Background: The transcriptional factor Prz1 is activated by calcineurin, whereas other regulators of Prz1 are unknown.
Results: Pka1, Rad24, Rad25, Msn5, Pac1, Ape2, and Tfs1 are isolated as dosage-dependent suppressors of the slow growth phenotype caused by Prz1 overexpression.
Conclusion: Prz1 is regulated by proteins involved in various biological processes.
Significance: Identification of regulators of Prz1 is crucial for understanding calcineurin-mediated signal transduction.
Keywords: Calcineurin, Protein-Protein Interactions, Transcription Factors, Yeast, Zinc Finger, Prz1
Abstract
Calcineurin phosphatase plays crucial roles in a wide variety of cell types and organisms. Dephosphorylation of the nuclear factor of activated T-cell (NFAT) family of transcriptional factors by calcineurin is essential for activating immune-responsive genes in mammals. NFAT activity is also regulated by diverse signaling pathways, which affect NFAT kinases and nuclear partner proteins. In fission yeast, calcineurin dephosphorylates and activates Prz1, a C2H2-type zinc finger transcriptional factor. Calcineurin-Prz1 signaling regulates the expression of the Pmc1 Ca2+ pump. Prz1-overexpressing cells showed extremely slow growth and high transcriptional activity of Prz1 in the absence of stimulation. Here, we isolated seven genes as dosage-dependent suppressors of this slow growth phenotype. These seven genes encode Rad24, Rad25, Pka1, Msn5 (SPAC328.01c), Pac1, Ape2, and Tfs1. All of them decreased the high transcriptional activity caused by Prz1 overexpression. Overexpression of Pka1, Rad24, and Rad25 also repressed the Ca2+-induced transcriptional activity in cells with Prz1 expressed at wild-type levels. Knock-out of rad24 or rad25 significantly enhanced the transcriptional activity of Prz1, whereas knock-out or mutation of other genes did not enhance the activity. The 14-3-3 proteins, Rad24 and Rad25, bound Prz1 and the Rad24-binding site located at residues 421–426 of Prz1. In msn5 deletion mutants, GFP-Prz1 localized at nucleus in the absence of Ca2+ stimulation, suggesting that Msn5 functions as an exportin for Prz1. In summary, our data suggest that Rad24 and Rad25 negatively regulate Prz1 and that Pka1, Msn5, Pac1, Tfs1, and Ape2 also regulate Prz1.
Introduction
The Ca2+/calmodulin-dependent protein phosphatase calcineurin is conserved in most eukaryotic species. In humans and other mammals, calcineurin plays important roles in various Ca2+-mediated processes including T-cell activation (1, 2), cardiac hypertrophy (3), neutrophil chemotaxis (4), apoptosis (5), angiogenesis (6), and memory development (7). For many of these cellular events, calcineurin exerts its functions by dephosphorylating NFAT3 family members. Dephosphorylated NFATs cause translocation from the cytoplasm to the nucleus (8). This event results in nuclear accumulation of NFAT and transcription of NFAT target genes. When Ca2+ entry is prevented or calcineurin is inhibited, NFAT is rephosphorylated by NFAT kinases, such as casein kinase 1 (9), glycogen synthase kinase 3 (GSK3) (10), dual specificity tyrosine phosphorylation regulated kinase (DYRK) (11), and MAP kinases p38 (12) and JNK (13). Then NFAT is rapidly exported to the nucleus by the nuclear export receptor CRM1 (14), and NFAT-dependent gene expression is terminated. Thus, many proteins interact with NFAT to negatively regulate the transcriptional activity.
We have been studying calcineurin signaling pathway in fission yeast Schizosaccharomyces pombe. Transcriptional factor Prz1 (Ppb1-responsive zinc finger protein) is dephosphorylated and activated by calcineurin and regulates the transcription of genes involved in calcium homeostasis (15). However, other regulatory mechanisms of Prz1 are not clarified.
In our previous study, we showed that overexpression of constitutively active calcineurin caused growth inhibition and aberrant cell morphology in wild-type cells but not in prz1 deletion cells (16). These results suggest that the slow growth phenotype caused by overexpression of constitutively active calcineurin is mediated by Prz1. In the present study, we demonstrated that overexpression of Prz1 also caused similar slow growth phenotype. Notably, our results revealed that calcineurin itself is not necessary (and is not sufficient) to cause the defective growth. We hypothesized that hyperactivity, caused either by constitutive calcineurin or Prz1, activates perhaps multiple target gene(s) that cause this phenotype. Thus, eliminating or reducing the expression or activity of overexpressed Prz1 provides an avenue to identify regulators of Prz1 activity. Then we performed a genetic screen to isolate regulators of Prz1. As a result of the screening, we identified seven genes that encode Pka1, Rad24, Rad25, Msn5 (SPAC328.01c), Ape2, Pac1, and Tfs1 as dosage-dependent suppressors of the slow growth phenotype.
EXPERIMENTAL PROCEDURES
Stains, Media, and Miscellaneous Procedures
The S. pombe strains used in this study are listed in Table 1. An S. pombe haploid strain in which the rad24+, rad25+, pka1+, ape2+, or tfs1+ gene had been deleted was purchased from Bioneer (Daejeon, Korea) or the Yeast Genetic Resource Center (Japan). The complete medium yeast extract/peptone/dextrose and the minimal medium EMM have been described previously (17). Standard methods for S. pombe genetics were followed according to Moreno et al. (18). FK506 (tacrolimus) was provided by the Astellas Pharmaceutical Co. (Osaka, Japan).
TABLE 1.
Strains used in this study
| Strain | Genotype | Reference |
|---|---|---|
| HM123 | h−leu1-32 | Our stock |
| KP1248 | h−leu1-32 ura4-294 | Our stock |
| KP1395 | h−leu1-32 ura4-294 ura4+::nmt1-GFP-prz1+ | Ref. 15 |
| KP2670 | h−leu1-32 ura4-294 arg1-1 ura4+::nmt1-GFP-ppb1ΔC arg1+::nmt1-GST-cnb1 | This study |
| KP2741 | h−leu1-32 arg1-1 arg1+::3xCRE::luc (R2.2) | This study |
| KP2755 | h−leu1-32 arg1-1 arg1+::3xCDRE::luc (R2.2) | Ref. 37 |
| KP2795 | h−leu1-32 ura4-D18 msn5::ura4+ | This study |
| KP2888 | h−leu1-32 ura4-D18 isp6::ura4+psp3::ura4+ | This study |
| KP2921 | h−leu1-32 ura4-D18 pka1::ura4+ | This study |
| KP3095 | h−leu1-32 ura4-294 isp6::KanMX6 ura4+:: nmt1-GFP-prz1+ | This study |
| KP3169 | h−leu1-32 ura4-294 arg1-1 isp6::KanMX6 pka1::ura4+arg1+::nmt1-GFP-prz1+ | This study |
| KP3170 | h−leu1-32 ura4-294 arg1-1prz1::ura4+arg1::3xCDRE::luc (R2.2) | This study |
| KP3380 | h−leu1-32 ura4-294 ura4+::nmt1-GFP-rst2+ | This study |
| KP4138 | h+ade6-M616 pac1-A324T | This study |
| KP4210 | h−leu1-32 pac1-A324T | Ref. 38 |
| KP4854 | h?leu1 arg1 ura4-D18 isp6::KanMX6 psp3::ura4+ | This study |
| arg1+::nmt1-GST-prz1+ | This study | |
| KP5376 | h−leu1-32 arg1-1 ura4-294 ura4+::nmt1-GFP-prz1+ | This study |
| arg1+::3xCDRE::luc (R2.2) | This study | |
| KP5511 | h−leu1-32 rad24::KanMX6 | This study |
| KP5512 | h−leu1-32 ape2::KanMX6 | This study |
| KP5513 | h−leu1-32 rad25::KanMX6 | This study |
| KP5514 | h−leu1-32 tfs1::KanMX6 | This study |
Gene disruptions are denoted by lowercase letters representing the disrupted gene followed by two colons and the wild-type gene marker used for disruption (for example, msn5::ura4+). Also, gene disruptions are denoted by an abbreviation of the gene preceded by Δ (for example, Δmsn5). Proteins are denoted by roman letters, and only the first letter is capitalized (for example, Msn5). Database searches were performed using the National Center for Biotechnology Information BLAST network service and the Sanger Center S. pombe database search service.
Cloning and Tagging of rad25+ and rad24+ Gene
The rad25+ and rad24+ genes were amplified by PCR with the genomic DNA of S. pombe as a template. For the rad25+ gene, the sense primer used PCR was 5′-GAAGATCTCATGAGTAATTCTCGTGAAAAC-3′, and the antisense primer was 5′-GGAATTCGCGGCCGCCAGCTTTAACAGTGTCAGTCG-3′. For the rad24+ gene, the sense primer was 5′-CGGGATCCCATGTCTACTACTTCTCGTGAAG-3′, and the antisense primer was 5′-GGAATTCGCGGCCGCCTGCGTCCGCCTTGGGCTCAG-3′. The PCR fragment was ligated to the C terminus of GST and subcloned into the pREP1 expression vector, containing a thiamine-repressible nmt1 promoter (19). Expression was induced by the incubation of the cells in EMM lacking thiamine.
Generation of Truncated Prz1 Mutants and Site-directed Mutagenesis
A series of truncated GFP-tagged Prz1 mutants was generated by the PCR technique. Ser-422 and Ser-424 were mutated to Ala by using a QuikChange mutagenesis kit (Stratagene). The primers used are summarized in Table 2.
TABLE 2.
Primer used in the Prz1-Rad24 binding experiment
| Primer | |
|---|---|
| Prz11–570 | |
| Sense | 5′-GGGATCCTAATGTTCAAATTGCCTGGATGG-3′ |
| Antisense | 5′-CGGGATCCTAATTTCCTTGAGACTTAGAC-3′ |
| Prz11–400 | |
| Sense | 5′-GGGATCCTAATGTTCAAATTGCCTGGATGG-3′ |
| Antisense | 5′-CGGGATCCTCATTCAAAACGGAATTGCTTAAAGCGG-3′ |
| Prz1400–681 | |
| Sense | 5′-CGGGATCCATGAATTATGATTCAAACAACTTTTC-3′ |
| Antisense | 5′-AACTGCAGGCGGCCGCTCATTTTTGTTTGCTTGTCGAGGC-3′ |
| Prz1S422A/S424A | |
| Sense | 5′-CCTTCATCTCCTTCCAAAGCCCAAGCTGGACCTAGTTTGCCAGCAAATCC-3′ |
| Antisense | 5′-GGATTTGCTGGCAAACTAGGTCCAGCTTGGGCTTTGGAAGGAGATGAAGG-3′ |
Deletion of msn5+ Gene
The SPAC3A11.01 gene, which we named msn5+, encodes a homologue of budding yeast Msn5p. To delete the msn5+ gene, a one-step gene disruption by homologous recombination (20) was performed. The msn5::ura4+ disruption was constructed as follows. The cloned open reading frame of the msn5+ gene in pBluescript SK(+) (Stratagene) was digested with HindIII, and the resulting fragment containing the msn5+ gene was subcloned into the HindIII site of the pBluescript vector. Then a BamHI fragment containing the ura4+ gene was inserted into the BamHI site of the previous construct, causing the interruption of the open reading frame. The fragment containing the disrupted msn5+ gene was transformed into haploid cells. Stable integrants were selected on medium lacking uracil, and disruption of the gene was checked by genomic Southern hybridization (supplemental Fig. S1).
Gene Cloning of Negative Regulators for Prz1
Prz1-overexpressing cells (KP1395) were transformed with an S. pombe genomic DNA library constructed in the vector pDB248. Leu+ transformants were selected on EMM plates with thiamine and then replica-plated onto EMM plates containing 5 μg/ml phloxine B red dye at 30 °C. Colonies that were able to grow on EMM plates and not stained red by phloxin B were selected. The plasmid DNA was recovered from transformants that showed plasmid-dependent rescue. These plasmids suppressed the slow growth phenotype caused by overexpression of Prz1. By DNA sequencing, the suppressing plasmids fell into six classes, which are described under “Results.”
Microscopic Analysis
The cells were grown on EMM plates with thiamine at 30 °C for 2 days and shifted to various conditions as indicated in the figure legends. The cells were microscopically examined under an Axioskop microscope (Carl Zeiss Inc.). Photographs were taken with a SPOT2 digital camera (Diagnostic Instruments Inc.). Images were processed with CorelDRAW software (Corel Corporation Inc.).
Cell Extract Preparation and Immunoblot Analysis
For the analysis of electrophoretic mobility shift of Prz1, whole cell extracts were prepared from cultures of wild-type cells harboring pREP1-GFP-prz1+ plasmid grown at 30 °C for 24 h. Total cell lysates were prepared as follows. Approximately 2 × 107 cells were resuspended in 500 μl of NaOH lysis buffer (1.85 m NaOH, 7.5% 2-mercaptoethanol). After incubation on ice for 10 min, the proteins were precipitated by the addition of 500 μl of 50% trichloroacetic acid. The pellets were washed and solubilized in SDS sample buffer (10% glycerol, 5% 2-mercaptoethanol, 2% SDS, 62.5 mm Tris-HCl, pH 6.8). Protein extracts (10–20 μg/5 μl) were subjected to immunoblot analysis with anti-GFP or phospho-PKA substrate antibodies (Cell Signaling Technology).
In Vivo GST Pulldown Assay
Cells expressing GFP-Prz1 from the chromosomally integrated gene were transformed with pREP1-rad24+-GST, pREP1-rad25+-GST, or pREP1-GST and were cultured at 30 °C for 24 h. Cell extract preparation and immunoblot analysis were performed as described (16).
In Vitro GST Pulldown Assay
The KP2888 cells (two protease-deficient strains) were transformed with pREP1-GFP-prz1+ or pREP1-rad24+-GST, and each tagged protein was expressed as described above. The cell lysates were prepared using lysis buffer (40 mm Tris-HCl, pH 8.0, 2 mm EDTA, 1% Triton X-100, and 0.1% 2-mercaptoethanol). The cell lysates from KP2888 cells overexpressing Rad24-GST or GST alone were incubated with a 50-μl bed volume of glutathione-Sepharose 4B (GE Healthcare) at 4 °C for 2 h with rotation. The beads were precipitated and washed three times with lysis buffer. Then lysates from the cells expressing truncated mutants of Prz1 tagged with GFP were incubated with the Rad24-GST or control GST beads. The beads were precipitated and washed five times with lysis buffer containing 1 m NaCl. The protein were eluted and analyzed by SDS-polyacrylamide gel electrophoresis followed by immunoblot analysis.
In Vitro Kinase Assay
The two protease-deficient cells expressing GST-Prz1 (KP4854) were cultured at 30 °C for 24 h in the absence of thiamine. Then the cells were treated with 0.2 m CaCl2 for 30 min. GST-Prz1 was purified from the cell lysates by glutathione-Sepharose 4B column (GE Healthcare). GST-Prz1 and myelin basic protein (Sigma-Aldrich) were incubated for 10 min at 37 °C in a 25-μl reaction mixture (50 mm Tris-Cl, pH 7.5, 100 mm, 10 mm MgCl2, 0.1 mm ATP, 5 μCi of [γ-32P]ATP, and 250 units of cAMP-dependent kinase subunit (New England Biolabs, P6000S)) in the presence or absence of 20 μm PKI(6–22) amide (Enzo Life Science). The reactions were stopped by adding SDS sample buffer. The radiolabeled proteins were separated by SDS-PAGE and visualized using autoradiography.
Calcineurin-dependent Response Element (CDRE)-dependent Reporter Assay
The cells were transformed or integrated with the reporter plasmid (3×CDRE::luc(R2.2)) (21) and were untreated or treated with 0.1 m CaCl2. The CDRE transcriptional activity was measured as described previously (21). For the experiments with overexpression of Prz1, Prz1-overexpressing cells (KP1395) were transformed with the reporter plasmid and were incubated in EMM with (Promoter OFF) or without (Promoter ON) 4 μm thiamine at 30 °C for 24 h.
CRE-dependent Reporter Assay
The cells were integrated with reporter plasmid (3×CRE::luc(R2.2)) (22) and were treated with 0.3 m KCl. The real time monitoring assay was measured as described previously (22).
RESULTS
Overexpression of Prz1 Caused Growth Inhibition and Aberrant Cell Morphology
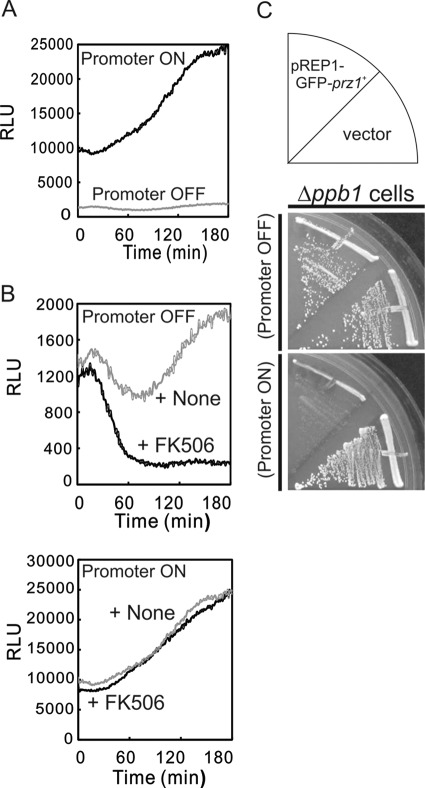
We previously reported that the overexpressed constitutively active form of calcineurin caused growth arrest and aberrant cell morphology. Furthermore, we revealed that the growth inhibition was mediated by Prz1 (16). In this study, we first tested whether overexpression of Prz1 also causes growth inhibition. We controlled the expression of Prz1 by using an expression vector pREP1 with nmt1 promoter. The nmt1 promoter is repressed when cells are grown in medium supplemented with thiamine and is derepressed in thiamine-free medium (19). As shown in Fig. 1A, the wild-type cells harboring pREP1-GFP-prz1+ plasmid could not grow on EMM plate without thiamine, whereas the cells could grow on an EMM plate containing thiamine. Microscopic analysis revealed that Prz1-overexpressing cells showed small cell morphology (Fig. 1B). The morphology is similar to that of cells overexpressing calcineurin (16).
FIGURE 1.
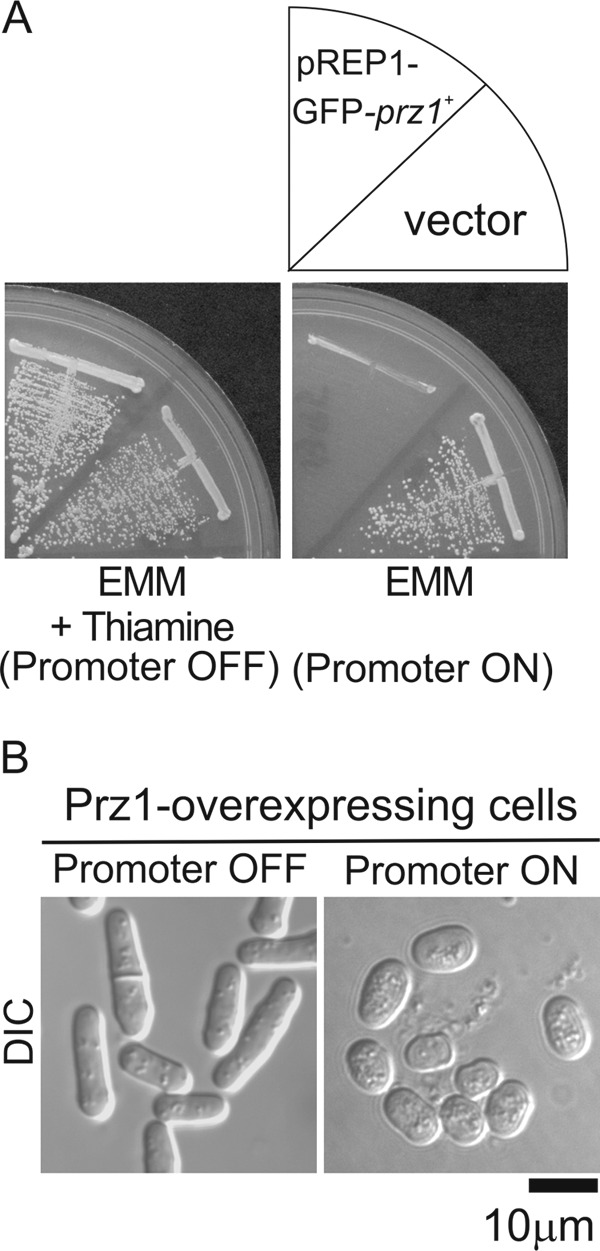
Overexpression of Prz1 caused slow growth and aberrant cell morphology. A, growth arrest caused by overexpression of Prz1. The wild-type cells transformed with the expression plasmid pREP1-GFP vector (vector) and the vector carrying the prz1+ gene were streaked onto EMM plate with (Promoter OFF) or without (Promoter ON) 4 μm thiamine at 30 °C for 3 days. B, overexpression of Prz1 caused aberrant cell morphology. The wild-type cells overexpressing GFP-Prz1 from the gene chromosomally borne under the nmt1 promoter (Prz1-overexpressing cells) were grown on EMM plate with 4 μm thiamine at 30 °C for 2 days and then shifted to liquid EMM with (Promoter OFF) or without (Promoter ON) 4 μm thiamine and were incubated for 24 h at 30 °C. The cells were examined under the differential interference contrast (DIC) microscope as described under “Experimental Procedures.” Bar, 10 μm.
We then monitored the CDRE transcriptional activity of the cells overexpressing Prz1 by 3×CDRE-fused R2.2-destabilized luciferase (3×CDRE::luc(R2.2)) (21). Prz1-overexpressing cells (Promoter ON) showed an ∼10-fold increase in the basal transcriptional activity as compared with Prz1-nonoverexpressing cells (Promoter OFF) (Fig. 2A). Then we examined whether the high transcriptional activity or the growth inhibition is dependent on calcineurin. The elevated transcriptional activity caused by overexpression of Prz1 was not affected by the addition of FK506, whereas the activity was inhibited when the expression was attenuated (Fig. 2B). The growth inhibition by Prz1 overexpression was similarly observed in calcineurin knock-out cells (Fig. 2C). Collectively, overexpression of Prz1 caused calcineurin-independent high CDRE transcriptional activity and growth inhibition.
FIGURE 2.
The growth inhibition and the high CDRE transcriptional activity caused by overexpression of Prz1 were independent of the activity of calcineurin. A, overexpression of Prz1 caused the high CDRE transcriptional activity. Prz1-overexpressing cells harboring the reporter plasmid (3×CDRE::luc(R2.2) reporter vector) were cultured in liquid EMM with (Promoter OFF) or without (Promoter ON) 4 μm thiamine at 30 °C and were harvested in the early logarithmic growth phase. Each cell was incubated with d-luciferin. The relative light units (RLU) were measured using a luminometer at 1-min intervals for 3 h. B, the high transcriptional activity was independent of the activity of calcineurin. Prz1-overexpressing cells were transformed with reporter plasmid, cultured as described in Fig. 2A, and treated with 0.5 μg/ml FK506 that was added at the start of the reporter assay. C, the growth inhibition was also independent of calcineurin. The Δppb1 cells (ppb1+ encodes single catalytic subunit of calcineurin) were transformed with pREP1-GFP-prz1+ and streaked, as described in Fig. 1A. The data shown are representative of multiple experiments.
Isolation of Multicopy Suppressor Genes for Prz1 Overexpression
To isolate genes that negatively regulate Prz1, we screened for suppressors that can rescue the slow growth phenotype caused by Prz1 overexpression. Prz1-overexpressing cells were transformed with a multicopy genomic library, and then we selected the transformants that grew better than the cells transformed with control plasmid. Twenty-three plasmids reproducibly suppressed the slow growth phenotype of the Prz1-overexpressing cells. Sequence analysis revealed that the suppressing plasmids fell into six classes: rad25+, pka1+, msn5+ (SPAC328.01c), pac1+, ape2+, and tfs1+. As shown in Fig. 3A, the overexpression of Rad25, Msn5, and Pac1 markedly suppressed the growth inhibition caused by overexpression of Prz1. The overexpression of Pka1, Ape2, and Tfs1 moderately suppressed the growth phenotype. Then we assessed whether the overexpression of the six genes can suppress the growth defect caused by a constitutive active calcineurin. The overexpression of these genes failed to suppress the growth defect (supplemental Fig. S2). Then we examined whether overexpression of these genes affects the CDRE transcriptional activity in Prz1-overexpressing cells. All of these suppressor genes, when overexpressed, repressed the transcriptional activity in Prz1-overexpressing cells (Fig. 3B).
FIGURE 3.
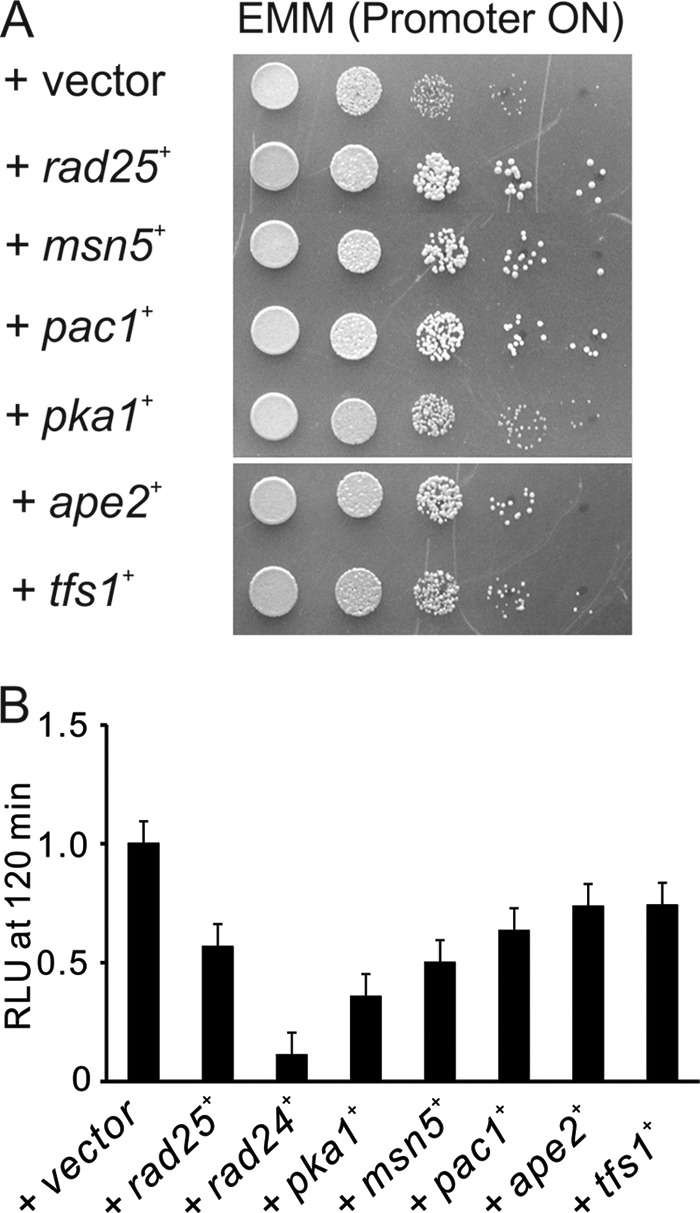
Isolation of multicopy suppressor genes for Prz1 overexpression. A, overexpression of Rad25, Msn5, Pac1, Pka1, Ape2, or Tfs1 suppressed the slow growth phenotype. Prz1-overexpressing cells containing the multicopy plasmid carrying various genes as indicated were spotted onto EMM plate without thiamine and were incubated for 3 days at 30 °C. The cells were spotted in serial 10-fold dilution starting with A600 = 0.3 of log phase cells (5 μl). B, the effects of multicopy suppressor genes on the CDRE transcriptional activity of Prz1-overexpressing cells. Prz1-overexpressing cells harboring the integration plasmid (3×CDRE::luc(R2.2)) were transformed with the multicopy plasmid carrying various genes and cultured in liquid EMM without thiamine. The vertical axis shows the ratio of the RLU of each cell to that of control. Error bars, means ± S.E. (n = 4 to 6).
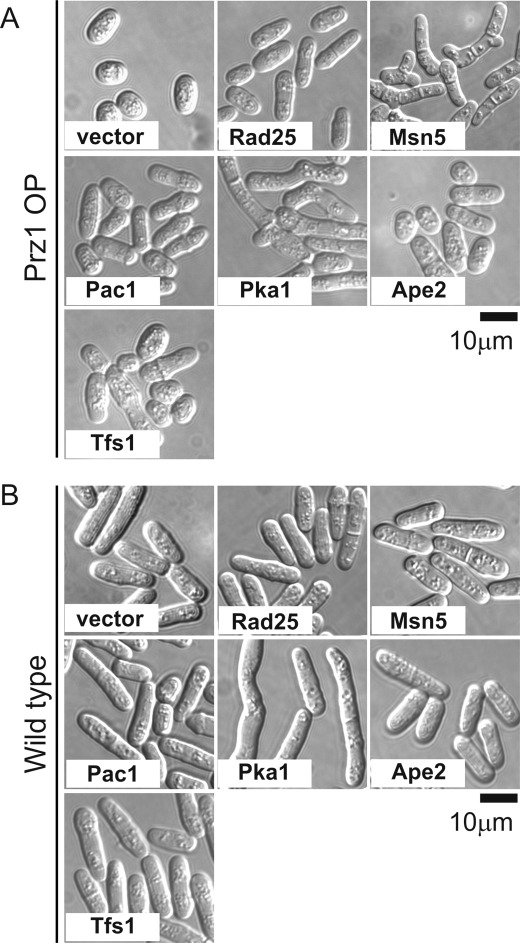
Effect of Suppressor Genes Overexpression on Aberrant Morphology of Prz1-overexpressing Cells
Then we examined whether the aberrant morphology caused by overexpression of Prz1 was restored to normal morphology by overexpression of these genes. The cells were completely restored to normal morphology by the overexpression of Rad25 or Pac1, whereas Ape2 and Tfs1 partially suppressed the aberrant morphology. Pka1 and Msn5 caused the long and branched morphology (Fig. 4A). When Pka1 was overexpressed in wild-type cells, the cells also became elongated (Fig. 4B), indicating that this phenotype is typical for high expression of Pka1. On the other hand, the wild-type cells overexpressing Msn5 showed normal morphology (Fig. 4B). These results indicated that the phenotype caused by Msn5 overexpression is specific to Prz1-overexpressing cells.
FIGURE 4.
The effect of Rad25, Msn5, Pac1, Pka1, Ape2, or Tfs1 on the aberrant cell morphology caused by overexpression of Prz1. Prz1-overexpressing (A) or wild-type (B) cells were transformed with the multicopy plasmid carrying various genes as indicated, grown on EMM plate with 4 μm thiamine at 30 °C for 2 days, shifted to liquid EMM without thiamine, and incubated for 24 h at 30 °C. The cells were examined under the differential interference contrast microscope as described under “Experimental Procedures.” Bar, 10 μm.
Effect of Multicopy Suppressor Genes on Other Transcriptional Factors
To exclude general effects on transcription, we examined the effect of the suppressor gene overexpression on the transcription mediated by Rst2, a zinc finger protein that regulates stress response element-dependent transcription (23). As shown in Fig. 5A, the overexpression of Rst2 also caused growth inhibition, and although Rad25 and Rad24 were able to weakly suppress the slow growth phenotype, other genes failed to suppress the phenotype. Then to further exclude general effects on transcription, we examined the transcriptional activity of Atf1 (22). Atf1 transcriptional factor plays a vital role in the stress response of S. pombe and is not regulated by calcineurin (22). As shown in Fig. 5B, only overexpression of Rad24 significantly repressed the KCl-stimulated Atf1 transcriptional activity.
FIGURE 5.
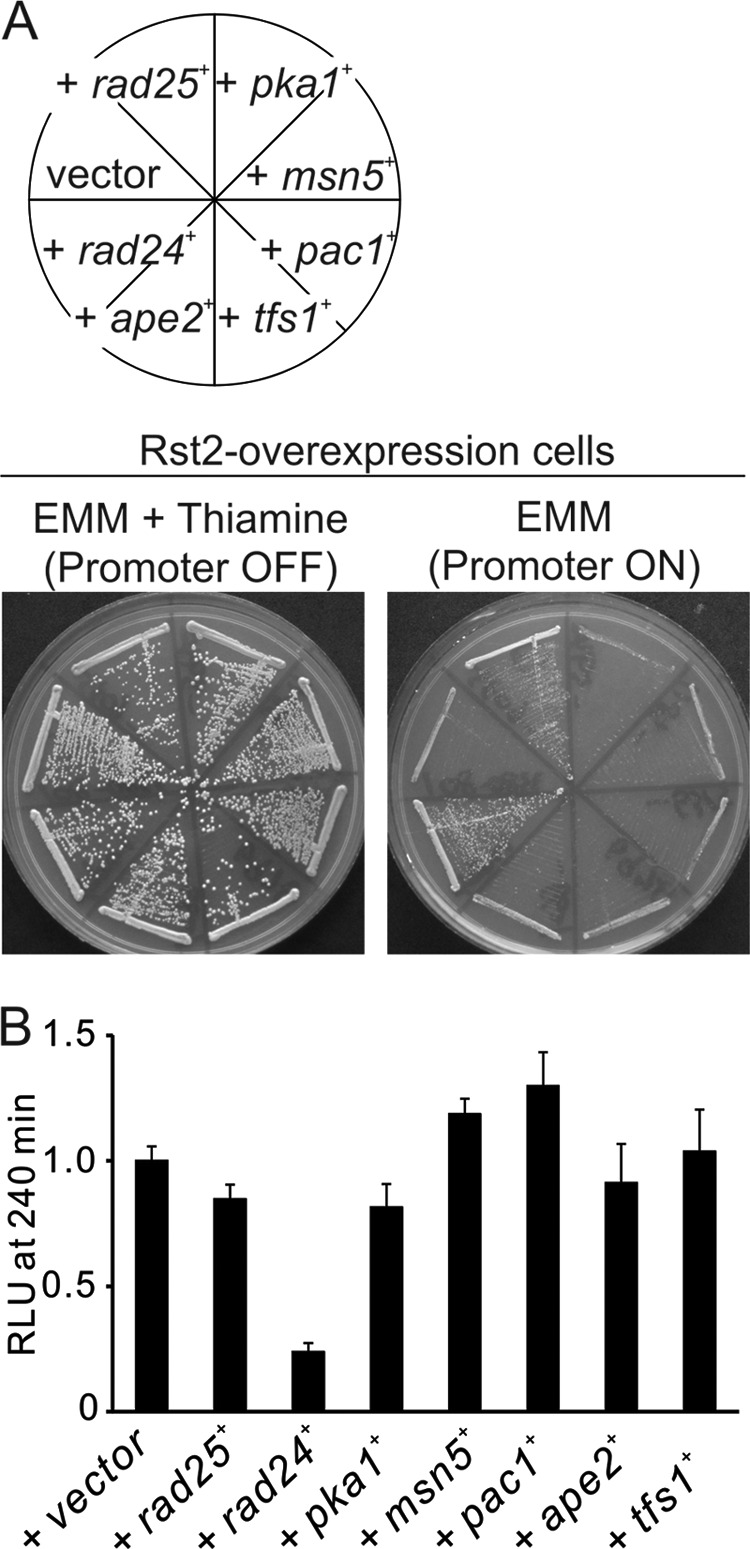
The effect of multicopy suppressor genes on other transcriptional factors. A, effect of suppressor genes on the growth defects caused by overexpression of Rst2. Rst2-overexpressing cells containing the multicopy plasmid carrying various genes as indicated were streaked onto EMM plate with (Promoter OFF) or without (Promoter ON) 4 μm thiamine at 30 °C for 3 days. B, effect of suppressor genes on the activity of Atf1 expressed at wild-type levels. The wild-type cells harboring the integration plasmid (3×CRE::luc(R2.2)) were transformed with the multicopy plasmid carrying various genes and cultured in liquid EMM. The cells were stimulated with 0.3 m KCl to measure the Atf1-mediated transcriptional activity. The vertical axis shows the ratio of the RLU of each cell to that of control. Error bars, means ± S.D.
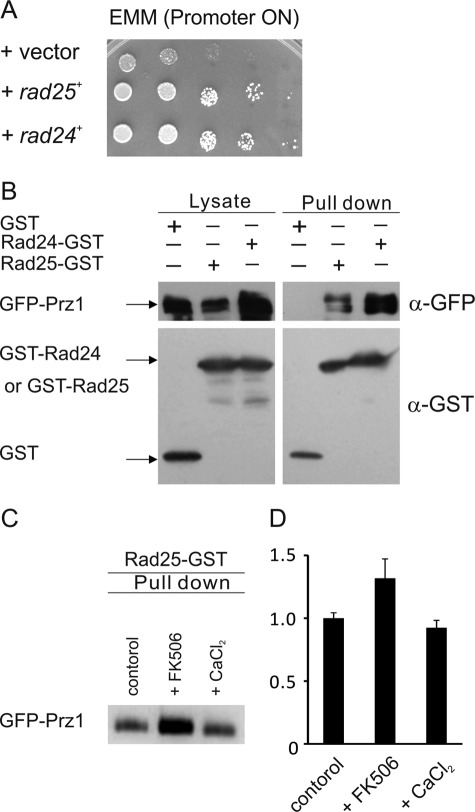
14-3-3 Proteins Rad24 and Rad25 Bind Prz1
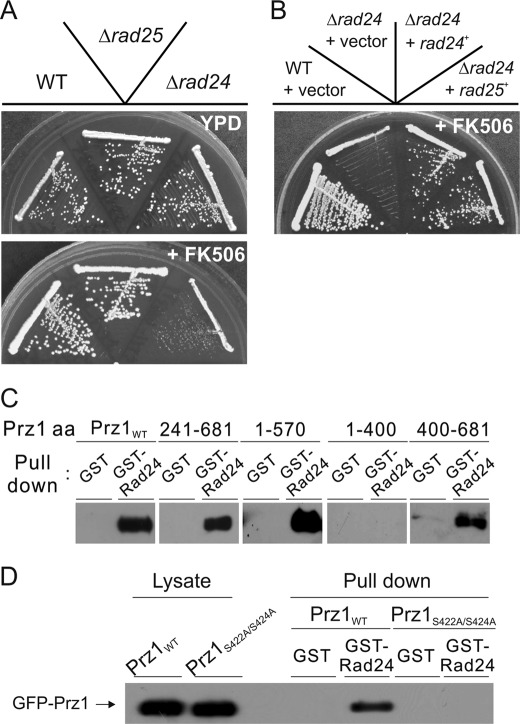
The 14-3-3 proteins are highly conserved in amino acid sequence from yeast to mammals. The 14-3-3 proteins are involved in the regulation of a variety of biological systems. The primary function of the 14-3-3 proteins is to bind phosphorylated proteins (24). In budding yeast, the 14-3-3 proteins Bmh1p and Bmh2p bind to the Snf1 (AMP-activated protein kinase)-dependent transcription factor Adr1 and inhibit its transcriptional activity (25). There are also two 14-3-3 proteins, Rad24 and Rad25, in fission yeast. We showed that overexpression of Rad24 also suppressed the growth phenotype and the high transcriptional activity of Prz1 overexpression (Figs. 3B and 6A). Then we examined whether Rad24 or Rad25 binds Prz1. In the GST pulldown assay, GFP-Prz1 associated with Rad24- or Rad25-GST but not with GST alone (Fig. 6B). Furthermore, we demonstrated that the binding of Rad25 to Prz1 was enhanced in the presence of FK506 (Fig. 6C). The binding of Rad24 to Prz1 was also enhanced in the presence of FK506 (data not shown). These results indicated that Rad24 and Rad25 bound Prz1 at the phosphorylated residues that might be dephosphorylated by calcineurin. In a previous report (26), the loss of rad24 rendered cells highly sensitive to DNA-damaging agents, whereas the rad25 null strain was modestly sensitive, suggesting that Rad25 does not play as significant a role in the DNA damage response. We also revealed that deletion of rad24+, but not rad25+, resulted in FK506-sensitive phenotype (Fig. 7A). Furthermore, overexpression of Rad25 suppressed the FK506-sensitive phenotype of rad24 null strain (Fig. 7B). These results indicate that Rad24 plays a major role in the cellular processes regulated by calcineurin and that these two 14-3-3 proteins are redundant. Then we searched the Rad24-binding site of Prz1. Several N- or C-terminally truncated Prz1 mutants were constructed and expressed in KP2888 cells. Then we confirmed the binding of the truncated mutants to Rad24-GST. As shown in Fig. 7C, GFP-Prz11–570, GFP-Prz1241–681, and GFP-Prz1400–681 retained the ability to interact with Rad24-GST. In contrast, GFP-Prz11–400 failed to bind Rad24-GST. These results indicated that the critical region of Prz1 for binding of Rad24 is contained within the region encompassing the Prz1 residues 400–570. Furthermore, we identified a 14-3-3 recognition motif ((R/K)XXSXP) (27) within the residues 421–426 (KSQSGP) of Prz1. Then we examined whether this region is necessary for the binding of Rad24 to Prz1. As shown in Fig. 7D, GFP-Prz1S422A/S424A could not bind with Rad24-GST. Collectively, these results suggest that Rad24 binds directly to Prz1 at the region of amino acid 421–426.
FIGURE 6.
The 14-3-3 proteins, Rad25 and Rad24, bound Prz1. A, overexpression of Rad24 also suppressed the slow growth phenotype caused by overexpression of Prz1. Prz1-overexpressing cells were transformed with a control vector (vector) or multicopy plasmid carrying the rad24+gene (rad24+) and rad25+ gene (rad25+). The transformants were spotted onto EMM plate without thiamine and then were cultured for 3 days at 30 °C. B, Rad25-GST and Rad24-GST bound GFP-Prz1. The KP3095 cells were transformed with pREP1-GST vector, pREP1-rad25+-GST, or pREP1-rad24+-GST and then were cultured in liquid EMM without thiamine for 24 h. GST and GST fusion proteins were precipitated by glutathione beads, washed extensively, subjected to SDS-PAGE, and immunoblotted using anti-GFP or anti-GST antibodies. C, the interaction between Rad25-GST and GFP-Prz1 was enhanced in the presence of FK506. The KP3095 cells were transformed with pREP1-rad25+-GST. The transformants were grown in liquid EMM without thiamine for 20 h and then treated with 0.5 μg/ml FK506 for 1 h. Pulldown assay was performed as described for B. D, quantification of pulldown assay. The bands as in C were quantified by densitometry using Multi Gauge v3.0 software (Fujifilm). The bars represent the means ± S.E. of the ratio of densitometric values of the each bands relative to control bands.
FIGURE 7.
Rad24 and Rad25 are negative regulators of Prz1. A, the Δrad24 cells, but not Δrad25 cells, showed FK506-sensitive phenotype. WT, Δrad24, and Δrad25 cells were streaked onto yeast extract/peptone/dextrose plate containing 0.5 μg/ml FK506 and cultured for 3 days at 30 °C. B, two 14-3-3 proteins, Rad24 and Rad25, are redundant. The Δrad24 cells were transformed with a control vector (vector), multicopy plasmid carrying the rad24+gene (rad24+), or rad25+ gene (rad25+). These cells were streaked onto yeast extract/peptone/dextrose plate containing 0.5 μg/ml FK506 and cultured for 3 days at 30 °C. C and D, the binding between Prz1 and Rad24 required residues 421–426 of Prz1. The cell lysates from KP2888 cells expressing GFP-Prz1wt, GFP-Prz11–570, GFP-Prz1241–681, GFP-Prz11–400, or GFP-Prz1400–681 (C) or GFP-Prz1S422A/S424A (D) were incubated with 50 μl of Rad24-GST or GST alone bound to glutathione-Sepharose beads. The pulldown assay is described under “Experimental Procedures”.
Cyclic AMP Repressed CDRE Transcriptional Activity
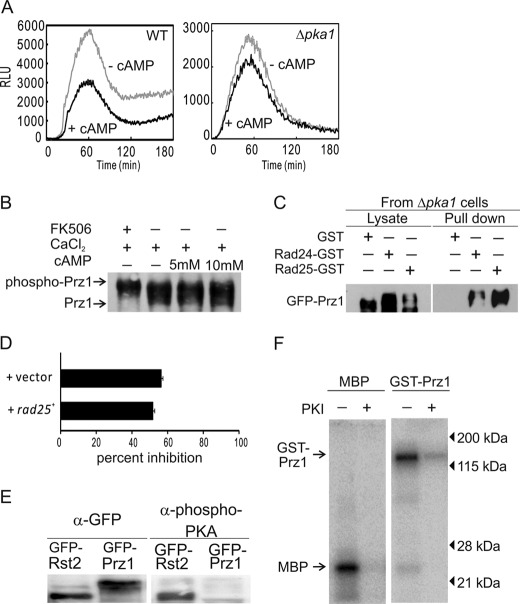
The fission yeast has a single gene, pka1+, encoding a PKA catalytic subunit. As described above, we isolated pka1+ as a gene dosage-dependent suppressor of the slow growth phenotype of Prz1-overexpressing cells. In Saccharomyces cerevisiae, activation of PKA by the addition of cAMP inhibits Crz1p activation by calcium (28). Therefore, we monitored whether the Ca2+-stimulated the CDRE transcriptional activity is repressed by cAMP addition. As shown in Fig. 8A, the transcriptional activity was repressed in the presence of cAMP. In Δpka1 cells, the effect of cAMP on the transcriptional activity was lower than in wild-type cells (Fig. 8A). The results indicate that cAMP also inhibits the CDRE transcriptional activity and that Pka1 may serve as a negative regulator of Prz1.
FIGURE 8.
The effect of Pka1 on phosphorylation of Prz1. A, cyclic AMP also suppressed the CDRE transcriptional activity. The wild-type or Δpka1 cells transformed with the reporter plasmid (3×CDRE::luc(R2.2) reporter vector) were grown to early log phase in liquid EMM. Then cells were incubated with d-luciferin and treated with 10 mm cAMP and 100 mm CaCl2. The data shown are representative of multiple experiments. B, the analysis of electrophoretic mobility shift of Prz1. The wild-type cells were transformed with pREP1-GFP-prz1+. The transformants were grown in liquid EMM without thiamine for 23 h and then pretreated with 0.5 μg/ml FK506 or 5–10 mm of cAMP for 30 min before the addition of 0.2 m CaCl2 for 30 min. The lysates of cells were analyzed by immunoblotting. C, Rad25 and Rad24 bound Prz1 in the pka1 deletion cells. The KP3169 cells were transformed with pREP1-GST, pPRE1-rad25+-GST, or pREP1-rad24+-GST. Pulldown assay was performed as described for Fig. 7 (C and D). D, the effect of cAMP on the CDRE transcriptional activity in Rad25-overexpressing cells. The wild-type cells harboring the integration plasmid (3×CDRE::luc(R2.2)) were transformed with the multicopy plasmid carrying rad25+ or control plasmid (vector) and were grown to early log phase in liquid EMM. Then cells were incubated with d-luciferin, with or without 10 mm cAMP and treated with 100 mm CaCl2. The data shown are representative of multiple experiments. The percentage of inhibition was determined by the formula (RLU at 60 min with cAMP/RLU at 60 min without cAMP) × 100. Error bars, means ± S.E. (n = 5). E, the Western blotting using phospho-PKA-specific substrate antibodies. The wild-type cells transformed with pREP1-GFP-Rst2 or pREP1-GFP-Prz1. The cell extract preparation and immunoblot analysis are described under “Experimental Procedures.” F, Prz1 was phosphorylated by mammalian PKA in vitro. The purification of GST-Prz1 and in vitro kinase assay are described under “Experimental Procedures.” The kinase assay was performed in presence or absence of 20 μm PKI(6–22) amide. Myelin basic protein (MBP) was used as a control substrate for PKA.
The Effect of Pka1 on Phosphorylation of Prz1
Then we examined the effect of cAMP on the mobility shift of Prz1 in vivo. The mobility shift of Prz1 was detected when cells were stimulated by Ca2+, whereas such a mobility shift was not detected when FK506 was added to the medium (Fig. 8B). The addition of cAMP did not affect the Ca2+-induced mobility shift (Fig. 8B). In the previous reports, some PKA phosphorylation sites have been reported to bind 14-3-3 proteins (29, 30). These reports prompted us to investigate whether the binding of 14-3-3 proteins to Prz1 is dependent on Pka1. As shown in Fig. 8C, in Δpka1 cells, Rad24 and Rad25 still bound Prz1. Furthermore, we showed that cAMP also suppressed the CaCl2-induced CDRE transcriptional activity in Rad25-overexpressing cells, and the magnitude of inhibition was similar between wild-type and Rad25-overexpressing cells (Fig. 8D). These results indicate that the binding of Rad24 or Rad25 to Prz1 is independent of Pka1. Furthermore, we examined whether phosphorylated Prz1 is detected by phospho-(Ser/Thr) PKA substrate antibody (Cell Signaling Technology). Phosphorylated Rst2, a fission yeast PKA-phosphorylated transcriptional factor, was detected by the antibody (23) (Fig. 8E). However, the same antibody failed to detect phosphorylated Prz1 (Fig. 8E). On the other hand, in vitro kinase assay using mammalian PKA demonstrated that GST-Prz1 was phosphorylated by PKA, and the phosphorylation was inhibited by PKI (Fig. 8F).
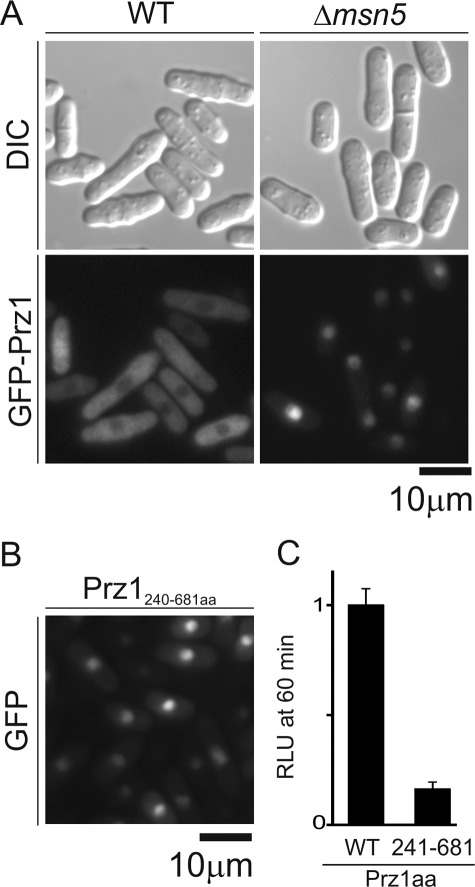
Msn5 Is Required for Export of Prz1 from Nucleus
The SPAC328.01c gene encodes a homologue of Msn5p in S. cerevisiae, and we named SPAC328.01c gene as msn5+. In S. cerevisiae, Msn5p had been identified as the exportin for Crz1p (31). Based on this report, we examined whether the localization of GFP-Prz1 is affected by the deletion of msn5+. The cells were analyzed under attenuated GFP-Prz1 expression. In wild-type cells, GFP-Prz1 largely localized to cytosol in the absence of stimulation. However, in Δmsn5 cells, GFP-Prz1 was observed mostly in the nucleus in the absence of Ca2+ stimulation (Fig. 9A), suggesting that Msn5 is involved in export of Prz1 from nucleus. When we examined the CaCl2-mediated CDRE transcriptional activity in Δmsn5 cells, the cells showed a significant decrease in the activity (Fig. 10B). We hypothesized that nucleocytoplasmic shuttling of Prz1 may be important for its transcriptional activity. In our previous study, we showed that the localization of Prz1 to the cytoplasm requires its N-terminal 240 resides (15) (Fig. 9B). Consistent with our hypothesis, the transcriptional activity of the truncated Prz1 also showed a significant decrease in the activity (Fig. 9C).
FIGURE 9.
Msn5 was required for Prz1 nuclear export. A, the localization of GFP-Prz1 in Δmsn5 cells. The WT or Δmsn5 cells transformed with pREP1-GFP-prz1+. The cells were grown in liquid EMM with 4 μm thiamine to attenuate the expression for 24 h at 30 °C. Then the cells were examined under the fluorescence microscope (GFP-Prz1) and as described under “Experimental Procedures.” Bar, 10 μm. B, the localization of GFP-Prz1241.681. The prz1 deletion mutants expressing GFP-Prz1241.681 were grown in liquid EMM with 4 μm thiamine for 24 h at 30 °C. Then cells were examined as described in Fig. 9A. Bar, 10 μm. C, the effect of GFP-Prz1241.681 on the CaCl2-induced CDRE transcriptional activity. The KP3170 cells expressing GFP-Prz1wt or -Prz1241.681 were cultured in liquid EMM with 4 μm thiamine. The cells were stimulated with CaCl2 to measure the CDRE transcriptional activity. The vertical axis shows the ratio of the RLU of each cell to that of control. Error bars, means ± S.E. (n = 4). DIC, differential interference contrast.
FIGURE 10.
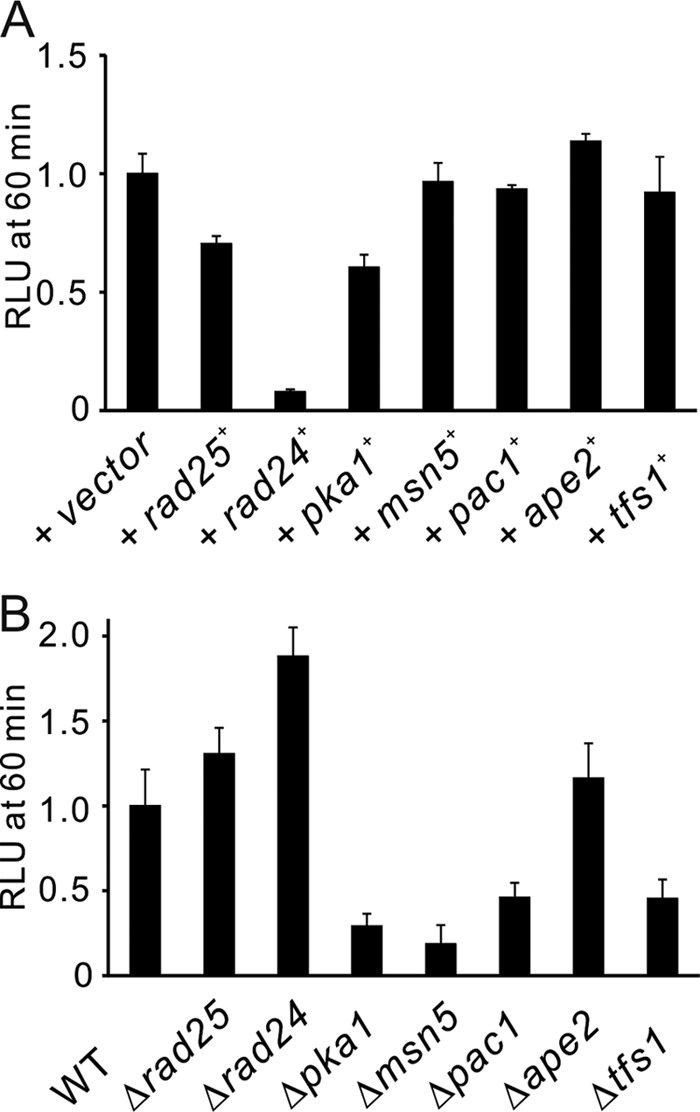
Overexpression or mutation of the suppressor genes altered the CaCl2-induced CDRE transcriptional activity. A, the effects of suppressor genes on the activity of Prz1 expressed at wild-type levels. The wild-type cells harboring the integration plasmid (3×CDRE::luc(R2.2)) were transformed with the multicopy plasmid carrying various genes and cultured in liquid EMM. The cells were stimulated with CaCl2 to measure the CDRE transcriptional activity. B, the effects of knock-out or mutation of the suppressor genes on the activity of Prz1 expressed at wild-type levels. The rad24, rad25, msn5, pka1, ape2, or tfs1 deletion mutants and pac1 mutants were transformed with the reporter plasmid (3×CDRE::luc(R2.2) reporter vector) and treated with 0.1 m CaCl2. The vertical axis shows the ratio of the RLU of each cell to that of control. Error bars, means ± S.E. (n = 4 to 9).
Overexpression or Mutation of Suppressor Genes Altered the CaCl2-induced CDRE Transcriptional Activity
To confirm whether these high copy suppressors influence the activity of Prz1 expressed at wild-type levels, we examined the effects of overexpression or mutation of these suppressor genes on the CaCl2-induced CDRE transcriptional activity in wild-type cells. As shown in Fig. 10A, in wild-type cells, overexpression of Rad24, Rad25, or Pka1 suppressed the transcriptional activity, whereas overexpression of Pac1, Tfs1, or Ape2 did not significantly suppress the transcriptional activity. On the other hand, Δrad24 and Δrad25 cells showed significantly higher transcriptional activity compared with wild-type cells, whereas Δpka1, Δmsn5, pac1 mutant and Δtfs1 cells showed low transcriptional activity, and Δape2 did not alter the activity (Fig. 10B). Pac1 is a member of the RNase III family of double-stranded specific ribonucleases (32). Ape2 is a homologue of the leucine aminopeptidase Ape2p and arginine/alanine aminopeptidase Aap1p in S. cerevisiae (Ref. 33; S. pombe GeneDB). Tfs1 is a transcriptional elongation factor TFIIS that induces the cleavage of nascent RNA at the pause or arrest sites and thereby enhances transcription elongation (34). These results suggest that Rad24 and Rad25 are negative regulators of Prz1, and Pka1, Msn5, Pac1, and Tfs1 are also involved in the regulation of Prz1 activity that is dependent on calcineurin, whereas Ape2 is involved in the calcineurin-independent activity of Prz1.
DISCUSSION
Overexpression of Prz1 showed a slow growth phenotype similar to that of constitutively active calcineurin. It also showed high CDRE transcriptional activity. In the present study, we demonstrated that this Prz1-mediated slow growth phenotype and the high transcriptional activity do not depend on the activity of calcineurin. This suggests that Prz1-overexpressing cells express the protein at levels that exceed the capacity of endogenous negative regulators to neutralize its high activity. As a result of a genetic screen for negative regulators of Prz1, we identified seven genes that were able to reverse the slow growth and suppress the high CDRE transcriptional activity caused by overexpression of Prz1. On the other hand, only three suppressors, Rad24, Rad25, and Pka1, repressed the Ca2+-induced activity of Prz1 expressed at wild-type level. These results suggest that Prz1 regulates transcription of various genes with CDRE via calcineurin-dependent and -independent mechanisms.
In this screen, although we expected to isolate kinases that phosphorylate Prz1 and counteract the stimulatory effect of calcineurin, we could not isolate such genes, except for pka1+. Therefore, we examined whether overexpression of known kinases such as GSK3 homologue (GSK3 and GSK31), DYRK family kinases (Pom1, Ppk15 and Prp4), p38 MAPK homologue (Sty1), PKC homologue (Pck2), ERK MAPK homologue (Pmk1) and pheromone-responsive MAPK (Spk1), could repress the slow growth phenotype caused by overexpression of Prz1. However, all of them could not repress the phenotype (supplemental Fig. S3). These results suggest that other kinase(s) may be involved in the phosphorylation of Prz1, alternatively, phosphorylation of the key residues that are dephosphorylated by calcineurin requires multiple kinases or factors.
The addition of cAMP or overexpression of Pka1 suppressed the CDRE transcriptional activity. However, in Δpka1 cells, the transcriptional activity was lower than that of wild-type cells. Interestingly, cAMP repressed the transcriptional activity in Δpka1 cells, but the extent of repression was lower than that in wild-type cells. This result suggests that cAMP inhibits the CDRE transcriptional activity through Pka1-dependent and -independent mechanisms.
In the previous report by Kafadar et al. (28), it is reported that Crz1p calcineurin-activated transcriptional factor, a Prz1 homologue in S. cerevisiae, is phosphorylated by PKA. However, in our present study, cAMP had no effect on the electrophoretic mobility of Prz1, and phosphorylated Prz1 was not detected by phospho-PKA substrate-specific antibodies. These results raise the possibility that Pka1 does not phosphorylate Prz1 directly. However, the use of overexpressed Prz1 to analyze its phosphorylation and binding to Rad25 could allow it to be phosphorylated by a different kinase, and thus its binding would be independent on PKA. The phospho-(Ser/Thr) PKA substrate antibody might fail to recognize the PKA-phosphorylated sites. Furthermore, we showed that Prz1 is phosphorylated by mammalian PKA in vitro. Therefore, we could not exclude the possibility that Prz1 is directly phosphorylated by Pka1.
In Δmsn5 cells, GFP-Prz1 is accumulated in the nucleus, and the CDRE transcriptional activity was significantly attenuated. In other studies, the efficient nucleocytoplasmic shuttling of transcriptional factors is necessary for their transcriptional activation (35, 36). Furthermore, we showed that GFP-Prz1241.681 was also mostly localized in the nucleus, and the truncated Prz1 showed significant decrease in the activity. Thus, the efficient nucleocytoplasmic shuttling of Prz1 may be important for its transcriptional activity.
In our screening, we showed that Pac1, Ape2, and Tfs1 are the novel regulators of Prz1. The regarding the results of the Ca2+-induced CDRE transcriptional activity using the mutated cells, pac1 and Δtfs1 cells showed the lower transcriptional activity, whereas Δape2 cells did not alter the transcriptional activity. These results suggest that pac1 and tfs1 are involved in the regulation of calcineurin-dependent Prz1 activity.
In summary, a genetic screening for multicopy suppressors of Prz1 overexpression identified two negative regulators (Rad24 and Rad25) and five putative regulators (Pka1, Msn5, Pac1, Tfs1, and Ape2) of Prz1. The present study suggests the following: 1) the 14-3-3 proteins, Rad24 and Rad25, bind phosphorylation sites of Prz1 and inhibit the dephosphorylation of Prz1 by calcineurin; 2) cAMP inhibits the transcriptional activity through Pka1-dependent and -independent mechanisms, and Pka1 represses the CDRE transcriptional activity, but the mechanism may not directly; 3) Msn5 regulates Prz1-mediated transactivation by promoting its export from the nucleus; and 4) Pac1, Ape2, and Tfs1 are identified as novel regulators of Prz1. Further study is needed to clarify the role of Pac1, Ape2, or Tfs1 in the regulation of Prz1.
Supplementary Material
Acknowledgment
We thank Astellas Pharma Inc. for the kind gift of the FK506 (tacrolimus) compound.
This work was supported by research grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan.

This article contains supplemental Figs. S1–S3.
- NFAT
- nuclear factor of activated T-cells
- EMM
- Edinburgh minimal medium
- RLU
- relative light units
- GSK
- glycogen synthase kinase
- PKI
- PKA-specific inhibitor
- CDRE
- calcineurin-dependent response element
- CRE
- cAMP response element.
REFERENCES
- 1. Clipstone N. A., Crabtree G. R. (1992) Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature 357, 695–697 [DOI] [PubMed] [Google Scholar]
- 2. O'Keefe S. J., Tamura J., Kincaid R. L., Tocci M. J., O'Neill E. A. (1992) Journals as policemen. Nature 357, 2–694 [DOI] [PubMed] [Google Scholar]
- 3. Molkentin J. D., Lu J. R., Antos C. L., Markham B., Richardson J., Robbins J., Grant S. R., Olson E. N. (1998) A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93, 215–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hendey B., Klee C. B., Maxfield F. R. (1992) Inhibition of neutrophil chemokinesis on vitronectin by inhibitors of calcineurin. Science 258, 296–299 [DOI] [PubMed] [Google Scholar]
- 5. Wang H. G., Pathan N., Ethell I. M., Krajewski S., Yamaguchi Y., Shibasaki F., McKeon F., Bobo T., Franke T. F., Reed J. C. (1999) Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science 284, 339–343 [DOI] [PubMed] [Google Scholar]
- 6. Graef I. A., Chen F., Chen L., Kuo A., Crabtree G. R. (2001) Signals transduced by Ca(2+)/calcineurin and NFATc3/c4 pattern the developing vasculature. Cell 105, 863–875 [DOI] [PubMed] [Google Scholar]
- 7. Mansuy I. M., Mayford M., Jacob B., Kandel E. R., Bach M. E. (1998) Restricted and regulated overexpression reveals calcineurin as a key component in the transition from short-term to long-term memory. Cell 92, 39–49 [DOI] [PubMed] [Google Scholar]
- 8. Crabtree G. R. (2001) Calcium, calcineurin, and the control of transcription. J. Biol. Chem. 276, 2313–2316 [DOI] [PubMed] [Google Scholar]
- 9. Zhu J., Shibasaki F., Price R., Guillemot J. C., Yano T., Dötsch V., Wagner G., Ferrara P., McKeon F. (1998) Intramolecular masking of nuclear import signal on NF-AT4 by casein kinase I and MEKK1. Cell 93, 851–861 [DOI] [PubMed] [Google Scholar]
- 10. Beals C. R., Sheridan C. M., Turck C. W., Gardner P., Crabtree G. R. (1997) Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science 275, 1930–1934 [DOI] [PubMed] [Google Scholar]
- 11. Gwack Y., Sharma S., Nardone J., Tanasa B., Iuga A., Srikanth S., Okamura H., Bolton D., Feske S., Hogan P. G., Rao A. (2006) A genome-wide Drosophila RNAi screen identifies DYRK-family kinases as regulators of NFAT. Nature 441, 646–650 [DOI] [PubMed] [Google Scholar]
- 12. Gómez del Arco P., Martínez-Martínez S., Maldonado J. L., Ortega-Pérez I., Redondo J. M. (2000) A role for the p38 MAP kinase pathway in the nuclear shuttling of NFATp. J. Biol. Chem. 275, 13872–13878 [DOI] [PubMed] [Google Scholar]
- 13. Chow C. W., Rincón M., Cavanagh J., Dickens M., Davis R. J. (1997) Nuclear accumulation of NFAT4 opposed by the JNK signal transduction pathway. Science 278, 1638–1641 [DOI] [PubMed] [Google Scholar]
- 14. Kehlenbach R. H., Dickmanns A., Gerace L. (1998) Nucleocytoplasmic shuttling factors including Ran and CRM1 mediate nuclear export of NFAT in vitro. J. Cell Biol. 141, 863–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hirayama S., Sugiura R., Lu Y., Maeda T., Kawagishi K., Yokoyama M., Tohda H., Giga-Hama Y., Shuntoh H., Kuno T. (2003) Zinc finger protein Prz1 regulates Ca2+ but not Cl− homeostasis in fission yeast. Identification of distinct branches of calcineurin signaling pathway in fission yeast. J. Biol. Chem. 278, 18078–18084 [DOI] [PubMed] [Google Scholar]
- 16. Sio S. O., Suehiro T., Sugiura R., Takeuchi M., Mukai H., Kuno T. (2005) The role of the regulatory subunit of fission yeast calcineurin for in vivo activity and its relevance to FK506 sensitivity. J. Biol. Chem. 280, 12231–12238 [DOI] [PubMed] [Google Scholar]
- 17. Toda T., Dhut S., Superti-Furga G., Gotoh Y., Nishida E., Sugiura R., Kuno T. (1996) The fission yeast pmk1+ gene encodes a novel mitogen-activated protein kinase homolog which regulates cell integrity and functions coordinately with the protein kinase C pathway. Mol. Cell. Biol. 16, 6752–6764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moreno S., Klar A., Nurse P. (1991) Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194, 795–823 [DOI] [PubMed] [Google Scholar]
- 19. Maundrell K. (1993) Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene 123, 127–130 [DOI] [PubMed] [Google Scholar]
- 20. Rothstein R. J. (1983) One-step gene disruption in yeast. Methods Enzymol. 101, 202–211 [DOI] [PubMed] [Google Scholar]
- 21. Deng L., Sugiura R., Takeuchi M., Suzuki M., Ebina H., Takami T., Koike A., Iba S., Kuno T. (2006) Real-time monitoring of calcineurin activity in living cells. Evidence for two distinct Ca2+-dependent pathways in fission yeast. Mol. Biol. Cell 17, 4790–4800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhou X., Ma Y., Sugiura R., Kobayashi D., Suzuki M., Deng L., Kuno T. (2010) MAP kinase kinase kinase (MAPKKK)-dependent and -independent activation of Sty1 stress MAPK in fission yeast. J. Biol. Chem. 285, 32818–32823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kunitomo H., Higuchi T., Iino Y., Yamamoto M. (2000) A zinc-finger protein, Rst2p, regulates transcription of the fission yeast ste11+ gene, which encodes a pivotal transcription factor for sexual development. Mol. Biol. Cell 11, 3205–3217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shen Y. H., Godlewski J., Bronisz A., Zhu J., Comb M. J., Avruch J., Tzivion G. (2003) Significance of 14-3-3 self-dimerization for phosphorylation-dependent target binding. Mol. Biol. Cell 14, 4721–4733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Parua P. K., Ratnakumar S., Braun K. A., Dombek K. M., Arms E., Ryan P. M., Young E. T. (2010) 14-3-3 (Bmh) proteins inhibit transcription activation by Adr1 through direct binding to its regulatory domain. Mol. Cell. Biol. 30, 5273–5283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ford J. C., al-Khodairy F., Fotou E., Sheldrick K. S., Griffiths D. J., Carr A. M. (1994) 14-3-3 protein homologs required for the DNA damage checkpoint in fission yeast. Science 265, 533–535 [DOI] [PubMed] [Google Scholar]
- 27. Yaffe M. B., Rittinger K., Volinia S., Caron P. R., Aitken A., Leffers H., Gamblin S. J., Smerdon S. J., Cantley L. C. (1997) The structural basis for 14-3-3:phosphopeptide binding specificity. Cell 91, 961–971 [DOI] [PubMed] [Google Scholar]
- 28. Kafadar K. A., Cyert M. S. (2004) Integration of stress responses. Modulation of calcineurin signaling in Saccharomyces cerevisiae by protein kinase A. Eukaryot. Cell 3, 1147–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim J. S., Diebold B. A., Babior B. M., Knaus U. G., Bokoch G. M. (2007) Regulation of Nox1 activity via protein kinase A-mediated phosphorylation of NoxA1 and 14-3-3 binding. J. Biol. Chem. 282, 34787–34800 [DOI] [PubMed] [Google Scholar]
- 30. Palmer D., Jimmo S. L., Raymond D. R., Wilson L. S., Carter R. L., Maurice D. H. (2007) Protein kinase A phosphorylation of human phosphodiesterase 3B promotes 14-3-3 protein binding and inhibits phosphatase-catalyzed inactivation. J. Biol. Chem. 282, 9411–9419 [DOI] [PubMed] [Google Scholar]
- 31. Boustany L. M., Cyert M. S. (2002) Calcineurin-dependent regulation of Crz1p nuclear export requires Msn5p and a conserved calcineurin docking site. Genes Dev. 16, 608–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rotondo G., Huang J. Y., Frendewey D. (1997) Substrate structure requirements of the Pac1 ribonuclease from Schizosaccharomyces pombe. RNA 3, 1182–1193 [PMC free article] [PubMed] [Google Scholar]
- 33. Kim D. U., Hayles J., Kim D., Wood V., Park H. O., Won M., Yoo H. S., Duhig T., Nam M., Palmer G., Han S., Jeffery L., Baek S. T., Lee H., Shim Y. S., Lee M., Kim L., Heo K. S., Noh E. J., Lee A. R., Jang Y. J., Chung K. S., Choi S. J., Park J. Y., Park Y., Kim H. M., Park S. K., Park H. J., Kang E. J., Kim H. B., Kang H. S., Park H. M., Kim K., Song K., Song K. B., Nurse P., Hoe K. L. (2010) Analysis of a genome-wide set of gene deletions in the fission yeast Schizosaccharomyces pombe. Nat. Biotechnol. 28, 617–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Williams L. A., Kane C. M. (1996) Isolation and characterization of the Schizosaccharomyces pombe gene encoding transcript elongation factor TFIIS. Yeast 12, 227–236 [DOI] [PubMed] [Google Scholar]
- 35. Xiao Z., Brownawell A. M., Macara I. G., Lodish H. F. (2003) A novel nuclear export signal in Smad1 is essential for its signaling activity. J. Biol. Chem. 278, 34245–34252 [DOI] [PubMed] [Google Scholar]
- 36. Herrmann A., Vogt M., Mönnigmann M., Clahsen T., Sommer U., Haan S., Poli V., Heinrich P. C., Müller-Newen G. (2007) Nucleocytoplasmic shuttling of persistently activated STAT3. J. Cell Sci. 120, 3249–3261 [DOI] [PubMed] [Google Scholar]
- 37. Ma Y., Sugiura R., Koike A., Ebina H., Sio S. O., Kuno T. (2011) Transient receptor potential (TRP) and Cch1-Yam8 channels play key roles in the regulation of cytoplasmic Ca2+ in fission yeast. PLoS One 6, e22421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhou D., Frendewey D., Lobo Ruppert S. M. (1999) Pac1p, an RNase III homolog, is required for formation of the 3′ end of U2 snRNA in S. pombe. RNA 5, 1083–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.