Background: A molecular mechanism of pulmonary metastasis through chondroitin sulfate remains unclear.
Results: Receptor for advanced glycation end products (RAGE) was identified as a receptor for chondroitin sulfate and heparan sulfate.
Conclusion: RAGE functions as a receptor for metastatic cancer cells.
Significance: This work provides insights into new therapeutic approaches for lung metastasis through RAGE, chondroitin sulfate, and heparan sulfate.
Keywords: Chondroitin Sulfate, Dermatan Sulfate, Glycobiology, Glycosaminoglycan, Heparan Sulfate, Heparin-binding Protein, Lung, Proteoglycan, Tumor Metastases, RAGE
Abstract
Altered expression of chondroitin sulfate (CS) and heparan sulfate (HS) at the surfaces of tumor cells plays a key role in malignant transformation and tumor metastasis. Previously we demonstrated that a Lewis lung carcinoma (LLC)-derived tumor cell line with high metastatic potential had a higher proportion of E-disaccharide units, GlcUA-GalNAc(4,6-O-disulfate), in CS chains than low metastatic LLC cells and that such CS chains are involved in the metastatic process. The metastasis was markedly inhibited by the pre-administration of CS-E from squid cartilage rich in E units or by preincubation with a phage display antibody specific for CS-E. However, the molecular mechanism of the inhibition remains to be investigated. In this study the receptor molecule for CS chains containing E-disaccharides expressed on LLC cells was revealed to be receptor for advanced glycation end products (RAGE), which is a member of the immunoglobulin superfamily predominantly expressed in the lung. Interestingly, RAGE bound strongly to not only E-disaccharide, but also HS-expressing LLC cells. Furthermore, the colonization of the lungs by LLC cells was effectively inhibited by the blocking of CS or HS chains at the tumor cell surface with an anti-RAGE antibody through intravenous injections in a dose-dependent manner. These results provide the clear evidence that RAGE is at least one of the critical receptors for CS and HS chains expressed at the tumor cell surface and involved in experimental lung metastasis and that CS/HS and RAGE are potential molecular targets in the treatment of pulmonary metastasis.
Introduction
Chondroitin sulfate (CS)3 and heparan sulfate (HS) are sulfated glycosaminoglycans (GAGs) that are covalently attached to core proteins to form proteoglycans (CS- and HS-PGs, respectively). They are ubiquitous in extracellular matrices and at cell surfaces in various tissues. CS/HS-PGs regulate various physiological events such as cytokinesis, morphogenesis, viral and bacterial infections, tumor growth, and tumor metastasis (1–7).
PGs with HS side chains play important roles in tumor proliferation, metastasis, invasion, adhesion, and angiogenesis (1, 2). The production of syndecans, a class of cell-surface-bound HS-PGs, is up-regulated in a range of malignancies, including pancreatic, gastric, and breast hepatocellular carcinomas and malignant mesotheliomas (8). Syndecans bind to fibronectin and laminin and enhance the function of β1 integrins during cell spreading on a matrix (9). Increasing evidence suggests that CS-PGs are also related to metastatic potential in addition to HS-PGs (10–12). CS-PGs at the tumor cell surface and in the extracellular matrix facilitate tumor invasion by enhancing integrin-mediated cell adhesion, motility, and intracellular signaling (12). The expression of versican is up-regulated in many tumors including lung cancer, as a macrophage activator that acts through Toll-like receptor-2 (TLR2) and its co-receptors TLR6 and CD14 (13).
Furthermore, recent studies revealed that the expression of E unit-containing structures recognized by an anti-CS-E phage display antibody was increased in ovarian and pancreatic cancers, resulting in alterations in tumor growth and tumor cell motility through regulation of the signaling of the vascular endothelial growth factor and the cleavage of CD44, respectively (14, 15). We demonstrated that the expression of the disulfated E-disaccharides was greater in the highly metastatic than low metastatic LLC cells (16) as well as metastatic osteosarcoma cells (17). The colonization of the mouse lung by intravenously injected LLC cells and the osteosarcoma cells was efficiently inhibited by preinjected CS-E polysaccharides derived from squid cartilage and by the anti-CS-E phage display antibody (16). However, the molecular mechanism underlying the inhibition of the metastatic process remains obscure. In view of these observations, it was hypothesized that some specific receptor(s) that interacts with CS chains with E units expressed at the surface of tumor cells exists in the vascular endothelial cells of mouse lung.
In this study we isolated the receptor for the metastasis of LLC and B16 melanoma cells mediated by CS chains with E-disaccharides from mouse lungs by affinity chromatography using a CS-E-immobilized column and identified it as receptor for advanced glycation end products (RAGE) (18). RAGE strongly bound not only to CS-E but also to HS in vitro and to cell surface CS chains containing E units and HS with presumably unique structural motifs expressed by LLC cells, suggesting that GAGs at the cell surface play crucial roles in the lung metastasis of LLC cells and that at least one of the potential receptors for such metastasis-mediating GAG chains is RAGE.
EXPERIMENTAL PROCEDURES
Materials
The following sugars and enzymes were purchased from Seikagaku Corp. (Tokyo, Japan): chondroitin, a chemically desulfated derivative of CS-A, CS-A from whale cartilage, CS-B (DS) from porcine skin, CS-C and CS-D from shark cartilage, CS-E from squid cartilage, HS from bovine kidney, highly purified chondroitinase ABC (protease-free) from Proteus vulgaris (EC 4.2.2.20). Chondroitinase B (EC 4.2.2.19), and heparinase-I (EC 4.2.2.7) and -III (EC 4.2.2.8) from Flavobacterium heparinum were purchased from IBEX Technologies (Montreal, Canada). Porcine intestinal mucosal HS and heparin were obtained from Sigma and Nacalai Tesque (Kyoto, Japan), respectively. The monoclonal anti-mouse/rat RAGE antibody, clone#175410 (catalog no. MAB1179), and the recombinant mouse RAGE/Fc chimera were purchased from R&D Systems (Minneapolis, MN). Even numbered, saturated oligosaccharide (tetra- to tetradecasaccharide) fractions were prepared by a partial enzymatic digestion of a commercial CS-E from squid cartilage with sheep testicular hyaluronidase, and structurally defined CS-E octa- and decasaccharides were prepared as described by Deepa et al. (19). All other chemicals and reagents were of the highest quality available.
Animals and Cell Lines
Seven-week-old male C57BL/6 mice were obtained from Japan SLC (Hamamatsu, Japan) and kept in standard housing. All the experiments were performed under the experimental protocol approved by the local animal care committee of Hokkaido University. LLC and B16 melanoma cells were obtained from RIKEN Cell Bank (Tsukuba, Japan) and Japanese Collection of Research Bioresources (Osaka, Japan), respectively. Mouse fibroblast L-cells and their mutants, Gro2C and Sog9, which are deficient in exostosin 1 and exostosin 1/chondroitin 4-O-sulfotransferase-1 (20–22), were kindly provided by Frank Tufaro (Allera Health Products, Inc., St. Petersburg, FL) and cultured in Dulbecco's modified Eagle's medium (DMEM, Wako, Osaka, Japan) supplemented with 10% (v/v) fetal bovine serum (Invitrogen) at 37 °C in a humidified 5% CO2 atmosphere.
Affinity Chromatography of Homogenate from Mouse Lung
The coupling of CS-E from squid cartilage, CS-A from whale cartilage, and heparin from porcine intestine to amino-cellulofine gel was individually carried out as described by Funahashi et al. (23). The extracts from a mouse lung of postnatal week 7 were prepared using 50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1 mm CaCl2, 1% Nonidet P-40, and a protease inhibitor mixture. Briefly, a mouse lung was homogenized with the buffer and rotated overnight at 4 °C. The homogenate was centrifuged at 7000 rpm for 30 min at 4 °C, and the supernatant was used for the following experiments. The affinity chromatography based on the electrostatic and/or conformational interactions was carried out as described previously (24). Briefly, the GAG-immobilized columns were equilibrated with 50 mm Tris-HCl, pH 7.4, containing 1 mm CaCl2, 1% Nonidet P-40 (buffer A), and 0.15 m NaCl. An extract was loaded to the column, and the bound proteins were eluted stepwise with 0.5, 1.0, and 2.0 m NaCl-containing buffer A.
Matrix-assisted Laser Desorption/Ionization Time of Flight/Mass Spectrometry (MALDI-TOF/MS) and Data Base Searches
Fractions obtained by affinity chromatography were separated by 12% SDS-PAGE, and the protein bands were stained with Coomassie Brilliant Blue or silver solution. Bands of interest were manually excised and digested in-gel with trypsin according to published procedures (25, 26).
Delayed extraction MALDI-TOF-MS was carried out using a Voyger DE-STR-H (Applied Biosystems, Framingham, MA) in the reflection mode according to the manufacturer's instructions. Spectra were calibrated externally using a standard mixture. The resulting spectra were used to search for matching sequences in the NCBI data base with the MASCOT program (Matrix Science, Boston, MA).
Surface Plasmon Resonance Analysis
The interaction of RAGE with various types of GAGs was examined using a BIAcore 2000 system (BIAcore AB, Uppsala, Sweden) as reported (27). Briefly, the binding reactions were carried out at 25 °C using streptavidin-derivatized sensor chips. Biotinylated GAGs (∼0.6 ng each) were immobilized on the surface of the streptavidin-derivatized sensor chip. RAGE in the running buffer, 10 mm HEPES, 0.15 m NaCl, 3 mm EDTA, and 0.005% (w/v) Tween 20, pH 7.4, was injected onto the surface of the GAG-immobilized sensor chips. Kinetic parameters were evaluated with BIAevaluation software 4.1 (BIAcore AB) using a 1:1 binding model with mass transfer, and association and dissociation rate constants (ka and kd) as well as dissociation equilibrium constants (Kd) were determined.
Enzyme-linked Immunosorbent Assay (ELISA) of Binding of RAGE-derived Peptides to Immobilized GAGs
To assess the potential binding site on RAGE for GAGs, ELISA was performed using GAGs, RAGE-derived peptides, and biotinylated GAGs. Biotinylated GAGs (0.5 μg each) were immobilized on 96-well streptavidin-coated microtiter plates (Nunc) at room temperature. The wells were blocked with 3% bovine serum albumin (BSA) and/or 3% blocking reagent (Roche Applied Science) in PBS for 1 h at room temperature. After washing with PBS containing 0.005% Tween 20 (PBS-T), RAGE-derived peptides, CKGAPKKPPQQLEWKLNTGRTEA (Cys-38–Ala-60) and GTFRCRATNRRGKEVKSNYRVRVY (Gly-94–Tyr-117) (0.25 μg each), chemically synthesized by Hokudo Co., Ltd (Sapporo, Japan), were added, and the plate was incubated for 1 h at room temperature. The reaction was carried out in PBS. Peptides bound through electrostatic or hydrophobic interactions were detected using antisera raised in rabbits against the peptides obtained from Hokudo Co. followed by incubation with alkaline phosphatase-conjugated goat anti-rabbit IgG/IgM. Alkaline phosphatase activity was detected using p-nitrophenyl phosphate as a substrate, and the absorbance was measured at 415 nm.
The reactivity of the commercial CS-E with the RAGE protein was evaluated by inhibition ELISA, where the RAGE-Fc chimera was preincubated with test CS-E oligo- and polysaccharides or Cys-38–Ala-60 and Gly-94–Tyr-117 peptides for 30 min at room temperature, and then the mixture was added to CS-E-immobilized microtiter plates. After the incubation, each well was washed with PBS-T and incubated with protein G-conjugated alkaline phosphatase (Pierce) to detect the RAGE-Fc chimera.
Immunofluorescence Flow Cytometry
Cultured cells including LLC cells, LLC cells treated individually with chondroitinase ABC, or a mixture of heparinase-I and -III, L-cells, Gro2c cells, and Sog9 cells were detached with 2 mm EDTA and suspended in Tris-buffered saline (TBS) at a concentration of 106 cells/ml. After 3 washes with TBS, the cells were incubated in TBS containing 0.1% BSA with the recombinant RAGE-Fc chimera or BSA as the control (1 μg/ml) at 4 °C for 20 min. The cells were washed with TBS three times and incubated with Alexa Fluor 488®-labeled Protein G (1 μg/ml). After three more washes with TBS, the cells were analyzed by immunofluorescence flow cytometry in a BD FACSCanto (BD Biosciences). Flow cytometric data were analyzed using Flowjo software (Tree Star, Inc., Ashland, OR).
Assays for Lung Metastasis
To investigate the involvement of RAGE in experimental tumor metastasis, the LLC cells and B16 melanoma cells were pretreated with either DMEM (control) or DMEM containing the anti-RAGE antibody (monoclonal rat IgG2, 1–80 μg/mouse) or GAGs (100 μg/mouse) 30 min before their injection into mice. IgG2 derived from rats was also used as a control. Each cell suspension (1∼4 × 105 cells/mouse) in a total volume of 200 μl was injected into a lateral tail vein of C57BL/6 mice as described (16). Three weeks after the injection, the animals were sacrificed, and the number of visible tumor cell parietal nodules in the lung was counted by two observers in a blinded fashion.
RESULTS
Identification of CS-E-binding Protein
To isolate the CS-E-binding protein involved in the tumor metastasis, a CS-E-immobilized gel was prepared and mixed with a tissue extract prepared from adult mouse lungs. The mixture was rotated overnight and poured into an open column. After the column was washed with a buffer containing 0.15 m NaCl, the bound proteins were eluted stepwise with buffers containing 0.5, 1.0, or 2.0 m NaCl, analyzed by SDS-PAGE, and detected with Coomassie Brilliant Blue or by silver-staining (Fig. 1A and supplemental Fig. S1). Three significant protein bands (46, 52, 80 kDa) and one faint band at 30 kDa were detected in the fraction eluted from the CS-E column with 0.5 m NaCl (Fig. 1A). In contrast, there were no detectable bands in the 0.5 m NaCl-eluted fractions obtained from a CS-A-immobilized column or a no GAG-immobilized control (supplemental Fig. S1), suggesting specific interactions between these proteins and CS-E. These three bands were also found in the 0.5 m NaCl-eluted fraction from a heparin-coupled column (supplemental Fig. S1), indicating that these proteins are heparin-binding proteins.
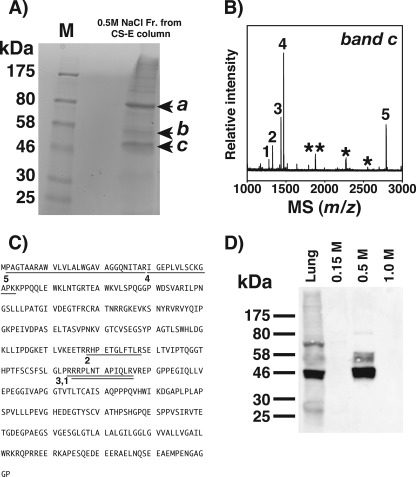
FIGURE 1.
Identification of CS-E-binding proteins from mouse lung. A, a lung homogenate from a 7-week-old mouse was applied to the CS-E-immobilized column, which was washed stepwise with a buffer containing 0.15 m NaCl followed by those containing 0.5, 1.0, and 2.0 m NaCl. The 0.5 m NaCl eluate was separated by 12% SDS-PAGE and visualized with Coomassie Brilliant Blue. Three protein bands at 46, 52, and 80 kDa were detected (arrowheads a–c). B, shown is the delayed extraction MALDI-TOF mass spectrum of the tryptic peptides obtained from band c in A. Numbered signals were used for a data base search using the MASCOT program. Asterisks indicate the peptide signals from trypsin itself. C, MS signals matched to the peptides derived from RAGE. The numbered underlines correspond to the respective MS signals in B. D, shown is Western blotting using anti-RAGE antibody of the lung homogenate (30 μg as total protein) and 0.15–1.0 m NaCl-eluted fractions (1 μg as total protein) recovered from the CS-E immobilized column.
Protein band c in Fig. 1A was excised from the gel and trypsinized, and the resulting peptides were analyzed by MALDI-TOF-MS, as described under “Experimental Procedures.” The resulting spectrum (Fig. 1B) was used to search for a matching protein(s) in the NCBI data base, with the Mascot program. The search yielded a top score of 87 for RAGE. The peptide sequences identified by MALDI-TOF-MS comprised amino acids 2–29, 30–43, 178–188, 215–226, and 216–226 (Fig. 1C), well consistent with the mass spectrometric data for trypsinate obtained from the authentic recombinant RAGE (supplemental Fig. S2, C and D).
Interestingly, band b in Fig. 1A was also identified as RAGE (supplemental Fig. S2, A and B). Because mouse RAGE contains two potential N-glycosylation sites (28, 29), bands b and c are most likely RAGE with and without N-linked glycosylation, respectively. The data obtained by mass spectrometry and the Mascot search were confirmed by Western blotting using specific antibodies against RAGE (Fig. 1D). Taken together, RAGE is a potential receptor in the lung for CS chains containing E-disaccharides and/or HS expressed at the surface of LLC cells as it also bound a heparin column. Consistent with our data, RAGE has been reported to be predominantly expressed in the mouse lung (30). The results of the quantitative real-time polymerase chain reaction also demonstrated the specific expression of RAGE in mouse lung (supplemental Fig. S3). Thus, we focused on the identification of RAGE in the present study.
A protein band a (Fig. 1A) was also subjected to MALDI-TOF-MS and the data base search, which revealed the membrane-organizing extension spike protein, moesin (supplemental Fig. S4), a member of the ERM protein family including ezrin and radixin (31). Although the RAGE was specifically distributed in the lung of mouse, the moesin is ubiquitously expressed (32). In this study we focused on RAGE. Because, however, the possibility of the involvement of moesin cannot be excluded, an investigation of its correlation is now in progress.
RAGE Strongly Interacts with Immobilized CS-E, HS, and Heparin
To verify the GAG-specific receptor function of RAGE, a quantitative kinetic analysis of the interaction of RAGE with immobilized GAG isoforms was carried out using a surface plasmon resonance biosensor, BIAcore. Biotinylated CS, HS, and heparin were individually immobilized on the streptavidin-coated sensor chip, and a recombinant soluble RAGE at varying concentrations was injected over the sensor surface to evaluate direct binding. Overlaid sensorgrams disclosed the strong binding of RAGE not only to HS/heparin but also to CS-E (Fig. 2), E-disaccharide units of which account for ∼67% of all the disaccharide units in the polysaccharides (33, 34). In addition, CS-B (also known as dermatan sulfate) and CS-D, which contain predominantly iduronic acid-GalNAc(4S) (iA units) and C units/D units, respectively (33, 35), showed moderate binding yet weaker binding than CS-E. In contrast, CS-A and CS-C polysaccharides, which mainly consist of mono-sulfated A- and C-disaccharide units (33), respectively, showed no significant binding to RAGE (Fig. 2). These results suggest RAGE to share HS-like and CS-E-like structures as ligands and recognize the sulfation pattern of CS and that the interaction may also involve iduronic acid and/or D-disaccharide-containing structures arranged in an additive or alternative manner.
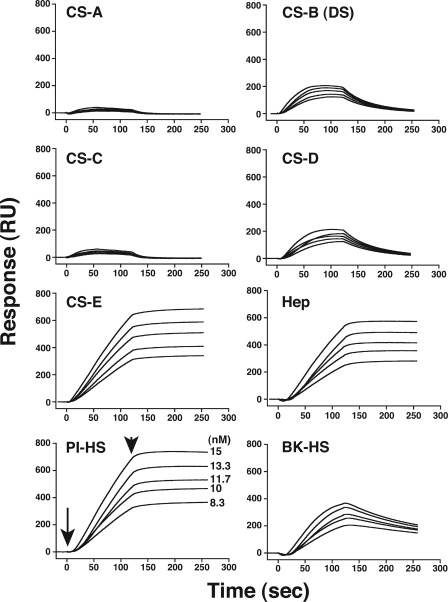
FIGURE 2.
Sensorgrams for the binding of recombinant RAGE to CS, DS, heparin, and HS. Various concentrations, 8–15 nm, of a recombinant soluble RAGE-Fc fusion protein were injected onto the surface of the sensor chips immobilized with CS-A, CS-B (DS), CS-C, CS-D, CS-E, heparin (Hep), and HS (from porcine intestine and bovine kidney). The sensorgrams obtained with each GAG preparation were overlaid in the respective panel using the BIAevaluation software. The arrow indicates the beginning of the association phase initiated by the injection of RAGE, and the arrowhead indicates the beginning of the dissociation phase initiated with the running buffer (10 mm HEPES, pH 7.4, 150 mm NaCl, 3 mm EDTA, 0.005% Tween20). PI-HS and BK-HS stand for HS derived from porcine intestine and bovine kidney, respectively.
Overlaid sensorgrams were further analyzed collectively by using “the 1:1 Langmuir binding model with mass transfer” of the BIAevaluation 4.1 software to calculate the kinetic parameters, which are summarized in Table 1. The evaluation of kinetic parameters revealed that CS-E interacted with RAGE with strong affinity (the apparent equilibrium dissociation constant, Kd = 0.2 nm) (Table 1). The high affinity of CS-E with RAGE may be not only due to strong interaction but also due to the reduced dissociation as shown in Fig. 2. In contrast, CS-B and CS-D showed weaker affinity for RAGE (360 and 300 nm, respectively), as reflected in the Kd values listed in Table 1. These differences in affinity support the high specificity of the interaction of RAGE with CS-E unit-containing structures. Reportedly, RAGE binds to heparin and HS chains (36) and to heparin-immobilized columns as described in the legend to supplemental Fig. S1. To compare the affinity of heparin/HS and CS for RAGE, the molecular interaction of RAGE with a heparin and HS preparations was also investigated. Based on the kinetic data, the interactions of heparin and HS with RAGE were comparable with that of CS-E (Fig. 2 and Table 1), implying that RAGE expressed in lung is a functional receptor for E-disaccharide-containing CS chains as well as HS chains with a RAGE binding motif.
TABLE 1.
Kinetic parameters for the interaction of RAGE with immobilized CS isoforms, HS, and heparin
The ka, kd, and Kd values were determined using a 1:1 Langmuir binding model with mass transfer as described under “Experimental Procedures.” The value for each CS variant, HS, and heparin preparation were expressed as the mean ± S.E. of five different concentrations. Note that the Kd values of CS-A and CS-C could not be determined due to the weak interaction with RAGE (Fig. 2).
| Immobilized GAG | ka | kd | Kd |
|---|---|---|---|
| m−1s−1 | s−1 | nm | |
| CS-B (DS) from porcine skin | (9.4 ± 3.6) × 104 | (1.9 ± 0.02) × 10−2 | 357 ± 136 |
| CS-D from shark fin cartilage | (1.5 ± 0.7) × 105 | (1.6 ± 0.03) × 10−2 | 306 ± 163 |
| CS-E from squid cartilage | (2.8 ± 0.1) × 104 | (5.4 ± 1.9) × 10−6 | 0.19 ± 0.06 |
| HS from bovine kidney | (2.5 ± 0.5) × 104 | (4.5 ± 0.5) × 10−3 | 203 ± 48 |
| HS from porcine intestine | (2.4 ± 0.4) × 104 | (1.5 ± 1.2) × 10−5 | 0.6 ± 0.4 |
| Heparin from porcine intestine | (1.6 ± 0.3) × 104 | (5.0 ± 3.0) × 10−4 | 3.1 ± 1.8 |
Binding Region on RAGE with CS-E and Heparin and Minimal Size of CS-E
Recently, RAGE was crystallized, and x-ray crystallography revealed an elongated molecule with a large positively charged region on the surface with direct implications for the binding of ligands (37, 38). Based on these observations, to assess whether the basic amino acid regions on the surface of RAGE interacted with GAGs, ELISA was performed using two chemically synthesized peptides (Cys-38–Ala-60 and Gly-94–Tyr-117) that contain basic amino acids and the antisera raised against these peptides. The Gly-94–Tyr-117 peptide interacted with both CS-E and heparin, whereas Cys-38–Ala-60 bound only to heparin (Fig. 3, A and B). Furthermore, this trend was confirmed by the inhibition ELISA using these peptides (Fig. 3, C and D), both of which contain basic amino acids including the double lysines or arginines. Hence, to further identify the critical residues in the peptides and show electrostatic interaction, mutant peptides CKGAPAAPPQQLEWKLNTGRTEA and GTFRCRATNAAGKEVKSNYRVRVY, were chemically synthesized and examined for their interactions with CS-E and heparin. The substitution of double basic amino acid residues in Gly-94–Tyr-117 had no apparent effect on the reactivity of the peptide to either heparin or CS-E, whereas the interaction of the Cys-38–Ala-60 peptide with heparin or CS-E was significantly reduced (supplemental Fig. S6). These observations indicate that not only double arginine residues but also other basic residues in the Gly-94–Tyr-117 peptide region of RAGE may be required for their interactions and that the double lysines in Cys-38–Ala-60 peptide region are critical for the binding. Note that CS-E did not interact with the Cys-38–Ala-60 peptide, from the ELISA assay, using the peptide antibody (Fig. 3A), whereas the binding was detected from the results of binding assay (supplemental Fig. S6B). This discrepancy might be dependent on the peptide antibody, which cannot recognize the peptide bound to CS-E for an unknown reason. In addition, hydrophobic interactions may also be involved. Thus, further analyses would be necessary to identify the critical residue(s) of Gly-94–Tyr-117 in RAGE for the interactions with GAGs.
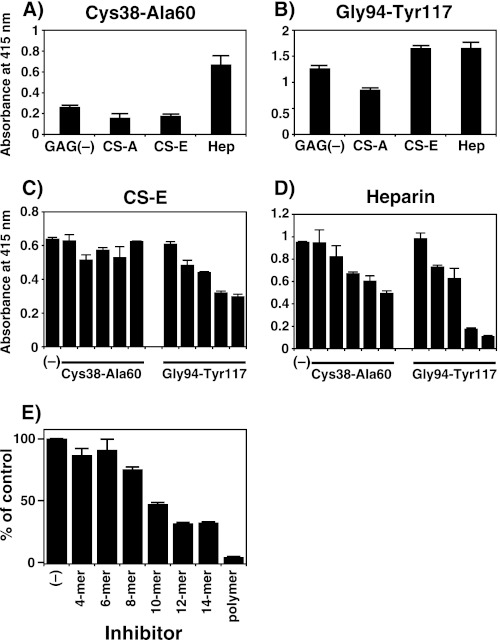
FIGURE 3.
Interaction of the RAGE peptides with GAGs and inhibition of the binding of a soluble form of recombinant RAGE to immobilized CS-E polysaccharides by RAGE-derived peptides and CS-E oligosaccharides. A and B, the binding activity of two peptides from RAGE with various GAG species was analyzed by ELISA, in which authentic commercial GAGs (CS-A, CS-E, and heparin (Hep)) were included. Biotinylated GAGs (0.5 μg each) were individually immobilized to wells of a streptavidin-coated plastic plate and processed for incubation with the two RAGE-derived peptides, CKGAPKKPPQQLEWKLNTGRTEA (Cys-38–Ala-60) and GTFRCRATNRRGKEVKSNYRVRVY (Gly-94–Tyr-117) (5 μg each). The amounts of GAGs immobilized onto the plate were comparable (∼0.1 μg) as estimated by a quantitative analysis using a dye 1,9-dimethylmethylene blue (49). Bound peptides were visualized by subsequent incubation with antisera against the respective peptide followed by alkaline phosphatase-linked goat anti-rabbit IgG/IgM (diluted 5000-fold). Enzymatic activity was measured using p-nitrophenylphosphate as a substrate at 415 nm. Negative controls received no primary antibody. Bars, mean ± S.E. (n = 3). Note that each antiserum recognized the respective peptide bound to GAGs (supplemental Fig. S5). C and D, shown is inhibition of the binding of soluble RAGE to immobilized CS-E and heparin by two RAGE-derived peptides, Cys-38–Ala-60 and Gly-94–Tyr-117. The recombinant RAGE-Fc chimera (20 ng) was added with or without either peptide to the CS-E- and heparin (∼0.1 μg)-coated microtiter plates. The amounts of both peptides were 50, 100, 200, 500, and 1000 ng (bars from the left). Bound RAGE protein was quantified using Protein G-alkaline phosphatase as described under “Experimental Procedures.” E, inhibition of the binding of soluble RAGE to immobilized CS-E polysaccharides by CS-E oligosaccharides is shown. The recombinant RAGE-Fc chimera (50 ng) was added with or without the indicated amounts of CS-E hexa-, deca-, tetradeca-, or polysaccharides to the CS-E-coated microtiter plates. Bound RAGE protein was quantified using protein G alkaline phosphatase as described under “Experimental Procedures.” The mean absorbance (at 415 nm) for binding of RAGE to CS-E in the absence of inhibitor was 1.46, and this value was taken as 100% (positive control). All other values are expressed as percentages of this control quantity. Values and the S.E. were obtained from the average of two separate experiments.
In addition, to identify the smallest fragment of the CS-E chain required for interfering with the binding of RAGE to immobilized CS-E, a panel of size-defined even-numbered CS-E oligosaccharides was examined. The CS-E oligosaccharides (5 μg of each) composed of 10 monosaccharide units reduced the binding of RAGE to CS-E by ∼50% (Fig. 3E). Together, these findings suggest that the interaction of RAGE with CS-E requires at least a nona- or decasaccharide length with a CS-E-like structure and a cluster of basic amino acids and that these peptides and/or dodecasaccharides containing the E unit(s) appear to be potent competitive inhibitors against several ligands of RAGE.
RAGE Binds to Cell-surface CS and HS Chains
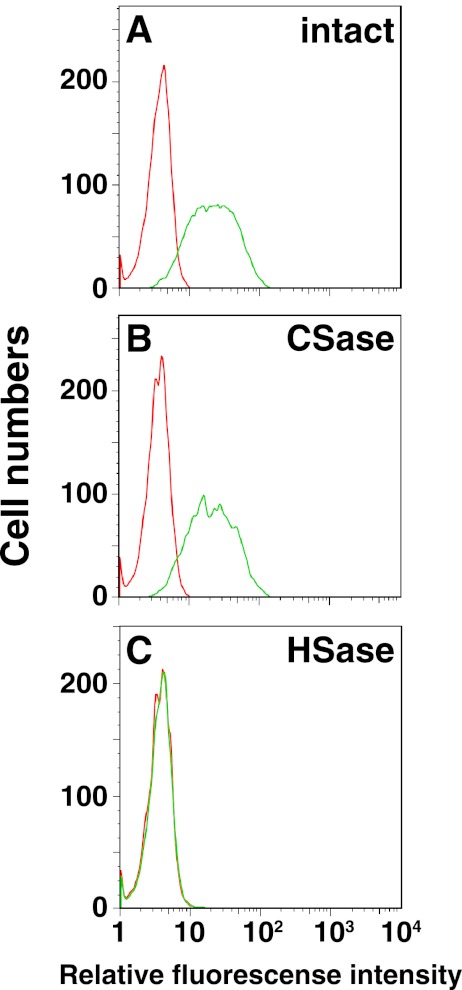
Based on the kinetic data in vitro, the binding of RAGE to cell-surface CS and HS chains was examined by flow cytometry using LLC cells and various mutant cells defective in the synthesis of HS, CS, or both (20–22). First, LLC cells were separately treated with chondroitinase or heparinase to remove the cell surface CS or HS, respectively, and the binding of a recombinant soluble RAGE to these cells was examined by flow cytometry. As shown in Fig. 4, the removal of the HS chain drastically reduced the binding of RAGE to LLC cells compared with that to control cells without treatment (Fig. 4), suggesting an important role of cell surface HS in the interaction with RAGE. Unexpectedly, chondroitinase treatment resulted in comparable RAGE binding compared with and without the treatment (Fig. 4), from which it was speculated that the defect in CS can partially be compensated by HS in vivo, whereas the defect in HS cannot be compensated by CS in vivo. In fact, Gly-94–Tyr-117 peptide interacted with both CS-E and heparin, whereas Cys-38–Ala-60 bound only to heparin (Fig. 3, A and B), which may support the above notion.
FIGURE 4.
Interaction of RAGE with cell-surface CS and HS by flow cytometry. The binding potential of RAGE was assessed by immunofluorescence flow cytometry toward LLC cells treated with no enzymes (A), chondroitinase (CSase, B), or with a mixture of heparinases I and III (HSase; C). Each cell line was treated with a RAGE/Fc chimera followed by Alexa Fluor 488-conjugated protein G. Green and red histograms represent the RAGE binding and the background fluorescence, respectively.
We also analyzed the binding of recombinant soluble RAGE to cell-surface CS/HS using L-cells derived from mouse connective tissues or to L-cell-derived mutant cell lines, Gro2C and Sog9 (20–22). Gro2C cells are partially deficient in HS side chains on the HS-PGs due to a dysfunctional exostosin 1, which encodes a HS polymerase essential for the synthesis of HS chains (21). Sog9 cells are defective in the expression of chondroitin 4-O-sulfotransferase-1, which transfers sulfate from 3′-phosphoadenosine-5′-phosphosulfate to the C-4 position of GalNAc residues in CS chains, in addition to the defect in exostosin 1 (20). The deficiency in the expression of chondroitin 4-O-sulfotransferase-1 results in a drastic decrease not only in A units but also in E units because A units are the biosynthetic precursor of E units (20, 35). RAGE bound to the surface of wild-type L-cells and to that of Gro2C and Sog9 cells to a lesser extent (supplemental Fig. S7). Although HS-defective Gro2c cells did not show binding to the RAGE, Sog9 cells defective in both CS and HS showed subtle yet appreciable binding (supplemental Fig. S7). In fact, the amounts of HS chain in Sog9 cells were up-regulated by the defective of CS chain compared with Gro2c cells (data not shown). These results altogether suggest that not only CS-PGs containing E units but also HS-PGs of the tumor cell surface contribute to the colonization of the lung through binding to RAGE. Although various ligands have been reported for RAGE such as amphotelin/high mobility group box protein-1 and advanced glycation end products (18, 30, 39), our data have provided clear evidence that GAG chains are additional functional RAGE binding molecules expressed in the lungs.
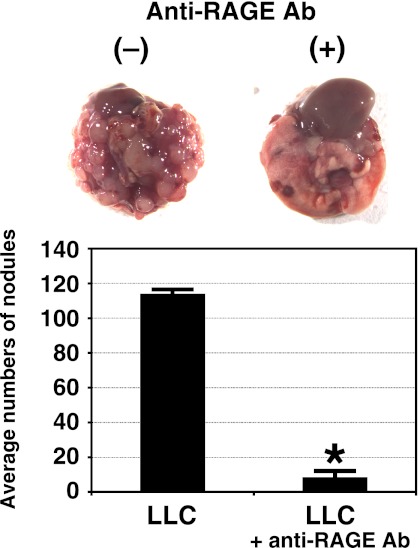
Anti-metastatic Activity of RAGE and Anti-RAGE Antibody
The increased expression of E units at the surface of LLC cells with a higher metastatic potential, and the strong anti-metastatic activity of pre-injected CS-E has been reported (16). These findings led us to hypothesize that the receptor for CS-E, RAGE, expressed in mouse lung is involved in the initial process of metastasis including tumor cell adhesion to vascular endothelial cells of the lung. To test this hypothesis, the antibody against RAGE was used for anti-metastasis assays. The pre-inoculation of mice with anti-RAGE antibody (80 μg/mouse) intravenously into the tail 30 min before the injection of the LLC cells, to mask RAGE protein expressed in the lung tissue, markedly reduced pulmonary metastasis when examined at 21 days after injection of the LLC cells, in contrast to a control experiment where the negative control antibody was pre-injected (Fig. 5). Because LLC cells were originally isolated from mouse lung, the observed colonization may represent simple cell adhesion of the LLC cells to the lung tissue. Therefore, another tumor cell line derived from mouse skin, B16 melanoma cells, which also colonize the mouse lung after injection into a tail vein (40), was utilized to investigate whether these cells have CS chains containing E-disaccharides and/or HS chains and bind to RAGE. Thus, GAGs were extracted from the B16 melanoma cells, as described under “supplemental Methods,” and the amounts and disaccharide compositions of CS and HS in the GAG preparation were determined after digestion with chondroitinase ABC or a mixture of heparinase-I and -III, respectively, followed by anion-exchange HPLC (supplemental Tables S1 and S2). CS chains expressed by B16 melanoma cells contained O-disaccharide units (GlcUA-GalNAc) (35), A units, and C units as the major disaccharides, with E units below the detection limit (supplemental Table S1). However, B16 melanoma cells expressed 1.5 times more DS chains, which may interact with RAGE (Fig. 2), than did LLC cells (supplemental Table S1). Hence, anti-metastasis assays were performed using DS and HS as well as CS-E polysaccharides to determine whether the metastasis of B16 melanoma cells depends on interactions of DS and/or HS chains and the anti-RAGE antibody with RAGE. The pre-injection of not only CS-E and HS polysaccharides but also anti-RAGE into the tail of mice suppressed the colonization of the lungs by tumor cells (supplemental Fig. S8). Although the highly sulfated disaccharide such as E unit (GlcUA-GalNAc(4S,6S)) in CS chains was not contained or was undetectable in B16 melanoma cells, there is a tri-sulfated disaccharide unit (GlcUA(2S)-GlcNS(6S)) in HS chains. Thus, HS rather than CS chains may be involved in the pulmonary metastasis of the B16 cells mediated by RAGE expressed in the lung. Furthermore, it is speculated that injected CS-E and HS quickly bind to lung endothelial RAGE and, therefore, block the attachment of LLC and the B16 cells in the lung. Collectively, these findings indicate that GAG chains on not only mouse LLC cells but also B16 melanoma cells and their presumed receptor, RAGE, expressed in mouse lung tissue play key roles in the pulmonary metastasis of tumor cells.
FIGURE 5.
Effects of pre-injection of an anti-RAGE antibody on the pulmonary metastasis of LLC cells. LLC-cell suspensions of 4 × 105 cells in 200 μl of DMEM were injected into a tail vein of C57BL/6 mice, and after 21 days the number of foci in the lungs was recorded. Six mice were used per group. Representative lungs from mice treated with a control buffer and anti-RAGE antibody (80 μg/mouse), which were injected into the tail of C57BL/6 mice 30 min before the injection of LLC cells, and metastasis was analyzed as described under “Experimental Procedures.” There was no significant difference between a control rat IgG and a buffer solution-only (data not shown). Measurement of the lung-based colonies of LLC cells is shown. Data represent the mean ± S.D. for two independent experiments. *, p < 0.01 versus control by one-way analysis of variance with Dunnett's adjustment.
DISCUSSION
In this study one of the receptors for E-disaccharide-containing CS and HS chains expressed at the surface of tumor cells with high metastatic potential was identified as RAGE, a member of the immunoglobulin superfamily of cell surface receptors that is predominantly expressed in the lung. A single pre-administration of anti-RAGE antibody or CS-E and HS suppressed the experimental lung metastasis of not only LLC but also B16 melanoma cells.
PGs and GAGs are frequently overexpressed in tumor stromata and tumor fibrotic tissues compared with the surrounding normal tissues (2, 12, 14). Changes of glycosylation in the tumor environment are thought to allow neoplastic cells to accelerate and/or usurp a number of cellular events such as receptor activation, cell adhesion, and cell motility and result in the spread of tumor cells throughout the suffering body (2). Recent studies have revealed that the expression of the epitope recognized by a phage display antibody, which strongly reacts with E unit-containing CS and iE unit (iduronic acid-GalNAc(4S,6S)-containing DS (35), is significantly up-regulated in ovarian and pancreatic carcinomas and highly metastatic LLC cells but not in normal tissues or cells (14–16). These findings suggest that not only CS-containing E-disaccharides but also RAGE would be therapeutic targets for treating lung metastasis and/or tumor development.
Although CS-PGs as well as HS-PGs expressed at cell surfaces or in the extracellular matrix have been shown to function as co-receptors for specific ligands or the assembly of extracellular matrix proteins (1, 2), recent evidence indicates that CS chains containing E units are a selective ligand for contactin-1 and P-selectin involved in neurite outgrowth and breast cancer metastasis, respectively (41, 42). Extending these observations, we also demonstrated that CS chains containing E units on metastatic tumor cells are critical ligands for RAGE expressed in mouse lung tissue, which the tumor cells colonize. It has also been reported that CS-E from squid cartilage interacts with the adhesion molecules L- and P-selectin and CD44 as well as chemokines (43), and herpes simplex virus rigidly binds to CS chains containing E units through a positively charged domain of glycoprotein C expressed on the envelope of the virus during the interaction (20, 44). CS chains containing E units have now been revealed to be involved in tumor cell adhesion in addition to adhesion to immune cells and viruses.
RAGE bound to CS-D and CS-B (DS) as well, but not to CS-A or CS-C among the CS isoforms examined, suggest the possible involvement of D-disaccharides and iduronic acid-containing DS motifs in the CS/DS chains in the binding to RAGE (Fig. 2). Consistent with the finding that a considerable number of heparin-binding proteins bind to CS-E as well (45), HS and heparin also strongly bound to RAGE (Figs. 1 and 2). In fact, large scale purification of RAGE from lung tissue has been achieved using a heparin-Sepharose gel (29, 46), suggesting RAGE to be a heparin-binding protein. Most recently, Rao et al. (36) reported that low anticoagulant heparin binds to RAGE and inhibits its interaction with ligands such as high mobility group box protein-1 and S100 calgranulins and that the inhibition of this binding leads to anti-inflammatory effects on leukocyte-mediated inflammation. Furthermore, Xu et al. (47) showed that HS was involved in the signaling of HMGB1 through RAGE. The interactions of RAGE with GAGs including CS-E and heparin are consistent with these observations and may primarily depend on their sulfation pattern, electrostatic potential, and conformation. Hence, it is proposed that CS-E, in addition to heparin showing low anticoagulant activity, is a therapeutic target not only as an anti-metastatic molecule but also as an anti-inflammatory molecule.
RAGE is a member of the immunoglobulin superfamily originally identified for its ability to bind advanced glycation end products, which are adducts formed by glycoxidation that accumulate in patients with disorders such as diabetes (18, 30, 39). Although RAGE is expressed at a low basal level in the majority of healthy adult tissues, the up-regulation of RAGE expression has been associated with a diverse range of pathological conditions including lung cancer, pulmonary fibrosis, and acute respiratory distress syndrome in addition to physiological events like neurite outgrowth (28, 30). Particularly, accumulating evidence indicates that RAGE plays an important role in tumor cell migration, proliferation, and metastasis (18, 30). Here, we demonstrated that the colonization of the lung by LLC and B16 cells was dramatically suppressed by a single pre-injection of anti-RAGE antibody (Fig. 5 and supplemental Fig. S8). In addition, we previously found that the experimental lung metastasis of LLC cells was strongly inhibited by the pretreatment of the cells with chondroitinase or the pre-administration of CS-E to mice (16). Although RAGE may provide the major mechanism for GAG-dependent colonization of tumor cells in the lung, the possibility cannot be excluded that other RAGE-binding ligands and/or proteins are also involved. In support of this notion, CS chains at the surface of metastatic breast cancer cells are a major ligand of P-selectin involved in pro-metastatic heterotypic adhesion to platelets and endothelium (42), and acquisition of the sialyl Lewis X carbohydrate, neuraminic acid α2-3Galβ1-4(Fucα1-3)GlcNAc-, depending on its amount, leads to the formation of lung tumors by B16 melanoma cells (40). Taken together, our results suggest that GAG and/or PGs further endow metastatic potential through the specific interaction of cell surface CS containing E units and HS chains with RAGE specifically expressed at the surface of the vascular endothelium in the lung.
RAGE plays an essential role in numerous pathological conditions such as cancers, inflammatory diseases, diabetes, fibrosis, acute respiratory distress syndrome, inflammation, osteoclast maturation, and physiological neurite outgrowth through its respective ligands (18, 28, 30, 36, 48). Further study of the pathogenic mechanisms behind disorders involving RAGE, CS/DS, and/or HS-PGs will provide insights into new therapeutic approaches for not only lung metastasis but various other diseases.
Supplementary Material
Acknowledgment
We thank Akane Miyasaka, Hokkaido University, for technical assistance.
This work was supported in part by Grant-in-aid for Scientific Research (B) 23390016 (to K. S.), Future Drug Discovery and Medical Care Innovation Program (to K. S.) and Young Scientists (B) 23790066 (to S. M.) from The Ministry of Education, Culture, Sports, Science, and Technology, Japan (MEXT).

This article contains supplemental Methods, Tables S1 and S2, and Figs. S1–S8.
- CS
- chondroitin sulfate
- DS
- dermatan sulfate
- HS
- heparan sulfate
- GAG
- glycosaminoglycan
- PG
- proteoglycan
- RAGE
- receptor for advanced glycation end products
- LLC
- Lewis lung carcinoma.
REFERENCES
- 1. Bishop J. R., Schuksz M., Esko J. D. (2007) Heparan sulfate proteoglycans fine-tune mammalian physiology. Nature 446, 1030–1037 [DOI] [PubMed] [Google Scholar]
- 2. Fuster M. M., Esko J. D. (2005) The sweet and sour of cancer. Glycans as novel therapeutic targets. Nat. Rev. Cancer 5, 526–542 [DOI] [PubMed] [Google Scholar]
- 3. Sasisekharan R., Shriver Z., Venkataraman G., Narayanasami U. (2002) Roles of heparan-sulfate glycosaminoglycans in cancer. Nat. Rev. Cancer 2, 521–528 [DOI] [PubMed] [Google Scholar]
- 4. Eccles S. A., Welch D. R. (2007) Metastasis. Recent discoveries and novel treatment strategies. Lancet 369, 1742–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fidler I. J. (2003) The pathogenesis of cancer metastasis. The “seed and soil” hypothesis revisited. Nat. Rev. Cancer 3, 453–458 [DOI] [PubMed] [Google Scholar]
- 6. Tímár J., Lapis K., Dudás J., Sebestyén A., Kopper L., Kovalszky I. (2002) Proteoglycans and tumor progression. Janus-faced molecules with contradictory functions in cancer. Semin. Cancer Biol. 12, 173–186 [DOI] [PubMed] [Google Scholar]
- 7. Sugahara K., Mikami T., Uyama T., Mizuguchi S., Nomura K., Kitagawa H. (2003) Recent advances in the structural biology of chondroitin sulfate and dermatan sulfate. Curr. Opin. Struct. Biol. 13, 612–620 [DOI] [PubMed] [Google Scholar]
- 8. Beauvais D. M., Rapraeger A. C. (2004) Syndecans in tumor cell adhesion and signaling. Reprod. Biol. Endocrinol. 2, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beauvais D. M., Burbach B. J., Rapraeger A. C. (2004) The syndecan-1 ectodomain regulates αvβ3 integrin activity in human mammary carcinoma cells. J. Cell Biol. 167, 171–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Faassen A. E., Schrager J. A., Klein D. J., Oegema T. R., Couchman J. R., McCarthy J. B. (1992) A cell surface chondroitin sulfate proteoglycan, immunologically related to CD44, is involved in type I collagen-mediated melanoma cell motility and invasion. J. Cell Biol. 116, 521–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Günthert U., Hofmann M., Rudy W., Reber S., Zöller M., Haussmann I., Matzku S., Wenzel A., Ponta H., Herrlich P. (1991) A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell 65, 13–24 [DOI] [PubMed] [Google Scholar]
- 12. Iida J., Meijne A. M., Knutson J. R., Furcht L. T., McCarthy J. B. (1996) Cell surface chondroitin sulfate proteoglycans in tumor cell adhesion, motility, and invasion. Semin. Cancer Biol. 7, 155–162 [DOI] [PubMed] [Google Scholar]
- 13. Kim S., Takahashi H., Lin W. W., Descargues P., Grivennikov S., Kim Y., Luo J. L., Karin M. (2009) Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature 457, 102–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. ten Dam G. B., van de Westerlo E. M., Purushothaman A., Stan R. V., Bulten J., Sweep F. C., Massuger L. F., Sugahara K., van Kuppevelt T. H. (2007) Antibody GD3G7 selected against embryonic glycosaminoglycans defines chondroitin sulfate-E domains highly up-regulated in ovarian cancer and involved in vascular endothelial growth factor binding. Am. J. Pathol. 171, 1324–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sugahara K. N., Hirata T., Tanaka T., Ogino S., Takeda M., Terasawa H., Shimada I., Tamura J., ten Dam G. B., van Kuppevelt T. H., Miyasaka M. (2008) Chondroitin sulfate E fragments enhance CD44 cleavage and CD44-dependent motility in tumor cells. Cancer Res. 68, 7191–7199 [DOI] [PubMed] [Google Scholar]
- 16. Li F., ten Dam G. B., Murugan S., Yamada S., Hashiguchi T., Mizumoto S., Oguri K., Okayama M., van Kuppevelt T. H., Sugahara K. (2008) Involvement of highly sulfated chondroitin sulfate in the metastasis of the Lewis lung carcinoma cells. J. Biol. Chem. 283, 34294–34304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Basappa, Murugan S., Sugahara K. N., Lee C. M., ten Dam G. B., van Kuppevelt T. H., Miyasaka M., Yamada S., Sugahara K. (2009) Involvement of chondroitin sulfate E in the liver tumor focal formation of murine osteosarcoma cells. Glycobiology 19, 735–742 [DOI] [PubMed] [Google Scholar]
- 18. Sims G. P., Rowe D. C., Rietdijk S. T., Herbst R., Coyle A. J. (2010) HMGB1 and RAGE in inflammation and cancer. Annu. Rev. Immunol. 28, 367–388 [DOI] [PubMed] [Google Scholar]
- 19. Deepa S. S., Kalayanamitra K., Ito Y., Kongtawelert P., Fukui S., Yamada S., Mikami T., Sugahara K. (2007) Novel sulfated octa- and decasaccharides from squid cartilage chondroitin sulfate E. Sequencing and application for determination of the epitope structure of the monoclonal antibody MO-225. Biochemistry 46, 2453–2465 [DOI] [PubMed] [Google Scholar]
- 20. Uyama T., Ishida M., Izumikawa T., Trybala E., Tufaro F., Bergström T., Sugahara K., Kitagawa H. (2006) Chondroitin 4-O-sulfotransferase-1 regulates E disaccharide expression of chondroitin sulfate required for herpes simplex virus infectivity. J. Biol. Chem. 281, 38668–38674 [DOI] [PubMed] [Google Scholar]
- 21. Gruenheid S., Gatzke L., Meadows H., Tufaro F. (1993) Herpes simplex virus infection and propagation in a mouse L cell mutant lacking heparan sulfate proteoglycans. J. Virol. 67, 93–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Banfield B. W., Leduc Y., Esford L., Schubert K., Tufaro F. (1995) Sequential isolation of proteoglycan synthesis mutants by using herpes simplex virus as a selective agent. Evidence for a proteoglycan-independent virus entry pathway. J. Virol. 69, 3290–3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Funahashi M., Matsumoto I., Seno N. (1982) Preparation of three types of heparin-Sepharose and their binding activities to thrombin and antithrombin III. Anal. Biochem. 126, 414–421 [DOI] [PubMed] [Google Scholar]
- 24. Bao X., Mikami T., Yamada S., Faissner A., Muramatsu T., Sugahara K. (2005) Heparin-binding growth factor, pleiotrophin, mediates neuritogenic activity of embryonic pig brain-derived chondroitin sulfate/dermatan sulfate hybrid chains. J. Biol. Chem. 280, 9180–9191 [DOI] [PubMed] [Google Scholar]
- 25. Hellman U., Wernstedt C., Góñez J., Heldin C. H. (1995) Improvement of an “in-gel” digestion procedure for the micropreparation of internal protein fragments for amino acid sequencing. Anal. Biochem. 224, 451–455 [DOI] [PubMed] [Google Scholar]
- 26. Rosenfeld J., Capdevielle J., Guillemot J. C., Ferrara P. (1992) In-gel digestion of proteins for internal sequence analysis after one- or two-dimensional gel electrophoresis. Anal. Biochem. 203, 173–179 [DOI] [PubMed] [Google Scholar]
- 27. Bao X., Nishimura S., Mikami T., Yamada S., Itoh N., Sugahara K. (2004) Chondroitin sulfate/dermatan sulfate hybrid chains from embryonic pig brain, which contain a higher proportion of l-iduronic acid than those from adult pig brain and exhibit neuritogenic and growth factor binding activities. J. Biol. Chem. 279, 9765–9776 [DOI] [PubMed] [Google Scholar]
- 28. Srikrishna G., Huttunen H. J., Johansson L., Weigle B., Yamaguchi Y., Rauvala H., Freeze H. H. (2002) N-Glycans on the receptor for advanced glycation end products influence amphoterin binding and neurite outgrowth. J. Neurochem. 80, 998–1008 [DOI] [PubMed] [Google Scholar]
- 29. Hanford L. E., Enghild J. J., Valnickova Z., Petersen S. V., Schaefer L. M., Schaefer T. M., Reinhart T. A., Oury T. D. (2004) Purification and characterization of mouse soluble receptor for advanced glycation end products (sRAGE). J. Biol. Chem. 279, 50019–50024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Buckley S. T., Ehrhardt C. (2010) The receptor for advanced glycation end products (RAGE) and the lung. J. Biomed. Biotechnol. 2010, 917108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Frame M. C., Patel H., Serrels B., Lietha D., Eck M. J. (2010) The FERM domain. Organizing the structure and function of FAK. Nat. Rev. Mol. Cell Biol. 11, 802–814 [DOI] [PubMed] [Google Scholar]
- 32. Lankes W. T., Furthmayr H. (1991) Moesin. A member of the protein 4.1-talin-ezrin family of proteins. Proc. Natl. Acad. Sci. U.S.A. 88, 8297–8301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kinoshita A., Sugahara K. (1999) Microanalysis of glycosaminoglycan-derived oligosaccharides labeled with a fluorophore 2-aminobenzamide by high performance liquid chromatography. Application to disaccharide composition analysis and exosequencing of oligosaccharides. Anal. Biochem. 269, 367–378 [DOI] [PubMed] [Google Scholar]
- 34. Kinoshita A., Yamada S., Haslam S. M., Morris H. R., Dell A., Sugahara K. (1997) Novel tetrasaccharides isolated from squid cartilage chondroitin sulfate E contain unusual sulfated disaccharide units GlcA(3-O-sulfate)β1–3GalNAc(6-O-sulfate) or GlcA(3-O-sulfate) β1–3GalNAc. J. Biol. Chem. 272, 19656–19665 [DOI] [PubMed] [Google Scholar]
- 35. Sugahara K., Mikami T. (2007) Chondroitin/dermatan sulfate in the central nervous system. Curr. Opin. Struct. Biol. 17, 536–545 [DOI] [PubMed] [Google Scholar]
- 36. Rao N. V., Argyle B., Xu X., Reynolds P. R., Walenga J. M., Prechel M., Prestwich G. D., MacArthur R. B., Walters B. B., Hoidal J. R., Kennedy T. P. (2010) Low anticoagulant heparin targets multiple sites of inflammation, suppresses heparin-induced thrombocytopenia, and inhibits interaction of RAGE with its ligands. Am. J. Physiol. Cell Physiol. 299, C97–C110 [DOI] [PubMed] [Google Scholar]
- 37. Koch M., Chitayat S., Dattilo B. M., Schiefner A., Diez J., Chazin W. J., Fritz G. (2010) Structural basis for ligand recognition and activation of RAGE. Structure 18, 1342–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Park H., Adsit F. G., Boyington J. C. (2010) The 1.5 Å crystal structure of human receptor for advanced glycation endproducts (RAGE) ectodomains reveals unique features determining ligand binding. J. Biol. Chem. 285, 40762–40770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Neeper M., Schmidt A. M., Brett J., Yan S. D., Wang F., Pan Y. C., Elliston K., Stern D., Shaw A. (1992) Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J. Biol. Chem. 267, 14998–15004 [PubMed] [Google Scholar]
- 40. Ohyama C., Tsuboi S., Fukuda M. (1999) Dual roles of sialyl Lewis X oligosaccharides in tumor metastasis and rejection by natural killer cells. EMBO J. 18, 1516–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mikami T., Yasunaga D., Kitagawa H. (2009) Contactin-1 is a functional receptor for neuroregulatory chondroitin sulfate-E. J. Biol. Chem. 284, 4494–4499 [DOI] [PubMed] [Google Scholar]
- 42. Monzavi-Karbassi B., Stanley J. S., Hennings L., Jousheghany F., Artaud C., Shaaf S., Kieber-Emmons T. (2007) Chondroitin sulfate glycosaminoglycans as major P-selectin ligands on metastatic breast cancer cell lines. Int. J. Cancer 120, 1179–1191 [DOI] [PubMed] [Google Scholar]
- 43. Kawashima H., Atarashi K., Hirose M., Hirose J., Yamada S., Sugahara K., Miyasaka M. (2002) Oversulfated chondroitin/dermatan sulfates containing GlcAβ1/IdoAα1–3GalNAc(4,6-O-disulfate) interact with L- and P-selectin and chemokines. J. Biol. Chem. 277, 12921–12930 [DOI] [PubMed] [Google Scholar]
- 44. Bergefall K., Trybala E., Johansson M., Uyama T., Naito S., Yamada S., Kitagawa H., Sugahara K., Bergström T. (2005) Chondroitin sulfate characterized by the E-disaccharide unit is a potent inhibitor of herpes simplex virus infectivity and provides the virus binding sites on gro2C cells. J. Biol. Chem. 280, 32193–32199 [DOI] [PubMed] [Google Scholar]
- 45. Deepa S. S., Umehara Y., Higashiyama S., Itoh N., Sugahara K. (2002) Specific molecular interactions of oversulfated chondroitin sulfate E with various heparin binding growth factors. Implications as a physiological binding partner in the brain and other tissues. J. Biol. Chem. 277, 43707–43716 [DOI] [PubMed] [Google Scholar]
- 46. Englert J. M., Ramsgaard L., Valnickova Z., Enghild J. J., Oury T. D. (2008) Large scale isolation and purification of soluble RAGE from lung tissue. Protein Expr. Purif. 61, 99–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xu D., Young J., Song D., Esko J. D. (2011) Heparan sulfate is essential for high mobility group protein 1 (HMGB1) signaling by the receptor for advanced glycation end products (RAGE). J. Biol. Chem. 286, 41736–41744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhou Z., Immel D., Xi C. X., Bierhaus A., Feng X., Mei L., Nawroth P., Stern D. M., Xiong W. C. (2006) Regulation of osteoclast function and bone mass by RAGE. J. Exp. Med. 203, 1067–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Klompmakers A. A., Hendriks T. (1986) A spectrophotometric microassay for sulfated glycosaminoglycans using a laser densitometer. Anal. Biochem. 153, 80–84 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.