Abstract
Post-translational modifications of α-synuclein occur in the brain of patients affected by Parkinson's disease and other α-synucleinopathies, as indicated by the accumulation of Lewy inclusions containing phosphorylated (at serine 129) and nitrated α-synuclein. Here we found that phospho-Ser 129 and nitrated α-synuclein are also formed within dopaminergic neurons of the monkey substantia nigra as a result of normal aging. Dopaminergic cell bodies immunoreactive for phospho-Ser 129 and nitrated α-synuclein were rarely seen in adult mature animals but became significantly more frequent in the substantia nigra of old primates. Dual labeling with antibodies against phospho-Ser 129 and nitrated α-synuclein revealed only limited colocalization and mostly stained distinct sub-populations of dopaminergic neurons. Age-related elevations of modified protein paralleled an increase in the number of neurons immunoreactive for unmodified α-synuclein, supporting a relationship between higher levels of normal protein and enhanced phosphorylation/nitration. Other mechanisms were also identified that likely contribute to α-synuclein modifications. In particular, increased expression of Polo-like kinase 2 within neurons of older animals could contribute to phospho-Ser 129 α-synuclein production. Data also indicate that a pro-oxidant environment characterizes older neurons and favors α-synuclein nitration. Aging is an unequivocal risk factor for human α-synucleinopathies. These findings are consistent with a mechanistic link between aging, α-synuclein abnormalities and enhanced vulnerability to neurodegenerative processes.
Keywords: monkey, nitration, Parkinson, phosphorylation, Polo-like kinase, synuclein
Pathological changes of α-synuclein are hallmarks of idiopathic Parkinson's disease (PD) and other age-related neurodegenerative disorders such as dementia with Lewy bodies and multiple system atrophy.1, 2 Intraneuronal accumulation of insoluble α-synuclein, as reflected by the formation Lewy bodies and Lewy neurites, is typically observed in postmortem brains of all PD patients, and the spreading of these inclusions throughout the brainstem, meso- and neo-cortex underlies a pathological staging of PD progression that was first proposed by Braak et al.3 Post-translational α-synuclein modifications are also a feature of PD: specific kinases catalyze α-synuclein phosphorylation (particularly at serine 129), and oxidative/nitrative reactions lead to the accumulation of nitrated α-synuclein within Lewy inclusions.4, 5, 6 Interestingly, protein deposition, phosphorylation and nitration may be interrelated, as suggested by findings showing that α-synuclein's tendency to aggregate is affected by its post-translational modifications.4, 7, 8 Furthermore, because modified forms of α-synuclein possess toxic properties, accumulation of insoluble, phosphorylated and/or nitrated protein could be a key event linking α-synuclein to neuronal dysfunction and, ultimately, neuronal demise in the pathogenesis of human α-synucleinopathies.9, 10, 11
Aging is an unequivocal PD risk factor, although the precise mechanisms by which neuronal susceptibility to degenerative processes is augmented by age remain unclear.12 Interestingly, recent work showing increased levels of α-synuclein protein in both human and non-human primate substantia nigra supports a relationship between aging and α-synuclein. Using a semiquantitative immunoblot analysis, Li et al.13 found a 100% increase in nigral α-synuclein in individuals >80 years of age as compared with subjects <60 years old; in contrast, α-synuclein levels were unaffected by age in the frontal cortex and caudate nucleus. Subsequent studies also reported an increase in the number, optical density and fluorescence intensity of α-synuclein-immunoreactive neurons as a function of age in the substantia nigra but not the ventral tegmental area of humans and rhesus monkeys.14, 15 Finally, experimental evidence indicates that age-related α-synuclein changes are rather unique to primates as levels of this protein actually decline in the mouse substantia nigra.16 The primate substantia nigra is highly vulnerable to both α-synuclein pathology and neurodegeneration, raising the intriguing possibility that this enhanced susceptibility is due, at least in part, to age-related α-synuclein elevation.
Elevated α-synuclein could itself promote pathological modifications of the protein, underscoring the relevance of studies on the effects of aging on α-synuclein aggregation, phosphorylation and nitration. Two previous investigations reported lack of α-synuclein deposition in the substantia nigra of young, middle-aged and old humans and monkeys.14, 15 No study to date, however, has assessed whether protein phosphorylation and nitration are affected by age and vary in parallel with changes in soluble and/or insoluble α-synuclein. In the present report, the number of neuronal cell bodies immunoreactive for normal, phospho-Ser 129 and nitrated α-synuclein was compared in the substantia nigra of young adult versus old squirrel monkeys. The focus on neuronal cell bodies is justified by the consideration that they are normally devoid of detectable immunoreactivity for nitrated or phosphorylated α-synuclein.4, 6 Data indicate an age-related increase in both normal and modified protein in the absence of overt α-synuclein aggregation. Results are also consistent with the interpretation that besides the increase in normal α-synuclein levels enhanced kinase expression and pro-oxidant/nitrative conditions contribute to the production of phospho-Ser 129 and nitrated α-synuclein, respectively, in older nigral neurons.
Results
Age-related α-synuclein accumulation within dopaminergic cell bodies
Aging was accompanied by an increase in nigral cell bodies immunoreactive for α-synuclein. Quantification of this effect revealed a significant difference between mature (<10 years of age) and old (>16 years old) monkeys. The total number of α-synuclein-immunoreactive cells was 60% greater in the latter than the former age group, and α-synuclein-positive cell bodies constituted 17% and 27% of the total count of nigral dopaminergic neurons in mature and old squirrel monkeys, respectively (Table 1). Interestingly, α-synuclein immunoreactivity was consistently associated with neuromelanin-containing neurons and, in fact, the number of unpigmented cell bodies expressing detectable levels of α-synuclein was negligible (<1% of the total count of dopaminergic cells) in either mature or old animals (Table 1).
Table 1. α-Synuclein-i.r. nigral cell counts in mature versus old monkeys.
| DAergic cells | α-Synuclein-i.r. cells | |||||
|---|---|---|---|---|---|---|
|
Age (years) |
Total |
NM containing |
No NM |
Total |
NM containing |
No NM |
| <10 (n=4) | 213±7.0 | 164±10 (77%) | 49±12 (23%) | 36.5±1.2 (17.1%) | 35.7±1.1 (16.7%) | 0.8±0.3 (0.4%) |
| >16 (n=3) | 218±3.0 | 206±1.0 (95%)* | 12±2.0 (5%)* | 58±4.6 (26.6%)* | 57.3±4.6 (26.3%)* | 0.7±0.3 (0.3%) |
Abbreviations: DAergic, dopaminergic; i.r., immunoreactive; NM, neuromelanin
For each animal, counts were made in two midbrain sections at the level of the third nerve, and values from the two sections were averaged. The total number of DAergic neurons was the sum of NM-containing cells plus tyrosine hydroxylase-i.r. neurons devoid of NM. α-Synuclein immunoreactivity was detected within pigmented neuronal cell bodies, i.e., NM-containing cells and, rarely, within neurons devoid of NM. Results are the means±S.E.M.; *P<0.01 versus the corresponding value in younger animals. Values in parenthesis show the percent of the total number of dopaminergic cells
Age-related α-synuclein phosphorylation
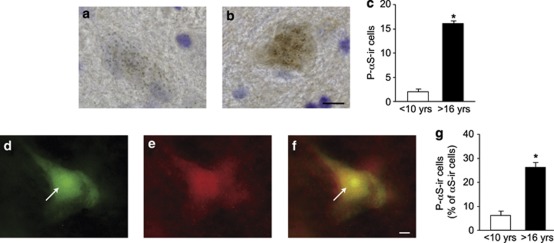
Phosphorylation at Ser129 has been reported to be the predominant modification of α-synuclein in Lewy bodies.17 An antibody that specifically recognizes phospho-Ser 129 α-synuclein4 was used to determine whether neuronal cell bodies in the primate substantia nigra contain this modified form of the protein and whether α-synuclein phosphorylation is enhanced by aging. Phospho-Ser 129 α-synuclein-immunoreactive neurons were rarely detected in midbrain sections from mature monkeys but became a more frequent feature of nigral specimens from old animals (Figures 1a and b). In the former, only ∼1% of dopaminergic neurons displayed detectable immunoreactivity for phospho-Ser 129 α-synuclein. In old animals, counts of these cells revealed an eightfold increase (Figure 1c), and nigral neurons immunoreactive for phospho-Ser 129 α-synuclein represented 7.3% of the total number of dopaminergic cells. To ensure specificity of these effects, tissue sections were stained with a second anti-phospho-Ser 129 α-synuclein antibody.17 A similar pattern of immunoreactivity and age-related changes was observed (data not shown).
Figure 1.
The number of dopaminergic cell bodies immunoreactive for phosphorylated α-synuclein is increased in the substantia nigra of old monkeys. Four mature and three old squirrel monkeys were used for these experiments. Representative midbrain sections from a mature (a) and an old (b) animal were immunostained for phospho-Ser 129 α-synuclein (brown) and counterstained with cresyl violet (purple). (c) The number of pigmented nigral neurons immunoreactive for phospho-Ser 129 α-synuclein was counted in mature (<10 years of age) and old (>16 years old) monkeys. Data are the means±S.E.M.; *P<0.001 versus the mature age group. A representative midbrain section from an aged monkey was dual labeled for phospho-Ser 129 α-synuclein (d) and (unmodified) α-synuclein (e). The merged image (f) shows coimmunoreactivity within a nigral neuron. Arrows indicate nuclear immunoreactivity. (g) In double-labeled sections, the number of neurons immunoreactive for phospho-Ser 129 α-synuclein was counted and expressed as percent of the total number of neurons stained for (unmodified) α-synuclein. Results are the means±S.E.M.; *P<0.001 versus the mature age group. Scale bar for panels a and b (in panel b)=10 μm. Scale bar for panels d–f (in panel f)=5 μm
In a second set of experiments, midbrain sections were double stained with antibodies against phospho-Ser 129 α-synuclein and unmodified α-synuclein. Fluorescence microscopy on sections stained for phospho-Ser 129 α-synuclein confirmed the formation of phosphorylated protein within dopaminergic neurons. Earlier investigations in vitro (e.g., HeLa cells) have reported a nuclear enrichment of phospho-Ser 129 α-synuclein, and data in mice (e.g., transgenic animals overexpressing α-synuclein) also showed nuclear anti-phospho-Ser 129 α-synuclein immunostaining, particularly in cortical brain regions.5, 18 Our present observations were consistent with the presence of both cytosolic and nuclear phospho-Ser 129 α-synuclein in the monkey substantia nigra (Figure 1d). Colocalization of total and phospho-Ser 129 α-synuclein immunoreactivities was also assessed. In all instances, cell bodies stained for phospho-Ser 129 α-synuclein were also immunoreactive for unmodified α-synuclein, consistent with the interpretation that immunoreactivity with the former antibody indeed detected phosphorylated α-synuclein. Approximately 5% of neuronal cell bodies immunoreactive for total (i.e., unmodified) α-synuclein also contained phosphorylated protein in mature monkeys; this percentage dramatically increased in old animals in which immunoreactivity for phospho-Ser 129 α-synuclein characterized 25% of nigral neurons expressing normal α-synuclein (Figures 1d–g).
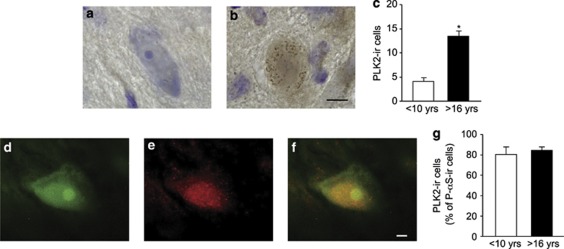
Members of the Polo-like kinase (PLK) protein family and, in particular, PLK2 have an important role in α-synuclein phosphorylation at Ser129.5, 19, 20 Using immunohistochemistry to identify and count PLK2-expressing neurons, we found that the number of cells displaying robust staining was >3-fold greater in the substantia nigra of old as compared with mature monkeys (Figures 2a–c). As these neurons also contained neuromelanin, data are consistent with a marked age-related enhancement of PLK2 levels within dopaminergic cell bodies. Dual staining and fluorescence microscopy allowed us to evaluate whether expression of PLK2 was associated with α-synuclein phosphorylation within the same neurons. Data revealed a substantial colocalization of phospho-Ser129 α-synuclein and PLK2, with >80% of phosphorylated α-synuclein-immunoreactive neurons also staining for PLK2 in the substantia nigra of either mature or old animals (Figures 2d–g).
Figure 2.
PLK2 immunoreactivity is enhanced in the substantia nigra of old (n=3) as compared with mature (n=4) squirrel monkeys. Representative midbrain sections from a mature (a) and an old (b) animal were immunostained for PLK2 (brown) and counterstained with cresyl violet (purple). (c) Pigmented PLK2-immunoreactive neurons were counted in the substantia nigra of mature (<10 years of age) and old (>16 years old) monkeys. Data are the means±S.E.M.; *P<0.001 versus the mature age group. A representative midbrain section from an old animal was dual labeled for phospho-Ser 129 α-synuclein (d) and PLK2 (e). The merged image (f) shows colabeling within a nigral neuron. (g) In double-labeled sections, the number of neurons immunoreactive for PLK2 was counted and expressed as percent of the total number of neurons stained for phospho-Ser 129 α-synuclein. Results are the means±S.E.M. Scale bar for panels a and b (in panel b)=10 μm. Scale bar for panels d–f (in panel f)=5 μm
α-Synuclein nitration in aging nigral neurons
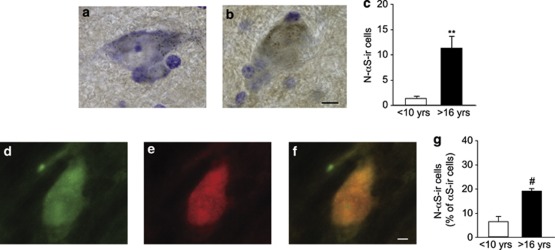
Antibodies that react with nitrated tyrosine residues of α-synuclein label Lewy inclusions in PD and other human α-synucleinopathies.6 Midbrain sections from mature and old monkeys were therefore stained with an anti-nitrated α-synuclein antibody to detect potential age-related modifications. Similar to the results with phosphorylated protein, immunoreactivity for nitrated α-synuclein was rarely detected within nigral neurons of mature animals; indeed, only 0.6% of dopaminergic neurons displayed detectable immunoreactivity for nitrated α-synuclein in this age group. In contrast, the anti-nitrated α-synuclein antibody robustly stained neuromelanin-containing cell bodies scattered throughout the substantia nigra of old monkeys (Figures 3a and b). Cell counts using bright-field microscopy confirmed that the number of neurons containing nitrated α-synuclein was nine times higher in the older age group (Figure 3c). In these animals, the count of neurons positive for nitrated α-synuclein was 5.2% of the total number of nigral dopaminergic neurons (data not shown).
Figure 3.
The number of dopaminergic cell bodies immunoreactive for nitrated α-synuclein is increased in the substantia nigra of old (n=3) as compared with mature (n=4) squirrel monkeys. Representative midbrain sections from a mature (a) and an old (b) animal were immunostained with an anti-nitrated α-synuclein antibody (brown) and counterstained with cresyl violet (purple). (c) The number of pigmented nigral neurons immunoreactive for nitrated α-synuclein was counted in mature (<10 years of age) and old (>16 years old) monkeys. Data are the means±S.E.M.; **P<0.005 versus the mature age group. A representative midbrain section from an aged monkey was dual labeled with anti-α-synuclein (d) and anti-nitrated α-synuclein (e) antibodies. The merged image (f) shows coimmunoreactivity within a nigral neuron. (g) In double-labeled sections, the number of neurons immunoreactive for nitrated α-synuclein was counted and expressed as percent of the total number of neurons stained for (unmodified) α-synuclein. Results are the means±S.E.M.; #P<0.01 versus the mature age group. Scale bar for panels a and b (in panel b)=10 μm. Scale bar for panels d–f (in panel f)=5 μm
To determine what percentage of cell bodies immunoreactive for unmodified α-synuclein contained detectable levels of nitrated protein, neuronal counts were performed using fluorescence microscopy on sections double labeled for total and nitrated α-synuclein: data indicate that the count of cell bodies immunoreactive for nitrated α-synuclein, expressed as a percentage of the total number of α-synuclein-positive neurons, increased from 6.3% in mature monkeys to 19.4% in old animals (Figures 3d–g).
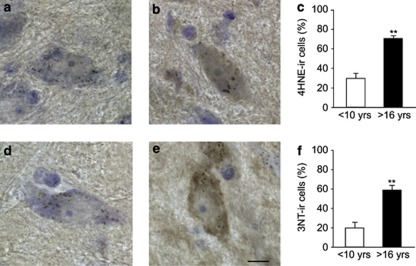
The formation of nitrated α-synuclein is the result of nitrative reactions that could be promoted within a pro-oxidant environment. The presence of such an environment within aging dopaminergic neurons was supported by experiments in which midbrain monkey tissues were immunostained with antibodies against 4-hydroxy-2-nonenal, a product of lipid peroxidation, or 3-nitrotyrosine, a marker of protein oxidation/nitration. In both instances, the number of immunoreactive nigral neurons was significantly increased with age. The percentage of neuromelanin-containing cells that were also immunoreactive for 4-hydroxy-2-nonenal was 25% and 70% in mature and old animals, respectively (Figures 4a–c); staining with anti-3-nitrotyrosine characterized 20 and 60% of nigral dopaminergic neurons in the two age groups (Figures 4d–f).
Figure 4.
Immunolabeling for markers of oxidative stress is enhanced within older nigral neurons. Four mature and three old squirrel monkeys were used for these experiments. Representative midbrain sections from a mature (a and d) and an old (b and e) monkey were immunostained for either 4-hydroxy-2-nonenal (a and b) or 3-nitrotyrosine (d and e; brown) and counterstained with cresyl violet (purple). Pigmented cell bodies with robust immunoreactivity for these markers of oxidative stress (b and e) are more typically seen in the substantia nigra of old monkeys. (c and f) The number of neurons immunoreactive for either 4-hydroxy-2-nonenal (c) or 3-nitrotyrosine (f) was counted in sections from mature (<10 years of age) and old (>16 years old) monkeys, and expressed as percent of the total number of neuromelanin-containing nigral neurons. Results are the means±S.E.M.; **P<0.005 versus the corresponding mature age group. Scale bar for panels a–e (in panel e)=10 μm
Colocalization of phosphorylated and nitrated α-synuclein
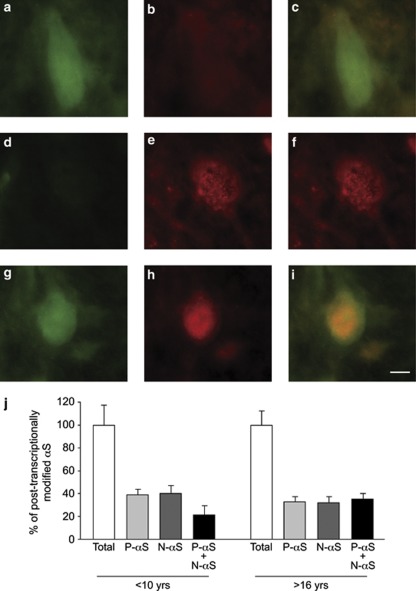
The formation of phosphorylated and nitrated α-synuclein and their parallel accumulation in the aging substantia nigra raise the question of whether these modified forms of the protein colocalize within the same dopaminergic neurons. To address this question, midbrain sections from mature and old monkeys were double immunostained with specific antibodies against phospho-Ser 129 and nitrated α-synuclein. Neurons characterized by single or double staining were observed throughout the nigral tissues of monkeys from the two age groups (Figures 5a–i). These single- or double-labeled cells were counted separately; then, the number of neurons immunoreactive for (i) phospho-Ser 129 α-synuclein only, (ii) nitrated α-synuclein only, or (iii) both phospho-Ser 129 and nitrated α-synuclein were expressed as percent of the total count (single+double stained) of immunoreactive cells. Results revealed that 30–40% of cells were single labeled for phospho-Ser 129 α-synuclein, whereas another 30–40% of neurons were stained with the anti-nitrated α-synuclein antibody (Figure 5j). Only the remaining 20–30% of cell bodies displayed immunoreactivity for both forms of modified α-synuclein, indicating that protein phosphorylation and nitration did not necessarily occur within the same population of nigral neurons. This percentage of single- and double-stained cells was not significantly different in tissues from either mature or old monkeys (Figure 5j). Thus, although the number of neurons immunoreactive for phospho-Ser 129 or nitrated α-synuclein augments with age (Figures 1 and 3), the proportion of cells in which these modified forms of the protein colocalize remains relatively constant (Figure 5j).
Figure 5.
Immunoreactivities for phosphorylated and nitrated α-synuclein colocalize only in part within nigral neuronal cell bodies. Four mature and three old squirrel monkeys were used for these experiments. A representative midbrain section from an old monkey was dual labeled for phospho-Ser 129 (a, d and g) and nitrated (b, e and h) α-synuclein. Merged images show different neurons in which phosphorylated and nitrated α-synuclein do (i) or do not (c and f) colocalize. (j) In dual labeled sections, the number of neurons immunoreactive for (i) phosphorylated α-synuclein only (P-αS), (ii) nitrated α-synuclein only (N-αS) or (iii) both phosphorylated and nitrated α-synuclein was counted in the substantia nigra of mature (<10 years of age) and old (>16 years old) monkeys. Results (means±S.E.M.) are expressed as percent of the total count of single- and double-stained cells, i.e., P-αS, N-αS and P-αS+N-αS. Scale bar for panels a–i (in panel i)=10 μm
Lack of α-synuclein aggregation
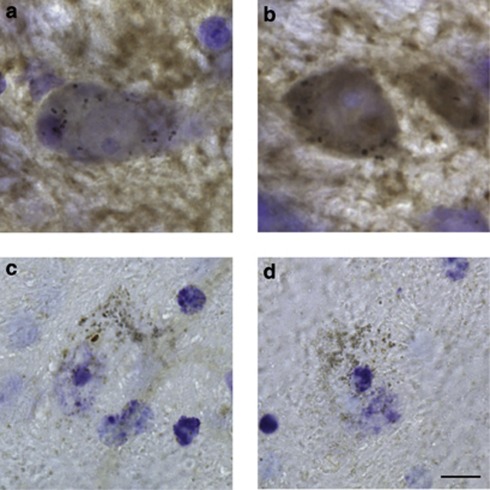
In tissues immunostained for α-synuclein, immunoreactivity labeled the neuropil as well as a few dopaminergic (neuromelanin-containing) neuronal cell bodies (Figures 6a and b). To determine if age-related changes in the number of cell bodies stained for normal, phosphorylated and nitrated α-synuclein was accompanied by formation of insoluble protein, midbrain sections were incubated with proteinase K before staining with the anti-α-synuclein antibody. Immunoreactivity was completely eliminated by proteinase K pre-treatment in samples from either mature or old monkeys, indicating a lack of age-related accumulation of insoluble/aggregated (proteinase K resistant) α-synuclein (Figures 6c and d).
Figure 6.
Lack of insoluble α-synuclein within nigral dopaminergic cell bodies of aging monkeys. Representative midbrain sections from a mature (a and c) and an old (b and d) animal were immunostained for α-synuclein (a and b) or incubated with proteinase K before α-synuclein immunolabeling (c and d). Sections were counterstained with cresyl violet (purple). Dopaminergic neurons are characterized by their content of neuromelanin (black granules). Pigmented cell bodies with robust α-synuclein immunoreactivity (brown) (b) are more typically seen in the substantia nigra of old monkeys. Proteinase K pre-treatment removes α-synuclein immunoreactivity, leaving only neuromelanin granules. Scale bar for panels a–d (in panel d)=10 μm
Discussion
The purpose of this study was to investigate age-related modifications of α-synuclein that specifically occur within dopaminergic cell bodies in the primate substantia nigra. Biochemical assays (e.g., western blot analysis) performed on tissue homogenates would have not been suitable to detect changes within distinct neuronal populations. Therefore, the effects of aging on nigral dopaminergic neurons were assessed after immunostaining monkey midbrain sections with specific antibodies against unmodified, phosphorylated or nitrated α-synuclein. Phosphorylation and nitration generate pathological forms of α-synuclein observed in the brain of PD patients.4, 6 A significant outcome of this study is the demonstration that phosphorylated and nitrated α-synuclein also accumulate within neuronal cell bodies in the primate substantia nigra as a consequence of normal aging.
In agreement with earlier investigations,14, 15 we found a significant increase in the number of dopaminergic cell bodies immunoreactive for unmodified α-synuclein in the substantia nigra of old as compared with adult mature monkeys. Previous work has also shown that older neurons are characterized by enhanced immunoreactivity for unmodified α-synuclein, consistent with an increase in intraneuronal protein concentration.14 Post-translational modifications of α-synuclein as a function of aging, which were revealed in the present study, were primarily reflected by changes in the number of immunoreactive neurons. Indeed, dopaminergic cell bodies positive for phospho-Ser 129 or nitrated α-synuclein were rarely seen in adult monkeys; in contrast, a sizable sub-population of cells immunoreactive for phosphorylated and/or nitrated α-synuclein became evident in old animals. In the latter, ∼30% of all dopaminergic (neuromelanin-containing) neurons stained positive for unmodified α-synuclein; 25% and 20% of these α-synuclein-positive cells were co-immunoreactive for phospho-Ser 129 and nitrated α-synuclein, respectively.
Results also provide important clues on the mechanisms leading to α-synuclein phosphorylation and nitration. As already mentioned, both the current and earlier studies have shown elevated α-synuclein in the substantia nigra of humans and non-human primates as a function of age.13, 14, 15 It is quite conceivable therefore that changes in α-synuclein expression and post-translational modifications of the protein are related events, with higher α-synuclein resulting in more pronounced phosphorylation and/or nitration. The reasons for marked α-synuclein elevation within older dopaminergic neurons remain unclear. An intriguing possibility, however, concerns the role of age-related changes in protein degradation pathways and, in particular, the lysosomal clearance system. Strong experimental evidence indicates that soluble monomeric α-synuclein is a substrate for chaperone-mediated autophagy (CMA) and that CMA activity declines as a result of aging as well as in some age-related diseases, including PD.21, 22 It is also noteworthy that phosphorylated and nitrated α-synuclein are less susceptible to CMA degradation than the unmodified protein,23 a feature that could contribute to their intraneuronal accumulation.
Our present findings indicate that other mechanisms besides increased levels of unmodified α-synuclein contribute to its phosphorylation and nitration. A number of kinases (e.g., casein kinases and G-protein-coupled receptor kinases) have been reported to partially phosphorylate α-synuclein in vitro. More recently, however, a primary role of PLK2 in catalyzing α-synuclein phosphorylation at serine 129 has been underscored by evidence of its specific and quantitative (>95%) effect on α-synuclein conversion.5, 20 Furthermore, PLK2 levels have been reported to be enhanced in postmortem brains of patients affected by Alzheimer's disease and Lewy body disease.5 In view of these considerations, we assessed a possible relationship between increased α-synuclein phosphorylation and age-related PLK2 changes. Indeed, a significantly greater number of PLK2-immunoreactive dopaminergic cells characterized the substantia nigra of old monkeys. Double staining of midbrain tissue sections with antibodies against PLK2 and phospho-Ser 129 α-synuclein revealed substantial colocalization in both adult and old monkeys. The number of colabeled neurons increased in older monkeys, further supporting a relationship between enhanced PLK2 expression and age-dependent α-synuclein phosphorylation.
A pro-oxidant environment characterizes dopaminergic neurons and is reflected by their accumulation of neuromelanin, a product of dopamine oxidative metabolism.24 Evidence from earlier investigations suggests that, in the presence of neuromelanin and under oxidative conditions, α-synuclein may precipitate around pigment-associated lipid droplets.25, 26 In this study, the effect of aging in promoting oxidative/nitrative reactions and α-synuclein/neuromelanin interactions was supported by findings showing (i) an increased number of neuromelanin-loaded cells with age, (ii) the occurrence of α-synuclein elevation almost exclusively within pigmented neurons and (iii) enhanced counts of neurons immunoreactive for 4-hydroxy-2-nonenal and 3-nitrotyrosine, two markers of oxidative/nitrative reactions. Taken together, these results are also compatible with the interpretation that α-synuclein accumulation in a setting favoring oxidative modifications leads to the formation of nitrated protein within aging dopaminergic cells.
Parallel increases in phosphorylated and nitrated α-synuclein raised the possibility that these modified forms of the protein may be generated within the same sub-population of nigral dopaminergic neurons. However, colocalization experiments did not support this hypothesis and, in the majority of instances, antibodies against phospho-Ser 129 and nitrated α-synuclein-labeled distinct neurons. This finding bears implications for the mechanisms of α-synuclein phosphorylation and nitration. If protein modifications were a mere consequence of enhanced α-synuclein, a greater degree of phospho-Ser 129 and nitrated α-synuclein colocalization might have been expected. Instead, limited coimmunostaining suggests that formation of phosphorylated and nitrated protein, although promoted by a common setting of age-related α-synuclein elevation, involves distinct mechanisms. As discussed above, older neurons with higher kinase expression would produce phospho-Ser 129 α-synuclein, whereas enhanced pro-oxidant conditions would favor the formation of nitrated protein.
Post-translational modifications affect the biological activity and toxic potential of α-synuclein. For example, phosphorylation has been suggested to modulate α-synuclein's interaction with phospholipids and other proteins (e.g., tau), and nitrated α-synuclein is capable of inducing adaptive immune responses and may exacerbate microglial activation.8, 27, 28, 29 Thus, neuronal α-synuclein accumulation and formation of phospho-Ser 129 and nitrated α-synuclein are age-related features of likely pathophysiological relevance. They could contribute to the progressive decline that characterizes the nigrostriatal system of older primates and have an important role in rendering aging dopaminergic cells increasingly vulnerable to neurodegenerative processes.14, 30 Similarities between α-synuclein modifications in the substantia nigra of old primates and in the brain of PD patients, as described in this study, strongly support these two possibilities.
An important property of α-synuclein is its tendency to aggregate, which could underlie the pathogenesis of Lewy inclusions in PD and may cause neuronal injury via the formation of toxic oligomeric and fibrillar species.10 Several lines of experimental evidence indicate that phosphorylation and nitration are likely to affect α-synuclein aggregation, although the precise relationship linking protein modifications to aggregate formation remains unclear. Initial studies reported that phospho-Ser 129 α-synuclein promoted deposition of insoluble protein, whereas subsequent investigations showed opposite results.4, 8 Similar inconsistencies have been found with nitrated α-synuclein, perhaps suggesting that the relationship between protein modifications and α-synuclein fibrillation/oligomerization may vary under different experimental conditions.7, 31 Our present findings do not support a direct role of phospho-Ser 129 and/or nitrated α-synuclein in inducing aggregation. In fact, despite the substantial increase in unmodified, phosphorylated and nitrated protein, no overt evidence of insoluble α-synuclein was found in the substantia nigra of aged monkeys. It is possible that small (e.g., oligomeric) aggregates may be formed but remained undetected under our experimental conditions. An alternative interpretation, however, is that other factors in addition to age-related changes are necessary to trigger α-synuclein aggregation. Potential culprits include (i) α-synuclein mutations,32 (ii) destabilization of aggregation-resistant forms of the protein,33 (iii) impairment of neuronal mitochondrial function34 and (iv) toxic dopaminergic cell injury.35 All these conditions are capable of promoting aggregation and, on the background of normal aging, could enhance α-synuclein pathogenicity and ultimately have a role in neurodegenerative processes.
Materials and Methods
Animals and tissue preparation
A total of seven squirrel monkeys (Saimiri sciureus) of both sexes were obtained from Osage Research Primates (Osage Beach, MO, USA). The animals were individually housed in a room with a 13/11-h light/dark cycle, with free access to water and a daily diet of monkey chow and fresh fruit. Animals were divided into two different age groups: <10 years of age (6–9 years, n=4) and >16 years old (17–19 years, n=3). These two age groups, representing mature adult and old animals, were chosen based on several observations in squirrel monkeys, including their average life span (18–22 years) and the age at which they reach sexual maturity and attain mature brain weight (2.5–3.5 years).30 All experimental protocols were in accordance with the standards established by the National Institutes of Health and the Office of the Prevention of Research Risks and were approved by the Institutional Animal Care and Use Committee.
The animals were euthanized using procedures consistent with the recommendations of the Panel of Euthanasia of the American Veterinary Medical Association. Animals were injected first with ketamine hydrochloride (15–20 mg/kg, i.m.) to provide restraint, and then with 0.22 ml/kg euthanasia solution (390 mg sodium pentobarbital and 50 mg phenytoin sodium/ml, i.v.). The brains were rapidly removed and dissected on ice. A tissue block encompassing the entire substantia nigra was fixed in 4% paraformaldehyde in 0.01 M phosphate-buffered saline (PBS, pH 7.4), cryoprotected in graded sucrose solutions and frozen in cold iso-pentane. Each midbrain block was cryostat-cut into 40-μm-thick sections through the full extent of the substantia nigra.
Immunohistochemistry
For bright-field microscopy, tissue sections were washed in PBS, and endogenous peroxidase was quenched by incubation in hydrogen peroxide solution. Sections were then blocked in 10% normal serum and incubated overnight at 4 °C in primary antibody. The list of antibodies and the dilution used for each antibody is shown in Table 2. The anti-nitrated α-synuclein antibody used in this study has been previously characterized.6 Briefly, the binding of this antibody is dependent on nitration of tyrosine residues at positions 125 and 136, with the latter being a stronger recognition epitope. Two different antibodies against phospho-Ser 129 α-synuclein were used to demonstrate specificity of the age-dependent effects.
Table 2. List of antibodies used for bright-field and fluorescence microscopy.
| Antigen | Supplier | Species | Dilution |
|---|---|---|---|
| Tyrosine hydroxylase | Pel Freez | Rabbit | 1 : 600 |
| α-Synuclein | Millipore | Mouse | 1 : 1000 |
| Phospho-Ser 129 α-synuclein | Wako | Mouse | 1 : 3000 |
| Phospho-Ser 129 α-synuclein | Gifta | Mouse | 1 : 3000 |
| Polo-like kinase 2 | Santa Cruz | Rabbit | 1 : 100 |
| Nitrated α-synuclein | Upstate | Mouse | 1 : 3000 |
| 4-Hydroxy-2-nonenal | Oxis Research | Mouse | 1 : 300 |
| 3-Nitrotyrosine | Upstate | Rabbit | 1 : 100 |
This antibody was generously provided by Elan Pharmaceuticals
Immunostaining was detected using the avidin–biotin immunoperoxidase method with 3,3′-diaminobenzadine as the chromagen (Vector Laboratories, Burlingame, CA, USA). Sections were lightly stained in cresyl violet (FD Neurotechnologies, Ellicott City, MD, USA), dehydrated and mounted in Depex mounting medium (EM Sciences, Hatfield, PA, USA). Sections from mature and old monkeys were always processed in parallel following identical procedures (e.g., 3,3′-diaminobenzadine incubation time). For immunolabeling with anti-phospho-Ser 129 α-synuclein, sections were incubated in 30% formic acid for 30 s and rinsed in PBS before immunostaining. Proteinase K treatment was performed as previously reported.35 Briefly, mounted sections were incubated at 55 °C in 50 mg/ml proteinase K (Invitrogen, Carlsbad, CA, USA) for 60 min, washed in PBS and then stained for α-synuclein as described above.
Separate sets of tissues were dual labeled for fluorescence microscopy with different combinations of primary antibodies (Table 2). Sections were incubated in the appropriate fluorescent secondary antibody conjugated to either FITC or Cy-3 (1 : 500, Jackson ImmunoResearch Laboratories, West Grove, PA, USA) and then mounted onto glass slides. Neuromelanin autofluoresence was blocked using the autofluorescence eliminator reagent (Millipore, Billerica, MA, USA) according to Kanaan et al.36 Tissues were observed using an Olympus BH2 light microscope (Olympus, Center Valley, PA, USA) equipped for epifluoresence. For dual labeling with anti-phospho-Ser 129 α-synuclein and anti-nitrated α-synuclein, two primary antibodies from the same host species (mouse) were used. Sections were therefore blocked with unconjugated donkey anti-mouse Fab IgG (H+L) fragment (1 : 100, Jackson ImmunoResearch Laboratories) after immunostaining for phospho-Ser 129 α-synuclein and before immunolabeling with anti-nitrated α-synuclein36; a distinct pattern of staining was observed for each primary antibody, confirming the effectiveness of the blocking procedure.
For both bright-field and fluorescence microscopy, control sections incubated in the appropriate IgG in lieu of primary antibody were devoid of staining. Control experiments were also carried out to ensure the specificity of the antibody used to detect phospho-Ser 129 α-synuclein. A set of midbrain sections was treated with alkaline phosphatase (Sigma, St. Louis, MO, USA) overnight at 37 °C and washed with PBS before incubation with hydrogen peroxide and primary antibodies against either phospho-Ser 129 α-synuclein or tyrosine hydroxylase.37 Sections immunostained for tyrosine hydroxylase retained their immunoreactivity, whereas immunolabeling for phospho-Ser 129 α-synuclein was no longer detectable.
Quantification
For each measurement, two midbrain sections at the level of the third nerve were chosen and immunostained with the appropriate antibody or combination of antibodies. The substantia nigra was delineated at low magnification ( × 1) using StereoInvestigator software (MBF Bioscience, Williston, VT, USA); the number of immunoreactive neurons was counted at higher magnification ( × 100) using the software's meander scan function. For each animal, values from the two sections were averaged. Data are presented as mean±S.E.M. Differences among means were analyzed using one-way ANOVA. Newman–Keuls post hoc analysis was used when differences were observed in ANOVA testing (P<0.05).
Acknowledgments
This work is supported by grants from the Parkinson's Disease Foundation and the Backus Foundation. We thank Drs. Amy Manning-Bog and Sarah Jewell for assistance with the experiments and manuscript review, and Dr. John P Anderson (Elan Pharmaceuticals, South San Francisco, California) for providing an antibody against phospho-Ser 129 α-synuclein.
Glossary
- CMA
chaperone-mediated autophagy
- PBS
phosphate-buffered saline
- PD
Parkinson's disease
- PLK
Polo-like kinase
The authors declare no conflict of interest.
Footnotes
Edited by A Verkhratsky
References
- Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goerdert M. Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with Lewy bodies. Proc Natl Acad Sci USA. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu PH, Galvin JE, Baba M, Giasson B, Tomita T, Leight S, et al. Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble α-synuclein. Ann Neurol. 1998;44:415–422. doi: 10.1002/ana.410440324. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, et al. α-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- Mbefo MK, Paleologou KE, Boucharaba A, Oueslati A, Schell H, Fournier M, et al. Phosphorylation of synucleins by members of the Polo-like kinase family. J Biol Chem. 2010;285:2807–2822. doi: 10.1074/jbc.M109.081950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, et al. Oxidative damage linked to neurodegeneration by selective α-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- Hodara R, Norris EH, Giasson BI, Mishizen-Eberz AJ, Lynch DR, Lee VM, et al. Functional consequences of α-synuclein tyrosine nitration: diminished binding to lipid vesicles and increased fibril formation. J Biol Chem. 2004;279:47746–47753. doi: 10.1074/jbc.M408906200. [DOI] [PubMed] [Google Scholar]
- Paleologou KE, Schmid AW, Rospigliosi CC, Kim HY, Lamberto GR, Fredenburg RA, et al. Phosphorylation at Ser-129 but not the phosphomimics S129E/D inhibits the fibrillation of α-synuclein. J Biol Chem. 2008;283:16895–16905. doi: 10.1074/jbc.M800747200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Feany MB. α-Synuclein phosphorylation controls neurotoxicity and inclusion formation in a Drosophila model of Parkinson disease. Nat Neurosci. 2005;8:657–663. doi: 10.1038/nn1443. [DOI] [PubMed] [Google Scholar]
- Uversky VN. Neuropathology, biochemistry, and biophysics of α-synuclein aggregation. J Neurochem. 2007;103:17–37. doi: 10.1111/j.1471-4159.2007.04764.x. [DOI] [PubMed] [Google Scholar]
- Oueslati A, Fournier M, Lashuel HA. Role of post-translational modifications in modulating the structure, function and toxicity of α-synuclein: implications for Parkinson's disease pathogenesis and therapies. Prog Brain Res. 2010;183:115–145. doi: 10.1016/S0079-6123(10)83007-9. [DOI] [PubMed] [Google Scholar]
- Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, et al. Incidence of Parkinson's disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003;157:1015–1022. doi: 10.1093/aje/kwg068. [DOI] [PubMed] [Google Scholar]
- Li W, Lesuisse C, Xu Y, Troncoso JC, Price DL, Lee MK. Stabilization of α-synuclein protein with aging and familial Parkinson's disease-linked A53T mutation. J Neurosci. 2004;24:7400–7409. doi: 10.1523/JNEUROSCI.1370-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Kordower JH. Age-associated increases of α-synuclein in monkeys and humans are associated with nigrostriatal dopamine depletion: is this the target for Parkinson's disease. Neurobiol Dis. 2007;25:134–149. doi: 10.1016/j.nbd.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Xuan Q, Xu SL, Lu DH, Yu S, Zhou M, Ueda K, et al. Increase expression of α-synuclein in aged human brain associated with neuromelanin accumulation. J Neural Transm. 2011;118:1575–1583. doi: 10.1007/s00702-011-0636-3. [DOI] [PubMed] [Google Scholar]
- Mak SK, McCormack AL, Langston JW, Kordower JH, Di Monte DA. Decreased α-synuclein expression in the aging mouse substantia nigra. Exp Neurol. 2009;220:359–365. doi: 10.1016/j.expneurol.2009.09.021. [DOI] [PubMed] [Google Scholar]
- Anderson JP, Walker DE, Goldstein JM, de Laat R, Balducci K, Caccavello RJ, et al. Phosphorylation of Ser-129 is the dominant pathological modification of α-synuclein in familial and sporadic Lewy body disease. J Biol Chem. 2006;281:29739–29752. doi: 10.1074/jbc.M600933200. [DOI] [PubMed] [Google Scholar]
- Schell H, Hasegawa T, Neumann M, Kahle PJ. Nuclear and neuritic distribution of serine-129 phosphorylated α-synuclein in transgenic mice. Neuroscience. 2009;160:796–804. doi: 10.1016/j.neuroscience.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Inglis KJ, Chereau D, Brigham EF, Chiou SS, Schöbel S, Frigon NL, et al. Polo-like kinase 2 (PLK2) phosphorylates α-synuclein at serine 129 in central nervous system. J Biol Chem. 2009;284:2598–2602. doi: 10.1074/jbc.C800206200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi M, Trashi E, Marin O, Negro A, Sarno S, Pinna LA. Superiority of PLK-2 as α-synuclein phosphorylating agent relies on unique specificity determinants. Biochem Biophys Res Commun. 2012;418:156–160. doi: 10.1016/j.bbrc.2011.12.152. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant α-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- Kaushik S, Cuervo AM. Chaperone-mediated autophagy. Methods Mol Biol. 2008;445:227–244. doi: 10.1007/978-1-59745-157-4_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Vicente M, Talloczy Z, Kaushik S, Massey AC, Mazzulli J, Mosharov EV, et al. Dopamine-modified α-synuclein blocks chaperone-mediated autophagy. J Clin Invest. 2008;118:777–788. doi: 10.1172/JCI32806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DG. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol Pharmacol. 1978;14:633–643. [PubMed] [Google Scholar]
- Fasano M, Giraudo S, Coha S, Bergamasco B, Lopiano L. Residual substantia nigra neuromelanin in Parkinsons's disease is cross-linked to α-synuclein. Neurochem Int. 2003;42:603–606. doi: 10.1016/s0197-0186(02)00161-4. [DOI] [PubMed] [Google Scholar]
- Halliday GM, Ophof A, Broe M, Jensen PH, Kettle E, Fedorow H, et al. α-Synuclein redistributes to neuromelanin lipid in the substantia nigra early in Parkinson's disease. Brain. 2005;128:2654–2664. doi: 10.1093/brain/awh584. [DOI] [PubMed] [Google Scholar]
- Pronin AN, Morris AJ. Surguchov, Benovic JL. Synucleins are a novel class of substrates for G protein-coupled receptor kinases. J Biol Chem. 2000;275:26515–26522. doi: 10.1074/jbc.M003542200. [DOI] [PubMed] [Google Scholar]
- Benner EJ, Banerjee R, Reynolds AD, Sherman S, Pisarev VM, Tsiperson V, et al. Nitrated α-synuclein immunity accelerates degeneration of nigral dopaminergic neurons. PLoS One. 2008;3:e1376. doi: 10.1371/journal.pone.0001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AD, Stone DK, Mosley RL, Gendelman HE. Nitrated α-synuclein-induced alterations in microglial immunity are regulated by CD4+ T cell subsets. J Immunol. 2009;182:4137–4149. doi: 10.4049/jimmunol.0803982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack AL, Di Monte DA, Delfani K, Irwin I, DeLanney LE, Langston WJ, et al. Aging of the nigrostriatal system in the squirrel monkey. J Comp Neurol. 2004;471:387–395. doi: 10.1002/cne.20036. [DOI] [PubMed] [Google Scholar]
- Yamin G, Uversky VN, Fink AL. Nitration inhibits fibrillation of human α-synuclein in vitro by formation of soluble oligomers. FEBS Lett. 2002;542:147–152. doi: 10.1016/s0014-5793(03)00367-3. [DOI] [PubMed] [Google Scholar]
- Li J, Uversky VN, Fink AL. Effect of familial Parkinson's disease point mutations A30P and A53T on the structural properties, aggregation, and fibrillation of human α-synuclein. Biochemistry. 2001;40:11604–11613. doi: 10.1021/bi010616g. [DOI] [PubMed] [Google Scholar]
- Bartels T, Choi JG, Selko DJ. α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477:107–110. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Shin SY, Choi C, Lee YH, Lee SJ. Formation and removal of α-synuclein aggregates in cell exposed to mitochondrial inhibitors. J Biol Chem. 2002;277:5411–5417. doi: 10.1074/jbc.M105326200. [DOI] [PubMed] [Google Scholar]
- McCormack AL, Mak SK, Shenasa M, Langston WJ, Forno LS, Di Monte DA. Pathologic modifications of α-synuclein in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated squirrel monkeys. J Neuropathol Exp Neurol. 2008;67:793–802. doi: 10.1097/NEN.0b013e318180f0bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan NM, Kordower JH, Collier TJ. Age-related accumulation of Marinesco bodies and lipofuscin in rhesus monkey midbrain dopamine neurons: relevance to selective neuronal vulnerability. J Comp Neurol. 2007;502:683–700. doi: 10.1002/cne.21333. [DOI] [PubMed] [Google Scholar]
- Mandell JW, Banker GA. A spatial gradient of tau protein phosphorylation in nascent axons. J Neurosci. 1996;16:5727–5740. doi: 10.1523/JNEUROSCI.16-18-05727.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]