Abstract
Purpose
Intimal hyperplasia (IH), a well-recognized cause of dialysis vascular access failure, is generally believed to be an acquired pathologic lesion. Recent data suggests that IH is present prior to AVF creation. We sought to determine whether pre-existing inflammation and oxidation co-exist with IH prior to their incorporation into an AVF conduit, as their presence may predispose the AVF to further IH following AVF creation.
Methods
At the time of first AV access surgery, vein segments were collected from ten Stage 4 and 5 CKD patients undergoing AVF creation 6-12 months prior to anticipated dialysis initiation. Morphometry and immunohistochemistry was performed to detect inflammatory markers IL-6, TGF-β1, and TNFa, and markers of DNA oxidative damage (8-Hydroxy-2′-deoxyguanosine [HNE]) and lipid peroxidation (4-Hydroxy-2-Nonenal [8OHdG]).
Results
The degree of IH severity was variable. IL-6, TGF-β1, and TNFa co-localized with a-smooth muscle actin prominently within the venous intima and media. Although more diffuse, HNE and 8OHdG were intensely expressed in parallel with the inflammatory markers. In spite of these findings, however, neither extant IH nor the intensity of inflammatory or oxidative markers were associated with primary or secondary AVF failure at 12 month follow-up.
Conclusions
Not only does venous IH pre-exist, but inflammation and oxidation markers are present within veins used for the AVF conduit prior to its creation in CKD patients as early as one year before dialysis is commenced. Nevertheless, short and long-term AVF outcomes were not associated with the inflammatory or oxidative burden, suggesting the complexity of AVF dysfunction in humans with CKD.
Keywords: AVF, CKD, Dialysis access, Inflammation, Oxidation
INTRODUCTION
The arteriovenous fistula (AVF) is the first choice for hemodialysis vascular access. While long-term patency rates for AVF are superior to prosthetic materials, up to 60% of AVF fail to mature (1).
Intimal hyperplasia (IH) is a well-recognized cause of dialysis vascular access dysfunction. Previous studies report intimal lesions and stenosis within dysfunctional AV access following surgery (2,3), which are commonly attributed to alterations in blood flow, pressure, and shear stress (4-6), consequences of the AV access configuration and arterial flow through a low resistance vein. While the pathologic changes in AVF conduits have been reported previously, little attention has been directed to the vein condition prior to AVF surgery. Data suggests that IH is present prior to AVF creation. While co-morbid conditions including hypertension could promote IH, antihypertensive medication use is not associated with improved AVF patency (7). Furthermore, cells of the pre-existing IH lesion are quiescent, (8,9) only appearing to become active following vessel injury. This may occur because vascular smooth muscle cells have already accumulated in the venous intima, and become activated by vessel injury, resulting in further IH progression. Alternatively, the presence of inflammation and oxidation could predispose the AVF to further IH once vessel injury from AVF creation occurs, by means of the stimulation of growth factors that promote vascular smooth muscle cell proliferation.
We sought to determine whether pre-existing inflammation and oxidation are coexistent with IH in veins prior to their incorporation into an AVF conduit. We performed a pilot study of upper extremity outflow veins obtained from chronic kidney disease (CKD) patients with stage 4-5 CKD who were 6-12 months away from commencing dialysis and who underwent AVF creation.
MATERIALS AND METHODS
Study population
Subjects were enrolled as part of a study to assess the cardiovascular effects of AVF creation. Adult Stage 4 and 5 CKD patients were identified between May 7, 2008 and July 14, 2010 who: 1) were not anticipated to require commencing hemodialysis for >6 months; 2) had no previous dialysis vascular access, including peripherally inserted and central venous catheters, and 3) had received venous duplex mapping at the Emory Dialysis Access Center of Atlanta, Emory University Hospital, and Emory Midtown Hospital. All patients had been instructed to limit venipuncture to superficial hand and distal forearm veins. Suitability of a patient for AVF creation was based on vein diameter ≥2.5 mm, and inflow artery diameter of ≥ 2 mm (10). A baseline visit occurred prior to AVF creation, at which time a blood sample, echocardiogram, and six-minute walk test were obtained. In addition, demographic data, clinical history, and medication use were obtained via direct patient interview. Subjects underwent AVF creation within two days to three weeks following the baseline visit. Subjects were prospectively assessed at 1, 3, and 9-12 month time-points following AVF creation. Determination of primary and secondary AVF patency was made by direct patient examination and review of surgical and outpatient procedural records. The Institutional Review Board (IRB) of Emory University Medical Center approved the study protocol and informed consent was obtained from each patient prior to study enrolment.
Tissue collection
Remnants of surgically excised circumferential cephalic and basilic veins were collected prospectively from 10 patients who underwent AVF creation at Emory University between May 2008 and July 2010. Remnant vein segments, which are normally discarded, were harvested from the cephalic or basilic vein to be used as the AVF conduit by one of four vascular surgeons participating in our study. Remnant vein segments were not collected on obviously diseased, thickened or otherwise abnormal or unsuitable veins. Vein specimens were placed immediately into formalin without use of forceps. Specimens were transferred to 70% ethanol 24 hours after initial placement in formalin. Venous segments were processed and embedded in paraffin blocks. Hematoxylin and eosin stain (H and E) was used to reveal cellularity and general morphologic characteristics.
Immunohistochemistry for inflammatory and oxidative markers
Paraffin-embedded vein segments were sectioned into 3.0-μm-thick serial sections. After heat-mediated antigen retrieval, slides were immunohistochemically stained on the DAKO Autostainer Plus Immunostainer (DAKO Corp, Carpinteria, CA, USA.) using a labeled streptavidin-biotin method for rabbit polycolonal antibody interleukin 6 (IL-6) (Abcam, Cambridge, Massachusetts, USA) (1:200 dilution), rabbit polyclonal IgG TGF-β1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) (1:25 dilution), and rabbit polyclonal antibody TNF-α (Abcam, Cambridge, Massachusetts, USA) (1:100 dilution) as cell markers for inflammation. Adjacent sections were reacted with mouse monoclonal antibody 8-Hydroxy-2′-deoxyguanosine (8OHdG) (Abcam, Cambridge, Massachusetts, USA) (1:100 dilution), and mouse monoclonal antibody 4-Hydroxy-2-Nonenal antibody (HNE) (Abcam, Cambridge, Massachusetts, USA)(1:10 dilution) as cell markers for DNA oxidative damage and lipid oxidative injury, respectively. The monoclonal mouse anti-human antibody Ki-67 (DAKO Corp, Carpinteria, CA, USA.), was tested on adjacent sections to examine cell cycle activity within the venous neointimal lesion.
After processing, the slides were dehydrated and cleared on the Leica Autostainer XL and coverslipped on the Leica CV5000 Coverslipper (Leica Microsystems, Inc.). We included a negative control (lacking primary antibody) for each immunohistochemical analysis. Intensity values obtained from controls were subtracted from intensity values from immunohistochemical analyses for each vein tissue section and each primary antibody. Values reported are the corrected relative antigen expression intensity/μm2. Bright field images were acquired using a Leica DM6000B epifluorescence microscope (Leica Microsystems). The background corrected relative expression/μm2 values from each section were quantified using NIS Elements AR 3.0 software (Nikon Corporation).
Morphometric assessment
Morphometric analysis was performed on Verhoeff-van Gieson (VvG)-stained venous section images to enable optimal differentiation of intima versus media layers by internal and external elastic laminas, and the pathologist was blinded to the subject clinical data.
Bioquant® Image Analysis Software (Nashville, TN) was used for morphometric analyses. Values for luminal area (La, mm2), intimal area (Ia, mm2), medial area (Ma, mm2), percentage luminal stenosis, ratio of intimal to medial area, average intima to medial thickness, and maximal intima to medial thickness were obtained from a tissue block of each venous specimen from each individual patient.
Luminal area (La) was measured by tracing around the edge of the luminal space. Intimal area (Ia) was measured by tracing around the internal elastic lamina, then subtracting the La. Medial area (Ma) was measured by tracing around the external elastic lamina, then subtracting the La and Ia. The ratio of intima to medial area was calculated by the formula Ia/Ma. The percentage of luminal stenosis was calculated for each specimen using the formula [1-La/(La+Ia+Ma)] × 100 (2).
The average thickness of intima (It) and medial (Mt) layers was obtained by averaging the multiple measurements on each incremental distance between the tracings used for separating each layers. The average number of data points for each specimen was approximately 500, using the same incremental distance. The ratio of average intima to medial thickness was calculated by the formula It/Mt. The maximal intima thickness (Max It) was determined by sorting out all the data points of It from each specimen, then obtaining the ratio of Max It/Mt.
Statistical analysis
All data are expressed as mean + standard error of the mean. The Wilcoxon rank sum test was used to compare the difference in inflammation and oxidation between the patent and failed AVF groups.
RESULTS
Clinical characteristics
Of the 15 subjects recruited, tissue specimens were obtained at the time of first vascular access surgery from 10 pre-dialysis Stage 4-5 CKD subjects, all of whom received an AVF. Clinical characteristics of the subjects are listed in Table I. Average age was 62.4 years, 60% were men, 40% were white, 50% had diabetes, 100% had hypertension, 90% had hyperlipidemia, and 70% had a history of tobacco use. The average Glomerular Filtration Rate (GFR) was 14 ± 3.1 mL/min (range 10 to 20), mean systolic blood pressure (SBP) was 143 ± 22.2 mmHg, and mean diastolic blood pressure (DBP) was 66 ± 25 mmHg. Average Body Mass Index (BMI) was 31.4 ± 10.4 and mean albumin/creatinine ratio was 882 ± 1048 mg/g.
TABLE I.
CLINICAL CHARACTERISTICS OF STUDY SUBJECTS
| Characteristics | A | B | C | D | E | F | G | H | I | J |
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | M | F | M | M | M | M | F | F | M | F |
| Age | 57 | 76 | 68 | 65 | 67 | 71 | 55 | 48 | 57 | 60 |
| Race | black | white | white | black | white | black | black | black | black | white |
| SBP | 156 | 115 | 130 | 162 | 136 | 187 | 128 | 146 | 147 | 125 |
| DBP | 82 | 55 | 63 | 70 | 73 | 104 | 81 | 65 | 72 | 61 |
| GFR | 12 | 11 | 14 | 20 | 12 | 14 | 15 | 17 | 10 | 11 |
| BMI | 25.3 | 20.7 | 26.5 | 31.7 | 27.5 | 20.1 | 45.1 | 49.1 | 25.5 | 42.3 |
| Diabetes | yes | no | no | yes | no | no | yes | yes | no | yes |
| Hypertension | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes |
| Tobacco Use** | no | yes | yes | yes | yes | yes | yes | no | no | yes |
| Hyperlipidemia | yes | yes | yes | yes | yes | yes | yes | yes | yes | no |
| Alb/creat mg/g | - | 33.3 | 413 | 831 | 17.1 | 227 | 830 | 312.5 | 2337 | 2938 |
current or former
BMI, body mass index; DBP, diastolic blood pressure; F, female; GFR, glomerular filtration rate; M, male; SBP, systolic blood pressure
Vascular access morphometry
Of the vein segments examined, the degree of IH was variable, and at times severe (Fig. 1). As is standard, the average intima-to-media ratio (IMR) was examined as the quantitative variable for severity of IH. As Table II demonstrates, IMR did not consistently reflect IH lesion severity. For example, patient B has a moderate IMR (IMR=0.34) that belies severe neointimal thickening (average intima thickness=109 μm), while patient F has a moderate IMR (0.35), yet has moderate neointimal thickening (average intima thickness=62 μm). Intimal hyperplasia was characterized by proliferation of vascular smooth muscle cells and fibroblast deposition within both the intimal and medial layers, as indicated by strong expression of α-smooth muscle actin and vimentin, respectively (data not shown).
Fig. 1.
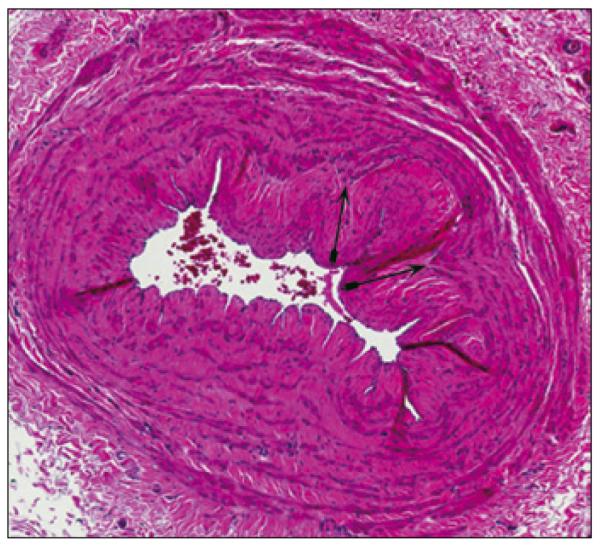
Marked intimal hyperplasia (indicated by double arrows, H and E staining) and severe luminal stenosis in the basilic vein of a pre-dialysis Stage 4 CKD (GFR=20 mL/min) patient prior to AVF creation.
TABLE II.
VENOUS SEGMENT MORPHOMETRY BY STUDY SUBJECT
| Morphometry Variables | A | B | C | D | E | F | G | H | I | J |
|---|---|---|---|---|---|---|---|---|---|---|
| Avg Intimal thickness (It) μm | 154.6 | 109.4 | 107.8 | 151 | 7.3 | 62.2 | 25.5 | 1.4 | 28.9 | 11 |
| Avg Media thickness (Mt) μm | 234.6 | 323.9 | 227.5 | 338.3 | 327.8 | 178.2 | 254.2 | 154.4 | 178.3 | 310 |
| Avg It/Mt Ratio | 0.66 | 0.34 | 0.47 | 0.45 | 0.02 | 0.35 | 0.1 | 0.01 | 0.16 | 0.04 |
| Intima Max (μm) | 276.3 | 206.9 | 215.6 | 368.7 | 22.8 | 335.1 | 85.8 | 6.2 | 113.3 | 33.2 |
| Max It /Mt | 1.18 | 0.64 | 0.95 | 1.09 | 0.07 | 1.88 | 0.34 | 0.04 | 0.64 | 0.11 |
| Intimal Area (Ia) (mm2) | 0.66 | 0.57 | 0.28 | 0.28 | 0.06 | 0.56 | 0.09 | 0.003 | 0.16 | 0.02 |
| Medial Area (Ma) (mm2) | 1.38 | 1.88 | 0.73 | 0.99 | 1.38 | 0.94 | 0.88 | 0.35 | 0.88 | 0.94 |
| Ia/ Ma | 0.48 | 0.3 | 0.38 | 0.29 | 0.04 | 0.6 | 0.1 | 0.01 | 0.19 | 0.02 |
Local inflammation and oxidation
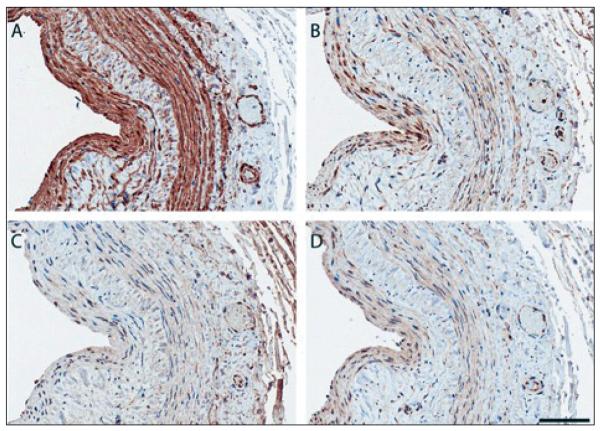
Makers of inflammation (Fig. 2) consistently co-localized with prominently expressed a-smooth muscle actin (panel A), reflecting vascular smooth muscle cells within the vascular neointima and media. TNF-α was the most intensely expressed inflammatory marker (panel B), followed by TGF-β (panel C), and IL-6 expression (panel D). TGF-β was more diffuse, with expression within the adventitia. All three inflammatory markers were present in the adventitia microvessels.
Fig. 2.
Representative photomicrographs of inflammatory biomarker immunoreactivity in vein segment of CKD patient obtained at the time of AVF creation. (A) a-smooth muscle actin (B) TNF-a (C) TGF-b and (D) IL-6. Note that neointima and media show abundant staining (dark brown) of a-SMA, reflecting VSMC’s and that a similar pattern of staining is present for inflammatory markers.
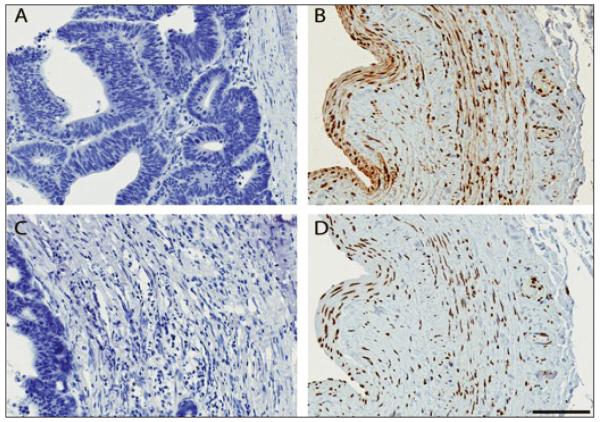
The distribution of oxidative markers were somewhat more diffuse than those of inflammation, but were expressed most prominently in parallel with the inflammatory markers. HNE and 8OHdG indices of colon tissue served as the negative controls (Fig. 3, panels A, C). Within the same venous specimen as used for the inflammatory markers, HNE (panel B) and 8OHdG (panel D) staining were observed within the neointima and thickened media of serial venous sections. Ki-67 expression was not observed in venous sections.
Fig. 3.
Local lipid peroxidation (HNE) and DNA oxidation (8-OHdG) are present in vein of CKD patient prior to vascular injury. (A, C). Colon control tissue with no evidence of HNE or 8-OHdG. (B) Localization of HNE (brown) in venous tissue. Note pattern of increased neointimal and media staining, with staining of the adventitia and neovasculature (magnification, x20). (D) Expression of 8-OHdG in neointima and media, with somewhat more diffuse pattern of staining.
Vascular access outcomes, intimal hyperplasia, and local inflammation
Over the 12-month follow-up, six of the 10 patients commenced hemodialysis using their original AVF, while the AVF was abandoned in four patients. The average I/M ratio at the time of AVF creation was similar (P=.38) among patients experiencing loss of primary AVF patency who required PTA (n=5, I/M ratio 0.33 ± 0.24) or thrombectomy (n=2, I/M ratio 0.06 ± 0.06) compared to patients who did not lose primary patency (n=3, I/M ratio 0.28 ± 0.23). There was also no statistically significant difference in average I/M ratio at AVF creation between patients with secondary AVF failure (n=4) versus those without secondary failure (n=6) (I/M ratio 0.16 ± 0.14 vs. 0.33 ± 0.25) (P=.25). Furthermore, average intimal or media thickness, or maximal intimal area within the collected veins did not significantly differ between patients with and without AVF failure.
The intensity of individual inflammatory and oxidative markers as well as their collective intensity was then compared between patients with and without AVF failure (Fig. 4). Neither 1) the level of individual inflammatory markers nor 2) the collective inflammatory burden (via rank sum) within the collected veins, at the time of AVF creation, was associated with primary or secondary AVF failure. Of note, the youngest patient (patient H), with obesity and no pre-existing intimal thickening experienced AVF thrombosis three months after AVF creation, and commenced dialysis one month later using an AVG.
Fig. 4.
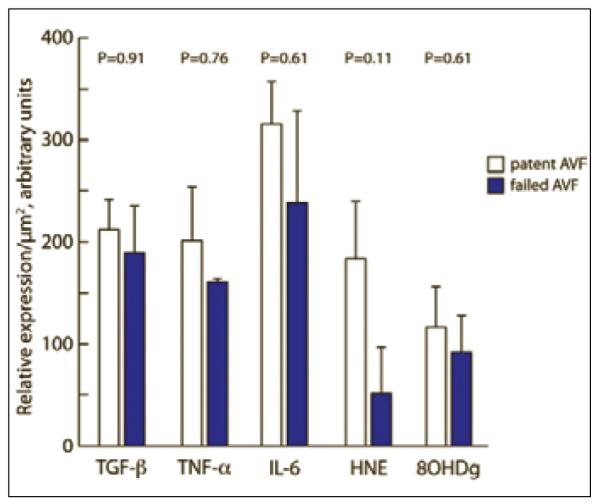
Inflammatory and oxidative mediators (relative expression/μm2) within veins of CKD patients (n=10) at the time of AVF creation. Note the lack of difference in mediator expression in veins used for AVF which remained patent or failed during a 12 month follow-up period.
DISCUSSION
Studies from animal models of AVF formation that generally model either the injury or CKD have suggested an important role for individual IH mediators (e.g. TGF-β). Here, we report that several inflammatory and oxidative markers associated with IH formation are prevalent in veins used for AVF conduits at the time of AVF creation in CKD patients. This finding complements previous reports that veins at the time of AVF failure also have elevated levels of IL-6, total TGF-β, and HNE (11-13). Based on the presence of inflammatory and oxidative mediators at the time of AVF failure (12,13), inflammation and oxidation have been suggested to predispose the AVF to future failure. By contrast, our findings reveal that the pre-existing inflammatory and oxidative burden in veins is not associated with future AVF failure. Moreover, it has previously been found that extant IH exists in veins at the time of AVF creation in CKD patients (9) However, the additional finding that cells within this lesion are quiescent has cast some doubt as to whether extant IH is predictive of future AVF failure. Our findings corroborate this view and collectively suggest that the complexities of AVF dysfunction in humans are more complicated than simple animal models.
Although it has been established that IH and inflammatory mediators are present within veins at the time of AVF failure, our studies were designed to ask whether these mediators were present at the time of AVF creation and whether they were associated with subsequent AVF failure. Although we found increased levels of pre-existing venous inflammation and oxidation as compared to historic controls, surprisingly, there was a trend towards lower levels of mediators in AVF veins that ultimately failed. This trend was not statistically significant, however.
We selected several mediators that can individually cause IH in experimental models (14,15). First, we analyzed the association of each individual mediator on primary and secondary AVF failure. Next, to estimate the inflammatory burden within each vein, we assigned ranks to each individual vein by mediator intensity to determine the cumulative rank. Neither the individual mediator nor the combined inflammatory burden was associated with primary or secondary AVF failure.
Our study has several limitations. As our sample size was limited, we are not able to examine the interaction between venous inflammation and oxidation, progression of comorbid conditions, medication use, and AVF outcomes. We were also unable to ascertain the impact of circulating inflammatory mediators on blood vessels used for the AVF conduit. Our analysis of venous TGFβ expression was limited to examining total rather than active TGFβ, and future studies are required to examine the role of active versus inactive TGFβ. Furthermore, we are not able to ascertain the impact of factors such as AVF blood flow, shear stress, and turbulence on IH development and AVF outcome.
In conclusion, our study found that pre-existing IH, inflammation, and oxidation are present within veins used for the AVF conduit prior to its creation. However, in patients with CKD, extant inflammation and oxidation within veins used for the AVF conduit did not predispose the AVF to future primary or secondary failure. Given the complexity of AVF dysfunction, our findings suggest that an anti-inflammatory approach alone may not improve AVF outcomes without determining the contribution of other multiple variables to AVF failure.
ACKNOWLEDGEMENTS
We would like to thank the physicians of Peachtree Vascular Surgery, Atlanta, GA for helping us to collect venous tissue for this study.
Footnotes
Financial support: This study was supported by a Harold Amos Faculty Development Award, Robert Wood Johnson Foundation (H.W.), an NIH R01HL79040 (A.H.), and a PHS Grant (UL1 RR02008, KL2 RR025009 or TL1 RR025010) from the Clinical and Translational Science Award Program, National Institutes of Health, National Center for Research Resources.
Conflict of interest: None.
REFERENCES
- 1.Dember LM, Beck GJ, Allon M, et al. Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: a randomized controlled trial. JAMA. 2008;299:2164–2171. doi: 10.1001/jama.299.18.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roy-Chaudhury P, Arend L, Zhang J, et al. Neointimal hyperplasia in early arteriovenous fistula failure. Am J Kidney Dis. 2007;50:782–790. doi: 10.1053/j.ajkd.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 3.Roy-Chaudhury P, Kelly BS, Miller MA, et al. Venous neointimal hyperplasia in polytetrafluoroethylene dialysis grafts. Kidney Int. 2001;59:2325–2334. doi: 10.1046/j.1523-1755.2001.00750.x. [DOI] [PubMed] [Google Scholar]
- 4.Budu-Grajdeanu P, Schugart RC, Friedman A, Valentine C, Agarwal AK, Rovin BH. A mathematical model of venous neointimal hyperplasia formation. Theor Biol Med Model. 2008;5:2. doi: 10.1186/1742-4682-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krueger U, Zanow J, Scholz H. Computational fluid dynamics and vascular access. Artif Organs. 2002;26:571–575. doi: 10.1046/j.1525-1594.2002.07078.x. [DOI] [PubMed] [Google Scholar]
- 6.Ene-Iordache B, Mosconi L, Remuzzi G, Remuzzi A. Computational fluid dynamics of a vascular access case for hemodialysis. J Biomech Eng. 2001;123:284–292. doi: 10.1115/1.1372702. [DOI] [PubMed] [Google Scholar]
- 7.yevzlin AS, Conley EL, Sanchez RJ, young HN, Becker BN. Vascular access outcomes and medication use: a USRDS study. Semin Dial. 2006;19:535–539. doi: 10.1111/j.1525-139X.2006.00218.x. [DOI] [PubMed] [Google Scholar]
- 8.Chang CJ, Ko PJ, Hsu LA, et al. Highly increased cell proliferation activity in the restenotic hemodialysis vascular access after percutaneous transluminal angioplasty: implication in prevention of restenosis. Am J Kidney Dis. 2004;43:74–84. doi: 10.1053/j.ajkd.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Lee T, Chauhan V, Krishnamoorthy M, et al. Severe venous neointimal hyperplasia prior to dialysis access surgery. Nephrol Dial Transplant. 2011;26:2264–2270. doi: 10.1093/ndt/gfq733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clinical Practice Guidelines for Vascular Access. Am J Kidney Dis. 2006;48:S176–S273. doi: 10.1053/j.ajkd.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 11.Marrone D, Pertosa G, Simone S, et al. Local activation of interleukin 6 signaling is associated with arteriovenous fistula stenosis in hemodialysis patients. Am J Kidney Dis. 2007;49:664–673. doi: 10.1053/j.ajkd.2007.02.266. [DOI] [PubMed] [Google Scholar]
- 12.Stracke S, Konner K, Kostlin I, et al. Increased expression of TGF-beta1 and IGF-I in inflammatory stenotic lesions of hemodialysis fistulas. Kidney Int. 2002;61:1011–1019. doi: 10.1046/j.1523-1755.2002.00191.x. [DOI] [PubMed] [Google Scholar]
- 13.Weiss MF, Scivittaro V, Anderson JM. Oxidative stress and increased expression of growth factors in lesions of failed hemodialysis access. Am J Kidney Dis. 2001;37:970–980. doi: 10.1016/s0272-6386(05)80013-7. [DOI] [PubMed] [Google Scholar]
- 14.Vindis C, Escargueil-Blanc I, Uchida K, Elbaz M, Salvayre R, Negre-Salvayre A. Lipid oxidation products and oxidized low-density lipoproteins impair platelet-derived growth factor receptor activity in smooth muscle cells: implication in atherosclerosis. Redox Rep. 2007;12:96–100. doi: 10.1179/135100007X162248. [DOI] [PubMed] [Google Scholar]
- 15.Lim S, Moon MK, Shin H, et al. Effect of S-adenosylmethionine on neointimal formation after balloon injury in obese diabetic rats. Cardiovasc Res. 2011 Feb 4; doi: 10.1093/cvr/cvr009. [DOI] [PubMed] [Google Scholar]