Abstract
Orbital apex and skull base masses often present with neuro-ophthalmic signs and symptoms. Though the localization of these syndromes and visualization of the responsible lesion on imaging is typically straightforward, definitive diagnosis usually relies on biopsy. Immunohistochemistry is important for categorization and treatment planning. IgG4 –related disease is emerging as a pathologically defined inflammatory process that can occur in multiple organ systems. We present two patients with extensive inflammatory mass lesions of the central nervous system with immunohistochemistry positive for IgG4 and negative for ALK-1 as examples of meningeal based IgG4-related inflammatory pseudotumors. In both patients, there was treatment response to mycophenolate mofetil.
Keywords: IgG4-related disease, mycophenolate mofetil, intracranial mass, optic neuropathy, inflammatory pseudotumor
1. Introduction
The term inflammatory pseudotumor has been applied to a heterogeneous group of mass-forming lesions in various anatomic regions and organs characterized by a proliferation of fibroblasts or myofibroblasts admixed with an inflammatory infiltrate composed mainly of lymphocytes and plasma cells. The term is sometimes used interchangeably with plasma cell granuloma and inflammatory myofibroblastic tumor (IMT), which leads to confusion both clinically and in the literature. Unlike IMT, which is considered neoplastic with ALK-1 expression as a distinguishing feature (1), many inflammatory pseudotumors of the orbit and central nervous system likely represent a manifestation of inflammatory fibrosclerosis or idiopathic sclerosing inflammation. This process is analogous to retroperitoneal fibrosis, sclerosing mediastinitis, sclerosing cholangitis, Riedel sclerosing thyroidits and sclerosing pancreatitis, which have been linked to IgG4 sclerosing diseases(2). IgG4 staining in inflammatory mass lesions has been proposed as a marker of lesions with an autoimmune or primary inflammatory etiology(3).
2. Case Reports
2.1 Patient 1
A 36-year-old woman presented with headaches, double vision worse in left gaze, and numbness of the left forehead and cheek. Visual acuity, color vision and computerized static perimetry were normal. There was 1mm of anisocoria (left larger than right) with brisk reaction bilaterally and no ptosis or ocular misalignment. There was a partial left VIth nerve palsy. MRI of the orbits revealed a T1 isointense, T2 isointense, homogenously enhancing extra-axial mass in the left middle cranial fossa involving the left cavernous sinus. Partial resection of the mass was performed. Pathology was felt to be nonspecific and non-diagnostic. Post-operative MRI of the brain revealed additional meningeal based masses in the right posterior fossa, left frontal region and left occipital region (Fig. 1, top row). Chest CT did not show any masses. Cerebral spinal fluid (CSF) contained 4 wbc/µL (44% lymphocytes), 1 rbc/µL (tube 4), 42 mg/dL protein and 30 mg/dL glucose. CSF cytology did not reveal malignant cells and CSF flow cytometry did not reveal a monoclonal B-cell population or aberrant expression of T-cell antigens. She was evaluated by the pulmonary and neuro-oncology services. A second biopsy was recommended. However, the patient’s symptoms had resolved following the surgery and post-operative steroid taper (dexamethasone 40mg daily tapered over 6 weeks), so she declined.
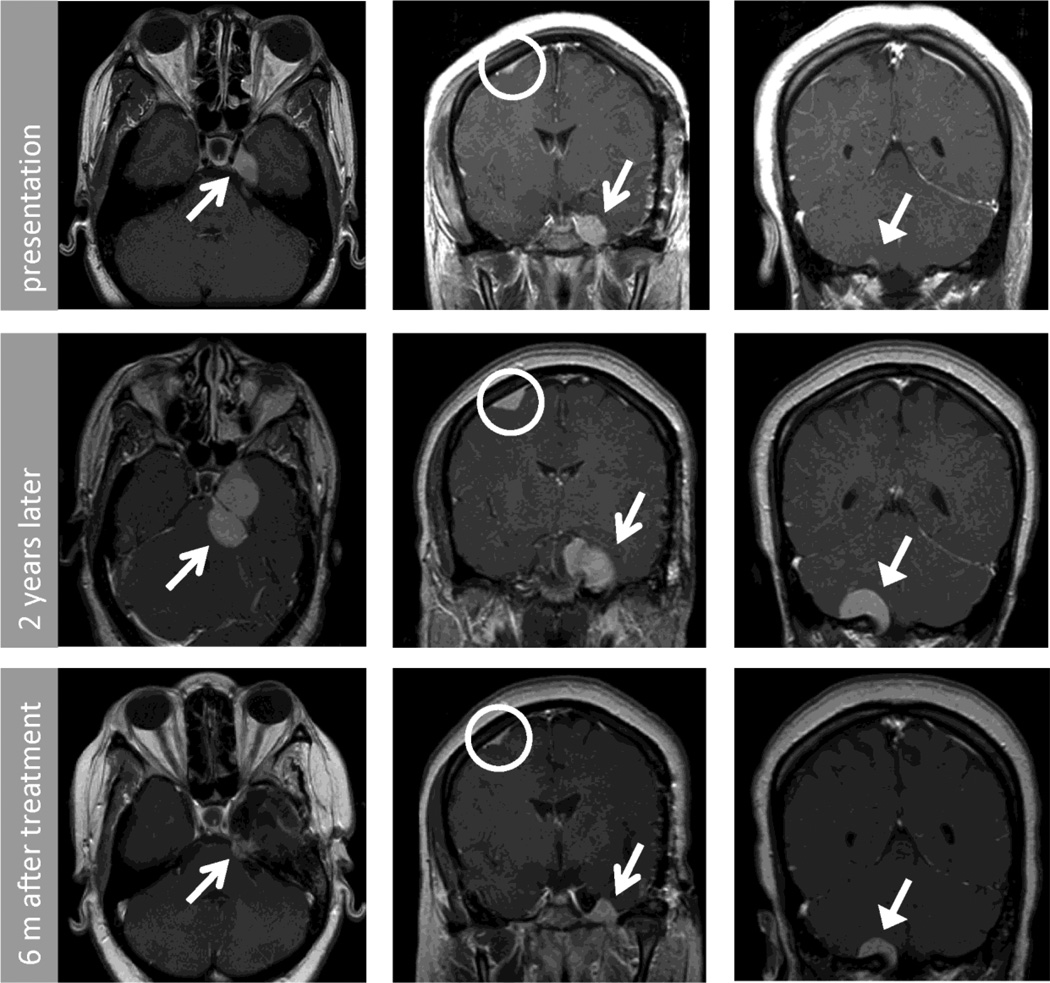
Figure 1.
Post contrast T1 MRI of brain axial (left column) and coronal (center and right column) images from patient 1. These demonstrate multifocal extra-axial lesions including middle cranial fossa (closed arrow), foramen magnum (open arrow) and superior frontal (circle). Top row shows initial imaging, middle row shows imaging obtained at representation 2 years later, and bottom row shows imaging 6 months after repeat resection of cavernous sinus lesion and medical therapy.
Two years later, she presented with blurred vision in the left eye and pain in the left cheek and chin that evolved over 3 months. MRI of the brain demonstrated enlargement of all the meningeal masses and segmental dural enhancement (Fig. 1, middle row). MRI of the spine did not reveal additional abnormalities. Repeat partial resection of the left cavernous sinus mass was performed.
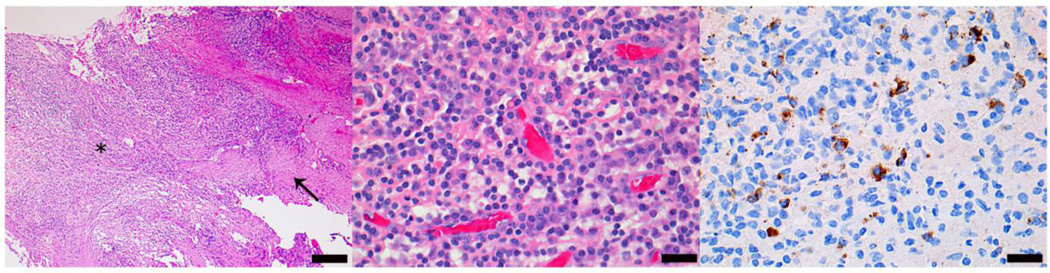
Microscopic examination of both biopsy specimens showed fragments of leptomeninges and dura involved by dense chronic inflammatory infiltrates comprised predominantly of plasma cells with abundant macrophages and lymphocytes (Fig. 2). The inflammatory infiltrates are organizing with moderate leptomeningeal fibrosis, but granulomatous inflammation is not observed. Gram, Grocott and Ziehl-Nielsen stains for bacterial, fungal and acid fast microorganisms were negative. The lymphocytic infiltrates consist largely of CD3-positive T-cells with scattered CD79a and CD20-positive B-cells. Immunohistochemistry for kappa and lambda light chains, and flow cytometry of representative biopsy tissue did not reveal a monoclonal B-cell cell population. Moreover aberrant expression of T-cell antigens was not observed. An immunohistochemical stain for anaplastic lymphoma kinase-1 (ALK-1) was negative. IgG4 staining was prominent. Examination of multiple high power fields (10 fields at 40X) revealed up to 43 plasma cells with strong IgG4 staining per high power field (range 27–43), and an IgG4/IgG ratio of 20%. She was diagnosed with IgG4-related inflammatory pseudotumor of the central nervous system.
Figure 2.
Histologic features of middle cranial fossa biopsy from patient one. Hematoxylin and eosin stain at low (10X) (left) and high (middle) (40X) power depict abundant macrophages, lymphocytes and plasma cells. Immunohistochemical stain for IgG4 (right) demonstrates multiple immunoreactive plasma cells. (scale bars: left 200 µm, middle, right 50 µm).
Dexamethasone 4 mg a day was continued beyond the post-operative course. There was no reduction in size of mass lesions on MRI after 2 months and she developed facial fullness attributable to steroid therapy. Therefore mycophenolate mofetil (MMF) was added and steroids were weaned over 3 months to dexamethasone 1 mg daily. MMF was well tolerated and side effects attributable to steroids resolved. Her clinical exam improved over 3 months. On brain MRI, the cavernous sinus mass lesion decreased in size by 70% and the cerebellar mass decreased by 50% (Fig. 1, bottom row). Over the past year, her mass lesions have remained stable on MMF 1000mg twice a day and dexamethasone 1mg a day. She did suffer a basilar artery distribution stroke of uncertain etiology.
2.2 Patient 2
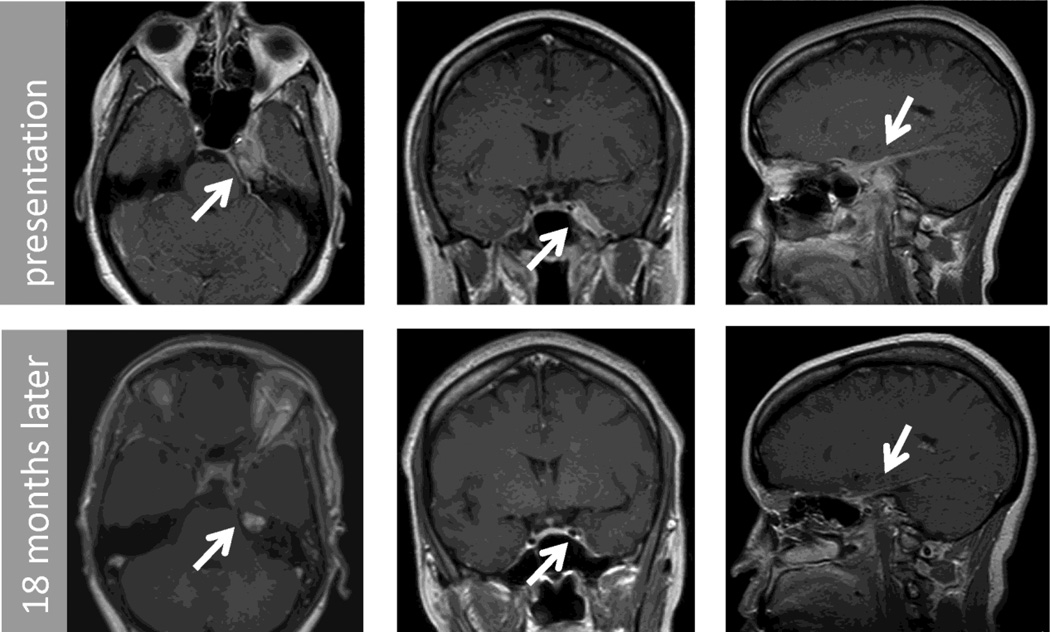
A 50-year-old woman presented with frequent headaches for 5 months, as well as double vision worse in left gaze and visual loss in the left eye of 2-week duration. The visual acuity was decreased to count fingers in the left eye with a relative afferent pupillary defect and normal fundus appearance. There was a partial left VIth nerve palsy. MRI of the brain and orbits revealed abnormal enhancement of the left petrous bone extending into the left cavernous sinus, orbital apex, middle cranial fossa, floor of the anterior cranial fossa, cerebellopontine angle, tentorium and superior nasopharynx (Fig. 3, top row). Cerebral spinal fluid (CSF) contained 12 wbc/µL (95% monocytes), 2 rbc/µL, 45 mg/dL protein and 40 mg/dL glucose. CSF cytology did not reveal malignant cells. She was evaluated by the otolaryngology and oncology services. Bilateral functional endoscopic sinus surgery including bilateral sphenoidotomies was performed with biopsy of the left petrous apex lesion. The pathology was interpreted as consistent with an inflammatory etiology. A bone marrow biopsy was normal. She received intravenous methylprednisolone for three days followed by daily oral steroid treatment. In the subsequent weeks, her headaches resolved, the left VIth nerve palsy improved and her vision in the left eye remained at count fingers level. A second biopsy of the left petrous apex lesion to obtain a larger specimen was performed using a similar approach.
Figure 3.
Post contrast T1 MRI of brain axial (left column), coronal (center column) and saggital (right column) images from patient 2. These demonstrate an extra-axial lesion involving left middle and anterior cranial fossa, petrous apex, cavernous sinus, cerebellopontine angle and tentorium. Top row shows initial imaging and bottom row shows 18 month follow up.
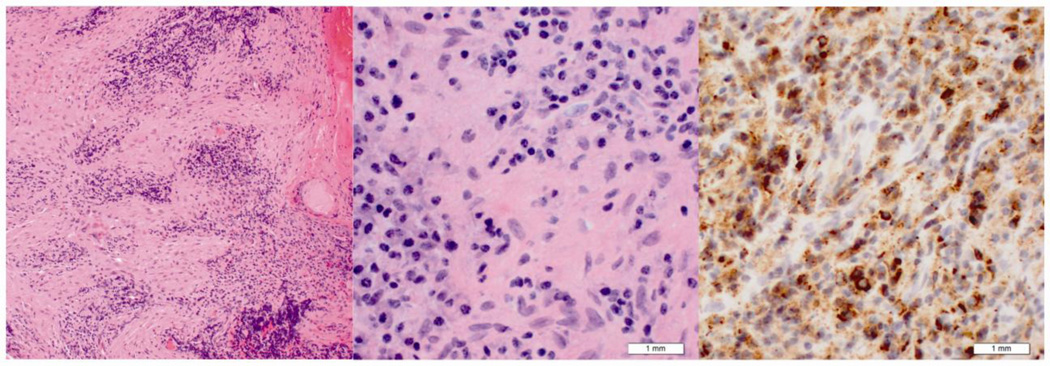
Microscopic examination of both biopsies showed an intense lymphoplasmacytic infiltrate in a background of dense fibrosis or sclerosis. Immunohistochemical studies showed a mixed population of CD20 (B-cells) and CD3-positive cells (T-cells) (Fig. 4) , plasma cells with cytoplasmic reactivity with both kappa and lambda immunoglobulin light chains, lack of reactivity with ALK, and strong reactivity with IgG4 with over 50 IgG 4 positive plasma cells per high power field and an IgG4/IgG ratio of 80%. The lack of monoclonality in plasma cells was also confirmed by the presence of kappa and lambda mRNA by in-situ hybridization. No immunoglobulin heavy chain gene rearrangement was detected by polymerase chain reaction (PCR) analysis consistent with a polyclonal B-cell reaction. She was diagnosed with IgG4-related inflammatory pseudotumor of the central nervous system.
Figure 4.
Histologic features of middle cranial fossa biopsy from patient two. Hematoxylin and eosin stain at low (10× objective lens) (left) and high (40× objective lens) (middle) power. These demonstrate a mixed inflammatory infiltrate and fibrosis. IgG4 immunohistochemical stain (right) demonstrates multiple immunoreactive plasma cells.
Treatment with a prolonged course of oral steroids was initiated. At the 4-month follow-up visit, vision with the left eye had improved to 20/40 and she had an incomplete inferior altitudinal field defect with a mean deviation of −13.37 dB on automated perimetry Humphrey central 24-2 threshold test (Zeiss Humphrey Systems, Dublin, California). The left VIth nerve palsy had resolved and optic atrophy was appreciated on ophthalmoscopic examination. A second pulse of intravenous steroids had no additional therapeutic effect on her vision. Due to weight gain of 10 kg, MMF was introduced and steroids were weaned over 3 months. The patient tolerated MMF without side effects and her weight returned to her previous baseline. MMF was discontinued after one year of treatment. At the 18-month follow-up visit her clinical examination had not changed and the lesion was reduced by approximately 90% on MRI.
3. Discussion
We present two patients with meningeal-based intracranial inflammatory mass lesions that were IgG4-positive and responded to a regimen including mycophenolate mofetil. In both cases, extensive care was taken to exclude neoplastic etiologies, in particular inflammatory myofibroblastic tumor (IMT). Prominent spindle cells typically seen in IMT were not seen in our cases. Both of our cases showed immunoreactivity with IgG4, but not with ALK-1. Yamamoto et al (4) demonstrated minimal overlap between IgG4 sclerosing disease and IMT. Lymphoproliferative disorders were excluded through multiple techniques including flow cytometry, immunohistochemistry, and gene rearrangement studies.
A challenge in the treatment of inflammatory central nervous system lesions is their relative rarity(5), which limits systematic study. One approach to classifying intracranial inflammatory mass lesions is to consider them along a spectrum of idiopathic inflammatory disorders of the central nervous system (6). The challenge with this approach is the heterogeneity that results from many of these entities being defined by what they are not rather than what they are. Immunohistochemistry offers a complimentary approach for characterization of intracranial inflammatory mass lesions by allowing us to draw connections between pathologies in other organ systems, for which there is greater treatment experience due to higher incidence. With these cases we contribute to the growing literature of diffuse manifestations of IgG4-related sclerosing disease.
To our knowledge there are nine other meningeal-based cases of IgG4 disease reported in the English literature (Table 1)(7–11). Our first case is unique in the literature due to the multifocal nature of the dural masses. Only two of the reported cases have evidence to support of systemic IgG4-related disease involving other organ systems. This supports a notion that most cases of IgG4-related disease involving the central nervous system (CNS) are locally limited. We believe that our cases are examples of local disease based on the lack of systemic symptoms and the lack of systemic mass lesions on imaging. IgG4 serum levels are not available for our patients.
Table 1.
Reported cases of meningeal-based IgG4 disease.
| Reference | Age /gender |
Location | Systemic IgG 4 |
Systemic Inflammation |
Treatment |
|---|---|---|---|---|---|
| Choi (8) | 46/F | dura (spine) | normal | none | steroids |
| Chan (9) | 37/M | dura (spine) | NR | Submandibular sialadenitis |
NR |
| Kosakai(10) | 54/F | Uveitis Orbital apex Dura (skull base) |
Elevated | Lung mass Nephritis |
steroids |
| Katsura(11) | 59/F | Dura (skull base) | NR | none | none |
| Lindstrom(7) | 74/F | leptomeningeal | NR | juvenile RA | steroids |
| 55/M | dura (spine) | NR | None | XRT, steroids |
|
| 60/F | dura (skull base) | NR | None | Anti-TNF Steroids |
|
| 63/M | dura | NR | Crohn's disease |
NR | |
| 53/M | Dura (posterior fossa) |
NR | None | Steroids | |
| Moss | 36/F | dura (multiple) | NR | None | MMF, steroids |
| 50/F | dura (skull base) | NR | none | MMF, steroids |
NR: not reported, RA: rheumatoid arthritis, XRT: radiation therapy, TNF: tumor necrosis factor, MMF: mycophenolate mofetil
Reported treatments of meningeal based IgG4-related disease has consisted mainly of steroids (Table 1)(7–11). Radiation therapy and anti-tumor necrosis factor use have been reported in one case each(7). We present evidence for effectiveness of mycophenolate mofetil (MMF) for treatment of IgG4-related disease of the central nervous system in the setting of corticosteroid dependence and side effects. MMF is an immunosuppressive agent with a favorable toxicity profile that has been in use for over 15 years(12). Treatment response to MMF has been reported in IgG4 associated autoimmune pancreatitis(13) and cholangitis(14), but not in intracranial disease. Efficacy has been reported in other inflammatory diseases of the central nervous system such as neurosarcoidosis(15) and of the eye, including inflammatory pseudotumor of the eye (16). MMF was chosen in our patients as a steroid sparing agent in the setting of recurrence and steroid side effects due to the favorable long term toxicity profile of MMF in comparison with corticosteroids as well as reported success in control of other inflammatory disease [2]. Studies of MMF use in inflammatory eye disease have shown a complication rate of 36.9%, discontinuation rate due to side effects of 8.3% and monotherapy failure rate of 10.7% [2]. This profile compares favorably with other antimetabolite immunosuppressive agents (i.e. methotrexate and azathioprine).
We present two patients with inflammatory intracranial mass lesions, both ALK-1 negative and IgG4-positive, with treatment response to mycophenolate mofetil. Future treatment studies grouping disease processes by similar histology or by molecular signatures have the potential to facilitate systematic investigation of therapeutic strategies.
Acknowledgements
The authors wish to thank Dr.’s Miguel Guzman and Caterina Giannini for their assistance with initial interpretation of the pathological specimens and Lisa Birmingham for her assistance with the figures. Dr. Moss receives support from K12EY021475 (National Eye Institute).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors disclose no financial or personal relationships with other people or organizations that could inappropriately influence this work
References
- 1.Swain RS, Tihan T, Horvai AE, Di Vizio D, Loda M, Burger PC, et al. Inflammatory myofibroblastic tumor of the central nervous system and its relationship to inflammatory pseudotumor. Human pathology. 2008;39(3):410–419. doi: 10.1016/j.humpath.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Bateman AC, Deheragoda MG. IgG4 related systemic sclerosing diseases: an emerging and under diagnosed condition. Histopathology. 2009;55(4):373–383. doi: 10.1111/j.1365-2559.2008.03217.x. [DOI] [PubMed] [Google Scholar]
- 3.Lui PCW, Fan YS, Wong SS, Chan ANH, Wong G, Chau TKF, et al. Inflammatory pseudotumors of the central nervous system. Human pathology. 2009;40(11):1611–1617. doi: 10.1016/j.humpath.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto H, Yamaguchi H, Aishima S, Oda Y, Kohashi K, Oshiro Y, et al. Inflammatory myofibroblastic tumor versus IgG4-related sclerosing disease and inflammatory pseudotumor: a comparative clinicopathologic study. The American journal of surgical pathology. 2009;33(9):1330. doi: 10.1097/pas.0b013e3181a5a207. [DOI] [PubMed] [Google Scholar]
- 5.Hausler M, Schaade L, Ramaekers VT, Doenges M, Heimann G, Sellhaus B. Inflammatory pseudotumors of the central nervous system: report of 3 cases and a literature review. Human pathology. 2003;34(3):253–262. doi: 10.1053/hupa.2003.35. [DOI] [PubMed] [Google Scholar]
- 6.McKinney A, Short J, Lucato L, SantaCruz K, McKinney Z, Kim Y. Inflammatory myofibroblastic tumor of the orbit with associated enhancement of the meninges and multiple cranial nerves. American journal of neuroradiology. 2006;27(10):2217. [PMC free article] [PubMed] [Google Scholar]
- 7.Lindstrom KM, Cousar JB, Lopes MBS. IgG4-related meningeal disease: clinicopathological features and proposal for diagnostic criteria. Acta neuropathologica. 2010:1–12. doi: 10.1007/s00401-010-0746-2. [DOI] [PubMed] [Google Scholar]
- 8.Choi SH, Lee SH, Khang SK, Jeon SR. IgG4-related sclerosing pachymeningitis causing spinal cord compression. Neurology. 2010;75(15):1388. doi: 10.1212/WNL.0b013e3181f73614. [DOI] [PubMed] [Google Scholar]
- 9.Chan SK, Cheuk W, Chan KT, Chan JKC. IgG4-related sclerosing pachymeningitis: a previously unrecognized form of central nervous system involvement in IgG4-related sclerosing disease. The American journal of surgical pathology. 2009;33(8):1249. doi: 10.1097/PAS.0b013e3181abdfc2. [DOI] [PubMed] [Google Scholar]
- 10.Kosakai A, Ito D, Yamada S, Ideta S, Ota Y, Suzuki N. A case of definite IgG4-related pachymeningitis. Neurology. 2010;75(15):1390–1392. doi: 10.1212/WNL.0b013e3181f73685. [DOI] [PubMed] [Google Scholar]
- 11.Katsura M, Morita A, Horiuchi H, Ohtomo K, Machida T. IgG4-Related Inflammatory Pseudotumor of the Trigeminal Nerve: Another Component of IgG4-Related Sclerosing Disease? American journal of neuroradiology. 2011;32(8):E150–E152. doi: 10.3174/ajnr.A2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zwerner J, Fiorentino D. Mycophenolate mofetil. Dermatologic therapy. 2007;20(4):229–238. doi: 10.1111/j.1529-8019.2007.00136.x. [DOI] [PubMed] [Google Scholar]
- 13.Mannion M, Cron RQ. Successful treatment of pediatric IgG4 related systemic disease with mycophenolate mofetil: case report and a review of the pediatric autoimmune pancreatitis literature. Pediatric Rheumatology Online Journal. 2011;9:1. doi: 10.1186/1546-0096-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghazale A, Chari ST, Zhang L, Smyrk TC, Takahashi N, Levy MJ, et al. Immunoglobulin G4-associated cholangitis: clinical profile and response to therapy. Gastroenterology. 2008;134(3):706–715. doi: 10.1053/j.gastro.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Androdias G, Maillet D, Marignier R, PinËde L, Confavreux C, Broussolle C, et al. Mycophenolate mofetil may be effective in CNS sarcoidosis but not in sarcoid myopathy. Neurology. 2011;76(13):1168. doi: 10.1212/WNL.0b013e318212aafb. [DOI] [PubMed] [Google Scholar]
- 16.Thorne JE, Jabs DA, Qazi FA, Nguyen QD, Kempen JH, Dunn JP. Mycophenolate mofetil therapy for inflammatory eye disease. Ophthalmology. 2005;112(8):1472–1477. doi: 10.1016/j.ophtha.2005.02.020. [DOI] [PubMed] [Google Scholar]