Background: Patients with insulin resistance respond poorly to interferon therapy.
Results: Induction of insulin resistance increased protein-tyrosine phosphatase (PTP) activity and provoked interferon resistance. PTP inhibition enhanced the interferon response in these experiments.
Conclusion: Resistance to interferon associated with insulin resistance can be ascribed to an increase in PTP activity.
Significance: PTP inhibition enhances the interferon response in these experimental models.
Keywords: Antiviral Agents; Insulin Resistance; Signal Transduction; Tumor Necrosis Factor (TNF); Tyrosine-protein Phosphatase (Tyrosine Phosphatase); 2′,5′ Oligoadenylate Synthase; Interferon α; Metformin
Abstract
Insulin resistance is a risk factor for non-response to interferon/ribavirin therapy in patients with chronic hepatitis C. The aim of this study was to determine the role played by protein-tyrosine phosphatases (PTPs) in the absence of interferon-α (IFNα) response associated with insulin resistance. We induced insulin resistance by silencing IRS-2 or by treating HepG2 cells with tumor necrosis factor-α (TNFα) and analyzed insulin response by evaluating Akt phosphorylation and IFNα response by measuring Stat-1 tyrosine phosphorylation and 2′,5′-oligoadenylate synthase and myxovirus resistance gene expression. The response to IFNα was also measured in insulin-resistant obese mice (high fat diet and ob/ob mice) untreated and treated with metformin. Silencing IRS-2 mRNA induces insulin resistance and inhibits IFNα response. Likewise, TNFα suppresses insulin and IFNα response. Treatment of cells with pervanadate and knocking down PTP-1B restores insulin and IFNα response. Both silencing IRS-2 and TNFα treatment increase PTP and PTP-1B activity. Metformin inhibits PTP and improves IFNα response in insulin-resistant cells. Insulin-resistant ob/ob mice have increased PTP-1B gene expression and activity in the liver and do not respond to IFNα administration. Treatment with metformin improves this response. In HepG2 cells, insulin resistance provokes IFNα resistance, which is associated with an increased PTP-1B activity in the liver. Inhibition of PTP-1B activity with pervanadate and metformin or knocking down PTP-1B reestablishes IFNα response. Likewise, metformin decreases PTP-1B activity and improves response to IFNα in insulin-resistant obese mice. The use of PTP-1B inhibitors may improve the response to IFNα/ribavirin therapy.
Introduction
The current standard treatment for chronic hepatitis C is the combined pegylated IFNα plus ribavirin therapy. However, this treatment achieves a sustained virological response in less than 50% of patients infected with type 1 genotype of hepatitis C virus (1). Several host factors have been found to be associated with a reduced rate of sustained virological response to this therapy; many of them are associated with insulin resistance (2). Mechanisms involved in this resistance to IFNα therapy in patients with insulin resistance are not well known.
Binding of insulin to its receptor is followed by tyrosine phosphorylation of its own receptor and insulin receptor substrates IRS-1 and IRS-2, among others. Activation of these substrates is followed by activation of downstream targets such as Akt/PKB, which is involved in almost all actions of insulin (3). Suppressor of cytokine signaling (SOCS)2 proteins, protein phosphatase 2A (PP2A), and protein-tyrosine phosphatases (PTPs) can negatively modulate insulin signals. SOCS proteins decrease insulin sensitivity by binding to the insulin receptor and reducing its ability to phosphorylate IRS proteins (4). PP2A, whose expression is increased in liver biopsies from patients with chronic hepatitis C, inhibits insulin signaling by dephosphorylating Akt/PKB (5). PTPs constitute a large family of phosphatases that can attenuate insulin, growth factors, and cytokine signaling by dephosphorylating tyrosine residues located in the tyrosine kinase domain of the corresponding receptors and other components of their signaling pathways (6). Thus, PTP-1B dephosphorylates tyrosine residues involved in recruiting and activating insulin signaling molecules (7). Levels of PTP-1B are increased in patients and animal models with insulin resistance (8), whereas absence of PTP-1B is associated with insulin hypersensitivity in mice (9).
IFNα exerts its effects by binding to cell surface receptors, which triggers the recruitment and activation of Janus-tyrosine kinases (JAK) by autophosphorylation. Signal transducer and activator of transcription-1 (Stat1) is then recruited to high affinity binding sites on phosphorylated JAK, leading to its activation (10). Phosphorylated Stat1 binds Stat2 to form a heterodimer, which translocates to the nucleus where it recognizes specific DNA sequences in promoter regions of target genes, including 2′5′ oligoadenylate synthase (2′5′OAS) and myxovirus-resistance (Mx) genes, leading to induction of an antiviral state (11). This signal can be negatively regulated mainly by the SOCS proteins, protein inhibitor of activated Stat1 (PIAS1), PP2A, and some PTPs that interact with JAK and TYK (12). SOCS proteins have been found to be significantly elevated in patients with chronic hepatitis C non-responders to antiviral therapy (13). PIAS1 prevents binding of unmethylated Stat1 to the promoter of target genes (14). PP2A inhibits Stat1 methylation by protein arginine methyl transferase 1, increases unmethylated Stat1-PIAS1 association, and consequently reduces the binding of Stat1 to IFNα-stimulated gene promoters (15). In liver biopsies from patients with chronic hepatitis C, PP2A expression was found to be increased (5, 15), which may interfere with IFNα signaling via Stat1 hypomethylation, increased Stat1-PIAS1 association, and reduced binding of Stat1 to target genes. PTP can also dephosphorylate tyrosine residues located in some components of IFNα signaling pathway, including JAK family of tyrosine kinase and Stat factors (6, 16). Thus, overactivation of PTP, particularly PTP-1B, is a possible mechanism of blocking both insulin and IFNα signaling, as PTP-1B can suppress not only insulin signaling but also signal transduction of IFNs, leptin, and other factors (17). As far as we know, no information is available on the PTP activity in situations of IFNα resistance, including hepatitis C virus-infected patients resistant to insulin and IFNα therapy.
The aim of this study was to determine the role played by PTP, particularly PTP-1B, in the suppression of IFNα signaling associated with insulin resistance induced by silencing IRS-2 or by treating HepG2 cells with TNFα.
EXPERIMENTAL PROCEDURES
In Vitro Experiments
We induced insulin resistance in hepatocellular carcinoma cell line (HepG2) (American Type Culture Collection) using two different methods; (a) by silencing IRS-2 with appropriated siRNA and (b) by treating cells with 20 ng/ml TNFα for 18 h. In control and insulin-resistant cells, we measured response to 100 nm insulin or 250 units/ml IFNα in the presence or absence of 10 μg/ml metformin (Sigma) or 0.1 mm pervanadate. Pervanadate was prepared by mixing equal volumes of freshly prepared 0.1 m H2O2 and 0.1 m Na3VO4 (Sigma). The mixture was incubated for 10 min before use. Some experiments were performed using protein-tyrosine phosphatase 1B-deficient neonatal hepatocytes obtained as described elsewhere (18). Likewise, in some experiments we used IRS-2-deficient hepatocytes (19). IFNα response was evaluated by quantifying Stat-1 tyrosine phosphorylation and 2′,5′-oligoadenylate synthase 1A (2′5′OAS) (20) and myxovirus-resistant (Mx) gene expression. In RT-PCR experiments, each experimental condition was repeated five times. Insulin response was evaluated by measuring tyrosine and serine phosphorylation of IRS-2 or Akt/PKB, respectively. We used 100 nm insulin to ensure that cells were insulin-resistant.
In Vivo Experiments
Sixty male mice, 6 weeks old, (Charles River Laboratories España, SA. Santa Perpetua de la Mogada. Spain) were distributed in 12 groups; group I included 5 C57BL/6J (wild-type) mice treated with 50 μl of 0.8% saline intraperitoneally for 12 weeks. Group II was formed by five C57BL/6J mice treated subcutaneously with 2 μg/kg/week pegylated IFNα-2a (PegasysTM, Roche Farma, SA, Madrid, Spain) for 12 weeks subcutaneously. Group III was composed by five wild-type mice treated intraperitoneally with IFNα and 24 mg/kg/day metformin intraperitoneally for 12 weeks. Group IV was composed with five wild-type mice treated with metformin for the same period of time. Groups V, VI, VII, and VIII were formed by C57BL/6J ob/ob mice, five animals each group, treated as groups I, II, III, and IV, respectively. Groups IX, X, XI, and XII were formed by C57BL/6J mice fed with a high fat diet for 5 months (Harlan Laboratories, Models, SL., San Feliu de Codinas, Spain), 5 mice each group, and treated as groups I, II, III, IV, respectively. All procedures were carried out in accordance with the Spanish Guide for the Care and Use of Laboratory Animals. In the liver of these animals, we measured (a) insulin resistance by detecting serine phosphorylation of Akt 15 min after injecting 0.5 units/kg insulin intraperitoneal, (b) PTP-1B activity, (c) PTP-1B, 2′5′OAS, and Mx gene expression, and (d) TNFα concentration.
Protein-tyrosine Phosphatase Assay
PTP activity was measured using a commercially available assay following the manufacturer's instructions (Promega, Madison, WI) and employing the tyrosine-phosphorylated peptide DADEpYLIPQQG as substrate (21). Activity of protein-tyrosine phosphatase-1B (PTP-1B) was measured using a colorimetric assay kits (Calbiochem) according to the manufacturer's protocol.
Immunoprecipitation
The immunoprecipitation assays were performed as previously described (22). Cellular proteins were immunoprecipitated with appropriated polyclonal antibodies bound to agarose beads (anti-Jak1, anti-Stat-1, anti-IRS-1, and anti-IRS-2) (Santa Cruz Biotechnology, Santa Cruz, CA). Immune complexes were recognized using specific antibodies (JAK1, Stat-1 (Santa Cruz), PTP-1B (Millipore Ibérica Madrid, Spain). Signals were detected using the ECL Prime Western blotting detection reagent (Amersham Biosciences).
Western Blot Analysis
Whole cellular protein extracts were prepared from HepG2 cells cultured until confluence as previously described (23).
RNA Interference
IRS-1 and IRS-2-specific siRNA and nonspecific control RNA used as a negative control were purchased from Santa Cruz Biotechnology. For the transfection experiments we followed the procedure described elsewhere (24).
Real-time Quantitative Polymerase Chain Reaction (PCR)
PCR was performed on a Light Cycle 1.0 (Roche Applied Science) in 20 μl with 50 ng of cDNA, 0.5 μm primers, 2 μl of FastStart DNA Master SYBR Green I (Roche Applied Science). Data from the real-time, quantitative PCR were analyzed following the method as described elsewhere (24). Sequences of primers used in these experiments are shown in the supplemental table.
Hepatic concentrations of TNFα were measured using high sensitivity enzyme-linked immunosorbent assays according to the manufacturer's instructions (Mouse TNF ELISA. BLK Diagnostics, Badalona, Spain).
Statistical Analyses
Quantitative data are presented as the mean ± S.D. Each in vitro experimental condition was repeated three to four times. Statistical analysis was based on Student's t test for comparison of two groups. A p value less than 0.05 was used to determine statistical significance.
RESULTS
Insulin Resistance Induced by Silencing IRS-2 mRNA Inhibited IFNα Response
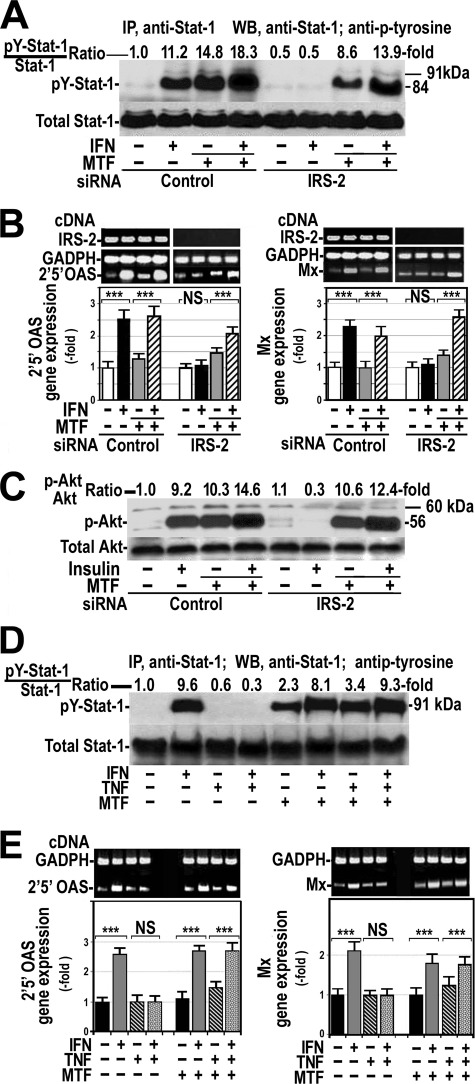
To assess whether insulin resistance was involved in the absence of response to IFNα, we induced insulin resistance by silencing IRS-2 gene expression using an appropriated siRNA in HepG2 cells. IRS-2 gene expression was decreased by about 80% in cells with silenced gene (Fig. 1A). As expected (19), silencing IRS-2 provoked insulin resistance as indicated by the absence of Akt serine-phosphorylation after insulin treatment (Fig. 1B).
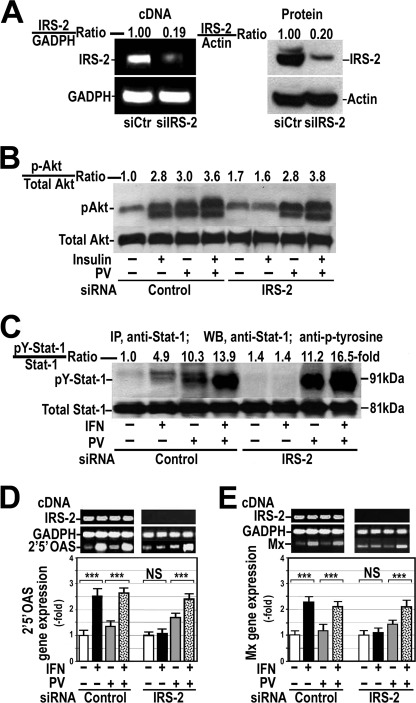
FIGURE 1.
Silencing IRS-2 inhibits the response of cultured hepatocytes to insulin and IFNα. A, in HepG2 cells, IRS-2 protein expression was silenced, and RT-PCR was performed for IRS-2 (IRS-2 cDNA) and GAPDH (GAPDH cDNA) transcripts. Decreased IRS-2 protein expression was examined by Western blot. B, control (siRNA Control) and insulin-resistant cells (siRNA IRS-2) were treated with 100 nm insulin for 15 min in the absence or presence of 0.1 mm pervanadate (PV). Whole cellular proteins were analyzed by Western blot using specific antibodies against Akt or serine-phosphorylated Akt (pAkt). Control, untreated cells. C, control and insulin-resistant cells were treated with 250 units/ml IFNα for 60 min in the absence or presence of 0.1 mm pervanadate. Cellular proteins were immunoprecipitated (IP) with anti-Stat-1 antibody, and total and tyrosine phosphorylated Stat-1 levels were analyzed by Western blot (WB). D and E, 2′5′OAS (D) and Mx (E) gene expression was measured by quantitative RT-PCR using endogenous GAPDH as reference. Control cells and cells with silenced IRS-2 were treated with IFNα in the presence or absence of 0.1 mm pervanadate. These blots are representative of experiments that were repeated three times. ***, p < 0.001; NS, not significant.
Using Stat-1 tyrosine phosphorylation as a marker for the IFNα signaling pathway activation, Fig. 1C shows that IFNα did not provoke any tyrosine phosphorylation of Stat-1 in cells with silenced IRS-2 insulin-resistant. Similar results were obtained when IFNα response was evaluated by measuring 2′5′OAS or Mx gene expression (Fig. 1, D and E).
As PTPs reduce tyrosine phosphorylation of some proteins, we investigated the role played by these phosphatases in this IFNα resistance by pretreating cells with 0.1 mm pervanadate, an unspecific tyrosine phosphatase inhibitor. Fig. 1C shows that inhibition of PTPs increased basal levels of tyrosine-phosphorylated Stat-1 and restored the response to IFNα in these insulin-resistant cells. Likewise, 2′5′OAS and Mx gene expression increased significantly after IFNα addition in insulin-resistant cells pretreated with pervanadate (Fig. 1, D and E). Similarly, these cells recovered insulin response (Fig. 1B).
TNFα Induced Insulin and IFNα Resistance, and These Effects Were Abrogated by Pervanadate
Treatment of HepG2 cells with 100 nm insulin resulted in a prompt serine phosphorylation of Akt that was particularly marked at 15 min (Fig. 2A). Treatment of cells with 20 ng/ml TNFα for 18 h provoked insulin resistance as indicated by the absence of additional Akt phosphorylation over the control level after insulin addition.
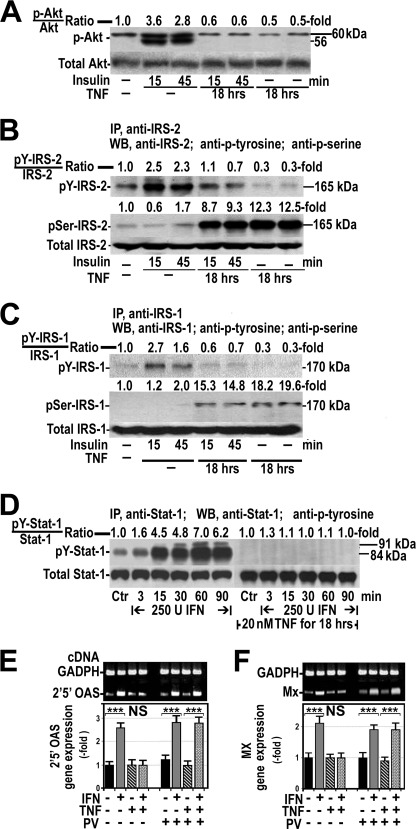
FIGURE 2.
TNFα induces insulin and IFNα resistance in HepG2 cells. A, effects of 100 nm insulin on Akt phosphorylation (p-Akt) in the absence or presence of 20 ng/ml TNFα for 18 h are shown. The blot is representative of one experiment that was repeated four times with similar results. B, shown is the effect of insulin on tyrosine (pY-IRS-2) and serine (pSer-IRS-2) phosphorylation of IRS-2 in the absence or presence of TNFα. Blots are representative of one experiment that was repeated four times with similar results. IP, immunoprecipitation. WB, Western blot. C, shown are the effects of insulin on tyrosine and serine phosphorylation of IRS-1 in the absence or presence of TNFα. Experimental conditions and procedures were identical to those described for IRS-2. D, shown are the effects of IFNα on tyrosine phosphorylation of Stat-1 (pY-Stat-1) in HepG2 cells in the absence or presence of TNFα. U, units. E and F, shown are the effects of IFNα on 2′5′OAS (E) and Mx (F) gene expression in the presence or absence of TNFα or pervanadate (PV). ***, p < 0.001; NS, not significant.
As insulin-induced activation of Akt is mediated by tyrosine phosphorylation of IRS-1 and IRS-2, we also analyzed the effect of 20 ng/ml TNFα for 18 h on insulin-induced tyrosine phosphorylation of these insulin substrates. Fig. 2B shows that 100 nm insulin induced tyrosine phosphorylation of IRS-2 that was particularly marked at 15 min. As expected, insulin did not have any effect on serine phosphorylation of IRS-2 at the same times. Treatment of cells with TNFα had no effects on tyrosine phosphorylation of IRS-2 but resulted in a striking increase in serine phosphorylation of this substrate. Finally, pretreatment of cells with TNFα phosphorylated IRS-2 at serine and prevented the effect of insulin on tyrosine phosphorylation of IRS-2. Similar results were obtained when phosphorylation at serine and tyrosine of IRS-1 was studied after treatment of cells with insulin, TNFα, or both (Fig. 2C).
In these TNFα-treated cells resistant to insulin, we studied the effects of IFNα on tyrosine phosphorylation of Stat-1 and gene expression of 2′5′OAS and Mx. For this purpose, HepG2 cells were cultured in the absence or presence of 20 ng/ml TNFα for 18 h followed by the exposition to 250 IU/ml IFNα for 3–90 min. As Fig. 2D shows, IFNα markedly stimulated tyrosine phosphorylation of Stat-1, particularly at 60 min. By contrast, this effect of IFNα was totally absent in cells pretreated with TNFα. Likewise, pretreatment of cells with TNFα abrogated the IFNα-induced gene expression of 2′5′OAS and Mx (Fig. 2, E and F).
In cells pretreated with TNFα, the addition of pervanadate increased markedly basal tyrosine phosphorylation of Stat-1 (Fig. 3A) and recovered the response of cells to IFNα (Figs. 2, E and F, and 3A), suggesting that PTPs were involved in the TNFα-induced resistance to IFNα. Similarly, pervanadate recovered response to insulin in cells previously treated with TNFα (Fig. 3B).
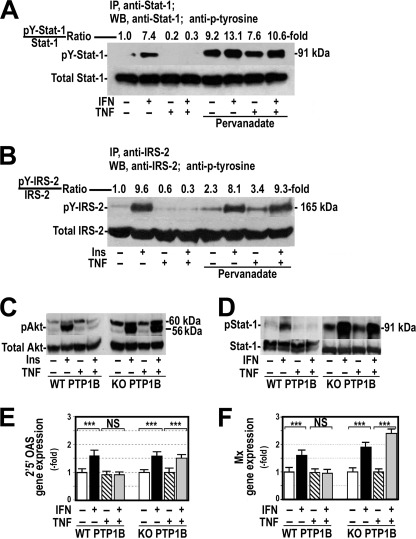
FIGURE 3.
Inhibition or absence of PTP1B restored the response to IFNα in cells treated with TNFα. A, HepG2 cells were exposed to 250 units/ml IFNα for 60 min, 20 ng/ml TNFα for 18 h, or both in the absence or presence of 0.1 mm pervanadate added to the cells 60 min before treatment with IFNα. Cellular proteins were immunoprecipitated (IP) as indicated in Fig. 1C, and tyrosine-phosphorylated Stat-1 was analyzed (pY-Stat1) by Western blot (WB). B, HepG2 cells were exposed to 100 nm insulin (Ins) for 15 min, 20 ng/ml TNFα for 18 h, or both in the absence or presence of 0.1 mm pervanadate added to the cells before insulin. Cellular proteins were immunoprecipitated with anti-IRS-2 antibody analyzed by Western blot using specific antibody against IRS-2 and antiphosphotyrosine. pY-IRS-2, IRS-2 tyrosine-phosphorylated. C, neonatal PTP-1B-deficient hepatocytes (KO PTP1B) and their counterpart wild-type cells were cultured (WT PTP1B) in the presence or absence of 20 ng/ml TNFα for 18 h and treated with 100 nm insulin for 15 min. Total and serine phosphorylated Akt was analyzed by Western blot. D, total and tyrosine-phosphorylated Stat-1 were measured in neonatal wild-type and PTP-1B-deficient hepatocytes cultured in the conditions described in panel C and treated with 250 units/ml IFNα instead of insulin. E and F, neonatal PTP-1B-deficient hepatocytes were treated as indicated in panel D. 2′5′ OAS (E) and Mx (F) gene expression were measured by quantitative RT-PCR using endogenous GAPDH as reference. ***, p < 0.001; NS, not significant.
To analyze whether PTP-1B was implicated in the IFNα resistance associated with the insulin resistance, we induced insulin resistance by incubating PTP-1B-deficient hepatocytes and their counterpart wild-type cells with 20 nm TNFα for 18 h and measured the response of these cells to 100 nm insulin or to 250 units/ml IFNα. As Fig. 3, C and D, show, response to both insulin and IFNα was absent in wild-type hepatocytes exposed to TNFα but was normal in hepatocytes with PTP-1B knocked down and treated with TNFα. In these PTP-1B-deficient hepatocytes pretreated with TNFα, 2′5′OAS and Mx gene expression increased significantly after treatment with IFNα (Fig. 3, E and F).
Silencing IRS-2 and Treatment of Cells with TNFα Increased PTP Activity
Because these results suggested that PTPs might be involved in the IFNα resistance associated with insulin resistance, we also investigated the impact of silencing IRS-2 on the PTP activity. Fig. 4A illustrates that PTP activity was markedly increased in cells with silenced IRS-2. IFNα treatment did not affect PTP activity in control cells and in insulin-resistant cells. Likewise, our study confirmed that pervanadate, at the doses used in these experiments, reduced PTP activity under the control levels and prevented its increase after silencing IRS-2.
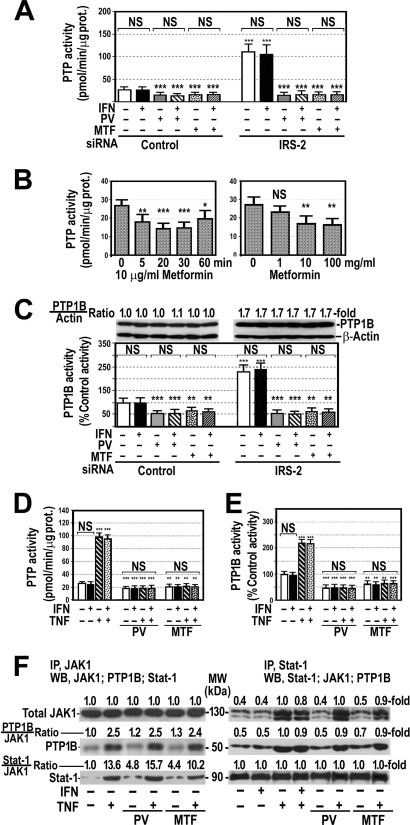
FIGURE 4.
Silencing IRS-2 and treatment of cells with TNFα increase PTP activity in HepG2 cells. A, PTP activity was measured in HepG2 cells with or without silenced IRS-2 treated with 250 units/ml IFNα for 60 min in the absence or presence of 0.1 mm pervanadate (PV) or 10 μg/ml metformin (MTF) added to the cells 60 or 30 min, respectively, before IFNα. ***, p < 0.001 as compared with not silenced control cells. NS, not significant. B, shown is a time- and dose-dependent effect of metformin on PTP activity. *, p < 0.05; **, p < 0.01; ***, p < 0.001; NS, not significant. C, PTP-1B enzyme activity was measured in HepG2 cells in the same experimental conditions as indicated in panel A. Proteins were extracted, and PTP-1B and β-actin were analyzed by Western blot. **, p < 0.01; ***, p < 0.001 as compared with not silenced control cells. NS, not significant. D, PTP activity was measured in cells treated with 20 ng/ml TNFα for 18 h, 250 units/ml IFNα for 60 min, or both in the absence or presence of 0.1 mm pervanadate or 10 μg/ml metformin. **, p < 0.01; ***, p < 0.001 as compared with cells untreated with pervanadate or metformin. NS, not significant. E, PTP1B activity was measured under the same conditions indicated in panel D. F, HepG2 cells were treated with TNFα in the absence and presence of pervanadate or metformin. After treatment, proteins were immunoprecipitated (IP) using a specific antibody either against JAK1 or Stat-1. Immunoprecipitated proteins were analyzed by Western blot (WB) using specific antibodies against JAK1, Stat-1, or PTP1B. Blots are representative of three separate experiments.
Because it is well established that metformin increases insulin sensitivity (25) and it has been reported that this drug improves response to IFNα therapy in some chronic hepatitis C patients (26), we wanted to know whether metformin had any effect on the PTP activity in cells with silenced IRS-2. As Fig. 4A shows, 10 μg/ml metformin, like pervanadate, decreased significantly PTP activity in these cells and avoided the increase caused by silencing IRS-2. This effect was time- and dose-dependent (Fig. 4B).
As PTP-1B has been involved in the inhibition of insulin signaling (9), we wanted to know whether silencing IRS-2 modified the activity of this particular PTP. Fig. 4C shows that PTP-1B activity was significantly enhanced in cells with silenced IRS-2 and that 0.1 mm pervanadate or 10 μg/ml metformin prevented this increase. Treatment of cells with IFNα did not modify PTP-1B activity either in control or insulin-resistant cells. This effect of silencing IRS-2 on PTP-1B activity was associated with a marked increase in PTP-1B protein expression, but this increase was not abrogated by treating cells with pervanadate or metformin (Fig. 4C).
As previous data suggested that PTPs are also implicated in the TNFα-induced inhibition of IFNα signaling, we measured the effects of TNFα and IFNα on PTP activity. As shown in Fig. 4D, IFNα did not modify PTP activity in HepG2 cells, but TNFα increased this activity 4-fold. Pretreatment of cells with pervanadate or metformin at the dose used in previous experiments reduced PTP activity under control levels. Effects of TNFα, IFNα, pervanadate, and metformin on PTP-1B activity were similar to those observed on PTP activity (Fig. 4E).
TNFα Induced Physical Interaction of PTP-1B with JAK1 and Stat-1
As dephosphorylation of JAK1 and Stat-1 by PTP-1B requires the association of this phosphatase to its substrates, we wanted to examine whether TNFα promoted a physical interaction of PTP-1B with JAK1 and Stat-1. Therefore, we immunoprecipitated JAK1 and analyzed the presence of PTP-1B and Stat-1 in the immunoprecipitates. Fig. 4F shows that JAK1 coimmunoprecipitated with PTP-1B and Stat-1 in control cells and that TNFα enhanced the physical interaction between these proteins. Likewise, immunoprecipitation of Stat-1 was associated with the coimmunoprecipitation of PTP-1B and JAK1 (Fig. 4F). Treatment of cells with pervanadate or metformin did not prevent the formation of this JAK1-PTP-1B-Stat-1 complex (Fig. 4F).
Metformin Restored Sensitivity to IFNα and Insulin in Insulin-resistant Hepatocytes
Because metformin inhibits PTP-1B activity, we investigated whether metformin was able to improve IFNα response in cells with silenced IRS-2 or treated with TNFα. Fig. 5 shows that the addition of 10 μg/ml metformin to the cells with silenced IRS-2 restored the response to IFNα as shown by the increase in tyrosine phosphorylation of Stat-1 (Fig. 5A) and in 2′5′OAS and Mx gene expression (Fig. 5B). Similarly, metformin restored the sensitivity to insulin (Fig. 5C). Likewise, metformin annulled the suppressor effects of TNFα on IFNα-induced tyrosine phosphorylation of Stat-1 (Fig. 5D) and gene expression of 2′5′OAS and Mx. (Fig. 2E).
FIGURE 5.
Metformin prevents IFNα and insulin resistance induced by silencing IRS-2 or TNFα treatment. A, control cells and cells with silenced IRS-2 protein expression were treated with 250 units/ml IFNα for 30 min in the absence or presence of 10 μg/ml metformin (MTF). After treatment, cellular proteins were analyzed as described in Fig. 1C. The blot is representative of one experiment that was repeated three times. Control, untreated cells. IP, immunoprecipitation; WB, Western blot. B, control cells and cells with silenced IRS-2 were treated with 250 units/ml IFNα in the presence or absence of 10 μg/ml metformin. 2′,5′-Oligoadenylate synthase-1a and Mx gene expression was measured by quantitative RT-PCR using endogenous GAPDH as reference. These blots are representative of experiments that were repeated three times. C, cells with silenced IRS-2 were treated with 100 nm insulin for 15 min in the absence or presence of 10 μg/ml metformin. After treatment, cellular proteins were analyzed as described in Fig. 1B. Shown is a representative blot of an experiment that was repeated twice. D, HepG2 cells were exposed to 250 units/ml IFNα for 60 min, 20 ng/ml TNFα for 18 h, or both in the absence or presence of 10 μg/ml metformin added to the cells 30 min before the addition of IFNα. After treatment, cellular proteins were extracted and immunoprecipitated as indicated in Fig. 1C. Blots are representative of one experiment that was repeated three times with similar results. pY-Stat-1, Stat-1-phosphorylated at tyrosine. E, shown are the effects of IFNα on 2′,5′ oligoadenyl synthase and Mx gene expression in the presence or absence of TNFα or metformin. ***, p < 0.001; NS, not significant.
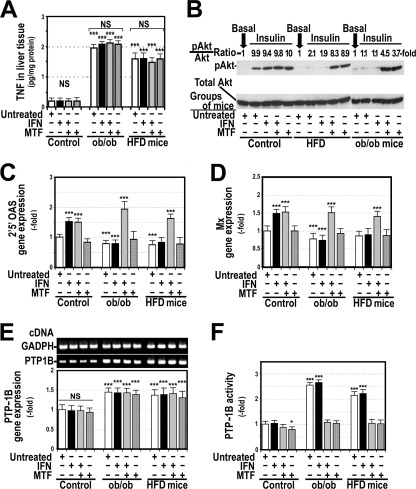
Insulin-resistant Obese Mice Did Not Respond to IFNα and Had Increased PTP-1B Activity and Gene Expression
Because obesity is associated with IFNα resistance (27) and TNFα levels are elevated in the liver of ob/ob mice (28) and in mice fed with a high fat diet (Fig. 6), we studied the response to 2 μg/kg/week pegylated IFNα2a in wild-type mice and in two different groups of obese mice, genetic ob/ob mice and mice fed with a high fat diet. As shown in Fig. 6A, hepatic levels of TNFα were significantly increased in both groups of obese mice, and administration of IFNα or 24 mg/kg/day metformin intraperitoneal for 12 weeks did not modify these levels. In addition, we confirmed that obese mice, particularly ob/ob mice, were insulin-resistant, as indicated by the absence of additional Akt phosphorylation in the liver 15 min after intraperitoneal administration of 0.5 units/kg insulin (Fig. 6B). Fig. 6, C and D, show that, compared with control mice, both obese groups of mice had decreased levels of 2′5′OAS and Mx gene expression and that this expression did not increase in obese mice receiving IFNα. PTP-1B gene expression was significantly elevated in both groups of untreated obese mice and in those treated with IFNα, metformin, or both (Fig. 6E). PTP-1B activity was also significantly increased in obese mice, but treatment with metformin decreased these levels to those in wild-type mice (Fig. 6F) and restored the response to IFNα (Fig. 6, C and D).
FIGURE 6.
Obese mice are resistant to insulin and IFNα. Treatment of ob/ob mice with metformin (MTF) decreased PTP-1B activity and restored insulin and IFNα sensitivity. C57BL/6J, ob/ob mice, and mice fed with a high fat diet (HFD) were treated with IFNα2a, metformin, or both as indicated under “Experimental Procedures.” A, shown are concentrations of TNFα in liver tissue of the 12 groups of mice. B, shown is total Akt and serine phosphorylated Akt (pAkt) in the liver before (basal) and 15 min after injecting 0.5 units/Kg insulin intraperitoneally. C, shown is 2′,5′OAS gene expression. D, shown is Mx gene expression. E, shown is PTP-1B gene expression. F, shown is PTP-1B activity. *, p<0.05; ***, p < 0.001 as compared with control mice. NS, not significant.
DISCUSSION
Our study shows that hepatic insulin resistance induced by silencing IRS-2 mRNA (Fig. 1B) resulted in resistance to IFNα, as shown by the absence of tyrosine phosphorylation of Stat-1 (Fig. 1C) and the lack of induction of 2′,5′OAS and Mx gene expression (Fig. 1, D and E). This absence of Stat-1 phosphorylation and activation can be ascribed either to a reduced phosphorylation by a diminished tyrosine kinase activity or to an increased tyrosine dephosphorylation by an enhanced activity of PTPs. These phosphatases provoke tyrosine dephosphorylation of insulin receptors and substrates and consequently are able to interrupt their signal resulting in insulin resistance (6, 29). Thus, whereas increased PTP-1B activity is associated with an insulin-resistant state (8), PTP-1B deficiency is linked to insulin hypersensitivity (7, 9).
The present study offers data indicating that these PTPs are also involved in the loss of IFNα response associated with insulin resistance. Thus, inhibition of PTPs with pervanadate, a commonly used general PTP inhibitor, resulted in the recovery of response to IFNα in cells with silenced IRS-2, resistant to insulin (Fig. 1).
Chronic hepatitis C patients, particularly non-responders to IFN/ribavirin therapy, frequently have elevated levels of TNFα (30), a cytokine that can induce insulin resistance (31). Moreover, this cytokine is also elevated in patients with obesity, liver steatosis, and other conditions associated with insulin resistance and poor response to IFN/ribavirin therapy (31). Therefore, we wanted to know whether TNFα induces resistance to insulin and IFNα in HepG2 cells. In the present study, we confirm that TNFα induced insulin resistance in these cells (Fig. 2A) and that this effect was associated with serine phosphorylation of IRS-1 and IRS-2 (Fig. 2, B and C). Our study shows that these insulin-resistant TNFα-treated cells were also resistant to IFNα (Fig. 2, D and F).
The molecular mechanisms by which TNFα inhibits IFNα effects are likely not unique. SOCS proteins have been implicated in the resistance to IFNα therapy in some patients with chronic hepatitis C (13). SOCS proteins, particularly SOCS3 (32), inhibit the interaction of insulin and cytokine receptors, including IFNα receptors, with signaling proteins (4, 32) and consequently are able to suppress insulin and IFNα effects (4, 33). However, recently, Franceschini et al. (34) showed that silencing SCOC3 did not restore IFNα signaling. The role of these proteins in the resistance to IFNα is supported by the fact that patients with chronic hepatitis C non-responder to antiviral therapy have increased expression of SOCS3 (13). Indeed, our data do not exclude that SCOS3 may act as an inhibitor of IFNα signaling in chronic hepatitis C resistant to antiviral therapy. However, our study indicates that an increased PTP activity is also implicated in the TNFα-induced suppression of IFNα and insulin signaling. As already mentioned, PTPs are able to interrupt signal transduction of both insulin and IFNα. The role of these phosphatases in the TNFα-induced suppression of insulin and IFNα signaling is supported by the fact that inhibition of PTP activity by pervanadate (Fig. 2) or knocking down of PTP-1B rescued the response to IFNα and insulin in TNFα-treated cells (Fig. 3).
Our study also demonstrates that insulin resistance provoked by silencing IRS-2 mRNA or by incubating cells with TNFα increased PTP and PTP-1B activity 2–4-fold (Fig. 4). The mechanism of this stimulation can be ascribed to the inhibitory effect of insulin on PTP activity. Indeed, PTP-1B is a substrate for Akt, and serine phosphorylation of PTP-1B by Akt negatively modulates its phosphatase activity (35). In fact, it has been shown that PTP-1B expression is elevated in the liver of IRS-2-deficient mice (36). Moreover, our study shows that TNFα increased the physical interaction of PTP-1B with JAK1 and Stat1 (Fig. 4F). Association of PTP-1B with JAK is needed for JAK being dephosphorylated by PTP-1B. Although some controversy does exist concerning interaction of these molecules (37), some studies have clearly demonstrated that PTP-1B binds JAK1 (38). Even though very little information is available on the effect of TNFα on PTP activity, a number of authors have demonstrated that TNFα induces PTP-1B gene expression in several experimental conditions (39, 40). These enhanced PTP-1B protein expression may favor its association with JAK. Our experiments also show that pervanadate, although inhibited PTP-1B activity, did not avoid coimmunoprecipitation of PTP-1B with JAK1 and Stat-1 (Fig. 5). Persistence of a physical interaction between these factors despite the loss of activity can be ascribed to the fact that pervanadate inhibits PTP-1B activity by oxidizing its catalytic cysteine (41).
Our results open the possibility of increasing the response rate of chronic hepatitis C patients to the combined therapy with IFNα/ribavirin through the use of PTP inhibitors. In this respect, our study demonstrates that administration of metformin, an oral antihyperglycemic agent used in the treatment of type 2 diabetes, to insulin-resistant cells or cells treated with TNFα restored both insulin and IFNα response (Figs. 1, 2, 3, and 5). Exact mechanisms of metformin action are not fully understood. Metformin reduces gluconeogenesis, hepatic glucose output, increases glucose uptake in muscle, insulin binding to its receptor, and activity of the AMP-activated protein kinase (42). Because metformin increases tyrosine phosphorylation of insulin receptor (43), it has been suggested that metformin stimulates its tyrosine kinase activity (44). However, metformin might also increase this phosphorylation by reducing PTP activity. In our study we clearly show that metformin decreased PTP and PTP-1B activity and prevented the increase in PTP activity that followed silencing IRS-2 synthesis or TNFα treatment (Fig. 4).
Because ob/ob mice and mice fed with a high fat diet are insulin-resistant (45) (Fig. 6), we used these two animal models to study the response to IFNα and the effects of metformin. We found that obese mice have increased hepatic levels of PTP-1B activity and that it was associated with an absence of response of 2′5′OAS and Mx gene expression to IFNα administration. Our study also shows that treatment of obese mice with metformin decreased PTP-1B activity and restored the response of 2′5′OAS and Mx gene expression to IFNα (Fig. 6). Overexpression of PTP-1B has been found in mice with diet-induced obesity and in ob/ob mice (45). In this study we also show that this inhibitory effect of metformin on PTP-1B is exerted, like pervanadate, without avoiding physical interactions of this phosphatase with its substrates (Fig. 4). These results are consistent with those reported by Holland et al. (46) showing that metformin inhibits PTPs. Therefore, metformin may favor IFNα signaling by attenuating PTP activity. These results allow us to speculate that metformin, like other PTP inhibitors, might increase the efficacy of IFNα/ribavirin therapy in patients with chronic hepatitis C and insulin resistance. Although little information is available on this subject, a pilot study showed that metformin improved response to this therapy, particularly in women (26). The use of metformin in these patients with mild liver dysfunction has been considered to be safe (47). Metformin-associated lactic acidosis is mostly reported in patients with liver cirrhosis, many of them actively using alcohol (48). Nevertheless, more studies are mandatory to establish the real value of metformin as adjuvant treatment in chronic hepatitis C and its safety.
Limitations of this study arise from the fact that it has been performed in cellular and animal models of insulin resistance, and although all experiments indicate that resistance to IFNα is closely associated with an increased activity and gene expression of PTP-1B, these results do not allow assurance that this is the cause of the poor response to IFNα/ribavirin therapy in patients with chronic hepatitis C and insulin resistance. Other factors, in addition to PTPs, may also interfere with IFNα signaling, some of them acting downstream of Stat1 activation. This is the case of PP2A and PIAS1 (14). It is well documented that hepatitis C virus proteins induce PP2A expression (15), which in turn inhibits protein arginine methyl transferase 1 and, consequently, results in hypomethylation of Stat1. Hypomethylated Stat1 binds to PIAS1, which prevents Stat1 from binding target gene promoters and IFNα from exerting its antiviral effects. Interestingly, it has been shown that PP2A is inactivated by tyrosine phosphorylation and that PTP1B can dephosphorylate PP2A (49). Therefore, we can hypothesize that PTP1B inhibition might also inactivate PP2A and thereby block the anti-IFNα effects of hepatitis C virus. Therefore, it is mandatory to demonstrate that insulin-resistant patients with chronic hepatitis C nonresponders to IFNα/ribavirin therapy have increased PTP-1B activity and gene expression in the liver and that inhibition of this phosphatase improves the response to antiviral therapy. Current research is actually being focused in this direction. As shown above, metformin inhibits PTP activity and improves antiviral response in these patients; nevertheless, other PTP inhibitors might also be considered. PTP-1B is a promising therapeutic target in the management of type 2 diabetes, and many research groups are interested in the development of potent and specific inhibitors for this enzyme that might benefit not only diabetes patients but also patients with chronic hepatitis C, insulin resistance, and poor response to IFNα therapy.
In conclusion, our study shows that insulin resistance induced by silencing IRS-2 gene expression or TNFα treatment provokes IFNα resistance and that this effect is associated with an enhanced PTP-1B activity. The role of PTP-1B in the IFNα hyposensitivity associated with insulin resistance is supported by the fact that inhibition of PTP-1B activity with pervanadate, metformin, or RNA interference rescues the response to insulin and IFNα. Thus, use of PTP inhibitors such as metformin may improve the response of chronic hepatitis C patients to combined therapy with IFNα/ribavirin.
Supplementary Material
Acknowledgment
We thank Cristina Rodríguez-Juan (Research Center University Hospital “12 de Octubre,” Madrid, Spain) for technical support.
This study was supported in part by Grants from the “Fundación Mutua Madrileña” and from the “Fondo de Investigación Sanitaria” (PI07/0052; PI10/0312), Spain.

This article contains a supplemental table.
- SOCS
- suppressor of cytokine signaling
- Mx
- myxovirus resistance
- 2′5′OAS
- 2′,5′-oligoadenylate synthase
- p-Akt
- serine-phosphorylated Akt
- PIAS1
- protein inhibitor of activated Stat1
- PP2A
- protein phosphatase 2A
- PTP
- Protein-tyrosine phosphatase.
REFERENCES
- 1. Jacobson I. M., Brown R. S., Jr., Freilich B., Afdhal N., Kwo P. Y., Santoro J., Becker S., Wakil A. E., Pound D., Godofsky E., Strauss R., Bernstein D., Flamm S., Pauly M. P, Mukhopadhyay P., Griffel L. H., Brass C. A., and WIN-R Study Group (2007) Peginterferon alfa-2b and weight-based or flat-dose ribavirin in chronic hepatitis C patients. A randomized trial. Hepatology 46, 971–981 [DOI] [PubMed] [Google Scholar]
- 2. Romero-Gómez M., Del Mar Viloria M., Andrade R. J., Salmerón J., Diago M., Fernández-Rodríguez C. M., Corpas R., Cruz M., Grande L., Vázquez L., Muñoz-De-Rueda P., López-Serrano P., Gila A., Gutiérrez M. L., Pérez C., Ruiz-Extremera A., Suárez E., Castillo J. (2005) Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology 128, 636–641 [DOI] [PubMed] [Google Scholar]
- 3. Taguchi A., White M. F. (2008) Insulin-like signaling, nutrient homeostasis, and life span. Annu. Rev. Physiol. 70, 191–212 [DOI] [PubMed] [Google Scholar]
- 4. Emanuelli B., Peraldi P., Filloux C., Chavey C., Freidinger K., Hilton D. J., Hotamisligil G. S., Van Obberghen E. (2001) SOCS-3 inhibits insulin signaling and is up-regulated in response to tumor necrosis factor-α in the adipose tissue of obese mice. J. Biol. Chem. 276, 47944–47949 [DOI] [PubMed] [Google Scholar]
- 5. Bernsmeier C., Duong F. H., Christen V., Pugnale P., Negro F., Terracciano L., Heim M. H. (2008) Virus-induced over-expression of protein phosphatase 2A inhibits insulin signaling in chronic hepatitis C. J. Hepatol. 49, 429–440 [DOI] [PubMed] [Google Scholar]
- 6. David M., Chen H. E., Goelz S., Larner A. C., Neel B. G. (1995) Differential regulation of the α/β interferon-stimulated Jak/Stat pathway by the SH2 domain-containing tyrosine phosphatase SHPTP1. Mol. Cell. Biol. 15, 7050–7058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gum R. J., Gaede L. L., Koterski S. L., Heindel M., Clampit J. E., Zinker B. A., Trevillyan J. M., Ulrich R. G., Jirousek M. R., Rondinone C. M. (2003) Reduction of protein-tyrosine phosphatase 1B increases insulin-dependent signaling in ob/ob mice. Diabetes 52, 21–28 [DOI] [PubMed] [Google Scholar]
- 8. Ahmad F., Goldstein B. J. (1995) Increased abundance of specific skeletal muscle protein-tyrosine phosphatases in a genetic model of insulin-resistant obesity and diabetes mellitus. Metabolism 44, 1175–1184 [DOI] [PubMed] [Google Scholar]
- 9. Elchebly M., Payette P., Michaliszyn E., Cromlish W., Collins S., Loy A. L., Normandin D., Cheng A., Himms-Hagen J., Chan C. C., Ramachandran C., Gresser M. J., Tremblay M. L., Kennedy B. P. (1999) Increased insulin sensitivity and obesity resistance in mice lacking the protein-tyrosine phosphatase-1B gene. Science 283, 1544–1548 [DOI] [PubMed] [Google Scholar]
- 10. O'Shea J. J., Gadina M., Schreiber R. D. (2002) Cytokine signaling in 2002. New surprises in the Jak/Stat pathway. Cell 109, S121–S131 [DOI] [PubMed] [Google Scholar]
- 11. Meyer T., Vinkemeier U. (2004) Nucleocytoplasmic shuttling of Stat transcription factors. Eur. J. Biochem. 71, 4606–4612 [DOI] [PubMed] [Google Scholar]
- 12. Hong F., Nguyen V. A., Gao B. (2001) Tumor necrosis factor α attenuates interferon α signaling in the liver. Involvement of SOCS3 and SHP2 and implication in resistance to interferon therapy. FASEB J. 15, 1595–1597 [DOI] [PubMed] [Google Scholar]
- 13. Walsh M. J., Jonsson J. R., Richardson M. M., Lipka G. M., Purdie D. M., Clouston A. D., Powell E. E. (2006) Non-response to antiviral therapy is associated with obesity and increased hepatic expression of suppressor of cytokine signaling 3 (SOCS-3) in patients with chronic hepatitis C, viral genotype 1. Gut 55, 529–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu B., Mink S., Wong K. A., Stein N., Getman C., Dempsey P. W., Wu H., Shuai K. (2004) PIAS1 selectively inhibits interferon-inducible genes and is important in innate immunity. Nat. Immunol. 5, 891–898 [DOI] [PubMed] [Google Scholar]
- 15. Duong F. H., Christen V., Filipowicz M., Heim M. H. (2006) S-Adenosylmethionine and betaine correct hepatitis C virus-induced inhibition of interferon signaling in vitro. Hepatology 43, 796–806 [DOI] [PubMed] [Google Scholar]
- 16. You M., Yu D. H., Feng G. S. (1999) Shp-2 tyrosine phosphatase functions as a negative regulator of the interferon-stimulated Jak/STAT pathway. Mol. Cell. Biol. 19, 2416–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xu H., An H., Hou J., Han C., Wang P., Yu Y., Cao X. (2008) Phosphatase PTP1B negatively regulates MyD88- and TRIF-dependent proinflammatory cytokine and type I interferon production in TLR-triggered macrophages. Mol. Immunol. 45, 3545–3552 [DOI] [PubMed] [Google Scholar]
- 18. Gonzalez-Rodriguez A., Clampit J.E., Escribano O., Benito M., Rondinone C. M., Valverde A. M. (2007) Developmental switch from prolonged insulin action to increased insulin sensitivity in protein-tyrosine phosphatase 1B-deficient hepatocytes. Endocrinology 148, 594–608 [DOI] [PubMed] [Google Scholar]
- 19. Valverde A. M., Burks D. J., Fabregat I., Fisher T. L., Carretero J., White M. F., Benito M. (2003) Molecular mechanisms of insulin resistance in IRS-2-deficient hepatocytes. Diabetes 52, 2239–2248 [DOI] [PubMed] [Google Scholar]
- 20. Mashimo T., Glaser P., Lucas M., Simon-Chazottes D., Ceccaldi P. E., Montagutelli X., Desprès P., Guénet J. L. (2003) Structural and functional genomics and evolutionary relationships in the cluster of genes encoding murine 2',5'-oligoadenylate synthetases. Genomics 82, 537–552 [DOI] [PubMed] [Google Scholar]
- 21. Zhang Z. Y., Maclean D., Thieme-Sefler A. M., Roeske R. W., Dixon J. E. (1993) A continuous spectrophotometric and fluorimetric assay for protein-tyrosine phosphatase using phosphotyrosine-containing peptides. Anal. Biochem. 211, 7–15 [DOI] [PubMed] [Google Scholar]
- 22. Lang A., Schrum L. W., Schoonhoven R., Tuvia S., Solís-Herruzo J. A., Tsukamoto H., Brenner D. A., Rippe R. A. (2000) Expression of small heat shock protein αB-crystallin is induced after hepatic stellate cell activation. Am. J. Physiol. Gastrointest. Liver Physiol.. 279, G1333–G1342 [DOI] [PubMed] [Google Scholar]
- 23. Solís-Herruzo J. A., Rippe R. A., Schrum L. W., de La Torre P., García I., Jeffrey J. J., Muñoz-Yagüe T., Brenner D. A. (1999) Interleukin-6 increases rat metalloproteinase-13 gene expression through stimulation of activator protein 1 transcription factor in cultured fibroblasts. J. Biol. Chem. 274, 30919–30926 [DOI] [PubMed] [Google Scholar]
- 24. Díaz-Sanjuán T., García-Ruiz I., Rodríguez-Juan C., Muñoz-Yagüe T., Solís-Muñoz P., Solís-Herruzo J. A. (2009) Interferon α increases metalloproteinase-13 gene expression through a polyomavirus enhancer activator 3-dependent pathway in hepatic stellate cells. J. Hepatol. 50, 128–139 [DOI] [PubMed] [Google Scholar]
- 25. Gin H., Messerchmitt C., Brottier E., Aubertin J. (1985) Metformin improved insulin resistance in type I, insulin-dependent, diabetic patients. Metabolism 34, 923–925 [DOI] [PubMed] [Google Scholar]
- 26. Romero-Gómez M., Diago M., Andrade R. J., Calleja J. L., Salmerón J., Fernández-Rodríguez C. M., Solà R., García-Samaniego J., Herrerías J. M., De la Mata M., Moreno-Otero R., Nuñez O., Olveira A., Durán S., Planas R. (2009) Treatment of insulin resistance with metformin in naïve genotype 1 chronic hepatitis C patients receiving peginterferon alfa-2a plus ribavirin. Hepatology 50, 1702–1708 [DOI] [PubMed] [Google Scholar]
- 27. Bressler B. L., Guindi M., Tomlinson G., Heathcote J. (2003) High body mass index is an independent risk factor for nonresponse to antiviral treatment in chronic hepatitis C. Hepatology 38, 639–644 [DOI] [PubMed] [Google Scholar]
- 28. García-Ruiz I., Rodríguez-Juan C., Díaz-Sanjuan T., del Hoyo P., Colina F., Muñoz-Yagüe T., Solís-Herruzo J. A. (2006) Uric acid and anti-TNF antibody improve mitochondrial dysfunction in ob/ob mice. Hepatology 44, 581–591 [DOI] [PubMed] [Google Scholar]
- 29. Kenner K. A., Anyanwu E., Olefsky J. M., Kusari J. (1996) Protein-tyrosine phosphatase 1B is a negative regulator of insulin- and insulin-like growth factor-I-stimulated signaling. J. Biol. Chem. 271, 19810–19816 [DOI] [PubMed] [Google Scholar]
- 30. Larrea E., Garcia N., Qian C., Civeira M. P., Prieto J. (1996) Tumor necrosis factor α gene expression and the response to interferon in chronic hepatitis C. Hepatology 23, 210–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peraldi P., Spiegelman B. (1998) TNF-α and insulin resistance. Summary and future prospects. Mol. Cell Biochem. 182, 169–175 [PubMed] [Google Scholar]
- 32. Song M. M., Shuai K. (1998) The suppressor of cytokine signaling (SOCS) 1 and SOCS3 but not SOCS2 proteins inhibit interferon-mediated antiviral and antiproliferative activities. J. Biol. Chem. 273, 35056–35062 [DOI] [PubMed] [Google Scholar]
- 33. Sakai I., Takeuchi K., Yamauchi H., Narumi H., Fujita S. (2002) Constitutive expression of SOCS3 confers resistance to IFN-α in chronic myelogenous leukemia cells. Blood 100, 2926–2931 [DOI] [PubMed] [Google Scholar]
- 34. Franceschini L., Realdon S., Marcolongo M., Mirandola S., Bortoletto G., Alberti A. (2011) Reciprocal interference between insulin and interferon-α signaling in hepatic cells. A vicious circle of clinical significance? Hepatology 54, 484–494 [DOI] [PubMed] [Google Scholar]
- 35. Ravichandran L. V., Chen H., Li Y., Quon M. J. (2001) Phosphorylation of PTP1B at Ser-50 by Akt impairs its ability to dephosphorylate the insulin receptor. Mol. Endocrinol. 15, 1768–1780 [DOI] [PubMed] [Google Scholar]
- 36. González-Rodríguez A., Mas Gutierrez J. A., Sanz-González S., Ros M., Burks D. J., Valverde A. M. (2010) Inhibition of PTP1B restores IRS1-mediated hepatic insulin signaling in IRS2-deficient mice. Diabetes 59, 588–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Myers M. P., Andersen J. N., Cheng A., Tremblay M. L., Horvath C. M., Parisien J. P., Salmeen A., Barford D., Tonks N. K. (2001) TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. J. Biol. Chem. 276, 47771–47774 [DOI] [PubMed] [Google Scholar]
- 38. Lu X., Malumbres R., Shields B., Jiang X., Sarosiek K. A., Natkunam Y., Tiganis T., Lossos I. S. (2008) PTP1B is a negative regulator of interleukin 4-induced STAT6 signaling. Blood 112, 4098–4108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nieto-Vazquez I., Fernández-Veledo S., de Alvaro C., Rondinone C. M., Valverde A. M., Lorenzo M. (2007) Protein-tyrosine phosphatase 1B-deficient myocytes show increased insulin sensitivity and protection against tumor necrosis factor-α-induced insulin resistance. Diabetes 56, 404–413 [DOI] [PubMed] [Google Scholar]
- 40. Zabolotny J. M., Kim Y. B., Welsh L. A., Kershaw E. E., Neel B. G., Kahn B. B. (2008) Protein-tyrosine phosphatase 1B expression is induced by inflammation in vivo. J. Biol. Chem. 283, 14230–14241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huyer G., Liu S., Kelly J., Moffat J., Payette P., Kennedy B., Tsaprailis G., Gresser M. J., Ramachandran C. (1997) Mechanism of inhibition of protein-tyrosine phosphatases by vanadate and pervanadate. J. Biol. Chem. 272, 843–851 [DOI] [PubMed] [Google Scholar]
- 42. Andújar-Plata P., Pi-Sunyer X., Laferrère B. (2012) Metformin effects revisited. Diabetes Res. Clin. Pract. 95, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gunton J. E., Delhanty P. J., Takahashi S., Baxter R. C. (2003) Metformin rapidly increases insulin receptor activation in human liver and signals preferentially through insulin receptor substrate 2. J. Clin. Endocrinol. Metab 88, 1323–1332 [DOI] [PubMed] [Google Scholar]
- 44. Stith B. J., Goalstone M. L., Espinoza R., Mossel C., Roberts D., Wiernsperger N. (1996) The antidiabetic drug metformin elevates receptor-tyrosine kinase activity and inositol 1,4,5-trisphosphate mass in Xenopus oocytes. Endocrinology 137, 2990–2999 [DOI] [PubMed] [Google Scholar]
- 45. Chua S. C., Jr., Chung W. K., Wu-Peng X. S., Zhang Y., Liu S. M., Tartaglia L., Leibel R. L. (1996) Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science 271, 994–996 [DOI] [PubMed] [Google Scholar]
- 46. Holland W., Morrison T., Chang Y., Wiernsperger N., Stith B. J. (2004) Metformin (glucophage) inhibits tyrosine phosphatase activity to stimulate the insulin receptor-tyrosine kinase. Biochem. Pharmacol. 67, 2081–2091 [DOI] [PubMed] [Google Scholar]
- 47. Emslie-Smith A. M., Boyle D. I., Evans J. M., Sullivan F., Morris A. D., and DARTS/MEMO Collaboration (2001) Contraindications to metformin therapy in patients with type 2 diabetes. A population-based study of adherence to prescribing guidelines. Diabet. Med. 18, 483–488 [DOI] [PubMed] [Google Scholar]
- 48. Brackett C. C. (2010) Clarifying metformin's role and risks in liver dysfunction. J. Am. Pharm. Assoc. 50, 407–410 [DOI] [PubMed] [Google Scholar]
- 49. Chen J., Parsons S., Brautigan D. L. (1994) Tyrosine phosphorylation of protein phosphatase 2A in response to growth stimulation and v-src transformation of fibroblasts. J. Biol. Chem. 269, 7957–7962 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.