Abstract
Background
Numerous studies find that socially disadvantaged women are more likely than socially advantaged women to deliver infants that weigh less than normal and/or are born weeks prior to their due date. However, little is known about the pathways that link maternal social disadvantage to birth outcomes. Using data from a prospective cohort study, we examined whether antenatal psychosocial stress, substance use, and maternal health conditions in pregnancy mediated the pathway between maternal social disadvantage and birth outcomes.
Methods
Analyses used structural equation modeling to examine data from a community clinic-based sample (n=2168) of pregnant women who completed questionnaires assessing psychosocial functioning and health behaviors as well as sociodemographic characteristics, which were matched with subsequent birth outcome data.
Results
Analyses revealed maternal social disadvantage predicted poorer birth outcomes through a mediated pathway including maternal health conditions in pregnancy.
Conclusions
The findings demonstrate that maternal social disadvantage is associated with poor health status in pregnancy, which in turn adversely affects birth outcomes. Results argue for more systematic attention to the roles of social disadvantage, including life course perspectives that trace social disadvantage prior to and through pregnancy.
Introduction
The purpose of this study was to examine the pathways that link maternal social disadvantage and birth outcomes including infant birth weight and gestational age at birth. In the United States, infants born weighing less than 2500 grams have higher mortality and morbidity rates than infants born at normal weight.1,2 Despite improvements in obstetric care over the past two decades, the overall rate of low birth weight increased from 6.9% in 1990 to 8.2% in 2009.3 Low birth weight has also been linked with health and chronic disease risk across the life course. Studies show that low weight at birth in combination with weight gain across the life course is associated with cardiovascular events in adulthood.4,5 Low birth weight has also been linked to chronic health conditions in adulthood including obesity,6 type 2 diabetes,7 and hypertension.8,9
Infants born prior to 37 completed weeks of gestation represent a significant public health concern because premature birth is the leading cause of neonatal deaths not associated with birth defects.10 In the United States more than one third of all infant deaths are estimated to be preterm related.11 The risk of adverse outcome declines as gestational age increases; however, infants born between 34 and 36 weeks are less healthy than infants born at term.12,13 Notably, over the period 1990–2009 the percentage of infants born early has increased in the United States from 10.6% to 12.1%.14 It has been suggested that the increase in infants born prior to 37 completed weeks gestation is due to the rise in multiple births, changes to labor and delivery procedures, advanced maternal age, and maternal health conditions (e.g., obesity). However, the exact causes of the increase in rates of early term infants are unclear.13
Infants with decreased fetal growth and lower gestational age at birth are frequently born to women with certain sociodemographic and health behavioral characteristics, including younger and older maternal age, African American race, chronic health conditions, low socioeconomic status (SES), smoking, and illicit drug use.2 However, the independent effects of these maternal factors in pregnancy explain only a small proportion of disparities in birth outcomes.
One factor that may in part explain disparities in birth outcomes is stress. Stress has been a frequently studied risk factor for decreased fetal growth and lower gestational age at birth because maternal psychological health has the potential to directly or indirectly affect the uteroplacental/fetal environment.15,16 Studies predominantly find a positive association between antenatal stress and adverse birth outcomes. However, research also shows that not all women who report high levels of antenatal stress deliver infants with lower birth weight and lower gestational age.17 This finding suggests these outcomes may in part result from differential vulnerability to stress.
Evidence is mounting that among certain subpopulations of pregnant women, exposure to social and economic disadvantage across the life course may compromise reproductive health, thus reducing the chances of delivering healthy infants.18–20 From this perspective, the prevalence of decreased birth weight and lower gestational age may be related not only to stress exposure during pregnancy, but also to the stress response that has been patterned by exposures to chronic and repeated stress across the life course.21 Specifically, this relationship may be the case among women from socially and economically disadvantaged backgrounds, who are more likely to report higher levels of psychosocial stress.22
In the present study we examine whether maternal social disadvantage operates in conjunction with known risk factors or independent of them to influence birth weight and gestational age at birth. Numerous studies have highlighted the indicators of maternal social disadvantage during the antenatal period (e.g., poverty, low education, and not living with a partner) that are associated with decreased fetal growth and lower gestational age at birth.23–26 However, less is known about the pathways that link these indicators of maternal social disadvantage to birth outcomes.27 Our ability to effectively address social and economic disparities in birth outcomes may be improved if studies model the independent as well as the interrelated pathways that link social, psychological, behavioral, and biological factors during and prior to pregnancy to adverse birth outcomes.28
Several potential pathways linking maternal social disadvantage and offspring birth outcomes have been suggested.29 First, repeated exposure to psychosocial stress may “weather” a woman's endocrine, immune, and reproductive systems, increasing the likelihood that she will deliver a premature and low birth weight infant.30 Both animal and experimental models have indicated that exposure to chronic and repeated stress result in elevated basal cortisol levels and dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis.31 Hyperactivity of the HPA axis may reflect the inability of a “worn-out” HPA axis to self-regulate owing to chronically elevated cortisol levels.31 Maternal exposures to psychosocial stress can result in increased release of norepinephrine and cortisol, which then activates placental corticotropin-releasing hormone gene expression. Elevated maternal plasma levels of this hormone have been linked to spontaneous preterm delivery and fetal growth restriction.32
Second, women experience psychosocial stress to varying degrees based on their position in the social and economic hierarchy.33 Social patterning indicates that individuals of low SES are exposed to a greater number and variety of stressful experiences, adverse interpersonal relationships, and lower perceptions of control.34 Psychosocial processes involved in responding to stressful events can amplify effects, such as diminishing optimism and fostering negative cognitive and affective states to a greater extent among those in lower SES conditions.35,36 Further, maternal depression, a condition related to stress, is prevalent among women of childbearing age because the onset of depression is often intertwined with reproductive events.37 There is evidence that antenatal depression is linked to negative birth outcomes.38 Further, women with low SES who reported antenatal depression tended to give birth to infants who were born prior to 37 weeks of completed gestation,38 which may be related to the higher prevalence of antenatal depression among economically disadvantaged women.39
Antidepressants are the most commonly prescribed medication in pregnancy.40 Almost 8% of pregnant women were prescribed antidepressants between 2004 and 2005, reflecting a nearly fourfold increase in use since 1996.41 There is growing evidence that antenatal exposure to antidepressants is associated with negative birth outcomes.42,43 Also, studies have shown women who use antidepressants during pregnancy are more likely to have lower levels of education attainment, are more often non-cohabitating, and are less likely to be employed full-time.42,44
A third potential pathway links the effects of social disadvantage to birth outcomes via maternal substance use. Maternal smoking during pregnancy, for example, is considered an important modifiable cause of adverse birth outcomes.45,46 In the United States, antenatal smoking is strongly associated with low levels of maternal education,46 with women reporting 12 or less years of education having the highest prevalence.47 During pregnancy, non-cohabitation is also associated with antenatal smoking.48 Chronic exposure to psychosocial stress as a function of social disadvantage may also contribute to the adoption or reinforce behavioral coping mechanisms such as cigarette smoking and drug use49,50 that may influence birth weight and gestational age at birth.46,51
Another pathway exists between maternal social disadvantage and maternal health conditions. Women with low SES report higher levels of chronic illnesses during pregnancy.52,53 Studies have also shown that pregnancy-related health conditions and maternal health conditions in pregnancy contribute to an increased risk of adverse birth outcomes.54–59
Using data from a prospective cohort study, we examined the pathways linking maternal social disadvantage to birth outcomes. Weathering theory posits that social disadvantage influences health outcomes through physical embodiment mechanisms as well as psychosocial and health behavior pathways. Consistent with this theorizing, we investigated whether antenatal psychosocial stress, antenatal substance use, or maternal health conditions in pregnancy mediated the pathway between maternal social disadvantage and offspring birth outcomes.
Materials and Methods
Procedures
Questionnaires measuring antenatal psychiatric disorders, including major depression symptoms based on DSM-IV diagnostic criteria, as well as sociodemographic and psychosocial factors, were distributed by clinical staff as part of routine clinical care. Questionnaires were self-administered and patients could complete them in either English or Spanish, with interpreters available to patients who spoke neither language. Exclusion criteria for the study included age less than 15 years at the time of delivery, no ongoing prenatal care, and inability to complete the clinical questionnaire due to mental incapacitation or language difficulties (i.e., no interpreter available). Written consent was obtained in order to examine questionnaire data as well as automated medical records. All study procedures were approved by the University of Washington Institutional Review Board.
Sample
Data stem from a longitudinal study of women who received prenatal care at a single university academic medical center from January 2004 to March 2010. The clinic serves a diverse group of women with respect to race, SES, and medical risk, with 46.5% reporting private health insurance coverage and 51.6% reporting publically funded health insurance.60 The percentage of those who declined to participate was low (6.8%). The final sample for the present analysis included 2168 women with complete information on infant birth outcomes. Due to Health Insurance Portability and Accountability Act regulations, it was not possible to compare characteristics of participants with the women who declined enrollment or were not approached by clinic staff to participate in the study.
Measures
Birth outcomes
Infant birth weight was recorded at the time of delivery. Gestational age at birth was estimated from the date of the first day of the last menstrual period (LMP) and verified by a first or second trimester ultrasound. If LMP was unknown or differed significantly from ultrasound, the ultrasound age was given preference. Analyses were conducted on the birth weight in grams and gestational age at birth in weeks for infants. Based on its distribution in the study, birth weight was standardized.
Social disadvantage
Indicators included partner status (living or not living with a partner/spouse), educational attainment (less than high school, some high school, high school graduate, some college, college graduate, and graduate school or more), and employment status (employed part or full time, electively not in labor force, or unemployed). Coding was in a negative direction to indicate social disadvantage (e.g., lower education reflected greater disadvantage). Maternal age was not conceptualized to be a social disadvantage indicator, but it was included separately as a measured variable to control for its contribution to the model.
Psychosocial stress
The Patient Health Questionnaire (PHQ) short form (15 items) was used to assess the severity of antenatal depression symptoms based on DSM-IV diagnostic criteria.61 As a depression symptoms severity measure, PHQ scores can range from 0 to 27, based on the nine items that comprise the depression module, which are scored from “not at all” (0) to “nearly every day” (3).62 Antenatal stress was measured using the Prenatal Psychosocial Profile stress scale, which is a validated measure for use in pregnant populations.63,64 The stress scale had 11 items, including financial worries, family problems, and recent loss of a loved one. Respondents were asked to what extent each event was an issue for them, ranging from no stress (0) to severe stress (3). In the present study, both antenatal depression symptoms and stress were summed and treated as continuous variables. Antenatal psychiatric medication use, self-reported at the time of the questionnaire, distinguished women who used prescribed antidepressants (n=140) or other psychiatric medication (n=40) during pregnancy from those taking no psychiatric medication (1=yes, 0=no).
Antenatal substance use
Antenatal smoking and drug use were measured using the Smoke-Free Families Prenatal Screen65 and the Drug CAGE.66 In the present study, tobacco use response categories were coded as 0=never smoked/prior smoker and 1=current smoker. Drug use was also coded as 0=never used illicit drugs during pregnancy and 1=used drugs during this period.
Maternal health conditions in pregnancy
Women were asked to self-report if in the 3 years prior to their pregnancy they had one or more of the following chronic medical conditions: asthma, hypertension, arthritis, diabetes, thyroid disorders, migraines, gastrointestinal disorders, cancer, seizure disorders, heart failure/other heart disease, or a physical disability. Data from automated medical records were used to identify women who experienced hypertensive or diabetic conditions during pregnancy. Women with pregnancy-related hypertension or pre-existing hypertension in combination with pregnancy-related hypertension were considered to have antenatal hypertensive conditions. Women with pregnancy-related diabetes were considered to have diabetic conditions in pregnancy. Self-report of chronic medical conditions has been shown to have validity compared with physician report.67 For this study, we included all the listed medical conditions in the measure, which was coded as an index with a range from 0 (no health conditions in pregnancy) to 8.
Analytic strategy
The hypothesized intergenerational relations between maternal social disadvantage, the mediators, and birth outcomes were evaluated using maximum likelihood estimation in Mplus 5.68 Modeling was done in two stages. First, we evaluated the measurement model by conducting a confirmatory factor analysis (CFA) of maternal social disadvantage, psychosocial stress, antenatal substance use, and offspring birth outcomes. Second, we analyzed the hypothesized structural relations between latent maternal factors and birth outcomes. We report three indicators of model fit assessment: the chi-square estimate (χ2), the comparative fit index (CFI), and the root mean square error of approximation (RMSEA).
Missing data strategy
Multiple imputation procedures in Stata69 were not used to generate the descriptive statistics with missing data (Table 1). However, we evaluated the CFA and the structural relations among model constructs in Mplus using weighted least squares, which employs maximum likelihood estimation with robust standard errors using numeric integration to impute missing data.
Table 1.
Descriptive Statistics for Study Variables in the Sample
| Variablea | Mean (SD) or % |
|---|---|
| Infant characteristics | |
| Gestational age at birth (weeks) | 38.65 (2.55) |
| Birth weight (g) | 3250 (693.66) |
| Maternal characteristics | |
| Age (years) | 30.5 (6.13) |
| Marital status | |
| Married or living with a partner | 87.7% |
| Not living with a partner | 12.2% |
| Employment status | |
| Full or part-time | 55.5% |
| Not in the labor force | 44.5% |
| Educational attainment (years) | |
| ≤8 | 1.8% |
| 9–11 | 8.4% |
| 12 | 9.4% |
| 13–15 | 22.0% |
| 16 | 25.7% |
| ≥17 | 32.4% |
| Antenatal psychosocial stress | 14.73 (3.89) |
| Antenatal depression | 3.57 (3.94) |
| Antenatal psychiatric medication use | 8.3% |
| Antenatal smoking | 7.1% |
| Antenatal drug use | 1.7% |
| Health conditions in pregnancy | 1.81 (1.81) |
Gestational age range, 18.57–46.42 weeks; birth weight range, 109–5198 grams; maternal age range, 15–53 years; psychosocial stress scores range, 11–41; Patient Health Questionnaire (depression) scores range, 0–24; health conditions in pregnancy range, 0–8.
Results
Descriptive and confirmatory factor analyses
Table 1 shows descriptive statistics for all study variables. On average, gestational age of respondents' infants at birth was 38.65 weeks (SD=2.55) and mean birth weight was 3250 g (SD=693.66). At enrollment, respondents were 30.5 years of age on average (SD=6.13, range: 13–53 years). Most of the women in our sample were married or living with a partner, and about half were employed full or part-time in the early stage of their pregnancy. Approximately 20% had a high school education or less, and about one third reported advanced education (beyond 4 years of college). The mean PHQ depression symptoms score was 3.57 (SD=3.94) and the mean stress score was 14.73 (SD=3.89), indicating that there were low levels of depression symptoms and antenatal stress among the sample. The prevalence of psychiatric medication use in pregnancy was 8.3% and 7.1% of the respondents reported smoking during pregnancy. The prevalence of drug use during pregnancy was low (1.7%). On average, respondents reported nearly two chronic health conditions during pregnancy.
The CFA of the constructs indicates support for the hypothesized measurement model. Model fit was adequate (χ2=59.13, df=19, CFI=0. 98, RMSEA=0.031) and all indicators loaded significantly onto their respective latent factors. Table 2 presents the results of the bivariate correlation analyses of the study variables. In general, variables anticipated to have significant association with variables in theorized pathways were consistent with expectations (e.g., social disadvantage indicators and psychosocial stress indicators).
Table 2.
Bivariate Correlations Among Study Variablesa
| Latent variables and mediators | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1. Maternal social disadvantage | — | |||||
| 2. Offspring birth outcomes | −0.139*** | — | ||||
| 3. Psychosocial stress | 0.485*** | −0.119*** | — | |||
| 4. Antenatal substance use | 0.787*** | −0.180** | 0.561*** | — | ||
| 5. Health conditions in pregnancy | 0.162*** | −0.262*** | 0.262*** | 0.180*** | — | |
| 6. Maternal age | −0.612*** | 0.019 | −0.164*** | −0.423*** | 0.054* | — |
All standardized correlation coefficients were estimated using maximum likelihood estimation.
p<0.05.
p<0.01.
p<0.001.
Structural equation model
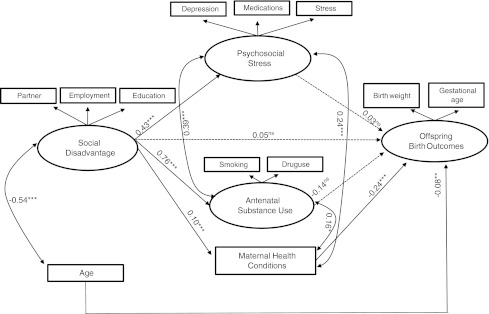
The structural equation model examined the effects of social disadvantage on birth outcomes, as well as psychosocial stress, antenatal substance use, and health conditions in pregnancy as mediators. Figure 1 presents the final model showing the effects of these constructs on birth outcomes. Fit of this model was satisfactory (CFI=0.951, RMSEA=0.050, χ2=184.57, df=29, p<0.001).
FIG. 1.
The SEM and the standardized model parameter estimates. Coefficients are standardized regression estimates (β). Solid lines represent regression paths that are significant at p<0.05 or greater; dotted lines are not significant. Single-headed arrows are regression paths; double-headed arrows are correlations. ns, nonsignificant; *p<0.05; ** p<0.01; *** p<0.001.
Social disadvantage significantly predicted greater psychosocial stress in pregnancy (β=0.43, p<0.001), substance use in pregnancy (β=0.76, p<0.001), and more health conditions in pregnancy (β=0.10, p<0.001). Psychosocial stress was significantly correlated with antenatal substance use (β=0.39, p<0.001) and maternal health conditions (β=0.24, p<0.001) as was substance use with maternal health (β=0.16, p<0.05). Social disadvantage showed a significant indirect pathway to offspring birth weight and gestational age at birth through health conditions in pregnancy (β=−0.025, p<0.001).
Health conditions in pregnancy predicted lower offspring weight and gestational age at birth (β=−0.24, p<0.001). Social disadvantage, psychosocial stress, and antenatal substance use were all significantly correlated with birth outcomes at the bivariate level (Table 2). Although in the expected direction, they did not achieve significance in the multivariate model (Fig. 1).
Discussion
This paper supports the importance of stress factors as significant contributors to women's reproductive health, including risk of adverse birth outcomes represented by low birth weight and lower gestational age at birth. Findings supported hypotheses that distal stress stemming from maternal social disadvantage conveys significant risk to health during pregnancy and to adverse birth outcomes. This study extends epidemiological findings, adding to our understanding of the erosive pathways through which stress contributes to persistent health disparities related to offspring low birth weight and lower gestational age at birth.
The finding that maternal social disadvantage was not directly associated with birth outcomes is consistent with some studies,23–26 but not other earlier findings that demonstrated direct effects.20,70 One reason for our data showing no direct effect may be the magnitude of effects represented through mediated pathways. Specifically, social disadvantage manifests significant pathways directly to maternal psychosocial stress, substance use, and health, as well as indirectly to birth outcomes mediated through maternal health. Cumulatively, social disadvantage demonstrated considerable influence in this model on pregnancy health and birth outcomes. Thus, although social disadvantage characteristics are not always changeable within the context of pregnancy, these characteristics are often associated with factors that are mutable,71,72 as is the case with our findings related to stressful event exposures, depression, and substance use.
Worsening trends in birth outcomes have been associated with increasing maternal age, particularly for women with socially disadvantaged backgrounds.33,73 We too found that older mothers had more negative birth outcomes, indicated by the direct pathway from maternal age, a trend most likely due to health conditions that affect offspring outcomes. Moreover, younger mothers also appeared at risk for negative birth outcomes. Bivariate correlations demonstrated that younger women were more likely to use illicit substances, have elevated psychosocial stress, and have higher levels of social disadvantage. Thus, younger and older women are likely to need special, yet differential attention to address their risk considerations. Educational, support, and care coordination services that attend to psychosocial and socioeconomic factors in addition to clinical health indicators appear promising.74
Use of psychiatric medications as a stress intervention requires caution. There is evidence suggesting that antenatal exposure to antidepressant medication is associated with increased risk of adverse birth outcomes.75,76 Our findings indicate a fit for antenatal psychiatric medications within a psychosocial stress factor, which is significantly associated with both substance use and impaired maternal health. Our findings suggest that social interventions, instead of or in concert with the use of psychopharmaceutical medications, may provide more direct stress relief stemming from social disadvantage and proximal life stressors, thus attenuating the strength of these risk factors.
The significant associations of social disadvantage to pregnancy health, and indirectly to adverse birth outcomes through pregnancy health, are consistent with the literature on allostatic load, the wear and tear on the body's adaptive systems leading to dysfunction over time.17,77 Exposure to chronic and repeated stress results in elevated basal cortisol levels and dysregulation of the HPA axis,78 which in turn may lead to immune-inflammatory dysregulation.31 Dysregulation of the HPA axis and the immune system are two possible mechanisms by which exposure to chronic stress may contribute to preterm labor.79 Women experience allostatic load to varying degrees based on their position in the economic and social hierarchy. For example, women from low SES backgrounds and those who are unmarried (not living with a partner) appear to be more likely to experience maternal health conditions in pregnancy, which may contribute to their increased risk of delivering lower birth weight and/or lower gestational age infants.54–59
The presence of significant effects here by both social disadvantage and health conditions in pregnancy reflect this dual layering of stress sources and the adverse implications for birth outcomes. As noted, cumulative stress models point to dysregulatory cascades across physiological systems, transferring effects of stress to birth outcomes. In this light, conditions such as lower offspring birth weight and lower gestational age at birth are profitably viewed as chronic diseases,80 both in their own right as outcomes but also as “wayside markers” of the chronic disease process and eroding health status that leads to subsequent health disorders. From a life course stress load perspective, adverse birth outcomes are a manifestation of the chronic disease process by which endocrine and immune systems may be affected by the cumulative effect of maternal social disadvantage prior to conception.
Although both maternal psychosocial stress and social disadvantage were significantly associated with substance use during pregnancy, the pathway from substance use to birth outcomes did not achieve significance. This stands in contrast to findings that have demonstrated important links between smoking and drug use to adverse birth outcomes.45,46,51 Possible explanations for this divergent result include measurement limitations (e.g., the inability to compare self-reports of antenatal drug use to biological data) that did not adequately capture substance use in pregnancy, as well as shared variance with psychosocial distress, such as self-medicating as a stress coping behavior. Therefore, the pathways from psychosocial stress to birth outcomes may be indirectly capturing correspondence with substances such as tobacco and illicit substance use. Histories of smoking and alcohol and drug use prior to pregnancy have demonstrated significant prediction of subsequent adverse birth outcomes,18 suggesting the value of their inclusion in research and screening on the basis of life course contributors.
These findings argue for the utility of considering adverse birth outcomes in stress embodiment terms. As with other health outcomes, there are multiple pathways through which cumulative stressors may impact pregnancy outcomes, such as behavioral, neuroendocrine, immune, and vascular mechanisms, that have established links to adverse health outcomes. Like other stress-related dysregulation of physiologic systems, the female reproductive axis is vulnerable to the “wear and tear” of cumulative stress. Rich-Edwards and Grizzard80 point to cascade effects that may be involved, such as adverse kindling or priming effects of stress on the HPA axis that yield stress arousal pathology, leading to problematic vascular responses to stress. Although an argument that “more bad things are worse” has intuitive logic, stress is neither inherently nor universally injurious. The stress response system is designed to galvanize and support systems to cope with threats, serving to protect the organism and preserve health, even potentially fostering adversarial growth.81,82 Our findings suggest this nuance, with both positive and negative pathways from stress to birth outcomes. For example, women with higher levels of stress and lower levels of coping and support resources are more likely to be prescribed psychiatric medications, whereas those with greater supports and less impaired stress response systems are more likely to effectively manage their stress and experience better health outcomes. To date, limited inclusion of protective factors in research related to adverse birth outcomes precludes full testing of constructs such as health hardiness or resilience relative to birth outcomes.
The findings from this study should be interpreted in light of several limitations. First, respondents were recruited from a community-based sample from a single geographic region. Although the sample characteristics included a range of social disadvantage characteristics, the representation of women with low SES and single women is lower than in some regions. Given the role of clinic staff in consenting women to participate in the study and Health Insurance Portability and Accountability Act regulations, we did not have data on who was approached to participate in the study. Thus, our study results may not be fully generalizable to other populations of pregnant women. In addition, detailed information was not available on prior mental health conditions, medications, income, and nonparticipants. Further, self-reported antenatal drug use in the sample was low. However, we were unable to compare maternal self reports with biological data, which may have resulted in under-reporting. Finally, we did not have complete information on antenatal medication use for the entire cohort, and therefore we could not determine the specific link between individual medication and risk of adverse birth outcome.
These findings direct attention to accessible targets during pregnancy—such as social and behavioral interventions to reduce economic strain stemming from social disadvantage, reduce psychosocial stressors and associated emotional distress, and augment personal resources that serve to buffer stress (e.g., reducing social isolation, increasing instrumental supports). One set of interventions focuses on stress relief and management. Supportive evidence found in the general population is being mirrored in applications with pregnant women. Examples include muscle and guided imagery relaxation, breathing retraining, meditative exercises such as yoga, and mindfulness interventions.83–87 Social disadvantage and stress obviously carry a range of potential targets. Services to support smoking and drug or alcohol cessation, interpersonal treatment for depression or other emotional disorders, specialized services such as shelter or case management for violence-exposed women, and medical assistance or other economic supports are among those suggested by the current findings.88,89 Pivotal to success is routine screening for economic and psychosocial issues (e.g., stress, depression, violence) during each trimester in pregnancy as well as the postpartum period.
Conclusions
Although adverse birth outcomes continue to rise and interventions designed to reduce antenatal stress are in their infancy, the role of stress and its potential as an intervention target have not yet been sufficiently explored. The current results support the premise that maternal social disadvantage prior to and during pregnancy is likely to affect inequalities in relation to pregnancy health and adverse birth outcomes. Moreover, social disadvantage is strongly associated with psychosocial stress and antenatal substance use, which significantly contribute to greater maternal health conditions. Moreover, these adverse birth outcomes set the stage for inequalities in infant health as well as subsequent life course health impairment.90
Acknowledgments
This publication was made possible by grant number 1KL2RR025015-01 from the National Center for Research Resources, a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research.
Author Disclosure
No competing financial interests exist.
References
- 1.Hamilton B. Martin J. Ventura S. Births: preliminary data for 2007. National Vital Statistics Reports. http://www.cdc.gov/nchs/data/nvsr/nvsr57/nvsr57_12.pdf. [Jul 17;2011 ]. http://www.cdc.gov/nchs/data/nvsr/nvsr57/nvsr57_12.pdf [PubMed]
- 2.Goldenberg R. Culhane J. Low birth weight in the United States. Am J Clin Nutr. 2007;85(Suppl):584S–590S. doi: 10.1093/ajcn/85.2.584S. [DOI] [PubMed] [Google Scholar]
- 3.Martin J. Hamilton B. Sutton P. Ventura S. Mathews M. Osterman M. Births: final data for 2008. National Vital Statistics Reports. http://www.cdc.gov/nchs/data/nvsr/nvsr59/nvsr59_01.pdf. [Jul 17;2011 ]. http://www.cdc.gov/nchs/data/nvsr/nvsr59/nvsr59_01.pdf [PubMed]
- 4.Rich-Edwards J. Kleinman K. Michels K, et al. Longitudinal study of birth weight and adult body mass index in predicting risk of coronary heart disease and stroke in women. BMJ. 2005;330:e1–e6. doi: 10.1136/bmj.38434.629630.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker D. Eriksson J. Forsen T. Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31:1235–1239. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- 6.Oken E. Gillman M. Fetal origins of obesity. Obes Res. 2003;11:496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- 7.Whincup P. Kaye S. Owen C. Birth weight and risk of type 2 diabetes: a systematic review. JAMA. 2008;300:2886–2897. doi: 10.1001/jama.2008.886. [DOI] [PubMed] [Google Scholar]
- 8.Gamborg M. Byberg L. Rasmussen F, et al. Birth weight and systolic blood pressure in adolescence and adulthood: meta-regression analysis of sex- and age-specific result from 20 Nordic studies. Am J Epidemiol. 2007;166:634–645. doi: 10.1093/aje/kwm042. [DOI] [PubMed] [Google Scholar]
- 9.Mzayek F. Hassig S. Sherwin R, et al. The association of birth weight with developmental trends in blood pressure from childhood through mid-adulthood. Am J Epidemiol. 2007;166:413–420. doi: 10.1093/aje/kwm098. [DOI] [PubMed] [Google Scholar]
- 10.Centers for DiseaseControl and Prevention. Healthy People 2000 Review 1998–99. Hyattsville, MD: National Center for Health Statistics; 2000. [Google Scholar]
- 11.Behrman RE, editor; Butler AS, editor. Preterm birth: causes, consequences, and prevention. Committee on understanding premature birth and assuring healthy outcomes, Board on Health Sciences Policy. Institute of Medicine. Washington, DC: National Academies Press; 2005. [Google Scholar]
- 12.Ramachandrappa A. Jain L. Health issues of the late preterm infant. Pediatr Clin North Am. 2009;56:565–588. doi: 10.1016/j.pcl.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Engle W. Kominiarek M. Late preterm infants, early term infants and timing of elective deliveries. Clin Perinatol. 2006;35:325–341. doi: 10.1016/j.clp.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton B. Martin J. Ventura S. National Vital Statistics Reports. 3. Vol. 59. Hyattsville, MD: National Center for Health Statistics; 2010. Births: Preliminary Data for 2009. [PubMed] [Google Scholar]
- 15.Austin M. Leader L. Maternal stress and obstetric and infant outcomes: epidemiological findings and neuroendocrine mechanisms. Aust NZ J Obstet Gynaecol. 2000;40:331–337. doi: 10.1111/j.1479-828x.2000.tb03344.x. [DOI] [PubMed] [Google Scholar]
- 16.Dole N. Savitz DA. Hertz-Picciotto IH. Siega-Riz AM. McMahon MJ. Beukens P. Maternal stress and preterm birth. Am J Epidemiol. 2003;157:14–24. doi: 10.1093/aje/kwf176. [DOI] [PubMed] [Google Scholar]
- 17.Hobel C. Culhane J. Role of psychosocial and nutritional stress on poor pregnancy outcome. J Nutr. 2003;133:1709S–1717S. doi: 10.1093/jn/133.5.1709S. [DOI] [PubMed] [Google Scholar]
- 18.Gavin AR. Hill KG. Hawkins DJ. Maas C. The role of maternal early life and later life risk factors on offspring low birth weight: findings from a three-generational study. J Adolesc Health. 2011;49:166–171. doi: 10.1016/j.jadohealth.2010.11.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins JW. Wambach J. David RJ. Rankin KM. Women's lifelong exposure to neighborhood poverty and low birth weight: a population-based study. Matern Child Health J. 2009;13:326–333. doi: 10.1007/s10995-008-0354-0. [DOI] [PubMed] [Google Scholar]
- 20.Colen C. Geronimus A. Bound J, et al. Maternal upward mobility and black-white disparities in infant birthweight. Am J Public Health. 2006;96:2032–2039. doi: 10.2105/AJPH.2005.076547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu M. Haflon N. Racial and ethnic disparities in birth outcomes: a life-course perspective. Matern Child Health J. 2003;7:13–30. doi: 10.1023/a:1022537516969. [DOI] [PubMed] [Google Scholar]
- 22.Bloch JR. Webb DA. Mathews L. Fitzpatrick E. Bennett IM. Culhance JF. Beyond marital status: the quality of the mother-father relationship and its influence on reproductive health behaviors and outcomes among unmarried low income pregnant women. Matern Child Health J. 2010;14:726–734. doi: 10.1007/s10995-009-0509-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah P. Zao J. Ali S. Maternal marital status and birth outcomes: a systematic review and meta-analyses. Matern Child Health J. 2010:e1–e13. doi: 10.1007/s10995-010-0654-z. [DOI] [PubMed] [Google Scholar]
- 24.Young R. Declercq E. Implications of subdividing marital status: are unmarried mothers with partners different from unmarried mothers without partners? An exploratory analysis. Matern Child Health J. 2010;14:209–214. doi: 10.1007/s10995-009-0450-9. [DOI] [PubMed] [Google Scholar]
- 25.Mortensen L. Diderichsen F. Davey Smith G. Nybo Andersen A. Time is on whose side? Time trends in the association between maternal social disadvantage and offspring fetal growth. A study of 1,409,399 births in Denmark, 1981–2004. J Epidemiol Community Health. 2009;63:281–285. doi: 10.1136/jech.2008.076364. [DOI] [PubMed] [Google Scholar]
- 26.Kramer M. Séguin L. Lydon J. Goulet L. Socio-economic disparities in pregnancy outcome: why do the poor fare so poorly? Paediatr Perinat Epidemiol. 2000;14:194–210. doi: 10.1046/j.1365-3016.2000.00266.x. [DOI] [PubMed] [Google Scholar]
- 27.Goldenberg R. Culhane J. Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Misra D. Guyer B. Allston A. Integrated perinatal health framework: a multiple determinants model with a life span approach. Am J Prev Med. 2003;25:65–75. doi: 10.1016/s0749-3797(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 29.Love C. David RJ. Rankin KM. Collins JW., Jr Exploring weathering: effects of lifelong economic environment and maternal age on low birth weight, small for gestational age, and preterm birth in African-American and white women. Am J Epidemiol. 2010;172:127–134. doi: 10.1093/aje/kwq109. [DOI] [PubMed] [Google Scholar]
- 30.McEwen B. Stellar E. Stress and the individual. Mechanisms leading to disease. Arch Intern Med. 1993;153:2093–2101. [PubMed] [Google Scholar]
- 31.Chrousos GP. The stress response and immune function: clinical implications. The 1999 Novera H. Spector Lecture. Ann NY Acad Sci. 2000;917:38–67. doi: 10.1111/j.1749-6632.2000.tb05371.x. [DOI] [PubMed] [Google Scholar]
- 32.Erickson K. Thorsen P. Chrousos G, et al. Preterm birth: associated neuroendocrine, medical, and behavioral risk factors. J Clin Endocrinol Metab. 2001;86:2544–2552. doi: 10.1210/jcem.86.6.7607. [DOI] [PubMed] [Google Scholar]
- 33.Geronimus AT Black/white differences in the relationship of maternal age to birthweight: a population-based test of the weathering hypothesis. Soc Sci Med. 1996;42:589–597. doi: 10.1016/0277-9536(95)00159-x. [DOI] [PubMed] [Google Scholar]
- 34.Evans GW. Kim P. Multiple risk exposure as a potential explanatory mechanism for the socioeconomic-status-health gradient. Ann NY Acad Sci. 2010;1186:174–189. doi: 10.1111/j.1749-6632.2009.05336.x. [DOI] [PubMed] [Google Scholar]
- 35.Adler NE. Snibbe AC. The role of psychosocial processes in explaining the gradient between socioeconomic status and health. Curr Dir Psychol Sci. 2004;12:119–123. [Google Scholar]
- 36.Gallo LC. Matthews KA. Understanding the association between socioeconomic status and physical health: do negative emotions play a role? Psych Bull. 2003;129:10–51. doi: 10.1037/0033-2909.129.1.10. [DOI] [PubMed] [Google Scholar]
- 37.Melville J. Gavin A. Guo Y. Fan M-Y. Katon W. Depressive disorders during pregnancy: prevalence and risk factors in a large urban sample. Obstet Gynecol. 2010;116:1064–1070. doi: 10.1097/AOG.0b013e3181f60b0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grote NK. Bridge JA. Gavin AR. Melville JL. Iyengar S. Katon WJ. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67:1012–1024. doi: 10.1001/archgenpsychiatry.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scholle SH. Haskett RF. Hanusa BH. Pincus HA. Kupfer DJ. Addressing depression in obstetrics/gynecology practice. Gen Hosp Psychiatry. 2003;25:83–90. doi: 10.1016/s0163-8343(03)00006-9. [DOI] [PubMed] [Google Scholar]
- 40.Kendall-Tackett K. Hale T. The use of antidepressants in pregnant and breastfeeding women: a review of recent studies. J Hum Lact. 2010;26:187–195. doi: 10.1177/0890334409342071. [DOI] [PubMed] [Google Scholar]
- 41.Andrade S. Raebel M. Brown J. Lane K, et al. Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol. 2008;198:194.e1–194.e5. doi: 10.1016/j.ajog.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 42.Reis M. Kallen B. Delivery outcome after maternal use of antidepressant drugs in pregnancy: an update using Swedish data. Psychol Med. 2010;40:1723–1733. doi: 10.1017/S0033291709992194. [DOI] [PubMed] [Google Scholar]
- 43.Lund N. Pedersen L. Henriksen T. Selective serotonin reuptake inhibitor exposure in utero and pregnancy outcomes. Arch Pediatr Adolesc Med. 2009;163:949–954. doi: 10.1001/archpediatrics.2009.164. [DOI] [PubMed] [Google Scholar]
- 44.Calderon-Margalit R. Qiu C. Ornoy A. Siscovick D. Williams M. Risk of preterm delivery and other adverse perinatal outcomes in relation to maternal use of psychotropic medications during pregnancy. Am J Obstet Gynecol. 2009;201(6):e1–e8. doi: 10.1016/j.ajog.2009.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Behrman RE, editor; Butler AS, editor. Preterm Birth: Causes, Consequences, and Prevention. Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes. Washington, DC: National Academies Press; 2007. [PubMed] [Google Scholar]
- 46.Cnattingius S. The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob Res. 2004;6(Suppl 2):S125–S140. doi: 10.1080/14622200410001669187. [DOI] [PubMed] [Google Scholar]
- 47.Tong V. Jones J. Dietz P. D'Angelo D. Bombard J. Trends in smoking before, during, and after pregnancy—Pregnancy Risk Assessment Monitoring System (PRAMS), United States, 31 sites, 2000–2005. MMWR Surveill Summ. 2009;58:1–29. [PubMed] [Google Scholar]
- 48.Orr S. Blazer D. Orr C. Maternal prenatal depressive symptoms, nicotine addiction, and smoking-related knowledge, attitudes, beliefs, and behaviors. Matern Child Health J. 2011:e1–e6. doi: 10.1007/s10995-011-0822-9. [DOI] [PubMed] [Google Scholar]
- 49.Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lindenberg CS. Reiskin HK. Gendrop SC. The social stress model of substance abuse among childbearing-age women: a review of the literature. J Drug Educ. 1994;24:253–268. doi: 10.2190/HH29-4Q1V-WK1D-DT4H. [DOI] [PubMed] [Google Scholar]
- 51.Schempf AH. Strobino DM. Illicit drug use and adverse birth outcomes: is it drugs or context? J Urban Health. 2008;85:858–873. doi: 10.1007/s11524-008-9315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silva L. Coolman M. Steegers E, et al. Maternal educational level and risk of gestational hypertension: the Generation R Study. J Human Hypertension. 2008;22:483–492. doi: 10.1038/jhh.2008.22. [DOI] [PubMed] [Google Scholar]
- 53.Anna V. van der Ploeg H. Cheung N. Huxley R. Bauman A. Sociodemographic correlates of the increasing trend in prevalence of gestational diabetes mellitus in a large population of women between 1995 and 2005. Diab Care. 2008;31:2288–2293. doi: 10.2337/dc08-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Josefsson A. Kernell K. Nielsen N. Bladh M. Sydsjo G. Reproductive patterns and pregnancy outcomes in women with congenital heart disease—a Swedish population- based study. Acta Obstet Gynecol Scand. 2011;90:659–665. doi: 10.1111/j.1600-0412.2011.01100.x. [DOI] [PubMed] [Google Scholar]
- 55.Potti S. Jain N. Mastrogiannis D. Dandolu V. Obstetric outcomes in pregnant women with diabetes versus hypertensive disorders versus both. J Matern Fetal Neonatal Med. 2011:e1–e4. doi: 10.3109/14767058.2011.580403. [DOI] [PubMed] [Google Scholar]
- 56.Cripe S. Frederick I. Qiu C. Williams M. Risk of preterm delivery and hypertensive disorders of pregnancy in relation to maternal co-morbid mood and migraine disorders during pregnancy. Paediatr Perinat Epidemiol. 2011;25:116–123. doi: 10.1111/j.1365-3016.2010.01182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Borthen I. Eide M. Veiby G. Daltveit A. Gilhus N. Complications during pregnancy in women with epilepsy: population-based cohort study. BJOG. 2009;116:1736–1742. doi: 10.1111/j.1471-0528.2009.02354.x. [DOI] [PubMed] [Google Scholar]
- 58.Dombrowski M. Asthma and pregnancy. Obstet Gynecol. 2006;108:667–681. doi: 10.1097/01.AOG.0000235059.84188.9c. [DOI] [PubMed] [Google Scholar]
- 59.Allen V. Joseph K. Murphy K. Magee L. Ohlsson A. The effect of hypertensive disorders in pregnancy on small for gestational age and stillbirth: a population based study. BMC Pregnancy Childbirth. 2004;4:17. doi: 10.1186/1471-2393-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bentley SM. Melville JL. Berry BD, et al. Implementing a clinical and research registry in obstetrics: overcoming the barriers. Gen Hosp Psychiatry. 2007;29:192–198. doi: 10.1016/j.genhosppsych.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 61.Spitzer R. Kroenke K. Williams J. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 62.Kroenke K. Spitzer RL. Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Curry MA. Burton D. Fields J. The prenatal psychosocial profiles: a research and clinical tool. Res Nurs Health. 1998;21:211–219. doi: 10.1002/(sici)1098-240x(199806)21:3<211::aid-nur4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 64.Curry MA. Campbell RA. Christian M. Validity and reliability testing of the prenatal psychosocial profile. Res Nurs Health. 1994;17:127–135. doi: 10.1002/nur.4770170208. [DOI] [PubMed] [Google Scholar]
- 65.Melvin CL. Tucker P. Measurement and definition for smoking cessation intervention research: the smoke-free families experience; smoke-free families common evaluation measures for pregnancy and smoking cessation projects working group. Tob Control. 2000;9(Suppl III):87–90. doi: 10.1136/tc.9.suppl_3.iii87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Midanik LT. Zahnd EG. Klein D. Alcohol and drug CAGE screeners for pregnant, low- income women: the California perinatal needs assessment. Alcohol Clin Exp Res. 1998;22:121–125. [PubMed] [Google Scholar]
- 67.Oksanen T. Kivimaki M. Pentti J. Virtanen M. Klaukka T. Vahtera J. Self-report as an indicator of incident disease. Ann Epidemiol. 2010;20:547–554. doi: 10.1016/j.annepidem.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 68.Muthén LK. Muthén BO. Mplus Users Guide. 5th. Los Angeles: Muthén & Muthén; 1998–2007. [Google Scholar]
- 69.StataCorp. Stata Statistical Software: Release 10. College Station, TX: StataCorpLP; 2007. [Google Scholar]
- 70.Astone N. Misra D. Lynch C. The effect of maternal socio-economic status throughout the lifespan on infant birthweight. Paediatr Perinat Epidemiol. 2007;21:310–318. doi: 10.1111/j.1365-3016.2007.00821.x. [DOI] [PubMed] [Google Scholar]
- 71.Hall R. Prevention of premature birth: do pediatricians have a role? Pediatrics. 2000;105:1137–1140. doi: 10.1542/peds.105.5.1137. [DOI] [PubMed] [Google Scholar]
- 72.Kramer M. Seguin L. Lydon J. Goulet L. Socio-economic disparities in pregnancy outcome: why do the poor fare so poorly? Paediatr Perinat Epidemiol. 2001;14:194–210. doi: 10.1046/j.1365-3016.2000.00266.x. [DOI] [PubMed] [Google Scholar]
- 73.Collins JW. Simon DM. Jackson TA, et al. Advancing maternal age and infant birth weight among urban African Americans: the effect of neighborhood poverty. Ethn Dis. 2006;16:180–186. [PubMed] [Google Scholar]
- 74.Salihu HM. Mbah AK. Jeffers D. Alio AP. Berry L. Healthy start program and feto-infant morbidity outcomes: evaluation of program effectiveness. Matern Child Health J. 2009;13:56–65. doi: 10.1007/s10995-008-0400-y. [DOI] [PubMed] [Google Scholar]
- 75.Davis R. Rubanowixe D. McPhillips H. Raebel M. Andrade S. Smith D, et al. Risks of congenital malformations and perinatal events among infants exposed to antidepressant medications during pregnancy. Pharmacoepidemiol Drug Saf. 2007;16:1086–1094. doi: 10.1002/pds.1462. [DOI] [PubMed] [Google Scholar]
- 76.Oberlander T. Warburton W. Misri S. Aghajanian J. Hertzman C. Effects of timing and duration of gestational exposure to serotonin reuptake inhibitor antidepressants: population-based study. Br J Psychiatry. 2008;193:338–343. doi: 10.1192/bjp.bp.107.037101. [DOI] [PubMed] [Google Scholar]
- 77.Stewart JA. The detrimental effect of allostatic: allostatic load as a measure of cumulative stress. J Physiol Anthropol. 2006;25:133–145. doi: 10.2114/jpa2.25.133. [DOI] [PubMed] [Google Scholar]
- 78.Kristenson M. Kucinskiene Z. Bergdahl B, et al. Increased psychosocial strain in Lithuanian versus Swedish men: the LiVicordia study. Psychosom Med. 1998;60:277–282. doi: 10.1097/00006842-199805000-00011. [DOI] [PubMed] [Google Scholar]
- 79.Lu M. Kotelchuck M. Hogan V. Jones L. Wright K. Halfon N. Closing the black-white gap in birth outcomes: a life-course approach. Ethn Dis. 2010;20(Suppl 2):s2-62–s2-76. [PMC free article] [PubMed] [Google Scholar]
- 80.Rich-Edwards J. Grizzard T. Psychosocial stress and neuroendocrine mechanisms in preterm delivery. Am J Obstet Gyncol. 2005;90:S30–35. doi: 10.1016/j.ajog.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 81.Linley PA. Joseph S. Positive change following trauma and adversity: a review. J Trauma Stress. 2004;17:11–21. doi: 10.1023/B:JOTS.0000014671.27856.7e. [DOI] [PubMed] [Google Scholar]
- 82.McEwan B. Protective and damaging effects of stress mediators: central role of the brain. New Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 83.Jallo N. Bourguignon C. Taylor AG. Utz SW. Stress management during pregnancy: designing and evaluating a mind-body intervention. Fam Community Health. 2008;31:190–203. doi: 10.1097/01.FCH.0000324476.48083.41. [DOI] [PubMed] [Google Scholar]
- 84.Narendran S. Nagarathna R. Narendran V, et al. Efficacy of yoga on pregnancy outcomes. J Altern Complementary Med. 2005;11:237–244. doi: 10.1089/acm.2005.11.237. [DOI] [PubMed] [Google Scholar]
- 85.Teixeira J. Martin D. Prendiville O, et al. The effects of acute relaxation on indices of anxiety during pregnancy. J Psychosom Obstet Gynecol. 2005;26:271–276. doi: 10.1080/01674820500139922. [DOI] [PubMed] [Google Scholar]
- 86.Urizar GG. Milazzo M. Le H, et al. Impact of stress reduction instructions on stress and cortisol levels during pregnancy. Biol Psychiatry. 2004;67:275–282. doi: 10.1016/j.biopsycho.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 87.Vieten C. Astin J. Effects of a mindfulness-based intervention during pregnancy on prenatal stress and mood: results of a pilot study. Arch Womens Ment Health. 2008;11:67–74. doi: 10.1007/s00737-008-0214-3. [DOI] [PubMed] [Google Scholar]
- 88.Grote NK. Swartz HA. Geibel SL. Zuckoff A. Houck PR. Frank E. A randomized controlled trial of culturally relevant, brief interpersonal psychotherapy for perinatal depression. Psychiatr Serv. 2009;60:313–321. doi: 10.1176/appi.ps.60.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hobel C. Goldstein A. Barrett E. Psychosocial stress and pregnancy outcome. Clin Obstet Gynecol. 2008;51:333–348. doi: 10.1097/GRF.0b013e31816f2709. [DOI] [PubMed] [Google Scholar]
- 90.Gisselmann M. The influence of maternal childhood and adulthood social class on the health of the infant. Soc Sci Med. 2006;63:1023–1033. doi: 10.1016/j.socscimed.2006.03.015. [DOI] [PubMed] [Google Scholar]