Abstract
Purpose
To assess the relationship between nocturnal plasma melatonin and serum estradiol (E2) and progesterone (P4) levels in depressed pregnant women (DW) and matched healthy women (HW).
Methods
We used analysis of variance (ANOVA) and linear regression analyses on data obtained from pregnant HW and DW.
Results
Log E2 and log P4 were positively correlated with measures of melatonin quantity in HW (p<0.05) but not DW, controlling for age. Log E2 and log P4 were positively correlated with melatonin offset and duration in DW (p<0.01) but not HW.
Conclusions
Pregnant DW may be less sensitive than HW to modulation of melatonin secretion by E2 and P4. That melatonin timing measures are more sensitive to E2 and P4 variation in DW may reflect a circadian system more attuned to the need for realignment in DW than in HW. These altered sensitivities to reproductive hormones may reflect a biologic vulnerability that predisposes some pregnant women to depression.
Introduction
Depressive disorders, which are approximately twice as common in women as in men, often occur in conjunction with changes during the reproductive cycle, for example, premenstrual dysphoric disorder (PMDD) and major depression during pregnancy, postpartum, and at the menopausal transition.1 Why women's depressive episodes often occur in association with changes in reproductive status is not understood. Some researchers propose that circadian rhythm dysregulation plays a role in mood disorders2,3 and reproductive hormones may modulate circadian rhythm amplitude and phase.4 The dramatic fluctuations in reproductive hormones that occur during pregnancy and the transition from pregnancy to postpartum occur together with alterations in diurnal melatonin timing and quantity.5 We hypothesize that the changes in reproductive hormones occurring during pregnancy may alter circadian rhythms and thereby precipitate depressive symptoms in vulnerable women.
Melatonin is an endocrine circadian rhythm marker synthesized primarily in the mammalian pineal gland, regulated by neural inputs from the suprachiasmatic nucleus (SCN). Plasma melatonin levels are very low in daylight but become elevated in darkness. In humans, melatonin is the most reliable biologic marker of circadian rhythms.6 Melatonin mediates the changes in reproductive hormones and behavior in seasonally breeding animals.7
Evidence for a role for reproductive hormones in circadian rhythm dysregulation and mood comes from our recent studies of depressed and nondepressed pregnant and postpartum women. We found significantly phased-advanced melatonin secretion and baseline offsets in pregnant women with personal and family histories of depression, regardless of their current diagnosis, relative to women without positive histories.8 We also found significantly lower nocturnal plasma melatonin levels in pregnant depressed women (DW) relative to matched healthy women (HW).8 Notably, whereas plasma melatonin quantity increased approximately linearly with the number of weeks pregnant in HW, it did not in DW; that is, melatonin levels became increasingly elevated as pregnancy progressed in healthy but not in depressed women. Additionally, our previous findings that pregnant HW and DW did not differ significantly in serum estradiol (E2) or progesterone (P4)8 suggested that the observed differences in melatonin quantity were not simply due to differences in quantity of reproductive hormones.
The primary aim of the present report is to characterize the relationship of serum E2 and P4 levels to the abnormal melatonin timing and quantitative measures we found previously in pregnant DW. Based on the earlier studies, we hypothesized that plasma melatonin levels would be positively correlated with serum E2 and P4 levels in HW but not in DW. We also expected to find positive correlations between measures of melatonin timing and E2 and P4 levels.
Materials and Methods
Subjects
Data for this report were collected between November 1989 and August 2008 and represent an extension of our earlier work8 by the addition of 7 HW and 1 DW. Participant recruitment procedures and research procedures are described in detail elsewhere.8 Briefly, potential subjects residing in San Diego, California, were screened via telephone. Women were eligible to participate if they did not smoke or use medications or herbal or over the-counter (OTC) preparations that would interfere with neuroendocrine measures. Participants agreed to attend multiple overnight hospital stays in the General Clinical Research Center. Eligible women underwent the following laboratory tests: a chemistry panel, thyroid indices, complete blood count, urinalysis, and urine toxicology screening. Eligible women could not have significant medical illness, and women who were receiving drug treatment were required to discontinue any medication that would interfere with melatonin measures. We required women with major depression to discontinue antidepressant treatment ≥2 weeks (≥4 weeks for fluoxetine treatment) before the start of the study. Both DW and HW could not have had alcohol abuse or dependency within the past year. Women with bipolar or primary anxiety disorders were excluded from the study.
To establish DSM-IV-TR entrance and baseline criteria, trained clinicians administered the following assessments to each participant: the Structured Clinical Interview for DSM-IV (SCID)9 and at least two baseline evaluation ratings, scheduled 1 week apart, using objective ratings with the 21-item Hamilton Depression Rating Scale (HAM-D)10 and an atypical depressive symptom inventory11; subjective ratings with the Beck Depression Inventory (BDI)12; and the Edinburgh Postnatal Depression Scale (EPDS),13 which has been validated for use during pregnancy.14 For study inclusion, DW were required to have a mean HAM-D score ≥14 and BDI and EPDS scores ≥10 for 2 weeks. Healthy comparison subjects were required to have a mean HAM-D score ≤8 and a mean BDI score ≤5.
Procedure
The University of California San Diego (UCSD) Institutional Review Board approved the study protocol. All subjects gave written informed consent after study procedures were explained fully.
Women who met entrance criteria were admitted to the UCSD General Clinical Research Center at 16:00 hours. After a night of adaptation to the sleep room, licensed nurses inserted an intravenous catheter at 17:00 hours and drew blood (3 mL) every 30 minutes from 18:00 to 11:00 hours for measurement of overnight plasma melatonin. To minimize sleep disturbances, nurses drew blood samples through an intravenous catheter threaded through a porthole in the wall into an adjoining room. Serum for E2 and P4 was obtained at 18:00 hours and again at 06:00 hours. Participants remained at bed rest in a single room with double doors, with the windows covered with heavy drapery to block extraneous light from entering between 16:00 hours and 11:00 hours. Light panels in the room allowed daytime light exposure to remain relatively dim (<30 lux). We considered this light intensity to be too dim to substantially suppress melatonin in undilated pupils, disrupt sleep, or shift circadian rhythms but not dim enough to serve as a dark pulse.15 Subjects slept in the dark with an eye mask. Nurses entered the room only when necessary (recorded by infrared camera), using a pen-sized dim red flashlight.
Assays
Estradiol and progesterone
Serum E2 was measured using a solid-phase radioimmunoassay (RIA) (Diagnostic Products Corporation, Los Angeles, CA) that has a sensitivity of 8 pg/mL and a working range of 10–3600 pg/mL. The cross-reactivities of the E2 antibody are as follows: estrone 10.0%, estriol 0.32%, dehydroepiandrosterone (DHEA) 0.001%, 5α-dihydrotestosterone 0.004%, testosterone 0.0001%, and P4, 11-deoxycortisol, cortisol, androsterone, androsenedione, and aldosterone <0.001%. At the 50% intercept, the intraassay and interassay coefficients of variation (CV) for this assay are 4% and 7%, respectively. Serum concentrations of P4 were measured using a solid-phase RIA (Diagnostic Products). The sensitivity of this assay is 0.5 ng/mL, and its working range is 0.1–40 ng/mL. The intraassay and interassay CVs for this assay are 5% and 8%, respectively. Cross-reactivity with E2 was 0.0%, 11-deoxycortisol 2.4%, 20 alpha-dihydroprogesterone 2.0%, 11-deoxycorticosterone 1.7%; beta-pregnan-3, 20-dione 1.3%, other related steroid and hormone species ≤0.3%.
Melatonin
Blood samples for melatonin were placed into plastic tubes containing ethylenediaminetetraacetic acid, centrifuged, frozen immediately, and stored at −70°C until assayed. All blood samples for the same subject were run in the same assay. Initial assays for melatonin and E2 and P4 have been described elsewhere.16,17 We assayed plasma melatonin for the first 24 subjects by RIA using a kit manufactured by Immuno-Biological Laboratories (Hamburg, Germany). After the manufacturer discontinued this kit, we assayed plasma for the remaining 9 subjects with a direct melatonin RIA kit manufactured by Bühlmann Laboratories (ALPCO Diagnostics, Windham, NH), using calibrators ranging from 1 to 81 pg/mL and reporting intraassay and interassay CVs of 6.7% and 10.4%, respectively. The standard range has an analytical sensitivity of 0.8 pg/mL. For melatonin statistical analyses, assay method type (Immuno-Biological vs. Bühlmann kit) was included as a covariate to correct for differences between assays.
Estimation of melatonin parameters
Using a visual inspection method described previously,18 we defined (1) the dim light melatonin onset (DLMO) as the time when the slope (dy/dt) of the log-transformed melatonin concentration curve first became steeply positive for at least three consecutive time points relative to the slope of the points immediately preceding it, (2) melatonin offset (DLMOff) as the time when the slope of the descending melatonin curve first approached zero for at least three consecutive time points, (3) synthesis offset (SynOff) as the time after the melatonin peak when the slope of the descending melatonin curve first became steeply negative for three consecutive time points, (4) melatonin standard duration as the difference in hours between the DLMO and DLMOff, (5) synthesis duration as the difference in hours between DLMO and SynOff, (6) the peak concentration as the single highest concentration during the nocturnal secretory episode, (7) the standard area under the curve (AUC) as the integrated melatonin concentration (pg/mL/h) from DLMO to DLMOff, and (8) synthesis AUC as the integrated melatonin concentration (pg/mL/h) from DLMO to SynOff.
Statistical analyses
Because the variances in melatonin AUCs and in reproductive hormone levels were nonhomogeneous across weeks of pregnancy, we log-transformed raw melatonin and reproductive hormone values (means of the 18:00 and 06:00 hour draws) for E2 (range 230–34,700 pg/mL) and P4 (range 14–231 ng/mL) before statistical analyses. We used separate, one-factor, between-subjects analyses of variance (ANOVA) to test the effects of diagnosis (healthy vs. depressed) on variables of interest. To characterize the relationships of E2 and P4 to melatonin measures, we used stepwise linear regression analyses, with the backward entry method, setting p=0.100 for elimination of variables from the model.19
Results
Subject characteristics
Complete datasets were obtained from 22 pregnant HW (self-reported ethnicity: Caucasian 13, 59%, Hispanic 6, 27%, Asian 1, 5%, African American 2, 9%) and 11 DW (Caucasian 5, 45%, Hispanic 3, 27%, African American 1, 9%, multiethnic 1, 9%). A chi-square analysis showed the HW and DW did not differ significantly in their distributions within ethnic groups (chi-square (df=4)=3.25, p=0.52). The distribution within groups also was approximately equal across seasons of testing, with the percentages of HW/DW subjects being: winter (9%/9%), spring (32%/18%), summer (32%/27%), fall (27%/45%); a chi-square analysis showed the HW and DW did not differ significantly in their distributions across seasons (chi-square (df=3)=1.23, p=0.74).
Table 1 shows other relevant characteristics of the HW and DW groups at the time of data collection. The HW and DW groups were approximately matched and did not differ significantly in age, F(1,31)=0.14, p=0.711; weeks pregnant at the time of testing, F(1,31)=0.177, p=0.677; or body mass index (BMI), F(1,31)=0.263, p=0.612. As we found previously,8 the HW and DW groups did not differ significantly in log E2 concentrations, F(1,31)=0.348, p=0.560, and log P4 concentrations, F(1,31)=0.846, p=0.365, when the number of weeks of pregnancy was included as a covariate in the analyses. As reported previously,8 however, melatonin log AUC was significantly lower in DW vs. HW, F(1,32)=5.730, p=0.023, as was log peak melatonin concentration, F(1,31)=4.72, p=.038. Melatonin onset time (DLMO) was marginally earlier in DW (mean=19:54±1:01 hh:mm (hours:minutes) vs. HW (mean=20:36±0:59 hh:mm), F(1,32)=3.66, p=0.065. The SynOff, DLMOff, and melatonin standard and synthesis durations did not differ significantly between groups (p>0.05). However, when groups were compared based on personal or family history of depression rather than current diagnosis, women with positive personal or family histories had significantly earlier DLMOs (mean=19:54±0:58 vs. 20:44±0:58 hh:mm, F(1,31)=6.19, p=0.018) and DLMOffs (mean=11:06±1:06 vs. 9:47±1:38 hh:mm, F(1,31)=7.48, p=0.010) than women with negative personal or family histories. SynOff was also marginally earlier in history-positive vs. history-negative women (5:15±1:27 vs. 6:03±0:54 hh:mm, F(1,31)=3.85, p=0.059). The groups did not differ significantly in synthesis duration, F(1,31)=0.01, p=0.92.
Table 1.
Subject Characteristics of 22 Healthy and 11 Depressed Women Studied During Pregnancy
| |
Healthy women |
Depressed women |
|
||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | p | |
| Age, years | 25.7 | 5.2 | 19–38 | 26.4 | 5.4 | 20–37 | 0.140 |
| Weeks pregnant | 30.3 | 8.0 | 8–38 | 29.1 | 7.6 | 14–38 | 0.677 |
| Body mass index | 30.1 | 5.6 | 22–41 | 31.1 | 5.2 | 25–40 | 0.096 |
| Estradiol | 3.9 | 0.5 | 2.4–4.5 log pg/mL | 3.8 | 0.2 | 3.4–4.1 log pg/mL | 0.348 |
| Progesterone | 2.0 | 0.3 | 1.2–2.3 log ng/mL | 1.9 | 0.2 | 1.6–2.4 log ng/mL | 0.846 |
| Melatonin AUC | 3.2 | 0.3 | 2.6–3.6 log ng/mL/h | 3.0 | 0.1 | 2.8–3.2 log ng/mL/h | 0.019 |
AUC, area under the curve; SD, standard deviation.
Correlations of melatonin quantity and timing with serum E2 and P4
Quantitative measures
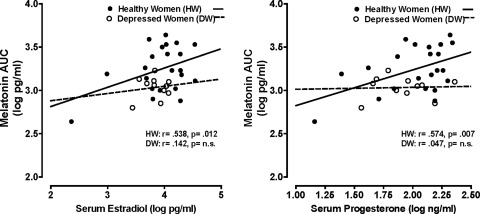
Log E2 and log P4 values were highly correlated with each other in both HW (r=0.911, p<0.0001) and DW (r=0.778, p=0.005). Tables 2 and 3 summarize results of regression analyses performed on melatonin variables with log E2 and log P4, with age included as an independent variable. These analyses showed serum log E2 and log P4 were significantly positively correlated with melatonin AUC in HW when age was controlled for. In contrast, the correlations between melatonin AUC and log E2 and log P4 were nonsignificant in DW (p>0.05) (Fig. 1). Thus, progressively elevated serum E2 and P4 were associated with increased nocturnal plasma melatonin quantity in HW but not in DW. All other melatonin quantity and timing measures (peak, DLMO, DLMOff, synthesis offset, standard, and synthesis durations) were not significantly correlated with log E2 or log P4 in HW (p>0.05).
Table 2.
Correlations Between Log Estradiol and Melatonin Measures in Healthy and Clinically Depressed Pregnant Women
| |
Healthy women (n=22) |
Depressed women (n=11) |
||
|---|---|---|---|---|
| |
Correlation with log E2 |
|
Correlation with Log E2 |
|
| r | p | r | p | |
| Peak | 0.282 | NS | −0.147 | NS |
| Log AUC | 0.538* | 0.012 | 0.142 | NS |
| Log synthesis AUC | 0.480* | 0.028 | 0.227 | NS |
| DLMO | −0.011 | NS | 0.075 | NS |
| DLMOff | 0.068* | NS | 0.904 | 0.0001 |
| Standard duration | 0.192* | NS | 0.801 | 0.003 |
| Synthesis offset | 0.095* | NS | 0.564 | 0.071 |
| Synthesis duration | 0.071* | NS | 0.514 | NS |
Tabled values are Pearson correlations except for those with asterisks (*), which are partial correlations controlling for age, in those cases where age was correlated with the melatonin measure at p<0.10, at least. Significant correlations (p<0.05, two-tailed) are highlighted in bold.
DLMO, dim light melatonin onset; DLMOff, melatonin offset; E2, estradiol; NS, not significant.
Table 3.
Correlations Between Log Progesterone and Melatonin Measures in Healthy and Clinically Depressed Pregnant Women
| |
Healthy women (n=22) |
Depressed women (n=11) |
||
|---|---|---|---|---|
| |
Correlation with log P4 |
|
Correlation with log P4 |
|
| r | p | r | p | |
| Peak | 0.317 | NS | −0.249 | NS |
| Log AUC | 0.574* | 0.007 | 0.047 | NS |
| Log synthesis AUC | 0.513* | 0.017 | 0.163 | NS |
| DLMO | 0.052 | NS | −0.084 | NS |
| DLMOff | 0.176* | NS | 0.884 | 0.0003 |
| Standard duration | 0.327* | NS | 0.857 | 0.001 |
| Synthesis offset | 0.128* | NS | 0.597 | 0.053 |
| Synthesis duration | 0.045* | NS | 0.642 | 0.033 |
Tabled values are Pearson correlations except for those with asterisks (*), which are partial correlations controlling for age, in those cases where age was correlated with the melatonin measure at p<0.100, at least. Significant correlations (p<0.05, two-tailed) are highlighted in bold.
P4, progesterone.
FIG. 1.
Scatterplot of serum estradiol and progesterone levels in relation to melatonin area under the curve (AUC) in healthy vs. depressed women.
Timing measures
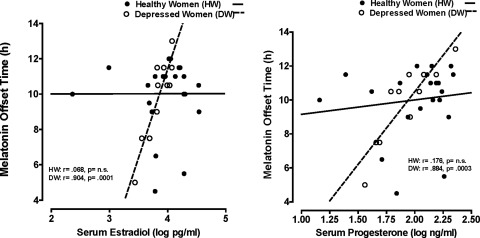
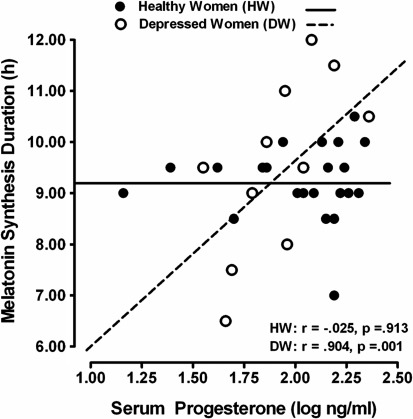
We observed a different pattern of results regarding melatonin timing measures: DLMOff and melatonin duration measures were positively correlated with both log E2 and log P4 in DW but not in HW (Fig. 2). All other melatonin quantity and timing measures were not significantly correlated with log E2 or log P4 in DW, with the exception of synthesis duration, which was significantly and positively correlated with log P4 in DW but not in HW (Fig. 3 and Tables 2 and 3).
FIG. 2.
Scatterplot of serum estradiol and progesterone levels in relation to melatonin offset (DLMOff) in healthy vs. depressed women.
FIG. 3.
Scatterplot of serum progesterone levels in relation to melatonin synthesis duration in healthy vs. depressed women.
Discussion
The most important findings of this study are that (1) nocturnal plasma melatonin quantity in pregnant women was significantly correlated with serum E2 and P4 levels in HW but not in DW and (2) melatonin offset time and synthesis duration were significantly correlated with E2 and P4 levels in DW but not in HW. Notably, as we found previously,8 HW and DW did not differ significantly in serum E2 and P4 levels (Table 1). Thus, the differences in the correlations of reproductive hormones with melatonin quantity and timing were not associated with differences between HW and DW in the absolute levels of E2 and P4. We propose that these group variations may reflect differences between DW and HW in E2 and P4 receptor sensitivity and signal transduction during pregnancy.
Melatonin quantity measures
The present results are consistent with earlier work showing that melatonin and serum P4 levels were positively correlated in human follicular cells.20 Adriaens et al.21 found that variations in melatonin levels could influence P4 concentrations. Testing the effects of a range of melatonin doses on in vitro cultured mouse ovarian follicles, they found increased P4 levels after administration of a low melatonin dose (100 μM); E2 levels were unaffected. More recently, Taketani et al.22 found that melatonin concentrations in the follicular fluid of cycling women were positively correlated with P4 levels; melatonin treatment in women with low endogenous P4 levels increased P4 concentrations during the midluteal phase.
In contrast, a body of literature implicates P4 in the modulation of melatonin in humans. In cycling women, Webley and Leidenberger23 found that increased P4 during the luteal vs. follicular menstrual phase was associated with significantly increased melatonin levels; furthermore, in women taking three-phase oral contraceptives, increased P4 dosage also was associated with elevated total 24-hour melatonin exposure. Increased melatonin secretion in association with oral contraceptive use has been reported elsewhere.24 Notably, Parry et al.25,26 found reduced luteal phase melatonin amplitude, peak, and AUC values, relative to healthy controls, in women who experienced depressive symptoms during the luteal menstrual phase.
Our current findings confirm and extend our earlier report8 showing that nocturnal melatonin AUC increased across weeks of pregnancy in HW but not in DW. Nakamura et al.27 also found that serum melatonin level increased as gestation progressed in normal pregnant women. Thus, E2 and P4 may be part of an important mechanism associated with increased melatonin quantity during pregnancy in HW. The magnitude of change of reproductive hormones during pregnancy, however, is quite large, 1,000–10,000-fold, whereas for melatonin, it is relatively small, only 2–3-fold.
Concerning the direction of association, the fact that E2 and P4 levels were not significantly different in HW and DW suggests that the observed alterations in endogenous melatonin quantity did not modulate E2 and P4 levels. Rather, these data suggest that E2 and P4 modulate melatonin production. During pregnancy, the organism preserves levels of E2 and P4 that sustain pregnancy; these levels are not compromised by alterations in melatonin levels in DW vs. HW, which would be maladaptive for the offspring. Along those lines, Zhao et al.28 showed that melatonin binding sites in rats were reduced by 70% after ovariectomy. Injection of E2 and P4 into the rat uteri partially restored melatonin receptor density, confirming a modulating influence of these reproductive hormones on melatonin receptor density. Additionally, Masana et al.29 observed that treatment of cultured ovarian hamster cells with E2 activated melatonin type 1 (MT1) receptors and increased melatonin type 2 (MT2) receptor density. Thus, a plausible inference from the dissociation between melatonin and reproductive hormones in DW in the present study is that pregnant women with depression have reduced E2 and P4 receptor sensitivity and signaling relative to pregnant HW.
It is likely that this reduced sensitivity in the depressed women may be at the steroid receptor. As melatonin is synthesized from serotonin in the pineal gland under the rate-limiting influence of norepinephrine (NE),30 the effect of E2 and P4 levels on melatonin production could occur through catecholamine pathways.31,32 Reduced sensitivity to the stimulating effects of reproductive hormones on melatonin may represent a biologic vulnerability that predisposes some women to develop the circadian dysregulation and depression.
Melatonin timing measures
We found that elevated serum E2 and P4 levels were associated with a phase-delayed DLMOff and longer synthesis duration in DW but not in HW. These results are interpretable in light of the fact that human circadian rhythms are the product of the interaction of multiple oscillators.35 As E2 generally advances circadian rhythms, whereas P4 delays them,36–38 our data suggest that sensitivity of morning and evening oscillators to E2 and P4 may be differentially altered in pregnant DW. That E2 and P4 were positively correlated with delayed DLMOff in DW may reflect increased sensitivity to P4 but decreased sensitivity to E2 of the morning oscillator, which controls DLMOff. The increased synthesis duration associated with greater P4 in DW would follow from the phase-delay in DLMOff, not a phase-advance of DLMO. This finding parallels our previous study of women with PMDD, in which increased melatonin duration was associated with depressive symptoms in the luteal phase, when P4 is elevated, but not in the follicular phase, when P4 is absent and women with PMDD are asymptomatic.39 In a previous study of menopausal women, we also found that DLMOff was delayed in DW compared with HW.18 Notably, delayed DLMOff is associated with an evening chronotype commonly found in depressive disorders.40,41 Photoperiodic information as measured by melatonin and influenced by reproductive hormones may be inadequately conveyed from the environment to the organism in persons with depressive disorders. Increased sensitivity of pregnant DW to the effects of E2 and P4 on melatonin timing measures may reflect a corrective influence of the circadian system on misalignment.
Implications for treatment and research
If the circadian rhythm dysregulation observed in some DW reflects reduced sensitivity to the stimulatory effects of reproductive hormones on melatonin secretion, treatments that alter melatonin quantity and timing may ameliorate depression. For example, critically timed exposure to bright light (light treatment) has been shown to relieve even nonseasonal depressions, possibly by synchronizing circadian rhythms.42–44 In the case of decreased melatonin quantity and delayed melatonin offset, morning light would be the indicated treatment, as it may increase melatonin quantity at night and phase-advance the morning DLMOff, whereas evening light could benefit women with phase-advanced melatonin rhythms or increased nighttime melatonin secretion. Luteinizing hormone, which weakly stimulates E2 production, increases during bright light treatment in healthy young men.45,46 Light treatment provides a potentially viable option for women who do not wish to use pharmacologic means to alter mood. Critically timed therapeutic sleep restriction (wake therapy) also has been reported to improve mood and alter melatonin timing. For example, morning/late-night, but not evening/early-night, wake therapy advances melatonin offset in PMDD.39
The strengths of this study include careful screening and diagnostic assessments of depression using objective, interview-based assessments by trained mental health professionals in women selected from a broad range of ages, parity, ethnicities, and socioeconomic status, with sampling distributed across the seasons of the year and precise circadian sampling of plasma melatonin. Study limitations include reliance on relatively small samples of women. Further research is needed to identify the mechanism(s) by which reproductive hormones regulate melatonin circadian rhythms. The abnormal melatonin profiles that characterize DW during different reproductive epochs8,18,26,47 implicate reproductive hormones in circadian rhythm dysregulation and depression. In particular, the differing reproductive hormonal milieus that characterize each reproductive epoch (menstrual cycle, pregnancy, postpartum, and menopause) have distinguishable melatonin profiles associated with each depressive state. For example, phase-advanced and low melatonin quantities characterize PMDD and pregnant DW, whereas phase-delayed and increased melatonin AUC characterize postpartum and menopausal depression. 8,18,26,47 Understanding the relationship of reproductive hormones to circadian rhythm dysregulation may elucidate important mechanisms in women's depressions.
Acknowledgments
This work was supported by NIH grants RO1 MH070788 and R01 MH080159 and NIH Clinical Research Center (CRC) grant M01 RR00827. We thank Alan Turken, B.S., for his excellent work in performing the assays of melatonin and reproductive hormones.
Disclosure Statement
The authors have no conflicts of interest to report.
References
- 1.Parry BL. Meliska CJ. Martinez LF, et al. Neuroendocrine abnormalities in women with depression linked to the reproductive cycle. In: Sibley D, editor; Hanin I, editor; Kuhar M, editor; Skolnick P, editor. The handbook of contemporary neuropharmacology. New York: John Wiley & Sons; 2007. pp. 843–857. [Google Scholar]
- 2.Wehr TA. Wirz-Justice A. Internal coincidence model for sleep deprivation and depression. In: Koella WP, editor. Sleep 1980. Basel: Karger; 1981. pp. 26–33. [Google Scholar]
- 3.Wirz-Justice A. Biological rhythm disturbances in mood disorders. Int Clin Psychopharmacol. 2006;21(Suppl 1):S11–15. doi: 10.1097/01.yic.0000195660.37267.cf. [DOI] [PubMed] [Google Scholar]
- 4.Leibenluft E. Do gonadal steroids regulate circadian rhythms in humans? J Affect Disord. 1993;29:175–181. doi: 10.1016/0165-0327(93)90031-e. [DOI] [PubMed] [Google Scholar]
- 5.Parry BL. Martinez LF. Maurer EL, et al. Sleep rhythms and women's mood. Part I: Menstrual cycle, pregnancy and postpartum. Sleep Med Rev. 2006;10:129–144. doi: 10.1016/j.smrv.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Arendt J. Melatonin and human rhythms. Chronobiol Int. 2006;23:21–37. doi: 10.1080/07420520500464361. [DOI] [PubMed] [Google Scholar]
- 7.Reiter RJ. The pineal and its hormones in the control of reproduction in mammals. Endocr Rev. 1980;1:109–131. doi: 10.1210/edrv-1-2-109. [DOI] [PubMed] [Google Scholar]
- 8.Parry BL. Meliska CJ. Sorenson DL, et al. Plasma melatonin circadian rhythm disturbances during pregnancy and postpartum in depressed women and women with personal or family histories of depression. Am J Psychiatry. 2008;165:1551–1558. doi: 10.1176/appi.ajp.2008.08050709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.First MB. Gibbon M. Spitzer RL. Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders—research version. New York: Biometerics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- 10.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 11.Rosenthal NE. Heffernan MM. Bulimia, carbohydrate craving and depression: A central connection? In: Wurtman RJ, editor; Wurtman JJ, editor. Nutrition and the brain. New York: Raven Press; 1986. [Google Scholar]
- 12.Beck AT. Ward CH. Mendelson M. Mock J. Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 13.Cox JL. Holden JM. Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 14.Green J. Murray D. The use of the Edinburgh Postnatal Depression Scale in research to explore the relationship between antenatal and postnatal dysphoria. In: Cox J, editor. Perinatal psychiatry: Use and misuse of the Edinburgh Postnatal Depression Scale. London: Gaskell; 1994. pp. 180–198. [Google Scholar]
- 15.Benloucif S. Burgess HJ. Klerman EB, et al. Measuring melatonin in humans. J Clin Sleep Med. 2008;4:66–69. [PMC free article] [PubMed] [Google Scholar]
- 16.Brzezinski A. Lynch HJ. Seibel MM, et al. The circadian rhythm of plasma melatonin during the normal menstrual cycle and in amenorrheic women. J Clin Endocrinol Metab. 1988;66:891–895. doi: 10.1210/jcem-66-5-891. [DOI] [PubMed] [Google Scholar]
- 17.Anderson DC. Hopper BR. Lasley BL. Yen SS. A simple method for the assay of eight steroids in small volumes of plasma. Steroids. 1976;28:179–196. doi: 10.1016/0039-128x(76)90108-2. [DOI] [PubMed] [Google Scholar]
- 18.Parry BL. Meliska CJ. Sorenson DL, et al. Increased melatonin and delayed offset in menopausal depression: Role of years past menopause, follicle-stimulating hormone, sleep end time, and body mass index. J Clin Endocrinol Metab. 2008;93:54–60. doi: 10.1210/jc.2006-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miles J. Shevlin M. Applying regression and correlation: A guide for students and researchers. London: Sage Publications; 2001. [Google Scholar]
- 20.Nakamura Y. Tamura H. Takayama H. Kato H. Increased endogenous level of melatonin in preovulatory human follicles does not directly influence progesterone production. Fertil Steril. 2003;80:1012–1016. doi: 10.1016/s0015-0282(03)01008-2. [DOI] [PubMed] [Google Scholar]
- 21.Adriaens I. Jacquet P. Cortvrindt R. Janssen K. Smitz J. Melatonin has dose-dependent effects on folliculogenesis, oocyte maturation capacity and steroidogenesis. Toxicology. 2006;228:333–343. doi: 10.1016/j.tox.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 22.Taketani T. Tamura H. Takasaki A, et al. Protective role of melatonin in progesterone production by human luteal cells. J Pineal Res. 2011;51:207–213. doi: 10.1111/j.1600-079X.2011.00878.x. [DOI] [PubMed] [Google Scholar]
- 23.Webley GE. Leidenberger F. The circadian pattern of melatonin and its positive relationship with progesterone in women. J Clin Endocrinol Metab. 1986;63:323–328. doi: 10.1210/jcem-63-2-323. [DOI] [PubMed] [Google Scholar]
- 24.Kostoglou-Athanassiou I. Athanassiou P. Treacher DF. Wheeler MJ. Forsling ML. Neurohypophysial hormone and melatonin secretion over the natural and suppressed menstrual cycle in premenopausal women. Clin Endocrinol (Oxf) 1998;49:209–216. doi: 10.1046/j.1365-2265.1998.00504.x. [DOI] [PubMed] [Google Scholar]
- 25.Parry BL. Berga SL. Mostofi N. Klauber MR. Resnick A. Plasma melatonin circadian rhythms during the menstrual cycle and after light therapy in premenstrual dysphoric disorder and normal control subjects. J Biol Rhythms. 1997;12:47–64. doi: 10.1177/074873049701200107. [DOI] [PubMed] [Google Scholar]
- 26.Parry BL. Berga SL. Kripke DF, et al. Altered waveform of plasma nocturnal melatonin secretion in premenstrual depression. Arch Gen Psychiatry. 1990;47:1139–1146. doi: 10.1001/archpsyc.1990.01810240059010. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura Y. Tamura H. Kashida S, et al. Changes of serum melatonin level and its relationship to feto-placental unit during pregnancy. J Pineal Res. 2001;30:29–33. doi: 10.1034/j.1600-079x.2001.300104.x. [DOI] [PubMed] [Google Scholar]
- 28.Zhao H. Pang SF. Poon AM. Variations of mt1 melatonin receptor density in the rat uterus during decidualization, the estrous cycle and in response to exogenous steroid treatment. J Pineal Res. 2002;33:140–145. doi: 10.1034/j.1600-079x.2002.02898.x. [DOI] [PubMed] [Google Scholar]
- 29.Masana MI. Soares JM., Jr Dubocovich ML. 17Beta-estradiol modulates hMT1 melatonin receptor function. Neuroendocrinology. 2005;81:87–95. doi: 10.1159/000084897. [DOI] [PubMed] [Google Scholar]
- 30.Klein DC. Coon SL. Roseboom PH, et al. The melatonin rhythm-generating enzyme: Molecular regulation of serotonin N-acetyltransferase in the pineal gland. Recent Prog Horm Res. 1997;52:307–357. [PubMed] [Google Scholar]
- 31.Wilkinson M. Arendt J. Effects of oestrogen and progesterone on rat pineal N-acetyltransferase activity and melatonin production. Experientia. 1978;34:667–669. doi: 10.1007/BF01937023. [DOI] [PubMed] [Google Scholar]
- 32.Cagnacci A. Zanni AL. Veneri MG, et al. Influence of exogenous melatonin on catecholamine levels in postmenopausal women prior and during oestradiol replacement. Clin Endocrinol (Oxf) 2000;53:367–372. doi: 10.1046/j.1365-2265.2000.01099.x. [DOI] [PubMed] [Google Scholar]
- 33.Bunney WE., Jr Davis JM. Norepinephrine in depressive reactions. A review. Arch Gen Psychiatry. 1965;13:483–494. doi: 10.1001/archpsyc.1965.01730060001001. [DOI] [PubMed] [Google Scholar]
- 34.Souetre E. Salvati E. Belugou JL, et al. Circadian rhythms in depression and recovery: Evidence for blunted amplitude as the main chronobiological abnormality. Psychiatry Res. 1989;28:263–278. doi: 10.1016/0165-1781(89)90207-2. [DOI] [PubMed] [Google Scholar]
- 35.Dijk DJ. von Schantz M. Timing and consolidation of human sleep, wakefulness, and performance by a symphony of oscillators. J Biol Rhythms. 2005;20:279–290. doi: 10.1177/0748730405278292. [DOI] [PubMed] [Google Scholar]
- 36.Morin LP. Fitzgerald KM. Zucker I. Estradiol shortens the period of hamster circadian rhythms. Science. 1977;196:305–307. doi: 10.1126/science.557840. [DOI] [PubMed] [Google Scholar]
- 37.Axelson JF. Gerall AA. Albers HE. Effect of progesterone on the estrous activity cycle of the rat. Physiol Behav. 1981;26:631–635. doi: 10.1016/0031-9384(81)90137-2. [DOI] [PubMed] [Google Scholar]
- 38.Albers HE. Gerall AA. Axelson JF. Effect of reproductive state on circadian periodicity in the rat. Physiol Behav. 1981;26:21–25. doi: 10.1016/0031-9384(81)90073-1. [DOI] [PubMed] [Google Scholar]
- 39.Parry BL. Meliska CJ. Martinez LF, et al. Late, but not early, wake therapy reduces morning plasma melatonin: Relationship to mood in premenstrual dysphoric disorder. Psychiatry Res. 2008;161:76–86. doi: 10.1016/j.psychres.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaspar-Barba E. Calati R. Cruz-Fuentes CS, et al. Depressive symptomatology is influenced by chronotypes. J Affect Disord. 2009;119:100–106. doi: 10.1016/j.jad.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 41.Meliska CJ. Martinez LF. Lopez AM, et al. Relationship of morningness-eveningness questionnaire score to melatonin and sleep timing, body mass index and atypical depressive symptoms in peri- and post-menopausal women. Psychiatry Res. 2011;188:88–95. doi: 10.1016/j.psychres.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wirz-Justice A. Terman M. Oren DA, et al. Brightening depression. Science. 2004;303:467–469. doi: 10.1126/science.303.5657.467c. [DOI] [PubMed] [Google Scholar]
- 43.Wirz-Justice A. Benedetti F. Berger M, et al. Chronotherapeutics (light and wake therapy) in affective disorders. Psychol Med. 2005;35:939–944. doi: 10.1017/s003329170500437x. [DOI] [PubMed] [Google Scholar]
- 44.Parry BL. Maurer EL. Light treatment of mood disorders. Dialogues Clin Neurosci. 2003;5:353–365. doi: 10.31887/DCNS.2003.5.4/bparry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoon IY. Kripke DF. Elliott JA. Youngstedt SD. Luteinizing hormone following light exposure in healthy young men. Neurosci Lett. 2003;341:25–28. doi: 10.1016/s0304-3940(03)00122-8. [DOI] [PubMed] [Google Scholar]
- 46.Kripke DF. Elliott JA. Youngstedt SD, et al. Weak evidence of bright light effects on human LH and FSH. J Circadian Rhythms. 2010;8:5. doi: 10.1186/1740-3391-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parry BL. LeVeau B. Mostofi N, et al. Temperature circadian rhythms during the menstrual cycle and sleep deprivation in premenstrual dysphoric disorder and normal comparison subjects. J Biol Rhythms. 1997;12:34–46. doi: 10.1177/074873049701200106. [DOI] [PubMed] [Google Scholar]