Abstract
Background
More than a quarter of ischemic strokes (IS) are excluded from thrombolysis due to unknown time of symptom onset. Recent evidence suggests that a mismatch between DWI and FLAIR imaging could be used as a surrogate for the time of stroke onset. We compared used the DWI–FLAIR mismatch and the FLAIR/DWI ratio to estimate the time of onset in a group of patients with nocturnal strokes and unknown time of onset.
Methods
We used a prospectively collected acute IS patient database with MRI as the initial imaging modality. Nineteen selected nocturnal stroke patients with unknown time of onset were compared with 22 patients who had an MRI within 6 hours from stroke onset (control A) and 19 patients who had an MRI between 6 and 12 hours (control B). DWI and FLAIR signal was rated as normal or abnormal. FLAIR/DWI ratio was calculated from independent DWI and FLAIR ischemic lesion volumes using semiautomatic software.
Results
The DWI–FLAIR mismatch was different among groups (unknown 43.7%, control A 63.6%, control B 10.5%; FFH p=0.001). There were significant differences in FLAIR/DWI ratio among the 3 groups (unknown: 0.05±0.12, control A: 0.17±0.15, control B: 0.04±0.06; KW p<0.0001). Post-hoc pair wise comparisons showed that FLAIR/DWI ratio from the unknown group was significantly different from control B (p=0.0045), but not different from control A. DWI volumes were not different among the 3 groups.
Conclusion
A large proportion of nocturnal IS patients with unknown time of stroke initiation have a DWI-FLAIR mismatch suggesting a recent stroke onset.
Keywords: Acute ischemic stroke, Fluid Attenuated Inversion Recovery, Diffusion-Weighted Imaging, circadian pattern, sleep
BACKGROUND
Intravenous tissue plasminogen activator (t-PA) is the only proven treatment for acute ischemic stroke (AIS) improving neurological outcome when administered within 4.5 hours of symptom onset.[1] Approximately a quarter of AIS present upon awakening from sleep.[2] These patients are denied thrombolytic therapy and excluded from acute clinical trials because the time of symptom onset is uncertain. To date, there is no specific biomarker to age stroke and clinicians still depend on the information provided by patients or bystanders to decide if the patient is eligible for thrombolysis.
Recent studies have demonstrated that a “mismatch” between an abnormal Diffusion-Weighted Imaging (DWI) signal and a normal Fluid-Attenuated Inversion Recovery (FLAIR) on brain MRI may function as a surrogate for determining the age of the stroke in patients with AIS with uncertain time of symptom onset, permitting reperfusion therapies.[3–7] These methods show high specificity and positive predictive value for identifying patients within 4·5 h of symptom onset; however, it has not proved adequate sensitivity.[6] Most of these studies used visual inspection of the MRI images to determine the DWI–FLAIR mismatch. Nonetheless, volumetric analysis of the diffusion– perfusion mismatch has been shown to more accurate than simple visual assessment in determining the ischemic penumbra. [8]
In this study we used visual inspection and volumetric analyses of the DWI–FLAIR mismatch to estimate the age of acute ischemic stroke in patients who presented with stroke symptoms upon awakening from sleep. We also studies whether the ratio of calculated volumes from hyperintense FLAIR and DWI signals would be a better method than the DWI–FLAIR mismatch for estimating the age of a stroke of unknown time onset.
METHODS
Patients
A prospectively collected AIS imaging database of patient presented within the 12 hours of the stroke onset was screened for patients who had MRI as initial brain imaging modality. We chose group of patients with unknown time of onset with high probability of having their stroke while sleep. The selection criteria for these patients was: presented with AIS symptoms, unknown time of stroke onset, last seen normal more than 6 hours ago, with an arrival to the Emergency Department (ED) between 4am and 10am and having an initial brain MRI performed within 3 hours from ED arrival. Two patient control groups (A and B) with known time from stroke onset were chosen as controls. Control A received brain MRI within 6 hours from stroke onset and control B received brain MRI between 6 and 12 hours. Signed consent to provide imaging data was obtained from patients or patient’s surrogates. The study was approved by the University of California San Diego Institutional Review Board.
Magnetic Resonance Protocol
All patients underwent brain MRI (1.5T, Siemens Medical System) before any type of recanalization therapy. The MRI protocol included DWI (b=1000), Gradient-Recalled Echo, FLAIR, and Perfusion Weighted Imaging sequences, with previously described MRI methodology.[9] Only FLAIR and DWI (b=1000) sequences were used for this study.
Imaging Analysis
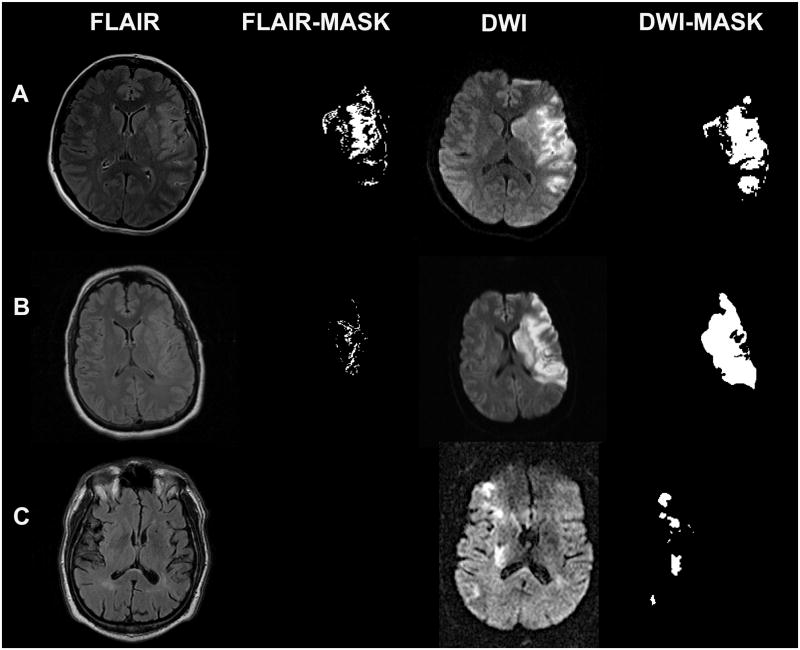
Four stroke physicians (BH, BCM, TH, BMM) with ample experience in analyzing acute stroke MRI imaging, blinded to all clinical data, independently interpreted FLAIR and DWI sequences. For rating images, the dichotomized choices were presence or absence of abnormal hyperintense signal suggestive of acute brain ischemia. Raters were allowed modify the contrasting level to display images with optimal contrast for lesion identification. They were instructed to judge lesion visibility using the DWI signal to identify only the changes of FLAIR parenchymal hyperintensity, disregarding other possible imaging signs of acute ischemic stroke, such as hyperintense vessels on FLAIR. For the volumetric analysis, first areas of abnormal DWI and FLAIR signal were obtained semi-automatically using maximal entropy threshold (Image J v1.42). (Figure 1) Then, manual thresholds were used in cases when the software was not able to depict the correct area (0% in DWI and 30% in FLAIR sequences). The FLAIR and DWI lesion volumes were calculated by adding all areas of abnormal hyperintense signal, multiplying by the distance between the slices. The FLAIR/DWI ratio was defined as the FLAIR lesion volume divided by the DWI lesion volume. Patients with DWI volumes equal to zero were excluded from the mismatch and imaging analysis. FLAIR imaging with extensive leukoaraiosis and imaging sequences with significant motion or artifacts were also excluded from the analysis. Neither small strokes nor lacunars were excluded.
Figure 1.
Lesion mask from abnormal hyperintense signal obtained using max threshold entropy (Image J v1.42). Row A corresponds to a patient imaging at 6hrs and 50 minutes from the onset. It shows an abnormal FLAIR signal that was identified by all raters. The FLAIR/DWI volume ratio was 8.2%. Row B corresponds to a patient imaging at 5hrs from the symptoms onset, FLAIR was rated as normal by 2 physicians and as abnormal by 2 others. The calculation of the FLAIR/DWI volume ratio was of 2.2%. The FLAIR imaging in row C was rated as normal by all physicians. No abnormal signal was obtained using automatic or manual thresholds. Patient had MRI at 2hrs from the stroke onset.
Statistical Analysis
Associations were assessed using the Kruskal-Wallis (KW) test for continuous data with Holm-adjusted pair wise Wilcoxon Rank Sum (WRS) for multiple pair comparisons and Fisher-Freeman-Halton (FFH) test categorical data. The final lesion score was obtained when consensus from at least three raters was reached. In cases of 50% or greater disagreement between the four raters, we used the imaging software as a fifth rater to obtain consensus. The final DWI–FLAIR mismatch and FLAIR/DWI ratio were plotted over time of stroke onset, and the associations between these analyzed using the Spearman correlation coefficient. Percentage of agreement and Fleiss Kappa ( ) was used to obtain the concordance and inter-rater agreement among the four raters. All analyses were performed using the statistical software program, R 2.9.2.
RESULTS
There were 22 nocturnal stroke patients with unknown time of onset, 19 patients in control A and patients 22 control B. Admission NIHSS was higher in the control A (11.2±9.3 vs. 17.1± 8.3 vs. 11.7±6.7; p=0.028) and hypertension more prevalent the control B (81.8% vs. 59.1% vs. 36.8%; p=0.012). All other baseline characteristics were similar among groups (Table 1)
Table 1.
Baseline Characteristics
| Wake-up N=22 |
CONTROL - A (0–6 hrs.) N=22 |
CONTROL - B (6–12 hrs.) N=19 |
P-value | |
|---|---|---|---|---|
| Age | 69.9±14.2 | 66.4±19.8 | 57.7±16.4 | ns† |
| Female No,% | 13 (59.1) | 9 (40.9) | 9 (47.3) | ns‡ |
| Hypertension | 18 (81.8) | 13 (59.1) | 7 (36.8) | 0.01‡ |
| Diabetes | 6 (27.3) | 5 (22.7) | 2 (10.5) | ns‡ |
| Baseline NIHSS | 11.2±9.3 | 17.10±8.3 | 11.67±6.7 | 0.03† |
| Median | 8.5 | 19 | 11.5 | |
| Q1 | 3 | 11 | 6.5 | |
| Q3 | 18.25 | 21 | 17 | |
| H/o stroke | 6 (27.3) | 3 (13.6) | 3 (15.8) | ns‡ |
| CAD | 5 (22.7) | 3 (13.6) | 2 (10.5) | ns‡ |
| Dyslipidemia | 5 (22.7) | 5 (22.7) | 4 (21.1) | ns‡ |
| Tobacco use | 1 (4.6) | 2 (9.1) | 5 (26.3) | ns‡ |
| Time from stroke onset symptoms to MRI | n/a | 193.0±97.2 | 427.0±81.0 | < 0.0001* |
| Time from arrival to ED to MRI scan | 71.6±46.0 | 63.4±57.4 | 65.9±71.3 | ns† |
Kruskal Wallis Test
Fisher-Freeman-Halton Test
ns: non-significant
Wilcoxon Rank-Sum Test
DWI–FLAIR mismatch FLAIR/DWI ratio
Three patients from the unknown group were excluded from the imaging analysis due to unsuitable FLAIR images. Twenty-two (100%) control A patients, 19 (100%) control B patients and 16 (84.2%) patients in unknown group had an abnormal hyperintense DWI signal. DWI volumes were not different among the three groups (Unknown group 22.4±35 ml; control A: 33.3±42.2 ml, control B: 26.3±39.1ml; KW p=0.21). The DWI lesion volume was smaller in the unknown group when comparing against combined control groups but such differences did not reach statistical significance (22.4±35 ml vs. 30.1±40.4 ml; WRS p=0.082).
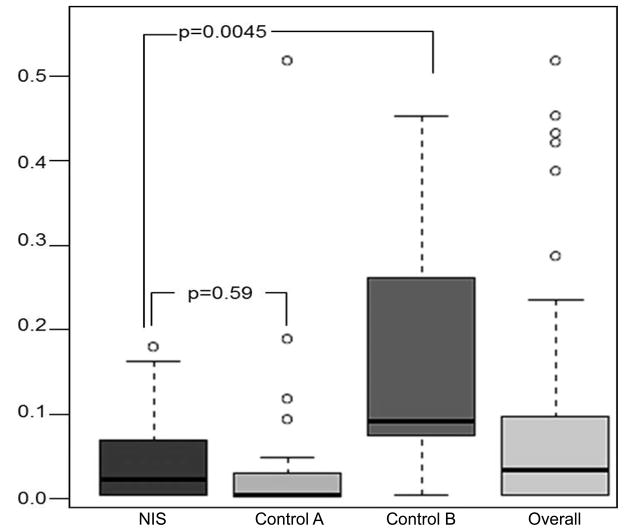
FLAIR was considered normal or negative in 52.6% patients from the unknown group, in 63.6% of control A and 10.5% of control B. The DWI FLAIR mismatch was different among groups (unknown stroke time group 43.7%, 6-hour control group A 63.6%, 12-h control group B 10.5%; FFH p=0.001). There were significant differences in FLAIR/DWI ratio among the three groups (unknown group: 0.05±0.12, control A: 0.17±0.15, control B 0.04±0.06; KW p<0.0001). Post-hoc pair wise comparisons showed that FLAIR/DWI ratio from the unknown stroke time group was significantly different from control A (p=0.0045), but not different from the control B (p=0.59) (Figure 3).
Figure 3. FLAIR/DWI signal ratio between unknown onset stroke patients and controls.
Control A: 0 to 6 hours from stroke onset to brain MRI.
Control B: 6 to 12 hours from stroke onset to brain MRI.
Pair wise group comparison using Kruskal Wallis test shows a significant difference between the wake-up groups versus Control B.
Time of onset and FLAIR hyperintense signal
There was a moderate correlation of DWI–FLAIR mismatch with the time of stroke onset (r = 0.61; p< 0.0001) within the 41 patients with known time of stroke onset.(Figure 2) The use of FLAIR/DWI ratio did not improve the correlation with the time of stroke onset (r = 0.58; p< 0.001).
Figure 2. FLAIR hyperintense signal to time form stroke onset.
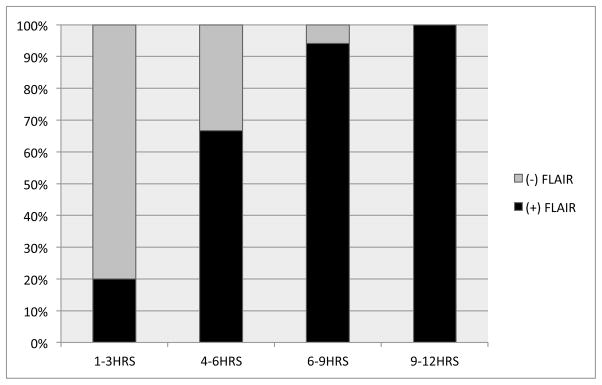
Rater’s consensus of FLAIR lesion visibility plotted against time from symptoms onset in the 41 patients with known time of onset.
Inter-Observer reliability
Inter-rater agreement was better for the DWI sequence than FLAIR. DWI abnormal hyperintense signal agreement was 86% with a κ coefficient of 0.57, FLAIR abnormal hyperintense signal agreement was 50% with a κ coefficient of 0.45 and the DWI–FLAIR mismatch agreement was 53% with a κ coefficient of 0.46.
DISCUSSION
In this study, more than 40% of patients in whom the time of stroke onset was uncertain had a DWI-FLAIR mismatch and nearly half had a negative FLAIR image for acute ischemic stroke. These findings were similar to those seen in patients whose stroke onset time was < 6 hours. In patients with unknown time of stroke onset, this criterion might identify those in who thrombolytic therapies might be considered. Intriguingly, we found that the mismatch and the FLAIR/DWI ratio within the unknown group were not different from the group patients presented within the 6 hours but differed from the group of patients scanned after 6 hours. Since our selection of patients with unknown time of stroke include the ones presented early in the morning and last seen normal more than 6 hours before, they likely comprise mainly strokes occurred while sleep (wake-up strokes).
Epidemiological studies reveal a circadian pattern for ischemic stroke with a higher frequency of stroke throughout first morning hours and decreased frequency of stroke during sleep.[2, 10–13] Brain imaging in patients with wake-up ischemic stroke suggest a possible pattern of late stroke during sleep.[14] Two separate studies assessing early ischemic changes by computer tomography (CT) within the 6 hours of the initial stroke symptoms, found no significant differences between patients who awoke with deficits versus the ones with a known stroke onset time, suggesting that the time from onset in both groups were similar.[15, 16]
The quantification the early ischemic changes by the Alberta Stroke Program Early CT Score (ASPECTS) found also no differences between wake-up strokes and the ones within the four hours from the stroke onset.[14] Alike CT studies, MRI perfusion imaging, reported a 73% favorable perfusion-diffusion mismatch imaging pattern in patients who awoke with stroke symptoms, similar to stroke patients who presented within 6 hours of known onset of symptoms.[17] Still, the reasons for these imaging findings remain unclear.
Our study concurs with separate observations of previous investigators that FLAIR signal in human brain ischemia increases with time. [3–5, 7] However, the use of DWI - FLAIR mismatch as clinical surrogate of time of stroke onset, can overestimate the real time of onset in more than 40% of patients due to the low sensitivity and poor negative predictive value.[4, 5] In human and animal brains the T2 relaxation time increases continuously during the first hours of brain ischemia but the detection of the abnormal ischemic signal can be subtle for the human eye.[18] On the present study, the visual estimation of the DWI–FLAIR mismatch had only a fair inter-rater reliability (Kappa: 0.45) barely lower the range of the observed on previous studies (0.46 to 0.65).[3–6] The observed inter-rater variability might account for the low sensitivity and predictive value of the visual method. Patient age, lesion volume, leukoaraiosis, motion artifact and different MRI field strengths are proposed reasons that explain the fair intrarater variability.[6] The use of software for the quantification of the FLAIR signal and standardized training in imaging could enhance the prediction of stroke onset.[7, 18, 19] In our study, the FLAIR/DWI signal intensity ratio was used as an attempt to homogenize the stroke heterogeneity and to correct for the rater variability. Contrary to what we hypothesize, the FLAIR/DWI ratio did not improve the correlation with time of stroke onset and it was not better than the visual estimation of the DWI–FLAIR mismatch. Likely, the FLAIR volume estimation is not as critical as the quantitative measurements of T2 signal. The use the difference of the T2 relaxation times between normal and lesional areas it promises to improve the association with time from stroke symptoms initiation.[18, 19] However, such approach requires rapid processing and interpretation to avoid delay on reperfusion therapies.
Based on our results and independent previous studies, nocturnal stroke patients have a high chance to be within the 3 to 6 hours of their stroke initiation. These findings have important implications for the calculation of a sample sizes and development of MRI selection thrombolytic clinical trials.
Few trials have included wake up strokes patients, thus data on clinical outcome after acute thrombolytic therapy is still uncertain. In a subgroup analysis of the Abciximab Emergent Stroke Treatment Trial (AbBEST) II study, the wake-up stroke group had more intracranial hemorrhage and poorer outcome that the awake group.[20] Others few retrospective studies have shown feasibility and safety on treating wake-up strokes with intravenous or intra-arterial thrombolytic therapy,[21, 22] but to date; there is no data from any randomized trial. Our study strength the use of DWI - FLAIR mismatch on wake-up stroke as promising approach to select patients for IV thrombolysis. Whether the DWI–FLAIR mismatch proves to be a better patient selection tool for thrombolysis, needs to be proven in a prospective clinical trial. Clinical trials are currently being designed to test the hypothesis that thrombolysis can be safe on patients with DWI–FLAIR. Hopefully this method could result in a large expansion of thrombolytic therapy for acute ischemic stroke.
Our study is limited to the small sample size, stroke hererogenity, and retrospective approach. We did not prospectively collect data of wake-up stroke patient and since our patient selection was only an approximation to wake–up subjects, our results should be taken only as exploratory and as hypothesis generating. We anticipate our findings to be corroborated on a larger prospective wake - up stroke study cohort.
Compelling data have shown that DWI–FLAIR mismatch is an optimal approach to estimate time of stroke onset in patients with unknown time of onset.[3, 5–7, 19] Furthermore, our results suggest that a large group of these patients have a DWI - FLAIR mismatch which could be amenable to reperfusion therapies. If these findings are replicated and validated in subsequent studies, it may be possible to define an MRI signature for selection of wake-up stroke patients for acute reperfusion trials.
Table 2.
DWI and FLAIR signal.
| Unknown N=19 |
CONTROL - A (0–6 hrs.) N=22 |
CONTROL - B (6–12 hrs.) N=19 |
P-value | |
|---|---|---|---|---|
| Negative FLAIR (%) | 10 (52.6) | 14 (63.6) | 2 (10.5) | 0.001‡ |
| Positive DWI (%) | 16 (84.2) | 22 (100) | 19 (100) | ns‡ |
| DWI - FLAIR mismatch | 43.7%* | 63.6% | 10.5% | |
| DWI lesion (cc) | 22.4.±35.0 | 33.3±42.2 | 26.3±39.1 | ns† |
| Q1 | 0.51 | 3.65 | 3.58 | |
| Median | 4.61 | 18.17 | 10.14 | |
| Q3 | 27.3 | 39.7 | 32.2 | |
| FLAIR/DWI ratio | 0.04±0.06 | 0.05±0.12 | 0.17±0.15 | <0.001† |
| Q1 | 0.00 | 0.00 | 0.07 | |
| Median | 0.02 | 0.00 | 0.09 | |
| Q3 | 0.07 | 0.03 | 0.26 |
ns: non-significant
Kruskal Wallis Test
Fisher-Freeman-Halton Test
ns: non-significant
Using only positive DWI subjects.
Acknowledgments
Source of funding: This study was supported by the National Institutes of Health (K23NS054084 and P50NS044378) to D.S.L. and educational NIH grant to UCSD Stroke Center (3P50 NSO44148-07S2).
Footnotes
Disclosures: Authors have nothing to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
David S Liebeskind, Email: davidliebeskind@yahoo.com.
Rema Raman, Email: reraman@ucsd.edu.
Qing Hao, Email: sunnyqhao@gmail.com.
Brett C Meyer, Email: bcmeyer@ucsd.edu.
Dawn M Meyer, Email: dmmeyer@ucsd.edu.
Thomas M Hemmen, Email: themmen@ucsd.edu.
References
- 1.Hacke W, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317–29. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 2.Elliott WJ. Circadian variation in the timing of stroke onset: a meta-analysis. Stroke. 1998;29(5):992–6. doi: 10.1161/01.str.29.5.992. [DOI] [PubMed] [Google Scholar]
- 3.Aoki J, et al. FLAIR can estimate the onset time in acute ischemic stroke patients. J Neurol Sci. 293(1–2):39–44. doi: 10.1016/j.jns.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Ebinger M, et al. Clinical and radiological courses do not differ between fluid-attenuated inversion recovery-positive and negative patients with stroke after thrombolysis. Stroke. 41(8):1823–5. doi: 10.1161/STROKEAHA.110.583971. [DOI] [PubMed] [Google Scholar]
- 5.Thomalla G, et al. Negative fluid-attenuated inversion recovery imaging identifies acute ischemic stroke at 3 hours or less. Ann Neurol. 2009;65(6):724–32. doi: 10.1002/ana.21651. [DOI] [PubMed] [Google Scholar]
- 6.Thomalla G, et al. DWI-FLAIR mismatch for the identification of patients with acute ischaemic stroke within 4.5 h of symptom onset (PRE-FLAIR): a multicentre observational study. Lancet Neurol. 10(11):978–986. doi: 10.1016/S1474-4422(11)70192-2. [DOI] [PubMed] [Google Scholar]
- 7.Petkova M, et al. MR imaging helps predict time from symptom onset in patients with acute stroke: implications for patients with unknown onset time. Radiology. 257(3):782–92. doi: 10.1148/radiol.10100461. [DOI] [PubMed] [Google Scholar]
- 8.Campbell BC, et al. Visual assessment of perfusion-diffusion mismatch is inadequate to select patients for thrombolysis. Cerebrovasc Dis. 29(6):592–6. doi: 10.1159/000311080. [DOI] [PubMed] [Google Scholar]
- 9.Liebeskind DS, Kidwell CS. Advanced MR imaging of acute stroke: the University of California at Los Angeles endovascular therapy experience. Neuroimaging Clin N Am. 2005;15(2):455–66. xiii. doi: 10.1016/j.nic.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Casetta I, et al. Patient demographic and clinical features and circadian variation in onset of ischemic stroke. Arch Neurol. 2002;59(1):48–53. doi: 10.1001/archneur.59.1.48. [DOI] [PubMed] [Google Scholar]
- 11.Chaturvedi S, Adams HP, Jr, Woolson RF. Circadian variation in ischemic stroke subtypes. Stroke. 1999;30(9):1792–5. doi: 10.1161/01.str.30.9.1792. [DOI] [PubMed] [Google Scholar]
- 12.Lago A, et al. Circadian variation in acute ischemic stroke: a hospital-based study. Stroke. 1998;29(9):1873–5. doi: 10.1161/01.str.29.9.1873. [DOI] [PubMed] [Google Scholar]
- 13.Marler JR, et al. Morning increase in onset of ischemic stroke. Stroke. 1989;20(4):473–6. doi: 10.1161/01.str.20.4.473. [DOI] [PubMed] [Google Scholar]
- 14.Huisa BN, et al. Alberta Stroke Program Early CT Score (ASPECTS) in patients with wake-up stroke. J Stroke Cerebrovasc Dis. 19(6):475–9. doi: 10.1016/j.jstrokecerebrovasdis.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serena J, et al. Stroke on awakening: looking for a more rational management. Cerebrovasc Dis. 2003;16(2):128–33. doi: 10.1159/000070592. [DOI] [PubMed] [Google Scholar]
- 16.Todo K, et al. Early CT findings in unknown-onset and wake-up strokes. Cerebrovasc Dis. 2006;21(5–6):367–71. doi: 10.1159/000091545. [DOI] [PubMed] [Google Scholar]
- 17.Fink JN, et al. The stroke patient who woke up: clinical and radiological features, including diffusion and perfusion MRI. Stroke. 2002;33(4):988–93. doi: 10.1161/01.str.0000014585.17714.67. [DOI] [PubMed] [Google Scholar]
- 18.Jokivarsi KT, et al. Estimation of the onset time of cerebral ischemia using T1rho and T2 MRI in rats. Stroke. 41(10):2335–40. doi: 10.1161/STROKEAHA.110.587394. [DOI] [PubMed] [Google Scholar]
- 19.Siemonsen S, et al. Quantitative t2 values predict time from symptom onset in acute stroke patients. Stroke. 2009;40(5):1612–6. doi: 10.1161/STROKEAHA.108.542548. [DOI] [PubMed] [Google Scholar]
- 20.Adams HP, Jr, et al. Emergency administration of abciximab for treatment of patients with acute ischemic stroke: results of an international phase III trial: Abciximab in Emergency Treatment of Stroke Trial (AbESTT-II) Stroke. 2008;39(1):87–99. doi: 10.1161/STROKEAHA.106.476648. [DOI] [PubMed] [Google Scholar]
- 21.Barreto AD, et al. Thrombolytic therapy for patients who wake-up with stroke. Stroke. 2009;40(3):827–32. doi: 10.1161/STROKEAHA.108.528034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Breuer L, et al. Feasibility and safety of magnetic resonance imaging-based thrombolysis in patients with stroke on awakening: initial single-centre experience. Int J Stroke. 5(2):68–73. doi: 10.1111/j.1747-4949.2010.00410.x. [DOI] [PubMed] [Google Scholar]