Background: COUP-TFII, an orphan nuclear receptor, regulates the differentiation process in various cell types during development.
Results: COUP-TFII inhibits Runx2-dependent osteocalcin transcription through physical interaction with Runx2 and matrix mineralization.
Conclusion: COUP-TFII is a negative regulator of osteoblast differentiation.
Significance: COUP-TFII has therapeutic potential for controlling bone-related disease.
Keywords: Bone, Cell Differentiation, Nuclear Receptors, Osteoblasts, Transcription Factors
Abstract
Chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII) is an orphan nuclear receptor of the steroid-thyroid hormone receptor superfamily. COUP-TFII is widely expressed in multiple tissues and organs throughout embryonic development and has been shown to regulate cellular growth, differentiation, and organ development. However, the role of COUP-TFII in osteoblast differentiation has not been systematically evaluated. In the present study, COUP-TFII was strongly expressed in multipotential mesenchymal cells, and the endogenous expression level decreased during osteoblast differentiation. Overexpression of COUP-TFII inhibited bone morphogenetic protein 2 (BMP2)-induced osteoblastic gene expression. The results of alkaline phosphatase, Alizarin Red staining, and osteocalcin production assay showed that COUP-TFII overexpression blocks BMP2-induced osteoblast differentiation. In contrast, the down-regulation of COUP-TFII synergistically induced the expression of BMP2-induced osteoblastic genes and osteoblast differentiation. Furthermore, the immunoprecipitation assay showed that COUP-TFII and Runx2 physically interacted and COUP-TFII significantly impaired the Runx2-dependent activation of the osteocalcin promoter. From the ChIP assay, we found that COUP-TFII repressed DNA binding of Runx2 to the osteocalcin gene, whereas Runx2 inhibited COUP-TFII expression via direct binding to the COUP-TFII promoter. Taken together, these findings demonstrate that COUP-TFII negatively regulates osteoblast differentiation via interaction with Runx2, and during the differentiation state, BMP2-induced Runx2 represses COUP-TFII expression and promotes osteoblast differentiation.
Introduction
Osteoblast differentiation is a highly regulated process controlled by a complex signaling pathway. Bone morphogenetic proteins (BMPs),3 members of the transforming growth factor-β superfamily, are the primary regulators of osteoblast differentiation (1). Among the BMPs, BMP2 is the most effective inducer of osteoblast differentiation and has been shown to regulate Runx2 activity via Smad signaling to promote osteoblast differentiation (2).
Runx2, a member of the Runt domain gene family, plays a major role in osteoblast differentiation by promoting the commitment of pluripotent mesenchymal cells to the osteoblast lineage through regulation of osteoblastic genes, such as type I collagen, osteopontin, bone sialoprotein (BSP), and osteocalcin (OC) (3). During osteoblast differentiation, Runx2 interacts with diverse transcription factors and recruits cofactors to form a complex on its target genes (4, 5).
The orphan nuclear receptors COUP-TFs, which are members of the steroid-thyroid hormone receptor superfamily, were first identified as activators of the chicken ovalbumin gene and were shown to bind to an imperfect direct repeat of the AGGTCA motif (6). COUP-TFs are widely expressed during embryonic development and have been shown to regulate many key biological processes, including angiogenesis, organogenesis, adipogenesis, neurogenesis, cell fate determination, and metabolic homeostasis (7–12). Among the three mammalian orthologs of COUP-TFs including COUP-TFI, COUP-TFII, and EAR2, COUP-TFII can act as either a positive or negative regulator of transcription. COUP-TFII-deficient mice die in utero with defects in heart development and angiogenesis. Tissue-specific knock-out studies have shown that COUP-TFII is required for the development of limb, skeletal muscles, and stomach and plays a critical role in determining vein identity (11, 13, 14). Even though the ligands of COUP-TFII are still unknown, the roles of COUP-TFII during angiogenesis, female reproduction, and neurogenesis have been well established. However, the roles of COUP-TFII in bone formation have not yet been elucidated.
This study was undertaken to determine the functional roles of COUP-TFII in osteoblast differentiation. Here, we show that COUP-TFII acts as a novel negative regulator of osteoblast differentiation via inhibiting Runx2 transactivity.
EXPERIMENTAL PROCEDURES
Materials
Recombinant human BMP2 protein was purchased from R&D Systems (Minneapolis, MN). Antibody against COUP-TFII was from Abcam (Cambridge, UK), and antibody against Runx2 was from Santa Cruz Biotechnology (Santa Cruz, CA). Hemagglutinin (HA) antibody was from Sigma-Aldrich. Actin and Myc antibodies were obtained from Cell Signaling Technology (Beverly, MA). Adenoviral (Ad) vector expressing COUP-TFII and HA-tagged COUP-TFII expression vector were kindly provided by Dr. Kee-Sook Lee (Chonnam National University, Gwangju, Korea). Ad-BMP2 and Ad-Runx2 were kindly provided by Dr. Renny Franceschi (University of Michigan School of Dentistry, Ann Arbor, MI). The COUP-TFII promoter-luciferase reporter was kindly provided by Dr. Juro Sakai (15). The shRNA expression vector pSIREN-RetroQ-ZsGreen (Clontech) for COUP-TFII (shCOUP-TFII) and firefly luciferase, which served as the control (shLuc), were kindly donated by Dr. Zhao Xu (Harvard Catalyst, The Harvard Clinical and Translational Science Center) (10). The target sequence of the COUP-TFII shRNA construct was 5′-AGCTCTTGCTTCGTCTCCC-3′. The COUP-TFII mutant constructs for this study were COUP-TFIIΔDBD (DNA binding domain deletion mutant), COUP-TFIIΔN75 (N-terminal deletion mutant), and COUP-TFIIΔ179 (C-terminal deletion mutant), which were described previously (16, 17).
Cell Culture and Viral Infection
Primary calvaria cells were isolated as described previously (18). MC3T3-E1 and isolated primary osteoblasts were cultured in α-minimal essential medium (α-MEM; Invitrogen); and C3H10T1/2, C2C12, and ST-2 were cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) containing 10% fetal bovine serum (FBS), 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen) in humidified air containing 5% CO2 at 37 °C. Differentiation of osteoblasts was induced by the addition of osteogenic medium containing 2% FBS, 50 μg/ml ascorbic acid and 5 mm β-glycerophosphate. The culture medium was replaced every other day. For viral infection, the cells were treated with the indicated viruses at the designated multiplicity of infection (m.o.i.) under serum-free conditions. After 4 h, an equivalent volume of medium containing 4% FBS was added, and the cells were incubated for an additional 24 h before the osteogenic medium was changed.
Transient Transfection Assay
Transient transfections were carried out using Lipofectamine 2000 (Invitrogen) as described previously (19). As an internal control, cytomegalovirus (CMV) β-galactosidase plasmid was cotransfected in each transfection experiment, and the luciferase activity was normalized to β-galactosidase activity.
RT-PCR and Real-time PCR
Total RNA was isolated from the cultures using the TRIzol reagent (Invitrogen) according to the manufacturer's instructions. RT-PCR was performed using 0.8 μg of total RNA. Each reaction consisted of an initial denaturation at 94 °C for 1 min followed by three-step cycling: denaturation at 94 °C for 30 s, annealing at a temperature optimized for each primer pair for 30 s, and extension at 72 °C for 30 s. After the required number of cycles (25–30 cycles), the reactions underwent a final extension at 72 °C for 5 min. The primer sequences were as follows: COUP-TFII, forward 5′-GACTCCGCCGAGTATAGCTG-3′ and reverse 5′-GCCCAACACAGGAGTTGTTT-3′; alkaline phosphatase (ALP), forward 5′-TACATTCCCCATGTGATGGC-3′ and reverse 5′-ACCTCTCCCTTGAGTGTGGG-3′; BSP, forward 5′-ACACTTACCGAGCTTATGAG-3′ and reverse 5′-TTGCGCAGTTAGCAATAGCA-3′; OC, forward 5′-CTCCTGAGTCTGACAAAGCC-3′ and reverse 5′-GCTGTGACATCCATTACTTG-3′; β-actin, forward 5′-TGGATGGCTACGTACATGGCTGGG-3′ and reverse 5′-TTCTTTGCAGCTCCTTCGTTGCCG-3′. PCRs were conducted using the QuantiTect SYBR PCR kit (Qiagen, Valencia, CA) in triplicate in the Rotor-Gene 6000 (Corbett Research, Sydney, Australia). The thermal cycling conditions were as follows: 15 min at 95 °C, followed by 40 cycles of 95 °C for 10 s, 58 °C for 15 s, and 72 °C for 20 s. All quantitation were normalized to an endogenous control β-actin. Data were analyzed by the ΔΔ-Ct method (20).
Chromatin Immunoprecipitation (ChIP) Assay
C3H10T1/2 cells were transfected with the indicated constructs for 48 h, and the ChIP assay was performed as described previously (19). The primer sequences were as follows: containing Runx2 binding region on the OC promoter (OG2), forward 5′-GAGGACATTACTGAACAC-3′ and reverse 5′-CAGTGGGTCAAACCCAAA-3′; containing Runx2 binding region on COUP-TFII promoter Runx2 consensus motif I, 1 forward 5′-TTTTCAGAGGACCCAACTCTG-3′ (120 bp): −4354/−4334 bp and 1 reverse 5′-TGAATAGAGAAGGGGCTTCCA-3′: −4235/−4254 bp; Runx2 consensus motif II, 2 forward, 5′-CTTGGAAGTTCAGAGGGACACAA-3′ (120 bp): −4278/−4256 bp and 2 reverse 5′-TCCAGGATCCATTGAATTTCTC-3′: −4159/−4180 bp; Runx2 consensus motif III, 3 forward 5′-TTCGGCTGTCCTGTCTCTTC-3′ (127 bp): −3789/−3770 bp and 3 reverse 5′-GGTGCTTCACTTTGTATTTTAGGA-3′: −3663/−3686 bp; Runx2 consensus motif IV, 8 forward 5′-GGCTGGCCAGTTAGCTAAAAC (122 bp): −2059/−2039 bp and 8 reverse 5′-TGCTCAAATGAAGCATCTCG-3′: −1938/−1957 bp.
Immunoprecipitation (IP)
C3H10T1/2 cells were transiently transfected with different DNA constructs. After 48 h, the cells were washed twice and treated with lysis buffer (150 mm Tris-HCl, pH 7.4, 100 mm NaCl, 1% Nonidet P-40), which contained protease inhibitors, for 30 min. After centrifugation at 4 °C for 15 min, supernatants were precleared with protein G-agarose beads (Invitrogen). Specific antibodies were then added, and the samples were incubated overnight at 4 °C with rotation. The protein G-agarose beads were added and incubated for 2 h. The immune complexes were washed five times with lysis buffer, and immunoprecipitated proteins were boiled for 5 min in SDS sample buffer. Western blot analysis was performed after the IP procedure.
Western Blot Analysis
Total cell extracts were harvested in lysis buffer (Cell Signaling) and then centrifuged at 12,000 × g for 15 min at 4 °C. Quantification of total protein was performed using the BCA protein assay reagent (Bio-Rad Laboratories). Proteins were resolved on a 10% SDS-PAGE and transferred to a PVDF membrane. After blocking in 5% milk in Tris-buffered saline with 0.1% Tween 20 (TBST), the membrane was incubated with specific primary antibodies. Signals were detected using an enhanced chemiluminescence (ECL) reagent (Santa Cruz Biotechnology) according to the manufacturer's instructions. Densitometric analysis was conducted directly from the blotted membrane using a LAS-4000 luminoimage analyzer system (Fujifilm, Tokyo, Japan).
ALP Staining and Activity and OC Production Assay
For ALP staining, the cultured cells were fixed with 70% ethanol, rinsed three times with deionized water, and then treated with a 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium solution (Sigma-Aldrich) for 15 min. The stained cultures were then photographed. For quantitative analysis, the stains were extracted with 10% (w/v) cetylpyridinium chloride in 10 mm sodium phosphate, pH 7.0, for 15 min. The ALP stain was quantified by measuring the absorbance at 540 nm using a multiplate reader (Bio-Tek Instruments, Winooski, VT). The enzyme activity of ALP in the cell layers was measured using the p-nitrophenyl phosphate substrate method, as described by Manolagas et al. (21). The ALP activity was normalized to the total amount of protein. The level of OC secreted into the culture medium was determined using a mouse OC ELISA kit (Biomedical Technologies Inc., Stoughton, MA) according to the manufacturer's instructions.
Alizarin Red (AR-S) Staining
Cells were fixed as described for ALP staining and then treated with a 40 mm AR-S stain solution, pH 4.2, for 10 min. Stained cultures were imaged and then quantified as described for ALP staining.
Statistical Analysis
All experiments were repeated at least three times, and statistical analysis was performed using a Student's t test or analysis of variance analyses followed by a Duncan's multiple comparison test. Differences with p < 0.05 were considered significant. The results are expressed as the mean ± S.D. of triplicate independent samples.
RESULTS
Expression Profiles of COUP-TFII during Osteoblast Differentiation
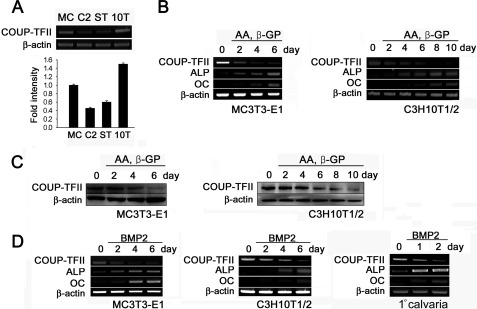
COUP-TFII is expressed in various tissues (22). To examine whether COUP-TFII is expressed in osteogenic cells, the endogenous expression of COUP-TFII was evaluated in various osteogenic progenitor cells, including MC3T3-E1 preosteoblast cells, C2C12 myoblastic cells, ST-2 bone marrow stromal cells, C3H10T1/2 mesenchymal cells. As shown in Fig. 1A, COUP-TFII was expressed in most cell lines at different levels. Among them, C3H10T1/2 cells showed the highest level of COUP-TFII expression.
FIGURE 1.
Expression profiles of COUP-TFII in osteogenic progenitor cells and during osteoblast differentiation. A, expression of COUP-TFII mRNA in osteoblast progenitor cells. MC, MC3T3-E1 preosteoblasts; C2, C2C12 myoblastic cells; ST, ST-2 bone marrow stromal cells; 10T, C3H10T1/2 multipotential mesenchymal cells. B, expression of COUP-TFII during osteoblast differentiation by ascorbic acid (AA, 50 μg/ml) and β-glycerophosphate (β-GP, 5 mm). At the designated time points, the cells were harvested for total RNA isolation, and RT-PCR was performed. C, Western blot analysis of COUP-TFII expression during osteoblast differentiation. D, COUP-TFII expression during BMP2-induced osteoblast differentiation. The cells were cultured with BMP2 (200 ng/ml), and the culture medium was replaced every other day. After the indicated days, the cells were collected for RT-PCR. 1°calvaria, primary calvaria cells.
During the osteoblast differentiation process induced by ascorbic acid and β-glycerophosphate, the expression levels of ALP and OC, which are typical osteoblast differentiation markers, were increased. These findings were consistent with other reports. In contrast, the levels of COUP-TFII mRNA and proteins were significantly decreased (Fig. 1, B and C). The COUP-TFII expression also consistently decreased during BMP2-dependent osteoblast differentiation (Fig. 1D). These results indicate that COUP-TFII is regulated during osteoblast differentiation, suggesting that COUP-TFII may play a specific role during osteoblast differentiation.
COUP-TFII Negatively Regulates BMP2-induced Osteoblastic Genes Expression
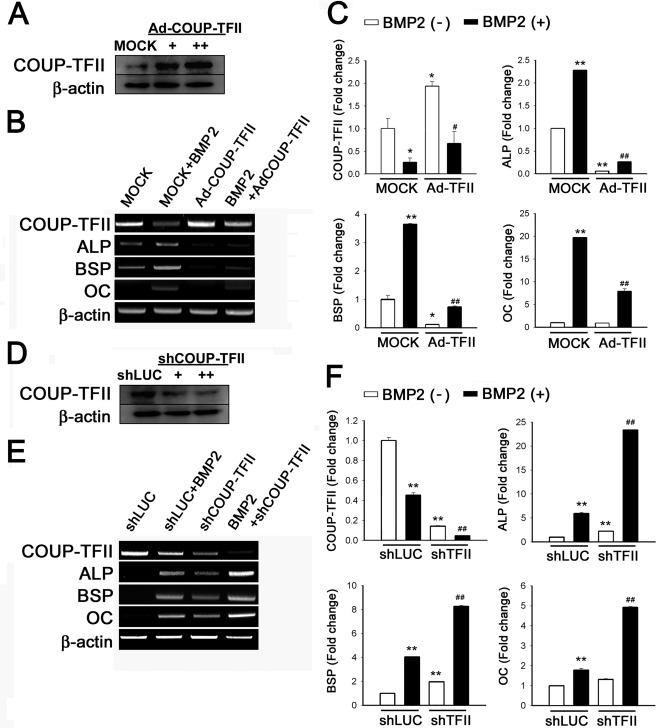
To examine the roles of COUP-TFII in osteoblast differentiation, COUP-TFII was overexpressed using adenovirus encoding COUP-TFII (Ad-COUP-TFII). Ad-COUP-TFII increased COUP-TFII expression (Fig. 2A). BMP2 increased the expression of osteoblastic genes such as ALP, BSP, and OC, and decreased expression of COUP-TFII (Fig. 2, B and C). Interestingly, overexpression of COUP-TFII significantly reduced BMP2-induced expression of osteoblastic genes.
FIGURE 2.
COUP-TFII inhibits BMP2-induced expression of osteoblast marker genes. A, adenovirus-mediated overexpression of COUP-TFII. C3H10T1/2 cells were infected with Ad-COUP-TFII (+, 50 m.o.i.; ++, 100 m.o.i.) or Ad-GFP (50 m.o.i., MOCK), and after 24 h, the culture medium was changed. After 3 days, the cells were harvested for Western blot analysis to confirm the efficiency of Ad-COUP-TFII. B, COUP-TFII overexpression inhibiting expression of osteoblastic marker genes induced by BMP2. C3H10T1/2 cells were infected with Ad-COUP-TFII (50 m.o.i.) or MOCK virus (50 m.o.i.) in the absence or presence of BMP2 for 4 days, and RT-PCR was performed. C, PCR results quantified using ImageJ. The band density of each gene was normalized by density of β-actin as a control. The graphs represent the -fold change. Data are expressed as the mean ± S.D. (error bars) of triplicate samples. D, inhibition of COUP-TFII expression by shCOUP-TFII. C3H10T1/2 cells were transfected with shCOUP-TFII (+, 50 ng/well; ++, 200 ng/well) or shLUC (as a control) for 3 days, and the cells were harvested for Western blot analysis. E, COUP-TFII down-regulation enhancing expression of osteoblastic marker genes induced by BMP2. The cells were transfected with shCOUP-TFII or shLUC in the absence or presence of BMP2 (200 ng/ml) for 3 days, and RT-PCR was performed. F, quantified PCR results using ImageJ. Data are expressed as the mean ± S.D. of triplicate samples (*, p < 0.05; **, p < 0.01 versus control group (MOCK). #, p < 0.05; ##, p < 0.01 versus BMP2-treated group).
To assess the effects of COUP-TFII further, COUP-TFII was down-regulated by transfecting short hairpin (sh) COUP-TFII. The efficiency of shCOUP-TFII was confirmed by Western blot analysis (Fig. 2D) and RT-PCR (Fig. 2, E and F). As shown in Fig. 2, E and F, down-regulation of COUP-TFII synergistically increased the expression of osteoblastic genes induced by BMP2. Also, shCOUP-TFII transfection slightly increased the expression of osteoblastic genes. Taken together, these findings indicate that COUP-TFII may act as a negative regulator of osteoblastic marker genes expression induced by BMP2.
COUP-TFII Inhibits BMP2-induced Osteoblast Differentiation and Mineralization
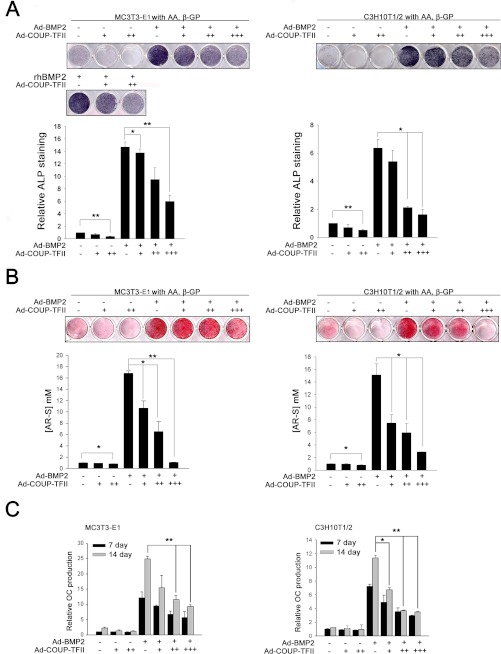
To better understand the role of COUP-TFII in osteoblast differentiation, the effect of overexpression of COUP-TFII was examined. As shown in Fig. 3A, BMP2 led to an increase in ALP staining. However, the increased ALP staining by BMP2 was dramatically decreased by COUP-TFII overexpression. Also, the overexpression of COUP-TFII alone slightly inhibited osteoblast differentiation induced by ascorbic acid and β-glycerophosphate.
FIGURE 3.
COUP-TFII decreases BMP2-induced osteoblast differentiation and mineralized nodule formation. C3H10T1/2 and MC3T3-E1 cells were infected with MOCK virus or Ad-BMP2 (50 m.o.i.) with or without Ad-COUP-TFII (+, 50 m.o.i.; ++, 100 m.o.i.; +++, 150 m.o.i.) and maintained in osteogenic medium in the absence or presence of BMP2 (200 ng/ml). After 7 days, ALP staining and after 14 days, AR-S were conducted. A, COUP-TFII overexpression inhibited BMP2-induced ALP staining levels. B, overexpression of COUP-TFII blocked BMP2-induced mineralization. The lower panels of A and B depict the eluted ALP staining or AR-S concentrations. C, the production of OC proteins was measured from culture medium in A and B using ELISA kits. Data are expressed as the mean ± S.D. (error bars) of triplicate samples (*, p < 0.05; **, p < 0.01).
Extracellular matrix mineralization is the most important process during bone formation. To examine the effects of COUP-TFII on mineralized nodule formation in vitro, AR-S staining was performed. As shown in Fig. 3B, BMP2 stimulated nodule formation, whereas COUP-TFII inhibited nodule formation induced by BMP2 in a dose-dependent manner. The overexpression of COUP-TFII alone slightly decreased nodule formation compared with the control group.
OC is a small, noncollagenous, highly conserved and secreted protein that is associated with the mineralized matrix of bone. To investigate osteoblast differentiation, OC production in the culture medium was measured by ELISA. In these experiments, COUP-TFII decreased BMP2-induced OC secretion (Fig. 3C). These data demonstrate that COUP-TFII may inhibit BMP2-induced osteoblast differentiation and mineralization.
Loss of Function of COUP-TFII Enhances Osteoblast Differentiation
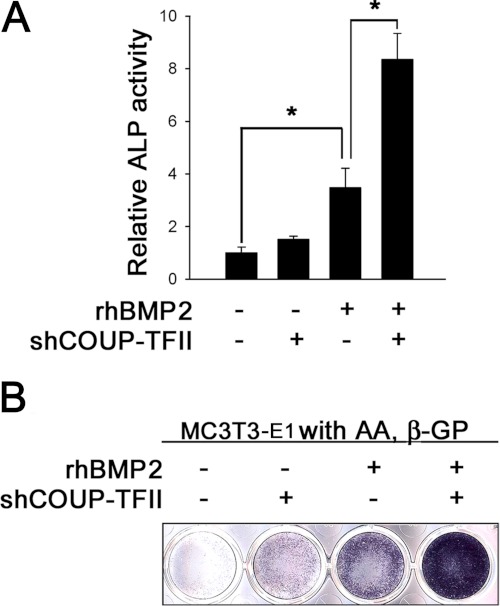
The down-regulation of COUP-TFII expression synergistically increased BMP2-induced osteoblastic gene expression. We also investigated whether up-regulation of osteoblastic genes by shCOUP-TFII led to the induction of osteoblast differentiation. As shown in Fig. 4, the down-regulation of COUP-TFII synergistically increased BMP2-induced ALP activity and staining. These results confirmed that COUP-TFII negatively impacts BMP2-induced osteoblast differentiation.
FIGURE 4.
Inhibition of COUP-TFII enhances BMP2-induced osteoblast differentiation. A, MC3T3-E1 cells were transfected with 200 ng of shCOUP-TFII or shLUC (as an internal control) in the presence or absence of BMP2 (200 ng/ml). After 3 days, the cell lysates were used for determining ALP activity. B, ALP staining was performed under the same condition as A. Data are expressed as the mean ± S.D. (error bars) of triplicate samples (*, p < 0.05). AA, ascorbic acid; β-GP, β-glycerophosphate.
COUP-TFII Suppresses Runx2 Transactivation through Protein Interaction with Runx2
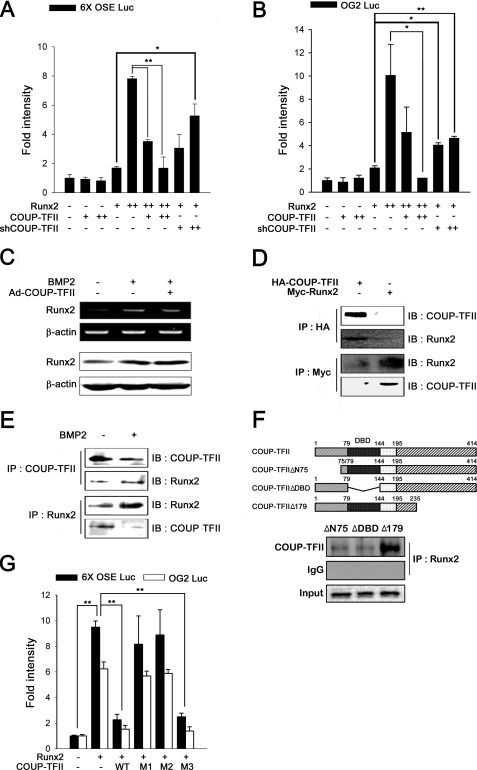
The mechanisms behind COUP-TFII regulation of BMP2-induced osteoblast differentiation were examined. Runx2, which is a key target of the BMP2 signaling pathway for osteoblast differentiation, is a main regulator of osteoblast differentiation. To examine whether COUP-TFII represses Runx2 transactivity, we used a 6xOSE-Luc promoter, which harbors six copies of the Runx2 binding region. COUP-TFII strongly repressed the promoter activity induced by Runx2 (Fig. 5A). Bone-specific expression of OC, which is a late marker of osteoblast differentiation, is regulated principally by Runx2 (23). Based on these facts, we examined the effect of COUP-TFII on Runx2-dependent transactivity of a −1.3-kb OG2-Luc promoter. As shown in Fig. 5B, Runx2 activated the OC promoter, but COUP-TFII alone had no effect. However, cotransfection with COUP-TFII and Runx2 suppressed Runx2-induced activity in a dose-dependent manner, and shCOUP-TFII transfection synergistically enhanced Runx2 activity. These results showed that inhibition of osteoblast differentiation by COUP-TFII occurs through the repression of Runx2 transactivity.
FIGURE 5.
COUP-TFII represses Runx2 transcriptional activity through protein interaction. A and B, C3H10T1/2 cells were cotransfected with 100 ng of the indicated luciferase reporter constructs and Runx2 constructs (+, 50 ng/well; ++, 200 ng/well) together with COUP-TFII (+, 50 ng/well; ++, 200 ng/well). Each value is the mean ± S.D. (error bars) of three independent experiments (*, p < 0.05; **, p < 0.01). C, C3H10T1/2 cells were infected with MOCK virus or Ad-COUP-TFII (50 m.o.i.) with or without BMP2 (200 ng/ml). To evaluate the alteration of BMP2-induced Runx2 expression by overexpression of COUP-TFII, RT-PCR (upper panels) and Western blot analysis (lower panels) were performed. D and E, COUP-TFII physically interacts with Runx2. C3H10T1/2 cells were transfected with 200 ng of Myc-tagged Runx2, HA-tagged COUP-TFII, or control construct (empty vector). D, IP was performed with Myc and HA antibodies, and Western blot (IB) analysis was performed with the indicated antibodies. E, to confirm the endogenous protein interaction, IP and Western blot analysis were performed with Runx2 and COUP-TFII antibodies. F, schematic represents COUP-TFII and its mutant constructs (upper panel). To evaluate the effect of COUP-TFII mutant constructs on the physical interaction between COUP-TFII and Runx2, the C3H10T1/2 cells were transfected with COUP-TFII or the indicated COUP-TFII mutants (200 ng/well), and the IP assay was performed (lower panel). G, C3H10T1/2 cells were cotransfected with 100 ng of the indicated luciferase reporter constructs and Runx2 constructs together with COUP-TFII or its mutants (WT, wild type; M1, COUP-TFIIΔN75; M2, COUP-TFIIΔDBD; M3, COUP-TFIIΔ179). Each value is the mean ± S.D. of three independent experiments (**, p < 0.01).
To determine whether COUP-TFII could regulate the expression of Runx2, RT-PCR and Western blot analysis were performed (Fig. 5C). The results showed that COUP-TFII did not affect BMP2-induced Runx2 expression. Therefore, we hypothesized that COUP-TFII just affected Runx2 transactivity instead of inhibiting Runx2 expression. To better understand the molecular mechanism by which COUP-TFII interferes with Runx2 transactivity, the potential physical interaction between COUP-TFII and Runx2 protein was investigated. This was assessed using an IP assay. As shown in Fig. 5D, exogenous COUP-TFII protein interacted with Runx2 in C3H10T1/2 cells. In addition, Fig. 5E also shows that endogenous COUP-TFII protein interacted with Runx2 protein regardless of BMP2 presence. BMP2 treatment increased the Runx2 protein level but decreased the COUP-TFII expression level in C3H10T1/2. These findings demonstrate that COUP-TFII physically interacts with Runx2.
To identify which domains of COUP-TFII are responsible for interacting with Runx2, the IP assay was performed using COUP-TFII deletion mutants (Fig. 5F). The results showed that the N-terminal deletion mutant (COUP-TFIIΔN75) and DNA binding domain (DBD) deletion mutant (COUP-TFIIΔDBD) did not interact with the Runx2 protein. In contrast, deletion of the C-terminal (COUP-TFIIΔ179) did not affect its ability to interact with the Runx2 protein. Thus, the N-terminal and DBD domains, but not the C-terminal domain, are responsible for the interaction between the COUP-TFII protein and Runx2.
We also analyzed the effect of COUP-TFII deletion mutants on transactivity of Runx2 (Fig. 5G). As observed in the IP assay (Fig. 5F), the C-terminal deletion mutant effectively inhibited the Runx2-dependent transactivation of the OG2-Luc promoter to levels similar to wild-type COUP-TFII. However, mutants that did not contain the N-terminal domain and DBD did not inhibit the transactivation of Runx2. These results were also confirmed using the 6xOSE-Luc promoter. Thus, our present data demonstrate that the N-terminal domain and DBD of COUP-TFII are important for inhibition of Runx2 transactivity via physical interactions.
COUP-TFII Inhibits Runx2 Binding on OC Promoter
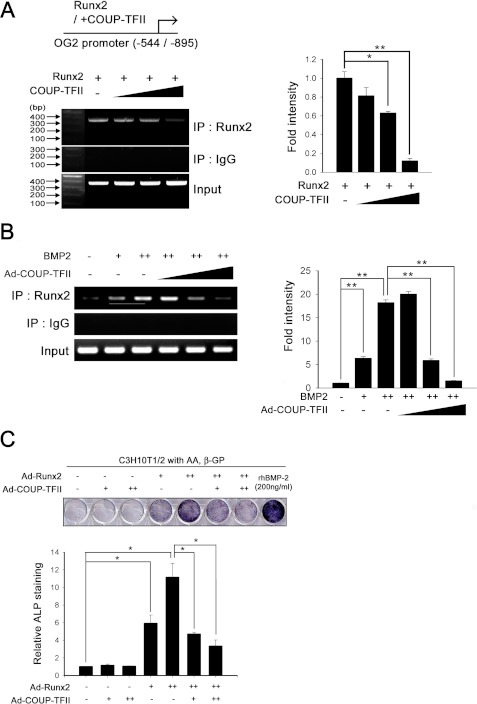
We examined whether the interaction between COUP-TFII and Runx2 inhibited binding between Runx2 and the OC promoter using the ChIP assay. As shown in Fig. 6A, overexpression of COUP-TFII significantly inhibited Runx2 binding to the OC promoter in C3H10T1/2 cells. We also investigated the effect of COUP-TFII on the function of endogenous Runx2 induced by BMP2 (Fig. 6B). The results showed that the overexpression of COUP-TFII also decreased binding of endogenous Runx2 to the OC promoter. This effect was confirmed by ALP staining (Fig. 6C). The ALP staining data showed that overexpressed COUP-TFII dose-dependently decreased Runx2-induced osteoblast differentiation. Overall, these results suggest that COUP-TFII inhibits osteoblast differentiation via inhibiting Runx2 binding to the OC promoter.
FIGURE 6.
COUP-TFII blocks Runx2 binding on OC gene. A, C3H10T1/2 cells were transfected with 200 ng of Myc-Runx2 with or without HA-COUP-TFII. After 48 h, the ChIP assay was carried out using the anti-Runx2 antibody. Immunoprecipitated products were amplified by PCR (left panel) with primers specific for the Runx2 binding sites on the OC (OG2) promoter. Right panel shows the band density (Runx2 band density/Input). Input chromatin controls and nonspecific IgG controls (as negative controls) are also shown after PCR amplification. B, for the ChIP assay, C3H10T1/2 cells were infected with Ad-COUP-TFII (50, 100, and 200 m.o.i.) in the presence of BMP2 (+, 100 ng/ml; ++, 200 ng/ml), and the ChIP assay was performed as described previously. Right panel shows the band density. C, C3H10T1/2 cells were infected with Ad-Runx2 (+, 50 m.o.i.; ++, 100 m.o.i.) and/or Ad-COUP-TFII (+, 50 m.o.i.; ++, 100 m.o.i.) or Ad-GFP (50 m.o.i.). After 24 h, the medium was replaced with osteogenic medium and changed every other day. The BMP2 (200 ng/ml)-treated group was used as a positive control. After 7 days, ALP staining was performed. Lower panels depict the eluted ALP staining concentrations. Data are expressed as the mean ± S.D. (error bars) of triplicate samples (*, p < 0.05).
Runx2 Inhibits COUP-TFII Promoter Activity and Expression
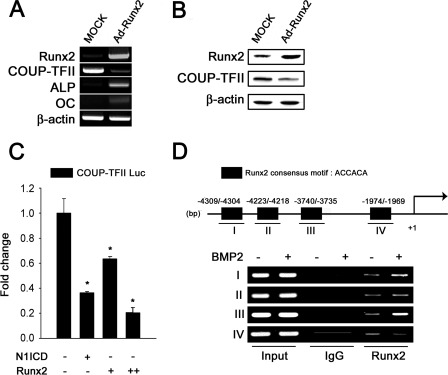
In mesenchymal cells, COUP-TFII is highly expressed and decreased during the process of osteoblast differentiation. To investigate how COUP-TFII was down-regulated during osteoblast differentiation, we hypothesized that activation of Runx2, which induces osteoblast differentiation, also affects the transcription of COUP-TFII. As shown in Fig. 7, A and B, Runx2 overexpression significantly decreased COUP-TFII expression. The Notch1 intracellular domain was used as a control to reduce the basal promoter activity of COUP-TFII (24). Transient transfection studies showed that Runx2 decreased the basal activity of the COUP-TFII promoter (Fig. 7C).
FIGURE 7.
Runx2 suppresses COUP-TFII promoter activity and mRNA expression. A, C3H10T1/2 cells were infected with Ad-Runx2 or MOCK (50 m.o.i.). Total cellular RNA was isolated, and semiquantitative RT-PCR was conducted to estimate the expressions of ALP, BSP, OC, and COUP-TFII mRNA. B, cells were prepared as described above. After 3 days, the cells were harvested for Western blot analysis to confirm the efficiency of Runx2 overexpression on COUP-TFII protein expression. C, C3H10T1/2 cells were transfected with 100 ng of the COUP-TFII luciferase reporter constructs and Runx2 constructs (+, 50 ng/well; ++, 200 ng/well) or Notch1 intracellular domain (N1ICD, 50 ng/well). Transfected Runx2 decreased the COUP-TFII basal promoter activity in a dose-dependent manner in C3H10T1/2 cells. The Notch1 intracellular domain was used as the control to reduce the basal promoter activity of COUP-TFII. D, upper panel represents the diagram for Runx2 binding motifs in COUP-TFII promoter. Lower panel shows ChIP assay for binding of endogenous Runx2 to Runx2 consensus motifs on the COUP-TFII promoter. C3H10T1/2 cells were treated with or without BMP2 (200 ng/ml) for 24 h. Soluble chromatin was prepared and immunoprecipitated with the antibody against Runx2 or IgG only as indicated. 10% of the soluble chromatins were used as the input. RT-PCR was performed to determine and quantify the complex of the COUP-TFII promoter with endogenous Runx2.
To further identify whether Runx2 binds directly to the COUP-TFII promoter and represses its expression, we analyzed the sequence of the COUP-TFII promoter and four binding region for Runx2 were identified (Runx2 consensus motif: ACCACA). As shown in Fig. 7D, Runx2 binds to Runx2 consensus motifs I and III, but not II and IV in the COUP-TFII promoter. The Runx2 consensus motifs I and III in the COUP-TFII promoter were responsible for inhibition of COUP-TFII expression by Runx2. Taken together, these results indicate that Runx2 induces osteoblast differentiation with down-regulation of COUP-TFII expression.
DISCUSSION
Osteoblast differentiation and bone formation are regulated by various transcription factors. In this study, we describe a new effector, COUP-TFII, which plays a regulatory role in osteoblast differentiation and mineralization. Based on the results of this study we came to the following conclusions: (i) Endogenous COUP-TFII is highly expressed in mesenchymal cell lines, C3H10T1/2 and decreases during osteoblast differentiation. (ii) By gain- and loss-of-function approaches, COUP-TFII inhibits osteoblast differentiation and mineralization. (iii) COUP-TFII interacts with Runx2 and represses Runx2 binding to the OC gene. (iv) During BMP2-induced osteoblast differentiation, Runx2 represses COUP-TFII expression by binding to the COUP-TFII promoter.
COUP-TFII is highly expressed in mesenchymal cells (8, 25). In our analysis, COUP-TFII was also shown to be strongly expressed in C3H10T1/2 mesenchymal cells and decreased during osteoblast differentiation. Also, overexpression of Runx2 inhibited COUP-TFII expression. These findings support the hypothesis that COUP-TFII blocks the differentiation of mesenchymal cells into osteoblasts, but if the BMP2 signal is transferred to the cells and activates Runx2, COUP-TFII expression is inhibited by the activated Runx2 via direct binding of Runx2 to the COUP-TFII promoter. However, further studies are needed to clarify the detailed mechanism of how Runx2 suppressed COUP-TFII transcription by directly binding to the COUP-TFII promoter. We suggest that Runx2 may recruit corepressors or inhibit the binding of positive transcription factor to the COUP-TFII promoter (26).
COUP-TFs have been shown to affect gene expression and development by multiple mechanisms, some of which require DNA binding and some of which do not. COUP-TFII can activate or repress gene expression by binding to COUP-TF motifs, such as those found in the ovalbumin gene (27). Also, COUP-TFII can influence gene expression by binding to other transcription factors (6). The results of this study suggest that COUP-TFII represses Runx2 binding to the OC gene through a protein interaction between COUP-TFII and Runx2. Previous studies reported that COUP-TFs can directly inhibit vitamin D receptor (VDR)-dependent gene expression by competition for occupancy of DNA binding sites (6). Because OCalso contains the VDRE (vitamin D-responsive element), COUP-TF might inhibit OC gene expression by binding to the VDRE of OC (22). In this study, the binding between COUP-TFII and the OC gene was not detected in the ChIP assay (data not shown). However, this does not mean that COUP-TFII cannot bind directly with the OC gene. As was shown in a previous report, although mouse OC encodes the VDRE-like sequence, the rat OC gene contains the exact VDRE sequence located in the distal region of the promoter (28–30). Therefore, there is still the possibility that COUP-TFII, in rat, inhibits OC gene expression via direct binding. However, further studies will be needed to test this hypothesis. COUP-TFII can also repress gene expression by regulating other cofactors, such as N-CoR, SMRT, or p300 (12, 31). Meanwhile, Runx2 by itself is not sufficient to induce its target gene expression, and various coregulators of Runx2 have been identified, such as p300 (32). These findings suggest that COUP-TFII could recruit corepressors or negatively mediate p300, resulting in antiosteogenic effects. We anticipate that the antiosteogenic effects of COUP-TFII require more than one of these mechanisms.
COUP-TFI and COUP-TFII, which are members of COUP-TFs, display overlapped expression patterns during development (25). We analyzed COUP-TFI expression during osteoblast differentiation. COUP-TFI showed a similar pattern as COUP-TFII (data not shown). The roles of COUP-TFI and COUP-TFII in osteogenesis may be similar or unique. However, further studies are needed to understand better the exact role of COUP-TFI and its relationship with COUP-TFII during osteogenesis.
Because nuclear receptors are important regulators of human physiology and pathology, ligands that interact with nuclear receptors to modulate the activity of the receptors have direct implications in therapy using drugs (33). The ligands of nuclear receptors have been used in many important clinical areas, for example, the estrogen receptor antagonist, tamoxifen, is currently used in the treatment of breast cancers (34). The identification of selective small molecule ligands of COUP-TFII will allow for the development of new therapeutic agents for a variety of bone diseases. Interestingly, recent structural analysis of COUP-TFII revealed a potential ligand binding pocket within the putative ligand binding domain of COUP-TFII (35). These findings suggest that the function of COUP-TFII could potentially be regulated by ligands, which strengthens the notion that COUP-TFII could be a promising therapeutic target for the development of bone-related disease therapies.
This study revealed that COUP-TFII negatively regulates osteoblast differentiation. In future studies, the ligand of COUP-TFII will be elucidated, and COUP-TFII will be evaluated as a potential new pharmacological target to regulate bone formation or defects.
This work was supported by the National Research Foundation of Korea Grants 2011-0010666 and 2011-0030759 funded by the Korea government (Ministry of Education, Science and Technology, MEST).
- BMP
- bone morphogenetic protein
- Ad
- adenoviral
- ALP
- alkaline phosphatase
- AR-S
- Alizarin Red S
- BSP
- bone sialoprotein
- COUP-TFII
- chicken ovalbumin upstream promoter-transcription factor II
- DBD
- DNA binding domain
- IP
- immunoprecipitation
- Luc
- luciferase
- m.o.i.
- multiplicity of infection
- OC
- osteocalcin
- Runx
- Runt-related gene
- sh
- short hairpin
- VDR
- vitamin D receptor
- VDRE
- vitamin D-responsive element.
REFERENCES
- 1. Wozney J. M., Rosen V., Celeste A. J., Mitsock L. M., Whitters M. J., Kriz R. W., Hewick R. M., Wang E. A. (1988) Novel regulators of bone formation: molecular clones and activities. Science 242, 1528–1534 [DOI] [PubMed] [Google Scholar]
- 2. Javed A., Bae J. S., Afzal F., Gutierrez S., Pratap J., Zaidi S. K., Lou Y., van Wijnen A. J., Stein J. L., Stein G. S., Lian J. B. (2008) Structural coupling of Smad and Runx2 for execution of the BMP2 osteogenic signal. J. Biol. Chem. 283, 8412–8422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Banerjee C., McCabe L. R., Choi J. Y., Hiebert S. W., Stein J. L., Stein G. S., Lian J. B. (1997) Runt homology domain proteins in osteoblast differentiation: AML3/CBFA1 is a major component of a bone-specific complex. J. Cell. Biochem. 66, 1–8 [DOI] [PubMed] [Google Scholar]
- 4. Harada H., Tagashira S., Fujiwara M., Ogawa S., Katsumata T., Yamaguchi A., Komori T., Nakatsuka M. (1999) Cbfa1 isoforms exert functional differences in osteoblast differentiation. J. Biol. Chem. 274, 6972–6978 [DOI] [PubMed] [Google Scholar]
- 5. Huang W., Yang S., Shao J., Li Y. P. (2007) Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front. Biosci. 12, 3068–3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cooney A. J., Tsai S. Y., O'Malley B. W., Tsai M. J. (1992) Chicken ovalbumin upstream promoter transcription factor (COUP-TF) dimers bind to different GGTCA response elements, allowing COUP-TF to repress hormonal induction of the vitamin D3, thyroid hormone, and retinoic acid receptors. Mol. Cell. Biol. 12, 4153–4163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Warnecke M., Oster H., Revelli J. P., Alvarez-Bolado G., Eichele G. (2005) Abnormal development of the locus coeruleus in Ear2(Nr2f6)-deficient mice impairs the functionality of the forebrain clock and affects nociception. Genes Dev. 19, 614–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pereira F. A., Qiu Y., Tsai M. J., Tsai S. Y. (1995) Chicken ovalbumin upstream promoter transcription factor (COUP-TF): expression during mouse embryogenesis. J. Steroid Biochem. Mol. Biol. 53, 503–508 [DOI] [PubMed] [Google Scholar]
- 9. Park J. I., Tsai S. Y., Tsai M. J. (2003) Molecular mechanism of chicken ovalbumin upstream promoter-transcription factor (COUP-TF) actions. Keio J. Med. 52, 174–181 [DOI] [PubMed] [Google Scholar]
- 10. Xu Z., Yu S., Hsu C. H., Eguchi J., Rosen E. D. (2008) The orphan nuclear receptor chicken ovalbumin upstream promoter-transcription factor II is a critical regulator of adipogenesis. Proc. Natl. Acad. Sci. U.S.A. 105, 2421–2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pereira F. A., Qiu Y., Zhou G., Tsai M. J., Tsai S. Y. (1999) The orphan nuclear receptor COUP-TFII is required for angiogenesis and heart development. Genes Dev. 13, 1037–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bailey P., Sartorelli V., Hamamori Y., Muscat G. E. (1998) The orphan nuclear receptor, COUP-TF II, inhibits myogenesis by post-transcriptional regulation of MyoD function: COUP-TF II directly interacts with p300 and myoD. Nucleic Acids Res. 26, 5501–5510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Qiu Y., Tsai S. Y., Tsai M. J. (1994) COUP-TF: an orphan member of the steroid/thyroid hormone receptor superfamily. Trends Endocrinol. Metab. 5, 234–239 [DOI] [PubMed] [Google Scholar]
- 14. Takamoto N., You L. R., Moses K., Chiang C., Zimmer W. E., Schwartz R. J., DeMayo F. J., Tsai M. J., Tsai S. Y. (2005) COUP-TFII is essential for radial and anteroposterior patterning of the stomach. Development 132, 2179–2189 [DOI] [PubMed] [Google Scholar]
- 15. Okamura M., Kudo H., Wakabayashi K., Tanaka T., Nonaka A., Uchida A., Tsutsumi S., Sakakibara I., Naito M., Osborne T. F., Hamakubo T., Ito S., Aburatani H., Yanagisawa M., Kodama T., Sakai J. (2009) COUP-TFII acts downstream of Wnt/β-catenin signal to silence PPARγ gene expression and repress adipogenesis. Proc. Natl. Acad. Sci. U.S.A. 106, 5819–5824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lin B., Chen G. Q., Xiao D., Kolluri S. K., Cao X., Su H., Zhang X. K. (2000) Orphan receptor COUP-TF is required for induction of retinoic acid receptor β, growth inhibition, and apoptosis by retinoic acid in cancer cells. Mol. Cell. Biol. 20, 957–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin F., Kolluri S. K., Chen G. Q., Zhang X. K. (2002) Regulation of retinoic acid-induced inhibition of AP-1 activity by orphan receptor chicken ovalbumin upstream promoter-transcription factor. J. Biol. Chem. 277, 21414–21422 [DOI] [PubMed] [Google Scholar]
- 18. Jeong B. C., Lee Y. S., Bae I. H., Lee C. H., Shin H. I., Ha H. J., Franceschi R. T., Choi H. S., Koh J. T. (2010) The orphan nuclear receptor SHP is a positive regulator of osteoblastic bone formation. J. Bone Miner. Res. 25, 262–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee Y. S., Kim D. K., Kim Y. D., Park K. C., Shong M., Seong H. A., Ha H. J., Choi H. S. (2008) Orphan nuclear receptor SHP interacts with and represses hepatocyte nuclear factor-6 (HNF-6) transactivation. Biochem. J. 413, 559–569 [DOI] [PubMed] [Google Scholar]
- 20. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔCT) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 21. Manolagas S. C., Burton D. W., Deftos L. J. (1981) 1,25-Dihydroxyvitamin D3 stimulates the alkaline phosphatase activity of osteoblast-like cells. J. Biol. Chem. 256, 7115–7117 [PubMed] [Google Scholar]
- 22. Pereira F. A., Tsai M. J., Tsai S. Y. (2000) COUP-TF orphan nuclear receptors in development and differentiation. Cell. Mol. Life Sci. 57, 1388–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ducy P., Zhang R., Geoffroy V., Ridall A. L., Karsenty G. (1997) Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89, 747–754 [DOI] [PubMed] [Google Scholar]
- 24. Diez H., Fischer A., Winkler A., Hu C. J., Hatzopoulos A. K., Breier G., Gessler M. (2007) Hypoxia-mediated activation of Dll4-Notch-Hey2 signaling in endothelial progenitor cells and adoption of arterial cell fate. Exp. Cell Res. 313, 1–9 [DOI] [PubMed] [Google Scholar]
- 25. Tsai S. Y., Tsai M. J. (1997) Chick ovalbumin upstream promoter-transcription factors (COUP-TFs): coming of age. Endocr. Rev. 18, 229–240 [DOI] [PubMed] [Google Scholar]
- 26. Javed A., Guo B., Hiebert S., Choi J. Y., Green J., Zhao S. C., Osborne M. A., Stifani S., Stein J. L., Lian J. B., van Wijnen A. J., Stein G. S. (2000) Groucho/TLE/R-esp proteins associate with the nuclear matrix and repress RUNX (CBFα/AML/PEBP2α) dependent activation of tissue-specific gene transcription. J. Cell Sci. 113, 2221–2231 [DOI] [PubMed] [Google Scholar]
- 27. Sagami I., Tsai S. Y., Wang H., Tsai M. J., O'Malley B. W. (1986) Identification of two factors required for transcription of the ovalbumin gene. Mol. Cell. Biol. 6, 4259–4267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang R., Ducy P., Karsenty G. (1997) 1,25-dihydroxyvitamin D3 inhibits Osteocalcin expression in mouse through an indirect mechanism. J. Biol. Chem. 272, 110–116 [DOI] [PubMed] [Google Scholar]
- 29. Aslam F., McCabe L., Frenkel B., van Wijnen A. J., Stein G. S., Lian J. B., Stein J. L. (1999) AP-1 and vitamin D receptor (VDR) signaling pathways converge at the rat osteocalcin VDR element: requirement for the internal activating protein-1 site for vitamin D-mediated trans-activation. Endocrinology 140, 63–70 [DOI] [PubMed] [Google Scholar]
- 30. Lian J. B., Stein G. S., Stein J. L., van Wijnen A. J. (1999) Regulated expression of the bone-specific osteocalcin gene by vitamins and hormones. Vitam. Horm. 55, 443–509 [DOI] [PubMed] [Google Scholar]
- 31. Shibata H., Nawaz Z., Tsai S. Y., O'Malley B. W., Tsai M. J. (1997) Gene silencing by chicken ovalbumin upstream promoter-transcription factor I (COUP-TFI) is mediated by transcriptional corepressors, nuclear receptor-corepressor (N-CoR) and silencing mediator for retinoic acid receptor and thyroid hormone receptor (SMRT). Mol. Endocrinol. 11, 714–724 [DOI] [PubMed] [Google Scholar]
- 32. Sierra J., Villagra A., Paredes R., Cruzat F., Gutierrez S., Javed A., Arriagada G., Olate J., Imschenetzky M., Van Wijnen A. J., Lian J. B., Stein G. S., Stein J. L., Montecino M. (2003) Regulation of the bone-specific osteocalcin gene by p300 requires Runx2/Cbfa1 and the vitamin D3 receptor but not p300 intrinsic histone acetyltransferase activity. Mol. Cell. Biol. 23, 3339–3351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shi Y. (2007) Orphan nuclear receptors in drug discovery. Drug Discov. Today 12, 440–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Enmark E., Gustafsson J. A. (1996) Orphan nuclear receptors: the first eight years. Mol. Endocrinol. 10, 1293–1307 [DOI] [PubMed] [Google Scholar]
- 35. Kruse S. W., Suino-Powell K., Zhou X. E., Kretschman J. E., Reynolds R., Vonrhein C., Xu Y., Wang L., Tsai S. Y., Tsai M. J., Xu H. E. (2008) Identification of COUP-TFII orphan nuclear receptor as a retinoic acid-activated receptor. PLoS Biol. 6, e227. [DOI] [PMC free article] [PubMed] [Google Scholar]