Abstract
Establishment of a rapid, highly specific, and accurate method for diagnosis of Toxoplasma gondii infection is essential to control and prevent zoonotic toxoplasmosis. In this study, a novel diagnostic strategy using magnetic bead-based serum peptide profiling by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) was developed. The serum peptides (samples I, II, and III) from T. gondii RH strain-infected mice at days 3, 6, and 9 post-infection (p.i.), and healthy mice were enriched by the optimized magnetic bead-based hydrophobic interaction (MB-HIC8). The mass spectrograms were acquired by MALDI-TOF MS, and analyzed by ClinProTools bioinformatics software from Bruker Daltonics. The diagnostic models from T. gondii RH-infected serum peptide profiling of samples I, II, and III were produced by genetic algorithms, and verified by cross-validation. The sample II model could correctly recognize T. gondii RH strain infection in mice at days 3, 6, and 9 p.i. with a sensitivity of 91.1% and a specificity of 96.7%., and also detect T. gondii ME49 strain-infected serum samples at days 3, 6, 9, and 12 p.i. with a sensitivity of 91.7%. The results of the present study suggest that serum peptide profiling by MALDI-TOF MS is a novel potential tool for the clinical diagnosis of acute T. gondii infection.
Key Words: MALDI-TOF MS, Mice, Serum peptide profiling, Toxoplasmosis
Introduction
Toxoplasmosis, caused by the protozoan parasite Toxoplasma gondii, is one of the most prevalent parasitic diseases of humans and other warm-blooded animals in the world. It has been reported that about one-third of the human population has been infected with this parasite (Kijlstra and Jongert 2008). Though T. gondii infection does not usually present with any clinical symptoms, it can cause serious outcomes, or disease in immunocompromised patients, pregnant women, and congenitally-infected children (Bohne et al. 1999; Yuan et al. 2007; Liu et al. 2009). The infection in animals often results in abortion, causes considerable economic losses, and is an important source of transmission to humans (Tenter et al. 2000). Therefore, surveillance of the infection by sensitive and specific methods is a key step toward control and prevention of toxoplasmosis in humans and animals.
In fact, detection of T. gondii infection is very difficult, due to its various clinical manifestations. Many diagnostic methods, including identification of the agent by molecular techniques, inoculation of laboratory animals and cell cultures, and immunologic assays, have been used for the diagnosis of toxoplasmosis in humans and animals (Buxton 1998). However, isolation of T. gondii is expensive and time-consuming (Salant et al. 2007). Polymerase chain reaction-based assays are not widely available for clinical applications because of the high false-positive and false-negative rates (Jalal et al. 2004). The specific antibodies to parasite antigens are detected to demonstrate T. gondii infection in humans and animals, but their sensitivity and specificity vary widely, due to the lysate antigen of the parasite used in the tests (Sensini 2006). It is necessary to develop more effective screening tests for toxoplasmosis.
Serum peptide levels can be altered in disease conditions, implicating that these molecules may be used as potential biomarkers and in the construction of diagnostic methods (Karpova et al. 2010). The mass spectrometer has been regarded as a highly accurate analysis of proteins or peptides, making high-throughput sample preparation possible. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) analysis of peptide or protein profiling in biological fluids is a new tool for the diagnosis of human disease, with the advantages of speed, ease of use, and accuracy (Fiedler et al. 2007; Mantini et al. 2010; Taneja et al. 2011; Xiao et al. 2011).
The objective of this study was to assess the feasibility of magnetic bead-based serum peptide profiling by MALDI-TOF MS in detecting T. gondii infection in a mouse model.
Materials and Methods
T. gondii infection in mice
Six-week-old BALB/c female mice, purchased from the Center of Experimental Animals, Jilin University, Changchun, China, were divided into two groups, including infection and healthy control groups. All mice in the infection group were intramuscularly injected with 100 μL of PBS containing 1000 T. gondii RH or ME49 strain tachyzoites, and the groups were divided into 3 and 4 subgroups, respectively. The mice in the healthy control group were administered PBS without parasite by the same route. All the infection subgroups and control group included a total of 30 mice. The animal experiments were performed and monitored in accordance with the guidelines established by the Animal Ethics Committee of the Academy of Military Medical Sciences in China.
Serum sample
Thirty serum samples from the infection subgroups and control group were collected at days 3, 6, 9, and 12 post-infection (p.i.), respectively. After sample collection, the sera were allowed to clot at room temperature for 1 h and centrifuged at 1500 g for 15 min. The recovery sera were divided into aliquots immediately and frozen at −80°C until use.
Serum pretreatment with magnetic beads
All serum samples were fractionated using different types of ClinProt™ microparticle beads (Bruker Daltonik GmbH, Bremen, Germany), including magnetic bead hydrophobic interaction (MB-HIC8), magnetic bead weak cation ion exchange (MB-WCX), and magnetic bead immobilized metal-affinity chromatography containing copper ions (MB-IMAC-Cu), to enrich serum peptides and generate a diversity of biomarker patterns. The serum samples were purified according to the manufacturer's instructions. Briefly, for MB-HIC8, 5 μL of homogenous magnetic beads were added to a PCR tube containing 10 μL binding buffer and 5 μL serum sample, and the solution was gently mixed. For MB-IMAC-Cu, 5 μL of homogenous magnetic beads were pretreated with 50 μL of binding buffer 3 times, and resuspended in 20 μL binding buffer, then 5 μL of serum sample was added. For MB-WCX, 10 μL of magnetic beads and 10 μL of binding buffer was transferred into a PCR tube and thoroughly mixed, then 5 μL of serum sample was added to the solution. The magnetic beads bound with peptide were separated from the supernatant by a magnetic separator, and washed twice with washing buffer. The peptides were eluted from the magnetic beads using elution buffer, and were transferred to a fresh tube for MS analysis.
Data acquisition with FlexControl software
Spectra from the infection and control groups were acquired by the software FlexControl using an Autoflex III smartbeam-MALDI-TOF MS. The instrument was operated in positive ion linear mode. Voltages of the ion source 1, ion source 2, and lens were set to 20.05, 18.95, and 6.52 kV, respectively, and the pulsed ion extraction time was 200 nsec; 200 shots were automatically accumulated for each spectrum.
The mass spectrometer was calibrated using the Bruker Protein Calibration Standard I Calibration kit after each 10 spectra.
Model generation and validation
ClinProTools software version 2.2 for Biomarker Detection and Evaluation (Bruker Daltonik) was used for normalization of a set of spectra derived from the T. gondii infection and control groups, internal signal alignment using prominent internal signal peaks, and a peak picking procedure using default settings was performed automatically. The pretreated data were used for visualization and statistical analysis in ClinProTools. A p value less than 0.05 was considered statistically significant.
The classification models for murine toxoplasmosis were generated by a genetic algorithm within the software suite, and cross-validation was performed to evaluate the reliability, and the specificity was also evaluated by detection of 30 serum samples from Leishmania donovani-positive mice.
Results
Serum samples
After the mice were injected with the T. gondii RH strain, they presented with clinical symptoms at day 4 p.i., including loss of appetite and depression. Some of the mice died at day 8 p.i., and the others presented with swollen abdomens, messy hair, and shortness of breath. The mice injected with the T. gondii ME49 strain showed clinical signs at day 8 p.i. and thereafter. All mice in the control group were healthy. The serum samples from the T. gondii RH-infection group collected at days 3, 6, and 9 p.i. were grouped into samples I, II, and III, which included all 30 samples. The serum samples from the T. gondii ME49-strain infection group were collected at days 3, 6, 9, and 12 p.i. The control group also contained 30 serum samples.
Screening of magnetic beads
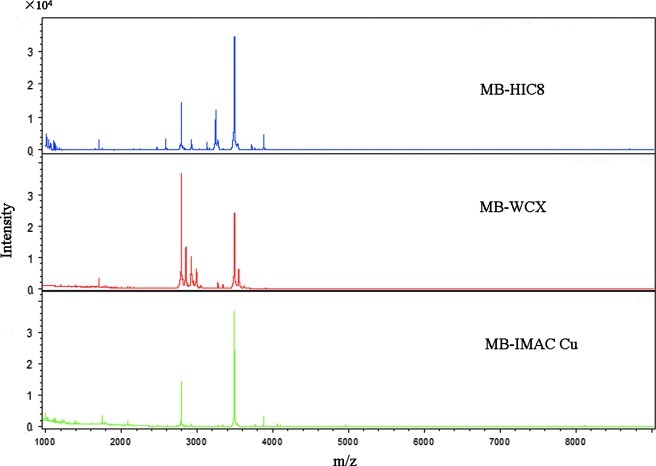
The serum samples from the T. gondii RH-infected sample III and control groups were purified using MB-HIC8, MB-WCX, and IMAC-Cu. Mass spectrometry analysis indicated that there were 69, 55, and 68 peaks, with signal-to-noise ratios >5, detected between mass to charge (m/z) 1000 and 10,000 in serum samples from the control group (Fig. 1).
FIG. 1.
MALDI-TOF mass spectra of serum peptides from the control group captured by MB-HIC8, MB-WCX, and MB-IMAC-Cu. Color images available online at www.liebertpub.com/vbz
By observing the sample distribution chart of the infection and control groups, there were small overlapping areas from the serum samples pretreated with MB-HIC8, which could accurately distinguish T. gondii infection from the control group (Supplementary Fig. 1; see online supplementary material at http://www.liebertonline.com). Therefore, MB-HIC8 was used to enrich serum peptides in this study.
Reproducibility of mass spectrometry
To check the reproducibility of the magnetic bead MALDI-TOF approach, serum samples from T. gondii-infected and healthy control mice was split into 5 subfractions. Each subfraction was processed separately using MB-HIC8, and MALDI-TOF spectra were acquired automatically. The spectra obtained were highly reproducible (data not shown), indicating that the mass spectra obtained following magnetic bead sample preparation are highly reproducible.
Model setting and validation
In order to generate a model that can discriminate T. gondii-infected from the healthy control mice, we explored serum peptide profiling of T. gondii RH strain samples I, II, and III, which were optimized by genetic algorithms and verified by cross-validation. The sample I model could not detect sample III, with sensitivity of only 3.3%, and the sample III model could not detect sample I, with sensitivity of only 13.3%, but the sample II model could detect sample I with sensitivity of 86.7%, and sample III with sensitivity of 93.3% (data not shown). As shown in Table 1, the sample II model could correctly distinguish T. gondii infection from healthy controls with high accuracy of 97.4% and sensitivity of 91.1%, better than the results from the sample I and III models (p<0.05).
Table 1.
Cross Validation of Models Generated from Samples I, II, and IIIa
| Model | Group | No. of positive | No. of negative | No. of invalid spectrab | Correct classified (%)c | Sensitivity (%)d | Specificity (%)e |
|---|---|---|---|---|---|---|---|
| Sample I | Infection | 41 | 44 | 5 | 61.4 | 45.6 | 96.7 |
| Control | 29 | 1 | |||||
| Sample II | Infection | 82 | 3 | 5 | 97.4 | 91.1 | 96.7 |
| Control | 29 | 1 | |||||
| Sample III | Infection | 61 | 21 | 8 | 81.1 | 67.8 | 96.7 |
| Control | 29 | 1 |
Samples I, II, and III are the sera collected from the T. gondii-infected mice at days 3, 6, and 9, respectively.
Number of invalid spectra is the percentage of the correctly classified part of valid spectra.
Correct classified is the percentage of the correctly classified part of valid spectra.
The sensitivity is the percentage of the correctly classified positives.
The specificity is the percentage of the correctly classified negatives.
All sera from L. donovani-infected mice were classified as “0” (not infected with T. gondii) using the sample II model, suggesting high specificity of this model. The serum samples from T. gondii ME49-infected mice were detected by the method, with an overall sensitivity of 91.7% (110/120), and sensitivities of 83.3% (25/30) in the serum samples at day 3 p.i., 96.7% (29/30) in the serum samples at day 6 p.i., and 93.3% (28/30) in the serum samples at days 9 and 12 p.i.
Discussion
Conventional methods, such as ELISA and rapid immunochromatography, can detect excretory/secretory antigens, which account for the majority of the circulating antigens in sera from hosts with acute toxoplasmosis, with a sensitivity of 80% at day 3 p.i. (Wang et al. 2010). Using magnetic bead-based serum peptide profiling by MALDI-TOF MS, we could detect T. gondii infection in mice at days 3, 6, and 9 p.i., with sensitivities of 86.7%, 96.7%, and 93.3%, respectively. In addition, it takes only 20 min to complete the detection of a single sample, and 384 samples can be detected in one test using serum peptide profiling by MALDI-TOF MS. These results suggest that it is a rapid, highly-specific, and accurate method for the diagnosis of T. gondii infection.
MALDI-TOF MS has been used for the early diagnosis of infection and disease from biological samples such as serum, urine, cerebrospinal fluid, saliva, and tears (Pitarch et al. 2004; Castano et al. 2006; Seng et al. 2010). However, highly concentrated components may suppress minor components, and similar m/z ratio peptides and proteins may result in overlapping peaks, so direct mass spectrometry analysis of serum samples often produces spectra of poor quality. Therefore, selective enrichment of specific peptides according to their biological, chemical, or physical properties can significantly improve spectrum quality by using magnetic beads with different types of surface characteristics (Qiu et al. 2009). We used MB-HIC8 to enrich serum peptides, and this produced a reliable diagnostic model.
MALDI-TOF MS can also be used to identify biomarkers. In the diagnostic model for T. gondii infection in mice, 69 peaks in the mass range 1000–10,000 m/z were detected, and a proteomic pattern containing five different protein peaks was established, which allowed detection of T. gondii infection at day 3 p.i. Among the five peaks, a peak with m/z of 2535.56 was upregulated, while the other four peaks with m/z of 3297.00, 3726.07, 3904.43, and 5022.20 were downregulated, in T. gondii-infected mice. Identification of these biomarkers might provide new insights into the development of toxoplasmosis, which could contribute to the development of new diagnostic strategies. Unfortunately, these enriched protein peaks are too low to be further identified by MS/MS. We will separate the differential peaks for MS/MS, which may reveal the origin and true nature of the peaks.
The established diagnostic model for murine toxoplasmosis cannot be used in the detection of T. gondii infection in other animals, due to the differing serum peptide profiles in different animals. Diagnostic models based on serum peptide profiling from other animals, including humans and farm animals, will be established and evaluated for their potential as a novel diagnostic strategy for toxoplasmosis in the future.
Supplementary Material
Acknowledgments
This work was supported by grants from the Chinese National Key Technology R&D Program (no. 2010BAD04B01), the Chinese National Nature Science Foundation (no. 30972178, 31001057, and 31072127), and the Chinese National Programs for High Technology Research and Development (no. 2011AA10A215).
Author Disclosure Statement
No competing financial interests exist.
References
- Bohne W. Holpert M. Gross U. Stage differentiation of the protozoan parasite Toxoplasma gondii. Immunobiology. 1999;201:248–254. doi: 10.1016/S0171-2985(99)80065-5. [DOI] [PubMed] [Google Scholar]
- Buxton D. Protozoan infections (Toxoplasma gondii, Neospora caninum and Sarcocystis spp.) in sheep and goats: recent advances. Vet Res. 1998;29:289–310. [PubMed] [Google Scholar]
- Castano EM. Roher AE. Esh CL, et al. Comparative proteomics of cerebrospinal fluid in neuropathologically-confirmed Alzheimer's disease and non-demented elderly subjects. Neurol Res. 2006;28:155–163. doi: 10.1179/016164106X98035. [DOI] [PubMed] [Google Scholar]
- Fiedler GM. Baumann S. Leichtle A, et al. Standardized peptidome profiling of human urine by magnetic bead separation and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Clin Chem. 2007;53:421–428. doi: 10.1373/clinchem.2006.077834. [DOI] [PubMed] [Google Scholar]
- Jalal S. Nord CE. Lappalainen M, et al. Rapid and sensitive diagnosis of Toxoplasma gondii infections by PCR. Clin Microbiol Infect. 2004;10:937–939. doi: 10.1111/j.1469-0691.2004.00948.x. [DOI] [PubMed] [Google Scholar]
- Karpova MA. Moshkovskii SA. Toropygin IY, et al. Cancer-specific MALDI-TOF profiles of blood serum and plasma: biological meaning and perspectives. J Proteomics. 2010;73:537–551. doi: 10.1016/j.jprot.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Kijlstra A. Jongert E. Control of the risk of human toxoplasmosis transmitted by meat. Int J Parasitol. 2008;38:1359–1370. doi: 10.1016/j.ijpara.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Liu Q. Wei F. Gao S, et al. Toxoplasma gondii infection in pregnant women in China. Trans R Soc Trop Med Hyg. 2009;103:162–166. doi: 10.1016/j.trstmh.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Mantini D. Petrucci F. Pieragostino D, et al. A computational platform for MALDI-TOF mass spectrometry data: application to serum and plasma samples. J Proteomics. 2010;73:562–570. doi: 10.1016/j.jprot.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Pitarch A. Abian J. Carrascal M, et al. Proteomics-based identification of novel Candida albicans antigens for diagnosis of systemic candidiasis in patients with underlying hematological malignancies. Proteomics. 2004;4:3084–3106. doi: 10.1002/pmic.200400903. [DOI] [PubMed] [Google Scholar]
- Qiu F. Liu HY. Zhang XJ, et al. Optimization of magnetic beads for MALDI-TOF MS analysis. Front Biosci. 2009;14:3712–3723. doi: 10.2741/3482. [DOI] [PubMed] [Google Scholar]
- Salant H. Markovics A. Spira DT, et al. The development of a molecular approach for coprodiagnosis of Toxoplasma gondii. Vet Parasitol. 2007;146:214–220. doi: 10.1016/j.vetpar.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Seng P. Rolain JM. Fournier PE, et al. MALDI-TOF-mass spectrometry applications in clinical microbiology. Future Microbiol. 2010;5:1733–1754. doi: 10.2217/fmb.10.127. [DOI] [PubMed] [Google Scholar]
- Sensini A. Toxoplasma gondii infection in pregnancy: opportunities and pitfalls of serological diagnosis. Clin Microbiol Infect. 2006;12:504–512. doi: 10.1111/j.1469-0691.2006.01444.x. [DOI] [PubMed] [Google Scholar]
- Taneja S. Ahmad I. Sen S, et al. Plasma peptidome profiling of acute hepatitis E patients by MALDI-TOF/TOF. Proteome Sci. 2011;9:5. doi: 10.1186/1477-5956-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenter AM. Heckeroth AR. Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30:1217–1258. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YH. Li XR. Wang GX, et al. Development of an immunochromatographic strip for the rapid detection of Toxoplasma gondii circulating antigens. Parasitol Int. 2010;60:105–107. doi: 10.1016/j.parint.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Xiao D. Meng FL. He LH, et al. Analysis of the urinary peptidome associated with Helicobacter pylori infection. World J Gastroenterol. 2011;17:618–624. doi: 10.3748/wjg.v17.i5.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z. Gao S. Liu Q, et al. Toxoplasma gondii antibodies in cancer patients. Cancer Lett. 2007;254:71–74. doi: 10.1016/j.canlet.2007.02.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.