Abstract
Brown-Norway rats (n = 113) sensitized and challenged with nDer f 1 allergen were used to examine the contribution of lung sensory nerves to ozone (O3) exacerbation of asthma. Prior to their third challenge rats inhaled 1.0 ppm O3 for 8 hours. There were three groups: 1) control; 2) vagus perineural capsaicin treatment (PCT) with or without hexamethonium; and 3) vagotomy. O3 inhalation resulted in a significant increase in lung resistance (RL) and an exaggerated response to subsequent allergen challenge. PCT abolished the O3-induced increase in RL and significantly reduced the increase in RL induced by a subsequent allergen challenge, while hexamethonium treatment reestablished bronchoconstriction induced by allergen challenge. Vagotomy resulted in a significant increase in the bronchoconstriction induced by O3 inhalation and subsequent challenge with allergen. In this model of O3 exacerbation of asthma, vagal C-fibers initiate reflex bronchoconstriction, vagal myelinated fibers initiate reflex bronchodilation, and mediators released within the airway initiate bronchoconstriction.
Keywords: ozone, Der f 1, Brown-Norway rat, airway reactivity, vagus nerve
1. Introduction
The epidemiologic link between the photochemical air pollutant, ozone, and the exacerbation of asthma is supported by a wealth of research findings (EPA, 1996). Observations from controlled clinical exposure studies indicate that asthmatic subjects respond in a dose-dependent manner to ozone with an exaggerated bronchoconstrictive response (Horstman et al., 1995), an increased responsiveness to inhaled allergen (Jorres et al., 1996; Kehrl et al., 1999) and the exacerbation of eosinophilic inflammation (Hiltermann et al., 1997; Peden et al., 1997), while the ozone-induced reflex-mediated reduction in forced vital capacity was similar to healthy contols As important as these observations are in identifying asthmatic patients as a susceptible population to ozone inhalation these studies provide no information regarding the underlying mechanisms involved in ozone-induced asthma exacerbation.
The present study had two specific aims. The first specific aim was to examine whether acute ozone exposure of Brown-Norway rats sensitized and challenged with house dust mite (nDer f 1) allergen resulted in a greater ozone-induced bronchoconstriction and an exacerbated airway response to nDer f 1 allergen challenge. The second specific aim was to examine whether these ozone-induced airway responses are the result of an alteration in the activity profile of lung sensory nerves. Specifically, we determined which group of lung vagal afferents (myelinated vs. nonmyelinated) contributes to the airway responses (reflex vs non-reflex) associated with ozone-asthma interactions. This was accomplished by combining allergen sensitization and challenge, and acute ozone exposure with: 1) blocking vagal C-fiber conduction with perineural capsaicin treatment; 2) blocking parasympathetic ganglionic neurotransmission with hexamethonium; and 3) abolishing both myelinated and nonmyelinated sensory and parasympathetic conduction by bilateral vagotomy.
2. Materials and Methods
The animal care and use committee of the University of California, Davis, approved all procedures. One hundred thirteen 8 to 10 week old, male Brown-Norway rats were used in this study. The study had two phases, the first phase was designed to examine whether ozone-induced bronchoconstriction is exacerbated in Brown-Norway rats sensitized and challenged with Dermatophagoides farinae allergen (nDer f 1) and whether ozone inhalation exacerbates the airway responses to allergen challenge (n = 42). The second phase of the study examined the role of vagal afferent and efferent nerves in the responses identified in the first phase of the experiment (n = 71).
2.1 Sensitization to nDer f 1 allergen
Eight to 10 week old, male Brown-Norway rats received 5 ug of nDer f 1 in 250 ul of sterile saline (sensitized) or saline alone (sham). Rats were sensitized and challenged with nDer f 1 via intratracheal instillation as previously described (Singh et al., 2003). Two weeks later, rats were challenged with 10 ug of nDer f 1 in 250 ul of sterile saline (sensitized and challenged, SC), or saline alone (sham, SH), 3 times with each successive challenge separated by 1 week. Rats were studied on the day of the 3rd challenge. Natural Der f 1 allergen purified from D. farinae culture medium via affinity chromatography was obtained from Indoor Biotechnologies, Inc (Charlottesville, VA).
2.2 Ozone Exposure
Rats received either 8 hrs of 1-ppm ozone in filtered air (O3) or filtered air alone (Air) followed by Air for an additional 8 hours immediately prior to being studied. This exposure regimen resulted in 4 study groups: Air/SC; Air/SH; O3/SC; O3/SH. In brief, rats were placed singly in one of two 8L glass exposure chambers and estimates of respiratory frequency (f) and tidal volume (VT) were used to calculate minute ventilation (VE) as previously described (Schelegle et al., 2001).
2.3 Study Protocol
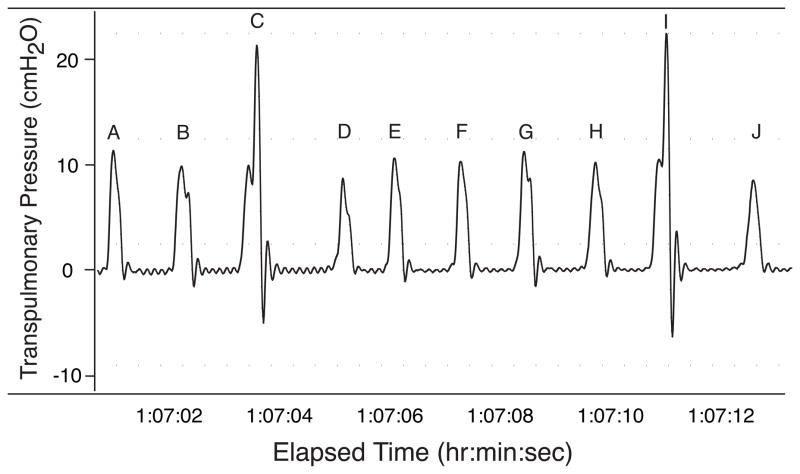
Immediately following the 8 hour post-ozone period or air exposure the rats were anesthetized with a solution of 2% alpha chloralose, 12.5% urethane and 5% borate (0.48 ml/100 g, i.p.). Arterial and venous catheters were placed for measurement of arterial blood gases, arterial blood pressure and injection of drugs. A14 gauge stainless-steel endotracheal cannula was surgically placed into the trachea just distal to the larynx. The tracheal cannula was attached to a Hans-Rudolph pneumotachometer (Series 8300) attached to a Validyne pressure transducer (model DP15-26). Transpulmonary pressure (PTP) was measured using a Validyne differential pressure transducer with one port attached to a water-filled cannula placed in the esophagus at mid-chest level and the other port attached to a side port of the pneumotachometer. Filtered air (2 L/min) was delivered to the rat using a blow-by system, consisting of a flowmeter and a plastic T that was attached to the pneumotachometer and flowmeter using plastic tubing. The remaining end of the T was vented to the room. Analog signals were sent to a Po-Ne-Mah data acquisition system (Gould Instrument Systems) and a MacLab data acquisition system (AD Instruments). Body temperature was recorded using a Physitemp rectal thermistor (model TH-8) and was maintained between 36 and 37 C through the use of a warm water blanket (American Medical Systems model K-20). The rats breathed spontaneously for the duration of the protocols. Breathing frequency (fb), tidal volume (VT), minute ventilation (VE), lung resistance (RL), heart rate (HR), and mean arterial blood pressure (Pa) were calculated and continuously monitored using the Po-Ne-Mah data acquisition system. Augmented breaths were counted prior to and following the instillation of nDer f 1 by replaying the digital signals recorded using MacLab data acquisition system. Augmented breaths were identified using the shape of the PTP signal. An augmented breath was defined as a breath that begins with a normal inspiratory effort that was then rapidly increased to a peak PTP (Fig. 1). Peak PTP was followed immediately with a rapid expiratory decrease in PTP (Fig. 1).
Figure 1.
Trace of transpulmonary pressure obtained from a Brown-Norway that has been sensitized and challenged with nDer f 1 and then exposed to 1.0 ppm ozone for eight hours. The trace contains ten breath excursions (a-j), two of these (c and i) meet the criteria for an augmented breath as a breath that begins with a normal inspiratory effort that was then followed by an additional rapid increased to a peak PTP.
2.4 Early and Late Phase Response to nDer f 1
The rats were given 30 minutes to stabilize after surgery before receiving their 3rd nDer f 1 or saline challenge as described above. Data were collected continuously for 6 hours following allergen challenge. Thirty-minute averages were utilized for data analysis.
2.5 Vagus Nerve Treatment
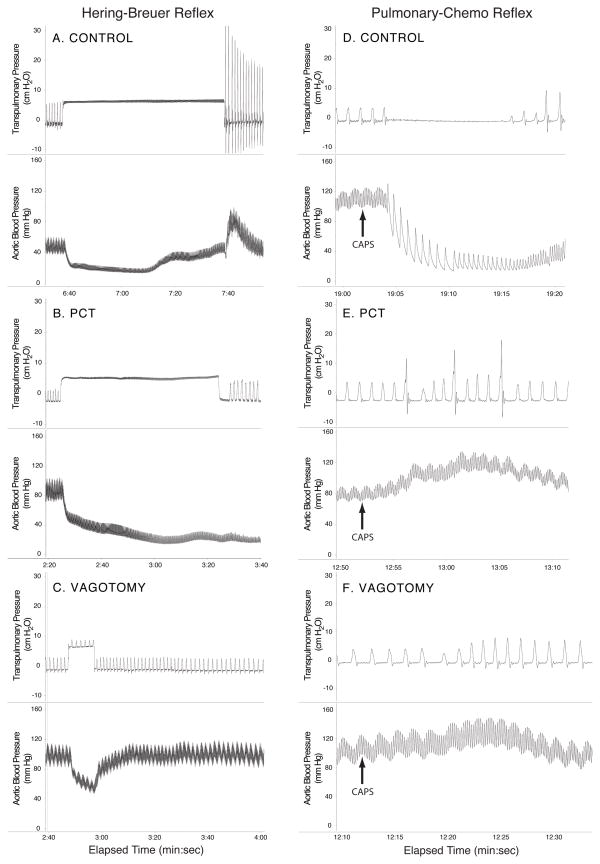
During surgical instrumentation both vagus nerves were dissected free of the surrounding tissue at mid-cervical level. Once exposed the vagus nerves were either left untreated (Control), topically treated with 1% capsaicin in olive oil for 1 minute (PCT), or surgically cut (Vagotomy) for the duration of the study. Breathing pattern was allowed to stabilize and a series of Hering-Breuer inflation reflexes (HBR) and pulmonary chemoreflex (PCR) were evoked to guarantee the selectivity of the nerve treatment (Fig. 2)(Mansoor et al., 1997). The Hering-Breuer inflation reflex was evoked by applying step-wise increases of 2.5, 5, and 10 cm H2O lung inflation pressures at the end of inspiration by occluding the outflow of the blow-by system and redirecting it through a pop-off valve set at the appropriate pressure. Ten ug/kg of capsaicin in 100 ul of saline was injected into the right atrium to evoke the PCR with 100 ul of saline serving as a control. After recovering from the HBR and PCR, data were collected for a 30min period before the rats were given their 3rd nDer f 1 or saline challenge. The early and late phase response to nDer f 1 instillation was examined in the Control groups by continuously collecting data for 6 hours following allergen challenge. In rats in which the effect of nerve treatment (PCT and Vagotomy) was examined data were collected continuously for 2 hours following allergen challenge. Thirty-minute averages were utilized for data analysis.
Figure 2.
Hering-Breuer inflation reflex (A–C) and pulmonary chemoreflex (D–F) obtained from Brown-Norway rats that were sensitized and challenged with the house dust mite allergen nDer f 1. Transpulmonary pressure (in cmH2O); Aortic blood pressure (in mmHg); and A and D: normal reflexes before perineural capsaicin treatment. C and F: reflexes after bilateral vagotomy. Note that perineural capsaicin abolished pulmonary C-fiber-induced pulmonary chemoreflex but not slowly adapting pulmonary stretch receptor induced Hering-Breuer reflex.
In a follow-up study the contributions of reflex mediated mechanisms in SC/O3/PCT treated rats were further examined using the ganglionic blocker, hexamethonium (Sigma-Aldrich Corp., St. Louis, MO, USA). In this study 20 mg/kg hexamethonium chloride (i.v.) was delivered 30 minutes after the HBR and PCR. After receiving hexamethonium data were collected for a 30 min period before the rats were given their 3rd nDer f 1 challenge. Data were collected continuously for 2 hours following allergen challenge.
2.6 Bronchoalveolar Lavage
Immediately following the experimental protocol the rats’ lungs were lavaged with 12 ml of sterile phosphate buffered saline using the method as previously described (Sterner-Kock et al., 1996). Lavage fluid was centrifuged and total protein in lavage was determined using a colormetric assay (BioRad, Inc, Hercules, CA, USA).
2.7 Calculations and Statistical Analysis
All data are expressed as mean ± standard error of the mean (SEM). The strengths of the HBR and PCR were determined as previously described (Mansoor et al., 1997). The strength of the HBR was expressed as an inhibitory ratio (IRHBR). The strength of the PCR was expressed as a percent change from baseline in HR (∂HR) and Pa (∂Pa) measured immediately prior to the injection of 10 ug/kg capsaicin into the right atrium.
In order to examine whether sensitization and challenge with nDer f 1 resulted in a greater ozone-induced bronchoconstriction, the mean RL data collected in the 30 minutes prior to the final allergen or saline instillation were compared using a two-way ANOVA. Grouping factors in this analysis were allergen treatment (SH versus SC) and exposure (Air versus O3). The values of RL following the final allergen or saline instillation were expressed as a percent change from pre-instillation baseline. To examine whether acute ozone exposure resulted in an exacerbated airway response to nDer f 1 allergen challenge, we compared the 12 consecutive 30 min (6 hours) mean RL values following the final allergen or saline instillation using a three-way ANOVA with repeated measures. As before, grouping factors in this analysis were allergen treatment (SH versus SC) and exposure (Air versus O3), while time after the final instillation was the repeated or within factor.
The contribution of lung vagal afferents (myelinated vs. nonmyelinated) to the airway responses (reflex vs non-reflex) associated with ozone-allergen interactions was examined using a three-way ANOVA. The three-way ANOVA compared the change in RL from 30 min prior to the final allergen or saline instillation to 30 min immediately following the final allergen or saline instillation. Grouping factors in this analysis were allergen treatment (SH versus SC), exposure (Air versus O3) and vagal treatment (Control versus PCT versus Vagotomy).
The contribution of ganglionic neurotransmission, hexamethonium treatment (HEX), to the airway responses associated with ozone-allergen interactions was examined using a one-way ANOVA to compare the change in RL. The groups compared in this one-way ANOVA were SC/O3, SC/O3/PCT and SC/O3/PCT/HEX.
The effects of ozone inhalation and/or allergen challenge on ventilatory and cardiovascular parameters were examined using MANOVA. The augmented breath data were evaluated using the nonparametric Kruskal-Wallis and Mann-Whitney U statistical tests (Statview).
Post hoc analysis was done only if we obtained a significant interaction term in the ANOVA or MANOVA tests. Mean differences between groups and mean differences within groups (repeated measures) were examined with test for least square difference with Tukey-Kramer correction applied for repeated tests (SAS). The result of a statistical test was considered significant if the calculated p value was equal to or less than 0.05.
3. Results
3.1 Sensitized and Challenge Brown-Norway Rats as a Model of Ozone-Induced Exacerbated Airway Responses
A total of 42 rats were studied in this phase of the investigation, divided into four groups: SH/Air (n = 11); SH/O3 (n = 11); SC/Air (n = 10); and SC/O3 (n = 10).
3.1.1 Breathing pattern response to ozone inhalation
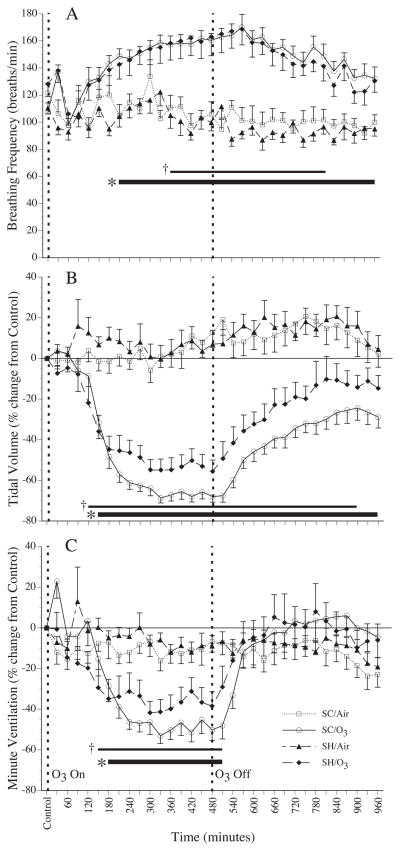
The inhalation of ozone resulted in the development of rapid shallow breathing in rats sham treated with saline and in rats sensitized and challenged with nDer f 1 allergen (Figure 3). In both groups there was a significant increase in fb, a decrease in VT and decrease in VE. Interestingly the decrease in VT in the rats sensitized and challenged with nDer f 1 allergen tended to be greater than that observed in rats sham treated with saline (p = 0.063). The observed ozone effects on breathing pattern in the SH and SC groups returned toward baseline in the 8 hour post-exposure period following the end of exposure. VE recovered completely, whereas fb and VT only partially recovered. The significant difference in VT between SC and SH groups exposed to ozone persisted through the post-exposure period.
Figure 3.
Mean breathing frequency (A), percent change in estimated tidal volume (B), and percent change in estimated minute ventilation (C) in Brown-Norway rats that were sensitized and challenged with nDer f 1 (SC, n=10) or sham treated with saline (SH, n=11) during 8 hours of exposure to 1.0 ppm ozone (O3) or filtered air (Air) that was followed by an 8 eight hour period in Air. Each point is a 30 minute average. * indicate a significant difference (p ≤ 0.05) between O3 and Air within SC and SH groups. † indicate a significant difference (p ≤ 0.05) between SC and SH groups exposed to O3. Values are mean ± sem.
3.1.2 Lung Resistance
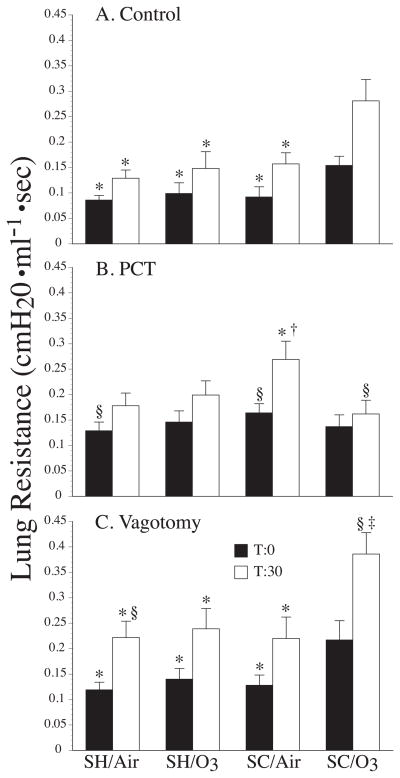
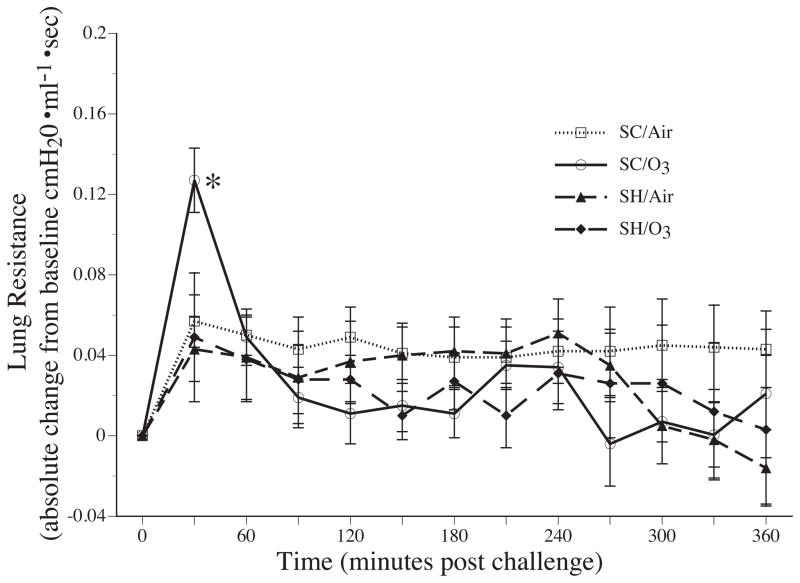
Ozone inhalation prior to the third nDer f 1 challenge in sensitized and challenged rats resulted in a significant increase in RL when compared to sensitized and challenged rats that inhaled filtered air (p = 0.014), and both groups of saline sham treated rats (with, p = 0.034 and without ozone exposure, p = 0.014) (Fig 4A).
Figure 4.
Mean lung resistance of Brown-Norway rats that were sensitized and challenged with nDer f 1 (SC) or sham treated with saline (SH) before (black columns) and 30 minutes after (white columns) the tracheal instillation of saline (in the SH groups) or nDer f 1 in saline (in the SC groups). The data in panel A are from rats whose vagus nerves were intact. The data in panel B are from rats whose vagus nerves were treated with perineural capsaicin (PCT). The data in panel C are from rats whose vagus nerves were cut (Vagotomy). * indicates a significant difference (p ≤ 0.05) from SC/O3 for before and after intratracheal instillation of nDer f 1. † indicates a significant difference (p ≤ 0.05) from SH/O3 for before and after intratracheal instillation. ‡ indicates a significant difference (p ≤ 0.05) from PCT. § indicates a significant difference (p ≤ 0.05) from Control. Values are mean ± sem. N’s for each group are reported in the results section of the text.
The instillation of allergen resulted in a significant early airway response in the sensitized and challenged group exposed to ozone, only (Fig. 4A and 5). The increase in RL following the instillation of nDer f 1 is significantly greater in the SC/O3 group compared to the other groups. The instillation of nDer f 1 allergen in the sensitized and challenged rats that inhaled ozone resulted in a significant increase in RL compared to the sensitized and challenged group exposed to air (p = 0.030) and saline instillation in the sham groups (with, p = 0.003 and without ozone exposure, p = 0.003) (Fig 4A and 5). In contrast, none of the experimental groups developed a late airway response (Fig 5).
Figure 5.
Change in mean lung resistances of Brown-Norway rats that were sensitized and challenged with nDer f 1 (SC) or sham treated with saline (SH) after the tracheal instillation of 250 ul saline (in the SH groups) or nDer f 1 in 250 ul saline (in the SC groups). Each point is a 30 minute average. * indicate a significant difference (p ≤ 0.05) between SC/O3 and all other groups. Note that there is a distinct early airway response following nDer f 1 instillation in the Brown-Norway rats that were sensitized and challenged with nDer f 1 and subsequently exposed to 1.0 ppm ozone. Values are mean ± sem. N’s for each group are reported in the results section of the text.
3.1.3 Augmented Breaths
No augmented breaths were observed during the baseline recording period before either nDer f 1 or saline instillation in the SC and SH rats exposed to air (Table 1). The inhalation of ozone resulted in a significant increase in the number of augmented breaths during the baseline recording period in the SC and SH rats (Table 1). Instillation of nDer f 1 in the SC rats exposed to Air resulted in a significant increase in augmented breaths compared to baseline and SH/Air, whereas nDer f 1 instillation in the SC rats exposed to ozone did not result in a change in the number of augmented breaths (Table 1).
Table 1.
Number of augmented breaths during 20 mins of spontaneous breathing prior to and 30 minutes after saline or nDer f 1 challenge.
| Control | PNCT | Vagotomy | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Baseline | ΔT30 | Baseline | ΔT30 | Baseline | ΔT30 | |
| SH/FA | 0 | 1 (0:2) | 0.29 (0:1) | 1.14 (−1:3) | 0 | 0.11§◇ (0:1) |
| SH/O3 | 4.82* (0:18) | 1.36 (−8:6) | 2.88* (0:7) | 3.38 (−3:10) | 0§◇ | 0.273§ (0:2) |
| SC/FA | 0 | 3.14† (1:6) | 0.50 (0:2) | 3.33 (0:8) | 0 | 0§◇ |
| SC/O3 | 6.56*‡ (0:17) | 1.78 (−9:10) | 6.00*‡ (1:10) | 3.88 (−1:13) | 0§◇ | 0◇ |
Values are means with range in parenthesis. Groups are: sham treated exposed to filtered air (SH/FA); sham treated exposed to ozone (SH/O3); sensitized and challenged with nDer f 1 exposed to filtered air (SC/FA); and sensitized and challenged with nDer f 1exposed to ozone (SC/O3). The number of augmented breaths are reported at baseline before nDer f 1 challenge or as an absolute change from baseline at 30 minutes following nDer f 1 challenge (ΔT30). Significant difference p ≤ 0.05:
O3 vs FA for SC or SH;
SC vs SH for O3 or FA;
SH-FA vs SC-O3;
Different from Control;
PNCT vs Vagotomy
3.1.4 Lavage Data
Ozone inhalation in combination with sham treatment or sensitized and challenge did not effect total lavage cell count or the percent lymphocytes, macrophages, neutrophils, eosinophils or monocytes. Sensitization and challenge with nDer f 1 allergen in combination with Air or ozone inhalation resulted in a significant increase in percent lymphocytes and eosinophils (Table 2). By comparison, ozone inhalation in SH or SC rats resulted in a significant elevation in lavage protein (Table 2).
Table 2.
Bronchoalveolar lavage total cells, differential cell counts and protein.
|
|
|||||||
|---|---|---|---|---|---|---|---|
| Total Cells | % Lymph | %MAC | %PMN | %EOS | %MONO | Protein | |
| SH/Air | 617270 ± 121133 | 2.8 ± 0.84 | 71.4 ± 9.49 | 13.2 ± 5.28 | 2.0 ± 0.42 | 0.7 ± 0.30 | 205 ± 18 |
| SH/O3 | 458146 ± 68859 | 5.6 ± 0.74 | 64.7 ± 4.73 | 20.8 ± 3.81 | 7.1 ± 1.93 | 1.9 ± 0.54 | 258* ± 12 |
| SC/Air | 566717 ± 50925) | 15.6† ± 1.49 | 47.2 ± 5.54 | 18.4 ± 4.89 | 17.7† ± 3.76 | 1.2 ± 0.24 | 231 ± 21 |
| SC/O3 | 641994 ± 104462 | 14.3†‡ ± 2.18 | 54.7 ± 6.54 | 11.7 ± 2.06 | 18.5†‡ ± 3.96 | 0.8 ± 0.30 | 283* ± 7 |
Values are means ± standard error. Differential cell counts are reported as percent for lymphocytes (Lymph), macrophages (Mac), neutrophils (PMN), eosinophils (Eos) and monocytes (Mono). Groups are: sham treated exposed to filtered air (SH/Air); sham treated exposed to ozone (SH/O3); sensitized and challenged with nDer f 1exposed to filtered air (SC/Air); and sensitized and challenged with nDer f 1exposed to ozone (SC/O3). Significant difference p ≤ 0.05:
O3 vs Air for SC or SH;
SC vs SH for O3 or Air;
SH/Air vs SC/O3
3.2 Vagus Nerve Treatments
A total of 71 rats were studied in this phase of the investigation. Forty-one rats received PCT and were distributed between four groups: SH/Air (n = 10); SH/O3 (n = 10); SC/Air (n = 11); and SC/O3 (n = 10). Twenty-three rats were vagotomized and distributed between four groups: SH/Air (n = 5); SH/O3 (n = 6); SC/Air (n = 6); and SC/O3 (n = 6). An additional seven SC/O3 rats received perineural capsaicin treatment and were treated with hexamethonium.
3.2.1 Vagal Perineural Capsaicin Treatment
Vagal perineural capsaicin treatment abolished the increase in RL associated with ozone exposure in the sensitized and challenged rats (Fig 4B). In contrast to the rats that received no vagal treatment, the instillation of nDer f 1 allergen in the sensitized and challenged group exposed to ozone and treated with perineural capsaicin resulted in a significantly (p = 0.015) smaller increase in RL compared to the sensitized and challenged group exposed to air (Fig 4B). The pattern of augmented breaths produced by ozone exposure in the SH and SC rats in the control condition persisted with perineural capsaicin treatment (Table 1).
3.2.2 Bilateral Vagotomy
Ozone inhalation in sensitized and challenged rats that were vagotomized resulted in a significant increase in RL compared to the sensitized and challenged rats exposed to air (p = 0.010) and saline instillation in the sham groups (with, p = 0.006 and without ozone exposure, p = 0.007) (Fig 4C). Across the groups that were vagotomized instillation of nDer f 1 allergen in the sensitized and challenged rats exposed to ozone resulted in a significant increase in RL compared to the sensitized and challenged rats exposed to air (p = 0.030) and saline instillation in the sham groups (with, p = 0.003 and without ozone exposure, p = 0.003) (Fig 4C). The pattern of augmented breaths produced by ozone exposure in the SH and SC rats in the control and PCT condition was abolished with bilateral vagotomy. The pattern of augmented breaths induced by saline or nDer f 1 allergen instillation in SH or SC with and without perineural capsaicin treatment was also abolished with bilateral vagotomy (Table 1).
3.2.3 Hexamethonium
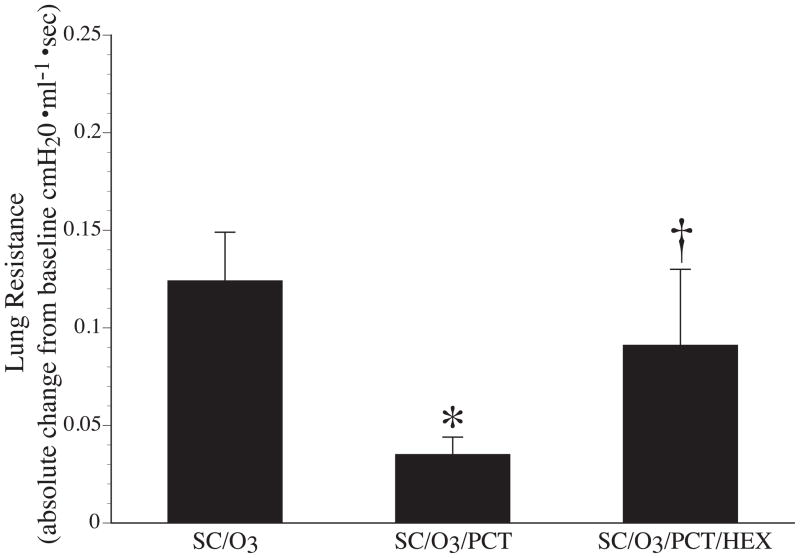
Treatment with 20 mg/kg HEX in a group of SC/O3/PCT rats reestablished the bronchoconstriction seen in the SC/O3 group. The change in RL from T0 to T30 was significantly greater in the SC/O3/PCT/HEX group compared to the SC/O3/PCT group (p = 0.044), while the SC/O3/PCT/HEX group and SC/O3 group were not significantly different (p = 0.839) (Fig 6).
Figure 6.
Change in mean lung resistances of Brown-Norway rats that were sensitized and challenged with nDer f 1 exposed to O3 and receive sham treatment (SC/O3), perineural capsaicin treatment SC/O3/PCT or PCT in combination with intravenous 20 mg/kg hexamethonium (SC/O3/PCT/HEX). * indicates a significant difference (p ≤ 0.05) between SC/O3 and SC/O3/PCT. † indicates a significant difference (p ≤ 0.05) between SC/O3/PCT and SC/O3/PCT/HEX.
4. Discussion
Several clinical exposure studies have examined the effects of a single acute ozone exposure on pulmonary functions, inflammation and airway response to allergen challenge in human asthmatics (Chen et al., 2004; Horstman et al., 1995; Kehrl et al., 1999; Peden et al., 2002). A synthesis of these studies strongly supports the thesis that asthmatics show similar reflex initiated decrements in inspiratory capacity and forced vital capacity as age matched normal subjects, but have a greater ozone-induced bronchoconstrictive responses (Horstman et al., 1995). In addition, it has been shown that this enhanced ozone-induced bronchoconstrictive response in asthmatics is associated with a greater neutrophilic inflammation (Scannell et al., 1996) and an enhanced eosinophilic inflammation (Peden et al., 2002). Other studies have shown that if the total inhaled dose of ozone is sufficient to produce significant restrictive and obstructive changes in pulmonary function allergic asthmatics will also show an increased responsiveness to inhaled allergen (Chen et al., 2004; Kehrl et al., 1999). Our data indicates that the ozone-induced enhanced functional changes and specific airway reactivity observed in human asthmatics can be mimicked in Brown-Norway rats sensitized and challenged with the house dust mite allergen nDer f 1. SH and SC rats had similar ozone-induced reflex alterations in breathing pattern, while only SC had a significant increase in RL following ozone exposure. In addition, only sensitized and challenged rats that inhaled ozone demonstrated a significant increase in RL with a subsequent challenge to nDer f 1. In contrast, we did not observe an enhancement of neutrophilic or eosinophilic airway inflammation in SC compared to SH rats exposed to ozone or in SC rats exposed to ozone compared to SC rats that inhaled filtered air. Our inability to observe an enhancement of ozone-induced airway inflammation may be due in part by the timing of our protocol. This contention is supported by the observations that prolonged acute exposure of asthmatic subjects to low levels of ozone (0.16 ppm) induced significant increases in airway eosinophils recovered by bronchoalveolar lavage 18 hours after exposure (Peden et al., 1997) and that ozone inhalation (1.2 ppm for 6 hr) in Brown Norway rats resulted in a 4-fold increase in eotaxin mRNA 20 hr following exposure (Ishii et al., 1998). suggesting that the time frame of our study was not long enough to study the effect of ozone on allergen-induced airway eosinophilic inflammation.
While the epidemiological and clinical studies clearly suggest that ozone exposure of sufficient magnitude may augment allergen-induced airway responses in some asthmatics, the mechanism by which ozone augments this response is unknown. The current study examined the role that vagal afferent and efferent nerves play in ozone-enhanced bronchoconstriction and specific airway reactivity in nDer f 1 sensitized and challenged Brown-Norway rats. These responses were examined using perineural capsaicin treatment that has previously been shown to produce a selective conduction block of vagal C-fibers in the rat (Mansoor et al., 1996; Schelegle et al., 2001), hexamethonium to block or reduce ganglionic neurotransmission, and bilateral vagotomy to eliminate all vagal afferent and efferent effects. The link between lung C-fibers and ozone inhalation come from numerous studies. Ozone inhalation has been shown to increase the discharge frequency of bronchial C-fibers in the dog (Coleridge et al., 1993) and pulmonary C-fiber activity in the rat (Ho and Lee, 1998). In the mouse, ozone activates C-fibers via TRPA1 ion channels (Taylor-Clark and Undem, 2010). Furthermore, a link between lung C-fibers and airway responses in asthma is supported by those studies in which capsaicin desensitization of sensory nerves, has been shown to provide significant protection against antigen-induced airway hyperreactivity in the rat (Alving et al., 1987), guinea-pig (Gentilini et al., 1990) and pig (Alving et al., 1990). In addition, the allergen-induced activation of TRPA1 receptors on lung sensory nerves intiates a reflex bronchoconstriction that contributes to the late airway response in the Brown-Norway rat (Raemdonck et al., 2012). These observations suggest that lung C-fibers may be involved in the enhanced airway responses following ozone exposure. The comparison of the control versus the perineural capsaicin treatment conditions in the current study supports the modulatory role of a reflex bronchoconstriction mediated by vagal C-fibers in both the enhanced bronchoconstriction following ozone exposure and the enhanced specific airway reactivity in sensitized and challenged Brown-Norway rats. This unique observation is consistent with the established role of lung C-fibers in initiating reflex bronchoconstriction (Canning and Fischer, 2001)
The comparison of the perineural capsaicin treatment versus the vagotomy conditions in the sensitized and challenged rats exposed to ozone and challenged with nDer f 1 supports the presence of a vagal mediated reflex bronchodilation initiated by myelinated sensory afferents that survive PCT and in the intact animal act to counterbalance the C-fiber mediated reflex and non-vagal bronchoconstrictive responses. The presence of a reflex bronchodilation is confirmed by the observation that the blockade of ganglionic neurotransmission with hexamethonium reestablishes allergen-induced bronchoconstriction in sensitized and challenged rats exposed to ozone and treated with perineural capsaicin treatment. The nature of this reflex bronchodilation is unclear.
While several studies support the presence of neuropeptide dependent production of inhibitory mediators in the airway (Li et al., 2005; Szarek and Spurlock, 1997; Szarek et al., 1998), early investigations do not support the presence of a reflex bronchodilation in the rat (Doidge and Satchell, 1982; Satchell, 1982). Data supporting the presence of reflex bronchodilation in the rat is limited to the observation of Szarek et al. (Szarek et al., 1995) that the pretreatment of isolated airway rings obtained from Sprague-Dawley rats with a dose of capsaicin sufficient to abolish capsaicin induced bronchodilation did not completely abolish the bronchodilation induced by electric field stimulation suggesting the presence of a reflex bronchodilation. Unfortunately the reflex nature of the bronchodilation in this study was not confirmed with the combined treatment of capsaicin and tetrodotoxin. In the cat (Aizawa et al., 1999; Ichinose et al., 1987) and human (Ichinose et al., 1996) active reflex bronchodilation has been induced by the inhalation of capsaicin aerosol supporting the role of lung C-fibers in this response. Further, Aizawa et al (Aizawa et al., 1999) has shown in the cat this reflex bronchodilation is mediated by the release of nitric oxide from vagal inhibitory noncholinergic, nonadenergic nerve fibers. While the release of neuropeptides from C-fiber endings may play a role in our study the fact that reflex bronchodilation survives perineural capsaicin treatment supports the contention that the sensory arm involved in this reflex is not initiated by sensory C-fibers that travel in the vagus nerves.
While catecholimine containing nerves do not innervate airway smooth muscle in the rat (El-Berman AW, 1970), an increase in circulating epinephrine acting on beta2-adrenoceptors could contribute to the observed bronchodilation in this study (Zhang et al., 2011). Consistent with our observations, hexamethonium would block the increased release of epinephrine from adrenal chromaffin cells (Sala et al., 2008), but would be blocked by bilateral vagotomy only if the increase in circulating epinephrine was mediated by vagal myelinated afferents that are not affected by perineural capsaicin treatment.
It is well established that deep inspirations induce both bronchodialation and bronhoprotection in heathy human subjects and that these responses are reduced or not present in asthmatics when challenged with methacholine and histamine (Pyrgos et al., 2003; Skloot and Togias, 2003; Skloot et al., 2007). In contrast, deep inspiration-induced bronchodilation is still present in asthmatics when challenged with allergen and is greater during the early phase response when compared to the late phase (Pellegrino et al., 1990). Both ozone exposure and nDer f 1 challenge alone resulted in a significant increase in the number of augmented breaths that were not associated with an increase in RL (Table 1 and Fig. 4A). In contrast, ozone exposure in sensitized and challenged rats induced significant increases in both augmented breaths and RL, while subsequent nDer f 1 challenge in this group resulted in a mild further increase in augmented breaths that was associated with a significant increase in RL (Table 1 and Fig. 4A). Since allergen-induced bronchoconstriction was present only in the combined exposure group it is possible that the increase in augmented breaths secondary to the activation of rapidly adapting pulmonary stretch receptors in this group could then result in a bronchodialation that counterbalances the bronchoconstriction induced by the combined effects of ozone and allergen. Consistent with this pattern of response is the relative bronchodialation produced by perineural capsaicin treatment that keeps rapidly adapting pulmonary stretch receptor signaling intact and the abolition of bronchodialation with vagotomy in the combined exposure group. The role that any of the discussed mechanisms play in the bronchodilation observed in the current study requires further investigation.
The significantly greater constrictive response with nDer f 1 challenge in the vagotomized sensitized and challenged rats exposed to ozone indicates the presence of a marked non-reflex bronchoconstrictive response that contributes to the exacerbation of allergen-induced airway responses following ozone inhalation. The mechanism underlying the observed non-reflex bronchoconstrictive response was not studied but could be the result of an increased responsiveness of airway smooth muscle to mediators and/or an increase in the amount of mediators released following nDer f 1 challenge. It is possible that the local release of neuropeptides from airway C-fibers that is insensitive to perineural capsaicin treatment (Schelegle et al., 2000) may play a role in the non-reflex bronchoconstrictive response. In Fisher 344 rats the release of substance P is known to induce a neurokinin 1 receptor (NK1) mediated mast cell dependent contraction of airway smooth muscle, while in BDE rats the tachykinin-dependent contraction of airway smooth muscle is the result of neurokinin A (NKA) acting on neurokinin 2 receptors (NK2) located on airway smooth muscle (Joos et al., 1994). Both of these responses would be expected to survive perineural capsaicin treatment and vagotomy.
The release of neuropeptides from lung C-fibers may also play a role in the ozone enhanced responses observed in this study. It has been shown that the neuropeptides substance P and neurokinin A that are released from lung C-fiber endings during ozone inhalation (Hazbun et al., 1993), have been shown to have multiple immune modulatory effects (Lambrecht, 2001). Substance P especially has been shown to increase the proliferation of lymphocytes and enhance immunoglobulin synthesis in vitro and in vivo (Nio et al., 1993; Scicchitano et al., 1988), suggesting another possible mechanism for augmenting the immune and exaggerating airway responses to inhaled allergen following the acute inhalation of ozone. This potential immunomodulatory role of neuropeptides is further illustrated by the report (Maghni et al., 2000) in antigen-sensitized Brown-Norway rats that selective NK-1 and NK-2 receptor antagonists decrease allergen-induced late airway responses. In addition, the NK-2 receptor antagonist decreased allergen-induced eosinophilic inflammation and the in situ production of Th2 (Il-4 and –5) cytokine expression in BAL cells (Maghni et al., 2000).
5. Conclusion
The results of the perineural capsaicin, vagotomy and hexamethonium studies demonstrate that in this Brown-Norway rat model of ozone-induced exacerbation of asthma, input from lung sensory fibers initiate both bronchoconstrictor and bronchodilator responses. Lung C-fibers initiate a bronchoconstrictor reflex that is counterbalanced by a bronchodilator reflex initiated by vagal myelinated fibers. In turn, when vagus nerves are intact, the bronchodilatory response predominates, but not sufficiently to negate antigen-induced non-reflex bronchoconstriction.
Table 3.
Effect of vagal nerve treatments, perineural capsaicin treatment (PCT) and vagotomy on the apneic period of the Hering-Breuer Inflation Reflex (HBR) and the change in heart rate (HR) and arterial blood pressure (ABP) of the Pulmonary Chemoreflex (PCR) in Brown Norway Rats after 8 hours of 1 ppm ozone inhalation.
| Control | PCT | Vagotomy | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| HBR Apnea (sec) | PCR-dHR (beats/min) | PCR-dABP (mmHg) | HBR Apnea (sec) | PCR-dHR (beats/min) | PCR-dABP (mmHg) | HBR Apnea (sec) | PCR-dHR (beats/min) | PCR-dABP (mmHg) | |
| SH/Air | 56.83 ± 5.49 | −63.80 ± 5.43 | −54.33 ± 5.68 | 52.62 ± 6.46 | −10.29 ± 2.30 | 8.67 ± 3.09 | 0.83 ± 0.02 | −11.64 ± 1.33 | −3.88 ± 1.69 |
| SH/O3 | 50.95 ± 3.97 | −71.91 ± 1.72 | −63.55 ± 2.45 | 54.66 ± 2.27 | −6.74 ± 2.60 | 0.81 ± 1.34 | 0.84 ± 0.02 | −6.65 ± 2.63 | 3.07 ± 1.79 |
| SC/Air | 63.07 ± 5.37 | −66.19 3.99 | −53.00 ± 6.74 | 59.13 ± 2.54 | −10.56 ± 4.61 | −2.88 ± 4.39 | 0.77 ± 0.02 | −8.35 ± 1.83 | 1.38 ± 2.24 |
| SC/O3 | 55.15 ± 4.42 | −71.33 ± 2.04 | −67.00 ± 2.63 | 59.59 ± 3.15 | −0.71 ± 7.26 | 5.04 ± 2.05 | 0.82 ± 0.03 | −6.30 ± 3.28 | 1.44 ± 2.65 |
Values are means ± standard error. Groups are: sham treated exposed to filtered air (SH/Air); sham treated exposed to ozone (SH/O3); sensitized and challenged with nDer f 1exposed to filtered air (SC/Air); and sensitized and challenged with nDer f 1exposed to ozone (SC/O3).
Highlights.
Brown-Norway rats sensitized and challenged with der f 1 allergen are a good model of ozone-induced exacerbation of specific airway reactivity.
-
In this model of O3 exacerbation of asthma:
vagal C-fibers initiate reflex bronchoconstriction
vagal myelinated fibers initiate reflex bronchodilation
mediators released within the airway initiate bronchoconstriction.
Acknowledgments
A national institute of health grant NIEHS R01-006976 and an unrestricted gift from the American Petroleum Institute supported this research. The authors thank Lei Putney from Dallas Hyde’s laboratory at the California National Primate Research Center for her effort in processing bronchoalveolar lavage fluid. The authors thank Dr. Ian Gilmour at the National Institute of Environmental Health Sciences Laboratory, Research Triangle, NC for his helpful insights on sensitizing Brown-Norway rats and for providing us with our original vials of purified nDer f 1 allergen. The authors thank Emily Wong for reading and providing editorial comments. The authors also thank Shannon Hilliar, Billy Cheung and Elan Yang for their technical support in the laboratory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aizawa H, Takata S, Inoue H, Matsumoto K, Koto H, Hara N. Role of nitric oxide released from iNANC neurons in airway responsiveness in cats. Eur Respir J. 1999;13:775–780. doi: 10.1034/j.1399-3003.1999.13d13.x. [DOI] [PubMed] [Google Scholar]
- Alving K, Matran R, LaCroix JS, Lundberg JM. Capsaicin and histamine antagonist-sensitive mechanisms in the immediate allergic reaction of pig airways. Acta Anaesthesiol Scand. 1990;138:49–60. doi: 10.1111/j.1748-1716.1990.tb08811.x. [DOI] [PubMed] [Google Scholar]
- Alving K, Ulfgren AK, Lundberg JM, Ahlstedt S. Effect of capsaicin on bronchial reactivity and inflammation in sensitized adult rats. Int Arch Allergy Appl Immunol. 1987;82:377–379. doi: 10.1159/000234231. [DOI] [PubMed] [Google Scholar]
- Canning BJ, Fischer A. Neural regulation of airway smooth muscle tone. Respiration physiology. 2001;125:113–127. doi: 10.1016/s0034-5687(00)00208-5. [DOI] [PubMed] [Google Scholar]
- Chen LL, Tager IB, Peden DB, Christian DL, Ferrando RE, Welch BS, Balmes JR. Effect of ozone exposure on airway responses to inhaled allergen in asthmatic subjects. Chest. 2004;125:2328–2335. doi: 10.1378/chest.125.6.2328. [DOI] [PubMed] [Google Scholar]
- Coleridge JC, Coleridge HM, Schelegle ES, Green JF. Acute inhalation of ozone stimulates bronchial C-fibers and rapidly adapting receptors in dogs. J Appl Physiol. 1993;74:2345–2352. doi: 10.1152/jappl.1993.74.5.2345. [DOI] [PubMed] [Google Scholar]
- Doidge JM, Satchell DG. Adrenergic and non-adrenergic inhibitory nerves in mammalian airways. Journal of the autonomic nervous system. 1982;5:83–99. doi: 10.1016/0165-1838(82)90030-3. [DOI] [PubMed] [Google Scholar]
- El-Berman AWMW, Bradley D. The distribution of acetylcholinesterase and catecholamine containing nerves in the rat lung. Anatomic Record. 1970;167:205–212. doi: 10.1002/ar.1091670208. [DOI] [PubMed] [Google Scholar]
- EPA; Development, O.o.R. Air quality criteria for ozone and related photochemical oxidants. NTIS; Springfield, VA, Research Triangle Park, NC: 1996. [Google Scholar]
- Gentilini G, Franchi-Micheli S, Ciuffi M, Bindi D, Zilletti L. Capsaicin and anaphylactic reactions in the giunea-pig airways. Agents Actions. 1990;30:92–94. doi: 10.1007/BF01969007. [DOI] [PubMed] [Google Scholar]
- Hazbun ME, Hamilton R, Holian A, Eschenbacher WL. Ozone-induced Increases in Substance P and 8-Epi-Prostaglandin F2α in the Airways of Human Subjects. Am J Respir Cell Mol Biol. 1993;9:568–572. doi: 10.1165/ajrcmb/9.5.568. [DOI] [PubMed] [Google Scholar]
- Hiltermann TJN, deBruijne CR, Stolk J, Zwinderman AH, Spieksma FTM, Roemer W, Steerenerg PA, Fischer PH, vanBree L, Hiemstra PS. Effects of photochemical air pollution and allergen exposure on upper respiratory tract inflammation in asthmatics. Am J Crti Care Med. 1997;156:1765–1772. doi: 10.1164/ajrccm.156.6.9704127. [DOI] [PubMed] [Google Scholar]
- Ho CY, Lee LY. Ozone enhances excitabilities of pulmonary C fibers to chemical and mechanical stimuli in anesthetized rats. Journal of Applied Physiology. 1998;85:1509–1515. doi: 10.1152/jappl.1998.85.4.1509. [DOI] [PubMed] [Google Scholar]
- Horstman DH, Ball BA, Brown J, Gerrity T, Folinsbee LJ. Comparison of pulmonary responses of asthmatic and nonasthmatic subjects performing light exercise while exposed to a low level of ozone. Toxicology and industrial health. 1995;11(4):369–385. doi: 10.1177/074823379501100401. [DOI] [PubMed] [Google Scholar]
- Ichinose M, Inoue H, Miura M, Yafuso N, Nogami H, Takishima T. Possible sensory receptor of nonadrenergic inhibitory nervous system. J Appl Physiol. 1987;63:923–929. doi: 10.1152/jappl.1987.63.3.923. [DOI] [PubMed] [Google Scholar]
- Ichinose M, Miura M, Tomaki M, Oyake T, Kageyama N, Ikarashi Y, Maruyama Y, Shirato K. Incubation with IgE increases cholinergic neuotransmission in human airways in vitro. American journal of respiratory and critical care medicine. 1996;154:1272–1276. doi: 10.1164/ajrccm.154.5.8912735. [DOI] [PubMed] [Google Scholar]
- Ishii Y, Shirato M, Nomura A, Sakamoto T, Uchida Y, Ohtsuka M, Sagai M, Hasegawa S. Cloning of rat eotaxin: ozone inhalation icreases mRNA and protein expression in lung of Brown Norway rats. Am J Physiol Lung Cell Mol Physiol. 1998;274:L171–L176. doi: 10.1152/ajplung.1998.274.1.L171. [DOI] [PubMed] [Google Scholar]
- Joos GF, Lefebvre RA, Kips JC, Pauwels RA. Tachykinins contract trachea from Fischer 344 rats by interaction with a tachykinin NK1 receptor. European journal of pharmacology. 1994;271:47–54. doi: 10.1016/0014-2999(94)90263-1. [DOI] [PubMed] [Google Scholar]
- Jorres R, Nowak D, Magnussen H. The effect of ozone exposure on allergen responsiveness in subjects with asthma or rhinitis. Am Crit Care Med. 1996;153:56–64. doi: 10.1164/ajrccm.153.1.8542163. [DOI] [PubMed] [Google Scholar]
- Kehrl HR, Peden DB, Ball B, Folinsbee LJ. Increased specific airway reactivity of persons with mild allergic asthma after 7.6 hours of exposure to 0.16 ppm ozone. J Allergy Clin Immunol. 1999;104(6):1198–1204. doi: 10.1016/s0091-6749(99)70013-8. [DOI] [PubMed] [Google Scholar]
- Lambrecht BN. Immunologists getting nervous: neuropeptides, dendritic cells and T ell activation-Commentary. Respiratory Research. 2001;2:133–138. doi: 10.1186/rr49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li PC, Shaw CF, Kuo TF, Chien CT. Inducible nitric oxide synthase evoked nitric oxide counteracts capsaicin-induced airway smooth muscle contraction, but exacerbates plasma extravasation. Neuroscience letters. 2005;378:117–122. doi: 10.1016/j.neulet.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Maghni K, Taha R, Afif W, Hamid Q, Martin JG. Dichotomy between neurokinin receptor actions in modulating allergic airway responses in an animal model of helper T cell type 2 cytokine-associated inflamation. Am J Crit Care Med. 2000;162:1068–1074. doi: 10.1164/ajrccm.162.3.9910089. [DOI] [PubMed] [Google Scholar]
- Mansoor JK, Hyde DM, Schelegle ES. Contribution of vagal afferents to breathing pattern in rats with lung fibrosis. Respir Physiol. 1996 doi: 10.1016/s0034-5687(96)02505-4. [DOI] [PubMed] [Google Scholar]
- Mansoor JK, Hyde DM, Schelegle ES. Contribution of vagal afferents to breathing pattern in rats with lung fibrosis. Respiration physiology. 1997;108:45–61. doi: 10.1016/s0034-5687(96)02505-4. [DOI] [PubMed] [Google Scholar]
- Nio DA, Moylan RN, Roche JK. Modulation of T lymphocyte function by neuropeptides. Journal of Immunology. 1993;150:5281–5288. [PubMed] [Google Scholar]
- Peden DB, Boehlecke B, Horstman D, Devlin R. Prolonged acute exposure to 0.16 ppm ozone induces eosinophilic airway inflammation in asthmatic subjects with allergies. J Allergy Clin Immunol. 1997;100:802–808. doi: 10.1016/s0091-6749(97)70277-x. [DOI] [PubMed] [Google Scholar]
- Peden DB, Boehlecke B, Horstman D, Devlin R. Prolonged acute exposure to 0.16 ppm ozone induces eosinophil airway inflamationin asthamtic subjucts with allergies. J Allergy Clin Immunol. 2002;100(6):802–808. doi: 10.1016/s0091-6749(97)70277-x. [DOI] [PubMed] [Google Scholar]
- Pellegrino R, Violante B, Crimi E, Brusasco V. Effects of deep inhalation during early and late asthmatic reactions to allergen. The American review of respiratory disease. 1990;142:822–825. doi: 10.1164/ajrccm/142.4.822. [DOI] [PubMed] [Google Scholar]
- Pyrgos G, Scichilone N, Togias A, Brown RH. Bronchodilation response to deep inspirations in asthma is dependent on airway distensibility and air trapping. J Appl Physiol. 2003;110:472–479. doi: 10.1152/japplphysiol.00603.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raemdonck K, de Alba J, Birrell MA, Grace M, Maher SA, Irvin CG, Fozard JR, O'Byrne PM, Belvisi MG. A role for sensory nerves in the late asthmatic response. Thorax. 2012;67:19–25. doi: 10.1136/thoraxjnl-2011-200365. [DOI] [PubMed] [Google Scholar]
- Sala F, Nistri A, Criado M. Nicotinic acetylcholine receptors of adrenal chromaffin cells. Acta physiologica (Oxford, England) 2008;192:203–212. doi: 10.1111/j.1748-1716.2007.01804.x. [DOI] [PubMed] [Google Scholar]
- Satchell D. Non-adrenergic, non-cholinergic nerves in mammalian airways: their function and the role of purines. Comparative biochemistry and physiology. 1982;72:189–196. doi: 10.1016/0306-4492(82)90083-1. [DOI] [PubMed] [Google Scholar]
- Scannell C, Chen L, Aris RM, Tager I, Christian D, Ferrando R, Welch B, Kelly T, Balmes JR. Greater ozone-induced inflammatory responses in subjects with asthma. American journal of respiratory and critical care medicine. 1996;154:24–29. doi: 10.1164/ajrccm.154.1.8680687. [DOI] [PubMed] [Google Scholar]
- Schelegle ES, Alfaro MF, Putney L, Stovall M, Tyler N, Hyde DM. Effect of C-fiber-mediated, ozone-induced rapid shallow breathing on airway epithelial injury in rats. J Appl Physiol. 2001;91:1611–1618. doi: 10.1152/jappl.2001.91.4.1611. [DOI] [PubMed] [Google Scholar]
- Schelegle ES, Chen AT, Loh CY. Effects of vagal perineural capsaicin treatment on vagal efferent and airway neurogenic responses in anesthetized rats. Journal of basic and clinical physiology and pharmacology. 2000;11:1–16. doi: 10.1515/jbcpp.2000.11.1.1. [DOI] [PubMed] [Google Scholar]
- Scicchitano R, Bienenstock J, Stanisz AM. In vivo immunoregulation by the neuropeptide substance P. Immunology. 1988;63:733–735. [PMC free article] [PubMed] [Google Scholar]
- Singh P, Daniels M, Winsett DW, Richards J, Doerfler D, Hatch G, Adler KB, Gilmour MI. Phenotypic comparison of allergic airway responses to house dust mite in three rat strains. American journal of physiology. 2003;284:L588–598. doi: 10.1152/ajplung.00287.2002. [DOI] [PubMed] [Google Scholar]
- Skloot G, Togias A. Bronchodilation and bronchoprotection by deep inspiration and their relationship to bronchial hyperresponsiveness. Clinical reviews in allergy & immunology. 2003;24:55–72. doi: 10.1385/CRIAI:24:1:55. [DOI] [PubMed] [Google Scholar]
- Skloot GS, Chandy D, Schachter N, Togias A. The bronchoprotective effect of deep inspiration is flow rate dependent. Respiratory medicine. 2007;101:1376–1382. doi: 10.1016/j.rmed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Sterner-Kock A, Vesely KR, Stovall MY, Schelegle ES, Green JF, Hyde DM. Neonatal capsaicin treatment increases the severity of ozone-induced lung injury. American journal of respiratory and critical care medicine. 1996;153:436–443. doi: 10.1164/ajrccm.153.1.8542155. [DOI] [PubMed] [Google Scholar]
- Szarek JL, Spurlock B. Sensory nerve-mediated inhibitory responses in airways of F344 rats. Toxicology. 1997;122:101–110. doi: 10.1016/s0300-483x(97)00085-1. [DOI] [PubMed] [Google Scholar]
- Szarek JL, Spurlock B, Gruetter CA, Lemke S. Substance P and capsaicin release prostaglandin E2 from rat intrapulmonary bronchi. The American journal of physiology. 1998;275:L1006–1012. doi: 10.1152/ajplung.1998.275.5.L1006. [DOI] [PubMed] [Google Scholar]
- Szarek JL, Stewart NL, Spurlock B, Schneider C. Sensory nerve and neuropeptide mediated relaxation responses in airways of Sprague-Dawley rats. J Appl Physiol. 1995;78(5):1679–1687. doi: 10.1152/jappl.1995.78.5.1679. [DOI] [PubMed] [Google Scholar]
- Taylor-Clark TE, Undem BJ. Ozone activates airway nerves via the selective stimulation of TRPA1 ion channels. The Journal of physiology. 2010;588:423–433. doi: 10.1113/jphysiol.2009.183301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Shibamoto T, Kuda Y, Ohmukai C, Kurata Y. Pulmonary vasoconstrictive and bronchoconstrictive responses to anaphylaxis are weakened via beta2-adrenoceptor activation by endogenous epinephrine in anesthetized rats. Anesthesiology. 2011;114:614–623. doi: 10.1097/ALN.0b013e31820b8d34. [DOI] [PubMed] [Google Scholar]