Abstract
Background
While exposures to urban fine particulate matter (PM2.5) and soot-black carbon (soot-BC) have been associated with asthma exacerbations, there is limited evidence on whether these pollutants are associated with the new development of asthma or allergy among young inner city children. We hypothesized that childhood exposure to PM2.5 and the soot-BC component would be associated with the report of new wheeze and development of seroatopy in an inner city birth cohort.
Methods
As part of the research being conducted by the Columbia Center of Children’s Environmental Health (CCCEH) birth cohort study in New York City, two-week integrated residential monitoring of PM2.5, soot-BC (based on a multi-wavelength integrating sphere method), and modified absorption coefficient (Abs*; based on the smoke stain reflectometer) was conducted between October 2005 and May 2011 for 408 children at age 5–6 years old. Residential monitoring was repeated 6 months later (n=262) to capture seasonal variability. New wheeze was identified through the International Study of Asthma and Allergies in Childhood (ISAAC) questionnaires during up to 3 years of follow-up and compared to a reference group that reported never wheeze, remitted wheeze, or persistent wheeze. Specific immunoglobulin (Ig) E against cockroach, mouse, cat, and dust mite and total IgE levels were measured in sera at ages 5 and 7 years.
Results
PM2.5, soot-BC, and Abs* measured at the first visit were correlated moderately with those at the second visit (Pearson r > 0.44). Using logistic regression models, a positive association between PM2.5 and new wheeze was found with adjusted odds ratio [95% confidence intervals] of 1.51 [1.05–2.16] per interquartile range (IQR). Positive but nonsignificant association was found between the development of new wheeze and soot-BC and (OR 1.40 [0.96–2.05]), and Abs* (OR 1.57 [0.91–2.68]); Significantly positive associations were found between air pollutant measurements and new wheeze when restricting to those participants with repeat home indoor measurements 6 months apart. Associations between pollutants and IgE levels were not detected.
Conclusions
Our findings suggest that childhood exposure to indoor air pollution, much of which penetrated readily from outdoor sources, may contribute to the development of wheeze symptoms among children age 5 to 7 years.
Keywords: indoor air pollution, long-term exposure, PM2.5, black carbon, wheeze, asthma, young children
1. Introduction
The prevalence of pediatric asthma increased continuously from 8.7 to 9.6% during 2001–2009 in the United States (CDC, 2011) and is up to 28% in some areas like Northern Manhattan and the South Bronx (Nicholas et al., 2005). Children who reside in these communities have disproportionately high exposure to traffic emissions, in part related to neighboring truck routes and bus depots (Kinney et al., 2000; Spira-Cohen et al., 2009). Diesel emissions from traffic are composed mainly of elemental carbon (EC), organic carbon, and semi-volatile organic compounds, such as polycyclic aromatic hydrocarbons (PAHs), and are a major source for fine particulate matter less than 2.5 μm in diameter (PM2.5) (Zielinska et al., 2004; Qin et al., 2006).
Associations between exposures to traffic-related air pollution, in particular diesel soot and PM2.5, and asthma exacerbations and hospitalizations among children have been reported (McConnell et al., 2006; Bell et al., 2009; Patel et al., 2009; Spira-Cohen et al., 2011). However, only a limited number of studies have addressed prospectively whether such exposure is associated with the development of new childhood asthma over time. A prospective study in Southern California demonstrated that exposure to EC, PM2.5, acid vapors and nitrogen dioxide (NO2) was associated with subsequent deficits in lung function among children ages 10 to 18 years (Gauderman et al., 2004). A subsequent study revealed that traffic-related air pollution at school and home, estimated based on one central site monitor, increased the risk of developing incident asthma during 3 years of follow-up of kindergarten and first-grade children (McConnell et al., 2010). In a Mexico City cohort, exposure to PM less than 10 microns (PM10), NO2, and ozone, determined using stationary site monitors, was associated with lung function deficits over a three year follow-up period among elementary school aged children (Rojas-Martinez et al., 2007). Further a recent report from Health Effects Institute (HEI), that reviewed studies of traffic-related air pollution and asthma incidence in children, noted that there is “suggestive but insufficient” evidence for a causal relationship between exposure to traffic-related air pollution and asthma onset (HEI, 2010).
Long-term exposure to air pollution attributed to traffic emissions in relation to respiratory diseases has been based largely on indirect or derived indicators of exposure such as geographic information systems (GIS), monitoring at central sites, and calculations of distance to major roadways to estimate exposure ( Brauer et al., 2002; Janssen et al., 2003; Gauderman et al., 2005; Brauer et al., 2007; Nordling et al., 2008; Chang et al., 2009; Patel et al., 2011). These approaches may introduce exposure misclassification and attenuate effect estimates (Molitor et al., 2006). Further, they do not necessarily consider contributions from other sources such as emissions from industrial sites or indoor heating units. The use of direct measures of residential indoor air pollution could serve as a better prediction of actual exposure levels with less uncertainty. Also, our previous analysis of indoor to outdoor ratios within our cohort indicated a high penetration efficiency of traffic-related air pollutants originating outdoors, and the small presence of indoor sources of PM2.5 such as smoking, cooking, burning incense/candle and other activities (Jung et al., 2010a).
Soot, produced by incomplete combustion of fossil fuels, has been used as a surrogate tracer for urban diesel emissions (Janssen et al., 2001), although any incomplete combustion source can produce soot. Soot can be measured by different instrumental techniques. In thermo-optical methods, the resultant measurements are referred commonly to elemental carbon (EC). In optical methods, light reflectance measured using a smoke stain reflectometer is called typically an absorbance coefficient (Abs), while in wavelength dependent optical methods the term used is soot-black carbon (BC) (Janssen et al., 2001; Lawless et al 2004; Spira-Cohen et al., 2009). The terms EC, Abs, and BC often are used interchangeably in cohort studies because EC has been shown to correlate highly with the optical methods (Kinney et al., 2000; Janssen et al., 2001; Lena et al., 2002). However, at high filter loadings, the relationship between Abs and BC is not linear due to saturation of the filter (Taha et al., 2007; Yan et al 2011). The brown carbon generated from second hand smoke and traffic-related aromatics are indistinguishable to the smoke stain reflectometer method (Andreae and Gelencser, 2006). Novel approaches using multi-wavelength optical measurements to determine “soot-BC” concentrations from filters have been developed to capture a larger spectrum of exposure, especially in urban areas where BC may be high (Lawless et al., 2004; Yan et al., 2011). In this study, we measured soot-BC that could serve as a better marker for traffic-generated emissions.
We hypothesized that residential measurements of PM2.5, soot-BC and Abs among inner city young children living in Northern Manhattan and the South Bronx would be associated with the development of new wheeze and seroatopy. Our approach, using a longitudinal birth cohort being carried out by the Columbia Center for Children’s Environmental Health (CCCEH), was to conduct two-week residential monitorings for 5–6 year old children repeated six months apart to assess childhood exposure to traffic-related air pollutants using more novel approaches, including multi-wavelength optical measures. We then examined associations between pollutant levels and subsequent new onset of respiratory symptoms and indoor allergen specific immunoglobulin (Ig) E at age 7 years after controlling for known covariates.
2. Methods and Materials
2.1. Study population
727 children of Dominican or African American mothers who reside in Northern Manhattan or the Bronx were enrolled into the CCCEH cohort ( Perera et al., 2002; Miller et al., 2004). Children (n=408) who reached age 5–6 years between October 2005 and May 2011 underwent residential air pollution monitoring. Data were analyzed for those children (n=349) who underwent residential monitoring and had completed questionnaire data through age 6 or 7 years. The study was approved by the Institutional Review Board of Columbia University Medical Center.
2.2. Measurements and exposure assessment
For measurement of PM2.5, filters were pre- and post-weighed on a microbalance after equilibration under a temperature (72 ± 2 °F) and humidity (40% ± 2% Relative Humidity, RH) controlled environment for 48 hours. The metric of diesel exposure is based on optical measurements of soot-BC from Teflon filters. Soot-BC was determined by using a novel multi-wavelength integrating sphere method to achieve maximum specificity over a large range of ambient pollutants levels (Lawless et al., 2004; Yan et al., 2011). In addition, reflectance readings were obtained using the EEL Smoke Stain Reflectometer (Diffusion Systems Ltd., London), and converted to modified Abs (referred as Abs*) as described (Kinney et al., 2002).
Two-week integrated indoor PM2.5 measures, soot-BC using multi-wavelength techniques, and Abs* using reflectance measurement were collected at each of the first 262 homes between October 2005 and May 2011, with a repeat air sampling performed 6 months (6.3 months ± 0.7; mean ± standard deviation) later to capture the seasonal variability in air pollution levels. Indoor air monitors were placed in a room where the child spent most of his or her time (mostly the room where the child sleeps). The average of two indoor pollutant measurements (Time 1 versus Time 2) was calculated to represent yearlong exposure of PM2.5, soot-BC, and Abs*. For an additional n=146 homes, only one two-week measure was collected because of budgetary constraints and analyzed unless where specified. Details of exposure assessment have been reported previously (Jung et al., 2010a; Jung et al., 2010b; Yan et al., 2011)
2.3. Definition of new wheeze
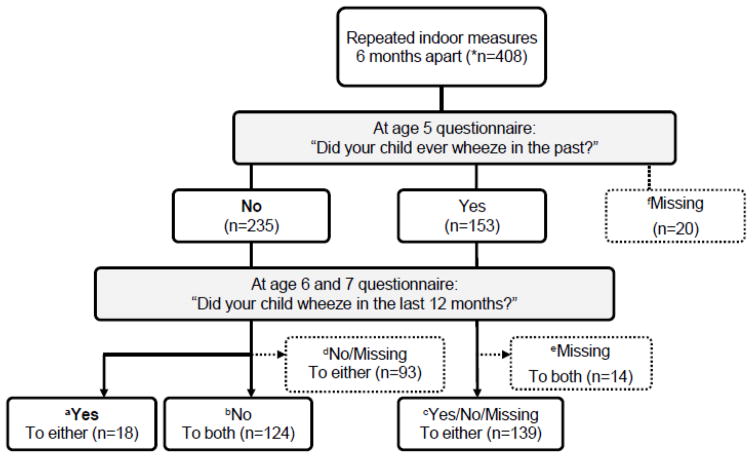
Respiratory symptoms at ages 5, 6, and 7 were queried from the International Study of Asthma and Allergy in Childhood (ISAAC) questionnaire administered to the mothers (Asher, 1998). The development of new wheeze was defined as reporting “no” at age 5 to the ISAAC question: “Did your child ever wheeze in the past?” AND “yes” to the question at age 6 or 7: “Did your child wheeze in the last 12 months?” (Fig. 1-a; n=18). The reference group was defined as breporting “no” to ever wheeze at age 5 and “no” to wheeze in the last 12 months at age 6 and 7 (Fig. 1-b; never wheeze n=124), or creporting “yes” to ever wheeze at age 5 and having at least one valid questionnaire data at age 6 or 7 (Fig. 1-c; persistent or remitted wheeze n=139). Because of the prospective design, completing at least one questionnaire at age 6 or 7 was required, and missing cases of 127 were excluded from the analyses (Fig. 1-d, e, f. Missing).
Fig. 1. Schematic demonstration of study definition of new wheeze at age 6 or 7 yr.
*n=408: Both Time 1 and 2 (n=262), Time 1 only (n=146); aCase of new wheeze (n=18) is defined as reporting “no” to “ever wheeze” at age 5 and “yes” to “wheeze in the last 12 months” at age 6 or 7. Reference group (n=263) is defined as breporting “no” to “ever wheeze” at age 5 and “no” to “wheeze in the last 12 months” at age 6 and 7 (Fig. 1-b; n=124), or creporting “yes” to “ever wheeze” at age 5 and having at least one valid questionnaire data at age 6 or 7 (Fig. 1-c; n=139). Missing case (n=127) is defined as dreporting “no” to “ever wheeze” at age 5 and missing data at age 6 or 7 (Fig. 1-d; n=93), or ereporting “yes” to “ever wheeze” at age 5 and missing both questionnaire data at age 6 and 7 (Fig. 1-e; n=14) or fmissing data at age 5 (Fig. 1-f; n=20).
Seventy percent (286/408) of Time 1 air monitorings were set up before the age 6 questionnaire was administered (average ± standard deviation; 9.2 months ± 3.7 prior to age 6 questionnaire).
2.4. Specific and total IgE
Sera allergen-specific IgE levels against cat, mouse, dog, Dermatophagoides farinae and German cockroach and total IgE levels at ages 5 (n=322) and 7 (n=266) were measured in duplicate using ImmunoCAP (Chang et al., 2010; Donohue et al., 2008). Children were considered seroatopic if they had a specific IgE ≥0.35 IU/mL to any of the indoor allergens tested. New seroatopy was defined as being seroatopic at age 7, but not age 5.
2.5. Statistical analysis
Chi-squared tests were used to compare prevalence between groups. Associations between indoor pollution levels and the development of new wheeze (yes/no) and specific seroatopy were analyzed by multiple logistic models adjusting for previously identified confounding variables (Miller et al., 2004; Patel et al., 2009). Odds ratios (OR) are presented for an interquartile range (IQR) increase in concentrations, equivalent to 8.75 μg/m3, 0.60 μg/m3 and 0.31 m−1×10−5 for PM2.5, soot-BC, and Abs*, respectively. Models examining the associations between indoor PM2.5, soot-BC and Abs* and the development of new wheeze were adjusted for the following confounding variables: ethnicity, sex, maternal asthma, prenatal environmental tobacco smoke (ETS), postnatal ETS, and cold/influenza (flu) season as described previously (Miller et al., 2004; Patel et al., 2009). A dichotomous variable that indicated whether the residential monitoring time occurred prior to or following the age 6 questionnaire also was included in the model to adjust for age-related differences in exposure assessment. Any controller or rescue medication reported at ages 5, 6, or 7 was identified, and two separate binary variables indicating whether children were on controller medication (yes/no) and on rescue medication (yes/no) were included in the models. Differences in total IgE levels, after log transformation, between ages 5 to 7 and pollutants were modeled using multiple linear regressions. When evaluating the associations between indoor air pollutants and IgE outcomes, the aforementioned confounding variables except flu season variable were included in the models.
Three sensitivity analyses were conducted as follows: 1) Reanalysis after removing two extreme pollution data points, one high and one low; 2) Exclusion of subjects that have a missing questionnaire record for “Did the child wheeze in the last 12 months?” either at age 6 or 7 when defining the reference group (Fig. 1-c. n=37/139) and 3) Exclusion of subjects with pre-existing wheeze because they answered “yes” to “Did your child ever wheeze in the past?” at age 5 (Fig. 1-c. n=139) from the reference group. All statistical analyses were performed using SPSS version 18 (Chicago, IL).
3. Results
3.1. Study cohort characteristics
Demographic characteristics of children are shown in Table 1. Children who had residential air monitoring and complete questionnaire data did not differ demographically from CCCEH children whose data were not analyzed in this study.
Table 1.
Demographic characteristic of the study cohort [no. (%)]a.
| Characteristic | Subjects includedb | Subjects excludedc |
|---|---|---|
| N=349 | N=378 | |
| N/total (%) | N/total (%) | |
| Maternal ethnicity | ||
| Dominican | 221/349 (63) | 252/378 (67) |
| African American | 128/349 (37) | 126/378 (33) |
| Girls | 192/349 (55) | 184/378 (49) |
| Maternal high school or greater degree | 221/348 (64) | 235/365 (64) |
| Maternal asthma (+) | 84/348 (24) | 79/378 (21) |
| On Medicaid during pregnancy | 320/348 (92) | 337/375 (90) |
| Prenatal ETS exposure (+) | 106/346 (31) | 140/371 (38) |
| Postnatal ETS exposure (+) | 153/349 (44) | 135/315 (43) |
None of the demographics differed significantly between the children included when compared to those excluded;
Includes participants who underwent residential monitoring and had questionnaire data at ages 5 and 6 or 7yrs;
Due to missing data. N=319 CCCEH subjects did not undergo childhood air monitoring study and n=59 had incomplete questionnaire data.
Thirty-nine percent (Fig. 1. 153/388) of the children were reported as having at least one wheezing episode during the first 5 years of life according to the ISAAC question “Did your child ever wheeze in the past?” at age 5. Among the 153 children with preexisting wheeze at age 5, 31.4% (48/153) of the children had wheeze prior to age 5. Six percent (18/281) of the children included in the models were identified as developing new wheeze when using a new “yes” to the ISAAC question “Did your child wheeze in the last 12 months?” at age 6 or 7 (Fig. 1, n=281; athe development of new wheeze (n=18), reference (n=263; i.e., never wheeze (Fig. 1-b. n=124) and, remitted or persistent wheeze (Fig. 1-c. n=139)). The proportion of new wheeze did not differ by sex, maternal asthma, prenatal ETS, and postnatal ETS exposure; however, Dominicans had a significantly higher proportion of new wheeze than African American (p=0.031).
3.2. Childhood exposure to PM2.5, soot-BC and Abs
Two-week averaged indoor concentrations of PM2.5, soot-BC, and Abs* measured at the first visit (Time 1) were correlated moderately with those measured at the second visit (Time 2) (Pearson r= 0.56, 0.44, 0.48 for PM2.5, soot-BC, and Abs*, p<0.001 for all, Table 2). When Time 1 and Time 2 measures are collected in the same season (either both in heating or nonheating season), stronger correlations of the measured air pollutant were found than those measured in the different season (e.g., Pearson r=0.57 in the same season; r=0.44 in the different season for soot-BC).
Table 2.
Correlation between time 1 (T1) and time 2 (T2) measures of indoor air pollutants over the 6 month time interval.
| N | PM2.5 | Soot-BC | Abs | |
|---|---|---|---|---|
| Overall | 259 | 0.557*** | 0.443*** | 0.482*** |
| Seasona | ||||
| Same season | 42 | 0.688*** | 0.572*** | 0.523*** |
| Different season | 217 | 0.530*** | 0.436*** | 0.490*** |
Pearson correlation coefficient performed on log-transformed data;
p-value < 0.001;
indicator of whether T1 and T2 measures were collected in the same season (heating(Oct.~Apr.) or nonheating(May~Sept.) season) or in the different seasons.
3.3. Associations between air pollution and health outcomes
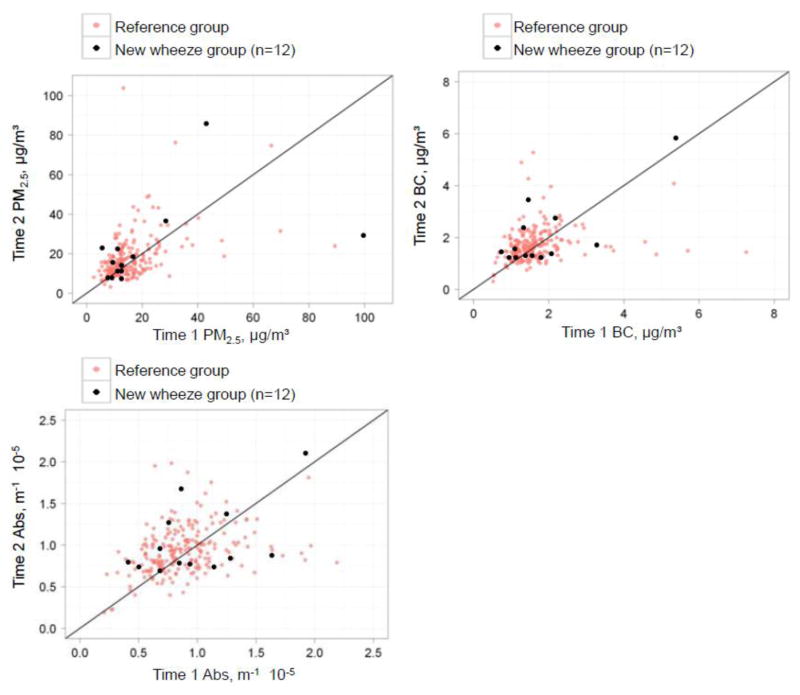
Cases of new wheeze and the reference are presented in Fig. 2. The median indoor PM2.5 and soot-BC concentrations for the new wheeze group were 12.2 μg/m3 and 1.42 μg/m3, and for the reference group were 13.8 μg/m3 and 1.50 μg/m3, respectively (Mann-Whitney test, p>0.05). Soot-BC measures correlated strongly with calculated Abs* (Pearson r=0.95, p<0.001); however, curvature was observed at high filter loadings (e.g., 5% of data) as reported previously (Yan et al., 2011). Median indoor Abs* for the new wheeze group was not statistically different from the reference group (0.86 vs. 0.85 m−1x10−5, Mann-Whitney test, p>0.05).
Fig. 2. Repeated indoor measures of traffic-related air pollutants: Time 1 versus Time 2, 6 months apart. There are 12 cases of new wheeze among those with both time 1 and time 2 available.
The development of new wheeze was defined as reporting “no” to ever wheeze at age 5 AND “yes” to wheeze in the last 12 months at age 6 and 7. Reference group was defined as reporting “no” to ever wheeze at age 5 and “no” to wheeze in the last 12 months at age 6 and 7, or reporting “yes” to ever wheeze at age 5 and having at least one valid questionnaire data at age 6 or 7.
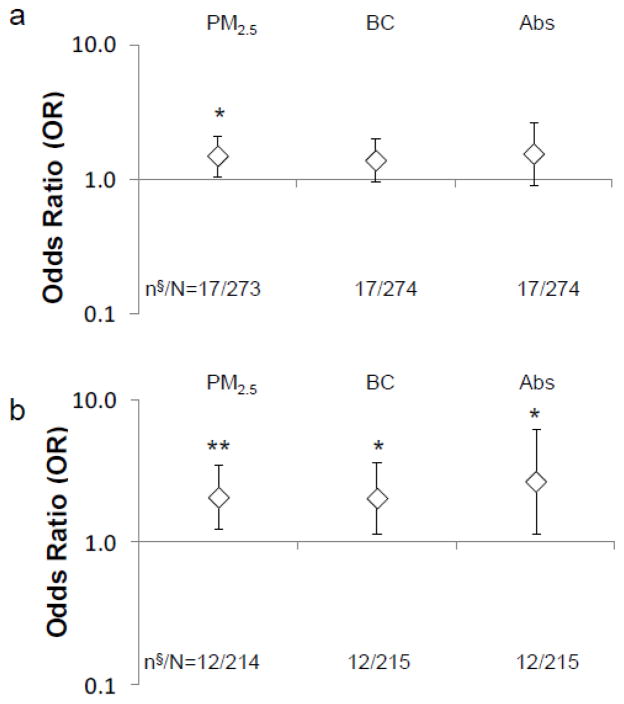
After controlling for confounding variables using logistic regression, significantly higher odds of new wheeze was associated with higher PM2.5 (OR 1.51, 95% CI [1.05–2.16], p=0.021) (Fig. 3-a). In addition, a positive but non-significant association was found between the development of new wheeze and soot-BC and (OR 1.40, 95% CI [0.96–2.05], p=0.085), and Abs* (OR 1.57, 95% CI [0.91–2.68], p=0.102). Furthermore, analyses were repeated after excluding subjects without repeat pollution measurements 6 months apart. Significantly positive associations with new wheeze were found for PM2.5 (OR 2.09, 95% CI [1.25–3.49], p<0.01), soot-BC (OR 2.03, 95% CI [1.16–3.56], p=0.013) and Abs* (OR 2.70, 95% CI [1.15–6.30], p=0.022) (Fig. 3-b).
Fig. 3. Associations between indoor air pollution concentrations and new wheeze between age 5 and age 6–7: (a) overall subjects (n§=17: cases of new wheeze; n§=1 lost due to a missing covariate; N=273 after excluding invalid exposure measures (n=8)) and (b) subjects with both time 1 and time 2 measures available (n§=12:cases of new wheeze; N=214 after excluding subjects with time 1 measure only (n=59) from overall subjects included in Fig. 3-a).
The data points and error bars describe the odds ratio (OR) and 95% confidence interval (CI), respectively, for an increase in risk of developing new wheeze at age 6–7, adjusting for ethnicity, sex, maternal education, maternal asthma, prenatal ETS exposure, postnatal ETS exposure, cold/flu season, residential monitoring conducted prior to age 6, controller medicines use at any time point between ages 5–7, and rescue medicines use at any time point between ages 5–7; *p<0.05 and **p<0.01
In addition, because previous literature reported an association between exposure to diesel exhaust particles and the development of seroatopy (Herr et al., 2010; Miller et al., 2009), we assessed whether residential levels of PM2.5, soot-BC and Abs* were associated with increases in total IgE or development of new seroatopy to a specific allergen. Seventy-two percent (117/163) of the children experienced any increase in total IgE from age 5 to 7, with the geometric mean of change of 1.73 IU/mL. Fourteen percent (25/176) of the children developed new seroatopy to any specific indoor allergen and nineteen percent (31/163) of children developed higher total IgE. Exposure to PM2.5, soot-BC and Abs* was not associated with increases in total IgE between ages 5 and 7 (effect estimates of −0.29, −0.32, and −0.54 and for PM2.5, soot-BC, and Abs* respectively) nor with a new positive IgE against indoor allergens.
3.4. Sensitivity analyses
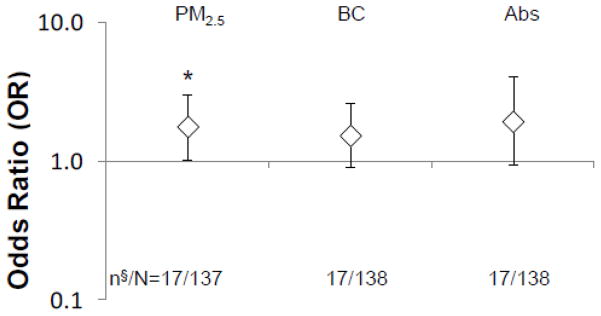
After one extremely high (96.6 μg/m3, 5.61 μg/m3 and 2.01 m−1x10−5 for PM2.5, soot-BC and Abs*) and one extremely low (3.53 μg/m3, 0.30 μg/m3 and 0.07 m−1x10−5 for PM2.5, soot-BC and Abs*) data points were removed from the dataset, a positive significant association with the development of new wheeze remained for PM2.5 (OR 1.69, 95% CI [1.10–2.59], p=0.018). After excluding 37 data points (Fig. 1-d: 24 cases with missing questionnaire data at age 7, 12 cases with missing questionnaire data at age 6, and Fig. 1-a: 1 new wheeze case identified at age 6 with missing questionnaire data at age 7), significant associations between PM2.5 and the development of new wheeze also remained (OR 1.74, 95% CI [1.11–2.71], p=0.017) and positive but non-significant associations for soot-BC (OR 1.45, 95% CI [0.96–2.20]) and Abs* (OR 1.62, 95% CI [0.90–2.91]) were found. Models displayed in Figure 3-(a) were robust to exclusion of pre-existing wheeze cases (Fig. 1-c; n=139) from the reference group (Fig. 4. OR 1.78, 95% CI [1.03–3.08], p=0.041 for PM2.5; OR 1.54, 95% CI [0.90–2.62], p=0.12 for soot-BC; OR 1.94, 95% CI [0.93–4.04], p=0.076 for Abs*).
Fig. 4. Sensitivity analysis for the development of new wheeze among wheeze-free children at age 5. n§=17: cases of the development of new wheeze; N=137 after excluding pre-existing wheeze at age 5 (n=139) and invalid exposure measures (n=8).
The data points and error bars describe the odds ratio (OR) and 95% confidence interval (CI), respectively, for an increase in risk developing new wheeze at age 6–7, adjusting for ethnicity, sex, maternal education, maternal asthma, prenatal ETS exposure, postnatal ETS exposure, cold/flu season, residential monitoring conducted prior to age 6, controller medicines use at any time point between ages 5–7, and rescue medicines use at any time point between ages 5–7; *p<0.01.
Discussion
In this prospective birth cohort of children from New York City (NYC), a positive association was found between measured residential indoor levels of PM2.5 and the development of new wheeze among children age 5 to 7 years old. Also a positive but non-significant association was observed between soot-BC exposure and new wheeze suggesting that the black carbon, used as a tracer for diesel exhaust particle (DEP) emission, may be an important constituent component of PM2.5. The larger effect size for PM2.5 when compared to soot/BC can be interpreted as that other chemical components of PM2.5 (such as PAHs and metals, that can be emitted from both traffic and space heating source emissions) may also contribute to the risk of developing wheeze (Miller et al., 2004; Patel et al., 2010; Jung, 2011a). Overall these results suggest that children who are exposed to high indoor air pollution during childhood may be at higher risk of developing new wheeze, a symptom characteristic of asthma, at young ages.
The strengths of this study include the longitudinal design. Another was the direct measurement of each child’s exposure based on home indoor samples to multiple air pollutants associated with traffic, industrial and heating sources. Analyses of median indoor to outdoor ratios (I/O ratio) reported previously by this group and others suggest that these pollutants readily penetrate from outside to inside (Kinney et al., 2002; Jung et al., 2010a). Specifically, the ratio that was slightly greater than 1.0 for PM2.5 and close to 1 for Abs* across season, indicated a small presence of indoor emission sources of PM2.5 and a high penetration efficiency of outdoor-originated black carbon into the indoor environment (Jung et al., 2010a) exposing children in this cohort. Few pediatric cohort studies to date have measured levels of PM2.5, BC, Abs* or other traffic related air pollutants as directly as performed in this study with the use of residential monitors. In one, a 6.2 ppb increase in residential outdoor NO2 concentration was associated with 1.29 times higher risk for incident asthma in children 10–18 years of age (Jerrett et al., 2008). A birth cohort study in Syracuse, NY reported that residential indoor PM2.5 levels, dichotomized at 15 μg/m3 (i.e. the US EPA long-term outdoor standard for PM2.5), was associated with elevated risk for infant wheezing (Hunt et al., 2011). Negative findings instead were reported in a birth cohort study from Denmark where 1-week integrated residential indoor PM2.5 and Abs levels were not associated with wheezing symptoms in infants (Raaschou Nielsen et al., 2010). While GIS variables have been used as a surrogate for air pollution mixtures, direct measurement of individual pollutants through residential monitoring conducted in the child’s major microenvironment (e.g., bed room, or playground) may reduce misclassification and possible confounders such as building types or floor height shown in our previous study (Jung et al., 2011b), allowing us to detect a smaller effect size, and to discern effects of multiple air pollutants that may be responsible for adverse respiratory health outcomes.
The present study employed repeat measures of two-week integrated indoor air pollution to represent long-term exposure in childhood. The two samples were collected 6 months apart to capture seasonal variations in air pollution exposure (Jung et al., 2010a). However, 36% (146/408) of the pollution exposure was based on a one time measure due to budgetary constraint. A moderate correlation between time 1 and 2 measures of PM2.5, BC, and Abs* was observed. Further, we found weaker correlations when the two sampling campaigns (time 1 and time 2) fell in different seasons (i.e. one in heating and the other one in nonheating season), indicating that indoor exposures to PM2.5, BC, and Abs* may vary by heating season. This result suggests that the use of two measurements may allow us to capture longer/year long exposure level. Supporting this explanation, stronger and significant positive associations for PM2.5, soot-BC, and Abs* were detected when the data analysis was restricted to those children who have two time measurements (Figure 3-b). Altogether, this result may indicate that the average of two time measurements 6 months apart produce better long-term exposure estimate especially for soot-BC and Abs* whose concentrations can be influenced significantly by heating season as shown in our previous study (Jung et al., 2010a).
Although measures of Abs* commonly are used as reliable surrogates for diesel traffic emission in cohort studies, the assumption of a linear dose-response relationship at higher filter loadings is not always valid (Taha et al., 2007; Yan et al. 2011). In this analysis, a new but validated direct measure of BC based on multi-wavelength optics was employed. The soot-BC measures correlated strongly with the Abs levels, and both trended towards an association with the development of new wheeze. But the confidence intervals were wider for analyses using Abs than soot-BC (Figure 3-a, b), suggesting that individual measures of soot-BC using multi-wavelength optics may yield less measurement error and better characterization of exposures in this urban environment.
Previous studies have reported potential associations between diesel exposure and allergic symptoms in children, presumably as a consequence of upregulation of allergic sensitization ( Diaz-Sanchez et al., 1997; Brauer et al., 2002; Brauer et al., 2007; Morgenstern et al., 2008). In the present study, associations between PM2.5, soot-BC, and Abs exposure and incidence of seroatopy to indoor allergens between age 5 and 7 were nonsignificant. These results are consistent with those reported by Morgenstern et al. who observed a lack of an association between ambient Abs, PM2.5, and distance to nearest main road and sensitization to indoor (house dust mite, cat, dog, and molds) allergens. Interestingly, they did observe an increased risk for sensitization with outdoor (timothy grass, rye, birch, and mugwort) allergens that were not assessed in our study (Morgenstern et al., 2008).
We acknowledge several limitations to this study. For one, exposure to pollution at nonresidential environments, such as schools, where children may spend considerable time, was not measured. However, young children at this age in general spend much of their time at home, especially during winter months (Xue et al., 2004). Second, the prevalence of new wheeze in this cohort during this period was low (6%), indicating that the analysis relied on very few cases. Similarly, it is possible that the observed associations may be driven particularly by extremely high and low concentrations and thus the estimates would not be stable. However, the association between PM2.5 and the development of new wheeze was sustained after removing extreme concentrations. Third, the majority of the monitorings were conducted at age 5 (68%); although 32% were monitored at age 6. To address this limitation, we controlled age-related differences in exposure assessment in the model. Also, only 70% of Time 1 air monitoring was conducted before age 6 questionnaire outcome was administered. Given moderate correlations between two measurements, average measures 6 months apart seem to still reflect chronic exposure with sufficient resolution. While it is also important to understand other wheeze phyenotypes (persistent wheeze, remitted (transient) wheeze, never wheeze), we did not compare each group with each other adequately due to an insufficient statistical power. Lastly, the analysis included pre-existing wheeze cases in the reference group, that may bias the result toward the null. However, the sensitivity analysis revealed that the associations of the development of new wheeze and air pollutions remained essentially the same even after excluding pre-existing wheeze cases.
Conclusions
An increased risk of developing new wheeze from higher indoor PM2.5 exposure among children age 5–7 was observed in this inner city cohort. The data suggest that the BC fraction of the PM 2.5 may be a relevant contributor, especially given their common sources of emissions in NYC from motor vehicles and space heating. This finding provides new evidence that childhood exposure to indoor air pollution, mainly penetrated from traffic emissions outside, may be associated with the development of new wheeze symptoms among children age 5 to 7 years.
Highlights.
Repeated measures, 6 months apart, may reflect reasonably chronic exposure to air pollution.
High PM2.5 levels may be associated with the development of new wheeze among young children.
Soot-BC may be the health relevant constituent of PM2.5.
Associations between traffic-air pollution and allergy symptoms were not found.
Acknowledgments
This work was supported by NIH (R01ES01363, P01ES09600, R01ES08977, P30ES09089), EPA (R827027), the Educational Foundation of America, the John & Wendy Neu Family Foundation, the New York Community Trust, and the Trustees of the Blanchette Hooker Rockefeller Fund.
Abbreviations
- Abs
Absorption Coefficient
- BC
Black Carbon
- CCCEH
Columbia Center for Children’s Environmental Health
- EC
Elemental Carbon
- ETS
Environmental Tobacco Smoke
- FEV1
Forced Expiratory Volume in One Second
- ISAAC
International Study of Asthma and Allergies in Childhood
- IgE
Immunoglobulin E
- IQR
Interquartile Range
- NO2
Nitrogen Dioxide
- OR
Odds Ratio
- PAHs
Polycyclic Aromatic Hydrocarbons
- PM2.5
Particulate Matter Less Than 2.5 μm in Diameter
- PM10
P articulate Matter Less Than 10 μm in Diameter
Footnotes
Conflicts of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kyung Hwa Jung, Email: kj2237@columbia.edu.
Shao-I Hsu, Email: sh2919cu@gmail.com.
Beizhan Yan, Email: yanbz@ldeo.columbia.edu.
Kathleen Moors, Email: km2347@columbia.edu.
Steven N. Chillrud, Email: chilli@ldeo.columbia.edu.
James Ross, Email: jross@ldeo.columbia.edu.
Shuang Wang, Email: sw2206@columbia.edu.
Matthew S. Perzanowski, Email: mp2217@columbia.edu.
Patrick L. Kinney, Email: plk3@columbia.edu.
Robin M. Whyatt, Email: rmw5@columbia.edu.
Frederica Perera, Email: fpp1@columbia.edu.
Rachel L. Miller, Email: rlm14@columbia.edu.
References
- Andreae M, Gelencser A. Black carbon or brown carbon? The nature of light-absorbing carbonaceous aerosols. Atmos Chem Phys. 2006;6:3131–3148. [Google Scholar]
- Asher W. The international study of asthma and allergies in childhood (ISAAC) Clinical & Experimental Allergy. 1998;28:52–66. doi: 10.1046/j.1365-2222.1998.028s5052.x. [DOI] [PubMed] [Google Scholar]
- Bell ML, Ebisu K, Peng RD, Samet JM, Dominici F. Hospital Admissions and Chemical Composition of Fine Particle Air Pollution. Am J Respir Crit Care Med. 2009;179:1115. doi: 10.1164/rccm.200808-1240OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer M, Hoek G, Smit HA, De Jongste JC, Gerritsen J, Postma DS, Kerkhof M, Brunekreef B. Air pollution and development of asthma, allergy and infections in a birth cohort. EUR RESPIR J. 2007;29:879–888. doi: 10.1183/09031936.00083406. [DOI] [PubMed] [Google Scholar]
- Brauer M, Hoek G, Van Vliet P, Meliefste K, Fischer PH, Wijga A, Koopman LP, Neijens HJ, Gerritsen J, Kerkhof M. Air pollution from traffic and the development of respiratory infections and asthmatic and allergic symptoms in children. Am J Respir Crit Care Med. 2002;166:1092–1098. doi: 10.1164/rccm.200108-007OC. [DOI] [PubMed] [Google Scholar]
- CDC. Asthma Prevalence, Disease Characteristics, and Self-Management Education—United States, 2001–2009. Centers for Disease Control and Prevention. Morbidity and Mortality Weekly Report (MMWR); 2011. [PubMed] [Google Scholar]
- Chang C, Gauvey-Kern K, Johnson A, Kelvin EA, Chew GL, Perera F, Miller RL. Cord blood versus age 5 mononuclear cell proliferation on IgE and asthma. Clinical and Molecular Allergy. 2010;8:11. doi: 10.1186/1476-7961-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Delfino RJ, Gillen D, Tjoa T, Nickerson B, Cooper D. Repeated respiratory hospital encounters among children with asthma and residential proximity to traffic. J Occup Environ Med. 2009;66:90–98. doi: 10.1136/oem.2008.039412. [DOI] [PubMed] [Google Scholar]
- Diaz-Sanchez D, Tsien A, Fleming J, Saxon A. Combined diesel exhaust particulate and ragweed allergen challenge markedly enhances human in vivo nasal ragweed-specific IgE and skews cytokine production to a T helper cell 2-type pattern. The Journal of Immunology. 1997;158:2406–2413. [PubMed] [Google Scholar]
- Donohue KM, Al-alem U, Perzanowski MS, Chew GL, Johnson A, Divjan A, Kelvin EA, Hoepner LA, Perera FP, Miller RL. Anti-cockroach and anti-mouse IgE are associated with early wheeze and atopy in an inner-city birth cohort. J Allergy Clin Immunol. 2008;122:914–920. doi: 10.1016/j.jaci.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauderman WJ, Avol E, Gilliland F, Vora H, Thomas D, Berhane K, McConnell R, Kuenzli N, Lurmann F, Rappaport E, Margolis H, Bates D, Peters J. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med. 2004;351:1057–1067. doi: 10.1056/NEJMoa040610. [DOI] [PubMed] [Google Scholar]
- Gauderman WJ, Avol E, Lurmann F, Kuenzli N, Gilliland F, Peters J, McConnell R. Childhood asthma and exposure to traffic and nitrogen dioxide. Epidemiology. 2005;16:737–743. doi: 10.1097/01.ede.0000181308.51440.75. [DOI] [PubMed] [Google Scholar]
- HEI. Traffic-related air pollution: A critical review of the literature on emission, exposure, and health effects. Health Effects Institute; 2010. Special report 17. [Google Scholar]
- Herr CEW, Ghosh R, Dostal M, Skokanova V, Ashwood P, Lipsett M, Joad JP, Pinkerton KE, Yap PS, Frost JD. Exposure to air pollution in critical prenatal time windows and IgE levels in newborns. Pediatric Allergy and Immunology. 2010 doi: 10.1111/j.1399-3038.2010.01074.x. [DOI] [PubMed] [Google Scholar]
- Hunt A, Crawford J, Rosenbaum P, Abraham J. Levels of household particulate matter and environmental tobacco smoke exposure in the first year of life for a cohort at risk for asthma in urban Syracuse, NY. Environment International. 2011;37:1196–1205. doi: 10.1016/j.envint.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Janssen NAH, Brunekreef B, Van Vliet P, Aarts F, Meliefste K, Harssema H, Fischer P. The relationship between air pollution from heavy traffic and allergic sensitization, bronchial hyperresponsiveness, and respiratory symptoms in Dutch schoolchildren. Environ Health Perspect. 2003;111:1512–1518. doi: 10.1289/ehp.6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen NAH, van Vliet PHN, Aarts F, Harssema H, Brunekreef B. Assessment of exposure to traffic related air pollution of children attending schools near motorways. Atmos Environ. 2001;35:3875–3884. [Google Scholar]
- Jerrett M, Shankardass K, Berhane K, Gauderman WJ, Kunzli N, Avol E, Gilliland F, Lurmann F, Molitor JN, Molitor JT, Thomas DC, Peters J, McConnell R. Traffic-related air pollution and asthma onset in children: a prospective cohort study with individual exposure measurement. Environ Health Perspect. 2008;116:1433–1438. doi: 10.1289/ehp.10968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KH, Patel MM, Moors K, Kinney PL, Chillrud SN, Whyatt R, Hoepner L, Garfinkel R, Yan B. Effects of heating season on residential indoor and outdoor polycyclic aromatic hydrocarbons, black carbon, and particulate matter in an urban birth cohort. Atmos Environ. 2010a;44:4545–4552. doi: 10.1016/j.atmosenv.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KH, Yan B, Chillrud SN, Perera FP, Whyatt R, Camann D, Kinney PL, Miller RL. Assessment of Benzo (a) pyrene-equivalent Carcinogenicity and Mutagenicity of Residential Indoor versus Outdoor Polycyclic Aromatic Hydrocarbons Exposing Young Children in New York City. International Journal of Environmental Research and Public Health. 2010b;7:1889. doi: 10.3390/ijerph7051889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KH, Yan B, Hsu S, Moors K, Chillrud SN, Whyatt R, Perzanowski M, Hoepner L, Goldstein I, Zhang B, Camann D, Kinney PL, Perera F, Miller RL. Prenatal and Early Childhood Exposure to Polycyclic Aromatic Hydrocarbons and Asthma: Effect of Seroatopy. 2011a doi: 10.1016/j.anai.2012.07.019. Submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KH, Bernabé K, Moors K, Yan B, Chillrud SN, Whyatt R, Camann D, Kinney PL, Perera FP, Miller RL. Effects of Floor Level and Building Type on Residential Levels of Outdoor and Indoor Polycyclic Aromatic Hydrocarbons, Black Carbon, and Particulate Matter in New York City. Atmosphere. 2011b;2:96–109. doi: 10.3390/atmos2020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney P, Aggarwal M, Northridge M, Janssen N, Shepard P. Airborne concentrations of PM (2.5) and diesel exhaust particles on Harlem sidewalks: a community-based pilot study. Environ Health Perspect. 2000;108:213. doi: 10.1289/ehp.00108213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney PL, Chillrud SN, Ramstrom S, Ross J, Spengler JD. Exposures to multiple air toxics in New York City. Environ Health Perspect. 2002;110(Suppl 4):539–546. doi: 10.1289/ehp.02110s4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawless PA, Rodes CE, Ensor DS. Multiwavelength absorbance of filter deposits for determination of environmental tobacco smoke and black carbon. Atmos Environ. 2004;38:3373–3383. [Google Scholar]
- Lena TS, Ochieng V, Carter M, Holguín-Veras J, Kinney PL. Elemental carbon and PM2.5 levels in an urban community heavily impacted by truck traffic. Environ Health Perspect. 2002;110:1009–1016. doi: 10.1289/ehp.021101009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell R, Berhane K, Yao L, Jerrett M, Lurmann F, Gilliland F, Künzli N, Gauderman J. Traffic, susceptibility, and childhood asthma. Environ Health Perspect. 2006;114:766. doi: 10.1289/ehp.8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell R, Islam T, Shankardass K, Jerrett M, Lurmann F, Gilliland F, Gauderman J. Childhood Incident Asthma and Traffic-Related Air Pollution at Home and School. Environ Health Perspect. 2010;118:1021. doi: 10.1289/ehp.0901232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RL, Garfinkel R, Horton M, Camann D, Perera FP, Whyatt RM, Kinney PL. Polycyclic aromatic hydrocarbons, environmental tobacco smoke, and respiratory symptoms in an inner-city birth cohort. Chest. 2004;126:1071–1078. doi: 10.1378/chest.126.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RL, Garfinkel R, Lendor C, Hoepner L, Li Z, Romanoff L, Sjodin A, Needham L, Perera FP, Whyatt RM. Polycyclic aromatic hydrocarbon metabolite levels and pediatric allergy and asthma in an inner-city cohort. Pediatric Allergy and Immunology. 2009;21:260–267. doi: 10.1111/j.1399-3038.2009.00980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitor J, Molitor NT, Jerrett M, McConnell R, Gauderman J, Berhane K, Thomas D. Bayesian modeling of air pollution health effects with missing exposure data. Am J Epidemiol. 2006;164:69. doi: 10.1093/aje/kwj150. [DOI] [PubMed] [Google Scholar]
- Morgenstern V, Zutavern A, Cyrys J, Brockow I, Koletzko S, Kramer U, Behrendt H, Herbarth O, von Berg A, Bauer CP. Atopic diseases, allergic sensitization, and exposure to traffic-related air pollution in children. Am J Respir Crit Care Med. 2008;177:1331–1337. doi: 10.1164/rccm.200701-036OC. [DOI] [PubMed] [Google Scholar]
- Nicholas SW, Jean-Louis B, Ortiz B, Northridge M, Shoemaker K, Vaughan R, Rome M. Addressing the childhood asthma crisis in Harlem: the Harlem Children’s Zone Asthma Initiative. Am J Public Health. 2005;95:245. doi: 10.2105/AJPH.2004.042705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordling E, Berglind N, Melén E, Emenius G, Hallberg J, Nyberg F, Pershagen G, Svartengren M, Wickman M, Bellander T. Traffic-related air pollution and childhood respiratory symptoms, function and allergies. Epidemiology. 2008;19:401–408. doi: 10.1097/EDE.0b013e31816a1ce3. [DOI] [PubMed] [Google Scholar]
- Patel M, Hoepner L, Garfinkel R, Chillrud S, Reyes A, Quinn J, Perera F, Miller R. Ambient Metals, Elemental Carbon, and Wheeze and Cough in New York City Children through Age 24 Months. Am J Respir Crit Care Med. 2009;180:1107–1113. doi: 10.1164/rccm.200901-0122OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MM, Chillrud SN, Correa JC, Hazi Y, Feinberg M, KCD, Prakash S, Ross JM, Levy D. Traffic-Related Particulate Matter and Acute Respiratory Symptoms among New York City Area Adolescents. Environ Health Perspect. 2010;118:1338–1343. doi: 10.1289/ehp.0901499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MM, Quinn JW, Jung KH, Hoepner L, Diaz D, Perzanowski M, Rundle A, Kinney PL, Perera FP, Miller RL. Traffic density and stationary sources of air pollution associated with wheeze, asthma, and immunoglobulin E from birth to age 5 years among New York City children. Environmental Research. 2011;111(8):1222–1229. doi: 10.1016/j.envres.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Illman SM, Kinney PL, Whyatt RM, Kelvin EA, Shepard P, Evans D, Fullilove M, Ford J, Miller RL. The challenge of preventing environmentally related disease in young children: community-based research in New York City. Environ Health Perspect. 2002;110:197–204. doi: 10.1289/ehp.02110197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Kim E, Hopke PK. The concentrations and sources of PM2.5 in metropolitan New York City. Atmos Environ. 2006;40:312–332. [Google Scholar]
- Raaschou Nielsen O, Hermansen M, Loland L, Buchvald F, Pipper CB, Sørensen M, Loft S, Bisgaard H. Long term exposure to indoor air pollution and wheezing symptoms in infants. Indoor Air. 2010;20:159–167. doi: 10.1111/j.1600-0668.2009.00635.x. [DOI] [PubMed] [Google Scholar]
- Rojas-Martinez R, Perez-Padilla R, Olaiz-Fernandez G, Mendoza-Alvarado L, Moreno-Macias H, Fortoul T, McDonnell W, Loomis D, Romieu I. Lung function growth in children with long-term exposure to air pollutants in Mexico City. Am J Respir Crit Care Med. 2007;176:377–384. doi: 10.1164/rccm.200510-1678OC. [DOI] [PubMed] [Google Scholar]
- Spira-Cohen A, Chen L, Kendall M, Lall R, Thurston G. Personal exposures to traffic-related air pollution and acute respiratory health among Bronx school children with asthma. Environ Health Perspect:- doi. 2011;119:559–565. doi: 10.1289/ehp.1002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira-Cohen A, Chen LC, Kendall M, Sheesley R, Thurston GD. Personal exposures to traffic-related particle pollution among children with asthma in the South Bronx, NY. J Expo Sci Env Epid. 2009;20:446–456. doi: 10.1038/jes.2009.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha G, Box G, Cohen D, Stelcer E. Black carbon measurement using laser integrating plate method. Aerosol Science and Technology. 2007;41:266–276. [Google Scholar]
- Xue J, McCurdy T, Spengler J, Özkaynak H. Understanding variability in time spent in selected locations for 7–12-year old children. J Expo Sci Env Epid. 2004;14:222–233. doi: 10.1038/sj.jea.7500319. [DOI] [PubMed] [Google Scholar]
- Yan B, Kennedy D, Miller RL, Cowin JP, Jung K, Perzanowski M, Balletta M, Perera FP, Kinney PL, Chillrud SN. Validating a nondestructive optical method for apportioning colored particulate matter into black carbon and additional components. Atmos Environ. 2011 doi: 10.1016/j.atmosenv.2011.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinska B, Sagebiel J, McDonald JD, Whitney K, Lawson DR. Emission rates and comparative chemical composition from selected in-use diesel and gasoline-fueled vehicles. J Air Waste Ma. 2004;54:1138–1150. doi: 10.1080/10473289.2004.10470973. [DOI] [PubMed] [Google Scholar]