Summary
Our objective was to investigate the safety, tolerability, and effects of tadalafil in children with pulmonary arterial hypertension after transition from sildenafil or receiving tadalafil as initial therapy. Thirty three pediatric patients with pulmonary arterial hypertension were retrospectively evaluated. Twenty nine of 33 patients were switched from sildenafil to tadalafil. The main reason for changing from sildenafil was once daily dosing. The average dose of sildenafil and tadalafil were 3.4+/−1.1 mg/kg/day and 1.0+/−0.4 mg/kg/day, respectively. In 14 of 29 patients undergoing repeat catheterization, statistically significant improvements were observed following transition from sildenafil to tadalafil, in mean pulmonary arterial pressure (mmHg) (53.2+/−18.3 versus 47.4+/−13.7, p<0.05) and pulmonary vascular resistance index (unitsxm2) (12.2+/−7.0 versus 10.6+/−7.2, p<0.05). In 4 patients treated with tadalafil as initial therapy, clinical improvement was noted. Side effect profiles were similar in patients who had transitioned from sildenafil to tadalafil and included headache, nausea, myalgia, nasal congestion, flushing, and allergic reaction. Two patients discontinued tadalafil due to migraine or an allergic reaction. One patient on sildenafil had no break through syncope after transition to tadalafil. Tadalafil can be safely used in pediatric patients with pulmonary arterial hypertension and may prevent disease progression.
Keywords: tadalafil, sildenafil, phosphodiesterase type 5 inhibitor, safety, tolerability, children
Introduction
Pulmonary arterial hypertension (PAH) is characterized by endothelial and smooth muscle cell proliferation and pulmonary vascular remodeling, resulting in progressive elevation of pulmonary arterial pressure, increase in pulmonary vascular resistance, right heart failure, and death [14]. The mechanism of progressive vascular dysfunction is caused in part by an over-expression of vasoconstrictors including endothelin-1 and a reduction of vasodilators including prostacyclin and nitric oxide. Conventional therapies, such as calcium channel blockers, have been used to improve the symptoms in patients with PAH. However, calcium channel blocker use has been limited due to few patients being candidates for this therapy [5]. Currently, specific targeted therapies have been approved for treatment including prostanoids [1,8,9,11,17], endothelin receptor antagonists [5,10], and phosphodiesterase type 5 inhibitors (PDE-5i) [4,7,13,15]. Two oral PDE-5i (sildenafil and tadalafil) are currently used for the treatment of PAH in adults. Tadalafil is a once daily dosing drug and has a longer half-life (about 17 hours) compared with sildenafil (4 hours) [16]. In 2009, Galiè et al. reported on a double-blind, placebo-controlled study on 405 adult patients with PAH during 16-weeks. They concluded that 40 mg of tadalafil was well tolerated and improved exercise capacity and quality of life measures and reduced clinical worsening [6]. Based on this study, tadalafil was approved by the Food and Drug Administration for treatment of adult patients with PAH. However, little is known of the use tadalafil in children with PAH. Our objective was to investigate the safety, tolerability, and effects of tadalafil in children with PAH after transition from sildenafil or receiving tadalafil as initial PDE-5i therapy.
Methods
This single-center study was a retrospective cohort study using clinical data from patients with PAH after transition from sildenafil or receiving tadalafil as initial PDE-5i therapy. We included 33 patients who were started with tadalafil from March, 2008 to December, 2010 and followed until March 2011, at Children’s Hospital Colorado, Denver. Twenty nine of 33 patients were switched from sildenafil to tadalafil. In the remaining 4 children, tadalafil was the initial PAH therapy or added as initial PDE-5i therapy in combination with other targeted therapy. All patients were enrolled in an IRB approved protocol entitled “A Prospective Evaluation of Adolescents and Children with Pulmonary Arterial Hypertension”. The clinical impact of tadalafil was evaluated by plasma brain natriuretic peptide levels, echocardiography, exercise capacity, cardiac catheterization, and World Health Organization functional class before and after initiation of tadalafil. Two-dimensional echocardiographic data included tricuspid regurgitant jet velocities for estimating right ventricular systolic pressure and right ventricular diastolic diameter. By right heart catheterization using a flow directed Swan-Ganz catheter and systemic arterial line for blood pressure monitoring, we measured mean right atrial pressure, mean pulmonary arterial pressure, mean systemic blood pressure, and pulmonary capillary wedge pressure. Accordingly, we calculated pulmonary vascular resistance index and pulmonary vascular resistance/systemic vascular resistance ratio. Cardiac output was obtained using thermo-dilution and cardiac index was calculated. If a significant intra-cardiac defect remained, cardiac output was obtained by the Fick method using the LaFarge estimation. Exercise capacity was assessed by 6-minute walk distance during follow up. Plasma brain natriuretic peptide was assayed on an i-STAT® system using the two-site enzyme-linked immunosorbant assay (Abbott Laboratories, IL, USA). Safety evaluations included the recording of adverse events, hemodynamic change, and laboratory tests. At each clinic visit, we routinely ask about clinical worsening including chest pain with exercise, dyspnea with exercise, abdominal pain after meals, dizziness, stomach pain, syncope, pre syncope, edema in the feet and hands, pallor, and cyanosis. In addition, after initiation of tadalafil therapy, all patients were asked about side effects of tadalafil such as headache, nasal congestion, flushing, myalgia, and any allergic reaction. Children >40kg were treated with the adult dose. Children 20 to 40kg were treated with ½ the adult dose and children <20kg were treated with ¼ of the adult dose.
All analyses included baseline and at least one post-baseline measure. All results are reported as median and range or mean ± standard deviation along with the 95% confidence interval as appropriate. Comparisons of 6-minute walk distance, World Health Organization functional class, natriuretic peptide levels, echocardiographic data and hemodynamic variables between before and after initiation of tadalafil were performed using Student t-tests when comparing data with a normal distribution. If data did not show a normal distribution, we used a non-parametric test for analysis (Mann-Whitney’s U test). For evaluating the difference in clinical variables, we picked the last data on sildenafil and the first data on tadalafil. The level of statistical significance was defined as p value of 0.05. Analyses were conducted using Statmate III for Windows (Atoms Co., Tokyo, Japan).
Results
Table 1 shows clinical characteristics of the pediatric patients. Among 16 patients with congenital heart disease, 6 patients with significant intra-cardiac shunts were classified as Eisenmenger syndrome due to mild to moderate desaturation. Fifteen patients received triple vasodilator therapy and 12 patients received double vasodilator therapy including tadalafil. The period of receiving concomitant therapies prior to initiation of tadalafil therapy was 54.3+/−21.3 months.
Table 1.
Patient demographics and clinical measurements
| Total number of patients | 33 |
| Transition / initial therapy (number) | 29/4 |
| Age (year-old) ; median(range) | 10 (4-18) |
| Gender (male/female) | 11/22 |
| Race (number) | Caucasian (14) |
| White (11) | |
| Hispanic (5) | |
| Asian (2) | |
| Black (1) | |
| Weight (kg) (median, IQR) | 33.9 (22.2-46.1) |
| Etiology (number) | congenital heart disease (16) |
| idiopathic (14) | |
| connective tissue disease (1) | |
| others (2) | |
| Concomitant therapy (number) | treprostinil (13) |
| epoprostenol (3) | |
| iloprost (2) | |
| ambrisentan (13) | |
| bosentan (6) | |
| calcium channel blocker (8) | |
| WHO functional class (number) | Class I (11) |
| Class II (13) | |
| Class III (8) | |
| Class IV (1) | |
| 6 minute walking distance (m) (28 patients) (median, range) |
512.5 (419.2-559.5) |
| Brain natriuretic peptide (pg/ml) (31 patients) (median, range) |
37.5 (15.0-607.0) |
| Echocardiographic data (30 patients) | (median, range) |
| tricuspid regurgitation velocity (m/s) | 3.9 (3.6-4.5) |
| right ventricular diastolic dimension (mm/m2) | 23.5 (12.6-57.1) |
| Right heart catheterization (30 patients) | (median, range) |
| mean right atrial pressure (mmHg) | 7 (3-8) |
| mean pulmonary arterial pressure (mmHg) | 45 (37-58) |
| mean systemic arterial pressure (mmHg) | 64 (56-71) |
| pulmonary vascular resistance index (unitxm2) | 8.3 (6.5-14.0) |
| pulmonary / systemic vascular resistance ratio | 0.64 (0.48-0.93) |
| cardiac index (l/min/m2) | 4.2 (3.3-5.1) |
Transition from sildenafil to tadalafil
The main reason for changing from sildenafil was once daily dosing. One patient had an allergic reaction to sildenafil (rash and clitoral swelling) and was trialed on tadalafil, but had a similar allergic reaction and tadalafil was discontinued. The mean follow-up period on sildenafil and tadalafil was 52.2+/−21.8 months and 9.0+/−7.2 months, respectively. The average dose of sildenafil and tadalafil were 3.4+/−1.1 mg/kg/day and 1.0+/−0.4 mg/kg/day, respectively.
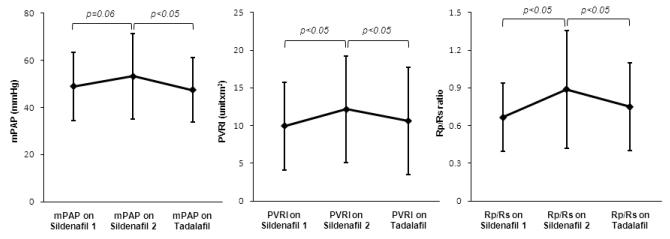
Fourteen of 29 patients had catheterization on sildenafil and tadalafil during follow-up. The time from previous catheterization on sildenafil to last catheterization on sildenafil was 15.2+/−8.8 months. The time from the initiation of tadalafil to the first catheterization on tadalafil was 7.6+/−3.4 months. The duration from the last catheterization on sildenafil to the first catheterization on tadalafil was 23.5+/−8.3 months. When previous catheterization data on sildenafil therapy was compared to last catheterization data on sildenafil, mean pulmonary arterial pressure, pulmonary resistance index, and pulmonary/systemic vascular index ratio were increased (49.0+/−14.5 mmHg versus 53.2+/−18.3 mmHg, p=0.06; 9.9+/−5.8 unitsxm2 versus 12.2+/−7.0 unitsxm2, p<0.05; 0.66+/−0.27 versus 0.89+/−0.47, p<0.05, respectively). After transition, statistically significant improvements were observed with tadalafil therapy compared with sildenafil therapy in mean pulmonary arterial pressure (53.2+/−18.3 mmHg versus 47.4+/−13.7 mmHg, p<0.05), pulmonary vascular resistance index (12.2+/−7.0 unitsxm2 versus 10.6+/−7.2 unitsxm2, p<0.05), and pulmonary-systemic vascular resistance ratio (0.89+/−0.47 versus 0.75+/−0.35, p<0.05) during follow-up (Figure 1). There was no change in sildenafil versus tadalafil, respectively, with regard to tricuspid regurgitant jet velocity (4.1+/−0.7 m/second versus 3.9+/−0.8 m/second, n=21), right ventricular diastolic dimension (24.5+/−10.1 mm/m2 versus 23.6+/−8.8 mm/m2, n=19), mean right atrial pressure (6.7+/−3.6 mmHg versus 6.6+/−2.4 mmHg, n=14), cardiac index (4.4+/−1.2 l/minutes/m2 versus 4.4 +/−1.3 l/minutes/m2, n=14), brain natriuretic peptide (102.2+/−238.3 pg/ml versus vs 100.2+/−160 pg/ml, n=24), and 6-minute walk distance (508.1+/−102.4 m versus 516.3+/−90.3 m, n=19) (paired t-test or Mann-Whitney’s U test). On sildenafil therapy, 11 patients (38%) were class I or II, 6 patients (21%) were class III, and only 1 patient (3%) was class IV. During follow-up, 4 patients improved functional class (class II to class I (n=1), class III to class II (n=3)) and 4 patients deteriorated functional class (class I to class II (n=1), class II to class III (n=3)) on tadalafil therapy.
Figure1. Hemodynamic improvement after transtion from sildenafil to tadalafil in 14 patients.
mPAP; mean pulmonary arterial pressure, PVRI; pulmonary vascular resistance index, Rp/Rs ratio; pulmonary/systemic vascular resistance ratio
on Sildenafil 1; previous catheterization on sildenafil therapy, on Sildenafil 2; last catheterization on sildenafil therapy
on Tadalafil; initial catheterization on tadalafil therapy
In 14 patients, mPAP, PVRI, and Rp/Rs ratio increased from the previous (sildenafil 1) to the last catheterization (sildenafil 2) on sildenafil therapy during follow-up (15.2+/−8.8 months). After transition to tadalafil, these hemodynamic data significantly improved compared to the last data on sildenafil therapy during follow-up (23.5+/−8.3 months).
Tadalafil as initial therapy
All patients had idiopathic PAH. Two patients had PAH therapy prior to initiation of tadalafil (inhaled treprostinil and ambrisentan, ambrisentan and epoprostenol) and 2 patients had tadalafil as the initial PAH therapy. Although statistical analysis was not performed due to the small number of the patients, tadalafil tended to improve 6-minute walk, tricuspid regurgitant jet velocity, brain natriuretic peptide, mean pulmonary arterial pressure, and pulmonary vascular resistance index compared with baseline during follow up (10.5+/−3.5 months) (Table 2). During the follow up period (13.3+/−13.2 months), 2 patients with class III improved to class I or II and the remaining 2 patients with class II did not deteriorate functional class. All 4 patients did not change the concomitant medications or add new vasodilator therapies after initiation of tadalafil.
Table 2.
Clinical profile in 4 patients treated with tadalafil as initial PDE-5i therapy
| case | age | diagnosis | 6MWD (m) |
WHO Functional class |
TR velocity (m/s) |
BNP (pg/ml) |
mPAP (mmHg) |
PVRI (unitxm2) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| before | after | before | after | before | after | before | after | before | after | before | after | |||
| 1 | 5 | IPAH |  |
 |
3 | 2 | 4.5 | 3.2 | 1289 | 66 | 76 |  |
16.7 |  |
| 2 | 8 | IPAH |  |
 |
3 | 1 | 3.6 | 2.9 | 163 | 124 | 58 |  |
14.4 |  |
| 3 | 8 | IPAH | 413 | 555 | 2 | 2 | 3.9 | 4.0 | 20 | 15 | 27 | 28 | 3.1 | 4.3 |
| 4 | 16 | IPAH | 581 | 652 | 2 | 2 | 3.9 | 3.1 | 46 | 15 | 38 |  |
8.2 |  |
| Mean +/−SD |
 |
 |
497+/− 118.8 |
603+/ −68.6 |
2.5+/− 0.6 |
1.5+/− 0.6 |
4.0+/− 0.4 |
3.3+/− 0.5 |
380+/− 609.5 |
55+/− 51.9 |
50+/− 21.7 |
28 | 11+/− 6.2 |
4.3 |
BNP; brain natriuretic peptid, IPAH; idiopathic pulmonary arterial hypertension, mPAP; mean pulmonary arterial pressure, PDE-5i; phosphodiesterase type 5 inhibitors, PVRI; pulmonary vascular resistance index, TR; tricuspid regurgitation, WHO; World Health Organization, 6MWD; 6-minute walking distance
Adverse events
Adverse events were assessed at each clinic visit. The number of clinic visits during the first year after initiation of tadalafil was 1.9+/−1.2. Side effect profiles were similar in patients on sildenafil or tadalafil (Table 3). Nasal congestion and myalgia were observed after transition, but these were very mild. One patient complained of erections as a side effect of sildenafil. He had received 40 mg/day of sildenafil for 3 years. After increasing the sildenafil dose to 60 mg/day, he complained of several episodes of erections during the school day. The erections resolved after transition of tadalafil. In the 33 patients, 2 patients discontinued tadalafil due to migraines or allergic reaction. Tadalafil dose in these 2 patients were 0.4 and 0.6 mg/kg/day which were lower than the average tadalafil dose (1.0 mg/kg/day) in our population. Over all, the discontinuation rates were 6%. Three patients on sildenafil had episodes of chest pain which resolved after transition of tadalafil. Only 1 patient had administration of other PAH drugs due to clinical worsening. This patient was started on subcutaneous treprostinil therapy due to worsening hemodynamic data and exercise intolerance after transition to tadalafil. No patients were hospitalized or died during the follow up period.
Table 3.
Adverse events on sildenafil and tadalafil in 29 patients
| sildenafil number (%) |
tadalafil number (%) |
|
|---|---|---|
| Symptoms due to PAH | ||
| fatigue | 7 (24) | 6 (21) |
| chest pain | 3 (10) | 0 (0) |
| dyspnea | 1 (3) | 1 (3) |
| syncope | 1 (3) | 0 (0) |
| Side effects | ||
| headache* | 4 (21) | 4 (21) |
| nausea | 2 (7) | 2 (7) |
| flushing | 1 (3) | 1 (3) |
| myalgia | 0 (0) | 1 (3) |
| nasal congestion | 0 (0) | 1 (3) |
| others | erection 1 | |
| clitoral and facial swelling 1 | clitoral and facial swelling 1 | |
PAH; pulmonary arterial hypertension
including migraine
Discussion
We found that orally administered tadalafil as a once daily dosing drug was well tolerated in pediatric patients. Most of the patients who switched from sildenafil (average dose; 3.4+/−1.1 mg/kg/day) to tadalafil (1.0+/−0.4 mg/kg/day) successfully continued tadalafil therapy without the need to switch back to sildenafil. Only 2 patients stopped tadalafil due to side effects including migraine and allergic reaction. Thus, the discontinuation rate of tadalafil therapy in children was 6%. In spite of concomitant therapy, tadalafil statistically improved hemodynamic data including mean pulmonary arterial pressure, pulmonary vascular resistance index, and pulmonary-systemic vascular resistance ratio compared with sildenafil in 14 of 29 patients with repeated catheterization. Importantly, clinical benefits of sildenafil were maintained in regards to echocardiographic data, brain natriuretic peptide level, 6-minute walk distance, and World Health Organization functional class after transition to tadalafil. There were no deaths in the short term follow-up.
The present study is helpful to guide tadalafil therapy in pediatric patients with PAH because our study suggests the safe use of tadalafil in pediatric patients with PAH. In addition, disease progression was rare during follow-up on tadalafil. Recently, tadalafil has been approved in the United States for the treatment of adults with group I pulmonary arterial hypertension [3]. In a large previous study, oral tadalafil of 40mg once daily was effective in improving exercise capacity even in the adult PAH patients receiving bosentan as background therapy [6]. Tadalafil has shown beneficial effects in a prospective cohort study in patients with Eisenmenger syndrome [12]. Despite several recent reports on tadalafil therapy, tadalafil is not approved yet in pediatric patients. The present report is the first to describe clinical benefits and safety in a dedicated cohort of pediatric patients with PAH. There is no currently established dosing of sildenafil or tadalafil in pediatric patients with PAH. We extrapolated the pediatric dose for this study from adult studies. Even though tadalafil doses were empiric, they appeared to be safe and effective. Our study provides important information on the usefulness of oral tadalafil therapy as an additional targeted therapy in pediatric PAH.
The reasons for hemodynamic improvement after transition to tadalafil from sildenafil are not clear, but it may be caused by differences in pharmacokinetics. Unlike sildenafil, the longer half life of tadalafil makes serum levels more consistent [13], which may have an advantage in regards to hemodynamics in patients with PAH. In addition, sildenafil therapy may have a risk for worse compliance due to multiple daily dosing. Our results provide initial data that pediatric patients may switch from sildenafil to tadalafil as a once daily dosing drug with a tolerable safety profile.
Limitations of the present study were the small sample size and relatively short observational duration. Due to the few patients, we could not assess the efficacy of tadalafil as initial therapy in the pediatric population. Therefore, the clinical findings tadalafil in children remain to be confirmed in a larger study. A further limitation may also be the relative heterogeneity of the study population. However, most pediatric PAH studies are comprised of children with idiopathic PAH and PAH associated with congenital heart disease. Thus, a larger study involving PAH patients will be needed to determine whether the results we observed in children PAH are generable to the larger pediatric population. Finally, we did not perform a pharmacokinetic analysis, which should be performed in future studies.
Conclusions
Convenience of once daily dosing prompted transition from sildenafil to tadalafil in pediatric patients. Tadalafil can be safely used in pediatric patients with PAH and may prevent disease progression.
Acknowledgement
This study was supported by the Jayden DeLuca Foundation, the Leah Bult Foundation, and UL1 RR025780 Colorado Clinical Translational Science Institute, National Center for Research Resources, and National Institutes of Health.
Footnotes
Disclosures: The University of Colorado is paid by United Therapeutics and Pfizer for Dr Ivy to be a consultant.
References
- 1.Barst RJ, McGoon M, McLaughlin V, Tapson V, Rich S, Rubin L, Wasserman K, Oudiz R, Shapiro S, Robbins IM, Channick R, Badesch D, Rayburn BK, Flinchbaugh R, Sigman J, Arneson C, Jeffs R. Beraprost therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2003;41:2119–2125. doi: 10.1016/s0735-1097(03)00463-7. [DOI] [PubMed] [Google Scholar]
- 2.Chin KM, Rubin LJ. Pulmonary arterial hypertension. J Am Coll Cardiol. 2008;51:1527–1538. doi: 10.1016/j.jacc.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 3.Eli Lilly and Company ADCIRCA (tadalafil) tablets for oral administration: US prescribing information [online. Oct 7, 2009.
- 4.Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, Grimminger F, Kurzyna M, Simonneau G. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 5.Galie N, Beghetti M, Gatzoulis MA, Granton J, Berger RM, Lauer A, Chiossi E, Landzberg M. Bosentan therapy in patients with eisenmenger syndrome: A multicenter, double-blind, randomized, placebo-controlled study. Circulation. 2006;114:48–54. doi: 10.1161/CIRCULATIONAHA.106.630715. [DOI] [PubMed] [Google Scholar]
- 6.Galie N, Brundage BH, Ghofrani HA, Oudiz RJ, Simonneau G, Safdar Z, Shapiro S, White RJ, Chan M, Beardsworth A, Frumkin L, Barst RJ. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119:2894–2903. doi: 10.1161/CIRCULATIONAHA.108.839274. [DOI] [PubMed] [Google Scholar]
- 7.Humpl T, Reyes JT, Holtby H, Stephens D, Adatia I. Beneficial effect of oral sildenafil therapy on childhood pulmonary arterial hypertension: Twelve-month clinical trial of a single-drug, open-label, pilot study. Circulation. 2005;111:3274–3280. doi: 10.1161/CIRCULATIONAHA.104.473371. [DOI] [PubMed] [Google Scholar]
- 8.Ivy DD, Claussen L, Doran A. Transition of stable pediatric patients with pulmonary arterial hypertension from intravenous epoprostenol to intravenous treprostinil. Am J Cardiol. 2007;99:696–698. doi: 10.1016/j.amjcard.2006.09.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ivy DD, Doran AK, Smith KJ, Mallory GB, Jr., Beghetti M, Barst RJ, Brady D, Law Y, Parker D, Claussen L, Abman SH. Short- and long-term effects of inhaled iloprost therapy in children with pulmonary arterial hypertension. J Am Coll Cardiol. 2008;51:161–169. doi: 10.1016/j.jacc.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ivy DD, Rosenzweig EB, Lemarie JC, Brand M, Rosenberg D, Barst RJ. Long-term outcomes in children with pulmonary arterial hypertension treated with bosentan in real-world clinical settings. Am J Cardiol. 2010;106:1332–1338. doi: 10.1016/j.amjcard.2010.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levy M, Celermajer DS, Bourges-Petit E, Del Cerro MJ, Bajolle F, Bonnet D. Add-on therapy with subcutaneous treprostinil for refractory pediatric pulmonary hypertension. J Pediatr. 2011;158:584–588. doi: 10.1016/j.jpeds.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 12.Mukhopadhyay S, Sharma M, Ramakrishnan S, Yusuf J, Gupta MD, Bhamri N, Trehan V, Tyagi S. Phosphodiesterase-5 inhibitor in eisenmenger syndrome: A preliminary observational study. Circulation. 2006;114:1807–1810. doi: 10.1161/CIRCULATIONAHA.105.603001. [DOI] [PubMed] [Google Scholar]
- 13.Rosenzweig EB. Tadalafil for the treatment of pulmonary arterial hypertension. Expert Opin Pharmacother. 2010;11:127–132. doi: 10.1517/14656560903413542. [DOI] [PubMed] [Google Scholar]
- 14.Rubin LJ. Primary pulmonary hypertension. N Engl J Med. 1997;336:111–117. doi: 10.1056/NEJM199701093360207. [DOI] [PubMed] [Google Scholar]
- 15.Singh TP, Rohit M, Grover A, Malhotra S, Vijayvergiya R. A randomized, placebo-controlled, double-blind, crossover study to evaluate the efficacy of oral sildenafil therapy in severe pulmonary artery hypertension. Am Heart J. 2006;151:851, e851–855. doi: 10.1016/j.ahj.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 16.von Keitz A, Rajfer J, Segal S, Murphy A, Denne J, Costigan T, Lockhart D, Beasley CM, Jr., Emmick JT. A multicenter, randomized, double-blind, crossover study to evaluate patient preference between tadalafil and sildenafil. Eur Urol. 2004;45:499–507. doi: 10.1016/j.eururo.2003.11.030. discussion 507-499. [DOI] [PubMed] [Google Scholar]
- 17.Yung D, Widlitz AC, Rosenzweig EB, Kerstein D, Maislin G, Barst RJ. Outcomes in children with idiopathic pulmonary arterial hypertension. Circulation. 2004;110:660–665. doi: 10.1161/01.CIR.0000138104.83366.E9. [DOI] [PubMed] [Google Scholar]