubMed] [Google Scholar]
- Kim J. W., Utada A. S., Fernandez-Nieves A., Hu Z. B., and Weitz D. A., Angew. Chem., Int. Ed. 46, 1819 (2007). 10.1002/anie.200604206 [DOI] [PubMed] [Google Scholar]
- Workman V. L., Dunnett S. B., Kille P., and Palmer D. D., Biomicrofluidics 1, 014105 (2007). 10.1063/1.2431860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Z. H., Li W., Seo M., Xu S. Q., and Kumacheva E., J. Am. Chem. Soc. 128, 9408 (2006). 10.1021/ja060882n [DOI] [PubMed] [Google Scholar]
- Hwang D. K., Dendukuri D., and Doyle P. S., Lab Chip 8, 1640 (2008). 10.1039/b805176c [DOI] [PubMed] [Google Scholar]
- Dendukuri D., Pregibon D. C., Collins J., Hatton T. A., and Doyle P. S., Nature Mater. 5, 365 (2006). 10.1038/nmat1617 [DOI] [PubMed] [Google Scholar]
- Dendukuri D., Hatton T. A., and Doyle P. S., Langmuir 23, 4669 (2007). 10.1021/la062512i [DOI] [PubMed] [Google Scholar]
- Liu K., Ding H. J., Liu J., Chen Y., and Zhao X. Z., Langmuir 22, 9453 (2006). 10.1021/la061729+ [DOI] [PubMed] [Google Scholar]
- Hwang D. K., Oakey J., Toner M., Arthur J. A., Anseth K. S., Lee S., Zeiger A., Van Vliet K. J., and Doyle P. S., J. Am. Chem. Soc. 131, 4499 (2009). 10.1021/ja809256d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X. H., Cheng W., Guo F., Liu W., Guo S. S., He Z. K., and Zhao X. Z., Lab Chip 11, 2561 (2011). 10.1039/c1lc20150f [DOI] [PubMed] [Google Scholar]
- Mukherjee S. and Sarkar K., Phys. Fluids 23, 013101 (2011). 10.1063/1.3533261 [DOI] [Google Scholar]
- Sostarecz M. C. and Belmonte A., J. Fluid Mech. 497, 235 (2003). 10.1017/S0022112003006621 [DOI] [Google Scholar]
- You R., Haj-Hariri H., and Borhan A., Phys. Fluids 21, 013102 (2009). 10.1063/1.3054156 [DOI] [Google Scholar]
- Hsu A. S. and Leal L. G., J. Non-Newtonian Fluid Mech. 160, 176 (2009). 10.1016/j.jnnfm.2009.03.004 [DOI] [Google Scholar]
- Baumann N., Phys. Fluids A 4, 567 (1992). 10.1063/1.858328 [DOI] [Google Scholar]
- Capretto L., Mazzitelli S., Balestra C., Tosi A., and Nastruzzi C., Lab Chip 8, 617 (2008). 10.1039/b714876c [DOI] [PubMed] [Google Scholar]
- Chan E. S., Lee B. B., Ravindra P., and Poncelet D., J. Colloid Interface Sci. 338, 63 (2009). 10.1016/j.jcis.2009.05.027 [DOI] [PubMed] [Google Scholar]
- Stone H. A., Annu. Rev. Fluid Mech. 26, 65 (1994). 10.1146/annurev.fl.26.010194.000433 [DOI] [Google Scholar]
- Frank X. and Li H. Z., Phys. Rev. E 74, 056307 (2006). 10.1103/PhysRevE.74.056307 [DOI] [PubMed] [Google Scholar]
- Saidi A., Martin C., and Magnin A., J. Non-Newtonian Fluid Mech. 165, 596 (2010). 10.1016/j.jnnfm.2010.02.020 [DOI] [Google Scholar]
- See supplementary material at http://dx.doi.org/10.1063/1.4720396 for detailed procedure of experiment and additional experiment data.