Background: Myofilament protein phosphorylation is central to cardiac muscle contractile regulation.
Results: AMP-activated protein kinase (AMPK) phosphorylates troponin I (TnI) to increase cardiac contraction and blunt the effects of TnI protein kinase A phosphorylation.
Conclusion: AMPK myofilament signaling represents a novel mechanism to regulate cardiac contraction.
Significance: AMPK links metabolics to TnI regulation of cardiac muscle function and adrenergic regulation.
Keywords: AMP-activated Kinase (AMPK), Cardiac Muscle, phosphorylation, Protein Kinase A (PKA), Troponin, Skinned Fiber Force
Abstract
AMP-activated protein kinase (AMPK) is an energy-sensing enzyme central to the regulation of metabolic homeostasis. In the heart AMPK is activated during cardiac stress-induced ATP depletion and functions to stimulate metabolic pathways that restore the AMP/ATP balance. Recently it was demonstrated that AMPK phosphorylates cardiac troponin I (cTnI) at Ser-150 in vitro. We sought to determine if the metabolic regulatory kinase AMPK phosphorylates cTnI at Ser-150 in vivo to alter cardiac contractile function directly at the level of the myofilament. Rabbit cardiac myofibrils separated by two-dimensional isoelectric focusing subjected to a Western blot with a cTnI phosphorylation-specific antibody demonstrates that cTnI is endogenously phosphorylated at Ser-150 in the heart. Treatment of myofibrils with the AMPK holoenzyme increased cTnI Ser-150 phosphorylation within the constraints of the muscle lattice. Compared with controls, cardiac fiber bundles exchanged with troponin containing cTnI pseudo-phosphorylated at Ser-150 demonstrate increased sensitivity of calcium-dependent force development, blunting of both PKA-dependent calcium desensitization, and PKA-dependent increases in length dependent activation. Thus, in addition to the defined role of AMPK as a cardiac metabolic energy gauge, these data demonstrate AMPK Ser-150 phosphorylation of cTnI directly links the regulation of cardiac metabolic demand to myofilament contractile energetics. Furthermore, the blunting effect of cTnI Ser-150 phosphorylation cross-talk can uncouple the effects of myofilament PKA-dependent phosphorylation from β-adrenergic signaling as a novel thin filament contractile regulatory signaling mechanism.
Introduction
AMP-activated protein kinase (AMPK)5 is a serine/threonine protein kinase that functions as a central physiological regulator of energy-generating pathways in multiple organ systems (1, 2). By responding to various metabolic changes, AMPK regulates key enzymes in metabolic pathways to restore cellular energy stores. Classical AMPK activation results from the increase in cellular AMP/ATP ratio-induced AMP binding during periods of high energy demands. This ability of AMPK to “sense” decreased ATP levels and its subsequent activation to increase ATP production have led AMPK to be termed the “master switch” for maintaining cellular energy levels (1). In the heart, AMPK signaling to increase ATP during elevated metabolic demand is necessary to maintain cardiac contractility. Recent evidence has demonstrated AMPK is activated by other stimuli in addition to AMP binding (3, 4), suggesting AMPK signaling may function to regulate other systems in addition to metabolism. In the absence of clear downstream signaling targets, the role of AMPK signaling directly at the myofilament is unclear.
Several lines of evidence indicate that cardiac troponin I (cTnI) may be a significant substrate for AMPK. Troponin I (TnI) plays a critical role in the regulation of muscle contraction (5). The phosphorylation of cTnI at Ser-23/24 through the β-adrenergic signaling pathway represents one of the primary myofilament mechanisms to regulate cardiac contractile dynamics (for review, see Ref. 6). In the normal heart cTnI Ser-23/24 are phosphorylated at a basal level with functional modulation resulting from alterations of this level (7–9). Cardiac TnI can also be phosphorylated at a number of other residues through different signaling pathways; however, the physiological relevance of the majority of these phosphorylations is not clearly understood. Oliveira et al. (10) reported AMPK can phosphorylate cTnI at Ser-150 in vitro, and Sancho Solis et al. (11) demonstrated the kinase domain of AMPK was sufficient to phosphorylate cTnI at Ser-150 in the myofilament lattice. Recently we demonstrated cTnI Ser-150 phosphorylation is nearly doubled in an adrenergic-induced model of hypertrophy (12). Serine 150 is located directly within the TnI switch peptide, a key element in the Ca2+ regulation of muscle contraction. Evidence supporting Ser-150 phosphorylation as functionally relevant has been demonstrated by Ouyang et al. (13) who reported cTnI pseudo-phosphorylation altered the interaction of cTnI with troponin C (TnC) to affect thin filament Ca2+ regulation. To date the phosphorylation of cTnI Ser-150 in vivo and its functional effect on contraction are not known.
To determine the role of AMPK as a common signaling molecule between cardiomyocyte cellular metabolism and contractile function, we investigated the role of AMPK to phosphorylate cTnI at Ser-150 and its effect on cardiac contraction. Consistent with previous findings, we demonstrate the AMPK holoenzyme phosphorylates cTnI at Ser-150 in vitro as well as within the muscle lattice. We further demonstrate that cTnI is endogenously phosphorylated at Ser-150 in the heart. Through the exchange of cardiac troponin (cTn) containing a pseudo-phosphorylated cTnI into cardiac-skinned fibers we demonstrate cTnI Ser-150 phosphorylation significantly increases cardiac muscle Ca2+ sensitivity. Importantly, this cTnI Ser-150 phosphorylation cross-talks through an intramolecular mechanism within cTnI to blunt the functional effects of β-adrenergic-induced cTnI Ser-23/24 PKA phosphorylation. Our findings support AMPK as a signaling molecule that links the cardiac myocyte metabolic needs to a direct enhancement of the myofilament contractile response through uncoupling the thin filament β-adrenergic response.
EXPERIMENTAL PROCEDURES
cDNA Constructs
The human cTnI Ser-150 to Asp (cTnI S150D), Ser-23/24 to Asp (S23D/S24D), and Ser-23/24/150 to Asp (S23D/S24D/S150D) pseudo phosphorylation mutant cDNA was generated by site-directed mutagenesis (QuikChange II kit, Agilent) according to the manufacturer's directions, and resultant constructs were verified by DNA sequencing.
Proteins
All cTnI residue numbers presented in this manuscript are presented according to the native human sequence including the first methionine. The individual recombinant human cTn subunits were expressed in Escherichia coli and purified to homogeneity as previously described (14). Troponin used for fiber exchange and kinase experiments contained human cardiac TnT (TnT) with an N terminal myc tag. Our laboratory and others have previously demonstrated the presence of this myc tag on TnT does not affect myofilament function (15, 16). Troponin used in Ca2+ binding experiments consisted of native human TnT, cTnI, and human cardiac TnC with the T53C, S35C/S84C mutations (17). Cardiac Tn complexes were reconstituted by sequential dialysis and column-purified as previously described (14). Column fractions containing pure cTn were dialyzed against exchange buffer (200 mm KCl, 5 mm MgCl2, 5 mm EGTA, 1 mm DTT, 20 mm MOPS, pH 6.5), and aliquots were stored at −80 °C until use. Myofibrils were prepared as described previously and endogenous cTn was partially exchanged for exogenous cTn as previously described (14).
Kinase Treatments
Purified cTn or exchanged myofibrils were treated with purified PAK, purified bovine protein kinase A catalytic subunit (Sigma), or active AMPK holoenzyme complex composed of α1/β1/γ2 or α2/β1/γ2 subunits (SignalChem). Kinase reaction conditions were 200 mm KCl, 10 mm MgCl2, 1 mm DTT, 20 mm MOPS, pH 7.0, in the presence of 2.5 mm EGTA or 0.25 mm CaCl2. The reaction was initiated by the addition of 1 mm ATP and carried out at 37 °C for varying time points before stopping the reaction with an equal volume of 2× urea sample buffer consisting of 8 m urea, 2 m thiourea, 75 mm DTT, 50 mm Tris-HCl, pH 6.8, containing 3% SDS and 0.05% bromphenol blue. Samples were stored at −80 °C until use.
Protein Electrophoresis, Staining, and Western Blot
Proteins (purified, myofibrils, or fiber bundles) were solubilized in denaturing sample buffer (50 mm Tris-HCl, pH 6.8, 2% SDS, 0.1% bromphenol blue, and 10% glycerol), heated for 5 min at 80 °C, and clarified by centrifugation for 5 min. Resultant protein was separated by SDS-PAGE on cooled 8 × 10-cm (Hoefer) 12% (29:1) polyacrylamide gels by methods previously described (18). Gels were either stained for phosphoproteins or total protein or transferred to membranes for Western blot as described in the figure legends. Phosphoproteins were identified by ProQ Diamond phosphoprotein staining (Invitrogen) and imaged on a Typhoon 9410 imager (GE Healthcare) with an excitation of 532 nm and 580BP30 emission filter according to the manufacturer's protocol. Total protein was visualized by Coomassie and digitized or Sypro Ruby (Invitrogen) staining imaged on a Typhoon 9410 (GE Healthcare) with an excitation of 457 nm and a 610BP30 emission filter (18). Western blot was conducted by wet transfer (Hoefer) of proteins to 0.45 μm PVDF at 10 °C for 90 min at 90 V as previously described (18). Cardiac TnI Ser-150 phosphorylation was identified with the rabbit pTnI 150 antibody, detected by a horseradish peroxidase-conjugated rabbit secondary, and developed with ECL Plus (GE Healthcare) on Hyperfilm (GE Healthcare). Subsequently, total cTnI was detected after blocking by re-probing the same membrane with the mouse cTnI antibody C5 (Fitzgerald) and detected with an alkaline phosphatase anti-mouse secondary antibody incubated in 5-bromo-4-chloro-3-indolyl-phosphate and nitro blue tetrazolium. This sequential development using different primary/secondary combinations and development methods is critical to avoid signal bleed from the first Western to the second. Alternately, myofibril gels probed for pTnI 150 were transferred to 0.22-μm low fluorescence PVDF (GE Healthcare) for fluorescent Dylight secondary (Jackson ImmunoResearch Laboratories) detection on a Typhoon 9410 imager (GE Healthcare).
Two-dimensional Isoelectric Focusing
Cardiac TnI was analyzed by two-dimensional isoelectric focusing on 18-cm 7–11 IPG strips (GE Healthcare) by methods previously described (18), except the first dimension of focusing was carried out on an Agilent 3100 OffGel fractionator (Agilent) run in the “in-gel” method. Resultant strips were separated in the second dimension on 18 × 8-cm (Hoefer) 12% (29:1) gels.
Quantification of cTnI Ser-150 Phosphorylation in Normal Heart
The percent of cTnI Ser-150 phosphorylated was determined by comparing a Western blot of rabbit and rat heart myofibrils to a maximally phosphorylated cTnI Ser-150 human cTn standard sample determined in a time course AMPK treatment probed with the pTnI 150 antibody. Unknown myofibrils and the Ser-150 maximally phosphorylated standard were run on the same gel, and Ser-150 phosphorylation was determined by Western blot with the pTnI 150 antibody. After total cTnI detection by differential fluorescent secondary antibody and quantification using ImageQuant TL 7.0 (GE Healthcare), the percent total cTnI Ser-150 phosphorylation was determined as (sample pTnI 150 signal/sample total cTnI)/(standard pTnI 150 signal/standard total cTnI) × 100.
Exchange of Recombinant cTn into Skinned Mouse Cardiac Bundles
Left ventricular papillary muscles were dissected from hearts of mice anesthetized with sodium pentobarbital as previously described (19). After dissection, muscles were cut in uniform strips of no greater than 150 μm in diameter and treated with 1% (v/v) Triton X-100 in relax buffer (41.89 mm potassium propionate, 10 mm EGTA, 10 mm creatine phosphate, 6.22 mm ATP, 6.57 mm MgCl2, 5 mm NaN3, and 100 mm BES, pH 7.0) at 4 °C for 4 h to extract membranes (skinned). The endogenous cTn from the resultant fiber bundles was then exchanged for exogenous cTn by overnight incubation at 4 °C in 13 μm recombinant cTn (14). Exogenous and endogenous TnT migrate at different mobilities when separated by SDS-PAGE; therefore, the percent of exogenous cTn exchange to total fiber cTn was determined in each fiber by Western blot for TnT with the mouse TnT monoclonal antibody CT3 (Developmental Studies Hybridoma Bank) as previously described (14). Animal care and use was performed in accordance with the guidelines of the Institutional Animal Care and Use Committee at the University of Illinois at Chicago and The Ohio State University.
Measurement of Isometric Tension
The measurement of steady-state isometric tension as a function of free Ca2+ was conducted as previously described (19). Fiber measurements to determine skinned fiber mechanical parameters were conducted at a sarcomere length of 2.2 μm as determined by laser diffraction, whereas fiber measurements to determine length-dependent activation were conducted at sarcomere lengths of 1.9 and 2.2 μm in the same fiber (20, 21). Fiber bundles were activated over a range of free Ca2+ concentrations to determine steady-state isometric tension. Only muscles that maintained greater then 85% maximal tension were included for analysis. After the mechanical experiment, each bundle was briefly dried and stored frozen for biochemical analysis.
Measurement of Thin Filament Steady-state Ca2+ Binding to TnC
Steady-state thin filament Ca2+ binding to TnC was measured as previously described (17). Briefly, thin filaments were reconstituted with various cTnI in the presence of 2-(4′-iodoacetamidoanilo)naphthalene-6-sulfonic acid (IAANS)-labeled C35S, C84S, and T53C TnC, and IAANS fluorescence was measured at various free Ca2+ concentrations as indicative of Ca2+ binding to TnC.
Data Processing and Statistical Analysis
Tension-Ca2+ relationships and steady-state Ca2+ binding were fit to a modified Hill equation to determine 50% maximal binding and Hill coefficient. PKA phosphorylation of cTn over time was fit with a single exponential to determine time to 50% maximal phosphorylation. Results of skinned fiber force measurements, length-dependent activation, and Ca2+ binding were compared by ANOVA with the Bonferroni post-hoc evaluation. Cardiac Tn phosphorylation was compared by Student's t test. A p < 0.05 was considered statistically significant. Data are presented as the mean ± S.E.
RESULTS
AMPK Holoenzyme Complex Phosphorylates cTnI at Ser-150
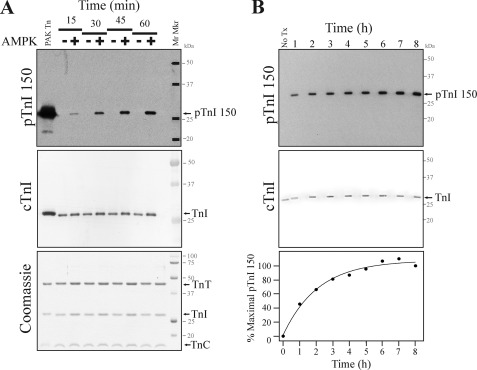
As a tool to establish the relevance of cTnI Ser-150 phosphorylation, we generated a custom antibody that specifically recognizes cTnI only when phosphorylated at Ser-150 (pTnI 150; data are reported in supplemental Figs. 1 and 2). To investigate the role of the AMPK holoenzyme to phosphorylate cTnI, we first sought to determine the effect of the functional AMPK holoenzyme (composed of α1/β1/γ2 subunits) to phosphorylate cTnI Ser-150 in purified recombinant human cTn in vitro. A Western blot with the pTnI 150 antibody demonstrates the time-dependent appearance of a single 25-kDa band after AMPK treatment that was of similar mobility to controls phosphorylated at Ser-150 with PAK (Fig. 1A). Subsequent re-probing of the membrane with a total cTnI antibody imaged by varied detection methods demonstrates the 25-kDa band is cTnI. Coomassie staining of an identical gel further demonstrates similar loading and integrity of the cTn complex. Next we conducted AMPK treatment over an extended time course to characterize AMPK holoenzyme phosphorylation of cTnI Ser-150 to saturation. Results in Fig. 1B demonstrate the phosphorylation of cTnI Ser-150 reached a maximum at 6 h without additional increased phosphorylation upon further addition of the AMPK holoenzyme. Similar to the α1/β1/γ2 AMPK holoenzyme, the α2/β1/γ2 holoenzyme also induced the time-dependent Ser-150 phosphorylation of cTnI (data not shown). These findings demonstrate cTnI Ser-150 in isolated cTn is a target of the AMPK holoenzyme.
FIGURE 1.
The AMPK holoenzyme complex phosphorylates cTnI Ser-150 in purified Tn. Recombinant human cTn was incubated with the AMPK holoenzyme (α1/β1/γ2). A, Tn incubated in the presence (+) or absence (-) of AMPK was stopped at 15-min intervals. Western blot with the pTnI 150 antibody that only recognizes cTnI when Ser-150 is phosphorylated demonstrates cTnI is phosphorylated by AMPK at Ser-150 in a time-dependent manner. Re-probing the same membrane with a cTnI antibody employing differential development and Coomassie staining demonstrates similar loading and integrity of the cTn samples. B, Tn was incubated in the presence of AMPK, and the reaction was stopped at 1-h intervals. At 6 h 25% more AMPK was added, and incubation was continued for an additional 2 h. A Western blot with the pTnI 150 antibody demonstrates AMPK phosphorylates Tn at Ser-150 to saturation at similar loading. The plot of percent maximal pTnI 150 phosphorylation over time demonstrates Ser-150 reached maximal phosphorylation at 6 h and was not further increased by the addition of more AMPK.
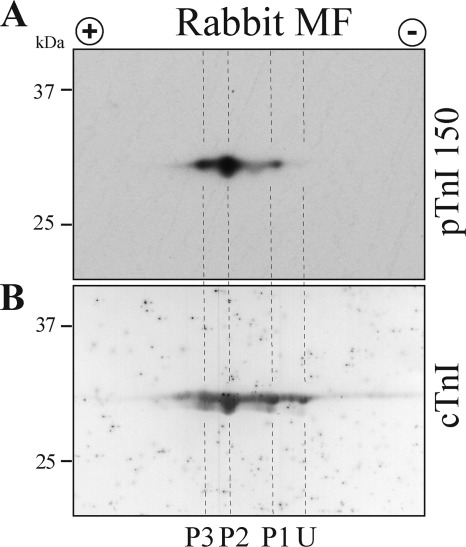
To establish the significance of cTnI Ser-150 phosphorylation in the normal heart, we conducted a Western blot of rabbit cardiac tissue separated by two-dimensional isoelectric focusing. A two-dimensional fractionated Western blot of rabbit ventricular myofibrils with the pTnI 150 antibody demonstrates three cTnI species with Ser-150 phosphorylation (Fig. 2A). After stripping, the membrane was re-probed with a total cTnI antibody. Differential detection of the total cTnI antibody identified four cTnI species (Fig. 2B). Alignment of the membranes demonstrates the pTnI 150 antibody solely recognized only the three most acidic spots (P1-P3) and not the most basic, unphosphorylated spot (U). Using the maximally Ser-150-phosphorylated sample from our AMPK treatment time course as a standard, we investigated the extent of cTnI Ser-150 phosphorylation in rabbit myofibril samples. By this method we determined normal rabbit myofibrils are Ser-150 phosphorylated at about 3% of total cTnI. These results demonstrate cTnI is endogenously phosphorylated at Ser-150 in the normal rabbit heart.
FIGURE 2.
Native rabbit cardiac muscle contains cTnI phosphorylated at Ser-150. A, Western blot of two-dimensional isoelectric focusing-separated rabbit ventricular myofibrils probed with the pTnI 150 antibody identified three cTnI species as containing Ser-150 phosphorylation (P1, P2, and P3). B, sequential probing of the same membrane with a total cTnI antibody identified four cTnI species. Alignment of the two membranes demonstrates the three most acidic, phosphorylated cTnI species were detected by the pTnI 150 antibody as containing Ser-150 phosphorylation without detection of the non-phosphorylated, basic species (U).
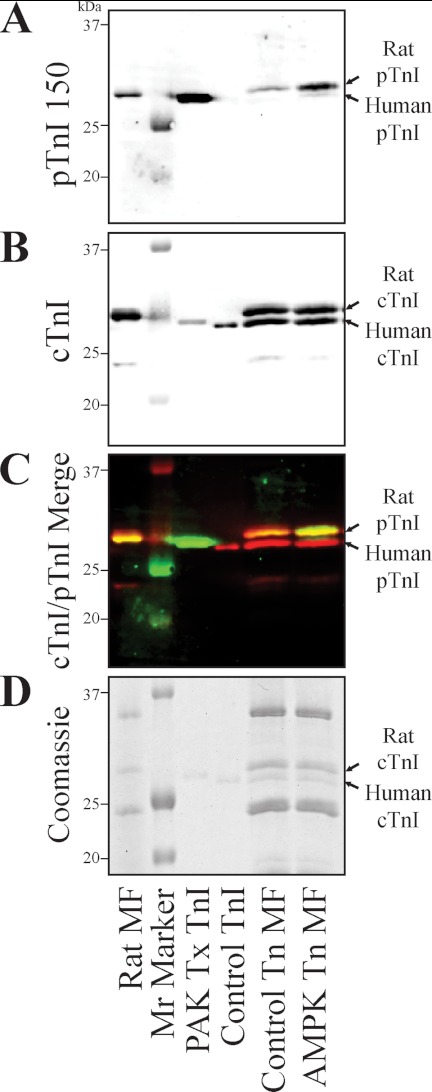
Next we sought to determine if the AMPK holoenzyme would phosphorylate cTnI at Ser-150 in the myofilament lattice. Employing similar methods described above, we first determined Ser-150 phosphorylation accounts for about 3% of the total cTnI in myofibrils from the normal rat heart. Endogenous cTn of rat myofibrils was then partially exchanged with purified, non-phosphorylated recombinant human cTn and treated with the AMPK holoenzyme complex (consisting of α1/β1/γ2 subunits). Western blot with a total cTnI antibody demonstrates that exogenous recombinant human cTnI migrates slightly faster than the endogenous rat cTnI (Fig. 3B). Taking advantage of this migration difference, quantification of the two cTnI species demonstrates myofibril exchange resulted in 46% recombinant human cTn that was similarly loaded in both sham (Control Tn MF) and AMPK-treated cTn-exchanged myofibrils (AMPK Tn MF). Sequential differential detection of Ser-150 phosphorylation with the pTnI 150 antibody demonstrates non-exchanged rat myofibrils (Rat MF) exhibit endogenous cTnI Ser-150 phosphorylation that was decreased after the exchange protocol (Control Tn MF) (Fig. 3A). After incubation with AMPK, endogenous rat cTnI Ser-150 phosphorylation was increased by 3.4 times, whereas exogenous human cTnI Ser-150 phosphorylation was also increased (Fig. 3A). Differential fluorescent Western imaging allowed for simultaneous detection of the pTnI 150 and total cTnI antibodies. Merge of the two antibody signals demonstrates pTnI 150 (green), and total cTnI (red) identified the same band (yellow) in the AMPK Tn MF sample (Fig. 3C). Note that AMPK-phosphorylated cTnI exhibits a slightly slower migration similar to that previously demonstrated to result from PAK Ser-150 phosphorylation (22). The AMPK cTnI Ser-150 phosphorylation in myofibrils was further verified by Western blots probed solely with the pTnI 150 antibody detected by enhanced chemiluminescence (data not shown). These findings demonstrate cTnI Ser-150 is endogenously phosphorylated in the rat heart, and AMPK further phosphorylates Ser-150 in the native muscle lattice.
FIGURE 3.
The AMPK holoenzyme phosphorylates cTnI Ser-150 in the cardiac muscle lattice. Rat endogenous cTn in ventricular myofibrils was partially exchanged with recombinant human cTn and incubated in the absence (Control Tn MF) or presence of the AMPK holoenzyme (AMPK Tn MF). A, myofibrils probed by Western blot with the pTnI 150 antibody demonstrates both the remaining endogenous cTnI and the exogenous exchanged human cTnI exhibited increased Ser-150 phosphorylation in the AMPK-treated myofibrils without antibody recognition of non-phosphorylated recombinant cTn (Control Tn). Western also identifies untreated, native rat cardiac myofibrils (Rat MF) as containing endogenous cTnI Ser-150 phosphorylation. B, simultaneous identification of total cTnI by differential detection identified two cTnI bands in exchanged myofibrils of similar size to endogenous rat and exogenous human cTnI at similar loading. C, merge of the pTnI 150 (green) and total cTnI (red) signals demonstrates the pTnI 150 band is identical in size to that of the total cTnI (overlap yellow). D, identical Coomassie-stained gel demonstrates similar loading and integrity of the samples. Mr Marker, molecular mass marker; PAK Tx TnI, Ser-150-phosphorylated human cTnI; Control TnI, unphosphorylated recombinant human cTnI.
cTnI Ser-150 Phosphorylation Increases Myofilament Ca2+-sensitive Force Development and Blunts PKA-dependent Ca2+ Desensitization
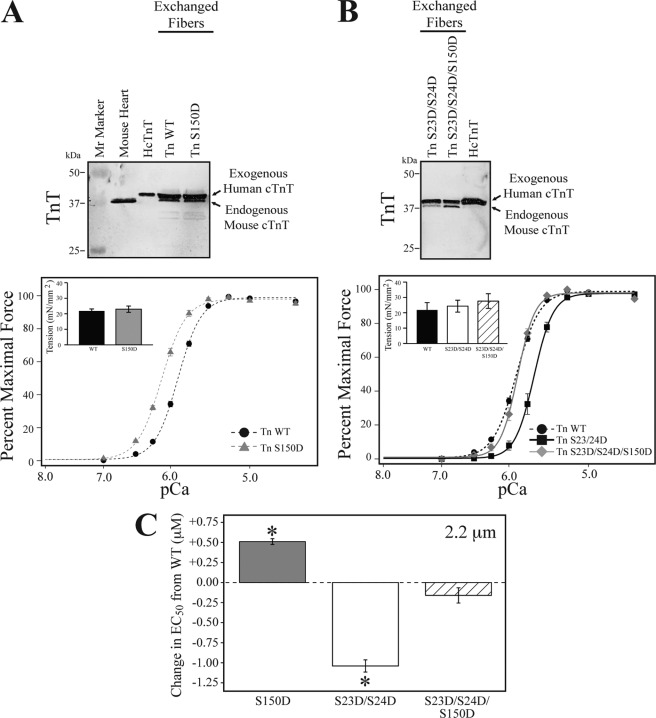
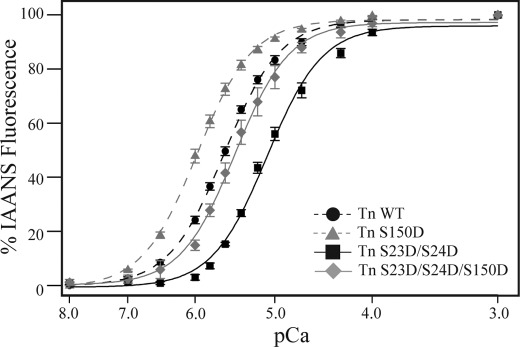
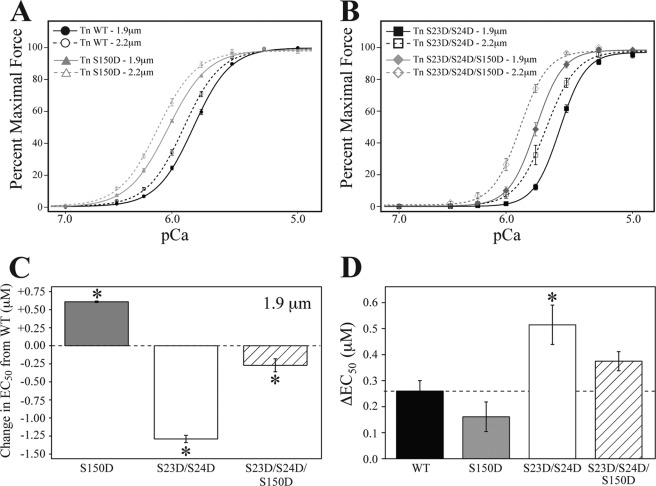
To determine the effect of cTnI Ser-150 phosphorylation on the regulation of force development, we exchanged the endogenous cTn in mouse skinned cardiac fiber bundles with either recombinant wild type (Tn WT) or Tn containing Ser-150 pseudo-phosphorylated cTnI (Tn S150D). Exchange efficiency of each fiber bundle after force measurements was evaluated by Western blot for exogenous human TnT. The results demonstrate recombinant cTn exchange averaged 70 ± 5% (Fig. 4). This percent exchange was the same for all the cTn exchange groups in the study. After exchange, fiber bundles were subjected to force-Ca2+ measurements at a sarcomere length of 2.2 μm. The results in Fig. 4A demonstrate that fibers exchanged with Tn S150D exhibit a significant 0.51 μm increase in EC50 compared with that of fibers exchanged with Tn WT (EC50; Tn WT = 1.27 ± 0.03, Tn S150D = 0.76 ± 0.02; Fig. 4, A and C, and Table 1). This change in Ca2+ sensitivity occurred in the absence of altered maximal tension development or Hill coefficient (Table 1). Thus, cTnI Ser-150 pseudophosphorylation significantly increases the ability of submaximal Ca2+ to activate the thin filament, resulting in increased force development at a given submaximal Ca2+ concentration.
FIGURE 4.
Cardiac TnI Ser-150 phosphorylation increases myofilament Ca2+-sensitive force development and blunts cTnI PKA dependent desensitization. Skinned mouse cardiac fiber bundles were exchanged with human cTn containing either wild-type (Tn WT), Ser-150 (Tn S150D), Ser-23/24 (Tn S23D/S24D), or combined Ser-150 and PKA (Tn S23D/S24D/S150D) pseudo-phosphorylated cTnI. Fiber bundles from individual force experiments were analyzed for cTn exchange by Western blot with a TnT antibody. Representative fibers demonstrate an average of 67% incorporation of the larger molecular mass, exogenous human TnT band that was not different in any of the exchanged groups. A, average tension-Ca2+ measurements of fiber bundles at 2.2 μm exchanged with Tn S150D (gray triangle, dashed line) exhibit increased Ca2+ sensitivity compared with Tn WT (black circles, dashed line) exchange in the absence of an effect on Hill coefficient or maximal force development (inset). B, fiber bundles exchanged with Tn S23D/S24D (black square, solid line) demonstrate the expected decrease in Ca2+ sensitivity compared with Tn WT. Exchange of the cTnI Tn S23D/S24D/S150D (gray diamond, solid line) did not exhibit altered Ca2+ sensitivity compared with Tn WT; however, Tn S23D/S24D/S150D Ca2+ sensitivity was increased compared with that of Tn S23D/S24D exchange. Tn S23D/S24D/S150D exchange did not alter the Hill coefficient or maximal force development (inset). C, the change in EC50 at 2.2 μm of the various exchanged cTn from that of WT demonstrates Tn S150D (gray bar) increased EC50 by 0.51 μm from Tn WT, whereas combination of S150D with S23D/S24D phosphorylation (hatched bar) was not different from Tn WT and blunted the −1.05 μm decrease in EC50 of cTnI S23D/S24D (white bar) exchange alone. Gray, Tn S150D; white, Tn S23D/S24D; hatched, Tn S23D/S24D/S150D. *, ANOVA Bonferroni EC50 p < 0.05 versus WT.
TABLE 1.
Mechanical characteristics of exchanged fiber bundles
Mechanical characteristics of skinned mouse cardiac fiber bundles exchanged with human cTn-containing wild type (Tn WT), Ser-150-pseudophosphorylated cTnI (Tn S150D), Ser-23/24-pseudophosphorylated cTnI (Tn S23D/S24D), or combined Ser-23/24- and Ser-150-pseudophosphorylated cTnI (Tn S23D/S24D/S150D). Values are the mean ± S.E. Fmax, maximal tension development in mN/mm2; EC50, the Ca2+ concentration in μm at 50% maximal force; Hill, slope of the tension-Ca2+ plot; ΔEC50, the difference in the calcium concentration at 50% maximal force from 1.9 to 2.2 μm sarcomere length; n, number of fibers in each group.
| Tn exchanged | 1.9 μm |
2.2 μm |
ΔEC50 | n | ||||
|---|---|---|---|---|---|---|---|---|
| Fmax | EC50 | Hill | Fmax | EC50 | Hill | |||
| Tn WT | 21.2 ± 1.0 | 1.53 ± 0.03a (P,PP,PPP) | 2.85 ± 0.10a (PP,PPP) | 21.6 ± 1.3 | 1.27 ± 0.03a (P,PP) | 2.84 ± 0.12a (PP,PPP) | 0.26 ± 0.04a (PP) | 7 |
| Tn S150D | 22.8 ± 2.2 | 0.92 ± 0.01a (W,PP,PPP) | 2.50 ± 0.10a (PP,PPP) | 24.8 ± 2.4 | 0.76 ± 0.02a (W,PP,PPP) | 2.65 ± 0.17a (PP,PPP) | 0.16 ± 0.06a (PP,PPP) | 7 |
| Tn S23D/S24D | 20.5 ± 2.6 | 2.82 ± 0.05a (W,P,PPP) | 4.47 ± 0.17a (W,P) | 26.3 ± 4.5 | 2.31 ± 0.07a (W,P,PPP) | 3.85 ± 0.12a(W,P) | 0.52 ± 0.08a (W,P) | 8 |
| Tn S23D/S24D/S150D | 23.7 ± 2.1 | 1.80 ± 0.09a (W,P,PP) | 4.18 ± 0.29a (W,P) | 30.7 ± 4.0 | 1.43 ± 0.09a (P,PP) | 3.87 ± 0.18a (W,P) | 0.38 ± 0.04a (P) | 9 |
a ANOVA = p < 0.05; W, significantly different vs. Tn WT; P, significantly different vs. Tn S150D; PP, significantly different vs. Tn S23D/S24D; PPP, significantly different vs. S23D/S24D/S150D.
Previously we demonstrated that single amino acid residue modifications within cTn can alter the effects of cTnI PKA phosphorylation function (14, 23). To investigate Ser-150 phosphorylation cross-talk on the effect of cTnI PKA phosphorylation, we exchanged mouse cardiac fiber bundles with human recombinant cTn containing cTnI pseudo-phosphorylated at the PKA sites (cTn S23D/S24D) or cTnI pseudo-phosphorylated at both the PKA and Ser-150 sites (cTn S23D/S24D/S150D). As expected, exchange of cTn S23D/S24D decreased Ca2+ sensitivity by 1.04 μm compared with Tn WT (Fig. 4, B and C, and Table 1) (14, 24). Upon combination with S150D, the Ca2+ sensitivity of Tn S23D/S24D/S150D-exchanged fibers was increased by 0.88 μm compared with Tn S23D/S24D exchange alone and was not different from Tn WT (EC50; Tn WT = 1.27 ± 0.03, Tn S23D/S24D = 2.31 ± 0.07, Tn S23D/S24D/S150D = 1.43 ± 0.09; Fig. 4, B and C, and Table 1). The combined Tn S23D/S24D/S150D fibers exhibited normal maximal tension development and Hill coefficient (Table 1). These data demonstrate cTnI Ser-150 phosphorylation blunts the Ca2+-desensitizing effects of PKA-dependent phosphorylation.
cTnI Ser-150 Phosphorylation Increases Ca2+ Sensitivity by Altering Ca2+ Binding to TnC
To investigate the mechanism of the cTnI Ser-150 phosphorylation-induced increase in Ca2+-dependent force and its combined effect to blunt PKA-dependent Ca2+ desensitization, we measured Ca2+ binding to TnC in reconstituted thin filaments. Similar to force development, thin filaments reconstituted with Tn S150D increased Ca2+ affinity compared with reconstitution with Tn WT (EC50; Tn WT = 2.5 ± 0.1, Tn S150D = 1.03 ± 0.16; Fig. 5 and Table 2). As expected, Tn S23D/S24D reconstitution decreased Ca2+ binding to TnC. This PKA-induced decrease in Ca2+ binding was blunted upon combination with Ser-150 pseudophosphorylation such that Ca2+ binding of Tn S23D/S24D/S150D was no longer different from Tn WT (EC50; Tn S23D/S24D = 8.3 ± 0.6, Tn S23D/S24D/S150D = 3.7 ± 0.8; Fig. 5 and Table 2). These data demonstrate Ser-150 phosphorylation increases Ca2+-dependent force development and blunts the Ca2+-desensitizing effects of cTnI PKA-dependent force desensitization through a mechanism directly influencing Ca2+ binding to TnC in the thin filaments.
FIGURE 5.
The cTnI Ser-150 phosphorylation-induced increase in submaximal force development results from increased Ca2+ binding to TnC. Steady-state Ca2+ binding to TnC was determined by IAANS-labeled TnC fluorescence of thin filaments reconstituted with cTn containing either wild-type (Tn WT; black circle, black dashed line), Ser-150 (Tn S150D; gray triangle, gray dashed line), PKA (Tn S23D/S24D; black square, black solid line), or combined Ser-150 with PKA (Tn S23D/S24D/S150D; gray diamond, gray solid line) pseudophosphorylated cTnI. Similar to Ca2+-regulated force development, Tn S150D-reconstituted thin filaments exhibit increased Ca2+ binding to TnC compared with WT. Calcium binding in thin filaments containing Tn S23D/S24D was decreased, whereas the combined Tn S23D/S24D/S150D filaments were not different from WT.
TABLE 2.
Thin filament TnC Ca2+ binding characteristics
Binding characteristics of Ca2+ to TnC in thin filaments reconstituted with human cTn containing wild type (Tn WT), Ser-150-pseudo-phosphorylated cTnI (Tn S150D), Ser-23/24-pseudophosphorylated cTnI (Tn S23D/S24D), or combined Ser-23/24- and Ser-150-pseudophosphorylated cTnI (Tn S23D/S24D/S150D). Values are the mean ± S.E. EC50, the Ca2+ concentration in μm at 50% maximal binding; Hill, slope of the binding-Ca2+ plot; n, number of fibers in each group.
| Thin filament Tn | EC50 | Hill | n |
|---|---|---|---|
| Tn WT | 2.52 ± 0.10a (P,PP) | 2.49 ± 0.08a (PP,PPP) | 10 |
| Tn S150D | 1.03 ± 0.16a (W,PP,PPP) | 1.07 ± 0.03a (PP,PPP) | 9 |
| Tn S23D/S24D | 8.33 ± 0.60a (W,P,PPP) | 8.39 ± 0.10a (W,P) | 5 |
| Tn S23D/S24D/S150D | 3.69 ± 0.80a (P,PP) | 3.47 ± 0.13a (W,P) | 4 |
a ANOVA Bonferroni p < 0.05; W, significantly different vs. Tn WT; P, significantly different vs. Tn S150D; PP, significantly different vs. Tn S23D/S24D; PPP, significantly different vs. S23D/S24D/S150D.
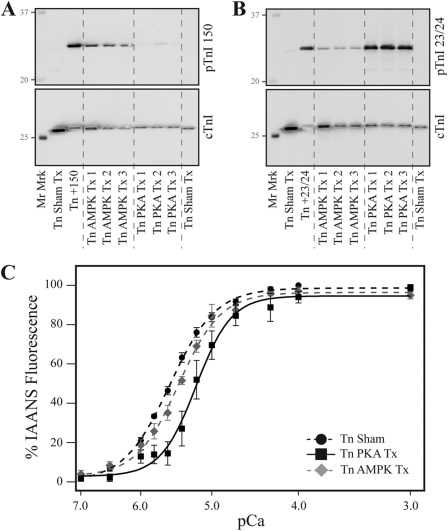
To validate our findings using the pseudo-phosphorylated cTnI, we treated recombinant human cTn separately with either PKA or AMPK before reconstituting thin filaments and the determination of Ca2+ binding to TnC. A representative Western blot of treated filaments after Ca2+ binding measurements demonstrated that PKA treatment of cTn for 20 min (Tn PKA Tx) induced significant cTnI Ser-23/24 native phosphate incorporation (Fig. 6B) with minimal native Ser-150 phosphorylation (Fig. 6A). Alternately, cTn AMPK treatment for 4 h (Tn AMPK Tx) induced significant levels of both Ser-150 and Ser-23/24 native phosphate incorporation (Fig. 6, A and B). Calcium binding to TnC in filaments reconstituted with PKA-treated cTn containing native Ser-23/24 phosphate demonstrated decreased Ca2+ affinity compared with filaments containing sham-incubated cTn as expected (EC50; Tn Sham Tx = 2.74 ± 0.10, n = 4; Tn PKA Tx = 7.03 ± 1.39, n = 4; p < 0.05; Fig. 6C). Importantly, Ca2+ binding affinity of thin filaments reconstituted with AMPK-treated cTn containing native phosphate at Ser-23/24/150 was not different from that of Sham Tn (EC50; Tn AMPK Tx = 3.72 ± 0.35, n = 4; Fig. 6C and Table 2). These data from experiments employing authentic phosphorylation of cTnI sites confirm findings with the pseudophosphorylated cTnI.
FIGURE 6.
The incorporation of native phosphate at cTnI Ser-150 blunts Ser-23/24-decreased Ca2+ binding to TnC. Recombinant human Tn was treated with PKA or AMPK and reconstituted into thin filaments to determine Ca2+ binding, and cTnI phosphorylation was determined. A, a representative Western blot of filaments with the pTnI 150 antibody demonstrates AMPK incorporation of native phosphate at Ser-150. B, a representative Western blot of filaments with the pTnI 23/24 antibody demonstrates that both AMPK and PKA induce Ser-23/24 phosphorylation at similarly loaded cTnI. C, steady-state Ca2+ binding to TnC demonstrates filaments reconstituted with PKA-treated Tn (Tn PKA Tx; black square, black solid line) exhibited decreased Ca2+ binding affinity to TnC compared with sham-treated fibers (Tn Sham; black circle, black dashed line), whereas Ca2+ binding was not different between filaments containing native phosphate at both the Ser-23/24 and Ser-150 (Tn AMPK Tx; gray diamond, gray dashed line) and sham-treated filaments. Mr Marker, molecular mass marker; Tn +150, cTnI Ser-150 phosphorylated positive control; Tn+23/24, cTnI Ser-23/24-positive control.
cTnI Ser-150 Phosphorylation Alone Does Not Alter Length-dependent Activation but Blunts cTnI PKA-dependent-induced Length-dependent Activation
The Ca2+ sensitivity of force development is altered by sarcomere length (20). To further investigate cTnI Ser-150 phosphorylation-induced Ca2+ sensitization, we measured the Ca2+-regulated force development at short (1.9 μm) and long (2.2 μm) sarcomere lengths in the same fiber bundle. At the short sarcomere length of 1.9 μm, the Ca2+ sensitivity of fibers exchanged with Tn S150D remained increased compared to Tn WT (EC50 at 1.9 μm; Tn WT = 1.53 ± 0.03; Tn S150D = 0.92 ± 0.01; Fig. 7, A and C, and Table 1). Similar to the 2.2-μm measurements, the exchange of Tn S23D/S24D decreased Ca2+ sensitivity at 1.9 μm compared with WT (EC50 at 1.9 μm; Tn S23D/S24D = 2.82 ± 0.05; Fig. 7, B and C, and Table 1). However, unlike at 2.2 μm, Ca2+ sensitivity at 1.9 μm of the combined Tn S23D/S24D/S150D exchange was slightly decreased from that of Tn WT (EC50 at 1.9 μm; Tn S23D/S24D/S150D = 1.80 ± 0.09; Fig. 7, B and C, and Table 1). Importantly, cTn S23D/S24D/S150D Ca2+ sensitivity was increased compared with Tn S23D/S24D exchange; however, this blunting of the PKA effect was less effective at 1.9 compared with 2.2 μm (difference in EC50 from Tn WT to Tn S23D/S24D/S150D; at 1.9 μm = 0.27 μm, at 2.2 μm = 0.16 μm). Another way to evaluate the effect of sarcomere length on Ca2+ sensitivity is through length-dependent activation by calculating the difference in half-maximally activating free Ca2+ from 1.9 to 2.2 μm sarcomere lengths (ΔEC50) (20, 21). In the case of length-dependent activation, the exchange of Tn S150D by itself did not alter ΔEC50, whereas exchange of the Tn S23D/S24D PKA pseudophosphorylation increased ΔEC50 by 2-fold compared with Tn WT (ΔEC50; Tn WT = 0.26 ± 0.04, Tn S150D = 0.16 ± 0.06, Tn S23D/S24D = 0.52 ± 0.08; Fig. 7D and Table 1). The combination of cTnI Ser-150 and PKA pseudophosphorylation blunted this PKA-induced length-dependent effect such that the ΔEC50 of Tn S23D/S24D/S150D fibers was not different from that of Tn WT (ΔEC50; Tn S23D/S24D/S150D = 0.38 ± 0.04; Fig. 7D and Table 1). These differences in the ability of the combined Tn S23D/S24D/S150D to blunt PKA-dependent Ca2+ desensitization at varied lengths suggests cTnI Ser-150 phosphorylation affects myofilament Ca2+ sensitivity by a mechanism that includes more than just Ca2+ binding to TnC.
FIGURE 7.
Cardiac TnI Ser-150 phosphorylation blunts the cTnI PKA-dependent increase in length-dependent activation. Skinned mouse cardiac fiber bundles were exchanged with human cTn containing either wild-type (Tn WT), Ser-150 (Tn S150D), Ser-23/24 (Tn S23D/S24D), or combined Ser-150 and PKA (Tn S23D/S24D/S150D) pseudo-phosphorylated cTnI- and Ca2+-dependent force development determined at 1.9 and 2.2 μm in the same fiber. A, average tension-Ca2+ measurements at sarcomere lengths of 1.9 and 2.2 μm demonstrate that the Ca2+ sensitivity of Tn S150D (gray triangle) exchanged fibers remains increased at both 1.9 (solid line) and 2.2 μm (dashed line) compared with Tn WT (black circle). B, similarly, the Ca2+ sensitivity of Tn S23D/S24D/S150D (gray diamond) exchanged fibers remained increased at both 1.9 and 2.2 μm compared with Tn S23D/S24D (black square) exchange. C, the change in EC50 of the various exchanged cTn from that of WT at 1.9 μm demonstrates Tn S150D (gray bar) increased EC50 by 0.61 μm from Tn WT, whereas combination of S150D with S23D/S24D phosphorylation (hatched bar) blunted the −1.29 μm decrease in EC50 of cTnI S23D/S24D (white bar) exchange alone. D, comparison of the change in half-maximal free activating Ca2+ from 1.9 to 2.2 μm (ΔEC50) demonstrates length-dependent activation of Tn S150D (gray bar) exchanged fiber bundles was not different from Tn WT (black bar). Although Tn S23D/S24D (white bar) exchange increased ΔEC50, the exchange of Tn S23D/S24D/S150D (hatched bar) was not different from that of Tn WT, demonstrating a Ser-150-induced blunting of the PKA-dependent increase in length-dependent activation. *, ANOVA Bonferroni p < 0.05 versus WT.
cTnI Ser-150 Phosphorylation Does Not Alter cTnI PKA Phosphorylation
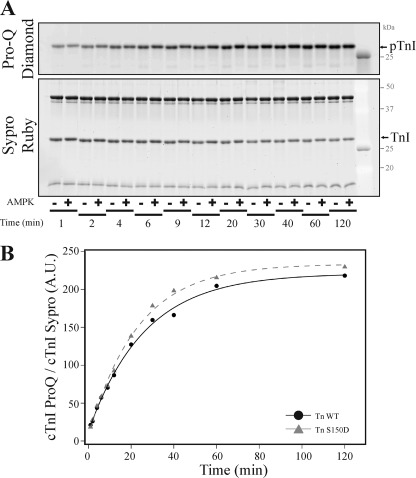
Finally we sought to investigate if cTnI Ser-150 phosphorylation affects upstream PKA function by affecting the ability of PKA to phosphorylate cTnI when in the cTn complex. Troponin containing WT or S150D cTnI was incubated with the catalytic subunit of PKA, and the reaction was stopped at varied time points. The resulting time-dependent phosphorylation of cTnI detected by ProQ Diamond phosphoprotein staining normalized to total cTnI demonstrates no significant difference in the rate that PKA phosphorylates either Tn WT or Tn S150D (time to 50% maximal phosphorylation; WT = 19.57 ± 1.17, Tn S150D = 18.43 ± 3.56; p > 0.05) (Fig. 8). This finding demonstrates cTnI S150D pseudophosphorylation does not affect the ability of PKA to phosphorylate cTnI when incorporated into the cTn complex.
FIGURE 8.
Cardiac TnI Ser-150 phosphorylation does not alter cTnI PKA-dependent phosphorylation. Recombinant human cTn containing wild-type or Ser-150 pseudophosphorylated cTnI (Tn S150D) was incubated with the PKA catalytic subunit for varied times before stopping the reaction. A, phosphorylation of cTnI detected by Pro-Q Diamond phosphoprotein-specific stained gels demonstrates the time-dependent increase in cTnI phosphorylation. After Pro-Q Diamond staining, gels were subjected to Sypro Ruby staining to determine total cTnI amount. B, fit of cTnI phosphorylation normalized to total cTnI demonstrates PKA-dependent phosphorylation of Tn S150D (black circle, solid line) over time was not different from that of Tn WT (gray triangle, dashed line). A.U., absorbance units.
DISCUSSION
The major findings of our studies include the following. 1) The AMPK holoenzyme phosphorylates cTnI at Ser-150. 2) Cardiac TnI Ser-150 is endogenously phosphorylated in normal rat and rabbit hearts. 3) The phosphorylation of cTnI Ser-150 increases myofilament Ca2+ sensitivity and blunts the Ca2+ desensitization induced by PKA Ser-23/24 phosphorylation of cTnI. 4) Cardiac TnI Ser-150 phosphorylation alone does not affect length-dependent activation but blunts the amplification of length-dependent activation by PKA phosphorylation of cTnI Ser-23/24.
AMPK cTnI Ser-150 Phosphorylation
Recent work demonstrated that AMPK associates with cTnI and further that an AMPK fragment phosphorylates this protein at Ser-150 (10, 11). To date the ability of the AMPK holoenzyme to phosphorylate cTnI has been unknown. AMPK is a heterotrimeric serine/threonine-protein kinase with the physiologically active AMPK holoenzyme consisting of a catalytic α subunit in complex with regulatory γ and β subunits (for review, see Refs. 2 and 26). The γ subunit contains regulatory nucleotide binding motifs, whereas the β subunit exhibits scaffold-like properties potentially localizing the AMPK catalytic α subunit. Sancho Solis et al. (11) demonstrated that the isolated AMPK α subunit kinase domain fragment was sufficient to phosphorylate cTnI at Ser-150. Our current data extends this finding, demonstrating that the physiologically relevant AMPK holoenzyme phosphorylates cTnI Ser-150 in vitro (Fig. 1) as well as within the myofilament lattice (Fig. 3). These findings establish the role of the intact AMPK α subunit as a signaling molecule that can phosphorylate cTnI Ser-150 when in complex with the regulatory β and γ subunits. The cTnI Ser-150 residue is known to be phosphorylated in vitro after kinase treatment with PKA (27), PAK (22), and AMPK (10, 11). Of these kinases, AMPK is likely the most physiologically relevant kinase to phosphorylate this site in vivo. Cardiac TnI Ser-150 represents a poor substrate for PKA (Fig. 6; Refs. 11 and 22). Likewise, PAK phosphorylates cTnI in vitro; however, in vivo PAK overexpression leads to the dephosphorylation of cTnI rather than direct phosphorylation (28). Although the ability of native AMPK to phosphorylate cTnI in vivo is currently unknown, Ser-150 phosphorylation is increased in a mouse cardiac hypertrophy model (12) and isolated perfused hearts treated with the AMPK-activating drug AICAR (5-aminoimidazole-4-carboxamide ribonucleotide) (29). These results support a direct role for AMPK as a signaling molecule to phosphorylate cTnI Ser-150 in cardiac muscle.
Effects of cTnI Ser-150 Phosphorylation on Ca2+ Regulation of Thin Filament
Cardiac TnI is central to regulation of the myosin interaction with actin, myocyte force development, and therefore, cardiac contractile dynamics (for review, see Ref. 6). The TnI C terminus surrounding Ser-150 is composed of two structural regions, the TnI inhibitory peptide (roughly composed of residues 129–148 (30)) and the TnI switch peptide (residues 148–164) (31). In the absence of Ca2+ binding to TnC, the TnI C terminus has a low probability of binding to the TnC N terminus, allowing the inhibitory peptide to bind actin, contributing to the inhibition of myosin interaction with actin (30, 32). Upon Ca2+ binding to TnC and exposure of the N-terminal binding site for TnI, the TnI inhibitory peptide is drawn from actin favoring binding of the switch peptide to TnC and stabilization of the TnC open, Ca2+ bound conformation (33). The balance between TnI inhibitory peptide binding to actin and switch peptide binding to TnC is critical to TnC Ca2+ binding and, therefore, regulation of myosin interaction with actin (17). Cardiac TnI Ser-150 is located in close proximity to the inhibitory peptide, leading us to hypothesize that the incorporation of a negatively charged phosphorylation at this site will weaken cTnI inhibitory peptide binding to actin, shift the cTnI C terminus away from its interaction with actin, and increase Ca2+-dependent force development. This hypothesis is supported by data demonstrating that the presence of cTn containing the negatively charged S150D-pseudophosphorylated cTnI in skinned fibers significantly increases Ca2+ sensitivity of force development (Figs. 4 and 7) and Ca2+ binding to TnC (Figs. 5 and 6). These findings are in agreement with others who have demonstrated that the treatment of skinned fibers with PAK (22) or cTnI S150E pseudophosphorylation exchange (13) increased thin filament Ca2+ sensitivity.
To probe the molecular mechanism of cTnI Ser-150 phosphorylation increased Ca2+-regulated force development, we investigated the Ca2+ binding to TnC. The binding of Ca2+ to TnC in the reconstituted thin filament is a direct measurement of Ca2+ ions ability to activate the filament in the absence of myosin. The increase in TnC Ca2+ binding of Tn S150D (Fig. 5) and native phosphate at Ser-150 (Fig. 6) demonstrates the negatively charged phosphate at Ser-150 directly affects the thin filament structure/function to alter Ca2+ binding in the absence of the thick filament. This direct effect of cTnI Ser-150 phosphorylation on thin filament Ca2+ binding occurs either through enhancement of switch peptide binding to TnC, stabilizing the TnC active state, or by shifting the balance of the cTnI C terminus binding away from actin. Data demonstrating Ser-150 pseudophosphorylation shortens the apparent distance between the TnI C terminus and cTnC (13, 34) support the latter, indicating Ser-150 phosphorylation shifts the cTnI inhibitory domain from actin toward that of TnC.
Previously we demonstrated the TnC Gly-159 to Asp mutation by itself did not alter Ca2+ regulation of force but blunted the cTnI PKA-dependent phosphorylation-induced Ca2+ desensitization, establishing the role of Tn modifications to feed back on cTnI PKA function (14). At the molecular level the PKA-dependent phosphorylation of the cTnI Ser-23/24 residues alter the interaction of the cTnI N terminus with TnC, destabilizing the TnC open conformation and desensitizing the thin filament to Ca2+ (35, 36). Cardiac TnI Ser-150 is located in close proximity to Ser-23 and Ser-24 in the cTn complex (37) and, therefore, may modulate the effect of cTnI PKA phosphorylation. Our results demonstrate cTnI Ser-150 phosphorylation cross-talks within cTnI to blunt PKA-induced Ca2+ desensitization (Figs. 4–6) through a thin filament-mediated mechanism (Figs. 5 and 6). In the myofilament, Ca2+-sensitive force development is further altered by length (20). Unlike activation of the reconstituted thin filament that solely depends upon Ca2+ binding to TnC, the force change resulting from increased length-dependent activation entails other mechanisms of the myofilament. The finding that cTnI Ser-150 pseudophosphorylation is more effective in blunting PKA-dependent Ca2+ desensitization at 2.2 μm compared with 1.9 μm (Fig. 7) suggests that in addition to its effects on TnC Ca2+ binding (Figs. 5–6), cTnI Ser-150 phosphorylation also effects the myofilament Ca2+ sensitivity response to length. The differential effect of cTnI Ser-150 phosphorylation activation of the myofilament versus reconstituted thin filament is of great interest and deserves further future investigation.
AMPK Signaling and Modulation of Myofilament Cardiac Contractility
The role of AMPK to regulate energy substrate availability as a modulator of heart function has been established (2). We demonstrate for the first time that the classical metabolic role of AMPK is directly coupled to a mechanical effect on contraction at the level of the myofilament through the phosphorylation of cTnI Ser-150. In the cardiac myocyte, AMPK signaling induces a number of metabolic changes to increase energy and maintain cardiac contraction (2). During cardiac stress that results in decreased ATP availability, the increased AMP/ATP ratio activates the AMPK signaling pathway. Correspondingly, in the non-stressed heart the level of cTnI Ser-150 phosphorylation resulting from AMPK would be predicted to be low but increased after stress-induced alteration of the AMP/ATP ratio. Although we suggest cTnI Ser-150 phosphorylation is relatively low in the normal heart, cardiac stress can nearly double Ser-150 phosphorylation (12), and AMPK treatment increased Ser-150 phosphorylation in the muscle lattice by about 3.5 times (Fig. 3). Similar magnitude changes of cTnI post-translational modifications have previously been demonstrated to exhibit a significant effect on cardiac contractile function (38, 39). Such coupling of AMPK-increased energy substrate availability to a cTnI Ser-150-enhanced myofilament response to Ca2+ (Figs. 4–6) is beneficial to maintaining enhanced contractility and cardiac output during periods of cardiac stress without the enhanced energetic cost of increased Ca2+ cycling (40). Although the physiological role of AMPK-induced cTnI Ser-150 phosphorylation remains to be demonstrated, it is clear this pathway can constitute a significant myofilament level contractile regulatory mechanism.
The effects of AMPK signaling to blunt cTnI PKA-dependent function and enhance contraction would likely be counterproductive to the normal and necessary cTnI-induced Ca2+ desensitization of β-adrenergic stimulation. The localized regulation of the AMPK and PKA signaling pathways is, therefore, necessary to avoid such AMPK-induced blunting of PKA-dependent myofilament desensitization. Although our data demonstrate cTnI Ser-150 phosphorylation by itself does not directly alter the ability of PKA to phosphorylate cTnI (Fig. 8), a number of other regulatory mechanisms are likely in place to differentially regulate these two signaling pathways. Of significance, PKA stimulation in adipocytes directly decreases AMPK activation (41, 42). Although PKA regulation of AMPK signaling has not been investigated in the cardiomyocyte, such PKA-mediated regulation would inhibit AMPK signaling-induced blunting of the cTnI PKA desensitization. In addition to Ser O-linked phosphorylation, cTnI Ser-150 can also be modified by β-N-acetyl-d-glucosamine, which decreases myofilament Ca2+ sensitivity (25). Such glycosylation of Ser-150 would block O-phosphorylation at this residue and enhance PKA-dependent Ca2+ desensitization. To date the differential regulation of the PKA- and AMPK-signaling pathways in the heart has not yet been thoroughly investigated; however, it is likely there are mechanisms that differentially regulate these pathways at the myofilament.
Significance
Our data demonstrate cTnI Ser-150 is endogenously phosphorylated in the normal heart, likely by AMPK. This cTnI Ser-150 phosphorylation significantly increases myofilament Ca2+ sensitivity and cross-talks within cTnI to blunt myofilament PKA-dependent functional effects, resulting in uncoupling of the myofilament β-adrenergic response from other non-myofilament β-adrenergic functional effects (i.e. Ca2+ handling). We propose the AMPK signaling pathway functions directly at the level of the myofilament to link the metabolic response of the cardiomyocyte to myofilament level function.
Supplementary Material
Acknowledgments
The CT3 monoclonal antibody developed by J. J.-C. Lin was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD, National Institutes of Health, and maintained by the University of Iowa Dept. of Biology, IA City, IA 52242.
This work was supported, in whole or in part, by National Institutes of Health Grant T32 007692.

This article contains supplemental Figs. 1 and 2.
- AMPK
- AMP-activated protein kinase
- PAK
- p21-activated kinase
- Tn
- troponin
- cTn
- cardiac Tn
- pTnI
- phosphorylated TnI
- BES
- N,N-bis[2-hydroxyethyl]-2-aminoethane sulfonic acid
- IAANS
- 2-(4′-iodoacetamidoanilo)naphthalene-6-sulfonic acid
- ANOVA
- analysis of variance.
REFERENCES
- 1. Hardie D. G., Carling D. (1997) The AMP-activated protein kinase. Fuel gauge of the mammalian cell? Eur. J. Biochem. 246, 259–273 [DOI] [PubMed] [Google Scholar]
- 2. Arad M., Seidman C. E., Seidman J. G. (2007) AMP-activated protein kinase in the heart. Role during health and disease. Circ. Res. 100, 474–488 [DOI] [PubMed] [Google Scholar]
- 3. Young L. H., Li J., Baron S. J., Russell R. R. (2005) AMP-activated protein kinase. A key stress signaling pathway in the heart. Trends Cardiovasc. Med. 15, 110–118 [DOI] [PubMed] [Google Scholar]
- 4. Birnbaum M. J. (2005) Activating AMP-activated protein kinase without AMP. Mol. Cell 19, 289–290 [DOI] [PubMed] [Google Scholar]
- 5. Perry S. V. (1999) Troponin I. Inhibitor or facilitator. Mol. Cell. Biochem. 190, 9–32 [PubMed] [Google Scholar]
- 6. Kobayashi T., Solaro R. J. (2005) Calcium, thin filaments, and the integrative biology of cardiac contractility. Annu. Rev. Physiol. 67, 39–67 [DOI] [PubMed] [Google Scholar]
- 7. Zakhary D. R., Moravec C. S., Stewart R. W., Bond M. (1999) Protein kinase A (PKA)-dependent troponin-I phosphorylation and PKA regulatory subunits are decreased in human dilated cardiomyopathy. Circulation 99, 505–510 [DOI] [PubMed] [Google Scholar]
- 8. Zhang J., Dong X., Hacker T. A., Ge Y. (2010) Deciphering modifications in swine cardiac troponin I by top-down high esolution tandem mass spectrometry. J. Am. Soc Mass Spectrom. 21, 940–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ayaz-Guner S., Zhang J., Li L., Walker J. W., Ge Y. (2009) In vivo phosphorylation site mapping in mouse cardiac troponin I by high resolution top-down electron capture dissociation mass spectrometry. Ser-22/23 are the only sites basally phosphorylated. Biochemistry 48, 8161–8170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oliveira S. M., Watkins H., Redwood C. S. (2007) Cardiac troponin I is a potential novel substrate for AMP-activated protein kinase. J. Muscle Res. Cell Motil. 28, 445 [Google Scholar]
- 11. Sancho Solis R., Ge Y., Walker J. W. (2011) A preferred AMPK phosphorylation site adjacent to the inhibitory loop of cardiac and skeletal troponin I. Protein Sci. 20, 894–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taglieri D. M., Monasky M. M., Knezevic I., Sheehan K. A., Lei M., Wang X., Chernoff J., Wolska B. M., Ke Y., Solaro R. J. (2011) Ablation of p21-activated kinase-1 in mice promotes isoproterenol-induced cardiac hypertrophy in association with activation of Erk1/2 and inhibition of protein phosphatase 2A. J. Mol. Cell. Cardiol. 51, 988–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ouyang Y., Mamidi R., Jayasundar J. J., Chandra M., Dong W. J. (2010) Structural and kinetic effects of PAK3 phosphorylation mimic of cTnI(S151E) on the cTnC-cTnI interaction in the cardiac thin filament. J. Mol. Biol. 400, 1036–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Biesiadecki B. J., Kobayashi T., Walker J. S., John Solaro R., de Tombe P. P. (2007) The troponin C G159D mutation blunts myofilament desensitization induced by troponin I Ser-23/24 phosphorylation. Circ. Res. 100, 1486–1493 [DOI] [PubMed] [Google Scholar]
- 15. Tardiff J. C., Factor S. M., Tompkins B. D., Hewett T. E., Palmer B. M., Moore R. L., Schwartz S., Robbins J., Leinwand L. A. (1998) A truncated cardiac troponin T molecule in transgenic mice suggests multiple cellular mechanisms for familial hypertrophic cardiomyopathy. J. Clin. Invest. 101, 2800–2811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sumandea M. P., Pyle W. G., Kobayashi T., de Tombe P. P., Solaro R. J. (2003) Identification of a functionally critical protein kinase C phosphorylation residue of cardiac troponin T. J. Biol. Chem. 278, 35135–35144 [DOI] [PubMed] [Google Scholar]
- 17. Davis J. P., Norman C., Kobayashi T., Solaro R. J., Swartz D. R., Tikunova S. B. (2007) Effects of thin and thick filament proteins on calcium binding and exchange with cardiac troponin C. Biophys. J. 92, 3195–3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Biesiadecki B. J., Tachampa K., Yuan C., Jin J. P., de Tombe P. P., Solaro R. J. (2010) Removal of the cardiac troponin I N-terminal extension improves cardiac function in aged mice. J. Biol. Chem. 285, 19688–19698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wolska B. M., Keller R. S., Evans C. C., Palmiter K. A., Phillips R. M., Muthuchamy M., Oehlenschlager J., Wieczorek D. F., de Tombe P. P., Solaro R. J. (1999) Correlation between myofilament response to Ca2+ and altered dynamics of contraction and relaxation in transgenic cardiac cells that express β-tropomyosin. Circ. Res. 84, 745–751 [DOI] [PubMed] [Google Scholar]
- 20. Konhilas J. P., Irving T. C., Wolska B. M., Jweied E. E., Martin A. F., Solaro R. J., de Tombe P. P. (2003) Troponin I in the murine myocardium. Influence on length-dependent activation and interfilament spacing. J. Physiol. 547, 951–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arteaga G. M., Palmiter K. A., Leiden J. M., Solaro R. J. (2000) Attenuation of length dependence of calcium activation in myofilaments of transgenic mouse hearts expressing slow skeletal troponin I. J. Physiol. 526, 541–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buscemi N., Foster D. B., Neverova I., Van Eyk J. E. (2002) p21-activated kinase increases the calcium sensitivity of rat Triton-skinned cardiac muscle fiber bundles via a mechanism potentially involving novel phosphorylation of troponin I. Circ. Res. 91, 509–516 [DOI] [PubMed] [Google Scholar]
- 23. Lu Q. W., Hinken A. C., Patrick S. E., Solaro R. J., Kobayashi T. (2010) Phosphorylation of cardiac troponin I at protein kinase C site threonine 144 depresses cooperative activation of thin filaments. J. Biol. Chem. 285, 11810–11817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Solaro R. J., Moir A. J., Perry S. V. (1976) Phosphorylation of troponin I and the inotropic effect of adrenaline in the perfused rabbit heart. Nature 262, 615–617 [DOI] [PubMed] [Google Scholar]
- 25. Ramirez-Correa G. A., Jin W., Wang Z., Zhong X., Gao W. D., Dias W. B., Vecoli C., Hart G. W., Murphy A. M. (2008) O-Linked GlcNAc modification of cardiac myofilament proteins. A novel regulator of myocardial contractile function. Circ. Res. 103, 1354–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dyck J. R., Lopaschuk G. D. (2006) AMPK alterations in cardiac physiology and pathology. Enemy or ally? J. Physiol. 574, 95–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moir A. J., Perry S. V. (1977) The sites of phosphorylation of rabbit cardiac troponin I by adenosine 3':5'-cyclic monophosphate-dependent protein kinase. Effect of interaction with troponin C. Biochem. J. 167, 333–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ke Y., Wang L., Pyle W. G., de Tombe P. P., Solaro R. J. (2004) Intracellular localization and functional effects of p21-activated kinase-1 (Pak1) in cardiac myocytes. Circ. Res. 94, 194–200 [DOI] [PubMed] [Google Scholar]
- 29. Behunin S. M., Chen H., Hidalgo C., Konhilas J. P. (2012) Biophys. J., Biophysical Society Meeting Abstracts, 1814-POS [Google Scholar]
- 30. Syska H., Wilkinson J. M., Grand R. J., Perry S. V. (1976) The relationship between biological activity and primary structure of troponin I from white skeletal muscle of the rabbit. Biochem. J. 153, 375–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li M. X., Spyracopoulos L., Sykes B. D. (1999) Binding of cardiac troponin-I 147–163 induces a structural opening in human cardiac troponin-C. Biochemistry 38, 8289–8298 [DOI] [PubMed] [Google Scholar]
- 32. Davis J. P., Tikunova S. B. (2008) Ca2+ exchange with troponin C and cardiac muscle dynamics. Cardiovasc. Res. 77, 619–626 [DOI] [PubMed] [Google Scholar]
- 33. Abbott M. B., Dong W. J., Dvoretsky A., DaGue B., Caprioli R. M., Cheung H. C., Rosevear P. R. (2001) Modulation of cardiac troponin C-cardiac troponin I regulatory interactions by the amino terminus of cardiac troponin I. Biochemistry 40, 5992–6001 [DOI] [PubMed] [Google Scholar]
- 34. Li M. X., Wang X., Lindhout D. A., Buscemi N., Van Eyk J. E., Sykes B. D. (2003) Phosphorylation and mutation of human cardiac troponin I deferentially destabilize the interaction of the functional regions of troponin I with troponin C. Biochemistry 42, 14460–14468 [DOI] [PubMed] [Google Scholar]
- 35. Howarth J. W., Meller J., Solaro R. J., Trewhella J., Rosevear P. R. (2007) Phosphorylation-dependent conformational transition of the cardiac specific N-extension of troponin I in cardiac troponin. J. Mol. Biol. 373, 706–722 [DOI] [PubMed] [Google Scholar]
- 36. Heller W. T., Finley N. L., Dong W. J., Timmins P., Cheung H. C., Rosevear P. R., Trewhella J. (2003) Small-angle neutron scattering with contrast variation reveals spatial relationships between the three subunits in the ternary cardiac troponin complex and the effects of troponin I phosphorylation. Biochemistry 42, 7790–7800 [DOI] [PubMed] [Google Scholar]
- 37. Warren C. M., Kobayashi T., Solaro R. J. (2009) Sites of intra- and intermolecular cross-linking of the N-terminal extension of troponin I in human cardiac whole troponin complex. J. Biol. Chem. 284, 14258–14266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kirk J. A., MacGowan G. A., Evans C., Smith S. H., Warren C. M., Mamidi R., Chandra M., Stewart A. F., Solaro R. J., Shroff S. G. (2009) Left ventricular and myocardial function in mice expressing constitutively pseudophosphorylated cardiac troponin I. Circ. Res. 105, 1232–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Murphy A. M., Kögler H., Georgakopoulos D., McDonough J. L., Kass D. A., Van Eyk J. E., Marbán E. (2000) Transgenic mouse model of stunned myocardium. Science 287, 488–491 [DOI] [PubMed] [Google Scholar]
- 40. Malik F. I., Hartman J. J., Elias K. A., Morgan B. P., Rodriguez H., Brejc K., Anderson R. L., Sueoka S. H., Lee K. H., Finer J. T., Sakowicz R., Baliga R., Cox D. R., Garard M., Godinez G., Kawas R., Kraynack E., Lenzi D., Lu P. P., Muci A., Niu C., Qian X., Pierce D. W., Pokrovskii M., Suehiro I., Sylvester S., Tochimoto T., Valdez C., Wang W., Katori T., Kass D. A., Shen Y. T., Vatner S. F., Morgans D. J. (2011) Cardiac myosin activation. A potential therapeutic approach for systolic heart failure. Science 331, 1439–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Djouder N., Tuerk R. D., Suter M., Salvioni P., Thali R. F., Scholz R., Vaahtomeri K., Auchli Y., Rechsteiner H., Brunisholz R. A., Viollet B., Mäkelä T. P., Wallimann T., Neumann D., Krek W. (2010) PKA phosphorylates and inactivates AMPK α to promote efficient lipolysis. EMBO J. 29, 469–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hurley R. L., Barré L. K., Wood S. D., Anderson K. A., Kemp B. E., Means A. R., Witters L. A. (2006) Regulation of AMP-activated protein kinase by multisite phosphorylation in response to agents that elevate cellular cAMP. J. Biol. Chem. 281, 36662–36672 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.