Mitochondria are crucial in cell life, as they are the main intracellular source of energy, and have a main role in cell death as they contain molecules able to trigger apoptosis. However, mitochondria can also release molecules called ‘damage-associated molecular patterns' (DAMPs) that trigger a potent innate immune response and cause inflammation through the engagement of the Toll-like receptors (TLR). During human immunodeficiency virus (HIV) infection, a proinflammatory status is present that is not completely explained by the activity of the virus per se, or by the effective immune response against the virus. However, the presence of significant amounts of DAMPs of mitochondrial origin, such as extracellular plasmatic mtDNA, in the peripheral blood of subjects with HIV infection suggests that part of the inflammation typical of such infection could be because of the activity of these molecules, and thus opens new therapeutic perspectives.
Mitochondria are intracellular organelles involved in a wide range of processes, as they are the main source of cellular energy, in the form of ATP, are deeply involved in the regulation of programmed cell death, in the homeostasis of intracellular calcium, and are the site of several biosynthesis pathways. Furthermore, they are the main source of reactive oxygen species. Mitochondria derive from ancestral endosymbiont bacteria; as a reminiscence of such origin, they contain several copies of a circular genome, the mtDNA, which encodes key proteins of the oxidative phosphorylation system. Like bacterial proteins, those synthetized within mitochondria contains formyl-methionin, and their degradation origin chemotactic formyl peptides,1 which behave as danger signals and can activate inflammasome.2 When cells are coping with a massive insult that is potentially harmful for the entire organism, mitochondria release into the cytoplasm molecules able to trigger cell death, such as cytochrome-c or apoptosis-inducing factor.
Mitochondria Contain and Release DAMPs
Although mitochondria are well known as key regulators of cell death, in the last few years new roles as a component of innate immune system have emerged. Mitochondria have been shown to participate in intracellular signaling of retinoic acid-inducible gene I-like receptors through engagement of mitochondrial antiviral signaling protein, an adaptor protein localized on the outer mitochondrial membrane,3 and to be a source of DAMPs. DAMPs are conceptually similar to a set of conserved molecules defined ‘pathogen-associated molecular patterns' (PAMPs). PAMPs are released by several microorganisms and trigger a potent innate immune response, causing inflammation through the engagement of the same TLR that are bound by DAMPs. This novel, expanding family includes hyaluronan fragments, heat shock proteins, S100 proteins, amyloid-β, uric acid, interleukin (IL)-1α, IL-33, and the DNA-binding nuclear protein ‘high mobility group box 1'.4
In a pivotal paper, Zhang et al.5 studied patients who survive a severe trauma, a condition that can origin a life-threatening complication known as the ‘systemic inflammatory response syndrome' (SIRS), with shock and compromised function of several organs. SIRS closely resembles sepsis, and is due to DAMPs release into circulation. Zhang et al.5 demonstrated that mtDNA and mtDNA-encoded formyl peptides, collectively named mitochondrial DAMPs (MTDs), are released by damaged tissues, and found in high amounts in patients' plasma. Formyl peptides bind the formyl peptide receptor-1 (FPR-1), and mtDNA binds TLR-9, a receptor that responds to bacterial or viral DNA as well as synthetic oligodeoxynucleotides, containing unmethylated CpG motifs. Activation of these receptors results in a potent inflammatory reaction. Injecting in rats MTDs equivalent to 5% of the liver resulted in a marked inflammation, with accumulation of albumin and elastase in lungs, influx of inflammatory cells in the airways, and appearance of tumor necrosis factor (TNF)-α followed by IL-6. In vitro, clinical plasma concentrations of mtDNA behave like GM-CSF in promoting the secretion of IL-8 by neutrophils.
These results was further confirmed in patients undergoing reamings of femur facture; bone tissue debris release MTDs in plasma, which in turn cause lung injury by the release of proinflammatory cytokines and neutrophil recruitment and activation.6 In vitro studies show that triggering of such cells is mediated by mtDNA through p38 MAPK intracellular phosphorylation, and can be inhibited by chloroquine,7 an inhibitor of endosomal acidification, which is prerequisite for TLR-9 activation.8 Mitochondria-derived formyl peptides are able to trigger oxidative burst and stimulate chemotaxis in neutrophils.9
MTDs and HIV infection
The discovery of the inflammatory role of MTDs opens several new perspectives. First, the quantification of MTDs in plasma could become a marker of tissue injury, useful when a massive cell death exists that has a pathogenetic role in promoting or sustaining sterile inflammation, such as in the case of autoimmune or cardiovascular diseases, chemo- and radiotherapy of different tumors.
Second, MTDs can explain, at least partially, pathogenetic mechanisms in different diseases. Several infections, such as that provoked by HIV-1, are characterized by an important immune activation. HIV-1 infects intestinal memory CD4+ T cells causing their death and, as a consequence of decreased immune defenses, that of intestinal cells. Profound functional damages occur to mucosal surfaces, which lose their integrity and allow the translocation of microbial products from the intestinal lumen into the circulation. Then, cells of innate immunity produce proinflammatory cytokines, causing activation and exhaustion of the immune system.
In this scenario antibiotics are quite ineffective, suggesting the presence of other mechanisms. The release of MTDs by damaged, apoptotic, or secondary necrotic cells could explain persistent immune activation, which in turn causes the release of MTDs from apoptotic immune cells and origins a vicious circle. In the primate model of retroviral infection, cell activation is crucial for the survival of the animal. Infected sooty mangabeys display a low activation and survive; rhesus macaques have a highly activate immune system and die of AIDS. Studies on MTDs and their receptors could help in understanding the genetic basis of such difference.
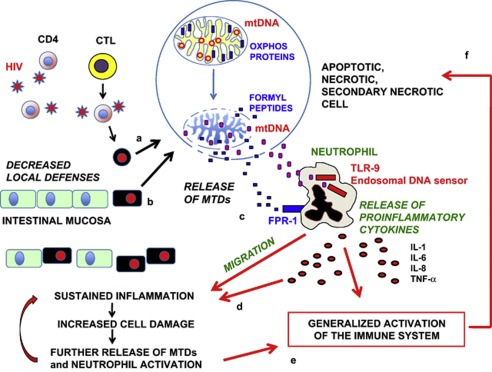
Few data are present on the role of soluble, plasma mtDNA in subjects with viral or bacterial infections. Very recently, we have measured plasma levels of mtDNA in different groups of HIV+ patients: those experiencing an acute HIV infection (AHI), long-term non progressors (LTNP), late presenters (LP) taking antiretroviral therapy for the first time, and healthy controls.10 In AHI and LP, mtDNA plasma levels were significantly (>10 times) higher than in healthy individuals or in LTNP; plasma mtDNA levels were neither correlated to peripheral blood CD4+ T cell count nor to markers of immune activation, but had a significant correlation with plasma viral load. Even if further studies are needed to confirm these observations, it might be that we have identified a possible, new role for mtDNA in the chronic inflammation that is typical of HIV infection, or, eventually, that soluble, extracellular mtDNA could be considered a new biomarker of virus-induced damage. A hypothetical mechanism by which MTDs sustain local inflammation and immune activation during HIV infection is shown in Figure 1.
Figure 1.
A possible, hypothetical mechanism by which MTDs sustain local inflammation and generalized immune activation during HIV infection. This schematic cartoon shows the hypothetical sequence of tissue damage caused by the release of MTDs during HIV-1 infection. Intestinal memory CD4+ T cells are infected by HIV-1 and, because of the direct action of the virus or the cytotoxicity exerted by cytotoxic T cells (CTL), are damaged and undergo cell death (a). This causes alterations in mitochondria that release MTDs, that is, mtDNA and formyl peptides that are products of the degradation of the proteins devoted to the synthesis of ATP by the process of oxidative phosphorylation (OXPHOS). Changes in the capacity to cope with the intestinal flora impair the local immune defenses, and this ultimately cause a damage to intestinal cells (b), with loss of the integrity of mucosal surfaces. MTDs released from damaged, apoptotic, necrotic, or secondary necrotic cells then bind their receptors on the surface of neutrophils; in particular, formyl peptides bind the FPR-1, whereas mtDNA bindsTLR-9 (c). Activation of neutrophils causes either the release of proinflammatory cytokines or the migration of these cells to the initial site of inflammation, with a further increase of the initial damage (d). Cytokines produced from neutrophils provoke a generalized activation of the immune system (e), and finally activated lymphocytes can undergo cell death/apoptosis (f), release MTDs, and thus continue the vicious circle. (Drawings are not in scale)
Concluding Remarks
Novel treatments could target either soluble MTDs or the receptors they use. Little is known about the possibility to block FPR-1 but molecules have been described that can inhibit such receptor.11 Immunoregulatory DNA sequences exist that bind and inhibit TLR-9.12 Molecules able to interfere with TLR-9 such as the antimalaria drugs chloroquine, hydrochloroquine, and quinacrine have been empirically used for 60 years in the treatment of immune-mediated inflammatory disorders, such as rheumatoid arthritis (RA), systemic lupus erythematosus, and Sjögren syndrome. The presence of mtDNA in synovial fluid of patients with RA and its capability act as proinflammatory molecule13 can explain the effects of such drugs in this context. The benefic effects of chloroquine that reduces the production/release of proinflammatory cytokines have been described in viral infections14 and recently confirmed in HIV+ patients;15 the well-known capability of such molecule to act as an inhibitor of TLR activity8, 16 can now be viewed not only as a mechanism to block PAMPs triggering of TLRs but also as a mechanism able to control DAMPs, and particularly MTDs, stimulation of innate immune system.
In conclusion, in diseases characterized by excessive inflammation, targeting FPR-1 and TLR-9 and/or interfering with soluble MTDs could reduce harmful immune activation. On the other hand, the use of MTDs for enhancing immune responses has to be explored.
Acknowledgments
This work was made possible by a grant awarded from Ministero della Salute, Roma, Italy (Programma Nazionale di Ricerca sull'AIDS 2009-2010, Istituto Superiore di Sanità, contract 40H71) to AC.
The authors declare no conflict of interest.
References
- Carp H. J Exp Med. 1982. pp. 264–275. [DOI] [PMC free article] [PubMed]
- Tschopp J. Eur J Immunol. 2011. pp. 1196–1202. [DOI] [PubMed]
- Seth RB, et al. Cell. 2005. pp. 669–682. [DOI] [PubMed]
- Sims GP, et al. Annu Rev Immunol. 2010. pp. 367–388. [DOI] [PubMed]
- Zhang Q, et al. Nature. 2010. pp. 104–107. [DOI] [PMC free article] [PubMed]
- Hauser CJ, et al. J Orthop Trauma. 2010. pp. 534–538. [DOI] [PMC free article] [PubMed]
- Zhang Q, et al. Shock. 2010. pp. 55–59. [DOI] [PubMed]
- Yasuda H, et al. Am J Physiol Renal Physiol. 2008. pp. F1050–F1058. [DOI] [PMC free article] [PubMed]
- Raoof M, et al. J Trauma. 2010. pp. 1328–1332. [DOI] [PubMed]
- Cossarizza A, et al. Mitochondrion. 2011. pp. 750–755. [DOI] [PubMed]
- Edwards BS, et al. Nat Protoc. 2006. pp. 59–66. [DOI] [PubMed]
- Barrat FJ, Coffman RL. Immunol Rev. 2008. pp. 271–283. [DOI] [PubMed]
- Collins LV, et al. J Leukoc Biol. 2004. pp. 995–1000. [DOI] [PubMed]
- Savarino A, et al. Lancet Infect Dis. 2003. pp. 722–727. [DOI] [PMC free article] [PubMed]
- Piconi S, et al. Blood. 2011. pp. 3263–3272. [DOI] [PubMed]
- Hong Z, et al. Int Immunopharmacol. 2004. pp. 223–234. [DOI] [PubMed]