Background: Borrelia burgdorferi contains a single superoxide dismutase (SOD).
Results: This is a Mn-SOD that is manganese-induced, zinc-repressed, and required for resistance to the metal-dependent redox compound streptonigrin.
Conclusion: Manganese is a key aspect in the defense against oxidative stress for B. burgdorferi.
Significance: Our work provides insight into mechanism of streptonigrin toxicity and metal-dependent gene regulation within an iron-lacking bacterial species.
Keywords: Bacteria, Manganese, Oxidative Stress, Superoxide Dismutase (SOD), Zinc, Borrelia burgdorferi, Lyme Disease
Abstract
Borrelia burgdorferi, the causative agent of Lyme disease, exists in nature through a complex life cycle involving ticks of the Ixodes genus and mammalian hosts. During its life cycle, B. burgdorferi experiences fluctuations in oxygen tension and may encounter reactive oxygen species (ROS). The key metalloenzyme to degrade ROS in B. burgdorferi is SodA. Although previous work suggests that B. burgdorferi SodA is an iron-dependent superoxide dismutase (SOD), later work demonstrates that B. burgdorferi is unable to transport iron and contains an extremely low intracellular concentration of iron. Consequently, the metal cofactor for SodA has been postulated to be manganese. However, experimental evidence to support this hypothesis remains lacking. In this study, we provide biochemical and genetic data showing that SodA is a manganese-dependent enzyme. First, B. burgdorferi contained SOD activity that is resistant to H2O2 and NaCN, characteristics associated with Mn-SODs. Second, the addition of manganese to the Chelex-treated BSK-II enhanced SodA expression. Third, disruption of the manganese transporter gene bmtA, which significantly lowers the intracellular manganese, greatly reduced SOD activity and SodA expression, suggesting that manganese regulates the level of SodA. In addition, we show that B. burgdorferi is resistant to streptonigrin, a metal-dependent redox cycling compound that produces ROS, and that SodA plays a protective role against the streptonigrin. Taken together, our data demonstrate the Lyme disease spirochete encodes a manganese-dependent SOD that contributes to B. burgdorferi defense against intracellular superoxide.
Introduction
The reactive oxygen species (ROS)3 superoxide anion (O2˙̄) is produced by the univalent reduction of dioxygen in aerobic habitats. Superoxide dismutases (SODs, EC 1.15.1.1) disproportionate O2˙̄ into hydrogen peroxide and oxygen at a diffusion-limited rate (1). This enzymatic activity requires a metal cofactor that defines different isozyme forms of SOD including Mn-SOD, Fe-SOD, Cu,Zn-SOD, Ni-SOD, or cambialistic SOD that can function with either manganese or iron (1–5). The different isozyme forms can be distinguished based on the sensitivity to H2O2 (Fe-SOD and Cu-Zn SOD) or cyanide (Cu,Zn-SOD) or enzyme activity that is resistant to both treatments (Mn-SOD) (6, 7). The inhibition of Fe-SOD and Cu,Zn-SOD by H2O2 is irreversible, whereas the inhibition of Cu,Zn-SOD by cyanide is reversible. In Escherichia coli, several key enzymes of biosynthetic reactions are sensitive to O2˙̄ (8–11). Moreover, SODs are widespread in bacteria, including those classified as anaerobes, which further suggests that intracellular targets may be universally damaged by O2˙̄ (12).
Borrelia burgdorferi, the causative agent of Lyme disease (13, 14), likely experiences a gradient of O2 exposure during its life cycle between the tick vector and mammalian hosts. This pathogen has evolved to exploit a tick protein that protects against ROS and enhances transmission from the arthropod vector to the mammalian host (15). In addition, B. burgdorferi lacks an electron transport chain, a known source of ROS, but does contain at least one putative flavoenzyme (BB 0812) that may contribute to endogenous O2˙̄ production during fluctuations of O2. The generation of O2˙̄ during the respiratory burst by phagocytic cells of the immune system contributes to the oxidative stress of bacteria. B. burgdorferi lacks catalase or peroxidase enzymes but encodes a single superoxide dismutase gene, sodA (bb0153) (16). In B. burgdorferi, SodA is essential for infectivity in a murine model (17), presumably because of host-derived O2˙̄.
B. burgdorferi SOD has previously been characterized as an Fe-SOD based on its enzymatic sensitivity to H2O2 and resistance to cyanide (18). However, a subsequent report by Posey and Gherardini (19) demonstrated the following for B. burgdorferi: 1) no growth requirement for iron, 2) lack of common iron containing proteins, 3) inability to transport iron, and 4) extremely low intracellular iron content inside the cell (less than 10 atoms/cell). These findings imply that a metal other than iron may be the cofactor for B. burgdorferi SodA. Despite the numerous publications maintaining that the B. burgdorferi SOD is a Mn-SOD (13, 17, 19), experimental evidence supporting this hypothesis has not been reported to date. In this study, we demonstrate that B. burgdorferi SodA is a Mn-SOD. We further show that B. burgdorferi likely contains intracellular targets that are sensitive to O2˙̄ damage and that SodA plays an important role in protecting B. burgdorferi from such damage. Furthermore, SodA expression is induced by manganese and repressed by zinc, which distinguishes it from E. coli and other bacteria (20–22). Our data suggest that the acquisition of manganese plays a crucial role in the defense against O2˙̄ for the Lyme disease spirochete. This work contributes to our growing knowledge of B. burgdorferi physiology, as well as our understanding on the mechanism of ROS response in bacteria that do not require iron.
EXPERIMENTAL PROCEDURES
Bacterial Strains, Growth Conditions, and Reagents
B. burgdorferi strains B31-A3, B31-M1 ML23/pBBE22, ΔsodA/pBBE22, ΔsodA/PsecA-sodA, and ΔsodA/PflgB-sodA from previous work were used throughout (17, 23–25). An additional set of strains 297, OY04/D4 (ΔbmtA), and OY06/D11 (ΔbmtA pbmtA) were used for experiments (26). Ferricytochrome c from equine heart, the 23-kDa Mn-SOD (pI 6.9) and 21-kDa Fe-SOD (pI 5.9) from E. coli, bovine xanthine oxidase, xanthine, manganese chloride, zinc sulfate, 2,2′-dipyridyl (dip), nitro blue tetrazolium, and streptonigrin were purchased from Sigma-Aldrich. Xanthine oxidase was dialyzed against 50 mm potassium phosphate, 0.1 mm EDTA buffer in 8,000-kDa molecular mass cutoff membranes (Fisher) prior to use. Kanamycin, ampicillin, and streptomycin (Sigma) were used at 300, 100, and 75 μg ml−1, respectively. Chelex 100 resin was purchased from Bio-Rad.
Generation of ΔsodA in B31-A3
To construct ΔsodA in B31-A3 genetic background, the sodA::aadA allele from ΔsodA in the ML23 genetic background (17) was amplified by PCR using the forward primer 1 (5′-CAAAACTTACAAAAAAGGCCAACC-3′) and reverse primer 2 (5′-ATCAGACCCACATACGAAGACAT-3′) and subsequently cloned into the StrataCloneTM PCR cloning vector pSC-A generating pSC-A-delsodA. pSC-A-delsodA was isolated from a clone from the StrataClone SoloPack E. coli strain, and 20 μg of DNA was electroporated into chemically competent B. burgdorferi strain B31-A3 as described previously (27). Selection of mutants was performed using a 96-well plate format as described previously (28). Forward primer 3 (5′-TGAGCCTTGTTATTGTGGAAGTG-3′) and reverse primer 4 (5′-GTAAAGGCTAATTAATCACTTC-3′) were used to screen for the sodA::aadA insertion. One clone (BT002) was used for further experiments.
Preparation of Chelex-100-treated BSK-II Medium
BSK-II medium was prepared and supplemented with 6% heat-inactivated rabbit serum as described previously (29). To reduce the divalent cations in BSK-II medium, Chelex 100 (Bio-Rad) was used to treat the medium as follows. BSK-II medium was prepared, and 50 g liter−1 of Chelex 100 resin was added to the medium followed by gentle stirring at 4 °C for 1 h. The Chelex-treated medium was centrifuged at 7,000 × g for 30 min, and the pH of the supernatant was reduced to 7.5 by the addition of HCl and then sterilized by filtration. This process removed the remaining Chelex 100 resin from the medium. Metal analysis by inductively coupled plasma MS confirmed that Chelex treatment reduced manganese concentration to below detection (data not shown).
Superoxide Dismutase Activity
Bacteria were grown in BSK-II medium at 37 °C to stationary phase (∼ 8 × 107 cells ml−1) and centrifuged at 10,000 × g and washed twice with 50 mm potassium phosphate, pH 7.8, 0.1 mm EDTA buffer. Cell pellets were concentrated 100-fold in fresh buffer and sonicated on ice for 10 min with a 3-s pulse and a 1-s rest. The unlysed cells and cell debris were cleared by centrifugation at 20,000 × g for 20 min, and the supernatant was considered as a cell-free extract (CFE). Protein concentration was determined by a Bio-Rad Bradford assay compared with a standard curve with bovine serum albumin.
Staining for superoxide dismutase activity following native PAGE was determined as described previously (7, 30). The samples were treated with 5 mm H2O2 or 2 mm sodium cyanide for 1 h prior to native PAGE and during activity staining of native gels. Quantification of SOD activity was determined by the xanthine/xanthine oxidase (X/XO) reduction of either cytochrome c or nitro blue tetrazolium (1, 30). One unit of SOD activity is defined as the amount required to inhibit the X/XO-mediated reduction of cytochrome c or nitro blue tetrazolium by 50%. Resistance of SOD activity to H2O2 and NaCN treatment was measured as described previously (6). Briefly, SOD activity from samples was determined, then samples were subjected to 0.5 mm H2O2, and SOD activity was determined throughout the duration of treatment up to 40 min. Activity is expressed as a percentage of inhibition by H2O2 normalized to activity at time 0. Care was taken to limit the introduction of H2O2 into the assay (less than 2.5 μm). NaCN (up to 10 mm) was included in the assay mixture to determine inhibition. Purified Mn-SOD and Fe-SOD from E. coli were used as controls for experiments (Sigma). Enzyme assays were conducted using a double-beam spectrophotometer (Thermo Scientific Evolution 160) maintained at 25 °C with an external circulating water bath.
Western Blotting Techniques
Samples were prepared as described above except the samples were resuspended in Laemmli sample buffer (Bio-Rad) and boiled instead of subjected to sonication. Denatured proteins were separated on Mini-PROTEAN TGXTM gels (12% acrylamide; Bio-Rad) and transferred to 0.45-μm nitrocellulose membranes (Bio-Rad). Transfer was confirmed by Ponceau S staining of the membrane. Primary antibodies to SodA (17) (polyclonal) and the loading control FlaB (monoclonal) were used at 1:2000 and 1:40, respectively. Secondary antibody (peroxidase-conjugated goat anti-mouse; Jackson ImmunoResearch Laboratories, West Grove, PA) was used at 1:1000. Detection of horseradish peroxidase activity was determined using 4-chloro-1-napthol and H2O2 (Fisher).
Sensitivity to Streptonigrin
B. burgdorferi strains were grown in BSK-II medium to log phase (∼1 × 107 cells ml−1) and diluted in BSK-II with either Me2SO (vehicle control) or 10 μg ml−1 of streptonigrin (prepared in a 50% Me2SO, 50% H2O solution, v/v) to 5 × 104 or 1 × 105 cells ml−1. Growth was monitored over time by dark field microscopy, and an untreated sample was included as a control for experiments. The samples were vortexed prior to and following sampling to homogenize the cells and maintain an adequate supply of O2. Two separate batches of BSK-II medium were prepared and used in separate experiments.
Metal Analysis by Inductively Coupled Plasma Mass Spectrometry
To determine the influence of ΔsodA on intracellular manganese content, strains were grown in BSK-II medium at 37 °C for 7 days (initial cell density at 105 cells ml−1). Samples (n = 3) were centrifuged, washed two times in phosphate/EDTA buffer as above, and concentrated 100-fold in buffer. The samples were placed in a drying oven at 95 °C for ∼ 24 h, and a dry weight measurement was recorded. Dry cell pellets were resuspended in 3 n nitric acid and heated in a drying oven as above. Acid-treated samples were resuspended in 0.5 ml of 3 n nitric acid and sent for analysis at the Analytical Spectroscopy Services Laboratory and analyzed using a Varian 820 inductively coupled plasma MS machine.
Statistical Analyses
Statistical significance was determined using a Student's t test, and when multiple comparisons were made the p value was corrected using the Bonferroni correction.
RESULTS
B. burgdorferi SOD Activity Is Resistant to H2O2 and Cyanide
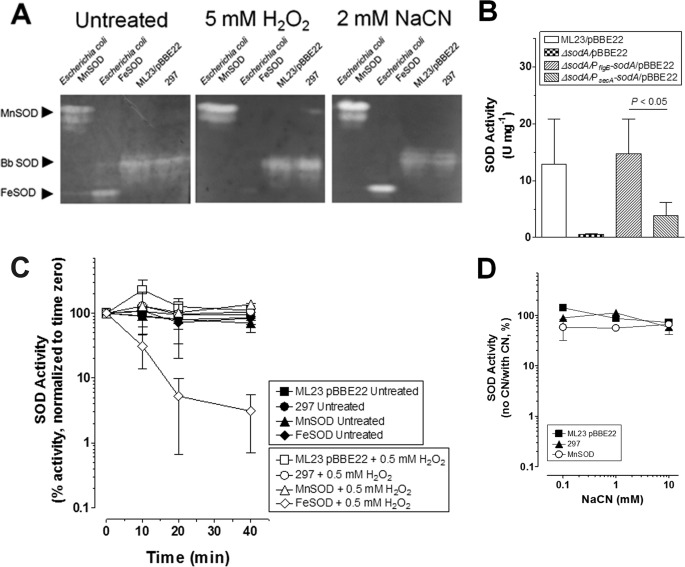
Previous work determined that B. burgdorferi SOD is a Fe-SOD based on its sensitivity to H2O2 and resistance to cyanide (18). However, subsequent work by Posey and Gherardini (19) demonstrated that B. burgdorferi is unable to transport iron and contains a very low quantity of iron, suggesting that iron is an unlikely cofactor for B. burgdorferi SodA. To resolve this contradiction, we first repeated earlier experiments using native PAGE followed by enzyme staining for SOD activity. We detected a single zone of SOD activity from B. burgdorferi strains 297 and B. burgdorferi ML23/pBBE22 (the parental strain of ΔsodA) (Fig. 1A). The zone of migration of SOD activity from the CFE of B. burgdorferi was slower than the Fe-SOD but faster than the Mn-SOD from E. coli. The migration differences can be attributed to the different pI for each protein (SodA from B. burgdorferi is 6.3, and SodA and SodB from E. coli are 6.9 and 5.9, respectively). Contrary to the earlier report showing that B. burgdorferi SOD activity is sensitive to H2O2, but resistant to cyanide treatment, a hallmark of Fe-SOD, we found that the SOD activity in both B. burgdorferi strains tested was resistant to H2O2 and cyanide (Fig. 1A).
FIGURE 1.
B. burgdorferi SOD activity is H2O2- and cyanide-resistant. A, cell-free extracts (400 μg) from ML23/pBBE22 (third lane) and 297 (fourth lane) were subjected to native PAGE followed by staining for SOD activity. Separate gels were left untreated or treated with 5 mm H2O2 or 2 mm NaCN for 1 h prior to and during staining to determine inhibition of SOD activity. Mn-SOD (first lane, 10 units) and Fe-Sod (second lane, 10 units) from E. coli were included as a controls. B, cell-free extracts from ML23/pBBE22 and derivatives were assayed for SOD activity by detecting the SOD-inhibited reduction of cytochrome c by X/XO. C, cell-free extracts from 297 and ML23/pBBE22 were assayed for H2O2-inhibitable SOD activity by detecting the SOD-inhibited reduction of nitro blue tetrazolium by X/XO. The samples were either left untreated or treated with 0.5 mm H2O2. The percentage of activity is shown compared with activity at time 0. Fe-SOD (H2O2-inhibited) and Mn-SOD (H2O2-resistant) from E. coli are shown as controls. Less than 2.5 μm of H2O2 was introduced into the assay for treated samples. D, cell-free extracts from 297 and ML23/pBBE22 were assayed for SOD activity that is inhibited by NaCN by detecting the SOD-inhibited reduction of nitro blue tetrazolium by X/XO. NaCN was included in the assay. The percentage of activity is shown compared with no NaCN addition. Mn-SOD (NaCN-resistant) from E. coli is shown as a control. The data shown in A are representative from separate experiments. The data in B and C are from separate biological samples prepared collected at different times (n = 3). A paired Student's t test was used to determine significance (p < 0.05).
To validate our result from the native PAGE method, we measured the SOD activity of B. burgdorferi in cell-free extracts based on the SOD inhibition of superoxide-dependent reduction of cytochrome c or nitro blue tetrazolium (6, 30). We first compared the SOD activity in wild-type (ML23/pBBE22), ΔsodA, and complemented strains (Fig. 1B). The SOD activity in the wild-type strain was readily detected; inactivation of the sodA gene virtually abolished such activity (Fig. 1B). This result validated the SOD activity assay and further suggests that SodA appears to be the major factor within B. burgdorferi for degrading O2˙̄. Interestingly, there was a significant difference between the two complemented strains (sodA driven by either secA or flaB promoter) (17).
Consistent with the result from native the PAGE experiment, the SOD activity from both B. burgdorferi strains showed resistance to a 0.5 mm H2O2 treatment (Fig. 1C). As controls, purified Fe-SOD from E. coli clearly demonstrated sensitivity to H2O2 treatment, whereas purified Mn-SOD from E. coli showed resistance to the same treatment (Fig. 1C). Similar to H2O2 treatment, B. burgdorferi SOD activity was highly resistant to cyanide treatment (Fig. 1D). The addition of high concentrations of cyanide did not inhibit the O2˙̄ reduction of nitro blue tetrazolium by the X/XO system (data not shown). These results strongly support B. burgdorferi SodA as a Mn-SOD, not a Cu-Zn or Fe-SOD.
The Manganese Transporter BmtA Influences B. burgdorferi SOD Activity and SodA Expression
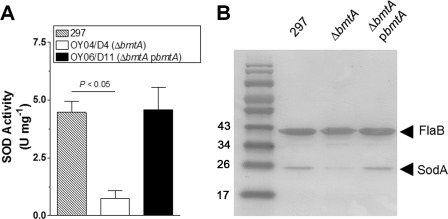
Recently, Ouyang et al. (26) reported BmtA as being responsible for manganese transport/homeostasis of B. burgdorferi. We postulated that if B. burgdorferi SodA is a Mn-SOD, BmtA should influence the SOD activity. We first replicated the findings by Ouyang et al. showing that deletion of bmtA reduces the intracellular manganese content by >12-fold compared with the parental and complemented strains (data not shown). To test the influence of BmtA on SOD activity in B. burgdorferi, the parental strain (297), ΔbmtA, and ΔbmtA pbmtA were cultivated in BSK-II medium, and cell-free extracts were prepared and assayed for SOD activity. The result showed that ΔbmtA had a significant reduction of ∼5-fold in activity, which was fully restored in the complemented strain (Fig. 2A). This result indicates that manganese is required for B. burgdorferi SOD activity, which is consistent with the above evidence that B. burgdorferi SodA is a Mn-SOD. In addition, we also determined the level of SodA in ΔbmtA. To our surprise, the level of SodA in ΔbmtA was greatly reduced (Fig. 2B). This finding indicates that unlike with E. coli and other bacteria (21, 22, 31), manganese level plays an important role in the regulation of SodA expression of B. burgdorferi.
FIGURE 2.
SOD activity and SodA expression is bmtA-dependent. A, samples were grown at 37 °C, and cell-free extracts were assayed for SOD activity (n = 3). B, expression of SodA was determined by Western blotting in samples from representative samples in A. A paired Student's t test was used to determine significance (p < 0.05).
Manganese in Medium Influences SodA Expression
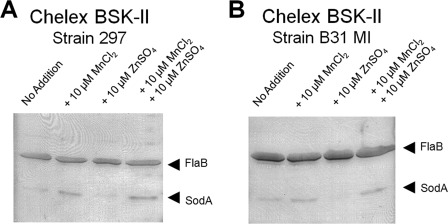
To gather additional evidence that B. burgdorferi SOD is a Mn-SOD, we altered exogenous manganese concentration in the medium and examined its impact on SodA expression. The medium for cultivation of B. burgdorferi, BSK-II medium is a complex, nutrient-rich environment that contains a variety of divalent cations including manganese. Thus, we first treated BSK-II medium with Chelex 100 resin to reduce the concentration of metals in the medium. The treatment of BSK-II medium did not significantly affect B. burgdorferi growth but reduced the concentration of manganese to below the limit of detection (which suggests that B. burgdorferi requires a very low level of manganese for its growth). B. burgdorferi strains 297 and B31-MI were inoculated into Chelex-treated BSK-II medium or the treated medium with addition of various concentrations of metals (Fig 3). Cultures were inoculated to an initial cell density of 105 cells ml−1, and SodA expression was determined after 5 days of growth (stationary phase). The results showed that SodA expression was induced in both strain 297 and B31-MI upon addition of MnCl2 to the Chelex-treated BSK-II medium (Fig. 3). The manganese affect is specific, as the addition of another metal, zinc, to Chelex-treated medium did not enhance SodA expression (rather it reduced its expression; Fig. 3). Furthermore, MnCl2 was sufficient to induce SodA expression in the presence of equimolar ZnSO4 in the medium (Fig. 3). These data further support the notion that B. burgdorferi SOD is a Mn-SOD and that manganese plays an important role in the regulation of SodA expression.
FIGURE 3.
SodA expression is manganese-dependent. A, strain 297 was grown for 7 days in Chelex-treated BSK-II medium with no metal addition (lane 1), with 10 μm MnCl2 (lane 2), with 10 μm ZnSO4 (lane 3), or with 10 μm MnCl2 and 10 μm ZnSO4 (lane 4). B, strain B31-MI was grown as in A with no metal addition (lane 1), with 10 μm MnCl2 (lane 2), with 10 μm ZnSO4 (lane 3), or with 10 μm MnCl2 and 10 μm ZnSO4 (lane 4). The samples were probed for FlaB and SodA expression.
SOD Activity Is Required for Resistance to Streptonigrin
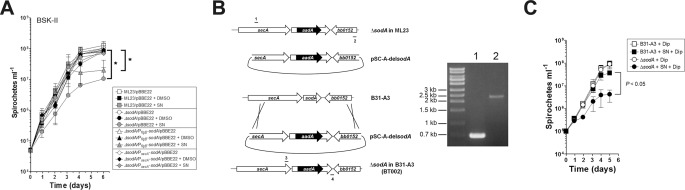
In E. coli, manganese transport is vital during oxidative stress and promotes Mn-SOD activity (22). Therefore, we tested this function via exposure to the redox cycling drug streptonigrin. Although earlier work demonstrated the importance of sodA in resistance to another redox cycling compound methyl viologen (17), such an experiment requires a high concentration of methyl viologen (20 mm). This is because entry of this compound into the cell is known to require a transporter (32) and B. burgdorferi appears to lack such a transporter (17). In contrary, streptonigrin is a hydrophobic, O2˙̄-generating compound (33), which can diffuse into the cell and elicits robust O2˙̄ production inside the cell. Indeed, we found that ΔsodA was sensitive to 10 μg ml−1 (19.7 μm) of streptonigrin (Fig. 4). This defect was partially complemented by expressing sodA under control of the promoter of flgB or was fully complemented by expressing sodA under control of the secA promoter.
FIGURE 4.
SOD activity is required for resistance to streptonigrin. A, ML23/pBBE22, sodA/pBBE22, and complemented strains were inoculated into BSK-II medium containing nothing, dimethyl sulfoxide (control, DMSO), or streptonigrin (10 μg ml−1, SN), and growth was monitored over time. The data are from separate experiments with different batches of BSK-II medium (n = 5). An asterisk indicates a significant difference, corrected with Bonferroni correction, in the final cell density of the sample compared with streptonigrin compared with the parental strain with streptonigrin (p < 0.025). B, schematic and confirmation of the generation of ΔsodA in a B31-A3 background (strain BT002). sodA::aadA was transferred from ΔsodA in the ML23 strain as described under “Experimental Procedures” (left panel). PCR with primers 1 and 3 confirmed the insertion of the aadA marker within the coding region of sodA. Lane 1, B31-A3; lane 2, strain BT002 (ΔsodA). The ∼2.3-kb DNA fragment corresponds to sodA::aadA, whereas the 0.7-kb DNA fragment is present in the parental strain B31-A3 (right panel). C, growth of B31-A3 with 300 μm dip in the presence or absence of streptonigrin (10 μg ml−1). Growth was monitored over time by cell enumeration with dark field microscopy. An asterisk indicates significant difference in the final cell density of sample with dip and streptonigrin compared with the parental strain with dip and streptonigrin (p < 0.05).
To confirm our results with strains derived from the ML23 background, we constructed another ΔsodA strain in B31-A3 background as described under “Experimental Procedures” and shown in Fig. 4B. To test whether iron is involved in the toxicity of streptonigrin, we included the ferrous iron chelator dip in our experiments. Research demonstrates that the toxicity of streptonigrin can be dramatically reduced when a ferrous iron chelator desferrioxamine or dip is added immediately prior to streptonigrin (34, 35). When B31-A3 and BT002 were grown in the presence of 300 μm of dip, no difference in growth between strains was observed (Fig. 4C). However, when streptonigrin was included, strain BT002 exhibited a significant defect in growth (Fig. 4C). These results support our findings with ΔsodA in the ML23 background regarding sensitivity to streptonigrin and support the hypothesis for the lack of iron in the toxicity of streptonigrin within B. burgdorferi (19). If iron was involved in the toxicity of ΔsodA, then dip should protect against streptonigrin. This was not our result; strain BT002 exhibited a growth defect similar to that of ΔsodA in the ML23 background.
We tested whether ΔbmtA was able to grow in the presence of streptonigrin, because this strain has reduced SOD activity and SodA expression. Indeed, similar to the ΔsodA strains, ΔbmtA exhibited a pronounced growth defect in the presence of streptonigrin (data not shown). Thus, manganese transport appears important in response to oxidative stress, as is the case in E. coli (22). We conclude that SodA and the manganese transporter BmtA are both important to the protection against the O2˙̄ produced by the redox cycling compound streptonigrin.
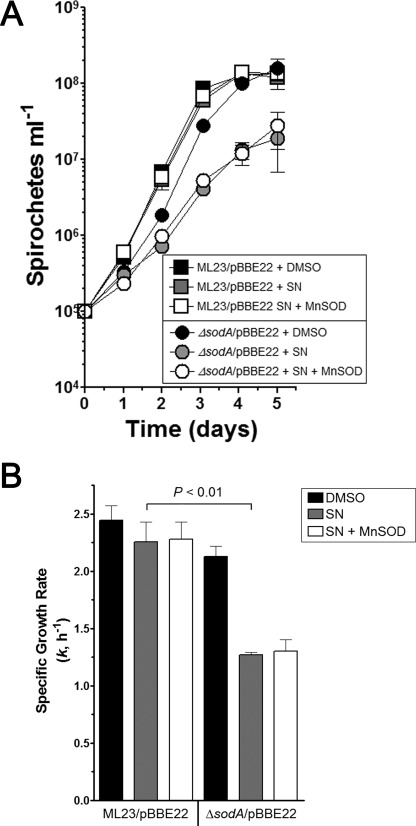
Membrane-associated polyunsaturated fatty acids have been shown to be the major target for ROS damage in B. burgdorferi (36). To generate ROS, streptonigrin needs to be reduced, which is favored by the intracellular environment (37–40). Furthermore, O2˙̄ has limited permeability of biological membranes, and the B. burgdorferi Mn-SOD is not membrane-associated (41). The results above suggest that B. burgdorferi Mn-SOD protects intracellular targets, rather than the membrane, from ROS damage. However, it is possible that reduced streptonigrin may pass from the intracellular compartment of B. burgdorferi to the extracellular milieu and interact with transition metals to form O2˙̄ (40). To test whether streptonigrin could damage an extracellular or intracellular component of B. burgdorferi, we conducted the same experiment by including Mn-SOD (75 units ml−1) from E. coli in the BSK-II medium in the presence of streptonigrin. As shown in Fig. 5, the addition of exogenous Mn-SOD provided no protection against the toxicity of streptonigrin, suggesting that the toxic effect of streptonigrin likely occurs within the cell.
FIGURE 5.
Exogenous Mn-SOD does not protect sodA from streptonigrin-dependent stress. ML23/pBBE22 and sodA strains were inoculated into BSK-II medium containing dimethyl sulfoxide (control, DMSO), streptonigrin (10 μg ml−1, SN) or streptonigrin plus Mn-SOD (75 units ml−1), and growth was monitored over time by cell enumeration with dark field microscopy. The data are from separate experiments from two with separate batches of medium (n = 3). B, data from A were used to determine the specific growth rate (k, h−1) for each sample. A paired Student's t test was used to determine significance between ML23/pBBE22 + SN and ΔsodA/pBBE22 + SN (p < 0.01).
The Mechanism of Toxicity of Streptonigrin in ΔsodA
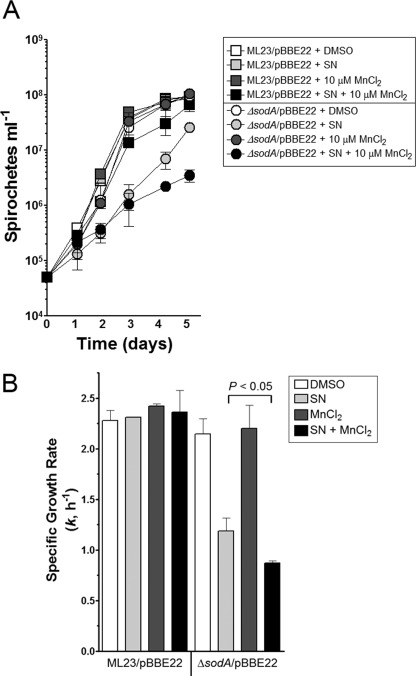
Streptonigrin is a metal-dependent redox cycling compound that produces ROS (38, 42–45). The most studied metal involved in streptonigrin toxicity is iron. Moreover, the study of the toxicity of streptongrin has been reserved for cells that require iron or contain high concentrations of iron (i.e., E. coli). Because B. burgdorferi appears not to require iron and does not actively transport iron (19), it is unlikely that iron is facilitating the toxicity of streptonigrin (Fig. 4C). However, B. burgdorferi does transport manganese (19, 26). Therefore, we reasoned that manganese could play a role in the toxicity of streptonigrin. Indeed, earlier work suggests that manganese can facilitate DNA binding of streptonigrin and increase the toxicity of streptonigrin (46). To test whether manganese contributes to the toxicity of streptonigrin, we used Chelex-treated BSK-II with or without added MnCl2. The samples were cultivated in Chelex-treated BSK-II with or without streptonigrin in the presence or absence of MnCl2. As shown in Fig. 6, when streptonigrin and manganese were added to the medium compared with streptonigrin alone, the ΔsodA strain exhibited a slower growth rate and reduced final cell density. As a control, the addition of manganese alone did not influence the parent strain or ΔsodA. Moreover, even in the wild-type parental strain, a slight reduction in growth was also observed when cultivated in the presence of both streptonigrin and manganese, suggesting that, in excess, manganese may overcome the protection by SOD against streptonigrin toxicity (Fig. 6).
FIGURE 6.
Manganese exacerbates the toxicity of streptonigrin in sodA. A, ML23/pBBE22 and sodA/pBBE22 were grown in Chelex-treated BSK-II medium containing dimethyl sulfoxide (control, DMSO), 10 μm MnCl2, streptonigrin (10 μg ml−1, SN), or 10 μm MnCl2 and SN. Growth was monitored over time. The data are from separate experiments with different batches of medium (n = 3). B, data from A were used to determine the specific growth rate (k, h−1) for each sample. A paired Student's t test was used to determine the significance between ΔsodA/pBBE22 + SN and ΔsodA/pBBE22 + SN + MnCl (p < 0.05).
The above data show that manganese contributes to the toxicity of streptonigrin in B. burgdorferi. Because SodA binds manganese, SodA may also protect cells against streptonigrin toxicity through sequestering manganese, thereby reducing redox cycling and production of ROS by streptonigrin. However, we found that ΔsodA has a significant reduction in intracellular manganese compared with that of the parental strain (0.21 ± 0.003 for ML23/pBBE22 and 0.13 ± 0.03 μmol/g of dry weight), and yet ΔsodA is more sensitive to streptonigrin compared with that of the wild-type strain. Thus, although manganese can contribute to the toxicity of streptonigrin, manganese is not the only metal associated with streptonigrin toxicity. These data suggest that the sensitivity of ΔsodA to streptonigrin is not due to a possible lacking of manganese sequestration, but rather it supports the conclusion that SOD protects against the O2˙̄ produced by streptonigrin.
DISCUSSION
Much work has been devoted to the role of ROS in pathogenic bacteria. However, most of these works have focused on the ROS response of bacteria that posses a TCA cycle and require iron. A key aspect of the ROS response revolves around the iron status within the pathogen because of the propagation of Fenton chemistry. This aspect of ROS response by bacterial pathogens is where the Lyme disease spirochete is unique. B. burgdorferi has apparently evolved without the need for iron (19). Thus, targets of ROS and the response by this pathogen may pose a unique system to discern aspects of oxidative stress in the absence of iron. To gain insight into ROS response in B. burgdorferi, we focused on the only SOD of B. burgdorferi, SodA. In this study, we provide biochemical and genetic evidence showing that the B. burgdorferi SOD is a manganese-dependent enzyme and that the manganese concentration dictates the level of SodA production in the cell. Furthermore, we have determined that streptonigrin, a redox cycling compound involved in metal-dependent toxicity, imposes an intracellular superoxide stress that requires SOD activity for protection. Our work indicates that this toxicity can be influenced by manganese in this iron-lacking pathogen.
Although earlier work demonstrated that Borrelia SOD activity was H2O2-sensitive and cyanide-resistant, characteristic of a Fe-SOD (18, 47), we were unable to reproduce these results. The reasons for this discrepancy are unclear. It has been reported that Mn-SODs can be partially inhibited by H2O2 during SOD activity staining following native PAGE, because of an uninvestigated interference (48). Therefore, the results obtained using native SOD staining should be confirmed with an independent method. In this study, we confirmed our H2O2-resistant results by measuring SOD activity quantitatively using cell-free extracts. Our results strongly suggest that B. burgdorferi SodA requires manganese for activity. Although we have not ruled out the possibility that SodA may be a cambialistic SOD (i.e., requiring either manganese or Fe for its activity), this is unlikely given the very low iron content of B. burgdorferi and the lack of any known iron uptake system encoded in the genome (16, 19).
It has been well established that SODs protect the key biosynthetic pathways necessary for growth of bacteria that posses iron sulfur cluster enzymes (9, 11). These enzymes are inhibited by aerobic conditions in minimal medium upon deletion of cytoplasmic SODs, which can be ablated by providing a nutrient-rich medium. However, in bacteria lacking iron-containing metabolic enzymes the targets of superoxide damage are subject to debate. Why does iron-free B. burgdorferi require SOD? Earlier work demonstrated that the polyunsaturated lipids of B. burgdorferi are damaged by ROS (36). If polyunsaturated fatty acids are the sole target of O2˙̄-mediated damage for B. burgdorferi, this selective pressure should have evolved for a periplasmic or membrane-bound SodA to protect. Although subcellular localization of SodA was not examined in this study, Mn-SOD has been well known to be an intracellular SOD in other bacteria (49), and several lines of evidence suggest that SodA is a cytoplasmic SOD. First, cell-free extracts used in this study were not enriched for membrane fractions, and SOD activity was readily detected. Second, sodA is required for resistance to streptonigrin, a redox cycling compound whose toxicity requires a continuous supply of electrons provided by the intracellular environment of a metabolically active cell (37, 50). Third, the addition of exogenous Mn-SOD to the growth medium did not rescue the growth defect of ΔsodA in the presence of streptonigrin. Finally, the presence of SodA was not identified in membrane-associated fractions from B. burgdorferi but was identified in the cytoplasm (41, 51).
The toxicity of redox cycling compounds, like methyl viologen, requires a carbon source that can be metabolized by the cell (50). This concept of a catabolic carbon source in the toxicity of another group of redox cycling compounds, aminoglycosides (52), has been recently studied (53). Our finding, along with the previous finding by Esteve-Gassent et al. (17), demonstrates that SOD protects against toxicity of streptonigrin or methyl viologen. These data suggest that the targets of superoxide for this iron-free organism may be intracellular.
Our finding that streptonigrin is toxic to B. burgdorferi is significant, because it is known that toxicity is dependent on iron (54). Streptonigrin is produced by the bacterium Streptomyces flocculus, possibly as a means to thwart competition among neighboring organisms (55, 56). Streptonigrin and many of these naturally produced compounds promote ROS within a target cell (38). NADH may serve to continue redox cycling of streptonigrin (37, 57), and the reduction in steady-state intracellular NADH may contribute to the toxicity of streptonigrin. Indeed, current work in our lab demonstrates that strains of B. burgdorferi are sensitive to another redox cycling compound, phenazine methosulfate (data not shown), which can be reduced by NADH. The reduction of phenazines by NADH is the basis for NAD+/NADH cycling assays used to measure the intracellular NAD+ and NADH concentrations (58, 59). Interestingly, Pseudomonas aeruginosa produces a phenazine compound, known as pyocyanin, to consume intracellular NADH and maintain redox homeostasis (60).
Although the metal-redox cycling events leading to O2˙̄ production by streptonigrin in B. burgdorferi remain to be elucidated, the results from this study show that manganese contributes to the toxicity of streptonigrin, but there may be at least one other metal that contributes to the redox cycling of streptonigrin. We routinely detect intracellular copper within several strains of B. burgdorferi during in vitro cultivation. Moreover, the intracellular content of copper appears regulated (data not shown). Therefore, the presence of copper within ΔbmtA can explain the toxicity of streptonigrin for ΔbmtA, which contains very low concentrations of manganese but is sensitive to streptronigrin, likely because of the reduced Mn-SOD activity.
Our results provide the first observation that B. burgdorferi, an “iron-free” organism, is sensitive to streptonigrin when the protectant SodA is abrogated. Our findings raise several intriguing questions. What are the intracellular targets of ROS in B. burgdorferi? Given the small genome and highly reduced biosynthetic capabilities of B. burgdorferi, the number of ROS targets are likely to be small. However, it is unknown whether there is a single target that upon damage creates a metabolic “bottleneck,” stunting growth, or whether there are multiple targets that contribute to an overall state of impaired metabolism. Regardless of the ROS targets, this pathogen presents a unique opportunity to the study of redox cycling compounds because of the near absence of cellular iron in B. burgdorferi.
What metals contribute to the toxicity of streptonigrin? We have shown that manganese can enhance the sensitivity of ΔsodA to streptonigrin, but this does not explain the results with ΔbmtA, which contains very low concentrations of manganese and Mn-SOD activity. Our ability to detect copper in several strains of B. burgdorferi suggests that this transition metal may be involved in streptonigrin toxicity. Furthermore, given the similarities of copper and iron in redox biology, it is intriguing to speculate that this pathogen may have evolved to utilize copper instead of iron to fulfill the requirement of transition metals in redox reactions. Future work in this pathogen may yield unique findings on the mechanism of redox cycling drugs and targets of ROS that are independent of iron.
Acknowledgments
We thank Drs. Zhiming Ouyang, Michael Norgard, and Janakiram Seshu for strains. We thank Dr. Robarge and Kim Hutchison at North Carolina State University for inductively coupled plasma MS analysis. Discussions with Drs. Xin Li, Greg A. Somerville, and Hosni M. Hassan regarding streptonigrin were appreciated. We thank Dr. Donald Becker for the suggestion to detect copper in B. burgdorferi. We thank Jolelyn Khoo and Junjie Zhang for careful reading of this manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 AI083640 (to X. F. Y.). This work was also supported by Indiana INGEN and METACyt grants from Indiana University, funded by the Lilly Endowment, Inc. (to X. F. Y.) and in part by Grant C06 RR015481-01 from National Center for Research Resources, National Institutes of Health.
- ROS
- reactive oxygen species
- SOD
- superoxide dismutase
- dip
- 2,2′-dipyridyl
- X/XO
- xanthine/xanthine oxidase
- CFE
- cell-free extract.
REFERENCES
- 1. McCord J. M., Fridovich I. (1969) Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 244, 6049–6055 [PubMed] [Google Scholar]
- 2. Yost F. J., Jr., Fridovich I. (1973) An iron-containing superoxide dismutase from Escherichia coli. J. Biol. Chem. 248, 4905–4908 [PubMed] [Google Scholar]
- 3. Keele B. B., Jr., McCord J. M., Fridovich I. (1970) Superoxide dismutase from Escherichia coli B. A new manganese-containing enzyme. J. Biol. Chem. 245, 6176–6181 [PubMed] [Google Scholar]
- 4. Youn H. D., Kim E. J., Roe J. H., Hah Y. C., Kang S. O. (1996) A novel nickel-containing superoxide dismutase from Streptomyces spp. Biochem. J. 318, 889–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martin M. E., Byers B. R., Olson M. O., Salin M. L., Arceneaux J. E., Tolbert C. (1986) A Streptococcus mutans superoxide dismutase that is active with either manganese or iron as a cofactor. J. Biol. Chem. 261, 9361–9367 [PubMed] [Google Scholar]
- 6. Asada K., Yoshikawa K., Takahashi M., Maeda Y., Enmanji K. (1975) Superoxide dismutases from a blue-green alga, Plectonema boryanum. J. Biol. Chem. 250, 2801–2807 [PubMed] [Google Scholar]
- 7. Benov L. T., Fridovich I. (1994) Escherichia coli expresses a copper- and zinc-containing superoxide dismutase. J. Biol. Chem. 269, 25310–25314 [PubMed] [Google Scholar]
- 8. Carlioz A., Touati D. (1986) Isolation of superoxide dismutase mutants in Escherichia coli. Is superoxide dismutase necessary for aerobic life? EMBO J. 5, 623–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gardner P. R., Fridovich I. (1991) Superoxide sensitivity of the Escherichia coli 6-phosphogluconate dehydratase. J. Biol. Chem. 266, 1478–1483 [PubMed] [Google Scholar]
- 10. Gardner P. R., Fridovich I. (1991) Superoxide sensitivity of the Escherichia coli aconitase. J. Biol. Chem. 266, 19328–19333 [PubMed] [Google Scholar]
- 11. Benov L., Fridovich I. (1999) Why superoxide imposes an aromatic amino acid auxotrophy on Escherichia coli. The transketolase connection. J. Biol. Chem. 274, 4202–4206 [DOI] [PubMed] [Google Scholar]
- 12. Gregory E. M., Moore W. E., Holdeman L. V. (1978) Superoxide dismutase in anaerobes. Survey. Appl. Environ. Microbiol. 35, 988–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Radolf J. D., Caimano M. J., Stevenson B., Hu L. T. (2012) Of ticks, mice and men. Understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat. Rev. Microbiol. 10, 87–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Steere A. C., Coburn J., Glickstein L. (2004) The emergence of Lyme disease. J. Clin. Invest. 113, 1093–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Narasimhan S., Sukumaran B., Bozdogan U., Thomas V., Liang X., DePonte K., Marcantonio N., Koski R. A., Anderson J. F., Kantor F., Fikrig E. (2007) A tick antioxidant facilitates the Lyme disease agent's successful migration from the mammalian host to the arthropod vector. Cell Host Microbe 2, 7–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fraser C. M., Casjens S., Huang W. M., Sutton G. G., Clayton R., Lathigra R., White O., Ketchum K. A., Dodson R., Hickey E. K., Gwinn M., Dougherty B., Tomb J. F., Fleischmann R. D., Richardson D., Peterson J., Kerlavage A. R., Quackenbush J., Salzberg S., Hanson M., van Vugt R., Palmer N., Adams M. D., Gocayne J., Weidman J., Utterback T., Watthey L., McDonald L., Artiach P., Bowman C., Garland S., Fuji C., Cotton M. D., Horst K., Roberts K., Hatch B., Smith H. O., Venter J. C. (1997) Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390, 580–586 [DOI] [PubMed] [Google Scholar]
- 17. Esteve-Gassent M. D., Elliott N. L., Seshu J. (2009) sodA is essential for virulence of Borrelia burgdorferi in the murine model of Lyme disease. Mol. Microbiol. 71, 594–612 [DOI] [PubMed] [Google Scholar]
- 18. Whitehouse C. A., Williams L. R., Austin F. E. (1997) Identification of superoxide dismutase activity in Borrelia burgdorferi. Infect. Immun. 65, 4865–4868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Posey J. E., Gherardini F. C. (2000) Lack of a role for iron in the Lyme disease pathogen. Science 288, 1651–1653 [DOI] [PubMed] [Google Scholar]
- 20. Barrière C., Brückner R., Talon R. (2001) Characterization of the single superoxide dismutase of Staphylococcus xylosus. Appl. Environ. Microbiol. 67, 4096–4104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Privalle C. T., Fridovich I. (1993) Iron specificity of the Fur-dependent regulation of the biosynthesis of the manganese-containing superoxide dismutase in Escherichia coli. J. Biol. Chem. 268, 5178–5181 [PubMed] [Google Scholar]
- 22. Anjem A., Varghese S., Imlay J. A. (2009) Manganese import is a key element of the OxyR response to hydrogen peroxide in Escherichia coli. Mol. Microbiol. 72, 844–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Casjens S., Palmer N., van Vugt R., Huang W. M., Stevenson B., Rosa P., Lathigra R., Sutton G., Peterson J., Dodson R. J., Haft D., Hickey E., Gwinn M., White O., Fraser C. M. (2000) A bacterial genome in flux. The twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35, 490–516 [DOI] [PubMed] [Google Scholar]
- 24. Elias A. F., Stewart P. E., Grimm D., Caimano M. J., Eggers C. H., Tilly K., Bono J. L., Akins D. R., Radolf J. D., Schwan T. G., Rosa P. (2002) Clonal polymorphism of Borrelia burgdorferi strain B31 MI. Implications for mutagenesis in an infectious strain background. Infect. Immun. 70, 2139–2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Burgdorfer W., Barbour A. G., Hayes S. F., Benach J. L., Grunwaldt E., Davis J. P. (1982) Lyme disease-a tick-borne spirochetosis? Science 216, 1317–1319 [DOI] [PubMed] [Google Scholar]
- 26. Ouyang Z., He M., Oman T., Yang X. F., Norgard M. V. (2009) A manganese transporter, BB0219 (BmtA), is required for virulence by the Lyme disease spirochete, Borrelia burgdorferi. Proc. Natl. Acad. Sci. U.S.A. 106, 3449–3454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Samuels D. S. (1995) Electrotransformation of the spirochete Borrelia burgdorferi. Methods Mol. Biol. 47, 253–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang X. F., Pal U., Alani S. M., Fikrig E., Norgard M. V. (2004) Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J. Exp. Med. 199, 641–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barbour A. G. (1984) Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol. Med. 57, 521–525 [PMC free article] [PubMed] [Google Scholar]
- 30. Beauchamp C., Fridovich I. (1971) Superoxide dismutase. Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 44, 276–287 [DOI] [PubMed] [Google Scholar]
- 31. Barrière C., Leroy-Sétrin S., Talon R. (2001) Characterization of catalase and superoxide dismutase in Staphylococcus carnosus 833 strain. J. Appl. Microbiol. 91, 514–519 [DOI] [PubMed] [Google Scholar]
- 32. Kao S. M., Hassan H. M. (1985) Biochemical characterization of a paraquat-tolerant mutant of Escherichia coli. J. Biol. Chem. 260, 10478–10481 [PubMed] [Google Scholar]
- 33. Hassan H. M., Fridovich I. (1977) Enzymatic defenses against the toxicity of oxygen and of streptonigrin in Escherichia coli. J. Bacteriol. 129, 1574–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. White J. R., Yeowell H. N. (1982) Iron enhances the bactericidal action of streptonigrin. Biochem. Biophys. Res. Commun. 106, 407–411 [DOI] [PubMed] [Google Scholar]
- 35. Ngok-Ngam P., Ruangkiattikul N., Mahavihakanont A., Virgem S. S., Sukchawalit R., Mongkolsuk S. (2009) Roles of Agrobacterium tumefaciens RirA in iron regulation, oxidative stress response, and virulence. J. Bacteriol. 191, 2083–2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Boylan J. A., Lawrence K. A., Downey J. S., Gherardini F. C. (2008) Borrelia burgdorferi membranes are the primary targets of reactive oxygen species. Mol. Microbiol. 68, 786–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. White H. L., White J. R. (1968) Lethal action and metabolic effects of streptonigrin on Escherichia coli. Mol. Pharmacol. 4, 549–565 [PubMed] [Google Scholar]
- 38. Hassan H. M., Fridovich I. (1979) Intracellular production of superoxide radical and of hydrogen peroxide by redox active compounds. Arch Biochem. Biophys. 196, 385–395 [DOI] [PubMed] [Google Scholar]
- 39. Hassett D. J., Charniga L., Bean K., Ohman D. E., Cohen M. S. (1992) Response of Pseudomonas aeruginosa to pyocyanin: mechanisms of resistance, antioxidant defenses, and demonstration of a manganese-cofactored superoxide dismutase. Infect. Immun. 60, 328–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cohen M. S., Chai Y., Britigan B. E., McKenna W., Adams J., Svendsen T., Bean K., Hassett D. J., Sparling P. F. (1987) Role of extracellular iron in the action of the quinone antibiotic streptonigrin. Mechanisms of killing and resistance of Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 31, 1507–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nowalk A. J., Nolder C., Clifton D. R., Carroll J. A. (2006) Comparative proteome analysis of subcellular fractions from Borrelia burgdorferi by NEPHGE and IPG. Proteomics 6, 2121–2134 [DOI] [PubMed] [Google Scholar]
- 42. Gregory E. M., Fridovich I. (1973) Oxygen toxicity and the superoxide dismutase. J. Bacteriol. 114, 1193–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bachur N. R., Gordon S. L., Gee M. V., Kon H. (1979) NADPH cytochrome P-450 reductase activation of quinone anticancer agents to free radicals. Proc. Natl. Acad. Sci. U.S.A. 76, 954–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gutteridge J. M. (1984) Streptonigrin-induced deoxyribose degradation. Inhibition by superoxide dismutase, hydroxyl radical scavengers and iron chelators. Biochem. Pharmacol. 33, 3059–3062 [DOI] [PubMed] [Google Scholar]
- 45. Hassett D. J., Britigan B. E., Svendsen T., Rosen G. M., Cohen M. S. (1987) Bacteria form intracellular free radicals in response to paraquat and streptonigrin. Demonstration of the potency of hydroxyl radical. J. Biol. Chem. 262, 13404–13408 [PubMed] [Google Scholar]
- 46. White J. R. (1977) Streptonigrin-transition metal complexes. Binding to DNA and biological activity. Biochem. Biophys. Res. Commun. 77, 387–391 [DOI] [PubMed] [Google Scholar]
- 47. Austin F. E., Barbieri J. T., Corin R. E., Grigas K. E., Cox C. D. (1981) Distribution of superoxide dismutase, catalase, and peroxidase activities among Treponema pallidum and other spirochetes. Infect. Immun. 33, 372–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chang S. K., Hassan H. M. (1997) Characterization of superoxide dismutase in Streptococcus thermophilus. Appl. Environ. Microbiol. 63, 3732–3735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gregroy E. M., Yost F. J., Jr., Fridovich I. (1973) Superoxide dismutases of Escherichia coli. Intracellular localization and functions. J. Bacteriol. 115, 987–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hassan H. M., Fridovich I. (1978) Superoxide radical and the oxygen enhancement of the toxicity of paraquat in Escherichia coli. J. Biol. Chem. 253, 8143–8148 [PubMed] [Google Scholar]
- 51. Jewett M. W., Byram R., Bestor A., Tilly K., Lawrence K., Burtnick M. N., Gherardini F., Rosa P. A. (2007) Genetic basis for retention of a critical virulence plasmid of Borrelia burgdorferi. Mol. Microbiol. 66, 975–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sha S. H., Schacht J. (1999) Stimulation of free radical formation by aminoglycoside antibiotics. Hear Res. 128, 112–118 [DOI] [PubMed] [Google Scholar]
- 53. Allison K. R., Brynildsen M. P., Collins J. J. (2011) Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature 473, 216–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yeowell H. N., White J. R. (1982) Iron requirement in the bactericidal mechanism of streptonigrin. Antimicrob. Agents Chemother. 22, 961–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. de Paiva S. R., Figueiredo M. R., Aragão T. V., Kaplan M. A. (2003) Antimicrobial activity in vitro of plumbagin isolated from Plumbago species. Mem. Inst. Oswaldo Cruz 98, 959–961 [DOI] [PubMed] [Google Scholar]
- 56. Inbaraj J. J., Chignell C. F. (2004) Cytotoxic action of juglone and plumbagin: a mechanistic study using HaCaT keratinocytes. Chem. Res. Toxicol. 17, 55–62 [DOI] [PubMed] [Google Scholar]
- 57. Cone R., Hasan S. K., Lown J. W., Morgan A. R. (1976) The mechanism of the degradation of DNA by streptonigrin. Can. J. Biochem. 54, 219–223 [DOI] [PubMed] [Google Scholar]
- 58. Bernofsky C., Swan M. (1973) An improved cycling assay for nicotinamide adenine dinucleotide. Anal. Biochem. 53, 452–458 [DOI] [PubMed] [Google Scholar]
- 59. Leonardo M. R., Dailly Y., Clark D. P. (1996) Role of NAD in regulating the adhE gene of Escherichia coli. J. Bacteriol. 178, 6013–6018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Price-Whelan A., Dietrich L. E., Newman D. K. (2007) Pyocyanin alters redox homeostasis and carbon flux through central metabolic pathways in Pseudomonas aeruginosa PA14. J. Bacteriol. 189, 6372–6381 [DOI] [PMC free article] [PubMed] [Google Scholar]