Abstract
Background
Volumetric measures of mesial temporal lobe structures on magnetic resonance imaging scans recently have been explored as potential biomarkers of dementia in patients with Parkinson's disease, with investigations primarily focused on hippocampal volume. Both in vivo magnetic resonance imaging and post-mortem tissue studies in Alzheimer's disease, however, demonstrate that the entorhinal cortex is involved earlier in disease-related pathology than the hippocampus. The entorhinal cortex, a region integral in declarative memory function, projects multimodal sensory information to the hippocampus via the perforant path. In Parkinson's disease, entorhinal cortex atrophy as measured on magnetic resonance imaging, however, has received less attention compared to hippocampal atrophy.
Methods
We compared entorhinal cortex and hippocampal atrophy in 12 subjects with Parkinson's disease dementia including memory impairment, 14 Parkinson's disease subjects with normal cognition, and 14 healthy controls with normal cognition, using manual segmentation methods on magnetic resonance imaging scans.
Results
While hippocampal volumes were similar in the two Parkinson's disease cognitive groups, entorhinal cortex volumes were substantially smaller in the demented Parkinson's disease subjects compared the cognitively normal Parkinson's disease subjects (p<0.05). In addition, normalized entorhinal cortex and hippocampal volumes for right and left hemispheres were significantly lower in the demented Parkinson's disease group compared to healthy controls.
Conclusions
Our findings suggest that entorhinal cortex atrophy differentiates demented and cognitively normal Parkinson's disease subjects, in contrast to hippocampal atrophy. Thus, entorhinal cortex atrophy on magnetic resonance imaging may be a potential biomarker for dementia in Parkinson's disease, particularly in the setting of memory impairment.
Keywords: Parkinson's disease, cognition, magnetic resonance imaging, mesial temporal lobe, Alzheimer's disease
Introduction
Dementia is a frequent and disabling outcome in the course of Parkinson's disease (PD) and greatly affects morbidity, mortality, and quality of life 1-4. Longitudinal studies demonstrate that about 80% of PD patients will develop dementia (PDD) 5, 6. Thus, it is critical to identify biomarkers associated with the development of PDD.
Structural magnetic resonance imaging (MRI) provides an in vivo method for the assessment of neuroanatomical substrates of dementia and associated neuropathological changes. For example, volumetric MRI has emerged as an important in vivo marker of Alzheimer's disease (AD) and amnestic mild cognitive impairment (MCI), with studies demonstrating hippocampal and entorhinal cortex atrophy on MRI in these patients 7-11. These mesial temporal lobe regions are important in declarative memory function 12, and the entorhinal cortex, which projects to the hippocampus via the perforant pathway, is particularly vulnerable to early AD-associated neuropathology 13. Atrophy in these regions on MRI correlates with histological changes found in post-mortem AD cases 14-16. MRI-derived volume loss in specific brain regions may also serve as a marker of incipient dementia, and entorhinal cortex atrophy on baseline MRI may better predict conversion to AD in those with amnestic MCI 11, 17, 18 or cognitive complaints 19. Furthermore, revised criteria for AD and MCI now incorporate MRI evidence of mesial temporal lobe atrophy into the diagnostic algorithms 20-22.
Hippocampal atrophy on MRI also occurs in PD dementia, though to a lesser degree than in AD 23-28. Non-demented PD patients also demonstrate hippocampal atrophy on MRI, though not in all studies 23, 24, 26, 29. Variable results in studies of non-demented PD patients may occur because some of these patients have normal cognition but others have mild cognitive impairment (i.e., cognitively impaired but not meeting dementia criteria). Thus, “non-demented” is not necessarily synonymous with “cognitively normal.” Overall, the presence of hippocampal atrophy in demented and non-demented PD patients suggests that it is not a specific marker for PDD.
Entorhinal cortex atrophy on MRI may be a better marker for dementia in PD since this region, which projects to the hippocampus, may be pathologically affected earlier than the hippocampus. Indeed, post-mortem PDD cases exhibit neuropathological changes in this structure 30-36, though the relative contributions from Lewy bodies, Lewy neurites, amyloid plaques, and neurofibrillary tangles remain controversial. Entorhinal cortex atrophy on MRI may reflect these underlying neuropathological changes. Only one volumetric MRI study has investigated entorhinal cortex atrophy in PDD and non-demented PD subjects 37. Entorhinal cortex volumes were significantly smaller in the PDD group compared to healthy controls, and while the PDD group had smaller mean entorhinal cortex volumes compared to the non-demented PD group, this difference was not significant on post-hoc comparisons. Since differences in methodologies and clinical groups may influence study results, entorhinal cortex atrophy on MRI warrants further investigation as an in vivo marker that can distinguish PDD patients, particularly those with memory impairment, from cognitively intact PD patients.
Our study aim was to examine entorhinal cortex and hippocampal atrophy using manual segmentation in two highly contrasting, PD cognitive groups (i.e., subjects categorized as PDD and PD with normal cognition [PD-NC]), compared to healthy controls (HC) with normal cognition. Although several semi-automated MRI techniques to detect brain atrophy are available, small areas with individual variability such as entorhinal cortex are best studied using manual segmentation 38. We hypothesized that entorhinal cortex volumes would not only distinguish PDD from HC subjects, but also would differentiate PDD from PD-NC subjects, in contrast to hippocampal volumes.
Methods
Subjects
Forty subjects (12 PDD, 14 PD-NC, and 14 HC) were studied. PD subjects were recruited from the Rush University Movement Disorders clinic and examined by a movement disorders neurologist (JGG). The PD subjects met United Kingdom Parkinson's Disease Society Brain Bank criteria 39. Atypical or secondary forms of parkinsonism (e.g., multiple system atrophy, progressive supranuclear palsy, corticobasal degeneration, dementia with Lewy bodies [DLB] or parkinsonism due to neuroleptic exposure, cerebrovascular disease, or known structural causes) were excluded. PD subjects were matched by age (+/- 3 years), gender, and education (+/- 3 years) to controls. Controls were recruited from the community as part of an ongoing, longitudinal neuroimaging study of aging and AD 18. As part of this study, the controls underwent evaluations including medical history, neurological examination, neuropsychological testing, blood tests, and MRI scans; in addition, these controls have been followed with annual clinical evaluations and MRI scans for over 10 years. Controls had normal neurologic examinations, normal cognition, and Mini-mental State Examination (MMSE) 40 scores ≥ 28. Controls were used solely as a reference for the neuroimaging comparisons across subject groups, not for comparisons of neuropsychological data. Exclusionary criteria for participants included: contraindications to MRI (e.g., cardiac pacemaker/defibrillator, surgical clips, foreign metallic implants); major depression; severe or unstable medical conditions; neurosurgery; seizures or other conditions that could cause cognitive impairment; or anticholinergic medications. Informed consent was obtained from all participants according to the Institutional Review Board rules of Rush University Medical Center, Chicago, IL.
Clinical and neuropsychological evaluations of PD subjects
The clinical evaluation of PD subjects included: assessments of demographic and disease-related variables, Unified Parkinson's Disease Rating Scale (UPDRS) Part III motor scores 41, Hoehn and Yahr stage, 42 and PD medications converted to levodopa equivalent doses (LEDD) 43. The neuropsychological evaluation assessed 4 cognitive domains (attention/executive function, language, memory, and visuospatial function) and depression 44. Raw scores for the cognitive tests were transformed to z-scores based on normative data 45, 46. Cognitive domain scores were calculated by averaging the z-scores for the neuropsychological tests within specific cognitive domains: (1) declarative memory - Consortium to Establish a Registry for Alzheimer's disease [CERAD] word list trials, delayed recall 47, 2) attention/executive function - Digit span 48, Symbol Digit Modalities Test 49, Category naming of animals 47, 3) language - 15-item Boston Naming Test 50, Similarities 48, and 4) visuospatial function - 15-item Judgment of Line Orientation 51, intersecting pentagon drawing item from the MMSE using an ordinal 6-point scale 52). PDD or PD-NC diagnoses were determined in a consensus conference (neurologist [JGG], neuropsychologists [GTS, BB]) incorporating Diagnostic and Statistical Manual of Mental Disorders, 4th edition criteria for PD dementia to include memory impairment 53, neuropsychological test performance, semi-structured interview with the subject and/or caregiver, and clinical impression.
MRI protocol and processing
MR images were acquired on a 1.5 Tesla General Electric Signa scanner, using the manufacturer's 3D Fourier transform spoiled gradient recalled pulse (SPGR) sequence with the following acquisition parameters: 124 contiguous coronal images, 1.6 mm thick, matrix=256x192, field of view=22 cm, TR/TE=33.3/7 msec, flip angle=35°, signals averaged=1.
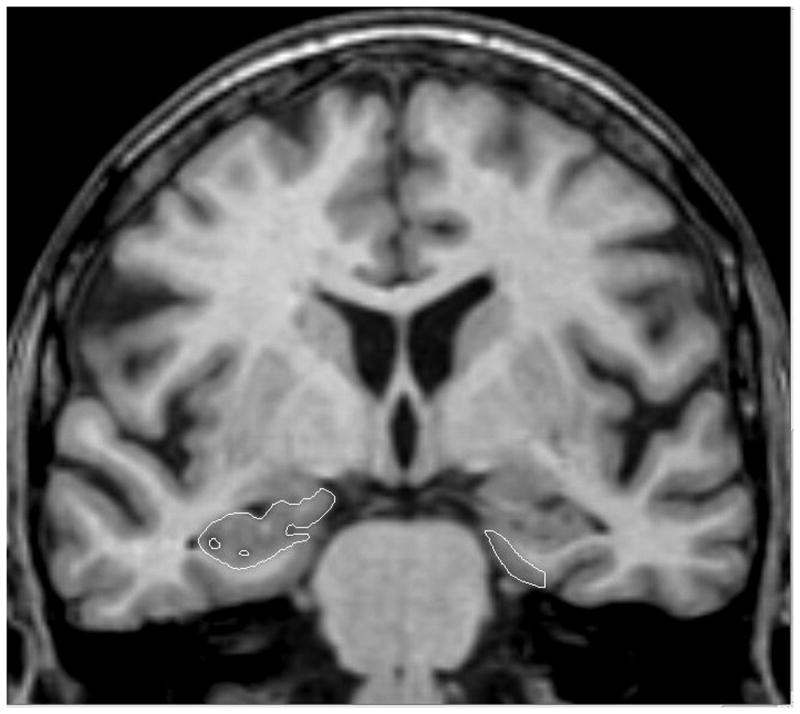
Entorhinal cortex and hippocampal volumes were manually segmented using Analyze software (Mayo Clinic Foundation, Rochester, MN) with each hemisphere computed separately from coronal slices oriented perpendicularly to the long axis of the hippocampal formation (Figure 1). Entorhinal cortex tracings began with the first section in which the gyrus ambiens, amygdala, and white matter of the parahippocampal gyrus were visible and ended two slices prior to where the lateral geniculate nucleus first appeared. The superomedial border rostrally was the sulcus semiannularis and caudally, the subiculum; the shoulder of the collateral sulcus was used as the ventrolateral border 54. Hippocampal volume included the fimbria, dentate gyrus, hippocampus proper, and subiculum, starting with the slice where the hippocampus could be clearly differentiated from the amygdala by the alveus and ending immediately rostral to the full appearance of the fornix 18.
Figure 1.
A coronal slice on a MRI scan illustrating the segmentation of the entorhinal cortex (outlined, right side) and Hippocampus (outlined, left side).
To correct for individual differences in brain size, volumes for each region of interest were divided by total intracranial volume (i.e., normalized). Intracranial volume was computed by tracing the inner table of the cranium in consecutive, sagittally-formatted, 5-mm sections spanning the entire brain with a straight line drawn from the inner surface of the clivus to the occipital bone, at the level of the foramen magnum. Normalized volumes were determined using the following formula: absolute volume (mm3)/intracranial volume (mm3) × 1000. Raters (JGG and TRS) were trained to be within 95% of each other and of LdeT-M; intra-rater correlation coefficients for entorhinal cortex and hippocampal volumes were high for TRS 55 and for JGG (0.98 and 0.99, respectively). Tracings were checked, slice-by-slice, by LdeT-M. Raters were blinded to participant identity and diagnosis.
Statistical analyses
Statistical analyses were performed using SPSS 18.0 (PASW 18, Chicago, IL). Demographic variables were compared across the 3 groups using one-way analysis of variance (ANOVA) and post-hoc comparisons with Scheffe tests. Gender differences were compared using Chi-square tests. Disease-related characteristics were compared using independent t-tests for continuous variables or non-parametric tests for ordinal variables. To assess group and hemispheric differences in entorhinal cortex and hippocampal volumes in the 3 groups, repeated measure ANOVAs were done separately for each region of interest, with groups as the between-subject factor and hemispheres as the within-subject factor, followed by post-hoc comparisons with Scheffe tests. Relationships between entorhinal cortex or hippocampal volumes and cognitive domain scores were examined using linear regressions, with region of interest as the dependent variable and cognitive domain z-scores as predictor variables. Statistical significance was set at p<0.05.
Results
Clinical features (Table 1)
Table 1. Demographic data and clinical characteristics of the groups.
| HC (n=14) | PD-NC (n=14) | PDD (n=12) | P value | |
|---|---|---|---|---|
| Age, years | 72.0 (6.1) | 70.6 (6.5) | 75.8 (7.1) | 0.129 |
| Gender (male), n | 7 | 9 | 10 | 0.206 |
| Education, years | 15.9 (3.1) | 15.2 (3.4) | 14.2 (3.5) | 0.444 |
| MMSE | 29.3 (0.6) | 28.9 (1.1) | 21.9 (4.1)* | 0.000 |
| PD duration, years | NA | 9.0 (3.3) | 12.5 (5.3) | 0.051 |
| LEDD, mg/d | NA | 915.3 (511.2) | 800.2 (246.5) | 0.464 |
| UPDRS total motor score | NA | 32.1 (9.8) | 37.8 (8.9) | 0.136 |
| Hoehn & Yahr stage, median (range) | NA | 2 (2-5) | 3 (2-5) | 0.012 |
| Hamilton Depression rating scale | NA | 4.5 (3.0) | 7.2 (3.9) | 0.08 |
There were no significant differences among the PDD, PD-NC or HC groups in age (p=0.129), gender (p=0.206), or education (p=0.444). There was a significant group effect for MMSE scores (F [2, 37] = 38.8, p<0.0001) with lower scores in the PDD group. Post-hoc comparisons for MMSE scores revealed that both the PD-NC and HC groups differed significantly from the PDD group (p<0.0001), but not from each other. PDD subjects had slightly longer disease durations than PD-NC subjects (9.0 +/- 3.3 vs. 12.5 +/- 5.3 years, p=0.051). Demented PD subjects had worse motor severity as measured by Hoehn and Yahr stage (p=0.012), but differences between PDD and PD-NC for UPDRS Part III total motor scores and LEDDs were not significant; neither were Hamilton depression rating scores which were low overall, reflecting a non-depressed PD cohort.
Neuropsychological features of the PD subjects (Table 2)
Table 2. Neuropsychological data for PD subjects.
| PD-NC | PDD | p value | |
|---|---|---|---|
| Cognitive domain z-scores | |||
| Memory | 0.78 (1.11) | -2.33 (0.81) | 0.000 |
| Attention/Executive function | 0.45 (0.79) | -1.09 (0.57) | 0.000 |
| Language | 0.69 (0.63) | -1.6 (0.93) | 0.000 |
| Visuospatial | 0.02 (0.92) | -4.10 (3.30) | 0.001 |
| Raw test scores | |||
| CERAD Word List trials | 22.0 (4.1) | 11.4 (2.7) | 0.000 |
| CERAD Delayed Recall | 7.5 (1.9) | 1.9 (1.8) | 0.000 |
| CERAD Recognition | 9.5 (0.9) | 6.1 (2.3) | 0.000 |
| Digits forwards | 10.1 (2.6) | 9.3 (2.0) | 0.391 |
| Digits backwards | 6.4 (1.9) | 4.1 (1.7) | 0.004 |
| Symbol Digit Modalities Test | 45.8 (10.3) | 10.0 (6.7) | 0.000 |
| Animal naming | 20.1 (3.9) | 10.2 (2.6) | 0.000 |
| Boston Naming Test | 14.6 (0.7) | 11.8 (2.0) | 0.001 |
| Similarities | 23.7 (5.4) | 11.9 (6.5) | 0.000 |
| Judgment of Line Orientation | 12.2 (2.6) | 6.5 (3.6) | 0.011 |
| Pentagons, median (range) | 6 (5-6) | 4 (1-6) | 0.000 |
Data presented as mean (SD) unless otherwise noted. Abbreviations: CERAD, Consortium to Establish a Registry for Alzheimer's Disease.
There were significant differences on raw neuropsychological test scores with worse cognitive performance in PDD subjects, compared to the PD-NC subjects. As often found in PD, performance was better on recognition tasks than on free recall 56; however, the PDD subjects performed significantly worse on both tasks compared to PD-NC subjects. Cognitive domain z-scores for memory as well as attention/executive function, language, and visuospatial function were significantly worse in PDD subjects compared to PD-NC subjects.
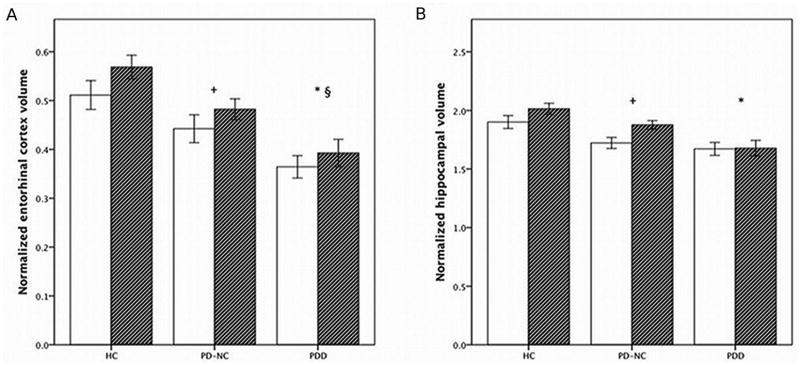
Entorhinal cortex and hippocampal volume measurements (Figure 2, Supplemental Table)
Figure 2.
Mean normalized volumes for A) entorhinal cortex and B) hippocampus.
Abbreviations: HC, healthy controls; PD-NC, PD with normal cognition; PDD, PD dementia. Hemispheric volumes (absolute volume in mm3/intracranial volume in mm3 × 1000) are depicted (left, white; right, hatched). Vertical bars represent the standard error of the mean. Significant differences: + PD-NC vs. HC (p=0.049, entorhinal cortex; p=0.053, hippocampus); *PDD vs. HC (p<0.0001, entorhinal cortex; p<0.0001, hippocampus), § PDD vs. PD-NC (p=0.04, entorhinal cortex).
Two factor repeated measures ANOVA revealed a significant effect for group (F [2, 37] = 13.11, p<0.0001) for the normalized entorhinal cortex volumes. On post-hoc comparisons, the PDD group had the smallest entorhinal cortex volumes and differed significantly from not only the HCs (p<0.0001), but also the PD-NCs (p=0.04). PD-NCs differed slightly, but significantly, from the HCs (p=0.049). There was also a significant effect for hemisphere (F [1, 37] = 6.73, p=0.014) due to larger right hemisphere volumes in all groups. However, there was no significant interaction between group and hemisphere (F [2, 37] = 0.27, p=0.764).
For the normalized hippocampal volumes, there were also significant group (F [2, 37] = 9.57, p<0.0001) effects, but in contrast to the entorhinal cortex volumes, post-hoc comparisons did not reveal significant differences between PDD and PD-NC subjects (p=0.173). However, there were significant differences between PDD and HCs (p<0.0001). Differences between PD-NC and HC groups were of borderline significance (p=0.053). Similar to the entorhinal cortex volumes, there were significant hemisphere effects due to larger right hemispheric volumes (F [1, 37] = 10.90, p=0.002), but no significant interaction between group and hemisphere (F [2, 37] = 2.47, p=0.098).
We also performed separate analyses of these two regions using age as a co-variate. Age was not significant in the model (entorhinal cortex, F [1, 36]=1.38, p=0.247 and hippocampus F [1, 36]=0.035, p=0.852). Controlling for age, volumetric results were similar to our original observations, such that the PDD group had significantly smaller entorhinal cortex volumes than the PD-NCs (p=0.04) and HCs (p<0.0001) and significantly smaller hippocampal volumes than the HCs (p<0.0001) but not the PD-NC group (p=0.09).
Relationship of volumes to cognitive domain z-scores in PD subjects
We performed linear regression analyses with normalized total entorhinal cortex and hippocampal volumes as dependent variables and cognitive domain z-scores (i.e., memory, attention/executive function, language, and visuospatial) as predictors. Models were run separately for entorhinal cortex and hippocampal volumes. Since we did not find any group by hemisphere effects on the repeated measure ANOVA analyses, normalized total volumes for the regions of interest were used in the linear regression analyses. Of the 4 cognitive domain z-scores entered in the models, only the memory domain z-score was a significant predictor of the volume. Memory domain z-scores accounted for 21% of the variance in normalized total entorhinal cortex volume (R2=0.21, F [1, 23] = 6.06, β = 0.457, p=0.02) and 18% of the variance in normalized total hippocampal volume (R2=0.18, F [1, 23] = 5.41, β = 0.427, p=0.03).
Discussion
The main finding of our study is that entorhinal cortex volumes on MRI are significantly smaller in PDD subjects compared to PD-NC subjects, whereas hippocampal volumes are not. These findings suggest that MRI-derived entorhinal cortex atrophy may provide an in vivo marker of dementia in PD. PDD subjects also had smaller entorhinal cortex and hippocampal volumes compared to HCs. We found larger right-side entorhinal cortex and hippocampal volumes in the PD-NC and HC groups; similar hemispheric asymmetry has been described before in volumetric MRI studies of the hippocampus 57. For PDD, however, hemispheric asymmetry (larger right side) was seen only in the entorhinal cortex, thereby suggesting that right-sided hippocampal dominance diminishes as dementia ensues. Furthermore, memory, but not non-memory, z-scores significantly predicted the regions of interest, thereby supporting a functional role for mesial temporal lobe structures.
Entorhinal cortex atrophy in PD may be more specific for dementia-related pathology including possible dual AD and PD pathology, particularly in the setting of memory deficits. We classified PDD subjects by DSM-IV criteria, which require impaired memory along with deficits in at least one other cognitive domain, to specifically assess the relationship between mesial temporal lobe regions of interest and memory function in PD. Not all PDD subjects, however, have memory impairment, and PDD criteria proposed by the Movement Disorder Society (MDS) allow impairment in non-memory domains 58. If these criteria were applied to our PDD cohort, all of our PD subjects with dementia would remain classified as demented, with memory being one of the impaired cognitive domains. While the presence of memory impairment in our PDD subjects may potentially reflect dual PD and AD pathology in these cases, future clinico-pathological studies will be needed to evaluate the underlying neuropathological contributions to PDD and relationships between neuroimaging and neuropathology. Few studies have investigated neuropathological correlates of MRI-derived mesial temporal lobe atrophy in Lewy body disorders 59. At present, the neuropathology of PDD remains heterogeneous with contributions from cortical and limbic Lewy bodies and Lewy neurites, brainstem degeneration, and amyloid plaques and neurofibrillary tangles 30-36.
Our findings of smaller entorhinal cortex volumes in PDD compared to PD-NC differ from the previous study examining entorhinal cortex volumes in PD by Kenny et al 37 and may be due to several methodological differences. Our studies utilized different inclusion/exclusion criteria and definitions of PDD. We matched our subject groups for age, gender, and education, whereas the other study did not. Manual segmentation protocols for measuring entorhinal cortex also differed in the neuroanatomical boundaries used and number of imaging slices included.
In addition, there were several differences in clinical features of our PD cohorts. Mean MMSE scores for the cognitively intact PD subjects were higher in our cohort (28.9 +/- 1.1 vs. 26.7 +/-1.8). Although the MMSE may not be a very sensitive test for PDD or PD-MCI 60, the higher MMSE scores in our non-demented PD cohort, along with their performance on detailed neuropsychological testing, support their diagnosis as cognitively normal PD and diminish the likelihood that they really have “mild cognitive impairment.” Furthermore, disease durations for the PD groups differed between the two studies. PD subjects in our cohort had long disease durations, ranging from 6-17 years, whereas Kenny et al reported durations as short as 1 year in demented and non-demented PD subjects. Our long PD duration solidifies our diagnostic confidence for PD. In addition, dementia was a later-onset complication in our PDD subjects, occurring after a mean of 10.5 years (+/- 5.0). These points underscore often subtle, clinical differences between non-demented PD groups (cognitively normal vs. mild cognitive impairment but not demented) and between PDD and DLB subjects that may be associated with heterogeneous neuroimaging and neuropathology findings.
The presence of hippocampal atrophy across the cognitive spectrum in PD is supported by other MRI studies 23-29, 61-63 and our study. Thus, hippocampal atrophy in PD may be the consequence of factors other than dementia-specific pathology including nigrostriatal dopaminergic depletion, disruption of noradrenergic or serotonergic projections to limbic structures, or concomitant, subsyndromal mood deficits. For example, infusion of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) into rodent substantia nigra pars compacta (SNPC) led not only to degeneration of nigrostriatal dopaminergic neurons, but also to cell loss in the hippocampal CA1 region 64, 65; neuroinflammation with microglial activation was detected in the SNPC, hippocampus, and amygdala. Also, degeneration of the locus ceruleus and raphe nuclei occurs in PD 30, 66 and may disrupt noradrenergic and serotonergic projections to limbic structures; clinically, this may manifest as anxiety and depression, which often present as subsyndromal disorders and predate PD-related motor symptoms 67. While our PD subjects were not depressed and did not have a history of major depressive episodes, we cannot fully exclude the occurrence of minor or subclinical depressive symptoms at some point in their lives. Hippocampal atrophy on MRI in depressed patients (without PD) has been attributed to hypothalamic-pituitary-adrenal axis dysfunction 68, 69. Indeed, hippocampal atrophy may be a downstream consequence of PD itself.
Our study's strengths include a well-defined PD cohort with detailed motor and cognitive characterizations, clinical diagnoses by Movement Disorders specialists, and cognitive diagnoses by consensus conference with clinicians and neuropsychologists. PDD and PD-NC subjects had similar disease durations and LEDDs and were carefully matched by age, education, and gender to healthy controls. Furthermore, volumetric analyses were performed in a blinded fashion, demonstrated good reliability, and utilized region of interest protocols developed at our center. Limitations include the small sample size, tertiary clinic referral pattern, and lack of clinico-pathological correlations to date. Manual segmentation techniques may be time consuming and operator dependent; while these features may limit its use in large scale, longitudinal studies, manual segmentation is well-suited for studying small brain regions such as the entorhinal cortex and for smaller-scale, hypothesis-generating studies.
We conclude that entorhinal cortex volumes on MRI are significantly smaller in PDD than PD-NC, whereas hippocampal volumes are not. Thus, entorhinal cortex atrophy on MRI may be a better in vivo marker for dementia in PD. These findings may shift our focus from the hippocampus to structures such as entorhinal cortex that are affected earlier in dementia and are more specific to memory deficits, possibly representing dual PD and AD pathologies. Studies with larger patient cohorts, detailed cognitive evaluations, cerebrospinal fluid biomarkers of beta-amyloid and tau proteins, and ultimately, post-mortem tissue will further our understanding of role of the entorhinal cortex in PD dementia and contributions from different pathologies. Prospective longitudinal studies that correlate clinical status and entorhinal cortex volumes for non-demented PD patients who convert to PDD will let us determine whether selective entorhinal cortex atrophy is a marker of incipient dementia.
Supplementary Material
Acknowledgments
This study was supported by grants K23NS060949 (JGG) and P01AG09466 (L deT-M) from the National Institutes of Health and from the Parkinson's Disease Foundation.
Financial Disclosure/Conflict of Interest: Dr. Goldman has received grant/research support from NIH K23NS060949 and the Parkinson's Disease Foundation. Dr. deToledo-Morrell has received grant/research support from NIH P01AG09466, T32AG00269, and U01AG024904.
Footnotes
Author Roles: Jennifer G. Goldman, MD, MS: 1) Research project: A. Conception, B. Organization, C. Execution; 2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3) Manuscript: A. Writing of the first draft, B. Review and Critique
Glenn Stebbins, PhD: 1) Research project: B. Organization; 2) Statistical Analysis: C. Review and Critique; 3) Manuscript: B. Review and Critique
Bryan Bernard, PhD: 1) Research project: C. Execution; 3) Manuscript: B. Review and Critique
Travis Stoub, PhD: 1) Research project: C. Execution; 3) Manuscript: B. Review and Critique
Christopher G. Goetz, MD: 1) Research project: A. Conception, B. Organization; 2) Statistical Analysis: C. Review and Critique; 3) Manuscript: B. Review and Critique
Leyla deToledo-Morrell, PhD: 1) Research project: A. Conception, B. Organization, C. Execution; 2) Statistical Analysis: C. Review and Critique; 3) Manuscript: B. Review and Critique
Financial Disclosures
Full Financial Disclosures of all Authors for the Past Year: Information concerning all sources of financial support and funding for the preceding twelve months, regardless of relationship to current manuscript. List sources or “none”:
| Jennifer G. Goldman, MD, MS: | |
|---|---|
| Stock Ownership in medically-related fields: none | Intellectual Property Rights: none |
| Consultancies: none | Expert Testimony: none |
| Advisory Boards: none | Employment: Rush University Medical Center |
| Partnerships: none | Contracts: none |
| Honoraria: Movement Disorders Society | Royalties: none |
| Grants: NIH K23NS060949 | Other: Parkinson's Disease Foundation |
| Glenn Stebbins, PhD: | |
| Stock Ownership in medically-related fields: none | Intellectual Property Rights: none |
| Consultancies: IMPAX Laboratories, Inc.; Ceregene, Inc.; Biovail Technologies, LTD; Santhera Pharmaceuticals; i3 | Expert Testimony: none |
| Advisory Boards: none | Employment: Rush University Medical Center |
| Partnerships: none | Contracts: none |
| Honoraria: none | Royalties: none |
| Grants: NIH, Michael J. Fox Foundation for Parkinson's Research, American Cancer Society, Fragile X Foundation | Other: Editorial Board, Journal of Clinical and Experimental Neuropsychology |
| Bryan Bernard, PhD: | |
| Stock Ownership in medically-related fields: none | Intellectual Property Rights: none |
| Consultancies: none | Expert Testimony: none |
| Advisory Boards: none | Employment: Rush University Medical Center |
| Partnerships: none | Contracts: none |
| Honoraria: none | Royalties: none |
| Grants: none | Other: none |
| Travis Stoub, PhD: | |
| Stock Ownership in medically-related fields: none | Intellectual Property Rights: none |
| Consultancies: none | Expert Testimony: none |
| Advisory Boards: none | Employment: Rush University Medical Center |
| Partnerships: none | Contracts: none |
| Honoraria: none | Royalties: none |
| Grants: none | Other: none |
| Christopher G. Goetz, MD: | |
| Stock Ownership in medically-related fields: none | Intellectual Property Rights: none |
| Consultancies: Addex Pharma SA, Asubio, Biovail Technologies, Cleveland Medical Devices, CNS Therapeutics, Curry Rockerfeller Group, Decision Resources, Dixon Group, ICON Clinical Research, Impax Pharmaceuticals, Ingenix (i3 Research), Intec Pharmaceuticals, Kenes International, Medical Education Global Solutions, Ono Pharmaceuticals, Oxford Biomedica, Santhera, United Bioscience Corporation, UCB. | Expert Testimony: none |
| Advisory Boards: Addex Pharma SA, Asubio, Biovail Technologies, Cleveland Medical Devices, CNS Therapeutics, Curry Rockerfeller Group, Decision Resources, Dixon Group, ICON Clinical Research, Impax Pharmaceuticals, Ingenix (i3 Research), Intec Pharmaceuticals, Kenes International, Medical Education Global Solutions, Ono Pharmaceuticals, Oxford Biomedica, Santhera, United Bioscience Corporation, UCB. | Employment: Rush University Medical Center |
| Partnerships | Contracts: None |
| Honoraria: Movement Disorder Society, American Academy of Neurology, University of Miami, University of Pennsylvania, University of Montreal. Neurological Society. | Royalties: Royalties: Oxford University Press, Elsevier Publishers, Wolters Kluwer Health, Lippincott, Wilkins and Williams. |
| Grants: Funding from NIH, Michael J. Fox Foundation, NIH. Dr. Goetz directs the Rush Parkinson's Disease Research Center that receives support from the Parkinson's Disease Foundation. He directs the translation program for the MDS-UPDRS and UDysRS and receives funds from the MDS for this effort. | Other: none |
| Leyla deToledo-Morrell, PhD: | |
| Stock Ownership in medically-related fields: None | Intellectual Property Rights: None |
| Consultancies: NIH | Expert Testimony: None |
| Advisory Boards: Neurobiology of Aging | Employment: Rush University |
| Partnerships: None | Contracts: None |
| Honoraria: NIH | Royalties: None |
| Grants: NIH P01 AG09466, NIH T32 AG00269 | Other: None |
References
- 1.Louis ED, Marder K, Cote L, Tang M, Mayeux R. Mortality from Parkinson disease. Arch Neurol. 1997;54(3):260–264. doi: 10.1001/archneur.1997.00550150024011. [DOI] [PubMed] [Google Scholar]
- 2.Aarsland D, Larsen JP, Tandberg E, Laake K. Predictors of nursing home placement in Parkinson's disease: a population-based, prospective study. J Am Geriatr Soc. 2000;48(8):938–942. doi: 10.1111/j.1532-5415.2000.tb06891.x. [DOI] [PubMed] [Google Scholar]
- 3.Hughes TA, Ross HF, Mindham RH, Spokes EG. Mortality in Parkinson's disease and its association with dementia and depression. Acta Neurol Scand. 2004;110(2):118–123. doi: 10.1111/j.1600-0404.2004.00292.x. [DOI] [PubMed] [Google Scholar]
- 4.Levy G, Tang MX, Louis ED, et al. The association of incident dementia with mortality in PD. Neurology. 2002;59(11):1708–1713. doi: 10.1212/01.wnl.0000036610.36834.e0. [DOI] [PubMed] [Google Scholar]
- 5.Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. 2003;60(3):387–392. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- 6.Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23(6):837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- 7.deToledo-Morrell L, Dickerson B, Sullivan M, Spanovic C, Wilson R, Bennett D. Hemispheric differences in hippocampal volume predict verbal and spatial memory performance in patients with Alzheimer's disease. Hippocampus. 2000;2000(10):136–142. doi: 10.1002/(SICI)1098-1063(2000)10:2<136::AID-HIPO2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 8.deToledo-Morrell L, Sullivan MP, Morrell F, Wilson RS, Bennett DA, Spencer S. Alzheimer's disease: in vivo detection of differential vulnerability of brain regions. Neurobiol Aging. 1997;18(5):463–468. doi: 10.1016/s0197-4580(97)00114-0. [DOI] [PubMed] [Google Scholar]
- 9.Jack CR, Jr, Petersen RC, Xu YC, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology. 1997;49(3):786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Killiany RJ, Hyman BT, Gomez-Isla T, et al. MRI measures of entorhinal cortex vs hippocampus in preclinical AD. Neurology. 2002;58(8):1188–1196. doi: 10.1212/wnl.58.8.1188. [DOI] [PubMed] [Google Scholar]
- 11.Stoub TR, Bulgakova M, Leurgans S, et al. MRI predictors of risk of incident Alzheimer disease: a longitudinal study. Neurology. 2005;64(9):1520–1524. doi: 10.1212/01.WNL.0000160089.43264.1A. [DOI] [PubMed] [Google Scholar]
- 12.Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253(5026):1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- 13.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 14.Bobinski M, de Leon MJ, Wegiel J, et al. The histological validation of post mortem magnetic resonance imaging-determined hippocampal volume in Alzheimer's disease. Neuroscience. 2000;95(3):721–725. doi: 10.1016/s0306-4522(99)00476-5. [DOI] [PubMed] [Google Scholar]
- 15.Jack CR, Jr, Dickson DW, Parisi JE, et al. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology. 2002;58(5):750–757. doi: 10.1212/wnl.58.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gosche KM, Mortimer JA, Smith CD, Markesbery WR, Snowdon DA. Hippocampal volume as an index of Alzheimer neuropathology: findings from the Nun Study. Neurology. 2002;58(10):1476–1482. doi: 10.1212/wnl.58.10.1476. [DOI] [PubMed] [Google Scholar]
- 17.deToledo-Morrell L, Goncharova I, Dickerson BC, Wilson RS, Bennett DA. From healthy aging to early Alzheimer's Disease: In vivo detection of entorhinal cortex atrophy. Ann N Y Acad Sci. 2000;911:240–253. doi: 10.1111/j.1749-6632.2000.tb06730.x. [DOI] [PubMed] [Google Scholar]
- 18.deToledo-Morrell L, Stoub TR, Bulgakova M, et al. MRI-derived entorhinal volume is a good predictor of conversion from MCI to AD. Neurobiol Aging. 2004;25(9):1197–1203. doi: 10.1016/j.neurobiolaging.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Dickerson BC, Goncharova I, Sullivan MP, et al. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer's disease. Neurobiol Aging. 2001;22(5):747–754. doi: 10.1016/s0197-4580(01)00271-8. [DOI] [PubMed] [Google Scholar]
- 20.Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6(8):734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 21.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Camicioli R, Moore MM, Kinney A, Corbridge E, Glassberg K, Kaye JA. Parkinson's disease is associated with hippocampal atrophy. Mov Disord. 2003;18(7):784–790. doi: 10.1002/mds.10444. [DOI] [PubMed] [Google Scholar]
- 24.Tam CW, Burton EJ, McKeith IG, Burn DJ, O'Brien JT. Temporal lobe atrophy on MRI in Parkinson disease with dementia: a comparison with Alzheimer disease and dementia with Lewy bodies. Neurology. 2005;64(5):861–865. doi: 10.1212/01.WNL.0000153070.82309.D4. [DOI] [PubMed] [Google Scholar]
- 25.Laakso MP, Partanen K, Riekkinen P, et al. Hippocampal volumes in Alzheimer's disease, Parkinson's disease with and without dementia, and in vascular dementia: An MRI study. Neurology. 1996;46(3):678–681. doi: 10.1212/wnl.46.3.678. [DOI] [PubMed] [Google Scholar]
- 26.Junque C, Ramirez-Ruiz B, Tolosa E, et al. Amygdalar and hippocampal MRI volumetric reductions in Parkinson's disease with dementia. Mov Disord. 2005;20(5):540–544. doi: 10.1002/mds.20371. [DOI] [PubMed] [Google Scholar]
- 27.Bouchard TP, Malykhin N, Martin WR, et al. Age and dementia-associated atrophy predominates in the hippocampal head and amygdala in Parkinson's disease. Neurobiol Aging. 2008;29(7):1027–1039. doi: 10.1016/j.neurobiolaging.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Burton EJ, McKeith IG, Burn DJ, Williams ED, O'Brien JT. Cerebral atrophy in Parkinson's disease with and without dementia: a comparison with Alzheimer's disease, dementia with Lewy bodies and controls. Brain. 2004;127(Pt 4):791–800. doi: 10.1093/brain/awh088. [DOI] [PubMed] [Google Scholar]
- 29.Bruck A, Kurki T, Kaasinen V, Vahlberg T, Rinne JO. Hippocampal and prefrontal atrophy in patients with early non-demented Parkinson's disease is related to cognitive impairment. J Neurol Neurosurg Psychiatry. 2004;75(10):1467–1469. doi: 10.1136/jnnp.2003.031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004;318(1):121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 31.Braak H, Rub U, Jansen Steur EN, Del Tredici K, de Vos RA. Cognitive status correlates with neuropathologic stage in Parkinson disease. Neurology. 2005;64(8):1404–1410. doi: 10.1212/01.WNL.0000158422.41380.82. [DOI] [PubMed] [Google Scholar]
- 32.Braak H, Braak E. Cognitive impairment in Parkinson's disease: amyloid plaques, neurofibrillary tangles, and neuropil threads in the cerebral cortex. J Neural Transm Park Dis Dement Sect. 1990;2(1):45–57. doi: 10.1007/BF02251245. [DOI] [PubMed] [Google Scholar]
- 33.Jellinger KA, Paulus W. Clinico-pathological correlations in Parkinson's disease. Clin Neurol Neurosurg. 1992;94(suppl):S86–88. doi: 10.1016/0303-8467(92)90033-y. [DOI] [PubMed] [Google Scholar]
- 34.Apaydin H, Ahlskog JE, Parisi JE, Boeve BF, Dickson DW. Parkinson disease neuropathology: later-developing dementia and loss of the levodopa response. Arch Neurol. 2002;59(1):102–112. doi: 10.1001/archneur.59.1.102. [DOI] [PubMed] [Google Scholar]
- 35.Aarsland D, Perry R, Brown A, Larsen JP, Ballard C. Neuropathology of dementia in Parkinson's disease: a prospective, community-based study. Ann Neurol. 2005;58(5):773–776. doi: 10.1002/ana.20635. [DOI] [PubMed] [Google Scholar]
- 36.Kovari E, Gold G, Herrmann FR, et al. Lewy body densities in the entorhinal and anterior cingulate cortex predict cognitive deficits in Parkinson's disease. Acta Neuropathol. 2003;106(1):83–88. doi: 10.1007/s00401-003-0705-2. [DOI] [PubMed] [Google Scholar]
- 37.Kenny ER, Burton EJ, O'Brien JT. A volumetric magnetic resonance imaging study of entorhinal cortex volume in dementia with lewy bodies. A comparison with Alzheimer's disease and Parkinson's disease with and without dementia. Dement Geriatr Cogn Disord. 2008;26(3):218–225. doi: 10.1159/000153432. [DOI] [PubMed] [Google Scholar]
- 38.Lehmann M, Douiri A, Kim LG, et al. Atrophy patterns in Alzheimer's disease and semantic dementia: a comparison of FreeSurfer and manual volumetric measurements. Neuroimage. 2010;49(3):2264–2274. doi: 10.1016/j.neuroimage.2009.10.056. [DOI] [PubMed] [Google Scholar]
- 39.Hughes AJ, Daniel SE, Blankson S, Lees AJ. A clinicopathologic study of 100 cases of Parkinson's disease. Arch Neurol. 1993;50(2):140–148. doi: 10.1001/archneur.1993.00540020018011. [DOI] [PubMed] [Google Scholar]
- 40.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 41.Fahn S, Elton RL, editors. the UPDRS Development Committee. Unified Parkinson's Disease Rating Scale. Florham Park, NJ: Macmillan Healthcare Information; 1987. [Google Scholar]
- 42.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 43.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. 2010;25(15):2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 44.Hamilton M. Development of a rating scale for primary depressive illness. Brit Jrl Soc Clin Psych. 1967;6:278–297. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 45.Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59(2):198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 46.Wilson R, Barnes L, Bennett D. Assessment of lifetime participation in cognitively stimulating activities. J Clin Exp Neuropsychol. 2003;25(5):634–642. doi: 10.1076/jcen.25.5.634.14572. [DOI] [PubMed] [Google Scholar]
- 47.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39(9):1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 48.Weschler D. Weschler Adult Intelligence Scale-III. New York: The Psychological Corporation; 1991. [Google Scholar]
- 49.Smith A. Symbol Digits Modality Test. Los Angeles: Western Psychological Services; 1973. [Google Scholar]
- 50.Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 51.Benton A, Varney N, Hamsher K. Visuospatial judgment: a clinical test. Arch Neurol. 1978;35:364–367. doi: 10.1001/archneur.1978.00500300038006. [DOI] [PubMed] [Google Scholar]
- 52.Bourke J, Castleden CM, Stephen B, Dennis M. A comparison of clock and pentagon drawing in Alzheimer's disease. Int J Geriatr Psychiatry. 1995;10:703–705. [Google Scholar]
- 53.Association AP. Diagnostic and statistical manual of mental disorders, text revision. 4th. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 54.Goncharova II, Dickerson BC, Stoub TR, deToledo-Morrell L. MRI of human entorhinal cortex: a reliable protocol for volumetric measurement. Neurobiol Aging. 2001;22(5):737–745. doi: 10.1016/s0197-4580(01)00270-6. [DOI] [PubMed] [Google Scholar]
- 55.Stoub TR, Rogalski EJ, Leurgans S, Bennett DA, deToledo-Morrell L. Rate of entorhinal and hippocampal atrophy in incipient and mild AD: relation to memory function. Neurobiol Aging. 2010;31(7):1089–1098. doi: 10.1016/j.neurobiolaging.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Higginson CI, Wheelock VL, Carroll KE, Sigvardt KA. Recognition memory in Parkinson's disease with and without dementia: evidence inconsistent with the retrieval deficit hypothesis. J Clin Exp Neuropsychol. 2005;27(4):516–528. doi: 10.1080/13803390490515469. [DOI] [PubMed] [Google Scholar]
- 57.Shi F, Liu B, Zhou Y, Yu C, Jiang T. Hippocampal volume and asymmetry in mild cognitive impairment and Alzheimer's disease: Meta-analyses of MRI studies. Hippocampus. 2009;19(11):1055–1064. doi: 10.1002/hipo.20573. [DOI] [PubMed] [Google Scholar]
- 58.Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord. 2007;22(12):1689–1707. doi: 10.1002/mds.21507. quiz 1837. [DOI] [PubMed] [Google Scholar]
- 59.Burton EJ, Mukaetova-Ladinska EB, Perry RH, Jaros E, Barber R, O'Brien JT. Neuropathological correlates of volumetric MRI in autopsy-confirmed Lewy body dementia. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 60.Hoops S, Nazem S, Siderowf AD, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. 2009;73(21):1738–1745. doi: 10.1212/WNL.0b013e3181c34b47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Apostolova LG, Beyer M, Green AE, et al. Hippocampal, caudate, and ventricular changes in Parkinson's disease with and without dementia. Mov Disord. 2010;25(6):687–688. doi: 10.1002/mds.22799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beyer MK, Janvin CC, Larsen JP, Aarsland D. A magnetic resonance imaging study of patients with Parkinson's disease with mild cognitive impairment and dementia using voxel-based morphometry. J Neurol Neurosurg Psychiatry. 2007;78(3):254–259. doi: 10.1136/jnnp.2006.093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nagano-Saito A, Washimi Y, Arahata Y, et al. Cerebral atrophy and its relation to cognitive impairment in Parkinson disease. Neurology. 2005;64(2):224–229. doi: 10.1212/01.WNL.0000149510.41793.50. [DOI] [PubMed] [Google Scholar]
- 64.Wang WF, Wu SL, Liou YM, Wang AL, Pawlak CR, Ho YJ. MPTP lesion causes neuroinflammation and deficits in object recognition in Wistar rats. Behav Neurosci. 2009;123(6):1261–1270. doi: 10.1037/a0017401. [DOI] [PubMed] [Google Scholar]
- 65.Sy HN, Wu SL, Wang WF, et al. MPTP-induced dopaminergic degeneration and deficits in object recognition in rats are accompanied by neuroinflammation in the hippocampus. Pharmacol Biochem Behav. 2010;95(2):158–165. doi: 10.1016/j.pbb.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 66.Dickson DW, Fujishiro H, Orr C, et al. Neuropathology of non-motor features of Parkinson disease. Parkinsonism Relat Disord. 2009;15(3):S1–5. doi: 10.1016/S1353-8020(09)70769-2. [DOI] [PubMed] [Google Scholar]
- 67.Aarsland D, Marsh L, Schrag A. Neuropsychiatric Symptoms in Parkinson's Disease. Movement Disorders. 2009;24(15):2175–2186. doi: 10.1002/mds.22589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A. 1996;93(9):3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gerritsen L, Comijs HC, van der Graaf Y, Knoops AJ, Penninx BW, Geerlings MI. Depression, Hypothalamic Pituitary Adrenal Axis, and Hippocampal and Entorhinal Cortex Volumes-The SMART Medea Study. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.01.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.