Abstract
Objectives/Aims
To examine whether morphine pharmacokinetics (PK) and/or genetic polymorphisms in opioid-related genes, underlie differences in analgesic response and side effects to morphine in Latino (L) vs non-Latino Caucasian (NL) children.
Background
Morphine has high interindividual variability in its analgesic response and side effects profile. Earlier studies suggest that morphine response may vary by race and ethnicity.
Methods
Prospective cohort study in L and NL children, 3–17 years of age comparing pain scores, occurrence of side effects, plasma morphine, morphine-6-and morphine-3-glucuronide concentrations measured after a single morphine IV bolus administration. Non-compartmental pharmacokinetic analysis and genotyping for 28 polymorphisms in 8 genes (UGT1A8, UGT2B7, ABCB1, COMT, STAT6, MC1R, OPRM1, and ARRB2) were done.
Results
We enrolled 68 children (33 L, 35 NL). There were no differences in pain scores or need for rescue analgesia. Statistically significant differences in the occurrence of side effects were documented: While 58% of L children experienced at least one side effect only 20% of NL did (p=0.001). Pruritus was 4 times (p=0.006) and emesis 7 times (p=0.025) more frequent in L compared to NL. PK parameters were similar between groups. None of the assessed polymorphisms mediated the association between ethnicity and side effects.
Conclusions
We found statistically significant differences in occurrence of side effects after morphine administration between L and NL children. Neither differences in morphine or metabolite concentrations, nor the genetic polymorphisms examined, explain these findings. Studies are needed to further investigate reasons for the increase in morphine side effects by Latino ethnicity.
Introduction
While morphine is the analgesic most widely used for treatment of pain in adults and children, high interindividual variability in its effect occurs. Earlier studies also suggest that morphine response may vary by race and ethnicity (1,2), evidenced by differences in pharmacokinetics and occurrence of side effects among adult volunteers of different ethnic/racial backgrounds.
In a study among adult volunteers of South American Indian, Latino and Caucasian origin, Cepeda et al. reported greater susceptibility for respiratory depression among South American Indians and Latinos when compared to Caucasians even with lower concentrations of the active M6G metabolite (1). Prior studies have reported that Latinos receive less opioids (3,4) for pain treatment when compared to Caucasian patients. While these findings are likely multifactorial, they are consistent with the documented susceptibility for respiratory depression and suggest a possible role of genetic variability in such responses.
More recent studies have focused on how interindividual differences in morphine response can be influenced by variability in genes coding for morphine metabolizing enzymes, opioid receptors and other opioid-related targets (5–16). It is also known that polymorphisms in such genes may vary by race/ethnicity.
To our knowledge there are no studies on morphine analgesic response and side effect profiles among Latino patients treated for pain. The present study aims to (1) evaluate possible ethnic differences in morphine pharmacokinetics, analgesic response and occurrence of side effects between Latino and non-Latino children, and (2) explore whether polymorphisms in genes coding for morphine metabolizing enzymes (UGT1A8, UGT2B7), opioid receptors (OPRM1) and other opioid-related targets (ARRB2, ABCB1, COMT, STAT6, MC1R) might underlie these differences.
Methods
Prospective cohort study conducted after Institutional Review Board approval from Seattle Children's Hospital (Seattle, WA). Children, with ASA status I–II, 3 to 17 years of age, who underwent tonsillectomy and adenoidectomy (T&A) in our institution from 2007–2010 were eligible for the study. Two groups were defined on the basis of ethnicity. The Latino group included children born to two parents who at the time of the child's admission self identified as Latinos. The Caucasian group included children born to two parents self-identified as non-Latino Caucasians.
We excluded developmentally delayed patients, patients currently being treated for asthma or gastro-esophageal reflux, patients with a history of moderate to severe obstructive sleep apnea (17) documented by sleep study, and children who were treated with opioid medication in the month prior to surgery.
Because some of the outcome variables measured can vary by age (i.e. nausea), to balance groups we did a stratified enrollment based on age (3–4, 5–7, 8–12, 13–17). Prior to surgery, we obtained written consent from parents and written assent from children 7 years of age and older. The consent had an optional section for genotyping. Genotyping was done only in patients where this section was also signed.
Assuming that differences in analgesic response and/or side effects will likely be due to PK differences, we calculated a sample size of 70 patients (35 per group) to detect a 10 unit difference in mean morphine clearance between groups. We used a median clearance of 49 ml·kg-1·min-1, range from 35 to 69 ml·kg−1·min−1 (18,19), with an estimated standard deviation (SD) of 12 units. We used NQuery Advisor 6.0 software with a two-sided α=0.05 level t-test and a power of 90%.
Procedures
Patients received a single intravenous (IV) dose of morphine (0.15 mg/kg) at induction of anesthesia. Plasma concentrations of morphine, and glucuronide metabolites M6G and M3G, were measured at baseline (immediately prior to morphine administration) and at five time points afterwards (10, 30, 60, 120 and 240 minutes).
The standardized anesthetic used included: inhalation induction and maintenance with sevoflurane, N20 and O2, two IV lines, one for clinical use and one for study blood samples in different extremities, acetaminophen orally (15 mg/kg, maximum dose 650 mg) pre-operatively or rectally (40 mg/kg) at the time of induction, and dexamethasone (0.2 mg/kg, maximum dose 8 mg). The use of dexamethasone as an anti-inflammatory and preventative antiemetic is considered standard of care for T&A's in our institution. Use of preventative ondansetron varies depending on surgeon's preference. To standardize prophylactic antiemetic administration, ondansetron (0.1 mg/kg, maximum dose 4 mg) and/or metoclopramide (0.15 mg/kg, maximum dose 10 mg) were administered only if patients presented with retching or vomiting in recovery. For postoperative analgesia, patients received hydromorphone rescue boluses IV (0.005 mg/kg) for pain scores ≥5 as assessed by the recovery nurse using the Face Legs Activity Cry and Consolability (FLACC) scale (20).
Independent of the recovery nurse, a research nurse assessed pain scores using FLACC scale, and sedation scores using Watcha scale (21) every 5 minutes in Phase I recovery. At these same points, data on hydromorphone and anti-emetic requirements as well as the presence of emesis, pruritus, and respiratory depression were also recorded. Emesis was defined as retching or actual vomiting; pruritus as active scratching of the nose or face; and respiratory depression as arterial oxygen saturation below 90% by pulse oximetry. English proficiency (using a 5 point Likert response scale) (22) of all parents and children was recorded at admission. For those of Latino origin, country of origin and time living in the US were also recorded.
Pharmacokinetic Analysis and Genotyping
Plasma concentrations of morphine, M3G and M6G were analyzed by liquid chromatography-mass spectrometry (details are provided in S1). The plasma concentration-time data from each subject was analyzed by non-compartmental pharmacokinetic analysis using WinNonlin software (Pharsight, Mountain View, CA). The area under the concentration time curve was calculated by the log-linear trapezoidal method from 0 to 240 minutes.
We assessed 28 genetic polymorphisms in 8 genes (Table 1) using DNA extraction from whole blood and subsequent genotyping at the Functional Genomics Laboratory of the Center for Ecogenetics and Environmental Health at the University of Washington (Seattle, WA); details are provided in S1. Genotyping was done blind to ethnicity and all outcomes.
Table 1.
Studied genes and polymorphisms
| Gene-symbol | Gene-name | Polymorphisms |
|---|---|---|
| ABCB1 | ATP-binding cassette (ABC) transporters | rs2032582, rs1128503, rs1045642 |
| COMT | catechol-O-methyltransferase | rs4680, rs740603, rs5746849, rs7290221, rs7287550, rs174680 |
| STAT6 | signal transducer and activator of transcription 6 | rs167769, rs3024974 |
| MC1R | melanocortin 1 receptor | rs1805009, rs1805007, rs1805008 |
| OPRM1 | opioid receptor, mu 1 | rs1799972, rs1799971 |
| ARRB2 | arrestin, beta 2 | rs1045280, rs2271167, rs2036657, rs3786047, rs28365158, rs35741745, rs11868227, rs28365160 |
| UGT1A8 | UDP glucuronosyltransferase 1 family | rs4399719, rs10929303, rs887829, rs7439366 |
| UGT2B7 | UDP glucuronosyltransferase 2 family | |
Statistical analysis
Individual plasma concentrations of morphine and its metabolites were graphically examined, along with descriptive statistics by study group. Mean plasma concentrations of morphine and morphine metabolites were compared using two sample t-test. P<0.05 was considered statistically significant. Analysis of morphine analgesic response and occurrence of side effects was restricted to the initial post anesthetic recovery period (i.e. Phase I), where patients remain NPO (nil per os, nothing by mouth), in order to avoid confounding by oral intake and side effects secondary to hydromorphone administration for rescue analgesia.
Wilcoxon rank sum test was used to compare pain scores between groups. Occurrence of side effects was compared between groups using chi-square test (or Fisher's Exact test). To examine if receiving hydromorphone doses for pain rescue could influence the comparison of side effects between groups, we performed a longitudinal Generalized Estimating Equation model to adjust for the number of doses of hydromorphone the patient had received prior to each assessment time point.
To investigate whether genotype could mediate any observed associations between ethnicity and clinical response, we used stratified analysis with Mantel-Haenszel methods, to assess if crude relative risk (RR) and 95% confidence interval (CI), comparing Latino and non-Latino children for each of the outcome variables were meaningfully attenuated (>10% reduction) by adjustment for genotype. For the stratified analysis, genotype was retained as observed, unless the variant allele frequency necessitated dichotomization of genotype (homozygous wildtype, any mutant allele). For genotypes in which only one ethnic group carried a variant allele, we examined the potential for that difference to mediate the associations observed by restricting analyses to those children who were homozygous wildtype.
Results
We initially enrolled 70 patients; two patients were excluded after enrollment because the standardized protocol was not followed. Both children were Latino, and both were excluded at the time of induction of anesthesia. One presented laryngospasm at induction and the second one received only half of the dose of morphine. Thus, 68 patients completed the study (33 Latino, 35 non-Latino), of which 55 patients, 26 (79%) Latino and 29 (83%) non-Latino consented for genotyping.
Latino and non-Latino children were similar in age and sex (Table 2). All Latino parents were primarily Spanish speakers, with only 18% of them being bilingual. In contrast 51% of Latino children were English proficient. All non-Latino patients were native English speakers. (Table 2) Most (85%) of the Latino families were from Mexico, and 3 (9%) were from Central America.
Table2.
Demographic and clinical characteristics of study participants, by ethnicity.
| Non-Latino (n=35) | Latino (n=33) | p-value‡ | |
|---|---|---|---|
| Sex: N (%) male | 17 (49%) | 12 (36%) | 0.31 |
| Age (years): mean (SD) | 8.2 (3.4) | 8.3 (3.7) | 0.85 |
| English-speaking family | 35 (100%) | 6 (18%) | -- |
| English- speaking child | 35 (100%) | 17 (51%) | -- |
| Midazolam given | 1 (3%) | 2 (6%)** | 0.61 |
| Surgical time (minutes): mean (SD) | 19.9 (13.9) | 23.6 (12.6) | 0.25 |
| Time in Phase I recovery (minutes): mean (SD) | 44.5 (14.4) | 47.8 (19.7) | 0.40 |
Age, surgical time and time in phase I recovery were tested using two-sample t-test; all other comparisons were done via Fisher's Exact or Chi-square test
Excludes 2 individuals for whom data were not available
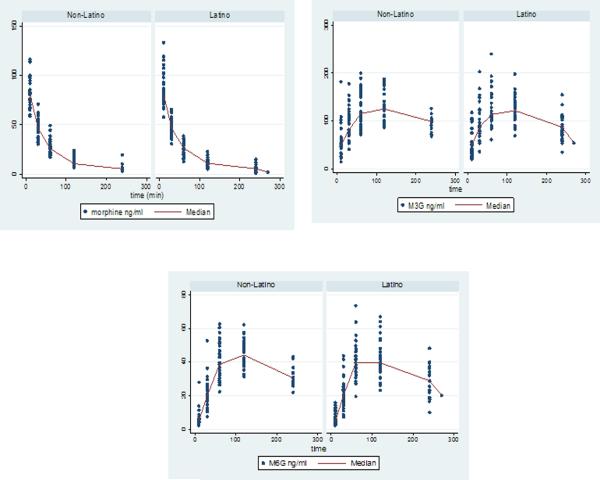
There were no differences in plasma concentrations of morphine or its metabolites between the two groups at each of the sampled time points (Figure 1). Area under the curve from 0 to 240 minutes (AUC0–240) for morphine and morphine metabolites was similar between groups. There were no differences either in morphine Cmax (sample at 10 min) or morphine metabolites Cmax or Tmax. (Table 3).
Figure 1.
Individual plasma concentrations of morphine and morphine metabolites by ethnic group.
Table 3.
Morphine pharmacokinetic parameters for Latino and non-Latino patients
| Non-Latino (n=35) | Latino (n=33) | p-value | |
|---|---|---|---|
| Morphine‡ | |||
| AUC( min*ng/ml) | 5309 (129) | 5528(1122) | 0.51 |
| Cmax (ng/ml) | 82 (15) | 87 (18) | 0.21 |
| M3G‡ | |||
| AUC (min*ng/ml) | 27105 (6360) | 26957 (7231) | 0.83 |
| Cmax (ng/ml) | 138 (33) | 135 (38) | 0.73 |
| Tmax (min) | 91 (36) | 86 (32) | 0.67 |
| M3G:morphine AUC ratio | 5.3 (2.1) | 4.9 (1.5) | 0.51 |
| M6G‡ | |||
| AUC (min*ng/ml) | 8418 (1552) | 8134 (2182) | 0.87 |
| Cmax (ng/ml) | 47 (9) | 45 (9) | 0.43 |
| Tmax (min) | 101 (29) | 95 (30) | 0.45 |
| M6G:morphine AUC ratio | 1.6(0.5) | 1.5 (0.4) | 0.75 |
Data is given as mean (sd). Comparisons were done using two-sample t-test.
Area under the curve (AUC), morphine-3-glucuronide (M3G), morphine-6-glucuronide (M6G), maximum plasma concentration (Cmax) and time at maximum plasma concentration (Tmax).
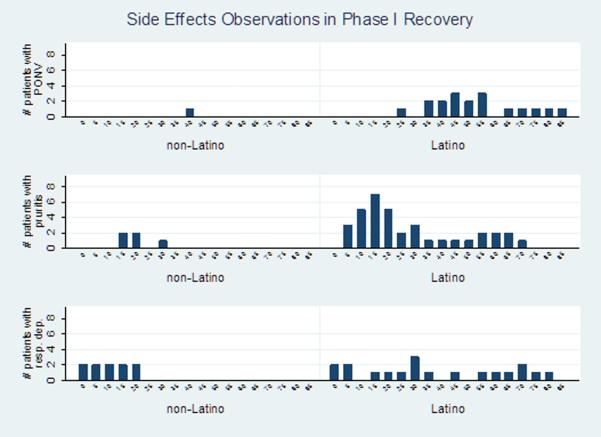
Morphine analgesia measured by pain scores and number of rescue boluses of hydromorphone, was not different between groups. (Table 4) In contrast, there were statistically significant differences in the occurrence of side effects. Occurrence of any side effect (emesis, pruritus and/or respiratory depression) was observed in 19 (58%) of Latino children but in only 7 (20%) of non-Latino children (p=0.001, RR=2.88, 95% CI 1.40–5.94). Emesis and pruritus were statistically significantly greater among Latinos than non-Latinos; and respiratory depression, while not statistically significant, was also more common in Latinos (Table 4). Seven Latino and 1 non-Latino patients received anti-emetic treatment with ondansetron. Four Latino patients required unplanned hospitalization: 3 for treatment of severe emesis and 1 for monitoring for respiratory depression. We analyzed separately the individuals who presented severe side effects and who required hospitalization and their morphine and morphine metabolites concentrations were not different from the mean group. Adjustment for time of administration and number of hydromorphone boluses given did not change the differences in side effects between groups. (Figure 2)
Table 4.
Morphine analgesic response and side effects
| Non-Latino N=35 | Latino N=33 | p-value | |
|---|---|---|---|
| Pain scores mean (SD) | 2.9 (2.9) | 3.0 (2.6) | 0.84 |
| Peak agitation score: mean (SD) | 1.5 (1.0) | 1.4 (0.9) | 0.56 |
| Any hydromorphone rescue boluses n (%) | 14 (40%) | 16 (48%) | 0.48 |
| Any side effect* n (%) | 7 (20%) | 19 (58%) | 0.001 |
| Emesis n (%) | 1 (3%) | 7 (21%) | 0.025 |
| Pruritus n (%) | 3 (9%) | 12 (36%) | 0.006 |
| Respiratory depression n (%) | 3 (9%) | 8 (24%) | 0.079 |
| Hospitalization for side effects n (%) | 0 (0%) | 4 (12%) | 0.05 |
Emesis, pruritus and/or respiratory depression; patients who experienced more than one side effect were counted only once.
Figure 2.
Side effects over time by ethnic group Y axis number of patients presenting the symptoms, X axis time in minutes. Note: One subject can be counted multiple times if symptoms persist over time. Analysis adjusted by number and timing of hydromorphone boluses.
In each group, all polymorphisms were in Hardy-Weinberg equilibrium. The RR between ethnicity and having any side effect was not attenuated by adjustment for any assessed polymorphism for which variant alleles were observed in both ethnic groups. Further, after adjustment Latino children remained at a statistically significant increased risk of having any side effect relative to non-Latino children (data not shown). Some polymorphisms were only observed in one ethnic group (rs1805007, rs1805008, rs1805009 in non-Latinos; and rs1799972 in Latinos); for these we confirmed RRs remained similar while excluding the children (n of 1 to 6) carrying a variant allele.
Discussion
Our study found similar post-operative morphine and morphine metabolite pharmacokinetic parameters in Latino and non-Latino children. However, we observed statistically significant differences in the occurrence of side effects between ethnic groups. The occurrence of any side effect was nearly three times more common in Latino patients than in their non-Latino counterparts. Furthermore these differences persisted when we looked at each side effect individually. These differences were not likely to be a result of key factors such as hydromorphone rescue bolus use or time in Phase I follow up, as these were similar between groups, and results did not change with adjustment for these factors.
Our findings are in part consistent with the results from Cepeda et al. (1) in that they document an increased susceptibility for respiratory depression among Latinos compared to non-Latino patients. In contrast we found a greater risk for Latino vs. non-Latino patients to present other side effects, differing from Cepeda's findings on no differences in the occurrence of emesis and pruritus between ethnic groups. Possibly the discrepancies between the two studies can be explained by differences in the study populations. Our study was done in a clinical setting with patients receiving morphine for pain treatment instead of volunteer subjects. In particular, our study included patients having T&As, a procedure associated with increased risk for nausea and vomiting, increasing our statistical power to observe such differences.
Despite the differences in the occurrence of side effects, analgesic response was similar in both groups. This finding is consistent with previous reports showing that analgesic response and side effects to morphine and morphine metabolites are independent pharmacodynamic outcomes (16,23).
The reasons behind such marked differences in the occurrence of side effects between the two ethnic groups are not entirely clear from this study. Since plasma concentrations of morphine and morphine metabolites were similar in the two groups they do not explain differences in the side effect profiles. Additionally, in the stratified analyses by genotype, genotype-adjusted RRs were not markedly weaker than the crude estimate. Therefore the studied genetic polymorphisms cannot explain the differences we observed in the occurrence of side effects between groups, even though we included genes believed important to morphine metabolism and response, and selected polymorphisms with known functional effects (e.g. amino acid changes or transcription factor binding sites in gene promoters).
Our study has limitations. Although it was powered to detect differences in pharmacokinetic parameters, our sample sizes were modest for the other outcomes. Nonetheless, we were able to observe marked differences in the occurrence of side effects between groups. We also could conclusively determine that in our data none of the genetic polymorphisms examined were the sole source of a nearly three-fold difference in the occurrence of any side effects in Latino vs. non-Latino children. Due to sample size we cannot rule out the possibility that some of these polymorphisms could contribute to some degree in combination with other polymorphisms or non-genetic factors.
Another potential limitation is that race and ethnicity were defined by self identification instead of markers of ancestry. While this is true, this is unlikely to account for the ethnicity-side effects associations we observed, as self identification occurred prior to the assessment of side effects, and any error would be more likely to attenuate the associations we report. Lastly it is a limitation that we measured morphine and morphine metabolite concentrations over a period of 240 minutes, which does not provide a full characterization of plasma drug metabolite concentration-time profile. We based our decision on reducing participant burden and on prior experience with pharmacokinetic analyses in similar study groups. It was estimated that with concentrations collected out to 240 minutes post-IV dose, we would be able to accurately estimate more than 75% of the total morphine AUC0→∞, given that morphine is administered intravenously and the AUC depends mainly on clearance. As for the morphine metabolites, it is known from studies in adults with normal renal function, that the removal of morphine glucuronide metabolites is rate-limited by their formation; hence, their time course of washout parallels that of the parent drug morphine (24). Our study sample purposefully included only patients older than 3 years of age with the assumption that renal and metabolic functions have reached adult levels (19).
In conclusion our study found significant differences in the occurrence of side effects between Latino and non-Latino children after morphine administration for pain treatment. In particular the prevalence and severity of emesis and pruritus were greater in Latino children than non-Latino children. These differences did not appear to be due to differences in age or sex, nor any of the genetic polymorphisms examined. Further, morphine and morphine metabolite concentrations were similar between groups and are therefore also unlikely to explain these findings. Future studies are needed to elucidate possible factors to explain these differences in morphine side effects by ethnicity.
Supplementary Material
Acknowledgements
The authors would like to thank Zahra Afsharinejad and Jasmine Wilkerson, for completing the genotyping assays; and Megan Kelton-Rehkopf and Linda Risler for completing the Pharmacokinetic assays.
Research Funding This work was supported by the National Center for Research Resources (NCRR), a component of the National Institutes of Health [CTSA grant number UL1RR025014], the National Institute of Diabetes and Digestive and Kidney Diseases [3U01DK082325-0381] and the Department of Health and Human Services [1T32GM086270-01].
Footnotes
Disclosures All authors declare that they have no conflicts of interest of any kind.
References
- 1.Cepeda MS, Farrar JT, Roa JH, et al. Ethnicity influences morphine pharmacokinetics and pharmacodynamics. Clin Pharmacol. 2001;70:351–361. [PubMed] [Google Scholar]
- 2.Zhou HH, Sheller JR, Nu H, et al. Clin Pharmacol Ther. 1993;54:507–513. doi: 10.1038/clpt.1993.182. [DOI] [PubMed] [Google Scholar]
- 3.Todd KH, Samaroo N, Hoffman JR. Ethnicity as a risk factor for inadequate emergency department analgesia. JAMA. 1993;269:1537–1539. [PubMed] [Google Scholar]
- 4.Tamayo-Sarver JH, Hinze SW, Cydulka RK, et al. Racial and ethnic disparities in emergency department analgesic prescription. Am J Public Health. 2003;93:2067–2073. doi: 10.2105/ajph.93.12.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sawyer MB, Innocenti F, Das Scheng C, et al. A pharmacogenetic study of uridine diphosphate-glucuronyltransferase 2B7 in patients receiving morphine. Clin Pharmacol Ther. 2003;73:566–574. doi: 10.1016/S0009-9236(03)00053-5. [DOI] [PubMed] [Google Scholar]
- 6.Duguay Y, Baar C, Skorpen F, et al. A novel functional polymorphism in the uridine diphosphate glusuronosyltransferase 2B7 promoter with significant impact on promoter activity. Clin Pharmacol Ther. 2004;75:223–233. doi: 10.1016/j.clpt.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Fujita K, Ando Y, Yamamoto W, et al. Association of UGT2B7 and ABCB1 genotypes with morphine-induced adverse drug reactions in Japanese patients with cancer. Cancer Chemother Pharmacol. 2010;65:251–258. doi: 10.1007/s00280-009-1029-2. [DOI] [PubMed] [Google Scholar]
- 8.Araki K, Fujita K, Ando Y, et al. Pharmacogenetic impact of polymorphisms in the coding region of UGT1A1 gene on SN-38 glucuronidation in Japanese patients with cancer. Cancer Sci. 2006;97:1255–1259. doi: 10.1111/j.1349-7006.2006.00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klepstad P, Dale O, Skorpen F, et al. Genetic variability and clinical efficacy of morphine. Acta Anesthesiol Scand. 2005;49:902–908. doi: 10.1111/j.1399-6576.2005.00772.x. [DOI] [PubMed] [Google Scholar]
- 10.Sia AT, Lim Y, Lim EC, et al. A118G Single Nucleotide Polymorphisms of human μ-Opioid receptor gene influences pain perception and patient-controlled intravenous morphine consumption after intrathecal morphine for postcesarean analgesia. Anesthesiology. 2008;109:520–526. doi: 10.1097/ALN.0b013e318182af21. [DOI] [PubMed] [Google Scholar]
- 11.Coulbault L, Beaussier M, Verstuyft C, et al. Environmental and genetic factors associated with morphine response in the postoperative period. Clin Pharmacol Ther. 2006;79:316–324. doi: 10.1016/j.clpt.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Rakvag TT, Ross JR, Sato H, et al. Genetic variation in the catechol-o-methyltransferase gene and morphine requirements in cancer patients with pain. Mol Pain. 2008;18:64. doi: 10.1186/1744-8069-4-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campa D, Gioia A, Tomei A, et al. Association of ABCB1/MDR1 and OPRM1 gene polymorphisms with morphine pain relieve. Clin Pharmacol Ther. 2008;83:559–566. doi: 10.1038/sj.clpt.6100385. [DOI] [PubMed] [Google Scholar]
- 14.Ross JR, Rutter D, Welsh K, et al. Clinical response to morphine in cancer patients and genetic variation in candidate genes. Pharmacogenomics J. 2005;5:324–336. doi: 10.1038/sj.tpj.6500327. [DOI] [PubMed] [Google Scholar]
- 15.Mogil JS, Ritchie J, Smith SB, et al. Melanocortin-1 receptor gene variants affect pain and μ-opioid analgesia in mice and humans. J Med Genet. 2005;42:583–587. doi: 10.1136/jmg.2004.027698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan EC, Lim EC, Teo YY, et al. Ethnicity and OPRM variant independently predict pain perception and patient-controlled analgesia usage for post-operative pain. Mol Pain. 2009;23:32. doi: 10.1186/1744-8069-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strauss SG, Lynn AM, Bratton SL, et al. Ventilatory response to CO2 in children with obstructive sleep apnea from adenotonsillar hypertrophy. Anesth Analg. 1999;89:328–32. doi: 10.1097/00000539-199908000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Lynn AM, Nespeca MK, Bratton SL, et al. Intravenous morphine in postoperative infants: intermittent bolus dosing versus targeted continuous infusions. Pain. 2000;88:89–95. doi: 10.1016/S0304-3959(00)00313-4. [DOI] [PubMed] [Google Scholar]
- 19.McRorrie TI, Lynn AM, Nespeca MK, et al. The maturation of morphine clearance and metabolism. Am J Dis Child. 1992;146:972–976. doi: 10.1001/archpedi.1992.02160200094036. [DOI] [PubMed] [Google Scholar]
- 20.Merkel SI, Voepel-Lewis T, Shayevitz JR, et al. The FLACC: a behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997;23:293–297. [PubMed] [Google Scholar]
- 21.Galinkin JL, Fazi LM, Cuy RM, et al. Use of intranasal fentanyl in children undergoing myringotomy and tube placement during halothane and sevoflurane anesthesia. Anesthesiology. 2000;93:1378–1383. doi: 10.1097/00000542-200012000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez A, Schillinger D, Grumbach K, et al. Physician language ability and cultural competence. An exploratory study of communication with Spanish-speaking patients. J Gen Intern Med. 2004;19:167–174. doi: 10.1111/j.1525-1497.2004.30266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romberg RR, Olofsen E, Biji H, et al. Polymorphisms of μ-Opioid receptor gene (OPRM1:c:118A>G) does not protect against opioid induced respiratory depression despite reduced analgesic response. Anesthesiology. 2005;102:522–530. doi: 10.1097/00000542-200503000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Osborne R, Joel S, Grebenik K, et al. The pharmacokinetics of morphine and morphine glucuronides in kidney failure. Clin Pharmacol Ther. 1993;54:158–167. doi: 10.1038/clpt.1993.127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.