Background: The contribution of TRPV1 trafficking to vanilloid-induced desensitization and tachyphylaxis remains unexplored.
Results: Agonist exposure promotes TRPV1 internalization and degradation in nociceptors and HEK293 cells, in a time-, dose-, and Ca2+-dependent manner.
Conclusion: Agonist-induced TRPV1 internalization and degradation notably contribute to long-term nociceptor desensitization.
Significance: Modulation of surface TRPV1 levels could be a therapeutic approach for pain treatment.
Keywords: Intracellular Trafficking, Pain, Receptor Desensitization, Receptor Endocytosis, TRP Channels
Abstract
TRPV1 receptor agonists such as the vanilloid capsaicin and the potent analog resiniferatoxin are well known potent analgesics. Depending on the vanilloid, dose, and administration site, nociceptor refractoriness may last from minutes up to months, suggesting the contribution of different cellular mechanisms ranging from channel receptor desensitization to Ca2+ cytotoxicity of TRPV1-expressing neurons. The molecular mechanisms underlying agonist-induced TRPV1 desensitization and/or tachyphylaxis are still incompletely understood. Here, we report that prolonged exposure of TRPV1 to agonists induces rapid receptor endocytosis and lysosomal degradation in both sensory neurons and recombinant systems. Agonist-induced receptor internalization followed a clathrin- and dynamin-independent endocytic route, triggered by TRPV1 channel activation and Ca2+ influx through the receptor. This process appears strongly modulated by PKA-dependent phosphorylation. Taken together, these findings indicate that TRPV1 agonists induce long-term receptor down-regulation by modulating the expression level of the channel through a mechanism that promotes receptor endocytosis and degradation and lend support to the notion that cAMP signaling sensitizes nociceptors through several mechanisms.
Introduction
Persistent chemical or physical noxious stimulation attenuates nociceptive sensory neuron excitability, making nociceptors partially or totally refractory to subsequent stimuli. Depending on the refractory period, which can range from minutes and hours up to days, this phenomenon is known as acute desensitization, down-regulation, or defunctionalization. Based on its ability to desensitize nociceptors, the irritant vanilloid capsaicin is therapeutically used in the treatment of postherpetic and trigeminal neuralgia, diabetic neuropathy, and osteoarthritic pain (1, 2). On the other hand, peripheral injury and inflammation can elicit an increase in peripheral nociceptor sensitivity or responsiveness, thereby leading to hyperalgesia and/or allodynia (3). The cellular and molecular mechanisms that elicit either type of adaptation, i.e. nociceptor sensitization and refractoriness, substantially overlap with those underlying neuronal plasticity in learning and memory (2, 3).
The transient receptor potential vanilloid TRPV13 is a nonselective cation channel, highly permeable to Ca2+, that plays a key role in the generation and maintenance of nociceptor sensitization and desensitization (for review, see Refs. 4 and 5). TRPV1 integrates multiple and diverse noxious stimuli including physical (T ≥42 °C and voltage) and chemical (protons, vanilloid compounds, and toxins) stimuli. Although under normal conditions TRPV1 activity is low and individual activators gate the channel with low efficacy and potency, simultaneous release of several mediators during inflammation synergistically acts on TRPV1, leading to enhanced nociceptor excitability. Increased excitability can be achieved by several signaling pathways that may lead to: (i) phosphorylation of TRPV1 that reduces its activation threshold, (ii) recruitment of TRPV1 receptor to the plasma membrane, and (iii) long-term transcriptional/transductional modification (4).
TRPV1 activation by capsaicin is followed by nociceptor desensitization, a state characterized by the inability of the receptor to respond to the vanilloid capsaicin or other noxious stimuli. TRPV1 desensitization is a process markedly depending on Ca2+ and involves various intracellular signaling pathways (6). Thus, dephosphorylation by the phosphatase calcineurin of TRPV1 previously phosphorylated by protein kinase A (PKA) or Ca2+-calmodulin-dependent kinase II leads to TRPV1 desensitization (7–10). Several other mechanisms like binding of molecules such as calmodulin (11–13), ATP (13), or AKAP150 (14) or the depletion of phosphoinositol 4,5-diphosphate from the plasma membrane (15–18) also modulate receptor desensitization. A simplified hypothesis considers that Ca2+-dependent binding of calmodulin to TRPV1 modulates the fast component of desensitization that relies on Ca2+ influx through the channel, whereas Ca2+-dependent phosphorylation/dephosphorylation processes may mediate the slow component of TRPV1 desensitization or tachyphylaxis (7).
In contrast to acute TRPV1 desensitization, the mechanisms involved in capsaicin-induced long-term TRPV1 desensitization are poorly understood. Because proinflammatory sensitization of nociceptors results in TRPV1 recruitment to the plasma membrane, we hypothesized that capsaicin-induced neuronal desensitization may involve TRPV1 withdrawal from the cell surface. Similar to members of other receptor families (19, 20), an activity-dependent tight control of plasma membrane-resident receptors may involve TRPV1 endocytosis followed either by receptor recycling to the plasma membrane or by its degradation through the proteasomal or lysosomal pathway. This would lead to short- or long-term down-regulation, respectively. Here, we report that dose- and time-dependent capsaicin-induced endocytosis of TRPV1 is involved in its pharmacological desensitization. Vanilloid-evoked receptor internalization required TRPV1 channel activity and the influx of Ca2+ and was modulated by PKA phosphorylation. Analysis of the vanilloid-induced internalization mechanism showed that it occurs through a clathrin-independent pathway that targets the channel for lysosomal degradation in both DRG neurons and TRPV1-expressing HEK293 cells. Taken together, these results indicate that capsaicin-induced TRPV1 endocytosis and degradation in nociceptors notably contribute to the long-term neuronal tachyphylaxis induced by vanilloids.
EXPERIMENTAL PROCEDURES
Chemicals and Antibodies
All drugs and antibodies were from Sigma unless otherwise stated. Anti-TRPV1 serum (21), anti-TRPV1 (extracellular) (α-TRPV1e) (Alomone), monoclonal Na+-K+ ATPase (Abcam, Cambridge, UK), and polyclonal anti-calnexin (Chemicon, Billerica, MA) were used. Secondary antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA).
Plasmids
Rat wild type TRPV1 (TRPV1-WT) and TRPV1PORELESS mutant were as described in Ref. 22. The nondesensitizing mutant TRPV1-S116D was obtained by site-directed mutagenesis. GFP-tagged wild type and dominant-negative dynamin constructs (GFP-Dyn-WT and Dyn-K44A) were gifts from M. McNiven (Mayo Clinic), and clathrin light chain (mLCA-DsRed) was from I. Pérez-Otaño Centro de Investigación Médica Aplicada (CIMA).
Cell Culture
Primary cultures of neonatal DRG neurons were prepared as in Ref. 23. HEK293 cells were cultured as described (24) and used 48 h after transfection with TurboFect (Fermentas Life Sciences) following the manufacturer's instructions.
Immunofluorescence-based Internalization Assays
Differential labeling of surface and internalized TRPV1 was obtained as in Ref. 25. Briefly, surface receptors of live cells in DRG primary cultures or transiently TRPV1-expressing (TRPV1+) HEK293 cells were labeled 1 h at 4 °C with α-TRPV1e, an antibody recognizing an extracellular epitope. Cells were then incubated 20 min at 37 °C with different capsaicin concentrations (10 nm to 1 μm) or vehicle (0.01% EtOH) to allow internalization. Control cultures were maintained at 4 °C, a condition nonpermissive for internalization. Surface receptors were stained with an A488 secondary antibody for 1 h at 4 °C. After washing at 4 °C, cells were fixed with 4% paraformaldehyde and permeabilized, and internalized receptors were stained with an A568 secondary antibody, mounted, and analyzed by confocal microscopy (Leica TCS; Leica Microsystems, Wetzlar, Germany).
For subcellular colocalization of internalized receptors, HEK293 cells were co-transfected with TRPV1-WT or TRPV1-S116D and DNA encoding organelle-specific fluorescent fusion proteins (mLCA-Red, GFP-Dyn-WT, or GFP-Dyn-K44A). Surface receptors, previously labeled at 4 °C with TRPV1e antibody, were incubated at 37 °C with different capsaicin concentrations or vehicle. Later, cells were fixed with 4% paraformaldehyde, permeabilized, and stained with a fluorescently labeled secondary antibody. Samples were analyzed by confocal microscopy.
Biotin Labeling of Surface Proteins
Adult rat DRG cultures or HEK293 cells transiently expressing TRPV1-WT or the mutants TRPV1PORELESS (22) or TRPV1-S116D (7) were exposed to the agonist capsaicin (or resiniferatoxin (RTX)) for different time spans at 37 °C, in the presence or absence of different drugs. Control samples were incubated with vehicle in the same conditions. Surface proteins were biotinylated as in Ref. 24. The absence of contaminating intracellular proteins in membrane fractions was verified by testing the presence of actin. Immunoblots were digitized and quantified.
Ca2+ Imaging
TRPV1-WT- or TRPV1-S116D-transfected HEK293 cells were loaded for 1 h at 37 °C with 10 μm Fura-2/AM ester (Biotium, Hayward, CA) in Buffer Solution (in mm: 140 NaCl, 4 KCl, 2 CaCl2, 2 MgCl2, 10 HEPES, 5 d-glucose, pH = 7.4, and osmolarity set at 320 mosm/kg with mannitol) as in Ref. 24. For acid solution, HEPES was replaced with MES, and pH was adjusted to 5.5. The applied protocol consisted of two 1-min pulses of acid solution interspersed by a 20-min incubation with either capsaicin or vehicle. For testing endocytosis, cells were pretreated for 30 min (and then maintained throughout the experiment) with 50 μm chlorpromazine or with a hyperosmotic solution (600 mosm/kg), created by supplementing 250 mm sucrose in the Buffer Solution. Fluorescence measurements were performed as in Ref. 24.
Electrophysiology
Currents were recorded at 22 °C in the perforated patch configuration using an EPC-10 amplifier (HEKA, Lambrecht, Germany). Continuous voltage linear ramps from −60 to +60 mV applied every 3 s, from a holding potential of 0 mV, were recorded throughout the entire protocol (two 1-min pulses of pH = 6.0 interspersed by a 20-min treatment with capsaicin or vehicle). Buffer Solution was used as control bath solution. For acid pH solution, 50 μm amiloride was included to block Acid-sensing ion channels. Pipette solution contained (in mm): 144 KCl; 2 MgCl2; 10 HEPES; 5 EGTA, pH 7.2, with KOH; ∼295 mosm/kg, supplemented with 240 μg/ml amphotericin B. The desensitization rate was calculated from normalizing the current magnitude of the second acid application (IpH2) obtained at +60 mV to the first (IpH1), IpH2/pH1. The Ca2+-free external solution (0Ca2+) contained no added Ca2+ and 1 mm EGTA to chelate ambient Ca2+. The role of intracellular Ca2+ was tested after a 1-h preincubation with 50 μm BAPTA-AM (Calbiochem) in serum-free medium at 37 °C before recording in 0Ca2+.
Statistics
Data are given as mean ± S.E. with n = number of cells and N = number of experiments. For statistical analysis, the nonparametric Mann-Whitney test or Student's t test was applied. *, p < 0.05; **, p < 0.01, ***, p < 0.001.
RESULTS
Capsaicin Induced TRPV1 Internalization in a Dose- and Time-dependent Manner
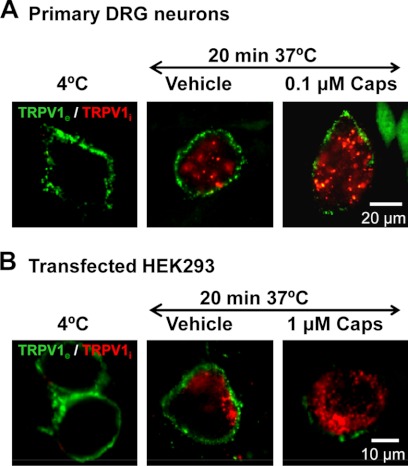
Exposure of nociceptors to capsaicin produces an initial excitation followed by a subsequently attenuated, or even abolished, response to additional noxious stimuli (1, 26). To evaluate the contribution of TRPV1 endocytosis to nociceptor desensitization, we initially used a differential immunocytochemical approach to discriminate surface-resident from internalized TRPV1 (25). Specifically, agonist-induced TRPV1 internalization in live DRG neurons was visualized by prelabeling surface receptors at 4 °C in nonpermeabilized cells with an antibody that recognizes an extracellular epitope and then incubating cells with vehicle (0.01% EtOH) or with 100 nm capsaicin for 20 min at 37 °C. Plasma membrane-resident and internalized receptors were differentially stained by first using an A488 secondary antibody in nonpermeabilized cells and, after fixation and permeabilization, labeling internalized receptors with an A568-labeled secondary antibody (25). Although at 4 °C labeled TRPV1 remained at the plasma membrane, incubation at 37 °C led to a partial internalization of receptors from the plasma membrane, as evident from a clear intracellular staining pattern (red) (Fig. 1A). Importantly, receptor endocytosis was significantly accelerated when neurons were exposed to 100 nm capsaicin. Note that although only small fluorescent clusters were observed close to the membrane under control conditions at 37 °C, exposure to capsaicin led to a drastic decrease of plasma membrane receptors (green), and large intracellular clusters containing TRPV1 emerged (red). Similar effects were observed in TRPV1-expressing HEK293 cells. Because different cell-specific factors/pathways might shift the EC50 for capsaicin and hence heterologously expressed TRPV1 may not be as sensitive as the native receptor in neurons, TRPV1+ HEK293 cells were exposed to 1 μm capsaicin (Fig. 1B). Altogether, these results indicate that exposure to capsaicin induces a significant TRPV1 channel internalization in both native and recombinant systems.
FIGURE 1.
Prolonged exposure to capsaicin promotes cell surface TRPV1 internalization. A, representative images of neonatal rat DRG neurons show that incubation with 0.1 μm capsaicin for 20 min redistributes TRPV1 from the cell surface to an intracellular reservoir of large deep clusters as compared with vehicle (0.01% EtOH). A differential labeling method based on Ref. 25 was used. Nonpermeabilized live cell membranes were immunolabeled with anti-TRPV1e (23) and incubated for 20 min at 37 °C in the presence or absence of 0.1 μm capsaicin, and surface receptors (green) were stained with an A488-labeled secondary antibody at 4 °C. Cells were then fixed, permeabilized, and internalized receptors stained in red. Bar, 20 μm. B, representative images in HEK293 cells transiently expressing the TRPV1 receptor and incubated in the absence or presence of 1 μm capsaicin for 20 min at 37 °C. The same labeling procedure was employed to differentially label internalized receptor from surface receptor. As control, cells were incubated at a temperature (4 °C) that blocks endocytosis. Bar, 10 μm.
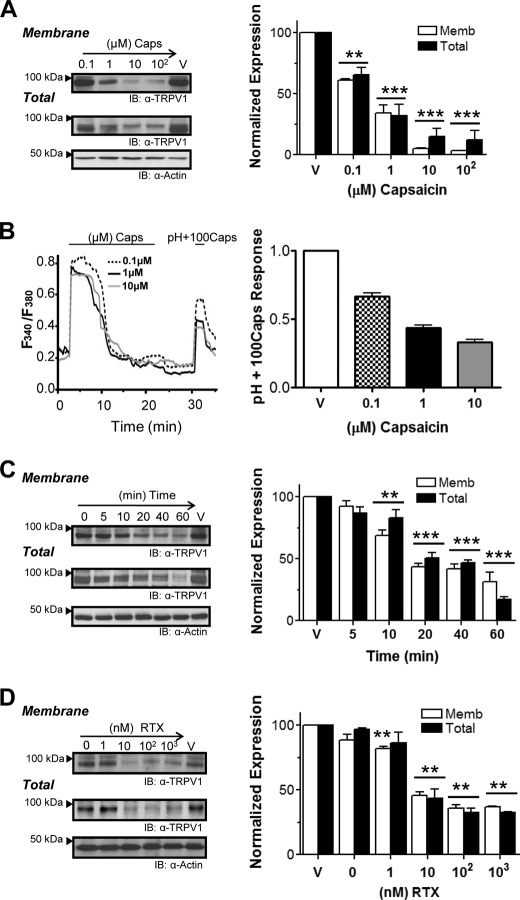
We then evaluated the kinetics of TRPV1 internalization and its dependence on the agonist concentration in TRPV1+ HEK293 cells (24). These cells were exposed to increasing capsaicin concentrations ranging from 0.1 to 100 μm for 20 min at 37 °C, and surface proteins were then biotinylated with a cleavable biotin label and pulled down with streptavidin-agarose. As shown in Fig. 2A, the vanilloid produced a noticeable decrement of surface TRPV1 in a dose-dependent manner, with an apparent EC50 of 0.5 μm and a maximum surface receptor loss of 90%. The internalization kinetics of plasma membrane TRPV1 at 1 μm capsaicin is shown in Fig. 2C. Receptor endocytosis displayed relatively slow kinetics with an apparent time constant of about 15 min and reached a plateau at roughly 30 min. Extended times did not provoke additional receptor endocytosis, suggesting a tight dependence on the capsaicin concentration.
FIGURE 2.
Extended incubation with vanilloids leads to TRPV1 down-regulation. A, dose-dependent effects of capsaicin on surface and total TRPV1 expression resolved by cell surface biotinylation assays. TRPV1+ HEK293 cells were incubated with capsaicin in 0.01% EtOH (ranging from 0.1 to 100 μm) or as control in vehicle (V)(0.01% EtOH) for 20 min at 37 °C. Representative blot (IB) from four independent experiments shows TRPV1 plasma membrane and total expression (left) and band density quantification (right). **, p < 0.01, ***, p < 0.001. B, left, representative recordings from Ca2+ influx measurements in TRPV1+ cells illustrate the maximum responsiveness, obtained by short pulse of high capsaicin (100 μm) plus low pH (5.5) (pH+100Caps), after incubation for 20 min at various capsaicin concentrations. Right, correlation of the pH+100Caps responsiveness to increasing agonist concentrations (n = 150, n = 3). C, time course effect of 1 μm capsaicin incubation from 5 min up to 1 h. n = 4. **, p < 0.01, ***, p < 0.001. D, dose-dependent effects of RTX on TRPV1 surface and total expression were assayed applying RTX concentrations from 1 nm to 1 μm for 20 min (n = 3). Data represent the mean ± S.E., and nonparametric Student's t test was applied. **, p < 0.01.
The responsiveness of remaining receptors, which may have been partially desensitized by capsaicin application, was assessed by Ca2+ microfluorography using an experimental paradigm based on the combined application of a supramaximal capsaicin concentration (100 μm) plus acid pH (pH 5.5), pH+100Caps (18), after a 20-min incubation with different capsaicin concentrations. Fig. 2B (left) shows representative traces illustrating a dose-dependent reduction of the transient Ca2+ influx obtained upon pH+100Caps pulse that correlates with reduced membrane receptors.
A similar internalization effect was observed when RTX was used (Fig. 2D). Because RTX is a higher affinity ligand, lower RTX concentrations induced TRPV1 internalization (apparent EC50 of 5 nm). Also note that RTX induced the endocytosis of up to 60% of membrane-resident TRPV1, as compared with the 90% removal promoted by capsaicin. This difference may be attributed to the stronger desensitization promoted by RTX. Other TRPV1 agonists such as phorbol esters also evoked receptor endocytosis similar to vanilloids (not shown). Receptor endocytosis evoked by prolonged exposure to TRPV1 agonists was not due to unspecific membrane perturbations of these compounds as evidenced by their lack of effect on the endoplasmic reticulum membrane protein calnexin, a marker of endoplasmic reticulum integrity, or by the lacking effect on other plasma membrane proteins such as the Na+-K+-ATPase (supplemental Fig. 1). Taken together, these results indicate that agonist-evoked TRPV1 endocytosis is dose- and time-dependent.
Unexpectedly, prolonged exposure of TRPV1+ cells to either capsaicin or RTX drastically decreased the total amount of receptors in a dose- and time-dependent fashion, roughly correlating with the proportion removed from the membrane (Fig. 2, A, C, and D). This observation implies that recruited receptors are targeted for degradation through the proteasomal or lysosomal pathways.
Capsaicin-induced Lysosome-dependent Down-regulation of TRPV1 Receptor Expression
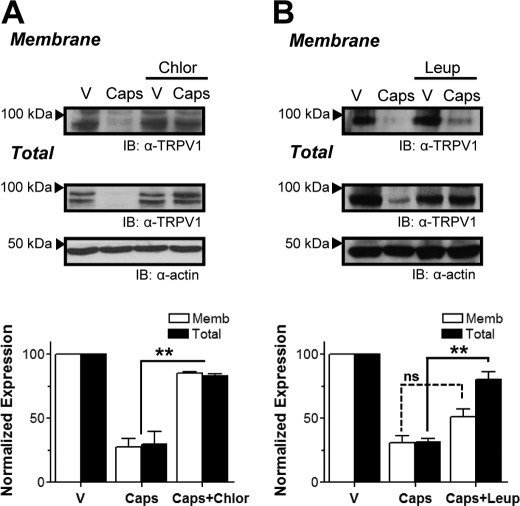
Potential pathways possibly involved in agonist-promoted degradation of TRPV1 protein were tested using specific inhibitors. Because the proteasome inhibitor MG132 did not inhibit TRPV1 degradation during capsaicin exposure (not shown), we investigated whether internalized receptors were targeted to lysosomes by preincubating TRPV1+ cells with chloroquine or NH4Cl, which alkalinizes lysosomes and other acidic compartments, or with the protease inhibitor leupeptin. As shown in Fig. 3B, leupeptin largely (80%) stabilized total receptor protein. Interestingly, preincubation with chloroquine also inhibited TRPV1 internalization (Fig. 3A). Because excitotoxic insults in some neuronal primary cultures lead to autophagy (27) and vanilloid exposure produced a marked decrease in the expressed TRPV1 protein, the potential role of an autophagic response in TRPV1+ cells challenged with capsaicin was examined by monitoring the ratio of Phosphatidylethanolamine-conjugated microtubule-associated protein light chain 3 (LC3-II) to endogenous LC3-I (28). Comparable LC3-II/LC3-I ratios were obtained in vehicle- and capsaicin-treated cells in three independent experiments, suggesting that autophagy was not involved in this process (not shown). Collectively, these results support the view that upon prolonged exposure to agonists, membrane TRPV1 receptors are endocytosed and targeted to lysosomes for degradation.
FIGURE 3.
TRPV1 internalized in response to vanilloids is targeted to lysosomes. A and B, representative blot (IB) from cell surface biotinylation assays showing membrane (Memb) and total TRPV1 expression (upper panel) and total band density quantification (lower panel) of TRPV1+ cells exposed to vehicle (V) or 1 μm capsaicin in the absence or presence of 200 μg/ml chloroquine (Chlor) (A) or 75 μm leupeptin (Leup) (B) to inhibit lysosomal proteases. Data represent mean ± S.E., n = 3. **, p < 0.01. ns, not significant.
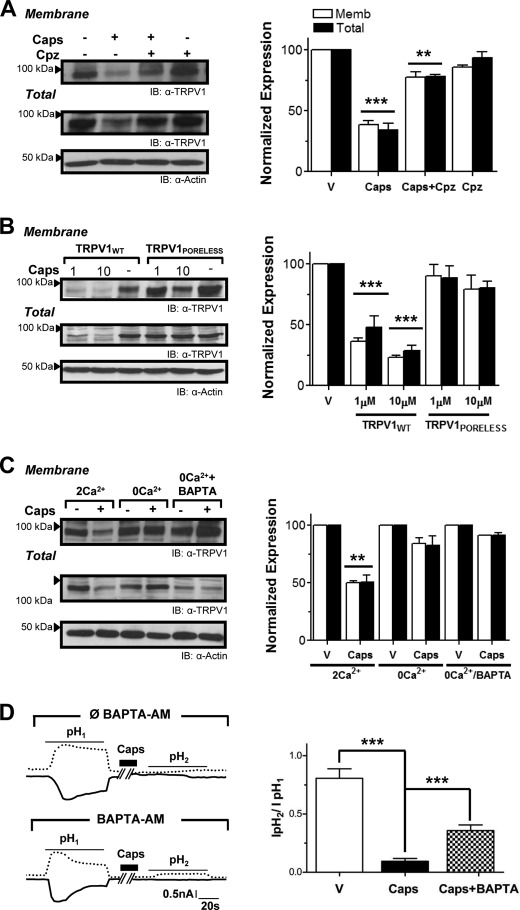
Agonist-evoked Membrane TRPV1 Internalization Follows a Clathrin- and Dynamin-independent Pathway
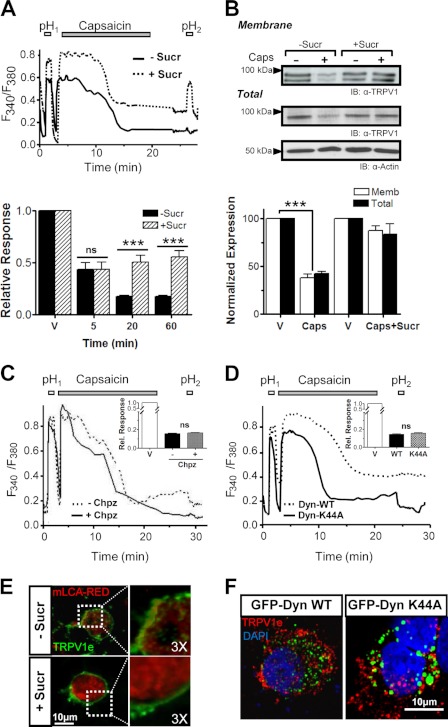
The contribution of TRPV1 internalization and kinetics was functionally evaluated by Ca2+ imaging using a hypertonic solution (+Sucr). Our experimental paradigm was based in the application of two short acid pH pulses that activate TRPV1, interspersed by exposure to 1 μm capsaicin or vehicle for different time spans. The ratio of pH responses (RpH2/pH1) was used as an indicator of the degree of desensitization. Fig. 4A illustrates representative traces obtained upon a 20-min capsaicin incubation in normosmotic and hyperosmotic conditions. Although control incubations caused only minimal desensitization (data not shown), 5 min of exposure to capsaicin reduced the relative pH response by 50% in both osmotic conditions (Fig. 4A, lower panel). Notably, incubations from 20 min onwards produced drastic differences in the RpH2/pH1, virtually abolishing the second pH response (RpH2/pH1 < 0.10) under isotonic conditions (−Sucr), whereas RpH2/pH1 persisted close to 50% when exposed to hyperosmotic medium (+Sucr).
FIGURE 4.
Agonist-induced TRPV1 is internalized through a clathrin- and dynamin-independent pathway. A, upper panel, representative Ca2+ imaging traces in TRPV1+ cells in the absence (black) or presence (dotted line) of a hyperosmotic sucrose solution. Two 1-min pH 5.5 pulses, pH1 and pH2, were applied interspersed by a 20-min incubation period in the absence (vehicle (V)) or presence of 1 μm capsaicin. Although a significant desensitization (expressed as RpH2/pH1) was evident under normosmotic conditions (−Sucr), the constant presence of hyperosmotic solution (+Sucr) significantly reduced capsaicin-induced TRPV1 desensitization. Lower panel, the time course of desensitization, which reaches its maximum after 20 min. ***, p < 0.001. ns, not significant. B, biotinylation of cell surface proteins in the presence of hyperosmotic 250 mm sucrose solution (600 mosm)) reveals a block of capsaicin-induced TRPV1 internalization. n = 3. ***, p < 0.001. IB, representative blot; Memb, membrane. C, measurement of Ca2+ influx in TRPV1-WT, using the same protocol as in A, in the presence of 50 μm Chpz (solid line) as compared with 0.1% DMSO used as Chpz vehicle (dotted line). Rel. Response, relative response. D, Ca2+ influx in TRPV1-WT, using the same protocol as in A, in cells co-transfected with GFP-fused WT dynamin (dotted trace) or the dominant-negative mutant GFP-Dyn-K44A (solid trace). Capsaicin-induced internalization occurs through a dynamin-independent process. E, representative images of TRPV1+ cells expressing red fluorescent fusion protein-mouse clathrin light A (mLCA-RED). TRPV1 was surface-labeled (green) and then allowed to internalize upon incubation with 1 μm capsaicin for 20 min in the presence of hyperosmotic solution (bottom) as compared with normosmotic conditions (top) and then fixed and visualized. Bar, 10 μm. F, immunocytochemistry of extracellularly labeled TRPV1 (red) internalized in the presence of 1 μm capsaicin for 20 min in the presence of GFP-fused WT dynamin (left) or the dominant-negative dynamin mutant GFP-Dyn-K44A (right) shows that capsaicin-induced TRPV1 internalization occurs through a dynamin-independent process.
Blockade of agonist-stimulated TRPV1 internalization under hypertonic conditions was further corroborated by both biotinylation and immunocytochemistry of surface receptors in live cells (Fig. 4, B and E). Note that hypertonic solution also reduced receptor degradation (Fig. 4B). Collectively, these results suggest that upon long exposure to capsaicin, at least ∼30% of the current loss was because of receptor withdrawal from the plasma membrane.
Because hypertonic solution inhibits both clathrin-mediated and clathrin-independent endocytosis (29–31), we investigated next whether capsaicin-induced TRPV1 internalization occurs through a clathrin-dependent pathway. For this purpose, we first evaluated the effect of 50 μm chlorpromazine (Chpz), a reagent that prevents clathrin-mediated endocytosis (32). As seen in Fig. 4C, Chpz did not affect the capsaicin-evoked desensitization ratio estimated from Ca2+ imaging measurements as compared with Chpz vehicle (0.1% DMSO). The implication of a clathrin-independent pathway was further substantiated by evaluating the effect of coexpressing TRPV1 with a GFP-tagged dominant-negative dynamin mutant (GFP-Dyn-K44A), which we compared with GFP-Dyn-WT as control (33). The correct functionality of both dynamin constructs was corroborated in our cell system by evaluating rhodamine-labeled transferrin endocytosis (not shown). As illustrated in Fig. 4D, neither dynamin construct significantly affected capsaicin-induced desensitization, as evident from the desensitization ratio (RpH2/pH1 = 0.16 ± 0.01 and 0.15 ± 0.01 in GFP-Dyn-K44A and GFP-Dyn-WT, n = 180, n = 5). Furthermore, colocalization image analysis of labeled internalized TRPV1 with both dynamin constructs displayed a clear distribution consistent with the independence of a clathrin-mediated pathway (Fig. 4F). Therefore, our results imply that agonist-induced TRPV1 membrane removal follows a clathrin-independent internalization mechanism.
Agonist-stimulated TRPV1 Down-regulation Requires Channel Activation and Ca2+ Influx
Given that agonist-induced TRPV1 internalization/degradation could be accomplished either by vanilloid binding itself or/and by TRPV1 channel activation and subsequent Ca2+ influx, we next explored whether ion flux through the TRPV1 pore was required in this process. First, capsazepine, a specific competitive antagonist with similar affinity as capsaicin (34), was used as a TRPV1 ligand that does not open its pore. As shown in Fig. 5A, incubation with capsazepine alone evoked neither TRPV1 internalization nor its degradation. However, when applied in combination with capsaicin, capsazepine was able to prevent the capsaicin-induced TRPV1 endocytosis. Together, these results indicate that activation of TRPV1 was required to drive its endocytosis and degradation. This notion was further supported by using a nonconducting TRPV1 mutant (TRPV1PORELESS), previously shown to be assembly-competent and to be targeted to the plasma membrane (22). As expected, prolonged exposure of this mutant TRPV1 channel with either 1 μm or 10 μm capsaicin did not affect its surface and total expression (Fig. 5B).
FIGURE 5.
TRPV1 down-regulation requires channel activation and Ca2+ influx. A, the competitive antagonist capsazepine (Cpz; 10 μm) did not elicit TRPV1 endocytosis and competed with capsaicin to induce receptor withdrawal and degradation. Surface-expressed TRPV1 was examined in transfected HEK cells by biotinylation. n = 4. **, p < 0.01, ***, p < 0.001. IB, representative blot; Memb, membrane; V, vehicle. B, dose-dependent effects of capsaicin on TRPV1PORELESS, a nonfunctional mutant capable of reaching the cell surface, show the significant resistance to internalization and degradation, even at higher doses of capsaicin. n = 4. ***, p < 0.001. C, to assess the involvement of both extracellular Ca2+ influx and Ca2+ release from intracellular stores, TRPV1-expressing cells were incubated with capsaicin without extracellular Ca2+ (0Ca2+) and by chelating intracellular Ca2+ with BAPTA (0Ca2+/BAPTA-AM). n = 3. Data are shown as mean ± S.E. **, p < 0.01. D, left, representative recordings of TRPV1 expressed in HEK293 cells using perforated patch clamp configuration after prolonged vanilloid exposure. Two 1-min pulses to pH 6 (shown as pH1 and pH2) were applied interspersed by a 20-min incubation period with 1 μm capsaicin. To assess the involvement of both extracellular Ca2+ influx and Ca2+ release from intracellular stores, cells were preincubated with the high affinity Ca2+ chelator BAPTA-AM 50 μm for 1 h (Lower trace). Right, desensitization rate was calculated from dividing the second acid-evoked current density (IpH2) by the first (IpH1). n = 5 for ∅ BAPTA and n = 9 for +BAPTA. ***, p < 0.001.
Because TRPV1 channels are highly permeable to Ca2+ and the divalent cation plays a pivotal role in acute desensitization and tachyphylaxis, we addressed the role of receptor-mediated Ca2+ influx in TRPV1 down-regulation upon prolonged agonist exposure. As shown in Fig. 5C, capsaicin produced minor TRPV1 internalization (∼15%) in the absence of extracellular Ca2+ (0Ca2+) as compared with >50% endocytosis in 2 mm Ca2+ (2Ca2+). We further evaluated a possible contribution of Ca2+ released from intracellular stores by preincubating cells with BAPTA-AM to chelate cytosolic Ca2+ (0Ca2+/BAPTA). This procedure reduced capsaicin-induced TRPV1 internalization to less than 10% of controls.
The contribution of endocytosis to overall receptor desensitization was also assessed using the perforated patch configuration to allow electrical access while maintaining the cellular trafficking machinery virtually undisturbed. As shown in Fig. 5D, we characterized TRPV1 current responses by applying the same protocol as employed for Ca2+ imaging recordings. As expected, although the desensitization rate with vehicle was ∼20% (not shown), it decreased up to 90% after capsaicin incubation (IpH2/IpH1 = 0.80 ± 0.08, n = 10, versus 0.09 ± 0.02, n = 5, respectively). Interestingly, when internalization was mostly inhibited (+BAPTA), prolonged application of capsaicin reduced the desensitization by up to 60% (IpH2/IpH1 = 0.36 ± 0.05, n = 9) (Fig. 5D). Collectively, these findings demonstrate that capsaicin-induced TRPV1 internalization requires receptor activation and Ca2+ influx, thus contributing to nociceptor desensitization.
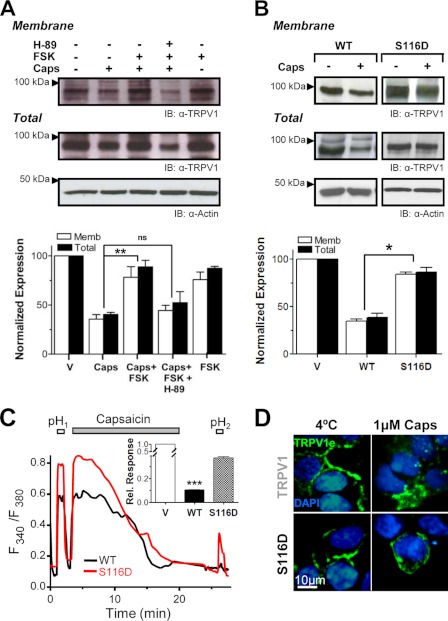
PKA Activity Modulates Agonist-induced TRPV1 Internalization
cAMP-dependent phosphorylation by PKA, as well as inhibition of the phosphatase calcineurin, potentiates TRPV1 activity (8). PKA phosphorylation has been also shown to attenuate TRPV1 Ca2+-dependent desensitization (7, 8, 13). More recently, PKA activation was reported to facilitate membrane insertion of functional TRPV1 multimers (35). We therefore investigated the role of PKA in modulating agonist-induced TRPV1 internalization in DRG neurons using surface biotinylation. A 15-min pretreatment with 20 μm forskolin (FSK) reduced agonist-induced TRPV1 internalization ∼2-fold (Fig. 6A). This effect was abolished by a 1-h preincubation with the PKA inhibitor H89 (10 μm). To determine whether PKA inhibits TRPV1 internalization by directly phosphorylating the receptor, we mutated its known major PKA phosphorylation site at Ser-116 located in its cytoplasmic N-terminal domain. Specifically, we studied TRPV1-S116D, a point mutation that emulates the PKA-phosphorylated receptor and that has been reported to reduce desensitization (7–9). As illustrated in Fig. 6C, when applying our prolonged agonist exposure protocol (1 μm capsaicin for 20 min), TRPV1-S116D-expressing cells displayed ∼60% desensitization (RpH2/pH1 = 0.37 ± 0.01, n = 506, n = 5) as compared with 90% observed with TRPV1-WT (RpH2/pH1 = 0.10 ± 0.01%, n = 409, n = 8). We then asked whether the observed decrease in desensitization, which agrees with results reported by others (8, 9), is because of decreased internalization of the S116D mutant. Using surface biotinylation/avidin pulldown assays, we found that in contrast to wild type channels, surface levels of TRPV1-S116D were not affected by capsaicin incubation, as revealed by the levels of the mutant protein, which remained similar to those of cells exposed to vehicle (Fig. 6B). This result was further substantiated by immunofluorescence labeling that illustrated that vanilloid had no effect on the internalization of surface-expressed TRPV1-S116D receptors (Fig. 6D). Taken together, these results suggest that capsaicin-induced endocytosis of TRPV1 channels is modulated by PKA phosphorylation of the cytosolic N terminus domain of the protein.
FIGURE 6.
PKA activation reduces agonist-induced TRPV1 internalization. A, cell surface biotinylation in nociceptor neurons challenged with 0.1 μm capsaicin for 20 min reveals increases in total and plasma membrane (Memb) levels of TRPV1 when cells were pretreated with the cAMP-increasing agent FSK (20 μm) (lane 3) as compared with non-FSK (lane 2) or cells incubated with FSK plus the PKA inhibitor H89 (10 μm) (lane 4). IB, representative blot. Lower, densitometric quantification of three Western blots. V, vehicle. B, surface biotinylation experiments in TRPV1-WT- or TRPV1-S116D-expressing HEK293 cells exposed to 1 μm capsaicin for 20 min. TRPV1 mutant displayed significant resistance to capsaicin-induced internalization and degradation as compared with WT channels. Data represent the mean ± S.E. with n = 3. *, p < 0.05; **, p < 0.01. ns, not significant. C, measurement of Ca2+ influx by Fura-2 imaging in HEK293 cells expressing TRPV1-WT (black trace) or TRPV1-S116D (red trace) after the two 1-min acidic pH pulses interspersed by a 20-min incubation with 1 μm capsaicin. TRPV1-S116D exhibited significant less desensitization than WT channels. Inset, average desensitization of TRPV1-WT and TRPV1-S116D estimated from RpH2/pH1 values (RpH2/pH1 = 0. 37 ± 0.01 n = 506 in S116D mutant versus RpH2/pH1 = 0.10 ± 0.01 n = 409 in WT, ***, p value < 0.001). D, representative images of surface-expressed TRPV1-WT or TRPV1-S116D exposed for 20 min to capsaicin were labeled with TRPV1e antibody at 4 °C, fixed, and permeabilized. Nuclei were stained with DAPI (blue). Bar = 10 μm.
DISCUSSION
Signaling events involved in TRPV1 tachyphylaxis have been extensively investigated. However, the contribution of TRPV1 mobilization from the plasma membrane to intracellular reservoirs as a mechanism to attenuate nociceptor overactivity has not yet been evaluated in detail. We and others have shown that TRPV1 sensitization is achieved at least in part by rapid recruitment of a subcellular population of vesicular TRPV1 that becomes translocated to the plasma membrane by regulated exocytosis under inflammatory conditions (21, 23, 36). Therefore, the opposite mechanism, i.e. endocytic removal of TRPV1 from the plasma membrane, seems a plausible mechanism to control nociceptor excitability when exposed to noxious stimuli. Interestingly, TRPV1 internalization triggers mustard oil-induced TRPA1 receptor desensitization (37). Moreover, in RTX toxicity, large and sustained Ca2+ influx (38) was accompanied by a ≥70% plasma membrane reduction and vesiculation, whereas capsaicin and heat produced less damaging effects and <10% of membrane capacitance changes (39). The aim of the present study was to determine whether long agonist exposure regulates cell surface expression of TRPV1 and thereby underlies nociceptor desensitization.
The most salient contribution of this study is the demonstration that prolonged incubation with capsaicin produces a drastic reduction of TRPV1 receptors. Although we cannot exclude that these treatments may additionally affect newly synthesized receptors, we found that the down-regulation of TRPV1 affected both its total cellular pool, as well as the plasma membrane population, i.e. the receptors that are directly relevant for signaling. Indeed, a large proportion of internalized TRPV1 was degraded. These effects were observed in both primary DRG neurons and recombinant systems. To gain insights into the mechanisms involved, we used cells transiently expressing TRPV1. They reproduced the robust TRPV1 nociceptor internalization and down-regulation upon long exposure to the agonist. Interestingly, we previously demonstrated in our cell system that in the absence of agonist, less than 5% of plasma membrane TRPV1 was internalized even after 90 min (24). We now found that TRPV1 internalization and down-regulation were mediated not only by natural vanilloids such as capsaicin and RTX, but also by both PKC-stimulating and nonstimulating forms of phorbol 12-myristate 13-acetate (not shown). In the presence of agonist, TRPV1 down-regulation was dose- and time-dependent and required channel activation and Ca2+ entry to the cell. Once internalized, TRPV1 is targeted for lysosomal degradation because treatment with different lysosomal inhibitors inhibits TRPV1 degradation.
We found here that upon prolonged agonist exposure, TRPV1 internalization is mediated by a clathrin-independent pathway. This was indicated by agonist-induced receptor endocytosis in cells expressing the dominant-negative dynamin K44A mutant, which blocks pinching of clathrin-coated pits. This conclusion was further bolstered by other experiments using chlorpromazine, a compound that inhibits the formation of clathrin-coated invaginations. Interestingly, TRPV1 has been shown to interact with the tyrosine kinase epidermal growth factor (EGF) receptor (EGFR), facilitating EGFR ubiquitylation (40), which seems to sequester EGFR in clathrin-independent routes (41). Whether prolonged agonist exposure mediates TRPV1 internalization by clathrin-independent endocytosis pathways like caveolae or involves small GTPases (RhoA, CDC-42, or ARF-6) remains to be clarified. Importantly, dynamin-independent endocytotic routes have been described in different cell types, e.g. in astrocytes, as the major pathway that is tightly regulated by cytosolic Ca2+ concentration and rab5 (42). Moreover, a dynamin- but Ca2+-independent endocytic mechanism was described in DRG neurons. It was promoted by high frequency or extreme physiological stimulation (42).
PKA signaling seems to play a key role in synaptic plasticity for a number of receptors and ion channels and acts not only by direct receptor or channel phosphorylation, but also by modulating channel trafficking through other means (34, 43, 44). TRPV1 is directly sensitized by cAMP-dependent PKA activation. Thus, PKA phosphorylation of amino acid Ser-116 in TRPV1 attenuates desensitization (tachyphylaxis) (7), and TRPV1 is dephosphorylated upon capsaicin activation. Furthermore, PKA facilitates the insertion of functional TRPV1 multimers into the plasma membrane (35). We now found in native nociceptor cultures that PKA activation significantly inhibited the capsaicin-induced reduction of membrane-resident TRPV1. Although this effect might also be accounted for by an increased membrane insertion rate, we found that plasma membrane levels of TRPV1 were not increased in nociceptors incubated only with FSK, suggesting that indeed agonist-induced internalization is inhibited. Our use of the TRPV1-S116D mutant revealed a direct role of receptor phosphorylation and pinpointed the targeted amino acid. Hence, similar to other ionotropic receptors, it seems likely that PKA phosphorylation/dephosphorylation of TRPV1 plays a key role in specifying trafficking to or from the plasma membrane, thus contributing to sensitization/desensitization of nociceptors.
In conclusion, here we have reported that agonist exposure of DRG neurons and TRPV1-expressing cells promotes receptor endocytosis and down-regulation through lysosomal degradation. This process requires Ca2+ entry and appears mediated by an endocytotic mechanism that is independent of clathrin and can be modulated by a PKA-dependent phosphorylation of serine 116. Our findings lend strong support to the notion that the analgesic activity of capsaicin and RTX is at least in part due to reduction of TRPV1 expression in nociceptors and substantiate that modulation of receptor levels at the plasma membrane as a valid therapeutic strategy for pain treatment.
Supplementary Material
Acknowledgments
We thank M. McNiven from Mayo Clinic, Rochester, MN for providing GFP-tagged WT dynamin and mutant K44A constructs and mLCA-RED, and we thank I. Pérez-Otaño from Centro de Investigación Médica Aplicada (CIMA), Navarra, Spain.
This work was supported by Spanish Ministry of Science and Innovation Grants SAF2007-63193 and SAF2010-17045 (to R. P. C.), BFU2009-08346 and PROMETEO/2010/046 (to A. F. M.), and Consolider-Ingenio 2010 CSD2008-00005 (to R. P. C. and A. F. M.).

This article contains supplemental Fig. 1.
- TRPV1
- transient receptor potential vanilloid
- DRG
- dorsal root ganglion
- RTX
- resiniferatoxin
- Dyn
- dynamin
- BAPTA
- 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- BAPTA-AM
- 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid-acetoxymethyl ester
- LC3
- light chain 3
- Sucr
- sucrose
- Chpz
- chlorpromazine
- FSK
- forskolin
- EGFR
- epidermal growth factor receptor
- Caps
- capsaicin
- DMSO
- dimethyl sulfoxide.
REFERENCES
- 1. Cortright D. N., Szallasi A. (2009) TRP channels and pain. Curr. Pharm. Des. 15, 1736–1749 [DOI] [PubMed] [Google Scholar]
- 2. Ji R. R., Woolf C. J. (2001) Neuronal plasticity and signal transduction in nociceptive neurons: implications for the initiation and maintenance of pathological pain. Neurobiol. Dis. 8, 1–10 [DOI] [PubMed] [Google Scholar]
- 3. Ji R. R., Kohno T., Moore K. A., Woolf C. J. (2003) Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 26, 696–705 [DOI] [PubMed] [Google Scholar]
- 4. Planells-Cases R., Garcìa-Sanz N., Morenilla-Palao C., Ferrer-Montiel A. (2005) Functional aspects and mechanisms of TRPV1 involvement in neurogenic inflammation that leads to thermal hyperalgesia. Pflugers Arch. 451, 151–159 [DOI] [PubMed] [Google Scholar]
- 5. Planells-Cases R., Valente P., Ferrer-Montiel A., Qin F., Szallasi A. (2011) Complex regulation of TRPV1 and related thermo-TRPs: implications for therapeutic intervention. Adv. Exp. Med. Biol. 704, 491–515 [DOI] [PubMed] [Google Scholar]
- 6. Koplas P. A., Rosenberg R. L., Oxford G. S. (1997) The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. J. Neurosci. 17, 3525–3537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bhave G., Zhu W., Wang H., Brasier D. J., Oxford G. S., Gereau R. W. (2002) cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron 35, 721–731 [DOI] [PubMed] [Google Scholar]
- 8. Mohapatra D. P., Nau C. (2005) Regulation of Ca2+-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J. Biol. Chem. 280, 13424–13432 [DOI] [PubMed] [Google Scholar]
- 9. Mohapatra D. P., Nau C. (2003) Desensitization of capsaicin-activated currents in the vanilloid receptor TRPV1 is decreased by the cyclic AMP-dependent protein kinase pathway. J. Biol. Chem. 278, 50080–50090 [DOI] [PubMed] [Google Scholar]
- 10. Jung J., Shin J. S., Lee S. Y., Hwang S. W., Koo J., Cho H., Oh U. (2004) Phosphorylation of vanilloid receptor 1 by Ca2+/calmodulin-dependent kinase II regulates its vanilloid binding. J. Biol. Chem. 279, 7048–7054 [DOI] [PubMed] [Google Scholar]
- 11. Numazaki M., Tominaga T., Takeuchi K., Murayama N., Toyooka H., Tominaga M. (2003) Structural determinant of TRPV1 desensitization interacts with calmodulin. Proc. Natl. Acad. Sci. U.S.A. 100, 8002–8006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rosenbaum T., Gordon-Shaag A., Munari M., Gordon S. E. (2004) Ca2+/calmodulin modulates TRPV1 activation by capsaicin. J. Gen. Physiol. 123, 53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lishko P. V., Procko E., Jin X., Phelps C. B., Gaudet R. (2007) The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron 54, 905–918 [DOI] [PubMed] [Google Scholar]
- 14. Chaudhury S., Bal M., Belugin S., Shapiro M. S., Jeske N. A. (2011) AKAP150-mediated TRPV1 sensitization is disrupted by calcium/calmodulin. Mol. Pain 7, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu B., Zhang C., Qin F. (2005) Functional recovery from desensitization of vanilloid receptor TRPV1 requires resynthesis of phosphatidylinositol 4,5-bisphosphate. J. Neurosci. 25, 4835–4843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lukacs V., Thyagarajan B., Varnai P., Balla A., Balla T., Rohacs T. (2007) Dual regulation of TRPV1 by phosphoinositides. J. Neurosci. 27, 7070–7080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stein A. T., Ufret-Vincenty C. A., Hua L., Santana L. F., Gordon S. E. (2006) Phosphoinositide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J. Gen. Physiol. 128, 509–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yao J., Qin F. (2009) Interaction with phosphoinositides confers adaptation onto the TRPV1 pain receptor. PLoS Biol. 7, e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moser E., Kargl J., Whistler J. L., Waldhoer M., Tschische P. (2010) G protein-coupled receptor-associated sorting protein 1 regulates the postendocytic sorting of seven-transmembrane-spanning G protein-coupled receptors. Pharmacology 86, 22–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murphy J. E., Padilla B. E., Hasdemir B., Cottrell G. S., Bunnett N. W. (2009) Endosomes: a legitimate platform for the signaling train. Proc. Natl. Acad. Sci. U.S.A. 106, 17615–17622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morenilla-Palao C., Planells-Cases R., García-Sanz N., Ferrer-Montiel A. (2004) Regulated exocytosis contributes to protein kinase C potentiation of vanilloid receptor activity. J. Biol. Chem. 279, 25665–25672 [DOI] [PubMed] [Google Scholar]
- 22. García-Sanz N., Fernández-Carvajal A., Morenilla-Palao C., Planells-Cases R., Fajardo-Sánchez E., Fernández-Ballester G., Ferrer-Montiel A. (2004) Identification of a tetramerization domain in the C terminus of the vanilloid receptor. J. Neurosci. 24, 5307–5314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Camprubí-Robles M., Planells-Cases R., Ferrer-Montiel A. (2009) Differential contribution of SNARE-dependent exocytosis to inflammatory potentiation of TRPV1 in nociceptors. FASEB J. 23, 3722–3733 [DOI] [PubMed] [Google Scholar]
- 24. Laínez S., Valente P., Ontoria-Oviedo I., Estévez-Herrera J., Camprubí-Robles M., Ferrer-Montiel A., Planells-Cases R. (2010) GABAA receptor associated protein (GABARAP) modulates TRPV1 expression and channel function and desensitization. FASEB J. 24, 1958–1970 [DOI] [PubMed] [Google Scholar]
- 25. Grampp T., Sauter K., Markovic B., Benke D. (2007) γ-Aminobutyric acid type B receptors are constitutively internalized via the clathrin-dependent pathway and targeted to lysosomes for degradation. J. Biol. Chem. 282, 24157–24165 [DOI] [PubMed] [Google Scholar]
- 26. Cortright D. N., Szallasi A. (2004) Biochemical pharmacology of the vanilloid receptor TRPV1. An update. Eur. J. Biochem. 271, 1814–1819 [DOI] [PubMed] [Google Scholar]
- 27. Williams A., Sarkar S., Cuddon P., Ttofi E. K., Saiki S., Siddiqi F. H., Jahreiss L., Fleming A., Pask D., Goldsmith P., O'Kane C. J., Floto R. A., Rubinsztein D. C. (2008) Novel targets for Huntington's disease in an mTOR-independent autophagy pathway. Nat. Chem. Biol. 4, 295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mizushima N., Yoshimori T. (2007) How to interpret LC3 immunoblotting. Autophagy 3, 542–545 [DOI] [PubMed] [Google Scholar]
- 29. Roseberry A. G., Hosey M. M. (2001) Internalization of the M2 muscarinic acetylcholine receptor proceeds through an atypical pathway in HEK293 cells that is independent of clathrin and caveolae. J. Cell Sci. 114, 739–746 [DOI] [PubMed] [Google Scholar]
- 30. Cinar H., Barnes E. M., Jr. (2001) Clathrin-independent endocytosis of GABAA receptors in HEK 293 cells. Biochemistry 40, 14030–14036 [DOI] [PubMed] [Google Scholar]
- 31. Fourgeaud L., Bessis A. S., Rossignol F., Pin J. P., Olivo-Marin J. C., Hémar A. (2003) The metabotropic glutamate receptor mGluR5 is endocytosed by a clathrin-independent pathway. J. Biol. Chem. 278, 12222–12230 [DOI] [PubMed] [Google Scholar]
- 32. Wang L. H., Rothberg K. G., Anderson R. G. (1993) Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 123, 1107–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ricks T. K., Trejo J. (2009) Phosphorylation of protease-activated receptor-2 differentially regulates desensitization and internalization. J. Biol. Chem. 284, 34444–34457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bevan S., Hothi S., Hughes G., James I. F., Rang H. P., Shah K., Walpole C. S., Yeats J. C. (1992) Capsazepine: a competitive antagonist of the sensory neuron excitant capsaicin. Br. J. Pharmacol. 107, 544–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vetter I., Wyse B. D., Monteith G. R., Roberts-Thomson S. J., Cabot P. J. (2006) The μ opioid agonist morphine modulates potentiation of capsaicin-evoked TRPV1 responses through a cyclic AMP-dependent protein kinase A pathway. Mol. Pain 2, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang X., Huang J., McNaughton P. A. (2005) NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 24, 4211–4223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Akopian A. N., Ruparel N. B., Jeske N. A., Hargreaves K. M. (2007) Transient receptor potential TRPA1 channel desensitization in sensory neurons is agonist-dependent and regulated by TRPV1-directed internalization. J. Physiol. 583, 175–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tóth A., Wang Y., Kedei N., Tran R., Pearce L. V., Kang S. U., Jin M. K., Choi H. K., Lee J., Blumberg P. M. (2005) Different vanilloid agonists cause different patterns of calcium response in CHO cells heterologously expressing rat TRPV1. Life Sci. 76, 2921–2932 [DOI] [PubMed] [Google Scholar]
- 39. Caudle R. M., Karai L., Mena N., Cooper B. Y., Mannes A. J., Perez F. M., Iadarola M. J., Olah Z. (2003) Resiniferatoxin-induced loss of plasma membrane in vanilloid receptor expressing cells. Neurotoxicology 24, 895–908 [DOI] [PubMed] [Google Scholar]
- 40. Bode A. M., Cho Y. Y., Zheng D., Zhu F., Ericson M. E., Ma W. Y., Yao K., Dong Z. (2009) Transient receptor potential type vanilloid 1 suppresses skin carcinogenesis. Cancer Res. 69, 905–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sigismund S., Woelk T., Puri C., Maspero E., Tacchetti C., Transidico P., Di Fiore P. P., Polo S. (2005) Clathrin-independent endocytosis of ubiquitinated cargos. Proc. Natl. Acad. Sci. U.S.A. 102, 2760–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jiang M., Chen G. (2009) Ca2+ regulation of dynamin-independent endocytosis in cortical astrocytes. J. Neurosci. 29, 8063–8074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hammond R. S., Lin L., Sidorov M. S., Wikenheiser A. M., Hoffman D. A. (2008) Protein kinase a mediates activity-dependent Kv4.2 channel trafficking. J. Neurosci. 28, 7513–7519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Man H. Y., Sekine-Aizawa Y., Huganir R. L. (2007) Regulation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proc. Natl. Acad Sci. U.S.A. 104, 3579–3584 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.