Background: Although Gβγ is known to activate GIRK, Gαi/o also modulates GIRK gating.
Results: The α2/α3 helices of Gαi3 in the GTP-bound state directly bind to the αA helix of GIRK.
Conclusion: The complex model explains how Gαi/o sequesters Gβγ efficiently from GIRK upon GTP hydrolysis.
Significance: The structural basis for the rapid closure of GIRK by Gαi/o is provided.
Keywords: Heterotrimeric G Proteins, NMR, Potassium Channels, Protein-Protein Interactions, Signal Transduction, G Protein α Subunit, G Protein Signaling, GIRK
Abstract
G protein-gated inwardly rectifying potassium channel (GIRK) plays a crucial role in regulating heart rate and neuronal excitability. The gating of GIRK is regulated by the association and dissociation of G protein βγ subunits (Gβγ), which are released from pertussis toxin-sensitive G protein α subunit (Gαi/o) upon GPCR activation in vivo. Several lines of evidence indicate that Gαi/o also interacts directly with GIRK, playing functional roles in the signaling efficiency and the modulation of the channel activity. However, the underlying mechanism for GIRK regulation by Gαi/o remains to be elucidated. Here, we performed NMR analyses of the interaction between the cytoplasmic region of GIRK1 and Gαi3 in the GTP-bound state. The NMR spectral changes of Gα upon the addition of GIRK as well as the transferred cross-saturation (TCS) results indicated their direct binding mode, where the Kd value was estimated as ∼1 mm. The TCS experiments identified the direct binding sites on Gα and GIRK as the α2/α3 helices on the GTPase domain of Gα and the αA helix of GIRK. In addition, the TCS and paramagnetic relaxation enhancement results suggested that the helical domain of Gα transiently interacts with the αA helix of GIRK. Based on these results, we built a docking model of Gα and GIRK, suggesting the molecular basis for efficient GIRK deactivation by Gαi/o.
Introduction
G protein-gated inwardly rectifying potassium ion channel (GIRK)3 (1) is a member of the inwardly rectifying potassium ion channel (Kir) family, regulating heart rate, neuronal excitability, and other physiological events (2, 3). GIRK functions as a tetramer in which a long, ∼88 Å K+ permeation pathway is formed by the transmembrane (TM) and cytoplasmic pore (CP) regions (4, 5). The crystal structure of GIRK revealed two K+-ion gates (5): the helix bundle crossing on the cytoplasmic side of the TM region and the loops on the membrane side of the CP region, referred to as G-loops. Although the gating of Kir family proteins is generally regulated by phosphatidylinositol 4,5-bisphosphate, the opening of the GIRK gate is also triggered by the direct binding of the G protein βγ subunits (Gβγ). The stimulation of G protein-coupled receptors (GPCRs) causes the exchange of GDP with GTP on the α subunit (Gα), which decreases the affinity of Gα for Gβγ. Gβγ then dissociates from Gα in the GTP bound state (Gα(GTP)) and associates with GIRK, resulting in the opening of the GIRK gate (2, 3, 6–11). Recently, our NMR analyses revealed that Gβγ binds to the border region of the two neighboring subunits of the GIRK tetramer. This Gβγ binding causes the reorientation of the tetramer subunits and the conformational change of the gate in the CP region (G-loops), which probably leads to the opening of the TM gate (12). When the GPCR stimulation ends, the GTP on Gα is hydrolyzed to GDP, and then Gα in the GDP-bound state (Gα(GDP)) removes Gβγ from GIRK, resulting in the closure of the GIRK gate.
Although Gβγ directly binds to and activates GIRK, Gα also plays important roles in regulating GIRK. In native tissues, GIRK is activated only through the stimulation of GPCRs that couple to the G proteins belonging to the i/o-family (Gi/o) (13). The activation and the deactivation of GIRK are also accelerated when the channels are co-expressed with Gαi/o (14–16).
Efficient GIRK activation seems to be attributed to the preassociation of the heterotrimeric G protein in the i/o-family (Gαi/o(GDP)βγ) with GIRK, in which Gαi/o is directly associated with both GPCR and GIRK before GPCR activation (17, 18). Furthermore, the direct association of Gαi/o(GDP)βγ with GIRK in the absence of GPCR-stimulation reportedly suppresses the basal activity of GIRK while maintaining the maximal evoked current by Gβγ in the presence of GPCR stimulation (14, 15, 19–22). This regulation mechanism is called “priming,” by which Gαi/o reportedly plays an important role by facilitating a better response to GPCR activation.
On the other hand, the deactivation is accelerated by the direct interaction of Gαi/o(GTP) with GIRK (18). The interaction enables Gαi/o to rapidly bind to Gβγ upon GTP hydrolysis, which is presumably assisted by the regulator of G protein signaling (RGS) (23), resulting in the closure of the GIRK gate. Because the re-association of Gαi/o(GDP) and Gβγ allows Gαi/o(GDP)βγ to enter the next cycle of GIRK activation, the rapid reassociation would also accelerate the activation of GIRK (15). Thus, the direct interaction between GIRK and Gαi/o(GTP) could contribute to the efficient activation and deactivation of the GIRK channel. In a FRET study investigating the conformational changes of the GIRK1/2 channel in Xenopus oocytes, the coexpression of Gαi/o(GTP) with Gβγ conferred different FRET patterns, and the maximal GIRK current, as compared with those observed when Gβγ and Gαi/o, were separately expressed (19).
Altogether, it is now evident that Gαi/o not only functions as the donor and acceptor of Gβγ but also modulates the gating property of GIRK. To reveal the underlying molecular mechanism by which the Gαi/o modulates GIRK, several studies have proved the interactions between Gαi/o and the cytoplasmic region of GIRK. Gαi/o binding to the N- and C-terminal fragments of GIRK was examined by pulldown assays in vitro (14, 15, 19, 20, 22, 24, 25), indicating that the C-terminal region of GIRK is essential for the i/o-family-specific binding to GIRK. On the other hand, little is known about the GIRK binding site on Gαi/o. Gα consists of a GTPase domain and a helical domain (26, 27) in which GDP or GTP is sandwiched by both domains and GPCRs bind to the GTPase domain (28). An electrophysiological study using a Gαi1/Gαq chimera, in which the helical domain was replaced, revealed that the helical domain of Gα is responsible for the specific activation of the GIRK channel (25). Thus, it remains unclear how Gαi/o modulates the GIRK gating property, and therefore, the elucidation of the molecular recognition mode of Gαi/o and GIRK is required.
In this study we performed the NMR analyses of the interaction between the cytoplasmic region of GIRK1 and Gαi3(GTP), which accelerates the deactivation of GIRK. The NMR spectral changes of Gα upon the addition of GIRK as well as the transferred cross-saturation (TCS) results indicated that Gα and the cytoplasmic region of GIRK1 directly bind to each other, with an estimated Kd value of ∼1 mm. We identified the binding sites on Gα and GIRK, respectively, and examined the binding mode by paramagnetic relaxation enhancement (PRE) experiments. Based on these results, we built a docking model of Gα and the cytoplasmic region of GIRK, suggesting the molecular basis for the efficient deactivation of the channel by Gαi/o.
EXPERIMENTAL PROCEDURES
Expression and Purification of Cytoplasmic Regions of Mouse GIRK1
The N- and C-terminal cytoplasmic regions of mouse GIRK1 (residues 41–63 and 190–386) were fused into a single polypeptide, which is hereafter referred to as GIRKCP-L. The C-terminal region of GIRKCP-L is 15 residues longer than that of GIRKCP (residues 41–63 and 190–371). We previously analyzed the interaction of GIRKCP with Gβγ and confirmed its validity by the crystal structure (29). Preliminary TCS experiments using GIRKCP and Gαi3 indicated that the C-terminal region of GIRKCP was involved in the interaction with Gαi3, suggesting that some interacting residues are missing in GIRKCP. Therefore, we extended the GIRKCP construct for 15 amino acids to obtain GIRKCP-L, which was used for the further analyses.
GIRKCP-L was expressed in Escherichia coli cells. The uniformly 2H,15N-labeled GIRKCP-L samples for the NMR analyses were prepared by growing E. coli in M9 minimal medium containing 15NH4Cl, [2H]glucose, and [2H/15N]Celtone® base powder in 99% 2H2O. GIRKCP-L was purified according to the same procedure as for GIRKCP (30). GIRKCP-L mainly eluted as a tetramer from the size exclusion chromatography column. The uniformly 2H,15N-labeled and uniformly 2H-labeled GIRKCP-L samples for the TCS experiments were prepared without the denaturing and refolding procedure to preserve the amide hydrogen atoms as 2H in the core of the protein. The GIRKCP-L samples were incubated at 303 K for >48 h before the TCS experiments to prevent 2H/1H exchange during the TCS measurements.
Assignments of NMR Signals of GIRKCP-L
The 1H,15N transverse relaxation-optimized spectroscopy (TROSY) signals of GIRKCP-L were well dispersed and mostly overlapped with those of GIRKCP. The backbone assignments of residues 41–63 and 190–366 of GIRKCP-L were transferred from those of GIRKCP (BioMagResBank accession number 11067) (30). The assignments of residues 367–377 were established by triple resonance experiments (HNCACB and HNCA) (31, 32) using uniformly 2H,13C,15N-labeled GIRKCP-L as shown in supplemental Table 1. The backbone amide resonances for Asn-378–Val-386 could not be assigned because their signals were very weak or not observed, probably due to either the fast exchange of their amide hydrogen atoms with those of water molecules or the line-broadening due to conformational exchange.
Expression and Purification of Gαi3
The Gαi3 protein, with an N-terminal decahistidine tag followed by an HRV3C protease cleavage site, was expressed in E. coli cells. The uniformly 2H,15N-labeled Gαi3 samples for NMR analyses were prepared by growing E. coli in M9 minimal medium containing 15NH4Cl, [2H]glucose, and [2H/15N]Celtone® base powder in 99% 2H2O. Gαi3 was purified as described (12, 33). Briefly, Gαi3 was purified to homogeneity by chromatography on a HIS-Select® Nickel Affinity Gel (Sigma) column, His-tag cleavage by PreScissionTM Protease (GE Healthcare), and removal of the cleaved His tags and the PreScissionTM Protease (GE Healthcare) by HIS-Select® Nickel Affinity Gel (Sigma).
Spin Labeling of Gαi3
For PRE experiments, we first prepared the Gαi3 mutant referred to as Hexa III, in which all six exposed Cys residues were substituted (Gαi3 (C3S-C66A-C214S-C305S-C325A-C351I)), according to the previous report (34). Using this construct as a template, cysteine substitutions were separately introduced to Ile-82 and Ser-153 by the QuikChange® system (Stratagene). All mutations were confirmed by DNA sequencing.
Spin-labeling was performed in a buffer containing 10 mm Hepes-NaOH (pH 7.0), 50 mm KCl, 10 mm MgCl2, and 0.60 mm GTPγS. The Gαi3 mutants were incubated with S-(1-oxy-2,2,5,5-tetramethylpyrroline-3-methyl)-methanethiosulfonate (MTSL) at a molar ratio of 1:3 for each protein:MTSL at room temperature for 4 h. Under these conditions we confirmed that only the most reactive cysteine residue was modified, whereas the remaining buried native cysteine residues were not modified. The excess MTSL was removed by extensive washes with the buffer by ultrafiltration using an Amicon® Ultra filter unit (Millipore). For the diamagnetic state experiments, ascorbate was added to the MTSL-labeled Gαi3 mutants at a molar ratio of 1:3 protein:ascorbate, and the solution was incubated at 4 °C for 12 h. The ascorbate was then removed by ultrafiltration. Thus, we prepared the MTSL-labeled Hexa III in the diamagnetic state.
NMR Analyses
All NMR experiments were performed on a Bruker Avance 600 spectrometer equipped with a cryogenic probe. The 1H,15N TROSY spectra of Gαi3 in the presence of various amounts of GIRKCP-L were observed at 308K. TCS and PRE experiments were performed at 303 K. All spectra were processed by the Bruker TopSpin 2.1 software, and the data were analyzed by Sparky (T. D. Goddard and D. G. Kneller, Sparky 3, University of California, San Francisco, CA). The error bars are based on the signal-to-noise ratio calculated by the Sparky software. The backbone NMR signal assignments of Gαi3 were reported previously (35).
To examine the spectral changes of Gαi3 induced by the presence of GIRKCP-L, we prepared five samples, each containing the uniformly 2H,15N-labeled Gαi3 (0.28 mm) mixed with GIRKCP-L at molar ratios (Gαi3:GIRKCP-L tetramer) of 1:0, 1:0.9, 1:1.8, 1:2.7, and 1:3.6 in the NMR sample buffer (10 mm HEPES-NaOH (pH 7.0), 50 mm KCl, 10 mm MgCl2, 0.6 mm GTPγS, 5 mm DTT), 1 mm sodium 2,2-dimethyl-2-silapentane-5-sulfonate (DSS), 10% 2H2O, 90% 1H2O). We then observed the 1H,15N TROSY spectrum of each sample. The residues with overlapping resonances were omitted from the analyses.
The TCS experiments were performed as described with minor modifications (12, 36). In the TCS experiments observing Gαi3, a solution containing uniformly 2H,15N-labeled Gαi3 (0.3 mm) and unlabeled GIRKCP-L (0.25 mm as a tetramer) was prepared in buffer (10 mm HEPES-NaOH (pH 6.5), 50 mm KCl, 5 mm DTT, 1 mm DSS, 0.8 mm GTPγS, 20% 1H2O, 80% 2H2O). The saturation frequency was set at 0.83 ppm, and the maximum radiofrequency amplitude was 0.17 kHz for WURST-2 (adiabatic factor Q0 = 1). The saturation duration and the relaxation delay were set at 1.5 and 2.5 s, respectively. To evaluate the effect of the residual aliphatic protons within Gαi3, TCS experiments were also performed under the same conditions as those mentioned above, with the sample containing uniformly 2H,15N-labeled Gαi3 (0.3 mm) and uniformly 2H-labeled GIRKCP-L (0.25 mm as a tetramer). It should be noted that the residues with signal overlapping and/or signal-to-noise ratios less than 10 were excluded from the analyses.
In the TCS experiments observing GIRKCP-L, a solution containing uniformly 2H,15N-labeled GIRKCP-L (0.25 mm as a tetramer) and unlabeled Gαi3 (0.4 mm) was prepared in buffer (10 mm HEPES-NaOH (pH 6.5), 50 mm KCl, 5 mm DTT, 1 mm DSS, 0.8 mm GTPγS, 20% 1H2O, 80% 2H2O). The saturation frequency was set at 0.83 ppm, and the maximum radiofrequency amplitude was 0.17 kHz for WURST-2 (adiabatic factor Q0 = 1). The saturation duration and the relaxation delay were set at 3.0 and 2.0 s, respectively. To evaluate the effect of the residual aliphatic protons within Gαi3, TCS experiments were also performed under the same conditions as those mentioned above, with the sample containing uniformly 2H,15N-labeled GIRKCP-L (0.25 mm as a tetramer) and uniformly 2H-labeled Gαi3 (0.4 mm). It should be noted that the residues with signal overlapping and/or signal-to-noise ratios less than 10 were excluded from the analyses.
In the PRE experiments of the paramagnetic state, samples containing uniformly 2H,15N-labeled GIRKCP-L (0.075 mm as a tetramer) mixed with 0.2 mm oxidized Hexa III-Cys-MTSL(ox) were prepared. In the experiments of diamagnetic state, samples containing uniformly 2H,15N-labeled GIRKCP-L (0.075 mm as a tetramer) mixed with reduced Hexa III-Cys-MTSL(red) in buffer (10 mm HEPES-NaOH (pH 7.0), 50 mm KCl, 1 mm DSS, 10% 2H2O, 90% 1H2O) were prepared. The 1H,15N TROSY spectrum of each sample was recorded. The residues with overlapping resonances were omitted from the analyses. PRE was calculated as paramagnetic to diamagnetic signal intensity ratios (Ipara/Idia) (37).
Construction of Complex Models
The complex models of Gα-GIRK and Gα-GIRK-Gβγ were obtained with the HADDOCK software (38).
First, we built a homology model of Gαi3(GTP) by the MODELLER software (39) using the crystal structure of Gαi1(GTPγS) (PDB code 1GIA) as a template, whose amino acid sequence is 94% identical to that of Gαi3. The crystal structure of GIRKCP (PDB code 1N9P) and the structure of Gβγ in the crystal structure of Gαi1(GDP)β1γ2 (PDB code 1GP2) were used, respectively, for dockings. The active residues used in the definition of the ambiguous interaction restraints for docking are listed in supplemental Table 2.
The Gα-GIRK-Gβγ ternary complex model was built as follows. First, we built a docking model of the Gβγ-GIRK complex with parameters listed in supplemental Table 2. Then, the Gα-GIRK and Gβγ-GIRK complex models were superimposed by the GIRK structure.
RESULTS
NMR Spectral Changes of Gαi3 upon Binding to GIRKCP-L
To investigate the direct binding between Gαi3 and GIRKCP-L, we observed a series of 1H,15N TROSY spectra of 0.28 mm uniformly 2H,15N-labeled Gαi3 in the absence or presence of 0.25, 0.50, 0.75, and 1.0 mm GIRKCP-L as a tetramer. As the concentration of GIRKCP-L increased, most signals exhibited decreased intensity due to line-broadening without changing their chemical shifts, whereas a number of signals exhibited further intensity reductions and eventually disappeared in the presence of 0.75 mm GIRKCP-L. In addition, several signals exhibited small but significant chemical shift changes (Fig. 1, A and B). Although the overall intensity reductions are caused by the slowing of the overall tumbling motion upon binding to GIRKCP-L, the further intensity reductions and apparent chemical shift changes reflect the direct binding of GIRKCP-L to Gαi3. The weighted averages of the chemical shift differences (Δδ) were calculated using the equation (Δδ = [(ΔδHN)2 + (ΔδN/6.5)2]1/2). The titrations curves of Δδ were fit to the following theoretical formula to obtain the value of the dissociation constant (Kd).
 |
where Δδsat is the Δδ value when a saturating amount of GIRKCP-L is added (ppm), and [Gαi3]tot = 0.28 mm. The fitting of the titration curves of the chemical shift changes for the signals from Lys-209 and Trp-258 resulted in dissociation constant (Kd) values of 0.6 and 1.1 mm, respectively (Fig. 1C).
FIGURE 1.
NMR spectral change of Gαi3 upon the addition of GIRKCP-L. A, shown is an overlay of the 1H,15N TROSY spectra of the uniformly 2H,15N-labeled Gαi3 (0.28 mm) in the absence (black) and presence (red) of 2.7 eq of GIRKCP-L (0.75 mm as a tetramer). The signals with chemical shift differences larger than 0.008 ppm are labeled. The signal from Gly-45, which is one of the residues showing significant intensity reduction without a significant chemical shift change, is also labeled in parentheses. B, overlays of the spectra in the presence of 0–2.7 eq of GIRKCP-L (as a tetramer) are displayed for Gly-45 (left) and Trp-258 (right) as typical examples of the signals with significant intensity reductions and chemical shift changes, respectively. C, titration curves of the chemical shift differences for Lys-209 (left) and Trp-258 (right) are shown. D, shown are plots of the chemical shift differences of Gαi3 in the absence and presence of 2.7 eq of GIRKCP-L (as a tetramer). The error bars were calculated based on the digital resolutions of the spectra. The minimum values of Δδ within the error ranges (Δδmin) were utilized for the evaluation. Bars corresponding to the residues with significant chemical shift differences, which have the minimum values of Δδ within the error ranges (Δδmin) larger than 0.008 ppm, are colored red. Asterisks in D and E indicate the residues with no data due to signal overlapping, lack of assignments, or insufficient signal-to-noise ratio. The secondary structure elements of Gαi3 are depicted in gray below the sequence, and α2, α3, α4, and β6 are colored green. E, shown are plots of the normalized intensity ratios (R) of uniformly 2H,15N-labeled Gαi3 upon the addition of 1.8 eq of the GIRKCP-L tetramer to Gαi3. The intensities of the free Gαi3 were divided by a scaling factor of 1.78 (see supplemental Methods) and then were used for the calculation of the intensity ratios. The error bars were calculated based on the signal-to-noise ratios. Bars corresponding to the signals with the maximum value of R, within the error ranges (Rmax) lower than 0.42 are colored cyan. F, mapping of the affected residues on the Gαi1 structure in which 333 of 354 residues (94%) are identical to those of Gαi3 (PDB code 1GIA) is shown. The amide nitrogen atoms of the residues with apparent chemical shift differences and intensity reductions are shown as balls colored red and cyan, respectively. Proline residues and residues with no data are colored black. The α2, α3, α4, and β6 are colored green, and GTPγS is depicted by teal sticks.
We evaluated the apparent chemical shift differences (Δδ) of Gαi3 in the absence of GIRKCP-L and the presence of 0.75 mm GIRKCP-L, in which 37–50% of Gαi3 was in the GIRKCP-L-bound state, as estimated by the Kd value of 0.6–1.1 mm (Fig. 1D). The residues with significant chemical shift changes are Glu-207, Lys-209, Trp-211, His-213, Phe-215, Glu-216, Ser-246, Trp-258, Arg-312, and Thr-316, for which the minimum values of Δδ within the error ranges (Δδmin = Δδ − error) are larger than 0.008 ppm. It should be noted that the threshold, 0.008 ppm, corresponds to 0.016–0.022 ppm between the free and GIRKCP-L-bound states.
We also evaluated the signal intensity reductions, as the accelerated intensity reductions are caused by the differential line broadening, i.e. the chemical shift changes in the slow to intermediate exchange regime, upon binding to GIRKCP-L (Fig. 1B) (40). Fig. 1E shows the intensity ratios (R) in the presence and absence of GIRKCP-L, which were corrected by multiplying by the scaling factor of 1.78, for the increase in the molecular weight upon binding (see supplemental Methods for details). The scaling factor of 1.78 is obviously too large for the N- and C-terminal residues (residues 1–31 and 349–354), because these residues tumble faster than those in the other regions of Gαi3, as evidenced by the small line widths and the small values of the chemical shift indices (35, 41), resulting in the R values larger than 1.0. Except for these signals, most signals showed the R values ranging from 0.40 to 0.80, suggesting that the tumbling motion is slowed in the presence of GIRKCP-L, presumably due to the increase in sample viscosity. The residues with significantly larger signal intensity reductions, which exhibited the maximum R value within the error range (Rmax = R + error) lower than 0.42, are Ala-41, Ser-44, Gly-45, Lys-46, Ser-47, Thr-177, Gly-203, and Ile-253.
Fig. 1F shows the mapping of these affected residues on the crystal structure of Gα (PDB code 1GIA), where the residues with significantly large Δδmin and small Rmax are colored red and blue, respectively. Most of the residues with chemical shift changes are located in two regions; that is, the region from the α2 helix and the following loop (Glu-207, Lys-209, Trp-211, His-213, Phe-215, and Glu-216) and the region from the α3 helix and the following loop (Ser-246 and Trp-258). The other residues, Arg-312 and Thr-316, are located on the loop between the α4 helix and the β6 strand. On the other hand, the residues with significant intensity reductions are located around the GTP binding site, in which Ser-44, Gly-45, Lys-46, Ser-47, and Gly-203 directly interact with the phosphate group of GTPγS.
As shown in Fig. 1F, the affected residues exhibited significant chemical shift changes, indicating that they are involved in the direct GIRKCP-L binding site and/or in the site(s) exhibiting a conformational change upon binding.
GIRKCP-L Binding Site on Gαi3 Revealed by Transferred Cross-saturation Experiments
To identify the GIRKCP-L binding site on Gαi3, TCS experiments were performed. The saturation of the GIRKCP-L resonances by the irradiation with radio frequency pulses caused the signal intensity reductions of the 1H,15N TROSY signals of the Gαi3 residues, which should be located in the GIRKCP-L binding site (Fig. 2A) (42, 43).
FIGURE 2.
TCS from GIRKCP-L to Gαi3. A, shown is a selected portion of the 1H,15N TROSY spectra of the uniformly 2H,15N-labeled Gαi3 (0.30 mm) in the presence of GIRKCP-L (0.25 mm as a tetramer), which was recorded without (left) and with (right) radio frequency irradiation. Cross-sections are also shown for the signals from Ala-99 and Trp-258. B, procedures were the same as A, except that 2H-labeled GIRKCP-L was used instead of unlabeled (1H-labeled) GIRKCP-L, as a negative control. C, shown is a plot of the difference in the reduction ratios (ΔRR) originating from the backbone amide groups with and without irradiation in the presence of unlabeled GIRKCP-L and 2H-labeled GIRKCP-L (see also supplemental Fig. 1). The residues indicated by asterisks are those with no data mostly due to overlapping of the resonances or insufficient signal-to-noise ratio. The error bars were calculated based on the signal-to-noise ratios. Bars corresponding to the residues with significant intensity reductions (minimum values of ΔRR (ΔRRmin) > 0.08) are colored red. The secondary structure elements of Gαi3 are depicted in gray below the sequence, and αA, αB, α2, and α3 are colored green. D, mapping of the affected residues in the TCS experiment on the Gα structure (PDB code 1GIA) is shown. The backbone nitrogen atoms of the affected residues are shown as red balls in the ribbon diagram of the Gα structure (left). The affected residues are colored red on the surface representations of the Gα structure, whereas the residues with no data, including proline residues, are colored black (center and right). The αA, αB, α2, and α3 are colored green.
In the case of a larger protein system, such as Gαi3 and GIRKCP-L, with molecular masses of 41 and 103 kDa, respectively, the enhanced 1H homonuclear dipolar-dipolar interactions might cause intramolecular saturation transfer from the residual protons in Gαi3 (the exchangeable hydrogen atoms in the NH, OH, and SH groups and/or the hydrogen atoms, due to the incomplete 2H-labeling of 2H,15N-labeled Gαi3). To exclude these effects, we also performed a control experiment by using 2H-labeled GIRKCP-L instead of the unlabeled (i.e. 1H-labeled) protein to reflect only the artificial effects described above (Fig. 2B, supplemental Fig. 1, gray) and subtracted the intensity reduction ratios of this control experiment from those obtained by using unlabeled GIRKCP-L (supplemental Fig. 1, orange). The differences in the reduction ratios, ΔRR, are shown in Fig. 2C, with the error bars calculated based on the signal-to-noise ratios. The minimum values of ΔRR within the error ranges (ΔRRmin = ΔRR − error) were utilized for the evaluation.
The residues with large intensity reductions (ΔRRmin > 0.08, Fig. 2C) are located on the helical domain (Gly-89 in the αA helix; Gly-112 and Ala-114 in the αB helix) and the GTPase domain (Arg-208, Lys-209, Trp-211, Ile-212, His-213, Glu-216, and Gly-217 in the α2 helix and the following loop; Met-240, Lys-248, Leu-249, Ile-253, Asn-256, and Trp-258 in the α3 helix and the flanking loops; Glu-186 in the β2 strand). The mapping of these residues on the structure of Gα revealed that the residues identified on the GTPase domain of Gαi3 are clustered, indicating that this site (hereafter, referred to as the “α2/α3 site”) mainly contributes to the GIRKCP-L binding (Fig. 2D). This is also supported by the two alanine mutants of the identified residues (I212A and W258A) that exhibited impaired binding affinity for GIRKCP-L (supplemental Fig. 2). It should be noted that Glu-186 seems separated from the cluster by the intervening residue, Phe-199 (supplemental Fig. 3). The amide group of Phe-199 is buried in the protein and is more than 6 Å away from the contiguous surface for GIRKCP-L binding, resulting in the lack of cross-saturation for Phe-199. Therefore, we conclude that the α2/α3 site is the major GIRKCP-L binding site.
The residues identified on the helical domain (Gly-89, Gly-112, and Ala-114), which are distant from the α2/α3 site, do not form a contiguous surface but are located on the same side of the protein surface as the α2/α3 site, suggesting that these residues transiently contact GIRKCP-L. We further investigated the interactions of the helical domain with GIRKCP-L (see below).
Gαi3 Binding Site on GIRKCP-L Revealed by TCS Experiments
Conversely, to identify the Gαi3 binding site on GIRKCP-L, TCS experiments observing GIRKCP-L were performed. In the same manner as the TCS experiments to identify the binding site on Gαi3, we evaluated the cross-saturation from unlabeled Gαi3 (supplemental Fig. 4, orange) by subtracting the intensity reduction ratios of the control experiment in the presence of 2H-labeled Gαi3 (supplemental Fig. 4, gray). The differences in the reduction ratios, ΔRR, are shown in Fig. 3A, with the error bars calculated based on the signal-to-noise ratios. The minimum values of ΔRR within the error ranges (ΔRRmin) were utilized for the evaluation.
FIGURE 3.
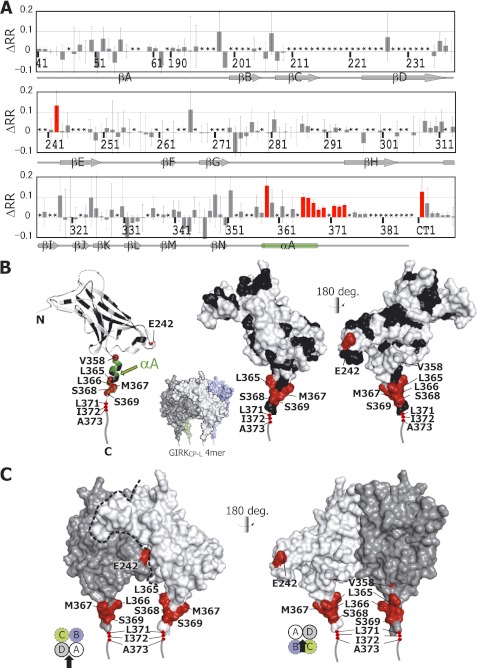
TCS from Gαi3 to GIRKCP-L. A, shown is a plot of the difference in the reduction ratios (ΔRR) originating from the backbone amide groups with and without radio frequency irradiation in the presence of unlabeled Gαi3 and 2H-labeled Gαi3 (see also supplemental Fig. 3). The residues indicated by asterisks are those with no data, mostly due to overlapping resonances or insufficient signal-to-noise ratio. The error bars were calculated based on the signal-to-noise ratios. Bars corresponding to the residues with significant intensity reductions, which have the minimum values of ΔRR within the error ranges (ΔRRmin) larger than 0.02, are colored red. The primary sequence of GIRKCP-L (residues 41–63 and 190–386) is displayed in the single-letter amino acid code followed by the residue number. The secondary structure elements of GIRKCP-L are depicted in gray below the sequence, based on the crystal structure (PDB code 1N9P), and the αA helix is colored green. B, mapping of the affected residues in the TCS experiment on a single subunit of the GIRKCP tetramer (PDB code 1N9P) is shown. Residues 371–386 are depicted by gray tubes. Side views of the GIRKCP-L structures parallel to the membrane plane, where the membrane side is above the molecules, are depicted by ribbon diagrams with balls for the amide nitrogen atoms of the affected residues (left). The affected residues are colored red on the surface representations of the GIRKCP-L structure, whereas the residues with no data, including proline residues, are colored black (center and right). The αA helix is colored green in the ribbon diagrams. Views from the outside (left and center) and the inside of the tetramer (right) are shown. The four subunits of the GIRKCP tetramer are shown in a surface representation for reference. C, mapping of the affected residues (colored red) in the TCS experiment on both of the two adjacent subunits of a GIRKCP tetramer (white and gray), viewed from the outside (left) and the inside (right) of the tetramer. Schematic drawings of the GIRKCP tetramer (subunits A, B, C, and D), viewed from the membrane side, are included to indicate the view of the surface models. The dashed blue line indicates the boundary between the two neighboring subunits.
The residues with large intensity reductions (ΔRRmin > 0.02) are Glu-242, Val-358, Leu-365, Leu-366, Met-367, Ser-368, Ser-369, Leu-371, Ile-372, and Ala-373 as well as one of the C-terminal unassigned residues (hereafter, referred to as CT1). These residues were mapped on a single subunit (Fig. 3B) and two adjacent subunits (Fig. 3C) of GIRKCP (PDB code 1N9P). All of these residues, except for Glu-242 are located on the C-terminal region around the αA helix of GIRKCP-L (Fig. 3). Therefore, we concluded that these residues are the Gαi3 binding residues. Glu-242 would be involved in the direct binding to the Gαi3, as explained under “Discussion.”
It should be noted that the first two residues, Ile-372 and Ala-373, and CT1 were detected among the extended C-terminal 15 residues from GIRKCP. The other C-terminal residues showed no significant intensity reductions. Thus, we concluded that the extension for 15 residues is sufficient for the interaction with Gαi3.
Contribution of Helical Domain of Gαi3 to Interaction with GIRKCP-L, as Investigated by PRE Experiments
The interaction of the helical domain of Gαi3 with GIRKCP-L was investigated by PRE experiments. PRE arises from magnetic dipolar interactions between the nuclear spin and the unpaired electron spin of the paramagnetic center, which enhances the relaxation of the nuclear spins, leading to the line-broadening and thus the intensity reduction of the NMR signals of the residues within about 20 Å of the spin label (37, 44). In addition, PRE can detect a transient interaction, because the PRE effect occurs within 250–500 μs (45), which is a much shorter duration than that of the cross-saturation (the effective saturation time of 200–300 ms for the TCS experiments in this study) (43).
The spin-labeling reagent, MTSL, which can be chemically introduced to cysteine side chains, was used to label Gαi3. First, we prepared a mutant of Gαi3 (C3S-C66A-C214S-C305S-C325A-C351I) in which the six natively existing cysteine residues that are exposed and reactive are substituted with other residues (hereafter, referred to as Hexa III). It should be noted that the corresponding mutant of Gαi1 (C3S-C66A-C214S-C305S-C325A-C351I), named Hexa I, was folded properly and functional (34). We also confirmed the proper folding of Hexa III by the 1H,15N TROSY spectrum of 2H,15N-labeled Hexa III (data not shown). Then, either Ile-82 or Ser-153 was substituted by Cys and chemically modified by MTSL (Hexa III-I82C-MTSL and Hexa III-S153C-MTSL). The Ile-82 residue is located at the center of the three residues identified by TCS (Gly-89, Gly-112, and Ala-114), and Ser-153 exists on the opposite side from I82. Because the paramagnetism of MTSL disappears upon reduction by the addition of ascorbate, the PRE effects were evaluated by the signal intensity ratios of the 1H,15N TROSY spectra of 2H,15N-labeled GIRKCP-L between the oxidized and reduced states in the presence of Hexa III-I82C-MTSL or Hexa III-S153C-MTSL.
Fig. 4A shows that the PRE effects from Hexa III-I82C-MTSL were observed for 13 signals of GIRKCP-L: Thr-353, Tyr-356, Ser-357, Leu-365, Leu-366, Met-367, Ser-368, Ser-369, Leu-371, Ile-372, Ala-373, A375, and CT1, which reside in the C-terminal region of GIRKCP-L. It should be noted that most of the other residues of the C-terminal region could not be analyzed due to signal overlapping, and thus these residues might also be affected by MTSL. On the other hand, only Cys-53 was detected for Hexa III-S153C-MTSL (Fig. 4B), which might be caused by the partial MTSL modification of Cys-53. Because the spin label was introduced at I82C, which is at the center of the three residues identified by TCS, we conclude that the Ile-82 side of the helical domain can approach within 20 Å of the C-terminal region of GIRKCP-L.
FIGURE 4.
PRE results of the interaction between GIRKCP-L and Gαi3. A and B, left, shown is a plot of the signal intensity RRs of paramagnetic to diamagnetic for Hexa III-I82C-MTSL (A) and Hexa III-S153C-MTSL (B). The residues indicated by asterisks are those with no data due to overlapping resonances or insufficient signal-to-noise ratio. The error bars were calculated based on the signal-to-noise ratios. Bars corresponding to the residues with significant intensity reductions, which have the minimum values of RR within the error ranges (RRmin) larger than 0.20, are colored red. The primary sequence of GIRKCP-L (residues 41–63 and 190–386) is displayed in the single-letter amino acid code followed by the residue number. Center, mapping of the affected residues in the PRE experiment on a single subunit of the GIRKCP structure (PDB code 1N9P) is shown. Residues 371–386 are depicted by gray tubes. Side views of the GIRKCP structures parallel to the membrane plane are depicted by surface representations viewed from the outside of the tetramer, where the membrane side is above the molecules. The affected residues are colored red. The proline residues and the residues with no data are colored black. Right, shown is mapping of the spin-labeled site of Gαi3 on the structure of Gα (PDB code 1GIA).
DISCUSSION
Direct Interaction between Gαi3 and Cytoplasmic Region of GIRK
NMR analyses were performed to probe the interaction between GIRKCP-L and Gαi3. Because cross-saturation is a phenomenon depending on the intermolecular 1H-1H distances, the TCS results from GIRKCP-L to Gαi3 (Fig. 2) and vice versa (Fig. 3) indicated that the α2/α3 site of Gαi3 and the αA helix of GIRKCP-L directly interact with each other. Our relaxation matrix calculations (43, 46) in which the on and off rates of the GIRKCP-L-Gαi3 interaction are also considered, suggested that under the current experimental conditions the cross-saturation effect should be observed for the amide hydrogen atoms of GIRKCP-L within 6 Å from Gαi3 and for those of Gαi3 within 5 Å from GIRKCP-L. Furthermore, the direct interaction was verified by the mutations of the interacting residues on Gαi3, which impaired the affinity for GIRKCP-L (I212A and W258A, supplemental Fig. 2).
Most of the residues with apparent chemical shift changes are located in the GIRKCP-L binding site consisting of the α2 and α3 helices of Gαi3, whereas the other residues, Arg-312 and Thr-316, exist on the α4/β6-loop that is adjacent to the α3 helix, reflecting the conformational changes of these residues upon GIRKCP-L binding (Fig. 1). The fitting of the titration curves of the chemical shift changes of Lys-209 and Trp-258, the GIRKCP-L binding residues, resulted in the Kd values of 0.6 and 1.1 mm, respectively, which are within the range of the fitting error (Fig. 1C). Thus, the Kd value for the binding of Gαi3 and GIRKCP-L was estimated as 1 mm. Although this Kd value is quite large as a value for a protein-protein interaction, it would fall in the nanomolar to micromolar range on the cell membrane, considering the reduced dimensionality effects (12, 47, 48). The Kd value on the order of 1 mm is 4 times of the Kd value of 0.25 mm that we previously reported for the GIRKCP-Gβγ interaction (12), which is consistent with the report that the affinity of the cytoplasmic region of GIRK1 for Gβγ was 4–5-fold stronger than that for Gαi3 (15).
As shown in Fig. 1, E and F, accelerated signal intensity reductions were observed for the Gαi3 residues at the GTP binding region, which reflect the larger chemical shift changes for the residues in the intermediate exchange regime. These residues are distant from the α2/α3 site, which is the direct GIRKCP-L binding interface, suggesting that the conformational change around the GTP binding site is induced by GIRKCP-L binding to the α2/α3 site. In particular, Ser-44, Gly-45, Lys-46, Ser-47, and Gly-203 are the residues directly interacting with the β- or γ-phosphate groups of GTP. This might be related to the report that the GTPase activity of Gαs is enhanced upon binding to its effector, adenylate cyclase (49), which also binds to the α2/α3 site of Gαs (50). However, the significance of the conformational change at the GTP binding site in terms of GIRK regulation remains unclear and is beyond the scope of this paper.
Binding Mode of Gα and Cytoplasmic Region of GIRK
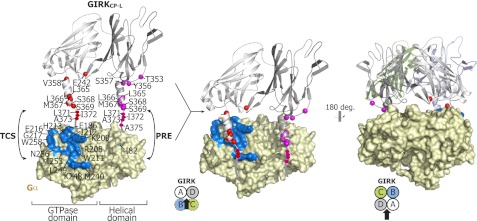
Although the TCS results revealed that the αA helix of GIRKCP-L directly binds to the α2/α3 site of Gαi3, the PRE results indicated the αA helix of GIRKCP-L is within 20 Å of Ile-82 of Gαi3. However, the αA helix of GIRKCP-L, which binds to the α2/α3 site, should be more than 20 Å away from the spin-labeled site, suggesting that another αA helix in a neighboring subunit of the GIRKCP-L tetramer must come close to the spin-labeled site (Fig. 5).
FIGURE 5.
The proposed model of Gα(GTP) and the cytoplasmic region of the GIRK complex. Left, two adjacent subunits of a GIRKCP tetramer (PDB code 1N9P), viewed from the inside of the tetramer, are depicted by a ribbon diagram, and a homology model of Gαi3 built from the crystal structure of Gαi1(GTPγS) (PDB code 1GIA) by the MODELLER software (39) is shown in a surface representation. Residues 371–386 of GIRK1 are depicted schematically. The nitrogen atoms of the GIRKCP-L residues identified in the TCS experiments and the residues with PRE effects from MTSL, modified to I82C on the helical domain of Gαi3 (green), are shown as red and magenta balls, respectively. The α2/α3 residues of Gαi3 identified in the TCS experiments are colored blue. Center, shown is the binding model of Gαi3 in the GTP-bound state and the cytoplasmic region of GIRK1 (PDB code 1N9P), obtained with HADDOCK software (38). The residues on GIRKCP-L and the α2/α3 residues on Gαi3 identified by TCS were defined as the active residues in the program so as to form the interface of the complex. The residues 358–370 on GIRK were defined as “semi-flexible segments” to allow them to move during the simulated annealing. Only the two adjacent subunits of the GIRK tetramer are shown from the inside of the tetramer. Right, the model displayed at the center is rotated by 180 degrees, and all of the subunits of the GIRK tetramer are shown.
To build a model of the Gαi3-GIRKCP-L complex satisfying the NMR-derived structural information, rigid body docking was performed by using the HADDOCK program (38). Because the structure of Gαi3(GTP) is not available, we built a homology model by the MODELLER software of Gαi3(GTP) from the crystal structure of Gαi1(GTPγS) (PDB code 1GIA) in which 94% of the 354 residues are identical to Gαi3 (39) and used it for the construction of the complex model. The residues in the α2/α3 site of Gα and in the αA helix of GIRKCP-L, identified by the TCS experiments, were specified as the “active residues” in the program so that they formed the interface of the complex. Although the information about the residues on the helical domain derived from the TCS and PRE experiments was not used for the calculation, the helical domain of Gα was calculated to be proximal to the αA helix in a neighboring subunit of GIRKCP-L, probably due to the restraint of Glu-242 on the subunit interface of GIRKCP-L, as determined from the TCS experiments (Fig. 5). In this binding mode, Glu-242 of GIRKCP-L at a distance from the αA helix, approaches the side chain of Glu-186 of Gαi3, which accounts for its intensity reduction in the TCS experiment.
The three residues that TCS identified on the helical domain of Gαi3 (Gly-89, Gly-112, and Ala-114) showed relatively weak cross-saturation, and they do not form a continuous binding surface, presumably because the αA helix of GIRKCP-L transiently accesses the Gαi3 helical domain. This can be accounted for by the conformational flexibility of the αA helix. In the crystal structure, the αA helix of GIRKCP is stabilized by the crystal contacts with the αA helix of another tetramer. In addition, the C-terminal part of the helix (residues 367–368) exhibited very weak or no NOEs that are typical for an α helix (data not shown), and the chemical shift index calculated by the 13C chemical shifts indicated that the C-terminal residues after Met-367 are unstructured. Altogether, we concluded that the α2/α3 site of Gαi3 mainly recognizes the αA helix of GIRKCP-L in one subunit of the tetramer, whereas the helical domain of Gαi3 transiently approaches the αA helix in another neighboring subunit.
The N terminus of Gα is modified by a lipid moiety in vivo and anchored to the cell membrane (51), which can be accounted for by this binding mode. Although the most N-terminal Gα residue in the crystal structure, Val-34, resides at about 35 Å away from the possible intracellular cell membrane in the current binding mode (supplemental Fig. 5), the N-terminal residues 1–33 of Gα, which would form a 40 Å-long α helix in the complex with Gβγ, are presumably unstructured and adopt a longer length than 40 Å in the GTP-bound state.
Molecular Recognition Mode between Gαi3 and GIRKCP-L
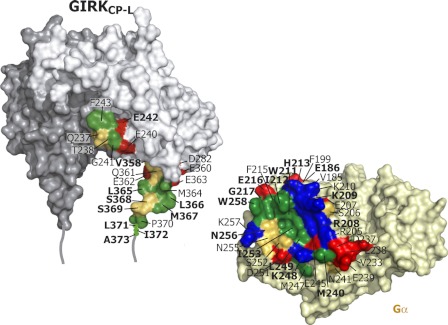
Fig. 6 shows the surface properties of the α2/α3 site of Gαi3 and the αA helix of GIRKCP-L. The α2/α3 site possesses a hydrophobic cleft at its center, which is surrounded by polar residues, whereas the αA helix also contains a cluster of hydrophobic residues that are exposed to the outside. This suggests that the complementarity of the shape at the direct binding sites, formed by these hydrophobic residues, is important for the binding. Indeed, the mutations of the hydrophobic residues of Gαi3, I212A and W258A, resulted in the impaired affinity for GIRKCP-L (supplemental Fig. 2).
FIGURE 6.
Properties of the binding interfaces on Gαi3 and GIRK1. An “open-book” display of the Gα-GIRK complex model (Fig. 5) is shown in surface representations. Residues 371–386 of GIRKCP-L are depicted schematically. The residues on GIRKCP-L and the α2/α3 residues on Gαi3 identified by TCS (labeled by bold letters) and their surrounding residues with Cα atoms within 6.0 Å, are colored according to their side-chain properties: acidic, basic, hydrophobic, and hydrophilic residues are colored red, blue, green, and yellow, respectively.
The α2/α3 site on Gα, which is highly conserved among the Gα family members, is known to be the effector binding surface of Gα in the GTP-bound form, as revealed by the crystal structures of Gα in complex with its effectors such as Gαs-adenylate cyclase (50) and Gαq-G protein receptor kinase (52), and with peptides (53). In these complexes, the hydrophobic and acidic side chains from the effectors and peptides are inserted into the hydrophobic cleft of the α2/α3 site on Gα. GIRK is also recognized in a similar manner to those of the effectors and the peptides. It should be noted that the conformational flexibility of the αA helix might play a role in its interaction with the hydrophobic cleft of α2/α3 on Gα.
The helical domain of Gα is reportedly important for the i/o-family-specific activation of the GIRK channel, as determined with a Gαi1/Gαq chimera (25). However, the main GIRK binding site for the Gαi3(GTP) revealed in this study was the α2/α3 site on the GTPase domain, not on the helical domain. The activation specificity should be accomplished by the binding of Gαi/o(GDP)βγ to GIRK, which seems to occur at a different site from that for Gαi/o(GTP).
Previously, the Gαi/o binding regions in GIRK1 were investigated by pulldown assays using the cytoplasmic fragments of GIRK (14, 15, 20, 22, 24). The cytoplasmic C-terminal residues 320–369 of GIRK1 were indicated to be important for strong binding to Gαi3 (14), whereas the N-terminal fragment of GIRK1 (residues 1–84) also bound to Gαi/o(GTP) (15, 20). The Gαi3 binding residues of GIRK1 revealed in this study are involved in the previously defined C-terminal region, and we identified three additional residues, Leu-371, Ile-372, and Ala-373. These residues vary among the GIRK subtypes, and GIRK1 possesses more hydrophobic and fewer charged residues than the other subtypes (supplemental Fig. 6), suggesting that the Gαi/o binding affinity might differ among the subtypes. Unfortunately, the N-terminal binding region was not identified here, as the construct used in our study lacks residues 1–40 and 64–84.
Modulation of GIRK by Gi/o
GIRK is activated by the Gβγ binding, and Gαi/o modulates the gating property of GIRK. Gαi/o(GDP)βγ is assumed to be precoupled with GPCRs and GIRK, which facilitates the efficient gate opening of GIRK, with the high specificity of GPCR signaling to GIRK (17, 18). Gαi/o(GDP)βγ binding to GIRK suppresses the basal K+ current of GIRK while maintaining the maximal current evoked by the GPCR stimulation, which is referred to as priming (14, 15, 19–22). On the other hand, Gαi/o(GTP) binding to GIRK accelerates its deactivation (15).
Recently, a two-site model was proposed for the G protein binding sites on GIRK (“anchoring site” and “activation site”) (19, 22). The anchoring site is relevant to precoupling and priming for GIRK when the site accommodates Gαi/o(GDP)βγ and to the binding of Gαi/o(GTP) when Gβγ shifts to the activation site, which was revealed by our NMR analyses as the border of the two neighboring subunits of the GIRK tetramer (12). In this study we have identified the anchoring site for Gαi/o(GTP) on GIRK (Figs. 5 and 7). Notably, it is unknown whether the site is identical to the one for Gαi/o(GDP)βγ. Upon ligand stimulation of the precoupled GPCR, the GDP-GTP exchange on Gαi/o in the complex with Gβγ at the anchoring site for Gαi/o(GDP)βγ decreases the affinity of Gαi/o for Gβγ, allowing Gβγ to activate GIRK by binding to the activation site. When the GPCR stimulation ends, GTP on Gαi/o at the anchoring site for Gαi/o(GTP) is rapidly hydrolyzed to GDP with assistance from RGS (23). Thus, Gαi/o, at the anchoring site for Gαi/o(GTP) immediately re-associates with Gβγ, leading to the closure of the GIRK gate.
FIGURE 7.
A model of the Gαi/o-GIRK-Gβγ ternary complex. Left, the nitrogen atoms of the Gβγ binding residues on GIRK1 (12) are mapped as magenta balls on the complex model of Gα and the GIRK tetramer (Fig. 5). Right, shown is a ternary complex model of Gα-GIRK-Gβγ. First, the binding model of Gβγ-GIRK was built by using HADDOCK with parameters listed in supplemental Table 2. The Gβγ binding residues on GIRKCP-L identified in our previous study (12) and the residues on Gβγ reported to be important for GIRK binding (54, 55) were defined as the active residues in the software. Then the Gα-GIRK complex model shown in Fig. 5 and the Gβγ-GIRK complex model were superimposed by the GIRK structure, rendering the Gα-GIRK-Gβγ ternary complex model. Gβ and Gγ are colored magenta and violet, respectively. The residues involved in the intermolecular interactions between Gα and Gβγ, within a distance of 4 Å in the Gαi1(GDP)β1γ2 (PDB code 1GP2) structure, are colored orange.
In this study we revealed that the cytoplasmic region of GIRK1 and the GTP-bound Gαi3 directly bind to each other, and the αA helix of GIRK1 corresponds to the anchoring site for Gαi/o(GTP). The mapping of the Gβγ binding residues (12) on the structure of the Gα-GIRK complex indicated that the binding sites of Gαi3(GTP) and Gβγ on GIRK do not overlap with each other, and therefore, Gαi3(GTP) and Gβγ can simultaneously bind to GIRK (Fig. 7). Furthermore, the mapping of the RGS binding site on the structure of Gα suggested that RGS can bind to Gαi3 in the complex with GIRK (supplemental Fig. 7). Therefore, the Gαi/o-GIRK-Gβγ ternary complex model obtained here provides the structural basis for the rapid closure of the GIRK channel upon signal termination through the efficient removal of the proximal Gβγ from GIRK.
It should be noted that the α2 helix of Gαi3 in the GIRK binding site (the α2/α3 site) is located in the switch II region, which is known to alter its conformation upon GDP-GTP exchange and is included in the Gβγ binding site. Thus, the anchoring site of Gαi/o(GDP)βγ is different from that of Gαi/o(GTP) revealed here. Structural analyses of the interaction between Gαi/o(GDP)βγ and GIRK will provide a complete understanding of the modulation mechanism of the GIRK-gating by Gi/o proteins.
Supplementary Material
This work was supported in part by grants from the Japan New Energy and Industrial Technology Development Organization (NEDO) and the Ministry of Economy, Trade, and Industry (METI) (to I. S.), a grant-in-aid for Scientific Research on Priority Areas from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (MEXT) (to M. O. and I. S.), Global COE Program “Medical System Innovation on Multidisciplinary Integration” from MEXT (to Y. M.), and a grant from Takeda Science Foundation (to M. O.).

This article contains supplemental Methods, Tables 1 and 2, and Figs. 1–7.
- GIRK
- G protein-gated inwardly rectifying potassium ion channel
- Gα
- G protein α subunit
- Gαi/o
- Gα in the i/o-family
- Gαi3
- Gα in the i3-family
- Gα(GTP)
- Gα in the GTP-bound state
- Gα(GDP)
- Gα in the GDP-bound state
- Gβγ
- G protein βγ subunits
- Gαi/o(GDP)βγ
- heterotrimeric G protein αβγ subunits of the i/o-family in the GDP-bound state
- GPCR
- G protein-coupled receptor
- TM
- transmembrane
- CP
- cytoplasmic pore
- GIRKCP-L
- cytoplasmic pore region of mouse GIRK1 (residues 41–63 and 190–386)
- GIRKCP
- cytoplasmic pore region of mouse GIRK1 (residues 41–63 and 190–371)
- TROSY
- transverse relaxation-optimized spectroscopy
- DSS
- sodium 2,2-dimethyl-2-silapentane-5-sulfonate
- TCS
- transferred cross-saturation
- PRE
- paramagnetic relaxation enhancement
- MTSL
- S-(1-oxy-2,2,5,5-tetramethylpyrroline-3-methyl)-methanethiosulfonate
- GTPγS
- guanosine 5–3-O-(thio)triphosphate
- RGS
- regulator of G protein signaling
- PDB
- Protein Data Bank
- RR
- reduction ratios.
REFERENCES
- 1. He C., Zhang H., Mirshahi T., Logothetis D. E. (1999) Identification of a potassium channel site that interacts with G protein βγ subunits to mediate agonist-induced signaling. J. Biol. Chem. 274, 12517–12524 [DOI] [PubMed] [Google Scholar]
- 2. Bichet D., Haass F. A., Jan L. Y. (2003) Merging functional studies with structures of inward-rectifier K(+) channels. Nat. Rev. Neurosci. 4, 957–967 [DOI] [PubMed] [Google Scholar]
- 3. Hibino H., Inanobe A., Furutani K., Murakami S., Findlay I., Kurachi Y. (2010) Inwardly rectifying potassium channels. Their structure, function, and physiological roles. Physiol. Rev. 90, 291–366 [DOI] [PubMed] [Google Scholar]
- 4. Kuo A., Gulbis J. M., Antcliff J. F., Rahman T., Lowe E. D., Zimmer J., Cuthbertson J., Ashcroft F. M., Ezaki T., Doyle D. A. (2003) Crystal structure of the potassium channel KirBac1.1 in the closed state. Science 300, 1922–1926 [DOI] [PubMed] [Google Scholar]
- 5. Whorton M. R., MacKinnon R. (2011) Crystal structure of the mammalian GIRK2 K+ channel and gating regulation by G proteins, PIP2, and sodium. Cell 147, 199–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clapham D. E., Neer E. J. (1993) New roles for G-protein βγ-dimers in transmembrane signaling. Nature 365, 403–406 [DOI] [PubMed] [Google Scholar]
- 7. Kurachi Y. (1995) G protein regulation of cardiac muscarinic potassium channel. Am. J. Physiol. 269, C821–C830 [DOI] [PubMed] [Google Scholar]
- 8. Ito H., Tung R. T., Sugimoto T., Kobayashi I., Takahashi K., Katada T., Ui M., Kurachi Y. (1992) On the mechanism of G protein βγ subunit activation of the muscarinic K+ channel in guinea pig atrial cell membrane. Comparison with the ATP-sensitive K+ channel. J. Gen. Physiol. 99, 961–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Logothetis D. E., Kurachi Y., Galper J., Neer E. J., Clapham D. E. (1987) The βγ subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature 325, 321–326 [DOI] [PubMed] [Google Scholar]
- 10. Reuveny E., Slesinger P. A., Inglese J., Morales J. M., Iñiguez-Lluhi J. A., Lefkowitz R. J., Bourne H. R., Jan Y. N., Jan L. Y. (1994) Activation of the cloned muscarinic potassium channel by G protein βγ subunits. Nature 370, 143–146 [DOI] [PubMed] [Google Scholar]
- 11. Wickman K. D., Iñiguez-Lluhl J. A., Davenport P. A., Taussig R., Krapivinsky G. B., Linder M. E., Gilman A. G., Clapham D. E. (1994) Recombinant G-protein βγ-subunits activate the muscarinic-gated atrial potassium channel. Nature 368, 255–257 [DOI] [PubMed] [Google Scholar]
- 12. Yokogawa M., Osawa M., Takeuchi K., Mase Y., Shimada I. (2011) NMR analyses of the Gβγ binding and conformational rearrangements of the cytoplasmic pore of G protein-activated inwardly rectifying potassium channel 1 (GIRK1). J. Biol. Chem. 286, 2215–2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hille B. (1992) G protein-coupled mechanisms and nervous signaling. Neuron 9, 187–195 [DOI] [PubMed] [Google Scholar]
- 14. Ivanina T., Varon D., Peleg S., Rishal I., Porozov Y., Dessauer C. W., Keren-Raifman T., Dascal N. (2004) Gαi1 and Gαi3 differentially interact with, and regulate, the G protein-activated K+ channel. J. Biol. Chem. 279, 17260–17268 [DOI] [PubMed] [Google Scholar]
- 15. Berlin S., Tsemakhovich V. A., Castel R., Ivanina T., Dessauer C. W., Keren-Raifman T., Dascal N. (2011) Two distinct aspects of coupling between Gαi and G protein-activated K+ channel (GIRK) revealed by fluorescently labeled Gαi3 subunits. J. Biol. Chem. 286, 33223–33235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Benians A., Leaney J. L., Milligan G., Tinker A. (2003) The dynamics of formation and action of the ternary complex revealed in living cells using a G-protein-gated K+ channel as a biosensor. J. Biol. Chem. 278, 10851–10858 [DOI] [PubMed] [Google Scholar]
- 17. Kovoor A., Lester H. A. (2002) Gi Irks GIRKs. Neuron 33, 6–8 [DOI] [PubMed] [Google Scholar]
- 18. Slesinger P. A., Reuveny E., Jan Y. N., Jan L. Y. (1995) Identification of structural elements involved in G protein gating of the GIRK1 potassium channel. Neuron 15, 1145–1156 [DOI] [PubMed] [Google Scholar]
- 19. Berlin S., Keren-Raifman T., Castel R., Rubinstein M., Dessauer C. W., Ivanina T., Dascal N. (2010) Gαi and Gβγ jointly regulate the conformations of a Gβγ effector, the neuronal G protein-activated K+ channel (GIRK). J. Biol. Chem. 285, 6179–6185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Peleg S., Varon D., Ivanina T., Dessauer C. W., Dascal N. (2002) Gαi controls the gating of the G protein-activated K+ channel, GIRK. Neuron 33, 87–99 [DOI] [PubMed] [Google Scholar]
- 21. Rubinstein M., Peleg S., Berlin S., Brass D., Dascal N. (2007) Gαi3 primes the G protein-activated K+ channels for activation by coexpressed Gβγ in intact Xenopus oocytes. J. Physiol. 581, 17–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rubinstein M., Peleg S., Berlin S., Brass D., Keren-Raifman T., Dessauer C. W., Ivanina T., Dascal N. (2009) Divergent regulation of GIRK1 and GIRK2 subunits of the neuronal G protein gated K+ channel by GαiGDP and Gβγ. J. Physiol. 587, 3473–3491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Doupnik C. A., Davidson N., Lester H. A., Kofuji P. (1997) RGS proteins reconstitute the rapid gating kinetics of gβγ-activated inwardly rectifying K+ channels. Proc. Natl. Acad. Sci. U.S.A. 94, 10461–10466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clancy S. M., Fowler C. E., Finley M., Suen K. F., Arrabit C., Berton F., Kosaza T., Casey P. J., Slesinger P. A. (2005) Pertussis-toxin-sensitive Gα subunits selectively bind to C-terminal domain of neuronal GIRK channels. Evidence for a heterotrimeric G-protein-channel complex. Mol. Cell Neurosci. 28, 375–389 [DOI] [PubMed] [Google Scholar]
- 25. Rusinova R., Mirshahi T., Logothetis D. E. (2007) Specificity of Gβγ signaling to Kir3 channels depends on the helical domain of pertussis toxin-sensitive Gα subunits. J. Biol. Chem. 282, 34019–34030 [DOI] [PubMed] [Google Scholar]
- 26. Sprang S. R., Chen Z., Du X. (2007) Structural basis of effector regulation and signal termination in heterotrimeric Gα proteins. Adv. Protein Chem. 74, 1–65 [DOI] [PubMed] [Google Scholar]
- 27. Oldham W. M., Hamm H. E. (2006) Structural basis of function in heterotrimeric G proteins. Q Rev. Biophys. 39, 117–166 [DOI] [PubMed] [Google Scholar]
- 28. Rasmussen S. G., DeVree B. T., Zou Y., Kruse A. C., Chung K. Y., Kobilka T. S., Thian F. S., Chae P. S., Pardon E., Calinski D., Mathiesen J. M., Shah S. T., Lyons J. A., Caffrey M., Gellman S. H., Steyaert J., Skiniotis G., Weis W. I., Sunahara R. K., Kobilka B. K. (2011) Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature 477, 549–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nishida M., MacKinnon R. (2002) Structural basis of inward rectification. Cytoplasmic pore of the G protein-gated inward rectifier GIRK1 at 1.8 Å resolution. Cell 111, 957–965 [DOI] [PubMed] [Google Scholar]
- 30. Yokogawa M., Muramatsu T., Takeuchi K., Osawa M., Shimada I. (2009) Backbone resonance assignments for the cytoplasmic regions of G protein-activated inwardly rectifying potassium channel 1 (GIRK1). Biomol. NMR Assign. 3, 125–128 [DOI] [PubMed] [Google Scholar]
- 31. Salzmann M., Pervushin K., Wider G., Senn H., Wüthrich K. (1998) TROSY in triple-resonance experiments. New perspectives for sequential NMR assignment of large proteins. Proc. Natl. Acad. Sci. U.S.A. 95, 13585–13590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Salzmann M., Wider G., Pervushin K., Senn H., Wuthrich K. (1999) TROSY-type triple-resonance experiments for sequential NMR assignments of large proteins. J. Am. Chem. Soc. 121, 844–848 [Google Scholar]
- 33. Yoshiura C., Kofuku Y., Ueda T., Mase Y., Yokogawa M., Osawa M., Terashima Y., Matsushima K., Shimada I. (2010) NMR analyses of the interaction between CCR5 and its ligand using functional reconstitution of CCR5 in lipid bilayers. J. Am. Chem. Soc. 132, 6768–6777 [DOI] [PubMed] [Google Scholar]
- 34. Medkova M., Preininger A. M., Yu N. J., Hubbell W. L., Hamm H. E. (2002) Conformational changes in the amino-terminal helix of the G protein αi1 following dissociation from Gβγ subunit and activation. Biochemistry 41, 9962–9972 [DOI] [PubMed] [Google Scholar]
- 35. Mase Y., Yokogawa M., Osawa M., Shimada I. Backbone resonance assignments for G protein αi3 subunit in the GTP-bound state. Biomol. NMR Assign. in press [DOI] [PubMed] [Google Scholar]
- 36. Nakanishi T., Miyazawa M., Sakakura M., Terasawa H., Takahashi H., Shimada I. (2002) Determination of the interface of a large protein complex by transferred cross-saturation measurements. J. Mol. Biol. 318, 245–249 [DOI] [PubMed] [Google Scholar]
- 37. Battiste J. L., Wagner G. (2000) Utilization of site-directed spin labeling and high resolution heteronuclear nuclear magnetic resonance for global fold determination of large proteins with limited nuclear Overhauser effect data. Biochemistry 39, 5355–5365 [DOI] [PubMed] [Google Scholar]
- 38. de Vries S. J., van Dijk M., Bonvin A. M. (2010) The HADDOCK web server for data-driven biomolecular docking. Nat. Protoc. 5, 883–897 [DOI] [PubMed] [Google Scholar]
- 39. Sali A., Potterton L., Yuan F., van Vlijmen H., Karplus M. (1995) Evaluation of comparative protein modeling by MODELLER. Proteins 23, 318–326 [DOI] [PubMed] [Google Scholar]
- 40. Matsuo H., Walters J., Teruya K., Tanaka T., Gasser G., Lippard S., Kyogoku Y., Wagner G. (1999) Identification by NMR spectroscopy of residues at contact surfaces in large, slowly exchanging macromolecular complexes. J. Am. Chem. Soc. 121, 9903–9904 [Google Scholar]
- 41. Mal T. K., Masutomi Y., Zheng L., Nakata Y., Ohta H., Nakatani Y., Kokubo T., Ikura M. (2004) Structural and functional characterization on the interaction of yeast TFIID subunit TAF1 with TATA-binding protein. J. Mol. Biol. 339, 681–693 [DOI] [PubMed] [Google Scholar]
- 42. Shimada I., Ueda T., Matsumoto M., Sakakura M., Osawa M., Takeuchi K., Nishida N., Takahashi H. (2009) Cross-saturation and transferred cross-saturation experiments. Prog. Nucl. Magn. Reson. Spectrosc. 54, 123–140 [Google Scholar]
- 43. Matsumoto M., Ueda T., Shimada I. (2010) Theoretical analyses of the transferred cross-saturation method. J. Magn. Reson. 205, 114–124 [DOI] [PubMed] [Google Scholar]
- 44. Clore G. M., Iwahara J. (2009) Theory, practice, and applications of paramagnetic relaxation enhancement for the characterization of transient low-population states of biological macromolecules and their complexes. Chem. Rev. 109, 4108–4139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Clore G. M. (2011) Exploring sparsely populated states of macromolecules by diamagnetic and paramagnetic NMR relaxation. Protein Sci. 20, 229–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Takahashi H., Miyazawa M., Ina Y., Fukunishi Y., Mizukoshi Y., Nakamura H., Shimada I. (2006) Utilization of methyl proton resonances in cross-saturation measurement for determining the interfaces of large protein-protein complexes. J. Biomol. NMR 34, 167–177 [DOI] [PubMed] [Google Scholar]
- 47. Ozcan F., Klein P., Lemmon M. A., Lax I., Schlessinger J. (2006) On the nature of low and high affinity EGF receptors on living cells. Proc. Natl. Acad. Sci. U.S.A. 103, 5735–5740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stratikos E., Mosyak L., Zaller D. M., Wiley D. C. (2002) Identification of the lateral interaction surfaces of human histocompatibility leukocyte antigen (HLA)-DM with HLA-DR1 by formation of tethered complexes that present enhanced HLA-DM catalysis. J. Exp. Med. 196, 173–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Scholich K., Mullenix J. B., Wittpoth C., Poppleton H. M., Pierre S. C., Lindorfer M. A., Garrison J. C., Patel T. B. (1999) Facilitation of signal onset and termination by adenylyl cyclase. Science 283, 1328–1331 [DOI] [PubMed] [Google Scholar]
- 50. Tesmer J. J., Sunahara R. K., Gilman A. G., Sprang S. R. (1997) Crystal structure of the catalytic domains of adenylyl cyclase in a complex with Gαs·GTPγS. Science 278, 1907–1916 [DOI] [PubMed] [Google Scholar]
- 51. Wedegaertner P. B., Wilson P. T., Bourne H. R. (1995) Lipid modifications of trimeric G proteins. J. Biol. Chem. 270, 503–506 [DOI] [PubMed] [Google Scholar]
- 52. Tesmer V. M., Kawano T., Shankaranarayanan A., Kozasa T., Tesmer J. J. (2005) Snapshot of activated G proteins at the membrane. the Gαq-GRK2-Gβγ complex. Science 310, 1686–1690 [DOI] [PubMed] [Google Scholar]
- 53. Johnston C. A., Lobanova E. S., Shavkunov A. S., Low J., Ramer J. K., Blaesius R., Fredericks Z., Willard F. S., Kuhlman B., Arshavsky V. Y., Siderovski D. P. (2006) Minimal determinants for binding activated Gα from the structure of a Gαi1-peptide dimer. Biochemistry 45, 11390–11400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Albsoul-Younes A. M., Sternweis P. M., Zhao P., Nakata H., Nakajima S., Nakajima Y., Kozasa T. (2001) Interaction sites of the G protein β subunit with brain G protein-coupled inward rectifier K+ channel. J. Biol. Chem. 276, 12712–12717 [DOI] [PubMed] [Google Scholar]
- 55. Ford C. E., Skiba N. P., Bae H., Daaka Y., Reuveny E., Shekter L. R., Rosal R., Weng G., Yang C. S., Iyengar R., Miller R. J., Jan L. Y., Lefkowitz R. J., Hamm H. E. (1998) Molecular basis for interactions of G protein βγ subunits with effectors. Science 280, 1271–1274 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.