Abstract
The mechanism of autosomal dominant retinitis pigmentosa (ADRP) caused by the P23H mutation in rhodopsin is tightly associated with misfolded rhodopsin (RHO) which causes endoplasmic reticulum overload (ER stress), activates the unfolded protein response (UPR) and triggers apoptosis. In efforts to create a therapy for ADRP caused by the P23H mutation, we have explored different approaches leading to survival of photoreceptor (PR) cells. The direct approach involves the modulation of the level of wild-type RHO, while the indirect approach involves reprogramming the UPR and increasing the expression of heat shock proteins (HSPs). Taking the direct approach, we found that over-expression of wild-type RHO rescues scotopic ERG responses partially. However, greater therapeutic effects were obtained by manipulation of the UPR in P23H RHO rat PRs treated with the endoplasmic reticulum protein BiP/Grp78. In vitro study revealed that the pro-survival effect of Bip gene was not associated with its function as a molecular chaperone, but rather with its regulation of the UPR. Another indirect approach was the over-expression of the Hsf-1 gene, a transcriptional regulator of the heat shock response. AAV-delivery of Hsf-1 resulted in an increase of scotopic ERG amplitudes by over 35%. Taken together these data suggest viable therapeutic treatments for ADRP.
XX1. Introduction
Autosomal dominant Retinitis Pigmentosa (ADRP) is an inherited retinal disorder, which leads to progressive death of PR and significant visual impairment. The rhodopsin-linked form of RP affects approximately in 1 in 30,000 people and is associated with over 100 different genetic modifications within the RHO gene (www.sph.uth.tmc.edu/RetNet). Progress in developing gene therapies for dominant RP has been slower compared to recessive forms of this retinopathy, but some advances in this field have been made lately. As any dominant disease, the treatment of ADRP correcting the primary genetic defect most likely requires a removal of the defective gene product in PR cells. However, alternative therapeutic approaches exist, such as modulation of cellular signaling caused by the accumulation of misfolded RHO. In the current study, we set out to determine what effects over-expressing wild-type (WT) RHO, the ER resident chaperone BiP/GRP78, and transcriptional activator of heat shock proteins, HSF1, would have on retinal function in P23H transgenic rats, as assessed by electroretinography (ERG).
The idea of testing the WT RHO in P23H RHO PR was originated from studies on transgenic mice, carrying mutated rhodopsin (RHO) transgene on different genetic backgrounds, suggesting that the increased amount of WT RHO in ADRP PRs might be beneficial for vision of these animals (Frederick et al., 2001,). Justification of over-expression of BiP protein was based on its chaperoning (Hoshino et al., 2007) and anti-apoptotic activities in vivo and in vitro (Miyake et al., 2000). The master regulator of chaperone gene transcription, HSF1, has also been shown to suppress polyglutamine aggregates in vivo and cause a therapeutic effect (Fujikake et al., 2008). Therefore, we employed a gene delivery approach with a help of adeno-associate viruses (AAV) to validate these therapeutic genes as candidates for ADRP gene therapy in P23H RHO transgenic rats.
XX.2 Materials and Methods
XX.2.1 Animals
Homozygous transgenic P23H line 3 RHO rats were used in this experiment. All animal procedures were performed according to the guidelines of the ARVO statement for the “Use of Animals in Ophthalmic and Vision Research.” Subretinal injection of P23H RHO-3 rats with 2 ul of AAV expressing mouse Rho resistant gene 301 (referred to WT), human GRP78 (BiP) and HSF-1 cDNA with titer of 1011–1012 /per genome was performed on postnatal day (P) 15 in P23H RHO pups, as described before (Gorbatyuk et al., 2010). Control eyes were injected with AAV-GFP.
XX.2.2 Transient Transfection of HeLa Cells and Immunohistochemistry
We used Lipofectamine 2000 and pcDNA3.1 plasmids expressing mouse P23H RHO, WT RHO, and human GRP78 under control of the CMV promoter to perform transfection in HeLa cells. At 48 h, cells were fixed with Pen-Fix, and immunohistochemistry was performed by using the 1D4 antibody against rhodopsin and anti-Flag antibody. Cy-2 and Cy-3 conjugated secondary antibodies were applied to detect RHO and the Flag epitope tag, respectively.
XX.2.3 ERG
The procedure was performed as described before (Gorbatyuk et al., 2010). Scotopic ERG responses were measured with 10 µsec flashes of white light at 2.68 cd-s/m2 intensity. Differences in a- and b-wave amplitudes between simultaneously recorded test and control eyes were the primary measure of outcome.
XX.2.4 Histology
Rats were intracardially perfused with 2% paraformaldehyde and 2.5% glutaradehyde, and eyes were removed from euthanized animals, post-fixed and embedded in epoxy resin (Gorbatyuk et al., 2010). Mean ONL thickness of the entire retina or specific region of the eye were compared between the AAV-BiP and control-injected eyes.
XX.3 Results
XX.3.1 Functional Preservation of P23H RHO Photoreceptors as a Result of Over-expression of Mouse RHO 301
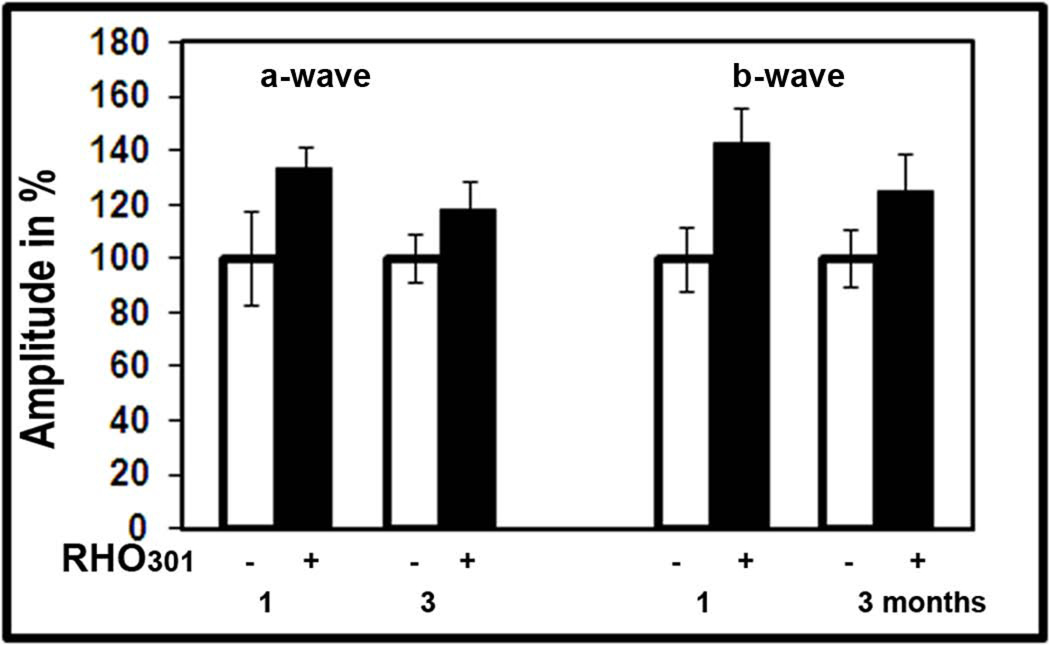
We used the Rho 301 gene in a “cut-and-replace” experiment as a resistant target for siRNA301. Rho 301 encodes the WT mouse rod opsin protein. In rats, we tested this gene independently to validate the therapeutic effect of WT RHO. We injected pups at P15 to analyze them by scotopic ERG at 1 and 3 months post injection. Results of this analysis demonstrated that both a- and b-waves responses were elevated in Rho301-treated eyes (Fig. XX.1) at 1 month post-treatment. A-wave amplitude was increased by 33% and b-wave amplitude by 42%. However, at 3 months following injection, the therapeutic effect was only 18% and 34% (p<0.05), respectively, for a- and b-wave amplitudes compared to the GFP-treated eye.
Fig. XX.1.
Over-expression of mouse RHO301 leads to functional preservation of P23H RHO-3 photoreceptors.
XX.3.2 Functional Preservation of P23H RHO Photoreceptors as a Result of Increased Expression of Human BiP/Grp78
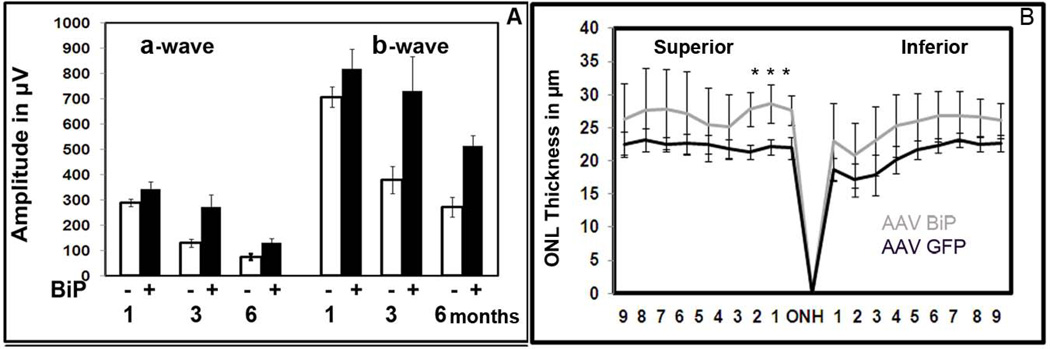
Following injection, we analyzed animals over 6 months by ERG and then euthanized them for histological analysis. The results of this experiment are shown in. Fig. XX.2A. AAV5-BiP treatment led to the rescue of retinal activity in P23H RHO rats. In BiP-treated eyes, a- and b-wave response amplitudes were higher than in control (GFP-injected) eyes, and this protection was consistent over 6 months. A-wave amplitudes were increased from 18% to 78% and b-wave amplitudes were amplified by 15% to 89%. The peak of therapeutic activity occurs at 3 months post-injection; the a-wave was increased by 110% and b-wave was increased by 92%, p<0.05, relative to the control-injected eyes.
Fig. XX.2.
Over-expression of human BiP/GRP78 protein leads to steady therapeutic effect over 6 months measured A: by scotopic ERG and B: by Histological analysis.
XX.3.3 Preservation of Retinal Integrity in P23H RHO Rats as a Result of Over-expression of Human BiP/GRP78
To determine if this therapeutic effect corresponded to preservation of retinal integrity, we enucleated euthanized rats and processed the retinas for histological analysis in order to measure the thickness of the outer nuclear layer (ONL). Morphometric analysis of individual retinas showed small but significant changes in individual sectors of central inferior hemisphere in BiP-treated retina (Fig.XX. 2B). The differences in the lengths of ONL in 3 individual sectors in the superior hemisphere were from 25% to 30%, P< 0.05.
XX.3.4 Elevation of BiP Protein Level Does Not support the Trafficking of P23H RHO to the Cell Membrane
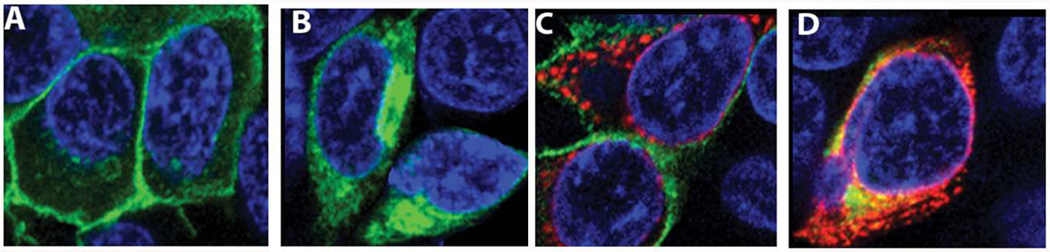
To determine if the increase in the level of BiP would promote opsin folding and localization to the cell membrane, we performed co-transfection of HeLa cells with plasmids expressing P23H opsin and BiP-Flag protein. The Flag epitope sequence was placed in front of KDEL fragment and served as a tag for detection of exogenous BiP protein. Results of the experiment are presented in Fig.XX.3 and suggest that the WT RHO, which served as a control, localized to the plasma membrane and the BiP-Flag over-expression did not interfere with this distribution. However, the co-expression of P23H RHO and BiP-Flag proteins in cells had no effect on the mis-localization of P23H RHO in the cytoplasm. This experiment indicated that extra BiP protein in the ER did not permit exit of P23H RHO from the ER and delivery to the cell membrane.
Fig. XX.3.
Immunostaining of HeLa cells co-transfected with wild-type RHO, P23H RHO, and BiP-Flag. A: Immunostaining analysis demonstrated the localization of wild-type RHO to the cell membrane and B: P23H RHO retained within the ER (green). C: Over-expression of BiP-Flag (red) did not affect the trafficking of WT RHO and D: did not promote the distribution of P23H RHO to the cytoplasm.
XX.3.5 Functional Preservation of P23H RHO Photoreceptors as a Result of Over-expression of HSF1
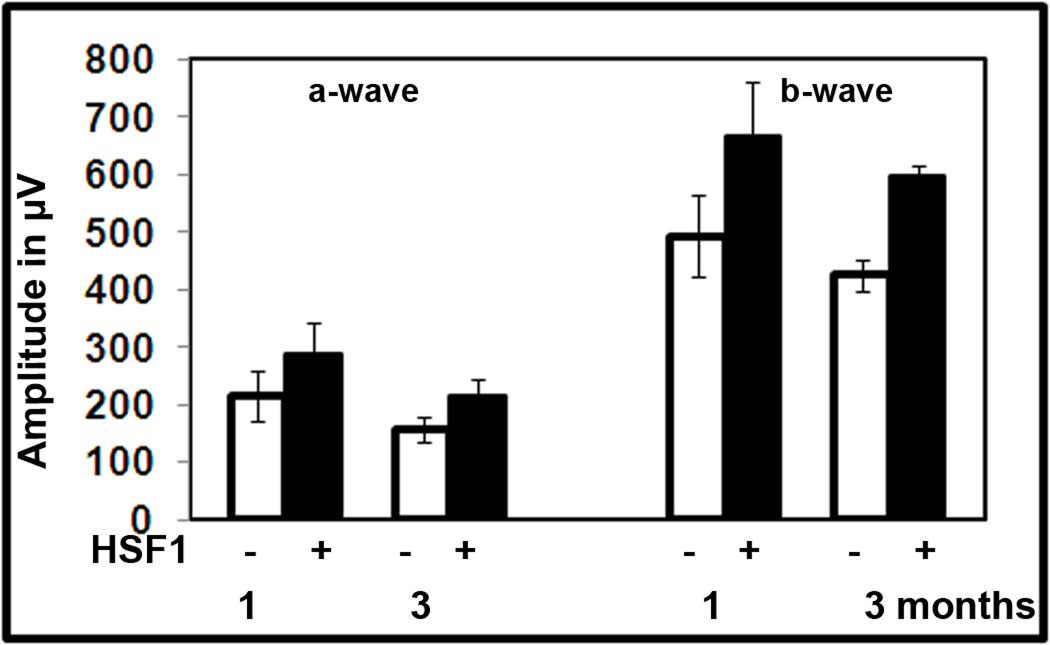
To determine if the HSF1 over-expression had a therapeutic effect in P23H RHO retina, we injected pups with AAV-HSF1 and then monitored them by ERG recording for 3 months (Fig. XX.4). We observed an increase of over 35% for both a- and b-wave amplitudes (P<0.05) in HSF1-treated eyes relative to the AAV-GFP treated eyes.
Fig. XX.4.
Over-expression of HSF1 in P23H RHO-3 photoreceptors leads to sustained preservation of a- and b-wave amplitudes of the scotopic ERG.
XX.4 Discussion
The P23H mutation of rhodopsin belongs to a class (Class II) that is retained in the ER and is later translocated to the cytoplasm for degradation. It causes photoreceptor cell death by a dominant negative mechanism. The dominant negative effect of misfolded RHO is associated with its ability to recruit the WT RHO to form aggresomes, therefore keeping the WT RHO from trafficking to the disk membrane. Therefore, we tested the hypothesis that increased expression of WT RHO would lead to enhancement of photoreceptor function in P23H transgenic rats, assuming that newly synthesized RHO escapes from the ER and tranlocates to the outer segments of PRs. Analysis of ERG results demonstrated that the highest therapeutic effect was observed at 1 month post-injection and was slightly diminished after that. It suggests that the deleterious effect of mutated RHO overcomes the boost normal RHO in RPs, first and second, that expression of exogenous RHO has to be optimized by manipulations with expression cassette, viral dose and time of injection. We note, however, that in P23H transgenic mice, we have seen a beneficial effect of over-expression of RHO for up to 6 months (Mao et al. submitted for publication.)
Gene therapy for photoreceptors expressing P23H RHO can be addressed either by directly targeting the mutant protein or by reprogramming different pathways in the cellular response to misfolded protein. For example, it has been show that pharmacological chaperones such as geldanamycin, radicicol and 17-AAG cause the induction of HSPs and are able to lessen the deleterious impact of P23H RHO (Mendes and Cheetham, 2008). Moreover, the impact of misfolded protein can be overcome by reducing protein aggregation and promoting degradation of the aggregation-prone species. Consequently, we decided to validate new therapeutic targets and to manipulate the level of molecular chaperones in P23H RHO ADRP.
Over-expression of ER resident BiP/Grp78 protein led to sustained elevation of ERG a- and b-wave amplitudes by almost 2-fold compared to control eyes and abolished the retinal degeneration in P23H RHO PR. This therapeutic effect was not necessarily linked to promoting the trafficking of misfolded rhodopsin to the cell membrane, but likely was associated with modulation of cellular signaling (Gorbatyuk et al., 2010). Currently we are testing this chaperone in other models of neurodegenerative diseases.
Another inducer of molecular chaperones, HSF1, has been reported to be involved in a prevention of ADRP in RP10 mouse model via inhibition of Hsp90 and an induction of Hsp70 (Tam et al., 2010). Therefore, it was not surprising that the gene delivery of HSF1 preserved the ERG response in P23H RHO transgenic rats. We hypothesize that due to over-expression of HSF1, the levels of HSP70, HSP 40 and other chaperones were elevated and that this elevation activates pro-survival cellular pathways in P23H RHO RP cells.
Thus, our data suggest that it may not be necessary to improve RHO folding to improve PR viability. Manipulation of cell death and pro-survival pathways and shifting the balance in PR cells toward cell survival could be a reliable therapeutic approach for preserving vision in people with ADRP caused by RHO mutations.
Acknowledgments
This study was supported by FFB: TA-GT-4090-0479-UFL, TA-GT-0507-0384, by C-NP-0706-0353-UCSF, by NIH: EY020905, EY11123, EY08571, EY02162, EY01919, EY06842, EY018313 and EY020846.
References
- Frederick JM, Krasnoperova NV, Hoffmann K, et al. Mutant rhodopsin transgene expression on a null background. Invest Ophthalmol Vis Sci. 2001;42:826–833. [PubMed] [Google Scholar]
- Fujikake N, Nagai Y, Popiel HA, et al. Heat shock transcription factor 1-activating compounds suppress polyglutamine-induced neurodegeneration through induction of multiple molecular chaperones. J Biol Chem. 2008;283:26188–26197. doi: 10.1074/jbc.M710521200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbatyuk MS, Knox T, LaVail MM, et al. Restoration of visual function in P23H rhodopsin transgenic rats by gene delivery of BiP/Grp78. Proc Natl Acad Sci U S A. 2010;107:5961–5966. doi: 10.1073/pnas.0911991107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T, Nakaya T, Araki W, et al. Endoplasmic reticulum chaperones inhibit the production of amyloid-beta peptides. Biochem J. 2007;402:581–589. doi: 10.1042/BJ20061318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes HF, Cheetham ME. Pharmacological manipulation of gain-of-function and dominant-negative mechanisms in rhodopsin retinitis pigmentosa. Hum Mol Genet. 2008;17:3043–3054. doi: 10.1093/hmg/ddn202. [DOI] [PubMed] [Google Scholar]
- Miyake H, Hara I, Arakawa S, et al. Stress protein GRP78 prevents apoptosis induced by calcium ionophore, ionomycin, but not by glycosylation inhibitor, tunicamycin, in human prostate cancer cells. J Cell Biochem. 2000;77:396–408. doi: 10.1002/(sici)1097-4644(20000601)77:3<396::aid-jcb5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Tam LC, Kiang AS, Campbell M, et al. Prevention of autosomal dominant retinitis pigmentosa by systemic drug therapy targeting heat shock protein 90 (Hsp90) Hum Mol Genet. 2010 doi: 10.1093/hmg/ddq369. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]