Summary
Background
Recent results have indicated that polyphosphate, released by activated platelets, can function as a procoagulant to modulate the proteolytic activity of serine proteases of the blood clotting cascade.
Objective
To determine whether polyphosphate is involved in inducing signal transduction in cellular and animal models.
Methods
The effect of polyphosphate on HUVECs was examined by monitoring cell permeability, apoptosis, and activation of NF-κB after treating cells with different concentrations of polyphosphate. Moreover, the expression of cell surface adhesion molecules (VCAM-1, ICAM-1 and E-selectin) and the adhesion of THP-1 cells to polyphosphate-treated cells were monitored by established methods. In the in vivo model, the proinflammatory effect of polyphosphate was assessed by monitoring vascular permeability and migration of leukocytes to the peritoneal cavity of mice injected with polyphosphate.
Results
Polyphosphate, comprised of 45, 65 and 70 phosphate units, enhanced the barrier permeability and apoptosis in cultured endothelial cells and up-regulated the expression of cell adhesion molecules, thereby mediating the adhesion of THP-1 cells to polyphosphate-treated endothelial cells. These effects of polyphosphate were mediated through the activation of NF-κB and could not be recapitulated by another anionic polymer, heparin. Polyphosphate also increased the extravasation of the BSA-bound Evans blue dye and the migration of leukocytes to the mouse peritoneal cavity, which was prevented when activated protein C was intravenously injected 2h prior to the challenge.
Conclusion
Polyphosphate, in addition to up-regulation of coagulation, can elicit potent proinflammatory responses through the activation of NF-κB, possibly contributing to the proinflammatory effect of activated platelets.
Introduction
Polyphosphate (polyP) is a linear polymer of inorganic phosphate, linked together through ATP-like phosphoanhydride bonds [1]. PolyP is stored in the dense granule of human platelets at high concentrations and can be released into the circulation upon the activation of platelets by various stimuli [2]. Recent results have indicated that polyP can modulate both blood clotting and inflammatory pathways [3]. Thus, it has been demonstrated that, polyP of a similar size to that found in platelets (60-100 phosphate units), can exert a procoagulant effect through the activation of the contact pathway as well as by enhancing the activation of the procoagulant factors V and XI by thrombin [3-5]. Moreover, a contact pathway-dependent proinflammatory role for polyP has been reported in an in vivo model of oedema where a subcutaneous injection of polyP has been shown to increase vascular leakage in the skin microvessels of mice [3]. The procoagulant effect of polyP appears to be primarily mediated through a template mechanism in which the anionic polymer, by simultaneous binding to the basic exosites of coagulation proteases and their target zymogens, decreases the dissociation constants for the interaction of these molecules in appropriate substrate activation complexes [5-7]. This is the same mechanism through which the anticoagulant heparin accelerates the inhibition of thrombin by antithrombin and other heparin-binding serpin inhibitors [8]. Interestingly, however, unlike heparin, polyP does not accelerate the inhibition of coagulation proteases by plasma inhibitors, but rather it binds to selected plasma proteins including thrombin and its substrates factors V and XI, thereby promoting the thrombin activation of these procoagulant substrates during the initiation and amplification of the blood clotting cascade [4,5]. This function of polyP resembles heparin since polysaccharides are also known to accelerate the thrombin activation of factor XI by a similar mechanism [9].
In light of increasing evidence that blood coagulation and inflammation are closely intertwined pathways [10], we speculated that, in addition to its ability to regulate coagulation and inflammation through the activation of the contact pathway [3], polyP may also directly elicit intracellular signalling responses when released from activated platelets during the initiation of the blood clotting cascade. Thus, we undertook this study to monitor the modulatory effect of polyP on human umbilical vein endothelial cells (HUVECs) by employing several established cell signalling assays. Moreover, we assessed the proinflammatory effect of polyP after its intraperitoneal injection into mice by monitoring its effect on the vascular leakage and on the migration of activated leukocytes to the peritoneal cavity. Our results, in both cellular and animal models, demonstrate that polyP comprised of 45, 65 and 70 phosphate units elicit potent proinflammatory responses through the activation of NF-κB that can not be recapitulated by the anionic polymer, unfractionated heparin. Further studies revealed that activated protein C (APC) has a protective effect against the cytotoxic effect of polyP in both cellular and animal models. Our results suggest that, in addition to up-regulation of coagulation, polyP can up-regulate inflammatory pathways and contribute to the proinflammatory function of activated platelets during the blood coagulation process.
Materials and Methods
Reagents
Bacterial lipopolysaccharide (LPS), polyP45, polyP65, 2-mercaptoethanol, carboxymethylcellulose-sodium (CMC-Na) and antibiotics (penicillin G and streptomycin) were purchased from Sigma (St. Louis, MO). Foetal bovine serum (FBS) and Vybrant DiD were purchased from Invitrogen (Carlsbad, CA, USA). PolyP70 was a generous gift from Dr. James Morrissey. Phosphatase (Psp) was purchased from Promega (WI, USA). Recombinant APC was prepared as described [11]. Mouse APC was obtained from Haematologic Technologies (Essex Junction, VT). Unfractionated heparin (average MW ~15 kDa) was purchased from Quintiles Clinical Supplies (Mt. Laurel, NJ). Six weeks old female ICR mice were obtained from Orient, South Korea.
Cell culture
Primary HUVECs were obtained from Cambrex Bio Science Inc. (Charles City, IA) and maintained as described [12]. The human monocytic leukaemia cell line, THP-1 (ATCC, Manassas, VA), was maintained at a density of 2 × 105 to 1 × 106 cells/mL in RPMI 1640 with L-glutamine and 10% heat-inactivated FBS supplemented with 2-mercaptoethanol (55 μM) and antibiotics (penicillin G and streptomycin as described [12]. All cell-based assays described below are conducted under serum-free conditions as described [12-14].
Permeability assay
Endothelial cell permeability in response to increasing concentrations of either polyP (0-75 μM for 4h) (the polyP concentration is expressed in terms of phosphate monomer throughout the manuscript) or unfractionated heparin (0-100 μg/mL for 4h) was quantitated by spectrophotometric measurement of the flux of Evans blue-bound albumin across functional cell monolayers using a modified 2-compartment chamber model as described [12-14]. Permeability was also monitored with the phosphatase (Psp)-treated polyP. For this, polyP was pretreated with 0.05 U/mg Psp for 2h prior to incubation with endothelial cells. Results are expressed as mean ±SEM and all experiments were repeated at least three times. In some experiments, the endothelial cell permeability was induced with LPS (10 ng/mL) for 4 h as described [14].
Apoptosis assay
The signalling effect of polyP (50 μM for 4h) and LPS (10 ng/mL for 4h) on cellular apoptosis was evaluated as described [13,14]. The number of apoptotic cells was expressed as the percentage of TUNEL-positive cells of the total number of nuclei determined by Hoechst staining as described [13,14]. Results are expressed as mean ±SEM and all experiments were repeated three times. DNA fragmentation assay was evaluated by using Cell Death Detection ELISA PLUS from Roche Diagnostics (Mannheim, Germany) according to the manufacturer's protocol.
Analysis of expression of cell surface receptors
The expression of vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1) and E-selectin on HUVECs was determined by a whole-cell ELISA as described [12-14]. Briefly, cell monolayer was treated for 4h with increasing concentrations of polyP (0-75 μM) and then fixed in 1% paraformaldehyde. After washing three times, mouse anti-human monoclonal antibodies to VCAM-1, ICAM-1, and E-selectin (Temecula, CA, USA) were added. After 1h (37 °C, 5% CO2), cells were washed and peroxidase-conjugated anti-mouse IgG (Sigma, St. Louis, MO) was added for 1h. Cells were washed again and developed using o-phenylenediamene substrate (Sigma, St. Louis, MO). All measurements were performed in triplicate wells and repeated at least twice.
Cell adhesion assay
The adherence of the monocytic THP-1 cell to endothelial cells was evaluated by fluorescent labelling of THP-1 cells as described [12]. Briefly, THP-1 cells were labelled with the Vybrant DiD dye followed by their addition to the washed and polyP65 (50 μM) or LPS (10 ng/mL) stimulated HUVECs. Cells were allowed to adhere and the non-adherent THP-1 cells were washed off and the fluorescence of the adherent cells was measured. The percentage of adherent THP-1 cells was calculated by the formula: % adherence = (adherent signal/total signal) × 100 as described [12]. All data are expressed as means ±SD from at least three independent experiments.
ELISA for NF-κB
A commercially available ELISA kit (Cell Signaling Technology, Inc, Danvers, MA) was used to measure the concentration of NF-κB in nuclear lysates of polyP (50 μM) stimulated cells according to the manufacturers’ protocol and as described [12].
In vivo permeability and leukocyte migration assays
Female ICR mice (6 weeks old upon receipt, from the Orient, South Korea) were used for in vivo studies after a 12-day acclimatisation period. The animals were housed in polycarbonate cages with free access to normal rodent pellet; and kept under controlled temperature (20-25 °C), humidity (40%-45%) and on a 12 h light/dark cycle. All animals were treated in accordance with the Guidelines for Care and Use of Laboratory Animals of Kyungpook National University. The vascular permeability assay was carried out according to previously described methods [15]. Briefly, 1% Evans blue dye solution in normal saline was injected intravenously in each mouse, immediately followed by an intraperitoneal injection of polyP (300 μg/g body weight) or 0.7% acetic acid as a positive control. Thirty minutes later, mice were sacrificed, and the peritoneal exudates were collected after being washed with 5 mL of normal saline, and centrifuged at 200 × g for 10 min. The absorbance of the supernatant was read at 650 nm using Sunrise ELISA Analyzer (Tecan Company, Austria). The vascular permeability was expressed in terms of dye (μg/mouse), which leaked into the peritoneal cavity according to the standard curve of Evans blue as described [15].
For assessing the leukocyte migration, animals were intraperitoneally injected with either polyP65 or polyP70 (300 μg/g body weight) dissolved in normal saline. Four hours later, mice were sacrificed and the peritoneal cavities were washed with 5 mL of the normal saline. Twenty microliters of peritoneal fluid was mixed with 0.38 mL of Turk's solution (0.01% crystal violet in 3% acetic acid) and the number of leukocytes was counted under a light microscope. In animals receiving APC, mouse APC (0.2 μg/g body weight) was injected intravenously 2h prior to the injection of polyP. In these studies, CMC-Na was administrated to mice as a positive control for polyP as described [15].
Statistical analysis
Data are expressed as means ± SD from at least three independent experiments. Statistical significance between 2 groups was determined by Student's t-test. The significance level was set at p < 0.05.
Results
PolyP enhances vascular cell permeability and apoptosis
The results presented in Fig. 1A demonstrate that polyP polymers containing 45, 65 and 70 phosphate units can all enhance the barrier permeability of vascular endothelial cells in a concentration dependent manner. A significant barrier permeability-inducing effect for polyP could be observed at a concentration of 10 μM (Fig. 1A). This concentration of polyP is physiologically relevant since its concentration in plasma has been reported to be higher than 10 μM [16,17], and it can attain much higher levels in the vicinity of activated platelets (the polyP concentration is expressed in terms of phosphate monomer) [18]. In all assays described below, the activities of both polyP65 and polyP70 were tested. Both polyP derivatives yielded essentially identical results. For this reason, only polyP65 data are presented in the figures. To determine whether this effect of polyP is specific, or whether increase in endothelial barrier permeability can also be mediated by other anionic polymers, the effect of unfractionated heparin was evaluated in this assay under the same experimental conditions. The results presented in Fig. 1B indicate that, unlike polyP, heparin does not increase the cell permeability, but rather exhibits a barrier protective effect to endothelial cells by reversing the barrier disruptive effect of polyP in a concentration dependent manner, with its optimal protective effect occurring at a concentration of ~10-20 μg/mL. Interestingly, the same concentration of heparin also reversed the hyperpermeability effect of LPS in endothelial cells (Fig. 1C). By contrast, polyP, under the same experimental conditions, exhibited an additive proinflammatory effect in the LPS-stimulated endothelial cells, possibly suggesting that LPS and polyP exert their proinflammatory effects through different mechanisms (Fig. 1C). In agreement with this hypothesis, the siRNA knockdown of the LPS receptor, toll-like receptor 4, had no effect on the proinflammatory signalling effect of polyP in endothelial cells (data not presented). It is known that APC protects endothelial cells from the hyperpermeability effect of LPS [12,19]. As shown in Fig. 1D, similar to heparin, APC exhibited a protective effect and reduced the barrier permeability of endothelial cells irrespective of whether the cells were stimulated with polyP or LPS. Pretreatment of polyP with Psp abrogated its ability to induce endothelial hyperpermeability, suggesting that the barrier disruptive effect is specifically mediated through polyP (Fig. 1D).
Figure 1. Effect of polyP on the barrier permeability of endothelial cells.
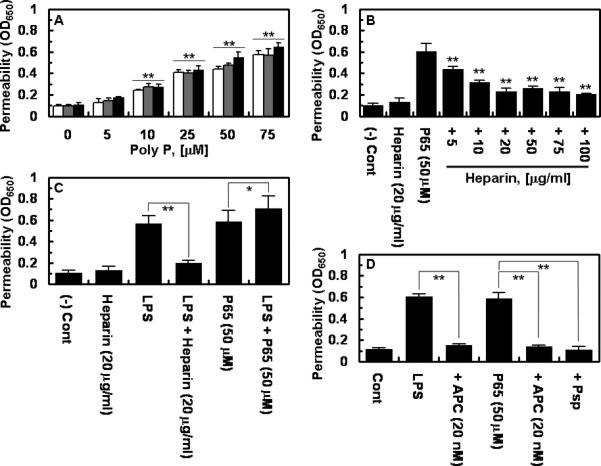
(A) Endothelial cells were incubated with indicated concentrations of polyP comprised of 45 (white bars), 65 (grey bars) and 70 (black bars) phosphate units for 4h followed by measuring permeability as described in Methods. (B) The same as panel A except that permeability was monitored with polyP65 after treating endothelial cells with indicated concentrations of unfractionated therapeutic heparin for 4h. (C) The same as above except that cells were incubated with LPS (10 ng/mL for 4h) with or without prior incubation with either heparin (20 μg/mL for 4h) or polyP65 (50 μM for 4h). (D) The same as above except that cells were pre-incubated with APC (20 nM for 3h) before treating cells with either LPS (10 ng/mL for 4h) or polyP65 (50 μM for 4h) in the absence or presence of 0.05 U/mg phosphatase (Psp). All results are means ± SD of three different experiments. *p < 0.05; **p < 0.01 compared to 0 (A), polyP65 (B), LPS (C).
LPS stimulates apoptosis in endothelial cells [19]. To determine whether polyP exerts a similar effect, endothelial cells were treated with polyP with or without stimulation with LPS. The results presented in Fig. 2 demonstrate that, similar to LPS, polyP induces apoptosis in endothelial cells and that APC exhibits a cytoprotective effect by inhibiting the effect of both LPS and polyP. Consistent with the results presented above, treatment with Psp eliminated the proapoptotic effect of polyP (Fig. 2A, analysis by the TUNEL assay; 2B analysis by the DNA fragmentation assay).
Figure 2. Pro-apoptotic effect of polyP and LPS on endothelial cells.
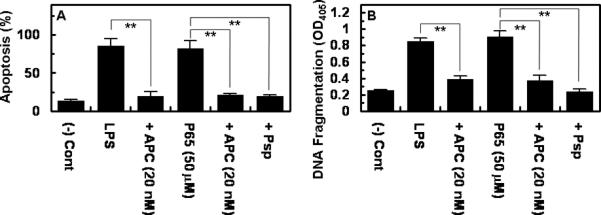
(A) Endothelial cells were incubated with LPS (10 ng/mL for 4h) or polyP65 (50 μM for 4h) followed by the analysis of apoptosis by a TUNEL assay (A) or DNA fragmentation (B) as described in Methods. The number of apoptotic cells is expressed as the percentage of TUNEL-positive cells of the total number of nuclei. The number of TUNEL-positive cells in panel A in the absence of LPS was 10-15%. When APC was present, the cell monolayer was pretreated with 20 nM APC for 3h prior to induction of apoptosis by either LPS or polyP65. **p < 0.01.
Effects of polyP on cell adhesion molecule expression, THP-1 adhesion and NF-κB activation
The effect of polyP on the expression of cell adhesion molecules, ICAM-1, VCAM-1 and E-selectin on the surface of endothelial cells was evaluated. The results presented in Fig. 3 demonstrate that polyP up-regulates the cell surface expression of all three adhesion molecules. The elevated expression of adhesion molecules correlated well with the enhanced binding of THP-1 cells to both polyP- and LPS-activated endothelial cells. Furthermore, APC down-regulated the proinflammatory function of both LPS and polyP in this assay (Fig. 3B). It is known that LPS up-regulates inflammatory pathways by activating NF-κB in endothelial cells [12,19]. As presented in Fig. 3C, polyP also specifically activated NF-κB in endothelial cells and APC effectively inhibited this function of polyP.
Figure 3. Effect of polyP on expression of cell adhesion molecules, adhesion of THP-1 cells and induction of NF-κB in endothelial cells.
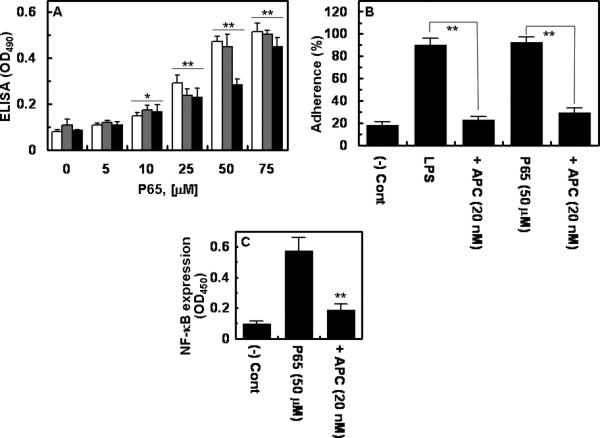
(A) Confluent cells were incubated with indicated concentrations of polyP65 for 4h followed by monitoring the cell surface expression of VCAM-1 (white bars), ICAM-1 (grey bars) and E-selectin (black bars) by a cell-based ELISA as described in Methods. (B) Confluent cells were incubated with LPS (10 ng/mL for 4h) or polyP (50 μM for 4h) and the THP-1 cell adherence to HUVECs was monitored as described in Methods. (C) Confluent cells were incubated with polyP (50 μM for 4h) followed by analysis of the NF-κB activation by an ELISA. When the effect of APC was assessed, cell monolayers were pretreated with 20 nM APC for 3h prior to their incubation with LPS or polyP65. *p < 0.05; **p < 0.01 compared to 0 (A) or polyP65 (C).
Analysis of permeability and leukocyte migration in vivo
The in vivo significance of the proinflammatory function of polyP was evaluated by intraperitoneal injection of either polyP65 or polyP70 to mice followed by measuring vascular permeability from the extent of extravasation of BSA-bound Evans blue dye from plasma into the peritoneal cavity. The results presented in Fig. 4 (shown for polyP65 only) suggest that polyP specifically increases the leakiness of the endothelium, thereby facilitating the passage of dye from plasma into the peritoneal cavity. The proinflammatory effect of polyP was specific since its treatment with Psp abrogated this effect (Fig. 4A). In agreement with results in the cellular model, both polyP65 and polyP70 markedly enhanced the binding of leukocytes to the vascular endothelium and their subsequent migration to the peritoneal cavity (Fig. 4B, shown for polyP65 only). The intravenous administration of APC abrogated the proinflammatory effect of polyP (Fig. 4B).
Figure 4. In vivo analysis of the effect of polyP on vascular leakage and the migration of leukocyte to peritoneal cavity.
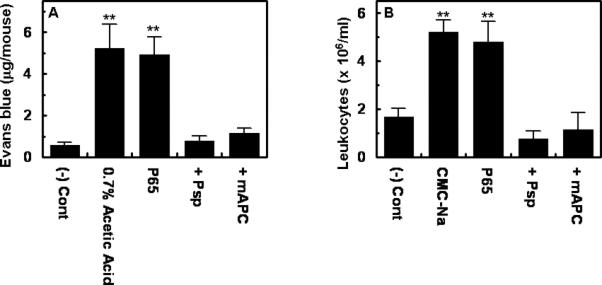
(A) Six weeks old female mice were intravenously injected with 1% BSA-bound Evans blue dye followed by an immediate intraperitoneal injection of polyP (300 μg/g body weight) or 0.7% acetic acid. Vascular permeability was determined from the extent of extravasation of Evans blue to the peritoneal cavity as described in Methods. (B) Leukocyte infiltration to peritoneal cavity was monitored after intraperitoneal injection of polyP65 (300 μg/g body weight) or CMC-Na (1.5%) as described in Methods. When the effect of APC was evaluated, mouse APC (0.2 μg/g body weight) was administered intravenously 2h prior to their challenge with polyP65 treated or not treated with phosphatase (Psp). **p < 0.01 compared to (-) Cont.
Discussion
In this study, we have demonstrated that polyP, similar in size to the platelet polyP, can elicit proinflammatory signalling responses in both cellular and animal models. We found that polyP comprised of either 45, 65 or 70 phosphate units, enhanced cell permeability, apoptosis and up-regulated the expression of cell surface adhesion molecules, VCAM-1, ICAM-1 and E-selectin, thereby supporting the binding of the monocytic THP-1 cells to the polyP-treated endothelial cells. The proinflammatory effect of polyP was mediated through the activation of NF-κB and was specific for polyP since Psp treatment completely abolished the cellular signalling function of the molecule. The in vivo relevance of these results was demonstrated by the observation that the intraperitoneal injection of polyP into mice resulted in vascular leakage and recruitment/migration of activated leukocytes to the peritoneal cavity. The mechanism through which polyP elicits proinflammatory responses is not known. Noting its polyanionic nature and similarity in function to LPS in eliciting proinflammatory responses, we decided to determine whether polyP can induce signal transduction through toll-like receptors (TLR) on vascular endothelial cells. However, the siRNA mediated knockdown of TLR2, TLR4 and the receptor for advanced glycation end products had no effect on the signalling function of polyP (data not presented), excluding the possibility that polyP induces signal transduction through interaction with these pathogen-associated molecular pattern recognition receptors. In agreement with these results, the incubation of endothelial cells with both LPS and polyP exhibited an additive proinflammatory effect, suggesting that the LPS receptor, TLR4, is not involved in the intracellular signalling function of polyP.
Unlike the unknown intracellular signalling mechanism of polyP, it has been demonstrated that polyP up-regulates coagulation primarily by functioning as a template on which procoagulant proteases and their substrates assemble in ternary complexes, thereby enhancing the rate of coagulation reactions [4,5]. For instance, it was recently shown that polyP could simultaneously bind to basic exosites of thrombin and its substrates factors XI and V to promote the activation of these substrates by thrombin [4-6]. Since other anionic polymers such as dextran sulphate and heparin can also enhance the protease activation of coagulation zymogens by this mechanism [9,20,21], we decided to compare the intracellular signalling function of heparin with polyP in endothelial cells. However, in all cellular assays described above, we found that heparin elicits a paradoxical protective effect in endothelial cells in a concentration dependent manner, suggesting that the two anionic polymers employ different mechanisms to elicit intracellular signalling responses. It should however be noted that, unlike heparin which dramatically enhances the reactivity of coagulation proteases with their target serpins (i.e., antithrombin), polyP has no effect on the inhibition of thrombin by antithrombin in either the presence or absence of heparin, suggesting that the two anionic polymers bind non-overlapping sites on thrombin to exert their biological functions [6].
That heparin elicits a protective response in endothelial cells in response to inflammatory mediators is not a new finding and has been demonstrated in several other reports [22-24]. It has been hypothesised that the signalling function of heparin may be mediated through its ability to bind and sequester the local concentrations of growth factors/cytokines in the vicinity of their cell surface receptors [22,24]. Another hypothesis is that heparin may electrostatically interact with cell membranes to internalize and bind to the cationic nuclear localization domain of NF-κB, thereby preventing the translocation of the transcription factor from the cytoplasm to the nucleus [22,25]. Whether polyP functions as a second messenger to mediate its proinflammatory effect directly through a cell surface receptor or if similar to heparin it sequesters proinflammatory mediators and/or internalizes to the cytoplasm in order to modulate the NF-κB pathway requires further investigation. There is however some evidence in support of the hypothesis that polyP can modulate cellular functions through binding to fibroblast growth factors, thereby facilitating their interactions with cell surface receptors [26,27]. Given the observation that polyP recruits activated leukocytes to the peritoneal cavity, it is possible that polyP interacts with distinct chemokines involved in neutrophil trafficking. Such chemokines are known to contain clusters of basic residues, which bind to glycosaminoglycans on the endothelium and to their cell surface chemokine receptors [28.29]. Thus, the possibility that polyP interacts with basic residues on distinct chemokines to modulate inflammatory responses warrants further investigation.
Finally, our results demonstrated that APC has a potent protective activity against the proinflammatory effect of polyP in both cellular and animal models. APC prevented both vascular leakage and accumulation of activated immune cells to the peritoneal cavity of experimental animals. These results are consistent with an antiinflammatory role for APC observed in other similar in vitro and in vivo inflammatory models [19]. APC is thought to inhibit the proinflammatory pathways, at least partly, through the endothelial protein C receptor-dependent activation of protease-activated receptor 1 [19,30]. Whether APC exerts its protective effect in response to polyP by the same mechanism was not investigated. In a recent study, it was shown that the proinflammatory activity of polyP requires the factor XIIa-dependent activation of the contact pathway [3]. It is not known if the activation of the contact pathway also contributes to the proinflammatory effect of polyP in our model system.
Acknowledgements
We would like to thank Audrey Rezaie for proofreading the manuscript. This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0003410 and 2011-0026695) to JSB and grants awarded by the National Heart, Lung, and Blood Institute of the National Institutes of Health HL 101917 and HL 68571 to ARR.
References
- 1.Kumble KD, Kornberg A. Inorganic polyphosphate in mammalian cells and tissues. J Biol Chem. 1995;270:5818–5822. doi: 10.1074/jbc.270.11.5818. [DOI] [PubMed] [Google Scholar]
- 2.Ruiz FA, Lea CR, Oldfield E, Docampo R. Human platelet dense granules contain polyphosphate and are similar to acidocalcisomes of bacteria and unicellular eukaryotes. J Biol Chem. 2004;279:44250–44257. doi: 10.1074/jbc.M406261200. [DOI] [PubMed] [Google Scholar]
- 3.Müller F, Mutch NJ, Schenk WA, Smith SA, Esterl L, Spronk HM, Schmidbauer S, Gahl WA, Morrissey JH, Renné T. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139:1143–1156. doi: 10.1016/j.cell.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith SA, Morrissey JH. Polyphosphate as a general procoagulant agent. J Thromb Haemost. 2008;6:1750–1756. doi: 10.1111/j.1538-7836.2008.03104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi SH, Smith SA, Morrissey JH. Polyphosphate is a cofactor for the activation of factor XI by thrombin. Blood. 2011;118:6963–6970. doi: 10.1182/blood-2011-07-368811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mutch NJ, Myles T, Leung LL, Morrissey JH. Polyphosphate binds with high affinity to exosite II of thrombin. J Thromb Haemost. 2010;8:548–555. doi: 10.1111/j.1538-7836.2009.03723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith SA, Choi SH, Davis-Harrison R, Huyck J, Boettcher J, Rienstra CM, Morrissey JH. Polyphosphate exerts differential effects on blood clotting, depending on polymer size. Blood. 2010;116:4353–4359. doi: 10.1182/blood-2010-01-266791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gettins PG, Olson ST. Exosite determinants of serpin specificity. J Biol Chem. 2009;284:20441–20445. doi: 10.1074/jbc.R800064200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gailani D, Broze GJ., Jr Factor XI activation in a revised model of Blood coagulation. Science. 1991;253:909–912. doi: 10.1126/science.1652157. [DOI] [PubMed] [Google Scholar]
- 10.Esmon CT. Inflammation and thrombosis. J Thromb Haemost. 2003;1:1343–1348. doi: 10.1046/j.1538-7836.2003.00261.x. [DOI] [PubMed] [Google Scholar]
- 11.Yang L, Manithody C, Rezaie AR. Contribution of basic residues of the 70-80-loop to heparin binding and anticoagulant function of activated protein C. Biochemistry. 2002;41:6149–6157. doi: 10.1021/bi015899r. [DOI] [PubMed] [Google Scholar]
- 12.Bae JS, Rezaie AR. Activated protein C inhibits high mobility group box 1 signaling in endothelial cells. Blood. 2011;118:3952–3959. doi: 10.1182/blood-2011-06-360701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bae JS, Yang L, Manithody C, Rezaie AR. Engineering a disulfide bond to stabilize the calcium-binding loop of activated protein C eliminates its anticoagulant but not its protective signaling properties. J Biol Chem. 2007;282:9251–9259. doi: 10.1074/jbc.M610547200. [DOI] [PubMed] [Google Scholar]
- 14.Bae JS, Yang L, Manithody C, Rezaie AR. The ligand occupancy of endothelial protein C receptor switches the PAR-1-dependent signaling specificity of thrombin from a permeability-enhancing to a barrier-protective response in endothelial cells. Blood. 2007;110:3909–3916. doi: 10.1182/blood-2007-06-096651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JD, Huh JE, Jeon G, Yang HR, Woo HS, Choi DY, Park DS. Flavonol-rich RVHxR from Rhus verniciflua Stokes and its major compound fisetin inhibits inflammation-related cytokines and angiogenic factor in rheumatoid arthritic fibroblast-like synovial cells and in vivo models. Int Immunopharmacol. 2009;9:268–276. doi: 10.1016/j.intimp.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Han KY, Hong BS, Yoon YJ, Yoon CM, Kim YK, Kwon YG, Gho YS. Polyphosphate blocks tumour metastasis via anti-angiogenic activity. Biochem J. 2007;406:49–55. doi: 10.1042/BJ20061542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lorenz B, Leuck J, Köhl D, Muller WE, Schröder HC. Anti-HIV-1 activity of inorganic polyphosphates. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:110–118. doi: 10.1097/00042560-199702010-00003. [DOI] [PubMed] [Google Scholar]
- 18.Smith SA, Mutch NJ, Baskar D, Rohloff P, Docampo R, Morrissey JH. Polyphosphate modulates blood coagulation and fibrinolysis. Proc Natl Acad Sci USA. 2006;103:903–908. doi: 10.1073/pnas.0507195103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C pathway. Blood. 2007;109:3161–3172. doi: 10.1182/blood-2006-09-003004. [DOI] [PubMed] [Google Scholar]
- 20.Rezaie AR. Rapid activation of protein C by factor Xa and thrombin in the presence of polyanionic compounds. Blood. 1998;91:4572–4580. [PubMed] [Google Scholar]
- 21.Nicolaes GA, Sørensen KW, Friedrich U, Tans G, Rosing J, Autin L, Dahlbäck B, Villoutreix BO. Altered inactivation pathway of factor Va by activated protein C in the presence of heparin. Eur J Biochem. 2004;271:2724–2736. doi: 10.1111/j.1432-1033.2004.04201.x. [DOI] [PubMed] [Google Scholar]
- 22.Young E. The anti-inflammatory effects of heparin and related compounds. Thromb Res. 2008;122:743–752. doi: 10.1016/j.thromres.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 23.Gori AM, Attanasio M, Gazzini A, Rossi L, Lucarini L, Miletti S, Chini J, Manoni M, Abbate R, Gensini GF. Cytokine gene expression and production by human LPS-stimulated mononuclear cells are inhibited by sulfated heparin-like semi-synthetic derivatives. J Thromb Haemost. 2004;2:1657–1662. doi: 10.1111/j.1538-7836.2004.00866.x. [DOI] [PubMed] [Google Scholar]
- 24.Thourani VH, Brar SS, Kennedy TP, Thornton LR, Watts JA, Ronson RS, Zhao ZQ, Sturrock AL, Hoidal JR, Vinten-Johansen J. Nonanticoagulant heparin inhibits NF-kappaB activation and attenuates myocardial reperfusion injury. Am J Physiol Heart Circ Physiol. 2000;278:H2084–2093. doi: 10.1152/ajpheart.2000.278.6.H2084. [DOI] [PubMed] [Google Scholar]
- 25.Lin YZ, Yao SY, Veach RA, Torgerson TR, Hawiger J. Inhibition of nuclear translocation of transcription factor NF-kappa B by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J Biol Chem. 1995;270:14255–14258. doi: 10.1074/jbc.270.24.14255. [DOI] [PubMed] [Google Scholar]
- 26.Shiba T, Nishimura D, Kawazoe Y, Onodera Y, Tsutsumi K, Nakamura R, Ohshiro M. Modulation of mitogenic activity of fibroblast growth factors by inorganic polyphosphate. J Biol Chem. 2003;278:26788–26792. doi: 10.1074/jbc.M303468200. [DOI] [PubMed] [Google Scholar]
- 27.Kawazoe Y, Katoh S, Onodera Y, Kohgo T, Shindoh M, Shiba T. Activation of the FGF signaling pathway and subsequent induction of mesenchymal stem cell differentiation by inorganic polyphosphate. Int J Biol Sci. 2008;4:37–47. doi: 10.7150/ijbs.4.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Segerer S, Johnson Z, Rek A, Baltus T, von Hundelshausen P, Kungl AJ, Proudfoot AE, Weber C, Nelson PJ. The basic residue cluster (55)KKWVR(59) in CCL5 is required for in vivo biologic function. Mol Immunol. 2009;46:2533–2538. doi: 10.1016/j.molimm.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 29.Severin IC, Gaudry JP, Johnson Z, Kungl A, Jansma A, Gesslbauer B, Mulloy B, Power C, Proudfoot AE, Handel T. Characterization of the chemokine CXCL11-heparin interaction suggests two different affinities for glycosaminoglycans. J Biol Chem. 2010;285:17713–17724. doi: 10.1074/jbc.M109.082552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296:1880–1882. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]


