Abstract
This aim of this study was to assess the potential role of IL-33 in the pathogenic process of chronic hepatitis B (CHB). The levels of serum IL-33 and soluble ST2 (sST2) in CHB patients and healthy controls (HC) were determined using enzyme-linked-immunosorbent serologic assay, and the Th1 (IFN-γ, TNF–α, IL-2) and Th2 (IL-4, IL-6, IL-10) cytokines by cytometric bead array. It was found that the levels of serum IL-33 in CHB patients were significantly higher than that of HC at the base line, but decreased after treatment with adefovir dipivoxil for 12 weeks. The levels of serum sST2, as a decoy receptor of IL-33, were significantly higher in CHB patients than the HC. There was no correlation between the levels of serum sST2 and IL-33. The concentrations of serum Th1 (IFN-γ, IL-2) and Th2 (IL-6, IL-10) cytokines in CHB patients significantly increased after treatment compared to the baseline. These results suggest that IL-33 is involved in the pathogenesis of CHB and that adefovir dipivoxil therapy can attenuate the production of IL-33 in patients with CHB.
Introduction
Hepatitis B virus (HBV) is one of the major causes of chronic liver disease in the world. There are about 130 million patients with HBV infection and 20% of them develop chronic hepatitis B (CHB) (Lavanchy 2004). More importantly, many of those patients with CHB eventually develop cirrhosis and hepatocellular carcinoma (Crockett and Keefe 2005; Rehermann and Nascimbeni 2005). Previous studies have revealed that the persistence of viral infection and chronic inflammation are dependent on the interaction between the virus, hepatocytes, and the host immune system (Rehermann 2000). The viral infection and related hepatocyte injuries are known to suppress the immune system (Gonzalez and others 2005; Martini and others 2005). Although experimental evidence suggests that antigen-specific Th1 immunity and pro-inflammatory cytokines play an important role in the HBV related liver injury and clearance of viruses (Falasca and others 2006; Katia and others 2006; Grzegorzewska and others 2011), the pathogenesis of chronic HBV infection has not been fully understood.
Interleukin-33 (IL-33) is one of the newly described members in the IL-1 family and can be produced by epithelial tissues and vascular endothelial cells (Schmitz and others 2005). IL-33 binds to its heterodimer receptors composed of IL-1 receptor-related protein ST2 and IL-1 receptor accessory protein (IL-1RaP), and can activate the MyD88 and NF-κB-related signal pathway (Schmitz and others 2005). ST2 has transmembrane form of ST2 (ST2 or ST2L) and soluble form of ST2 (sST2). ST2 is expressed on Th2 and mast cells, and functions as a mediator of IL-33 bioactivities, while sST2 acts as a decoy receptor for IL-33. Biologically, IL-33 induces Th2 cell differentiation and activates mast cells, leading to Th2 cytokine production and Th2 response and pulmonary and mucosal Th2 inflammation (Schmitz and others 2005). Further, IL-33 can antagonize the lipopolysaccharide (LPS)-mediated mortality in a model of septic shock (Chackerian and others 2007), and the levels of IL-33 were elevated in systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) patients (Yang and others 2011). Recently, IL-33 has been found to be an important factor in the pathogenesis of human immunodeficiency virus (HIV) infection and dengue virus infection (Becerra and others 2008; Miyagaki and others 2011). However, little is known whether IL-33 could participate in the pathogenic process of HBV infection.
In the current study, we examined the concentrations of serum IL-33 and sST2 in patients with CHB and healthy controls (HC) to evaluate the potential role of IL-33/ST2 axis in the pathogenic process of CHB. Further, we determined the concentrations of serum IL-33 before and after antiviral therapies in patients with CHB.
Materials and Methods
Patients
A total of 33 patients with CHB were sequentially recruited at the inpatient service in the Second Part of First Hospital. Another 20 gender-, age- and ethnic-matched HC who had no history of liver diseases and no evidence of hepatitis B virus (HBV), hepatitis C virus (HCV), and hepatitis D virus (HDV), infections were recruited. Subjects with HBV infection were confirmed positive for HBsAg and detectable HBV virions for at least 12 months (Yim and Lok 2006). Individuals with history of infection, current hepatitis C, D virus or human immunodeficiency virus (HIV) infection, autoimmune hepatitis, or metabolic liver disease, who had received immunosuppressive therapy or antiviral therapy during the past 12 months before entry, were excluded. All patients denied drug use or exposure to obvious hepatotoxin.
Patients with CHB were orally treated with 10 mg adefovir dipivoxil (Gilead Science) daily for 12 weeks. The concentrations of serum IL-33, sST2, HBV DNA, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were measured before and after treatment. Individuals with 100-fold reduced serum viral load were defined as drug-responsive patients; otherwise, individuals were defined as drug nonresponsive. Written informed consent was obtained from individual participants, and the study was approved by the First Hospital Ethical Committee of Jilin University.
Blood samples were obtained from individual subjects, sera were prepared and then stored at −80°C till needed. The levels of serum IL-33 were measured before and after treatment, and the concentrations of serum IFN-γ, TNF-α, IL-2, IL-4, IL-10 and IL-6 were determined.
Measurement of IL-33 and sST2 by enzyme-linked-immunosorbent serologic assay
The concentrations of serum IL-33 and sST2 in individual patients and HC were determined by enzyme-linked-immunosorbent serologic assay (ELISA) using human IL-33 and sST2 ELISA Kits, according to the manufacturer's instruction (Roche Diagnostics). Briefly, individual sera at 1:4 dilutions were subjected to ELISA analysis, and the concentrations of serum IL-33 in individual samples were calculated according to the standard curve established using the recombinant IL-33 and sST2 provided. The detection limit of IL-33 and sST2 ELISA kit was 0–16 ng/L and 1.6 ng/L, respectively.
Cytometric bead array of serum cytokines
The concentrations of serum cytokine levels (IFN-γ, TNF-α, IL-2, IL-4, IL-10, and IL-6) were determined by cytometric bead array (CBA) (Morgan and others 2004), according to the manufacturer's protocol (BD Biosciences) with minor modifications. Briefly, 25 μL of individual sera was used in duplicate for analysis, as previously described (Tárnok and others 2003). The concentrations of serum cytokines were quantified using the CellQuestPro and CBA software (Becton Dickinson) on a FACSCalibur cytometry (BD Biosciences).
Serological analysis of hepatitis
HBV-related HBsAg, HBsAb, HBeAg, and HBeAb were detected by a chemiluminescent microparticle immunoassay (CMIA) using an Abbott I 2000 automated chemiluminescence immunoassay analyzer (Abbott Laboratories) (Li and others 2010). The levels of serum ALT and AST were detected using a Biochemistry Automatic Analyzer (Roche Diagnostics). The amount of serum HBV DNA was measured by quantitative PCR assay using a luciferase quantization detection kit, following the protocols (Roche Amplicor). The detection limit of viral DNA was 300 copies/mL (Jiang and others 2010).
Treatment of HepG2.2.15 cells with IL-33
The human hepatoma cell line, HepG2.2.15, was maintained in Dubecco's modified Eagle's medium with 10% fetal bovine serum and 200 μg/mL G418 in 5% CO2-humidified air as previously described. Cells were cultured at a density of 1×105 cells per well in 12-well plates. Twenty-four hours after incubation, the culture medium was removed, and fresh culture media containing 1, 10, 50 and 100 ng/mL of IL-33 (eBioscience) were added, while the control group was refreshed with new culture media. After 2 days, supernatant from 3 flasks of each group were independently collected for determination of HBsAg, HBeAg, and HBV DNA, and cells were harvested by trypsin digestion and washed 3 times with phosphate buffered solution (pH 7.3).
Determination of HBsAg, HBeAg, and HBV DNA in supernatant
Supernatant from each flask was collected at different times after oxymatrine treatment and stored at −20°C until measurement. HBsAg and HBeAg were simultaneously detected by a CMIA using Abbott I 2000 automated chemiluminescence immunoassay analyzer (Abbott Laboratories). HBV DNA in the supernatant was purified with QIAamp DNA Mini Kit (QIAGEN Inc.) following the manufacturer's instruction.
Statistical analysis
The data are expressed as median and range unless specified. The differences between the groups were analyzed by Wilcoxon-rank sum test and Chi-square tests using SPSS 16.0 software. The relationship between variables was evaluated using the Spearman rank correlation test. A two-side P value <0.05 was considered statistically significant.
Results
The potential role of IL-33 in the pathogenic process of CHB was studied in a total of 33 CHB patients and 20 HC. There was no significant difference in the distribution of age and gender among both groups of subjects, but the concentrations of serum ALT and AST in patients with CHB were significantly higher than the HC (Table 1).
Table 1.
The Demographic and Clinical Characteristics of Subjects
| Parameters | CHB patients | Healthy controls |
|---|---|---|
| NO | 33 | 20 |
| Age (years) | ||
| Mean±SD | 35.7±9.8 | 32.4±8.1 |
| Median (range) | 37 (19–62) | 34 (21–55) |
| Sex N (%) | ||
| Male | 27 (88) | 16 (80) |
| Female | 6 (12) | 4 (20) |
| HBV DNA (log10 copies/mL) | ||
| Median (range) | 7.39a (5.6–9.9) | NA |
| ALT (U/L) | ||
| Median (range) | 135a (12–914) | 14 (5–26) |
| AST (U/L) | ||
| Median (range) | 104.5a (22–445) | 13 (8–21) |
| HBsAg,pos/neg | 33/0 | 0/20 |
| HBeAg,pos/neg | 33/2 | 0/20 |
| Anti-HBe,pos/neg | 1/32 | 0/20 |
Normal values: ALT ≤40 IU/L; AST ≤40 IU/L; HBV DNA ≤3 log10 copies/mL.
P<0.05 versus HC Data were expressed as median and range.
HBV, Hepatitis B virus; CHB, chronic hepatitis B.
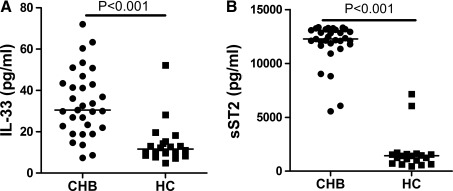
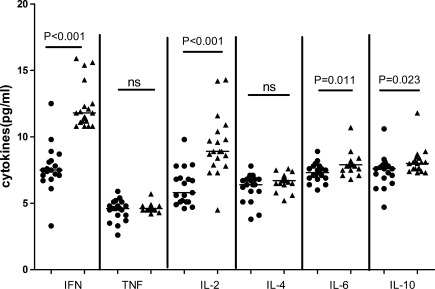
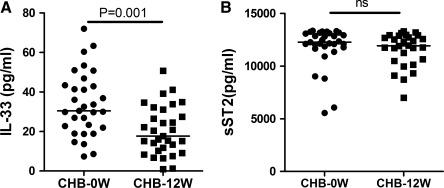
Analysis of serum cytokines indicated that the concentrations of serum IL-33 in CHB patients were significantly higher than the HC (P<0.001, Fig. 1A). Further, the study didn't found any correlation between serum IL-33 and abnormal levels of serum ALT or AST (r=0.113, P=0.552; r=0.123, P=0.519, respectively). Further analysis revealed that the levels of serum sST2 were significantly higher in CHB patients than the HC (P<0.001, Fig. 1B), but there was no significant correlation between the levels of serum IL-33 and sST2 (r=−0.011, P=0.952). Further, the concentrations of serum IFN-γ, IL-6, IL-2, and IL-10, but not TNF-α and IL-4, in CHB patients were significantly lower after 12 weeks of treatment (Fig. 2). Following treatment of CHB patients with adefovir dipivoxil for 12 weeks, 30 out of 33 CHB patients had dramatically reduced levels of serum HBV virus. The levels of serum IL-33 in patients with CHB significantly decreased after treatment with adefovir dipivoxil (P=0.001, Fig. 3A). Meanwhile, the 12 weeks treatment did not significantly change the levels of serum sST2 in CHB patients (P=0.202, Fig. 3B).
FIG. 1.
The levels of serum IL-33 and sST2. The concentrations of serum IL-33 and sST2 in individual CHB patients and HC determined by enzyme-linked-immunosorbent serologic assay. Data are expressed as the mean values of individual participants from two separate experiments. The horizontal lines indicate the median values of different groups. (A) The basal levels of serum IL-33; (B) The basal levels of serum sST2; HC, Healthy controls; CHB: Patients with CHB; CHB, chronic hepatitis B; sST2, soluble ST2.
FIG. 2.
The changes of serum Th1 and Th2 cytokines. The concentrations of serum Th1(IFN-γ, IL-2, TNF-α) and Th2(IL-4, IL-6, IL-10) in individual participants were determined by CBA. Data are expressed as the mean values of individual samples from two separate experiments. The horizontal lines show the median. • represent the level of serum cytokines in CHB patients before treatment; ▴ represent the level of serum cytokines in CHB patients after treatment.
FIG. 3.
The changes in the levels of serum IL-33 and sST2 in CHC patients with IFN treatment. Data are expressed as the mean values of individual participants from two separate experiments. The horizontal lines show the median. (A) The changes in the levels of serum IL-33. (B) The changes in the levels of serum sST2.
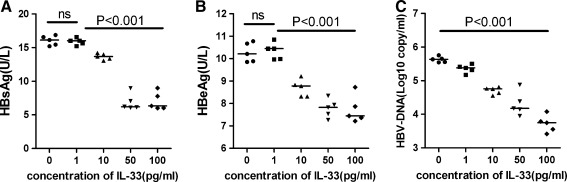
After treatment with different concentrations of IL-33 for 2 days, HBsAg and HBeAg secreted into the medium were significantly reduced in a dose-dependent manner (P=0.004, P<0.001, respectively, Fig. 4A, B). As demonstrated in Fig. 4C, treating HepG2.2.15 cells with IL-33 resulted in a significant reduction in hepatitis B virions in the supernatant in a dose-dependent manner (P<0.001).
FIG. 4.
Effect of IL-33 on the secretion of HBsAg and HBeAg and the replication of HBV-DNA in the HepG2.2.15 cell line. Data are expressed as the mean values of individual participants from the separate experiments. (A) After treatment with different concentration of IL-33 for 2 days, HBsAg in the supernatant significantly decreased in a manner of dose-independent; (B) HBsAg in the supernatant significantly decreased in a dose-independent manner; (C) the HBV-DNA significantly decreased in a dose-independent manner. HBV, Hepatitis B virus.
Discussions
IL-33 is a multifunctional cytokine involved in various disease conditions (Barksby and others 2007; Liew and others 2010; Mok and others 2010). IL-33, through the receptor complex composed of ST2 and IL-1RaP, can activate the MAP-kinase and NF-κB signaling pathways and promote Th2 response and cytokine production (Schmitz and others 2005). Indeed, intra-nasal administration of IL-33 triggered an immediate allergic response in the airway and endogenous IL-33 contributes to airway inflammation (Louten and others 2011). A recent study revealed that the levels of serum IL-33 were elevated in SLE and RA patients, and correlated with the levels of serum ESR and CRP, the two inflammation markers, indicating that IL-33 may participate in the acute phase response. (Yang and others 2011). In addition, IL-33 can protect against septic shock by enhancing neutrophil infiltration at the site of inflammation (Chackerian and others 2007). Other studies suggest that IL-33 participates in the pathogenic process of acute hepatitis induced by Con-A (Arshad and others 2011; Volarevic and others 2011) and IL-33 overexpression is associated with the development of HBV/HCV-related liver fibrosis (Marvie and others 2010). We are interested in further examining the mechanisms underlying the action of IL-33/sST2 axis in HBV-related liver injury. We found that the levels of serum IL-33 in CHB patients were significantly higher than the HC. Our results are consistent with recent studies demonstrating that IL-33 production is associated with chronic inflammation such as Crohn's disease (Carriere and others 2007). In addition, treatment with adefovir dipivoxil to inhibit the replication of HBV dramatically decreased the levels of serum IL-33 in CHB patients. These confirm that IL-33 can play a significant role in the progression of CHB and our data suggest that IL-33 may be a pathogenic factor in the pathogenic process of CHB patients. A recent study revealed that the level of IL-33 was correlated with activity of SLE (Yang and others 2011), but no correlation was found between the levels of IL-33 and ALT or AST in our study. Further, the levels of serum sST2 were significantly higher in CHB patients than the HC, but were not correlated with the levels of IL-33 in the patients. Treatment with adefovir dipivoxil for 12 weeks did not significantly change the levels of serum sST2. A previous study has shown that the levels of serum sST2 in acute liver failure patients are higher than patients with chronic liver failure and HC (Roth and others 2010). It is possible that high levels of serum sST2 are an early marker for liver injury, while high levels of serum IL-33 may be associated with the development and progression of liver fibrosis and damage (Marvie and others 2010). We are interested in further examining the mechanisms underlying the action of IL-33/sST2 axis in HBV-related liver injury.
We found that the levels of serum IFN-γ, IL-2, IL-6, and IL-10 in CHB patients were significantly higher after 12 weeks of treatment than the baseline, supporting the notion that pro-inflammatory cytokines, such as IFN-γ and IL-6 are not only important factors for the clearance of infected HBV, but also for liver injury (Frese and others 2002; Ghoneum and Matsuura 2004; Li and others 2004). The lower levels of IFN-γ, IL-2, IL-6, and IL-10 in CHB patients at baseline were unlikely to have come from the antagonization of IL-33-induced Th2 responses in those patients, because we failed to detect significant difference in the levels of serum IL-4 and TNF-α in CHB patients between baseline and after 12 weeks of treatment. Given that IL-6 is a critical factor in the functional development of Th17 cells (Chun and others 2007), and IFN-γ and IL-2 are effectors of Th1 response (Jiang and others 2010), the lower levels of serum IFN-γ, IL-2, and IL-6 in CHB patients at baseline indicated continual viral replication and pathogenic progression. IL-10 promotes the development of type 2 cytokine pattern by inhibiting IFN-γ production of T lymphocytes, particularly via suppression of IL-12 synthesis in accessory cells (Knapp and others 2003), and the low levels of serum IL-10 may be the result of multi-cytokines interaction. Although IL-33 has been shown to promote IFN-γ production by invariant NKT and NK cells (Bourgeois and others 2009), IL-33 may, through an unknown pathway, downregulate the functional development of HBV-related Th1 response and inhibit IFN-γ production. However, the precise mechanisms remain to be further investigated.
As indicated by our study results, secretion of HBsAg and HBeAg from HepG2.2.15 cells could be inhibited after incubation with a low concentration of IL-33 for 2 days, and this decrease was more significant following the increase of the dose of IL-33. The copy of viral DNA can also be inhabited by the IL-33 in a dose-dependent manner. HBsAg and HBeAg could be rapidly secreted into the blood and elicits T-cell tolerance (Rehermann and Nascimbeni 2005). Our research indicates that IL-33could alleviate host immune tolerance induced during HBV infection.
In conclusion, our data indicate that the concentrations of serum IL-33 are significantly higher in HBV infected patients than HC and are significantly decreased after 12 weeks of treatment, suggesting that IL-33 may be a pathogenic factor in HBV-related liver injury in CHB patients. The study found that IL-33 can inhabit the replication of HBV-DNA and the secretion of HBsAg and HBeAg by HepG2.2.15. The limitations of the study include the absence of source for IL-33 and histopathological examinations of liver tissues were not conducted. Although more detailed studies are necessary to determine the role and mechanisms of IL-33 in the pathogenic process of CHB, our novel findings may provide new insights into understanding the pathogenesis of CHB.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (No. 30972610), Jilin Province Science and Technology Agency (No. 200705128 and 20110716), the Health Department Research Projects in Jilin Province (2009Z054), the Cutting-edge Science and Interdisciplinary Innovation Projects of Jilin University, and the Novartis Sebivo Post Approval Commitment study.
Author Disclosure Statement
No competing financial interests exist.
References
- Arshad MI. Rauch M. L'helgoualc'h A. Julia V. Leite-de-Moraes MC. Lucas-Clerc C. Samson M. NKT cells are required to induce high IL-33 expression in hepatocytes during ConA-induced acute hepatitis. Eur J Immunol. 2011;41:2341–2348. doi: 10.1002/eji.201041332. [DOI] [PubMed] [Google Scholar]
- Barksby HE. Lea SR. Preshaw PM. Taylor JJ. The expanding family of interleukin-1 cytokines and their role in destructive inflammatory disorders. Clin Exp Immunol. 2007;149:17–25. doi: 10.1111/j.1365-2249.2007.03441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra A. Warke RV. de Bosch N. Rothman AL. Bosch I. Elevated levels of soluble ST2 protein in dengue virus infected patients. Cytokine. 2008;41:114–120. doi: 10.1016/j.cyto.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois E. Van LP. Samson M. Diem S. Barra A. Roga S. Gombert JM. Schneider E. Dy M. Gourdy P. Girard JP. Herbelin A. The pro-Th2 cytokine IL-33 directly interacts with invariant NKT and NK cells to induce IFN-gamma production. Eur J Immunol. 2009;39:1046–1055. doi: 10.1002/eji.200838575. [DOI] [PubMed] [Google Scholar]
- Carriere V. Roussel L. Ortega N. Lacorre DA. Americh L. Aguilar L. Bouche G. Girard JP. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci USA. 2007;104:282–287. doi: 10.1073/pnas.0606854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chackerian AA. Oldham ER. Murphy EE. Schmitz J. Pflanz S. Kastelein RA. IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. J Immunol. 2007;179:2551–2555. doi: 10.4049/jimmunol.179.4.2551. [DOI] [PubMed] [Google Scholar]
- Chun HY. Chung JW. Kim HA. Yun JM. Jeon JY. Ye YM. Kim SH. Park HS. Suh CH. Cytokine IL-6 and IL-10 as biomarkers in systemic lupus erythematosus. J Clin Immunol. 2007;27:461–466. doi: 10.1007/s10875-007-9104-0. [DOI] [PubMed] [Google Scholar]
- Crockett SD. Keefe EB. Natural history and treatment of hepatitis B virus and hepatitis C virus coinfection. Ann Clin Microbiol Antimicrob 13. 2005;4:13. doi: 10.1186/1476-0711-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falasca K. Ucciferri C. Dalessandro M. Zingariello P. Mancino P. Petrarca C. Pizzigallo E. Conti P. Vecchiet J. Cytokine patterns correlate with liver damage in patients with chronic hepatitis B and C. Ann Clin Lab Sci. 2006;36:144–150. [PubMed] [Google Scholar]
- Frese M. Schwärzle V. Barth K. Krieger N. Lohmann V. Mihm S. Haller O. Bartenschlager R. Interferon-gamma inhibits replication of subgenomic and genomic hepatitis C virus RNAs. Hepatology. 2002;35:694–703. doi: 10.1053/jhep.2002.31770. [DOI] [PubMed] [Google Scholar]
- Ghoneum M. Matsuura M. Augmentation of macrophage phagocytosis by modified arabinoxylan rice brain. Int J Immunopathol Pharmacol. 2004;17:283–392. doi: 10.1177/039463200401700308. [DOI] [PubMed] [Google Scholar]
- Gonzalez MI. Rubinstein N. Ilarregui JM. Toscano MA. Sanjuan NA. Rabinovich GA. Regulated expression of Galectin-1 after in vitro productive infection with herpes simplex virus type 1: implications for T cell apoptosis. Int J Immunopathol Pharmacol. 2005;18:615–623. doi: 10.1177/039463200501800402. [DOI] [PubMed] [Google Scholar]
- Grzegorzewska AE. Wobszal P. Jagodziński PP. Interleukin-18 promoter polymorphism and development of antibodies to surface antigen of hepatitis B virus in hemodialysis patients. Kidney Blood Press Res. 2011;35:1–8. doi: 10.1159/000329932. [DOI] [PubMed] [Google Scholar]
- Jiang Y. Ma Z. Xin G. Yan H. Li W. Xu H. Hao C. Niu J. Zhao P. Th1 and Th2 immune response in chronic hepatitis B patients during a long-term treatment with adefovir dipivoxil. Mediators Inflamm. 2010;2010:143026. doi: 10.1155/2010/143026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp S. Hennig BJ. Frodsham AJ. Zhang L. Hellier S. Wright M. Goldin R. Hill AV. Thomas HC. Thursz MR. Interleukin-10 promoter polymorphisms and the outcome of hepatitis C virus infection. Immunol Genetics. 2003;55:362–369. doi: 10.1007/s00251-003-0594-5. [DOI] [PubMed] [Google Scholar]
- Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, arid current and emerging prevention and control measures. J Viral Hepatitis. 2004;11:97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- Li A. Yuan Q. Huang Z. Fan J. Guo R. Lou B. Zheng Q. Ge S. Chen Y. Su Z. Yeo AE. Chen Y. Zhang J. Xia N. Novel double-antigen sandwich immunoassay for human hepatitis B core antibody. Clin Vaccine Immunol. 2010;17(3):464–469. doi: 10.1128/CVI.00457-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DH. Kumanogoh A. Cao TM. Parnes JR. Cullen JM. Woodchuck interleukin-6 gene: structure, characterization and biologic activity. Gene. 2004;342:157–164. doi: 10.1016/j.gene.2004.07.034. [DOI] [PubMed] [Google Scholar]
- Liew FY. Pitman NI. McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol. 2010;10:103–110. doi: 10.1038/nri2692. , Review. [DOI] [PubMed] [Google Scholar]
- Louten J. Rankin AL. Li Y. Murphy EE. Beaumont M. Moon C. Bourne P. McClanahan TK. Pflanz S. Endogenous IL-33 enhances Th2 cytokine production and T-cell responses duringallergic airway inflammation. Int Immunol. 2011;23:307–315. doi: 10.1093/intimm/dxr006. [DOI] [PubMed] [Google Scholar]
- Martini F. Agrati C. D'Offizi G. Poccia F. HLA-E up-regulation induced by HIV infection may directly contribute to CD94-mediated impairment of NK cells. Int J Immunopathol Pharmacol. 2005;18:269–276. doi: 10.1177/039463200501800209. [DOI] [PubMed] [Google Scholar]
- Marvie P. Lisbonne M. L'helgoualc'h A. Rauch M. Turlin B. Preisser L. Bourd-Boittin K. Theret N. Gascan H. Piquet-Pellorce C. Samson M. Interleukin-33 overexpression is associated with liver fibrosis in mice and humans. J Cell Mol Med. 2010;14:1726–1739. doi: 10.1111/j.1582-4934.2009.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagaki T. Sugaya M. Yokobayashi H. Kato T. Ohmatsu H. Fujita H. Saeki H. Kikuchi Y. Tamaki T. Sato S. High levels of soluble ST2 and low levels of IL-33 in sera of patients with HIV infection. J Invest Dermatol. 2011;131(3):794–796. doi: 10.1038/jid.2010.366. [DOI] [PubMed] [Google Scholar]
- Mok MY. Huang FP. Ip WK. Lo Y. Wong FY. Chan EY. Lam KF. Xu D. Serum levels of IL-33 and soluble ST2 and their association with disease activity in systemic lupus erythematosus. Rheumatology. 2010;49:520–527. doi: 10.1093/rheumatology/kep402. [DOI] [PubMed] [Google Scholar]
- Morgan E. Varro R. Sepulveda H. Ember JA. Apgar J. Wilson J. Lowe L. Chen R. Shivraj L. Agadir A. Campos R. Ernst D. Gaur A. Cytometric bead array: a multiplexed assay platform with applications in various areas of biology. Clin Immunol. 2004;110:252–266. doi: 10.1016/j.clim.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Rehermann B. Interaction between the hepatitis C virus and the immune system. Semin Liver Dis. 2000;20:127–141. doi: 10.1055/s-2000-9946. [DOI] [PubMed] [Google Scholar]
- Rehermann B. Nascimbeni M. Immunology of hepatitis b virus and hepatitis c virus infection. Nat Rev. 2005;5(3):215–229. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- Roth GA. Zimmermann M. Lubsczyk BA. Pilz J. Faybik P. Hetz H. Hacker S. Mangold A. Bacher A. Krenn CG. Ankersmit HJ. Up-regulation of interleukin 33 and soluble ST2 serum levels in liver failure. J Surg Res. 2010;163:e79–e83. doi: 10.1016/j.jss.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Schmitz J. Owyang A. Oldham E. Song Y. Murphy E. McClanahan TK. Zurawski G. Moshrefi M. Qin J. Li X. Gorman DM. Bazan JF. Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–484. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Tárnok A. Hambsch J. Chen R. Varro R. Cytometric bead array to measure six cytokines in twenty-five microliters of serum. Clin Chem. 2003;49:1000–1002. doi: 10.1373/49.6.1000. [DOI] [PubMed] [Google Scholar]
- Volarevic V. Mitrovic M. Milovanovic M. Zelen I. Nikolic I. Mitrovic S. Pejnovic N. Arsenijevic N. Lukic ML. Protective role of IL-33/ST2 axis in con A-Induced Hepatitis. J Hepatol. 2012;56:26–33. doi: 10.1016/j.jhep.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Yang Z. Liang Y. Xi W. Li C. Zhong R. Association of increased serum IL-33 levels with clinical and laboratory characteristics of systemic lupus erythematosus in Chinese population. Clin Exp Med. 2011;11:75–80. doi: 10.1007/s10238-010-0115-4. [DOI] [PubMed] [Google Scholar]
- Yim HJ. Lok AS. Natural history of chronic hepatitis B virus infection: what we knew in 1981 and what we know in 2005. Hepatology. 2006;43:S173–S181. doi: 10.1002/hep.20956. [DOI] [PubMed] [Google Scholar]