Abstract
p57 (Kip2, cyclin-dependent kinase inhibitor 1C), often found downregulated in cancer, is reported to hold tumor suppressor properties. Originally described as a cyclin-dependent kinase (cdk) inhibitor, p57KIP2 has since been shown to influence other cellular processes, beyond cell cycle regulation, including cell death and cell migration. Inhibition of cell migration by p57KIP2 is attributed to the stabilization of the actin cytoskeleton through the activation of LIM domain kinase-1 (LIMK-1). Furthermore, p57KIP2 is able to enhance mitochondrial-mediated apoptosis. Here, we report that the cell death promoting effect of p57KIP2 is linked to its effect on the actin cytoskeleton. Indeed, whereas Jasplakinolide, an actin cytoskeleton-stabilizing agent, mimicked p57KIP2's pro-apoptotic effect, destabilizing the actin cytoskeleton with cytochalsin D reversed p57KIP2's pro-apoptotic function. Conversely, LIMK-1, the enzyme mediating p57KIP2's effect on the actin cytoskeleton, was required for p57KIP2's death promoting effect. Finally, p57KIP2-mediated stabilization of the actin cytoskeleton was associated with the displacement of hexokinase-1, an inhibitor of the mitochondrial voltage-dependent anion channel, from the mitochondria, providing a possible mechanism for the promotion of the mitochondrial apoptotic cell death pathway. Altogether, our findings link together two tumor suppressor properties of p57KIP2, by showing that the promotion of cell death by p57KIP2 requires its actin cytoskeleton stabilization function.
Keywords: p57KIP2, cell migration, cancer, cytoskeleton
p57 (Kip2, cyclin-dependent kinase inhibitor 1C) has been described as a potential tumor suppressor gene due to its ability to inhibit several of the hallmarks of cancer.1 The importance of p57KIP2 in the suppression of cancer is highlighted by its mutation/inactivation in Beckwith-Wiedemann Syndrome, a cancer pre-disposing syndrome,2 and its reported decrease in protein expression in cancers of the breast, lung, liver, and prostate, among others.3, 4, 5, 6, 7, 8 Inhibition of p57KIP2 expression in cancer cells is mediated by promoter DNA methylation at CpG islands and/or by histone modifications, such as methylation and deacetylation.1 Worth a notice, the lack of p57KIP2 expression in tumors is associated with poor survival, and has been identified as prognostic marker for many of the aforementioned cancer types.1
Initially identified as a member of the Cip/Kip family of Cdk (cyclin-dependent kinase) inhibitors,9, 10 p57KIP2 has since been shown to have diverse cellular roles aside from cell cycle inhibition, for example, promotion of cell death,11, 12, 13 inhibition of cell migration,14 and regulation of cell differentiation.15, 16, 17 Indeed, considering that p57KIP2 null mice have increased embryonic lethality and gross developmental defects as compared with p21CIP1/CDKN1A and p27KIP2/CDKN1B null mice, it appears that the cellular roles of p57KIP2 surpass that of the other Cdk inhibitors.18, 19, 20
The ease at which cancer cells migrate and invade a neighboring tissue demonstrates the importance of regulators of cell cytoskeleton structure in the cancer progression. The cytoskeleton remodeling factor cofilin disassembles actin filaments and thereby facilitates cell migration through increasing the actin mobile fraction.21 Active unphosphorylated cofilin is associated with increased tumor metastasis.22 Cofilin can be inactivated by LIM domain kinase-1/2 (LIMK-1/2),23, 24 which are themselves activated by phosphorylation on Thr50825 through the Rho/Rho kinase (ROCK) signaling pathway.26 Interestingly, p57KIP2 interacts with LIM domain kinase-1 (LIMK-1) and increases its kinase activity independently of Rho/ROCK signaling and thereby contributes to the stabilization of actin filaments and the inhibition of cell migration.14
p57KIP2 expression can enhance cell death induced by staurosporine (STS) and DNA damaging agents such as etoposide and cisplatin, all of which induce apoptotic mitochondrial events.11, 12 In contrast, p57KIP2 has no potentiating effect on apoptosis induced by death receptor ligand,12 indicating that p57KIP2 enhances cell death specifically at the level of the mitochondria. Indeed, overexpression of Bcl-2 or inhibition of the mitochondrial voltage-dependent anion channel (VDAC) inhibited p57KIP2's cell death promoting effect.12 Interestingly, there are reports that actin can influence the conductance of VDAC channels. In Neurospora crassa, an abundance of monomeric actin facilitated the closure of VDAC channels, whereas stable filamentous actin (F-actin) was unable to do the same.27 In fact, the presence of F-actin led to an increased ion conductance across the outer mitochondrial membrane. This indicated a possible link between the stability of actin cytoskeleton and the integrity of the mitochondrial transmembrane potential. In this study, we investigated the possibility that the cell death promoting effect of p57KIP2 requires its actin cytoskeleton-stabilizing effect.
Results
p57KIP2 stabilizes the actin cytoskeleton and promotes apoptosis
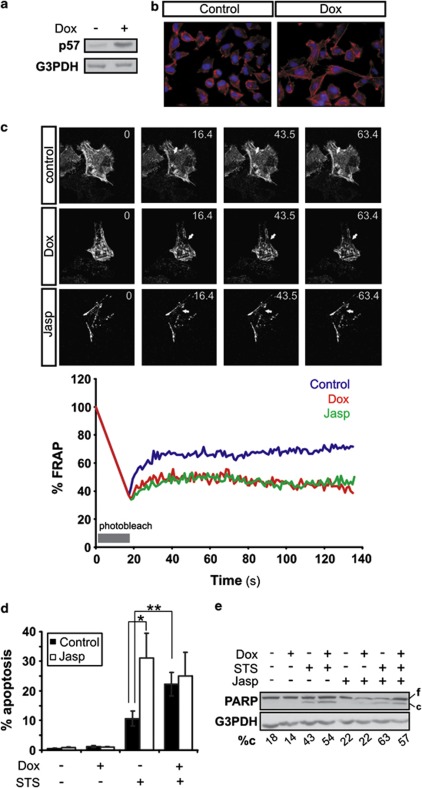
Two of the tumor suppressor characteristics of p57KIP2 include inhibition of cell migration and promotion of apoptosis.11, 12, 14 In this study, we took advantage of HeLa Tet-On derived cell line stably transfected with tetracycline-inducible p57KIP2, HeLa-p57KIP2 cells. These cells can be induced to express p57KIP2 selectively, upon administration of the tetracycline analog doxycycline (Dox) (Figure 1a). Induction of p57KIP2 expression in HeLa-p57KIP2 cells led to an increased formation of actin stress fibers as visualized by staining with phalloidin, which binds F-actin (Figure 1b). Additionally, fluorescence recovery after photobleaching (FRAP) analysis of green fluorescence protein (GFP)-labeled-actin revealed a reduced actin-GFP mobile fraction in HeLa-p57KIP2 cells treated with Dox as compared with untreated cells, providing additional evidence for the actin cytoskeleton-stabilizing function of p57KIP2 (Figure 1c). Although, induction of p57KIP2 expression did not provoke cell death per se, it sensitized cells to apoptosis triggered by STS (Figures 1d and e, Supplementary Figure S1).
Figure 1.
Cytoskeleton stabilization enhances STS-induced apoptosis. (a) HeLa-p57 TET-on cells were treated with Dox for 24 h. p57KIP2 expression was analyzed by immunoblotting, and (b) actin filaments were stained with phalloiden. (c) The mobile actin fraction following exposure to Dox or Jasp (3 h) was measured by FRAP analysis of cells transfected with actin-GFP. Values represent the % fluorescence recovery over time of actin-GFP after bleaching. Arrows indicate the photobleached area. (d) HeLa-p57KIP2 cells were treated with Jasp for 30 min, followed by treatment with STS for 3 h with or without exposure to Dox. Apoptotic nuclear morphology was quantified after Hoechst staining and expressed as the percentage of the total cells counted. (e) PARP cleavage was assessed by immunoblotting. G3PDH was used as a loading control. Full length (f) and cleaved (c) PARP was analyzed by densitometry and cleaved PARP was expressed as a % of total PARP (%c)
It has already been established that stabilization of the actin cytoskeleton can induce apoptotic cell death.28, 29 Jasplakinolide (Jasp), an actin cytoskeleton-stabilizing agent, was used to investigate whether actin cytoskeleton stabilization increases cell death induced by STS, thereby mimicking the effect of p57KIP2.30 At the concentration used in this study, Jasp stabilized the actin cytoskeleton, as shown by FRAP measurement of the mobility of actin-GFP (Figure 1c), but did not increase cell death (Figures 1d and e, Supplementary Figure S1). Pre-treatment of HeLa-p57KIP2 cells with Jasp 30 min before STS treatment resulted in an increase in cells exhibiting apoptotic nuclear morphology (Figure 1d, Supplementary Figure S1) and poly ADP-ribose polymerase (PARP) cleavage (Figure 1e), similar to levels observed in cells co-treated with Dox and STS. Importantly, induction of p57KIP2 expression by Dox treatment did not significantly increase the cell death caused by Jasp and STS co-treatment indicating that indeed Jasp mimics the pro-apoptotic effect of p57KIP2.
Destabilization of actin filaments inhibits p57KIP2 enhancement of STS-induced cell death
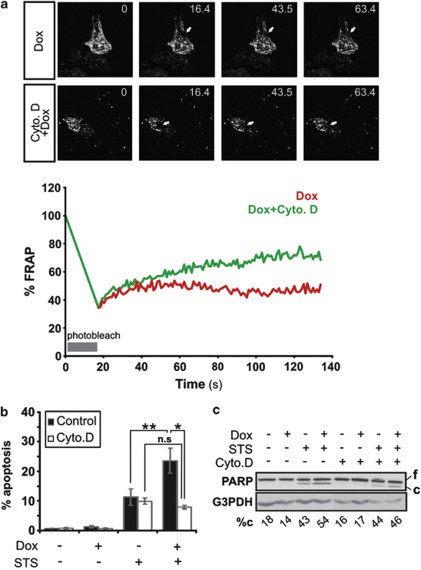
As actin cytoskeleton stabilization mimicked the pro-apoptotic effects of p57KIP2, an actin cytoskeleton destabilizing agent cytochalasin D (Cyto. D),31 was used to investigate whether depolymerization of actin filaments could prevent the promotion of cell death by p57KIP2. Cyto. D was used at a concentration that does not induce cell death, but yet retains the ability to destabilize the actin cytoskeleton (Figures 2a–c). FRAP analysis of actin-GFP mobility in HeLa-p57 cells treated with Dox showed that treatment with Cyto. D for 3 h successfully reversed the actin cytoskeleton-stabilizing effect of p57KIP2 (Figure 2a). To assess the effect of actin depolymerization on the p57KIP2-death promoting effect, HeLa-p57KIP2 cells were treated with Dox and STS with or without Cyto. D pre-treatment. Cells co-treated with Dox and STS presented reduced apoptotic nuclear morphology in the presence of Cyto. D (Figure 2b: 7.2% with Cyto. D versus 22.2% without Cyto. D). Furthermore, the increase in cleaved PARP in HeLa-p57KIP2 cells treated with STS was reduced in the presence of Cyto. D (Figure 2c). Therefore, one could conclude that actin cytoskeleton destabilization inhibits the ability of p57KIP2 to enhance STS-induced apoptotic cell death.
Figure 2.
Cytoskeleton destabilization prevents the enhancement of apoptosis by p57KIP2. (a) FRAP analysis of HeLa-p57KIP2 cells treated with or without cytochalsin D for 3 h. Values represent the % fluorescence recovery over time of actin-GFP after bleaching. Arrows indicate the photobleached area. (b and c) HeLa-p57KIP2 cells were treated with cytochalsin D for 1 h, followed by treatment with STS for 3 h. (b) Apoptotic nuclear morphology was quantified after Hoechst staining and expressed as a percentage of the total cells counted. (c) PARP cleavage was assessed by immunoblotting. G3PDH was used as a loading control. Full length (f) and cleaved (c) PARP was analyzed by densitometry and cleaved PARP was expressed as a % of total PARP (%c)
LIMK-1 is required for p57KIP2-induced cell death
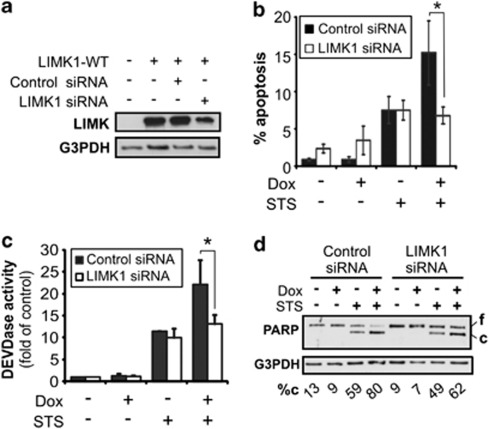
Activation of LIMK-1 kinase results in decreased cofilin activity through phosphorylation and as such, increased actin cytoskeleton stabilization.26 It has previously been shown that p57KIP2 directly interacts with LIMK-1 resulting in an increase in LIMK-1 kinase activity, which is required for p57KIP2-mediated actin cytoskeleton stabilization.14 To investigate whether LIMK-1 is required for p57KIP2-mediated apoptosis, small interfering RNA (siRNA) directed against LIMK-1 were used, which resulted in the specific knockdown of LIMK-1 protein levels (Figure 3a).
Figure 3.
LIMK-1 is required for p57KIP2-induced apoptosis. HeLa-p57KIP2 cells were transfected with scrambled sequence siRNA or LIMK-1 siRNA in the presence or absence of Dox (24 h) and STS (3 h). (a) LIMK-1 knockdown was confirmed by immunoblotting against LIMK-1, using G3PDH as a loading control. (b) Apoptotic nuclear morphology was quantified by Hoechst staining and expressed as a percentage of the total cells counted. (c) Activation of effector caspases was measured by DEVDase assay, expressed as fold increase of control. Values represent the mean +/− S.D. of three separate experiments. (d) PARP cleavage was assessed by immunoblotting, using G3PDH as a protein loading control. Full length (f) and cleaved (c) PARP was analyzed by densitometry and cleaved PARP was expressed as a % of total PARP (%c)
In HeLa-p57KIP2 cells co-treated with Dox and STS, there was a reduction in the apoptotic nuclei to 6.7% in LIMK-1 knockdown cells as compared with 15.2% in LIMK-1 expressing cells (Figure 3b). In fact, the number of apoptotic nuclei in LIMK-1 knockdown cells co-treated with Dox and STS were similar to those observed in cells treated only with STS (Figure 3b). Similarly, measurement of DEVDase activity also showed a decrease caspase-3 like activity in cells co-treated with Dox and STS when LIMK-1 expression was suppressed as compared with the control (Figure 3c). Furthermore, analysis of PARP cleavage by immunoblot confirmed that in LIMK-1 deficient cells, p57KIP2 was unable to enhance STS-mediated cleavage of this caspase-3 substrate (Figure 3d). Together, these results demonstrated that silencing of LIMK-1 prevented p57KIP2 enhancement of STS-induced apoptosis. Thus, this additionally established that loss of the actin-stabilizing effect of p57KIP2 is sufficient to prevent its pro-apoptotic effect.
Actin cytoskeleton stabilization by p57KIP2 positively modulates apoptosis at the mitochondrial level
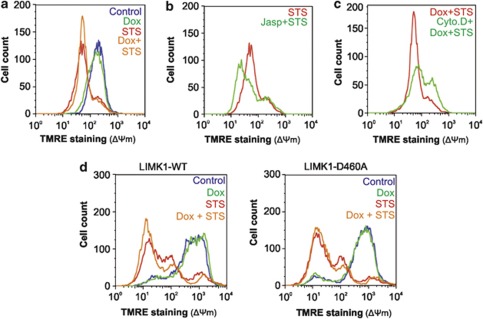
So far, it has been established that the pro-apoptotic effect of p57KIP2 requires its ability to stabilize the actin cytoskeleton. This death promoting effect of p57KIP2 was previously shown to be mediated through the mitochondria.12 Indeed treatment of HeLa-p57KIP2 cells with Dox and STS increased the loss of mitochondrial transmembrane potential (ΔΨm) compared with STS treatment alone (Figure 4a). The importance of actin dynamic for maintenance of ΔΨm is demonstrated by the ability of Jasp to enhance STS-induced loss in ΔΨm (Figure 4b), and of Cyto. D to reduce the loss of ΔΨm induced by Dox and STS co-treatment (Figure 4c). Furthermore, in the presence of a kinase-inactive mutant of LIMK-1 (LIMK-1-D460A), p57KIP2 expression did not enhance STS-induced loss of ΔΨm (Figure 4d). In all, these results show that a stable actin cytoskeleton, induced by p57KIP2, positively modulates apoptosis at the mitochondrial level.
Figure 4.
Actin cytoskeleton stabilization by p57KIP2 promotes ΔΨm. HeLa-p57KIP2 cells were treated STS in the presence or absence of Dox. (a) The ΔΨm was measured by flow cytometry after staining with TMRE. (b) HeLa-p57KIP2 cells were treated with Jasp for 30 min, followed by treatment with STS for 5 h. The ΔΨm was measured by flow cytometry after staining with TMRE. (c) HeLa-p57KIP2 cells were treated with Cytochalsin D for 30 min, followed by treatment with STS for 3 h in the presence of Dox. The ΔΨm was measured by flow cytometry after staining with TMRE. (d) HeLa-p57KIP2 cells were transfected with wild-type LIMK (LIMK-WT) or a kinase-inactive mutant of LIMK (LIMK-D460A) before treatment with Dox and STS. The ΔΨm was measured by flow cytometry after staining with TMRE
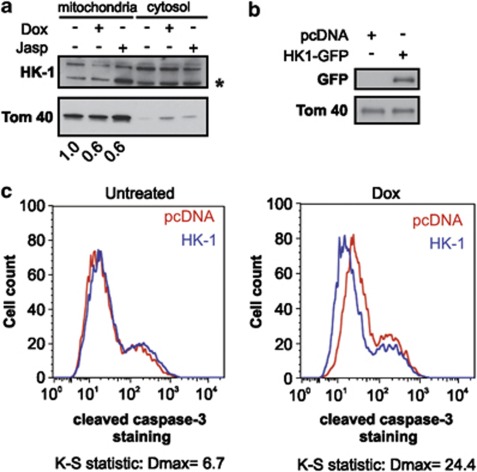
Actin stabilization by p57KIP2 displaces hexokinase-1 (HK-1) from mitochondria
Previous work has shown that inhibition of VDAC channel opening using 4,4'-diisothiocyanatostilbene-2,2'-disulfonic acid (DIDS) counteracts the p57KIP2-death promoting effect.14 Therefore, the effect of p57KIP2 on the mitochondrial protein levels of HK-1, an important regulator of VDAC channel conductance, was examined. A decrease in mitochondrial localization of HK-1 was observed upon Dox treatment of HeLa-p57KIP2 cells (Figure 5a), Actin cytoskeleton stabilization by Jasp also resulted in a decrease in mitochondrial HK-1 (Figure 5a). Finally, the ability of HK-1 to rescue cells from apoptosis induced by p57KIP2 was investigated. Accordingly, overexpression of HK-1 in p57KIP2 expressing cells resulted in a decrease in cleaved caspase-3 staining compared with the pcDNA-transfected control (Figures 4b and c).
Figure 5.
Actin stabilization by p57KIP2 promotes displacement of HK-1 from mitochondria. (a) HeLa-p57KIP2 cells were treated with either Dox or Jasp. Mitochondrial and cytosolic protein fractions were analyzed by immunoblotting using antibodies against HK-1. Tom40 was used as a mitochondrial loading control. Asterisk (*) indicates a cleavage product of HK-1. Values represent the fold decrease of full-length HK-1 compared with control. (b) HeLa-p57KIP2 cells were transfected with HK-1-GFP or pcDNA and grown in the presence or absence of Dox for 24 h. Overexpression of HK-1-GFP was analyzed by immunoblotting with GFP, using Tom40 as a loading control, and (c) cleaved caspase-3 staining was assessed by flow cytometry
Discussion
p57KIP2 inhibits many of the hallmarks of cancer and as such has been hailed as a potential tumor suppressor gene.1 The results in this study corroborate previous studies showing firstly that p57KIP2 inhibits cancer cell migration through stabilization of the actin cytoskeleton, and secondly that p57KIP2 enhances apoptotic cancer cell death through mitochondrial depolarization. This study examines the interplay between these two seemingly disparate functions of p57KIP2, and shows that the pro-apoptotic effect of p57KIP2 depends on its ability to stabilize the actin cytoskeleton. Disturbing actin cytoskeleton dynamics affects the ability of p57KIP2 to promote cell death. We here show that stabilizing the actin cytoskeleton mimics the death promoting effect of p57KIP2, and destabilizing actin filaments reverses this effect. Significantly, inhibition of the actin cytoskeleton-stabilizing effect of p57KIP2 (through LIMK-1 knockdown) also abrogates the death promoting effect of p57KIP2, providing a direct link between the actin cytoskeleton-stabilizing function of p57KIP2 and the death promoting function of p57KIP2. Stabilization of actin cytoskeleton has been shown to induce cell death.28, 32 Although to date, the mechanism by which F-actin sensitizes to cell death is not fully described, evidence suggests that it is through mitochondrial depolarization.27, 32 Further investigation into how p57KIP2 sensitizes cells to death revealed a link between actin cytoskeleton stabilization and mitochondrial hexokinase levels. Hexokinase is often found overexpressed in cancer cells, where its binding to mitochondria enhances the cell's rate of glycolysis through easy access to mitochondrial-generated ATP.33 The closure of VDAC channels upon hexokinase binding is one mechanism used by cancer cells to evade death.34 This study shows that actin cytoskeleton stabilization either by Jasp or by p57KIP2 expression displaces HK-1 from the mitochondrial membrane. In fact, overexpression of HK-1 inhibits the promotion of cell death by p57KIP2. This describes a scenario whereby stabilization of actin by p57KIP2 promotes cell death through the displacement of HK-1 from the mitochondria. Many cancer cells exhibit decreased p57KIP2 expression either through promoter DNA methylation, histone modifications, genetic mutation, silencing by microRNA's, or proteasomal degradation.1 Interestingly, induced p57KIP2 expression in tumors forces regression of the cancer.35 Therefore, as with many tumor suppressors, it is important for the cancer to keep expression of p57KIP2 at a reduced level. Consequently, p57KIP2 protein expression is being tested as a prognostic indicator in cancer. In this study, we have shown that the negative regulation of at least two cancer hallmarks, increased cell migration-metastasis and evasion of cell death, can be traced back to the ability of p57KIP2 to stabilize actin cytoskeleton. Potentially, other hallmarks of cancer could also be regulated by p57KIP2 regulation of the actin cytoskeleton dynamic. Already, increased actin mobility is known to enhance tumor angiogenesis.36 These findings should increase interest in therapies that reactivate p57KIP2 expression in cancer cells.
Materials and Methods
Plasmids and siRNA
Myc-LIMK-1 and Myc-LIMK-1 D640A mutant in the FPC1 vector were gifts from K Mizuno (Tohoku University, Japan). Actin-GFP plasmid was a gift from Pirta Hotulainen (University of Helsinki, Finland). GFP plasmid was from Clontech (Invitrogen, Carlsbad, CA, USA). SMARTPool siRNA against LIMK-1 and non-targeted sequence were purchased from Dharmacon (Lafayette, CO, USA).
Cell culture, transfections, and treatments
Human cervical carcinoma HeLa cells expressing p57KIP2 under the control of the tetracycline promoter (HeLa-p57KIP2) were a gift from S Okret, Karolinska Institutet and were cultured in MEM medium (Gibco, Carlsbad, CA, USA) supplemented with 10% Fetal bovine serum, penicillin/streptomycin, non-essential amino acids, sodium pyruvate, and L-glutamine. 2 μg/ml Dox was added to the culture medium for 24 h to induce expression of p57KIP2. Transfections were performed with Lipofectamine and Plus reagent (Invitrogen) according to the manufacturer's protocol. Cells were treated with 0.1 μM Jasp, 1 μM Cytochalsin D, or 0.1 μM STS for the indicated times.
Protein extracts and immunoblotting
Cells were harvested and lysed by sonication in NP40 lysis buffer containing protease inhibitors.17 Protein lysates were heated to 95 °C for 5 min in Laemmli buffer. In all, 20–40 μg proteins were electrophoresed on 12% SDS-PAGE acrylamide gels, and then immunoblotted onto nitrocellulose membrane. Membranes were blocked in 5% milk and incubated with anti-PARP (BD Pharmingen, Franklin Lakes, NJ, USA), anti-p57KIP2 (C-20, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-HK-1 (N-19, Santa Cruz Biotechnology), anti-GFP (Santa Cruz Biotechnology), anti-translocase of outer membrane 40 (tom40; Santa Cruz Biotechnology), or anti-glycerol 3 phosphate dehydrogenase (G3PDH; Trevigen, Gaithersburg, MD, USA) overnight at 4 °C, followed by incubation with the appropriate horseradish peroxidase secondary antibody (1 : 10 000) for 1 h at room temperature. Bands were visualized by enhanced chemiluminescence (Pierce, Rockford, IL, USA) following the manufacturers protocol.
Fluorescence recovery after photobleaching (FRAP)
FRAP was used to assess the amount of mobile actin-GFP monomers. HeLa- p57KIP2 cells, grown on glass chamber slides, were transfected with actin-GFP and treated as indicated in the figure legends. Chamber slides were then placed in the POC-chamber/CTI Controller/heating insert P system for live cell imaging. After bleaching the region of interest (ROI) with 100% intensity of Argon/2 laser 488 nm (current 4.7 Amp), the time course of fluorescence recovery in the bleached ROI was monitored with 20 ms intervals. Samples were analyzed with Axiovert 200 m microscope (Zeiss, Oberkochen, Germany).
Measurement of effector caspase-3/7 (DEVDase) activity
DEVDase activity in cell lysates was calculated by measuring the rate of cleavage of the fluorogenic DEVD-MCA substrate (Peptide Institute Inc., Osaka, Japan). Free AMC was monitored in a Fluoroscan II plate reader (Labsystems, Waltham, MA, USA) using 355 nm excitation and 460 nm emission wavelengths.
Measurement of mitochondrial transmembrane potential
Cells were incubated with 25 nM tetramethylrhodamine ethyl ester (TMRE) (Molecular Probes, Carlsbad, CA, USA) at 37 °C for 30 min before harvesting. Cells were washed twice in phosphate-buffered saline (PBS), trypsinised, resuspended in PBS and analyzed by flow cytometry on a FACSCalibur (Becton Dickinson, Franklin Lakes, NJ, USA) flow cytometer. Results were analyzed using CellQuest (Becton Dickinson) software.
Subcellular fractionation
Harvested cells were washed twice in PBS and resuspended for 10 min on ice in buffer A (10 mM Hepes, pH 7.9 at 4 °C, 1.5 mM MgCl2, 10 mM KCl and 0.5 mM DTT). Cells were centrifuged at 2000, r.p.m. for 5 min at 4 °C. Cell pellet was resuspended in buffer A containing 0.2% NP40 and protease inhibitors for 2 min on ice. Cells were centrifuged at 500 r.p.m. for 2 min. The supernatant was collected and further centrifuged at 14 000 r.p.m. for 30 min. The supernatant and pellet contained the cytosolic and mitochondrial fractions, respectively.
Quantification of apoptotic nuclear morphology
Cells were grown on glass coverslips, treated, and fixed with 4% paraformaldehyde for 15 min at 4 °C. Fixed cells were then incubated in Hoechst (1 μg/ml) for 10 min at room temperature to stain nuclei. At least 200 cells were counted per sample.
Detection of F-actin
To visualize F-actin, cells grown on glass coverslips were fixed and incubated with rhodamine-conjugated phalloidin (1 : 8000) for 30 min at room temperature. Nuclei were stained by incubating coverslips in 1 μg/ml Hoechst (Molecular Probes) for 10 min at room temperature. Cells were visualized using a 510 Meta confocal microscope (Zeiss).
Measurement of cleaved caspase-3
Cells were fixed with 4% paraformaldehyde for 10 min at 37 °C and permeabilized with 90% methanol for 30 min at −20 °C. Cells were incubated in blocking solution (0.5% BSA in PBS) for 10 min at room temperature, followed by anti-cleaved caspase-3 (Cell signaling, Danvers, MA, USA; 1 : 3200 in blocking solution) for 1 h at room temperature, and then with goat anti-rabbit Alexa 488 (Invitrogen; 1 : 200 in blocking solution) for 30 min. Cells were washed once in blocking solution between all antibody incubations, and were finally resuspended in PBS for analysis on a FACSCalibur (Becton Dickinson) flow cytometer. Results were analyzed using CellQuest software.
Statistical analysis
Statistical analyses were performed using two tailed, paired student's t-test where *P<0.05 and **P<0.01 were considered to be significant. Bars and error bars represent mean with S.E.M.
Acknowledgments
This work has been supported by grants from the Swedish Research Council and the Swedish Cancer Society.
Glossary
- Cdk
cyclin-dependent kinase
- Cyto. D
cytochalsin D
- DIDS
4,4'-diisothiocyanatostilbene-2,2'-disulfonic acid
- Dox
doxycycline
- F-actin
filamentous actin
- FRAP
flourescence recovery after photobleaching
- G3PDH
glycerol 3 phosphate dehydrogenase
- GFP
green fluorescent protein
- HK-1
hexokinase-1
- Jasp
jasplakinolide
- LIMK-1
LIM domain kinase-1
- p21/Cip1/CDKN1A
cyclin-dependent kinase inhibitor 1A
- p27/Kip1/CDKN1B
cyclin-dependent kinase inhibitor 1B
- p57/KIP2/CDKN1C
cyclin-dependent kinase inhibitor 1C
- PARP
poly ADP-ribose polymerase
- PBS
phosphate-buffered saline
- ROCK
rho kinase
- ROI
region of interest
- STS
staurosporine
- TMRE
tetramethylrhodamine ethyl ester
- Tom40
translocase of outer membrane 40
- VDAC
voltage-dependent anion channel
- ΔΨm
loss of mitochondrial transmembrane potential
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by P Salomoni
Supplementary Material
References
- Kavanagh E, Joseph B. The hallmarks of CDKN1C (p57, KIP2) in cancer. Biochim Biophys Acta. 2011;1816:50–56. doi: 10.1016/j.bbcan.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Lam WW, Hatada I, Ohishi S, Mukai T, Joyce JA, Cole TR, et al. Analysis of germline CDKN1C (p57KIP2) mutations in familial and sporadic Beckwith-Wiedemann syndrome (BWS) provides a novel genotype-phenotype correlation. J Med Genet. 1999;36:518–523. [PMC free article] [PubMed] [Google Scholar]
- Oya M, Schulz WA. Decreased expression of p57(KIP2)mRNA in human bladder cancer. Br J Cancer. 2000;83:626–631. doi: 10.1054/bjoc.2000.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JQ, Wu F, Usuki H, Kubo A, Masaki T, Fujita J, et al. Loss of p57KIP2 is associated with colorectal carcinogenesis. Int J Oncol. 2003;23:1537–1543. [PubMed] [Google Scholar]
- Larson PS, Schlechter BL, King CL, Yang Q, Glass CN, Mack C, et al. CDKN1C/p57kip2 is a candidate tumor suppressor gene in human breast cancer. BMC Cancer. 2008;8:68. doi: 10.1186/1471-2407-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pateras IS, Apostolopoulou K, Koutsami M, Evangelou K, Tsantoulis P, Liloglou T, et al. Downregulation of the KIP family members p27(KIP1) and p57(KIP2) by SKP2 and the role of methylation in p57(KIP2) inactivation in nonsmall cell lung cancer. Int J Cancer. 2006;119:2546–2556. doi: 10.1002/ijc.22214. [DOI] [PubMed] [Google Scholar]
- Ito Y, Takeda T, Sakon M, Tsujimoto M, Monden M, Matsuura N. Expression of p57/Kip2 protein in hepatocellular carcinoma. Oncology. 2001;61:221–225. doi: 10.1159/000055378. [DOI] [PubMed] [Google Scholar]
- Atasoy P, Yilmaz E, Bozdogan O, Ayva S, Batislam E. Expression profile and prognostic importance in prostate lesions of the reverse transcriptase component of human telomerase (hTERT) and of cyclin-dependent kinase inhibitor p57 (p57kip2a) Int Urol Nephrol. 2009;41:55–60. doi: 10.1007/s11255-008-9399-7. [DOI] [PubMed] [Google Scholar]
- Matsuoka S, Edwards MC, Bai C, Parker S, Zhang P, Baldini A, et al. p57KIP2, a structurally distinct member of the p21CIP1 Cdk inhibitor family, is a candidate tumor suppressor gene. Genes Dev. 1995;9:650–662. doi: 10.1101/gad.9.6.650. [DOI] [PubMed] [Google Scholar]
- Lee MH, Reynisdottir I, Massague J. Cloning of p57KIP2, a cyclin-dependent kinase inhibitor with unique domain structure and tissue distribution. Genes Dev. 1995;9:639–649. doi: 10.1101/gad.9.6.639. [DOI] [PubMed] [Google Scholar]
- Samuelsson MK, Pazirandeh A, Okret S. A pro-apoptotic effect of the CDK inhibitor p57(Kip2) on staurosporine-induced apoptosis in HeLa cells. Biochem Biophys Res Commun. 2002;296:702–709. doi: 10.1016/s0006-291x(02)00912-9. [DOI] [PubMed] [Google Scholar]
- Vlachos P, Nyman U, Hajji N, Joseph B. The cell cycle inhibitor p57(Kip2) promotes cell death via the mitochondrial apoptotic pathway. Cell Death Differ. 2007;14:1497–1507. doi: 10.1038/sj.cdd.4402158. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Perez-Perez MM, Hernando E, Serrano M, Cordon-Cardo C. p73beta-Mediated apoptosis requires p57kip2 induction and IEX-1 inhibition. Cancer Res. 2005;65:2186–2192. doi: 10.1158/0008-5472.CAN-04-3047. [DOI] [PubMed] [Google Scholar]
- Vlachos P, Joseph B. The Cdk inhibitor p57(Kip2) controls LIM-kinase 1 activity and regulates actin cytoskeleton dynamics. Oncogene. 2009;28:4175–4188. doi: 10.1038/onc.2009.269. [DOI] [PubMed] [Google Scholar]
- Jia J, Lin M, Zhang L, York JP, Zhang P. The Notch signaling pathway controls the size of the ocular lens by directly suppressing p57Kip2 expression. Mol Cell Biol. 2007;27:7236–7247. doi: 10.1128/MCB.00780-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph B, Andersson ER, Vlachos P, Sodersten E, Liu L, Teixeira AI, et al. p57Kip2 is a repressor of Mash1 activity and neuronal differentiation in neural stem cells. Cell Death Differ. 2009;16:1256–1265. doi: 10.1038/cdd.2009.72. [DOI] [PubMed] [Google Scholar]
- Joseph B, Wallen-Mackenzie A, Benoit G, Murata T, Joodmardi E, Okret S, et al. p57(Kip2) cooperates with Nurr1 in developing dopamine cells. Proc Natl Acad Sci USA. 2003;100:15619–15624. doi: 10.1073/pnas.2635658100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Frisen J, Lee MH, Massague J, Barbacid M. Ablation of the CDK inhibitor p57Kip2 results in increased apoptosis and delayed differentiation during mouse development. Genes Dev. 1997;11:973–983. doi: 10.1101/gad.11.8.973. [DOI] [PubMed] [Google Scholar]
- Zhang P, Liegeois NJ, Wong C, Finegold M, Hou H, Thompson JC, et al. Altered cell differentiation and proliferation in mice lacking p57KIP2 indicates a role in Beckwith-Wiedemann syndrome. Nature. 1997;387:151–158. doi: 10.1038/387151a0. [DOI] [PubMed] [Google Scholar]
- Susaki E, Nakayama K, Yamasaki L, Nakayama KI. Common and specific roles of the related CDK inhibitors p27 and p57 revealed by a knock-in mouse model. Proc Natl Acad Sci USA. 2009;106:5192–5197. doi: 10.1073/pnas.0811712106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Troys M, Huyck L, Leyman S, Dhaese S, Vandekerkhove J, Ampe C. Ins and outs of ADF/cofilin activity and regulation. Eur J Cell Biol. 2008;87:649–667. doi: 10.1016/j.ejcb.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Wang W, Mouneimne G, Sidani M, Wyckoff J, Chen X, Makris A, et al. The activity status of cofilin is directly related to invasion, intravasation, and metastasis of mammary tumors. J Cell Biol. 2006;173:395–404. doi: 10.1083/jcb.200510115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, et al. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393:805–809. doi: 10.1038/31729. [DOI] [PubMed] [Google Scholar]
- Yang N, Higuchi O, Ohashi K, Nagata K, Wada A, Kangawa K, et al. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 1998;393:809–812. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- Ohashi K, Nagata K, Maekawa M, Ishizaki T, Narumiya S, Mizuno K. Rho-associated kinase ROCK activates LIM-kinase 1 by phosphorylation at threonine 508 within the activation loop. J Biol Chem. 2000;275:3577–3582. doi: 10.1074/jbc.275.5.3577. [DOI] [PubMed] [Google Scholar]
- Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, et al. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- Xu X, Forbes JG, Colombini M. Actin modulates the gating of Neurospora crassa VDAC. J Membr Biol. 2001;180:73–81. doi: 10.1007/s002320010060. [DOI] [PubMed] [Google Scholar]
- Posey SC, Bierer BE. Actin stabilization by jasplakinolide enhances apoptosis induced by cytokine deprivation. J Biol Chem. 1999;274:4259–4265. doi: 10.1074/jbc.274.7.4259. [DOI] [PubMed] [Google Scholar]
- Cioca DP, Kitano K. Induction of apoptosis and CD10/neutral endopeptidase expression by jaspamide in HL-60 line cells. Cell Mol Life Sci. 2002;59:1377–1387. doi: 10.1007/s00018-002-8515-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubb MR, Senderowicz AM, Sausville EA, Duncan KL, Korn ED. Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J Biol Chem. 1994;269:14869–14871. [PubMed] [Google Scholar]
- Casella JF, Flanagan MD, Lin S. Cytochalasin D inhibits actin polymerization and induces depolymerization of actin filaments formed during platelet shape change. Nature. 1981;293:302–305. doi: 10.1038/293302a0. [DOI] [PubMed] [Google Scholar]
- Gourlay CW, Carpp LN, Timpson P, Winder SJ, Ayscough KR. A role for the actin cytoskeleton in cell death and aging in yeast. J Cell Biol. 2004;164:803–809. doi: 10.1083/jcb.200310148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante E, Pedersen PL. High aerobic glycolysis of rat hepatoma cells in culture: role of mitochondrial hexokinase. Proc Natl Acad Sci USA. 1977;74:3735–3739. doi: 10.1073/pnas.74.9.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzoine L, Zilberberg N, Ben-Romano R, Shoshan-Barmatz V. Voltage-dependent anion channel 1-based peptides interact with hexokinase to prevent its anti-apoptotic activity. J Biol Chem. 2009;284:3946–3955. doi: 10.1074/jbc.M803614200. [DOI] [PubMed] [Google Scholar]
- Jin RJ, Lho Y, Wang Y, Ao M, Revelo MP, Hayward SW, et al. Down-regulation of p57Kip2 induces prostate cancer in the mouse. Cancer Res. 2008;68:3601–3608. doi: 10.1158/0008-5472.CAN-08-0073. [DOI] [PubMed] [Google Scholar]
- Thoenes L, Gunther M. Novel approaches in anti-angiogenic treatment targeting endothelial F-actin: a new anti-angiogenic strategy. Curr Opin Mol Ther. 2008;10:579–590. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.