Abstract
Objective
It is now recognized that mean circulating DHEAS concentrations in most midlife women exhibit a positive inflection starting in the early perimenopause, continuing through the early post menopause and returning to early perimenopausal levels by late post menopause. This rise in mean DHEAS is accompanied by concomitant rises in testosterone (T), dehydroepiandrosteone (DHEA), androstenedione (Adione), and an equal rise androstenediol (Adiol). These observations suggest that there is a specific relationship between the circulating levels of steroids emanating from the adrenal, declining ovarian function and stages of the menopausal transition (MT). This study was designed to test the hypothesis that the menopausal stage-specific change in circulating DHEAS is associated with concomitant changes in the circulating pattern of adrenal steroids and that some of these adrenal androgens could influence the circulating estrogen/androgen balance.
Methods
Stored annual serum samples (n=120) were first selected to represent four longitudinal DS profiles of individual women in order to assess and compare changes in the adrenal contribution to circulating steroids.
Results
Changes in mean circulating DHEAS levels in midlife women during the MT is associated with changes in mean circulating Testosterone (T), androstendione (Adione), and androstenediol (Adiol). Mean Adione and T concentrations changed the least while mean DHEAS and Adiol changed the most.
Conclusion
Changes in circulating steroid hormone emanating from the adrenal during the menopausal transition may be more important than the decline of ovarian function in terms of altering the estrogen/androgen balance.
Keywords: DHEAS, androstenediol, estrogen, estrogenicity, menopause, adrenal
Introduction
The decline in circulating dehydroepiandrostenedione sulfate (DHEAS) evident from early adulthood, in both men and women, in both cross-sectional and longitudinal studies has been interpreted as an indicator of somatic aging independent of gonadal function and is utilized clinically in that capacity. The classical and expected profile of mean DHEAS is a continuous, gradual decline with advancing age. However, we have reported that, in contrast to the prevailing dogma (1–6), serum concentrations of adrenal androgens in midlife women do not always demonstrate a continuous, age-related decrease but variably exhibit a transient increase in the late perimenopause (7–9). At the present time, the underlying mechanism responsible for this increase in a circulating adrenal steroid and its relationship to the decline in ovarian function during the menopausal transition (MT) remains to be elucidated. Understanding this phenomenon may be important because of its potential as an endocrine contributor to the variability in peri-menopausal symptoms, differences in circulating steroid hormone concentrations, and/or control of adrenal function. The present study was designed to test the hypothesis that the early to late peri-menopausal change in mean circulating DHEAS is closely associated with similar changes in other circulating adrenal androgens.
Methods
This study reports results from serum samples selected from the overall SWAN population that has been previously reported (10). Briefly, SWAN is a multi-site, longitudinal cohort study being conducted in community-based groups of women who belonged to one of 5 ethnic/racial groups. Eligibility criteria for the SWAN longitudinal cohort were: age 42 to 52 years; intact uterus and at least 1 ovary; no current use of estrogens or other medications known to affect ovarian function; at least one menstrual period in the 3 months before screening; and self-identification in one of the 5 eligible ethnic groups. Institutional Review Board approval was obtained at each study site.
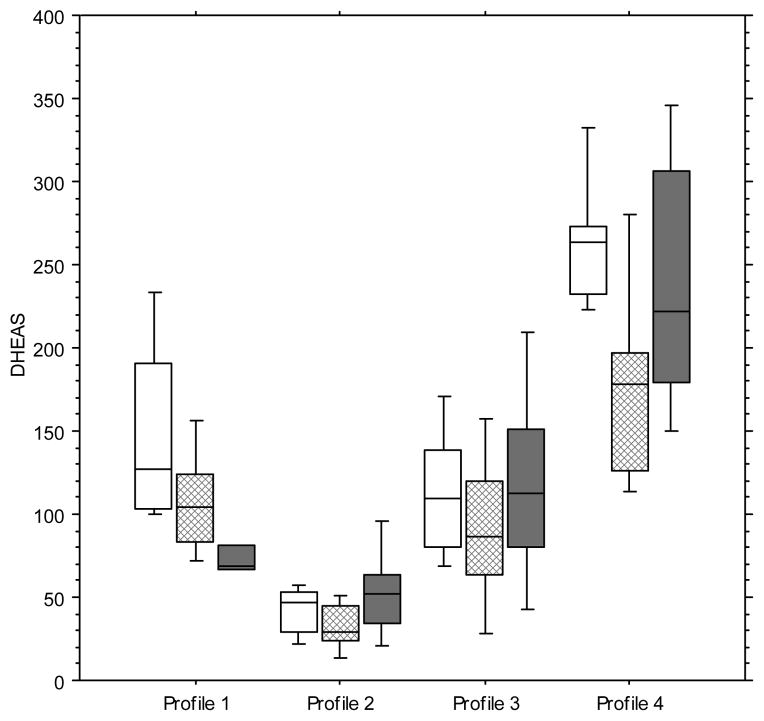
A total of 120 stored serum samples were first selected to represent four longitudinal DHEAS profiles of individual women to assess annual changes in mean adrenal steroid hormones (Figure 1). Annual Profile 1 (N=30), the profile used as reference, includes data from women that exhibited the classical and expected mean DHEAS concentration at baseline followed by a subsequent gradual decline over the next two years (Table 1). In Profiles 2, 3 and 4 (N=30 each), the selected data were from women who had an initial transitory fall and then rise in circulating mean DHEAS initially from their in SWAN baseline assessment (11). Profile 2 is unique in that it has a mean DHEAS baseline that is more than 1 standard deviation (SD) below the overall population mean and has a DHEAS change profile characterized by a DHEAS fall from year 0 to year 1, with a rise in the subsequent year. Profile 3 is unique in that it has a mean baseline that is within 1 SD of the overall mean that is followed by a mean DHEAS decline the next year and followed by a rise in year 2. Profile 4 has a mean DHEAS baseline that is greater than 1 SD above the population mean and has a DHEAS change profile whereby mean DHEAS falls from year 0 to year 1 followed by a rise in year 2. Thus, Profiles 2, 3, and 4 all have profiles that represent a fall followed by a rise in mean DHEAS concentration, but all profiles link the subsequent change to a different baseline (year 0) DHEAS mean concentration (Figure 1).
FIG. 1.
Serum DHEAS patterns for the selected study cohort. Reference profile 1 (n = 30) depicts the mean and range of DHEAS at baseline with the classical anticipated continued decline from year 0 (open boxes) to year 1 (hatched boxes) and year 2 (solid boxes). Profiles 2 (selected to represent the baseline mean of >1 SD below the x̄ of reference DHEAS with a subsequent increase; n = 30), 3 (at the x̄ of reference DHEAS; n = 30), and 4 (>1 SD above the x̄ of the reference DHEAS with an immediate decline and recovery; n = 30) illustrate the samples in which there was the transitory fall and then rise, a profile that has been uniquely reported in SWAN enrollees. Boxes represent the SEM and bars the range. DHEAS, dehydroepiandrosterone sulfate.
Table 1.
Demographic and menopausal status characteristics of the women exhibiting the four profiles determined by baseline DHEAS values and changes in subsequent annual DHEAS values (N=30)a.
| Characteristic | Baseline DHEAS Reference Profile 1 (N=30) Mean (SD) |
Baseline DHEAS < 1 SD of DHEAS Profile 2 (N=30) Mean (SD) |
Baseline DHEAS at the Mean Profile 3 (N=30) Mean (SD) |
Baseline DHEAS > 1 SD of Mean Profile 4 (N=30) Mean (SD) |
p-value for overall subgroup difference |
|---|---|---|---|---|---|
| Age (years) | 45.3 (2.5) | 46.9 (3.6) | 46.6 (2.4) | 46.6 (2.8) | 0.62 |
| Body mass index (kg/m2) | 33.6 (9.1) | 31.8 (6.5) | 30.8 (8.7) | 29.9 (7.4) | 0.81 |
| Percent (N) | Percent (N) | Percent (N) | Percent (N) | ||
| Ethnicity: | 0.51 | ||||
| African American | 50% (5) | 50% (5) | 40% (4) | 20% (2) | |
| White | 50% (5) | 50% (5) | 60% (6) | 80% (8) | |
| Menopause status: | 0.15 | ||||
| Premenopausal | 20 (2) | 40 (4) | 60 (6) | 70 (7) | |
| Early perimenopausal | 80 (8) | 60 (6) | 40 (4) | 30 (3) | |
| Hot flashes in past 2 weeks (yes) | 50 (5) | 40 (4) | 0.0 (0) | 0.0 (0) | 0.004 |
| Current smoking (yes) | 40 (4) | 40 (4) | 0.0 (0) | 10 (1) | 0.06 |
| Perceived stress | 9.0 (2.4) | 6.6 (2.7) | 8.0 (2.4) | 7.6 (2.7) | 0.24 |
| Difficulty paying for basics (yes): | 0.07 | ||||
| Very hard | 10 (1) | 20 (2) | 0.0 (0) | 0.0 (0) | |
| Somewhat hard | 50 (5) | 0.0 (0) | 20 (2) | 30 (3) | |
| Not hard at all | 40 (4) | 80 (8) | 80 (8) | 70 (7) |
The Reference Profile shows a DHEAS fall in subsequent years, while Profiles 2–4 show a rise in the subsequent year and then fall.
Only White (59%) and African American (41%) women were included in the study. However, since circulating DHEAS concentrations at baseline are ethnically distributed, the proportion of the each ethnicity was not equal in the four profiles. The percentage of African Americans was greatest in Profile 2 (55%) and lowest in Profile 4 (22%).
DHEAS Assay
The DHEAS assay is a competitive immunoassay run on Bayer Diagnostic’s ACS-180 automated analyzer using chemiluminescent technology. This assay was developed de novo for SWAN and uses rabbit polyclonal anti-DHEAS antibody, goat anti-rabbit IgG labeled with superparamagnetic particles (PMP), and DHEAS labeled with dimethylacridinuim ester (DMAE). 10 μL of serum is required for the assay in addition to sufficient dead volume for aspiration and repeat. The assay range is 0.04–26.1 μM (1.52 to 1020 μg/dL) and is standardized against DHEAS obtained from Steraloids (Newport, RI). The inter-assay and intra-assay coefficients of variation were 13% and 10 %, respectively.
Cortisol Assay
The cortisol assay is a competitive immunoassay run on Bayer Diagnostic’s ACS-180 automated analyzer using chemiluminescence technology. The assay uses cortisol labeled with DMAE, a polyclonal rabbit anti-cortisol antibody, and a monoclonal mouse anti-rabbit antibody coupled to PMP. The inter-assay and intra-assay coefficients of variation were 4.8% and 3.6%, respectively.
Testosterone Assay
The testosterone (T) assay is a competitive immunoassay modified to run on Bayer Diagnostic’s ACS-180 automated analyzer using chemiluminescence technology (12). The assay uses T labeled with DMAE, a polyclonal rabbit anti-T antibody, and a monoclonal mouse anti-rabbit antibody coupled to PMP. 45 μL of serum is required for the assay in addition to sufficient dead volume for aspiration and repeat assay determinations. The SWAN reporting range for the T assay is 0.4–3.5 nM (10–100 ng/dL). The actual assay range is 0.07–16.6 nM (2–478 ng/dL). The ACS T assay is standardized analytically and confirmed by GC-MS. The inter-assay and intra-assay coefficients of variation were 12% and 7%, respectively.
Androstenedione and Androstenediol (Assays
Androstenedione (Adione) and 5-androstene-3β,17β-diol (Adiol) were analyzed in a single aliquot (0.8 ml) of serum by radioimmunoassay (RIA) methods (13,14). The analytes were extracted from plasma using hexane:ethyl acetate (3:2) and, following evaporation of the organic solvents, the extract was redisolved in isooctane and applied to a celite partition column impregnated with ethylene glycol. Adione and Adiol were eluted with isooctane and 40% toluene in isooctane, respectively. After evaporation of the eluates, each residue was reconstituted in assay buffer and appropriate aliquots were taken for RIA. Each RIA utilizes a highly specific antiserum in conjunction with an iodinated (Adione) or tritiated (Adiol) radioligand. Following an appropriate incubation period, antibody-bound vs unbound hormone were separated by use of either second antibody (Adione) or dextran-coated charcoal (Adiol). The antibody-bound hormone was then counted after centrifugation. Inter-assay and intra-assay coefficients of variation were 8% and 10%, at 460 and 1450 pg/ml, respectively, for Adione, and 13% and 11% at 460 and 1210 pg/ml, respectively, for Adiol.
SHBG Assay
The sex hormone-binding globulin (SHBG) assay is a competitive immunoassay run on Bayer Diagnostic’s ACS:180 automated analyzer using chemiluminescent technology (15). The assay was developed de novo and uses a rabbit polyclonal anti-SHBG antibody, SHBG labeled with DMAE, and goat anti-rabbit Immunoglobulin G (IgG) coupled with PMP. Forty μL of serum is required for the assay in addition to sufficient dead volume for aspiration and repeat. The expected values are from 20 to 130 nM. The reporting range for the SHBG assay is 10–150 nM (actual assay range: 1.95 to 250 nM). The assay is standardized against SHBG obtained from Wein Laboratories Inc. (Concord, MA). The inter-assay and intra-assay coefficients of variation were 12% and 9%, respectively.
Estradiol Assay
The off-line estradiol (E2) assay (16) is a semi-automated, competitive immunoassay with manual steps and an off-line incubation. Standards, quality control preparations, and serum samples are pipetted into 12×75 polypropylene tubes by hand with a dilution of antibody reagent, and then incubated. First, DMAE-labeled E2 and rabbit anti-estradiol antibody are added to the tubes and incubated. Then all tubes are placed on Bayer Diagnostic’s ACS-180 automated analyzer with monoclonal mouse anti rabbit IgG coupled to PMP for analysis. 1.0 mL of serum is required for the assay in addition to sufficient dead volume for aspiration and repeats. The SWAN reporting range for the E2 assay is 3.7–734 pM (1–200 pg/mL). The ACS E2-6 Master Curve standards are manufactured and evaluated by GC-MS. The inter-assay and intra-assay coefficients of variation were 14% and 9%, respectively.
Data Analysis
One way ANOVA was used to test whether the values of steroids (androgens, E2) as well as SHBG in the reference DHEAS profile (Profile 1) were different than those in the DHEAS profile with unique DHEAS rises patterns (Profiles 2–4). Correlation analysis was further applied to test the strength and the direction of associations of circulating Adione, Adiol, and T with DHEAS at the levels of baseline. Hormone values were log transformed prior to analysis. A p value of less than 0.05 was considered to be statistically significant. Data analysis was done using StatView Version 5.0.1.0 (1998, SAS Institute, Inc,) and SAS version 8.0. Simple linear regression was used to illustrate the relationships between T, DHEAS, Adione and Adiol. In the regression analyses all hormone concentrations were log transformed prior to analysis and then back transformed to remove skewness.
Results
While there was no statistically significant difference in comparing the age of the reference Profile 1 women to characteristics of the other 3 profiles, there was a nonsignificant trend that the reference women had a greater BMI as well as were more likely to be perimenopausal than premenopausal (Table 1). Women in the reference Profile 1 were more likely to report having hot flashes in the two weeks prior to the baseline examination as compared to the other profiles.
The four distinct hormone profiles contrast an expected continuing gradual decline in mean DHEAS as shown in Profile 1 (Figure 1 and Table 2) to the main patterns of increase and decline that were captured in the larger SWAN study. These patterns most likely represent the same basic physiology mechanism but appear as different patterns because the women were recruited at different times relative to an inflection in DHEAS production.
Table 2.
Hormonal characteristics of the women exhibiting the four profiles determined by baseline DHEAS values and changes in subsequent annual DHEAS values (N=30)a.
| Characteristic | Baseline DHEAS Reference Profile 1 (N=30) | Baseline DHEAS < 1 SD of Mean Profile 2 (N=30) | Baseline DHEAS at Mean Profile 3 (N=30) | Baseline DHEAS > 1 SD of Mean Profile 4 (N=30) | p-value for overall subgroup difference |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD, p profile 2 and 3) | Mean (SD) | Mean (SD, p profile 3 and 4) | ||
| Androstenedione (pg/mL) | 1515.1 (1174.0) | 973.2 (446.2, p=0.169) | 927.6 (350.6) | 1188.7 (344.7, p=0.210) | 0.19 |
| Androstenediol (pg/mL) | 865.4 (205.3) | 287.7 (139.1, p=0.117) | 703.6 (327.0) | 1023.8 (266.5, p=0.056) | < 0.0001 |
| DHEAS (ug/dL) | 144.6 (49.5) | 41.6 (14.1, p=0.004) | 112.2 (39.2) | 265.1 (44.3, p=0.001) | < 0.0001 |
| SHBG (nM) | 46.9 (17.6) | 48.7 (28.1, p=0.560) | 42.3 (22.8) | 32.2 (11.4, p=0.386) | 0.36 |
| Testosterone (ng/dL) | 61.7 (22.9) | 34.6 (23.9, p=0.553) | 51.0 (32.4) | 63.0 (24.1, p=8.828) | 0.008 |
| Estradiol (pg/mL) | 81.2 (36.3) | 79.9 (72.3, p=0.675) | 59.3 (49.4) | 66.4 (86.9, p=0.383) | 0.26 |
The Reference Profile shows a DHEAS fall in subsequent years, while Profiles 2–4 show a fall in the subsequent year and then rise. P values are provided to illustrate the significance between the mean (Profile 3) and < 1SD below the mean (Profile 2) and between the mean (Profile 3) and > 1SD above the mean (Profile 4).
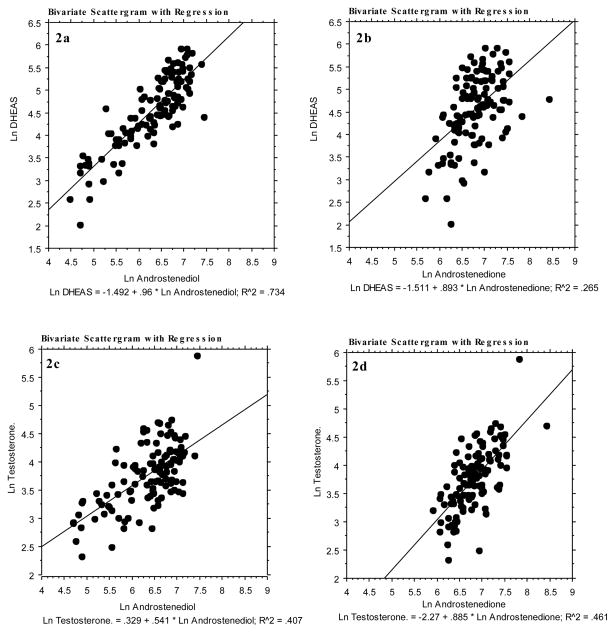
Baseline mean DHEAS were associated with different androgen profiles using regression analysis. The DHEAS decline and increase were attended by concomitant changes in Adiol and, to a lesser degree, T, and Adione (Figure 2). Further, a consistently stronger correlation was found between DHEAS and Adiol (0.71, p=<0.05), irrespective of the baseline level of DHEAS compared to T (0.20, p=<0.05) and Adione (0.28, p=<0.05). Circulating mean cortisol concentrations were not correlated with DHEAS and there was no difference in mean cortisol concentrations among the different profiles (data not shown).
FIG. 2.
Linear regressions of the four hormones described in this study (Ln DHEAS and Ln T versus Ln Adiol and Adione). Panels 2a and 2b show Ln DHEAS versus Ln Adiol (n = 119, P < 0.0001) and Ln Adione (n = 119, P < 0.0001), respectively. Panels 2c and 2d show Ln T vs Ln Adiol (n = 117, P < 0.0001) and Ln Adione (N = 117, P< 0.0001), respectively. Some samples were excluded for lack of T, Adiol, or Adione assay results. DHEAS, dehydroepiandrosterone sulfate; T, testosterone; Adiol, androstenediol; Adione, androstenedione.
Discussion
A menopausal stage-specific rise in mean serum DHEAS is observed in most women between the early peri-menopause and early post menopause stage of the MT (8), which also occurs following bilateral salpingo-oophorectomy during the early perimenopause (9). This change in DHEAS is accompanied by simultaneous and similar changes in circulating adrenal androgens but not cortisol. Biologically, an increase in these androgenic steroids could be important for women approaching menopause given the fact that DHEAS, resulting in a rise in DHEA, provides a reservoir of substrate for peripheral conversion into other bioactive steroids. Interestingly, one of these metabolites, Adiol, has both inherent estrogenic and androgenic activity (17, 18) and possibly contributes directly to the circulating estrogen pool and alterations in the circulating estrogen to androgen ratio (19). This previous report showed the relationship between the four adrenal androgens, DHEAS, T, Adiol and Adione (19) and we speculate that these hormone would move together; the present data show that increasing circulating levels of DHEAS are accompanied by concomitant increases in Adiol, Adione and T in peri-menopausal women. This is consistent with the literature, which indicates that circulating DHEAS is a reliable index of total androgens in women (20).
Circulating Adiol concentrations were more robustly correlated to DHEAS during all segments of the perimenopausal dynamics of DHEAS as compared to Adione and T in the longitudinal analysis presented here. This observation of a strong, positive correlation between DHEAS and Adiol is consistent with the concept that Adiol is a peripheral conversion product of DHEA. It is also possible that it is a direct secretory product of the adrenal. The lack of correlation between DHEAS and E2, however, does not support the contention that peripheral metabolism of DHEA contributes significantly to circulating E2. The absence of data demonstrating the conversion of exogenous DHEA to Adiol concentrations similar to those observed in this study also suggests that much or most of the circulating Adiol that occurs during the MT is a result of direct secretion by the adrenal glands. While Adiol is a weak estrogen agonist compared to E2, the serum concentrations of Adiol in mid-life women are substantially higher than levels of E2 during the menopausal transition in most women (19). The five-fold or greater range of circulating Adiol levels, from 689 pM (200 pg/mL) to greater than 3.8 nM (1100 pg/mL), presents the possibility that some women may experience estrogen action from a sufficient increase in Adiol. In contrast, the range of circulating E2 concentrations is only 3 fold (20–60 pg/mL) when measured during the early follicular phase of the menstrual cycle. Therefore, during the MT, the between-woman difference in circulating Adiol concentrations exceeds the between-woman difference in E2 by up to one thousand fold, potentially providing a more variable source of estrogenic, albeit weak, support.
Recent interest has arisen in identifying the direct effects of steroid hormone metabolites that may activate steroid hormone receptors (18). The present data provide additional and direct evidence for a potential role of steroids emanating from the adrenal in modulating symptoms and health outcomes of mid-life women. The circulating levels for Adione and T increased only two and three fold, respectively, and only a modest statistically significant difference was found in the mean Adione concentrations across the total range of DHEAS concentrations. This modest increase in Adione and T in association with the robust rise in DHEA supports the concept that while there is likely an increased conversion of DHEA to Adione and T in women during early-to-late MT, the much greater rise in DHEA suggests that changes in adrenal secretion rates rather than just peripheral conversion may be important in the support of sex-steroid sensitive tissues. To be clear, this report does not demonstrate a direct secretion of steroids by the adrenal gland is responsible, but in the face of no evidence in the literature to suggest changes in metabolism or clearance rates could account for such concentration changes, we interpret the observed increase as due to increased production rate. Thus, increased Adiol production (with inherent estrogenic activity), rather than an elevation of DHEA as a prohormone, suggests an alternative explanation for the positive physiological effects that have previously been attributed primarily to endogenous DHEA.
The grouping of women based on initial circulating DHEAS concentrations created trajectory groups that were ethnically divergent. This is to be expected, as previous reports have demonstrated ethnic differences in DHEAS in mid-aged women (8, 21), in which Whites have higher, and African Americans lower, baseline DHEAS. Despite this apparent initial, ethnic based difference in baseline DHEAS, the relative change in the complement of adrenal androgens during the MT was similar for all four trajectories. Thus, the absolute peak concentrations of adrenal androgens remain ethnically distinct.
This study failed to detect a clear pattern in circulating cortisol with respect to DHEAS profiles, which is consistent with a separate and selective acceleration of the Δ5-C19 androgen synthetic pathway and argues against a “spill-over” resulting from an increased ACTH drive. Increased ACTH drive would have selective preference for the Δ4 pathway, resulting in increases in Adione and T concentrations, which is opposite to that observed in the present study. The present data also argue that the observed increase in adrenal secretory activity is not a result of a stress response, either specific to the perimenopause or independent of it. Although cortisol was measured in these profiles, these results should be interpreted with caution because samples for the SWAN cohort were only collected between 6 and 10 am.
Conclusion
The data presented herein illustrate that there are wider between-woman differences in circulating adrenal steroids during the MT and immediately following menopause than previously appreciated. Since approximately 85% of all women have some rise in DHEAS during the menopausal transition (Crawford et al., 2009), then we expect that the increased contribution of adrenal steroids as demonstrated here is a common event for most women. While circulating E2 concentrations are known to decline in women over the course of the MT (12), the current data demonstrate that a modest decline in circulating levels of E2 in the late peri-menopause is associated with a much broader range of circulating levels of Adiol and DHEA. Thus the change in steroids emanating from the adrenal during the MT may be an important contributor to the overall change in sex steroid hormone balance over the MT and it may be a better indicator of phenotype than the smaller decline in ovarian steroid production. These observations raise the question as to the possibility that differences in adrenal steroid levels may predict difference in symptoms and health outcomes. However, since the rise in adrenal steroids was recognized only when a large number of longitudinal samples were evaluated (Crawford, et al., 2009), it is unlikely that parallel changes in symptoms or health outcomes would be detected in this relatively small cross-section investigation. Future longitudinal studies are planned that will address these interesting possibilities.
Acknowledgments
Funding Credits
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
We thank the study staff at each site and all the women who participated in SWAN
Footnotes
The authors have no conflicts of interest to declare.
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011, MaryFran Sowers, PI 1994-2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Sherry Sherman 1994 – present; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Kim Sutton-Tyrrell, PI 2001 – present; New England Research Institutes, Wate rtown, MA -Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair, Chris Gallagher, Former Chair
References
- 1.Burger HG, Dudley EC, Hopper JL, et al. The endocrinology of the menopausal transition: a cross-sectional study of a population-based sample. Journal of Clinical Endocrinology & Metabolism. 1995;80(12):3537–45. doi: 10.1210/jcem.80.12.8530596. [DOI] [PubMed] [Google Scholar]
- 2.Burger HG, Dudley EC, Cui J, Dennerstein L, Hopper JL. A prospective longitudinal study of serum testosterone, dehydroepiandrosterone sulfate, and sex hormone-binding globulin levels through the menopause transition. Journal of Clinical Endocrinology & Metabolism. 2000;85(8):2832–8. doi: 10.1210/jcem.85.8.6740. [DOI] [PubMed] [Google Scholar]
- 3.Labrie F, Belanger A, Cusan L, Gomez JL, Candas B. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. Journal of Clinical Endocrinology & Metabolism. 1997;82(8):2396–402. doi: 10.1210/jcem.82.8.4160. [DOI] [PubMed] [Google Scholar]
- 4.Longcope C, Franz C, Morello C, Baker R, Johnston CC., Jr Steroid and gonadotropin levels in women during the peri-menopausal years. Maturitas. 1986;8(3):189–96. doi: 10.1016/0378-5122(86)90025-3. [DOI] [PubMed] [Google Scholar]
- 5.Orentreich N, Brind JL, Rizer RL, Vogelman JH. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. Journal of Clinical Endocrinology & Metabolism. 1984;59(3):551–5. doi: 10.1210/jcem-59-3-551. [DOI] [PubMed] [Google Scholar]
- 6.Rannevik G, Jeppsson S, Johnell O, Bjerre B, Laurell-Borulf Y, Svanberg L. A longitudinal study of the perimenopausal transition: altered profiles of steroid and pituitary hormones, SHBG and bone mineral density. Maturitas. 1995;21(2):103–13. doi: 10.1016/0378-5122(94)00869-9. [DOI] [PubMed] [Google Scholar]
- 7.Lasley BL, Santoro N, Randolf JF, et al. The relationship of circulating dehydroepiandrosterone, testosterone, and estradiol to stages of the menopausal transition and ethnicity. Journal of Clinical Endocrinology & Metabolism. 2002;87(8):3760–3767. doi: 10.1210/jcem.87.8.8741. [DOI] [PubMed] [Google Scholar]
- 8.Crawford S, Santoro N, Laughlin GA, Sowers MF, McConnell D, Sutton-Tyrrell K, Weiss G, Vuga M, Randolph J, Lasley BL. Circulating Dehydroepiandrosterone Sulfate Concentrations during the Menopausal Transition. J Clin Endocrinol Metab. 2009;94(8):2945–51. doi: 10.1210/jc.2009-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lasley BL, Crawford SL, Laughlin GA, Santoro N, McConnell DS, Crandall C, Greendale GA, Polotsky AJ, Vuga M. Circulating dehydroepiandrosterone sulfate levels in women who underwent bilateral salpingo-oophorectomy during the menopausal transition. Menopause. 2011;18(5):494–498. doi: 10.1097/gme.0b013e3181fb53fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sowers M, Crawford S, Morgenstein D. Design and sampling methods of SWAN: a multi-center, multi-ethnic, community-based cohort study of women and the menopausal transition. In: Wren J, Lobo RA, Kelsey J, Marcus R, editors. Menopause: Biology and Pathobiology. Academic Press; 1999. [Google Scholar]
- 11.Sowers MF, Beebe JL, McConnell D, Randolph J, Jannausch M. Testosterone concentrations in women aged 25–50 years: associations with lifestyle, body composition, and ovarian status. American Journal of Epidemiology. 2001;153(3):256–64. doi: 10.1093/aje/153.3.256. [DOI] [PubMed] [Google Scholar]
- 12.Randolph JF, Jr, Sowers M, Gold EB, et al. Reproductive hormones in the early menopausal transition: relationship to ethnicity, body size, and menopausal status. Journal of Clinical Endocrinology & Metabolism. 2003;88(4):1516–22. doi: 10.1210/jc.2002-020777. [DOI] [PubMed] [Google Scholar]
- 13.Dorgan JF, Stanczyk FZ, Longcope C, et al. Relationship of serum dehydroepiandrosterone (DHEA), DHEA sulfate, and 5-androstene-3 beta, 17 betAdiol to risk of breast cancer in postmenopausal women. Cancer Epidemiology, Biomarkers & Prevention. 1997;6(3):177–81. [PubMed] [Google Scholar]
- 14.Goebelsmann U, Horton R, Mestman JH, et al. Male pseudohermaphroditism due to testicular 17 -hydroxysteroid dehydrogenase deficiency. Journal of Clinical Endocrinology & Metabolism. 1973;36(5):867–79. doi: 10.1210/jcem-36-5-867. [DOI] [PubMed] [Google Scholar]
- 15.Sowers M, Crawford S, Morgenstein D. Design and sampling methods of SWAN: a multi-center, multi-ethnic, community-based cohort study of women and the menopausal transition. In: Wren J, Lobo RA, Kelsey J, Marcus R, editors. Menopause: Biology and Pathobiology. Academic Press; 1999. [Google Scholar]
- 16.England BG, Parsons GH, Possley RM, McConnell DS, Midgley AR. Ultrasensitive semiautomated chemiluminescent immunoassay for estradiol. Clinical Chemistry. 2002;48(9):1584–1586. [PubMed] [Google Scholar]
- 17.Adams JB, Martyn P, Lee FT, Phillips NS, Smith DL. Metabolism of 17 beta-estradiol and the adrenal-derived estrogen 5-androstene-3 beta,17 betAdiol (hermaphrodiol) in human mammary cell lines. Annals of the New York Academy of Sciences. 1990;595:93–105. doi: 10.1111/j.1749-6632.1990.tb34285.x. [DOI] [PubMed] [Google Scholar]
- 18.Adams JB. Adrenal androgens and human breast cancer: a new appraisal. Breast Cancer Research & Treatment. 1998;51(2):183–8. doi: 10.1023/a:1006050720900. [DOI] [PubMed] [Google Scholar]
- 19.Lasley BL, Stanczyk FX, Gee NA, Chen J, El Khoudary, Crawford S, McConnell DS. Androstenediol Complements Estradiol During the Menopausal Transition. Menopause. doi: 10.1097/gme.0b013e31823df577. (submitted Oct 2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Labrie F, Luu-The V, Belanger A, et al. Is dehydroepiandrosterone a hormone? Journal of Endocrinology. 2005;187(2):169–96. doi: 10.1677/joe.1.06264. [DOI] [PubMed] [Google Scholar]
- 21.Spencer JB, Klein M, Kumar A, Azziz R. The age-associated decline of androgens in reproductive age and menopausal Black and White women. J Clin Endocrinol Metab. 2007;92(12):4730–3. doi: 10.1210/jc.2006-2365. [DOI] [PubMed] [Google Scholar]