Abstract
Although oligomeric β-amyloid (Aβ) has been suggested to have an important role in Alzheimer disease (AD), the mechanism(s) of how Aβ induces neuronal cell death has not been fully identified. The balance of pro- and anti-apoptotic Bcl-2 family proteins (e.g., Bcl-2 and Bcl-w versus Bad, Bim and Bax) has been known to have a role in neuronal cell death and, importantly, expression levels of these proteins are reportedly altered in the vulnerable neurons in AD. However, the roles of apoptotic proteins in oligomeric Aβ-induced cell death remain unclear in vivo or in more physiologically relevant models. In addition, no study to date has examined whether Bax is required for the toxicity of oligomeric Aβ. Here, we found that treatment with oligomeric Aβ increased Bim levels but decreased Bcl-2 levels, leading to the activation of Bax and neuronal cell death in hippocampal slice culture and in vivo. Furthermore, the inhibition of Bax activity either by Bax-inhibiting peptide or bax gene knockout significantly prevented oligomeric Aβ-induced neuronal cell death. These findings are first to demonstrate that Bax has an essential role in oligomeric Aβ-induced neuronal cell death, and that the targeting of Bax may be a therapeutic approach for AD.
Keywords: Alzheimer disease, neuronal cell death, Bax, Bim, Bcl-2, β-amyloid
Alzheimer disease (AD) is the most common neurodegenerative disorder, and β-amyloid (Aβ) has been suggested to have a critical role in the pathogenesis of AD. It has been shown that oligomeric Aβ is a main Aβ species inducing neurodegeneration in AD, yet the molecular mechanism(s) of its neurotoxicity remains elusive.1 At present, it is reported that oligomeric Aβ induces apoptotic neuronal death in the rat and mouse neurons in vitro and in vivo.2, 3
Previous studies found that expression levels of Bcl-2 family proteins, such as Bax, Bak, Bad, Bcl-2, Bim, Bcl-w and Bcl-x are altered in the vulnerable neurons in AD.4 Bcl-2 family is structurally defined by the presence of up to four conserved ‘BCL-2 homology' (BH) domains. The family proteins are the key regulators of evolutionally conserved pathway of apoptosis.5, 6 Bax and Bak belong to the multi-BH domains pro-apoptotic subfamily, which promotes apoptosis by translocating into the mitochondrial membrane and facilitating cytochrome c release whereas Bcl-2 and Bcl-XL belong to the prosurvival subfamily, which prevents apoptotic death in multiple cell types including neuron.7 It is hypothesized that the BH3-only proteins, such as Bim, Bid, Puma, Noxa and Bad induce the activation of Bax and Bak, either directly or indirectly by inactivating the prosurvival Bcl-2 proteins. In the presence of apoptotic stimuli, Bax is known to change its conformation. Specifically, as an early step of Bax activation, the N-terminus exposure is considered a prerequisite for membrane insertion of Bax at mitochondria and multimerization of Bax.8, 9 Ku70, a DNA repair factor, can prevent the conformational change by binding with Bax in the cytosol, which leads to the inhibition of Bax-mediated cell death.10 Previously, a cell permeable Bax-inhibiting peptide (BIP), designed to induce the Bax-binding domain of Ku70, was found to rescue cells from Bax-mediated cell death.10, 11, 12
Notably, overexpression of anti-apoptotic proteins, Bcl-w, or genetic ablation of a proapoptotic effecter, Bim, significantly protected neurons against fibrillar Aβ-induced apoptosis in neuroblastoma cell lines and primary neuron culture.13, 14 Giovanni et al.15 also reported that fibrillar Aβ-induced cell death is dependent on Bax in primary neuron culture. However, these results have never been confirmed in vivo or in a more physiologically relevant model, and all the previous studies examined the toxicity of fibrillar Aβ, not oligomeric Aβ. Therefore, to advance our understanding of the involvement of Bcl-2 protein family as the major mechanism of oligomeric Aβ-induced neuronal cell death, in this study, we examined the effect of oligomeric Aβ on the regulation of Bcl-2/Bim/Bax and its functional importance in neuronal cell death in the organotypic hippocampal culture and mouse model for Aβ toxicity.
Results
Oligomeric Aβ induces Bim upregulation and Bcl-2 downregulation in hippocampal slice culture
To characterize oligomeric Aβ in this study, we synthesized Aβ peptide, and then denatured the peptide and allowed oligomers to form, as described for Aβ-derived diffusible ligands.1 Consistent with previous findings for oligomeric Aβ,1, 16 our oligomeric Aβ preparations contain spherical particles visible by negative staining with transmission electron microscopy, and western blots show that the preparations contain various size of oligomeric proteins (Figure 1).
Figure 1.
Physicochemical and morphological features of the synthetic Aβ1–42. (a) Electron micrograph shows the typical pattern of oligomer formation of Aβ. The arrows indicate oligomers in the lower panel. Scale bar, 100 nm. (b) Synthetic Aβ1–42 was subjected to SDS-polyacrylamide gel and detected by western blotting with 6E10 antibody. Molecular weight markers in kDa are at left
Time-dependent changes of the levels of Bax, Bcl-2 and Bim were examined in oligomeric Aβ-treated brain slices. Oligomeric Aβ increased the expression of Bim but decreased the level of Bcl-2 (Figure 2a). However, the level of Bax was not affected by oligomeric Aβ (Figure 2a). As the N-terminus exposure is an early step of Bax activation that occurs in the cytosol, we analyzed this conformational change of Bax with a monoclonal antibody (6A7) recognizing the epitope in the N-terminus of Bax.9 Although the level of Bax expression was not affected by oligomeric Aβ, the number of 6A7-positive cells were significantly increased in oligomeric Aβ treated slice cultures (Figure 2b), suggesting that oligomeric Aβ induces Bax activation.
Figure 2.
Oligomeric Aβ differentially regulates Bim, Bcl-2 and Bax in the hippocampal slice culture. (a) Representative western blots showed oligomeric Aβ induced upregulation of Bim and downregulation of Bcl-2 in a time-dependent manner. Bax levels were not changed by oligomeric Aβ treatment. Actin was used as internal loading control. The values of each band were normalized to that of actin and shown as a relative value of each group compared with the non-treatment slices. The indicated comparisons are significant at *P<0.05, n=4. (b) Immunocytochemistry analysis demonstrated the increased number of positive cells stained with 6A7 antibody, which specifically detect the active form of Bax, 24 h after oligomeric Aβ treatment in hippocampal slice cultures. Conversely, control peptide (Aβ42–1) did not induce the active form of Bax (green: 6A7, blue: DAPI). Scale bar, 100 μm
Ablation of Bax reduces oligomeric Aβ-induced neuronal cell death in hippocampal slice culture
To further determine the functional role of Bax in oligomeric Aβ-induced neuronal cell death, hippocampal slice culture prepared from wild-type and bax−/− mice (Figures 3a and b) were used. Slices were treated with oligomeric Aβ in the presence of propidium iodide (PI), which penetrates cell membranes of dead or dying cells, and is widely used for evaluation of cell death. Although Aβ42–1 peptide, a reverse sequence of Aβ1–42, had no effect on PI uptake, the number of PI-positive cells was significantly increased in oligomeric Aβ-treated slices after 48 h of the treatment, indicating that oligomeric Aβ induces neuronal cell death in the hippocampal slice culture. However, the neuronal cell death induced by oligomeric Aβ was dramatically reduced in the slice culture from bax−/− mice (Figure 3c). Nissl staining analysis further confirmed neuronal cell death by oligomeric Aβ in wild–type (WT) mouse slice culture but not in bax−/− mouse slice culture (Figure 3d). These results indicate that Bax is a critical mediator for the neurotoxicity induced by oligomeric Aβ.
Figure 3.
bax−/− neurons are resistant to oligomeric Aβ neurotoxicity. (a) DNA was extracted from bax+/+ and bax−/− mice, and each genotype was identified by PCR with the primer sets specifically detecting each genotype as described in the previous study. bax+/+ yields a 304-bp PCR product and bax−/− yields a 507-bp PCR product. (b) Total protein (20 μg) from whole brain was analyzed by immunoblot with anti-Bax antibody. Immunoblot analysis showed Bax in bax+/+ mouse samples at the expected molecular weight of 21 kDa, and no expression of Bax in bax−/− samples. (c) Hippocampal slice culture were treated with oligomeric Aβ (500 nM) in the presence of PI (red) for 48 h. Representative data showed oligomeric Aβ-induced PI uptake was significantly reduced in the slice cultures from bax−/− mice compared with bax+/+ mice. The PI uptake was quantitatively analyzed (n=5). Control Aβ42–1 peptide had no effect on PI uptake. Scale bar, 500 μm. *P<0.05, **P<0.01. (d) Neuronal cell loss by oligomeric Aβ (arrows) was significantly reduced in the hippocampal slice cultures from bax−/− mice. Scale bar, 500 μm
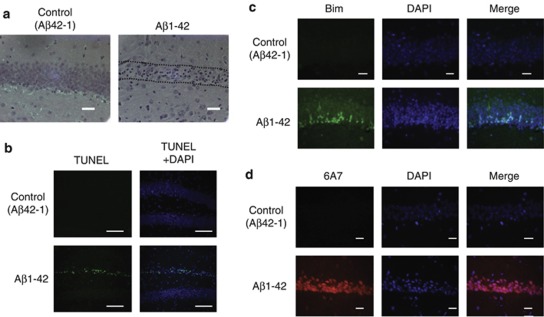
Intrahippocampal injection of oligomeric Aβ increases Bim expression and active forms of Bax
To further examine the involvement of pro-apoptotic proteins in oligomeric Aβ-induced cell death, we determined the change of the levels of Bim and the active form of Bax in vivo. We injected oligomeric Aβ into the hippocampus and assessed its neurotoxicity. Aβ42–1 was used as a control. Oligomeric Aβ injection into the WT mouse hippocampus induced neuronal cell death in 20 days after injection (Figure 4a). The number of terminal dUTP nick-end labeling (TUNEL)-positive neurons was significantly increased by Aβ injection (Figure 4b). To see the involvement of Bim and Bax, we measured the level of Bim and active form of Bax, and found that the level of Bim was dramatically increased as early as 10 days after oligomeric Aβ injection (Figure 4c). The number of neurons containing the active form of Bax recognized by 6A7 antibody was also dramatically increased by Aβ injection (Figure 4d). These data further support that both Bim and Bax have roles in oligomeric Aβ-induced neuronal cell death.
Figure 4.
Intrahippocampal injection of oligomeric Aβ upregulates Bim and activates Bax. C57BL/6J mice were killed and the levels of Bim and active Bax were analyzed at 10 or 20 days after oligomeric Aβ injection. (a) At 20 days after oligomeric Aβ injection, neuronal cell loss in hippocampus was evident in Aβ injected mice in H&E staining, but not in control peptide-injected mice. The region of neuronal cell loss is indicated by dotted lines. Scale bar, 100 μm. (b) The number of TUNEL-positive cells in hippocampus was dramatically increased after oligomeric Aβ injection whereas virtually no TUNEL-positive cell (green) was detected in control peptide-injected hippocampus tissues. Blue: DAPI. Scale bar, 200 μm. (c) Immunoreactivity for Bim (green) was increased in hippocampus at 10 days after the oligomeric Aβ injection. Scale bar, 100 μm. (d) The number of active Bax-positive cells detected by 6A7 antibody (red) was dramatically increased at 10 days after the oligomeric Aβ injection. Scale bar, 100 μm compared with control peptide-injected mice
BIP suppresses neuronal cell death and Bax activation induced by oligomeric Aβ in hippocampal slice culture
In this study, in addition to utilizing Bax knockout (KO) mice, we utilized BIP to examine the role of Bax in oligomeric Aβ-induced neurotoxicity. BIP used in this study consists of five amino acids, VPTLK, a sequence that is known to inhibit Bax activation.10, 11, 17 A mutated (scrambled) peptide, KLVPT, which does not bind Bax but has the same cell permeability, was used as a negative control. Both peptides were tagged with fluorescein isothiocyanate (FITC) so that intracellular delivery can be tracked by FITC signal. We first treated hippocampal slices with each peptide and analyzed green fluorescence to confirm their cell permeability, and confirmed that BIP and the control peptide equally penetrated neuronal cells after 24-h treatment (Figure 5a). To determine whether BIP is able to suppress oligomeric Aβ-induced neuronal cell death in slice culture, either BIP or control peptide were co-applied with oligomeric Aβ. After 24-h treatment of oligomeric Aβ and the peptide, the treatment of BIP significantly prevented PI uptake, whereas the control peptide did not affect the level of PI uptake (Figure 5b), suggesting BIP specifically blocks neuronal cell death induced by oligomeric Aβ. Consistent with the PI uptake results, both Nissl staining and caspase-3 immunoblot analyses also showed that BIP significantly suppressed oligomeric Aβ-induced neuronal loss and caspase-3 activation, but control peptide failed to prevent neuronal cell death (Figures 5c and d). We also found that the treatment of BIP suppresses oligomeric Aβ-induced conformational change of Bax (Figure 6). These results further support the hypothesis that the activation of Bax by oligomeric Aβ is an essential mechanism of oligomeric Aβ-induced neuronal cell death.
Figure 5.
Bax-inhibiting peptide (BIP) suppresses neuronal cell death induced by oligomeric Aβ. Either BIP (VPTLK) or control peptide (KLPVT) was added to hippocampal slice cultures at the same time with oligomeric Aβ to examine its neuroprotective effect. (a) Cell permeability of both peptides was confirmed by green fluorescence in hippocampal slice cultures. Strong green fluorescence of both BIP- and negative-control peptide was found at 24 h after the peptide treatment, indicating both peptides are penetrated to neurons. (b) The intensity of PI in slices treated with oligomeric Aβ for 48 h was quantified as a marker of cell death. BIP significantly prevented oligomeric Aβ-induced PI uptake (n=5). Scale bar, 500 μm. *P<0.01 versus control #P<0.05 versus Aβ only (−). Aβ42–1 peptide was used as a control. (c) Nissl staining demonstrated that the treatment of BIP significantly prevented neuronal cell loss induced by oligomeric Aβ. Arrows indicate the region of neuronal cell loss. (d) BIP suppressed the activation of caspase-3 (cleaved caspase-3) induced by oligomeric Aβ (n=4). *P<0.01 versus control, #P<0.05 versus Aβ only (−)
Figure 6.
BIP prevents the conformational change of Bax induced by oligomeric Aβ. BIP (VPTLK) was treated to hippocampal slice cultures with oligomeric Aβ for 48 h. Aβ42–1 was used as a control. The slices were stained with 6A7 antibody for detecting active conformational change of Bax. In the BIP-treated slices, the number and intensity of 6A7-positive signal (green) was significantly decreased compared with the slices treated with Aβ only. Scale bar, 100 μm
Discussion
In this study, we demonstrate that oligomeric Aβ altered the expression levels of Bcl-2, Bim and Bax, and that the genetic or pharmacological ablation of Bax activity suppresses oligomeric Aβ-mediated neurotoxicity in both ex vivo and in vivo. These results clearly indicate that Bax has an essential role in the induction of neuronal cell death caused by oligomeric Aβ.
In healthy cells, Bax is located in the cytosol or loosely associated to mitochondria and endoplasmic reticulum.18 Bax translocation to the mitochondria, which occurs in cells with apoptotic stresses, is thought to lead to mitochondrial dysfunction and release of cytochrome c and subsequent apoptosis.19 Before its translocation to mitochondria, Bax changes its conformation that exposes the N-terminal residues.20 This conformational change is believed to be necessary for membrane insertion of Bax at mitochondria and multimerization of Bax.21 The present study demonstrates that oligomeric Aβ induced the N-terminal exposure of Bax in neurons and that the inhibition of this event by BIP rescues neurons from oligomeric Aβ's neurotoxicity, suggesting the activation of Bax by its conformational change is a key element of oligomeric Aβ-induced neurotoxicity.
Although the molecular mechanism(s) of Bax activation has not been clearly defined, multiple pro-apoptotic proteins (e.g., Bim) and anti-apoptotic proteins (e.g., Bcl-2) are known to regulate the activation of Bax thorough heterodimerization.22 In fact, consistent with the previous findings, our current data demonstrate that the level of Bim is significantly upregulated after oligomeric Aβ treatment, whereas anti-apoptotic protein Bcl-2 is downregulated, suggesting that oligomeric Aβ induces neuronal cell death through the alteration of the balance of Bcl-2 family proteins and consequent activation of Bax. Unlike most ‘BH3-only' proteins, Bim can interact with all pro-survival Bcl-2 proteins with high affinity, and is one of the few BH3-only proteins that can directly activate Bax.23 For instance, several in vitro data suggested that the direct physical interaction between Bim and Bax can drive Bax activation.24, 25 Importantly, the ablation of Bim suppresses Aβ-induced cell death in primary neurons,13 and overexpression of anti-apoptotic proteins (Bcl-w) significantly protected neurons against Aβ-induced apoptosis.14 Previous reports suggested that overexpression of Bim in cultured neurons induces a rapid apoptotic death26 and Bim is highly expressed in AD neurons.13 Bim is also known to induce Bax activation directly or indirectly.27 Collectively, these data strongly suggest that Bim might be an upstream regulator of Bax activation in oligomeric Aβ-induced neurotoxicity and the pathological role of Bim and Bax in neuronal cell loss in AD.
However, it remains unknown how oligomeric Aβ activates Bim and consequent neuronal cell death. Although future study will be required to determine the mechanism, one potential pathway might be c-Jun N-terminal kinase (JNK)-dependent phosphorylation, as JNK-mediated phosphorylation promotes Bax-dependent apoptosis.28 As the activation of JNK by Aβ has been shown29 and the active form of JNK has been reported to be increased in vulnerable neurons in AD,30 it is plausible that the activation of JNK and subsequent activation of Bim/Bax-mediated apoptosis pathway might be the mechanism causing neuronal cell death in oligomeric Aβ-treated neurons and AD. Alternately, cyclin-dependent kinase 4 (Cdk4) has been suggested as an activator of Bim. Induction of Bim in nerve growth factor-deprived neuronal cells requires active Cdk4 and consequent reactivation of a series of genes suppressed by E2F.31 Among the reactivated genes, members of the myb transcription factor family, especially c-myb, seem to have a role in the activation of Bim. In previous studies, the inhibition of Cdk by various experimental approaches (i.e., chemical inhibitors, dominant-negative constructs, small interfering RNA) provide further evidence for the role of Cdk4 in the induction of Bim and neuronal cell death evoked by Aβ.13, 15, 32 Given that the elevated expression of cell cycle-related proteins including Cdk4 is evident in the vulnerable neurons in AD33, 34, 35 and Aβ-treated neuronal cells,32, 36 an abortive reentry into the cell cycle might induce neuronal cell death by upregulating Bim/Bax apoptotic pathway. Supporting the causal role of cell cycle reentry in neuronal cell death, we previously found that dysregulation of cell cycle reentry results in neurodegeneration in vivo.37
Although we did not examine mRNA level of Bim and its posttranslational change, a previous report demonstrated that fibrillar Aβ increases the level of Bim mRNA and protein.13 Thus, the increase of Bim expression by Aβ is likely mediated by transcriptional regulation. However, the increased translational modification such as phosphorylation of Bim might also be the reason for the increase of band intensity in our western blot data (Figure 2a). In fact, the western blot bands for upregulated Bim have a slightly higher molecular weight and this change may come from the phosphorylation of Bim, as Bim is known to be phosphorylated by several kinases. Cdk1/Cyclin B1 is one of the kinases to phosphorylate Bim.38 Interestingly, in our recent study (manuscript in preparation), we found that Aβ induced abnormal cell cycle upregulation as well as Cdk's activation and these events may have a role in posttranslational modification of Bim as seen in this study. Furthermore, the phosphorylation of Bim has been suggested to regulate Bax-dependent cell death.28 Therefore, the modification may regulate the apoptotic activity in our model. In future study, we will pursue the link between Cdk and Bim in Aβ neurotoxicity.
The present study showing Bax activation by oligomeric Aβ and the resistance of bax−/−neurons to oligomeric Aβ cell death suggest that Bax is a key mediator of oligomeric Aβ toxicity, and therefore BIP's protective effects against oligomeric Aβ can be translated as the result of Bax inhibition. BIP was developed as a Bax-inhibiting peptide, however, as usual in any type of drug, we cannot entirely exclude the possibility that BIP has unexpected target(s) in the cell, which can regulate cellular response to oligomeric Aβ. The future study developing therapeutics based on BIP will need further careful examination of the possibilities of the existence of the target(s) of BIP in addition to Bax.
In conclusion, in this study, we found that bax−/− mice are resistant to oligomeric Aβ-induced neuronal cell death, suggesting the essential role of Bax in neurotoxicity of oligomeric Aβ. Furthermore, we show for the first time that BIP application prevents oligomeric Aβ-induced neuronal cell death, suggesting that BIP and its mimetics may be utilized to mitigate the progress of AD by rescuing neurons from Bax-induced cell death. Our study also suggests that oligomeric Aβ regulates the activity of Bim, Bcl-2 and Bax in neurons. Taken all together, it is highly likely that the activation of Bax by the regulation of Bim and Bcl-2 family is crucial for oligomeric Aβ-induced neuronal cell death.
Materials and Methods
Materials
Anti-β-actin antibody was obtained from Millipore (Billerica, MA, USA) and anti-cleaved-caspase-3 antibody was obtained from Cell Signaling Technology (Danvers, MA, USA). Aβ peptide (Aβ1–42) and reverse control peptide (Aβ42–1) were purchased from AnaSpec (Fremont, CA, USA). PI and other chemicals were obtained from Sigma (St. Louis, MO, USA).
Preparation of oligomeric Aβ
Soluble oligomeric Aβ was prepared from synthetic peptide according to a previous paper.39 Briefly, 1 mg of Aβ1–42 peptide was dissolved in 120 μl of hexafluoroisopropanol for 60 min at room temperature, and placed back on ice for 5–10 min. After evaporation of hexafluoroisopropanol overnight in the hood at room temperature, the peptide was dissolved in 40 μl of fresh anhydrous DMSO, and further diluted to 5 mM stock solution. The stock peptide solution was then incubated for 24 h at 4 °C, and centrifuged at 14 000 × g for 10 min at 4 °C. Supernatant was used as oligomeric Aβ. Before we treated slice culture with oligomeric Aβ, the oligomers were incubated at room temperature for 20 h.
Mouse strains
Bax KO mice (strain name: B6.129 × 1-Bax tm1Sjk/J.) were purchased from The Jackson Laboratories (Bar Harbor, ME, USA). This Bax KO mouse has C57BL/6 genetic background. Bax KO mice were crossed with wild-type C57BL/6 mice, and bax+/− mouse colony was generated. Bax KO as well as WT mice used for this study were generated by crossing bax+/− mice in our mouse colony. Each experiment used a set of Bax KO and WT mice obtained from the same parent to minimize variations caused by genetical differences among mice. bax−/− mice were genotyped by PCR, as described previously. The primers for the wild-type and KO Bax alleles were 5′-GAGCTGATCAGAACCATCATG-3′ (sense) and 5′-CCGCTTCCATTGCTCAGCGG-3′ (antisense). Conditions were set as follows: 94° C, 3 min (1 cycle); 94° C, 30 s, 63° C, 1 min, 72° C, 1 min (35 cycles); 72° C, 2 min (1 cycle). All protocols involving the use of mice were approved by the Institutional Animal Care and Use Committee of Case Western Reserve University.
Electron microscopy
Aβ1–42 was adsorbed onto carbon films supported on Formvar (EMS, Hatfield, PA, USA) membrane-coated nickel grids. The excess buffered-protein solution was removed, and negatively stained with 2% uranyl acetate. Grids were then washed by touching the buffer and the excess buffer was immediately blotted using Whatman (Picataway, NJ, USA) filter paper. Grids were then air-dried and kept at room temperature. Negatively stained specimens were observed by a JEOL 1200EX electron microscope (JEOL, Tokyo, Japan) with 80 kV of electron acceleration voltage.
Preparation of organotypic hippocampal slice cultures
Organotypic hippocampal slice cultures were prepared as described previously.40 Briefly, hippocampal slice cultures were prepared using 7–10-day-old mouse pups. Slices were cut at 400 μm on a Mcllwain tissue chopper, transferred to Millicell membrane inserts (0.4 μm; Millipore), and placed in 6-well culture plates. The culture medium consisted of basal Eagle's medium with Earle's balanced salt solution, 20% heat-inactivated horse serum, enriched with 5.6 mM glucose. The medium was changed every other day. The effect of oligomeric Aβ (500 nM) was tested in the slices that had been maintained for 11–14 days in vitro. Aβ oligomer or reversed sequence of Aβ1–42 control peptide (Aβ42–1) was added to cultures in serum-free medium and, after the treatment, the hippocampal slices were rinsed twice in ice-cold phosphate-buffered saline (PBS), and then harvested by removing the Millicell membrane insert.
Assessment of neuronal cell death in organotypic hippocampal slice cultures
To determine neuronal cell death in the hippocampal slices, PI (5 μg/ml) was added to the slice culture medium. Images were acquired through an AxioCam camera on an Axiovert 200M microscope (Zeiss, Oberkochen, Germany). Fluorescent intensity was measured using Image J (NIH, Bethesda, MD, USA). Hematoxylin and eosin (H&E) and Nissl staining was also performed for routine histochemical and morphological analyses.
Protein extraction and western blot analysis
After oligomeric Aβ treatment, the slices were rinsed twice with ice-cold PBS and then lysed in ice-cold cell lysis buffer (Cell Signaling Technology) with protease and phosphatase inhibitor cocktail (Roche, Indianapolis, IN, USA). The protein concentration was determined by BCA assay (Pierce, Rockford, IL, USA). The extracted proteins were separated on 10 or 12% SDS-polyacrylamide gel and transferred onto polyvinylidene difluoride membranes. The blots were blocked with 10% non-fat milk in TBS-T for 1 h at room temperature, and then treated with primary antibodies diluted with 1% non-fat milk and incubated overnight at 4 °C. The following antibodies were used for western blot analysis: anti-caspase-3 (1 : 1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-Bim (1 : 1000; Stressgen, Farmingdale, NY, USA), anti-Bcl-2 (1 : 1000; Stressgen) and 6A7 (conformational specific Bax antibody) (1 : 1000; BD Pharmingen, San Diego, CA, USA).
Intrahippocampal injection of oligomeric Aβ
C57BL6/J mice (The Jackson Laboratories; 2–3-month-old) were anesthetized with pentobarbital and placed in a stereotaxic frame. Injection was made using a 10-μl microsyringe (Hamilton, Reno, NV, USA). A volume of 1 μl of oligomeric Aβ dissolved at 50 μM in PBS was injected into the left hippocampus. Control animals were prepared identically and injected with the same volume and concentration of Aβ42–1 in PBS. Injections were made at stereotaxic coordinates from bregma; antereoposterior (AP)=2.3 mm, mediolateral (ML)=2.5 mm and doroventral (DV)=−2.5 mm according to a previous report.41 This corresponds to a site in the dorsal hippocampus in the apical dendritic zones of the CA1 region near the hippocampal fissure. Mice were killed 10 or 20 days after injection, brains dissected out, fixed in 10% buffered-formalin and paraffin-embedded. For brain tissue sections, 6-μm-thick serial sections were cut, mounted onto slides and rehydrated according to standard protocols.
Immunocytochemistry
Cultured hippocampal slices were rinsed with ice-cold PBS once and fixed for 2 h with 4% paraformaldehayde in 0.1 M PBS.42 After washing with PBS, sections were permeabilized overnight with PBS containing 0.2% Triton X-100. At the end of the permeabilization blocking solution, 10% normal goat serum in PBS was applied for 4 h at room temperature. After washing with PBS, primary antibody was added and incubated for 24–48 h at 4 °C. After thorough washing of the sections in PBS, a secondary antibody labeled with either Alexa Fluor (Life Technologies, Grand Island, NY, USA) 488 or 568 (1 : 300) was added and incubated for 4 h at room temperature. All of the experiments contained at least one sample incubated without a primary antibody to exclude non-specific signal. Nuclei were visualized with DAPI. Images were acquired through an AxioCam camera on an Axiovert 200M microscope (Zeiss). Images were then analyzed with the Axiovision software (Zeiss).
TUNEL analysis
Detection of 3′-OH termini of DNA strand breaks was performed using in situ cell death detection kit (Roche). Briefly, the tissue sections were treated with proteinase K (20 μg/ml in 10 mmol/l Tris-HCl, pH 7.4) for 30 min at 37 °C after rehydration. After rinsing with PBS, TUNEL reaction mixture containing terminal deoxynucleotidyl transferase and fluorescence-labeled nucleotide was applied for 1 h 37 °C. The samples were then washed and mounted using Aquamount (Southern Biotech, Birmingham, AL, USA).
Statistical analysis
Data were expressed as the means±S.E.; the number of independent experiments is indicated in the corresponding figure legend. Differences between groups were examined for statistical significance using one-way analysis of variance with an unpaired Student's t-test. A P value of <0.05 indicated a statistically significant difference.
Acknowledgments
We thank Dr. Hisashi Fujioka for electron microscopy analysis. This work was supported by the National Institutes of Health (AG028679 to HL; AG031903 and P30CA043702 to SM); the Alzheimer's Association (IIRG-11-173471 to HL).
Glossary
- Aβ
β-amyloid
- AD
Alzheimer disease
- BH
BCL-2 homology
- BIP
Bax-inhibiting peptide
- PI
propidium iodide
- TUNEL
terminal dUTP nick-end labeling
- FITC
fluorescein isothiocyanate
- JNK
c-Jun N-terminal kinase
- cdk4
cyclin-dependent kinase 4
Dr. Xiongwei Zhu was a consultant for and received grant support from Medivation. Dr. Mark A Smith was a consultant for Anavex Life Sciences Corporation, Eisai, Medivation, Neurotez and Takeda Pharmaceuticals; owned stock options in Aria Neurosciences, Neurotez, Panacea and Voyager, and received lecture fees from GSK, Medivation and Pfizer.
Footnotes
Edited by D Bano
References
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, et al. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo W, Lee HP, Zou WQ, Wang X, Perry G, Zhu X, et al. Cellular prion protein is essential for oligomeric amyloid-beta-induced neuronal cell death. Hum Mol Genet. 2012;21:1138–1144. doi: 10.1093/hmg/ddr542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, Ferreira ST, et al. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282:11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Shimohama S, Kamoshima W, Ota T, Matsuoka Y, Nomura Y, et al. Alteration of proteins regulating apoptosis, Bcl-2, Bcl-x, Bax, Bak, Bad, ICH-1 and CPP32, in Alzheimer's disease. Brain Res. 1998;780:260–269. doi: 10.1016/s0006-8993(97)01202-x. [DOI] [PubMed] [Google Scholar]
- Davies AM. The Bcl-2 family of proteins, and the regulation of neuronal survival. Trends Neurosci. 1995;18:355–358. doi: 10.1016/0166-2236(95)93928-q. [DOI] [PubMed] [Google Scholar]
- Farrow SN, White JH, Martinou I, Raven T, Pun KT, Grinham CJ, et al. Cloning of a bcl-2 homologue by interaction with adenovirus E1B 19K. Nature. 1995;374:731–733. doi: 10.1038/374731a0. [DOI] [PubMed] [Google Scholar]
- Zornig M, Hueber A, Baum W, Evan G. Apoptosis regulators and their role in tumorigenesis. Biochim Biophys Acta. 2001;1551:F1–37. doi: 10.1016/s0304-419x(01)00031-2. [DOI] [PubMed] [Google Scholar]
- Hsu YT, Youle RJ. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J Biol Chem. 1998;273:10777–10783. doi: 10.1074/jbc.273.17.10777. [DOI] [PubMed] [Google Scholar]
- Nechushtan A, Smith CL, Hsu YT, Youle RJ. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 1999;18:2330–2341. doi: 10.1093/emboj/18.9.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez JA, Gama V, Yoshida T, Sun W, Hayes P, Leskov K, et al. Bax-inhibiting peptides derived from Ku70 and cell-penetrating pentapeptides. Biochem Soc Trans. 2007;35 (Pt 4:797–801. doi: 10.1042/BST0350797. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Tomioka I, Nagahara T, Holyst T, Sawada M, Hayes P, et al. Bax-inhibiting peptide derived from mouse and rat Ku70. Biochem Biophys Res Commun. 2004;321:961–966. doi: 10.1016/j.bbrc.2004.07.054. [DOI] [PubMed] [Google Scholar]
- Gomez JA, Chen J, Ngo J, Hajkova D, Yeh IJ, Gama V, et al. Cell-penetrating penta-peptides (CPP5s): measurement of cell entry and protein-transduction activity. Pharmaceuticals (Basel) 2010;3:3594–3613. doi: 10.3390/ph3123594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas SC, Shi Y, Vonsattel JP, Leung CL, Troy CM, Greene LA. Bim is elevated in Alzheimer's disease neurons and is required for beta-amyloid-induced neuronal apoptosis. J Neurosci. 2007;27:893–900. doi: 10.1523/JNEUROSCI.3524-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Wang Y, Ogawa O, Lee HG, Raina AK, Siedlak SL, et al. Neuroprotective properties of Bcl-w in Alzheimer disease. J Neurochem. 2004;89:1233–1240. doi: 10.1111/j.1471-4159.2004.02416.x. [DOI] [PubMed] [Google Scholar]
- Giovanni A, Keramaris E, Morris EJ, Hou ST, O'Hare M, Dyson N, et al. E2F1 mediates death of B-amyloid-treated cortical neurons in a manner independent of p53 and dependent on Bax and caspase 3. J Biol Chem. 2000;275:11553–11560. doi: 10.1074/jbc.275.16.11553. [DOI] [PubMed] [Google Scholar]
- Lauren J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Yokota T, Gama V, Yoshida T, Gomez JA, Ishikawa K, et al. Bax-inhibiting peptide protects cells from polyglutamine toxicity caused by Ku70 acetylation. Cell Death Differ. 2007;14:2058–2067. doi: 10.1038/sj.cdd.4402219. [DOI] [PubMed] [Google Scholar]
- Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- Putcha GV, Deshmukh M, Johnson EM. BAX translocation is a critical event in neuronal apoptosis: regulation by neuroprotectants, BCL-2, and caspases. J Neurosci. 1999;19:7476–7485. doi: 10.1523/JNEUROSCI.19-17-07476.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskes R, Desagher S, Antonsson B, Martinou JC. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol. 2000;20:929–935. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlich F, Banerjee S, Suzuki M, Cleland MM, Arnoult D, Wang C, et al. Bcl-x(L) retrotranslocates Bax from the mitochondria into the cytosol. Cell. 2011;145:104–116. doi: 10.1016/j.cell.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG, et al. BAX activation is initiated at a novel interaction site. Nature. 2008;455:1076–1081. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR, Chipuk JE. Apoptosis: stabbed in the BAX. Nature. 2008;455:1047–1049. doi: 10.1038/4551047a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield J, Neame SJ, Paquet L, Bernard O, Ham J. Dominant-negative c-Jun promotes neuronal survival by reducing BIM expression and inhibiting mitochondrial cytochrome c release. Neuron. 2001;29:629–643. doi: 10.1016/s0896-6273(01)00239-2. [DOI] [PubMed] [Google Scholar]
- Czabotar PE, Colman PM, Huang DC. Bax activation by Bim. Cell Death Differ. 2009;16:1187–1191. doi: 10.1038/cdd.2009.83. [DOI] [PubMed] [Google Scholar]
- Putcha GV, Le S, Frank S, Besirli CG, Clark K, Chu B, et al. JNK-mediated BIM phosphorylation potentiates BAX-dependent apoptosis. Neuron. 2003;38:899–914. doi: 10.1016/s0896-6273(03)00355-6. [DOI] [PubMed] [Google Scholar]
- Ma QL, Yang F, Rosario ER, Ubeda OJ, Beech W, Gant DJ, et al. Beta-amyloid oligomers induce phosphorylation of tau and inactivation of insulin receptor substrate via c-Jun N-terminal kinase signaling: suppression by omega-3 fatty acids and curcumin. J Neurosci. 2009;29:9078–9089. doi: 10.1523/JNEUROSCI.1071-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Ogawa O, Wang Y, Perry G, Smithzzz MA. JKK1, an upstream activator of JNK/SAPK, is activated in Alzheimer's disease. J Neurochem. 2003;85:87–93. doi: 10.1046/j.1471-4159.2003.01645.x. [DOI] [PubMed] [Google Scholar]
- Biswas SC, Liu DX, Greene LA. Bim is a direct target of a neuronal E2F-dependent apoptotic pathway. J Neurosci. 2005;25:8349–8358. doi: 10.1523/JNEUROSCI.1570-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanni A, Wirtz-Brugger F, Keramaris E, Slack R, Park DS. Involvement of cell cycle elements, cyclin-dependent kinases, pRb, and E2F x DP, in B-amyloid-induced neuronal death. J Biol Chem. 1999;274:19011–19016. doi: 10.1074/jbc.274.27.19011. [DOI] [PubMed] [Google Scholar]
- Webber KM, Raina AK, Marlatt MW, Zhu X, Prat MI, Morelli L, et al. The cell cycle in Alzheimer disease: a unique target for neuropharmacology. Mech Ageing Dev. 2005;126:1019–1025. doi: 10.1016/j.mad.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Zhu X, McShea A, Harris PL, Raina AK, Castellani RJ, Funk JO, et al. Elevated expression of a regulator of the G2/M phase of the cell cycle, neuronal CIP-1-associated regulator of cyclin B, in Alzheimer's disease. J Neurosci Res. 2004;75:698–703. doi: 10.1002/jnr.20028. [DOI] [PubMed] [Google Scholar]
- Bajic VP, Su B, Lee HG, Kudo W, Siedlak SL, Zivkovic L, et al. Mislocalization of CDK11/PITSLRE, a regulator of the G2/M phase of the cell cycle, in Alzheimer disease. Cell Mol Biol Lett. 2011;16:359–372. doi: 10.2478/s11658-011-0011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varvel NH, Bhaskar K, Patil AR, Pimplikar SW, Herrup K, Lamb BT. Abeta oligomers induce neuronal cell cycle events in Alzheimer's disease. J Neurosci. 2008;28:10786–10793. doi: 10.1523/JNEUROSCI.2441-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HG, Casadesus G, Nunomura A, Zhu X, Castellani RJ, Richardson SL, et al. The neuronal expression of MYC causes a neurodegenerative phenotype in a novel transgenic mouse. Am J Pathol. 2009;174:891–897. doi: 10.2353/ajpath.2009.080583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Fhearraigh S, Mc Gee MM. Cyclin B1 interacts with the BH3-only protein Bim and mediates its phosphorylation by Cdk1 during mitosis. Cell Cycle. 2011;10:3886–3896. doi: 10.4161/cc.10.22.18020. [DOI] [PubMed] [Google Scholar]
- Klein WL. Abeta toxicity in Alzheimer's disease: globular oligomers (ADDLs) as new vaccine and drug targets. Neurochem Int. 2002;41:345–352. doi: 10.1016/s0197-0186(02)00050-5. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Galimberti I, DePaola V, Caroni P. Preparation of organotypic hippocampal slice cultures for long-term live imaging. Nat Protoc. 2006;1:1165–1171. doi: 10.1038/nprot.2006.168. [DOI] [PubMed] [Google Scholar]
- Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11:909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogolla N, Galimberti I, DePaola V, Caroni P. Staining protocol for organotypic hippocampal slice cultures. Nat Protoc. 2006;1:2452–2456. doi: 10.1038/nprot.2006.180. [DOI] [PubMed] [Google Scholar]