Abstract
Anticoagulation is essential for maintaining the fluidity of extravascular blood on the apheresis circuit. Although both citrate and heparin are used as an anticoagulant during apheresis, citrate is preferred for the majority of exchange procedures because of its safety and effectiveness. Complications of citrate are primarily due to physiologic effects of hypocalcemia. Symptoms of hypocalcemia and other citrate-induced metabolic abnormalities affect neuromuscular and cardiac function and range in severity from mild dysesthesias (most common) to tetany, seizures and cardiac arrhythmias. Oral or intravenous calcium supplementation is advised for decreased ionized calcium levels and/or symptomatic management of hypocalcemia. Heparin-based anticoagulation is limited to certain apheresis procedures (membrane-based plasma exchange, LDL apheresis, or photopheresis) or is used in combination with citrate to reduce citrate load. While effective, heparin anticoagulation is associated with an increased frequency of bleeding complications and heparin-induced thrombocytopenia.
Keywords: apheresis, citrate, heparin, anticoagulation, hypocalcemia
Introduction
Anticoagulation is essential during apheresis to prevent clotting of the extracorporeal circuit. The foreign surface of the tubing potently activates platelets and leads to contact mediated activation of the hemostatic system. In this paper, we will provide an overview of the two commonly used anticoagulants in therapeutic apheresis, citrate and heparin, with an emphasis on citrate pharmacology, dosing considerations, and toxicity.
Citrate pharmacology
The sodium salt of citric acid has been used as an anticoagulant since 1914.1 Citrate is the anticoagulant of choice for blood additives and extracorporeal circuits involving low blood flow rates (30-70 ml/min), where citrate load is offset by clearance mechanisms. In apheresis, citrate-based anticoagulation is preferred over heparin therapy because of its low cost, safety, and rapid systemic clearance.
Citrate exerts its anticoagulant effect through reversible chelation of circulating divalent cations, including Ca2+ and Mg2+, and sequestration of these ions from their normal physiological function. In normal individuals, 40% of plasma Ca2+ is bound to proteins (albumin), 13% is complexed with small endogenous anions (such as phosphate and lactate), and 47% is free in solution as ionized calcium. This ionized calcium (iCa2+) is the physiologically active form of calcium and is necessary for many cellular processes, including hemostasis, regulation of muscle contraction and the stabilization of cellular membranes.2,3 Normal ionized calcium levels range from 1.1-1.4 mmol/L (4.5-5.6 mg/dL) and do not vary with albumin levels. During hemostasis, Ca2+ serves as a co-factor for phospholipid-dependent assembly of the tenase and prothrombinase complexes.4 In the extracorporeal circuit, citrate concentrations of 15-24 mmol/L reduce ionized Ca2+ levels sufficiently (to 0.2-0.3 mmol/L) to impair hemostasis and produce an anticoagulant effect.3
In healthy individuals, exogenous citrate is rapidly metabolized by the Kreb's tricarboxylic acid cycle in the mitochondria of the kidney, liver, and skeletal muscle. Metabolism of one citrate molecule results in release of bound calcium, consumption of three hydrogen ions and release of three molecules of bicarbonate,5 which contributes to blood alkalinity. Approximately 18-20% of infused citrate remains unmetabolized and is excreted by the kidneys.6,7 In the presence of normal hepatic metabolism, the half-life of infused citrate is 36 ± 18 minutes.8
Citrate formulations and dosing considerations
The most commonly used citrate formulations in apheresis are Acid-Citrate-Dextrose Formula A (ACD-A) and ACD-B, both of which contain citric acid, sodium citrate, and dextrose.9 These commercial solutions vary in citrate content. ACD-A contains 3% citrate (112 mmol/L of citrate or 21.3 mg/mL),3,10 whereas ACD-B has a reduced citrate concentration of 2% citrate (68 mmol/L or 12.8 mg/mL).3,11 Sodium citrate has a higher citrate concentration (4%; 136 mmol/L citrate or 25.6 mg/mL) and is, therefore, rarely used as an anticoagulant in apheresis procedures.12 The presence of additional citrate in blood products particularly in Fresh Frozen Plasma (FFP) and derivatives (Plasma Frozen within 24 Hours After Phlebotomy or Plasma Cryoprecipitate Reduced), but also in Red Blood Cells (RBCs), can augment citrate levels when used as replacement fluid during apheresis procedures.10
Citrate delivery in apheresis can be viewed in two contexts—the rate of anticoagulant delivery to the patient, which mediates the systemic effects of citrate, and the rate of anticoagulant delivery in the circuit, which is necessary for maintaining effective anticoagulation in the extracorporeal circuit. Generally, the minimum concentration of citrate necessary to provide adequate regional anticoagulation should be utilized, as this will in turn, reduce the systemic effects of the drug.
Citrate dosing in apheresis is based on the knowledge that citrate is infused at a higher rate than its removal. This is done because donors can generally tolerate up to a 20% decline in ionized calcium levels13 and because higher infusion rates permit shorter runs.11 Current instruments use the total blood volume (TBV) to calculate the anticoagulant (AC) infusion rate delivered to the patient (ml/min/L TBV). To minimize systemic fluctuations of ionized calcium levels, most instruments control AC infusion parameters to maintain citrate delivery rates between 1.0 to 1.8 mg/kg/min.14 In published studies, AC infusion rates of 0.8, 1.0 and 1.2 ml ACD-A/min/L TBV were associated with declines in ionized calcium values of 10-15%, 15-25% and 20-35%.11
Once parameters are determined for AC infusion rates based on patient TBV, the operator configures procedural parameters for anticoagulant delivery in the extracorporeal circuit. Three procedural settings influence citrate concentrate in the circuit: inlet pump rate, AC flow rate and/or the whole blood (WB) to AC ratio. The inlet pump controls the rate at which anticoagulated whole blood from the patient is delivered to the centrifuge. An increased inlet pump flow rate delivers increased amount of citrated blood to the centrifuge, which is then returned to the patient. The AC flow rate is the rate at which citrate is infused from the ACD-A bag to the inlet line containing the patient's blood. Increases in AC flow rate or the pump controlling the AC flow (AC pump rate) also increase citrate delivery to the circuit. The WB:AC ratio is the ratio of WB to citrate anticoagulant in the extracorporeal circuit. For example, a WB:AC ratio of 12:1 represents 12 parts WB to 1 part AC or an AC dilution of 1/13th. Either the WB: AC ratio or the reciprocal of the dilution factor is used by some instruments to calculate an “AC ratio” (AC ratio of 13 in this example). In general, the higher the AC ratio (>15), the less citrate anticoagulant is present in the circuit. A higher AC ratio favors development of platelet clumping and interface stability. The lower the AC ratio (<10), the greater the concentration of anticoagulant delivered to the patient, which can increase symptoms of citrate toxicity. The effect of these procedural settings on citrate delivery is summarized in Table 1.
Table 1. Procedural settings and influence on systemic citrate concentrations.
| Setting | Description | Effect |
|---|---|---|
| Inlet Pump | controls the rate of blood delivery from the patient to the centrifuge |
↑pump rate: increases citrate delivery ↓pump rate: decreases citrate delivery |
| AC flow rate or AC pump rate | rate at which citrate is infused from the anticoagulant bag to the inlet line containing the patient's blood |
↑AC flow rate: increases citrate delivery ↓AC flow rate: decreases citrate delivery |
| Whole blood (WB):AC ratio | ratio of WB to citrate anticoagulant in the extracorporeal circuit |
↑WB:AC ratio: decreased citrate delivery ↓WB:AC ratio: increased citrate delivery |
In addition to these technical variables, the amount of citrate reinfused to the patient is influenced by the centrifugation separation efficiency, the amount and type of blood component returned containing citrate (e.g FFP contains ∼7 mmol citrate/Unit and RBC contains ∼2-3mmol/Unit), the return speed, and the length of the entire procedure.15,16 Effects of citrate load based on various procedures and plasma replacement are provided in Table 2. Table 3 provides a listing of recommended AC ratios by manufacturer and type of procedure performed.
Table 2. Effects of exchange procedure and replacement fluid on citrate load.
| Amount of citrate administered for anticoagulation (A) | If FFP used as replacement fluid† (B) | Total amount of citrate administered (A+B) | |
|---|---|---|---|
| Centrifugal TPE using citrate | 14 mmol/hr | 50 mmol/hr | 64 mmol/hr |
| Membrane TPE using citrate | 28-56 mmol/hr | 50 mmol/hr | 80+ mmol/hr |
| Membrane TPE using heparin | 0 mmol/hr | 50 mmol/hr | 50 mmol/hr |
Based on following estimates: FFP infusion rate of 30 ml/min (or 1800 mL/hr), ∼ 7mmol citrate/FFP Unit, 250 mL/Unit FFP, yielding ∼50 mmol citrate/hr
Modified with permission from 2011 Therapeutic Apheresis Academy presentation by Dr. David M. Ward, Professor of Medicine, Division of Nephrology, University of San Diego
Table 3. Recommended AC ratios by Apheresis Instrument and Procedure.
| Apheresis machine (Vendor, Location) |
Apheresis procedure | Default AC ratio* | WB:AC dilution/ratio |
|---|---|---|---|
|
Cobe Spectra (Caridian BCT, Lakewood, CO) |
TPE | 10 | 9:1 |
| AutoPBSC procedures | 12 | 11:1 | |
| MNC procedures | 12 | 11:1 | |
| PMN procedures | 13 | 12:1 | |
|
Spectra Optia (Caridian BCT, Lakewood, CO) |
TPE | 10 | 9:1 |
|
Fresenius-Kabi AS104 (Fresenius-Kabi, Bad Homburg, Germany) |
TPE with albumin | 12 | 12:1 |
| TPE with FFP | 14 | 14:1 | |
| Platelet collection/depletion |
8 | 8:1 | |
| RBC exchange/depletion |
12 | 12:1 | |
| WBC collection/depletion |
10 | 10:1 | |
| Fresenius-Kabi COM.TEC | TPE with albumin | 12 | 12:1 |
| TPE with FFP | 14 | 14:1 |
AC ratio as provided by the manufacturer
Table modified with permission from 2011 Therapeutic Apheresis Academy presentation by Dr. Gay Wehrli, Associate Medical Director of Blood Bank and Transfusion Medicine Services and Assistant Professor of Pathology, University of Virginia, 2011
Citrate Toxicity
In general, apheresis performed with citrate anticoagulation is considered safe, and serious side effects are uncommon.17 However, as citrate containing blood is returned to the donor, chelation of cations can continue in the systemic circulation. Consequently, metabolic complications ensue (hypocalcemia, hypomagnesemia, metabolic alkalosis, and other electrolyte derangements) and are accompanied by symptoms. In several studies examining a variety of apheresis procedures, mild citrate related reactions (usually perioral tingling or paresthesias) have been reported as the most frequent complication. Citrate related complications have been reported to occur in: 1.2% of donors during voluntary donation,18 7.8% of patients undergoing therapeutic plasma exchange procedures,19 and 48% of patients undergoing large volume leukapheresis during peripheral blood progenitor cell collection.20 Although extremely rare, life-threatening hypocalcemia may develop during apheresis, particularly during large volume exchanges that are used for stem cell collection. The following sections describe the physiologic changes that commonly accompany citrate infusions, manifestations of citrate toxicity, and management of symptoms.
Citrate-Induced Hypocalcemia
Risk factors for citrate toxicity
The most common reactions during apheresis are principally related to effects of hypocalcemia.20-23 Factors influencing symptom development include rate of citrate infusion, the rate of decline in ionized calcium levels and hepatic metabolism of the infused citrate. A routine citrate infusion rate of 1-1.8 mg/kg/min is known to result in a 25-35% reduction in ionized calcium levels.11,24,25 A 0.5-0.6 mmol/L increase in plasma citrate lowers iCa2+ levels by 0.1 mmol/L.3 As ionized calcium levels decrease, protein bound calcium levels will also decrease by 35%, as Ca2+ dissociates from albumin to maintain homeostasis.26 As ionized calcium levels fall, signs and symptoms of hypocalcemia will invariably develop. Tetany is seen when the ionized calcium level falls below 1.1 mmol/L (4.4 mg/dL), and an ionized calcium level less than 0.8 mmol/L (3.2 mg/dL) have been associated with fatal arrhythmias.27
There is considerable individual variability in the development of symptoms and signs of citrate-induced hypocalcemia. Predictors of citrate toxicity include older age, female gender, lower body weight, and blood volume less than 4 liters.9,20,24,28,29 Patients with comorbid conditions such as hepatic or renal insufficiency may be at increased risk of citrate toxicity due to impaired metabolism of citrate.10,30 Patients with thrombotic thrombocytopenia purpura (TTP) or others with renal failure are at risk of developing metabolic alkalosis given decreased bicarbonate excretion, and they may benefit from closer monitoring of their acid-base status.10,31,32 Metabolic alkalosis is especially of concern in the mechanically ventilated patient where respiratory compromise cannot occur.2 Patients with pre-existing cardiac dysfunction33 or underlying neuromuscular disorders (such as myasthenia gravis)17,34 may be especially susceptible to the neurotoxic effects of citrate-induced hypocalcemia.33 A low baseline serum albumin also places patients at risk for developing citrate-related symptoms, as albumin-bound calcium normally disassociates to counter-act decreases in ionized calcium levels.28,29 Presence of these risk factors and/or comorbidities may identify patients who will benefit from procedural modifications or calcium supplementation.
Technical issues may also predispose a patient to developing citrate toxicity. Longer procedures will result in citrate accumulation.11,24 Large volume procedures will inevitably result in a larger volume of infused citrate.35,36 The final citrate load to the patient is also considerably higher when FFP is used as replacement fluid, as it contains approximately 15% citrate by volume.10,11,16,37-39 Not surprisingly, the risk of citrate toxicity and hypocalcemia will also be higher when apheresis is performed on consecutive days.9,31
Signs and symptoms of hypocalcemia
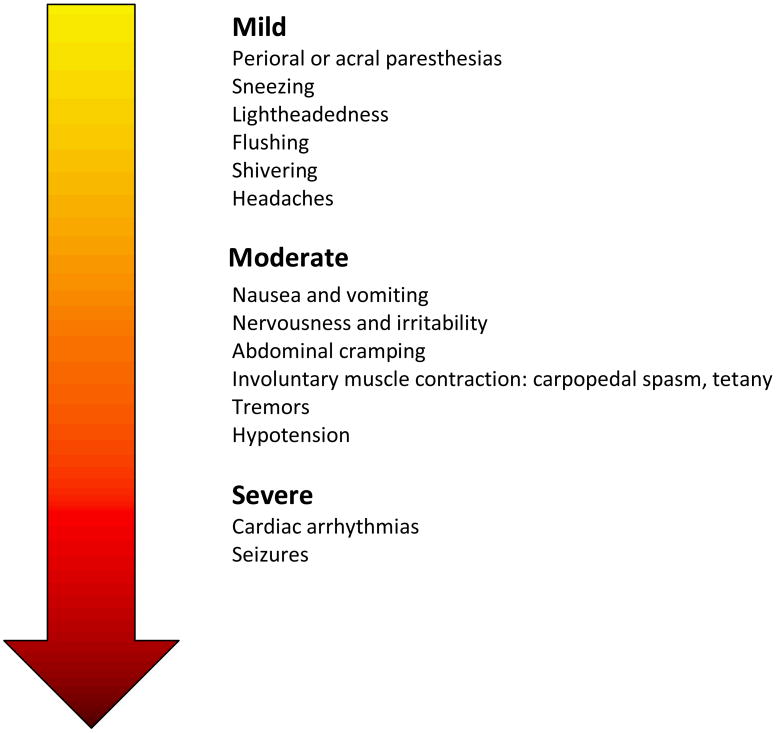
Hypocalcemia is defined as an iCa2+ of <4.5 mg/dL (1.1 mmol/L). Symptoms manifest variably when iCa2+ levels fall below normal range. Symptoms of hypocalcemia can be categorized as mild, moderate or severe (See Figure 1). Transient episodes of hypocalcemia are usually mild, well tolerated, and often inconsequential. Nonspecific mild symptoms of hypocalcemia in donors include headaches, nervousness, irritability, lightheadedness, flushing, shivering, nausea, vomiting, chest discomfort, and abdominal cramping. Hypocalcemia can also alter neuromuscular function by enhancing motor nerve excitability and by causing spontaneous depolarization of nerve membranes. These changes in neuromotor excitability range from mild (loss of deep tendon reflexes, dysesthesia, muscle cramps, tremors, perioral or acral paresthesias), to moderate (involuntary muscle contraction, carpopedal spasm), or severe symptoms (tetany, laryngospasm, or seizures).3,9,11,21,22,40 Of these neurologic symptoms, local paresthesias are by far the most common side effect, constituting 12.1%41-39%42 of adverse reactions reported by donors.
Figure 1. Severity of symptoms associated with hypocalcemia.
Hypocalcemia also affects cardiac function.6,43 A low ionized calcium level from citrate administration has been demonstrated to cause a prolonged QT interval on electrocardiography (ECG). This is a sensitive marker of citrate toxicity and has been found to occur in all patients during plateletpheresis.44 Other ECG abnormalities reported with citrate exposure include increases in ST segment duration and decreases in T wave amplitude.45 This is a direct result of hypocalcemia and subsequent prolongation of the plateau phase of myocardial depolarization which can lead to cardiac arrhythmias.3,19,46 Although citrate-related cardiac abnormalities are usually brief and self-limited, one case report of fatal cardiac arrest after plasma exchange has been reported, secondary to citrate toxicity.47 Decreases in myocardial contractility, decreases in cardiac output, and increased vascular smooth muscle relaxation from citrate-induced hypocalcemia have also been seen as serum citrate levels rise above 2.5 mmol/L and can result in systemic hypotension.21,44,48,49 Symptomatic hypocalcemia can be exacerbated when replacement fluids are administered which do not contain calcium (such as albumin) or when replacement fluids are given which contain additional citrate (such as FFP).3,17,23,39 Alkalosis can also accentuate citrate-induced symptoms.
Management of hypocalcemia
The presence of hypocalcemia (iCa2+ less than 4.5 mg/dL or 1.1 mmol/L) and severity of clinical symptoms dictates replacement strategies. Calcium replacement can be administered by the oral or parenteral route for prophylactic, intermittent, or continuous supplementation.
Mild symptomatic hypocalcemia
Strategies for management of mild hypocalcemia include using an anticoagulant solution with a lower citrate concentration (such as ACD-B), using a lower TBV estimate, altering citrate infusion rates through adjustment of the inlet pump or AC ratios, or using oral calcium supplements.7,11,19,49 Slowing the whole blood processing rate, inlet pump rate, citrate infusion rate, or reinfusion rate during the apheresis session has immediate effects and often suffices to mitigate symptoms.14,16,19,20,49 Simply pausing the apheresis procedure is also effective as it allows for citrate metabolism to catch up with citrate accumulation.14 Inevitably, these technical adjustments will extend procedure times.
Oral calcium supplements have been shown to be effective for management of mild symptomatic hypocalcemia. Oral calcium carbonate (2 grams in the form of an antacid, such as TUMS) can be given prophylactically 30 minutes before the initiation of apheresis. In a randomized, double blind, placebo controlled trial of 23 donors undergoing plateletpheresis, 2 grams of oral calcium carbonate was shown to increase total and ionized serum calcium levels during apheresis, while attenuating the compensatory rise in PTH.28 Additionally, prophylactic oral calcium significantly reduced the severity of paresthesias when compared to placebo, with a trend toward lower frequency of overall symptoms.28 Although oral supplements are recommended for patients with a history of paresthesias, routine prophylaxis is not recommended as 70-80% of donors will have minimal symptoms that do not require intervention.29,41 In another study, ingestion of 50-240 mL of an isotonic sport drink (containing ∼5 g of calcium) can significantly increase ionized calcium levels after 2-5 minutes and resolve ongoing clinical symptoms.9
Moderate to severe symptomatic hypocalcemia
Intravenous (IV) calcium supplementation is indicated for management of moderate to severe symptomatic hypocalcemia. IV calcium can be administered intermittently as scheduled boluses during apheresis or it can be given continuously throughout the entire procedure.
Two IV formulations are available for calcium replacement: calcium gluconate (CaGluc) or calcium chloride (CaCl2). Dosing guidelines for calcium supplementation is shown in Table 4. Calcium gluconate contains about 94 mg of elemental calcium per gram50, whereas CaCl2 has three times the elemental calcium content (270 mg elemental calcium/g) and has greater bioavailability.51 In one prospective study comparing the two formulations, continuous infusion of calcium chloride during leukapheresis resulted in higher total calcium and higher ionized calcium levels when compared to calcium gluconate.24 Administration of calcium chloride results in greater increases in serum ionized calcium and improvement in blood pressure when compared to calcium gluconate.51 However, the benefits of IV CaCl2 are offset by its vesicant properties (as 10% solution) and requirement for a central line for infusion.
Table 4. Calcium and Magnesium Supplement Guidelines.
| Formulation | Description | Dosing | Notes |
|---|---|---|---|
| Calcium Carbonate | Oral supplement (400 mg elemental calcium per g) |
2 grams po | |
| Calcium Gluconate | 10% solution (1gram/10mL: contains 90 mg elemental Ca2+ per g) |
|
|
| Calcium Chloride | 10% solution (1 g/10mL; contains 270 mg elemental Ca2+ per g) |
|
Vesicant if delivered via peripheral line; must be given by central venous access |
| Magnesium Sulfate PO | 3 grams | 3 g po every 6 hours for 4 doses | |
| Magnesium Sulfate IV | 1-2 grams | 1 to 2 g of IV MgSO4 over 5-60 minutes. For Torsades, 1-2 g may be given by IV push over 5-20 minutes | Pediatric dosing: 25-50 mg/kg IV or IM |
In certain situations, where the risk of citrate toxicity is high, continuous infusion of intravenous calcium may be indicated. For example, during large volume leukapheresis (involving a 3-volume or greater exchange) there is a high incidence of citrate related complications. Continuous infusion of IV calcium has been shown to be effective in raising total calcium levels,24 maintaining ionized calcium levels within the normal range,9 and reducing the PTH response to hypocalcemia.20,24 This results in a significant reduction in the incidence of citrate-related symptoms from 48%20-54%24 down to 17%20-20%,24 without affecting the technical performance or efficacy of the procedure. Prophylactic continuous infusion of IV calcium has also been shown to decrease the severity of citrate-induced symptoms,20 and it has been used successfully in other studies of peripheral blood progenitor cell harvesting9,43 or plateletpheresis.52 Similarly, supplementation of return fluid with a constant infusion of calcium gluconate has been demonstrated to significantly decrease the incidence of citrate reactions during therapeutic plasma exchange, although the severity of the reactions was not affected in this study.14 Dosing guidelines for IV calcium supplementation in the return fluid are shown in Table 4.
Infusions of IV calcium before the initiation of apheresis has demonstrated limited efficacy because of its transient nature.9 Likewise, intermittent boluses of 10% calcium gluconate during apheresis have not been shown to decrease the rate of citrate reactions when compared to simply adjusting the apheresis process or administering oral calcium carbonate.14 Patients with a significant cardiac history should not receive continuous IV calcium infusions during apheresis.20 In addition, calcium should never be added directly to citrated plasma as this may activate clotting factors and initiate clotting in the returned blood.11
Other Citrate-Induced Metabolic Abnormalities
Several metabolic complications other than hypocalcemia have been described with citrate administration including hypomagnesemia, metabolic alkalosis, hypokalemia, and changes in parathyroid hormone levels (PTH). These metabolic complications are citrate mediated and often related to citrate infusion rates or donor characteristics.29
Magnesium is a divalent cation which has a similar affinity to citrate as calcium. During apheresis, magnesium levels decrease by as much as 30-50% depending on the procedure performed and the citrate infusion rate, although the fall in magnesium levels is more rapid and takes longer to recover when compared to calcium.25,53 Hypomagnesemia may also result from increased urinary excretion of magnesium, which has been recorded during and after apheresis.24,29 Clinically, the symptoms of hypomagnesemia are very similar to the symptoms of hypocalcemia and include muscle spasm, muscle weakness, decreased cardiac contractility, and decreased vascular tone. In studies, decreased levels of ionized magnesium is significantly associated with the development of citrate-associated symptoms.24,29 Unlike the symptoms of hypocalcemia, symptoms of hypomagnesemia will not respond when calcium supplementation is administered. Administration of magnesium sulfate can improve paresthesias and other symptoms due to decreased ionized magnesium levels.49 Dosing guidelines for oral and IV magnesium supplementation are provided in Table 4.
Metabolic alkalosis can also develop as a direct result of apheresis. Normally, the liver metabolizes citrate, consuming hydrogen ions and producing sodium bicarbonate, thus raising serum pH levels.36 The subsequent development of metabolic alkalosis is usually tempered by renal excretion of excess bicarbonate, but in patients with renal failure or who are anuric, excess bicarbonate can accumulate resulting in a metabolic alkalosis.24,29,31,32 Still, no significant morbidity has been shown with the development of metabolic alkalosis10.
The generation of a metabolic alkalosis contributes to the development of hypokalemia. For example, the simultaneous development of metabolic alkalosis and hypokalemia, with serum potassium levels less than 3.0 mEq/L, has been found to occur frequently in patients with TTP after plasma exchange.10 Metabolic alkalosis directly induces hypokalemia, as high serum bicarbonate levels cause a compensatory shift of hydrogen ions out of the intracellular compartment in exchange for potassium. The concurrent presence of hypocalcemia and hypomagnesemia also contributes to the development of hypokalemia, as the development of citrate-induced hypocalcemia has been found to be strongly associated with the subsequent development of citrate-induced hypokalemia.36,53 Metabolic processing of the dextrose contained in citrate anticoagulants is also hypothesized to contribute to potassium shifts and to the development of hypokalemia. Clinically, hypokalemia may exacerbate neurologic and cardiac abnormalities causing muscle weakness and hypotonia, as low serum potassium levels and decreased potassium conductance contribute to unstable membrane potentials.36,54 Finally, serum glucose levels rise during apheresis because of the dextrose contained in ACD-A and ACD-B, prompting increased insulin secretion.28
Heparin Anticoagulation
Unfractionated heparin (UFH) can be used as an anticoagulant during apheresis; however, because of the efficacy of citrate based anticoagulation in routine exchange procedures, heparin use is limited to certain apheresis indications: combined citrate/heparin therapy in pediatric apheresis, large volume leukapheresis in adults, or as a stand alone agent in membrane based plasma exchange, LDL-apheresis, or photopheresis.20,24 When heparin is administered as a simultaneous continuous infusion with citrate,55 WB:citrate ratios are lowered to1:25 to 1:30 and heparin is given by bolus dosing (20-40 IU/kg) followed by continuous infusion (0.1-0.5 IU/kg/min) with monitoring of activated clotting time (ACT) to maintain clotting time to 180-210 seconds56. Alternatively, heparin can be added to ACD solution (5000 units to 500 mL ACD-A bag) and infused at a WB:AC ratio of 1:30.56 Combining heparin with citrate anticoagulation does not completely eliminate citrate reactions; in one study, 39% of patients still experienced paresthesias due to citrate-related hypocalcemia.42 For membrane based plasma exchange, LDL apheresis or photophoresis, we advise following manufacturer guidelines for heparin dosing as recommendations vary by membrane system and instrument.
All heparins exert their primary anticoagulant effect by binding antithrombin (AT) and altering its conformation to produce rapid inactivation of clotting factors, particularly thrombin and Factor Xa. Thus, the major complication of UFH therapy is bleeding (major bleeding 0-7%, fatal bleeding 0-3%).57 Hemorrhagic episodes are correlated with the intensity of anticoagulation, concomitant use of anti-platelet therapy, gender (F>M), comorbidities such as recent surgical or interventional procedures, and coexisting hemostatic defects.58 In patients receiving heparin therapy for continuous renal replacement, bleeding risk was shown to be increased by 50% for every 10 second rise in the aPTT.59 Other complications include heparin-induced thrombocytopenia (HIT) 1-5%,60 and osteoporosis (2-3% risk of vertebral fracture with >1 month treatment).57 Heparin-Induced Thrombocytopenia (HIT) is a life-threatening immune complication of heparin therapy caused by antibodies directed to complexes containing heparin and an autologous platelet protein, Platelet Factor 4 (PF4). The diagnostic hallmark of HIT is the development of thrombocytopenia during heparin therapy, usually within 5-7 days of drug initiation. Clinically, thrombocytopenia is mild (usually 50-100,000), rarely causes bleeding, and often times, is manifested only as a relative drop (30-50%) in platelet counts.60 Thrombocytopenia in HIT, however, serves as a marker for thrombotic complications that develop in approximately 20-50% of patients. Mortality from thrombotic complications in unrecognized HIT is exceptionally high (6-27%).60 HIT is diagnosed based on clinical features (temporal relationship of thrombocytopenia with recent heparin exposure, 30-50% drop in platelet counts, and exclusion of other causes). Clinical evaluation for HIT can be challenging in patients with other causes for thrombocytopenia, such as infection and/or underlying TTP.
Conclusion
Citrate is the preferred anticoagulant for maintaining patency of apheresis circuits. Symptoms of citrate toxicity are generally due to symptomatic hypocalcemia and can be prevented through monitoring of ionized calcium and monitoring of patients symptoms. Prophylactic oral calcium supplementation or a continuous infusion of intravenous calcium can be effective in reducing the incidence of citrate-induced symptoms during high risk procedures. Unfractionated heparin is reserved for procedures requiring high blood flow (membrane-based plasma exchange or LDL apheresis) or for use in conjunction with citrate to minimize symptoms of citrate toxicity.
Acknowledgments
This work is supported by NIH grant T32HL007057-35 (GL). Contents of this manuscript were presented at the 2011 Therapeutic Apheresis Academy
References
- 1.Mollison PL. The introduction of citrate as an anticoagulant for transfusion and of glucose as a red cell preservative. Br J Haematol. 2000;108:13–8. doi: 10.1046/j.1365-2141.2000.01827.x. [DOI] [PubMed] [Google Scholar]
- 2.Carlon GC, Howland WS, Goldiner PL, Kahn RC, Bertoni G, Turnbull AD. Adverse effects of calcium administration. Report of two cases. Arch Surg. 1978;113:882–5. doi: 10.1001/archsurg.1978.01370190104021. [DOI] [PubMed] [Google Scholar]
- 3.Strauss RG. Mechanisms of adverse effects during hemapheresis. J Clin Apher. 1996;11:160–4. doi: 10.1002/(SICI)1098-1101(1996)11:3<160::AID-JCA7>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 4.Hastings AB, M F, Eichelberger L, Hall Jl, Da Costa E. The ionization of calcium, magnesium, and strontium citrates. J Biol Chem. 1934;107:351–70. [Google Scholar]
- 5.Flanigan MJ, Pillsbury L, Sadewasser G, Lim VS. Regional hemodialysis anticoagulation: hypertonic tri-sodium citrate or anticoagulant citrate dextrose-A. Am J Kidney Dis. 1996;27:519–24. doi: 10.1016/s0272-6386(96)90162-6. [DOI] [PubMed] [Google Scholar]
- 6.Ludbrook J, Wynn V. Citrate intoxication; a clinical and experimental study. Br Med J. 1958;2:523–8. doi: 10.1136/bmj.2.5095.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farrokhi P, Farahmand H, Bismuth A, et al. How to stabilize the level of ionized calcium and citrate during plateletpheresis. Vox Sang. 1998;74:7–12. [PubMed] [Google Scholar]
- 8.Kramer L, Bauer E, Joukhadar C, et al. Citrate pharmacokinetics and metabolism in cirrhotic and noncirrhotic critically ill patients. Crit Care Med. 2003;31:2450–5. doi: 10.1097/01.CCM.0000084871.76568.E6. [DOI] [PubMed] [Google Scholar]
- 9.Kishimoto M, Ohto H, Shikama Y, Kikuta A, Kimijima I, Takenoshita S. Treatment for the decline of ionized calcium levels during peripheral blood progenitor cell harvesting. Transfusion. 2002;42:1340–7. doi: 10.1046/j.1537-2995.2002.00205.x. [DOI] [PubMed] [Google Scholar]
- 10.Marques MB, Huang ST. Patients with thrombotic thrombocytopenic purpura commonly develop metabolic alkalosis during therapeutic plasma exchange. J Clin Apher. 2001;16:120–4. doi: 10.1002/jca.1022. [DOI] [PubMed] [Google Scholar]
- 11.Hester JP, McCullough J, Mishler JM, Szymanski IO. Dosage regimens for citrate anticoagulants. J Clin Apher. 1983;1:149–57. doi: 10.1002/jca.2920010306. [DOI] [PubMed] [Google Scholar]
- 12.Crookston KP, Novak DJ. Physiology of Apheresis. In: McLeod BC, Szczepiorkowski ZM, Weinstein R, Winters JL, editors. Apheresis: Principles and Practice. Bethesda, MD: AABB Press; 2010. pp. 45–69. [Google Scholar]
- 13.Ladenson JH, Miller WV, Sherman LA. Relationship of physical symptoms, ECG, free calcium, and other blood chemistries in reinfusion with citrated blood. Transfusion. 1978;18:670–9. doi: 10.1046/j.1537-2995.1978.18679077948.x. [DOI] [PubMed] [Google Scholar]
- 14.Weinstein R. Prevention of citrate reactions during therapeutic plasma exchange by constant infusion of calcium gluconate with the return fluid. J Clin Apher. 1996;11:204–10. doi: 10.1002/(SICI)1098-1101(1996)11:4<204::AID-JCA5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 15.Hester JP, Ayyar R. Anticoagulation and electrolytes. J Clin Apher. 1984;2:41–51. doi: 10.1002/jca.2920020109. [DOI] [PubMed] [Google Scholar]
- 16.Penny A. Plasmapheresis procedure design operation: a consideration of citrate anticoagulant usage. Transfus Sci. 1989;10:51–6. [Google Scholar]
- 17.Uhl L, Maillet S, King S, Kruskall MS. Unexpected citrate toxicity and severe hypocalcemia during apheresis. Transfusion. 1997;37:1063–5. doi: 10.1046/j.1537-2995.1997.371098016446.x. [DOI] [PubMed] [Google Scholar]
- 18.Crocco A, D'Elia D. Adverse reactions during voluntary donation of blood and/or blood components. A statistical-epidemiological study. Blood Transfus. 2007;5:143–52. doi: 10.2450/2007.0005-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodnitzky RL, Goeken JA. Complications of plasma exchange in neurological patients. Arch Neurol. 1982;39:350–4. doi: 10.1001/archneur.1982.00510180028007. [DOI] [PubMed] [Google Scholar]
- 20.Buchta C, Macher M, Bieglmayer C, Hocker P, Dettke M. Reduction of adverse citrate reactions during autologous large-volume PBPC apheresis by continuous infusion of calcium-gluconate. Transfusion. 2003;43:1615–21. doi: 10.1046/j.1537-2995.2003.00571.x. [DOI] [PubMed] [Google Scholar]
- 21.Winters JL. Complications of donor apheresis. J Clin Apher. 2006;21:132–41. doi: 10.1002/jca.20039. [DOI] [PubMed] [Google Scholar]
- 22.Pulsipher MA, Chitphakdithai P, Miller JP, et al. Adverse events among 2408 unrelated donors of peripheral blood stem cells: results of a prospective trial from the National Marrow Donor Program. Blood. 2009;113:3604–11. doi: 10.1182/blood-2008-08-175323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korach JM, Petitpas D, Poiron L, Vincent N, Berger PH, Chillet P. 14 years of therapeutic plasma exchange in France. Transfus Apher Sci. 2001;25:73–7. doi: 10.1016/s1473-0502(01)00090-8. [DOI] [PubMed] [Google Scholar]
- 24.Bolan CD, Cecco SA, Wesley RA, et al. Controlled study of citrate effects and response to i.v. calcium administration during allogeneic peripheral blood progenitor cell donation. Transfusion. 2002;42:935–46. doi: 10.1046/j.1537-2995.2002.00151.x. [DOI] [PubMed] [Google Scholar]
- 25.Bolan CD, Greer SE, Cecco SA, Oblitas JM, Rehak NN, Leitman SF. Comprehensive analysis of citrate effects during plateletpheresis in normal donors. Transfusion. 2001;41:1165–71. doi: 10.1046/j.1537-2995.2001.41091165.x. [DOI] [PubMed] [Google Scholar]
- 26.Toffaletti J, Nissenson R, Endres D, McGarry E, Mogollon G. Influence of continuous infusion of citrate on responses of immunoreactive parathyroid hormone, calcium and magnesium components, and other electrolytes in normal adults during plateletapheresis. J Clin Endocrinol Metab. 1985;60:874–9. doi: 10.1210/jcem-60-5-874. [DOI] [PubMed] [Google Scholar]
- 27.Urban P, Scheidegger D, Buchmann B, Barth D. Cardiac Arrest and Blood Ionized Calcium Levels. Annals of Internal Medicine. 1988;109:110–3. doi: 10.7326/0003-4819-109-2-110. [DOI] [PubMed] [Google Scholar]
- 28.Bolan CD, Cecco SA, Yau YY, et al. Randomized placebo-controlled study of oral calcium carbonate supplementation in plateletpheresis: II. Metabolic effects. Transfusion. 2003;43:1414–22. doi: 10.1046/j.1537-2995.2003.00513.x. [DOI] [PubMed] [Google Scholar]
- 29.Bolan CD, Wesley RA, Yau YY, et al. Randomized placebo-controlled study of oral calcium carbonate administration in plateletpheresis: I. Associations with donor symptoms. Transfusion. 2003;43:1403–13. doi: 10.1046/j.1537-2995.2003.00514.x. [DOI] [PubMed] [Google Scholar]
- 30.Nowak MA, Campbell TE. Profound hypercalcemia in continuous veno-venous hemofiltration dialysis with trisodium citrate anticoagulation and hepatic failure. Clin Chem. 1997;43:412–3. [PubMed] [Google Scholar]
- 31.Nagai Y, Itabashi M, Mizutani M, et al. A case report of uncompensated alkalosis induced by daily plasmapheresis in a patient with thrombotic thrombocytopenic purpura. Ther Apher Dial. 2008;12:86–90. doi: 10.1111/j.1744-9987.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- 32.Pearl RG, Rosenthal MH. Metabolic alkalosis due to plasmapheresis. Am J Med. 1985;79:391–3. doi: 10.1016/0002-9343(85)90320-1. [DOI] [PubMed] [Google Scholar]
- 33.Smith RM. Blood replacement in thoracic surgery for children. J Am Med Assoc. 1956;161:1124–8. doi: 10.1001/jama.1956.02970120006002. [DOI] [PubMed] [Google Scholar]
- 34.Wirguin I, Brenner T, Shinar E, Argov Z. Citrate-induced impairment of neuromuscular transmission in human and experimental autoimmune myasthenia gravis. Ann Neurol. 1990;27:328–30. doi: 10.1002/ana.410270316. [DOI] [PubMed] [Google Scholar]
- 35.Humpe A, Riggert J, Munzel U, Kohler M. A prospective, randomized, sequential crossover trial of large-volume versus normal-volume leukapheresis procedures: effects on serum electrolytes, platelet counts, and other coagulation measures. Transfusion. 2000;40:368–74. doi: 10.1046/j.1537-2995.2000.40030368.x. [DOI] [PubMed] [Google Scholar]
- 36.Schlenke P, Frohn C, Steinhardt MM, Kirchner H, Kluter H. Clinically relevant hypokalaemia, hypocalcaemia, and loss of hemoglobin and platelets during stem cell apheresis. J Clin Apher. 2000;15:230–5. doi: 10.1002/1098-1101(2000)15:4<230::aid-jca3>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 37.Basic-Jukic N, Kes P, Glavas-Boras S, Brunetta B, Bubic-Filipi L, Puretic Z. Complications of therapeutic plasma exchange: experience with 4857 treatments. Ther Apher Dial. 2005;9:391–5. doi: 10.1111/j.1744-9987.2005.00319.x. [DOI] [PubMed] [Google Scholar]
- 38.Rock G, Sutton DM. Apheresis: man versus machine. Transfusion. 1997;37:993–5. doi: 10.1046/j.1537-2995.1997.371098016435.x. [DOI] [PubMed] [Google Scholar]
- 39.Antonic M, Gubensek J, Buturovic-Ponikvar J, Ponikvar R. Comparison of citrate anticoagulation during plasma exchange with different replacement solutions. Ther Apher Dial. 2009;13:322–6. doi: 10.1111/j.1744-9987.2009.00733.x. [DOI] [PubMed] [Google Scholar]
- 40.Juan D. Hypocalcemia. Differential diagnosis and mechanisms. Arch Intern Med. 1979;139:1166–71. doi: 10.1001/archinte.139.10.1166. [DOI] [PubMed] [Google Scholar]
- 41.Moog R. Adverse events in peripheral progenitor cell collection: a 7-year experience. J Hematother Stem Cell Res. 2001;10:675–80. doi: 10.1089/152581601753193896. [DOI] [PubMed] [Google Scholar]
- 42.Reik RA, Noto TA, Fernandez HF. Safety of large-volume leukapheresis for collection of peripheral blood progenitor cells. J Clin Apher. 1997;12:10–3. doi: 10.1002/(sici)1098-1101(1997)12:1<10::aid-jca3>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 43.Passos-Coelho JL, Braine HG, Wright SK, et al. Large-volume leukapheresis using regional citrate anticoagulation to collect peripheral blood progenitor cells. J Hematother. 1995;4:11–9. doi: 10.1089/scd.1.1995.4.11. [DOI] [PubMed] [Google Scholar]
- 44.Laspina SJ, Browne MA, McSweeney EN, et al. QTc prolongation in apheresis platelet donors. Transfusion. 2002;42:899–903. doi: 10.1046/j.1537-2995.2002.00144.x. [DOI] [PubMed] [Google Scholar]
- 45.Furman RA, Hellerstein HK, Startzman VV. Electrocardiographic changes occurring during the course of replacement transfusions. J Pediatr. 1951;38:45–50. doi: 10.1016/s0022-3476(51)80085-4. [DOI] [PubMed] [Google Scholar]
- 46.Pinnick RV, Wiegmann TB, Diederich DA. Regional citrate anticoagulation for hemodialysis in the patient at high risk for bleeding. N Engl J Med. 1983;308:258–61. doi: 10.1056/NEJM198302033080506. [DOI] [PubMed] [Google Scholar]
- 47.van der Meulen J, Janssen MF, Oe PL. Cardiac arrest during rapid plasma exchange for haemolytic uraemic syndrome. Nephrol Dial Transplant. 1994;9:1841. [PubMed] [Google Scholar]
- 48.Bunker JP, Bendixen HH, Murphy AJ. Hemodynamic effects of intravenously administered sodium citrate. N Engl J Med. 1962;266:372–7. doi: 10.1056/NEJM196202222660802. [DOI] [PubMed] [Google Scholar]
- 49.Olson PR, Cox C, McCullough J. Laboratory and clinical effects of the infusion of ACD solution during plateletpheresis. Vox Sang. 1977;33:79–87. doi: 10.1111/j.1423-0410.1977.tb02237.x. [DOI] [PubMed] [Google Scholar]
- 50.AHA. Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Part 10.1: Life-Threatening Electrolyte Abnormalities. Circulation. 2005;112:IV-121–IV-5. [Google Scholar]
- 51.Broner CW, Stidham GL, Westenkirchner DF, Watson DC. A prospective, randomized, double-blind comparison of calcium chloride and calcium gluconate therapies for hypocalcemia in critically ill children. J Pediatr. 1990;117:986–9. doi: 10.1016/s0022-3476(05)80151-9. [DOI] [PubMed] [Google Scholar]
- 52.Bastin G. The use of constant calcium infusion reduces side effects in high-yield platelet apheresis (abstract) J Clin Apher. 1993;8:32. [Google Scholar]
- 53.Mercan D, Bastin G, Lambermont M, Dupont E. Importance of ionized magnesium measurement for monitoring of citrate-anticoagulated plateletpheresis. Transfusion. 1997;37:418–22. doi: 10.1046/j.1537-2995.1997.37497265343.x. [DOI] [PubMed] [Google Scholar]
- 54.Perseghin P, Confalonieri G, Buscemi F, et al. Electrolyte monitoring in patients undergoing peripheral blood stem cell collection. J Clin Apher. 1999;14:14–7. doi: 10.1002/(sici)1098-1101(1999)14:1<14::aid-jca3>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 55.Diaz MA, Garcia-Sanchez F, Lillo R, Vicent MG, Vicario JL, Madero L. Large-volume leukapheresis in pediatric patients: pre-apheresis peripheral blood CD34+ cell count predicts progenitor cell yield. Haematologica. 1999;84:32–5. [PubMed] [Google Scholar]
- 56.Kim HC. Therapeutic pediatric apheresis. Journal of Clinical Apheresis. 2000;15:129–57. doi: 10.1002/(sici)1098-1101(2000)15:1/2<129::aid-jca7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 57.Hirsh J, Bauer KA, Donati MB, Gould M, Samama MM, Weitz JI. Parenteral Anticoagulants: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:141S–59. doi: 10.1378/chest.08-0689. [DOI] [PubMed] [Google Scholar]
- 58.Schulman S, Beyth RJ, Kearon C, Levine MN. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:257S–98S. doi: 10.1378/chest.08-0674. [DOI] [PubMed] [Google Scholar]
- 59.van de Wetering J, Westendorp RG, van der Hoeven JG, Stolk B, Feuth JD, Chang PC. Heparin use in continuous renal replacement procedures: the struggle between filter coagulation and patient hemorrhage. J Am Soc Nephrol. 1996;7:145–50. doi: 10.1681/ASN.V71145. [DOI] [PubMed] [Google Scholar]
- 60.Arepally G, Ortel TL. Heparin-induced thrombocytopenia. N Eng J Med. 2006;355:809–17. doi: 10.1056/NEJMcp052967. [DOI] [PubMed] [Google Scholar]
- 61.Kankirawatana S, Huang ST, Marques MB. Continuous infusion of calcium gluconate in 5% albumin is safe and prevents most hypocalcemic reactions during therapeutic plasma exchange. Journal of Clinical Apheresis. 2007;22:265–9. doi: 10.1002/jca.20142. [DOI] [PubMed] [Google Scholar]