Abstract
We characterized immune modulating functions of porcine γδ T cell subsets in rotavirus infection using a gnotobiotic pig model of human rotavirus infection and sort-purified lymphocyte autologous co-cultures. We demonstrated that CD2+CD8− and CD2−CD8− γδ T cells have mainly pro-inflammatory function as evident by directly secreting IFN-γ or promoting CD4+ αβ T cell proliferation and IFN-γ production, whereas CD2+CD8+ γδ T cells mainly exert regulatory T cell function by expressing FoxP3, secreting IL-10 and TGF-β or increasing IL-10 and TGF-β production by CD4+ αβ T cells. γδ T cells responded to rotavirus infection by increasing TLR2, TLR3, TLR9 expression and IFN-γ and/or TGF-β production. The CD8− subsets likely differentiate into CD8+ subset by acquiring CD8 expression, explaining in part the apparently dual functions of CD2+CD8+ and CD2+CD8− subsets. Thus, both CD8+ and CD8− γδ T cell subsets can contribute to anti-rotavirus immunity and to the maintenance and restoration of intestinal and systemic homeostasis.
Keywords: gammadelta (γδ) T cell subset, rotavirus, gnotobiotic pigs, Toll-like receptors, cytokines, T cell proliferation
1. Introduction
Studies of γδ T cells in humans, mice and cattle have revealed the diverse immune functions of different γδ T cell subsets; however the immune functions of porcine γδ T cell subsets have not been clearly identified. Pigs are the most important large animal models for human biomedical research and are also used as donors for xenotransplantation, thus characterization of the immune modulating functions of porcine γδ T cell subsets is vitally important. Because the lack of monoclonal antibodies distinguishing specific variable (V) and diversity (D) segments of porcine γδ T cell TCRs (i.e., the Vγ and Vδ antibodies used for studying γδ T cell subsets in humans and mice), the equivalent phenotypes between human and porcine γδ T cell subsets have not been determined. However, all mammalian species studied share two principal subsets of γδ T cells in terms of their distributions. One subset is predominant in tissues including mucosal and skin surfaces, (Vδ1 in humans and mice; WC1−CD8+ in cattle, and CD2+CD8+ in pigs) and the other subset is predominant in the blood (Vδ2 in humans; Vγ1 in mice, WC1+ in cattle, and CD2−CD8− in pigs) [1–5]. Functionally, γδ T cells in humans, mice and cattle have also been divided into two principal subsets: the pro-inflammatory and anti-inflammatory subsets [6]. Circulatory γδ T cells are mainly pro-inflammatory and act as primary responders for invading pathogens, including viruses, bacteria and parasites; whereas tissue-specific γδ T cells have pro62 inflammatory and anti-inflammatory dual functions depending on the anatomic locations and the microenvironment. γδ T cells located in mucosal and skin surfaces are largely anti-inflammatory and contribute to the maintenance of the epithelial integrity.
Phenotypically, porcine γδ T cells are defined by the monoclonal anti-porcine antibody Tcr1-N4 (clone PGBL22A) that recognizes a determinant on a constant region of porcine T cell receptor (TCR) δ chain [7]. The total γδ T cells are divided into three subsets based on the surface expression of CD2 and CD8αα (CD2+CD8+, CD2+CD8− and CD2−CD8−) [8]. Our previous study focusing on porcine γδ T cell subset distribution and kinetics in response to enteric virus infection showed that rotavirus infection significantly increased frequencies of CD2+CD8+ γδ T cell subset and decreased the CD8− subsets in ileum, spleen and blood of gnotobiotic (Gn) pigs at post-inoculation days (PID) 3–5 [4]. However, the exact immune functions of each porcine γδ T cell subset are yet to be identified.
γδ T cells can respond directly to pathogen-associated molecular patterns (PAMPs) without any help from antigen presenting cells (APCs) by increasing PAMP receptor expressions, such as Toll-like receptors (TLRs) [9]. Freshly isolated human γδ T cells have been stimulated via the TCR ligand in the presence of poly (I:C) without the presence of APCs to enhance IFN-γ production [10], which indicated that human γδ T cells express the poly (I:C) receptor TLR3. Also, human Vγ2Vδ2 T cells expressed TLR2 mRNA and produced IFN-γ under the stimulation of a TLR2 agonist (Pam3Cys) [11]. Pietschmann et al [12] compared the different TLR expression patterns among human Vδ1 and Vδ2 T cells and found that TLR2, TLR3 and TLR6 proteins were detected in both Vδ1 and Vδ2 T cells. Mouse γδ T cells were also found to express TLR2 and TLR4 [13]. However, it is unknown if porcine γδ T cells express TLRs and whether TLR expressing γδ T cells are involved in the immune responses to rotavirus infection.
The objective of the present study is to characterize the immunological functions of the three porcine γδ T cell subsets in the face of enteric virus infections. The frequencies of TLR2, TLR3, TLR9 and FoxP3 expression, and IFN-γ and TGF-β production among the three γδ T cell subsets in Gn pigs were determined after human rotavirus (HRV) inoculation. The cytokine production profiles of the three γδ T cell subsets after phosphoantigen isopentenyl pyrophosphate (IPP) stimulation and the influence of each γδ T cell subset on CD4+ αβ T cell cytokine production and proliferation in sort-purified autologous co-cultures were examined by using enzyme-linked immunosorbent (ELISA) and multi-color flow cytometry assays. Our findings indicate that, similar to other mammalian species studied previously (humans, mice and cattle), porcine γδ T cells express TLRs and can be divided into two major functional subsets, the pro-inflammatory (CD2+/−CD8−) and the anti-inflammatory (CD2+CD8+) subsets. CD2+/−CD8− γδ T cells can exert anti-viral effector cell functions by producing IFN-γ and promoting the proliferation and IFN-γ production by CD4+ αβ T cells. CD2+CD8+ γδ T cells from the intestine and spleen have characteristics of regulatory cell functions (express FoxP3 and produce TGF-β and IL-10). However, CD2+CD8+ γδ T cells also have anti-viral functions (produce IFN-γ) in early stage of rotavirus infection.
2. Materials and methods
2.1. Viruses and inoculums
The virulent Wa strain HRV (G1P1A[8]) (from Dr. Linda Saif, The Ohio State University) was passaged through Gn pigs and the pooled intestinal contents from the 27th passage were used for inoculation or challenge of Gn pigs at a dose of 1 × 105 fluorescent focus-forming units (FFU). The median infectious dose (ID50) of the HRV in Gn pigs was approximately 1 FFU [14]. The HRV infection in Gn pigs were confirmed by fecal virus shedding using ELISA and cell culture immunofluorescence (CCIF) assays as previously described [14].
The cell-culture adapted attenuated Wa strain HRV (from Dr. Linda Saif, The Ohio State University), derived from the 34th passage in African green monkey kidney cells (MA104) [15] was used for inoculation of a subgroup of Gn pigs at 5 × 107 FFU/dose and was used to prepare semi-purified Wa HRV antigen by centrifugation through a 40 % sucrose cushion as described [16]. The semi-purified Wa HRV antigen was used in the CD4+ T cell proliferation assay in the co-culture studies.
2.2. Inoculation of gnotobiotic pigs
Near-term pigs of Landrace and Large White cross breed were derived from pregnant sows by surgery and maintained in germ-free isolator units as described [17]. Pigs were fed with commercial ultra-high temperature (UHT)-treated sterile milk. All pigs were confirmed germ-free prior to rotavirus or norovirus exposure. Pigs were given 8 ml of 100 mM sodium bicarbonate to reduce gastric acidity 10 min before virus inoculation. All animal experimental procedures were conducted in accordance with protocols approved by Institutional Animal Care and Use Committees of Virginia Polytechnic Institute and State University.
For the study of TLR expressing γδ T cell responses [PID 0 (n = 7), 3 (n = 7) and 5 (n = 4)] and IFN-γ [PID 0 (n = 7), 3 (n = 4) and 5 (n = 4)] or TGF-β [PID 0 (n = 3) and 5 (n = 4)] producing γδ T cell responses, Gn pigs (both males and females) were randomly assigned to the HRV and mock control groups. At 5 days of age (PID 0), Gn pigs in HRV groups were orally inoculated with 1 × 105 FFU virulent Wa HRV in 5 ml of Dulbecco’s Modified Eagle’s Medium (DMEM). Control pigs were given an equal volume of the diluent. The pigs were euthanized on PID 0, 3 and 5 to isolate mononuclear cells (MNCs) from ileum, spleen and peripheral blood as described [15].
In the following studies, Gn pigs were inoculated with rotavirus or norovirus and colonized with or without a probiotic lactobacilli strain, as specified in each experiment. These Gn pigs were shared with other research projects. MNCs from these Gn pigs were used in the present study because the inoculations and ages do not affect the interpretation of the data regarding the function of each γδ T cell subset and the use of animal resources was maximized. In the studies of FoxP3 expression among γδ T cell subsets and antigen presenting cell (APC) function of γδ T cells, Gn pigs were mono-associated with (FoxP3 expression study, n = 3) or without (APC function study, n = 3) the Lactobacilli acidophilus NCFM strain as we previously described [18]. The pigs were inoculated with two oral doses of attenuated Wa HRV at 5 × 107 FFU/dose in 5 ml of DMEM at 5 (PID 0) and 15 (PID 10) days of age, challenged with 1 × 105 FFU of virulent Wa HRV on PID 28, and euthanized on PID 35 [post-challenge days (PCD) 7]. MNCs from ileum, spleen and peripheral blood were isolated as previously described [15] and intraepithelial lymphocytes (IEL) were collected by incubating ileum with EDTA buffer before processed for MNC isolation.
In the co-culture studies, human norovirus-inoculated Gn pigs were used for acquiring sort-purified γδ T cell subsets and CD4+ αβ T cells. Gn pigs were orally inoculated at 33 days of age with 2.74 × 104 to 2.74 × 106 copies of viral RNA (determined by qRT-PCR) of a norovirus GII.4 strain (10 % human stool sample 092895 suspension from Dr. Xi Jiang, Cincinnati Children’s Hospital Medical Center, OH). All the Gn pigs were infected by norovirus, which was confirmed by fecal virus shedding using RT-PCR and qRT-PCR (Bui and Yuan, unpublished data). MNCs from spleen and IEL were isolated from the pigs at PID3 or PID 4 for sort-purification of γδ T cell subsets and CD4+ αβ T cells.
2.3. Staining cells for flow cytometry analysis
For staining TLR expressing γδ T cells, MNCs (2 × 106 cells/tube) were stained on the same day of MNC isolation without in vitro stimulation [19]. For FoxP3 expression by γδ T cells, MNCs were incubated for 5 hrs with Brefeldin A (10 ug/ml, Sigma) in complete medium consisting of RPMI-1640 (Gibco, BRL) supplemented with 8 % fetal bovine serum, 20 mM HEPES (N-2-hydroxyethyl-piperazine-Nk-2-ethanesulphonic acid), 2 mM L-glutamine, 1 mM sodium pyruvate, 0.1 mM non-essential amino acids, 100 µg/ml of gentamicin, 10 µg/ml of ampicillin and 50 mM 2-mercaptoethanol (E-RPMI) at 37 °C. For IFN-γ or TGF-β production by γδ T cells, the MNCs were incubated in E-RPMI for 17 and 48 hrs, respectively, at 37 °C and Brefeldin A was added for the last 5 hrs as described previously [20].
For all staining, except when specifically noted, MNCs were incubated with antibodies for 15 min at 4 °C at each step and then washed once between steps with the staining buffer prepared according to BD Pharmingen™ BrdU Flow Kits Instruction Manual) and centrifuged at 500 × g for 5 min at 4 °C. The staining of γδ T cell subsets has been described previously [4]. Briefly, MNCs were first stained with mouse anti-porcine Tcr1-N4 (IgG1, VMRD, PGBL22A), mouse anti-porcine CD2 (IgG3, VMRD, PG168A) and SpectralRed™ SPRD) conjugated mouse anti-porcine CD8α (IgG2a, Southern Biotech, 76-2-11) antibodies followed by the allophycocyanin conjugated rat anti-mouse IgG1 (IgG1, BD Pharmingen, A85-1) and fluorescein isothiocyanate (FITC) conjugated rat anti-mouse IgG3 (IgM, Southern Biotech, LO-MG3). Intracellular TLR (TLR3 and TLR9) and extracellular TLR (TLR2) staining was performed as previously described [19]. Phycoerythrin (PE) conjugated mouse anti-porcine IFN-γ (IgG1, BD Pharmingen, P2G10) and PE conjugated mouse anti-human TGF-β1 (IgG1, R&D systems, 27232; cross reactive with porcine TGF-β1 [21]) antibodies were used to detect intracellular IFN-γ and TGF-β production, respectively, as described [20].
For staining of FoxP3 expressing γδ T cells, after surface staining of Tcr1-N4, CD2 and CD8, MNCs were permeabilized and washed with FoxP3 Staining Buffer Set following manufacturer’s instructions (eBiosciences, 00–5523) prior to staining with phycoerythrin-cyanine tandem fluorochrome (PE-Cy7) conjugated rat anti-mouse/rat FoxP3 (IgG2a, eBioscience, FJK-16s) for 30 min at 4 °C.
First sets of negative controls included the secondary antibodies: allophycocyanin conjugated rat anti-mouse IgG1 (IgG1, BD pharmingen, A85-1) (for Tcr1-N4); FITC conjugated rat anti-mouse IgG3 (IgM, Southern Biotech, LO-MG3) (for CD2), and the following isotype-matched irrelevant control antibodies: SRPD conjugated mouse IgG2a isotype control (Southern Biotech, HOPC-1) (for CD8); PE conjugated mouse IgG1 isotype control (eBioscience, P3.6.2.1) (for TLR2, TLR3, IFN-γ and TGF-β); PE conjugated rat IgG2a isotype control (eBioscience, 12–4321) (for TLR9); and PE-Cy7 conjugated rat IgG2a isotype control (eBioscience, 25–4321) (for FoxP3). The negative controls were included in each staining to set the quadrant markers for the bivariate dot plots. The second sets of control tubes were stained with all the antibodies to define γδ T cell subsets and the isotype-matched irrelevant control antibodies for detecting the non-specific reactions for TLRs, cytokines and FoxP3. All antibodies were titrated to provide the optimal signal to noise ratio.
Analysis of the stained cells was performed using a FACSAria flow cytometer (Becton Dickinson) and 100,000 cells were acquired. Data analysis was performed using FlowJo 7.2.2 (Tree Star, Inc) software. Data are presented as mean frequencies of TLRs, IFN-γ or TGF-β expressing/producing γδ T cells among each γδ T cell subset, and FoxP3 expressing γδ T cell subsets among total γδ T cells. The frequencies of sample tubes were subtracted by the frequencies of non-specific reactions in the second sets of control tubes for each tissue.
2.4. Sort-purification of γδ T subsets and CD4+ αβ T cells
For the in vitro cell co-culture studies, MNCs from spleen and IEL were collected from Gn pigs (n = 6) infected with human norovirus at PID 3 or 4. The three γδ T cell subsets and CD4+ αβ T cells were enriched from the MNCs by magnetic antibody cell sorting (MACS) and fluorescence-activated cell sorting (FACS) technology. Streptavidin conjugated Dynabeads (Invitrogen, 656.01) were primed before use according to the manufacturer’s instructions and then incubated with biotinylated rat anti-mouse IgG1 antibody (IgG1, BD Pharmingen, A85-1) at 4 °C for 1 hr followed by washing 4 times with the staining buffer. MNCs were stained with mouse anti-porcine Tcr1-N4 (IgG1, VMRD, PGBL22A) or mouse anti-porcine CD3 (IgG1, Southern Biotech, PPT3) at 4 °C for 30 min, followed by addition of the prepared Dynabeads and incubation at room temperature for 30 min. Total γδ T cells and CD3+ T cells were then enriched using the magnetic separation rack (Invitrogen) according to the manufacturer’s instructions.
Enriched total γδ T cells and CD3+ T cells were further sorted into three γδ T cell subsets, and CD3+CD4+ αβ T cells, respectively by FACS. Mouse anti-porcine CD2 (IgG3, VMRD, PG168A), FITC conjugated rat anti-mouse IgG3 (IgM, Southern Biotech, LO-MG3) and SPRD conjugated mouse anti-porcine CD8α (IgG2a, Southern Biotech, 76-2-11) antibodies were used for sorting γδ T cell subsets. PE conjugated mouse anti-porcine CD4 (IgG2b, BD Pharmingen, 74-12-4) were used for separating CD3+CD4+ αβ T cells (extrathymic porcine γδ T cells are CD4−). The purity of each T subset was determined by flow cytometry and only cells with > 99 % purity were used in the subsequent in vitro culture or autologous lymphocytes co-culture studies.
2.5. In vitro culture with IPP and IL-2 stimulation
To determine the cytokine production profile of each γδ T cell subset, each sort-purified γδ T cell subset (1.25 × 104 cells in 0.8 ml) was cultured for 5 days in E-RPMI media at 37 °C in 5 % CO2 humidified air with the stimulation of 7.5 µg/ml of IPP plus 1.25 µg/ml of IL-2 or mock stimulated. During the last 2 days, fresh media containing 7.5 µg/ml of IPP plus 1.25 µg/ml of IL-2 were added. The supernatants were collected and stored at −80 °C for subsequent measuring of cytokine concentrations by ELISA and the MNCs were harvested, washed with the staining buffer and stained with the following antibodies: SPRD conjugated mouse anti-porcine CD8α (IgG2a, Southern Biotech, 76-2-11) and mouse anti-porcine Tcr1-N4 (IgG1, VMRD, PGBL22A) followed by allophycocyanin conjugated rat anti-mouse IgG1 (IgG1, BD Pharmingen, A85-1). Flow cytometry analysis was performed to determine the frequencies of CD8+ γδ T cells among total γδ T cells using a FACSAria flow cytometer (Becton Dickinson) and FlowJo 7.2.2 (Tree Star, Inc) software.
2.6. In vitro co-culture and αβ T cell proliferation assay
To determine the influence of each γδ T cell subset on αβ T cell proliferation and cytokine production, sort-purified splenic CD3+CD4+ αβ T cells (1.25 × 104) were co-cultured at 1:1 ratio with or without each splenic γδ T cell subset for 5 days in E-RPMI media (total 2.5 × 104 cells in 0.8 ml) in 5 % CO2 at 37 °C with the stimulation of 12 µg/ml phytohaemagglutinin (PHA) or with 12 µg/ml of the semi-purified Wa HRV [20]. During the last 2 days, 0.2 ml of fresh media containing 12 µg/ml of PHA or Wa HRV antigen was added. 10 µg/ml of bromodeoxyuridine (BrdU) was added to the cell cultures for the last 18 hrs. The supernatants were collected and stored at −80 °C for subsequent measuring of cytokine concentrations by ELISA and the MNCs were harvested, washed with the staining buffer and stained with the following antibodies: PE conjugated mouse anti-porcine CD4 (IgG2b, BD Pharmingen, 74-12-4) and mouse anti-porcine CD3 (IgG1, Southern Biotech, PPT3) followed by allophycocyanin conjugated rat anti-mouse IgG1 (IgG1, BD Pharmingen, A85-1) and FITC conjugated anti-BrdU (BD Pharmingen, 557891) as described [20]. Flow cytometry analysis was performed to determine the frequencies of BrdU+CD3+CD4+ αβ T cells using a FACSAria flow cytometer (Becton Dickinson) and FlowJo 7.2.2 (Tree Star, Inc) software. The proliferating T cell responses were presented as the frequencies of BrdU+CD4+ αβ T cells among total CD3+ MNCs.
2.7. Cytokine assays
Supernatants from the in vitro culture or co-cultures were examined for IFN-γ, IL-10, TGF-β1 and IL-17 concentrations by ELISA as described [22] or following the manufacturer’s instructions (the IL-17 kit, Cat# E101–807, Bethyl Laboratories, Inc.). The OD value was measured at 450 nm using a spectrophotometer (Tecan Group Ltd.). The standard curves were calculated using a computer-generated two-parameter curve-fit for each cytokine. The minimal detection concentrations were 7.8 pg/ml for IFN-γ and IL-10, 15.6 pg/ml for TGF-β1 and 18.8 pg/ml for IL-17.
2.8. Statistical analysis
Non-parametric Kruskal-Wallis rank sum test was performed to compare frequencies of TLRs, cytokines and FoxP3 expressing/producing γδ T cell subsets in each tissue, concentrations of IFN-γ, IL-10 and TGF-β in the cell culture supernatants and frequencies of proliferating CD4+ αβ T cells in the co-cultures. When differences among these groups were detected, the same test was used in a pairwise fashion to clarify the nature of the differences. Correlations between TLR expression and cytokine production were analyzed using Spearman’s correlation coefficiency. All statistical analyses were performed using SAS program 9.2 (SAS Institute, NC, USA). All statistical significance was assessed at p < 0.05.
3. Results
3.1. TLR expression by all three γδ T cell subsets increased significantly after HRV infection
To assess which γδ T cell subsets respond to rotavirus infection, we investigated the frequencies of TLR2, TLR3 and TLR9 expression by each γδ T cell subset from ileum, spleen and blood of Gn pigs inoculated with HRV. We have previously demonstrated that HRV inoculation increased TLR2, TLR3 and TLR9 expression on CD14+ macrophages and conventional dendritic cells in spleen of Gn pigs [19].
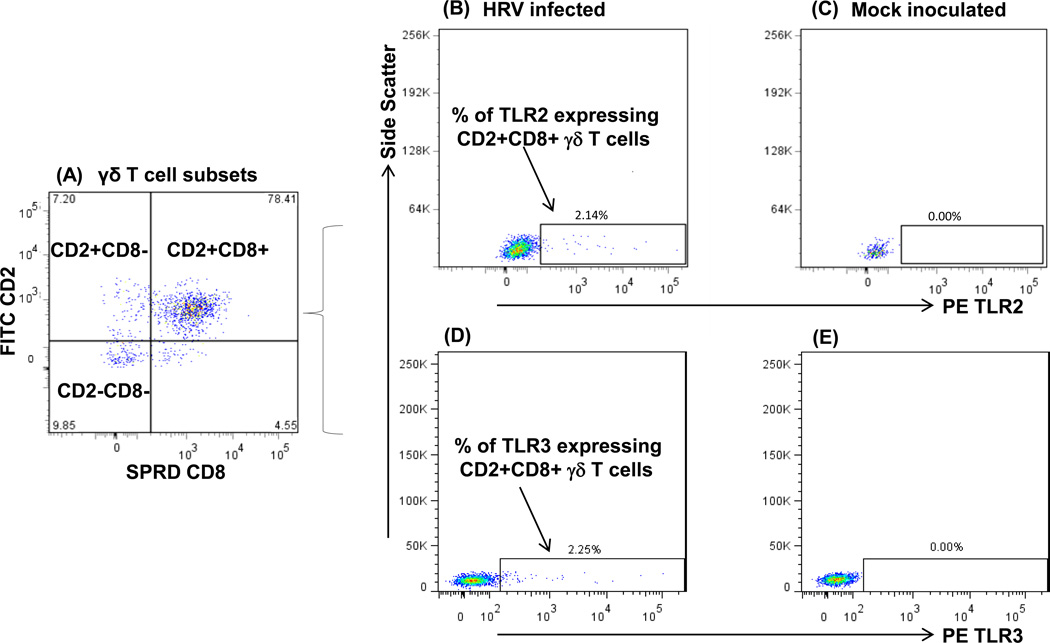
As shown in the representative dot plots in Fig. 1, CD2+CD8+ is the predominant γδ T cell subset in ileum of HRV-infected Gn pigs, followed by CD2−CD8− and CD2+CD8− subsets. Frequencies of TLR2 and TLR3 expressing CD2+CD8+ γδ T cells in ileum were substantially higher (Fig. 1B and 1D) in HRV-infected pigs compared to the mock-inoculated pigs (Fig. 1C and 1E). Mean frequencies of TLR2, TLR3 and TLR9 expressing γδ T cell subsets in ileum, TLR2 and TLR3 expressing γδ T cell subsets in spleen and TLR9 expressing γδ T cell subsets in blood of the HRV-infected Gn pigs at PID 0, 3 and 5 are summarized in Fig. 2. Because TLR9 expression in spleen and TLR2 and TLR3 expression in blood of the Gn pigs were low and did not differ significantly among PID 0, 3 and 5 in any γδ T cell subset, the data were not shown.
Fig. 1. Representative dot plots of TLR2 and TLR3 expressing CD2+CD8+ γδ T cell subset in ileum of Gn pigs infected with HRV at PID 5 or mock infected.
Gn pigs were infected with the virulent Wa HRV. The MNCs were isolated upon euthanasia at PID 5 and freshly stained and gated for γδ cells as described in our previous study [4]. Three defined γδ T cell subsets are shown in Fig. 1A with the frequencies among total γδ T cells at each corner. Fig. 1B to 1E show the representative dot plots for TLR2 and TLR3 expressing CD2+CD8+ γδ T cells in ileum of HRV infection and mock infected Gn pigs, respectively. The numbers above the rectangles are the frequencies of TLR2+ or TLR3+ cells among CD2+CD8+ γδ T cells.
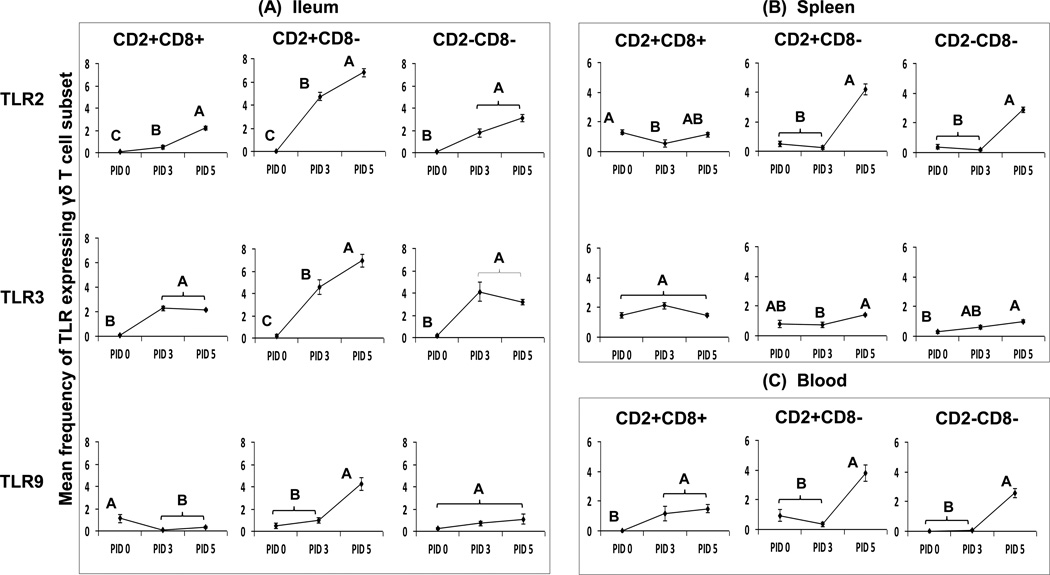
Fig. 2. Percentage of TLR expressing γδ T cells among each subset in ileum (A), spleen (B) and blood (C) of Gn pigs infected with HRV or mock infected.
The panels A, B and C depict the mean frequencies of TLR expressing cells among each γδ T cell subset in ileum (TLR2 TLR3 and TLR9), spleen (TLR3 and TLR3) and blood (TLR9) of Gn pigs infected with HRV (PID 3 and PID 5) or mock infection (PID 0). Data are presented as mean frequency ± standard error of the mean (n = 4–7). Different capital letters indicate significant differences in frequencies among the time points for the same γδ T cell subset and tissue (Kruskal-Wallis test, p < 0.05), while shared letters indicate no significant difference.
It is clear that all the three porcine γδ T cell subsets in ileum responded to HRV infection as evidenced by the total lack of TLR2 and TLR3 expression at PID 0 and the significantly increased expression of TLR2 and TLR3 by each subset post-inoculation. Compared to PID 0, Gn pigs had significantly higher frequencies of TLR2 and TLR3 expressing CD2+CD8+, CD2+CD8− and CD2−CD8− subsets in ileum at PID 3 and PID 5 (Fig. 2A). HRV infection also significantly increased frequencies of the TLR9 expressing CD2+CD8− subset in ileum at PID 5 compared to PID 0 (Fig. 2A).
In spleen, frequencies of TLR2 and TLR3 expressing γδ T cells were higher than those in ileum at PID 0 for all three subsets (Fig. 2A and 2B). Higher baseline of TLR2 and TLR3 expression in all γδ T cell subsets in spleen suggests a lower threshold for antigen recognition and activation of systemic γδ T cells. After HRV inoculation, frequencies of TLR2 and TLR3 expressing CD8− γδ T cell subsets in spleen and TLR9 expressing CD8− γδ T cell subsets in blood were higher or significantly higher at PID 5 compared to PID 0, suggesting that the CD8− subsets in spleen and blood were activated by HRV inoculation, albeit two days later than the TLR2 and TLR3 responses in ileum (Fig. 2B and 2C).
3.2. Both CD2+CD8+ and CD2+CD8− γδ T cell subsets generated a swift IFN-γ response after HRV inoculation
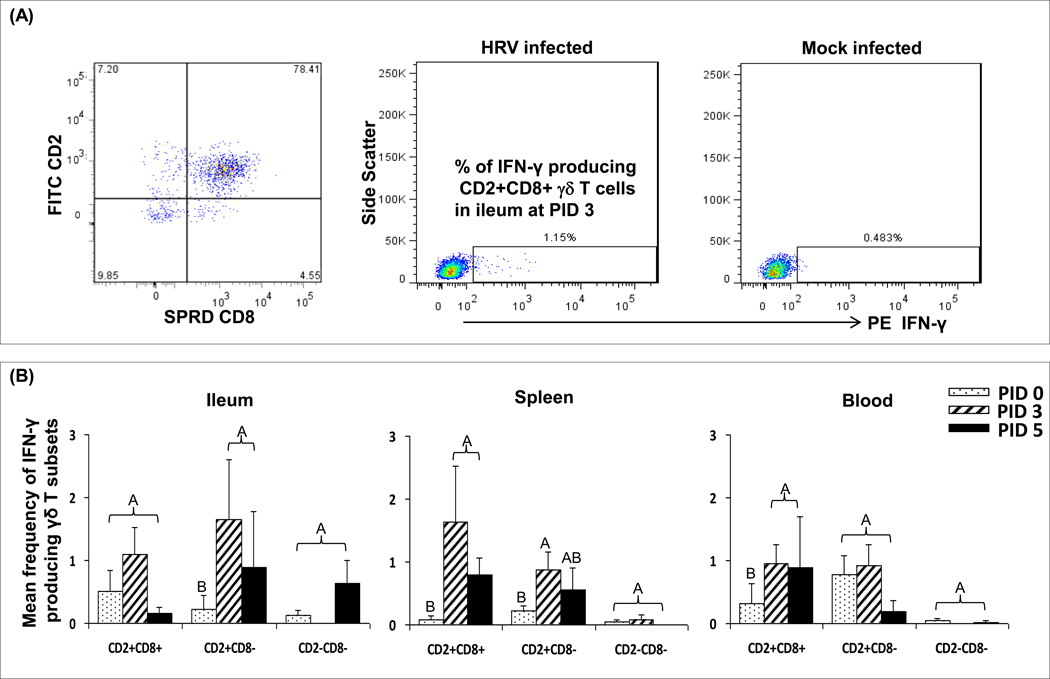
The frequencies of CD2+CD8+ γδ T cell subset increased significantly in ileum after HRV inoculation as reported in our previous study [4] and this study showed that this subset produced IFN-γ (Fig. 3A). Significant correlations were found between IFN-γ production and TLR3 expression in ileum (Spearman correlation coefficiency r = 0.54; p = 0.0371) and TLR9 expression in blood (r = 0.58; p = 0.0225) in the CD2+CD8+ subset. Frequencies of IFN-γ producing CD2+CD8+ subset in spleen and blood and IFN-γ producing CD2+CD8− subset in ileum and spleen of HRV-inoculated Gn pigs increased significantly at PID 3 compared to PID 0 (Fig. 3). At PID 5, the IFN-γ responses started to decline, but were still higher or significantly higher than those of pre-inoculation (PID 0). Frequencies of the CD2−CD8−γδ T cells in ileum and blood significantly reduced at PID 5 as shown in our previous study [4] and no significant IFN-γ response in this subset was observed after HRV inoculation (Fig. 3).
Fig. 3. Representative dot plots (A) and mean frequencies (B) of IFN-γ producing γδ T cell subsets in ileum, spleen and blood of Gn pigs inoculated with HRV.
MNCs were cultured in vitro for 17 hours without antigen stimulation before staining for flow cytometry analysis. The right two dot plots in panel (A) show the representatives of IFN-γ producing CD2+CD8+ subset in ileum of Gn pigs infected with HRV at PID 3 or mock infected. Figures in panel (B) compare the mean frequencies of IFN-γ producing γδ T cells among the three subsets in ileum, spleen and blood of Gn pigs infected with HRV (PID 3 and PID 5) or mock infected (PID 0). Data are presented as mean frequency ± standard error of the mean (n = 4–7). Different capital letters on top of bars indicate significant differences in frequencies among the time points for the same γδ T cell subset and tissue (Kruskal-Wallis test, p < 0.05), while shared letters indicate no significant difference.
3.3. CD2+CD8+ γδ T cell subset in ileum, spleen and blood produced TGF-β with or without HRV inoculation
The CD2+CD8+ subset in ileum had high frequencies of TGF-β production at both PID 0 and PID 5 (data not shown). The frequencies of TGF-β production in the CD2+CD8+ subset was higher compared to the CD8− γδ T subsets in ileum, spleen and blood. After HRV inoculation, there was a trend for reduced TGF-β production in the CD2+CD8+ subset in ileum and spleen. However, there were no statistically significant differences because of the low pig numbers in each group (n = 3–4).
3.4. CD2+CD8+ γδ T cells in intestinal and systemic sites expressed FoxP3
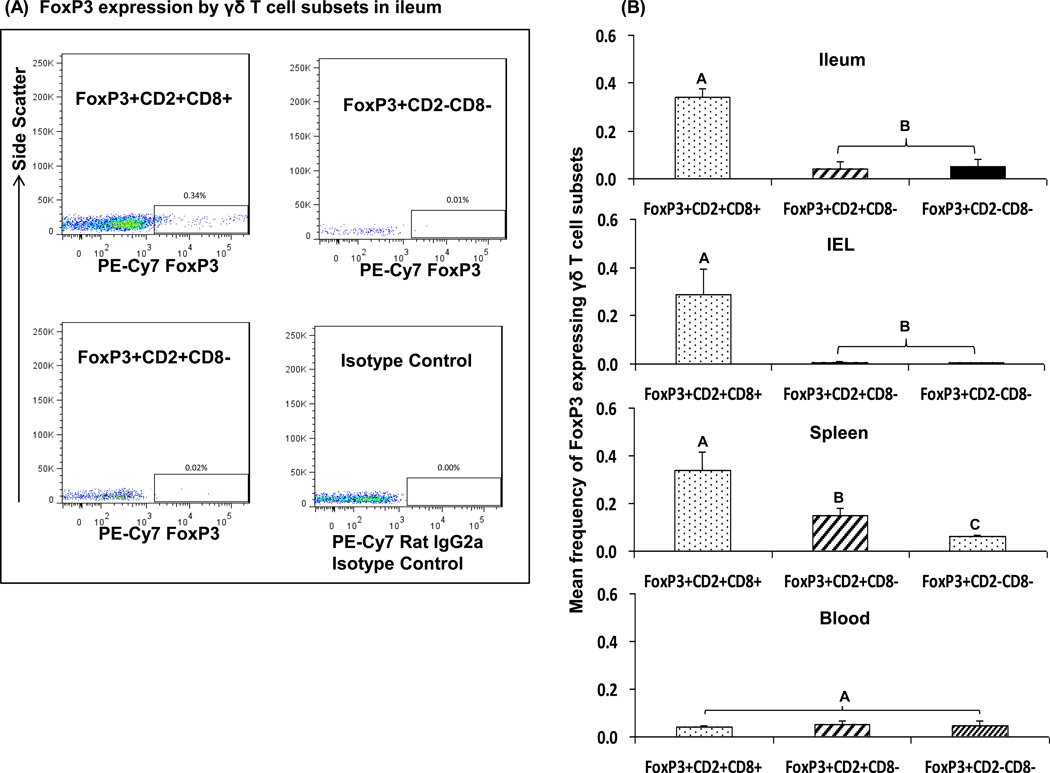
Transcription factor forkhead box P3 (FoxP3) acts as the regulatory T cell lineage specification factor [23]. Expression of FoxP3 is the marker of regulatory T cell function. To better clarify the potential regulatory function of the γδ T cell subsets, patterns of FoxP3 expression by each γδ T cell subset in Gn pigs were investigated. Fig. 4A depicts the representative dot plots of FoxP3 expression in each γδ T cell subset in ileum. Frequencies of FoxP3 expressing CD2+CD8+ γδ T cells in ileum, IEL and spleen were significantly higher compared to the CD8− subsets (Fig. 4B) confirming that tissue-specific CD2+CD8+ subset exhibits regulatory T cell function. The two CD8− subsets in all tissues had low or no FoxP3 expression, which is consistent with the low or non-existent TGF-β production by these two subsets with or without HRV inoculation. FoxP3 expression was low and the frequencies did not differ among the three γδ T cell subsets in blood.
Fig. 4. Representative dot plots (A) and mean frequencies (B) of FoxP3 expressing γδ T cell subsets in intestinal and systemic lymphoid tissues of Gn pigs challenged with virulent HRV at PCD7.
MNCs were cultured in vitro with Brefeldin A for 5 hrs before staining for flow cytometry analysis. Panel (A) shows the representative dot plots of FoxP3 expressing γδ T cells in ileum of Gn pigs. Figures in panel (B) depict the mean frequencies of FoxP3 expressing γδ T cell subsets among total γδ T cells in ileum, IEL, spleen and blood. Data are presented as mean frequency ± standard error of the mean (n = 3). Different capital letters on top of bars indicate significant differences in frequencies among the three γδ T cell subsets from the same tissue (Kruskal-Wallis test, p < 0.05), while shared letters indicate no significant difference.
3.5. Sort-purified CD2+CD8+ subset produced IL-10 and TGF-β whereas CD8− subsets produced IFN-γ after IPP plus IL-2 stimulation
To further differentiate immune functions among γδ T cell subsets, the cytokine production profiles of each sort-purified γδ T cell subset from spleen and IEL of Gn pigs were determined by ELISA after the cells were stimulated with the γδ T cell agonist phosphoantigen IPP for 5 days in cell culture. IPP plus IL-2 is known to activate human Vδ2 T cells [24, 25].
Mock-stimulated γδ T cells did not produce any detectable level of IFN-γ or TGF-β in any subset. Substantial amounts of IL-10 were produced by the CD2+CD8+ subset from spleen (~6000 pg/ml) and IEL (~2000 pg/ml) without IPP plus IL-2 stimulation (Fig. 5). No IFN-γ was produced following stimulation with IPP plus IL-2 by the CD2+CD8+ subset in spleen and IEL. In contrast, the CD8− subset from both spleen and IEL produced IFN-γ, although the variability was high. There were no significant differences in IFN-γ concentrations among the subsets or between the IPP plus IL-2 stimulated and mock stimulated cells due to the high variability.
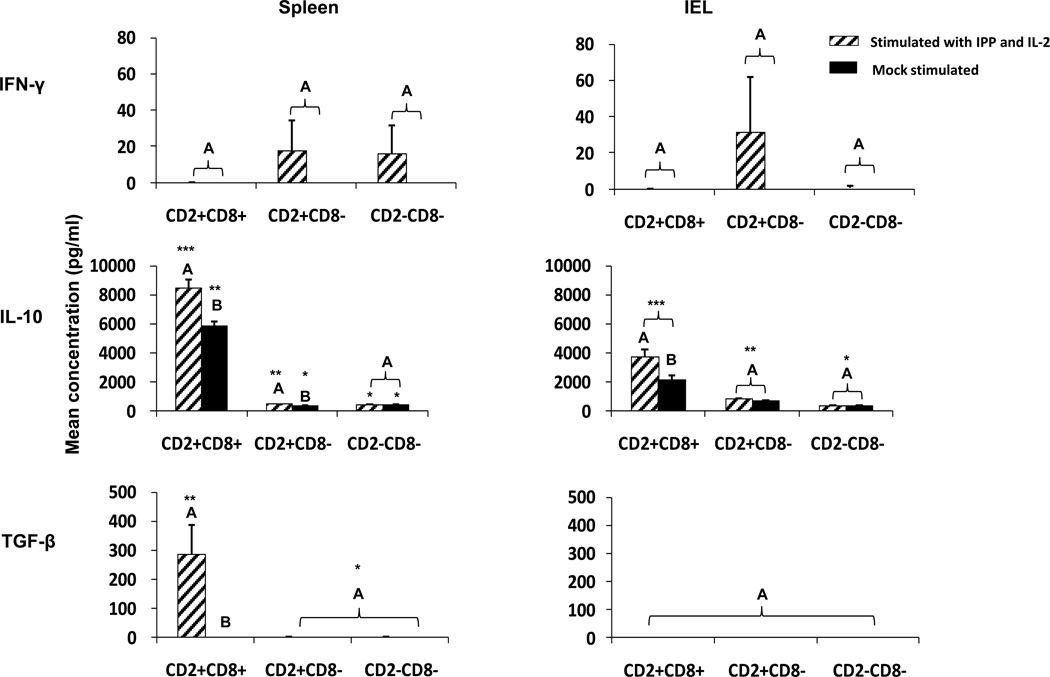
Fig. 5. Cytokine production by splenic and IEL γδ T cell subsets in cultures with or without IPP plus IL-2 stimulation.
MNCs isolated from spleen and IEL of Gn pigs infected with human norovirus GII.4 were sorted into three γδ T cell subsets by using MACS and FACS technologies. Each γδ T cell subset was cultured in vitro for 5 days with the stimulation of IPP plus IL-2 or mock stimulated. Concentrations of IFN-γ, IL-10 and TGF-β in the culture supernatants were measured by ELISA. Data are presented as mean concentrations ± standard error of the mean (n = 6). Different capital letters on top of bars indicate significant differences in cytokine concentrations between IPP plus IL-2 stimulated and mock stimulated cells for the same γδ T cell subset and tissue (Kruskal-Wallis test, p < 0.05), while shared letters indicate no significant difference. Different numbers of * indicates significant difference in cytokine concentrations among the three γδ T cell subsets under the same stimulation condition for the same tissue (Kruskal-Wallis test, p < 0.05), while same numbers of * indicate no significant difference.
The CD2+CD8+ subset from spleen and IEL produced significantly higher amounts of IL-10 in IPP plus IL-2 stimulated cells compared to the mock-stimulated cells. More importantly, the CD2+CD8+ subset produced a significantly higher amount of IL-10 than the two CD8− subsets in spleen (14–23 fold) and IEL (3–13 fold) with or without IPP plus IL-2 stimulation. Interestingly, IPP stimulation also significantly increased IL-10 production by the splenic CD2+CD8− subset. The CD2+CD8+ γδ T cells from spleen were the only subset producing any detectable amount of TGF-β (Fig. 5). IL-17 was undetectable in the culture supernatants of any γδ T cell subsets (data not shown).
3.6. CD8− γδ T cells acquire CD8 expression during in vitro culture
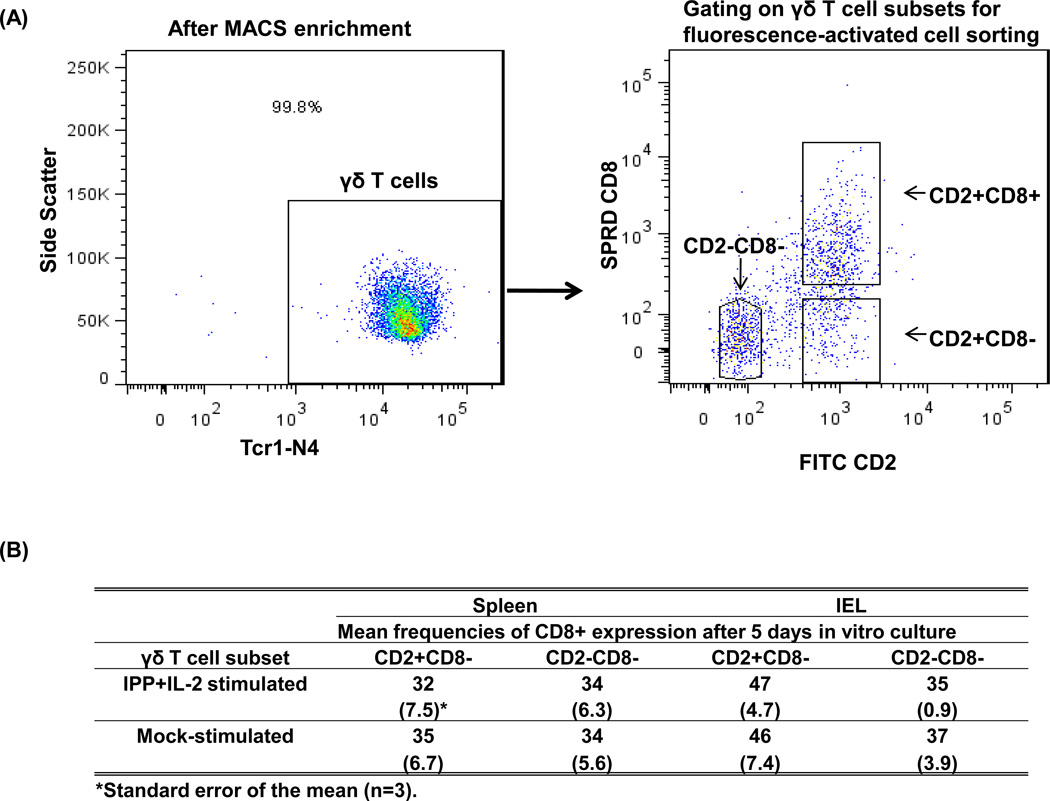
Our previous studies showed that frequencies of CD8− γδ T cells decreased and CD2+CD8+ γδ T cells increased significantly at PID 5 in HRV-inoculated and lactobacilli-colonized Gn pigs. Thus we postulated that CD8− γδ T cells acquired the CD8 marker upon activation and differentiated into CD2+CD8+ γδ T cells [4]. In this study, γδ T cell subsets were sort-purified by MACS and FACS and the purity of total γδ T cells was > 99 % for all the samples. Three γδ T cell subsets were collected by FACS using the gating method shown in Fig. 6A, which ensured that the purity of each subset was > 99 %. After the cells were cultured in vitro for 5 days with or without IPP plus IL-2 stimulation, the CD8− subsets were stained again for Tcr1-N4 and CD8 and were subjected to flow cytometry analysis. As shown in Fig. 6B, the γδ T cells that were originally > 99 % of CD8− at the beginning of the culture expressed CD8 in up to 47 % of the cells from IEL and up to 35 % of the cells from spleen at the end of the culture. There were more CD2+CD8− γδ cells acquiring CD8 expression than CD2−CD8− γδ cells. There were no significant differences in the frequencies of CD8+ γδ T cells between IPP plus IL-2 stimulated and mock stimulated γδ T cell cultures, suggesting that IPP plus IL-2 is not necessary for the differentiation.
Fig. 6. CD8− γδ T cells differentiation into CD8+ γδ T cells during in vitro culture.
MNCs isolated from spleen and IEL of Gn pigs (n = 3) infected with human norovirus GII.4 were sorted into three γδ T cell subsets by using MACS and FACS technologies. Each γδ T cell subset was cultured in vitro for 5 days with the stimulation of IPP plus IL-2 or mock stimulation. The culture supernatants were kept to measure IFN-γ, IL-10 and TGF-β by ELISA (See Fig. 5 legend) and the cells were collected for the Tcr1-N4 and CD8 staining. The left dot plot in panel (A) shows the purity of γδ T cells after enrichment by MACS and the right dot plot in panel (A) demonstrates the gating of three γδ T cell subsets to be collected by FACS. The table in panel (B) lists the mean frequencies of CD8 expression after 5 days in vitro culture of the CD2+CD8− and CD2−CD8− subsets from spleen and IEL.
3.7. Influence of sort-purified γδ T cell subsets on autologous CD4+ αβ T cell cytokine production and proliferation in co-cultures
All three γδ T cell subsets enhanced IFN-γ production by CD4+ αβ T cells in the co-cultures
To further identify the immune modulating functions of γδ T cell subsets on the development of adaptive immune response, sort-purified splenic γδ T cells were co-cultured with autologous CD4+ αβ T cells and stimulated with PHA for 5 days in cell culture. The final cytokine concentrations in the culture supernatants were measured by ELISA and the CD4+ αβ T cell proliferation was measured by BrdU staining and flow cytometry. As shown in Fig. 7, CD4+ αβ T cells cultured alone with PHA produced low levels of IFN-γ. The CD8− subsets, but not the CD2+CD8+ subset, cultured alone with PHA also produced low levels of IFN-γ. All three γδ T cell subsets enhanced IFN-γ production by CD4+ αβ T cells in the co-culture; however, the two CD8− γδ T cell subsets increased the concentrations of IFN-γ substantially (5 to 7-fold by CD8− subsets versus 2-fold by the CD2+CD8+ subset).
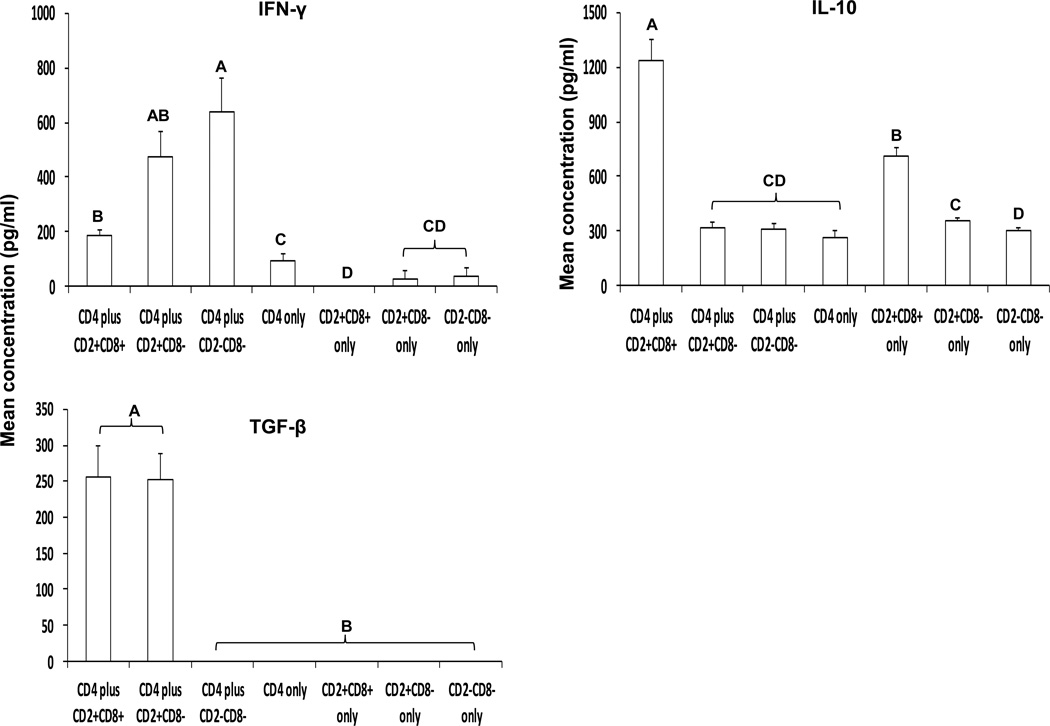
Fig. 7. Modulation of cytokine production of sort-purified splenic CD4+ αβ T cells by each γδ T cell subset.
Three γδ T cell subsets and CD4+ αβ T cells were sort-purified from splenic MNCs of Gn pigs by using MACS and FACS. γδ T cell subsets and CD4+ αβ T cells were cultured individually or co-cultured with PHA stimulation for 5 days. The cells from the co-cultures were collected for the BrdU staining (see Fig. 8 legend). Concentrations of IFN-γ, IL-10 and TGF-β in the culture supernatants were measured by ELISA. Data are presented as mean concentration ± standard error of the mean (n = 6). Different letters on top of bars indicate significant differences in cytokine concentrations among the cell cultures (Kruskal-Wallis test, p < 0.05), while shared letters indicate no significant difference.
The CD2+CD8+ subset significantly enhanced IL-10 production by CD4+ αβ T cells
All three γδ T cell subsets and CD4+ αβ T cells produced measurable levels of IL-10 when cultured alone with PHA. The CD2+CD8+ subset cultured alone produced significantly more IL-10 than the two CD8− subsets alone, a similar trend as the IPP plus IL-2 stimulated CD2+CD8+ γδ T cell subset (Fig. 5), but at a lower magnitude (~10-fold less) (Fig. 7. Note the scale differences in the y axis of the figures for IL-10 concentration). The CD2+CD8+ subset significantly enhanced IL-10 production by CD4+ αβ T cells.
The CD2+CD8+ subset significantly increased TGF-β production by CD4+ αβ T cells
Without γδ T cells in the culture, CD4+ αβ T cells did not produce any detectable TGF-β. Interestingly, the CD2+CD8− subset also significantly enhanced TGF-β production by CD4+ αβ T cells. Due to low yields of sort-purified γδ and αβ T cells from IEL, co-culture studies were not conducted for IEL.
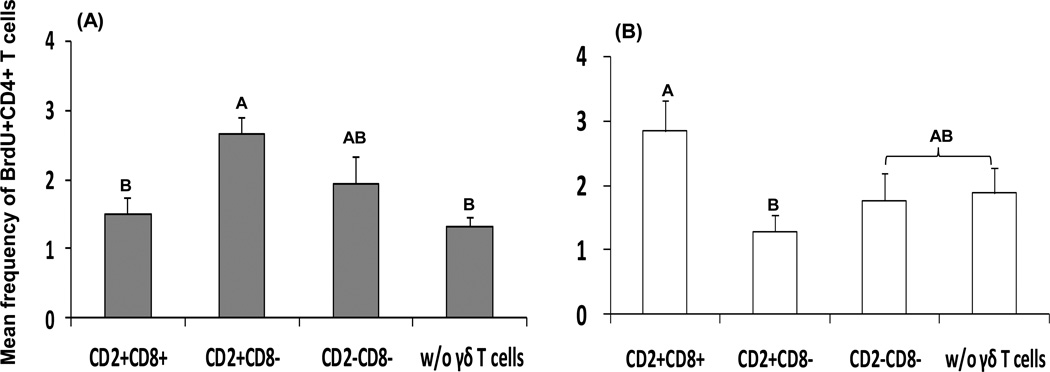
The CD2+CD8− subset significantly enhanced CD4+ αβ T cell proliferation with PHA stimulation
Consistent with the IFN-γ production profile, splenic CD4+ αβ T cells co-cultured with the CD2+CD8− γδ T cell subset had significantly higher frequencies of BrdU+CD4+ αβ T cells compared to co-cultures with the CD2+CD8+ γδ T cell subset or without γδ T cells (Fig. 8A). The CD2−CD8− subset also increased frequencies of BrdU+CD4+ αβ T cells in the co-culture, but the increase was not statistically significant.
Fig. 8. Proliferation of PHA-stimulated (A) and Wa HRV-stimulated (B) sort-purified splenic CD4+ T cells co-cultured with each γδ T cell subset.
Cells collected from the co-cultures (see Fig. 7 legend) were stained with antibodies to CD3, CD4, and BrdU to measure proliferation of CD4+ αβ T cells by flow cytometry. Frequencies of proliferating cells were defined as BrdU+CD4+ T cells among total CD3+ T cells (Fig. 8A) (n = 6). Three γδ T cell subsets and CD4+ αβ T cells were also sort-purified from MNCs isolated from spleen of Gn pigs inoculated with two oral doses of attenuated HRV and challenged with virulent HRV. The co-cultures of CD4+ αβ T cells with each γδ T cell subset or without were stimulated with Wa HRV antigen for 5 days and the cells were collected for CD3, CD4, and BrdU staining to measure proliferation of CD4+ αβ T cells among CD3+ T cells (Fig. 8B) (n = 3). Data are presented as mean concentration ± standard error of the mean. Different letters on top of bars indicate significant differences in frequencies of proliferating CD4+ αβ T cells among the cell cultures (Kruskal-Wallis test, p < 0.05), while shared letters indicate no significant difference.
3.8. The CD2+CD8+ subset increased HRV-specific CD4+ αβ T cell proliferation without the presence of APCs
Previous studies indicated that a minority of the porcine CD2+CD8+ γδ T cells could act as professional APCs and presented African swine fever virus antigens to CD4+ T cells [26, 27]. In this study, the sort-purified splenic CD4+ αβ T cells from attenuated HRV-vaccinated pigs collected on PID 28 were cultured in vitro for 5 days. Semi-purified Wa HRV antigen was added in all the cultures. When CD4+ αβ T cells were cultured alone or co-cultured with the CD8− γδ T cell subsets, frequencies of proliferating CD4+ αβ T cells were low and did not differ significantly (Fig. 8B). However, when CD4+ αβ T cells were co-cultured with CD2+CD8+ γδ T cells in the presence of Wa HRV antigen, frequencies of proliferating CD4+ αβ T cells increased substantially. This observation suggested that the CD2+CD8+ subset acted as APCs and presented Wa HRV antigen to HRV-specific CD4+ αβ T cells to promote proliferation.
4. Discussion
In this study, we characterized immunological functions of the three porcine γδ T cell subsets. γδ T cells play important roles in antigen recognition and innate immune responses. Antigen recognition by γδ T cells is not restricted to the γδ TCR. γδ T cells can sense invading pathogens or self-antigens via pattern recognition receptors such as TLRs, scavenger receptors, integrins and NK receptors [26]. TLR expression by γδ T cells has been demonstrated in humans [12], mice [13] and cattle [28]. TLR expression on porcine γδ T cell subsets was examined for the first time in this study. We demonstrated that rotavirus infection stimulated significant alternations in TLR expression in all three γδ T cell subsets, with the highest increases in the CD2+CD8− subset in ileum and spleen. TLR2 and TLR3 expression in all three γδ T cell subsets in ileum increased significantly at PID 3–5 after HRV infection, suggesting that all three subsets were involved in rotavirus recognition and in the innate mucosal immune responses to rotavirus infection. Splenic and circulating CD8− γδ T cells expressed increased frequencies of TLRs by PID 5. This TLR response, two days after those in ileum, suggests that the CD8− subsets in spleen and blood were activated when rotavirus entered circulation after the initial replication in the small intestinal epithelium. Rotavirus fecal shedding peaked at PID 1, whereas viremia peaked at PID 3 in virulent HRV orally inoculated Gn pigs [29], which explains the delayed TLR responses in splenic and circulating γδ T cells compared to ileal γδ T cells. At PID 0, there was no TLR2 or TLR3 expression in ileum and a higher baseline of TLR expression in spleen for all γδ T cell subsets of naïve Gn pigs. These observations suggest that intestinal γδ T cells are non-activated at homeostasis and are quickly activated upon recognition of invading pathogens, whereas systemic γδ T cells are constitutively activated at a low level and can be further activated during enteric virus infection.
Similar to other species, we can generally divide porcine γδ T cells into two major functional subsets: the pro-inflammatory (CD2+CD8− and CD2−CD8−) and the regulatory (CD2+CD8+) subsets based on their cytokine production profile, FoxP3 expression and immunomodulating effects on αβ T cells observed in this study. The CD2+CD8− γδ T cell subset had significantly increased frequencies of IFN-γ expression in ileum and spleen in Gn pigs after rotavirus infection and produced IFN-γ after stimulation with IPP plus IL-2. CD2+CD8− and CD2−CD8− γδ T cell subsets enhanced proliferation of and IFN-γ production by CD4+ αβ T cells in the co-cultures, confirming that CD2+CD8− and CD2− CD8− γδ T cells are pro-inflammatory effectors. In contrast, the CD2+CD8+ subset expressed FoxP3 in ileum, IEL and spleen and produced TGF-β in ileum, spleen and blood of Gn pigs, produced significantly higher amounts of IL-10 and TGF-β after stimulation with IPP plus IL-2, and significantly enhanced IL-10 and TGF-β production by PHA-stimulated αβ T cells in the co-cultures. These are characteristics of regulatory T cells. However, in addition to the anti-inflammatory/regulatory functions, the CD2+CD8+ subset also had significantly increased frequencies of IFN-γ expression in spleen and blood of HRV-infected Gn pigs and enhanced production of IFN-γ by splenic CD4+ αβ T cells in the co-cultures after PHA stimulation. Yet it did not enhance CD4+ αβ T cell proliferation, indicating that this subset in specific microenvironments (spleen and blood) has a pro-inflammatory function.
This dual function of porcine CD2+CD8+ γδ T cells (primarily anti-inflammatory) has also been reported for γδ T cells in other species. The condition of microenvironment could determine the immune regulatory function of a specific γδ T cell subset. Human Vδ2 T cells (pro-inflammatory) primed ex vivo towards IFN-γ and TNF-α production can be driven towards IL-4 production under appropriate Th2-priming in vitro culture conditions [30]. TGF-β and IL-15 can stimulate human Vδ2 T cells to express FoxP3 and acquire regulatory functions to suppress the proliferation of anti-CD3/CD28 stimulated peripheral blood mononuclear cells [31]. A model of inflammation regulation was proposed by Holderness [6] to explain the inducible pro-inflammatory responses of tissue-specific γδ T cells and at the same time, the anti-inflammatory, tissue repair responses commonly seen from the same cells during inflammation.
Interestingly, we found that co-culture with CD2+CD8− subset not only significantly enhanced IFN-γ, but also TGF-β production by CD4+ αβ T cells. Porcine CD2+CD8− γδ thymocytes were proposed to be the precursor of all three peripheral γδ T cell subsets [8], suggesting that extra-thymic porcine CD8− subsets can possibly differentiate into CD8+ subset and obtain regulatory function. Indeed we observed that a large percentage (32–47 %) of sort-purified splenic and IEL CD8− γδ T cells (more than 99 % purity) became CD8+ after 5 days of in vitro culture. Therefore the TGF-β inducing effect was likely exerted by the newly emerged CD8+ γδ T cells in the culture. It cannot be dismissed, although unlikely, that there was a small number (less than 1 %) of CD8+ γδ T cells among the sort-purified CD8− γδ T cells selectively expanded in the culture condition.
Although initial studies of mice showed that FoxP3 expression is highly restricted to αβ T cells and there is a lack of FoxP3 expression on any other cell populations including γδ T cells [23], FoxP3+ γδ T cells have been identified in mice and cattle [32, 33], Our study showed that FoxP3 was expressed, albeit in low frequencies (< 0.5 %), on the CD2+CD8+ T cells in ileum, IEL and spleen of Gn pigs. This observation corroborates with the regulatory cytokine production in Gn pigs (TGF-β) and after in vitro IPP stimulation (IL-10 and TGF-β) by the CD2+CD8+ subset. The CD2+CD8− subset from spleen of Gn pigs also expressed low levels of FoxP3, which is consistent with the findings from the co-culture study that this subset promoted TGF-β production by CD4+ αβ T cells and from the IPP stimulation study that this subset produced significantly higher levels of IL-10 after IPP stimulation in the cell culture.
Previous studies suggested that IPP directly activated pro-inflammatory γδ T cells (i.e. Vδ2Vγ9 T cells in humans) to proliferate and produce cytokines without requiring antigen presentation by APCs [34, 35]. Adding IL-2 increases the magnitude of their effector responses but IL-2 alone is not sufficient to induce effector responses by these γδ T cells [36]. In our study, IPP (plus IL-2) stimulated γδ T cells to produce IFN-γ (CD8− subsets) or TGF-β (CD2+CD8+ subset). Although IL-10 was produced constitutively by the CD2+CD8+ subset, IPP stimulation significantly enhanced IL-10 production. Thus, IPP can activate both pro-inflammatory and regulatory porcine γδ T cells to exert their respective immune effector functions. IPP stimulation did not have an effect on the differentiation of CD8− subsets into CD8+ γδ T cells. Taken together, the data suggest that IPP stimulation did not have a bias toward expanding the pro- or anti-inflammatory γδ T cell function. The factors that regulate the differentiation of extra-thymic γδ T cells require further study.
γδ T cells can present antigens to other cells to induce adaptive immune responses. Previous studies found that porcine CD2+CD8+ γδ T cells present antigens via MHC II to CD4+ T cells [27]. Bovine WC1 γδ T cells and human Vδ2 T cells expressed MHC II and potentially presented antigen in the context of MHC II [37, 38]. Our co-culture study with the presence of HRV antigen and without APCs provided additional evidence that porcine CD2+CD8+ γδ T cells have APC function and can present viral antigens to CD4+ T cells.
In summary, porcine CD2+CD8− and CD2−CD8− γδ T cells have mainly pro-inflammatory function by directly secreting IFN-γ or promoting CD4+ αβ T cell proliferation and IFN-γ production, whereas CD2+CD8+ γδ T cells mainly exert regulatory T cell function by expressing FoxP3, directly secreting IL-10 and TGF-β or increasing IL-10 and TGF-β production by CD4+ αβ T cells. γδ T cells responded to rotavirus infection by TLR expression and IFN-γ and TGF-β production with different patterns in CD2+CD8+ subset from CD8− subsets. The CD8− subsets can differentiate into CD8+ subset by acquiring CD8 expression. This phenomenon may explain, at least in part, the apparently dual functions of CD2+CD8+ and CD2+CD8− subsets. Thus, both CD8+ and CD8− γδ T cell subsets can contribute to anti-virus immunity as well as to the maintenance and restoration of intestinal and systemic homeostasis in the face of enteric virus infections.
Acknowledgements
We thank Dr. Marlice Vonck, Dr. Kevin Pelzer, Pete Jobst, Andrea Aman and Shannon Viers for animal care. We thank Melissa Makris for assistance in flow cytometry. We thank Dr. Linda Saif for the virulent and attenuated Wa HRV and Dr. Xi Jiang for human norovirus inoculums. This work was supported by grants from National Institutes of Health (R01AT004789 and R01AI089634-subaward) to L.Y.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hahn YS, Taube C, Jin N, et al. Different potentials of gamma delta T cell subsets in regulating airway responsiveness: V gamma 1+ cells, but not V gamma 4+ cells, promote airway hyperreactivity, Th2 cytokines, and airway inflammation. J Immunol. 2004;172(5):2894–2902. doi: 10.4049/jimmunol.172.5.2894. [DOI] [PubMed] [Google Scholar]
- 2.Holderness J, Hedges JF, Daughenbaugh K, Kimmel E, Graff J, Freedman B, Jutila MA. Response of gammadelta T Cells to plant-derived tannins. Critical reviews in immunology. 2008;28(5):377–402. doi: 10.1615/critrevimmunol.v28.i5.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saalmuller A, Hirt W, Reddehase MJ. Porcine gamma/delta T lymphocyte subsets differing in their propensity to home to lymphoid tissue. Eur J Immunol. 1990;20(10):2343–2346. doi: 10.1002/eji.1830201026. [DOI] [PubMed] [Google Scholar]
- 4.Wen K, Li G, Zhang W, et al. Development of gammadelta T cell subset responses in gnotobiotic pigs infected with human rotaviruses and colonized with probiotic lactobacilli. Vet Immunol Immunopathol. 2011;141(3–4):267–275. doi: 10.1016/j.vetimm.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson E, Hedges JF, Butcher EC, Briskin M, Jutila MA. Bovine gamma delta T cell subsets express distinct patterns of chemokine responsiveness and adhesion molecules: a mechanism for tissue-specific gamma delta T cell subset accumulation. J Immunol. 2002;169(9):4970–4975. doi: 10.4049/jimmunol.169.9.4970. [DOI] [PubMed] [Google Scholar]
- 6.Holderness J. Select procyanidins induce γδ T cell activation and proliferation (MS Thesis) Bozeman, Montana: Montana State University; 2008. pp. 30–40. [Google Scholar]
- 7.Davis WC, Zuckermann FA, Hamilton MJ, Barbosa JI, Saalmuller A, Binns RM, Licence ST. Analysis of monoclonal antibodies that recognize gamma delta T/null cells. Vet Immunol Immunopathol. 1998;60(3–4):305–316. doi: 10.1016/s0165-2427(97)00107-4. [DOI] [PubMed] [Google Scholar]
- 8.Sinkora M, Sinkorova J, Holtmeier W. Development of gammadelta thymocyte subsets during prenatal and postnatal ontogeny. Immunology. 2005;115(4):544–555. doi: 10.1111/j.1365-2567.2005.02194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hedges JF, Lubick KJ, Jutila MA. Gamma delta T cells respond directly to pathogen-associated molecular patterns. J Immunol. 2005;174(10):6045–6053. doi: 10.4049/jimmunol.174.10.6045. [DOI] [PubMed] [Google Scholar]
- 10.Wesch D, Beetz S, Oberg HH, Marget M, Krengel K, Kabelitz D. Direct costimulatory effect of TLR3 ligand poly(I:C) on human gamma delta T lymphocytes. J Immunol. 2006;176(3):1348–1354. doi: 10.4049/jimmunol.176.3.1348. [DOI] [PubMed] [Google Scholar]
- 11.Deetz CO, Hebbeler AM, Propp NA, Cairo C, Tikhonov I, Pauza CD. Gamma interferon secretion by human Vgamma2Vdelta2 T cells after stimulation with antibody against the T-cell receptor plus the Toll-Like receptor 2 agonist Pam3Cys. Infect Immun. 2006;74(8):4505–4511. doi: 10.1128/IAI.00088-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pietschmann K, Beetz S, Welte S, Martens I, Gruen J, Oberg HH, Wesch D, Kabelitz D. Toll-like receptor expression and function in subsets of human gammadelta T lymphocytes. Scandinavian journal of immunology. 2009;70(3):245–255. doi: 10.1111/j.1365-3083.2009.02290.x. [DOI] [PubMed] [Google Scholar]
- 13.Schwacha MG, Daniel T. Up-regulation of cell surface Toll-like receptors on circulating gammadelta T-cells following burn injury. Cytokine. 2008;44(3):328–334. doi: 10.1016/j.cyto.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ward LA, Rosen BI, Yuan L, Saif LJ. Pathogenesis of an attenuated and a virulent strain of group A human rotavirus in neonatal gnotobiotic pigs. J Gen Virol. 1996;77(Pt 7):1431–1441. doi: 10.1099/0022-1317-77-7-1431. [DOI] [PubMed] [Google Scholar]
- 15.Yuan L, Ward LA, Rosen BI, To TL, Saif LJ. Systematic and intestinal antibody-secreting cell responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J Virol. 1996;70(5):3075–3083. doi: 10.1128/jvi.70.5.3075-3083.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ward LA, Yuan L, Rosen BI, To TL, Saif LJ. Development of mucosal and systemic lymphoproliferative responses and protective immunity to human group A rotaviruses in a gnotobiotic pig model. Clin Diagn Lab Immunol. 1996;3(3):342–350. doi: 10.1128/cdli.3.3.342-350.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer RC, Bohl EH, Kohler EM. Procurement and Maintenance of Germ-Free Seine for Microbiological Investigations. Appl Microbiol. 1964;12:295–300. doi: 10.1128/am.12.4.295-300.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang W, Azevedo MS, Wen K, Gonzalez A, Saif LJ, Li G, Yousef AE, Yuan L. Probiotic Lactobacillus acidophilus enhances the immunogenicity of an oral rotavirus vaccine in gnotobiotic pigs. Vaccine. 2008;26(29–30):3655–3661. doi: 10.1016/j.vaccine.2008.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wen K, Azevedo MS, Gonzalez A, Zhang W, Saif LJ, Li G, Yousef A, Yuan L. Toll-like receptor and innate cytokine responses induced by lactobacilli colonization and human rotavirus infection in gnotobiotic pigs. Vet Immunol Immunopathol. 2009;127:304–315. doi: 10.1016/j.vetimm.2008.10.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan L, Wen K, Azevedo MS, Gonzalez AM, Zhang W, Saif LJ. Virus-specific intestinal IFN-gamma producing T cell responses induced by human rotavirus infection and vaccines are correlated with protection against rotavirus diarrhea in gnotobiotic pigs. Vaccine. 2008;26(26):3322–3331. doi: 10.1016/j.vaccine.2008.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez AM. Studies of human rotavirus candidate non-replicating vaccines and innate immunity in a gnotobiotic pig model of human rotavirus disease (PhD Dissertation) Columbus, Ohio: Ohio State University; 2007. pp. 281–284. [Google Scholar]
- 22.Azevedo MS, Yuan L, Pouly S, Gonzales AM, Jeong KI, Nguyen TV, Saif LJ. Cytokine responses in gnotobiotic pigs after infection with virulent or attenuated human rotavirus. J Virol. 2006;80(1):372–382. doi: 10.1128/JVI.80.1.372-382.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22(3):329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Morita CT, Beckman EM, Bukowski JF, Tanaka Y, Band H, Bloom BR, Golan DE, Brenner MB. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human gamma delta T cells. Immunity. 1995;3(4):495–507. doi: 10.1016/1074-7613(95)90178-7. [DOI] [PubMed] [Google Scholar]
- 25.Sicard H, Ingoure S, Luciani B, Serraz C, Fournie JJ, Bonneville M, Tiollier J, Romagne F. In vivo immunomanipulation of V gamma 9V delta 2 T cells with a synthetic phosphoantigen in a preclinical nonhuman primate model. J Immunol. 2005;175(8):5471–5480. doi: 10.4049/jimmunol.175.8.5471. [DOI] [PubMed] [Google Scholar]
- 26.Takamatsu HH, Denyer MS, Stirling C, Cox S, Aggarwal N, Dash P, Wileman TE, Barnett PV. Porcine gammadelta T cells: possible roles on the innate and adaptive immune responses following virus infection. Vet Immunol Immunopathol. 2006;112(1–2):49–61. doi: 10.1016/j.vetimm.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 27.Takamatsu HH, Denyer MS, Wileman TE. A sub-population of circulating porcine gammadelta T cells can act as professional antigen presenting cells. Vet Immunol Immunopathol. 2002;87(3–4):223–224. doi: 10.1016/s0165-2427(02)00083-1. [DOI] [PubMed] [Google Scholar]
- 28.Hedges JF, Graff JC, Jutila MA. Transcriptional profiling of gamma delta T cells. J Immunol. 2003;171(10):4959–4964. doi: 10.4049/jimmunol.171.10.4959. [DOI] [PubMed] [Google Scholar]
- 29.Azevedo MS, Yuan L, Jeong KI, et al. Viremia and nasal and rectal shedding of rotavirus in gnotobiotic pigs inoculated with Wa human rotavirus. J Virol. 2005;79(9):5428–5436. doi: 10.1128/JVI.79.9.5428-5436.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wesch D, Glatzel A, Kabelitz D. Differentiation of resting human peripheral blood gamma delta T cells toward Th1- or Th2-phenotype. Cell Immunol. 2001;212(2):110–117. doi: 10.1006/cimm.2001.1850. [DOI] [PubMed] [Google Scholar]
- 31.Casetti R, Agrati C, Wallace M, Sacchi A, Martini F, Martino A, Rinaldi A, Malkovsky M. Cutting edge: TGF-beta1 and IL-15 Induce FOXP3+ gammadelta regulatory T cells in the presence of antigen stimulation. J Immunol. 2009;183(6):3574–3577. doi: 10.4049/jimmunol.0901334. [DOI] [PubMed] [Google Scholar]
- 32.Kang N, Tang L, Li X, et al. Identification and characterization of Foxp3(+) gammadelta T cells in mouse and human. Immunol Lett. 2009;125(2):105–113. doi: 10.1016/j.imlet.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Gerner W, Stadler M, Hammer SE, Klein D, Saalmuller A. Sensitive detection of Foxp3 expression in bovine lymphocytes by flow cytometry. Vet Immunol Immunopathol. 2010 doi: 10.1016/j.vetimm.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 34.Janeway CA, Jr, Jones B, Hayday A. Specificity and function of T cells bearing gamma delta receptors. Immunol Today. 1988;9(3):73–76. doi: 10.1016/0167-5699(88)91267-4. [DOI] [PubMed] [Google Scholar]
- 35.De Libero G. Sentinel function of broadly reactive human gamma delta T cells. Immunol Today. 1997;18(1):22–26. doi: 10.1016/s0167-5699(97)80010-2. [DOI] [PubMed] [Google Scholar]
- 36.Li H, David Pauza C. Interplay of T-cell receptor and interleukin-2 signalling in Vgamma2Vdelta2 T-cell cytotoxicity. Immunology. 2011;132(1):96–103. doi: 10.1111/j.1365-2567.2010.03343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Price SJ, Hope JC. Enhanced secretion of interferon-gamma by bovine gammadelta T cells induced by coculture with Mycobacterium bovis-infected dendritic cells: evidence for reciprocal activating signals. Immunology. 2009;126(2):201–208. doi: 10.1111/j.1365-2567.2008.02889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gammadelta T Cells. Science. 2005;309(5732):264–268. doi: 10.1126/science.1110267. [DOI] [PubMed] [Google Scholar]