Background: Cyclic AMP signaling impairs megakaryopoiesis through an unknown mechanism.
Results: cAMP transcriptionally represses the transcription factor E2A with resultant impairment in the expression of its target CDKN1A.
Conclusion: In discovering a mechanism for cAMP-induced inhibition, E2A is identified clearly as critical megakaryocytic factor.
Significance: Elucidating the repertoire of necessary transcription factors is crucial for understanding control of megakaryocytic lineage.
Keywords: Cell Differentiation, Cyclic AMP (cAMP), Differentiation, Helix-Loop-Helix Transcription Factors, Hematopoiesis, Megakaryopoiesis
Abstract
Signaling via the intracellular second messenger cyclic AMP (cAMP) has long been implicated in the repression of megakaryocytic differentiation. However, the mechanisms by which cAMP signaling impairs megakaryopoiesis have never been elucidated. In a human CD34+ cell culture model, we show that the adenylyl cyclase agonist forskolin inhibits megakaryocytic differentiation in a protein kinase A-dependent manner. Using this system to screen for downstream effectors, we identified the transcription factor E2A as a key target in a novel repressive signaling pathway. Specifically, forskolin acting through protein kinase A-induced E2A down-regulation and enforced expression of E2A overrode the inhibitory effects of forskolin on megakaryopoiesis. The dependence of megakaryopoiesis on critical thresholds of E2A expression was confirmed in vivo in haploinsufficient mice and ex vivo using shRNA knockdown in human progenitors. Using a variety of approaches, we further identified p21 (encoded by CDKN1A) as a functionally important megakaryopoietic regulator residing downstream of E2A. These results thus implicate the E2A-CDKN1A transcriptional axis in the control of megakaryopoiesis and reveal the lineage-selective inhibition of this axis as a likely mechanistic basis for the inhibitory effects of cAMP signaling.
Introduction
Augmentation of intracellular cAMP levels by a variety of pharmacologic agents has been shown to impair megakaryocytic differentiation in cell lines and in primary progenitors (1, 2). Of clinical relevance, the phosphodiesterase inhibitor anagrelide serves as a first line therapy to lower platelet counts in patients with myeloproliferative disorders such as essential thrombocythemia (3–5). Although cAMP activates multiple known downstream effectors, including protein kinase A (PKA),3 the mechanisms by which elevations in cAMP impair megakaryocytic differentiation remain unknown.
Multiple transcription factors (TFs) have been implicated in programming the differentiation of megakaryocytes from the bipotential megakaryocyte-erythroid progenitor. Those making critical contributions include GATA-1, GATA-2, RUNX1, FLI1, and SCL (6, 7). Recent studies correlating global TF chromatin occupancy and gene expression patterns in primary human megakaryocytes showed that these five factors form a core regulatory circuit controlling many effector genes. However, the majority (67%) of megakaryocytic target genes showed no evidence of regulation by any of these factors (8), indicating the existence of additional megakaryocytic TFs that remain to be established. One candidate megakaryocytic TF suggested by that study was transcription factor 3, more commonly known as E2A, a member of the E-protein family of basic helix-loop-helix transcription factors and a master regulator of B- and T-cell lymphopoiesis (9–12). Indeed, indirect lines of published evidence have hinted at a potential role for E2A in programming megakaryopoiesis. Specifically, E2A may participate in the promegakaryocytic “pentameric” transcriptional complex containing SCL, GATA-1, LMO-2, and Ldb1 (13–15). Additionally, mice lacking E2A show decreased numbers of bone marrow megakaryocytic precursors (16). However, a direct, non-redundant promegakaryocytic role for E2A within or outside the pentameric complex remains unproven.
In the current study, we utilized ex vivo cultures of primary human progenitors to establish a role for E2A in the regulation of megakaryopoiesis and show that inhibition of human megakaryopoiesis by cAMP signaling results from PKA dependent down-regulation of E2A transcription. In addition, mice haploinsufficient for E2A were found to have deficiencies in marrow megakaryocytic maturation and stress thrombopoiesis responses. The ex vivo and in vivo loss of function studies thus confirmed a non-redundant, dosage-sensitive influence of E2A on the programming of megakaryopoiesis.
A transcriptional target of E2A likely to be relevant in this pathway consists of CDKN1A, encoding the cell cycle inhibitor p21. E2A directly transactivates CDKN1A through binding to intralocus E box elements (17), and deficiency of E2A in knock-out mice impairs p21 expression in hematopoietic cells, even in E2A haploinsufficient mice (16, 18–20). Intriguingly, p21 expression greatly increases during megakaryocytic differentiation of human hematopoietic progenitors (21), and its overexpression promotes megakaryocytic differentiation in multiple leukemic cells lines (22, 23). Accordingly, we showed that inhibition of E2A expression, by either forskolin treatment or direct shRNA knockdown, blocked p21 up-regulation during human megakaryopoiesis. Loss-of-function and gain-of-function studies in human progenitors confirmed p21 as a functionally relevant target of E2A in megakaryopoiesis. These results thus identify the E2A-CDKN1A transcriptional axis as an essential component of the regulatory circuitry dedicated to programming megakaryopoiesis. Furthermore, the lineage selective repression of this axis by cAMP-PKA signaling constitutes a novel mechanism that may underlie the selective platelet lowering effects of anagrelide.
EXPERIMENTAL PROCEDURES
Plasmids
The control and CDKN1A lentiviral expression constructs 670-1-Empty and 670-1-p21 (24) were provided by Dr. Judith Campisi (Buck Institute, Novato, CA). The E12 and E47 expression constructs, MSCV-MigR1-E12 and MSCV-MigR1-E47, were provided by Dr. Barbara Kee (University of Chicago, Chicago, IL). Lentiviral packaging vectors pCMV-dR8.74 and pMD2.G were provided by Dr. Didier Trono (School of Life Sciences, Swiss Institute of Technology, Lausanne, Switzerland). Lentiviral shRNA (pLKO.1) constructs targeting human E2A and CDKN1A were purchased from Open Biosystems (Huntsville, AL).
Cell Culture, Transfections, and Transductions
Culture conditions for all cells consisted of 37 °C, 5% CO2, and humidified air. HEK293T cells were cultured and transfected by calcium phosphate precipitation as described previously (25). Purified primary human CD34+ hematopoietic cells isolated from the peripheral blood of healthy donors were obtained from the Hematopoietic Cell Processing Core at the Fred Hutchinson Cancer Center (Seattle, WA) and after 72 h in serum-free multi-lineage culture, were cultured in serum free uni-lineage media for either megakaryopoiesis or erythropoiesis as described (25). As has been described (26), the extent of differentiation observed in this system displays some variability due to donor heterogeneity. Forskolin (Sigma-Aldrich), 1,9-dideoxyforskolin (Sigma-Aldrich), anagrelide (Tocris, Ellisville, MO), and H89 (Cell Signaling Technology, Beverley, MA) were dissolved in dimethyl sulfoxide, whereas N6-Benzoyl-cAMP (Sigma-Aldrich) was dissolved in water, and unless otherwise specified, compounds were added to the medium just prior to initiating 6-day megakaryocytic cultures. Dimethyl sulfoxide was used as a solvent control. In co-treatment experiments, H89 was added 3 h prior to the addition of forskolin. For lentiviral transduction, supernatants from HEK293T cells transiently co-transfected with pCMV-dR8.74, pMD2.G, and lentiviral constructs were used for spinoculation as described previously (25). Transduced cells were selected in 2 μg/ml of puromycin (Sigma-Aldrich) during an initial 48-hour expansion phase and 1 μg/ml during the subsequent differentiation phase. For immunoblot and quantitative PCR studies, viable transduced cells were isolated prior to the differentiation phase using Ficoll-Paque gradients (GE Healthcare). Transduced cells were screened by immunoblot for knockdown or overexpression. For retroviral transduction, supernatants from transiently transfected Phoenix and FlyRD18 cells were employed for spinoculation as described previously (27). Transduction efficiency was determined by GFP expression using flow cytometry. The capacity of human progenitors for megakaryocytic differentiation is somewhat inhibited by the process of retroviral transduction due to the extended expansion of the cells and the preferential transduction of highly proliferative cells.
Animal Model
All of the animal experiments were approved by the University of Virginia Animal Care and Use Committee and were performed in an American Association for Laboratory Animal Care-accredited facility. E2A+/− mice (28) on a C57BL/6 genetic background were provided generously by Dr. Cornelis Murre (University of California-San Diego, San Diego, CA). For stress thrombopoiesis studies, 5-fluorouracil (Sigma-Aldrich) prepared in sterile PBS was administered by intraperitoneal injection of a single dose of 150 mg/kg (29). Blood collected from the retro-orbital plexus with heparinized micro-capillary tubes was placed into EDTA-coated microtubes (BD Biosciences). A Hemavet 850FS automated analyzer (Drew Scientific, Dallas, TX) was used to determine complete blood counts.
Flow Cytometry and Immunohistochemistry
Flow cytometric analysis of ploidy and CD41 expression in human and murine progenitors was performed as described previously as was immunohistochemical staining for von Willebrand factor (25). For detection of murine CD42b, cells were stained with phycoerythrin-conjugated anti-GPIba (Emfret Analytics, Wurzburg, Germany). Data were analyzed with FlowJo software (TreeStar, Ashland, OR). For sorting, a FACSVantage SE Turbo sorter (Beckton Dickson, San Jose, CA) was used to isolate cells based on either CD41 or GFP expression.
Immunoblot and RNA Quantitation
Immunoblot analysis of whole cell lysates was performed as described previously (25). Primary antibodies included mouse anti-tubulin (Sigma-Aldrich); mouse anti-E2A (Santa Cruz Biotechnology, Santa Cruz CA); rabbit anti-CREB (Cell Signaling); rabbit anti-phospho-CREB-Ser-133, and mouse anti-p21 (Millipore, Billerica, MA). Densitometry data were acquired on a GS800 calibrated densitometer (Bio-Rad) and analyzed with Quantity One software (Bio-Rad). For quantitation of mRNA, total cellular RNA was isolated using the RNeasy plus mini kit (Qiagen, Valencia, CA) and converted to cDNA using the iScript cDNA synthesis kit (Bio-Rad). Quantitative PCR was performed using the iQ SYBR Green supermix on the iCycler platform (Bio-Rad). The ΔΔCT formula was used to calculate relative transcript levels using GAPDH for normalization. Quantitative PCR primers are listed in supplemental Table S1.
Statistical Analysis
Kaleidagraph software (version 4.0, Synergy Software, Reading, PA) was used for graphical representation of data and statistical analysis. Results were analyzed by unpaired two-tailed Student's t test for comparisons between two groups. For comparisons involving more than two groups, analysis of variance with Tukey's Honestly Significant Difference post hoc test was performed. p ≤ 0.05 were considered significant.
RESULTS
Inhibition of Megakaryopoiesis by cAMP Signaling Occurs through PKA
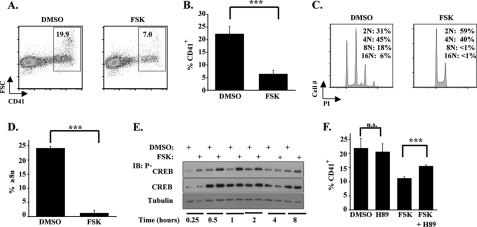
To analyze the effects of cAMP signaling on megakaryopoiesis, human CD34+ cells maintained in uni-lineage megakaryocytic medium were treated with the adenylyl cyclase agonist forskolin (FSK) at a standard dose of 10 μm (2, 30). This treatment reproducibly inhibited several parameters of megakaryocytic differentiation, including CD41 up-regulation, increase in forward light scatter (FSC), and polyploidization (Fig. 1, A–D). Further gating on small (FSClo) and large (FSChi) cell populations showed FSK impaired CD41 up-regulation in both populations to an equal extent (supplemental Fig. S1A). 1,9-Didoexyforskolin, an analog of FSK that does not stimulate adenylyl cyclase but does promote Protein Phosphatase 2A activity (31), had no effect on megakaryocytic differentiation (supplemental Fig. S1B). CD41 up-regulation also was inhibited by treatment with anagrelide at a standard dose of 500 nm (supplemental Fig. S1C). Activation of cAMP-PKA signaling in these cells by FSK and anagrelide was confirmed by determination of CREB phosphorylation on Ser-133 (Fig. 1E and supplemental Fig. S1D) (32). Robust Ser-133 phosphorylation was seen within 15 min of FSK treatment and peaked at 1 h (Fig. 1E). To determine the contribution of PKA to the inhibitory effects of FSK, cells were treated with H89, an inhibitor of PKA (33). H89 attenuated the repressive effects of FSK on CD41 up-regulation and diminished CREB phosphorylation on Ser-133 (Fig. 1F and supplemental Fig. S1E). Further support for involvement of PKA in repression of megakaryopoiesis was provided by treating cells with N6-Benzoyl-cAMP, a cAMP analog that selectively activates PKA but not Epac (34). Notably, N6-Benzoyl-cAMP inhibited megakaryocytic differentiation in a manner similar to FSK (supplemental Fig. S1F).
FIGURE 1.
Cyclic AMP-PKA signaling inhibits primary human megakaryocytic differentiation. A–D, purified human CD34+ cells underwent culture for 6 days in unilineage megakaryocytic medium ± 10 μm FSK. A, flow cytometric assessment of CD41 expression and cell size as reflected by FSC with gating on viable cells. Shown are percentages of CD41+ cells determined using FlowJo software. B, graph of CD41 percentages (mean ± S.E.) for multiple independent experiments (n = 5) conducted as in A. ***, p < 0.001. C, flow cytometric analysis of ploidy as reflected by propidium iodide (PI) intensity, with gating on CD41+ cells. Shown are percentages of cells with indicated ploidy levels. D, graph of percentages of cells with ploidy ≥ 8n (mean ± S.E.) for multiple independent experiments (n = 3) conducted as described in C. ***, p < 0.001. E, immunoblot (IB) analysis of PKA activation, as reflected by CREB phosphorylation on serine 133, in human progenitors treated with FSK. Cells cultured in megakaryocytic medium ± 10 μm FSK for the indicated times underwent immunoblotting of whole cell lysates. Shown are representative results from 1 of 2 independent experiments. F, graph depicting the effects of FSK and the PKA antagonist H89 on megakaryocytic differentiation. Human progenitors in megakaryocytic medium were treated with either 1.25 μm FSK, 10 μm H89, both compounds, or solvent and analyzed by flow cytometry as in A. Shown are mean ± S.E. for three independent experiments. **, p < 0.01; n.s., not significant; DMSO, dimethyl sulfoxide;.
cAMP-PKA Signaling Specifically Represses E2A Expression in Megakaryocytic Lineage
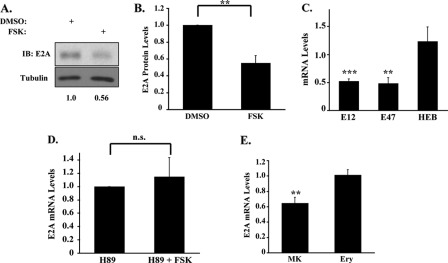
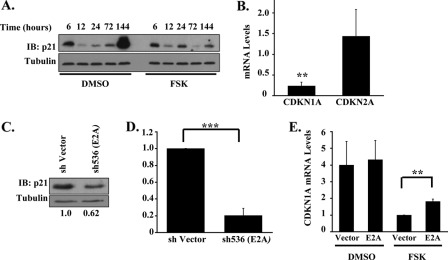
To identify transcriptional targets inhibited by cAMP-PKA signaling, primary progenitors in megakaryocytic medium ± FSK underwent screening for alterations in expression of megakaryocytic TFs (13, 14, 35, 36). Although most of the TFs analyzed showed no changes (data not shown), E2A protein levels consistently declined by ∼45% with FSK treatment (Fig. 2, A and B). This down-regulation also occurred at the transcript level, involving both E12 and E47 splice variants but sparing the paralogous HEB gene (Fig. 2C). Anagrelide induced a similar although slightly smaller decline (∼35%) in E2A transcript levels (supplemental Fig. S2A). The FSK-induced decline in E2A mRNA levels was not accompanied by alterations in transcript stability (supplemental Fig. S2B). Co-treatment with the PKA inhibitor H89 abrogated the repressive effects of FSK on E2A expression (Fig. 2D). Strikingly, FSK had no effects on E2A expression in cells undergoing erythroid culture (Fig. 2E), despite robust activation of PKA (supplemental Fig. S2C). These findings suggest that cAMP-PKA signaling in megakaryocytes regulates E2A by a lineage-specific mechanism involving transcriptional repression.
FIGURE 2.
E2A expression is selectively repressed in the megakaryocytic lineage by cAMP-PKA signaling. A, E2A protein expression in primary human progenitors subjected to FSK treatment for 6 h. Cells in megakaryocytic medium ± 10 μm FSK underwent immunoblotting of whole cell lysates with signal quantitation by scanning densitometry. Numbers below the blot represent relative E2A protein levels normalized to tubulin. B, fold changes in E2A protein levels following 4 h of FSK treatment. Graphic summary (mean ± S.E.) of three independent experiments conducted similarly to that described in A, with signal quantitation by scanning densitometry. **, p < 0.01. C, effects of FSK on transcript levels for members of the E-protein family of transcription factors. Human progenitors in megakaryocytic medium ± 10 μm FSK for 4 h were analyzed by quantitative RT-PCR. The graph depicts the fold change in mRNA levels associated with FSK treatment (mean ± S.E. for three independent experiments). **, p < 0.01; ***, p < 0.001, for FSK versus solvent control. D, role of PKA in FSK repression of E2A transcript levels. Human progenitors in megakaryocytic medium ± 2.5 μm FSK and H89 were analyzed by quantitative RT-PCR. The graph shows fold change in E2A mRNA levels associated with FSK treatment (mean ± S.E. for three independent experiments); n.s., not significant. E, lineage selectivity of E2A repression by FSK. Human progenitors grown in megakaryocytic (MK) or erythroid (Ery) medium for 4 days were treated for 4 h with 10 μm FSK followed by quantitative RT-PCR analysis. The graph shows fold change in E2A mRNA levels associated with FSK treatment versus solvent control (mean ± S.E. for three independent experiments). **, p < 0.01, comparing FSK treated to solvent control. DMSO, dimethyl sulfoxide; IB, immunoblot.
Dosage-dependent Influence of E2A on Megakaryopoiesis
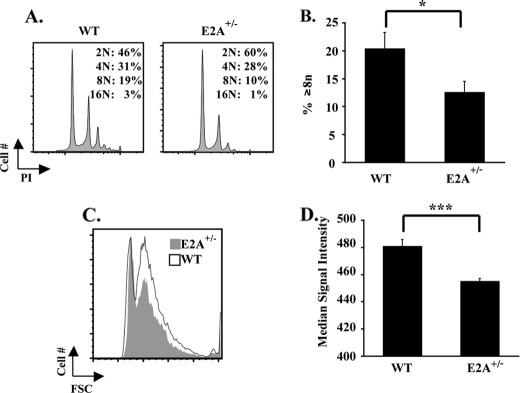
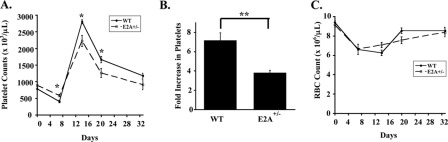
To assess whether the decline in E2A expression associated with cAMP-PKA signaling could contribute to megakaryocytic inhibition, multiple approaches were employed. First, mice haploinsufficient for E2A (E2A+/−) (28) were analyzed under resting conditions and during recovery from myelosuppression. We chose to study haploinsufficient mice because E2A+/− megakaryocytes demonstrated a 50% decrease in E2A mRNA levels as compared with wild type controls (supplemental Fig. S3A), a decline similar to that obtained with FSK treatment of human CD34+ cells (Fig. 2, A–C). Adult E2A+/− mice showed normal resting platelet counts and volumes (supplemental Table S2) but manifested multiple significant abnormalities in bone marrow megakaryocytes, including diminished CD42b expression (supplemental Fig. S3B), decreased polyploidization (Fig. 3, A and B), and diminished cell size (Fig. 3, C and D). In addition, E2A+/− megakaryocytes showed decreased expression of von Willebrand factor, with a subset of megakaryocytic cells completely devoid of von Willebrand factor on immunostaining of intact marrow (supplemental Fig. S3C, arrows). To analyze stress thrombopoiesis, animals were challenged with 5-fluorouracil. Despite their normal resting platelet counts, the E2A+/− mice revealed impairment in platelet recovery following a myelosuppressive challenge (Fig. 4, A and B). This defect was lineage-specific as their red cell recovery occurred normally (Fig. 4C). The various abnormalities seen in the E2A+/− mice thus support the idea that a 50% decrease in E2A levels is sufficient to cause impairments in megakaryocytic differentiation in vivo.
FIGURE 3.
Defective basal megakaryopoiesis in E2A haploinsufficient mice. A, comparison of ploidy distribution in wild type versus E2A+/− megakaryocytes. Flow cytometric assessment of ploidy in marrow cells with gating on CD41+ cells as described in Fig. 1C. B, percentages of cells with ploidy ≥ 8n (mean ± S.E.) in marrows from multiple animals (six per group) analyzed as described in A. *, p < 0.05. C, comparison of cell size in wild type versus E2A+/− megakaryocytes. Flow cytometric assessment of the size distribution of marrow CD41+ cells from both strains depicted by overlay of forward scatter profiles. Shown is representative overlay with one mouse from each group. D, graph of median signal intensity (mean ± S.E.) of the forward scatter profiles of CD41+ megakaryocytes from wild type and E2A+/− mice (six animals per group). ***, p < 0.001.
FIGURE 4.
Impaired stress thrombopoiesis but not erythropoiesis in E2A haploinsufficient mice. A–C, wild type and E2A+/− mice (four per group) received single intraperitoneal injections of 5-fluorouracil (150 mg/kg) followed by serial determinations of complete blood counts. A, platelet counts on days 0, 7, 14, 20, and 33 post-injection. Shown are means ± S.E. *, p = 0.05. B, fold changes in platelet counts from nadir on day 7 to peak on day 14. Shown are means ± S.E. **, p < 0.01. C, red blood cell counts on days 0, 7, 14, 20, and 33 post-injection. Shown are means ± S.E.
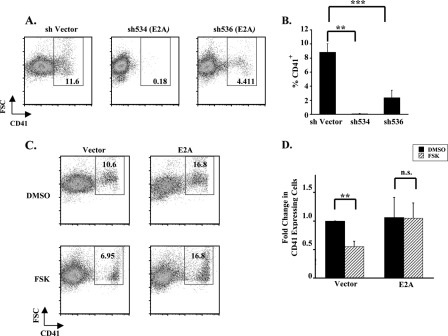
As a second approach, primary human progenitors were transduced with lentiviral shRNA vectors targeting E2A, using two distinct hairpins, each of which knocked down E2A protein levels >90% (supplemental Fig. S4A). Both hairpins strongly inhibited megakaryocytic differentiation, decreasing CD41 expression both overall and in size specific subpopulations by >70% (Fig. 5, A and B, and supplemental Fig. S4B), thereby confirming a critical, non-redundant role for E2A in human megakaryopoiesis. As a third approach, to determine the necessity of E2A down-regulation in human megakaryocytic inhibition by cAMP-PKA signaling, E2A expression was retrovirally enforced in progenitors undergoing megakaryocytic culture ± FSK. Although FSK prevented megakaryopoiesis in control vector-transduced cells, enforced expression of E2A rendered transduced cells resistant to the inhibitory effects of FSK (Fig. 5, C and D). Similar patterns of resistance were seen when the CD41+ population was subdivided on the basis of cell size (supplemental Fig. S4C). These findings suggest that megakaryopoiesis is highly sensitive to E2A levels and that E2A down-regulation is a mandatory step in the inhibitory pathway downstream of cAMP-PKA signaling.
FIGURE 5.
E2A levels dynamically influence human megakaryopoiesis. A, blockade of megakaryopoiesis by E2A knockdown. Primary human progenitors transduced with lentiviral shRNA constructs targeting E2A underwent unilineage megakaryocytic culture followed by flow cytometric assessment as described in Fig. 1A. B, graph of CD41 percentages (mean ± S.E.) for multiple independent experiments (n = 3) conducted as described in A. ***, p = 0.001; **, p < 0.01 with statistical analysis performed using analysis of variance with Tukey's Honetly Significant Difference post hoc test. C, enforced E2A expression prevents FSK repression of megakaryopoiesis. Human progenitors transduced with retroviral expression constructs were cultured in megakaryocytic medium ± 10 μm FSK followed by flow cytometry with gating on GFP+ cells. D, graph depicting the fold change in percentages of CD41+ cells associated with FSK treatment (mean ± S.E.) for three independent experiments conducted as described in C; **, p < 0.01; n.s., not significant; DMSO, dimethyl sulfoxide.
cAMP Signaling Impairs Expression of an E2A Target Gene, CDKN1A, Involved in Megakaryopoiesis
A potentially relevant downstream target of E2A in the cAMP-PKA inhibitory pathway consists of the cell cycle inhibitor p21 (CDKN1A) as it is up-regulated during megakaryopoiesis (21), transcriptionally activated by E2A (17), and has been implicated in promoting features of megakaryocytic differentiation in leukemic cell lines (22, 23, 37, 38). In primary human progenitors, p21 levels increased dramatically during late stages of megakaryocytic differentiation (Fig. 6A), an effect that was blocked at both the protein and mRNA levels by FSK treatment (Fig. 6, A and B). Anagrelide treatment resulted in a similar failure to up-regulate CDKN1A transcripts during late stages of megakaryopoiesis (supplemental Fig. S5A). FSK did not repress megakaryocytic expression of the related cell cycle inhibitor, CDKN2A, suggesting that the repression of p21 did not occur secondary to global changes in cell cycle regulation (Fig. 6B).
FIGURE 6.
CDKN1A (encoding p21) is an E2A target gene affected by cAMP-PKA signaling. A, FSK blockade of megakaryocytic up-regulation of p21 protein. Primary human progenitors cultured for the indicated durations in megakaryocytic medium ± 10 μm FSK underwent immunoblotting of whole cell lysates. Representative example from one of three independent experiments. B, FSK represses CDKN1A transcript levels but not those of the related CDKN2A cell cycle inhibitor. Primary human progenitors in megakaryocytic medium ± 10 μm FSK for 6 days were analyzed by quantitative RT-PCR. The graph depicts the fold change in mRNA levels associated with FSK treatment (mean ± S.E. for three independent experiments). ***, p < 0.001, for FSK treated versus solvent control. C, E2A regulation of p21 protein expression. Primary human progenitors transduced with shRNA constructs as in Fig. 5A were cultured in megakaryocytic medium followed by immunoblotting on whole cell lysates. Densitometry was performed as described in Fig. 2A. Shown is a representative example from 1 of two independent experiments. D, E2A regulation of CDKN1A transcript levels. Primary human progenitors transduced with shRNA constructs and grown in megakaryocytic medium were analyzed by quantitative RT-PCR. The graph depicts the fold change in CDKN1A mRNA levels associated with E2A knockdown (mean ± S.E. for three independent experiments). ***, p < 0.001. E, enforced E2A expression enhances CDKN1A transcript levels in FSK-treated cells. Primary human progenitors transduced with retroviral expression constructs, cultured in megakaryocytic medium, and isolated by cell sorting were analyzed by quantitative RT-PCR. The graph depicts relative CDKN1A mRNA levels using as a standard FSK-treated cells transduced with parent vector (mean ± S.E. of three independent experiments). **, p < 0.01. DMSO, dimethyl sulfoxide; IB, immunoblot.
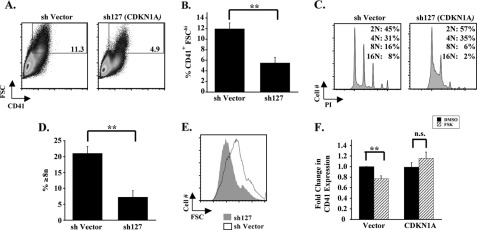
To determine the influence of E2A on p21 expression in megakaryocytes, knockdown of E2A was conducted in human progenitors as in Fig. 5, A and B, and resulted in the diminution of p21 protein and CDKN1A mRNA levels by ∼40 and ∼80%, respectively (Fig. 6, C and D). Conversely, enforced expression of E2A, as in Fig. 5, C and D, increased CDKN1A levels in FSK treated cells by ∼2-fold (Fig. 6E and supplemental Fig. S5B). Collectively, these results support CDKN1A as a target gene downstream of E2A in the cAMP-PKA-repressive pathway. To examine the role of p21 in megakaryocytic differentiation within primary human progenitors, shRNA knockdown of CDKN1A was performed (supplemental Fig. S5C). Consistent with previous results obtained in a thrombopoietin-responsive cell line (39), CDKN1A/p21 knockdown in primary human progenitors (supplemental Fig. S5C) inhibited several features of megakaryocytic differentiation (Fig. 7, A–E). Of note, although the total percentage of CD41+ cells was unchanged by CDKN1A/p21 knockdown, there was a significant decrease in the large (CD41+ FSChi) population (Fig. 7, A and B). To determine whether down-regulation of p21 contributed to megakaryocytic inhibition cAMP-PKA signaling, p21 expression was enforced by lentiviral transduction of primary human progenitors undergoing megakaryocytic culture ± FSK. Despite the low yield of CDKN1A-transduced cells due to p21-mediated growth inhibition, a discernable and reproducible desensitization to FSK was observed in association with enforced p21 expression (Fig. 7F), analogous to results obtained with enforced E2A expression (Fig. 5, C and D). Thus, CDKN1A/p21, in addition to E2A, appears to be a key target in the inhibitory pathway downstream of cAMP-PKA.
FIGURE 7.
p21 levels influence human megakaryopoiesis. A, effects of CDKN1A knockdown on CD41 expression. Primary human progenitors transduced with shRNA constructs and cultured in megakaryocytic medium were assessed by flow cytometry as described in Fig. 1A. Gating is on CD41+ FSChi population of cells. B, graph of CD41 percentages (mean ± S.E.) for multiple independent experiments (n = 3) conducted as described in A; **, p = 0.01. C, effects of CDKN1A knockdown on megakaryocytic ploidy. Primary human progenitors transduced with shRNA constructs and cultured in megakaryocytic medium were assessed by flow cytometry as described in Fig. 1C. D, percentages of cells with ploidy ≥ 8n (mean ± S.E.) for three independent experiments conducted as described in C; **, p < 0.01. E, size of megakaryocytes derived from transduced progenitors was compared as described in Fig. 3E. F, enforced CDKN1A expression counteracts repressive effects of FSK. Primary human progenitors transduced with lentiviral expression constructs were cultured in megakaryocytic medium ± 10 μm FSK. Flow cytometry was performed as described in Fig. 1A. The graph depicts the fold change in CD41 expression (mean ± S.E. for three independent experiments) associated with FSK treatment in cells transduced with lentiviral parent vector or lentivirus expressing CDKN1A. **, p < 0.01. n.s., not significant; DMSO, dimethyl sulfoxide.
DISCUSSION
The mechanisms by which cyclic AMP signaling inhibits megakaryocytic differentiation have remained poorly understood despite longstanding awareness of this phenomenon. In this study, we have identified a novel, lineage selective inhibitory pathway in which cAMP signals via PKA to repress the transcription factor E2A and its downstream target p21. In addition to providing a possible explanation for the lineage selective anti-megakaryocytic effects of anagrelide, these findings expand the limited repertoire of transcriptional pathways that actively regulate megakaryopoiesis.
The effects of cAMP signaling on E2A expression reflect a specialized pathway that does not globally affect megakaryocytic TFs or E-proteins (Fig. 2C and data not shown). This pathway also displays lineage selectivity in that erythroid cells failed to down-regulate E2A in response to PKA activation (Fig. 2E and supplemental Fig. S2C). Our data suggest that the down-regulation of E2A occurs at the transcriptional level and requires activation of PKA. Regulation of E2A expression has been described previously in thymocyte development (10) and in B-cells subjected to mitogenic stimuli (41). In B-cells, appropriate expression of E2A transcripts requires intact signaling via Rap family GTPases (42). Interestingly, Rap1 has been implicated in promoting megakaryocytic differentiation (43), and PKA has been shown to act as a negative feedback regulator of Rap1a signaling (44). Whether the repression of E2A by cAMP-PKA signaling in megakaryocytes involves inactivation of Rap proteins constitutes a topic of future investigation.
A role for E2A in programming megakaryopoiesis has been suggested by a previous study of marrow progenitor composition within E2A-deficient mice. This study showed that E2A−/− marrows contain fewer unilineage committed megakaryocytic progenitors and more erythroid-committed progenitors (CFU-E) as compared with wild type controls (16). Our data extend these findings by directly demonstrating a critical influence of E2A levels on megakaryocytic differentiation in isolated human progenitors as well as in vivo in mice (Figs. 3 and 5), thus linking E2A to a recently defined megakaryocytic TF network containing GATA-1, SCL, RUNX1, and FLI-1 (8). A precedent for E2A functioning as a core element within a network of lineage-specifying transcription factors has been observed in T- and B-lymphopoiesis, where its appropriate gene dosage is critical for progression through major developmental checkpoints (18).
E2A regulation of megakaryopoiesis most likely occurs through a mechanism involving transcriptional regulation of target genes. In hematopoietic cells, E2A binds to target genes either as a homodimeric complex, as a heterodimer with the tissue-specific basic helix-loop-helix factors SCL and LYL-1, or as part of higher order transcriptional complexes containing SCL, GATA-1, Ldb1, LMO-2, and ETO2 (13–15). The ∼50% decline in E2A levels associated with cAMP-PKA signaling most likely has disproportionate effects on subsets of these complexes, particularly those containing E2A homodimers. Furthermore, E2A homodimers are known to positively regulate expression of CDKN1A (17), whose expression is potently repressed by cAMP-PKA signaling (Fig. 6, A and B). Secondary compromise of SCL function associated with E2A down-regulation is less likely to be a relevant mechanism for several reasons. First, SCL-haploinsufficient mice show no defects in megakaryopoiesis (45). Second, SCL−/− megakaryocytes, although abnormal, show increases in ploidy (29) rather then the diminished ploidy seen with E2A haploinsufficiency (Fig. 3, A and B). Third, SCL represses rather than activates CDKN1A expression in megakaryocytes (46). And fourth, SCL−/− progenitors also show impaired erythroid development, in contrast to the enhanced erythroid commitment of E2A−/− progenitors (16, 45, 47). Thus, we postulate a novel promegakaryocytic function for E2A homodimers, mediated in part through activation of CDKN1A. The homologous E-protein HEB also is expressed in megakaryocytes and is not down-regulated by cAMP-PKA signaling (Fig. 2C), raising the possibility that E2A is unique among E proteins in its capacity to engage in megakaryocytic programming.
A variety of studies have implicated the product of CDKN1A, p21, in the regulation of megakaryocytic differentiation. In a cell line model of megakaryocytic differentiation employing UT7 cells engineered for high level expression of the thrombopoietin receptor, a megakaryocytic senescence/differentiation program induced by thrombopoietin absolutely depends on delayed p21 up-regulation (39). In K562 cells, enforced expression of p21 drives extensive megakaryocytic differentiation, whereas overexpression of a related cell cycle inhibitor, p27, results in erythroid differentiation (23). In primary megakaryocytic differentiation, p21 has been shown to undergo marked up-regulation in a p53-independent manner (21). Although the overall percentage of CD41+ cells was unchanged by p21 knockdown, a clear difference was seen in the percentage of large CD41+ cells (Fig. 7, A and B). Coupled with the corresponding impairments in cell size and polyploidization (Fig. 7, C and E), this highlights the importance of p21 in the enlargement and endomitosis of megakaryocytes during terminal differentiation. This finding raises the possibility that E2A regulates megakaryocytic size and polyploidy through its regulation of CDKN1A while affecting CD41 expression (see Fig. 5, A and B) through the regulation of currently undetermined target genes. A repressive role has also been described for p21 in megakaryopoiesis. Specifically, enforced expression of p21 in murine and human progenitors by retroviral transduction interferes with their subsequent polyploidization in culture (48). Furthermore, a critical promegakaryocytic function of SCL consists of repressing CDKN1A through binding to a conserved E-box element in the first intron (46). Thus, megakaryocytes derived from SCLfl/fl;PF4-Cre mice show a ∼3-fold increase in CDKN1A transcripts, and correcting CDKN1A levels in these cells by shRNA knockdown partially restores their normal differentiation. Our results as well as those of others (21, 48) have demonstrated that up-regulation of p21 occurs during later stages of megakaryocytic development (Fig. 6A). SCL most likely does not exert repression in these later stages, as ChIP-seq analysis of mature human megakaryocytes shows no binding peak for SCL within the CDKN1A locus (8). Thus, we postulate that premature induction of p21 may prevent further megakaryocytic development but that the strong up-regulation occurring in later stages plays a critical role in promoting differentiation. A similar paradigm is seen in B-lymphopoiesis where premature expression of Blimp-1, a factor absolutely critical for terminal differentiation of plasma cells, prohibits differentiation (40).
The capacity to blunt FSK repression through enforced expression of either E2A or p21 (Figs. 5D and 7F) suggests a transcriptional pathway in which p21 down-regulation, resulting from E2A down-regulation, contributes to megakaryocytic inhibition by cAMP-PKA signaling. That the E2A shRNA knockdown caused more inhibition than the CDKN1A knockdown (Figs. 5B and 7B) suggests the existence of additional E2A target genes involved in megakaryopoiesis. The identification of these targets, as well as the mechanism of E2A repression by cAMP-PKA signaling, constitutes key areas of future investigation. Similarly, understanding the physiological role of this pathway with regards to the fine tuning of platelet production will require specific approaches to disable the pathway in vivo. Although the identification of this pathway may provide a potential mechanism of action for the therapeutic agent anagrelide, our findings also offer a prototype for the unusual phenomenon of lineage-specific differentiation blockade.
Supplementary Material
Acknowledgments
We thank Drs. Barbara Kee and Judith Campisi for providing plasmids; Dr. Cornelis Murre for providing E2A+/− mice; Joanne Lannigan and the University of Virginia Flow Cytometry Core Facility for assistance with flow cytometry and cell sorting; Dr. Gail Wertz for use of the GS800 densitometer; and the University of Virginia Cardiovascular Research Center for use of the Hemavet analyzer.
This work was supported, in whole or in part, by National Institutes of Health Grants DK090926 (to A. N. G) and DK079924 (to A. N. G.).

This article contains supplemental “Methods,” Tables S1 and S2, Figs. S1–S5, and additional references.
- PKA
- protein kinase A
- TF
- transcription factor
- shRNA
- short hairpin RNA
- FSK
- forskolin
- CREB
- cAMP-responsive element-binding protein.
REFERENCES
- 1. Vittet D., Duperray C., Chevillard C. (1995) Cyclic Amp inhibits cell growth and negatively interacts with platelet membrane glycoprotein expression on the Dami human megakaryoblastic cell line. J. Cell. Physiol. 163, 645–655 [DOI] [PubMed] [Google Scholar]
- 2. Freson K., Peeters K., De Vos R., Wittevrongel C., Thys C., Hoylaerts M. F., Vermylen J., Van Geet C. (2008) PACAP and its receptor VPAC1 regulate megakaryocyte maturation: Therapeutic implications. Blood 111, 1885–1893 [DOI] [PubMed] [Google Scholar]
- 3. Seiler S., Arnold A. J., Grove R. I., Fifer C. A., Keely S. L., Jr., Stanton H. C. (1987) Effects of anagrelide on platelet cAMP levels, cAMP-dependent protein kinase, and thrombin-induced Ca++ fluxes. J. Pharmacol. Exp. Ther. 243, 767–774 [PubMed] [Google Scholar]
- 4. Solberg L. A., Jr., Tefferi A., Oles K. J., Tarach J. S., Petitt R. M., Forstrom L. A., Silverstein M. N. (1997) The effects of anagrelide on human megakaryocytopoiesis. Br. J. Haematol. 99, 174–180 [DOI] [PubMed] [Google Scholar]
- 5. Silverstein M. N., Petitt R. M., Solberg L. A., Jr., Fleming J. S., Knight R. C., Schacter L. P. (1988) Anagrelide–a new drug for treating thrombocytosis. New Engl. J. Med. 318, 1292–1294 [DOI] [PubMed] [Google Scholar]
- 6. Doré L. C., Crispino J. D. (2011) Transcription factor networks in erythroid cell and megakaryocyte development. Blood 118, 231–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goldfarb A. N. (2007) Transcriptional control of megakaryocyte development. Oncogene 26, 6795–6802 [DOI] [PubMed] [Google Scholar]
- 8. Tijssen M. R., Cvejic A., Joshi A., Hannah R. L., Ferreira R., Forrai A., Bellissimo D. C., Oram S. H., Smethurst P. A., Wilson N. K., Wang X., Ottersbach K., Stemple D. L., Green A. R., Ouwehand W. H., Göttgens B. (2011) Genome-wide analysis of simultaneous GATA1/2, RUNX1, FLI1, and SCL binding in megakaryocytes identifies hematopoietic regulators. Dev. Cell 20, 597–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beck K., Peak M. M., Ota T., Nemazee D., Murre C. (2009) Distinct roles for E12 and E47 in B cell specification and the sequential rearrangement of immunoglobulin light chain loci. J. Exp. Med. 206, 2271–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Engel I., Johns C., Bain G., Rivera R. R., Murre C. (2001) Early thymocyte development is regulated by modulation of E2A protein activity. J. Exp. Med. 194, 733–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lazorchak A., Jones M. E., Zhuang Y. (2005) New insights into E-protein function in lymphocyte development. Trends Immunol. 26, 334–338 [DOI] [PubMed] [Google Scholar]
- 12. Kee B. L. (2009) E and ID proteins branch out. Nat. Rev. Immunol. 9, 175–184 [DOI] [PubMed] [Google Scholar]
- 13. Hamlett I., Draper J., Strouboulis J., Iborra F., Porcher C., Vyas P. (2008) Characterization of megakaryocyte GATA1-interacting proteins: The corepressor ETO2 and GATA1 interact to regulate terminal megakaryocyte maturation. Blood 112, 2738–2749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schuh A. H., Tipping A. J., Clark A. J., Hamlett I., Guyot B., Iborra F. J., Rodriguez P., Strouboulis J., Enver T., Vyas P., Porcher C. (2005) ETO-2 associates with SCL in erythroid cells and megakaryocytes and provides repressor functions in erythropoiesis. Mol. Cell. Biol. 25, 10235–10250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tripic T., Deng W., Cheng Y., Zhang Y., Vakoc C. R., Gregory G. D., Hardison R. C., Blobel G. A. (2009) SCL and associated proteins distinguish active from repressive GATA transcription factor complexes. Blood 113, 2191–2201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Semerad C. L., Mercer E. M., Inlay M. A., Weissman I. L., Murre C. (2009) E2A proteins maintain the hematopoietic stem cell pool and promote the maturation of myelolymphoid and myeloerythroid progenitors. Proc. Natl. Acad. Sci. U.S.A. 106, 1930–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Prabhu S., Ignatova A., Park S. T., Sun X. H. (1997) Regulation of the expression of cyclin-dependent kinase inhibitor p21 by E2A and Id proteins. Mol. Cell. Biol. 17, 5888–5896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Herblot S., Aplan P. D., Hoang T. (2002) Gradient of E2A activity in B-cell development. Mol. Cell. Biol. 22, 886–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang Q., Esplin B., Borghesi L. (2011) E47 regulates hematopoietic stem cell proliferation and energetics but not myeloid lineage restriction. Blood 117, 3529–3538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang Q., Kardava L., St Leger A., Martincic K., Varnum-Finney B., Bernstein I. D., Milcarek C., Borghesi L. (2008) E47 controls the developmental integrity and cell cycle quiescence of multipotential hematopoietic progenitors. J. Immunol. 181, 5885–5894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taniguchi T., Endo H., Chikatsu N., Uchimaru K., Asano S., Fujita T., Nakahata T., Motokura T. (1999) Expression of p21(Cip1/Waf1/Sdi1) and p27(Kip1) cyclin-dependent kinase inhibitors during human hematopoiesis. Blood 93, 4167–4178 [PubMed] [Google Scholar]
- 22. Matsumura I., Ishikawa J., Nakajima K., Oritani K., Tomiyama Y., Miyagawa J., Kato T., Miyazaki H., Matsuzawa Y., Kanakura Y. (1997) Thrombopoietin-induced differentiation of a human megakaryoblastic leukemia cell line, CMK, involves transcriptional activation of p21(WAF1/Cip1) by STAT5. Mol. Cell. Biol. 17, 2933–2943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Muñoz-Alonso M. J., Acosta J. C., Richard C., Delgado M. D., Sedivy J., León J. (2005) p21(Cip1) and p27(Kip1) induce distinct cell cycle effects and differentiation programs in myeloid leukemia cells. J. Biol. Chem. 280, 18120–18129 [DOI] [PubMed] [Google Scholar]
- 24. Campeau E., Ruhl V. E., Rodier F., Smith C. L., Rahmberg B. L., Fuss J. O., Campisi J., Yaswen P., Cooper P. K., Kaufman P. D. (2009) A versatile viral system for expression and depletion of proteins in mammalian cells. Plos One 4, e6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Elagib K. E., Mihaylov I. S., Delehanty L. L., Bullock G. C., Ouma K. D., Caronia J. F., Gonias S. L., Goldfarb A. N. (2008) Cross-talk of GATA-1 and P-TEFb in megakaryocyte differentiation. Blood 112, 4884–4894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mathur A., Hong Y., Wang G., Erusalimsky J. D. (2004) Assays of megakaryocyte development: Surface antigen expression, ploidy, and size. Methods Mol. Biol. 272, 309–322 [DOI] [PubMed] [Google Scholar]
- 27. Elagib K. E., Xiao M., Hussaini I. M., Delehanty L. L., Palmer L. A., Racke F. K., Birrer M. J., Shanmugasundaram G., McDevitt M. A., Goldfarb A. N. (2004) Jun blockade of erythropoiesis: Role for repression of GATA-1 by HERP2. Mol. Cell. Biol. 24, 7779–7794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bain G., Maandag E. C., Izon D. J., Amsen D., Kruisbeek A. M., Weintraub B. C., Krop I., Schlissel M. S., Feeney A. J., van Roon M. (1994) E2a proteins are required for proper B-cell development and initiation of immunoglobulin gene rearrangements. Cell 79, 885–892 [DOI] [PubMed] [Google Scholar]
- 29. McCormack M. P., Hall M. A., Schoenwaelder S. M., Zhao Q., Ellis S., Prentice J. A., Clarke A. J., Slater N. J., Salmon J. M., Jackson S. P., Jane S. M., Curtis D. J. (2006) A critical role for the transcription factor Scl in platelet production during stress thrombopoiesis. Blood 108, 2248–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Keefer J. R., Schneidereith T. A., Mays A., Purvis S. H., Dover G. J., Smith K. D. (2006) Role of cyclic nucleotides in fetal hemoglobin induction in cultured CD34+ cells. Exp. Hematol. 34, 1151–1161 [DOI] [PubMed] [Google Scholar]
- 31. Neviani P., Santhanam R., Oaks J. J., Eiring A. M., Notari M., Blaser B. W., Liu S., Trotta R., Muthusamy N., Gambacorti-Passerini C., Druker B. J., Cortes J., Marcucci G., Chen C. S., Verrills N. M., Roy D. C., Caligiuri M. A., Bloomfield C. D., Byrd J. C., Perrotti D. (2007) FTY720, a new alternative for treating blast crisis chronic myelogenous leukemia and Philadelphia chromosome-positive acute lymphocytic leukemia. J. Clin. Invest. 117, 2408–2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Delghandi M. P., Johannessen M., Moens U. (2005) The cAMP signaling pathway activates CREB through PKA, p38, and MSK1 in NIH 3T3 cells. Cell Signal. 17, 1343–1351 [DOI] [PubMed] [Google Scholar]
- 33. Davies S. P., Reddy H., Caivano M., Cohen P. (2000) Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351, 95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roscioni S. S., Dekkers B. G. J., Prins A. G., Menzen M. H., Meurs H., Schmidt M., Maarsingh H. (2011) cAMP inhibits modulation of airway smooth muscle phenotype via the exchange protein activated by cAMP (Epac) and protein kinase A. Brit. J. Pharmacol. 162, 193–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stankiewicz M. J., Crispino J. D. (2009) ETS2 and ERG promote megakaryopoiesis and synergize with alterations in GATA-1 to immortalize hematopoietic progenitor cells. Blood 113, 3337–3347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jackers P., Szalai G., Moussa O., Watson D. K. (2004) Ets-dependent regulation of target gene expression during megakaryopoiesis. J. Biol. Chem. 279, 52183–52190 [DOI] [PubMed] [Google Scholar]
- 37. Kikuchi J., Furukawa Y., Iwase S., Terui Y., Nakamura M., Kitagawa S., Kitagawa M., Komatsu N., Miura Y. (1997) Polyploidization and functional maturation are two distinct processes during megakaryocytic differentiation: Involvement of cyclin-dependent kinase inhibitor p21 in polyploidization. Blood 89, 3980–3990 [PubMed] [Google Scholar]
- 38. Muñoz-Alonso M. J., Ceballos L., Bretones G., Frade P., León J., Gandarillas A. (2012) MYC accelerates p21CIP-induced megakaryocytic differentiation involving early mitosis arrest in leukemia cells. J. Cell. Physiol. 227, 2069–2078 [DOI] [PubMed] [Google Scholar]
- 39. Besancenot R., Chaligné R., Tonetti C., Pasquier F., Marty C., Lécluse Y., Vainchenker W., Constantinescu S. N., Giraudier S. (2010) A senescence-like cell-cycle arrest occurs during megakaryocytic maturation: Implications for physiological and pathological megakaryocytic proliferation. Plos Biol. 8, e1000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Messika E. J., Lu P. S., Sung Y. J., Yao T., Chi J. T., Chien Y. H., Davis M. M. (1998) Differential effect of B lymphocyte-induced maturation protein (Blimp-1) expression on cell fate during B cell development. J. Exp. Med. 188, 515–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Quong M. W., Harris D. P., Swain S. L., Murre C. (1999) E2A activity is induced during B-cell activation to promote immunoglobulin class switch recombination. EMBO J. 18, 6307–6318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Katayama Y., Sekai M., Hattori M., Miyoshi I., Hamazaki Y., Minato N. (2009) Rap signaling is crucial for the competence of IL-7 response and the development of B-lineage cells. Blood 114, 1768–1775 [DOI] [PubMed] [Google Scholar]
- 43. Delehanty L. L., Mogass M., Gonias S. L., Racke F. K., Johnstone B., Goldfarb A. N. (2003) Stromal inhibition of megakaryocytic differentiation is associated with blockade of sustained Rap1 activation. Blood 101, 1744–1751 [DOI] [PubMed] [Google Scholar]
- 44. Tsygankova O. M., Saavedra A., Rebhun J. F., Quilliam L. A., Meinkoth J. L. (2001) Coordinated regulation of Rap1 and thyroid differentiation by cyclic AMP and protein kinase A. Mol. Cell. Biol. 21, 1921–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mikkola H. K., Klintman J., Yang H., Hock H., Schlaeger T. M., Fujiwara Y., Orkin S. H. (2003) Haematopoietic stem cells retain long term repopulating activity and multipotency in the absence of stem-cell leukaemia SCL/tal-1 gene. Nature 421, 547–551 [DOI] [PubMed] [Google Scholar]
- 46. Chagraoui H., Kassouf M., Banerjee S., Goardon N., Clark K., Atzberger A., Pearce A. C., Skoda R. C., Ferguson D. J., Watson S. P., Vyas P., Porcher C. (2011) SCL-mediated regulation of the cell cycle regulator p21 is critical for murine megakaryopoiesis. Blood 118, 723–735 [DOI] [PubMed] [Google Scholar]
- 47. Hall M. A., Curtis D. J., Metcalf D., Elefanty A. G., Sourris K., Robb L., Gothert J. R., Jane S. M., Begley C. G. (2003) The critical regulator of embryonic hematopoiesis, SCL, is vital in the adult for megakaryopoiesis, erythropoiesis, and lineage choice in CFU-S12. Proc. Natl. Acad. Sci. U.S.A. 100, 992–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Baccini V., Roy L., Vitrat N., Chagraoui H., Sabri S., Le Couedic J. P., Debili N., Wendling F., Vainchenker W. (2001) Role of p2l(CiP1/Waf1) in cell-cycle exit of endomitotic megakaryocytes. Blood 98, 3274–3282 s [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.