INTRODUCTION
Osteoarthritis (OA) is the most common form of arthritis and a major cause of pain and disability in older adults (1). Often OA is referred to as degenerative joint disease “(DJD)”. This is a misnomer because OA is not simply a process of wear and tear but rather abnormal remodeling of joint tissues driven by a host of inflammatory mediators within the affected joint. The most common risk factors for OA include age, gender, prior joint injury, obesity, genetic predisposition, and mechanical factors, including malalignment and abnormal joint shape (2, 3). Despite the multifactorial nature of OA, the pathological changes seen in osteoarthritic joints have common features that affect the entire joint structure resulting in pain, deformity and loss of function.
The pathologic changes seen in OA joints (Figures 1 and 2) include degradation of the articular cartilage, thickening of the subchondral bone, osteophyte formation, variable degrees of synovial inflammation, degeneration of ligaments and, in the knee, the menisci, and hypertrophy of the joint capsule. There can also be changes in periarticular muscles, nerves, bursa, and local fat pads that may contribute to OA or the symptoms of OA. The findings of pathological changes in all of the joint tissues are the impetus for considering OA as a disease of the joint as an organ resulting in “joint failure”. In this review, we will summarize the key features of OA in the various joint tissues affected and provide an overview of the basic mechanisms currently thought to contribute to the pathological changes seen in these tissues.
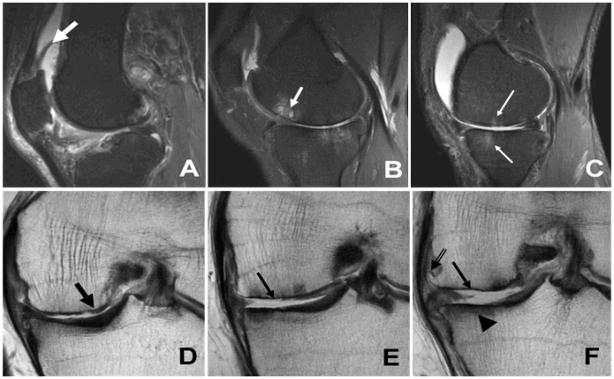
Figure 1.
Sagittal inversion recovery (A–C) and coronal fast spin echo (D–F) images illustrating the magnetic resonance imaging findings of osteoarthritis. (A) reactive synovitis (thick white arrow), (B) subchondral cyst formation (white arrow), (C) bone marrow edema (thin white arrows), (D) partial thickness cartilage wear (thick black arrow), (E–F) full thickness cartilage wear (thin black arrows), subchondral sclerosis (arrowhead) and marginal osteophyte formation (double arrow). Image courtesy of Drs. Hollis Potter and Catherine Hayter, Hospital for Special Surgery, New York, NY
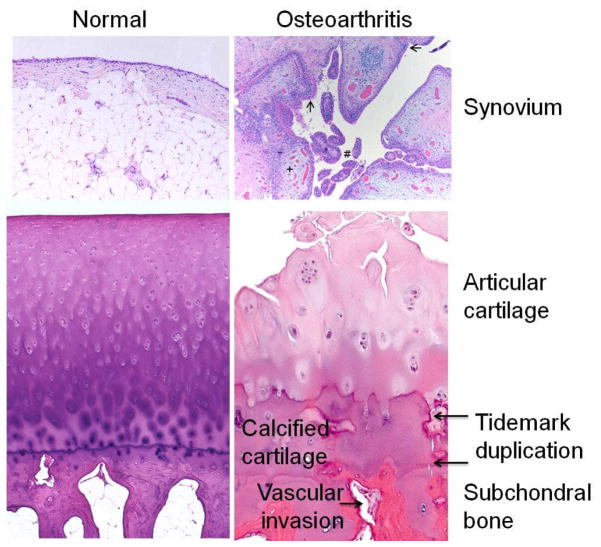
Figure 2.
Histologic features of osteoarthritis (OA). The normal synovium has a thin (1–2 cells thick) lining layer and a vascularized, loose connective tissue sublining layer. OA synovium demonstrates features of synovial villous hyperplasia (#), lining hyperplasia (arrows), increased vascularity (+) and perivascular mononuclear cell (inflammatory) infiltration. In OA articular cartilage, loss of cells and matrix is accompanied by areas of cell clusters. There is thickening of the calcified zone and duplication of the tidemark which normally separates articular cartilage from the underlying calcified cartilage. The subchondral bone is also thickened and vascular invasion, which can extend through the tidemark and into the base of the articular cartilage, is seen. Histology kindly provided by Ed DiCarlo, Hospital for Special Surgery, New York, NY.
ARTICULAR CARTILAGE
The articular cartilage is altered to some degree in all joints with OA. Cartilage provides a smooth surface with a very low coefficient of friction allowing for an efficient gliding motion during joint movement. This is facilitated by a boundary layer of lubricants on the articular surface provided by lubricin and hyaluronic acid produced by both chondrocytes and synovial cells (4). In OA, the earliest changes in cartilage appear at the joint surface in areas where mechanical forces, in particular shear stress, are greatest (5).
In normal adult cartilage in the resting, non-stressed steady state, chondrocytes are quiescent cells and there is very little turnover of the cartilage matrix. In OA the chondrocytes become “activated” characterized by cell proliferation, cluster formation, and increased production of both matrix proteins and matrix-degrading enzymes (6). Disruption of the normal resting state of chondrocytes may be viewed as an injury response involving the recapitulation of developmental programs, leading to matrix remodeling, inappropriate hypertrophy-like maturation, and cartilage calcification (6).
The matrix degrading enzymes found in the OA joint include aggrecanases and collagenases, which are members of the matrix metalloproteinase (MMP) family, as well as several serine and cysteine proteinases (7). Matrix degradation in early OA may be due to MMP-3 and A Disintegrin and Metalloproteinase with Thrombospondin Motifs 5 (ADAMTS-5), which degrade aggrecan, followed by increased activity of collagenases, in particular MMP-13, which is highly efficient at degrading type II collagen. Once the collagen network is degraded, it appears that a state is reached that cannot be reversed.
Chondrocytes have receptors for extracellular matrix (ECM) components, many of which are responsive to mechanical stimulation. Activation of these receptors stimulates the production of matrix-degrading proteinases and inflammatory cytokines and chemokines, either as initiating or feedback amplification events. The type II collagen-containing network in the interterritorial region is normally not accessible to degradation by proteinases because it is coated with proteoglycans. The importance of proteoglycan depletion in cartilage erosion was demonstrated in Adamts5 knockout mice, which are protected against progression in the surgical OA model (8). However, aggrecan depletion, by itself, does not drive OA progression, as suggested by recent studies in Mmp13 knockout mice showing that MMP-13 deficiency inhibits cartilage erosion, but not aggrecan depletion (9).
Recent studies suggest that biomechanical stress may initiate the disruption of the pericellular matrix through the serine proteinase, High Temperature Requirement A1 (HTRA1) (10). The receptor tyrosine kinase, discoidin domain receptor 2 (DDR2) is then exposed to its ligand, native type II collagen (Figure 3), and preferentially induces and activates MMP-13 (11). Syndecan-4, a trans-membrane heparan sulfate proteoglycan involved in the maintenance of homeostasis, is a positive effector of ADAMTS-5 activation through controlling the synthesis of the stromelysin, MMP-3 (12).
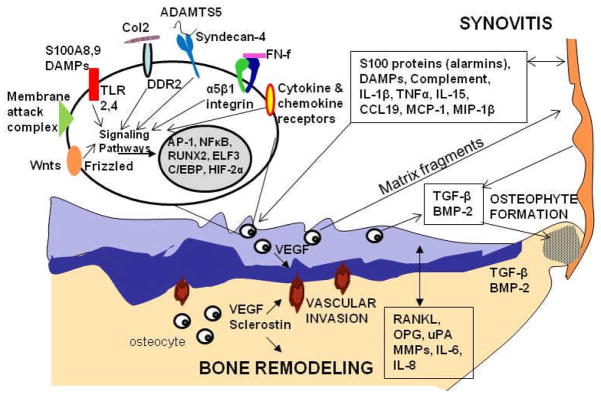
Figure 3.
Selected factors involved in the osteoarthritic process in the synovium, cartilage, and bone. Proteins including S100 proteins (alarmins) and damage-associated molecular pattern molecules (DAMPs), cytokines (interleukin (IL)-1β, tumor necrosis factorα (TNFα), IL-15), chemokines (C-C motif ligand 19 (CCL19), monocyte chemotactic protein-1 (MCP-1), monocyte inflammatory protein (MIP-1β)), and complement components released from the synovium can stimulate articular chondrocytes through activation of various cell surface receptors including toll-like receptors (TLRs), cytokine and chemokine receptors, or by formation of the complement membrane attack complex. Other factors which activate cartilage matrix destruction include binding of native type II collagen to discoidin domain receptor 2 (DDR2), fibronectin fragments to the α5β1 integrin, Wnt proteins to frizzled and binding of extracellular factors to syndecan-4. Syndecan-4 may also act by targeting A Disintegrin and Metalloproteinase with Thrombospondin Motifs-5 (ADAMTS-5) to the cell surface. Various signaling pathways lead to activation of a set of transcription factors that regulate expression of matrix degrading enzymes and inflammatory mediators. Matrix fragments released from the cartilage can stimulate further synovitis. Production of vascular endothelial growth factor (VEGF) in cartilage and bone stimulates vascular invasion from subchondral bone into the zone of calcified cartilage. VEGF, sclerostin, receptor activator of nuclear factor kappa-B ligand (RANKL), osteoprotegerin (OPG), urokinase-type plasminogen activator (uPA), matrix metalloproteinase (MMP)s, IL-6 and IL-8 mediate bone remodeling and potentially diffuse to the cartilage to also promote cartilage matrix destruction. Transforming growth factor-β (TGF-β) and bone morphogenic protein-2 (BMP-2) produced in the synovium, cartilage, and bone stimulate osteophyte formation.
Chondrocytes in OA cartilage, especially those in clonal clusters, express cytokine and chemokine receptors, MMPs, and a number of other genes that enhance or modulate inflammatory and catabolic responses, including cyclooxygenase (COX)-2, microsomal PGE synthase-1 (mPGES-1), soluble phospholipase A2 (sPLA2), and inducible nitric oxide synthase (NOS2). Activation of chondrocytes by mechanical and inflammatory stimuli occurs primarily through the NF-κB and stress- and mitogen-induced protein kinase (MAPK) pathways (6). Activation of canonical NF-κB (p65/p50) signaling is required for the chondrocytes to express MMPs, NOS2, COX2, and IL-1. Upon activation, the extracellular-regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and p38 MAPK cascades coordinate the induction and activation of transcription factors such as AP-1 (cFos/cJun), ETS, and C/EBPβ, that regulate expression of genes involved in catabolic and inflammatory events.
Another primary response factor for the regulation of cytokine-induced MMP-13 in chondrocytes is HIF2α (13), which is strongly induced by NF-κB signaling. Induction of both ADAMTS4 and 5 requires Runx2 (14), whereas NF-κB and HIF2α (13) mediate ADAMTS4 up-regulation. Recent studies indicate that epigenetic mechanisms also play a role through modulation of the DNA methylation status on promoters driving expression of, for example, IL1β and MMP13 genes (15) or through dysregulation of the microRNAs that are important for maintenance of homeostasis (16, 17).
Recent studies have also implicated synovial inflammation (discussed further below) and secreted damage-associated molecular patterns (DAMPs) or alarmins, that act as ligands of Toll-like receptors (TLR) or Receptor for Advanced Glycation Endproducts (RAGE), in the activation of inflammatory and catabolic events in articular cartilage. TLRs are expressed in chondrocytes activated by inflammatory stimuli (18), and TLR-2 and 4, which are present in OA cartilage lesional areas, may be activated by specific peptide ligands, leading to increased expression of inflammatory and catabolic genes, including MMP-3, MMP-13 and NOS2, through the cytosolic adaptor myeloid differentiation factor 88 (MyD88) and subsequent NF-κB signaling (19).
The high mobility group box (HMGB) protein 1 has been implicated in potentiating and contributing to OA, by acting on articular chondrocytes (20) or synoviocytes (21), and enhancing inflammatory insults. The alarmins, S100A4, A8, A9, and A11, along with HMGB1, also signal through RAGE and TLRs to drive inflammation-associated matrix catabolism and increase ROS through upregulating cytokines and chemokines (22, 23). Proinflammatory cytokines, prostaglandins, reactive oxygen species (ROS), and nitric oxide (NO) may also cause oxidative stress and chondrocyte apoptosis by altering mitochondrial function (24).
Wnt pathway signaling may play a role in cartilage destruction in OA through promotion of chondrocyte hypertrophy (25). Chondrocytes express multiple Wnt family members (26) and activation of canonical Wnt signaling through the frizzled receptors leading to increased β-catenin activity appears to promote matrix destruction, while inhibitors of Wnt activation, such as secreted frizzled-related proteins (FRZ), may be protective. Because of the important role of the Wnt pathway in regulating bone formation, alterations in Wnt signaling may be involved in both the cartilage and bone changes seen in OA. However, further studies are needed to better define the components of the Wnt pathways that promote OA and separate them from those that may be protective.
As articular cartilage matrix proteins are degraded, fragments of matrix proteins are produced which can feedback and stimulate further matrix destruction. Fragments found in OA cartilage include fibronectin (27, 28), small leucine-rich proteoglycans (29), and collagen (30). Fibronectin and collagen fragments can stimulate the production of inflammatory cytokines, chemokines, and MMPs (27, 31, 32). Inflammation also may be driven by cartilage matrix degradation products through activation of innate immune responses. Members of the small leucine-rich proteoglycan (SLRP) family such as fibromodulin and decorin may target the classic complement pathway and enhance or inhibit its activation (33). COMP, on the other hand, is a potent activator of the alternative complement pathway and complexes of COMP and C3b may be found in OA synovial fluids (34).
Increased age is the strongest risk factor for OA and aging-related changes in cartilage may contribute to the excessive matrix remodelling response. As reviewed recently (35), these aging changes include the accumulation of advanced glycation end-products that make the cartilage more “brittle” and the appearance of chondrocytes with features of the senescence-associated secretory phenotype, including increased production of many cytokines, chemokines, and MMPs.
Cell death has been observed during the development of OA and it may also be related to aging. There is evidence for a loss of cells starting in the superficial zone of cartilage that is associated with an age-related decrease in the HMGB protein 2 (36). Increased production of ROS mediated by mechanical injury or in response to cytokines and matrix fragments may also contribute to cell death (37) as well as a decline in autophagy which serves as a protective mechanism used by cells under stress (38). Proof of concept that cell death contributes to OA was provided by a study using caspase inhibitors to block cell death which resulted in decreased severity of cartilage lesions in a rabbit model of post-traumatic OA (39).
Calcification of the articular cartilage and meniscus (chondrocalcinosis), often accompanied by the presence of crystals (calcium pyrophosphate and/or hydroxyapatite) in the joint, is commonly seen in older adults with knee OA (40). A population-based study noted radiographic evidence of both chondrocalcinosis and knee OA in 18.2% of the population over the age of 65 years while 6.9% had knee OA without chondrocalcinosis (41). Crystals could play a role in the pathogenesis of OA by stimulating TLRs present on chondrocytes and synovial cells to promote production of inflammatory mediators (42). Hydroxyapatite crystals may stimulate production of inflammatory mediators, including IL-1 and IL-18, through activation of the NLRP3 inflammasome (43). Given that hydroxyapatite crystals are common in OA, these studies suggest that targeting the inflammasome may be a novel approach for preventing progression in a sub-set of people with OA.
MENISCUS AND LIGAMENTS
Pathologic changes in menisci and ligaments are common in people with knee OA. It is well accepted that injury to the meniscus and/or joint ligaments predisposes to the development of OA (2, 3) and MRI studies have revealed changes even in individuals without a known history of joint trauma changes. Meniscal damage occurs in 63% of adults with symptomatic knee OA (44) and in a longitudinal study, symptomatic subjects with significant meniscal damage had an odds ratio of 7.4 for the development of radiographic knee OA 30 months later (45). Likewise, anterior cruciate ligament (ACL) disruption is common in older adults with knee OA. In an MRI study, 22.8% of people with symptomatic knee OA had evidence of complete ACL rupture but less than half of those gave a history of trauma (46).
The pathologic changes in the menisci in both aging and OA have similarities to changes noted in the articular cartilage, including matrix disruption, fibrillation, cell clusters, calcification and cell death (47, 48). There is significant correlation between gross morphologic changes of OA in the knee cartilage and those in the menisci from the same joints (48). An increase in vascular penetration accompanied by increased sensory nerve densities has been noted in OA menisci, which may relate to the ability of menisci to serve as a source of pain in knee OA (49).
In addition to ligament injury, varus-valgus laxity, possibly related to aging changes in ligaments, is may play a role in the development of knee OA (50). Degenerative changes are common in ligaments from knee joints removed at the time of joint replacement for OA, particularly in the posterolateral bundle of the ACL, which in one study was severely affected in 78% of the joints (51). Similar to the meniscus, histologic changes include matrix disruption and collagen fiber disruption. ACL pathology, but not posterior cruciate disruption, was noted to correlate with radiographic severity (52). A recent study (53) of ACLs obtained at autopsy from 65 tissue donors with ages from 23–92 years found similar changes of collagen fiber disorganization and mucoid degeneration, as well as chondroid metaplasia and calcium deposition. These changes were more prevalent with increasing age and correlated with the presence of OA-like changes in the articular cartilage. In donors with grade II–IV cartilage lesions (on a scale of 0-IV) all had some abnormality in the ACL and 24.1% had ACL rupture. Further research on the pathogenesis of OA in these and other soft tissues of the joint will be important in order to know if therapies targeted at the articular cartilage will also target the changes in these tissues.
BONE
The structural and functional properties of peri-articular bone in OA represent the dynamic adaptation to biomechanical factors and the effects of soluble products generated in the adjacent joint tissues. The effects of mechanical load on bone are embodied in Wolff’s hypothesis, which states that the distribution and material properties of bone are determined by the magnitude and direction of applied load (54). In this paradigm, the changes in subchondral bone volume and density that characterize the osteoarthritic process are reflective of the prior loading history. The effects of loading may produce changes in subchondral bone height and contour, termed “attrition”. Bone remodeling in OA also may be initiated at sites of local bone damage resulting from excessive repetitive loading. This form of microdamage is associated with the appearance of microcracks, that initiate targeted remodeling, which likely accounts for the bone marrow lesions observed with MRI in patients with OA (Figure 1). Histological examination of the lesions reveals local fat necrosis and marrow fibrosis at various stages of healing (55). The correspondence of the bone marrow lesions with regions of bone and cartilage damage strongly supports a primary role for a mechanical and traumatic etiology for the subchondral bone marrow changes.
An additional mechanism for skeletal adaptation occurs at the joint margins and entheseal sites, where new bone is added by endochondral ossification, recapitulating the cellular mechanisms of skeletal growth and development (56). This process gives rise to the formation of osteophytes. Local production of growth factors, including transforming factor-β and bone morphogenic protein-2 have been implicated in this process (57, 58). Although there remains controversy regarding their functional role, osteophytes may serve to stabilize the joint rather than contributing to OA progression (56).
The subchondral bone plate beneath the articular cartilage is organized into cortical bone, whereas the deeper zones transition into a network of cancellous bone. The articular cartilage is separated from the subchondral bone by a zone of calcified cartilage, and the interface between the articular and calcified cartilage can be identified by the so-called “tide-mark” (Figure 2). The calcified cartilage undergoes marked alterations in cellular composition and structure in OA (59, 60). This process involves the penetration of calcified cartilage by vascular elements that extend from the subchondral bone and adjacent marrow space recapitulating the vascular invasion of the growth plate that occurs during the development. This results in duplication of the tidemark and advancement of the calcified cartilage into the deep zones of the articular cartilage leading to local cartilage thinning.
Walsh and coworkers have observed sensory nerve fibers expressing nerve growth factor (NGF) in the vascular channels associated with osteochondral angiogenesis and speculated that they could be a potential source of symptomatic pain (59–61). The regions of vascular invasion were associated with localized bone marrow replacement by fibrovascular tissue expressing vascular endothelial factor (VEGF). VEGF expression was also detected in chondrocytes in proximity to the angiogenesis, where VEGF could provide the signals for recruitment of the vascular elements.
The properties of subchondral bone also are influenced by the organization and composition of the organic bone matrix and mineral content (62, 63). The state of bone mineralization is highly dependent on the rate of bone remodeling. When the rate of bone remodeling is high, the “late” phase of mineral accretion is attenuated leading to a state of relative hypomineralization, which is associated with a reduction in the elastic modulus. In contrast, in conditions of low bone turnover, the continued deposition of mineral leads to an increase in the elastic modulus and the bone becomes resistant to deformation and more “brittle”, adversely affecting the overlying articular cartilage (63).
The detection of bone changes in OA prior to the appearance of detectible changes in the articular cartilage can be attributed, in part, to the marked differential capacity of cartilage and bone to adapt to altered mechanical loads and damage. Bone can rapidly alter its architecture and structure via cell-mediated processes of modeling and remodeling. In contrast, the capacity of chondrocytes to repair and modify their surrounding extracellular matrix is relatively limited in comparison to skeletal tissues (64).
Multiple studies have provided insights into the sequential structural changes in subchondral cortical and trabecular bone in OA. Karvonen et al. (65) analyzed the bone mineral density of the subchondral trabecular bone in the knee joints of patients with early OA and observed reduced levels deep to the thickened cortical bone. These findings were confirmed by utilizing a computerized method of textural image analysis (Fractal Signature Analysis) (66, 67). The osteoporotic changes in the subchondral trabeculae were speculated to be related to reduced transmission of load from the thickened cortical plate and to represent a form of so-called “stress shielding”. Recent studies on SOST and additional components of the Wnt-β-catenin pathway in osteochondral samples have provided potential mechanistic insights into the molecular signals by which mechanical factors modulate subchondral bone remodeling (68). The SOST gene encodes the protein sclerostin, which is a potent inhibitor of the Wnt pathway that contributes to the regulation of bone formation. SOST expression in osteocytes was locally decreased in regions of bone sclerosis. Increased mechanical loading in these regions could be responsible for the down-regulation of SOST with resultant increase in localized bone formation.
Changes in bone volume represent only one of the factors that determine the mechanical properties of bone. Day and co-workers (63) constructed finite element models from microCT scans of subchondral trabecular bone from the proximal tibiae from cadaver specimens with early cartilage damage. They found that the volume fraction of trabecular bone was increased, but observed that the tissue modulus of the bone was reduced in the condyles in which there was damage in the overlying articular cartilage. They attributed the reduction in modulus to a decrease in mineral density, which they speculated was related to incomplete mineralization due to an increase in the rate of bone remodeling. These observations indicate that the properties of the subchondral bone in certain stages of OA may be associated with decreased rather than increased bone tissue modulus and have significant implications with respect treatment strategies for targeting subchondral and peri-articular bone remodeling in OA. The lack of efficacy of a recent trial with risedronate in reducing progression of the cartilage changes in OA highlights the complexity of the issues surrounding the influences of bone adaptation and its effects on the natural history of OA (69).
SYNOVIUM
Synovial inflammatory infiltrates are identified in many OA patients, although they are generally of lower grade than those observed in RA (70) (Figure 2). Recent histologic surveys demonstrated that synovitis occurs even in early stages of disease (71) and after joint injuries (70, 72), which increase risk of OA. Specific aspects of synovial inflammation, such as numbers of infiltrating macrophages, may be higher in early disease (71), but the prevalence of synovitis increases with advancing disease stage (72, 73). The “synovitis” observed in OA and post-traumatic joint disease encompasses a variety of histologic patterns, including infiltration of macrophages and lymphocytes, either diffusely or in perivascular aggregates, which are detected in over 50% of patients with knee OA (74). Lining or villous hyperplasia is common, and fibrosis and cartilage/bone detritus are more typical of advanced stage disease. Increased vascularity also is seen, and may be a target for therapy (49).
Despite lower severity and greater variability in OA-associated synovitis compared to RA, many groups have reported that low-grade synovitis is associated with disease manifestations. For example, synovitis observed during arthroscopy was associated with progression of cartilage lesions in a prospective study of 422 patients (75), and those exhibiting synovial inflammation had both more severe baseline chondropathy and more severe progression of cartilage pathology. Although an earlier MRI study in patients with established knee OA (76) failed to confirm these findings, a more recent study of 514 patients with knee pain without radiographic knee OA provided evidence that effusion and synovitis were associated with subsequent development of cartilage erosion (77). Utilizing ultrasound imaging that detected synovial effusion also predicted progression to joint replacement (78).
A relationship between synovitis and symptoms was first noted by Torres and colleagues (79) who showed that synovitis, meniscal tears and bone marrow lesions detected by MRI all correlated with symptoms. Others (76) reported that change in pain scores over time varied with change in synovitis, suggesting a causal relationship. We recently observed a relationship between synovitis and knee symptoms exists even in patients without radiographic evidence of OA (72). In addition to subjectively measured symptoms, synovitis was recently associated with inferior knee joint function measured objectively by walking and stair-climbing times (80).
Soluble inflammatory mediators, including cytokines and chemokines which can promote synovitis, are increased in synovial fluid in OA and post-joint injury tissues. The most extensively studied are IL-1β and TNFα, which can suppress matrix synthesis and promote cartilage catabolism (6). However, attempts to block their activity in patients have demonstrated only minimal symptomatic efficacy (81, 82). Therefore, many other inflammatory mediators that can impact synovitis deserve investigation. The perivascular inflammatory infiltrates in OA synovium are largely comprised of lymphocytes (71), and the common γ-chain family of cytokines play important roles in activation, survival and function of T lymphocyte populations. Of these family members, serum levels of IL-15 have been associated with the incidence and progression of radiographic OA (83), and synovial fluid levels are elevated in early stage OA (84). IL-17, which is predominantly produced by T-lymphocytes, is an additional cytokine implicated in OA pathogenesis. In vitro studies have shown that IL-17 can induce chemokine production by both synovial fibroblasts and chondrocytes, particularly in synergy with IL-1 or TNFα (85), and IL-17 blockade has been demonstrated to decrease synovial thickening and IL-6 levels in a murine meniscectomy model, although no difference in cartilage appearance was noted (86).
Many chemokines are produced in joint tissues of patients with OA and after joint injury (87). In patients with early stage OA, synovial chemokine expression was associated with the presence of synovial inflammation (72), and expression of CCL19 and its receptor CCR7 was associated with greater symptoms. Other chemokines, including MCP-1 and MIP-1 β, have also been associated with knee pain levels (88). Certain chemokines, however, may play a positive role. For example, the chemokine SDF-1 recruits mesenchymal progenitors during tissue repair (89).
How synovitis is triggered in OA, in which evidence of a systemic immune response or infection is lacking, is an area of current investigation. Disruption of the articular extracellular matrix is a hallmark of OA, and molecular products of extracellular matrix catabolism have been linked to inflammation at least through two mechanisms: stimulation of TLRs and activation of the complement cascade. TLRs are implicated in triggering cellular inflammation and repair responses to both pathogenic and endogenous “danger” signals produced during infection or non-infectious tissue injury (90). There are many putative endogenous TLR ligands, some of which are modified in form or concentration in OA. These include matrix components such as tenascin C (91), fibronectin isoforms (27, 28), and fragments of hyaluronic acid (92). TLR activation results in production of many chemokines (i.e. IL-8 and CCL5) and cytokines (i.e. IL-1, IL-6 and TNF) (90). TLR-4 deficiency reduced disease severity in a model of inflammatory arthritis (93), but efficacy of specific TLR deficiency or blockade has not yet been reported in models of OA. Recent data implicate TLR-2 and TLR-4 in promoting catabolism of murine cartilage explants in vitro (19).
Certain matrix components can activate the complement cascade. Fibromodulin (94), and cartilage oligomeric matrix protein (COMP) (34) activate the classical or alternative complement pathways respectively, while other matrix components act as inhibitors (95). Synovial complement deposition in patients with cartilage degeneration has been reported and may be increased during acute flares (96). Complement components are identified in OA synovial fluid (97), and in vesicles released from osteoarthritic cartilage in vitro (98) and a recent work in murine OA models demonstrated that C5 and C6 deficient mice are partially protected from the development of OA (99).
CONCLUSIONS
The modern definition of OA must include both patient-reported symptoms as well as structural changes within the joint, including not only the remodeling of articular cartilage and neighboring bone but also the synovial inflammation and damage to ligaments and menisci. Driven by mechanical factors, OA is an active response to injury, rather than a degenerative process. Now that we have started to gain a better understanding of processes affecting the individual tissues in the OA joint, there is a need to determine mechanisms of cross-talk and feedback among the tissues that are relevant to disease progression. Which factors released from bone and synovium are promoting cartilage degradation and which factors released from cartilage drive synovitis and bone remodeling? Is the meniscus caught in the cross-fire or also an active contributor to the altered milieu in the joint fluid. Will targeting one process in one tissue be sufficient to slow or reverse the changes that have occurred in the other tissues? Answering these and other questions relevant to OA will most likely require a systems approach which can integrate data from the molecular level, including genomics, epigenetics, proteomics and metabolomics, with data obtained at the structural level, including joint tissue remodeling, as well as biomechanical measures. Advances in imaging and biochemical markers will also be needed to address many of these questions as will a structure modifying therapy with proven and unequivocal benefit. Although not there yet, we are getting much closer to realizing the dream with a growing list of targets (Table 1) being tested in pre-clinical and early phase human studies.
Table 1.
Biological processes and mediators responsible for joint tissue destruction in OA and potential therapeutic interventions
| Biological Process | Proposed Mediators | Potential Therapeutics |
|---|---|---|
| Matrix degradation | MMP-1,-3,-9,-13, ADAMTs-4, -5, cathepsin K, serine proteases (Htra1) driven by cytokines (IL- 1,-6,-7,-8,-17,-18, OSM), chemokines (IL-8, GRO-α,-γ, RANTES, MCP-1) and others (S100 proteins, TGFα, matrix fragments, leukotrienes and prostaglandins) | Protease inhibitors, TIMPs, anti- cytokine therapy, TLR inhibition, MAP kinase inhibition, NFκB inhibition, lipoxygenease and cyclooxygenase inhibitors |
| Reduced matrix repair | ↓ activity of IGF-1, TGF-β, BMP-7 (OP-1), FGF-18 | Growth factors (IA or by gene therapy) |
| Cell death | ↓ HMGB2, ↓ autophagy, reactive oxygen and nitrogen species | Caspase inhibitors, anti-oxidants, iNOS inhibitors |
| Chondrocyte hypertrophy | RUNX2, HIF2α, WNT/β-catenin, IL-8 | PTH, calcitonin |
| Calcification and crystals | Transglutaminase, inorganic pyrophosphate, TLRs, NLRP3 | Phosphocitrates, TLR and NLRP3 inhibition |
| Subchondral bone sclerosis | WNT/β-catenin, ↓ sclerostin (SOST), BMPs, IGF-1 | Wnt or BMP antagonists, slowing bone remodeling with bisphosphonates or anti-RANKL |
| Osteophyte formation | TGF-β, BMP-2 | Since these may stabilize the joint should probably not be targeted directly |
| Focal bone remodelling (bone marrow lesions) | RANKL, VEGF | Bisphosphonates, anti-RANKL |
| Synovitis | IL-1β, TNFα, IL-17, IL-15, IL-7, CCL19, MCP-1, MIP-1β, S100 proteins/alarmins | Anti-cytokine therapy, TLR antagonism, complement inhibition |
MMP=Matrix Metalloproteinase; ADAMTS= A Disintegrin and Metalloproteinase with Thrombospondin Motifs; IL=Interleukin; OSM=Oncostatin M; GRO= Growth-related Oncogene; RANTES= Regulated upon Activation, Normal T-cell Expressed, and Secreted; MCP-1=Monocyte Chemotactic Protein-1; TGF=Transforming Growth Factor; TIMP=Tissue Inhibitor of Metalloproteinase; TLR=Toll-like Receptor; MAP=Mitogen Activated protein; IGF=Insulin-like Growth Factor; BMP=Bone Morphogenic Protein; FGF=Fibroblast Growth Factor; IA=Intra-articular; HMGB= High-mobility Group Box protein; iNOS=inducible Nitric Oxide Synthetase; RUNX=Runt-related Transcription factor; HIF=Hypoxia-induced Factor; PTH=Parathyroid Hormone; NLRP3=NOD-like receptor family, pryin domain containing 3; RANKL= Receptor Activator of Nuclear factor Kappa-B Ligand; VEGF=Vascular Endothelial Growth Factor; TNF=Tumor Necrosis Factor; CCL=Chemokine (C-C motif) Ligand; MIP=Macrophage Inflammatory Protein.
Acknowledgments
Supported by grants from the National Institutes of Health (AG16697, AR49003, K08AR057859, AG022021, AR060546) and the Arthritis Foundation.
Contributor Information
Richard F. Loeser, Department of Internal Medicine, Section of Molecular Medicine, Wake Forest University School of Medicine, Winston-Salem, NC, USA.
Steven R. Goldring, Chief Scientific Officer and Richard L. Menschel Chair, The Hospital for Special Surgery and Department of Medicine, Weill Cornell Medical College, New York, New York, USA.
Carla R. Scanzello, Department of Internal Medicine, Section of Rheumatology, Rush Medical College, Chicago, IL, USA.
Mary B. Goldring, The Hospital for Special Surgery and Department of Cell and Developmental Biology, Weill Cornell Medical College, New York, New York, USA.
References
- 1.Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation --- United States, 2007–2009. MMWR Morb Mortal Wkly Rep. 2010;59:1261–5. [PubMed] [Google Scholar]
- 2.Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, et al. Osteoarthritis: New Insights. Part 1: The Disease and Its Risk Factors. Ann Intern Med. 2000;133:635–46. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 3.Blagojevic M, Jinks C, Jeffery A, Jordan KP. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2010;18:24–33. doi: 10.1016/j.joca.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Greene GW, Banquy X, Lee DW, Lowrey DD, Yu J, Israelachvili JN. Adaptive mechanically controlled lubrication mechanism found in articular joints. Proc Natl Acad Sci U S A. 2011;108:5255–9. doi: 10.1073/pnas.1101002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andriacchi TP, Mundermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32:447–57. doi: 10.1023/b:abme.0000017541.82498.37. [DOI] [PubMed] [Google Scholar]
- 6.Goldring MB, Marcu KB. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res Ther. 2009;11:224. doi: 10.1186/ar2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Troeberg L, Nagase H. Proteases involved in cartilage matrix degradation in osteoarthritis. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbapap.2011.06.020. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–8. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 9.Little CB, Barai A, Burkhardt D, Smith SM, Fosang AJ, Werb Z, et al. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009;60:3723–33. doi: 10.1002/art.25002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polur I, Lee PL, Servais JM, Xu L, Li Y. Role of HTRA1, a serine protease, in the progression of articular cartilage degeneration. Histol Histopathol. 2010;25:599–608. doi: 10.14670/hh-25.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu L, Servais J, Polur I, Kim D, Lee PL, Chung K, et al. Attenuation of osteoarthritis progression by reduction of discoidin domain receptor 2 in mice. Arthritis Rheum. 2010;62:2736–44. doi: 10.1002/art.27582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Echtermeyer F, Bertrand J, Dreier R, Meinecke I, Neugebauer K, Fuerst M, et al. Syndecan-4 regulates ADAMTS-5 activation and cartilage breakdown in osteoarthritis. Nat Med. 2009;15:1072–6. doi: 10.1038/nm.1998. [DOI] [PubMed] [Google Scholar]
- 13.Yang S, Kim J, Ryu JH, Oh H, Chun CH, Kim BJ, et al. Hypoxia-inducible factor-2alpha is a catabolic regulator of osteoarthritic cartilage destruction. Nat Med. 2010;16:687–93. doi: 10.1038/nm.2153. [DOI] [PubMed] [Google Scholar]
- 14.Tetsunaga T, Nishida K, Furumatsu T, Naruse K, Hirohata S, Yoshida A, et al. Regulation of mechanical stress-induced MMP-13 and ADAMTS-5 expression by RUNX-2 transcriptional factor in SW1353 chondrocyte-like cells. Osteoarthritis Cartilage. 2011;19:222–32. doi: 10.1016/j.joca.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto K, Oreffo RO, Gibson MB, Goldring MB, Roach HI. DNA demethylation at specific CpG sites in the IL1B promoter in response to inflammatory cytokines in human articular chondrocytes. Arthritis Rheum. 2009;60:3303–13. doi: 10.1002/art.24882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dudek KA, Lafont JE, Martinez-Sanchez A, Murphy CL. Type II collagen expression is regulated by tissue-specific miR-675 in human articular chondrocytes. J Biol Chem. 2010;285:24381–7. doi: 10.1074/jbc.M110.111328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyaki S, Sato T, Inoue A, Otsuki S, Ito Y, Yokoyama S, et al. MicroRNA-140 plays dual roles in both cartilage development and homeostasis. Genes Dev. 2010;24:1173–85. doi: 10.1101/gad.1915510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geurts J, van den Brand BT, Wolf A, Abdollahi-Roodsaz S, Arntz OJ, Kracht M, et al. Toll-like receptor 4 signalling is specifically TGF-beta-activated kinase 1 independent in synovial fibroblasts. Rheumatology (Oxford) 2011 doi: 10.1093/rheumatology/ker021. [DOI] [PubMed] [Google Scholar]
- 19.Liu-Bryan R, Terkeltaub R. Chondrocyte innate immune myeloid differentiation factor 88-dependent signaling drives procatabolic effects of the endogenous Toll-like receptor 2/Toll-like receptor 4 ligands low molecular weight hyaluronan and high mobility group box chromosomal protein 1 in mice. Arthritis Rheum. 2010;62:2004–12. doi: 10.1002/art.27475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinola T, Kouri VP, Clarijs P, Ciferska H, Sukura A, Salo J, et al. High mobility group box-1 (HMGB-1) in osteoarthritic cartilage. Clin Exp Rheumatol. 2010;28:511–8. [PubMed] [Google Scholar]
- 21.Garcia-Arnandis I, Guillen MI, Gomar F, Pelletier JP, Martel-Pelletier J, Alcaraz MJ. High mobility group box 1 potentiates the pro-inflammatory effects of interleukin-1beta in osteoarthritic synoviocytes. Arthritis Res Ther. 2010;12:R165. doi: 10.1186/ar3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yammani RR, Carlson CS, Bresnick AR, Loeser RF. Increase in production of matrix metalloproteinase 13 by human articular chondrocytes due to stimulation with S100A4: Role of the receptor for advanced glycation end products. Arthritis Rheum. 2006;54:2901–11. doi: 10.1002/art.22042. [DOI] [PubMed] [Google Scholar]
- 23.Zreiqat H, Belluoccio D, Smith MM, Wilson R, Rowley LA, Jones K, et al. S100A8 and S100A9 in experimental osteoarthritis. Arthritis Res Ther. 2010;12:R16. doi: 10.1186/ar2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blanco FJ, Rego I, Ruiz-Romero C. The role of mitochondria in osteoarthritis. Nat Rev Rheumatol. 2011;7:161–9. doi: 10.1038/nrrheum.2010.213. [DOI] [PubMed] [Google Scholar]
- 25.Luyten FP, Tylzanowski P, Lories RJ. Wnt signaling and osteoarthritis. Bone. 2009;44:522–7. doi: 10.1016/j.bone.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Blom AB, Brockbank SM, van Lent PL, van Beuningen HM, Geurts J, Takahashi N, et al. Involvement of the Wnt signaling pathway in experimental and human osteoarthritis: prominent role of Wnt-induced signaling protein 1. Arthritis Rheum. 2009;60:501–12. doi: 10.1002/art.24247. [DOI] [PubMed] [Google Scholar]
- 27.Homandberg GA, Wen C, Hui F. Cartilage damaging activities of fibronectin fragments derived from cartilage and synovial fluid. Osteoarthritis Cartilage. 1998;6:231–44. doi: 10.1053/joca.1998.0116. [DOI] [PubMed] [Google Scholar]
- 28.Zack MD, Arner EC, Anglin CP, Alston JT, Malfait AM, Tortorella MD. Identification of fibronectin neoepitopes present in human osteoarthritic cartilage. Arthritis Rheum. 2006;54:2912–22. doi: 10.1002/art.22045. [DOI] [PubMed] [Google Scholar]
- 29.Melrose J, Fuller ES, Roughley PJ, Smith MM, Kerr B, Hughes CE, et al. Fragmentation of decorin, biglycan, lumican and keratocan is elevated in degenerate human meniscus, knee and hip articular cartilages compared with age-matched macroscopically normal and control tissues. Arthritis Res Ther. 2008;10:R79. doi: 10.1186/ar2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bank RA, Krikken M, Beekman B, Stoop R, Maroudas A, Lafeber FP, et al. A simplified measurement of degraded collagen in tissues: application in healthy, fibrillated and osteoarthritic cartilage. Matrix Biol. 1997;16:233–43. doi: 10.1016/s0945-053x(97)90012-3. [DOI] [PubMed] [Google Scholar]
- 31.Pulai JI, Chen H, Im HJ, Kumar S, Hanning C, Hegde PS, et al. NF-{kappa}B Mediates the Stimulation of Cytokine and Chemokine Expression by Human Articular Chondrocytes in Response to Fibronectin Fragments. J Immunol. 2005;174:5781–8. doi: 10.4049/jimmunol.174.9.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fichter M, Korner U, Schomburg J, Jennings L, Cole AA, Mollenhauer J. Collagen degradation products modulate matrix metalloproteinase expression in cultured articular chondrocytes. J Orthop Res. 2006;24:63–70. doi: 10.1002/jor.20001. [DOI] [PubMed] [Google Scholar]
- 33.Heinegard D, Saxne T. The role of the cartilage matrix in osteoarthritis. Nat Rev Rheumatol. 2011;7:50–6. doi: 10.1038/nrrheum.2010.198. [DOI] [PubMed] [Google Scholar]
- 34.Happonen KE, Saxne T, Aspberg A, Morgelin M, Heinegard D, Blom AM. Regulation of complement by cartilage oligomeric matrix protein allows for a novel molecular diagnostic principle in rheumatoid arthritis. Arthritis Rheum. 2010;62:3574–83. doi: 10.1002/art.27720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loeser RF. Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthritis Cartilage. 2009;17:971–9. doi: 10.1016/j.joca.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taniguchi N, Carames B, Ronfani L, Ulmer U, Komiya S, Bianchi ME, et al. Aging-related loss of the chromatin protein HMGB2 in articular cartilage is linked to reduced cellularity and osteoarthritis. Proc Natl Acad Sci U S A. 2009;106:1181–6. doi: 10.1073/pnas.0806062106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Del Carlo M, Jr, Loeser RF. Cell death in osteoarthritis. Curr Rheumatol Rep. 2008;10:37–42. doi: 10.1007/s11926-008-0007-8. [DOI] [PubMed] [Google Scholar]
- 38.Carames B, Taniguchi N, Otsuki S, Blanco FJ, Lotz M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010;62:791–801. doi: 10.1002/art.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.D’Lima D, Hermida J, Hashimoto S, Colwell C, Lotz M. Caspase inhibitors reduce severity of cartilage lesions in experimental osteoarthritis. Arthritis Rheum. 2006;54:1814–21. doi: 10.1002/art.21874. [DOI] [PubMed] [Google Scholar]
- 40.Ea HK, Nguyen C, Bazin D, Bianchi A, Guicheux J, Reboul P, et al. Articular cartilage calcification in osteoarthritis: insights into crystal-induced stress. Arthritis Rheum. 2011;63:10–8. doi: 10.1002/art.27761. [DOI] [PubMed] [Google Scholar]
- 41.Musacchio E, Ramonda R, Perissinotto E, Sartori L, Hirsch R, Punzi L, et al. The impact of knee and hip chondrocalcinosis on disability in older people: the ProVA Study from northeastern Italy. Ann Rheum Dis. 2011 doi: 10.1136/ard.2011.150508. (in press) [DOI] [PubMed] [Google Scholar]
- 42.Liu-Bryan R, Pritzker K, Firestein GS, Terkeltaub R. TLR2 signaling in chondrocytes drives calcium pyrophosphate dihydrate and monosodium urate crystal-induced nitric oxide generation. J Immunol. 2005;174:5016–23. doi: 10.4049/jimmunol.174.8.5016. [DOI] [PubMed] [Google Scholar]
- 43.Jin C, Frayssinet P, Pelker R, Cwirka D, Hu B, Vignery A, et al. NLRP3 inflammasome plays a critical role in the pathogenesis of hydroxyapatite-associated arthropathy. Proc Natl Acad Sci U S A. 2011;108:14867–72. doi: 10.1073/pnas.1111101108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Englund M, Guermazi A, Gale D, Hunter DJ, Aliabadi P, Clancy M, et al. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med. 2008;359:1108–15. doi: 10.1056/NEJMoa0800777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Englund M, Guermazi A, Roemer FW, Aliabadi P, Yang M, Lewis CE, et al. Meniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons: The Multicenter Osteoarthritis Study. Arthritis Rheum. 2009;60:831–9. doi: 10.1002/art.24383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hill CL, Seo GS, Gale D, Totterman S, Gale ME, Felson DT. Cruciate ligament integrity in osteoarthritis of the knee. Arthritis Rheum. 2005;52:794–9. doi: 10.1002/art.20943. [DOI] [PubMed] [Google Scholar]
- 47.Katsuragawa Y, Saitoh K, Tanaka N, Wake M, Ikeda Y, Furukawa H, et al. Changes of human menisci in osteoarthritic knee joints. Osteoarthritis Cartilage. 2010;18:1133–43. doi: 10.1016/j.joca.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 48.Pauli C, Grogan SP, Patil S, Otsuki S, Hasegawa A, Koziol J, et al. Macroscopic and histopathologic analysis of human knee menisci in aging and osteoarthritis. Osteoarthritis Cartilage. 2011;19:1132–41. doi: 10.1016/j.joca.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ashraf S, Wibberley H, Mapp PI, Hill R, Wilson D, Walsh DA. Increased vascular penetration and nerve growth in the meniscus: a potential source of pain in osteoarthritis. Ann Rheum Dis. 2011;70:523–9. doi: 10.1136/ard.2010.137844. [DOI] [PubMed] [Google Scholar]
- 50.Sharma L, Lou C, Felson DT, Dunlop DD, Kirwan-Mellis G, Hayes KW, et al. Laxity in healthy and osteoarthritic knees. Arthritis Rheum. 1999;42:861–70. doi: 10.1002/1529-0131(199905)42:5<861::AID-ANR4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 51.Watanabe A, Kanamori A, Ikeda K, Ochiai N. Histological evaluation and comparison of the anteromedial and posterolateral bundle of the human anterior cruciate ligament of the osteoarthritic knee joint. The Knee. 2011;18:47–50. doi: 10.1016/j.knee.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 52.Mullaji AB, Marawar SV, Simha M, Jindal G. Cruciate ligaments in arthritic knees: a histologic study with radiologic correlation. The Journal of arthroplasty. 2008;23:567–72. doi: 10.1016/j.arth.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 53.Hasegawa A, Otsuki S, Pauli C, Miyaki S, Patil S, Steklov N, et al. Anterior cruciate ligament changes in human joint in aging and osteoarthritis. Arthritis Rheum. 2011 doi: 10.1002/art.33417. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frost HM. Perspective: genetic and hormonal roles in bone disorders: insights of an updated bone physiology. J Musculoskelet Neuronal Interact. 2003;3:118–35. [PubMed] [Google Scholar]
- 55.Taljanovic MS, Graham AR, Benjamin JB, Gmitro AF, Krupinski EA, Schwartz SA, et al. Bone marrow edema pattern in advanced hip osteoarthritis: quantitative assessment with magnetic resonance imaging and correlation with clinical examination, radiographic findings, and histopathology. Skeletal Radiol. 2008;37:423–31. doi: 10.1007/s00256-008-0446-3. [DOI] [PubMed] [Google Scholar]
- 56.van der Kraan PM, van den Berg WB. Osteophytes: relevance and biology. Osteoarthritis Cartilage. 2007;15:237–44. doi: 10.1016/j.joca.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 57.Zoricic S, Maric I, Bobinac D, Vukicevic S. Expression of bone morphogenetic proteins and cartilage-derived morphogenetic proteins during osteophyte formation in humans. J Anat. 2003;202:269–77. doi: 10.1046/j.1469-7580.2003.00158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blaney Davidson EN, van der Kraan PM, van den Berg WB. TGF-beta and osteoarthritis. Osteoarthritis Cartilage. 2007;15:597–604. doi: 10.1016/j.joca.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 59.Walsh DA, Bonnet CS, Turner EL, Wilson D, Situ M, McWilliams DF. Angiogenesis in the synovium and at the osteochondral junction in osteoarthritis. Osteoarthritis Cartilage. 2007;15:743–51. doi: 10.1016/j.joca.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 60.Walsh DA, McWilliams DF, Turley MJ, Dixon MR, Franses RE, Mapp PI, et al. Angiogenesis and nerve growth factor at the osteochondral junction in rheumatoid arthritis and osteoarthritis. Rheumatology (Oxford) 2010;49:1852–61. doi: 10.1093/rheumatology/keq188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ashraf S, Mapp PI, Walsh DA. Contributions of angiogenesis to inflammation, joint damage and pain in a rat model of osteoarthritis. Arthritis Rheum. 2011 doi: 10.1002/art.30422. (in press) [DOI] [PubMed] [Google Scholar]
- 62.Donnelly E, Chen DX, Boskey AL, Baker SP, van der Meulen MC. Contribution of mineral to bone structural behavior and tissue mechanical properties. Calcif Tissue Int. 2010;87:450–60. doi: 10.1007/s00223-010-9404-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Day JS, Ding M, van der Linden JC, Hvid I, Sumner DR, Weinans H. A decreased subchondral trabecular bone tissue elastic modulus is associated with pre-arthritic cartilage damage. J Orthop Res. 2001;19:914–8. doi: 10.1016/S0736-0266(01)00012-2. [DOI] [PubMed] [Google Scholar]
- 64.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626–34. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 65.Karvonen RL, Miller PR, Nelson DA, Granda JL, Fernandez-Madrid F. Periarticular osteoporosis in osteoarthritis of the knee. J Rheumatol. 1998;25:2187–94. [PubMed] [Google Scholar]
- 66.Messent EA, Ward RJ, Tonkin CJ, Buckland-Wright C. Tibial cancellous bone changes in patients with knee osteoarthritis. A short-term longitudinal study using Fractal Signature Analysis. Osteoarthritis Cartilage. 2005;13:463–70. doi: 10.1016/j.joca.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 67.Messent EA, Buckland-Wright JC, Blake GM. Fractal analysis of trabecular bone in knee osteoarthritis (OA) is a more sensitive marker of disease status than bone mineral density (BMD) Calcif Tissue Int. 2005;76:419–25. doi: 10.1007/s00223-004-0160-7. [DOI] [PubMed] [Google Scholar]
- 68.Chan BY, Fuller ES, Russell AK, Smith SM, Smith MM, Jackson MT, et al. Increased chondrocyte sclerostin may protect against cartilage degradation in osteoarthritis. Osteoarthritis Cartilage. 2011;19:874–85. doi: 10.1016/j.joca.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 69.Bingham CO, 3rd, Buckland-Wright JC, Garnero P, Cohen SB, Dougados M, Adami S, et al. Risedronate decreases biochemical markers of cartilage degradation but does not decrease symptoms or slow radiographic progression in patients with medial compartment osteoarthritis of the knee: results of the two-year multinational knee osteoarthritis structural arthritis study. Arthritis Rheum. 2006;54:3494–507. doi: 10.1002/art.22160. [DOI] [PubMed] [Google Scholar]
- 70.Pessler F, Dai L, Diaz-Torne C, Gomez-Vaquero C, Paessler ME, Zheng DH, et al. The synovitis of “non-inflammatory” orthopaedic arthropathies: a quantitative histological and immunohistochemical analysis. Ann Rheum Dis. 2008;67:1184–7. doi: 10.1136/ard.2008.087775. [DOI] [PubMed] [Google Scholar]
- 71.Benito MJ, Veale DJ, FitzGerald O, van den Berg WB, Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64:1263–7. doi: 10.1136/ard.2004.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scanzello CR, McKeon B, Swaim BH, DiCarlo E, Asomugha EU, Kanda V, et al. Synovial inflammation in patients undergoing arthroscopic meniscectomy: molecular characterization and relationship to symptoms. Arthritis Rheum. 2011;63:391–400. doi: 10.1002/art.30137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krasnokutsky S, Belitskaya-Levy I, Bencardino J, Samuels J, Attur M, Regatte R, et al. Quantitative MRI evidence of synovial proliferation is associated with radiographic severity of knee osteoarthritis. Arthritis Rheum. 2011 doi: 10.1002/art.30471. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pearle AD, Scanzello CR, George S, Mandl LA, DiCarlo EF, Peterson M, et al. Elevated high-sensitivity C-reactive protein levels are associated with local inflammatory findings in patients with osteoarthritis. Osteoarthritis Cartilage. 2007;15:516–23. doi: 10.1016/j.joca.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 75.Ayral X, Pickering EH, Woodworth TG, Mackillop N, Dougados M. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis -- results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage. 2005;13:361–7. doi: 10.1016/j.joca.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 76.Hill CL, Hunter DJ, Niu J, Clancy M, Guermazi A, Genant H, et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2007;66:1599–603. doi: 10.1136/ard.2006.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roemer FW, Guermazi A, Felson DT, Niu J, Nevitt MC, Crema MD, et al. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: the MOST study. Ann Rheum Dis. 2011 doi: 10.1136/ard.2011.150243. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Conaghan PG, D’Agostino MA, Le Bars M, Baron G, Schmidely N, Wakefield R, et al. Clinical and ultrasonographic predictors of joint replacement for knee osteoarthritis: results from a large, 3-year, prospective EULAR study. Ann Rheum Dis. 2010;69:644–7. doi: 10.1136/ard.2008.099564. [DOI] [PubMed] [Google Scholar]
- 79.Torres L, Dunlop DD, Peterfy C, Guermazi A, Prasad P, Hayes KW, et al. The relationship between specific tissue lesions and pain severity in persons with knee osteoarthritis. Osteoarthritis Cartilage. 2006;14:1033–40. doi: 10.1016/j.joca.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 80.Sowers M, Karvonen-Gutierrez CA, Jacobson JA, Jiang Y, Yosef M. Associations of anatomical measures from MRI with radiographically defined knee osteoarthritis score, pain, and physical functioning. J Bone Joint Surg Am. 2011;93:241–51. doi: 10.2106/JBJS.I.00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cohen SB, Proudman S, Kivitz AJ, Burch FX, Donohue JP, Burstein D, et al. A randomized, double-blind study of AMG 108 (a fully human monoclonal antibody to IL 1R1) in patients with osteoarthritis of the knee. Arthritis Res Ther. 2011;13:R125. doi: 10.1186/ar3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Magnano MD, Chakravarty EF, Broudy C, Chung L, Kelman A, Hillygus J, et al. A pilot study of tumor necrosis factor inhibition in erosive/inflammatory osteoarthritis of the hands. J Rheumatol. 2007;34:1323–7. [PubMed] [Google Scholar]
- 83.Ling SM, Patel DD, Garnero P, Zhan M, Vaduganathan M, Muller D, et al. Serum protein signatures detect early radiographic osteoarthritis. Osteoarthritis Cartilage. 2009;17:43–8. doi: 10.1016/j.joca.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scanzello CR, Umoh E, Pessler F, Diaz-Torne C, Miles T, Dicarlo E, et al. Local cytokine profiles in knee osteoarthritis: elevated synovial fluid interleukin-15 differentiates early from end-stage disease. Osteoarthritis Cartilage. 2009;17:1040–8. doi: 10.1016/j.joca.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 85.Honorati MC, Bovara M, Cattini L, Piacentini A, Facchini A. Contribution of interleukin 17 to human cartilage degradation and synovial inflammation in osteoarthritis. Osteoarthritis Cartilage. 2002;10:799–807. doi: 10.1053/joca.2002.0829. [DOI] [PubMed] [Google Scholar]
- 86.Chen L, Li DQ, Zhong J, Wu XL, Chen Q, Peng H, et al. IL-17RA aptamer-mediated repression of IL-6 inhibits synovium inflammation in a murine model of osteoarthritis. Osteoarthritis Cartilage. 2011;19:711–8. doi: 10.1016/j.joca.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 87.Endres M, Andreas K, Kalwitz G, Freymann U, Neumann K, Ringe J, et al. Chemokine profile of synovial fluid from normal, osteoarthritis and rheumatoid arthritis patients: CCL25, CXCL10 and XCL1 recruit human subchondral mesenchymal progenitor cells. Osteoarthritis Cartilage. 2010;18:1458–66. doi: 10.1016/j.joca.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 88.Cuellar JM, Scuderi GJ, Cuellar VG, Golish SR, Yeomans DC. Diagnostic utility of cytokine biomarkers in the evaluation of acute knee pain. J Bone Joint Surg Am. 2009;91:2313–20. doi: 10.2106/JBJS.H.00835. [DOI] [PubMed] [Google Scholar]
- 89.Miller RJ, Banisadr G, Bhattacharyya BJ. CXCR4 signaling in the regulation of stem cell migration and development. J Neuroimmunol. 2008;198:31–8. doi: 10.1016/j.jneuroim.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 91.Midwood K, Sacre S, Piccinini AM, Inglis J, Trebaul A, Chan E, et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med. 2009;15:774–80. doi: 10.1038/nm.1987. [DOI] [PubMed] [Google Scholar]
- 92.Belcher C, Yaqub R, Fawthrop F, Bayliss M, Doherty M. Synovial fluid chondroitin and keratan sulphate epitopes, glycosaminoglycans, and hyaluronan in arthritic and normal knees. Ann Rheum Dis. 1997;56:299–307. doi: 10.1136/ard.56.5.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abdollahi-Roodsaz S, Joosten LA, Koenders MI, Devesa I, Roelofs MF, Radstake TR, et al. Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J Clin Invest. 2008;118:205–16. doi: 10.1172/JCI32639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sjoberg A, Onnerfjord P, Morgelin M, Heinegard D, Blom AM. The extracellular matrix and inflammation: fibromodulin activates the classical pathway of complement by directly binding C1q. J Biol Chem. 2005;280:32301–8. doi: 10.1074/jbc.M504828200. [DOI] [PubMed] [Google Scholar]
- 95.Kalchishkova N, Furst CM, Heinegard D, Blom AM. NC4 Domain of Cartilage-specific Collagen IX Inhibits Complement Directly Due to Attenuation of Membrane Attack Formation and Indirectly through Binding and Enhancing Activity of Complement Inhibitors C4B-binding Protein and Factor H. J Biol Chem. 2011;286:27915–26. doi: 10.1074/jbc.M111.242834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Konttinen YT, Ceponis A, Meri S, Vuorikoski A, Kortekangas P, Sorsa T, et al. Complement in acute and chronic arthritides: assessment of C3c, C9, and protectin (CD59) in synovial membrane. Ann Rheum Dis. 1996;55:888–94. doi: 10.1136/ard.55.12.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gobezie R, Kho A, Krastins B, Sarracino DA, Thornhill TS, Chase M, et al. High abundance synovial fluid proteome: distinct profiles in health and osteoarthritis. Arthritis Res Ther. 2007;9:R36. doi: 10.1186/ar2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rosenthal AK, Gohr CM, Ninomiya J, Wakim BT. Proteomic analysis of articular cartilage vesicles from normal and osteoarthritic cartilage. Arthritis Rheum. 2011;63:401–11. doi: 10.1002/art.30120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang Q, Rozelle A, Lepus C, Scanzello C, Song J, Larsen D, et al. Identification of a central role for complement in osteoarthritis. Nature Medicine. 2011 doi: 10.1038/nm.2543. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]