Background: A trans-histone modification pathway is central to establishment of active chromatin in eukaryotes.
Results: Histone H2B ubiquitylation enhances activity of the Schizosaccharomyces pombe Set1C methyltransferase complex in vitro without affecting its assembly in vivo.
Conclusion: Direct enhancement of activity is a conserved feature of this pathway.
Significance: Deciphering the mechanism of trans-histone crosstalk is crucial for understanding how formation of active chromatin is regulated.
Keywords: Chromatin, Chromatin Histone Modification, Histone Methylation, Histones, Ubiquitin
Abstract
The methylation of histone H3 at lysine 4 (H3K4me) is critical for the formation of transcriptionally active chromatin in eukaryotes. In yeast, Drosophila, and some human cell lines, H3K4me is globally stimulated by the monoubiquitylation of histone H2B (H2Bub1), another histone modification associated with transcription. The mechanism of this “trans-histone” modification pathway remains uncertain, and studies carried out in different experimental systems have suggested that H2Bub1 could either influence the subunit composition of methyltransferase complexes or directly stimulate methyltransferase activity. We have reconstituted this pathway in vitro using the native H3K4-specific methyltransferase complex Set1C purified from the fission yeast Schizosaccharomyces pombe and chromatin substrates that contain semisynthetic H2Bub1. We found that the activity of S. pombe Set1C toward nucleosomal histone H3 is directly enhanced by H2Bub1 in vitro. Importantly, Set1C purified from cells lacking H2Bub1 retained activity on free histone substrates, suggesting that Set1C remains intact in the absence of H2Bub1. Chromatin immunoprecipitation assays revealed a defect in recruitment of intact Set1C to transcribed chromatin in H2Bub1-deficient mutants. Our data argue that trans-histone crosstalk in S. pombe involves direct enhancement of Set1C methyltransferase activity by H2Bub1 and suggest that this represents a conserved aspect of H2Bub1-H3K4me crosstalk in eukaryotes.
Introduction
Histone modifications play important roles in regulating transcription by RNA polymerase II. Methylation of histone H3 at lysine 4 (H3K4me) is a universal marker of active genes in eukaryotes and functions as a specific chromatin-binding site for numerous proteins that modulate transcription and chromatin structure (1). These include the NURF and CHD chromatin-remodeling enzymes, the NuA3 and SAGA histone acetyltransferase complexes, and the general transcription factor TFIID, implicating H3K4me as a critical determinant of the basic architecture of active chromatin (2, 3). Several lines of evidence also indicate a crucial role for H3K4me in maintenance of active chromatin states during metazoan development. H3K4me marks the promoters of key developmental regulators in mammalian embryonic stem cells (often in concert with histone H3 lysine 27 methylation, a mark associated with silent chromatin, as part of “bivalent” chromatin domains) and serves as a marker of future activation upon differentiation (4). Global depletion of H3K4me prevents in vitro differentiation of mammalian embryonic stem cells and also results in early developmental arrest in Xenopus laevis, arguing for a key functional role for the H3K4me mark in metazoan development (5, 6).
The mechanisms that regulate formation of H3K4me at active chromatin remain unclear. In both budding yeast and fission yeast, Set1 is the sole methyltransferase for H3K4, whereas in metazoans, H3K4me is catalyzed by additional enzymes that share the catalytic SET domain but differ in their domain organization (7–11). These include the mammalian MLL family proteins, implicated in pathogenesis of leukemia. All Set1/MLL enzymes characterized to date are associated with a conserved core group of interacting factors: Ash2, Dpy30, and the WD repeat proteins Swd3 (Wdr5 in mammals) and Swd1 (Rbbp5 in mammals) (12, 13). H3K4 methyltransferase complexes containing Set1 (referred to here as Set1C; also known as COMPASS) also contain orthologs of a third WD repeat protein (Swd2 in yeast and Wdr82 in mammals) and a CXXC zinc finger protein (Spp1 in yeast and Cpf1 in mammals) (9, 14, 15).
A fundamental aspect of H3K4me regulation is its relationship to histone H2B monoubiquitylation (H2Bub1), another modification universally linked to active gene transcription. Bulk levels of H3K4me are diminished by H2Bub1 loss in a variety of eukaryotes and in some transformed human cell lines, suggesting that the H2Bub1-H3K4me regulatory pathway is generally involved in formation of transcriptionally active chromatin (8, 16–21). Initial characterization of this “trans-histone” modification pathway, first discovered in the budding yeast Saccharomyces cerevisiae, indicated that H2Bub1 did not control the cellular levels of Set1 or the association of Set1 with active chromatin (16, 22). Remarkably, H2Bub1 was found to have a similar relationship to methylation of histone H3 lysine 79 (H3K79me) but not to methylation at lysine 36 (H3K36me), although both modifications are also abundantly present in the coding regions of active genes (23, 24). Thus, the H2Bub1-methylation pathway indicated specific interactions between H2Bub1 and histone methylation machinery or reflected the sensitivity of certain methyltransferases to a feature of chromatin structure brought about by H2Bub1.
Until recently, it had not been possible to ascertain whether the H2Bub1-methylation relationship involved a direct effect on activity of histone methyltransferase complexes or indirect effects on some other aspect of methyltransferase function (such as interaction with other factors). The advent of chemical methods to site-specifically introduce ubiquitin onto target peptides via expressed protein ligation has allowed large-scale semisynthesis of full-length H2Bub1 and generation of chromatin substrates that are uniformly monoubiquitylated at the correct site on histone H2B (25). In vitro methyltransferase assays performed with these substrates and purified methyltransferase enzymes have demonstrated a direct effect of H2Bub1 on the H3K79 methyltransferase activity of the human Dot1L enzyme and have suggested a similar effect on the H3K4 methyltransferase activity of human Set1C (25, 26). These experiments strongly argue that trans-histone crosstalk mediated by H2Bub1 involves direct enhancement of methyltransferase activity.
Despite these compelling in vitro results, studies of S. cerevisiae Set1C have suggested more complex alternative models for how H2Bub1-H3K4me crosstalk operates in vivo. In one report, H2Bub1 was found to be specifically required for the association of Swd2 with Set1C; in strains lacking H2Bub1, Swd2 was found to dissociate from the complex and was no longer bound to transcribed chromatin. Loss of this subunit from Set1C was found to dramatically decrease the in vitro methyltransferase activity of the complex toward recombinant histone H3 and led to a loss of H3K4me in vivo, leading to the proposal that H2Bub1 impacts H3K4me indirectly by promoting incorporation of Swd2 into Set1C (27). A second study reported that the H2Bub1-specific E3 ligase Bre1 catalyzed the monoubiquitylation of Swd2 in a manner dependent upon the presence of H2Bub1; this modification was shown to specifically promote the chromatin association of Spp1, thereby enhancing Set1C methyltransferase activity (28). These findings raise the possibility that multiple potential mechanisms govern H2Bub1-H3K4me crosstalk in vivo.
To date, the direct and indirect models for trans-histone crosstalk have each been supported by data obtained in different species and experimental systems. Thus, it is unclear whether distinct trans-histone crosstalk mechanisms operate in different species or whether multiple, potentially overlapping mechanisms collectively contribute to modification crosstalk in a given organism.
Here, we have investigated H2Bub1-H3K4me crosstalk regulation in the fission yeast Schizosaccharomyces pombe. Use of this tractable model system allowed us to study both the in vitro and in vivo properties of Set1C in relation to H2Bub1. Our data provide evidence that H2Bub1 directly enhances the activity of intact Set1C by stabilizing its interaction with chromatin.
EXPERIMENTAL PROCEDURES
Yeast Strains and Media
The yeast strains used in this study are listed in supplemental Table S1. All knock-out strains and tagged strains were constructed using standard techniques (29, 30). To construct strains with combinations of markers, haploid strains with the individual markers were crossed, and tetrads were dissected to isolate haploid progeny with both parental markers. Strains were cultured using standard media and conditions (31).
Set1C Purification
Purification of Spf1-TAP2 was performed as described with minor modifications (32). Briefly, 6 liters of culture grown in YES medium to 107 cells/ml was harvested and flash-frozen in small chunks. Cells were lysed under cryogenic conditions using a Spex freezer mill, and frozen cell powder was resuspended in 20–40 ml lysis buffer (20 mm HEPES (pH 7.6), 250 mm KCl, 5% glycerol, 10 mm magnesium acetate, 1 mm EDTA, 1 mm EGTA, 10 mm β-glycerophosphate, 0.1% Nonidet P-40, 10 mm β-mercaptoethanol, 1 mm PMSF, and protease inhibitor mixture (Roche Applied Science)). All subsequent steps were carried out at 4 °C. Extracts were centrifuged for 15 min at 15,000 × g, and supernatants were incubated with 0.5 ml of IgG-Sepharose resin (GE Healthcare) that was prewashed with buffer A. After 2 h, the resin was collected by centrifugation and transferred to a small column (Bio-Rad). The resin was washed with 10 ml of lysis buffer and 3 ml of tobacco etch virus buffer (20 mm HEPES (pH 7.6), 150 mm KCl, 5% glycerol, 1 mm EDTA, 10 mm β-mercaptoethanol, and 1 mm PMSF) and then incubated with 1.5 ml of cleavage buffer containing 15 μg of tobacco etch virus protease (Invitrogen) for 1 h at room temperature. Eluates were collected, and beads were washed with an additional further 4–5 ml of cleavage buffer. The combined eluates were adjusted to 3 mm CaCl2, 1 mm imidazole, and 1 mm magnesium acetate and incubated with 0.1 ml of calmodulin-Sepharose resin (GE Healthcare) for 1 h at 4 °C with rocking. Beads were washed with calmodulin buffer (10 mm HEPES (pH 7.6), 150 mm KCl, 5% glycerol, 2 mm CaCl2, 1 mm magnesium acetate, 1 mm imidazole, 0.1% Nonidet P-40, 10 mm β-mercaptoethanol, and 1 mm PMSF) and eluted with 1 ml of calmodulin buffer with 10 mm EGTA instead of 2 mm CaCl2. Eluates were dialyzed overnight against 2 liters of 20 mm HEPES (pH 7.6), 20% glycerol, 150 mm potassium acetate, 10 mm magnesium acetate, 1 mm EDTA, and 1 mm dithiothreitol. Typically, a 10-μl aliquot of purified material was analyzed by SDS-PAGE on 4–20% gradient acrylamide gels (Bio-Rad), followed by silver staining as described (18).
Recombinant Histones and Mononucleosome Assembly
Recombinant X. laevis histone octamers containing unmodified H2B or H2Bub1 were a generous gift from R. McGinty and T. Muir (25, 33).
The 601 DNA sequence used for mononucleosome assembly was PCR-amplified from plasmid pGEM301 (34) and purified by phenol/chloroform extraction and ethanol precipitation. Mononucleosomes were assembled by dilution from high salt in reactions containing 3 μg of octamer and 3 μg of 601 DNA (34). Assemblies containing >90% assembled DNA, as assessed by native gel analysis (see Fig. 2), were concentrated in Vivaspin 500 concentrators (GE Healthcare) and used for subsequent experiments.
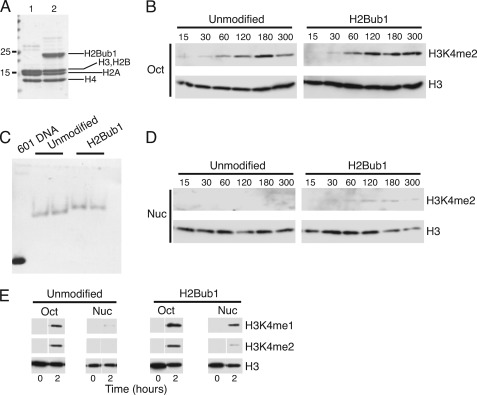
FIGURE 2.
H2Bub1 stimulates activity of S. pombe Set1C toward nucleosomal histone H3 in vitro. A, unmodified (lane 1) or H2Bub1 (lane 2) recombinant histone octamers were separated by SDS-PAGE and visualized by Coomassie Blue staining. B, Set1C methyltransferase reactions containing octamers were incubated at 30 °C for the indicated times (in minutes). Activity was assayed by Western blotting with the antibodies indicated on the right. C, mononucleosomes reconstituted with the indicated recombinant histone octamers (in duplicate) and the 601 DNA fragment were electrophoresed on a 5% native acrylamide gel and visualized by ethidium bromide staining. D, Set1C methyltransferase reactions containing mononucleosomes were incubated at 30 °C for the indicated times (in minutes). Activity was assayed by Western blotting with the antibodies indicated on the right. E, Set1C methyltransferase reactions containing either octamers (oct) or assembled mononucleosomes (nuc) were incubated at 30 °C for the indicated times and analyzed by Western blotting with the antibodies indicated on the right.
Methyltransferase Assays
Typical reactions contained 1 μg of histones (octamers or nucleosomes), 0.8 mm S-adenosylmethionine (New England Biolabs), ∼20 ng of purified Set1C, 10 mm HEPES (pH 7.6), 50 mm NaCl, 37.5 mm potassium acetate, 10% glycerol, 2.5 mm magnesium acetate, 1 mm EDTA, and 3 mm dithiothreitol in a final volume of 40 μl. Incubations were carried out at 30 °C for the indicated times, after which reactions were terminated by the addition of an equal volume of 2× SDS-PAGE sample buffer (100 mm Tris (pH 6.8), 2% SDS, and 20% glycerol). Reactions were analyzed by SDS-PAGE on 15% acrylamide gels, followed by Western blotting with antibody against total histone H3 (Abcam 1791), monomethyl histone H3 lysine 4 (Abcam 8895), or dimethyl histone H3 lysine 4 (Millipore 07-030).
Gel Filtration Chromatography
Cell pellets derived from a 50-ml logarithmic YES culture were resuspended in 0.4 ml of running buffer (20 mm HEPES (pH 7.6), 150 mm NaCl, 10% glycerol, 1 mm EDTA, 0.1% Nonidet P 40, 1 mm dithiothreitol, 1 mm PMSF, and protease inhibitor mixture) and lysed in a BioSpec BeadBeater (4 × 30-s pulses) at 4 °C. Lysates were collected and centrifuged at 15,000 × g for 15 min. Supernatants (typically 5–10 mg of total protein) were loaded onto a Superose 6 column connected to an ÅKTA purifier system (GE Healthcare). The column was equilibrated in running buffer lacking protease inhibitors. Elution was performed with 1.5 volumes of the same buffer; 1-ml fractions were collected. Even-numbered fractions were concentrated using Vivaspin 500 spin columns and analyzed by Western blotting with an anti-TAP antibody (Open Biosystems).
ChIP
Fifty ml of S. pombe cultures grown in YES medium was fixed with formaldehyde, washed, and harvested as described (18). Cell pellets were resuspended in 0.4 ml of lysis buffer (50 mm HEPES (pH 7.6), 150 mm NaCl, 1 mm EDTA, 1% Triton X-100, 0.1% sodium deoxycholate, 1 mm PMSF, and protease inhibitor mixture) and lysed by bead beating in the presence of glass beads. Lysates were collected and centrifuged at top speed for 15 min at 4 °C. The pellet fraction containing the chromatin was washed once with lysis buffer, collected again by centrifugation, and resuspended in 1 ml of lysis buffer. Extracts were sonicated for 6 × 300s pulses in a Bioruptor water bath sonicator (Diagenode) and centrifuged at top speed for 5 min at 4 °C. The supernatant containing soluble sheared chromatin was used for immunoprecipitation.
Immunoprecipitations contained 2 mg of total protein and 20 μl of IgG-Sepharose beads and were incubated for 4–16 h at 4 °C with rocking. Beads were collected by centrifugation and washed with 0.5 ml each of lysis buffer, lysis buffer + 0.1% SDS, lysis buffer + 500 mm NaCl + 0.1% SDS, LiCl buffer (10 mm Tris (pH 8), 250 mm LiCl, 1 mm EDTA, 0.5% sodium deoxycholate, and 0.5% Nonidet P-40), and Tris/EDTA buffer (10 mm Tris (pH 7.5) and 1 mm EDTA). Each wash was carried out at room temperature for 4 min with rocking. Beads were eluted with 100 μl of 50 mm Tris (pH 7.5), 10 mm EDTA, and 1% SDS for 65 °C for 15 min. Beads were then washed once with 150 μl of Tris/EDTA buffer + 0.67% SDS. The wash was combined with the eluate and incubated overnight at 65 °C (∼16 h) to reverse cross-links. Purification of DNA was carried out as described (18). Input and immunoprecipitated samples were analyzed by quantitative PCR using SYBR Green PCR Master Mix (Bio-Rad). Primers for act1+ were as described (35).
RESULTS
H2Bub1 Directly Enhances Activity of S. pombe Set1C toward Nucleosomal Histone H3 in Vitro
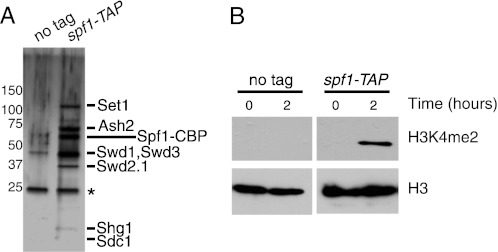
To test whether H2Bub1 directly stimulates the activity of Set1C in vitro, native Set1C was first purified from a strain of S. pombe carrying a TAP tag on Spf1, an ortholog of Spp1 (9, 36). The spf1-TAP strain retained wild-type levels of H3K4 methylation (supplemental Fig. S1), confirming that the tagged complex was fully functional. S. pombe Set1C has been extensively analyzed by the TAP method in previous studies and found to contain eight subunits: Set1, Ash2, Spf1, Swd3, Swd1, Swd2.1, Shg1, and Sdc1 (9, 36). Analysis of the composition of our purified material by SDS-PAGE and silver staining revealed bands at the predicted sizes of all of the known Set1C subunits (Fig. 1A), confirming that we had purified an intact Set1C.
FIGURE 1.
S. pombe Set1C has H3K4 methyltransferase activity in vitro. A, TAP purifications carried out in an untagged (JTB204) (supplemental Table S1) or spf1-TAP (JTB241) strain were analyzed by SDS-PAGE and silver staining. Molecular mass markers (in kilodaltons) are indicated on the left. Bands detected specifically in the spf1-TAP purification that correspond to the known components of Set1C are indicated on the right. Spf1-CBP, Spf1 tagged with calmodulin-binding peptide yielded by the TAP procedure (see “Experimental Procedures”). B, in vitro methyltransferase reactions containing TAP-purified material from the indicated strains and recombinant histone octamers were incubated at 30 °C and analyzed by Western blotting with the antibodies indicated on the right.
We next incubated purified Set1C with unmodified recombinant histone octamers in the presence of the methyl group donor S-adenosylmethionine and analyzed the reactions by Western blotting with antibodies specific for the dimethylated form of H3K4 (H3K4me2). We readily detected H3K4me2 in these assays. Material isolated from an untagged strain yielded no activity (Fig. 1B), confirming that the methylation was generated by Set1C.
We next tested the effects of H2Bub1 and nucleosome structure in our in vitro system. First, we compared the activity of Set1C on standard (unmodified) recombinant histone octamers with that on octamers reconstituted with semisynthetic H2Bub1 produced using the expressed protein ligation method (Fig. 2A) (25). In both cases, Set1C led to formation of H3K4me2 in a time-dependent manner over a 3-h period (Fig. 2B). Thus, H2Bub1 does not impact Set1C activity on “free” histones that have not been assembled into nucleosomes. We next tested Set1C activity toward nucleosomal histone H3 by assembling both the unmodified and H2Bub1 octamers onto a defined mononucleosome positioning sequence (the 601 sequence (37)) by salt dialysis (Fig. 2C). Methylation of histone H3 in unmodified nucleosomes was undetectable after 5 h (Fig. 2D) and after longer time periods (supplemental Fig. S2), indicating that Set1C was inactive on standard recombinant nucleosomes under these conditions. In contrast, H2Bub1 mononucleosomes were methylated by Set1C (Fig. 2D). Activity on nucleosomes was detectable after 30 min and appeared to plateau at 2–3 h. Prolonged incubations allowed a greater stimulation of activity by H2Bub1 (supplemental Fig. S2). Thus, H2Bub1 specifically promotes Set1C activity toward nucleosomal histone H3.
H2Bub1 has been shown to differentially influence the various methylation states of H3K4 in vivo (38, 39). In S. pombe, H2Bub1 is absolutely required for generation of trimethyl H3K4 (H3K4me3) and greatly enhances the levels of H3K4me2 and monomethyl H3K4 (H3K4me1) (18). We assayed the levels of all three forms of H3K4me in our in vitro system by Western blotting. We were unable to detect the formation of H3K4me3 in our assays, likely due to the inefficiency of the in vitro reactions (data not shown). As we found for H3K4me2, H3K4me1 was readily detected on both unmodified and ubiquitylated histone octamers, but formation of H3K4me1 on nucleosomal H3 was enhanced by H2Bub1 (Fig. 2E). These results recapitulate the trans-histone crosstalk pathway that operates in vivo and suggest that this pathway can involve direct enhancement of Set1C activity by H2Bub1.
S. pombe Set1C Is Largely Intact in Vivo in Absence of H2Bub1
Our in vitro results suggested that H2Bub1 directly impacts the activity of Set1C in S. pombe. We wanted to address whether H2Bub1 may also indirectly affect the activity of Set1C by influencing its assembly or composition in vivo. We first measured the expression levels of TAP-tagged versions of Set1, Spf1, and the S. pombe Swd2 ortholog Swd2.1 (36) in denaturing whole cell extracts derived from wild-type and H2Bub1-deficient strains (htb1-K119R, a strain in which the single ubiquitylation site on H2B is mutated (18), and brl2Δ, a strain lacking an E3 ubiquitin ligase enzyme that catalyzes H2Bub1 formation (18, 40)) by Western blotting. The overall levels of Set1C subunits were mostly unchanged upon loss of H2Bub1, although we did note a modest (15–40%) reduction in the total levels of all three Set1C subunits (supplemental Fig. S3, A and B) (data not shown).
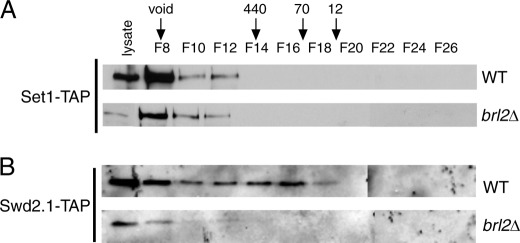
To assess the role of H2Bub1 in Set1C assembly, we analyzed the native size of Set1C components by gel filtration chromatography in both wild-type and brl2Δ strains. In the wild-type strain, Set1-TAP was detected in fractions corresponding to a native size of >1 MDa (Fig. 3A, upper panel), consistent with the size previously determined for S. pombe Set1C (9). The brl2Δ strain gave an identical elution profile for Set1-TAP (Fig. 3A, lower panel), suggesting that Set1C remained largely intact in the absence of H2Bub1. The overall levels of Set1-TAP were noticeably reduced in native extracts prepared from H2Bub1-deficient strains compared with wild-type extracts (compare lysate lanes). This contrasts with our findings in denaturing extracts, in which the levels of Set1C subunits were mostly unchanged by H2Bub1 loss (supplemental Fig. S3). We attribute this difference to increased protease activity in the mutant extracts, which was not apparent under denaturing conditions (data not shown).
FIGURE 3.
Analysis of Set1C by gel filtration chromatography in wild-type and brl2Δ strains. A, native whole cell extracts prepared from the indicated set1-TAP strains (JTB242 and JTB405) were fractionated by gel filtration, and even-numbered fractions (F) were analyzed by Western blotting with an anti-TAP antibody. The lysate lanes contained 2% of the unfractionated extract. The void volume and peak elution volumes of molecular mass standards (in kilodaltons) are denoted by arrows. B, same as described for A with the indicated swd2.1-TAP strains (JTB450 and JTB454).
We next followed the purification of Swd2.1-TAP, as Swd2 incorporation into Set1C was found to be sensitive to the presence of H2Bub1 in budding yeast (27). A portion of Swd2.1-TAP eluted with the same profile as Set1-TAP in the wild-type strain, suggesting its presence in the same complex (Fig. 3B, upper panel). Swd2.1-TAP was also detected in lower molecular mass fractions, perhaps indicating functions for this protein that are independent of Set1C. Importantly, most Swd2.1-TAP in the brl2Δ extracts eluted at the same size as Set1-TAP (although as we observed for Set1-TAP, the overall levels were lower than in the wild-type strain). Fig. 3B (lower panel) demonstrates that H2Bub1 was not required for Swd2.1 association with Set1C.
S. pombe Set1C Purified from Strains Lacking H2Bub1 Retains Activity toward Histone Octamer Substrates
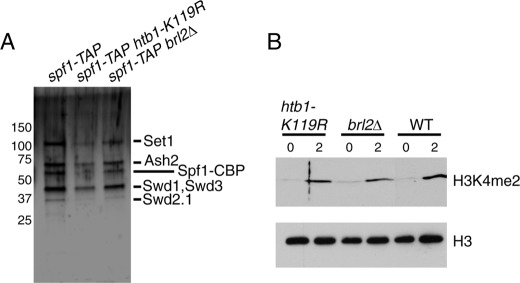
Our analysis in S. pombe suggested that Set1C was intact in vivo in the absence of H2Bub1. To confirm the functionality of Set1C in the absence of H2Bub1, we purified Set1C via Spf1-TAP from strains lacking H2Bub1. Analysis of subunit composition by silver staining did not reveal any differences between wild-type and H2Bub1-deficient Set1C, and all of the core Set1C subunits necessary for methyltransferase activity (9) were present at stoichiometries comparable with that observed for the wild-type complex (Fig. 4A). Moreover, all three complexes possessed similar activity toward recombinant histone octamers in vitro (Fig. 4B). These results further argue that the stimulation of H3K4me by H2Bub1 does not involve effects on the integrity of Set1C in vivo.
FIGURE 4.
Set1C purified from H2Bub1-deficient strains retains activity in vitro. A, TAP purifications carried out in the indicated strains (JTB241, JTB253-3, and JTB416) were analyzed by SDS-PAGE and silver staining. Molecular mass markers (in kilodaltons) are indicated on the left. Bands detected specifically in the spf1-TAP purification that correspond to the known components of Set1C are indicated on the right. B, in vitro methyltransferase reactions containing equal amounts of the TAP-purified material shown in A and recombinant histone octamers were incubated at 30 °C and analyzed by Western blotting with the antibodies indicated on the right.
H2Bub1 Is Required for Association of S. pombe Set1C with Transcribed Chromatin
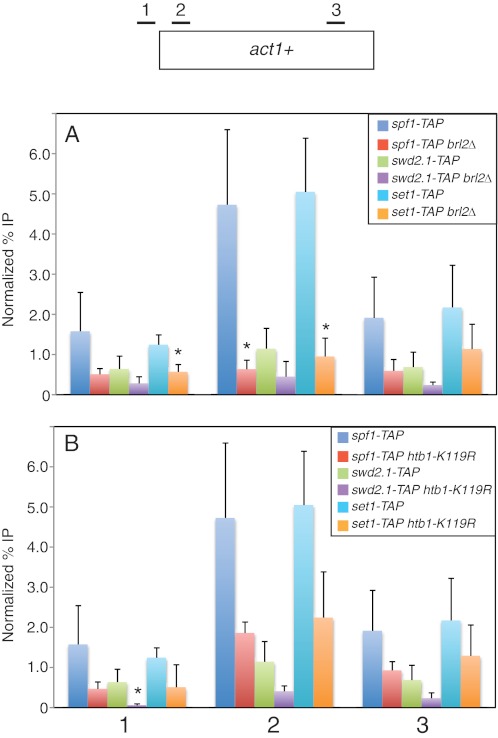
Our data suggested that Set1C remained intact in the absence of H2Bub1 in S. pombe. We thus expected that ChIP analysis would reveal similar effects of H2Bub1 loss on chromatin association of different Set1C subunits. ChIP analysis in set1-TAP, spf1-TAP, and swd2.1-TAP strains showed that this was indeed the case. Notably, cross-linking of all three Set1C subunits to the constitutively transcribed act1+ gene was reduced in brl2Δ strains relative to wild-type strains as measured by quantitative PCR analysis of the immunoprecipitated material (Fig. 5A). These differences were particularly significant just downstream of the transcription start site (primer pair 2), where we observed a 7-fold decrease for Spf1-TAP, a 2.5-fold decrease for Swd2.1-TAP, and a 5-fold decrease for Set1-TAP. This location coincides with the peak of H3K4me3 associated with the locus (supplemental Fig. S4). We noted that the cross-linking of Swd2.1-TAP to act1+ was consistently lower than that of the other subunits, perhaps reflecting its apparently substoichiometric presence in Set1C (Fig. 1A) or its low overall abundance relative to other Set1C subunits (supplemental Fig. S3).
FIGURE 5.
ChIP analysis of Set1C subunits in wild-type and H2Bub1-deficient strains. A, ChIP was carried out in the indicated TAP-tagged strains (JTB241, JTB416, JTB450, JTB454, JTB242, and JTB405) and analyzed by quantitative PCR as described under “Experimental Procedures.” Positions of primer pairs are diagrammed at the top. ChIP signals are expressed as a percentage of the input signal for each primer pair. Normalization was carried out by subtracting the background percentage obtained from an untagged strain. Error bars denote S.D. from at least three independent experiments. *, mutant values that are significantly different from wild-type values (p < 0.05; unpaired t test). B, same as described for A for the indicated TAP-tagged strains (JTB241, JTB253-3, JTB450, JTB478, JTB242, and JTB404).
Similar (albeit smaller) reductions in cross-linking of all three Set1C subunits were observed in the htb1-K119R mutant background (Fig. 5B), arguing that the decreases were specifically due to loss of H2Bub1. Furthermore, act1+ transcript levels did not change in H2Bub1 mutants (supplemental Fig. S5A), indicating that the observed changes are not a reflection of reduced transcription. As an additional control, we carried out ChIP analysis with TAP-tagged Rtf1, a subunit of the polymerase-associated factor complex predicted to be enriched at the constitutive act1+ locus. We found that cross-linking of Rtf1-TAP to act1+ was similar in wild-type and htb1-K119R strains (supplemental Fig. S5B), indicating that ChIP of TAP-tagged proteins is not generally impaired in H2Bub1-deficient strains. We conclude that H2Bub1 stabilizes the association of Set1C with transcribed chromatin in vivo.
DISCUSSION
Histone modifications are key components of chromatin-based signaling pathways that regulate transcription, DNA repair, and other aspects of chromosome function. Regulatory crosstalk between histone modifications can underlie crosstalk between these signaling pathways. Various modes of crosstalk between different modifications have been described, including functional antagonism and cooperativity (41). The H2Bub1-H3K4me modification pathway is unique in that it involves a specific dependence of a modification of one histone on the presence of a modification of another histone and thus represents a potentially novel form of communication between chromatin components.
In this study, we have characterized H2Bub1-H3K4me crosstalk both in vitro and in vivo using the model eukaryote S. pombe. Use of a combination of biochemical and genetic approaches provided us with the first comprehensive view of the crosstalk pathway in a single model system. Our data strongly support direct enhancement of Set1C activity, rather than Set1C assembly, as the basis for trans-histone crosstalk in this organism. Given the complementary recent in vitro findings with human Set1C (26), we suggest that this mechanism likely reflects a conserved aspect of trans-histone crosstalk acting in transcriptional regulation.
Alternative models of H2Bub1-H3K4me crosstalk derive largely from work in budding yeast and are based on effects of H2Bub1 on Set1C assembly. Our results obtained with S. pombe argue that Set1C remains intact in the absence of H2Bub1 because (i) the apparent stoichiometry of purified subunits is the same in the wild-type, brl2Δ, and htb1-K119R strains, (ii) Swd2.1 still co-fractionates with Set1 by gel filtration chromatography in brl2Δ, and (iii) complexes purified from all three strains have comparable activity on free histone substrates. Thus, Set1C complex assembly does not generally underlie the H2Bub1-H3K4me link in this organism. Further study will be needed to determine whether H2Bub1 influence on Set1C composition is a feature of the crosstalk pathway in other species. Interestingly, study of the human Swd2 ortholog Wdr82 has shown that its association with active chromatin is also reduced upon depletion of the H2B-ubiquitylating enzyme RNF20, although the impact of H2Bub1 on the composition of the human Set1 complexes and their association with chromatin has yet to be elucidated in detail (15).
Our ChIP analysis in S. pombe is the first to suggest that chromatin binding of intact Set1C is compromised in the absence of H2Bub1. Although our analysis included only three Set1C subunits, the fact that Set1 was among them is an indication that the entire complex, rather than individual subunits, is affected, given the known scaffolding role for Set1 within the complex (42). On the basis of this finding, one might predict that H2Bub1 would also promote nucleosome binding by Set1C in vitro. We have thus far been unable to detect such an effect (data not shown). In vivo, H2Bub1 is likely part of a combinatorial recruitment mechanism for Set1C, such that its effect on the chromatin association of the complex may be apparent only at certain loci. This view could also explain why Set1 chromatin association in S. cerevisiae has not been shown to be affected by H2Bub1 at select transcribed genes. Here again, species-specific aspects to histone crosstalk regulation may also be relevant, and further exploration of this issue is warranted.
The direct model for H2Bub1-H3K4me crosstalk is attractive given its parallel to the H2Bub1-H3K79me relationship, in which H2Bub1 directly stimulates methyltransferase activity of a recombinant human Dot1L enzyme toward nucleosomal H3 (25). The molecular details of this stimulation, for either H3K4me or H3K79me, are currently unclear. Enhancement of methyltransferase activity could result from direct contact between ubiquitylated H2B and Set1C that either stabilizes the enzyme-substrate complex or causes an allosteric effect (or a combination of both). In S. cerevisiae, mutations in histone H2A or H2B residues that are close to the H2Bub1 site on the nucleosome surface can reduce total H3K4me levels without affecting the levels of H2Bub1, arguing for the presence of a specific nucleosome-binding site for Set1C (43, 44). These data were interpreted in the context of the loss of specific Set1C subunits from chromatin; our data would predict that this binding site, including H2Bub1, can function to recruit the entire Set1C.
Changes to nucleosome structure induced by H2Bub1 could also facilitate the methylation reaction. The impact of H2Bub1 on chromatin structure remains poorly defined, as some reports suggest that it has a destabilizing effect, whereas others claim the opposite (45, 46). It is noteworthy that our results directly comparing the activity of native Set1C toward free histones with that on nucleosomes show that the complex has a clear preference for the former. This argues against the notion that nucleosome stabilization by H2Bub1 is an important factor in the H2Bub1-H3K4me crosstalk pathway. Chromatin structure effects may be particularly relevant to the human Dot1L activation mechanism because this enzyme can be stimulated by ubiquitin positioned at various locations on the nucleosome (33, 47), and H2Bub1 does not influence its affinity for nucleosomes in vitro (25). Clearly, a key issue to be addressed is whether or not Set1C interacts directly with the nucleosome and how this interaction is influenced directly by H2Bub1. The in vitro system described here will be ideal for further investigation of H2Bub1-H3K4me crosstalk and mechanistic studies on the formation of active chromatin states.
Supplementary Material
Acknowledgments
We thank R. McGinty and T. Muir for the generous gift of recombinant histone octamers containing monoubiquitylated histone H2B. We thank A. F. Stewart for providing S. pombe strains, M. Carey for providing plasmid pGEM601, and A. Verreault for the gift of anti-histone H3 antibody. We thank A. Verreault and members of the Tanny laboratory for discussion and D. Bernard, R. McGinty, and T. Muir for critical reading of the manuscript.
This work was supported in part by Canadian Institutes of Health Research Grant MOP-97890 (to J. C. T.) and startup funds from the Sackler Program in Epigenetics (to J. C. T.).

This article contains supplemental “Experimental Procedures,” Figs. S1–S5, Table S1, and additional references.
- TAP
- tandem affinity purification.
REFERENCES
- 1. Ruthenburg A. J., Allis C. D., Wysocka J. (2007) Methylation of lysine 4 on histone H3: intricacy of writing and reading a single epigenetic mark. Mol. Cell 25, 15–30 [DOI] [PubMed] [Google Scholar]
- 2. Shilatifard A. (2008) Molecular implementation and physiological roles for histone H3 lysine 4 (H3K4) methylation. Curr. Opin. Cell Biol. 20, 341–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vermeulen M., Eberl H. C., Matarese F., Marks H., Denissov S., Butter F., Lee K. K., Olsen J. V., Hyman A. A., Stunnenberg H. G., Mann M. (2010) Quantitative interaction proteomics and genome-wide profiling of epigenetic histone marks and their readers. Cell 142, 967–980 [DOI] [PubMed] [Google Scholar]
- 4. Lessard J. A., Crabtree G. R. (2010) Chromatin regulatory mechanisms in pluripotency. Annu. Rev. Cell Dev. Biol. 26, 503–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wysocka J., Swigut T., Milne T. A., Dou Y., Zhang X., Burlingame A. L., Roeder R. G., Brivanlou A. H., Allis C. D. (2005) WDR5 associates with histone H3 methylated at K4 and is essential for H3 K4 methylation and vertebrate development. Cell 121, 859–872 [DOI] [PubMed] [Google Scholar]
- 6. Jiang H., Shukla A., Wang X., Chen W. Y., Bernstein B. E., Roeder R. G. (2011) Role for Dpy30 in ES cell fate specification by regulation of H3K4 methylation within bivalent domains. Cell 144, 513–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Briggs S. D., Bryk M., Strahl B. D., Cheung W. L., Davie J. K., Dent S. Y., Winston F., Allis C. D. (2001) Histone H3 lysine 4 methylation is mediated by Set1 and required for cell growth and rDNA silencing in Saccharomyces cerevisiae. Genes Dev. 15, 3286–3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mohan M., Herz H. M., Smith E. R., Zhang Y., Jackson J., Washburn M. P., Florens L., Eissenberg J. C., Shilatifard A. (2011) The COMPASS family of H3K4 methylases in Drosophila. Mol. Cell. Biol. 31, 4310–4318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roguev A., Schaft D., Shevchenko A., Aasland R., Shevchenko A., Stewart A. F. (2003) High conservation of the Set1/Rad6 axis of histone 3 lysine 4 methylation in budding and fission yeasts. J. Biol. Chem. 278, 8487–8493 [DOI] [PubMed] [Google Scholar]
- 10. Xiao Y., Bedet C., Robert V. J., Simonet T., Dunkelbarger S., Rakotomalala C., Soete G., Korswagen H. C., Strome S., Palladino F. (2011) Caenorhabditis elegans chromatin-associated proteins SET-2 and ASH-2 are differentially required for histone H3 Lys 4 methylation in embryos and adult germ cells. Proc. Natl. Acad. Sci. U.S.A. 108, 8305–8310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eissenberg J. C., Shilatifard A. (2010) Histone H3 lysine 4 (H3K4) methylation in development and differentiation. Dev. Biol. 339, 240–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dou Y., Milne T. A., Ruthenburg A. J., Lee S., Lee J. W., Verdine G. L., Allis C. D., Roeder R. G. (2006) Regulation of MLL1 H3K4 methyltransferase activity by its core components. Nat. Struct. Mol. Biol. 13, 713–719 [DOI] [PubMed] [Google Scholar]
- 13. Takahashi Y. H., Westfield G. H., Oleskie A. N., Trievel R. C., Shilatifard A., Skiniotis G. (2011) Structural analysis of the core COMPASS family of histone H3K4 methylases from yeast to human. Proc. Natl. Acad. Sci. U.S.A. 108, 20526–20531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee J. H., Skalnik D. G. (2005) CpG-binding protein (CXXC finger protein 1) is a component of the mammalian Set1 histone H3 Lys-4 methyltransferase complex, the analog of the yeast Set1/COMPASS complex. J. Biol. Chem. 280, 41725–41731 [DOI] [PubMed] [Google Scholar]
- 15. Wu M., Wang P. F., Lee J. S., Martin-Brown S., Florens L., Washburn M., Shilatifard A. (2008) Molecular regulation of H3K4 trimethylation by Wdr82, a component of human Set1/COMPASS. Mol. Cell. Biol. 28, 7337–7344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sun Z. W., Allis C. D. (2002) Ubiquitination of histone H2B regulates H3 methylation and gene silencing in yeast. Nature 418, 104–108 [DOI] [PubMed] [Google Scholar]
- 17. Dover J., Schneider J., Tawiah-Boateng M. A., Wood A., Dean K., Johnston M., Shilatifard A. (2002) Methylation of histone H3 by COMPASS requires ubiquitination of histone H2B by Rad6. J. Biol. Chem. 277, 28368–28371 [DOI] [PubMed] [Google Scholar]
- 18. Tanny J. C., Erdjument-Bromage H., Tempst P., Allis C. D. (2007) Ubiquitylation of histone H2B controls RNA polymerase II transcription elongation independently of histone H3 methylation. Genes Dev. 21, 835–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bray S., Musisi H., Bienz M. (2005) Bre1 is required for Notch signaling and histone modification. Dev. Cell 8, 279–286 [DOI] [PubMed] [Google Scholar]
- 20. Zhu B., Zheng Y., Pham A. D., Mandal S. S., Erdjument-Bromage H., Tempst P., Reinberg D. (2005) Monoubiquitination of human histone H2B: the factors involved and their roles in HOX gene regulation. Mol. Cell 20, 601–611 [DOI] [PubMed] [Google Scholar]
- 21. Kim J., Hake S. B., Roeder R. G. (2005) The human homolog of yeast BRE1 functions as a transcriptional coactivator through direct activator interactions. Mol. Cell 20, 759–770 [DOI] [PubMed] [Google Scholar]
- 22. Ng H. H., Robert F., Young R. A., Struhl K. (2003) Targeted recruitment of Set1 histone methylase by elongating Pol II provides a localized mark and memory of recent transcriptional activity. Mol. Cell 11, 709–719 [DOI] [PubMed] [Google Scholar]
- 23. Ng H. H., Xu R. M., Zhang Y., Struhl K. (2002) Ubiquitination of histone H2B by Rad6 is required for efficient Dot1-mediated methylation of histone H3 lysine 79. J. Biol. Chem. 277, 34655–34657 [DOI] [PubMed] [Google Scholar]
- 24. Briggs S. D., Xiao T., Sun Z. W., Caldwell J. A., Shabanowitz J., Hunt D. F., Allis C. D., Strahl B. D. (2002) Gene silencing: trans-histone regulatory pathway in chromatin. Nature 418, 498. [DOI] [PubMed] [Google Scholar]
- 25. McGinty R. K., Kim J., Chatterjee C., Roeder R. G., Muir T. W. (2008) Chemically ubiquitylated histone H2B stimulates hDot1L-mediated intranucleosomal methylation. Nature 453, 812–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim J., Guermah M., McGinty R. K., Lee J. S., Tang Z., Milne T. A., Shilatifard A., Muir T. W., Roeder R. G. (2009) RAD6-mediated transcription-coupled H2B ubiquitylation directly stimulates H3K4 methylation in human cells. Cell 137, 459–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee J. S., Shukla A., Schneider J., Swanson S. K., Washburn M. P., Florens L., Bhaumik S. R., Shilatifard A. (2007) Histone crosstalk between H2B monoubiquitination and H3 methylation mediated by COMPASS. Cell 131, 1084–1096 [DOI] [PubMed] [Google Scholar]
- 28. Vitaliano-Prunier A., Menant A., Hobeika M., Géli V., Gwizdek C., Dargemont C. (2008) Ubiquitylation of the COMPASS component Swd2 links H2B ubiquitylation to H3K4 trimethylation. Nat. Cell Biol. 10, 1365–1371 [DOI] [PubMed] [Google Scholar]
- 29. Bähler J., Wu J. Q., Longtine M. S., Shah N. G., McKenzie A., 3rd, Steever A. B., Wach A., Philippsen P., Pringle J. R. (1998) Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14, 943–951 [DOI] [PubMed] [Google Scholar]
- 30. Sato M., Dhut S., Toda T. (2005) New drug-resistant cassettes for gene disruption and epitope tagging in Schizosaccharomyces pombe. Yeast 22, 583–591 [DOI] [PubMed] [Google Scholar]
- 31. Moreno S., Klar A., Nurse P. (1991) Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194, 795–823 [DOI] [PubMed] [Google Scholar]
- 32. Tanny J. C., Kirkpatrick D. S., Gerber S. A., Gygi S. P., Moazed D. (2004) Budding yeast silencing complexes and regulation of Sir2 activity by protein-protein interactions. Mol. Cell. Biol. 24, 6931–6946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chatterjee C., McGinty R. K., Fierz B., Muir T. W. (2010) Disulfide-directed histone ubiquitylation reveals plasticity in hDot1L activation. Nat. Chem. Biol. 6, 267–269 [DOI] [PubMed] [Google Scholar]
- 34. Carey M., Li B., Workman J. L. (2006) RSC exploits histone acetylation to abrogate the nucleosomal block to RNA polymerase II elongation. Mol. Cell 24, 481–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guiguen A., Soutourina J., Dewez M., Tafforeau L., Dieu M., Raes M., Vandenhaute J., Werner M., Hermand D. (2007) Recruitment of P-TEFb (Cdk9-Pch1) to chromatin by the cap methyltransferase Pcm1 in fission yeast. EMBO J. 26, 1552–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roguev A., Shevchenko A., Schaft D., Thomas H., Stewart A. F. (2004) A comparative analysis of an orthologous proteomic environment in the yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe. Mol. Cell. Proteomics 3, 125–132 [DOI] [PubMed] [Google Scholar]
- 37. Thåström A., Lowary P. T., Widlund H. R., Cao H., Kubista M., Widom J. (1999) Sequence motifs and free energies of selected natural and non-natural nucleosome positioning DNA sequences. J. Mol. Biol. 288, 213–229 [DOI] [PubMed] [Google Scholar]
- 38. Dehé P. M., Pamblanco M., Luciano P., Lebrun R., Moinier D., Sendra R., Verreault A., Tordera V., Géli V. (2005) Histone H3 lysine 4 monomethylation does not require ubiquitination of histone H2B. J. Mol. Biol. 353, 477–484 [DOI] [PubMed] [Google Scholar]
- 39. Shahbazian M. D., Zhang K., Grunstein M. (2005) Histone H2B ubiquitylation controls processive methylation but not monomethylation by Dot1 and Set1. Mol. Cell 19, 271–277 [DOI] [PubMed] [Google Scholar]
- 40. Zofall M., Grewal S. I. (2007) HULC, a histone H2B-ubiquitinating complex, modulates heterochromatin independent of histone methylation in fission yeast. J. Biol. Chem. 282, 14065–14072 [DOI] [PubMed] [Google Scholar]
- 41. Lee J. S., Smith E., Shilatifard A. (2010) The language of histone crosstalk. Cell 142, 682–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Roguev A., Schaft D., Shevchenko A., Pijnappel W. W., Wilm M., Aasland R., Stewart A. F. (2001) The Saccharomyces cerevisiae Set1 complex includes an Ash2 homolog and methylates histone 3 lysine 4. EMBO J. 20, 7137–7148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nakanishi S., Sanderson B. W., Delventhal K. M., Bradford W. D., Staehling-Hampton K., Shilatifard A. (2008) A comprehensive library of histone mutants identifies nucleosomal residues required for H3K4 methylation. Nat. Struct. Mol. Biol. 15, 881–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chandrasekharan M. B., Huang F., Chen Y. C., Sun Z. W. (2010) Histone H2B C-terminal helix mediates trans-histone H3K4 methylation independent of H2B ubiquitination. Mol. Cell. Biol. 30, 3216–3232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fierz B., Chatterjee C., McGinty R. K., Bar-Dagan M., Raleigh D. P., Muir T. W. (2011) Histone H2B ubiquitylation disrupts local and higher order chromatin compaction. Nat. Chem. Biol. 7, 113–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chandrasekharan M. B., Huang F., Sun Z. W. (2009) Ubiquitination of histone H2B regulates chromatin dynamics by enhancing nucleosome stability. Proc. Natl. Acad. Sci. U.S.A. 106, 16686–16691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wu L., Zee B. M., Wang Y., Garcia B. A., Dou Y. (2011) The RING finger protein MSL2 in the MOF complex is an E3 ubiquitin ligase for H2B K34 and is involved in crosstalk with H3 K4 and K79 methylation. Mol. Cell 43, 132–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.