Background: The melanocortin 1 receptor is known to regulate inflammation.
Results: The melanocortin 1 receptor inhibits activation of p38 MAPK, subsequently enhancing syndecan-2 expression and migration in melanoma cells.
Conclusion: The melanocortin 1 receptor regulates melanoma cell migration by controlling syndecan-2 expression.
Significance: This work shows the melanocortin 1 receptor plays a role during cell migration.
Keywords: Extracellular Matrix Proteins, MAP Kinases (MAPKs), Migration, Proteoglycan, Signal Transduction
Abstract
The melanocortin 1 receptor (MC1R), a key regulator of melanogenesis, is known to control inflammation, acting in concert with the MC1R ligand α-melanocyte-stimulating hormone. Although cell migration is a key event in inflammation, few studies have addressed the function of MC1R in this context. Using highly motile melanoma cells, we found that the expression level of MC1R was associated with the extent of migration of mouse melanoma cells, suggesting that MC1R plays a functional role in controlling this migration. Overexpression of MC1R enhanced melanoma cell migration, whereas the opposite was true when MC1R levels were knocked down using small inhibitory RNAs. Interestingly, MC1R expression enhanced the synthesis of syndecan-2, a cell surface heparan sulfate proteoglycan known to be involved in melanoma cell migration. Knockdown of syndecan-2 expression decreased MC1R-mediated cell migration. Further, MC1R inhibited the activation of p38 MAPK, subsequently enhancing expression of sydnecan-2, in parallel with an increase in the extent of cell migration. Consistently, activation of p38 by H2O2 inhibited syndecan-2 expression and cell migration, whereas inhibition of p38 activation enhanced syndecan-2 expression and cell migration. Finally, we found that α-melanocyte-stimulating hormone inhibited MC1R-mediated cell migration via activation of p38 and inhibition of syndecan-2 expression. Together, the data strongly suggest that MC1R regulates melanoma cell migration via inhibition of syndecan-2 expression.
Introduction
The melanocortin receptors, belonging to the G-protein-coupled receptor superfamily, include five members (1), each with a characteristic tissue distribution pattern and a unique biological function (2). Of the five members, the melanocortin 1 receptor (MC1R),2 expressed primarily in melanocytes, plays a crucial role in regulation of pigmentation (3). When MC1R interacts with ligands such as α-melanocyte stimulating hormone (α-MSH) or adrenocorticotropic hormone, the protein activates adenylate cyclase to increase cAMP production; this subsequently enhances phosphorylation of the cAMP responsive-element-binding protein (CREB) by PKA. Activated CREB regulates expression of microphthalmia-associated transcription factor, which in turn stimulates transcription of microphthalmia-associated transcription factor target genes including those encoding key enzymes involved in melanin synthesis; these are tyrosinase, dopachrome tautomerase, and tyrosinase-related protein-1 (4). MC1R employs these regulatory mechanisms to control skin and hair pigmentation.
Recent studies have shown that MC1R is involved in the inflammatory response, in addition to the role played by the protein in melanogenesis. It has been reported that both adrenocorticotropic hormone and α-MSH induce expression of IL-10, an anti-inflammatory cytokine, in keratinocytes (5). Other groups have found that α-MSH inhibits either expression or activation of proinflammatory cytokines including TNF-α and IL-1 in melanocytes and melanoma cells (6), suggesting that MC1R plays an additional role during inflammation. Because cells are highly motile under inflammatory conditions, we hypothesized that MC1R might be involved in the regulation of cell migration. Indeed, several studies have found that MC1R regulates such migration and that this activity is highly dependent on expression of MC1R ligands. For example, melanoma cell migration is reduced by α-MSH (7, 8), and this inhibition appears to be associated with development of an anti-inflammatory response (9). Conversely, Agouti signal protein (ASP), another MC1R ligand, promotes melanoma cell migration (10).
Cell migration is a crucial step in cancer development. Melanoma is very aggressive, and it is thus very important to clarify the migration mechanism of melanoma cells. In addition, melanoma is a cancer that is difficult to cure because the potential for malignancy is so high. Thus, melanoma is the most serious form of skin cancer. Accordingly, molecules regulating melanoma progression and factors promoting the aggressive phenotype of the cancer must be identified to allow prediction of the probability that metastasis will occur. Consistent with the role played by MC1R in the response to inflammation, MC1R expression is greatly increased in melanoma cells. More than 80% of human metastatic melanoma cell lines have been shown to display MC1R, and high level MC1R expression has been documented, via immunohistochemistry, in primary and metastatic melanoma tissue samples (11). Therefore, it is very possible that MC1R is involved in regulation of cell migration.
Syndecans are transmembrane cell surface heparan sulfate proteoglycans and act as receptors for extracellular matrix proteins (including fibronectin and laminin) and for growth factors such as FGF-2 and VEGF (12–14). In this manner, syndecan family members are involved in various cellular activities including adhesion, migration, cytoskeletal organization, and differentiation (15). In particular, it is well known that syndecan-2 regulates cell migration. Syndecan-2 is normally highly expressed in cells that are actively migrating. The protein plays a major role in the interaction of cells and extracellular matrix proteins and regulates cytoskeletal organization. Thus, syndecan-2 controls the migration of various types of cancer cells including different lines of colon cancer cells (16, 17) and fibrosarcoma cells (18). Recently, we reported that the syndecan-2 level was increased in melanoma cells and that this increase critically affected the migratory potential of such cells (19).
Because the expression levels of both MC1R and syndecan-2 increase in highly motile melanoma cells and because both proteins play important roles in cell migration, it seemed very possible that MC1R might be involved in regulation of melanoma cell migration. In this present report, we show that MC1R regulates melanoma cell migration via control of syndecan-2 expression.
EXPERIMENTAL PROCEDURES
Materials and Antibodies
Monoclonal antibody to syndecan-2 was produced by AdipoGen Inc. (19). mAb to phosphotyrosine was purchased from Millipore (Billerica, MA). mAbs to ERK, phospho-ERK and β-actin were purchased from Santa Cruz (Santa Cruz, CA). Polyclonal antibodies to phospho-p38, phospho-JNK, p38, and JNK were purchased from Cell Signaling (Danvers, MA). Polyclonal antibodies to phosphorylation site-specific FAK (Tyr(P)397), were purchased from Abcam (Cambridge, UK). SB239063 was purchased from Calbiochem (Darmstadt, Germany), and FGF-2 was purchased from Millipore. pGL3-basic vector was purchased from Promega (Madison, WI), and pCMV/β-galactosidase was purchased from Clontech. α-MSH and gelatin B was purchased from Sigma-Aldrich, and the Luciferase assay kit was from Promega. Effectene was purchased from Qiagen. Lipofectamine 2000 was purchased from Invitrogen.
Cell Culture and Transfection
Mouse melanoma cells B16, B16F10, and human melanoma cells A375 were purchased from the Korean Cell Line Bank. B16, B16F10, and A375 cells were maintained in DMEM (WelGene, Daegu-si, Korea), supplemented with 10% (v/v) FBS with gentamicin (50 μg/ml; Sigma) at 37 °C in 5% CO2 in a humidified atmosphere. B16G4F cell was kindly provided by Dr. A. N. Eberle (University of Basel) (20). B16G4F cell was maintained in DMEM (WelGene), supplemented with 10% (v/v) FBS and 5 mm l-glutamine with gentamicin (50 μg/ml; Sigma) at 37 °C in 5% CO2 in a humidified atmosphere. Transient transfections were carried out using either Effectene reagent (Qiagen) or Lipofectamine 2000 (Invitrogen) according to the provided manufacturer's instructions.
Synthesis of Small Interfering RNA Constructs
Oligonucleotides were designed targeting mouse MC1R. The sequences of the primers are as follows: mouse MC1R sense sequence, 5′-UGGUGAGUCUGGUGGAGAA-3′, and antisense sequence, 5′-UUCUCCACCAGACUCACCA-3′; mouse syndecan-2 sense sequence, 5′-GGAGAAACAUUCAGACAAU-3′ and antisense sequence, 5′-AUUGUCUGAAUGUUUCUCC-3′. Scrambled siRNA (siGENOME Non-Targeting siRNA #2) was purchased from Dharmacon (Chicago, IL) and used as a control. Oligonucleotides were designed targeting human syndecan-2, containing a 9-bp hairpin loop. Oligonucleotides were annealed and cloned into a pSUPER vector (Oligoengine, Seattle, WA). The sequences of the primers are as follows: human syndecan-2 sense primer, 5′-GATCCCCTGACGATGACTACGCTTCTTTCAAGAGAACTGCTACTGATGCGAAGATTTTTGGAAA-3′; human syndecan-2 antisense primer, 5′-AGCTTTTCCAAAAATGACGATGACTACGCTTCTTCTCTTGAAACTGCTACTGATGCGAAGAGGG-3′. Bold characters indicate human syndecan-2 sequences, and italics indicate the hairpin loop.
RNA Extraction and Reverse Transcription-PCR
Total RNA extracted from cultured cells was used as template for reverse transcriptase reaction. Aliquots of cDNA were amplified using the following primers: mouse syndecan-2 (forward) 5′-TGGCTACTTCGTTTGAGCAC-3′ and (backward) 5′-CACTACATTCTCAGCCTCCG-3′; rat syndecan-2 (forward) 5′-ATGCGGGTACGAGCCACGTC-3′ and (backward) 5′-CGGGAGCAGCACTAGTGAGG-3′; human syndecan-2 (forward) 5′-CATCTCCCCTTTGCTAACGGC-3′ and (backward) 5′-TAACTCCATCTCCTTCCCCAGG-3′; mouse MC1R (forward), 5′-CCTCTGCCTCAAGGGTGCTG-3′ and (backward) 5′-TCAACAGTGGAGCTGAGGACG-3′; GAPDH (forward) 5′-CCACCCATGGCAAATTCCATGGCA-3′ and (backward) 5′-TCTAGACGGCAGGTCAGGTCCACC-3′; and β-actin (forward) 5′-TGGAATCCTGTGGCATCCATGAAA-3′ and (backward) 5′-TAAAACGCAGCTCAGTAACAGTCCG-3′. After the initial denaturation at 94 °C for 5 min, 30 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 60 s were carried out. The reaction products were analyzed in 1% agarose gels. The amplified DNA fragments were cloned and sequenced to confirm the PCR products.
Immunoblotting
The cells were washed twice with PBS, and the cells were lysed in radioimmune precipitation assay buffer (50 mm Tris, pH 8.0, 150 mm NaCl, 1% Nonidet P-40, 10 mm NaF, and 2 mm Na3VO4) containing a protease inhibitor mixture (20 μg/ml 1,4-dithiotheritol, 1 μg/ml aprotinin, 1 μg/ml antipain, 5 μg/ml leupeptin, 1 μg/ml pepstatin A, and 20 μg/ml phenylmethylsulfonyl fluoride). The lysates were clarified by centrifugation at 18,000 × g for 15 min at 4 °C, denatured with SDS sample buffer, boiled, and analyzed by SDS-PAGE. The resolved proteins were transferred to PVDF membranes (Millipore) and probed with the appropriate antibodies. The signals were detected by enhanced chemiluminescence (iNtRON Biotechnology, Kyungki-Do, Korea).
Flow Cytometry
Cells cultured in 60-mm diameter tissue culture dishes were washed with PBS and released by 0.2% EDTA followed by the addition of 5% FBS in PBS. After pelleting, the cells were resuspended in PBS and counted. The cells were incubated overnight with anti-syndecan-2 antibody in 10% FBS in PBS on 4 °C. After washing three times with PBS containing 0.05% Tween 20, the cells were incubated FITC-conjugated anti-mouse antibody in 10% FBS in PBS for 30 min. Syndecan-2 expression was analyzed by flow cytometry.
Construction of Transcriptional Syndecan-2 Reporter Plasmids
The 3-kb-long promoter genes of syndecan-2 were amplified by PCR using the following mouse syndecan-2 primers: (forward) 5′-GGTACCATCTCATCAACACCAA-3′ and (backward) 5′-AGGGATATGAGGCCATGGTAAT-3′. After an initial denaturation at 94 °C for 5 min, 30 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 60 s were carried out. The syndecan-2 promoter gene was ligated into pGL3-basic vector at KpnI/XhoI cloning sites.
Luciferase Assay
The cells were plated in 24-well plates 24 h prior to transfection with 0.2 μg of pGL3-basic vector or syndecan-2 reporter constructs together with 0.1 μg of pCMV/β-galactosidase as the normalizing control. 24 h later from transfection, the cells were washed with PBS and lysed with reporter lysis buffer (Promega). Soluble extracts were harvested and assayed for both luciferase and β-galactosidase activities according to the manufacturer's instruction. We calculated the relative luciferase units/β-galactosidase activity to normalize the luciferase values for transfection efficiency. All of the transfections were repeated at least three times using different plasmid preparations.
Transwell Migration Assay
Gelatin B (10 μg/ml) was added to each well of a Transwell plate (Costar; 8-μm pore size), and then the membranes were allowed to dry at 25 °C for 1 h. The Transwell plates were assembled in a 24-well plate, and the lower chambers were filled with FGF-2 (100 ng/ml) in fresh medium. The cells were added to each upper chamber, and the plates were incubated at 37 °C in 5%CO2. The cells that had migrated to the lower surface of the filters were stained with 0.6% hematoxylin and 0.5% eosin and counted.
Statistical Analysis
The data are represented as the means from three independent experiments. Statistical analysis was performed using an unpaired Student's t test. A p value less than 0.01 or 0.05 was considered statistically significant.
RESULTS
MC1R Regulates Melanoma Cell Migration
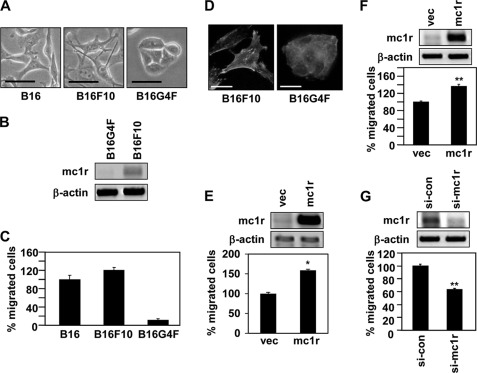
To explore the role played by MC1R in melanoma cell migration, we investigated whether the MC1R expression level affected the extent of such migration. We first compared the morphologies of various types of mouse melanoma cells, including the B16 line expressing native MC1R and B16G4F; the latter line is MC1R-deficient (21, 22). Of the three cell lines examined, B16F10 cells were morphologically the most dendrite- or spine-like. B16G4F cells were round in shape and were densely packed, reminiscent of epithelial cells (Fig. 1A). MC1R is known to regulate the dendricity of melanoma cells (23, 24), and a close association would thus be expected between the extent of dendrite-like morphology and MC1R expression level. As expected, the level of mRNA encoding MC1R was high in B16F10 cells, but not in B16G4F cells (Fig. 1B). In line with the observed morphological differences, B16F10 cells migrated much more rapidly than did B16G4F cells (Fig. 1C). Consistently, only B16F10 cells formed regions of focal adhesion; this is a crucial feature of migrating cells (Fig. 1D). These data suggest that MC1R might be involved in regulation of cell migration. To directly investigate this possibility, we compared the expression level of MC1R with the extent of cell migration. Overexpression of MC1R enhanced migration of both B16G4F (Fig. 1E) and B16F10 cells (Fig. 1F). On the other hand, reduction in MC1R expression, achieved via the use of siRNA targeting to MC1R, significantly reduced migration of B16F10 cells (Fig. 1G). Thus, it seemed very possible that MC1R regulated melanoma cell migration.
FIGURE 1.
MC1R expression levels affect melanoma cell migration. A, B16, B16F10 and B16G4F cells were distributed to tissue culture plates and incubated at 37 °C. After 48 h, digital photographs were taken using a phase contrast microscope. Scale bar, 20 μm. B, total RNA was extracted from exponentially growing B16G4F and B16F10 cells, and mRNA expressions were analyzed by RT-PCR, using β-actin as the loading control. C, Transwell migration assays were performed using FGF-2 (100 ng/ml) as a chemoattractant in the lower chamber. B16, B16F10, and B16G4F cells (5 × 104 cells/well) were allowed to migrate for 12 h, and migrated cells were stained with hematoxylin and eosin. The data shown are representative of three independent experiments. D, B16F10 cells (3.5 × 104 cells) or B16G4F cells (1.5 × 105 cells) were distributed to 12-well tissue culture plates and incubated for 48 h. The cells were fixed and immunostained with either anti-paxillin antibody (Texas Red) or phalloidin (FITC-conjugated). Scale bar, 20 μm. E, B16G4F cells were transfected with either vector (vec) or MC1R. Total RNA was extracted, and mRNA expressions were analyzed by RT-PCR. β-Actin was used as a control (top panel). Transwell migration assays were performed using FGF-2 (100 ng/ml) as a chemoattractant in the lower chamber. Transfected cells were allowed to migrate for 24 h, and migrated cells were stained with hematoxylin and eosin (bottom panel). The data shown are representative of three independent experiments. *, p < 0.01 versus vector. F, B16F10 cells were transfected with either vector or MC1R. After 24 h, total RNA was extracted, and mRNA expressions were analyzed by RT-PCR. β-Actin was used as a control (top panel). Migrated cells were analyzed by Transwell migration assay (bottom panel). G, B16F10 cells were transfected with siRNA targeting MC1R (si-mc1r). After 48 h, MC1R mRNA expression was analyzed by RT-PCR. β-Actin was used as a control (top panel). Migrated cells were analyzed by Transwell migration assay (bottom panel). si-con, control siRNA.
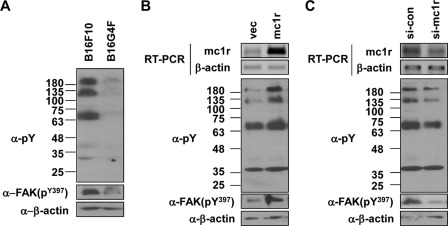
An increase in the extent of tyrosine phosphorylation, and activation of focal adhesion kinase (FAK), are the key signaling events during cell migration (25). In line with the observed increase in migratory activity, B16F10 cells showed elevated levels of overall tyrosine phosphorylation and of phosphorylation of FAK at tyrosine 397; the overall extent of tyrosine phosphorylation was much lower in B16G4F cells (Fig. 2A). Further, MC1R overexpression increased both the overall tyrosine phosphorylation level and FAK phosphorylation at tyrosine 397 in B16F10 cells (Fig. 2B); the opposite effects were observed when MC1R expression was reduced (Fig. 2C). These results suggested that MC1R regulated the migration of melanoma cells.
FIGURE 2.
MC1R regulates the activation of the focal adhesion kinase. A, B16G4F and B16F10 cells were distributed to tissue culture plates and incubated at 37 °C for 48 h. Total cell lysates were analyzed by Western blotting with either anti-phosphotyrosine (α-pY) or anti-phospho FAK (α-FAK(pY397)) antibodies; β-actin (α-β-actin) was detected as the loading control. B, B16G4F cells were transfected with either vector (vec) or MC1R. After 24 h, total RNA was extracted, and mRNA expressions were analyzed by RT-PCR, using β-actin as the loading control (top panel). Total cell lysates were analyzed by Western blotting as described for A (bottom panel). C, B16F10 cells were transfected with siRNA targeting MC1R (si-mc1r). After 48 h, MC1R mRNA expression was analyzed by RT-PCR. β-Actin was used as the control (top panel). Total cell lysates were analyzed by Western blotting as described for A (bottom panel). si-con, control siRNA.
MC1R Promotes Migration of Melanoma Cells by Controlling Syndecan-2 Expression
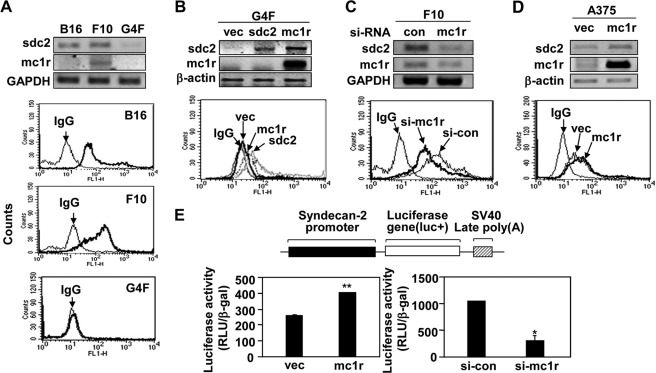
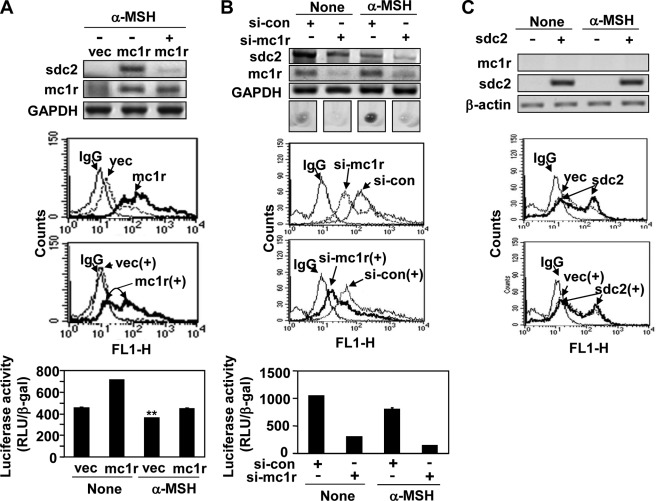
Previously, we showed that syndecan-2 controlled the migration of melanoma cells (19). Therefore, we explored whether syndecan-2 was involved in regulation of MC1R-mediated cell migration in melanoma cells. Interestingly, we found that the extent of cell surface expression of syndecan-2 was relatively high in B16 and B16F10 cells, which synthesized elevated levels MC1R, but lower in B16G4F cells (Fig. 3A). This suggested that the expression levels of syndecan-2 and MC1R were interdependent. Consistent with this notion, MC1R expression increased the level of syndecan-2 in B16G4F cells (Fig. 3B), and the cell surface level of syndecan-2 fell in B16F10 cells containing siRNA targeting MC1R (Fig. 3C). On the other hand, syndecan-2 expression had no affect on the level of MC1R (Fig. 3B). As in mouse melanoma cells, MC1R enhanced the levels of both mRNA encoding syndecan-2 and cell surface expression of the protein in human melanoma A375 cells (Fig. 3D). Consistently, our syndecan-2 promoter reporter assay revealed that increased MC1R expression enhanced transcription of the syndecan-2 gene, whereas reduction in the level of MC1R expression inhibited such transcription (Fig. 3E). These results strongly suggested that MC1R regulated expression of syndecan-2.
FIGURE 3.
MC1R increases syndecan-2 expression in melanoma cells. A, B16, B16F10, and B16G4F cells distributed to tissue culture plates and incubated at 37 °C. After 48 h, mRNA expression levels of MC1R (mc1r) and syndecan-2 (sdc2) were analyzed by RT-PCR, using GAPDH as the loading control (top panel). The cells were detached and harvested using 0.2% EDTA, 5% FBS in PBS and incubated with anti-syndecan-2 antibody. The cell surface expression of syndecan-2 was analyzed by flow cytometry. IgG was used as a control (bottom panel). B, B16G4F cells were transfected with either rat syndecan-2 (sdc2) or MC1R (mc1r), and mRNA expression levels were analyzed by RT-PCR. β-Actin was used as the loading control (top panel). The cell surface expression level of syndecan-2 was analyzed by flow cytometry described for A. IgG was used as a control (bottom panel). C, B16F10 cells were transfected with siRNA targeting MC1R (si-mc1r), and mRNA expression levels of syndecan-2 and MC1R were analyzed by RT-PCR. GAPDH was used as the loading control (con, top panel). The cell surface expression level of syndecan-2 was analyzed by flow cytometry as described for A. IgG was used as a control (bottom panel). D, A375 cells were transfected with either vector (vec) or MC1R (mc1r), and mRNA expression levels were analyzed by RT-PCR. β-Actin was used as the loading control (top panel). The cell surface expression level of syndecan-2 was analyzed by flow cytometry as described for A. IgG was used as a control (bottom panel). E, B16F10 cells were co-transfected with syndecan-2 reporter construct and MC1R (left panel) or siRNA targeting MC1R (right panel). Syndecan-2 promoter activity was analyzed by luciferase assay. *, p < 0.01; **, p < 0.05 versus vector or control siRNA (si-con).
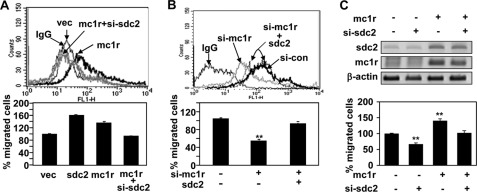
Because syndecan-2 is a key regulator of melanoma cell migration, we next investigated whether the protein was involved in MC1R-mediated cell migration. Consistent with the data described above, both syndecan-2 and MC1R enhanced migration of B16G4F cells. However, co-transfection of B16G4F cells with an MC1R expression vector and syndecan-2-targeting siRNA decreased the cell surface expression level of syndecan-2 and also reduced MC1R-induced migratory activity, compared with what was seen using cells transfected with the MC1R expression vector alone (Fig. 4A). On the other hand, the reduction in B16F10 cell migration induced by siRNA targeting MC1R was rescued by expression of syndecan-2 (Fig. 4B). Similarly, MC1R expression enhanced synthesis of syndecan-2 and increased the migration of human melanoma A375 cells; such MC1R-stimulated migration was reduced in the presence of siRNA targeting syndecan-2 (Fig. 4C). These results showed that syndecan-2 was involved in the MC1R-induced migration of melanoma cells, suggesting that MC1R-mediated cell migration was, at least in part, dependent on expression of syndecan-2.
FIGURE 4.
MC1R-mediated cell migration is dependent on syndecan-2 expression. A, B16G4F cells were transfected with indicated siRNA or cDNA. The expression level of cell surface syndecan-2 was analyzed by flow cytometry described for Fig. 3A. IgG was used as control (top panel). B16G4F cells (1 × 105 cells/well) transfected with indicated cDNA were seeded in the upper chamber of Transwell plates. Cell migration was analyzed as described in the legend to Fig. 1 (bottom panel). B, B16F10 cells were transfected with indicated siRNA or cDNA. The expression level of cell surface syndecan-2 was analyzed by flow cytometry described for Fig. 3A. IgG was used as control (top panel). Cell migration was analyzed as described in the legend to Fig. 1 (bottom panel). *, p < 0.01 versus control siRNA (si-con). C, A375 cells were transfected with indicated siRNA or cDNA. The expression mRNA levels of syndecan-2 or MC1R were analyzed by RT-PCR. β-Actin was used as the loading control (top panel). Cell migration was analyzed as described in the legend to Fig. 1 (bottom panel). **, p < 0.05 versus control. vec, vector; si-sdc2, siRNA syndecan-2; si-mc1r, siRNA targeting MC1R.
MC1R Regulates Syndecan-2 Expression via Inhibition of p38 Activity
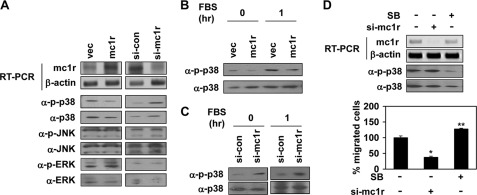
We next explored how MC1R regulated syndecan-2 expression. The MAPK signaling pathway is important in terms of regulation of various cell systems; such activities include gene expression, proliferation, and survival-specific actions (26). Therefore, we determined whether MC1R expression activated any MAPK. Of the three known MAPKs: ERK, p38, and JNK/SAPK, we found that MC1R expression inhibited activation of p38. B16F10 cells expressing MC1R had a reduced basal activity of this MAPK, but not of other MAPKs, whereas B16F10 cells expressing siRNA targeting MC1R showed enhanced phosphorylation of p38 (Fig. 5A). Serum enhanced p38 activation, but MC1R expression inhibited such serum-stimulated activation in B16F10 cells (Fig. 5B). The opposite effect was found in p38 activity of MC1R knocked down cells (Fig. 5C). Consistently, SB239063, a general p38 inhibitor, increased cell migration of B16F10 cells (Fig. 5D).
FIGURE 5.
MC1R inhibits activation of p38. A, B16F10 cells were transfected with either vector (vec) or MC1R (mc1r, left panels) and either control siRNA (si-con) or siRNA targeting MC1R (si-mc1r, right panels). Expression mRNA level of MC1R was analyzed by RT-PCR. β-Actin (α-β-actin) was detected as the loading control (top two panels). Total cell lysates were analyzed by Western blotting with indicated antibodies (bottom six panels). B, B16F10 cells were transfected with vector or MC1R (mc1r). After serum starvation, the cells were activated using 10% FBS for 1 h. The phosphorylation of p38 was analyzed by Western blotting with anti-phospho-p38 (α-p-p38) antibody. p38 (α-p38) was detected as the loading control. C, B16F10 cells were transfected with control siRNA or siRNA targeting MC1R (si-mc1r). After serum starvation, the phosphorylation of p38 was analyzed by Western blotting with anti-phospho-p38 (α-p-p38) antibody. p38 (α-p38) was detected as the loading control (left panel). After serum starvation, the cells were activated using 10% FBS for 1 h and analyzed p38 activity by Western blotting (right penal). D, B16F10 cells were transfected with siRNA targeting MC1R (si-mc1r). B16F10 cells pretreated with SB239063 (5 μm, 30 min) were incubated for 24 h. mRNA expression of these cells was analyzed by RT-PCR (top panel), and the phosphorylation of p38 was analyzed by Western blotting with anti-phospho-specific p38 antibody (α-p-p38, middle panel). Cell migration was analyzed as described in the legend to Fig. 1 (bottom panel). *, p < 0.01; **, p < 0.05 versus si-control.
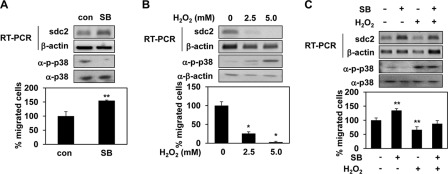
Next, we investigated whether inhibition of p38 activity was involved in regulation of syndecan-2 expression. As MC1R inhibited p38 activity, B16F10 cells were treated with SB239063. Interestingly, inhibition of p38 activity enhanced expression of syndecan-2 in and migration of B16F10 cells (Fig. 6A), suggesting that inhibition of p38 activity was important in terms of induction of syndecan-2 expression. When we treated B16F10 cells with H2O2, a well known activator of p38 (27), p38 activity increased in a dose-dependent manner, and such activation was inversely associated with the level of syndecan-2 expression. Further, H2O2 reduced the migratory behavior of B16F10 cells (Fig. 6B). In addition, inhibition of p38 activation using SB239063 rescued the decrease in both syndecan-2 expression and cell migration by H2O2 (Fig. 6C). Together, these results suggested that MC1R enhanced expression of syndecan-2 via inhibition of p38 activity; the extent of cell migration was subsequently elevated.
FIGURE 6.
p38 inhibition is required for syndecan-2 expression. A, B16F10 cells treated with SB239063 (SB) described as Fig. 5D and mRNA level of syndecan-2 (sdc2) was analyzed by RT-PCR. β-Actin was used as the loading control (con, top panel). The cells (1.5 × 105 cells) were harvested and seeded into upper chamber of Transwell plates. After 6 h, the migrated cells were stained with hematoxylin and eosin (bottom panel). **, p < 0.05 versus control. B, B6F10 cells were treated with the indicated amount of H2O2 in culture medium (1% FBS) for 24 h. Syndecan-2 mRNA expression was analyzed by RT-PCR, and the phosphorylation of p38 was analyzed by Western blotting with anti-phospho-specific p38 antibody (α-p-p38, top panel). Cell migration was analyzed as described for A (bottom panel). *, p < 0.01 versus 0 mm. C, B16F10 cells were pretreated with SB239063 (5 μm) for 30 min and then incubated an additional 24 h in the presence of H2O2 (2.5 mm). Syndecan-2 mRNA expression was analyzed by RT-PCR. The cells were lysed by radioimmune precipitation assay buffer and detected phospho-p38 (α-p-p38) by Western blotting (top panel). Cell mobility was measured by Transwell migration assay (bottom panel). **, p < 0.05 versus untreated cells.
α-MSH Negatively Regulates MC1R-mediated Syndecan-2 Expression and Cell Migration
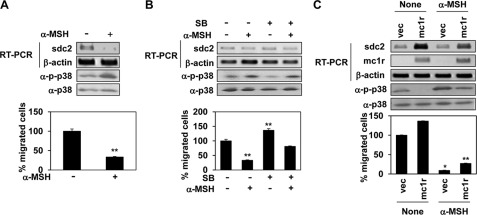
α-MSH, a ligand of MC1R during melanogenesis (4), is also known to regulate cell migration during inflammation (9). In addition, we previously reported that α-MSH inhibited syndecan-2 expression in and migration of melanoma cells (19). We therefore investigated whether α-MSH regulated syndecan-2 synthesis by controlling the signaling pathways affected by MC1R expression. Consistently, MC1R overexpression enhanced synthesis of both mRNA encoding syndecan-2 and the level of cell surface protein in B16F10 cells. However, α-MSH significantly inhibited the rise in syndecan-2 expression induced by MC1R (Fig. 7A). Reduction in the MC1R expression level enhanced the fall seen in the level of syndecan-2, and α-MSH further potentiated such a reduction in syndecan-2 expression (Fig. 7B). The color of cells showed that MC1R targeting siRNA and α-MSH work effectively in melanin synthesis, a well known function. On the other hand, α-MSH did not regulate syndecan-2 expression in B16G4F cells; such cells synthesize syndecan-2 only under the control of an artificial promoter (Fig. 7C). Thus, α-MSH inhibited syndecan-2 expression in melanoma cells, via binding to MC1R.
FIGURE 7.
α-MSH inhibits MC1R-mediated regulation of syndecan-2 expression in melanoma cells. A, B16F10 cells transfected with siRNA targeting MC1R (si-mc1r) were treated with α-MSH (1 μm) for 24 h. The expression levels of mRNA were analyzed by RT-PCR. GAPDH detected as the loading control (top panel). Cell surface expression of syndecan-2 (sdc2) was analyzed by flow cytometry described for Fig. 3A. The cells were treated with α-MSH (+), as was stated above. IgG was used as a control (middle panel). B16F10 cells were co-transfected with syndecan-2 reporter construct and MC1R (mc1r). The cells were treated with α-MSH (1 μm) for 24 h, and syndecan-2 promoter activity was analyzed by luciferase assay (bottom panel). **, p < 0.05 versus untreated vector (vec) cells. B, B16F10 cells transfected with siRNA targeting MC1R were treated with α-MSH (1 μm) for 24 h. The expression levels of the target mRNAs were analyzed by RT-PCR. Equal numbers of cells were harvested by centrifugation, and the cell pellets were photographed with a digital camera (top panel). The expression level of cell surface syndecan-2 was analyzed by flow cytometry (middle panel). B16F10 cells were co-transfected with syndecan-2 reporter construct and siRNA targeting MC1R (si-mc1r). The cells were treated with α-MSH (1 μm) for 24 h, and syndecan-2 promoter activity was analyzed by luciferase assay (bottom panel). C, B16G4F cells transfected with rat syndecan-2 were treated with α-MSH (1 μm) for 24 h. The expression mRNA levels of target mRNAs were analyzed by RT-PCR (top panel). Cell surface expression of syndecan-2 was analyzed by flow cytometry using anti-syndecan-2 antibody (bottom panel). si-con, control siRNA.
Interestingly, α-MSH stimulated p38 activation. When B16F10 cells were treated with α-MSH, p38 activation was enhanced, syndecan-2 expression was decreased, and cell migration fell (Fig. 8A). In addition, SB239063 significantly inhibited α-MSH-mediated effects including p38 activation, decreased syndecan-2 expression, and decreased cell migration (Fig. 8B). In addition, α-MSH-mediated p38 activation completely abolished all the effects of MC1R, resulting in p38 activation, decreased syndecan-2 expression, and decreased cell migration (Fig. 8, A and B).
FIGURE 8.
α-MSH inhibits syndecan-2 expression via stimulation of p38 activation. A, B16F10 cells were treated with α-MSH (1 μm) for 24 h. Syndecan-2 (sdc2) mRNA expression was analyzed by RT-PCR. β-Actin was used as the loading control. The cells were lysed using radioimmune precipitation assay buffer, and total cell lysates were analyzed by Western blotting with anti-phospho p38 (α-p-p38) antibody. Anti-p38 (α-p38) antibody was detected as a control (top panel). B16F10 cells were harvested and seeded into the upper chambers of Transwell plates. After 24 h, migrated cells were stained with hematoxylin and eosin (bottom panel). **, p < 0.05 versus untreated cells. B, B16F10 cells pretreated with SB239063 (SB, 5 μm) for 30 min were incubated with α-MSH (1 μm). After 24 h, syndecan-2 expression levels (top panel), phosphorylation of p38 (middle panel), and cell migration (bottom panel) were analyzed as described for A. **, p < 0.05 versus untreated cells. C, B16F10 cells transfected with MC1R were treated with α-MSH (1 μm). After 24 h, syndecan-2 and MC1R expression levels (top panel), phosphorylation of p38 (middle panel), and cell migration (bottom panel) were analyzed as described for A. *, p < 0.01; **, p < 0.05 versus control. vec, vector.
Further, α-MSH reduced MC1R-mediated inhibition of p38 activity and increased both syndecan-2 expression in and migration of melanoma cells (Fig. 8C). Together, the data suggested that α-MSH negatively regulated MC1R-mediated syndecan-2 expression and cell migration by activating p38.
DISCUSSION
MC1R is well known to play a role in regulation of melanin synthesis by melanocytes and melanoma cells. Recent studies have revealed other functions of MC1R in dendrites formation by skin cells (23, 24), inflammatory response (5, 6), oxidative response (28), and cell migration (7–10). In all instances, MC1R is located on the cell surface, transducing signals from extracellular functional ligands including α-MSH, adrenocorticotropic hormone, and ASP. In the present report, we provide evidence that MC1R itself is an important regulator of cell migration. Elevated levels of either endogenous MC1R or exogenously expressed MC1R enhanced migration of both human and mouse melanoma cells. The basal expression level of MC1R was higher in B16F10 cells, which migrated rapidly, than in B16G4F cells, which migrated less vigorously (Fig. 1). Elevated MC1R expression was associated with a rise in the extent of melanoma cell migration, whereas the opposite was true when MC1R expression was suppressed using siRNAs (Fig. 1). Therefore, MC1R appears to act as a receptor potentiating melanoma cell migration.
Although the mechanism of MC1R-mediated regulation of melanoma cell migration remains poorly elucidated, our results indicate that enhanced syndecan-2 expression plays a key role in such regulation. Overexpression of MC1R enhanced expression of mRNA encoding syndecan-2 and cell surface expression of the protein (Fig. 3), and reduction of syndecan-2 expression abolished the MC1R-mediated increase in the extent of cell migration (Fig. 4). Therefore, it is likely that syndecan-2 is an important component of the MC1R-mediated melanoma cell migration pathway. Consistent with the data of a previous report indicating that syndecan-2 regulates FAK activity (18, 19), MC1R enhanced FAK activity in melanoma cells (Fig. 2).
Interestingly, MC1R negatively regulated p38 activity. We found that the extent of p38 phosphorylation was decreased in MC1R-transfected cells and inhibition of p38 activity enhanced migration of melanoma cells (Figs. 5 and 6). In addition, inhibition of p38 via either MC1R expression or using a chemical inhibitor enhanced expression of syndecan-2 (Fig. 6A). On the other hand, activation of p38 using H2O2 enhanced p38 activity but reduced both syndecan-2 expression and subsequent cell migration (Fig. 6B). These results strongly suggest that MC1R-mediated inhibition of p38 activity regulates melanoma cell migration via expression of syndecan-2.
We also showed that the action of MC1R in terms of cell migration is regulated by several physiological ligands. α-MSH is a well known MC1R ligand. Via interaction with MC1R, α-MSH regulates various cellular functions including melanin synthesis, activation of survival pathways, inflammation, and proliferation (6, 29). Especially, it is well known that α-MSH stimulates MC1R activation of the melanin synthesis pathway in both melanocytes and melanoma cells. Briefly, α-MSH activates MC1R, which in turn activates adenylate cyclase to increase cAMP production and, subsequently, phosphorylation of CREB. Activated CREB promotes expression of microphthalmia-associated transcription factor, which is a transcription factor controlling the expression of genes encoding enzymes essential for melanin synthesis; these enzymes are tyrosinase, dopachrome tautomerase, and tyrosinase-related protein-1 (4). Our results show that α-MSH acts as an inhibitor of MC1R function in terms of cell migration. We verified that α-MSH reduced syndecan-2 expression via binding to MC1R (Fig. 7) and decreased cell migration (Fig. 8A). Further, the p38 activity level was increased by α-MSH (Fig. 8). In other words, α-MSH regulates syndecan-2-mediated migration via a mechanism involving p38. This is consistent with data obtained by others, showing that α-MSH inhibited migration of melanoma cells via down-regulation of syndecan-2 expression (19). Therefore, MC1R plays key roles in the regulation of various cellular functions, including melanin synthesis and cell migration; such activity is dependent on ligands of MC1R.
It is accepted that α-MSH has anti-inflammatory functions. α-MSH decreases either expression or activation of proinflammatory cytokines including IL-1 and TNF-α but increases the synthesis of anti-inflammatory cytokines such as IL-6 and IL-10. As a result, α-MSH reduces the migration of melanocytes, melanoma cells, and keratinocytes.
Interestingly, ASP, another ligand of MC1R, seems to act in a manner opposite to that of α-MSH. ASP inhibits melanin synthesis by decreasing cAMP levels in melanocytes and melanoma cells (30, 31). In addition, ASP promotes melanoma cell motility and up-regulates syndecan-2 expression in such cells (10). Therefore, ASP may function in vivo as an antagonist of α-MSH. However, it is not yet known whether ASP activates p38. Thus, further work on ASP signaling may aid in an understanding of MC1R function and signaling.
Many studies have identified variant MC1R alleles in the human. Representative polymorphic variant alleles of human MC1R are associated with red hair color (thus, the alleles carry the RHC descriptor), fair skin, and difficulty in tanning. The presence of these alleles is associated with high cancer risks (32). RHC variant cells differ in terms of cell signaling compared with that of wild-type MC1R cells. RHC variants synthesizing reduced levels of cAMP and eumelanin (33, 34) have been reported. Interestingly, RHC variant cell function in a manner similar to that seen when ASP is added to cells expressing wild-type MC1R. Therefore, to understand MC1R function, it will be important to explore regulation of migration and syndecan-2 expression in RHC variant cells. This will allow melanoma risks in subjects with RHC variants to be understood.
In summary, we herein show that MC1R is involved in regulation of melanoma cell migration. MC1R inhibits p38 activation, resulting in increased syndecan-2 expression and a subsequent enhancement in cell migration. In addition, α-MSH inhibits migration via negative regulation of MC1R action; syndecan-2 expression is thus down-regulated. However, further studies are required to clarify the precise regulatory roles played by MC1R during the response to inflammation or in carcinogenesis. The present work provides the evidence that MC1R plays a regulatory role during melanoma cell migration.
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by Ministry of Education, Science and Technology Grants 2009-0071381 and 2010-0028042 (to E.-S. O.).
- MC1R
- melanocortin 1 receptor
- MSH
- melanocyte-stimulating hormone
- CREB
- cAMP responsive-element-binding protein
- ASP
- Agouti signal protein
- FAK
- focal adhesion kinase.
REFERENCES
- 1. Abdel-Malek Z. A. (2001) Melanocortin receptors. Their functions and regulation by physiological agonists and antagonists. Cell. Mol. Life Sci. 58, 434–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cone R. D. (2006) Studies on the physiological functions of the melanocortin system. Endocr. Rev. 27, 736–749 [DOI] [PubMed] [Google Scholar]
- 3. Lin J. Y., Fisher D. E. (2007) Melanocyte biology and skin pigmentation. Nature 445, 843–850 [DOI] [PubMed] [Google Scholar]
- 4. Yamaguchi Y., Brenner M., Hearing V. J. (2007) The regulation of skin pigmentation. J. Biol. Chem. 282, 27557–27561 [DOI] [PubMed] [Google Scholar]
- 5. Redondo P., García-Foncillas J., Okroujnov I., Bandrés E. (1998) α-MSH regulates interleukin-10 expression by human keratinocytes. Arch. Dermatol. Res. 290, 425–428 [DOI] [PubMed] [Google Scholar]
- 6. Eves P. C., MacNeil S., Haycock J. W. (2006) α-Melanocyte stimulating hormone, inflammation and human melanoma. Peptides 27, 444–452 [DOI] [PubMed] [Google Scholar]
- 7. Zhu N., Eves P. C., Katerinaki E., Szabo M., Morandini R., Ghanem G., Lorigan P., MacNeil S., Haycock J. W. (2002) Melanoma cell attachment, invasion, and integrin expression is upregulated by tumor necrosis factor α and suppressed by α melanocyte stimulating hormone. J. Invest. Dermatol. 119, 1165–1171 [DOI] [PubMed] [Google Scholar]
- 8. Zhu N., Lalla R., Eves P., Brown T. L., King A., Kemp E. H., Haycock J. W., MacNeil S. (2004) Melanoma cell migration is upregulated by tumour necrosis factor-α and suppressed by α-melanocyte-stimulating hormone. Br. J. Cancer 90, 1457–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eves P., Haycock J., Layton C., Wagner M., Kemp H., Szabo M., Morandini R., Ghanem G., García-Borrón J. C., Jiménez-Cervantes C., Mac Neil S. (2003) Anti-inflammatory and anti-invasive effects of α-melanocyte-stimulating hormone in human melanoma cells. Br. J. Cancer 89, 2004–2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Le Pape E., Passeron T., Giubellino A., Valencia J. C., Wolber R., Hearing V. J. (2009) Microarray analysis sheds light on the dedifferentiating role of Agouti signal protein in murine melanocytes via the Mc1r. Proc. Natl. Acad. Sci. U.S.A. 106, 1802–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Salazar-Onfray F., López M., Lundqvist A., Aguirre A., Escobar A., Serrano A., Korenblit C., Petersson M., Chhajlani V., Larsson O., Kiessling R. (2002) Tissue distribution and differential expression of melanocortin 1 receptor, a malignant melanoma marker. Br. J. Cancer 87, 414–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mahalingam Y., Gallagher J. T., Couchman J. R. (2007) Cellular adhesion responses to the heparin-binding (HepII) domain of fibronectin require heparan sulfate with specific properties. J. Biol. Chem. 282, 3221–3230 [DOI] [PubMed] [Google Scholar]
- 13. Clasper S., Vekemans S., Fiore M., Plebanski M., Wordsworth P., David G., Jackson D. G. (1999) Inducible expression of the cell surface heparan sulfate proteoglycan syndecan-2 (fibroglycan) on human activated macrophages can regulate fibroblast growth factor action. J. Biol. Chem. 274, 24113–24123 [DOI] [PubMed] [Google Scholar]
- 14. Chen E., Hermanson S., Ekker S. C. (2004) Syndecan-2 is essential for angiogenic sprouting during zebrafish development. Blood 103, 1710–1719 [DOI] [PubMed] [Google Scholar]
- 15. Choi Y., Chung H., Jung H., Couchman J. R., Oh E. S. (2011) Syndecans as cell surface receptors. Unique structure equates with functional diversity. Matrix Biol. 30, 93–99 [DOI] [PubMed] [Google Scholar]
- 16. Choi Y., Kim H., Chung H., Hwang J. S., Shin J. A., Han I. O., Oh E. S. (2010) Syndecan-2 regulates cell migration in colon cancer cells through Tiam1-mediated Rac activation. Biochem. Biophys. Res. Commun. 391, 921–925 [DOI] [PubMed] [Google Scholar]
- 17. Lee H., Kim Y., Choi Y., Choi S., Hong E., Oh E. S. (2011) Syndecan-2 cytoplasmic domain regulates colon cancer cell migration via interaction with syntenin-1. Biochem. Biophys. Res. Commun. 409, 148–153 [DOI] [PubMed] [Google Scholar]
- 18. Park H., Han I., Kwon H. J., Oh E. S. (2005) Focal adhesion kinase regulates syndecan-2-mediated tumorigenic activity of HT1080 fibrosarcoma cells. Cancer Res. 65, 9899–9905 [DOI] [PubMed] [Google Scholar]
- 19. Lee J. H., Park H., Chung H., Choi S., Kim Y., Yoo H., Kim T. Y., Hann H. J., Seong I., Kim J., Kang K. G., Han I. O., Oh E. S. (2009) Syndecan-2 regulates the migratory potential of melanoma cells. J. Biol. Chem. 284, 27167–27175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chluba-de Tapia J., Bagutti C., Cotti R., Eberle A. N. (1996) Induction of constitutive melanogenesis in amelanotic mouse melanoma cells by transfection of the human melanocortin-1 receptor gene. J. Cell Sci. 109, 2023–2030 [DOI] [PubMed] [Google Scholar]
- 21. Poste G., Doll J., Brown A. E., Tzeng J., Zeidman I. (1982) Comparison of the metastatic properties of B16 melanoma clones isolated from cultured cell lines, subcutaneous tumors, and individual lung metastases. Cancer Res. 42, 2770–2778 [PubMed] [Google Scholar]
- 22. Solca F. F., Chluba-de Tapia J., Iwata K., Eberle A. N. (1993) B16-G4F mouse melanoma cells. An MSH receptor-deficient cell clone. FEBS Lett. 322, 177–180 [DOI] [PubMed] [Google Scholar]
- 23. Kauser S., Thody A. J., Schallreuter K. U., Gummer C. L., Tobin D. J. (2005) A fully functional proopiomelanocortin/melanocortin-1 receptor system regulates the differentiation of human scalp hair follicle melanocytes. Endocrinology 146, 532–543 [DOI] [PubMed] [Google Scholar]
- 24. Scott G. A., Cassidy L. (1998) Rac1 mediates dendrite formation in response to melanocyte stimulating hormone and ultraviolet light in a murine melanoma model. J. Invest. Dermatol. 111, 243–250 [DOI] [PubMed] [Google Scholar]
- 25. Parsons J. T. (2003) Focal adhesion kinase. The first ten years. J. Cell Sci. 116, 1409–1416 [DOI] [PubMed] [Google Scholar]
- 26. Wagner E. F., Nebreda A. R. (2009) Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer 9, 537–549 [DOI] [PubMed] [Google Scholar]
- 27. Zhuang S., Demirs J. T., Kochevar I. E. (2000) p38 mitogen-activated protein kinase mediates bid cleavage, mitochondrial dysfunction, and caspase-3 activation during apoptosis induced by singlet oxygen but not by hydrogen peroxide. J. Biol. Chem. 275, 25939–25948 [DOI] [PubMed] [Google Scholar]
- 28. Haycock J. W., Rowe S. J., Cartledge S., Wyatt A., Ghanem G., Morandini R., Rennie I. G., MacNeil S. (2000) α-Melanocyte-stimulating hormone reduces impact of proinflammatory cytokine and peroxide-generated oxidative stress on keratinocyte and melanoma cell lines. J. Biol. Chem. 275, 15629–15636 [DOI] [PubMed] [Google Scholar]
- 29. De Luca M., Siegrist W., Bondanza S., Mathor M., Cancedda R., Eberle A. N. (1993) α-Melanocyte stimulating hormone (α-MSH) stimulates normal human melanocyte growth by binding to high-affinity receptors. J. Cell Sci. 105, 1079–1084 [DOI] [PubMed] [Google Scholar]
- 30. Manceau M., Domingues V. S., Mallarino R., Hoekstra H. E. (2011) The developmental role of Agouti in color pattern evolution. Science 331, 1062–1065 [DOI] [PubMed] [Google Scholar]
- 31. Sakai C., Ollmann M., Kobayashi T., Abdel-Malek Z., Muller J., Vieira W. D., Imokawa G., Barsh G. S., Hearing V. J. (1997) Modulation of murine melanocyte function in vitro by Agouti signal protein. EMBO J. 16, 3544–3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Valverde P., Healy E., Jackson I., Rees J. L., Thody A. J. (1995) Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nat. Genet. 11, 328–330 [DOI] [PubMed] [Google Scholar]
- 33. Newton R. A., Roberts D. W., Leonard J. H., Sturm R. A. (2007) Human melanocytes expressing MC1R variant alleles show impaired activation of multiple signaling pathways. Peptides 28, 2387–2396 [DOI] [PubMed] [Google Scholar]
- 34. Roberts D. W., Newton R. A., Leonard J. H., Sturm R. A. (2008) Melanocytes expressing MC1R polymorphisms associated with red hair color have altered MSH-ligand activated pigmentary responses in coculture with keratinocytes. J. Cell. Physiol. 215, 344–355 [DOI] [PubMed] [Google Scholar]